Abstract
The objective of this study was to determine how individuals poststroke respond to user-driven treadmill (UDTM) control in terms of walking speeds, peak anterior ground reaction forces (AGRF), peak posterior ground reaction forces (PGRF), and trailing limb angles (TLA). Twenty individuals with chronic stroke walked overground during a 10-meter walk test to determine their self-selected (SS) speeds before walking on a treadmill in its fixed-speed (FSTM) and UDTM control modes at their SS and fastest comfortable (Fast) speeds. Paired t-tests were used to compare the walking speeds, peak AGRF, peak PGRF, and TLA among test conditions (α=0.05). Participants selected similar SS (p>0.05) and faster Fast walking speeds (p<0.05) with the UDTM control compared to the FSTM control. There were no changes in their peak AGRF or PGRF for either limb or speed between UDTM and FSTM conditions (p>0.05). Individuals used greater paretic TLA at SS speeds with UDTM control (p<0.05). There was no difference in the AGRF required at Fast speeds with FSTM and UDTM control even though participants selected faster speeds with UDTM control. In work with young, healthy adults, we found that the treadmill control condition did not affect the amount of forward propulsion needed. Therefore, it is likely that when walking with UDTM control, individuals poststroke adjust their posture to make better use of their forward propulsion. This means they can reach faster walking speeds without increasing their push-off forces. Future work should assess how to most effectively prescribe UDTM control for gait training programs.
Keywords: Stroke, User-driven treadmill control, Treadmill-based gait training
INTRODUCTION
Each year, nearly 800,000 Americans suffer a stroke (Benjamin et al., 2017), which changes their muscle activation and movement patterns and decreases their walking speeds (Mayo et al., 1999). Increased walking speeds correspond to improved quality of life for community-dwelling older adults and indicate decreased fall risks and increased community involvement for stroke survivors (Abellan van Kan et al., 2009; Fritz and Lusardi, 2009). Therefore, increasing walking speed is often a key outcome of poststroke rehabilitation (Bohannon et al., 1991).
Although increasing walking speed is often a goal of poststroke rehabilitation and previous work suggests gait deficits can be targeted to achieve increased speeds, there is no consensus on the best approach to improve walking function for stroke survivors (Dickstein, 2008). Treadmill-based training studies have shown mixed results, with only 50% of individuals poststroke able to ambulate independently (Jørgensen et al., 1995). It has been suggested that walking at fast speeds and directly targeting mechanisms of propulsion, such as anterior ground reaction forces and trailing limb angles, may improve outcomes after treadmill-based training (Awad et al., 2014b; Kesar et al., 2011b; Lamontagne and Fung, 2004).
“Traditional” or fixed-speed treadmill (FSTM) control is commonly used for treadmill-based training paradigms because it allows a high number of task-specific repetitions at a relatively low cost (Langhorne et al., 2009; Sullivan et al., 2002). However, it can limit stride-to-stride variation in motor patterns which has been linked to improved motor recovery (Stergiou and Decker, 2011). We have developed a user-driven treadmill (UDTM) control algorithm that retains the repetitive training nature of FSTM walking while responding to an individual’s propulsion mechanics in real-time and allowing increased kinematic variability compared to FSTM control (Kempski et al., 2018). This controller adjusts the speed of the treadmill belt(s) in real-time in response to the user’s anterior-posterior ground reaction forces, trailing limb angle in the form of step length, and their position relative to the center of the treadmill (Ray et al., 2018). In addition, young, healthy adults selected speeds with this UDTM control that were faster than their speeds with FSTM control and similar to their overground walking speeds (Ray et al., 2018). Training at faster walking speeds during poststroke rehabilitation can be linked to improved walking function (Dickstein, 2008).
This study implements novel real-time, adaptive UDTM treadmill control and compares the response of stroke survivors to FSTM and UDTM control. We examined the self-selected and fastest comfortable walking speeds individuals selected with each controller. We hypothesized that stroke survivors would select faster walking speeds with UDTM control than with FSTM control as did our cohort of young, healthy adults (Ray et al., 2018). To achieve these faster walking speeds with the UDTM control, we expected individuals to increase their forward propulsion by increasing their peak anterior ground reaction forces, peak posterior ground reaction forces, and trailing limb angles (Awad et al., 2014a; Moore et al., 1993; Peterson et al., 2010).
METHODS
Data Collection
20 individuals poststroke participated in this study (Table 1). The protocol was approved by the University of Delaware Institutional Review Board, and each participant completed a written consent form. The protocol included two sessions, a Baseline Session and an Evaluation Session. All participants were between 21 and 85 years of age and had experienced a single, unilateral stroke at least 6 months prior to enrolling (Table 2). During the Baseline Session, participants completed the 6-minute walk test (6MWT) without assistance and demonstrated passive ankle dorsiflexion range of motion to neutral with their knee extended and passive hip extension of at least 5 degrees.
Table 1:
Participant Information and Walking Speed Classification
| Subject | Sex | Age (Years) | Time Since Stroke (Months) | Paretic Side | Height (m) | Mass (kg) | 10m Test Speed from Evaluation Session (m/s) | Walking Speed Classification (Perry et al., 1995) |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 57 | 58 | Left | 1.98 | 107.99 | 0.75 | Limited Community |
| 2 | M | 63 | 29 | Left | 1.80 | 94.29 | 1.11 | Community |
| 3 | M | 55 | 114 | Right | 1.78 | 77.98 | 0.83 | Community |
| 4 | F | 75 | 94 | Left | 1.57 | 78.75 | 0.91 | Community |
| 5 | F | 70 | 19 | Right | 1.78 | 94.22 | 0.90 | Community |
| 6 | F | 60 | 30 | Right | 1.60 | 76.96 | 0.87 | Community |
| 7 | M | 66 | 9 | Left | 1.82 | 82.47 | 1.10 | Community |
| 8 | F | 63 | 117 | Left | 1.55 | 52.05 | 0.74 | Limited Community |
| 9 | M | 59 | 78 | Left | 1.70 | 100.70 | 0.77 | Limited Community |
| 10 | F | 58 | 14 | Right | 1.68 | 89.46 | 1.06 | Community |
| 11 | F | 66 | 33 | Left | 1.47 | 77.77 | 1.36 | Community |
| 12 | M | 27 | 87 | Left | 1.83 | 91.17 | 1.00 | Community |
| 13 | M | 75 | 14 | Left | 1.73 | 90.49 | 0.96 | Community |
| 14 | F | 60 | 35 | Left | 1.60 | 74.01 | 1.32 | Community |
| 15 | M | 64 | 62 | Right | 1.78 | 77.23 | 1.29 | Community |
| 16 | M | 74 | 20 | Right | 1.91 | 84.97 | 1.74 | Community |
| 17 | F | 43 | 30 | Right | 1.65 | 58.48 | 0.98 | Community |
| 18 | F | 77 | 21 | Left | 1.65 | 66.02 | 1.12 | Community |
| 19 | M | 69 | 21 | Left | 1.78 | 95.38 | 1.43 | Community |
| 20 | M | 57 | 24 | Left | 1.83 | 110.52 | 1.19 | Community |
| Average (Stdev) |
11 M 9 F |
62 (12) | 45 (35) |
13 Left 7 Right |
1.72 (0.13) | 84.04 (15.07) | 1.07 (0.26) | N/A |
Table 2:
Summary of Participant Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| – 21 to 85 years of age – Single, unilateral stroke at least 6 months prior – Ambulatory without assistance – No falls within the last 6 months – Resting heart rate 40–100 beats per minute – Resting blood pressure 90/60–170/90 mmHg – Able to complete 6 minute walk test (6MWT) without assistance – Passive hip extension of at least 5° – Passive ankle dorsiflexion to neutral with knee extended |
– BMI > 35 – Evidence of cerebellar stroke – Neurological conditions in addition to stroke – Lower limb Botulinum toxin injects within 4 months – Current participation in physical therapy – Pain that limited walking – Unable to communicate with investigators – Shows signs of neglect – Unexplained dizziness within past 6 months – Congestive heart failure – Chronic obstructive pulmonary disease – Severe peripheral vascular disease – Rash or open wound on shank of affected limb – Score > 1 on question 1b of NIH Stroke Scale – Score > 0 on question 1c of NIH Stroke Scale – Current enrollment in another research study – Use of a pacemaker – Currently pregnant |
The Baseline Session included a participant eligibility screening and time to practice walking with UDTM control. A physical therapist measured the individual’s resting blood pressure (90/60—170/90 mmHg), resting heart rate (between 40–100 bpm) and body mass index (BMI ≤ 35). The participants performed the 10-meter walk test and 6MWT (American Thoracic Society, 2002) and then practiced walking with UDTM control for no more than 5 minutes.
The Evaluation Session occurred at least 24 hours after the Baseline Session and included a second 10-meter walk test and another vitals screening. Then, a randomized series of walking trials were performed on the treadmill at the participants’ self-selected (SS) and fastest comfortable (Fast) speeds with both FSTM and UDTM control.
Participants were outfitted with 42 retroreflective markers for motion capture. 26 single markers defined anatomical landmarks and 16 markers on rigid plastic shells tracked the motion of their legs, while an 8-camera system sampled marker data at 100 Hz (Motion Analysis Corp., Santa Rosa, CA, USA).
Participants walked on an instrumented split-belt treadmill (Bertec Corp., Columbus, OH, USA) in FSTM and UDTM control modes with the belt speeds tied while kinetic data were sampled at 2000 Hz. Participants were allowed a “light touch” on the treadmill handrails. The SS and Fast speeds were determined as follows.
10-meter walk test: The time to walk 10 meters was averaged over 3 trials to compute the preferred SS speed. The 10-meter test speeds reported are from the Evaluation Session.
- Fixed-Speed Treadmill Control (FSTM)
- SS Speed: The treadmill speed was set at the participant’s SS speed from the 10-m test. The speed was then increased or decreased by 0.05 m/s according to the user’s preference. Once the preferred speed was set, data were collected for 1 minute.
- Fast Speed: The same procedure as the SS speeds, except the verbal instructions directed participants to walk at the “fastest speed you can safely maintain for 1–2 minutes.”
User-Driven Treadmill Control (UDTM): For both the SS and Fast speed conditions, participants took up to 1 minute to reach their chosen, steady state speed by altering their propulsive forces, step length, or position relative to the treadmill. Steady state occurred when the belt speed fluctuated less than 0.2 m/s from stride to stride. Then, data were collected for 1 minute at that steady state speed, which was not necessarily the same as those from the FSTM trials.
Data Analysis
Kinetic data and kinematic data were filtered at 30 Hz and 6 Hz respectively, and calculations were performed using Visual 3D software (C-Motion Inc., Germantown, MD, USA). The primary outcome variables for this study include the SS and Fast walking speeds, peak anterior ground reaction forces (AGRF), peak posterior ground reaction forces (PGRF) and trailing limb angles (TLA) at the instant of peak AGRF. TLA is the angle between a straight line connecting the calculated hip joint center and the 5th metatarsal of the trailing limb and the vertical axis of the lab.
Shapiro-Wilke tests (α = 0.05) were used to determine if each outcome variable belonged to a normal distribution. If so, a one-way repeated measures ANOVA was used to determine if significant differences existed between the FSTM and UDTM conditions (α = 0.05). If not, then Wilcoxon signed rank tests were used to compare between conditions and within subjects (α = 0.05). Bonferroni corrections were used to adjust for multiple comparisons.
RESULTS
Participants selected SS speeds of 1.07 ± 0.26 m/s, 0.88 ± 0.22 m/s, and 0.94 ± 0.25 m/s for the 10m walk test, FSTM, and UDTM conditions respectively. The 10m walk test speeds were significantly faster than those for either treadmill-based SS speed condition (Δ10m-FSTM = 0.19 m/s, p < 0.0001 || Δ10m-UDTM = 0.13 m/s, p = 0.004 || Figure 1). The participants selected Fast speeds of 1.16 ± 0.26 m/s and 1.22 ± 0.32 m/s for the FSTM control and UDTM control conditions respectively (ΔUDTM—FSTM = 0.06 m/s, p = 0.008).
Figure 1:
Group average SS and Fast walking speeds with FSTM and UDTM control. Statistical tests were used to compare within subject differences.
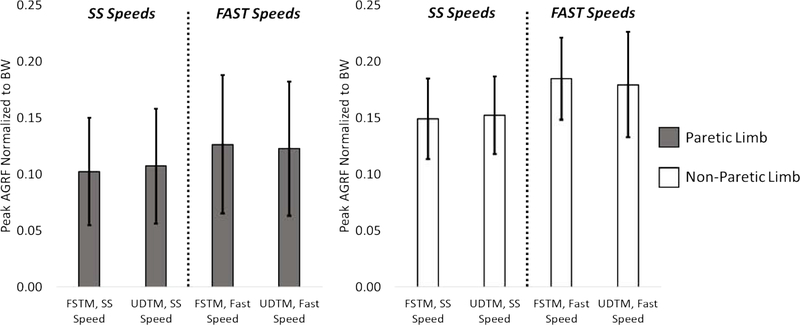
There were no differences in peak AGRF for either limb between the FSTM and UDTM SS speed conditions (ΔUDTM—FSTM, Paretic=0.49% BW, p=0.408 || ΔUDTM—FSTM, Non-Paretic=0.32% BW, p=0.545 || Figure 2). There were no significant differences in peak AGRF for either limb in the Fast speed trials, despite the faster speeds with the UDTM control (ΔUDTM—FSTM, Paretic=0.37% BW, p=0.533 || Δ UDTM—FSTM, Non-Paretic=0.52% BW, p=0.327).
Figure 2:
Group average peak AGRF values normalized to the participants’ body weight for the SS and Fast speed trials with FSTM and UDTM control. Statistical tests were used to compare within subject differences.
There were no differences in peak PGRF for either limb between the SS speed conditions with FSTM and UDTM control (ΔUDTM—FSTM, Paretic=0.40% BW, p=0.630 || Δ UDTM—FSTM, Non-Paretic=1.00% BW, p=0.207 || Figure 3). There were no significant differences in peak PGRF for either limb in the Fast speed trials, despite the faster speeds with the UDTM control (ΔUDTM—FSTM, Paretic=0.24% BW, p=0.772 || Δ UDTM—FSTM, Non-Paretic=0.95% BW, p=0.232).
Figure 3:
Group average peak PGRF values normalized to the participants’ body weight for the SS and Fast speed trials with FSTM and UDTM control. Statistical tests were used to compare within subject differences.
There were greater paretic limb TLA values for individuals walking with UDTM control than FSTM control at their SS speeds (ΔUDTM—FSTM, Paretic=1.21°, p = 0.038 || Δ UDTM—FSTM, Non-Paretic=0.54°, p=0.163 || Figure 4). There were no significant differences in TLA for either limb between the FSTM and UDTM Fast speed conditions (ΔUDTM—FSTM, Paretic=0.29°, p=0.611 || Δ UDTM—FSTM, Non-Paretic=0.01°, p=0.986).
Figure 4:
Group average TLA values at the instant of peak AGRF for the SS and Fast speed trials with FSTM and UDTM control. Statistical tests were used to compare within subject
DISCUSSION
This study demonstrates that individuals poststroke can tolerate walking on a treadmill with UDTM control. Participants selected slower SS speeds with FSTM and UDTM control than they did for the 10m walk test, which was expected since stroke survivors typically select slower walking speeds on treadmills than they do overground (Bayat et al., 2005). It was not expected that they would select similar SS speeds with FSTM and UDTM control because healthy adults selected faster speeds with UDTM control (Ray et al., 2018). However, individuals poststroke did select faster Fast speeds with UDTM control, which agreed with the results from work with young, healthy adults (Ray et al., 2018).
Since participants selected similar SS speeds with FSTM and UDTM control, they were expected to employ similar gait mechanics for both conditions. There were no significant changes in peak AGRF or PGRF for either limb as expected, but the increase in paretic TLA for the UDTM condition was surprising. Individuals increased their paretic TLA by 1.21° with UDTM control, which exceeds the minimum detectable change (MDC) of 1.0° (Kesar et al., 2011a). The wide variety of individual responses to UDTM control may obscure trends associated with either increasing or decreasing walking speeds. Therefore, it is possible that individuals use the same peak AGRF at a greater TLA to allow a more pure translation of AGRF into forward motion of the body’s center of mass, and the same amount of AGRF becomes more useful with a change in posture. Closer examination of the peak AGRF and PGRF shows individuals increased their non-paretic PGRF by 1.01% BW in the UDTM condition, which exceeds the MDC but did not reach statistical significance (Kesar et al., 2011a). This change in PGRF may balance the increased TLA and allow individuals to maintain similar SS speeds when walking with FSTM and UDTM control.
In the Fast speed condition, participants selected faster speeds with UDTM control, so they were expected to increase their AGRF, PGRF, and TLA for both limbs. However, there were no significant differences between the FSTM and UDTM conditions, which is surprising since AGRF and TLA are key mechanisms for increasing walking speed poststroke (Awad et al., 2014a). Healthy adults used the same AGRF and TLA to reach matched speeds with FSTM and UDTM control (Ray et al., 2018), so a change in treadmill control should not cause changes in mechanics without changes in walking speed. This suggests there is a large amount of variation in user responses and individuals may use different proportions of increased propulsion and decreased braking to change walking speeds. Future work will model individual strategies to gain insight into how users changed walking speeds.
The small size and homogeneous nature of the sample in this study may limit the interpretation of these results. To ensure that participants were able to complete the entire evaluation session, we recruited participants with high walking function. This study presents a conservative evaluation of the effect of the UDTM control in stroke survivors. We would expect individuals who are limited community ambulators (Perry et al., 1995) with more severe hemiparesis to have more dramatic changes in their walking speeds and gait mechanics when walking with UDTM control because this high functioning group may already have reached their optimal walking speeds. Future work should implement UDTM control in a clinical training program with a group of stroke survivors with more severe hemiparesis and gait impairments.
Since treadmill-based gait training provides a high number of task-specific repetitions at a relatively low cost, it is a popular choice for poststroke rehabilitation (Ada et al., 2003; Duncan et al., 2011; Macko et al., 2005; Sullivan et al., 2002). However, many individuals do not improve their walking function after training, and a more effective option is required (Dickstein, 2008). This study demonstrates that individuals poststroke can easily tolerate walking with UDTM control and may achieve increased Fast walking speeds, which are beneficial for training (Sullivan et al., 2002). Due to the wide variety of responses, future work will model how individuals generated forward propulsion with FSTM and UDTM control to determine how to most effectively prescribe UDTM control for use in gait training programs.
ACKNOWLEDGEMENTS
The authors acknowledge the support of NIH P30 103333, NIH U54-GM104941, Helwig Mechanical Engineering Fellowship & UDel Dissertation Fellowship. They would also like to thank DRI ResCore, Maggie French, Jessica Galgiani, Ryan Wilke, & Brian Knarr.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, Vellas B, 2009. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging 13, 881–9. [DOI] [PubMed] [Google Scholar]
- Ada L, Dean CM, Hall JM, Bampton J, Crompton S, 2003. A Treadmill and Overground Walking Program Improves Walking in Persons Residing in the Community After Stroke: A Placebo-Controlled, Randomized Trial. Arch. Phys. Med. Rehabil 84, 1486–1491. 10.1053/S0003-9993(03)00349-6 [DOI] [PubMed] [Google Scholar]
- American Thoracic Society, 2002. ATS Statement: Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care Med 166, 111–117. 10.1164/rccm.166/1/111 [DOI] [PubMed] [Google Scholar]
- Awad LN, Binder-Macleod SA, Pohlig RT, Reisman DS, 2014a. Paretic Propulsion and Trailing Limb Angle Are Key Determinants of Long-Distance Walking Function After Stroke. Neurorehabil. Neural Repair 29, 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad LN, Reisman DS, Kesar TM, Binder-Macleod SA, 2014b. Targeting paretic propulsion to improve poststroke walking function: a preliminary study. Arch. Phys. Med. Rehabil. 95, 840–8. 10.1016/j.apmr.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayat R, Barbeau H, Lamontagne A, 2005. Speed and Temporal-Distance Adaptations during Treadmill and Overground Walking Following Stroke. Neurorehabil. Neural Repair 19, 115–124. 10.1177/1545968305275286 [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics Committee and Stroke Statistics Subcommittee, O. behalf of the A.H.A.S.C. and S.S., 2017. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation e1–458. [Google Scholar]
- Bohannon RW, Horton MG, Wikholm JB, 1991. Importance of four variables of walking to patients with stroke. Int. J. Rehabil. Res 14, 246–50. [DOI] [PubMed] [Google Scholar]
- Dickstein R, 2008. Rehabilitation of Gait Speed After Stroke: A Critical Review of Intervention Approaches. Neurorehabil. Neural Repair 22, 649–660. 10.1177/1545968308315997 [DOI] [PubMed] [Google Scholar]
- Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, Dobkin BH, Rose DK, Tilson JK, Cen S, Hayden SK, 2011. Body-Weight–Supported Treadmill Rehabilitation after Stroke. N. Engl. J. Med 364, 2026–2036. 10.1056/NEJMoa1010790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz S, Lusardi M, 2009. White Paper: Walking Speed: the Sixth Vital Sign. J. Geriatr. Phys. Ther. 32, 1–5. [PubMed] [Google Scholar]
- Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS, 1995. Recovery of walking function in stroke patients: The copenhagen stroke study. Arch. Phys. Med. Rehabil 76, 27–32. 10.1016/S0003-9993(95)80038-7 [DOI] [PubMed] [Google Scholar]
- Kempski K, Ray NT, Knarr BA, Higginson JS, 2018. Dynamic Structure of Variability of Joint Angles and Center of Gravity Position during User-Driven Treadmill Walking. Gait Posture. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesar TM, Binder-Macleod SA, Hicks GE, Reisman DS, 2011a. Minimal detectable change for gait variables collected during treadmill walking in individuals post-stroke. Gait Posture 33, 314–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesar TM, Reisman DS, Perumal R, Jancosko AM, Higginson JS, Rudolph KS, Binder-Macleod SA, 2011b. Combined effects of fast treadmill walking and functional electrical stimulation on post-stroke gait. Gait Posture 33, 309–313. 10.1016/J.GAITPOST.2010.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne A, Fung J, 2004. Faster Is Better: Implications for Speed-Intensive Gait Training After Stroke. Stroke 35, 2543–2548. 10.1161/01.STR.0000144685.88760.d7 [DOI] [PubMed] [Google Scholar]
- Langhorne P, Coupar F, Pollock A, 2009. Motor recovery after stroke: a systematic review. Lancet Neurol. 8, 741–754. [DOI] [PubMed] [Google Scholar]
- Macko RF, Ivey FM, Forrester LW, Hanley D, Sorkin JD, Katzel LI, Silver KH, Goldberg AP, 2005. Treadmill Exercise Rehabilitation Improves Ambulatory Function and Cardiovascular Fitness in Patients With Chronic Stroke. Stroke 36, 2206–2211. 10.1161/01.STR.0000181076.91805.89 [DOI] [PubMed] [Google Scholar]
- Mayo NE, Wood-Dauphinee S, Ahmed S, Gordon C, Higgins J, McEwen S, Salbach N, 1999. Disablement following stroke. Disabil. Rehabil 21, 258–68. [DOI] [PubMed] [Google Scholar]
- Moore S, Schurr K, Wales A, Moseley A, Herbert R, 1993. Observation and analysis of hemiplegic gait: swing phase. Aust. J. Physiother 39, 271–278. 10.1016/S0004-9514(14)60487-6 [DOI] [PubMed] [Google Scholar]
- Perry J, Garrett M, Mulroy SJ, 1995. Classification of Walking Handicap after Stroke. Stroke 26, 982–989. [DOI] [PubMed] [Google Scholar]
- Peterson CL, Cheng J, Kautz SA, Neptune RR, 2010. Leg extension is an important predictor of paretic leg propulsion in hemiparetic walking. Gait Posture 32, 451–456. 10.1016/J.GAITPOST.2010.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray NT, Knarr BA, Higginson JS, 2018. Walking speed changes in response to novel user-driven treadmill control. J. Biomech 78, 143–149. 10.1016/j.jbiomech.2018.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiou N, Decker LM, 2011. Human movement variability, nonlinear dynamics, and pathology: Is there a connection? Hum. Mov. Sci 30, 869–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KJ, Knowlton BJ, Dobkin BH, 2002. Step training with body weight support: Effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Arch. Phys. Med. Rehabil 83, 683–691. [DOI] [PubMed] [Google Scholar]