Abstract
Background
Cystic fibrosis is an autosomal recessive inherited defect in the cystic fibrosis transmembrane conductance regulator (CFTR) gene resulting in abnormal regulation of salt and water movement across the membranes. In the liver this leads to focal biliary fibrosis resulting in progressive portal hypertension and end‐stage liver disease in some individuals. This can be asymptomatic, but may lead to splenomegaly and hypersplenism, development of varices and variceal bleeding, and ascites; it has negative impact on overall nutritional status and respiratory function in this population. Prognosis is poor once significant portal hypertension is established. The role and outcome of various interventions for managing advanced liver disease (non‐malignant end stage disease) in people with cystic fibrosis is currently unidentified. This is an updated version of a previously published review.
Objectives
To review and assess the efficacy of currently available treatment options for preventing and managing advanced liver disease in children and adults with cystic fibrosis.
Search methods
We searched the Cochrane Cystic Fibrosis Trials Register, compiled from electronic database searches and handsearching of journals and conference abstract books.
Date of last search: 19 November 2019.
We also searched the reference lists of relevant articles and reviews and online trials registries.
Date of last search: 01 January 2020.
Selection criteria
Any published and unpublished randomised controlled trials and quasi‐randomised controlled trials of advanced liver disease in cystic fibrosis with cirrhosis or liver failure, portal hypertension or variceal bleeding (or both).
Data collection and analysis
Authors independently examined titles and abstracts to identify potentially relevant trials, but none were eligible for inclusion in this review.
Main results
A comprehensive search of the literature did not identify any published eligible randomised controlled trials.
Authors' conclusions
In order to develop the best source of evidence, there is a need to undertake randomised controlled trials of interventions for preventing and managing advanced liver disease in adults and children with cystic fibrosis.
Plain language summary
Interventions for managing advanced liver disease in cystic fibrosis
Review question
We aimed to find the best evidence about preventing and managing advanced liver disease in adults and children with cystic fibrosis by comparing different treatment options.
Background
In advanced liver disease in cystic fibrosis, the normal liver tissue is replaced by scar tissue. As the disease progresses, the liver becomes hard and the blood cannot easily flow through the organ leading to increased pressure in an important blood vessel in the liver called the portal vein (portal hypertension). Later on, the veins around the lower part of the oesophagus (gullet) become swollen and torn, resulting in life‐threatening bleeding (variceal bleeding). There are several treatments currently available for variceal bleeding and portal hypertension; drug treatments (non‐selective beta blockers), endoscopic therapy (e.g. band ligation where tiny elastic bands are placed around the enlarged veins to tie them off so they can't bleed), or sclerotherapy (where a blood‐clotting solution is injected directly into a vein and irritates the lining of the blood vessel so it swells and sticks together). The insertion of a transjugular intrahepatic porto‐systemic shunt (also known as TIPSS) (an artificial channel within the liver that allows movement between the inflowing and out flowing veins) has been employed in recurrent bleeding or as a bridge to liver transplantation. Surgical porto‐systemic shunts have also been used in selected patients with preserved liver function. Liver transplantation is performed in cystic fibrosis patients with decompensated cirrhosis or end‐stage liver disease.
Guidelines for screening and managing portal hypertension have been available for the general population (without cystic fibrosis). However, the optimal treatment for advanced liver disease in cystic fibrosis has not yet been defined, leading to a wide variety of practice among different centres. This is an updated version of a previously published review.
Search date
The evidence is current to: 19 November 2019.
Study characteristics
We searched for high quality trials comparing the treatments described above in children and adults with advanced liver disease in cystic fibrosis. Unfortunately, we did not find any trials to include in this review.
Key results
Since we did not find any trials for this review, it is not possible to make any specific recommendations or to develop best‐practice guidelines at this stage. Our review highlighted a clear need for randomised controlled trials of treatments for the prevention and management of advanced liver disease in adults and children with cystic fibrosis.
Background
A subject‐specific glossary and a statistical glossary are available in the appendices (Appendix 1; Appendix 2).
Description of the condition
Cystic fibrosis (CF) is a life‐limiting autosomal recessive disorder caused by mutations in the CF transmembranes conductance regulator (CFTR) gene on chromosome 7, which is expressed on epithelial cells. It is a multi‐organ disease primarily affecting the lungs, pancreas, sweat glands, reproductive tract (Wolffian ducts in males), gastro‐intestinal tract and liver (Davis 2006). The incidence of CF varies between Asia, Europe and the USA. In Asia, while the condition is severely under‐diagnosed, its prevalence is rare. The incidence of CF is 1 in 2000 to 3000 newborns in the European countries; 1 in every 3500 births in the USA (WHO 2015). Further, a mean prevalence of CF was 0.737 per 10,000 people in some of the countries in the European Union, 0.797 per 10,000 in the USA, and 2.98 per 10,000 in the Republic of Ireland (Farrell 2008). The mortality data for the white population in the USA from 1999 to 2006 was 3708 people (CDC 2010).
Cystic fibrosis‐associated liver disease (CFLD) is an early complication of CF which occurs mostly in the first decade of life, particularly in people with a history of meconium ileus or pancreatic insufficiency and severe (class I to III) mutations (Colombo 2002; Nagel 1989). Advanced liver disease presents mainly during pre‐puberty and puberty with a median age at diagnosis of 12 years (Lindblad 1999). The incidence rate (number of new cases per year) of CFLD is 1.8% (Colombo 2002).
With increased life expectancy, CFLD has emerged as a significant cause for morbidity and mortality (Simmonds 2008) and evidence of clinically significant liver disease is found in around 25% of people with CF. Between 3% and 5% develop pulmonary hypertension (PHT) due to severe cirrhosis during the first decade of life, with a median age of diagnosis of 10 to 11 years of age; 90% of those affected are diagnosed before 20 years of age (Bartlett 2009; Debray 1999; Gooding 2005). A prevalence rate for CFLD of 41% at 12 years of age has been reported (Lamireau 2004). CFLD may progress to chronic obstructive cholangiopathy, focal biliary cirrhosis and multilobular cirrhosis with associated PHT with or without variceal bleeding and end‐stage liver disease. Cirrhosis and PHT can negatively impact on respiratory function due to organomegaly (abnormal enlargement of organs), ascites and intra‐pulmonary shunting (Smith 2004). PHT is an increase in the blood pressure within a system of veins called the portal venous system. Clinically significant PHT is defined as a hepatic venous pressure gradient of 10 mm Hg or more (Bari 2012). Most people with CFLD are asymptomatic. Approximately 10% of people with CFLD and PHT progress to advanced liver disease (Rowland 2011). Liver failure may occur subsequently (Melzi 2006).
Diagnosis of CFLD should be made when at least two of the following variables are present (Debray 2011):
abnormal physical examination: a palpable liver edge more than 2 cm below the bottom edge of the rib cage on the mid‐clavicular line and a prominent left lobe palpable in the epigastrium, confirmed by ultrasound;
abnormal liver function tests: an increase in transaminases (aspartate aminotransferase (AST) and alanine aminotransferase (ALT)) and gamma‐glutamyl transpeptidase (GGT) levels above the upper normal limits on at least three consecutive occasions over 12 months, after other causes of liver disease;
ultrasonographic evidence of liver involvement (an increased parenchymal echogenicity, irregular margins, nodularity) or PHT (splenomegaly, increased think ness of lesser omentum, spontaneous spleno‐renal anastomosis, large collateral veins, ascites) or biliary abnormalities (bile duct dilatation);
a liver biopsy may be indicated if there is diagnostic doubt.
There is a developing role for transient elastography (also known as Fibroscan®) for investigating CFLD, despite the absence of adequate validation against histology and other long‐term outcomes such as PHT, bleeding risk, cirrhosis, synthetic failure and transplantation (Ledder 2014). This method is widely used to stage liver fibrosis as an alternative to a liver biopsy; it measures liver stiffness, and it is also a valuable tool to detect and quantify CFLD in children and adults with CFLD (Ledder 2014; Malbrunot‐Wagner 2011; Menten 2010; Sadler 2015; Witters 2009). Liver stiffness is an accurate non‐invasive indicator in assessing the progression of liver disease in people with CF (Kitson 2013).
Description of the intervention
The principle of managing PHT in people with CFLD is not dissimilar to its management in those without CFLD.
In adults with evidence of PHT and the presence of varices, non‐selective beta‐blockers (NSBB) should be considered to prevent or reduce the number of bleeding events (Cheng 2005). The two most commonly used NSBB for preventing bleeding are propranolol and nadolol. Their dose is titrated to achieve the resting heart rate of 55 beats per minute or a reduction of heart rate by 25% from baseline, and adjusted to maximal tolerated doses. Propranolol is usually started at a dose of 20 mg twice daily and nadolol at a daily dose of 40 mg (Garcia‐Tsao 2007). However, in people with CF with a broncho‐constrictive element to their lung disease, NSBB may be contraindicated. Some studies in children suggest a possible benefit with few side effects, but there are no control data from these studies (Shashidhar 1999).
In adults and children with acute bleeding varices, the preferred initial intervention is upper gastro‐intestinal endoscopy with band ligation or sclerotherapy (Brigman 2006; Debray 1999; Efrati 2003; Price 1996; Stringer 1993). Endoscopic therapies have no effect on either portal flow or resistance (Garcia‐Tsao 2007). Endoscopic variceal band ligation is indicated as the primary variceal prophylaxis as well as the preferred therapy for variceal haemorrhage (Gluud 2012) and a single‐ or multi‐band ligator may be used (Wong 2000). Endoscopic variceal band ligation is very effective with a high success rate in both acute variceal bleeding episodes as well as for primary prophylaxis (Funakoshi 2012; Mileti 2011). Band ligation must be repeated periodically until the varices have been ablated (Gooding 2005). During sclerotherapy, a chemically irritating compound (ethanolamine or tetradecyl sulphate) is injected into (intravariceal) or adjacent to (paravariceal) a varix to control active bleeding or for variceal decompression (Gugig 2012; Kahan 1989). Sclerotherapy has been shown to be inferior to band ligation and is recommended only in specific cases, such as very young children, in whom banding is not possible (due to size of gastroscope and banding device) (Brigman 2006). Sclerotherapy is no longer used in the secondary prophylaxis of variceal haemorrhage in adults.
A balloon tamponade (Sengstaken Tube) is indicated only in cases of massive variceal bleeding in the absence, or failure, of definitive endoscopic treatment.
In a small number of individuals, for example those with severe PHT and refractory variceal haemorrhage in whom therapeutic endoscopy treatment has failed, a transjugular intrahepatic porto‐systemic stent shunt (TIPSS) should be considered either as a long‐term treatment option or as a bridge to transplant (Brigman 2006). An interventional radiologist generally inserts the TIPSS; a catheter is placed into the jugular vein and advanced into the hepatic vein, where a needle is used to form a tract between the left portal vein and the right hepatic vein. This tract is expanded with a balloon angioplasty catheter and a stent is then placed, forming a permanent porto‐systemic (PS) shunt (Efrati 2003; Gugig 2012). The diameter of the stent is generally between 8 mm and 10 mm.
Surgical PS shunting could be considered in selected individuals with preserved liver function and without severe respiratory disease or deterioration (Debray 1999; Efrati 2003). This procedure is indicated for large varices at a high risk of bleeding and failure to respond to a banding programme (Ledder 2014). For individuals with non‐cirrhotic PHT, in particular with extrahepatic portal vein thrombosis, PS shunt surgery represents the only effective therapy to prevent recurrent bleeding and repeated endoscopies for many years. Non‐selective shunts (mesocaval, portocaval), selective shunts (distal spleno‐renal) and intermediate shunts may be used (Gugig 2012).
Liver transplantation is the treatment of choice for decompensated or end‐stage CFLD or uncontrollable variceal bleeding despite an intensive therapeutic endoscopic programme (Cox 1987; Kobelska‐Dubiel 2014; Mack 1995; Noble‐Jamieson 1996). Whole‐liver, reduced‐liver or split‐liver transplant techniques are used (Melzi 2006).
How the intervention might work
Treatment with NSBB lowers cardiac output and causes vasoconstriction in the gastrointestinal tract, thereby reducing portal and collateral blood flow in the abdomen. However, this treatment is reserved for selected individuals only and should be avoided in those with CFLD who have a broncho‐constrictive element to their respiratory disease. These agents have been shown to prevent bleeding in more than 50% of people with medium or large varices (Garcia‐Tsao 2007).
Individuals undergoing variceal band ligation will have endoscopy sessions at regular intervals until the varices are ablated. In children this will generally be carried out under general anaesthesia and consequently, if frequent endoscopy sessions are required, may potentially have a negative impact on respiratory function.
A TIPSS places a stent between the intrahepatic portions of the portal vein and the hepatic vein and leads to a reduction of portal venous pressure and thus a reduction of pressure in oesophageal and gastric varices, preventing further variceal haemorrhage; it is reported to successfully control active and potentially life‐threatening variceal bleeding in more than 90% of cases. This technique is associated with low complication rates, but major risks include TIPSS dysfunction with stenosis, occlusion or thrombosis and new‐onset or worsened encephalopathy. In the long term, regular radiographic examination (ultrasound scans or TIPSS venogram, or both) are necessary to ensure that the TIPSS is functioning correctly and not suffering from blockages (Pozler 2003).
Surgical PS shunting aims to divert portal blood flow and as such decrease portal pressure leading to a reduced risk of bleeding (Ledder 2014). Surgical PS shunting improves hypersplenism without deteriorating liver function or encephalopathy (Wolff 2003). The non‐selective shunts, communicate with the entire portal system and have been associated with higher rates of hepatic encephalopathy compared with the selective shunts. The distal spleno‐renal shunt is a selective shunt which connects the distal end of the splenic vein to the left renal vein with interruption of all collateral vessels (e.g. coronary vein and gastroepiploic veins). In effect it separates the portal venous circulation into a decompressed gastrosplenic venous circuit (thus reducing the pressure on gastric and oesophageal varices) and a high‐pressure superior mesenteric venous system that continues to perfuse the liver. Liver function is preserved by a distal spleno‐renal shunt which is associated with a lower incidence of portal systemic encephalopathy (D’Amico 1995). Potential complications include onset or worsening of hepatic encephalopathy, shunt thrombosis or occlusion (Debray 1999; Efrati 2003). Hepatic encephalopathy is the most common adverse event developed after treatment with PS shunting for PHT (Flass 2013).
Liver transplantation is indicated in people with CFLD with progressive hepatic dysfunction, development of ascites and jaundice, intractable variceal bleeding, hepato‐pulmonary and porto‐pulmonary syndrome, severe malnutrition unresponsive to intensive nutritional support, deteriorating quality of life (QoL) related to liver disease or deteriorating pulmonary function or both (Debray 2011; Fridell 2003; Ikegami 2008; Melzi 2006; Nash 2008). Based on these factors, two scoring systems are currently available to evaluate the need for liver transplantation in adults with CFLD (Milkiewicz 2012; Noble‐Jamieson 1996). No such specific scoring systems exist for children. Liver transplantation in this group does not improve long‐term nutritional status, but short‐term improvement in pulmonary function after liver transplantation has been reported. Some of the initial improvement in lung function may reflect the resolution of the effects of severe PHT such as pulmonary oedema, intrapulmonary shunting ascites and organomegaly, in addition to reduced infection resulting from improved immune function, particularly neutrophil function (Dowman 2012). One further study showed there is improvement in QoL after liver transplantation (Moyer 2009).
Why it is important to do this review
Evidence‐based guidelines from the European and American Board of Gastroenterology recommend screening for and treating large varices in adults with severe PHT without a history of variceal bleeding (Shneider 2012). They suggest the use of NSBB as a first‐step therapy in adults with grade two or grade three varices, band ligation if beta‐blockers are contra‐indicated or have failed (secondary prophylaxis), and the placement of a TIPSS in cases of ligation failure (tertiary prophylaxis) (De Franchis 2005). In contrast, there is a paucity of data to recommend specific approaches for the management of PHT in children; and extrapolation of care models for adults to children may not be appropriate (Shneider 2012).
There are currently no clear recommendations for treating advanced liver disease in people with CF (Debray 2011). Furthermore, for people with CFLD, no specific recommendations exist with respect to the prevention and treatment of variceal haemorrhage. In addition, because of their lung disease, the use of beta‐blockers is usually contra‐indicated. Regular screening or therapeutic endoscopies may require general anaesthesia (particularly when carried out in children) and may lead to a reduction in lung function or predisposition to infection. This is an update of a previously published review (Than 2017).
Objectives
To assess the various treatment options for preventing and managing advanced liver disease in children and adults with CFLD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs.
Types of participants
Children and adults of all ages and either gender, diagnosed with CFLD with cirrhosis or liver failure, PHT or variceal bleeding (or both).
Types of interventions
We planned to evaluate the effects of the following comparisons.
Pharmacological interventions (e.g. NSBB) for any duration compared to placebo or no intervention
Endoscopic interventions (e.g. band ligation, sclerotherapy) compared to active control
TIPSS compared to active control
Surgical interventions (e.g. surgical PS shunt, liver transplantation) compared to active control
Types of outcome measures
We planned to assess the following outcome measures.
Primary outcomes
-
Change in variceal bleeding and portal pressure
development of first bleeding episode (primary prevention)
re‐bleeding following endoscopic treatment (secondary prevention)
-
Adverse effects
of non‐surgical interventions (e.g. aggravated congestive cardiac failure, respiratory distress, hypotension from non‐selective beta‐blockers, stenosis, occlusion, thrombosis, worsened encephalopathy after TIPSS, perforation of oesophagus, oesophageal ulceration, oesophageal stricture after sclerotherapy)
of surgical interventions (e.g. thrombosis, occlusion, onset or worsening of encephalopathy, infection, rejection)
Secondary outcomes
-
Nutritional status
body mass index (BMI) calculated as weight (kg)/height² (m)
standard deviation (SD) score (z score) for weight (zW)
SD score (z score) for height (zH)
-
Respiratory outcomes
forced expiratory volume in one second (FEV₁)
forced vital capacity (FVC)
SD score (z scores ) for FEV₁/FVC ratio
Quality of life (QoL) as measured by a validated scoring system, e.g. Cystic Fibrosis Questionnaire Revised (CFQ‐R) (Quittner 2009)
Need for liver transplantation
Mortality (bleeding‐related mortality, all‐cause mortality)
Search methods for identification of studies
There will be no restrictions regarding language or publication status.
Electronic searches
We attempted to identify relevant trials from the Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register using the search term: liver.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE,a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of the last search: 19 November 2019.
We also searched the following trial registries for the latest clinical investigations and treatment in order to identify unpublished studies, ongoing studies and potential relevant trials. Searched terms can be found in Appendix 3.
ClinicalTrials.gov (https://clinicaltrials.gov)
metaRegister of Current Controlled Trials (mRCT) (www.controlled‐trials.com/mrct/)
World Health Organisation International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/)
Date of last search: 01 January 2020
Searching other resources
We checked the reference lists of the trials we identified for further potential relevant trials.
Data collection and analysis
Selection of studies
Two review authors aimed to independently check the titles and abstracts identified from the searches. However, no eligible RCTs were found. For future updates, the authors will adhere to the protocol described below.
Two review authors (SK and AWT) will independently assess all the potentially relevant trials obtained from the Information Specialist as well as from the handsearches undertaken by the authors. If there is disagreement between two authors, the third review author (NNT) will arbitrate and the three authors will discuss and finalise the selection of trials. The authors will select the trials if they meet the inclusion criteria, regardless of publication status (published, unpublished, in press and in progress). They will also include trials recorded and published in languages other than English. The authors will record details of any excluded trials together with the reasons for exclusion (Higgins 2011a).
Data extraction and management
Two review authors (SK and SM) will independently extract and record the data from included trials using standardised data extraction forms; one review author (SK) will prepare the forms based on the checklist for data collection described in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). The data extraction form will include trial characteristics, such as trial design, participants, interventions, primary and secondary outcome measures and the analysis performed in original trials. If there are differences in the data extracted by the first two review authors, a third review author (SM) will check these and arbitrate. If there are insufficient or missing data, the authors will contact the corresponding trial investigators for additional information. One of the review authors (SK) will enter data into the Review Manager (RevMan) software for analysis, which another review author (IVM) will check (RevMan 2014).
We will analyse surgical and non‐surgical interventions separately.
Assessment of risk of bias in included studies
Two review authors (SK and SM) will independently assess the risk of bias for each included trial using the criteria outlined in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). If there is disagreement, the third author (NNT) will be involved and the three authors will discuss until they achieve consensus. The authors will use Cochrane's tool to assess risk of bias in the included studies with regard to: random sequence generation; blinding of participants, personnel, and outcome assessors; completeness of outcome data for each main outcome; selective outcome reporting; and other sources of bias. The authors will grade studies using judgements of 'low risk', 'high risk' or 'unclear risk' of bias according to the specific criteria for each domain. They will record all judgements in the 'Risk of bias' tables together with the characteristics of each included trial and will also prepare the 'Risk of bias' summary figure. The authors will contact the trial investigators for details of the procedures involved in the conduct of trials and will keep their replies as evidence.
Measures of treatment effect
Dichotomous data
The review authors will use the Mantel‐Haenszel risk ratio (RR) with 95% confidence intervals (CI) for dichotomous outcome data (such as number of active variceal bleeding, stenosis, occlusion, thrombosis, worsened encephalopathy and mortality). They will further calculate the number needed to treat (NNT) for dichotomous outcomes to reflect the number of participants necessary to obtain a beneficial or harmful outcome with the intervention.
Continuous data
The review authors will use the fixed‐effect mean differences (MD) with 95% CI for continuous data variables (such as biochemical investigation results, measurements from ultrasound, measurements from hepatic scintigraphy, QoL, nutritional status, respiratory outcomes) provided the same scales are used to measure the outcomes in all the included trials. If trials assess the same outcome, but measure it in different ways, then the authors will use the standardised mean difference (SMD) as a summary statistic in meta‐analysis. If this is the case, they will prefer to report the MD based on change from baseline over the MD based on absolute values (Deeks 2011).
Unit of analysis issues
For dichotomous data, the participant will be the unit of analysis. For continuous data, the authors will use MD which will be the average change from baseline and not the absolute mean. For outcomes that may occur more than once, such as active variceal bleeding, the review authors will analyse count data as continuous data to prevent any unit of analysis errors. They will not include any cross‐over trials or cluster‐randomised trials. For trials with two treatment arms of different interventions, the review authors will analyse the outcomes for each arm separately.
Dealing with missing data
If data are missing the review authors will contact trial investigators or sponsors in order to verify key trial characteristics and obtain any missing numerical outcome data whenever possible. If this is not possible and if they assume that these data are 'missing at random', they will perform an available case analysis (i.e. ignoring the missing data) and discuss the impact of missing data on our results. When the review authors assume that the missing outcome data are 'not missing at random', they will conduct an intention‐to‐treat (ITT) analysis by imputing the missing data with replacement values, and treating these as if they were observed (e.g. last observation carried forward, imputing an assumed outcome such as assuming all were poor outcomes, imputing the mean, imputing based on predicted values from a regression analysis). The review authors also plan to perform a sensitivity analysis to assess the impact of any unknown status or assumptions made about missing data on participants who withdrew from trials on the overall pooled result of the meta‐analysis (Higgins 2011d).
Assessment of heterogeneity
The review authors plan to assess heterogeneity between the included trials by checking for overlap of horizontal lines representing each trial on the forest plot and the Chi² test, with a 10% level of significance. They will use the I² statistic to measure the percentage of inconsistency in results due to inter‐trial variability in each analysis. In line with guidance in the Cochrane Handbook for Systematic Reviews of Interventions, the review authors will consider I² values from 0% to 40% to be not important; values from 30% to 60% as moderate heterogeneity; values from 50% to 90% as substantial heterogeneity; and values over 75% as considerable heterogeneity (Deeks 2011). If they find I² values over 50% (considered as substantial heterogeneity), they will explore it by pre‐specified subgroup analyses (see below).
Assessment of reporting biases
The review authors plan to use funnel plots to assess reporting biases if the meta‐analysis includes 10 or more trials (Sterne 2011). They will also attempt to trace the original protocol of the trial and compare the sample size; they will check that outcomes listed in the methods in the protocol appear in the final reports.
Data synthesis
The review authors will analyse any relevant data using RevMan (RevMan 2014). For the pooling of outcomes, the review authors will use a fixed‐effect model if the I² statistic is homogeneous (i.e. I² is under 50%). They will apply a random‐effects model for data synthesis when they identify substantial heterogeneity (I² at least 50%) which can not be explained by subgroup analyses.
Subgroup analysis and investigation of heterogeneity
If the review authors identify significant heterogeneity (i.e. I² greater than 50%), they will conduct the following subgroup analyses for each group of interventions.
Pharmalogical therapy
Children with CFLD (aged up to 18 years) versus adults with CFLD (aged over 18 years)
Different types of NSBB
Different doses of NSBB
Short‐term therapy (less than six months) versus long‐term therapy (six months and over)
Endoscopic therapy
Children with CFLD (aged up to 18 years) versus adults with CFLD (aged over 18 years)
Banding versus sclerotherapy
TIPSS
Children with CFLD (aged up to 18 years) versus adults with CFLD (aged over 18 years)
Surgical therapy
Children with CFLD (aged up to 18 years) versus adults with CFLD (aged over 18 years)
PS shunts (selective or nonselective or partial) versus liver transplantation
Sensitivity analysis
The review authors will perform a sensitivity analysis by repeating the meta‐analysis after exclusion of trials with an overall high risk of bias and those with unclear methodological data from the overall analysis.
They also plan to perform a sensitivity analysis to assess the impact of any unknown status or assumptions made about missing data on participants who withdrew from trials on the overall pooled result of the meta‐analysis (Higgins 2011d).
Summary of findings and assessment of the certainty of the evidence
For future updates, the authors will create a 'Summary of findings' table for each comparison presented using the methods and recommendations described in chapter 11 of theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c) and using GRADEpro software for overall grading of the quality of the evidence (Deeks 2011).
In the summary of the finding tables, the review authors will report change in variceal bleeding (first bleeding episode and re‐bleeding) and portal pressure after various interventions such as pharmacotherapy, endoscopy and surgical interventions, which included the development of first bleeding episode and re‐bleeding following endoscopic treatment.
They will also report the adverse effects after the interventions as detailed in the outcome measures above.
Lastly, they will report the all‐cause mortality and QoL in the summary of findings tables.
Results
Description of studies
Please see the tables for further details of the studies listed in this review (Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies).
Results of the search
No RCTs were identified in the comprehensive searches undertaken for this review (Figure 1).
1.
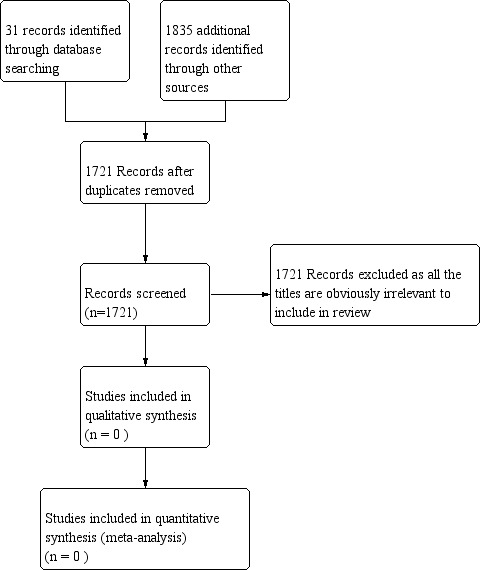
Figure 1 PRISMA study flow diagram
Included studies
The authors did not identify any trials which were eligible for inclusion in this review.
Excluded studies
We identified 25 potentially relevant trials, all of which were excluded after checking the abstracts or full text as these trials did not meet with the eligible criteria for inclusion in our review. Out of the 25 trials, nine were excluded as they were not RCTs. A further 16 trials were excluded as they did not examine the types of interventions we planned to evaluate.The reasons for exclusion of studies are described in the table of 'Characteristics of excluded studies'.
Risk of bias in included studies
No trials met the inclusion criteria; therefore, there were no trials for which risk of bias could be assessed in this review.
Allocation
There were no trials for which risk of bias could be assessed in this review.
Blinding
There were no trials for which risk of bias could be assessed in this review.
Incomplete outcome data
There were no trials for which risk of bias could be assessed in this review.
Selective reporting
There were no trials for which risk of bias could be assessed in this review.
Other potential sources of bias
There were no trials for which risk of bias could be assessed in this review.
Effects of interventions
There are no trials eligible for inclusion in this review.
Discussion
Summary of main results
No randomised controlled trials (RCTs) were identified which met the inclusion criteria for this review and we found no evidence in relation to the management of cystic fibrosis‐related liver disease (CFLD). The possibility of surgical intervention might be effective in cystic fibrosis liver disease but it needs further evaluation in RCTs.
Overall completeness and applicability of evidence
Although we searched for various articles and websites for eligible trials to include in the review which might demonstrate the effectiveness of interventions available for both prevention and management of advanced CFLD, we did not identify any eligible trials and hence there is no evidence currently available. The effective management of advanced CFLD with either medical or surgical intervention requires further research to provide data for clinically important outcomes including safety and long‐term effects.
Quality of the evidence
No trials have been included in this review and we have not been able to assess the quality of evidence.
Potential biases in the review process
We have undertaken comprehensive searches of a range of data sources, but not identified any relevant trials. That said, it remains possible that we may have failed to uncover some potentially eligible trials. Since there are no randomised control trials which meet inclusion criteria for this update, we are not able to determine any further potential sources of bias in the review process.
Agreements and disagreements with other studies or reviews
A relatively common, serious complication in CFLD) is portal hypertension and consequent acute bleeding of oesophageal varices. Cirrhosis and cholangiocarcinoma, while rare, are probably more serious. Studies related to preventing and managing of acute variceal bleeding secondary to portal hypertension in people with CFLD are scarce. We identified only some small retrospective studies on the management of CFLD. There are currently no clear guidelines or recommendations for treatment based on high‐level evidence for the management of CFLD.
Pharmacological interventions
The efficacy and safety of non‐selective beta blockers (NSBB) in preventing variceal bleeding has not been evaluated in people with cystic fibrosis (CF) because of their potential adverse effects on pulmonary disease. Moreover, the repeated general anaesthesia required for screening of therapeutic endoscopic procedures may also reduce lung function and predispose to infection unless managed with intravenous antibiotics and vigorous physiotherapy (Debray 2011). A few small studies and case series have shown no effects of NSBB in children with decompensated portal hypertension. (Ozsoylu 1985; Ozsoylu 2000; Shashidhar 1999).
Endoscopic interventions
Sclerotherapy
Prophylactic sclerotherapy is not beneficial in CF because it carries a risk of bleeding during or following the procedure (D’Amico 1995). The preferred initial intervention for bleeding varices is oesophagogastroduodenoscopy with injection sclerotherapy or band ligation (Brigman 2006; Debray 1999; Efrati 2003; Price 1996; Stringer 1993). Injection sclerotherapy is associated with an 86% success rate without significant morbidity in people with CF (D’Amico 1995; Stringer 1993).
Band ligation
Endoscopic variceal band ligation is the treatment of choice for primary variceal prophylaxis (Funakoshi 2012; Mileti 2011). Band ligation is also the preferred approach for secondary variceal prophylaxis (Flass 2013), especially in older children and adults (Brigman 2006). This method can also be used to manage the difficult variceal bleeding in people with CF (Garcia‐Tsao 2007). However, band ligation can also carry a risk of bleeding both during and following the procedure which may result in repeated general anaesthesia with increased morbidity in people with CF.
Transjugular intrahepatic porto‐systemic stent shunt (TIPSS)
Use of TIPSS may be reserved for individuals with variceal haemorrhage refractory to sclerotherapy. It can also be used on an emergency basis in individuals who are actively bleeding, or in those who exhibit rapid progression to liver failure (Debray 1999). Both as a long‐term therapy for portal hypertension and as a bridge for liver transplantation, TIPSS can effectively control variceal bleeding (Brigman 2006); indeed, TIPSS controls active variceal bleeding in more than 90% of cases. Shunt stenosis is the principal limiting factor of TIPSS. The technique and complications in children are comparable to those in adults.
Surgical porto‐systemic (PS) shunt
A surgical PS shunt may also be reserved for refractory cases as a secondary measure. Partial splenectomy with splenorenal shunt was reported to have a favourable outcome in 15 of 19 individuals with CF with portal hypertension, with the improvement in liver function and portal hypertensive symptoms, significantly delaying or obviating the need for liver transplantation (Louis 2007). Surgical PS shunting can permanently reduce portal pressure and relieve portal hypertension in those without progressive liver and lung failure (Debray 1999; Efrati 2003). Surgical PS shunting has been used to preserve liver function, but carries the risk of acute liver failure and hepatic encephalopathy (Debray 2011); it offers a prolonged alternative treatment for refractory bleeding (Efrati 2003).
Liver transplantation
The established indications for liver transplant in CF include cirrhosis with evidence for hepatic decompensation or uncontrollable variceal bleeding. Some retrospective reviews have shown that there was a significant survival advantage in both adults and children with CF cirrhosis receiving liver transplant compared to those with cirrhosis who did not receive a transplant (Lu 2010; Mendizabal 2011).
Authors' conclusions
Implications for practice.
Many randomised controlled trials (RCTs) have shown the efficacy and safety of interventions for preventing (primary as well as secondary) and controlling the bleeding of oesophageal varices in non‐cystic fibrosis (CF) adults. However, we were not able to deduce exactly the same results from these interventions and apply them in managing children and adults with advanced cystic fibrosis liver disease (CFLD).
Conclusions could not be drawn with regard to the efficacy and safety of interventions for advanced CFLD due to a lack of RCTs in this field. Currently, the decision about the choice of treatment should be based on the best available level of evidence, clinicians’ experience, and the clinical situation of individuals.
The authors will continue to search for clinical trials eligible to include in the review every two years and update the review if new evidence is found.
Implications for research.
Our systematic review has identified the need for a well‐designed, high‐powered, multicentre RCT to assess the efficacy and safety of the interventions for advanced liver disease in people with CF. For the future research, the primary outcome of the trials should be a change in variceal bleeding, in terms of development of first bleeding episode (primary prevention) and re‐bleeding following treatments (secondary prevention), a change in portal pressure with intervention that employed for portal decompression, the overall survival of both the graft and the patient in cases of liver transplantation, and adverse effects of interventions. The important secondary outcomes should include nutritional status (body mass index, standard deviation (SD) score (z score) for weight, SD score (z score) for height, respiratory outcomes (forced expiratory volume at one second (FEV1), forced vital capacity (FVC), SD score (z scores) for FEV1/FVC ratio), quality of life as measured by a validated scoring system, e.g. the Cystic Fibrosis Questionnaire Revised (CFQ‐R) (Quittner 2009), need for liver transplantation, mortality (bleeding‐related mortality, all‐cause mortality).
What's new
| Date | Event | Description |
|---|---|---|
| 19 March 2020 | New search has been performed | A search of the Cochrane Cystic Fibrosis and Genetic Disorders Review Group's Cystic Fibrosis Trials Register identified three new references potentially eligible for inclusion in this updated review. All were additional references to three already excluded trials (Colombo 1992; Colombo 1996; Van de Meeberg 1997). |
| 19 March 2020 | New citation required but conclusions have not changed | One co‐author has stepped down from the author team at this update. Since no new studies have been identified at this update, our conclusions remain the same. |
Acknowledgements
We would like to take this opportunity to express our gratitude to Mrs Nikki Jahnke, Managing Editor at the Cochrane Cystic Fibrosis and Genetic Disorders (CFGD) Group who supported us throughout this work. We are thankful for her inspiring guidance, and friendly advice on writing this review. We thank Dr. Soe Moe, Community Medicine Department, Melaka Manipal Medical College for her contribution in previous version of the review. We also would also like to express our warm thanks to Professor Jaspal Singh Sahota, Chief Executive of Melaka Manipal Medical College and Professor Adinegara Lutfi Abas, Dean of Faculty of Medicine, Melaka Manipal Medical College, for their support in writing this review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Subject specific glossary
| Term | Explanation |
| ascites | an abnormal accumulation of fluid within the abdomen |
| anastomosis | a connection that is created between tubular structures, such as blood vessels or loops of intestine, e.g. when part of an intestine is surgically removed, the two remaining ends are sewn or stapled together |
| cholangiopathy | any disease of the bile ducts |
| clavicle | the bone extending from the breastbone (sternum) at the base of the front of the neck to the shoulder, also known as the collarbone |
| collaterals | a side branch, as of a blood vessel or nerve |
| coagulation | (also known as clotting) is the process by which blood changes from a liquid to a gel |
| encephalopathy | a general term describing a disease that affects the function or structure of your brain |
| epigastrium | the part of the abdominal wall that is above the belly button |
| extrahepatic portal vein thrombosis | the development of a blood clot in the vein outside the liver that brings blood into the liver |
| focal biliary cirrhosis | inflammation and scarring of the liver tissue and bile ducts at one particular site of the liver, further impairing the release of bile and overall liver function |
| hepatic | relating to affecting, associated with, supplying or draining the liver |
| hepatic venous pressure | the venous pressure differences between portal vein and Inferior vena cava (a large vein which carries blood from lower part of the body to the heart ) distal to the liver |
| hepatic encephalopathy | brain dysfunction directly due to liver dysfunction, most often recognized in advanced liver disease |
| hepatic portal system | a group of veins that carry blood from blood vessels in the stomach, intestine, spleen and pancreas which merge into the portal vein, which then branches into smaller vessels and travels through the liver |
| heterogenous | different in kind, diverse, unlike each other |
| intrahepatic | within the liver |
| intravascular | within blood vessels |
| isotope | any of two or more types of atoms of a chemical element with the same atomic number and nearly identical chemical behavior but with a different number of neutrons (that is, a greater or lesser atomic mass) than the standard for that element and different physical properties |
| jugular | of or relating to the throat or neck or relating to the jugular vein |
| lesser omentum | part of the membrane that forms the lining of the abdominal cavity which is found in a double layer and goes from the beginning of the small intestine and stomach’s lesser curvature to the liver |
| mesenteric | relating to the peritoneal or membranous fold attaching the small intestine to the dorsal body wall |
| mid‐clavicular line | a vertical line passing through the midpoint of the collarbone |
| occlusion | a shutting off, blocking or obstruction of something |
| oesophagus | the tube that connects the pharynx (throat) with the stomach, in humans it is about 23 cm long |
| parenchymal | the essential and distinctive tissue of an organ or an abnormal growth as distinguished from its supportive framework |
| parenchymal echogenicity | In ultrasonography, the extent to which functional part of an organ or structure gives rise to reflections of ultrasonic waves |
| portal hypertension | an increase in the blood pressure within a system of veins called the portal venous system (or hepatic portal system ‐ see above) |
| porto‐systemic shunt | also known as a liver shunt, is a bypass of the liver by the body's circulatory system i.e. a blood vessel that carries blood around the liver instead of through it |
| splenomegaly | enlargement of the spleen |
| spleen | the spleen plays multiple supporting roles in the body, acting as a filter for blood as part of the immune system, recycling old red blood cells and storing platelets and white blood cells; it also helps fight certain kinds of bacteria that cause pneumonia and meningitis. |
| splenorenal | of relating to or joining the veins in the spleen veins or arteries in the kidneys |
| steatosis | a build up of fat in the liver |
| stenosis | a narrowing of a passage or vessel |
| scintigraphy | a diagnostic technique which uses small amounts of radioactive materials called radiotracers, a special camera and a computer to evaluate an organ's function and anatomy and determine whether it is working properly |
| technetium | a radioactive tracer isotope widely used in nuclear medicine e.g. scintigraphy (see above) |
| thrombosis | formation or presence of a blood clot in a blood vessel |
| varices (singular: varix) |
abnormally dilated and lengthened veins, arteries or lymph vessels, e.g. a varicose vein |
Appendix 2. Glossary of statistical terms
| Term | Explanation |
| Chi² test | a statistical test based on comparison of a test statistic to a Chi² distribution; used in RevMan analyses to test the statistical significance of the measure of heterogeneity |
| dichotomous | related with scale for grouping into two categories only |
| forest plot | a graphical representation of the individual results of each study included in a meta‐analysis together with the combined meta‐analysis result; it allows readers to see the variety between individual study results. The results of individual studies are shown as squares centred on each study’s point estimate. A horizontal line runs through each square to show each study’s confidence interval ‐ usually, but not always, a 95% confidence interval. The overall estimate from the meta‐analysis and its confidence interval are shown at the bottom, represented as a diamond. The centre of the diamond represents the pooled point estimate, and its horizontal tips represent the confidence interval. |
| I² statistic | a measure of heterogeneity (difference); it describes the percentage of the variability in effect estimates that is due to real differences rather than sampling error (chance). A value greater than 50% may be considered to represent substantial heterogeneity. |
| intention‐to‐treat (ITT) principle | a strategy for analysing data from a randomised controlled trial where all participants are included in the group to which they were allocated, whether or not they received (or completed) the treatment given to that group. ITT analysis prevents bias caused by the loss of participants, which may disrupt the equality between groups at the start of the study established by randomisation and which may reflect non‐adherence to the protocol. The term is often misused in trial publications when some participants are excluded. |
| odds ratio | the ratio of the odds of an event happening in one group compared to the odds of an event happening in another group. In studies of treatment effect, the odds in the treatment group are usually divided by the odds in the control group. An odds ratio of one indicates no difference between comparison groups. For undesirable outcomes an odds ratio that is less than one indicates that the intervention was effective in reducing the risk of that outcome. When the risk is small, odds ratios are very similar to risk ratios. |
| quasi‐randomised controlled trial | a trial using methods of allocating people to a particular group which are not random, but were intended to produce similar groups of participants. Quasi‐random methods include using the person's date of birth, the day of the week or month of the year, a person's medical record number, or just putting people into alternate groups. In practice, these methods of allocation are relatively easy to manipulate, introducing selection bias. |
| randomised controlled trial | an experiment where two or more interventions (possibly including a control intervention or no intervention) are compared by putting participants into intervention groups at random. In most trials each person is put into one intervention group, but sometimes assignment is to defined groups of individuals (for example, in a household) or interventions are assigned within individuals (for example, in different orders or to different parts of the body). |
| Review Manager 5 | software developed for Cochrane to help review authors to produce Cochrane Reviews |
| sensitivity analysis | an analysis used to determine how sensitive the results of a study or systematic review are to changes in how it was done, they are used to assess how robust the results are to uncertain decisions or assumptions about the data and the methods that were used |
Appendix 3. Search strategies
Cystic fibrosis AND Liver disease
Cystic fibrosis AND Cirrhosis of liver
Cystic fibrosis AND Portal hypertension
Cystic fibrosis AND Liver disease AND Portal hypertension
Cystic fibrosis AND Oesophageal varices
Cystic fibrosis AND Variceal bleeding
Cystic fibrosis AND Treatment of oesophageal varices
Cystic fibrosis AND Esophageal variceal banding
Cystic fibrosis AND Sclerotherapy of oesophageal varices
Cystic fibrosis AND beta‐blockers for oesophageal varices
Cystic fibrosis AND transjugular intrahepatic porto‐systemic stent shunt (TIPSS)
Cystic fibrosis AND Liver transplantation for advanced liver disease
www.controlled‐trials.com/mrct/
Cystic fibrosis
Cystic fibrosis AND Liver disease
Cystic fibrosis AND Cirrhosis of liver
Cystic fibrosis AND Portal hypertension
Cystic fibrosis AND Liver disease AND Portal hypertension
Cystic fibrosis AND Oesophageal varices
Cystic fibrosis AND Variceal bleeding
Cystic fibrosis AND Treatment of oesophageal varices
Cystic fibrosis AND Esophageal variceal banding
Cystic fibrosis AND Sclerotherapy of oesophageal varices
Cystic fibrosis AND beta‐blockers for oesophageal varices
Cystic fibrosis AND transjugular intrahepatic porto‐systemic stent shunt (TIPSS)
Cystic fibrosis AND Liver transplantation for advanced liver disease
www.who.int/ictrp/en/ or http://apps.who.int/trialsearch/
Cystic fibrosis
Cystic fibrosis AND Liver disease
Cystic fibrosis AND Cirrhosis of liver
Cystic fibrosis AND Portal hypertension
Cystic fibrosis AND Liver disease AND Portal hypertension
Cystic fibrosis AND Oesophageal varices
Cystic fibrosis AND Variceal bleeding
Cystic fibrosis AND Treatment of oesophageal varices
Cystic fibrosis AND Esophageal variceal banding
Cystic fibrosis AND Sclerotherapy of oesophageal varices
Cystic fibrosis AND beta‐blockers for oesophageal varices
Cystic fibrosis AND transjugular intrahepatic porto‐systemic stent shunt (TIPSS)
Cystic fibrosis AND Liver transplantation for advanced liver disease
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Belli 1987 | Examined the effects of taurine supplementation on the absorption of a fat meal. |
| Bittner 1991 | Evaluated the effect of UDCA in CF and hepatopathy. Treatment with UDCA is not a relevant treatment to this review as its main purpose of use is to delay the progression of CFLD. Our review evaluates the interventions for advanced liver disease in cystic fibrosis; i.e. interventions for prevention (primary and secondary) and treatment of oesophageal variceal bleeding due to portal hypertension in CFLD. |
| Colombo 2002 | Not a RCT. Prospective observational study on incidence, risk factors and outcome of CFLD. |
| Colombo 1992 | Evaluated the effect of UDCA in CF liver disease. Treatment with UDCA is not a relevant treatment to this review as its main purpose of use is to delay the progression of CFLD. Our review evaluates the interventions for advanced liver disease in cystic fibrosis; i.e. interventions for prevention (primary and secondary) and treatment of oesophageal variceal bleeding due to portal hypertension in CFLD. |
| Colombo 1996 | Long‐term follow‐up study evaluating UDCA for CFLD. Treatment with UDCA is not a relevant treatment to this review as its main purpose of use is to delay the progression of CFLD. Our review evaluates the interventions for advanced liver disease in cystic fibrosis; i.e. interventions for prevention (primary and secondary) and treatment of oesophageal variceal bleeding due to portal hypertension in CFLD. |
| Darling 1985 | Examined the effects of taurine supplementation on fat absorption in CF. |
| Fleet 2000 | Case study, not a RCT. |
| Kapustina 2000 | Evaluated the effect of UDCA on lipid metabolism in people with CF. Treatment with UDCA is not a relevant treatment to this review as its main purpose of use is to delay the progression of CFLD. Our review evaluates the interventions for advanced liver disease in cystic fibrosis; i.e. interventions for prevention (primary and secondary) and treatment of oesophageal variceal bleeding due to portal hypertension in CFLD. |
| Lamireau 2006 | Not a RCT. |
| Lepage 1997 | Examined the effect of UDCA on the hepatic metabolism of essential fatty acid in children with CF. Treatment with UDCA is not a relevant treatment to this review as its main purpose of use is to delay the progression of CFLD. Our review evaluates the interventions for advanced liver disease in cystic fibrosis; i.e. interventions for prevention (primary and secondary) and treatment of oesophageal variceal bleeding due to portal hypertension in CFLD. |
| Merli 1994 | Examined the effect of a medium dose of UDCA with or without taurine supplementation on the nutritional status of people with CF. Treatment with UDCA is not a relevant treatment to this review as its main purpose of use is to delay the progression of CFLD. Our review evaluates the interventions for advanced liver disease in cystic fibrosis; i.e. interventions for prevention (primary and secondary) and treatment of oesophageal variceal bleeding due to portal hypertension in CFLD. |
| Mieles 1991 | Not a RCT. |
| Narkewicz 1994 | Evaluated the effect of UDCA for CFLD on indirect tests of hepatic function. Treatment with UDCA is not a relevant treatment to this review as its main purpose of use is to delay the progression of CFLD. Our review evaluates the interventions for advanced liver disease in cystic fibrosis; i.e. interventions for prevention (primary and secondary) and treatment of oesophageal variceal bleeding due to portal hypertension in CFLD. |
| Nightingale 2010 | A retrospective non‐RCT. |
| Noble‐Jamieson 1994 | Non‐randomised study. |
| O'Brien 1992 | Assessed the effect of UDCA in CFLD. Treatment with UDCA is not a relevant treatment to this review as its main purpose of use is to delay the progression of CFLD. Our review evaluates the interventions for advanced liver disease in cystic fibrosis; i.e. interventions for prevention (primary and secondary) and treatment of oesophageal variceal bleeding due to portal hypertension in CFLD. |
| Pozler 2003 | Not a RCT. |
| Pukhalsky 2008 | Evaluated anti‐inflammatory treatment on low frequency hepato‐biliary abnormalities in people with CF. |
| Roland 2011 | Not a RCT, a cohort study. |
| Schuster 1977 | Not a RCT, a cohort study. |
| Smith 1991 | Examined the effects of taurine on faecal fatty acid and sterol excretion in CF. |
| Spray 1998 | Prospective study of the role of UDCA on histological changes in children with CFLD. Treatment with UDCA is not a relevant treatment to this review as its main purpose of use is to delay the progression of CFLD. Our review evaluates the interventions for advanced liver disease in cystic fibrosis; i.e. interventions for prevention (primary and secondary) and treatment of oesophageal variceal bleeding due to portal hypertension in CFLD. |
| Thomas 1995 | Examined the effect of UDCA on malabsorption of vitamin E in CF. Treatment with UDCA is not a relevant treatment to this review as its main purpose of use is to delay the progression of CFLD. Our review evaluates the interventions for advanced liver disease in cystic fibrosis; i.e. interventions for prevention (primary and secondary) and treatment of oesophageal variceal bleeding due to portal hypertension in CFLD. |
| Thompson 1987 | Assessed protein metabolism in CF, response to malnutrition and taurine supplementation. |
| Van de Meeberg 1997 | Long‐term follow up of low‐dose versus high‐dose UDCA in cholestasis related to CF. Treatment with UDCA is not a relevant treatment to this review as its main purpose of use is to delay the progression of CFLD. Our review evaluates the interventions for advanced liver disease in cystic fibrosis; i.e. interventions for prevention (primary and secondary) and treatment of oesophageal variceal bleeding due to portal hypertension in CFLD. |
CF: cystic fibrosis CFLD: cystic fibrosis liver disease RCT; randomised controlled trial UDCA: ursodeoxycholic acid
Differences between protocol and review
None
Contributions of authors
| Roles and responsibilities | |
| Task | Who will undertake the task? |
| Protocol stage: draft the protocol | SK, SM, NNT, IVM, AWT |
| Review stage: select which trials to include (2 + 1 arbiter) | SK, NNT, AWT |
| Review stage: extract data from trials (2 people) | SK, SM |
| Review stage: enter data into RevMan | SK, IVM, NNT |
| Review stage: carry out the analysis | SK, SM, NNT, AWT |
| Review stage: interpret the analysis | SK, SM, IVM, AWT |
| Review stage: draft the final review | SK, NNT, IVM, AWT |
| Update stage: update the review | SK, NNT, IVM, AWT |
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
All authors: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies excluded from this review
Belli 1987 {published data only}
- Belli DC, Levy E, Darling P, Leroy C, Lepage G, Giguere R, et al. Taurine improves the absorption of a fat meal in patients with cystic fibrosis. Pediatrics 1987;80(4):517‐23. [CENTRAL: 50249; CFGD Register: CO1; CRS: 5500100000000311; PUBMED: 3658570] [PubMed] [Google Scholar]
Bittner 1991 {published data only}
- Bittner P, Posselt HG, Sailer T, Ott H, Magdorf K, Wahn U, et al. The effect of treatment with ursodeoxycholic acid in cystic fibrosis and hepatopathy: results of a placebo‐controlled study. Bile Acids As Therapeutic Agents. From Basic Science to Clinical Practice. Falk Symposium 58. 1991;Falk Symposium 58:345‐8. [CENTRAL: 220490; CFGD Register: CO7b; CRS: 5500100000001144] [Google Scholar]
- Bittner P, Seiler T, Ott H, Posselt H‐G, Margdorf K, Kawinkel M. Therapeutical approach of ursodeoxycholic acid in cystic fibrosis and hepatopathy. 16th Annual Meeting of the European Working Group for Cystic Fibrosis; 1989; Prague, Czechoslovakia. 1989:72. [CENTRAL: 291213; CFGD Register: CO7a; CRS: 5500100000001287]
Colombo 1992 {published data only}
- Colombo C, Crosignani A, Assaisso M, Battezzati PM, Podda M, Giunta A, et al. Ursodeoxycholic acid therapy in cystic fibrosis‐associated liver disease: a dose‐response study. Hepatology (Baltimore, Md.) 1992;16(4):924‐30. [CENTRAL: 208420; CFGD Register: CO4b; CRS: 5500100000001071; PUBMED: 1398498] [DOI] [PubMed] [Google Scholar]
- Colombo C, Crosignani A, Castellani R, Balistreri WF, Setchell KDR, Giunta A. Ursodeoxycholic acid therapy for liver disease associated with cystic fibrosis. Bile Acids As Therapeutic Agents. From Basic Science to Clinical Practice. Falk Symposium 58 1991;43(43):349‐56. [CENTRAL: CN‐00362985; CFGD Register: CO4c; CRS: 1231564] [Google Scholar]
- Colombo C, Setchell KDR, Podda M, Crossignani A, Assaisso ML, Giunta A. Ursodeoxycholic Acid (UDCA) in CF associated liver disease (LD): A dose response study. 17th European Cystic Fibrosis Conference; 1991 Jun 18‐21; Copenhagen, Denmark. 1991:140. [CENTRAL: 291253; CFGD Register: CO4a; CRS: 5500100000001318]
Colombo 1996 {published data only}
- Colombo C, Allocca M, Quattrucci S, Traverso G, Farina S, Lucidi V, et al. Ursodeoxycholic acid for liver disease associated to cystic fibrosis: long‐term follow‐up of patients enrolled in the Italian multicenter trial. Pediatric Pulmonology 2005;40(Suppl 28):343. [CENTRAL: 593027; CFGD Register: CO8e; CRS: 5500100000002988] [Google Scholar]
- Colombo C, Battezzati PM, Podda M, Bettinardi N, Giunta A. Ursodeoxycholic acid for liver disease associated with cystic fibrosis: a double‐blind multicenter trial. The Italian Group for the Study of Ursodeoxycholic Acid in Cystic Fibrosis. Hepatology (Baltimore, Md.) 1996;23(6):1484‐90. [CENTRAL: 125471; CFGD Register: CO8c; CRS: 5500100000000751; PUBMED: 8675168] [DOI] [PubMed] [Google Scholar]
- Colombo C, Battezzati PM, Santini B, Iapichino L, Quattrucci S, Lucidi V, et al. Treatment with Ursodeoxycholic acid for patients with cystic fibrosis and liver disease. Clinical Ecology of Cystic Fibrosis 1993;18:275‐9. [CENTRAL: 444883; CFGD Register: CO8d; CRS: 5500100000002341] [Google Scholar]
- Colombo C, Battezzati PM, Santini B, Lapichino L, Quattrucci S, Lucidi V, et al. Ursodeoxycholic Acid (UDCA) for liver disease associated with cystic fibrosis (CF): a double‐blind multicenter trial. 18th European Cystic Fibrosis Conference; 1993 May 21‐26; Madrid, Spain. 1993:W6.1. [CENTRAL: 291252; CFGD Register: CO8a; CRS: 5500100000001317]
- Colombo C, Podda M, Battezzati PM, Santini B, Iapichino L, Quattrucci S, et al. Ursodeoxycholic acid for cystic fibrosis associated liver disease: final report of a multicenter trial. Hepatology (Baltimore, Md.) 1993;18:142A. [CENTRAL: 208167; CFGD Register: CO8b; CRS: 5500100000001048] [Google Scholar]
- Colombo C, Podda M, Battezzati PM, Santini B, Japichino L, Outtrucci S, et al. Ursodeoxycholic acid for cystic fibrosis‐associated liver disease: Final report of a multicenter trial. Hepatology (Baltimore, Md.) 1994;20(4 Pt 2):143A. [CENTRAL: CN‐00352579; CFGD Register: CO8f; CRS: 1223063] [Google Scholar]
Colombo 2002 {published data only}
- Colombo C, Battezzati PM, Crosignani A. Liver disease in cystic fibrosis: a prospective study on incidence, risk factors and outcome. Hepatology 2002;36:1374‐82. [DOI] [PubMed] [Google Scholar]
Darling 1985 {published data only}
- Darling PB, Lepage G, Leroy C, Masson P, Roy C. Effect of taurine supplements on fat absorption in cystic fibrosis. Pediatric Research 1985;19(6):578‐82. [CENTRAL: 38640; CFGD Register: CO12; CRS: 5500100000000231; PUBMED: 4011338] [DOI] [PubMed] [Google Scholar]
Fleet 2000 {published data only}
- Fleet M, Stanley AJ, Forrest EH, Hayes PC, Redhead DN. Transjugular intrahepatic portosystemic stent shunt placement in a patient with cystic fibrosis complicated by portal hypertension. Clinical Radiology 2000 May;55(3):236‐7. [DOI] [PubMed] [Google Scholar]
Kapustina 2000 {published data only}
- Kapustina TJ, Kashirskaia NJ, Kapranov NI, Neudakhin EV. Effect of ursodeoxycholic acid on lipid metabolism in patients with cystic fibrosis. 13th International Cystic Fibrosis Congress; 2000 Jun 4‐8; Stockholm, Sweden. 2000:132. [CENTRAL: 302959; CFGD Register: CO14; CRS: 5500100000001693]
Lamireau 2006 {published data only}
- Lamireau T, Martin S, Lallier M, Marcotte JE, Alvarez F. Liver transplantation for cirrhosis in cystic fibrosis. Canadian Journal of Gastroenterology 2006;20(7):475‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lepage 1997 {published data only}
- Lacaille F, Paradis K, Lenaerts C, Senechal L, Lepage G, Roy CC, et al. Ursodeoxycholic acid (UDCA) improves essential fatty acid (EFA) deficiency in cystic fibrosis. Hepatology (Baltimore, Md.) 1993;18(4 Pt 2):271A. [CENTRAL: 221437; CFGD Register: CO13b; CRS: 5500100000001145] [Google Scholar]
- Lepage G, Paradis K, Lacaille F, Senechal L, Ronco N, Champagne J, et al. Ursodeoxycholic acid improves the hepatic metabolism of essential fatty acids and retinol in children with cystic fibrosis. Journal of Pediatrics 1997;130(1):52‐8. [CENTRAL: 135934; CFGD Register: CO13a; CRS: 5500100000000805; PUBMED: 9003851] [DOI] [PubMed] [Google Scholar]
Merli 1994 {published data only}
- Merli M, Bertasi S, Servi R, Diamanti S, Martino F, Santis A, et al. Effect of a medium dose of ursodeoxycholic acid with or without taurine supplementation on the nutritional status of patients with cystic fibrosis: a randomised, placebo‐controlled, crossover trial. Journal of Pediatric Gastroenterology and Nutrition 1994;19(2):198‐203. [CENTRAL: 108869; CFGD Register: CO5; CRS: 5500100000000659; EMBASE: 1994299511; PUBMED: 7815243] [DOI] [PubMed] [Google Scholar]
Mieles 1991 {published data only}
- Mieles LA, Orenstein DM, Toussaint RM, Selby R, Gordon RD, Starzl TE. Outcome after liver transplantation for cystic fibrosis. Pediatric Pulmonology. Supplement 1991;11(S1):130‐1. [PMC free article] [PubMed] [Google Scholar]
Narkewicz 1994 {published data only}
- Narkewicz MR, Sokol RJ, Lear JL, Wagener JS, Accurso FJ. Effect of ursodeoxycholic acid (UDCA) therapy for CF liver disease on indirect tests of hepatic function. Pediatric Pulmonology 1994;Suppl 10:342. [CENTRAL: 291473; CFGD Register: CO10; CRS: 5500100000001500] [Google Scholar]
Nightingale 2010 {published data only}
- Nightingale S, O'Loughlin EV, Dorney SF, Shun A, Verran DJ, Strasser SI, et al. Isolated liver transplantation in children with cystic fibrosis‐‐an Australian experience. Pediatric Transplantation 2010;14(6):779‐85. [DOI] [PubMed] [Google Scholar]
Noble‐Jamieson 1994 {published data only}
- Noble‐Jamieson G, Valente J, Barnes ND, Friend PJ, Jamieson NV, Rasmussen A, et al. Liver transplantation for hepatic cirrhosis in cystic fibrosis. Archives of Disease in Childhood 1994;71(4):349‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 1992 {published data only}
- O'Brien S, Fitzgerald MX, Hegarty JE. A controlled trial of ursodeoxycholic acid treatment in cystic fibrosis‐related liver disease. European Journal of Gastroenterology & Hepatology 1992;4(10):857‐63. [CENTRAL: 196428; CFGD Register: CO11a; CRS: 5500100000001004; EMBASE: 1992292460] [PubMed] [Google Scholar]
- O'Brien S, Fitzgerald MX, Hegarty JE. Ursodeoxycholic acid treatment in cystic fibrosis related liver disease. Gut 1992;33:S14. [CENTRAL: 208507; CFGD Register: CO11b; CRS: 5500100000001089] [Google Scholar]
- O'Brien SM, Campbell GR, Burke AF, Maguire OC, Rowlands BJ, FitzGerald MX, et al. Serum bile acids and ursodeoxycholic acid treatment in cystic fibrosis‐related liver disease. European Journal of Gastroenterology & Hepatology 1996;8(5):477‐83. [CENTRAL: 129951; CFGD Register: CO11c; CRS: 5500100000000779; PUBMED: 8804877] [PubMed] [Google Scholar]
Pozler 2003 {published data only}
- Pozler O, Krajina A, Vanicek H. Transjugular intrahepatic portosystemic shunt in five children with cystic fibrosis: long‐term results. Hepatogastroenterology 2003;50:1111‐4. [PubMed] [Google Scholar]
Pukhalsky 2008 {published data only}
- Pukhalsky A, Shmarina G, Pukhalskaya D, Perederko L. Whether low frequency of hepatobiliary abnormalities in cystic fibrosis patients is associated with anti‐inflammatory treatment?. European Respiratory Society Annual Congress; 2008 Oct 4‐8; Berlin, Germany.. 2008:216s. [CENTRAL: 758640; CFGD Register: IB93; CRS: 5500100000003483]
Roland 2011 {published data only}
- Rowland M, Gallagher CG, Laoide RO, Canny G, Broderick A, Hayes R, et al. Outcome in cystic fibrosis liver disease. American Journal of Gastroenterology 2011;106:104‐9. [DOI] [PubMed] [Google Scholar]
Schuster 1977 {published data only}
- Schuster SR, Shwachman H, Toyam WM, Rubino A, Taik‐Khaw K. The management of portal hypertension in cystic fibrosis. Journal of Pediatric Surgery 1977;12(2):201‐6. [DOI] [PubMed] [Google Scholar]
Smith 1991 {published data only}
- Smith LJ, Lacaille F, Lepage G, Ronco N, Lamarre A, Roy CC. Taurine decreases fecal fatty acid and sterol excretion in cystic fibrosis. A randomized double‐blind trial. American Journal of Diseases of Children 1991;145(12):1401‐4. [CENTRAL: 98496; CFGD Register: CO3; CRS: 5500100000000606; PUBMED: 1669669] [DOI] [PubMed] [Google Scholar]
Spray 1998 {published data only}
- Spray C, Sinha B, Raman M, Ramani P, Weller P, Kelly D. Does ursodeoxycholic acid improve histological changes in liver disease in cystic fibrosis?. Journal of Pediatric Gastroenterology and Nutrition 1998;26(5):584. [CENTRAL: 543167; CFGD Register: CO6b; CRS: 5500050000000030] [Google Scholar]
- Spray C, Sinha B, Venkataraman M, Davies P, Ramani P, Weller P, et al. The role of ursodeoxycholic acid on histological changes in children with cystic fibrosis‐liver disease ‐ a prospective study. 13th International Cystic Fibrosis Congress; 2000 Jun 4‐8; Stockholm, Sweden. 2000:132. [CENTRAL: 302984; CFGD Register: CO6a; CRS: 5500100000001713]
Thomas 1995 {published data only}
- Thomas PS, Bellamy M, Geddes D. Malabsorption of vitamin E in cystic fibrosis improved after ursodeoxycholic acid. Lancet 1995;346(8984):1230‐1. [CENTRAL: 119931; CFGD Register: GN77; CRS: 5500100000000723; PUBMED: 7475686] [DOI] [PubMed] [Google Scholar]
Thompson 1987 {published data only}
- Thompson GN, Robb TA, Davidson GP. Taurine supplementation, fat absorption, and growth in cystic fibrosis. Journal of Pediatrics 1987;111(4):501‐6. [CENTRAL: 50200; CFGD Register: CO2a; CRS: 5500100000000309; EMBASE: 1987208025; PUBMED: 3309233] [DOI] [PubMed] [Google Scholar]
- Thompson GN, Tomas FM. Protein metabolism in cystic fibrosis: responses to malnutrition and taurine supplementation. American Journal of Clinical Nutrition 1987;46(4):606‐13. [CENTRAL: 50330; CFGD Register: CO2b; CRS: 5500100000000313; EMBASE: 1988005723; PUBMED: 3661477] [DOI] [PubMed] [Google Scholar]
Van de Meeberg 1997 {published data only}
- Meeberg PC, VanBerge Henegouwen GP, The DCF‐UDCATG. Long‐term follow‐up of low dose versus high dose ursodeoxycholic acid (UDCA) in cholestasis related to cystic fibrosis (CF). Gut 1996;39(Suppl 3):A118. [CENTRAL: 208505; CFGD Register: CO9b; CRS: 5500100000001087] [Google Scholar]
- Meeberg PC, Houwen RH, Sinaasappel M, Heijerman HG, Bijleveld CM, Vanberge Henegouwen GP. Low‐dose versus high‐dose ursodeoxycholic acid in cystic fibrosis‐related cholestatic liver disease. Results of a randomised study with 1‐ year follow‐up. Scandinavian Journal of Gastroenterolology 1997;32(4):369‐73. [CENTRAL: 139270; CFGD Register: CO9a; CRS: 5500100000000821; PUBMED: 9140160] [DOI] [PubMed] [Google Scholar]
- Meeberg PC, Houwen RHJ, Sinaasappel M, Bijleveld ChMA, Heijerman HGM, Berge Henegouwen GP. Long‐term follow‐up of low‐dose versus high‐dose ursodeoxycholic acid (UDCA) in cystic fibrosis related cholestasis. European Journal of Gastroenterology & Hepatology 1996;8(Suppl 12):A39. [CENTRAL: CN‐00222479; CFGD Register: CO9c; CRS: 1135014] [Google Scholar]
Additional references
Bari 2012
- Bari K, Garcia‐Tsao G. Treatment of portal hypertension. World J Gastroenterol 2012 Mar 21;18(11):1166‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bartlett 2009
- Bartlett JR, Friedman KJ, Ling SC, Pace RG, Bell SC, Bourke B, et al. Genetic modifiers of liver disease in cystic fibrosis. JAMA 2009;302(10):1076‐83. [DOI: 10.1001/jama.2009.1295] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brigman 2006
- Brigman C, Feranchak A. Liver involvement in cystic fibrosis. Current Treatment Options in Gastroenterology 2006;9(6):484‐96. [DOI] [PubMed] [Google Scholar]
CDC 2010
- Centers for Disease Control and Prevention. National Center for Health Statistics. CDC WONDER. wonder.cdc.gov/ (accessed prior to 01 September 2016).
Cheng 2005
- Cheng JW, Zhu L, Gu MJ, Song ZM. Meta‐analysis of propranolol effects on gastrointestinal haemorrhage in cirrhotic patients. World Journal of Gastroenterology 2003;9(8):1836‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colombo 2002
- Colombo C, Battezzati PM, Crosignani A, Morabito A, Costantini D, Padoan R, et al. Liver disease in cystic fibrosis: a prospective study on incidence, risk factors, and outcomes. Hepatology 2002;36(6):1374‐82. [DOI] [PubMed] [Google Scholar]
Cox 1987
- Cox KL, Ward RE, Furgiuele TL, Cannon RA, Sanders KD, Kurland G. Orthotopic liver transplantation in cystic fibrosis. Paediatrics 1987;80(4):571–4. [PubMed] [Google Scholar]
Davis 2006
- Davis PB. Cystic fibrosis since 1938. American Journal of Respiratory and Critical Care Medicine 2006;173(5):475‐82. [DOI] [PubMed] [Google Scholar]
De Franchis 2005
- Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. Journal of Hepatology 2005;43(1):167‐76. [DOI] [PubMed] [Google Scholar]
Debray 1999
- Debray D, Lykavieris P, Gauthier F, Dousset B, Sardet A, Munck A, et al. Outcome of cystic fibrosis associated liver cirrhosis: management of portal hypertension. Journal of Hepatology 1999;31(1):77‐83. [DOI] [PubMed] [Google Scholar]
Debray 2011
- Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis‐associated liver disease. Journal of Cystic Fibrosis 2011;10 Suppl 2:S29‐S36. [DOI: 10.1016/S1569-1993(11)60006-4] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks J, Higgins J, Altman D, editor(s). Chapter 9 Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dowman 2012
- Dowman JK, Watson D, Loganathan S, Gunson BK, Hodson J, Mirza DF, et al. Long‐term impact of liver transplantation on respiratory function and nutritional status in children and adults with cystic fibrosis. American Journal of Transplantation 2012;12(4):954‐64. [DOI] [PubMed] [Google Scholar]
D’Amico 1995
- D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta‐analytic review. Hepatology 1995;22(1):332‐54. [DOI] [PubMed] [Google Scholar]
Efrati 2003
- Efrati O, Barak A, Modan‐Moses D, Augarten A, Vilozni D, Katznelson D, et al. Liver cirrhosis and portal hypertension in cystic fibrosis. European Journal of Gastroenterology & Hepatology 2003;15(10):1073‐8. [DOI] [PubMed] [Google Scholar]
Farrell 2008
- Farrell PM. The prevalence of cystic fibrosis in the European Union. Journal of Cystic Fibrosis 2008;7(5):450‐53. [DOI] [PubMed] [Google Scholar]
Flass 2013
- Flass T, Narkewicz MR. Cirrhosis and other liver disease in cystic fibrosis. Journal of Cystic Fibrosis 2013;12(2):116‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fridell 2003
- Fridell JA, Bond GJ, Mazariegos GV, Orenstein DM, Jain A, Sindhi R, et al. Liver transplantation in children with cystic fibrosis: a long‐term longitudinal review of a single centre experience. Journal of Pediatric Surgery 2003;38(8):1152‐6. [DOI] [PubMed] [Google Scholar]
Funakoshi 2012
- Funakoshi N, Duny Y, Valats JC, Ségalas‐Largey F, Flori N, Bismuth M, et al. Meta‐analysis: beta‐blockers versus banding ligation for primary prophylaxis of oesophageal variceal bleeding. Annals of Hepatology 2012;11(3):369‐83. [PubMed] [Google Scholar]
Garcia‐Tsao 2007
- Garcia‐Tsao G, Sanyal AJ, Grace ND, Carey WD. Prevention and management of gastroesophageal varices and variceal haemorrhage in cirrhosis. American Journal of Gastroenterology 2007;102(9):2086‐102. [DOI] [PubMed] [Google Scholar]
Gluud 2012
- Gluud LL, Krag A. Banding ligation versus beta‐blockers for primary prevention in oesophageal varices in adults. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD004544.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gooding 2005
- Gooding I, Dondos V, Gyi KM, Hodson M, Westaby D. Variceal haemorrhage and cystic fibrosis: outcomes and implications for liver transplantation. Liver Transplantation 2005;11(12):1522‐6. [DOI] [PubMed] [Google Scholar]
Gugig 2012
- Gugig R, Rosenthal P. Management of portal hypertension in children. World Journal of Gastroenterology 2012;18(11):1176‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011).The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011).The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011).The Cochrane Collaboration 2011. Available from handbook.cochrane.org.
Higgins 2011d
- Higgins JPT, Deeks JJ, Altman DG on behalf of the CSMG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ikegami 2008
- Ikegami T, Sanchez EQ, Uemura T, Narasimhan G, Masannat O, Chinnakotla S, et al. Liver transplantation for cystic fibrosis in adults. Surgery Today 2008;38(1):26‐9. [DOI] [PubMed] [Google Scholar]
Kahan 1989
- Kahan D, Jones B, Bomman PC. Incidence and management of complications of injection sclerotherapy: a ten year prospective evaluation. Surgery 1989;105(2 Pt 1):160‐5. [PubMed] [Google Scholar]
Kitson 2013
- Kitson MT, Kemp WW, Iser DM, Paul E, Wilson JW, Roberts SK. Utility of transient elastography in the non‐invasive evaluation of cystic fibrosis liver disease. Liver International 2013;33(5):698‐705. [DOI] [PubMed] [Google Scholar]
Kobelska‐Dubiel 2014
- Kobelska‐Dubiel N, Klincewicz B, Cichy W. Liver disease in cystic fibrosis. Przegląd Gastroenterologiczny 2014;9(3):136‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamireau 2004
- Lamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. Journal of Hepatology 2004;41(6):920‐5. [DOI] [PubMed] [Google Scholar]
Ledder 2014
- Ledder O, Haller W, Couper RT, Lewindon P, Oliver M. Cystic fibrosis: an update for clinicians. Part 2: hepatobiliary and pancreatic manifestations. Journal of Gastroenterology and Hepatology 2014;29(12):1954‐62. [DOI] [PubMed] [Google Scholar]
Lindblad 1999
- Lindblad A, Glaumann H, Strandvik B. Natural history of liver disease in cystic fibrosis. Hepatology 1999;30:1151‐8. [DOI] [PubMed] [Google Scholar]
Louis 2007
- Louis D, Duc ML, Reix P. Partial splenectomy for portal hypertension in cystic fibrosis related liver disease. Pediatric Pulmonology 2007;42:1173‐80. [DOI] [PubMed] [Google Scholar]
Lu 2010
- Lu BR, Esquivel CO. A review of abdominal organ transplantation in patients with cystic fibrosis. Pediatric Transplantation 2010;14:954‐60. [DOI] [PubMed] [Google Scholar]
Mack 1995
- Mack DR, Traystman MD, Colombo JL, Sammut PH, Kaufman SS, Vanderhoof JA, et al. Clinical denouement and mutation analysis of patients with cystic fibrosis undergoing liver transplantation for biliary cirrhosis. Journal of Pediatrics 1995;127(6):881–7. [DOI] [PubMed] [Google Scholar]
Malbrunot‐Wagner 2011
- Malbrunot‐Wagner AC, Bridou XL, Nousbaum JB, Rio C, Dirou A, Ginies JL, et al. Transient elastography and portal hypertension in paediatric patient with cystic fibrosis. Journal of Cystic Fibrosis 2011;10(5):338‐42. [DOI] [PubMed] [Google Scholar]
Melzi 2006
- Melzi ML, Kelly DA, Colombo C, Jara P, Manzanares J, Colledan M, et al. Liver transplant in cystic fibrosis: a poll among European centre. A study from the European Liver Transplant Registry. Transplant International 2006;19(9):726‐31. [DOI] [PubMed] [Google Scholar]
Mendizabal 2011
- Mendizabal M, Reddy KR, Cassuto J. Liver transplantation in patients with cystic fibrosis: analysis of United Network for Organ Sharing data. Liver Transplantation 2011;17:243‐50. [DOI] [PubMed] [Google Scholar]
Menten 2010
- Menten R, Leonard A, Clapuyt P, Vincke P, Nicolae AC, Lebecque P. Transient elastography in patient with cystic fibrosis. Pediatric Radiology 2010;40(7):1231‐5. [DOI] [PubMed] [Google Scholar]
Mileti 2011
- Mileti E, Rosenthal P. Management of portal hypertension in children. Current Gastroenterology Reports 2011;13(1):10‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milkiewicz 2012
- Milkiewicz P, Skiba G, Kelly D, Weller P, Bonser R, Gur U, et al. Transplantation for cystic fibrosis: outcome following early liver transplantation. Journal of Gastroenterology and Hepatology 2012;17(2):208‐13. [DOI] [PubMed] [Google Scholar]
Moyer 2009
- Moyer K, Balistreri W. Hepatobiliary disease in patients with cystic fibrosis. Current Opinion in Gastroenterology 2009;25(3):272‐8. [DOI] [PubMed] [Google Scholar]
Nagel 1989
- Nagel RA, Javaid A, Meire HB, Westaby D, Kavari J, Lombard MG, et al. Liver disease and bile duct abnormalities in adult with cystic fibrosis. Lancet 1989;2(8677):1422‐25. [DOI] [PubMed] [Google Scholar]
Nash 2008
- Nash KL, Collier JD, French J, McKeon D, Gimson AE, Jamieson NV, et al. Cystic fibrosis liver disease: to transplant or not to transplant?. American Journal of Transplantation 2008;8(1):162‐9. [DOI] [PubMed] [Google Scholar]
Noble‐Jamieson 1996
- Noble‐Jamieson G, Barnes N, Jamieson N, Friend P, Calne R. Liver transplantation for hepatic cirrhosis in cystic fibrosis. Journal of the Royal Society of Medicine 1996;89 Suppl 27:31–7. [PMC free article] [PubMed] [Google Scholar]
Ozsoylu 1985
- Ozsoylu S, Kocak N, Yuce A. Propranolol therapy for portal hypertension in children. Journal of Pediatrics 1985;106:317‐21. [DOI] [PubMed] [Google Scholar]
Ozsoylu 2000
- Ozsoylu S, Kocak N, Demir H. Propranolol for primary and secondary prophylaxis of variceal bleeding in children with cirrhosis. Turkish Journal of Pediatrics 2000;42(1):31‐3. [PubMed] [Google Scholar]
Pozler 2003
- Pozler O, Krajina A, Vanicek H, Hulek P, Zizka J, Michl A, et al. Transjugular intrahepatic portosystemic shunt in five children with cystic fibrosis: long term results. Hepatogastroenterology 2003;50(52):1111‐4. [PubMed] [Google Scholar]
Price 1996
- Price MR, Sartorelli KH, Karrer FM, Narkewicz MR, Sokol RJ, Lilly JR. Management of oesophageal varices in children by endoscopic variceal ligation. Journal of Pediatric Surgery 1996;31(8):1056‐9. [DOI] [PubMed] [Google Scholar]
Quittner 2009
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire‐Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rowland 2011
- Rowland M, Gallagher CG, O'Laoide R, Canny G, Broderick A, Hayes R, Greally P, Slattery D, Daly L, Durie P, Bourke B. Outcome in cystic fibrosis liver disease. American Journal of Gastroenterology 2011;106:104‐9. [DOI] [PubMed] [Google Scholar]
Sadler 2015
- Sadler MD, Crotty P, Fatovich L, Wilson S, Rabin HR, Myers RP. Non invasive methods, including transient elastrography, for the detection of liver disease in adults with cystic fibrosis. Canadian Journal of Gastroenterology and Hepatology 2015;29(3):139‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shashidhar 1999
- Shashidhar HH, Langhans N, Grand RJ. Propranolol in prevention of portal hypertensive haemorrhage in children: a pilot study. Journal of Pediatric Gastroenterology and Nutrition 1999;29(1):12‐7. [DOI] [PubMed] [Google Scholar]
Shneider 2012
- Shneider BL, Bosch J, Franchis R, Emre SH, Groszmann RJ, Ling SC, et al. Portal hypertension in children: expert paediatric opinion on the report of the Baveno v Consensus Workshop on Methodology of Diagnosis and Therapy in Portal Hypertension. Pediatric Transplantation 2012;16(5):426‐37. [DOI: 10.1111/j.1399-3046.2012.01652.x] [DOI] [PubMed] [Google Scholar]
Simmonds 2008
- Simmonds NJ, Cullinan P, Hodson ME. Growing old with cystic fibrosis ‐ the characteristics of long‐term survivors of cystic fibrosis. Respiratory Medicine 2009;103(4):629‐35. [DOI: 10.1016/j.rmed.2008.10.011] [DOI] [PubMed] [Google Scholar]
Smith 2004
- Smith JL, Lewindon PJ, Hoskins AC, Pereira TN, Setchell KD, O'Connell NC, et al. Endogenous ursodeoxycholic acid and cholic acid in liver disease due to cystic fibrosis. Hepatology 2004;39(6):1673‐82. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne J, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Stringer 1993
- Stringer MD, Price JF, Mowat AP, Howard ER. Liver cirrhosis in cystic fibrosis. Archives of Disease in Childhood 1993;69(3):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2015
- World Health Organization (Genomic Resource Centre). Genes and human disease. www.who.int/genomics/public/geneticdiseases/en/index2.html (accessed 11 August 2015).
Witters 2009
- Witters P, Boeck K, Dupont L, Proesmans M, Vermeulen F, Servaes R, et al. Non‐invasive liver elastography (Fibroscan) for detection of cystic fibrosis‐associated liver disease. Journal of Cystic Fibrosis 2009;8(6):392‐9. [DOI] [PubMed] [Google Scholar]
Wolff 2003
- Wolff M, Hirner A. Current state of portosystemic shunt surgery. Langenbeck's Archives of Surgery 2003;388(3):141‐9. [DOI] [PubMed] [Google Scholar]
Wong 2000
- Wong T, Pereira SP, McNair A, Harrison PM. A prospective, randomised comparison of the ease and safety of variceal ligation using a multiband vs. a conventional ligation device. Endoscopy 2000;32:931‐4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Than 2017
- Palaniappan SK, Than NN, Thein AW, Moe S, Mourik I. Interventions for preventing and managing advanced liver disease in cystic fibrosis. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD012056.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


