Abstract
Objectives:
In the skeletal muscles, water metabolism is mainly regulated by water channel aquaporin 4 (AQP4). Although the expression level of AQP4 was reduced by long-term denervation, during denervation the relationship between muscle atrophy initiation and AQP4 expression decrease initiation remains unknown. The present study examined the relationship between the timing of muscle atrophy initiation and that of AQP4 expression decrease initiation, during the early stage of denervation.
Methods:
Female 344 rats (8 weeks of age) were randomly assigned to control (C), day 1 post-sciatic denervation (D1), day 4 post- sciatic denervation (D4) and day 7 post- sciatic denervation (D7) groups (n=6 per group). In the tibialis anterior (TA) muscles of each group, the expression levels of some target proteins were quantified by Western blot analysis.
Results:
The expression level of AQP4 significantly decreased on day 4 post-denervation (p<0.05). Moreover, the beginning of the decrease in AQP4 expression level was concurrent with the timing of muscle atrophy in the skeletal muscles during the early stage of denervation.
Conclusions:
The present study suggested that the progression of the decrease in the AQP4 expression level is partly related to the progression of muscle atrophy during the early stage of denervation.
Keywords: Denervation, AQP4, AQP1, Muscle Atrophy
Introduction
The regulation of water homeostasis is required to maintain the homeostasis of skeletal muscle in vivo. Indeed, muscle water content is markedly decreased in the skeletal muscles undergoing pathological atrophy such as myopathy and neuropathy[1]. In the skeletal muscles, water homeostasis is mainly regulated by aquaporin 4 (AQP4), which is a selective water channel specifically expressed on the plasma membrane of myofibers[2,3].
Although the physiological roles of AQP4 are not fully understood, it is thought that AQP4 is related to the regulation of the morphological changes of skeletal muscles[4-7]. Indeed, we previously demonstrated that the expression level of AQP4 was relatively maintained throughout muscle hypertrophy[8]. In contrast, the expression level of AQP4 markedly decreased in the atrophied muscles due to denervation of a certain period[9], while this decrease in the AQP4 expression level was not observed in hindlimb suspension-induced muscle atrophy[6,10]. Thus, unlike muscle hypertrophy, in case of muscle atrophy, the changes of AQP4 expression patterns in response to muscle atrophy remain controversial. Furthermore, even if denervation can induced the decrease of AQP4 expression in skeletal muscles, during denervation the relationship between the timing of muscle atrophy initiation and that of AQP4 expression decrease initiation remains unknown, actually.
In the skeletal muscles, water is metabolized between myofibers and capillaries, and the water molecules are transferred between AQP4 on myofibers and AQP1 expressed on the endothelial cells composing capillaries[3,11]. The changes in the capillary supply are induced in response to the skeletal muscle morphology[12]. For example, the capillary supply is facilitated by muscle hypertrophy, whereas muscle atrophy induces a decline in the capillary supply[13-16]. Recently, it was demonstrated that the expression pattern of AQP1 localized on the endothelial cells composing capillaries is not altered in the skeletal muscles by sciatic nerve freezing-induced denervation[17]. However, whether the changes in the AQP1 expression pattern are related to the regulation of the changes in skeletal muscle morphology remains unclear.
In the present study, we examined the relationship between the timing of muscle atrophy initiation and that of AQP4 expression decrease initiation, during the early stage of denervation. Moreover, the present study investigated the differences of time course of the changes in AQP4 and AQP1 expression patterns in the skeletal muscles during the early stage of denervation. The findings of the present study are useful to clarify the roles of water metabolism in regulating the morphological changes of skeletal muscle.
Materials and methods
Experimental design and surgical procedure
Adult female Fischer 344 rats (8 weeks of age) were used in this study, and randomly assigned to control (C), day 1 post-denervation (D1), day 4 post-denervation (D4) and day 7 post-denervation (D7) groups (n=6 per group). The rats were housed in individual cages at 22°C with a 12-h light/dark cycle, and were provided with food and water ad libitum. All experiments were carried out following approval from the Ethics Committee on Life Sciences of Osaka Institute of Technology.
Under pentobarbital sodium anesthesia (60 mg kg-1 i.p.) and inhalation anesthesia with 0.7% isoflurane, the right hindlimbs of the rates were denervated by cutting the sciatic nerve, and an approximately 3-mm segment of the nerve was removed to prevent nerve regeneration. The contralateral hindlimbs were used as sham operated controls (C group). The rats were sacrificed at day 1, 4 or 7 post-denervation. At death, the rats were anesthetized and the tibialis anterior muscles were removed. Muscle samples were quickly frozen in liquid nitrogen and stored at -80°C until use.
Western blot analysis
Sample preparation and Western blot analysis were performed according to the protocol described in a previous study8. In brief, the muscle samples were homogenized in 300 µl of RIPA buffer (50mM Tris-HCl pH 8.0, 150 mM sodium chloride, 1% NP-40, 0.5% sodium deoxycholate and 0.1% sodium dodecyl sulfate) (Sigma-Aldrich, St Louis, MO) containing 1x Protease Inhibitor Cocktail (Roche Diagnostic, Dubai, UAE). After the homogenate samples were centrifuged at 12000 ×g for 10 min at 4°C, the supernatants were collected. We used the BCA protein assay kit (Thermo scientific, Rockford, IL) to measure the protein concentration.
The homogenate samples (10 mg of protein) were resolved by SDS-PAGE using either a 12.5% polyacrylamide gel for AQP4, α1-syntrophin, MAFbx, AQP1 and GAPDH or a 7.5% gel for fast myosin heavy chain, and then transferred onto polyvinylidene difluoride membranes (ATTO, Tokyo, Japan) at 144 mA for 45 min. After transfer, to block any nonspecific immunoreactivity, the membranes were incubated with 20 mM Tris-buffered saline (TBS) (pH 7.6) containing 5% normal serum and 0.1% Tween-20 for 1 h at room temperature. The membranes were then incubated overnight at 4°C with primary antibodies. The primary antibodies were diluted with 20 mM TBS containing 5% normal serum and 0.1% Tween-20. The primary antibodies used in the present study were as follows: goat polyclonal anti-AQP4 (Santa Cruz biotechnology, Santa Cruz, CA), mouse monoclonal anti-GAPDH (internal control) (Cell Signaling Technology, Danvers, MA), rabbit polyclonal anti-syntrophin alpha 1 (Abcam, Cambridge, UK), mouse monoclonal anti-myosin (skeletal, Fast) (Sigma-Aldrich, Louis, MO) and mouse monoclonal anti-MAFbx (a marker protein of muscle atrophy[18,19]) (Santa Cruz biotechnology), rabbit monoclonal Anti-AQP1 (Abcam). Then, they were washed in 20 mM TBS containing 5% normal serum and 0.1% Tween-20, and incubated for 1 h at room temperature with secondary antibodies. The secondary antibodies used in the present study were as follows: biotinylated anti-rabbit IgG (Invitrogen, Camarillo, CA), biotinylated anti-goat IgG (Merck Millipore, Darmstadt, Germany) and biotinylated anti-mouse IgG (Vector Laboratories, Burlingame, CA). They were washed in 20 mM TBS containing 5% normal serum and 0.1% Tween-20, and incubated for 1 h at room temperature with TBS containing 5% normal serum and peroxidase conjugated streptavidin horseradish (GE Healthcare, Buckinghamshire, UK). Finally, immunoreactivity was detected by chemiluminescence using ImmunoStar Zeta (Wako, Osaka, Japan). The bands were quantified by densitometric analysis using ImageJ software (ver.1.48, http://rsb.info.nih.gov/ij/). The mean value for the control samples on each immunoblot, expressed relative to GAPDH as an internal control, was adjusted to equal 1.0, and each sample value was expressed relative to the adjusted mean value for the control group.
Statistical analysis
All data are presented as means ± SD and were analyzed using the StatView statistical-analysis program (SAS institute Inc., Cary, NC, USA). All data were tested by one-way ANOVA using Scheffé’s test. Differences were considered to be significant at a confidence level of 0.05.
Results
Time course changes in the relative tibialis anterior muscle weight after denervation
To identify the time course effects of denervation on muscle atrophy, we examined the relative tibialis anterior muscle weight in each group. As shown in Figure 1, the relative tibialis anterior muscle weight gradually decreased after denervation, and that in the D4 and D7 groups was significantly lower than that in the C and D1 groups (p<0.05). Furthermore, there was a significant difference between the D4 and D7 groups (p<0.05). Therefore, muscle atrophy was gradually progressing during the early stages of denervation.
Figure 1.
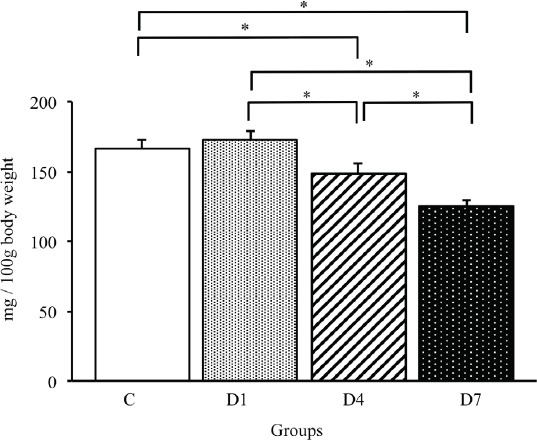
Relative tibialis anterior muscle weights in control, D1, D4 and D7 groups. Values are the mean ± SD. Relative muscle weights are presented as mg/100 g of body weight. C: control group. D1: day 1 post-denervation group. D4: day 4 post-denervation. D7: day 7 post-denervation. *p<0.05.
Time course changes in MAFbx expression patterns in the tibialis anterior muscles during the early stages of denervation
To confirm the progression of denervation-induced muscle atrophy, we examined the time course changes in MAFbx protein, which is a main regulator for the progression of denervation-induced muscle atrophy[18], by Western blot analysis (Figure 2). As a result, the expression level of MAFbx gradually increased after denervation, and a significant increase in the MAFbx expression level was observed on days 4 and 7 post-denervation compared with the C group (p<0.05) (Figure 3). Therefore, the muscle atrophy regulated by MAFbx was induced during the early stages of denervation.
Figure 2.
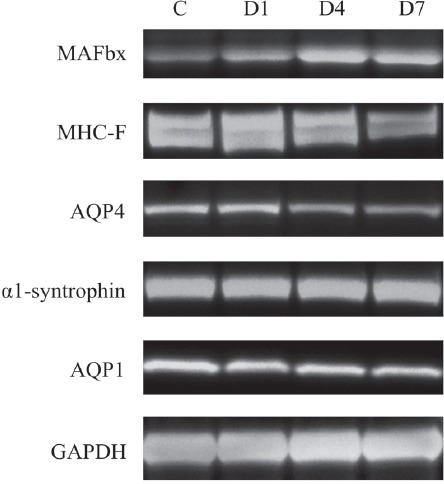
Representative images of Western blots for MAFbx, MHC-F, AQP4, α1-syntrophin, APQ1 and GAPDH in tibialis anterior muscles in control, D1, D4 and D7 groups. C: control group. D1: day 1 post-denervation group. D4: day 4 post-denervation. D7: day 7 post-denervation.
Figure 3.
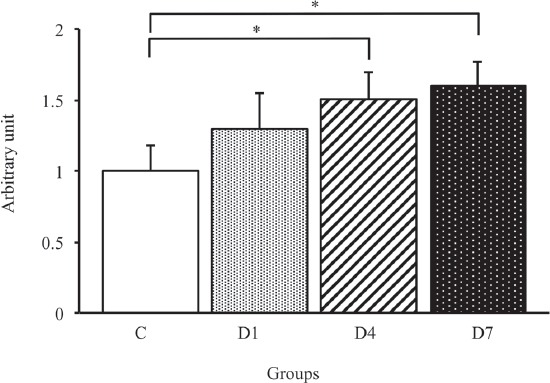
MAFbx protein expression level in the tibialis anterior muscles in control, D1, D4 and D7 groups. C: control group. D1: day 1 post-denervation group. D4: day 4 post-denervation. D7: day 7 post-denervation. MAFbx protein expression levels, normalized by the GAPDH protein expression level, were calculated by densitometric analysis. Values are the mean ± SD. Fold changes were expressed relative to the levels observed in the C group. *p<0.05.
The changes in expression patterns of fast myosin heavy chain in the tibialis anterior muscles in response to denervation
We performed Western blot analysis using the fast myosin heavy chain (MHC-F) antibody to examine the time course changes in the MHC-F expression level in skeletal muscles during the early stages of denervation (Figure 2). As shown in Figure 4, the expression level of MHC-F gradually decreased after denervation, and the expression level of MFC-F in the D7 group was significantly lower than that in the C and D1 groups (p<0.05). On the other hand, there were no significant differences among the C, D1 and D4 groups. Therefore, the expression level of MHC-F gradually decreased in skeletal muscles after denervation, and a significant decrease in the MHC-F expression level was only noted at 7 days post-denervation.
Figure 4.
MHC-F protein expression levels in the tibialis anterior muscles in control, D1, D4 and D7 groups. C: control group. D1: day 1 post-denervation group. D4: day 4 post-denervation. D7: day 7 post-denervation. MHC-F protein expression levels, normalized by the GAPDH protein expression level, were calculated by densitometric analysis. Values are the mean ± SD. Fold changes were expressed relative to the levels observed in the C group. *p<0.05.
Time course changes in AQP4 and a1-syntrophin expression patterns in the tibialis anterior muscles during the early stages of denervation
To identify the effects of early-stage denervation on the AQP4 and α1-syntrophin expression levels, we examined the time course changes in the AQP4 expression level by Western blot analysis (Figure 2). As shown in Figure 5, the expression level of AQP4 in the D4 and D7 groups significantly lower than that in the C and D1 groups (p<0.05). The expression level of AQP4 in the D7 group was similar to that in the D4 group. On the other hand, there were no significant differences in the α1-syntrophin expression level among all groups (Figure 6).
Figure 5.
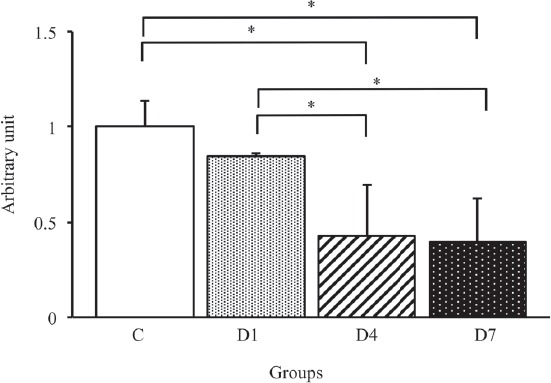
APQ4 protein expression levels in the tibialis anterior muscles in control, D1, D4 and D7 groups. C: control group. D1: day 1 post-denervation group. D4: day 4 post-denervation. D7: day 7 post-denervation. APQ4 protein expression levels, normalized by the GAPDH protein expression level, were calculated by densitometric analysis. Values are the mean ± SD. Fold changes were expressed relative to the levels observed in the C group. *p<0.05.
Figure 6.
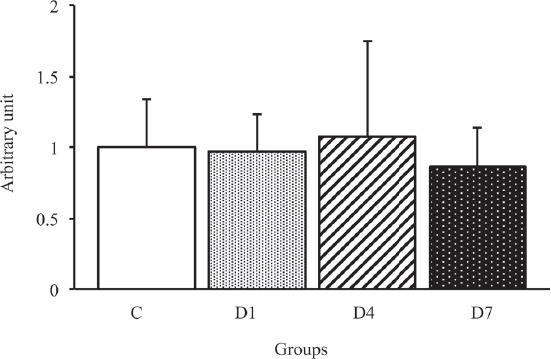
α1-syntrophin protein expression levels in the tibialis anterior muscles in control, D1, D4 and D7 groups. C: control group. D1: day 1 post-denervation group. D4: day 4 post-denervation. D7: day 7 post-denervation. α1-syntrophin protein expression levels, normalized by the GAPDH protein expression level, were calculated by densitometric analysis. Values are the mean ± SD. Fold changes were expressed relative to the levels observed in the C group. There were no significant differences in the expression level of α1-syntrophin protein among all groups.
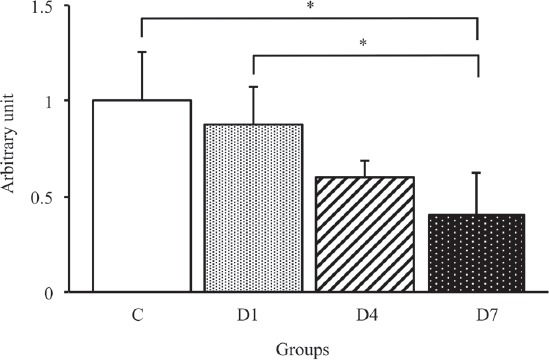
To examine the relationship between the AQP4 and α1-syntrophin expression levels, we calculated the ratio of AQP4/α1-syntrophin expression in each group. As a result, the ratio of AQP4/α1-syntrophin protein expression in the D4 and D7 groups was significantly lower than that in the C and D1 groups (p<0.05) (Figure 7). On the other hand, there was no significant difference between the D4 and D7 groups. Therefore, the expression level of AQP4, but not α1-syntrophin, significantly decreased after denervation, and consequently, the relationship between AQP4 and α1-syntrophin expression was lost during the early stages of denervation.
Figure 7.
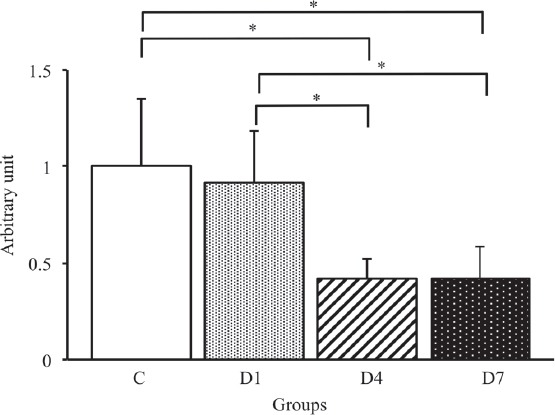
The ratio of AQP4/α1-syntrophin protein expression in the tibialis anterior muscles in control, D1, D4 and D7 groups. C: control group. D1: day 1 post-denervation group. D4: day 4 post-denervation. D7: day 7 post-denervation. Values are the mean ± SD. Fold changes were expressed relative to the levels observed in the C group. *p<0.05.
The effects of early-stage denervation on the expression patterns of AQP1 in the tibialis anterior muscles
We performed Western blot analysis using the AQP1 antibody to examine the effects of early-stage denervation on the AQP1 expression level in skeletal muscles (Figure 2). As shown in Figure 8, there were no significant differences among all groups. These results suggested that the expression level of AQP1 in the skeletal muscles is not influenced during the early stages of denervation.
Figure 8.
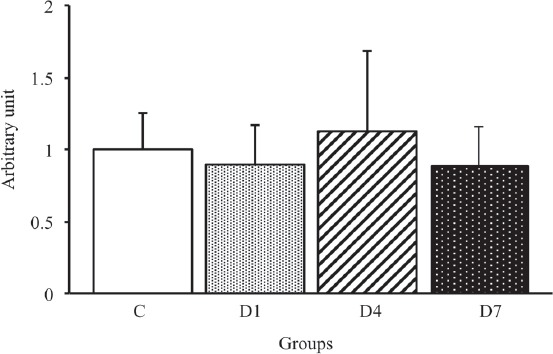
AQP1 protein expression levels in the tibialis anterior muscles in control, D1, D4 and D7 groups. C: control group. D1: day 1 post-denervation group. D4: day 4 post-denervation. D7: day 7 post-denervation. AQP1 protein expression levels, normalized by the GAPDH protein expression level, were calculated by densitometric analysis. Values are the mean ± SD. Fold changes were expressed relative to the levels observed in the C group. There were no significant differences in the expression level of AQP1 protein among all groups.
Discussion
AQP4 is a water channel localized on the plasma membrane of myofibers in the skeletal muscles[3,5]. We previously reported a marked reduction in the AQP4 expression level due to long-term denervation[9]. However, the relationship between the decrease in AQP4 expression and the progression of muscle atrophy is not fully understood. To our knowledge, the present study revealed for the first time that during the early stage of denervation, the decrease in the AQP4 expression level induced in the skeletal muscles was concurrent with the significant occurrence of muscle atrophy. In addition, the present study suggested that the decrease in the expression level of AQP4, but not AQP1, may be partly related to muscle atrophy during the early stage of denervation.
In the present study, the significant loss of relative muscle weight was detected at day 4 post-denervation, and this timing of the loss of relative muscle weight corresponded to that of the increase in MAFbx expression. MAFbx is a ubiquitin ligase required to promote denervation-induced protein degradation, and the expression of MAFbx was reported to rapidly increase at day 3 post-denervation[18]. Indeed, the enhancement of protein breakdown, rather than the inhibition of protein synthesis, is a major factor for the progression of muscle atrophy at the beginning of denervation[20]. Therefore, the present study suggested that muscle atrophy is induced by the promotion of protein degradation instead of the inhibition of protein synthesis during the early stage of denervation.
The present study revealed that the decrease in the AQP4 expression level coincided with the decrease in relative muscle weight. We previously demonstrated that the regulation of AQP4 expression is dependent on the nerve supply to skeletal muscles because the decrease in AQP4 expression due to denervation was followed by the restoration of the AQP4 expression level during re-innervation[17]. Moreover, the AQP4 expression level did not decrease in the hindlimb suspension-induced atrophied skeletal muscles[5]. Therefore, these findings suggested that the decrease in the AQP4 expression level and the progression of muscle atrophy are respectively induced by independent regulatory mechanisms during the early stage of denervation. On the other hand, as AQP4 is considered to be one of the main factors regulating the homeostasis of muscle morphology[4-8], the possibility that the decrease in the AQP4 expression level is partly involved in the progression of muscle atrophy in the denervated skeletal muscles was not completely ruled out in the present study.
The AQP4 expression level began to decrease at day 4 post-denervation. Furthermore, the expression level of AQP4 on day 4 post-denervation was similar to that on day 7 post-denervation. Of note, the ratio of decrease in the AQP4 expression level on days 4 and 7 post-denervation was similar to that at 2 weeks after denervation reported in the previous study[9]. Therefore, the decrease in the expression of AQP4 induced by the loss of the nerve supply may peak at day 7 post-denervation.
The present study demonstrated that the beginning of the decrease in the AQP4 expression level was similar that of the increase in MAFbx expression. This suggested that the decrease in AQP4 expression is due to the degradation of AQP4 protein during the early stage of denervation. On the other hand, to our knowledge, there are no reports on the changes in AQP4 mRNA in the skeletal muscles in response to denervation. However, it was reported that AQP4 mRNA was markedly reduced in the skeletal muscles with neurogenic atrophy such as amyotrophic lateral sclerosis[21]. Therefore, the present study did not rule out the possibility that the down-regulation of AQP4 mRNA transcription is involved in the reduction of AQP4 during the early stage of denervation. Further studies are needed to clarify the effects of the loss of the nerve supply on the transcription of AQP4 mRNA during the early stage of denervation.
AQP4 is specifically expressed on the membrane of myofibers containing MHC-F, and it is thought that the AQP4 expression patterns are correlated with the MHC-F expression patterns in the skeletal muscles[2,5,22]. However, the present study indicated that the decrease in AQP4 expression was followed by the decrease in MHC-F expression in skeletal muscles during the early stage of denervation. These findings suggested that the decrease in AQP4 expression patterns may be partly involved in the decrease in MHC-F expression patterns in the skeletal muscles during the early stage of denervation, rather than the decrease of MHC-F affected the decrease of AQP4 in denervated skeletal muscles. Although whether the changes of AQP4 and MHC-F affect regulatory mechanisms of each other’s expression remains unclear until now, the changes of water and electrolyte balance induced by the decrease of AQP4 may possibly affect the changes of MHC-F expression levels during the early stage of denervation. For example, it was reported that AQP4 knockout altered the Ca2+ oscillations in neural stem cells[23]. On the other hand, the profiles of myosin heavy chain were affected by Ca2+ oscillations in skeletal muscles[24]. Therefore, the changes of Ca2+ oscillations induced by AQP4 decrease may possibly affect the downregulation of MHC-F in skeletal muscles during early stage of denervation. Further studies are needed to evaluate whether the changes of AQP4 expression are directly involved in the regulation of MHC-F expression during early stage of denervation.
In general, the decrease in AQP4 expression is induced in response to reduced α1-syntrophin expression[25-27]. On the other hand, it was reported that the decrease in AQP4 occurred without the decrease in α1-syntrophin expression in the skeletal muscles in limb-girdle muscular dystrophin type 2B patients[28]. Similarly, in the present study, the expression level of AQP4, but not α1-syntrophin, gradually decreased after denervation, and the relationship between AQP4 and α1-syntrophin expression was consequently lost during the early stages of denervation. Therefore, the decrease in AQP4 may be induced independently of changes in α1-syntrophin expression in the skeletal muscles under certain conditions (e.g. loss of the nerve supply) in vivo. Moreover, the stable expression of AQP4 is essentially maintained by the binding of AQP4 to the PDZ domain of α1-syntrophin in the myofibers[25-27]. Therefore, the present study suggested that the relationship between AQP4 and α1-syntrophin may be readily lost in the myofibers due to block of the nerve supply.
In contrast to AQP4, the expression level of AQP1 did not change in the skeletal muscles during the early stage of denervation in the present study. The capillaries do not begin to change during the first week post-denervation in the skeletal muscles[16]. Therefore, as AQP1 is specifically expressed on the endothelial cells in the skeletal muscles[3,11], these results are reasonable. Furthermore, the present results are consistent with those of a previous study which found that the AQP1 expression level did not change in skeletal muscles lacking a nerve supply by sciatic nerve freezing[17]. Therefore, unlike AQP4, the regulatory mechanisms of AQP1 expression may be inherently independent of the condition of nerve supply and the progression of muscle atrophy in the skeletal muscles. On the other hand, AQP1 expresses in the red blood cells as well as vascular endothelial cells[27-29]. The present study did not evaluate whether the blood were completely removed from skeletal muscles, and thus it was not able to exclude the possibility that AQP1 expressing in residual red blood cells within skeletal muscles was included in the AQP1 expression levels indicated in the present study. Further studies are needed to reveal net effects of denervation on the expression levels of AQP1 in endothelial cells of skeletal muscles.
In conclusion, the present study demonstrated for the first time that the timing of AQP4 expression decrease initiation was concurrent with that of muscle atrophy initiation in the skeletal muscles during the early stage of denervation. These findings suggested that the progression of the decrease in AQP4 expression may be partly related to the progression of muscle atrophy during the early stage of denervation. On the other hand, unlike AQP4, it was suggested that the regulation of AQP1 expression may be inherently independent of the nerve supply and the morphological changes of skeletal muscles.
Acknowledgements
This study was supported by a Grant-in Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (JSPS KAKENHI Grant Number JP17K01771).
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Vignos PJ, Jr, Lefkowitz M. A biochemical study of certain skeletal muscle constituents in human progressive muscular dystrophy. J Clin Invest. 1959;38:873–881. doi: 10.1172/JCI103869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frigeri A, Nicchia GP, Verbavatz JM, Valenti G, Svelto M. Expression of aquaporin-4 in fast-twitch fibers of mammalian skeletal muscle. J Clin Invest. 1998;102(4):695–703. doi: 10.1172/JCI2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frigeri A, Nicchia GP, Balena R, Nico B, Svelto M. Aquaporins in skeletal muscle: reassessment of the functional role of aquaporin-4. FASEB J. 2004;18(7):905–907. doi: 10.1096/fj.03-0987fje. [DOI] [PubMed] [Google Scholar]
- 4.Crosbie RH, Dovico SA, Flanagan JD, Chamberlain JS, Ownby CL, Campbell KP. Characterization of aquaporin-4 in muscle and muscular dystrophy. FASEB J. 2002;16(9):943–949. doi: 10.1096/fj.01-0327com. [DOI] [PubMed] [Google Scholar]
- 5.Frigeri A, Nicchia GP, Nico B, Quondamatteo F, Herken R, Roncali L, Svelto M. Aquaporin-4 deficiency in skeletal muscle and brain of dystrophic mdx mice. FASEB J. 2001;15(1):90–98. doi: 10.1096/fj.00-0260com. [DOI] [PubMed] [Google Scholar]
- 6.Frigeri A, Nicchia GP, Desaphy JF, Pierno S, De Luca A, Camerino DC, Svelto M. Muscle loading modulates aquaporin-4 expression in skeletal muscle. FASEB J. 2001;15(7):1282–1284. doi: 10.1096/fj.00-0525fje. [DOI] [PubMed] [Google Scholar]
- 7.Wakayama Y, Jimi T, Inoue M, Kojima H, Murahashi M, Kumagai T, Yamashita S, Hara H, Shibuya S. Reduced aquaporin 4 expression in the muscle plasma membrane of patients with Duchenne muscular dystrophy. Arch Neurol. 2002;59(3):431–437. doi: 10.1001/archneur.59.3.431. [DOI] [PubMed] [Google Scholar]
- 8.Ishido M, Nakamura T. Aquaporin-4 Protein Is Stably Maintained in the Hypertrophied Muscles by Functional Overload. Acta Histochem Cytochem. 2016;49:89–95. doi: 10.1267/ahc.16005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishido M, Nakamura T. Marked decrease of aquaporin-4 protein is independent of the changes in alpha1-syntrophin and TRPV4 levels in response to denervation-induced muscle atrophy in vivo. J Muscle Res Cell Motil. 2017;38:175–181. doi: 10.1007/s10974-017-9471-y. [DOI] [PubMed] [Google Scholar]
- 10.Basco D, Nicchia GP, Desaphy JF, Camerino DC, Frigeri A, Svelto M. Analysis by two-dimensional Blue Native/SDS-PAGE of membrane protein alterations in rat soleus muscle after hindlimb unloading. Eur J Appl Physiol. 2010;110:1215–1224. doi: 10.1007/s00421-010-1592-6. [DOI] [PubMed] [Google Scholar]
- 11.Jimi T, Wakayama Y, Inoue M, Kojima H, Oniki H, Matsuzaki Y, Shibuya S, Hara H, Takahashi J. Aquaporin 1: examination of its expression and localization in normal human skeletal muscle tissue. Cells Tissues Organs. 2006;184:181–187. doi: 10.1159/000099625. [DOI] [PubMed] [Google Scholar]
- 12.Hudlicka O. Microcirculation in skeletal muscle. Muscles Ligaments Tendons J. 2011;1:3–11. [PMC free article] [PubMed] [Google Scholar]
- 13.Egginton S, Hudlicka O. Capillary growth in relation to blood flow and performance in overloaded rat skeletal muscle. J Appl Physiol. 1998;85(6):2025–2032. doi: 10.1152/jappl.1998.85.6.2025. [DOI] [PubMed] [Google Scholar]
- 14.Plyley MJ, Olmstead BJ. Time course of changes in capillarization in hypertrophied rat plantaris muscle. J Appl Physiol. 1998;84(3):902–907. doi: 10.1152/jappl.1998.84.3.902. [DOI] [PubMed] [Google Scholar]
- 15.Tyml K, Mathieu-Costello O, Cheng L, Noble EG. Differential microvascular response to disuse in rat hindlimb skeletal muscles. J Appl Physiol. 1999;87:1496–1505. doi: 10.1152/jappl.1999.87.4.1496. [DOI] [PubMed] [Google Scholar]
- 16.Wagatsuma A, Osawa T. Time course of changes in angiogenesis-related factors in denervated muscle. Acta Physiol (Oxf) 2006;187:503–509. doi: 10.1111/j.1748-1716.2006.01594.x. [DOI] [PubMed] [Google Scholar]
- 17.Ishido M, Nakamura T. The expression of aquaporin-4 is regulated based on innervation in skeletal muscles. J Muscle Res Cell Motil. 2018;39:17–23. doi: 10.1007/s10974-018-9494-z. [DOI] [PubMed] [Google Scholar]
- 18.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 19.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldspink DF. The effects of denervation on protein turnover of rat skeletal muscle. Biochem J. 1976;156(1):71–80. doi: 10.1042/bj1560071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jimi T, Wakayama Y, Matsuzaki Y, Hara H, Inoue M, Shibuya S. Reduced expression of aquaporin 4 in human muscles with amyotrophic lateral sclerosis and other neurogenic atrophies. Pathol Res Pract. 2004;200(3):203–209. doi: 10.1016/j.prp.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Nicchia GP, Mola MG, Pisoni M, Frigeri A, Svelto M. Different pattern of aquaporin-4 expression in extensor digitorum longus and soleus during early development. Muscle Nerve. 2007;35(5):625–631. doi: 10.1002/mus.20736. [DOI] [PubMed] [Google Scholar]
- 23.Kong H, Fan Y, Xie J, Ding J, Sha L, Shi X, Sun X, Hu G. AQP4 knockout impairs proliferation, migration and neuronal differentiation of adult neural stem cells. J Cell Sci. 2008;121(Pt 24):4029–4036. doi: 10.1242/jcs.035758. [DOI] [PubMed] [Google Scholar]
- 24.Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12(16):2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams ME, Mueller HA, Froehner SC. In vivo requirement of the alpha-syntrophin PDZ domain for the sarcolemmal localization of nNOS and aquaporin-4. J Cell Biol. 2001;155(1):113–122. doi: 10.1083/jcb.200106158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neely JD, Amiry-Moghaddam M, Ottersen OP, Froehner SC, Agre P, Adams ME. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc Natl Acad Sci U S A. 2001;98(24):14108–14113. doi: 10.1073/pnas.241508198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokota T, Miyagoe Y, Hosaka Y, Tsukita K, Kameya S, Shibuya S, Matsuda R, Wakayama Y, Takeda S. Aquaporin-4 is absent at the sarcolemma and at perivascular astrocyte endfeet in a1-syntrophin knockout mice. Proc Japan Acad. 2000;76(2):22–27. [Google Scholar]
- 28.Au CG, Butler TL, Egan JR, Cooper ST, Lo HP, Compton AG, North KN, Winlaw DS. Changes in skeletal muscle expression of AQP1 and AQP4 in dystrophinopathy and dysferlinopathy patients. Acta Neuropathol. 2008;116:235–246. doi: 10.1007/s00401-008-0369-z. [DOI] [PubMed] [Google Scholar]
- 29.Denker BM, Smith BL, Kuhajda FP, Agre P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J Biol Chem. 1988;263(30):15634–15642. [PubMed] [Google Scholar]
- 30.Preston GM, Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA. 1991;88(24):11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith BL, Agre P. Erythrocyte Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J Biol Chem. 1991;266(10):6407–6415. [PubMed] [Google Scholar]


