Abstract
The Maternal Vitamin D Osteoporosis (MAVIDOS) trial reported higher total body bone mineral content in winter-born infants of mothers receiving vitamin D supplementation [1000 IU/day cholecalciferol] compared with placebo from 14 weeks gestation until delivery. This sub-study aimed to determine whether antenatal vitamin D supplementation altered postnatal bone formation in response to mechanical stimulation. Thirty-one children born to MAVIDOS participants randomised to either placebo (n=19) or cholecalciferol (n=12) were recruited at age 4-5 years. Children received whole body vibration (WBV) for 10 minutes on 5 consecutive days. Fasting blood samples for bone homeostasis, 25 hydroxyvitamin D (25OHD), parathyroid hormone (PTH), and bone turnover markers (Pro-collagen Type 1 N-terminal propeptide, P1NP; Cross-linked C-telopeptide of Type I Collagen, CTX) were collected pre-WBV and on day 8 (D8). Mean changes (D) in P1NP (ng/ml) between baseline and D8 in the vitamin-D intervention and placebo groups were 40.6 and -92.6 respectively and mean changes (Δ) in CTX (ng/ml) were 0.034 (intervention) and -0.084 (placebo) respectively. Between-group DP1NP difference was 133.2ng/ml [95% CI 0.4, 266.0; p=0.049] and ΔCTX 0.05ng/ml (95% CI -0.159, 0.26ng/mL; p=0.62). Antenatal vitamin-D supplementation resulted in increased P1NP in response to WBV, suggesting early life vitamin D supplementation increases the anabolic response of bone to mechanical loading in children.
Keywords: Vitamin D, MAVIDOS, Whole Body Vibration, Mechanical Stimulation, Bone Turnover Markers
Introduction
Vitamin D is widely recognised as essential for normal skeletal metabolism. In childhood, maintenance of sufficient serum 25-hydroxyvitamin D (25OHD) levels is critical to prevent adverse outcomes, including hypocalcaemic seizures, cardiomyopathy, rickets and growth failure.
There is also increasing recognition that maternal vitamin D status might be an important determinant of offspring bone development[2-4]. Lower bone mass accrual in childhood resulting in bones that are of reduced size in relation to body size is predictive of increased fracture risk[5]. Observational studies have demonstrated that lower maternal serum 25OHD during pregnancy and at birth are associated with reduced bone width and mass in the offspring at 8-9 years age[6]. The Southampton Women’s Survey also reported a positive correlation between maternal 25OHD status and offspring bone mineral accrual[7] at age 6-7 years[8]. The study from the Australian Raine Cohort demonstrated a positive relationship between maternal vitamin D status and offspring bone mass at 20 years[9]. Equally there are observational data that do not support these findings[10]. The ALSPAC study demonstrated no association between maternal vitamin D status and offspring bone mass at 9 years[11]. Similarly, Garcia et al. reported no association between maternal 25OHD concentrations in mid-pregnancy and offspring bone mineral density[12].
The Maternal Vitamin D Osteoporosis Study (MAVIDOS), a multicentre, randomised, double-blind, placebo controlled trial of 1000 IU/day cholecalciferol vs. placebo from 14 weeks gestation to birth, was conducted at three UK centres (Southampton, Oxford and Sheffield)[4]. Although no significant differences in neonatal bone indices (bone area, bone mineral content (BMC), bone mineral density (BMD)) were detected between randomization groups overall, in a pre-specified analysis, an interaction between treatment group and offspring season of birth was detected. Whole body BMC and BMD were shown to be approximately 9% and 5% higher, respectively, in children born in winter (December to February) to mothers randomised to cholecalciferol compared to those randomised to placebo, a difference in BMC of around 0.5 standard deviations (p=0.004)[1].
The growing skeleton responds to mechanical stimulation with an increase in bone size and mass and that can persist into adult life[13,14]. In contrast, without mechanical stimulation, peak bone mass may not be fully achieved[15,16]. Data from our preclinical murine model study looking at the effect of reduced vitamin D intake during pregnancy and early life on the skeleton’s response to mechanical loading demonstrated that antenatal vitamin D depletion substantially reduces the loading-dependent increase in both cortical and trabecular bone mass of offspring mice[17]. We have also demonstrated in a cohort of healthy pre-pubertal boys aged between 9 and 11 years, that brief exposure to whole body vibration - 10 minutes daily for five days - increases the bone formation marker N-terminal propeptide of type I procollagen (PINP) by 25% and the bone resorption marker C-terminal telopeptide of type I collagen (CTX) by 11%[18].
The main aim of our study was to investigate whether antenatal vitamin D supplementation altered postnatal bone formation in response to mechanical stimulation by WBV. Our hypothesis was that children born to mothers who received vitamin D supplementation during pregnancy would have a greater increase in the bone formation marker P1NP in response to WBV than children whose mothers had received placebo.
Materials and methods
Study design
This was a prospective single centre interventional study in which each subject participated in the trial for a total of 8 days. The study sample consisted of children born to mothers who had participated in the MAVIDOS study. Details of the MAVIDOS study have been published previously[1,4], but briefly, women with a baseline 25(OH)D between 25 and 100 nmol/l at 11-14 weeks’ gestation were randomized to either placebo or 1000 IU/day cholecalciferol from 14 weeks gestation until delivery (n=1134). Women were recruited into the MAVIDOS study from three UK centres: Southampton, Oxford and Sheffield. All children born to mothers who participated in MAVIDOS trial in Sheffield (n=56) were invited to participate in this sub-study.
Prior to undertaking this study, we conducted a patient and public involvement (PPI) focus group. A total of six mothers from various occupational backgrounds ranging from homemaker to a mathematician took part in the focus group. A copy of the lay summary, patient information leaflet(s) and the consent form has been forwarded to all the volunteers 48 hours prior to the meeting. All the six volunteers actively took part in the discussion and shared their comments, views and suggestions openly to the group. They made suggestions to the wordings of the participant information sheet, timings and place of tests that are more comfortable for young children. We based our focus group discussion from our earlier work in this area[18].
All potential participants were contacted by methods discussed and agreed by the patient and public involvement (PPI) focus group of this study. Information sheets detailing the study procedures with our contact details were sent out to the families of potential participants who had expressed an interest in the study prior to their attendance. Parents and families were clearly informed that this study was an additional study to MAVIDOS, so they were free to decline without their participation in the core trial protocol being affected.
The Yorkshire and Humber South Yorkshire Research Ethics Committee approved the study. Written informed consent was obtained from the parents and guardians of all study participants. The ClinicalTrials.gov registration number is NCT02743559.
Study participants
There were a total of 56 potential participants. The children were aged between 4 and 5 years at the time of recruitment and they formed 2 groups, (i) an intervention group whose mothers had received antenatal vitamin D (cholecalciferol 1000 IU/day) supplements (n=29) and (ii) a placebo group (n=27) (Figure 1).
Figure 1.
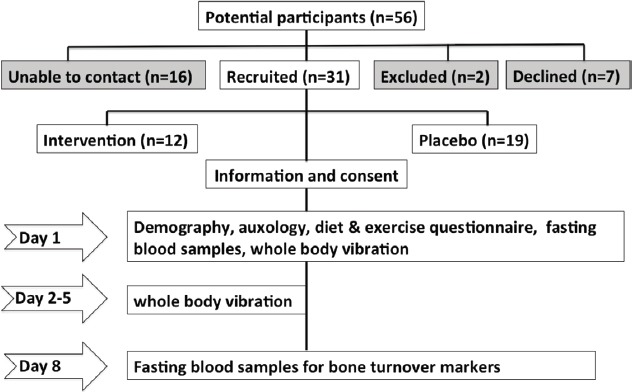
Study design.
Children with balance problems, existing or healing fractures, any chronic illness involving bone, liver or renal systems, with known disorders of growth or glucose metabolism, or with existing long-term use of steroids, anticonvulsants or any medication that might affect calcium and vitamin D metabolism were excluded.
Enrolment into the study and first day procedures took place in the Clinical Research Facility at Sheffield Children’s Hospital; other study procedures took place at the participant’s home.
Anthropometry, activity and diet
Anthropometry was undertaken with the participants wearing light clothing (vest and pants). Height (without shoes, to next succeeding 1 mm) by wall-mounted stadiometer (Holtain, Crymych), and weight (to nearest 0.1 kg) by electronic balance scales (Seca GmbH & Co, Hamburg, Germany) were measured. Body mass index (BMI) was derived as [weight (kg)/height (m)[2]]. A validated questionnaire regarding exercise[19] was used to ascertain the amount of physical activity undertaken over the 7 days prior to standing on the platform and then again on day 8 to determine whether undergoing WBV had had an effect on participant’s levels of physical activity. The frequency and intensity of the types of exercise given in answer to these questions were multiplied by the anticipated metabolic equivalents (METs) of nine, five and three for strenuous, moderate, and mild exercise respectively, to provide an activity score for comparison. The “Rapid assessment of dietary calcium intake” questionnaire was given to all participants and their parents/carers[20].
Laboratory methods
Fasting blood samples for bone homeostasis: calcium, phosphorus, alkaline phosphatase, magnesium, PTH, serum 25OHD; and for bone turnover markers (P1NP, CTX); were collected immediately pre-vibration on day 1 and again on day 8.
Serum calcium, phosphate, albumin, and alkaline phosphatase were measured using Micro Slide Technology Colorimetric/Rate by Reflectance Spectrophotometry in the Vitros 5, 1 FS System (Ortho Clinical Diagnostics; Raritan, New Jersey) analyser. The interassay CVs were: calcium (1.4%), phosphate (1.6%), albumin (2.9%), and alkaline phosphatase (2.4%). Intact parathyroid hormone (PTH) was measured using Immunoassay (Chemiluminescent Microparticle Immunoassay) in the Architect i 1000 System (Abbott; Abbott Park, Illinois) (PTH analytical sensitivity ≤1 ng/L). Serum total 25-hydroxyvitamin D (25OHD) levels were determined using an UPLC/Mass Spectrometer Semi-automated hexane extraction in the Acquity Ultra Performance LC/Quattro MS (Waters; Milford, Massachusetts) analyser. Lower limit of detection for 25OHD2 was 6 nmol/L and for 25OHD3 3.5 nmol/L. The interassay coefficient of variation (CV) for 25OHD2 and 25OHD3 were 5.7% and 5.4% respectively. Blood samples for bone formation marker P1NP was measured using automated immunoassay (Elecsys, Cobas E11, Roche Diagnostics, UK; intraassay % CV<1.7%) and bone resorption marker CTX (Elecsys β-CrossLaps/serum kit, Cobas E411, Roche Diagnostics, UK; intraassay % CV 2.8-8.4%). All samples were collected in the mornings approximately between 0800 and 0900 hours following an overnight fast. Samples were centrifuged, separated and stored at -80°C within 2 hours of collection.
Whole body vibration intervention
The participants were asked to stand barefoot and facing forwards on a portable Marodyne LivMD low magnitude (0.4g), high frequency (30-90 Hz) vibrating platform (Marodyne Medical, Inc., Lakeland, Florida) to receive whole body vibration (WBV). WBV was delivered in 4 cycles of 2 minutes 30 seconds each, separated by 30 seconds off the platform, providing 10 minutes of vibration every day for 5 consecutive days around the same time between 7 and 8 am. Both participants and their carers were taught to use the vibrating plate at home. Delivering vibration in this pattern allowed the participants to become accustomed to the platform in a more comfortable manner. Additionally, it has also been demonstrated that insertion of rest periods enhances the anabolic effect of loading on bone[21,22].
Statistical analysis
Sample size was estimated using the results from our previous WBV study done on healthy pre-pubertal boys, in which a mean difference of 175.6 (SD=162.7) in PINP between day 0 and day 8 was found[18]. To detect 50% of the difference in PINP between children of mothers who were deficient in vitamin D versus those replete in pregnancy, at the 5% significance level with 90% power, would require 20 observations at day 8 (10 per group).
We performed all our analyses using Statistical Package for the Social Sciences version 22 (SPSS by IBM), Data Desk™ v6.2.1, and Stata v15[23]. Groups were compared at baseline using either t-test (continuously distributed data) or proportions test (categorical data). Baseline data are summarized by median (25th/75th centiles) for continuously distributed data or n (%) for categorical data. Graphical presentation of bone turnover markers (P1NP and CTX) was illustrated using box and whisker plots, which have the following interpretation. The median is shown as the middle of the box, the 75th centile the top of the box, the 25th centile the lower of the box. Whiskers (above and below) the box represent values outside the main distribution. Outliers are shown as circles. Differences (post vibration - baseline) between randomised groups (intervention i.e vitamin D supplementation in pregnancy vs placebo) for PTH, 25OHD, bone profile, and bone turnover markers were analysed by an independent t-test using an arbitrary level of 5% statistical significance (two-tailed). Ninety five percent (95%) confidence intervals (CIs) were estimated.
Results
31 children (placebo group n=19; cholecalciferol group n=11) participated in the study. Baseline characteristics of the study subjects are shown in Table 1. Age, gender, ethnicity, height, weight, and BMI were similar between the groups. Bone profile including serum 25OHD and PTH were normal in the entire cohort and there were no significant differences between the groups.
Table 1.
Baseline characteristics. Data are median (25th/75th centiles) or n (%). Bold type indicates significant p value.
| Variable | Intervention | Placebo | p value |
|---|---|---|---|
| Age (years) | 4.9(4.4,5.2) | 5.1(4.5,5.4) | 0.318 |
| Gender (female) | 7(58%) | 8(42%) | 0.295 |
| Ethnicity (caucasian) | 12(100%) | 14(74%) | 0.21 |
| Height (cms) | 107.5(102.5, 113.4) | 108.1 (103.8, 114) | 0.43 |
| Weight (kg) | 18.3 (16.9, 21.7) | 17.8 (16.2, 18.8) | 0.68 |
| BMI (kg/m2) | 16.1 (15.7, 17.0) | 15.0 (14.1, 16.2) | 0.16 |
| Serum 25OHD (nmol/l) | 71(58,98) | 79(61,81) | 0.537 |
| Serum calcium (mmol/l) | 2.4(2.37,2.42) | 2.35(2.31,2.42) | 0.615 |
| Serum phosphate (mmol/l) | 1.52(1.5,1.62) | 1.61(1.55,1.69) | 0.058 |
| Serum alkaline Phosphatase (u/l) | 204.5(180,215) | 200(186,213) | 0.864 |
| Serum albumin (g/l) | 42(41,44) | 42(41.5,43.5) | 0.634 |
| Serum magnesium (mmol/l) | 0.85(0.85,0.89) | 0.84(0.82,0.88) | 0.379 |
| Serum parathyroid hormone (ng/l) | 27.9(24.0,34.1) | 36.5(22.3,40.0) | 0.685 |
| Serum P1NP (ng/ml) | 552.2(512.3,678.6) | 587.1(518.2,801.7) | 0.558 |
| Serum CTX (ng/ml) | 1.52(1.3,1.81) | 1.59(1.3,1.81) | 0.618 |
| Dietary calcium intake (gms/day) | 783(577,842) | 521(430,535) | 0.040 |
| Activity score (METS units) | 66 (44,83) | 104 (68,120) | 0.040 |
Data acquisition on diet and exercise shown in Table 1 were limited due to non-filling of questionnaires or failed recall of requested information in 30% of cases (similar in both groups). Dietary calcium intake (grams/day) was significantly higher in the intervention group (p=0.04). The activity score (METs units) was significantly higher in the placebo group when compared with the intervention group (p=0.04).
Median (25th/75th centiles) P1NP (ng/ml) at baseline was 552.2 (512.3, 678.6) for the intervention group and 587.1 (518.2, 801.7) for the placebo group. Comparison of P1NP at baseline and post vibration by randomisation group is shown in Figure 2. The mean change (Δ) in P1NP (ng/ml) between baseline and D8 in the intervention and placebo groups was 40.6 and -92.6, respectively. The between-group difference in ΔP1NP was 133.2 ng/mL (95% CI 0.4, 266.0; p=0.049). Mean (%) change in P1NP within the intervention group was 11% (pre vs post vibration by patient) and within the placebo group was -13.3%; difference between groups 24% (Table 2).
Figure 2.
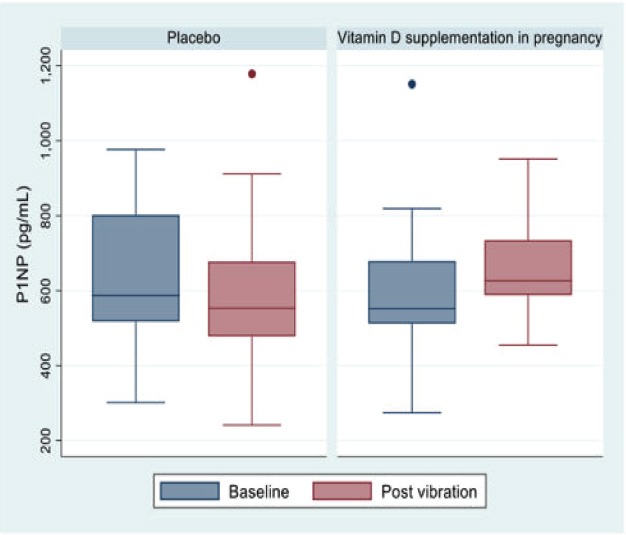
P1NP at baseline and post vibration.
Table 2.
Comparison of bone turnover markers at baseline and post vibration by randomisation group. Data are median (25th/75th centiles) or n (%). Bold type indicates significant p value.
| Variable | Intervention | Placebo | Between group difference (95% confidence interval) | P value |
|---|---|---|---|---|
| Median serum P1NP (ng/ml) at baseline | 552.2(512.3,678.6) | 587.1(518.2,801.7) | - | 0.558 |
| Mean change in P1NP (ng/ml) between baseline and D8 | 40.6 | -92.6 | 133.2 (0.4, 266.0) | 0.049 |
| Mean % change in P1NP between baseline and D8 | 11 | -13.3 | 24 | - |
| Median serum CTX (ng/ml) at baseline | 1.52(1.3,1.8) | 1.59(1.3,1.8) | - | 0.618 |
| Mean change in CTX (ng/ml) between baseline and D8 | -0.034 | -0.084 | 0.05 (-0.159,0.26) | 0.620 |
| Mean % change in CTX between baseline and D8 | -0.5 | -4.9 | 4.4 | - |
Median (25th/75th centiles) for CTX (ng/mL) at baseline was 1.52 (1.3, 1.8) for the intervention group and 1.59 (1.3, 1.8) and for the placebo group. Comparison of CTX at baseline and post vibration by randomisation group is shown in Figure 3. The mean change (Δ) in CTX (ng/ml) between baseline and D8 in the intervention and placebo groups was -0.034 and -0.084 ng/ml, respectively. The between-group difference in ΔCTX was 0.05 ng/ml (95% CI= -0.159,0.26 ng/mL; p=0.62). Mean (%) change in CTX within the intervention group was -0.5% and within the placebo group was -4.9%; difference between groups 4.4% (Table 2).
Figure 3.
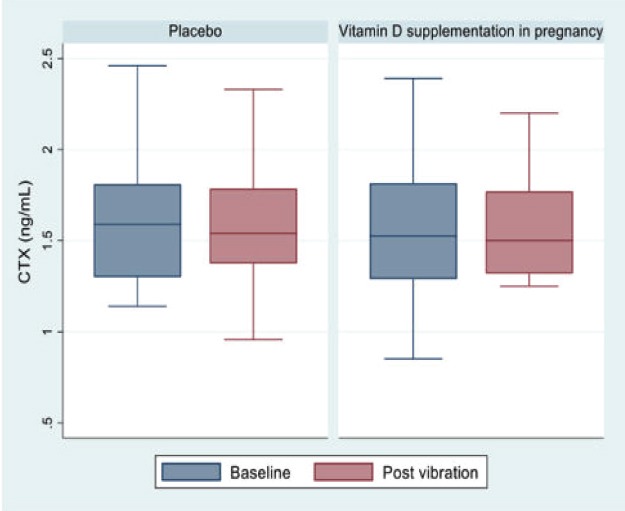
CTX at baseline and post vibration.
Discussion
In this study we have shown that antenatal vitamin D supplementation increases the anabolic response of growing bone to mechanical stimulation. We found that five consecutive days of low magnitude high frequency WBV increased the bone formation marker PINP by 11% between the pre-vibration baseline and the day 8 measurement in children whose mothers had received 1000 IU vitamin D supplementation during pregnancy. By contrast the P1NP decreased by 13% in the placebo group, showing a net benefit of 24% in the intervention group. We did not find a change in the bone resorption marker CTX in either the intervention or the placebo group.
These results are in contrast to the findings of our previous study in which the effect of WBV in 36 healthy pre pubertal boys was examined. Participants in that study received either low (<1 g, frequency 32-37 Hz, amplitude 0.085 mm) or high (>2 g, frequency 5 to 30 Hz, amplitude 0+/-4.5 mm) magnitude vibration for 1, 3 or 5 consecutive days. A significant rise in both P1NP 25.1% (p=0.005) and CTX 10.9% (p=0.009) from baseline to day 8 was observed in the group exposed to 5 days of WBV, irrespective of the mode of vibration[18]. However, subjects in that study were older (9-11 years) and had higher baseline P1NP and CTX levels. It is possible some of those participants, despite being Tanner stage 1, had some early hormonal changes of puberty resulting in a higher P1NP and CTX response to vibration. It may also be the case that there were simply insufficient numbers in this study to detect significant changes in CTX.
The increase in the bone formation marker but not the bone resorption marker in our current study suggests that there is an uncoupling of bone turnover favouring formation more than the resorption in response to mechanical stimulation. Similarly in the previously mentioned study of prepubertal boys a greater increase in bone formation than resorption marker was also observed. Studies have reported increased expression of osteoprotegerin [a factor affecting bone resorption] in serum in association with reduced bone resorption through the activation of the canonical wnt-signalling pathway through LRP5/6[24]; however we did not measure OPG in this study, as we had not previously demonstrated a significant difference in OPG levels between baseline and day 8 following WBV in prepubertal boys[18].
Soderpalm et al. studied the tolerability and effects of WBV on muscle and bone in six ambulatory children with Duchenne Muscular Dystrophy aged between 5-12 years. They did not find a significant change in bone mass or bone turnover markers. However a non-significant trend towards an increase in bone specific alkaline phosphatase was observed after 3 months of WBV, which returned to baseline, 3 months post discontinuation of WBV. Their study population differed to ours, having a significant muscle disease and also being treated with the steroid prednisolone and we did not measure alkaline phosphatase post vibration in our study to compare with Soderpalm’s results. However these results do suggest that the response to WBV of bone turnover markers may be transient and not sustained over long periods of time[25]. Kilebrant et al. studied the effects of WBV on bone mass, bone turnover markers and body composition in 19 children aged between 5 and 16 with severe motor disabilities who used wheelchairs for mobility, but also spent time each day in a standing frame. WBV was delivered 5-15 minutes per treatment twice a week for 6 months. All measurements were undertaken at baseline, 6 and 12 months. They found a significant increase in TBLH BMD and BMC after 6 months of WBV. There were no clear changes in any of the bone formation or resorption markers measured between baseline and 6 months; however, no “early” measurements i.e. within 1-2 weeks of commencing WBV, were made[26]. There are no published studies of the acute response to vibration in children with bone diseases.
The results obtained in our study are consistent with those from our studies of antenatal vitamin D depletion in a preclinical mouse model system. There we demonstrated that despite post-natal vitamin D repletion, the offspring of dams who had been made vitamin D deficient accrued substantially less bone in response to mechanical loading than the offspring of dams who were replete in vitamin D. These effects were apparent during growth affecting both cortical and trabecular bone and after growth had ceased affecting cortical bone accrual at skeletal maturity. It seems possible that this “programming” effect may also be operating in the human situation, given the results demonstrated here, and the observational data from other studies that suggest a positive association of maternal and early infant vitamin D status with later bone size and mass[17]. There are no similar previous experiments of this nature (i.e combining vitamin D depletion with mechanical loading) with which to compare our data.
Strengths and limitations
This is the first study to report the results of mechanical loading of bone in children exposed to high dose (1000 IU daily) vitamin D supplementation in pregnancy. The key strength of our study is the unique study cohort that has been randomised to vitamin D or placebo antenatally, combined with the novel technique of stimulating bone in a consistent way and measuring the response using serum markers of bone formation and resorption.
The focus of the study was only to measure the change in two specific bone turnover markers (PINP and CTX) in response to loading and therefore we were unable to determine any mechanism that might link antenatal vitamin D supplementation with this response. Muscle strength, a determinant of bone mass, was not measured. This study was conducted in only 1 out of 3 MAVIDOS centers, thus sample size was relatively small with a limited number of subjects in the intervention group. Dietary calcium intake (grams/day) was significantly higher in the intervention group (p=0.04). The activity score (METs units) was significantly higher in the placebo group when compared with the intervention group (p=0.04). These could potentially have interacted with the effect of any prenatal programming on bone formation and resorption markers. However, failure to ascertain dietary and exercise data in nearly a third of participants means that drawing conclusions about potential interactions of such factors with the response to vibration is difficult. Equally adjusting for both dietary calcium and exercise at baseline had little influence on the change in PINP and CTX.
Conclusions
Our study demonstrates that children born to mothers who received vitamin D supplementation during pregnancy have a greater bone formation response to mechanical stimulation. This implies early life vitamin D supplementation increases the anabolic response of bone to mechanical loading in children. We suggest that supplementation of pregnant women with a higher dose of vitamin D may improve bone health in early childhood, which in turn may increase lifetime bone accrual and reduce the risk of developing osteoporosis and fragility fractures in later life as adults. Given the limited sample size, confirmation in a larger cohort is needed, along with prospective data collection on fractures.
Footnotes
JSG-K, ASR, RCH, RJM, EMC, have nothing to disclose. CC reports personal fees from ABBH, AMGEN, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfzer, Roche, Servier and Takeda, outside the submitted work. NCH reports personal fees, consultancy, lecture fees and honoraria from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Servier, Shire, Consilient Healthcare and Internis Pharma, outside the submitted work. RE reports consulting fees from Amgen, AstraZeneca, Chronos, GSK, Immunodiagnostic Systems, Fonterra Brands, Ono Pharma, Lilly, Bayer, Janssen Research, Alere, CL Biosystems, Teijin Pharm, D-Star, Roche Diagnostics, Inverness Medical; grant support from Amgen, Alexion, Immunodiagnostic Systems, Roche, AstraZeneca. NJB reports personal fees from Internis, grants and personal fees from Alexion Pharma, personal fees from Mereo Biopharma, grants and personal fees from Amgen, personal fees from Novartis, outside the submitted work.
Edited by: G. Lyritis
References
- 1.Cooper C, Harvey N, Bishop N, Kennedy S, Papageorghiou A, Schoenmakers I, et al. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. The Lancet Diabetes & Endocrinology. 2016;4(5):393–402. doi: 10.1016/S2213-8587(16)00044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godfrey K, Walker-Bone K, Robinson S, Taylor P, Shore S, Wheeler T, et al. Neonatal Bone Mass: Influence of Parental Birthweight, Maternal Smoking, Body Composition, and Activity During Pregnancy. Journal of Bone and Mineral Research. 2001;16(9):1694–1703. doi: 10.1359/jbmr.2001.16.9.1694. [DOI] [PubMed] [Google Scholar]
- 3.Viljakainen H, Saarnio E, Hytinantti T, Miettinen M, Surcel H, Mäkitie O, et al. Maternal Vitamin D Status Determines Bone Variables in the Newborn. The Journal of Clinical Endocrinology & Metabolism. 2010;95(4):1749–1757. doi: 10.1210/jc.2009-1391. [DOI] [PubMed] [Google Scholar]
- 4.Harvey N, Javaid K, Bishop N, Kennedy S, Papageorghiou A, Fraser R, et al. MAVIDOS Maternal Vitamin D Osteoporosis Study: study protocol for a randomized controlled trial. The MAVIDOS Study Group. Trials. 2012:13. doi: 10.1186/1745-6215-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark E, Ness A, Bishop N, Tobias J. Association Between Bone Mass and Fractures in Children: A Prospective Cohort Study. Journal of Bone and Mineral Research. 2006;21(9):1489–1495. doi: 10.1359/jbmr.060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javaid M, Crozier S, Harvey N, Gale C, Dennison E, Boucher B, et al. Maternal Vitamin D Status During Pregnancy and Childhood Bone Mass at Age 9 Years: A Longitudinal Study. Obstetrical & Gynecological Survey. 2006;61(5):305–307. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 7.Harvey N, Javaid M, Poole J, Taylor P, Robinson S, Inskip H, et al. Paternal Skeletal Size Predicts Intrauterine Bone Mineral Accrual. The Journal of Clinical Endocrinology & Metabolism. 2008;93(5):1676–1681. doi: 10.1210/jc.2007-0279. [DOI] [PubMed] [Google Scholar]
- 8.Moon R, Harvey N, Davies J, Cooper C. Vitamin D and bone development. Osteoporosis International. 2014;26(4):1449–1451. doi: 10.1007/s00198-014-2976-y. [DOI] [PubMed] [Google Scholar]
- 9.Zhu K, Whitehouse A, Hart P, Kusel M, Mountain J, Lye S, et al. Maternal Vitamin D Status During Pregnancy and Bone Mass in Offspring at 20 Years of Age: A Prospective Cohort Study. Journal of Bone and Mineral Research. 2014;29(5):1088–1095. doi: 10.1002/jbmr.2138. [DOI] [PubMed] [Google Scholar]
- 10.Moon R, Davies J, Cooper C, Harvey N. Vitamin D, and Maternal and Child Health. Calcified Tissue International. 2020;106(1):30–46. doi: 10.1007/s00223-019-00560-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawlor D, Wills A, Fraser A, Sayers A, Fraser W, Tobias J. Association of maternal vitamin D status during pregnancy with bone-mineral content in offspring: a prospective cohort study. The Lancet. 2013;381(9884):2176–2183. doi: 10.1016/S0140-6736(12)62203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia A, Erler N, Jaddoe V, Tiemeier H, van den Hooven E, Franco O, et al. 25-hydroxyvitamin D concentrations during fetal life and bone health in children aged 6 years: a population-based prospective cohort study. The Lancet Diabetes & Endocrinology. 2017;5(5):367–376. doi: 10.1016/S2213-8587(17)30064-5. [DOI] [PubMed] [Google Scholar]
- 13.Bailey D, Mckay H, Mirwald R, Crocker P, Faulkner R. A Six-Year Longitudinal Study of the Relationship of Physical Activity to Bone Mineral Accrual in Growing Children: The University of Saskatchewan Bone Mineral Accrual Study. Journal of Bone and Mineral Research. 1999;14(10):1672–1679. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 14.Ducher G, Daly R, Bass S. Effects of Repetitive Loading on Bone Mass and Geometry in Young Male Tennis Players: A Quantitative Study Using MRI. Journal of Bone and Mineral Research. 2009;24(10):1686–1692. doi: 10.1359/jbmr.090415. [DOI] [PubMed] [Google Scholar]
- 15.Heaney R, Abrams S, Dawson-Hughes B, Looker A, Looker A, Marcus R, et al. Peak Bone Mass. Osteoporosis International. 2001;11(12):985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 16.Weaver C, Gordon C, Janz K, Kalkwarf H, Lappe J, Lewis R, et al. The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporosis International. 2016;27(4):1281–1386. doi: 10.1007/s00198-015-3440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borg S, Buckley H, Owen R, Marin A, Lu Y, Eyles D, et al. Early life vitamin D depletion alters the postnatal response to skeletal loading in growing and mature bone. PLOS ONE. 2018;13(1):e0190675. doi: 10.1371/journal.pone.0190675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison R, Ward K, Horne C, Bishop N. Acute bone response to whole body vibration in pre-pubertal boys. Bone. 2011;48 [PMC free article] [PubMed] [Google Scholar]
- 19.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141–146. [PubMed] [Google Scholar]
- 20.Nordblad M, Graham F, Mughal M, Padidela R. Rapid assessment of dietary calcium intake. Archives of Disease in Childhood. 2015;101(7):634–636. doi: 10.1136/archdischild-2015-308905. [DOI] [PubMed] [Google Scholar]
- 21.Gross T, Poliachik S, Ausk B, Sanford D, Becker B, Srinivasan S. Why Rest Stimulates Bone Formation: A Hypothesis Based on Complex Adaptive Phenomenon. Exercise and Sport Sciences Reviews. 2004;32(1):9–13. doi: 10.1097/00003677-200401000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaMothe J, Zernicke R. Rest insertion combined with high-frequency loading enhances osteogenesis. Journal of Applied Physiology. 2004;96(5):1788–1793. doi: 10.1152/japplphysiol.01145.2003. [DOI] [PubMed] [Google Scholar]
- 23.StataCorp 2017. Stata Statistical Software Release 15. StataCorp LLC: College Station, TX; [Google Scholar]
- 24.Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, et al. Sclerostin Binds to LRP5/6 and Antagonizes Canonical Wnt Signaling. Journal of Biological Chemistry. 2005;280(20):19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 25.Soderpalm AC, Kroksmark AK, Magnusson P, Karlsson J, Tulinius M, Swolin-Eide D. Whole body vibration therapy in patients with Duchenne muscular dystrophy--a prospective observational study. J Musculoskelet Neuronal Interact. 2013;13(1):13–8. [PubMed] [Google Scholar]
- 26.Kilebrant S, Braathen G, Emilsson R, Glansén U, Söderpalm A, Zetterlund B, et al. Whole-body vibration therapy in children with severe motor disabilities. Journal of Rehabilitation Medicine. 2015;47(3):223–228. doi: 10.2340/16501977-1921. [DOI] [PubMed] [Google Scholar]


