Abstract
Objective:
To investigate the clinical efficacy of autologous bone marrow mesenchymal stem cell (BMSC) transplantation in the treatment of knee osteoarthritis (OA) and its effect on the expression of serum TNF-α and IL-6.
Methods:
The clinical data of 86 patients with knee OA treated in the Shanghai East Hospital Tongji University from March 2015 to March 2017 were analyzed retrospectively. Observation group (treated with intraarticular injection of autologous BMSC); Control group (treated with arthroscopic debridement and injected with sodium hyaluronate). The patients were followed up for 1 year after treatment, the clinical efficacy was evaluated by the Lysholm Knee Scale (Lysholm), Visual analog scale (VAS), and the expression of serum TNF-α and IL-6 was detected by ELISA.
Results:
The expression of TNF-α and IL-6 at 6 and 12 months after surgery was significantly lower than that before surgery (P<0.05).
Conclusions:
The Lysholm score and total effective rate of knee OA patients underwent BMSC transplantation are better than that of joint debridement, while the VAS score was lower than that of joint debridement. Meanwhile, BMSC transplantation seems to reduce the postoperative inflammatory reaction.
Keywords: Autologous Bone Marrow Mesenchymal Stem Cells, IL-6, Knee Osteoarthritis, Lysholm, TNF-α, VAS
Introduction
Knee osteoarthritis (OA), the most common joint pathology of aging and occurs over time, is usually related to joint overloading, joint injury, and obesity[1]. The common characteristic of OA is articular cartilage damage, accompanied by synovial and subchondral bone lesions[2]. The more serious the degeneration of articular cartilage, the more serious the disease would be, which can cause the destruction of deep bone, synovitis, osteophytosis, subchondral bone hyperplasia and sclerosis, cystic disease[3], and can increase the secretion of pro-inflammatory factors, thus resulting in accelerated degradation of articular cartilage[4]. The knee joint appears repeatedly swelling, pain and rigidity. Patients with severe OA may have joint function constraints, which seriously affect the quality of life, while long-term treatment costs cause a heavy economic burden[5].
At present, drugs and physical therapy for knee OA can only alleviate the disease or relieve pain, and cannot effectively control the disease progression[6,7]. Total knee replacement may be necessary if joint function continues to degrade to the late stage[8]. To prevent the patient from getting worse, and to restore the joint function and reduce the economic loss, it is very important to choose an appropriate treatment method for the patient.
Arthroscopic debridement, a surgery that can be used to treat joints with different degrees of injury and improve the limb function of the patients, has the advantages of a minimally invasive, quick recovery and little interference to the joints, and can effectively delay the degeneration of joint function[9]. Injection of sodium hyaluronate into the articular cavity can effectively protect the soft tissue of the joint, lubricate the joints, alleviate the pain of the patients and delay the further degeneration of the knee[10]. Autologous bone marrow mesenchymal stem cell (BMSC) is derived from autologous bone marrow and can be used in minimally invasive surgery for early arthritis[11]. BMSCs are easy to culture, have the capacity for multipotent differentiation,and have immunosuppressive activity[12], and also plays an important role in cell regeneration, and participate in the regeneration, repair and inflammatory response of connective tissue[13].
Cytokines in human tissues play an important role in the development of OA, which can destroy synovium and articular cartilage[14]. IL-6 is a cytokine with many biological activities induced by TNF-α[15]. And IL-6, TNF-α can be found in macrophages, fibroblasts, and chondrocytes. Cartilage injury can stimulate the secretion of IL-6 and TNF-α[16], while TNF-α plays an important role in synovitis and promote the degradation of the cartilage matrix[17].
There is no comparative study on the clinical efficacy of arthroscopic debridement with the injection of sodium hyaluronate and BMSC transplantation. Therefore, the purpose of this study is to investigate the clinical efficacy of these two methods in the treatment of patients with knee OA and their effects on serum IL-6 and TNF-α, to provide data basis for patients to choose proper treatment methods.
Materials and methods
General information
The clinical data of 86 patients with knee joint OA admitted in the Shanghai East Hospital Tongji University from March 2015 to March 2017 were retrospectively analyzed, including 35 males and 51 females with an age range of 60-78 years old. 40 patients treated with intraarticular injection of autologous BMSC were the observation group, and 46 patients treated with arthroscopic debridement and sodium hyaluronate injection were the control group. There was no significant difference in the general information between the two groups (P>0.05), as shown in Table 1. All the patients were diagnosed with knee OA by X-ray, with the Kellgren-Lawrance grade of 0-II, and had significant joint pain and received the first surgical treatment after ineffective conservative treatment. All patients with clinical data deficiency, malignant tumor, heart, liver and kidney insufficiency, severe diabetes mellitus, osteoporosis, gout and rheumatic osteoarthritis were excluded from the study. This study was approved by the Medical Ethics Committee; all patients and their families were informed and signed consent.
Table 1.
Comparison of general information between two groups of patients.
| Factor | Observation group (n=40) | Control group (n=46) | t/χ2 | P | |
|---|---|---|---|---|---|
| Age (Years) | 67.28±5.35 | 66.52±6.33 | 0.596 | 0.553 | |
| Gender | 1.664 | 0.264 | |||
| Male | 12 (30.00) | 20 (43.48) | |||
| Female | 28 (70.00) | 26 (56.52) | |||
| Disease course (Years) | 7.24±2.86 | 7.13±3.01 | 0.173 | 0.863 | |
| BMI (kg/m2) | 25.27±2.93 | 25.81±3.01 | 0.840 | 0.403 | |
| Kellgren-Lawrance Grade | 0.834 | 0.659 | |||
| 0 | 12 (30.00) | 10 (21.74) | |||
| I | 17 (42.50) | 23 (50.00) | |||
| II | 11 (27.50) | 13 (28.26) | |||
| Living condition | 1.565 | 0.241 | |||
| Humid | 31 (77.50) | 30 (65.22) | |||
| Normal | 9 (22.50) | 16 (34.78) | |||
| History of joint trauma | 0.101 | 0.827 | |||
| Yes | 23 (57.50) | 28 (60.87) | |||
| No | 17 (42.50) | 18 (39.13) |
Treatment methods
The control group received arthroscopic debridement and injection of sodium hyaluronate. Articular cavity exploration and bursae suprapatellaris, meniscus, medial and lateral condyle of femur, patellar joint, lateral recess and ligaments clearance were conducted. Inflammatory synovium was flushed and cleaned, hyperplastic osteophyte was repaired and supporting ligament was released. 2 ml of sodium hyaluronate was injected intraarticularly (Shandong Bausch+lomb Fruida Pharmaceutical co., Ltd., SFDA Approval No. H10960136) after clearance, once a week for 5 weeks.
The observation group received autologous BMSC transplantation. Preparation of platelet lysate: 400mL of venous blood was centrifuged to obtain the supernatant, frozen at -20o for half a day, and then stored at -80o for reserve. After dissolved in water bath at 37o, the solution was centrifuged again to obtain the supernatant; then the platelet lysate was prepared. The solution dissolved in a 37o water bath was dispensed by sterile needle tube for use.
BMSC isolation culture: 50 ml of bone marrow was extracted from the patient’s posterior superior iliac spine. The nucleated cells were isolated by density gradient centrifugation, and then the isolated BMSCs were subjected to adherent culture and passage, and the cells were dispensed at the second passage and stored at -80o for use.
Injection of BMSC in the knee cavity: frozen BMSCs were resuscitated and cultured 3 days before surgery. The BMSCs were collected by 0.25% trypsin (Wuhan Hualianke Biotechnology Co., Ltd., cat No. TE2004Y) on the day of surgery, and centrifuged at 2000rpm for 6 min, then the supernatant was discarded and 3 mL of the platelet lysate was added for resuspension. A syringe was used to withdraw the joint fluid and the needle tube was removed, then the BMSC suspension sterile needle tube was inserted into the indwelling needle and slowly injected into the articular cavity. Pull out the needle and press the needle hole for 5 min, prone for 30 min and move the knee joint 8-10 times every 5 min, a total of 3 courses of treatment, with an interval of one month. And each course of treatment was divided into three steps, 3 mL of platelet lysate was injected on the 1st day of surgery, 3 mL of BMSC suspension was injected on the 4th day.
Postoperative care: Patients with no swelling pain, no abnormal blood routine, liver and kidney function discharged. After discharge from the hospital, patients avoided strenuous exercise and had bed rest, proper knee and muscle function training, regular blood routine monitoring, liver and kidney function exams and treatment according to doctor’s advice. Once adverse reactions occurred, treatment stopped immediately.
Observation index
The patients in both groups were followed up for 12 months after treatment, the clinical efficacy in the two groups was evaluated by The Lysholm Knee Scale (Lysholm), Visual analog scale (VAS) and total effective rate, and the expression level of serum TNF-α and IL-6 before and after treatment was detected by ELISA. Lysholm was used to evaluate the recovery of the patients. VAS was used to evaluate the degree of pain. WOMAC was used to access the effective number of patients: Patients with a WOMAC score of 0 and no clinical symptoms were considered cured; Patients with a decreased WOMAC score of 1-2 compared with that before treatment and mild clinical symptoms were considered significantly effective; Patients with a decreased WOMAC score compared with that before treatment and a remission were considered effective; Patients with an unchanged WOMAC score compared with that before treatment and unchanged or aggravated clinical symptoms were considered ineffective. Total effective rate was calculated as (number of cured + significantly effective + effective) / total number of patients.
Determination of serum TNF- α and IL-6 levels in two groups of patients
1mL of articular fluid was obtained before, 6 months, 12 months after treatment, respectively and centrifuged at 3000rpm for 8 min. The level of serum TNF-α (Shanghai Jingkang Bioengineering Co., Ltd., cat no. JK-(a)-0016) and IL-6 (Shanghai Jingkang Bioengineering Co., Ltd., cat no. JK-(a)-0023) was detected by ELISA, and OD value at wavelength of 450 nm was determined. The operation was carried out strictly according to the instruction.
Statistical analysis
SPSS 20.0 statistical software (Shanghai Cabit Information Technology Co., Ltd.) was used for analysis. Chi-square test was used for counting data, t-test was used for measurement data, and repeated measures ANOVA was used for comparison of intragroup before and after treatment. Any finding of P<0.05 was considered statistically significant.
Results
Lysholm score of patients in both groups before and after treatment
There was no significant difference in Lysholm score between the two groups before surgery (P>0.05), while the Lysholm score in the observation group was significantly higher than that in the control group at 6 months and 12 months after surgery (P<0. 05). The Lysholm score in the two groups before and after surgery was statistically significant (P<0.001). The Lysholm score in the observation group and the control group at 6 and 12 months after surgery was significantly higher than that before surgery, and the Lysholm score at 12 months after surgery was significantly higher than that at 6 months after surgery, the difference was statistically significant (P<0.05) (Figure 1, Table 2).
Figure 1.
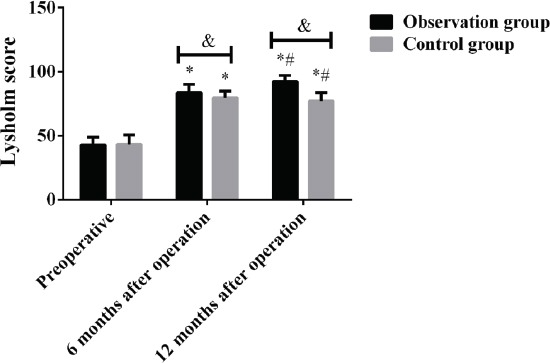
Lysholm score of patients in both groups before and after treatment. Results showed that the Lysholm score in the observation group was significantly higher than that in the control group at 6 months and 12 months after surgery, the difference was statistically significant (P<0.05). The Lysholm score in the observation group and the control group at 6 and 12 months after surgery was significantly higher than that before surgery, and the Lysholm score at 12 months after surgery was significantly higher than that at 6 months after surgery, the difference was statistically significant (P<0.05). &, P<0.05, *compared with that before surgery, P<0.05; #compared with that 6 months after surgery, P<0.05.
Table 2.
Lysholm score of patients in both groups before and after treatment.
| Group | Before surgery | 6 months after surgery | 12 months after surgery | F | P |
|---|---|---|---|---|---|
| Observation group (n=40) | 42.76±6.13 | 83.55±6.52* | 92.34±4.67*# | 824.200 | 0.000 |
| Control group (n=46) | 43.37±7.31 | 79.76±5.16* | 77.23±6.41*# | 470.300 | 0.000 |
| t | 0.416 | 3.006 | 12.330 | ||
| P | 0.679 | 0.004 | 0.000 |
Note: compared with before surgery, P<0.05; #compared with 6 months after surgery, P<0.05.
VAS score of patients in both groups before and after treatment
There was no significant difference in VAS score between the two groups before surgery (P>0.05), while the VAS score in the observation group was significantly lower than that in the control group at 6 months and 12 months after surgery (P<0.05). The VAS score in the two groups before and after surgery was statistically significant (P<0.001). The VAS score in the observation group and the control group at 6 and 12 months after surgery was significantly lower than that before surgery, there was no significant difference in VAS score between 12 months and 6 months after surgery in the observation group (P>0.05), and the VAS score at 12 months after surgery was significantly higher than that at 6 months after surgery in the control group, the difference was statistically significant (P<0.05) (Figure 2, Table 3).
Figure 2.
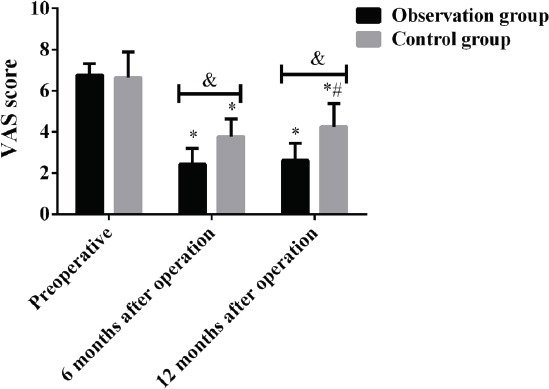
VAS score of patients in both groups before and after treatment. Results showed that the VAS score in the observation group was significantly lower than that in the control group at 6 months and 12 months after surgery, the difference was statistically significant (P<0.05). The VAS score in the observation group and the control group at 6 and 12 months after surgery was significantly lower than that before surgery (P>0.05), and the VAS score at 12 months after surgery was significantly higher than that at 6 months after surgery in the control group, the difference was statistically significant (P<0.05). &,P<0.05, *compared with that before surgery, P<0.05; #compared with that 6 months after surgery, P<0.05.
Table 3.
VAS score of patients in both groups before and after treatment.
| Group | Before surgery | 6 months after surgery | 12 months after surgery | F | P |
|---|---|---|---|---|---|
| Observation group (n=40) | 6.76±0.57 | 2.43±0.78* | 2.61±0.83* | 443.900 | 0.000 |
| Control group (n=46) | 6.65±1.24 | 3.76±0.86* | 4.25±1.12*# | 93.470 | 0.000 |
| t | 0.515 | 7.468 | 7.617 | ||
| P | 0.608 | 0.000 | 0.000 |
Note: compared with before surgery, P<0.05; #compared with 6 months after surgery, P<0.05.
Comparison of the total effective rate between the two groups
In the observation group, there was 1 case of cure, 14 cases of significantly effective, 13 cases of effective, 12 cases of ineffective; In the control group, there was 0 case of cure, 4 cases of significantly effective, 17 cases of effective, 25 cases of ineffective. The total effective rate in the observation group (70.00%) was significantly higher than that in the control group (45.65%), and the difference was statistically significant (P<0.05) (Table 4).
Table 4.
Comparison of the total effective rate between the two groups.
| Group | Cure | Significantly effective | Effective | Ineffective | Total effective rate |
|---|---|---|---|---|---|
| Observation group (n=40) | 1(2.50) | 14(35.00) | 13(32.50) | 12(30.00) | 28(70.00) |
| Control group (n=46) | 0 | 4(8.70) | 17(36.95) | 25(54.35) | 21(45.65) |
| χ2 | - | - | - | - | 5.174 |
| P | - | - | - | - | 0.030 |
Expression level of serum TNF-α and IL-6 of patients in the two groups before and after treatment
There was no significant difference in the expression of TNF-α and IL-6 between the two groups before surgery (P>0.05), while the expression of TNF-α and IL-6 in the observation group was significantly lower than that in the control group at 6 months and 12 months after surgery (P<0.05). The expression of TNF-α and IL-6 in the two groups before and after surgery was statistically significant (P<0.001). The expression of TNF-α and IL-6 in the observation group and the control group at 6 and 12 months after surgery was significantly lower than that before surgery, and the expression at 12 months after surgery was significantly lower than that at 6 months after surgery, the difference was statistically significant (P<0.05) (Figure 3, Tables 5 and 6).
Figure 3.
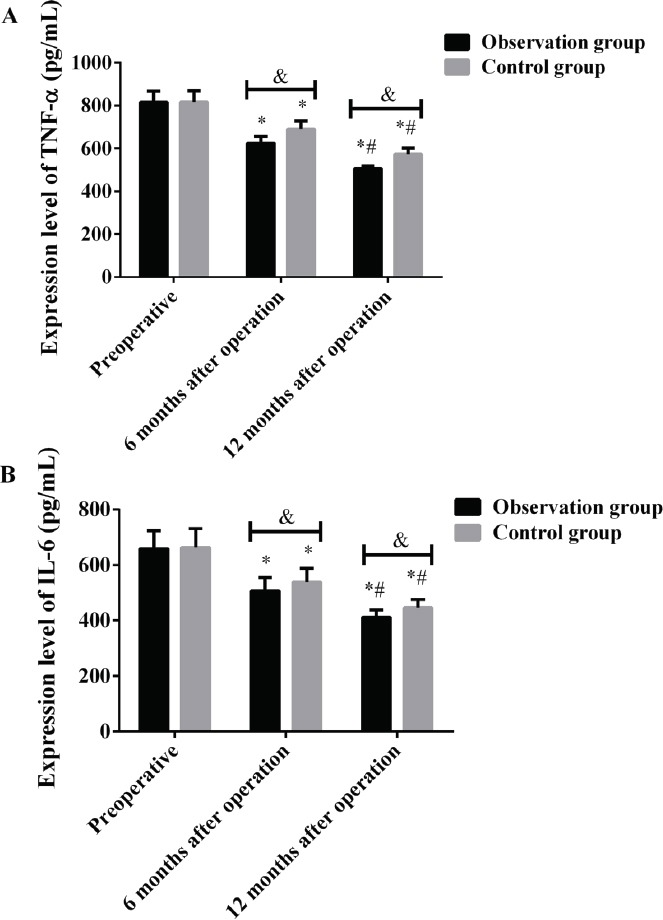
Expression level of serum TNF-α and IL-6 of patients in the two groups before and after treatment. Results of ELISA showed that, A: The expression of TNF-α in the observation group was significantly lower than that in the control group at 6 months and 12 months after surgery, the difference was statistically significant (P<0.05). The expression of TNF-α in the observation group and the control group at 6 and 12 months after surgery was significantly lower than that before surgery, and the expression at 12 months after surgery was significantly lower than that at 6 months after surgery, the difference was statistically significant (P<0.05). B: The expression of IL-6 in the observation group was significantly lower than that in the control group at 6 months and 12 months after surgery, the difference was statistically significant (P<0.05). The expression of IL-6 in the observation group and the control group at 6 and 12 months after surgery was significantly lower than that before surgery, and the expression at 12 months after surgery was significantly lower than that at 6 months after surgery, the difference was statistically significant (P<0.05). &, P<0.05, *compared with that before surgery, P<0.05; #compared with that 6 months after surgery, P<0.05.
Table 5.
Expression level of serum TNF-α of patients in the two groups before and after treatment (pg/mL).
| Group | Before surgery | 6 months after surgery | 12 months after surgery | F | P |
|---|---|---|---|---|---|
| Observation group (n=40) | 815.48±52.31 | 624.23±31.85* | 505.75±12.34*# | 750.900 | 0.000 |
| Control group (n=46) | 816.52±53.28 | 690.56±38.24* | 573.26±28.63*# | 398.900 | 0.000 |
| t | 0.091 | 8.663 | 13.83 | ||
| P | 0.928 | 0.000 | 0.000 |
Note: compared with before surgery, P<0.05; #compared with 6 months after surgery, P<0.05.
Table 6.
Expression level of serum IL-6 of patients in the two groups before and after treatment (pg/mL).
| Group | Before surgery | 6 months after surgery | 12 months after surgery | F | P |
|---|---|---|---|---|---|
| Observation group (n=40) | 658.26±65.23 | 506.52±48.77* | 411.34±26.75*# | 253.200 | 0.000 |
| Control group (n=46) | 663.64±67.58 | 537.85±49.63* | 446.13±29.51*# | 208.300 | 0.000 |
| t | 0.374 | 2.944 | 5.694 | ||
| P | 0.709 | 0.004 | 0.000 |
Note: compared with before surgery, P<0.05; #compared with 6 months after surgery, P<0.05.
Discussion
OA is one of the most frequent and highly devastating joint disease, with approximately 355 million patients worldwide (18) and approximately 8.5 million patients in the UK[19]. According to the statistics in 2004, among people aged over 60 years, the prevalence of symptomatic OA was 9.6% in males and 18% in females[20]. Studies of Garay[21] et al. showed that knee pain and quality of life were significantly improved in patients treated with BMSC. Yamasaki[22] showed that transplantation of BMSC into rabbit articular cartilage defect could regenerate cartilage tissue. The BMSC transplantation in human could alleviate clinical symptoms of cartilage defect of knee joint and elbow joint. According to Li[23], arthroscopic debridement combined with intraarticular injection of sodium hyaluronate could significantly improve the Lysholm score in patients with knee OA. Moreover, the study results of Sun[24] showed that arthroscopic debridement combined with intraarticular injection of sodium hyaluronate in the treatment of knee OA could significantly improve the symptoms of knee joint pain, facilitate the recovery of knee joint function, and have fewer complications.
The results showed that the Lysholm score in the observation group and the control group increased gradually, but the score of the patients in the observation group was significantly higher than that in the control group at 6 months and 12 months after operation (P<0.05), suggesting that the patients in both groups had different degrees of recovery and alleviated clinical symptoms after surgery, but the recovery of OA patients who underwent BMSC transplantation was better. The VAS score in the observation group was significantly lower than that in the control group at 6 months and 12 months after surgery (P<0.05). The VAS score in the observation group and the control group at 6 and 12 months after surgery was significantly lower than that before surgery, there was no significant difference in VAS score between 12 months and 6 months after surgery in the observation group (P>0.05), and the VAS score at 12 months after surgery was significantly higher than that at 6 months after surgery in the control group (P<0.05). These results showed that the pain was relieved in patients of both groups, but the pain of OA patients treated with sodium hyaluronate injection after debridement increased 12 months after surgery, which indicated a unstable therapeutic effect. The total effective rate in the observation group (70.00%) was significantly higher than that in the control group (45.65%) (P<0.05). Based on the results of Lysholm, VAS and total effective rate, the clinical efficacy of BMSC transplantation is better than that of arthroscopic debridement in the treatment of knee OA. It is speculated that OA is caused by the deficiency and degeneration of repairability of mesenchymal stem cells because of their inactivation[25]. And as the reduced number of mesenchymal stem cells in patients with OA, the ability of proliferation and differentiation is weakened[26]. Therefore, the way to repair cartilage and inhibit cartilage degeneration is to provide a certain number of active mesenchymal stem cells[27]. Studies have also shown that mesenchymal stem cells can repair damaged articular cartilage because they can secrete a variety of cytokines and growth factors with paracrine and autocrine activity, which can inhibit local immune system, inhibit fibrosis and apoptosis, increase angiogenesis, and stimulate intrinsic repair of tissue or mitosis and differentiation of stem cells[28,29].
There was no significant difference in the expression of TNF-α and IL-6 between the two groups before surgery (P>0.05). The expression of TNF-α and IL-6 in the observation group and the control group decreased continuously at 6 months and 12 months after operation, while the expression in the observation group was significantly lower than that in the control group at that time (P<0.05). Studies of Kaneko[30] et al. have shown that IL-6 is highly expressed in serum and synovial fluid of patients with knee arthritis. Min[31] et al have found that TNF-α is an independent predictor of severe knee OA. However, it has been reported that mesenchymal stem cells may act as cell sentinels for inflammation and tissue damage, responding by secreting excessive signaling molecules that may also contribute to tissue repair, including cytokines such as TNF-α and IL-6, growth factors and chemokines[32,33]. All these studies suggest that mesenchymal stem cells can regulate inflammatory response by secreting active substances or inhibiting endogenous cell growth factors from providing tissue regeneration environment.
In conclusion, the Lysholm score and total effective rate of knee OA patients underwent BMSC transplantation were better than that of joint debridement, while the VAS score was lower than joint debridement. Meanwhile, BMSC transplantation can reduce postoperative inflammatory reactions.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Shanghai East Hospital, Tongji University. Signed written informed consents were obtained from the patients and/or guardians.
Funding
This study was supported by the Scientific Research Project of Shanghai Science and Technology Committee (17411968400).
Authors’ contributions
JL and GS conceived and designed the study. JL, QS and XZ were responsible for the collection and analysis of the data. JL and QS interpreted the data and drafted the manuscript. GS revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25(6):815–823. doi: 10.1016/j.berh.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ, et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012;20(12):1484–1499. doi: 10.1016/j.joca.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–199. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis Cartilage. 2013;21(1):16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res. 2004;427(Suppl):S6–S15. doi: 10.1097/01.blo.0000143938.30681.9d. [DOI] [PubMed] [Google Scholar]
- 6.Vega A, Mrtín-Ferrero MA, Del Canto F, et al. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation. 2015;99(8):1681–1690. doi: 10.1097/TP.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 7.Orozco L, Munar A, Soler R, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: two-year follow-up results. Transplantation. 2014;97(11):e66–e68. doi: 10.1097/TP.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 8.Teuscher DD, Lieberman JR. A Randomized, Controlled Trial of Total Knee Replacement [published correction appears in N Engl J Med 2016 Feb 18;374(7):698] N Engl J Med. 2016;374(7):691–692. doi: 10.1056/NEJMc1514794. [DOI] [PubMed] [Google Scholar]
- 9.Thorlund JB, Juhl CB, Roos EM, Lohmander LS. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h–2747. doi: 10.1136/bmj.h2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen T, Hautopp H, Duus B, Juhl C. No Effect of Acupuncture as Adjunctive Therapy for Patients with Total Knee Replacement: A Randomized Controlled Trial. Pain Med. 2018;19(6):1280–1289. doi: 10.1093/pm/pnx317. [DOI] [PubMed] [Google Scholar]
- 11.Soler R, Orozco L, Munar A, et al. Final results of a phase I-II trial using ex vivo expanded autologous Mesenchymal Stromal Cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee. 2016;23(4):647–654. doi: 10.1016/j.knee.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Wright T. Biomechanical factors in osteoarthritis: the effects of joint instability. HSS J. 2012;8(1):15–17. doi: 10.1007/s11420-011-9249-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4(3):206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 15.Mabey T, Honsawek S. Cytokines as biochemical markers for knee osteoarthritis. World J Orthop. 2015;6(1):95–105. doi: 10.5312/wjo.v6.i1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stannus O, Jones G, Cicuttini F, et al. Circulating levels of IL-6 and TNF-a are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18(11):1441–1447. doi: 10.1016/j.joca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Pelletier JP, Martel-Pelletier J. Rôle de l'inflammation synoviale, des cytokines et de l'IGF-1 dans la physiopathologie de l'arthrose [Role of synovial inflammation, cytokines and IGF-1 in the physiopathology of osteoarthritis] Rev Rhum Ed Fr. 1994;61(9 Pt 2):103S–108S. [PubMed] [Google Scholar]
- 18.Brisson NM, Stratford PW, Maly MR. Relative and absolute test-retest reliabilities of biomechanical risk factors for knee osteoarthritis progression: benchmarks for meaningful change. Osteoarthritis Cartilage. 2018;26(2):220–226. doi: 10.1016/j.joca.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1145–1153. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silverwood V, Blagojevic-Bucknall M, Jinks C, Jordan JL, Protheroe J, Jordan KP. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23(4):507–515. doi: 10.1016/j.joca.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Garay-Mendoza D, Villarreal-Martínez L, Garza-Bedolla A, et al. The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. Int J Rheum Dis. 2018;21(1):140–147. doi: 10.1111/1756-185X.13139. [DOI] [PubMed] [Google Scholar]
- 22.Yamasaki S, Mera H, Itokazu M, Hashimoto Y, Wakitani S. Cartilage Repair With Autologous Bone Marrow Mesenchymal Stem Cell Transplantation: Review of Preclinical and Clinical Studies. Cartilage. 2014;5(4):196–202. doi: 10.1177/1947603514534681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guilak F, Nims RJ, Dicks A, Wu CL, Meulenbelt I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 2018;71-72:40–50. doi: 10.1016/j.matbio.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magnusson K, Turkiewicz A, Timpka S, Englund M. Prediction of midlife hand osteoarthritis in young men. Osteoarthritis Cartilage. 2018;26(8):1027–1032. doi: 10.1016/j.joca.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013;9(10):584–594. doi: 10.1038/nrrheum.2013.109. [DOI] [PubMed] [Google Scholar]
- 26.Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46(3):704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 27.Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48(12):3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 28.Kang KT, Son J, Koh YG, et al. Effect of femoral component position on biomechanical outcomes of unicompartmental knee arthroplasty. Knee. 2018;25(3):491–498. doi: 10.1016/j.knee.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Petursson G, Fenstad AM, Gøthesen Ø, et al. Computer-Assisted Compared with Conventional Total Knee Replacement: A Multicenter Parallel-Group Randomized Controlled Trial. J Bone Joint Surg Am. 2018;100(15):1265–1274. doi: 10.2106/JBJS.17.01338. [DOI] [PubMed] [Google Scholar]
- 30.Kaneko S, Satoh T, Chiba J, Ju C, Inoue K, Kagawa J. Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther. 2000;6(2):71–79. doi: 10.1080/13684730050515796. [DOI] [PubMed] [Google Scholar]
- 31.Min S, Wang C, Lu W, et al. Serum levels of the bone turnover markers dickkopf-1, osteoprotegerin, and TNF-a in knee osteoarthritis patients. Clin Rheumatol. 2017;36(10):2351–2358. doi: 10.1007/s10067-017-3690-x. [DOI] [PubMed] [Google Scholar]
- 32.Vézina Audette R, Lavoie-Lamoureux A, Lavoie JP, Laverty S. Inflammatory stimuli differentially modulate the transcription of paracrine signaling molecules of equine bone marrow multipotent mesenchymal stromal cells. Osteoarthritis Cartilage. 2013;21(8):1116–1124. doi: 10.1016/j.joca.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Wu Y, Goh EL, Wang D, Ma S. Novel treatments for osteoarthritis: an update. Open Access Rheumatol. 2018;10:135–140. doi: 10.2147/OARRR.S176666. [DOI] [PMC free article] [PubMed] [Google Scholar]


