Abstract
Background
Recent studies have suggested that hepatocyte senescence could contribute to hepatic steatosis and its progression in nonalcoholic fatty liver disease (NAFLD). However, the underlying mechanism causing hepatocyte senescence in this pathological condition is still unclear. A thorough understanding of the mechanism could provide a new target for therapeutic intervention. The purpose of this study was to investigate the role of p66shc in hepatocyte senescence and hepatocyte damage in NAFLD progression.
Material/Methods
We examined the expression levels of hepatic p66shc and senescence markers in rats and humans with NAFLD, and we assessed the effect of p66shc knockdown or overexpression on senescence and steatosis in human liver cells.
Results
In this study, we showed that increased hepatic p66shc expression was consistent with upregulated expression of the following senescence markers in NAFLD rats: heterochromatin protein-1-beta (HP1β), p16, p21, and p53. Furthermore, senescence and steatosis could be induced in hepatoblastoma cell line (HepG2) cells when cells were stimulated with a low concentration of H2O2, and this effect was significantly alleviated by knockdown of p66shc. However, overexpression of p66shc could promote senescence and steatosis in L02 cells. Finally, increased hepatic p66shc protein levels correlated with enhanced expression of the senescence marker p21 and mirrored the degree of disease severity in NAFLD patients.
Conclusions
Our findings indicated that the increase in hepatocyte senescence and steatosis in NAFLD may be caused by the upregulation of p66shc expression, implying that strategies for p66shc-mediated regulation of hepatocyte senescence may provide new therapeutic tools for NAFLD.
MeSH Keywords: Cell Aging, Fatty Liver, Shc Signaling Adaptor Proteins
Background
With the rapid increase in the prevalence of obesity and metabolic syndrome, nonalcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease worldwide [1,2]. However, the pathological mechanisms underlying NAFLD have not yet been fully clarified. The causes were originally ascribed to lipid metabolism disorders coupled with oxidative stress [3]. Currently, other factors and pathogenic mechanisms are likely responsible for tissue injury and the promotion of disease progression [4–7].
Cellular senescence has been demonstrated to be closely associated with NAFLD. Senescent cells have been found in the livers of patients and mice with NAFLD [8–11], and an increased proportion of senescent hepatocytes appears to be independently associated with disease progression [9,12–14]. Further studies have shown that hepatocyte senescence contributes to the pathogenesis of NAFLD by driving hepatic fat accumulation and steatosis [15]. In addition, senescent human hepatocytes can secrete a spectrum of proteins associated with inflammation and tissue damage, including proinflammatory cytokines, growth factors, and matrix-degrading enzymes, which have been demonstrated to have implications for the promotion of NAFLD progression [16–18]. These findings indicate that hepatocyte senescence plays a critical role in the pathophysiology of NAFLD. However, genes that may be critically involved in regulating hepatocyte senescence in NAFLD are still unknown.
The 66 kDa isoform of Shc (p66shc) is encoded by the ShcA gene locus that is expressed as 3 isoforms: p66shc, p46shc, and p52shc. Compared with the other 2 isoforms, p66shc mainly functions within the mitochondria and controls mitochondrial reactive oxygen species (ROS) production and ROS-dependent oxidative stress [19,20]. Oxidative stress, which is ubiquitous in progressive non-alcoholic steatohepatitis (NASH), appears to be a major driver of hepatocyte senescence [4,21,22]. However, the relationship between p66shc and senescence in hepatocytes is still unclear to date. Additionally, increased expression of p66shc has been observed in NAFLD patients and mice [23], but whether and how p66shc contributes to the progression of the disease remains unknown. Therefore, we hypothesize that p66shc is implicated in hepatocyte senescence and contributes to liver dysfunction during NAFLD via a senescence-dependent mechanism.
Material and Methods
Animal studies
All animal experimental protocols were approved by the Ethics Committee for Animal Experiments of Hangzhou Normal University, Hangzhou, China (approval number: 2016056; date of approval: 2016.02.29), and were carried out in accordance with the National Research Council’s Guide for The Care and Use of Laboratory Animals. A total of 20 male Sprague-Dawley rats weighing 180–200 g were purchased from Shanghai SLACCAS Laboratory Animal Co., Ltd. (Shanghai, China) and were maintained under specific pathogen-free conditions at a constant temperature of 21±2°C and humidity of 55±10%. The rats were randomly divided into 2 groups: a high-fat diet (HFD) group and a normal diet group, with 10 rats per group. Rat feed was obtained from Trophic Animal Feed High-Tech Co., Ltd. (Nantong, China), and the HFD included 82% basic feed, 10% lard oil, 5% yolk powder, 2% cholesterol, and 1% sodium deoxycholate. Rat body weight, behavior, appetite and fur color were monitored on a weekly basis. At the end of 8 weeks, all rats were fasted overnight and then euthanized by intraperitoneal injection of 1% pentobarbital sodium (0.5 mL/kg body weight). Blood samples were collected for biochemical analysis. Liver tissue samples were obtained for histological analysis and total RNA and protein extraction.
In vitro studies
In this study, we used the hepatoblastoma cell line (HepG2) and L02 cell lines as representative of primary human hepatocytes, since the isolation and culture of primary human hepatocytes are particularly difficult. Besides, HepG2 cells [24,25] and L02 cells [26,27] have been shown to be highly relevant and suitable for senescence studies in vitro model.
Induction of senescence in HepG2 cells
HepG2 was purchased from the Cell Resource Centre of the Chinese Academy of Sciences, Ltd. (Shanghai, China). The cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco) with 10% fetal bovine serum (FBS) (Gibco). Cells were preincubated in 6-well plates at a density of 5×105 cells per well for 24 hours and then treated with 0.5 mM hydrogen peroxide (H2O2) (Sigma) for 60 minutes to induce senescence; control cells were incubated in culture media alone. Then, the cells were incubated at 37°C and 5% CO2 for 5 days [24,25]. Senescence was confirmed using senescence-associated beta-galactosidase (SA-β-gal) activity and expression of the cell cycle phase markers p16, p21, and p53.
p66shc knockdown in HepG2 cells
A lentivirus-mediated short hairpin RNA (shRNA) vector was constructed by GenePharma (Shanghai, China). HepG2 cells were infected with either a lentiviral vector expressing a shRNA targeting p66shc mRNA (shp66shc) or a vector expressing a shRNA control (negative control lentivirus, NC). The shRNA sequences for p66shc RNA interference were as follows: #1 GCCACGGGAGCTTTGTCAATA, #2 GCAAACAGATCATCGCCAACC, and #3 CCGCTTTGAAAGTGTCAGTCA. The sequence for the control was a nontargeted scrambled shRNA sequence: TTCTCCGAACGTGTCACGT. Then, the cells were treated with 0.5 mM H2O2 to induce lipotoxicity and senescence for further analysis. Then, shp66shc efficiency was detected by quantitative real-time polymerase chain reaction (qRT-PCR) and western blot assays.
p66shc overexpression in L02 cells
The human hepatocyte cell line (L02) was provided by the Cell Resource Centre of the Chinese Academy of Sciences, Ltd. (Shanghai, China). The cells were incubated in RPMI 1640 medium (Gibco) containing 10% (v/v) FBS (Gibco) at 5% CO2 and 37°C. Then, L02 cells were transfected with pcDNA3.1-p66shc or pcDNA3.1-negative control (GenePharma) following the instructions provided with Lipofectamine™ 2000 (Invitrogen) for these experiments. Then, p66shc overexpression was confirmed by qRT-PCR and western blot assays.
Human studies
Liver tissue samples were obtained from 27 patients who had liver biopsies collected due to suspected NAFLD; patients were treated at the Diagnosis and Treatment Center of the Affiliated Hospital of Hangzhou Normal University (Hangzhou, China). In addition, histologically normal liver sections from 3 patients who underwent a partial hepatectomy for liver cancer were used as controls. All patients gave their informed consent for inclusion before they participated in the study. The study was carried out according to the rules of the Declaration of Helsinki (as revised in Brazil in 2013), and the experimental protocols were approved by the Ethics Committee of the Affiliated Hospital of Hangzhou Normal University (approval number: 2015-HS-001; date of approval: 2015. 02.05) in Hangzhou, China. All liver samples were assessed by the same single pathologist using the Steatosis, Activity, Fibrosis (SAF) score system [28]. Protein levels of p66shc and the senescence marker p21 were assessed by immunohistochemistry.
Evaluation of SA-β-gal activity
SA-β-gal activity was evaluated using a senescent cell histochemical staining kit (Beyotime). Senescent hepatocytes were counted as a percentage of all hepatocytes per field.
Oil Red O staining
Preparation of an Oil Red O (BASO) working solution and staining of slides were performed according to the manufacturer’s recommendations. The surfaces of lipid droplets were quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA) by measuring the area occupied by red pixels.
RNA isolation and qRT-PCR analysis
Total RNA was extracted with TRIzol reagent (Life Technologies). All samples were reverse transcribed to generate cDNA according to the directions of the reverse transcription kit (Roche, Basel, Switzerland). The cDNA samples were amplified using Fast SYBR Green Master Mix and an ABI PRISM 7900 system (both Applied Biosystems, Foster City, CA, USA). The results were normalized to the expression level of the housekeeping gene GAPDH (glyceraldehyde 3-phosphate dehydrogenase). The sequences of the primers are shown in Table 1.
Table 1.
Sequence of the Primers for Quantitative Real-Time Polymerase Chain Reaction.
| Gene | Forward sequence (5′-3′) | Reverse sequence (5′-3′) |
|---|---|---|
| Rat-GAPDH | GACATCAAGAAGGTGGTGAAGC | TGTCATTGAGAGCAATGCCAGC |
| Rat-P16 | CCGAGAGGAAGGCGAACTC | GCTGCCCTGGCTAGTCTATCTG |
| Rat-P21 | GAGCAAAGTATGCCGTCGTC | CTCAGTGGCGAAGTCAAAGTTC |
| Rat-P53 | GCCATCTACAAGAAGTCACAGC | GATGATGGTAAGGATAGGTCGG |
| Rat-P66shc | GGTTGCGTGGAGGTCTTA | GTCTGCTGCCATGAGGTT |
| Human-GAPDH | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
| Human- P16 | CAGTAACCATGCCCGCATAGA | AAGTTTCCCGAGGTTTCTCAGA |
| Human- P21 | TGGAGACTCTCAGGGTCGAAA | GGCGTTGGAGTGGTAGAAATC |
| Human- P53 | GAGGGATGTTTGGGAGATGTAA | CCCTGGTTAGTACGGTGAAGTG |
| Human- P66shc | GTATGTGCTCACTGGCTTGC | CTGACACTTTCAAAGCGGTG |
Western blot analysis
Proteins were extracted using radioimmunoprecipitation assay (RIPA) lysis buffer (Thermo Scientific), and then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Beyotime) and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad). After blocking nonspecific binding, the PVDF membranes were incubated with the following primary antibodies: GAPDH (Cell Signaling Technology), p66shc (Abcam), p21 (Santa Cruz Biotechnology), p16 (Abcam), nuclear heterochromatin protein-1-beta (HP1β) (Cell Signaling Technology), and the phosphorylated form of H2A histone family member X (γ-H2AX) (Cell Signaling Technology). Then, the membranes were immunostained with secondary antibodies (Santa Cruz Biotechnology). Finally, an enhanced chemiluminescence (ECL) system (Thermo Fisher Scientific) was used for protein detection.
Statistical analysis
Data are expressed as the mean±standard deviation. Comparisons between groups were performed using unpaired Student’s t-tests. Correlations were assessed by Pearson’s correlation coefficient between the normally distributed hepatic p66shc and p21 expression levels in NAFLD patients and by Spearman’s rank correlation coefficient between the nonnormally distributed hepatic p66shc expression and the senescence markers and biochemical parameters levels in rats. P<0.05 was indicative of a statistically significant difference. All analyses were performed using SPSS software (SPSS version 22, Chicago, IL, USA).
Results
Increased hepatic expression of p66shc in NAFLD rats correlated with increases in biochemical markers of senescence
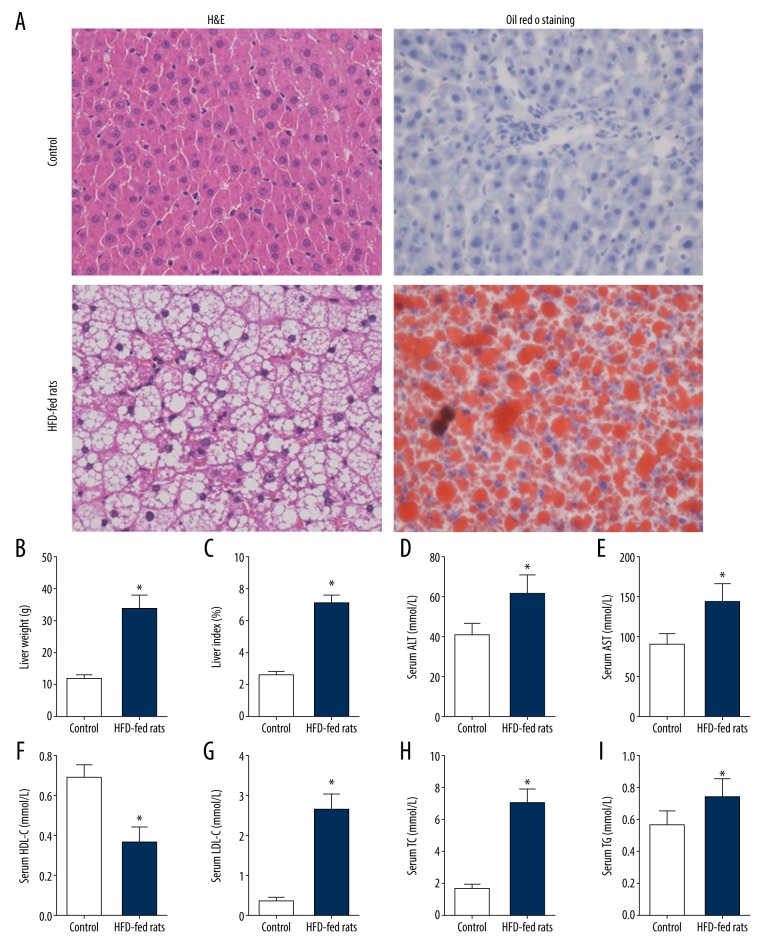
To understand the relationship between p66shc and hepatocyte senescence in NAFLD and the role of each in the disease pathogenesis, we first generated an HFD-induced rat model of NAFLD. HFD-fed rats displayed an obvious increase in liver weight and liver index compared with those in the control group (all P<0.05; Figure 1B, 1C). According to hematoxylin and eosin staining (H&E) and Oil Red O staining, HFD-fed rats presented with increased lipid droplet accumulation and inflammatory infiltration (Figure 1A). Consistent with the pathologic alterations, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), triglyceride (TG), and low-density lipoprotein cholesterol (LDL-C) levels were also significantly increased in response to the HFD, and high-density lipoprotein cholesterol (HDL-C) levels in the HFD group were decreased compared with those in the control group (all P<0.05; Figure 1D–1I).
Figure 1.
Characterization of the rat model of NAFLD. (A) Representative images of H&E and Oil Red O staining of liver sections (400×). (B) Liver weight. (C) Liver index. (D) Serum ALT level. (E) Serum AST level. (F) Serum HDL-C level. (G) Serum LDL-C level. (H) Serum TC level. (I) Serum TG level. The values represent means±SD (n=10 per group). Statistical significance: * P<0.05. NAFLD – nonalcoholic fatty liver disease; H&E – hematoxylin and eosin staining; ALT – alanine aminotransferase; AST – aspartate aminotransferase; HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; TC – total cholesterol; TG – triglyceride; SD – standard deviation.
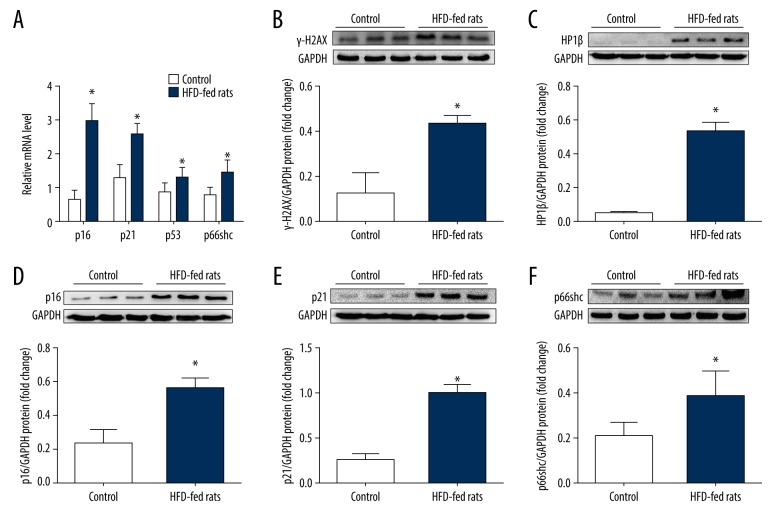
Subsequently, we found that a HFD diet increased the expression of a variety of senescence markers in the liver as follows: the cell cycle inhibitors p16, p21, and p53, and γ-H2AX (a marker of the DNA damage response and an initiator of cell senescence) and HP1β (a marker of senescence-associated heterochromatin foci), as shown in Figure 2 (all P<0.05). We then measured the p66shc expression level in the livers of the rats. As expected, hepatic p66shc expression was significantly increased in NAFLD rats (P<0.05; Figure 2A, 2F) and positively correlated with the senescence markers p16, p21, and p53 (r=0.593, r=0.544, and r=0.870, respectively, all P<0.05). Furthermore, p66shc mRNA levels were also found to be positively correlated with serum TC and TG levels and inversely correlated with serum HDL-C levels (r=0.532, r=0.563, and r=−0.621, respectively, all P<0.05). These results suggest that p66shc is closely related to hepatocyte senescence and that it may be involved in the pathogenesis of NAFLD.
Figure 2.
The expression levels of hepatic p66shc and senescence markers in HFD-induced NAFLD rats compared with controls. (A) Hepatic mRNA levels of p66shc and senescence related genes p16, p21, and p53. (B) γ-H2AX protein level. (C) HP1β protein level. (D) p16 protein level. (E) p21 protein level. (F) p66shc protein level. The values represent means±SD; n=10 (A) and 6 (B–F) per group, respectively. Statistical significance: * P<0.05. HFD – high fat diet; NAFLD – nonalcoholic fatty liver disease; γ-H2AX – phosphorylated form of H2A histone family member X; HP1β – nuclear heterochromatin protein-1-beta; SD – standard deviation.
p66shc expression and lipid accumulation are exacerbated in H2O2-induced senescent HepG2 cells
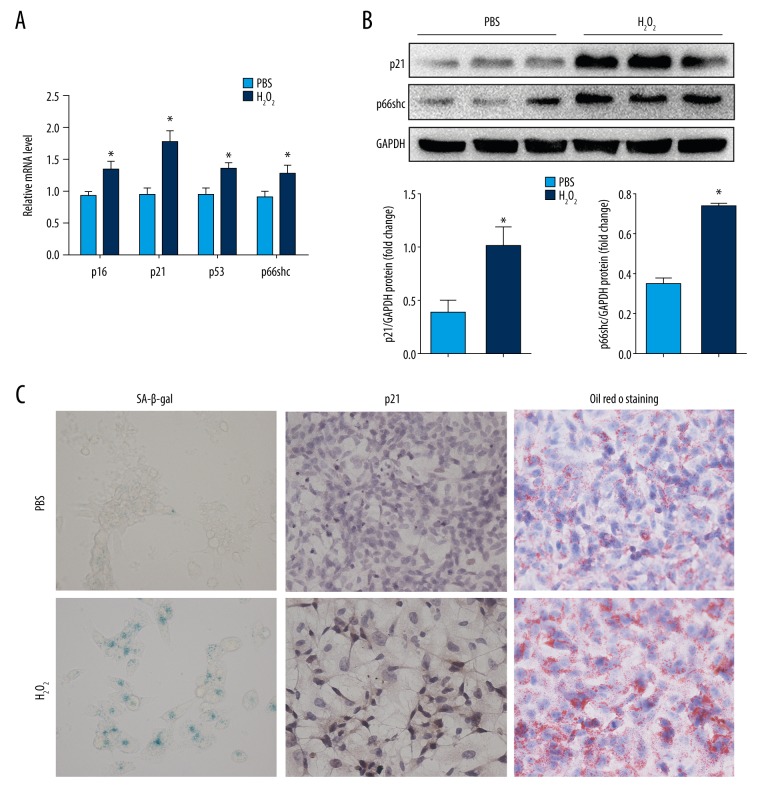
We further investigated the role of p66shc in regulating hepatocyte senescence and hepatocyte damage in vitro. Oxidative stress plays a critical role in the progression of NAFLD and appears to be a major driver of hepatocyte senescence; thus, a model of oxidative stress-induced senescence in HepG2 cells was established in our study. As shown in Figure 3, after exposure to low concentration of H2O2, the senescence markers p16, p21, and p53 and the proportion of SA-β-gal-positive cells showed significant increases in H2O2-treated HepG2 cells compared with those values in untreated cells (all P<0.05). Along with the increases in markers of senescence, H2O2-treated senescent HepG2 cells displayed higher expression levels of p66shc (Figure 3A, 3B) and a larger amount of severe lipid accumulation than control cells (all P<0.05, Figure 3C, right panel).
Figure 3.
p66shc expression and lipid accumulation in H2O2-induced senescent HepG2 cells. (A) mRNA levels of p66shc and senescence markers p16, p21, and p53. (B) p66shc and p21 protein levels as evaluated by western blot assays. (C) Representative images of SA-β-gal activity staining (left panel), p21 immunohistochemical staining (middle panel), and Oil Red O staining (right panel, 400×). Statistical significance: * P<0.05. H2O2 – hydrogen peroxide; HepG2 – hepatoblastoma cell line; mRNA – messenger RNA; SA-β-gal – senescence-associated beta-galactosidase.
p66shc deficiency inhibits H2O2-induced senescence and lipid accumulation in HepG2 cells
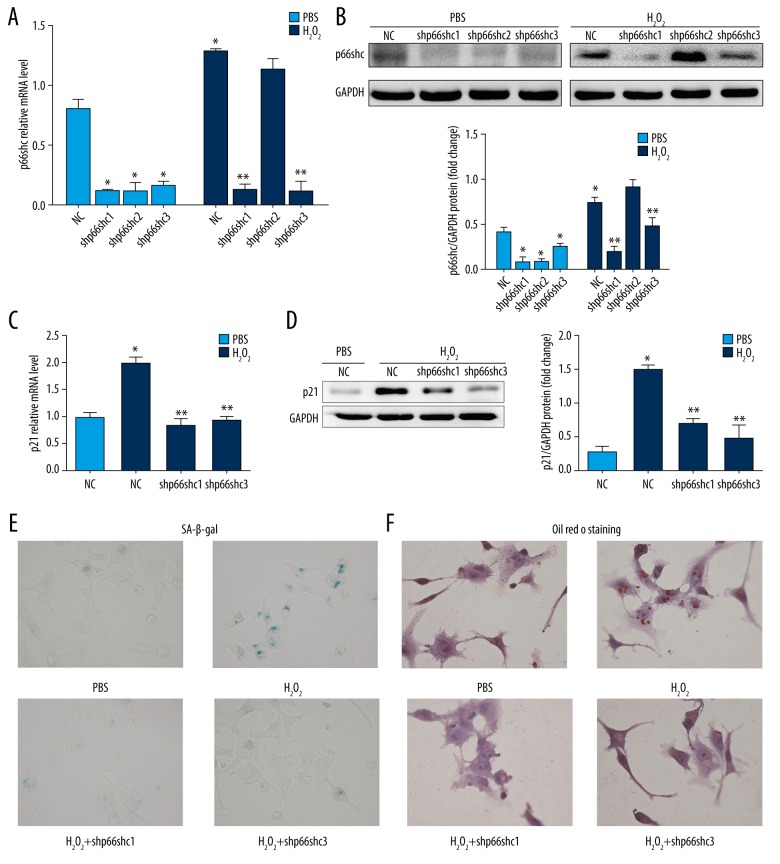
Based on the aforementioned findings, gene-specific lentiviral shRNAs were then designed to knockdown the expression of p66shc in HepG2 cells. As shown in Figure 4, both shp66shc #1 and #3 significantly inhibited p66shc mRNA and protein expression in HepG2 cells (P<0.05; Figure 4A, 4B). Loss of p66shc due to treatment with either shp66shc #1 or #3 resulted in significant inhibition of H2O2-induced increases in p21 expression and the percentage of SA-β-gal-positive cells (all P<0.05; Figure 4C–4E). In addition, Oil Red O staining analysis indicated that H2O2-induced intracellular lipid accumulation was attenuated by treatment with either shp66shc #1 or #3 (P<0.05; Figure 4F).
Figure 4.
p66shc deficiency inhibits H2O2-induced senescence and lipid accumulation in HepG2 cells. (A) p66shc mRNA level. (B) p66shc protein level as evaluated by western blot assays. (C) Senescence marker p21 mRNA level. (D) p21 protein level as evaluated by western blot assays. Representative images of SA-β-gal activity staining (E) and Oil Red O staining (F). (400×). Statistical significance: * P<0.05 versus control group and ** P<0.05 versus control group after incubation with H2O2. H2O2 – hydrogen peroxide; HepG2 – hepatoblastoma cell line; mRNA – messenger RNA; SA-β-gal – senescence-associated beta-galactosidase.
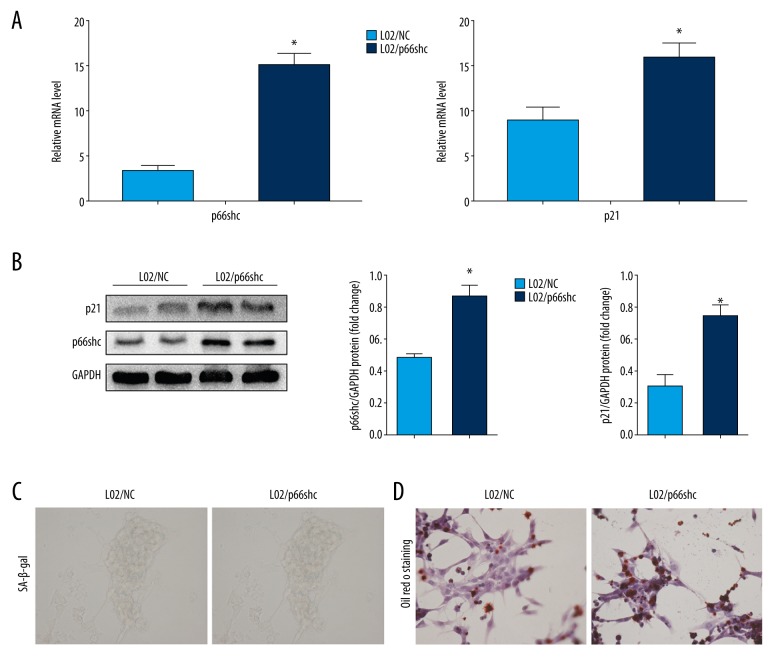
Overexpression of p66shc promotes senescence and steatosis in L02 cells
To further verify the regulation of hepatocyte senescence and steatosis by p66shc, we transfected L02 cells with either recombinant DNA encoding p66shc or a negative control (NC). Following transfection with the recombinant DNA (L02/p66shc cells), the expression of p66shc was significantly enhanced compared with that observed in the control cells (P<0.05; Figure 5A, 5B). As expected, p66shc overexpression enhanced p21 expression and SA-β-gal activity significantly in L02/p66shc cells compared with that in the control cells (all P<0.05; Figure 5A–5C). In addition, overexpression of p66shc resulted in a significant increase in hepatocellular fat deposition (P<0.05; Figure 5D). Overall, these data strongly indicate that p66shc promotes senescence and abnormal lipid accumulation in hepatocytes. The enhanced senescence and steatosis in the liver tissue of NAFLD patients may be caused by the increase in p66shc expression.
Figure 5.
Overexpression of p66shc promotes senescence and steatosis in L02 cells. (A) p66shc and senescence marker p21 mRNA levels. (B) p66shc and senescence marker p21 protein levels as evaluated by western blot assays. Representative images of SA-β-gal activity staining (C) and Oil Red O staining (D). (400×). Statistical significance: * P<0.05. SA-β-gal – senescence-associated beta-galactosidase.
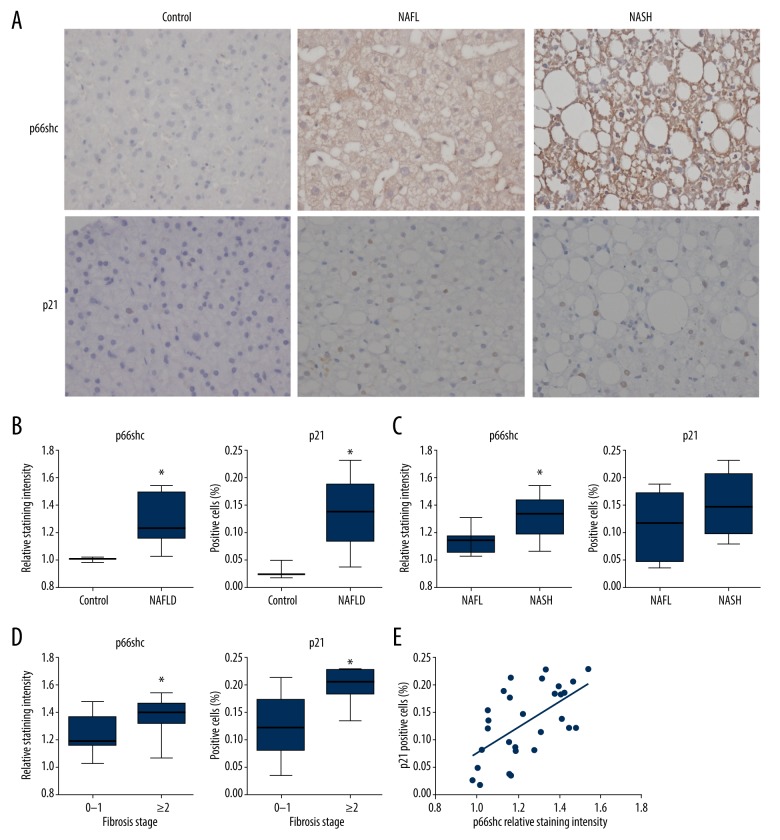
Increased hepatic p66shc expression positively correlated with increased expression of the senescence marker p21 and disease severity in patients with NAFLD
Finally, we examined hepatic p66shc and senescence markers in human NAFLD. The classification of patients according to histopathological type is presented in Table 2. The expression levels of p66shc and p21 protein were detected by immunohistochemistry (Figure 6A). Hepatic p66shc protein levels were enhanced in parallel with the severity of liver injury. There were significant differences in p66shc staining intensity when making the following comparisons of patients: NAFLD and control, NAFL and NASH, and fibrosis 0–1 and fibrosis ≥2 (all P<0.05; Figure 6B–6D). Furthermore, the percentage of p21-positive hepatocytes was also increased in patients with NAFLD compared with that of controls (P<0.05; Figure 6B). Moreover, patients with fibrosis ≥2 had higher expression of p21 than patients with fibrosis 0–1 (P<0.05; Figure 6D). In addition, scatter plot analysis demonstrated a positive correlation between p66shc expression and p21 expression (r=0.594, P<0.05; Figure 6E).
Table 2.
Histopathological Features of NAFLD Patients with Liver Biopsy.
| Histopathological feature | N | |
|---|---|---|
| Steatosis (grade: 0/1/2/3) | 0/9/6/12 | |
| Activity (grade: 0–4) | Lobular inflammation (grade: 0/1/2) | 1/14/12 |
| Hepatocyte ballooning (grade: 0/1/2) | 0/12/15 | |
| Fibrosis (stage: 0/1/2/3/4) | 5/7/8/6/1 | |
| NASH/NAFL | 19/8 | |
Steatosis, activity, fibrosis (SAF) score system was used to assess the pathological characteristics and histological severity of all liver samples. NAFLD – (S≥1AanyFany); NASH – (S≥1A≥2Fany); NAFL – (S≥1A<2F<2) [28]. NAFLD – nonalcoholic fatty liver disease; NASH – nonalcoholic steatohepatitis; NAFL – nonalcoholic fatty liver.
Figure 6.
Increased hepatic p66shc protein expression positively correlates with increased protein expression of the senescence marker p21 and disease severity in NAFLD patients. (A) Representative images of immunohistochemical staining of hepatic p66shc and p21 in a subset of patients (400×). Hepatic p66shc relative staining intensity and p21-positive cells are shown in NAFLD patients and control subjects (B), NAFL and NASH (C), fibrosis 0–1 and fibrosis ≥2 (D). Box plots demonstrate the interquartile range (box) as well as median and range. (E) Scatter plot analysis of the correlation between p66shc and p21 expression levels. Statistical significance: * P<0.05. NAFLD – nonalcoholic fatty liver disease; NAFL – nonalcoholic fatty liver; NASH – nonalcoholic steatohepatitis.
Discussion
Increasing evidence indicates that hepatocyte senescence plays a pivotal role in the progression of NAFLD [8–15]. Thus, potential endogenous regulators of hepatocyte senescence may represent a new therapeutic option for NAFLD. Excessive production of ROS, the majority of which are generated in mitochondria, contributes to the process of cellular senescence. The adapter protein p66shc is a recognized mediator of mitochondrial ROS production and moderates various types of oxidative stress-related damage under a variety of pathophysiological conditions, including hypertension [29], diabetes [30,31], ischemic injury [32], skeletal muscle damage [33], and kidney disease [34,35]. Thus, it is interesting to speculate whether p66shc plays an important role in regulating hepatocyte senescence and participates in the pathophysiology of NAFLD.
The role of p66Shc in hepatocyte and liver diseases has been identified. For instance, Tomita et al. found that the p66shc protein level was elevated in the livers of patients and mice with NASH [23]. Conversely, inhibition of p66shc attenuated ethanol-induced mitochondrial ROS generation and hepatocyte damage in mice [36], which may mechanistically resemble NAFLD. Moreover, overexpression of p66shc can increase sensitivity to oxidation-dependent DNA damage in hepatocytes [37,38]. In addition, a recent study demonstrated that p66shc could contribute to liver fibrosis through mitochondrial ROS [39]. In our study, we further demonstrated p66shc was a driver of lipid accumulation in human liver cells, and the increased hepatic p66shc in NAFLD patients was correlated with the histological severity. Together with our data, these evidences indicate that p66shc might play a critical role in hepatic lipid transportation and consumption and contribute to the progression of NAFLD. However, the mechanisms have not been fully elucidated.
In our study, we showed that hepatic p66shc and senescence markers were enhanced in a rat model of NAFLD. In in vitro experiments, H2O2 enhanced the expression of senescence markers and lipid accumulation in hepatocytes. Inhibiting p66shc signaling suppressed H2O2-induced senescence and steatosis in hepatocytes. We further demonstrated that overexpression of p66shc could promote senescence and steatosis in hepatocytes. Therefore, we hypothesized that hepatocyte senescence was regulated by p66shc to be involved in NAFLD pathogenesis. In addition, our analysis of human samples showed that increased p66shc-mediated senescence signaling might play an important role in the progression of NAFLD.
P66shc was demonstrated to serve as a redox protein that produces ROS in mitochondria. Our results showed that H2O2-mediated oxidative stress would further activate p66shc and result in senescence. It appears that p66shc regulates senescence via ROS generation but may also be activated by increased ROS. In addition, the p53/p21/Cip1/Waf1 pathway is one of the main driving forces behind the induction of the senescence program [40]. Recent studies suggested that p53 could promote p66shc transcription by binding to the putative p53 binding sequence in the p66shc promoter [23]. We further demonstrated that p66shc and p21 are genetically and functionally linked. Therefore, p66shc is very likely to be a critical component of the p53/p21 senescence pathway in hepatocytes.
However, some limitations should be considered for this research. First, data on the effects of p66shc silencing in a NAFLD animal model were absent, and these experiments will need to be done in future research. Second, we did not directly confirm that p21-mediated hepatocyte senescence was upstream or downstream of p66shc and cannot conclude that the effect of p66shc on lipid consumption and disease progression was mediated by this specific senescence pathway. The exact mechanisms will need to be further explored in future work. Third, the clinical sample size was not sufficiently large, and future studies with larger samples are warranted to gain more insight into the clinical correlation between p66shc and hepatocyte senescence and disease progression in NAFLD.
Conclusions
To our best knowledge, this was the first study demonstrating that p66shc regulated senescence and lipid accumulation in hepatocytes, which indicated that p66shc may explain the progression of NAFLD in part via a senescence-dependent mechanism. Inhibition of hepatic p66shc expression and its mediated hepatocyte senescence signaling pathway may thus provide a potential therapeutic target for impeding the progression of hepatocyte damage in NAFLD.
Acknowledgments
The authors would like to acknowledge the Center for Translational Medicine and the members of the laboratory for experimental support, and we also thank all of the participants in the study.
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- NAFL
nonalcoholic fatty liver
- NASH
nonalcoholic steatohepatitis
- SAF
steatosis, activity, fibrosis
- ROS
reactive oxygen species
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- TC
total cholesterol
- TG
triglyceride
- HP1β
nuclear heterochromatin protein-1-beta
- γ-H2AX
the phosphorylated form of H2A histone family member X
- SA-β-gal
senescence-associated beta-galactosidase
- SD-rat
Sprague-Dawley rat
- HFD
high-fat diet
- H&E
hematoxylin and eosin staining
- shRNA
short hairpin RNA
Footnotes
Conflict of interest
None.
Source of support: This work was supported by grants from the National Natural Science Foundation of China (81570524), Natural Science Foundation of Zhejiang Province (LQ16H290003) and Scientific Research Support Fund for Teachers of Jining Medical College (JYFC2018FKJ147)
References
- 1.Seto WK, Yuen MF. Nonalcoholic fatty liver disease in Asia: Emerging perspectives. J Gastroenterol. 2017;52(2):164–74. doi: 10.1007/s00535-016-1264-3. [DOI] [PubMed] [Google Scholar]
- 2.Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–33. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day CP, James OF. Steatohepatitis: A tale of two “hits”? Gastroenterology. 1998;114(4):842–45. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 4.Peverill W, Powell LW, Skoien R. Evolving concepts in the pathogenesis of NASH: Beyond steatosis and inflammation. Int J Mol Sci. 2014;15(5):8591–638. doi: 10.3390/ijms15058591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozaykut P, Sahin A, Karademir B, et al. Endoplasmic reticulum stress related molecular mechanisms in nonalcoholic steatohepatitis. Mech Ageing Dev. 2016;157:17–29. doi: 10.1016/j.mad.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Lebeaupin C, Vallée D, Hazari Y, et al. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J Hepatol. 2018;69(4):927–47. doi: 10.1016/j.jhep.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Czaja MJ. Function of autophagy in nonalcoholic fatty liver disease. Dig Dis Sci. 2016;61(5):1304–13. doi: 10.1007/s10620-015-4025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakajima T, Nakashima T, Okada Y, et al. Nuclear size measurement is a simple method for the assessment of hepatocellular aging in non-alcoholic fatty liver disease: Comparison with telomere-specific quantitative FISH and p21 immunohistochemistry. Pathol Int. 2010;60(3):175–83. doi: 10.1111/j.1440-1827.2009.02504.x. [DOI] [PubMed] [Google Scholar]
- 9.Aravinthan A, Scarpini C, Tachtatzis P, et al. Hepatocyte senescence predicts progression in non-alcohol-related fatty liver disease. J Hepatol. 2013;58(3):549–56. doi: 10.1016/j.jhep.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 10.Donati B, Valenti L. Telomeres, NAFLD and chronic liver disease. Int J Mol Sci. 2016;17(3):383. doi: 10.3390/ijms17030383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondo Y, Ishigami A. Involvement of senescence marker protein-30 in glucose metabolism disorder and non-alcoholic fatty liver disease. Geriatr Gerontol Int. 2016;16( Suppl 1):4–16. doi: 10.1111/ggi.12722. [DOI] [PubMed] [Google Scholar]
- 12.Aravinthan A, Mells G, Allison M, et al. Gene polymorphisms of cellular senescence marker p21 and disease progression in non-alcohol-related fatty liver disease. Cell Cycle. 2014;13(9):1489–94. doi: 10.4161/cc.28471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunt EM, Walsh SN, Hayashi PH, et al. Hepatocyte senescence in end-stage chronic liver disease: A study of cyclin-dependent kinase inhibitor p21 in liver biopsies as a marker for progression to hepatocellular carcinoma. Liver Int. 2007;27(5):662–71. doi: 10.1111/j.1478-3231.2007.01470.x. [DOI] [PubMed] [Google Scholar]
- 14.Park H, Ishigami A, Shima T, et al. Hepatic senescence marker protein-30 is involved in the progression of nonalcoholic fatty liver disease. J Gastroenterol. 2010;45(4):426–34. doi: 10.1007/s00535-009-0154-3. [DOI] [PubMed] [Google Scholar]
- 15.Ogrodnik M, Miwa S, Tchkonia T, et al. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017;8:15691. doi: 10.1038/ncomms15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davalos AR, Coppe JP, Campisi J, et al. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010;29(2):273–83. doi: 10.1007/s10555-010-9220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irvine KM, Skoien R, Bokil NJ, et al. Senescent human hepatocytes express a unique secretory phenotype and promote macrophage migration. World J Gastroenterol. 2014;20(47):17851–62. doi: 10.3748/wjg.v20.i47.17851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 19.Migliaccio E, Giorgio M, Mele S, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402(6759):309–13. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Kim YR, Vikram A, et al. Sirtuin1-regulated lysine acetylation of p66Shc governs diabetes-induced vascular oxidative stress and endothelial dysfunction. Proc Natl Acad Sci USA. 2017;114(7):1714–19. doi: 10.1073/pnas.1614112114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang MJ, Chen F, Lau JTY, et al. Hepatocyte polyploidization and its association with pathophysiological processes. Cell Death Dis. 2017;8(5):e2805. doi: 10.1038/cddis.2017.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arauz J, Ramos-Tovar E, Muriel P. Redox state and methods to evaluate oxidative stress in liver damage: From bench to bedside. Ann Hepatol. 2016;15(2):160–73. doi: 10.5604/16652681.1193701. [DOI] [PubMed] [Google Scholar]
- 23.Tomita K, Teratani T, Suzuki T, et al. p53/p66Shc-mediated signaling contributes to the progression of non-alcoholic steatohepatitis in humans and mice. J Hepatol. 2012;57(4):837–43. doi: 10.1016/j.jhep.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Aravinthan A, Challis B, Shannon N, et al. Selective insulin resistance in hepatocyte senescence. Exp Cell Res. 2015;331(1):38–45. doi: 10.1016/j.yexcr.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 25.Aravinthan A, Shannon N, Heaney J, et al. The senescent hepatocyte gene signature in chronic liver disease. Exp Gerontol. 2014;60:37–45. doi: 10.1016/j.exger.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Hu XN, Wang JF, Huang YQ, et al. Huperzine A attenuates nonalcoholic fatty liver disease by regulating hepatocyte senescence and apoptosis: An in vitro study. Peer J. 2018;6:e5145. doi: 10.7717/peerj.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W, Zhang Y, Hou J, et al. Tris (2-chloroethyl) phosphate induces senescence-like phenotype of hepatocytes via the p21-Rb pathway in a p53-independent manner. Environ Toxicol Pharmacol. 2017;56:68–75. doi: 10.1016/j.etap.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Bedossa P, Poitou C, Veyrie N, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56(5):1751–59. doi: 10.1002/hep.25889. [DOI] [PubMed] [Google Scholar]
- 29.Carney EF. Hypertension: Role of p66Shc in renal vascular dysfunction. Nat Rev Nephrol. 2016;12(8):442. doi: 10.1038/nrneph.2016.89. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Kim YR, Vikram A, et al. P66Shc-induced microRNA-34a causes diabetic endothelial dysfunction by downregulating Sirtuin1. Arterioscler Thromb Vasc Biol. 2016;36(12):2394–403. doi: 10.1161/ATVBAHA.116.308321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menini S, Amadio L, Oddi G, et al. Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress. Diabetes. 2006;55(6):1642–50. doi: 10.2337/db05-1477. [DOI] [PubMed] [Google Scholar]
- 32.Boengler K, Bencsik P, Palóczi J, et al. Lack of contribution of p66shc and its mitochondrial translocation to ischemia-reperfusion injury and cardioprotection by ischemic preconditioning. Front Physiol. 2017;8:733. doi: 10.3389/fphys.2017.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granatiero V, Gherardi G, Vianello M, et al. Role of p66shc in skeletal muscle function. Sci Rep. 2017;7(1):6283. doi: 10.1038/s41598-017-06363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller B, Palygin O, Rufanova VA, et al. p66Shc regulates renal vascular tone in hypertension-induced nephropathy. J Clin Invest. 2016;126(7):2533–46. doi: 10.1172/JCI75079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright KD, Staruschenko A, Sorokin A. Role of adaptor protein p66Shc in renal pathologies. Am J Physiol Renal Physiol. 2018;314(2):F143–53. doi: 10.1152/ajprenal.00414.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koch OR, Fusco S, Ranieri SC, et al. Role of the life span determinant p66(shcA) in ethanol-induced liver damage. Lab Invest. 2008;88(7):750–60. doi: 10.1038/labinvest.2008.44. [DOI] [PubMed] [Google Scholar]
- 37.Perrini S, Tortosa F, Natalicchio A, et al. The p66Shc protein controls redox signaling and oxidation-dependent DNA damage in human liver cells. Am J Physiol Gastrointest Liver Physiol. 2015;309(10):G826–40. doi: 10.1152/ajpgi.00041.2015. [DOI] [PubMed] [Google Scholar]
- 38.Haga S, Terui K, Fukai M, et al. Preventing hypoxia/reoxygenation damage to hepatocytes by p66(shc) ablation: Up-regulation of anti-oxidant and anti-apoptotic proteins. J Hepatol. 2008;48(3):422–32. doi: 10.1016/j.jhep.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Y, Wang Z, Feng D, et al. p66Shc contributes to liver fibrosis through the regulation of mitochondrial reactive oxygen species. Theranostics. 2019;9(5):1510–22. doi: 10.7150/thno.29620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Z, Trotman LC, Shaffer D, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436(7051):725–30. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]