Abstract
Effects of high-pressure processing (HPP, 100–600 MPa for 3 min at 30 °C) on the glucosinolate content, conversion to isothiocyanates, and color changes during storage in fresh broccoli sprouts were investigated. A mild heat treatment (60 °C) and boiling (100 °C) were used as positive and negative controls, respectively. Glucosinolates were quantified using liquid chromatography–mass spectrometry, and isothiocyanates were quantified using high-performance liquid chromatography–photodiode array detection. A formation of isothiocyanates was observed in all high-pressure-treated sprouts. The highest degree of conversion (85%) was observed after the 600 MPa treatment. Increased isothiocyanate formation at 400–600 MPa suggests an inactivation of the epithiospecifier protein. During storage, color changed from green to brownish, reflected by increasing a* values and decreasing L* values. This effect was less pronounced for sprouts treated at 100 and 600 MPa, indicating an influence on the responsible enzymes. In summary, HPP had no negative effects on the glucosinolate–myrosinase system in broccoli sprouts.
Keywords: Brassica oleracae, myrosinase, epithiospecifier protein
INTRODUCTION
Broccoli sprouts are a rich source of glucosinolates, a group of phytochemicals that may play a role in preventing multiple types of cancer.1 Broccoli sprouts have recently become popular as a result of their 10–100 times higher levels of glucoraphanin compared to that of mature broccoli. Thus, consuming smaller amounts of broccoli sprouts may exert the same efficacy in reducing cancer risk as much higher quantities of mature broccoli.2 The predominant glucosinolates in broccoli and broccoli sprouts are glucoraphanin and glucoerucin, which are hydrolyzed into the isothiocyanates sulforaphane and erucin, respectively. Glucosinolates themselves are not known to be bioactive, although they are bitter.3 The conversion to isothiocyanates is essential for their bioactivity.
The glucosinolate–myrosinase system is a well-studied plant chemical defense system. The endogenous plant enzyme myrosinase is released when the tissue of cruciferous vegetables is attacked by insects, herbivores, or microorganisms. As a result, glucosinolates are hydrolyzed to various breakdown products, including isothiocyanates, thiocyanates, and nitriles.4 The compartmentation of the biologically inactive substrate (glucosinolate) from the activating enzyme (myrosinase) represents a fundamental principle of the plant defense system, whereby defense compounds are generated on demand after tissue breakdown.4,5
The extent of hydrolysis and the structure and concentration of the metabolites depend upon intrinsic factors, such as substrate and cofactors of myrosinase (ascorbic acid and ferrous ions), and extrinsic factors, such as pH and temperature.6 The presence of the epithiospecifier protein (ESP), which is present in some cruciferous vegetables, including broccoli, drives the formation to nitriles.7 However, unlike isothiocyanates, these nitrile products have no biological activity.8
Both glucosinolates and isothiocyanates are of immense interest in food processing, given their bitter flavor, pungent taste and aroma, and relationship to human health.9,10 Furthermore, the content of glucosinolates and isothiocyanate formation are affected by food processing and preservation techniques.8,11 During cooking of cruciferous vegetables, the glucosinolate–myrosinase system may be modified as a result of inactivation of myrosinase, loss of cofactors, such as ESP, thermal breakdown, and/or leaching of glucosinolates. Additionally, the hydrolysis products of glucosinolates are volatile.6 Boiling of cruciferous vegetables resulted in significant losses (90%) of glucosinolates by leaching into the cooking water.11 Furthermore, microwaving and boiling of broccoli inactivates myrosinase, resulting in only residual sulforaphane production compared to raw broccoli.12
With regard to the thermal stability of myrosinase, one report suggests that myrosinase from broccoli sprouts is more heatresistant than that from mature broccoli. The enzyme was thermally inactivated in broccoli at ~70 °C, while in immature broccoli sprouts, myrosinase remained active at temperatures as high as 100 °C.13 Matusheski et al. determined that, in broccoli with active ESP, glucoraphanin hydrolysis produced the inactive sulforaphane nitrile as the main product.13 Interestingly, mild heating (60–70 °C) of broccoli or broccoli sprouts increased sulforaphane formation and decreased sulforaphane nitrile formation. As a result of the different thermal labilities of myrosinase and ESP, mild heating has been demonstrated to inactivate ESP while retaining myrosinase activity.
The rising popularity of sprouts brings increased challenges for maintaining food safety. Sprouts are usually consumed raw in Western countries to preserve the bioactive substances and, thus, maximize the beneficial health effects. However, the germination and sprouting process provide optimal conditions for bacterial growth, creating a high risk for microbial food spoilage. Broccoli sprouts contain mesophilic, psychrotrophic, total, and fecal coliform bacteria.14 Thus, it is necessary to develop hygienic methods to extend the shelf life and ensure safety of sprouts. Conventional heat treatment is an effective way to enhance the microbial safety of sprouts, although high temperatures may modify the glucosinolate–myrosinase system and alter the fresh texture and flavor of the product that is desirable for consumers.
High-pressure processing (HPP, 400–600 MPa at chilled or room temperature), can be employed to preserve heat-sensitive nutrients and other food quality attributes while inactivating pathogenic and spoilage microorganisms. Inactivation of bacterial spores requires a combination of pressure and moderate temperatures.15 Yang et al. while reviewing various intervention technologies for ensuring the microbiological safety of sprouts concluded that physical intervention methods, such as HPP, are more effective in reducing microbial populations. For example, 650 MPa treatment at 20 °C for 15 min reduced more than 5 logs of Escherichia coli O157:H7 population in alfalfa seeds. A 300 MPa treatment at 4 °C for 15 min reduced Salmonella typhimurium, E. coli, and Listeria innocua populations inoculated in garden cress seeds by 6 logs.16 A 5 log reduction of foodborne pathogens on seeds used for sprout production is recommended by the National Advisory Committee on Microbial Criteria for Foods.16,17 However, application of HPP for seed decontamination had a limited log reduction, mainly as a result of the low moisture content of the seeds.18 In contrast, sprouts are high-moisturecontent products, and pressure treatment shows commercial promise.19
The objective of this study was to investigate the contents of glucosinolates and isothiocyanates in high-pressure (HP)-treated broccoli sprouts because we anticipated HPP to lead to decompartmentalization and enzymatic conversion of glucosinolates to isothiocyanates and enzyme action on glucosinolates. We have previously reported similar HPPinduced decompartmentalization on polyglutamyl folates in a variety of vegetables.20 The effects of HPP were studied over a range of pressures (100–600 MPa) applied with a holding time of 3 min at 30 °C (Supporting Figure 1 of the Supporting Information). Additionally, changes in the color of the HPPtreated broccoli sprouts over a storage period of 11 days at 6 °C were recorded.
MATERIALS AND METHODS
Chemicals
All chemicals were of analytical grade. Solvents for chromatography were of high-performance liquid chromatography (HPLC) and liquid chromatography–mass spectrometry (LC–MS) quality. Glucosinolate standards (glucoiberin, glucoraphanin, and glucoerucin) were purchased from Oskar Tropitzsch (Marktredwitz, Germany). Isothiocyanate standards (iberin, sulforaphane, and erucin) were obtained from LKT Laboratories (St. Paul, MN, U.S.A.). Standards were stored at −20 °C.
Sample Material
Fresh, 6-day-old broccoli sprouts were bought from a local grocery store (Columbus, OH, U.S.A.) and stored refrigerated at 6 °C until processing.
Pressure Treatment
A custom-made HP kinetic tester (PT-1, Avure Technologies, Inc., Kent, WA, U.S.A.) was used for the HPP of the broccoli sprouts.21,22 The unit is rated to 700 MPa and 120 °C. It has a 54 mL stainless-steel pressure chamber immersed in a temperature-controlled glycol bath, and the system is pressurized by an intensifier (M-340 A, Flow International, Kent, WA, U.S.A.). The bath surrounding the pressure chamber was maintained at a suitable temperature, so that isothermal process conditions could be maintained throughout the pressure-holding time. Propylene glycol (43140 Brenntag Northeast, Inc., Reading, PA, U.S.A.) was used as a heating medium in the bath as well as a pressure-transmitting fluid. The temperature profile was monitored by a K-type thermocouple probe (KMQSS-040U-7, Omega Engineering, Inc., Norwalk, CT, U.S.A.).
Dependent upon the target pressure, pressure come-up ranged from 7 to 18 s, and this value is not reported as a part of the pressure-holding time. Depressurization occurred in less than 2 s, regardless of the pressurization level. The sample temperature and chamber pressure were recorded every second during the treatment cycle with K-type thermocouple sensors and pressure transducers, respectively. A data acquisition computer equipped with relevant hardware and software was used to record the data.
For each treatment condition, 1.5–2 g of broccoli sprouts were vacuum-packed in sterile filter bags (5 × 7 cm) and heat-sealed. Two packaged samples were loaded in a 10 mL polypropylene syringe covered with two layers of insulating material (sports tape, CVS). Water was used as a pressure-transmitting fluid within the syringe and helps to maintain a uniform temperature within the processed volume. Prior to pressurization experiments based on the knowledge of heat of compression (δCH) of the test material and target pressure, the sample pouches and carrier were precooled in a water bath to a suitable preprocessing temperature T1.23 The broccoli sprout samples were treated for 3 min at 100, 200, 300, 400, 500, and 600 MPa (±5 MPa) at 30 ± 2 °C. Treatments at 400–600 MPa at chilled or atmospheric conditions are representative pasteurization conditions for various high-moisture-content foods, including fruits and vegetables.15 After the pressurization, the pouches were flash-frozen in liquid nitrogen and stored at −80 °C until glucosinolate and isothiocyanate analysis.
For color measurement, the same HPP conditions were applied. The only difference was that the broccoli sprouts were not vacuum-packaged; the air was just removed out of the pouches to the practical extent possible. Immediately after the pressurization, the pouches were immersed in ice water and then stored at 6 °C for 12 days.
Treatment of Control Samples
Additional pouches with 1.5–2.0 g of fresh, raw broccoli sprouts were prepared by vacuum packaging the sprouts and heat sealing the samples. A mild heat treatment at 60 °C and boiling at 100 °C were used as positive and negative controls, respectively (Supporting Figure 1 of the Supporting Information). These process conditions were chosen based on the study by Bricker et al.1 The 60 °C mild heat treatment should inactivate ESP but retain myrosinase. It was performed by submerging the pouch with broccoli sprouts in a 60 °C water bath for 10 min. Boiling (corresponding to the steaming in the study by Bricker et al.1) should inactivate both myrosinase and ESP. For boiling, a pouch with broccoli sprouts was submerged in a boiling water bath for 5 min. These control sprouts were flash-frozen in liquid nitrogen and stored at −80 °C until analysis.
Glucosinolate and Isothiocyanate Quantitation
Extraction and LC–MS Analysis of Glucosinolates
The glucosinolates glucoraphanin, glucoerucin, and glucoiberin were extracted and quantified using a method described by Bricker et al., with slight modifications.1 The flash-frozen broccoli sprouts were ground in a mortar with liquid nitrogen to avoid thawing. For extraction, 0.4 ± 0.02 g of frozen broccoli sprouts were added to a glass vial, followed by immediate addition of 3 mL of boiling LC–MS-grade water to denature endogenous myrosinase and preserve glucosinolates. The loosely capped vial was immediately placed in a boiling water bath for 5 min. After that, the vial was alternately vortexed and sonicated for 5 min. The sample was centrifuged for 5 min at 600g, and the aqueous extract was removed and set aside. Twice more, the pellet was resuspended in 3 mL of room-temperature LC–MS-grade water and vortexed/sonicated for 10 min, followed by centrifugation and removal. The supernatants were brought up to 10 mL with LC–MS-grade water. The extracts were centrifuged at 20000g for 5 min and passed through 0.2 μm polytetrafluoroethylene (PTFE) filters into HPLC vials.
Glucoraphanin, glucoerucin, and glucoiberin were separated on a Zorbax SB-CN reversed-phase (RP) column (4.6 × 250 mm, 5 μm, Agilent Technologies, Santa Clara, CA, U.S.A.) at 30 ± 1 °C with Agilent 1200 series HPLC. A binary gradient, consisting of (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile (ACN), was used at a flow rate of 1.5 mL/min. Initial conditions consisted of 0% B for 3 min, followed by linear increases to 10% B by 4 min, 40% B by 12 min, and 95% B by 13 min, after which the column was equilibrated at 0% B for 3 min. An AB Sciex QTrap 5500 mass spectrometer (Concord, Ontario, Canada), operated with electrospray ionization in negative-ion mode, was used for quantitation. Selected reaction monitoring was based on the common liberation of the HSO4− anion from glucosinolate. Thus, the transitions for glucoraphanin (436 > 97), glucoerucin (420 > 97), and glucoiberin (422 > 97) were used. The mass spectrometer parameters were set as follows: source temperature, 550 °C; declustering potential, −70 V; collision energy, −30 eV; exit potential, −10 V; collision cell exit potential, −11 V; and curtain gas, 30 psi. An external standard curve of the glucoraphanin, glucoerucin, and glucoiberin potassium salts was used to quantify the glucosinolates.
Extraction and HPLC Analysis of Isothiocyanates
Sulforaphane, erucin, and iberin in the broccoli sprouts were measured using a method described by Bricker et al.,1 with minor modifications. Initially, 0.25 ± 0.02 g of broccoli sprouts were combined with 5 mL of water and 10 mL of dichloromethane (DCM). The mixture was mechanically shaken for 10 min to allow for glucosinolate conversion, centrifuged at 600g for 5 min, followed by removal of the DCM layer. Once more, the sample was extracted in DCM, and the DCM layers were brought up to 25 mL with DCM. A 3 mL aliquot was mixed with 1 mL of conjugating reagent (20 mM triethylamine and 200 mM 2-mercaptoethanol in DCM) to make the isothiocyanates non-volatile and imbue the isothiocyanate derivatives with a higher extinction coefficient. This mixture was incubated at 30 °C for 60 min and then dried under gaseous nitrogen. Samples were reconstituted in water/ACN (1:1, v/v), centrifuged at 20000g for 5 min, and then passed through 0.45 μm nylon filters into HPLC vials.
Separation and quantification of the 2-mercaptoethanol conjugates of the isothiocyanates were achieved using Waters 2695 HPLC coupled to a 996 photodiode array (PDA) detector (Milford, MA, U.S.A.). The column was a Waters Symmetry C18 column (4.6 × 75 mm, 3.5 μm), and the temperature was set to 30 ± 1 °C. A linear gradient, consisting of (A) 0.1% formic acid in water and (B) 0.1% formic acid in ACN, was employed at a flow rate of 1.2 mL/min. Initial conditions of 0% B increased to 50% B over 15 min. The 2-mercaptoethanol conjugates of sulforaphane, erucin, and iberin were quantified by creating an external standard curve. It was assumed that the isothiocyanates conjugated with equal efficiency in extract and standard solution.
The limit of detection (LOD) and limit of quantification (LOQ) of the glucosinolates and isothiocyanates were calculated by the signal-to-noise (S/N) ratio. A S/N ratio of 3 was used for estimating LOD, and a S/N ratio of 10 was used for estimating LOQ.
Color Measurement
At 1, 4, 6, and 11 days after pressure treatment, a color reading was taken using a ColorQuest XE spectrophotometer (HunterLab, Hunter Associates Laboratories, Inc., Reston, VA, U.S.A.).
All sample pouches were read as reflectance, with the specular component included, in quintuplicate with a D65 illuminant and 10° observer angle. Color measurements are expressed using the CIE L*a*b* color system. L* indicates lightness; a* corresponds to coloration from green (−) to red (+); and b* corresponds to coloration from blue (−) to yellow (+).
Statistical Analysis
From each processed pouch, experiments for glucosinolate and isothiocyanate quantitation were conducted in triplicate. The color readings were conducted in quintuplicate.
Results in tables and figures are expressed as the mean ± standard deviation based on the wet weight of the broccoli sprouts. Standard deviations in figures are shown as error bars. Statistical comparisons were performed using IBM SPSS Statistics 21 for Windows (SPSS, Inc., Chicago, IL, U.S.A.). Differences between means were assessed using analysis of variance (ANOVA), followed by the Student–Newman–Keuls (S–N–K) procedure. Differences were considered to be significant at p ≤ 0.05. The homogeneity of variances was checked using Levene’s test. In case of no homogeneity, data were transformed for statistical analysis. Standard distribution was tested with the Kolmogorov–Smirnov (K–S) test.
RESULTS AND DISCUSSION
For hydrolysis of glucosinolates and formation of health beneficial compounds by myrosinase, tissue disruption has to occur, e.g., by cutting or mastication. However, vegetables are often heat-treated before consumption, which can result in myrosinase inactivation.24–26 Health beneficial isothiocyanates cannot be formed in foods under these circumstances. The myrosinases in different Brassica vegetables are known to exhibit varying degrees of thermal stability.10,25–28 Rapeseed myrosinase has been shown to be the most heat-stable enzyme.27 Broccoli myrosinase had a low thermal stability compared to other myrosinase sources. The myrosinase in broccoli tissue was stable until 45 °C, and its activity was reduced by more than 95% after a 10 min treatment at 70 °C.29 In broccoli juice, myrosinase was stable up to 40 °C.25
Typically, vegetable products, including sprouts, require heat treatment (>70 °C) for demonstrating product microbiological safety (5 log reduction of target pathogen). However, this heat treatment also inactivates myrosinase. Thus, the use of minimal processing approaches, such as use of HPP, which inactivates harmful pathogens with minimal impact on desired nutrients and enzymes, has been proposed. Studies on thermal and pressure stability of myrosinase from broccoli25,28–30 and mustard seeds26,31 were conducted on a kinetic basis. Myrosinase activity and inactivation, cell leakage, and glucosinolate conversion reaction products were assessed. Van Eylen et al.29 showed that moderate pressures of 50–250 MPa at 30 °C had limited effects on the activity of broccoli myrosinase. Ludikhuyze et al.28 studied the pressure inactivation of myrosinase from lyophilized broccoli powder in phosphate buffer at 20 °C in the pressure range of 250–500 MPa. A significant inactivation was observed between 300 and 500 MPa. These results indicated that myrosinase from broccoli is pressure-sensitive. Furthermore, an antagonistic effect between low pressure and temperature was noted. At 35 °C, thermal inactivation could be retarded by application of pressures below 350 MPa. Antagonistic effects were also described in another study with broccoli juice and broccoli.25,29 In broccoli juice, pressures up to 200 MPa retarded thermal inactivation at 50–60 °C. In black, brown, and yellow mustard seeds, myrosinase enzyme inactivation also increased with pressure (600–800 MPa) and temperature (30–70 °C).10 Indeed, at combinations of lower pressures (200–400 MPa) and high temperatures (60–80 °C), there was less inactivation.
Effects of Thermal Processing on the Glucosinolate Contents
In the broccoli sprouts, the glucosinolates glucoraphanin, glucoerucin, and glucoiberin were determined (Table 1). Glucoraphanin was the predominant glucosinolate, followed by glucoiberin and glucoerucin. The raw, untreated sprouts had a total glucosinolate content of 1.38 μmol/g. In most reports, glucoraphanin is the predominant glucosinolate in broccoli and broccoli sprouts, consistent with this study.1,11,32,33 Only Bricker et al.1 found the concentration of glucoerucin to be slightly higher than that of glucoraphanin, which may be an effect of the variety of the broccoli. Tian et al.33 quantified additional glucosinolates in minor concentrations in broccoli sprouts, e.g., progoitrin, glucobrassicin, neoglucobrassicin, or 4-methoxyglucobrassicin.
Table 1.
Glucosinolate Contents in Raw and Thermal-Treated Broccoli Sproutsa
| treatment | glucoiberin (μmol/g) | glucoraphanin (μmol/g) | glucoerucin (μmol/g) | total glucosinolates (μmol/g) |
|---|---|---|---|---|
| raw/untreated | 0.32 ± 0.04 a | 0.84 ± 0.08 a | 0.22 ± 0.04 a | 1.38 ± 0.15 a |
| positive control (60 °C, 10 min) | 0.07 ± 0.02 b | 0.18 ± 0.05 b | 0.05 ± 0.01 b | 0.30 ± 0.07 b |
| negative control (100 °C, 5 min) | 0.82 ± 0.04 c | 2.16 ± 0.10 c | 1.08 ± 0.02 c | 4.06 ± 0.15 c |
Different letters in the same column indicate significantly different results (p ≤ 0.05; ANOVA, followed by S–N–K).
The glucosinolate content of the negative control (4.06 μmol/g) reflects the situation in the fresh sprouts where myrosinase was inactivated and no hydrolysis could have happened during the homogenization. The total glucosinolate content is comparable to reports in the literature.1,32,33 The mildly heated (60 °C) sprouts had significantly reduced levels of glucosinolates, as described in a previous work.1 In accordance with Bricker et al.,1 a loss in rigidity of the broccoli sprouts during the 60 °C treatment was observed. Broccoli sprouts are a produce with delicate superstructure and vulnerable to tissue damage. This could result in decompartmentalization, myrosinase release, and formation of hydrolysis products.
Effects of Thermal and HPP on the Isothiocyanate Content and Degree of Conversion of Glucosinolates to Isothiocyanates
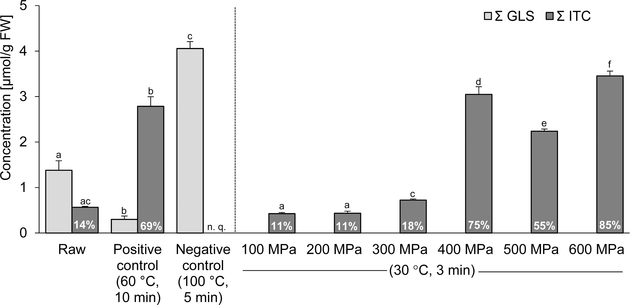
For the isothiocyanate analysis, the glucosinolates were hydrolyzed by adding water and allowing the endogenous myrosinase to react with the glucosinolates. The 2-mercaptoethanol conjugates of sulforaphane, erucin, and iberin were quantified (Table 2). The raw, untreated sprouts had a total isothiocyanate content of 0.57 μmol/g. The 60 °C mildly heated sprouts (positive control) had significantly increased levels. The boiling at 100 °C (negative control) resulted in concentrations that were not quantifiable. The isothiocyanate contents of the sprouts treated at 100–300 MPa were comparable to the raw, untreated sprouts. A significant increase of the isothiocyanate content was recorded for the sprouts treated at 400, 500, and 600 MPa. Figure 1 summarizes the total glucosinolate and isothiocyanate contents of the raw and heat-treated broccoli sprouts and the isothiocyanate contents of the HP-treated broccoli sprouts, including degree of conversion.
Table 2.
Isothiocyanate Contents in Raw and Thermal- and HP-Treated Broccoli Sproutsa
| treatment | iberin (μmol/g) | sulforaphane (μmol/g) | erucin (μmol/g) | total isothiocyanates (μmol/g) |
|---|---|---|---|---|
| raw/untreated | nqb | 0.16 ± 0.02 a | 0.41 ± 0.02 a | 0.57 ± 0.02 ac |
| positive control (60 °C, 10 min) | 0.47 ± 0.13 a | 1.66 ± 0.10 b | 0.66 ± 0.01 b | 2.79 ± 0.2 b |
| negative control (100 °C, 5 min) | nq | nq | ndc | |
| 100 MPa (30 °C, 3 min) | nq | 0.11 ± 0.01 a | 0.32 ± 0.02 c | 0.43 ± 0.02 a |
| 200 MPa | nq | 0.09 ± 0.01 a | 0.35 ± 0.04 c | 0.44 ± 0.04 a |
| 300 MPa | 0.02 ± 0.00 b | 0.18 ± 0.02 a | 0.52 ± 0.02 d | 0.72 ± 0.02 c |
| 400 MPa | 0.51 ± 0.04 a | 1.55 ± 0.09 c | 0.98 ± 0.05 e | 3.05 ± 0.17 d |
| 500 MPa | 0.41 ± 0.02 a | 1.11 ± 0.03 d | 0.72 ± 0.02 f | 2.24 ± 0.05 e |
| 600 MPa | 0.65 ± 0.03 c | 1.79 ± 0.09 e | 1.01 ± 0.01 e | 3.45 ± 0.11 f |
Different letters in the same column indicate significantly different results (p ≤ 0.05; ANOVA, followed by S–N–K).
nq = not quantifiable for LOQ (iberin, 3.39 × 10−9 mol/mL; sulforaphane, 4.46 × 10−9 mol/mL; and erucin, 5.42 × 10−9 mol/mL).
nd = not detectable for LOD (iberin, 1.02 × 10−9 mol/mL; sulforaphane, 1.34 × 10−9 mol/mL; and erucin, 1.63 × 10−9 mol/mL).
Figure 1.
Total glucosinolate (GLS) and total isothiocyanate (ITC) contents in raw and thermal-treated broccoli sprouts, total isothiocyanate contents in HP-treated broccoli sprouts, and indication of the degrees of conversion (in %). nq = not quantifiable for LOQ. Different letters indicate significantly different results within one substance group (p ≤ 0.05; ANOVA, followed by S–N–K).
The degrees of conversion of glucosinolates to isothiocyanates was calculated by expressing the amount of isothiocyanates formed as a percentage of the total glucosinolate content in the boiled broccoli sprouts. This value of 4.06 μmol/g is considered the initial glucosinolate concentration in all broccoli sprouts prior to any loss during processing.
The boiling should inactivate myrosinase, resulting in a low degree of conversion. This was accomplished because no isothiocyanates could be quantified in the boiled broccoli sprouts, meaning that the degree of conversion was negligible. In the untreated, raw broccoli sprouts, the myrosinase and ESP should retain activity. The moderate degree of conversion of 14% indicates that this was achieved. Bricker et al.1 observed a conversion of glucoraphanin to sulforaphane of 23%. Similar degrees were described by other reports.34,35 The mild heat treatment (60 °C) resulted in a considerably higher conversion of glucosinolates to isothiocyanates (69%). This has been shown by Matusheski et al.13 and Bricker et al.1 Although ESP activity and nitrile formation were not measured, a selective inactivation of ESP is assumed. ESP drives the formation of nitriles at the expense of isothiocyanates. In broccoli with active ESP, hydrolysis of glucoraphanin produced 9 times more inactive nitrile product than sulforaphane.13
In the HP-treated sprouts, differences in the degrees of conversion existed between the sprouts treated at 100–300 and 400–600 MPa. For the lower pressure levels, the degree of conversion ranged from 11 to 18%. From 400 MPa onward, the degree of conversion increased up to 85% for 600 MPa. With increasing pressure, more decompartmentalization was achieved, myrosinase remained active, and possibly ESP was selectively inactivated, to effect the conversion to isothiocyanates, as evidenced in Table 2. The formation of isothiocyanates was observed in all HP-treated broccoli sprouts, suggesting that myrosinase was active after HPP.
Van Eylen et al.24 investigated the hydrolysis products after the pressure treatment and reported that considerable amounts of glucosinolates were hydrolyzed into isothiocyanates. The highest degrees of conversion of glucoraphanin to sulforaphane were observed after the pressure treatments (200–500 MPa) at 40 °C for 15 min. After a 35 min treatment, the 300 MPa treatment exhibited the highest conversion. After the 500 MPa treatment, only a small amount of sulforaphane was formed, which is explained by myrosinase inactivation. When processing conditions became more intensive and where more sulforaphane was formed, less glucoraphanin was converted to sulforaphane nitrile. They concluded that the ESP was not only less thermostable but also less resistant to pressure treatments, which is consistent with the present data.13,24 After the pressure treatments at 100–300 MPa, the isothiocyanate contents of the broccoli sprouts were comparable to the content in the raw sprouts and significantly lower than after the pressure treatments at 400–600 MPa. This is reflected by the fact that the degrees of conversion in the sprouts treated at 400–600 MPa were 4–5 times higher than in the sprouts treated at 100–300 MPa. It also indicates an inactivation of the ESP, starting from 400 MPa.
Effects of Processing on the Color
Color is an important quality characteristic of fruits and vegetables. It affects sensory perception and consumer acceptance of foods. HPP has been shown to have limited effects on pigments (e.g., chlorophylls, carotenoids, and anthocyanins). Nevertheless, color compounds can change during storage as a result of incomplete inactivation of enzymes and microorganisms, which can lead to undesired chemical reactions in the food matrix.36 Chlorophyll is responsible for the green color of broccoli and broccoli sprouts. Thermal processing often results in the loss of chlorophyll. The conversion from green to olive brown of pheophytins is regarded as an index of the severity of heat treatment.37 Studies have shown that chlorophylls a and b are pressure-stable at room temperature. Reductions in chlorophyll contents were noticed only when the pressure was combined with temperatures exceeding 50 °C.37,38
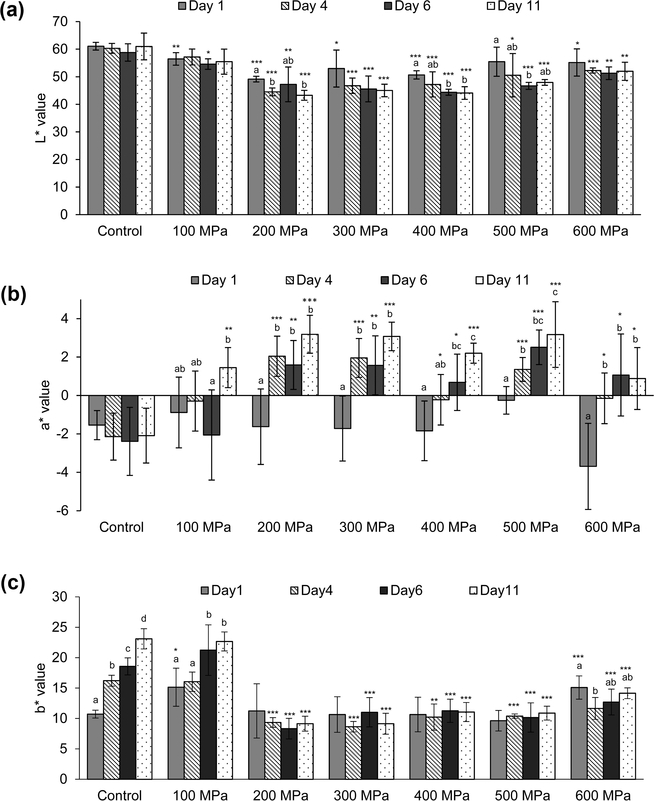
The L* and a* values of the untreated broccoli sprouts did not change over the storage time, while the b* value already increased after the fourth day (panels a–c of Figure 2), indicating a more yellow coloration of the sprouts. Broccoli is a highly perishable commodity, and its senescence is indicated by chlorophyll loss and yellowing of florets. Hansen et al.39 showed that broccoli florets stored in air had slightly yellow flower buds after 7 days. Low oxygen or low oxygen and high carbon dioxide atmospheres could delay the yellowing. An entire exclusion of oxygen was not obtained for the broccoli sprout pouches. The yellowing seems to be based on the formation of esterified xanthophylls of lutein, violaxanthin, and neoxanthin.40 In the broccoli sprouts treated at 100 MPa, an increase of the b* value was observed after the sixth day. Within the sprouts treated at 200–500 MPa, no considerable changes were observed for the b* values compared to the control sample on the first day after HPP. The b* value of the 600-MPa-treated sprouts was slightly higher on the first day after HPP but then decreased to the same level as the other HP-treated sprouts (Figure 2c).
Figure 2.
Effects of storage on the (a) L*, (b) a*, and (c) b* values of HP-treated broccoli sprouts. Control is the raw, untreated broccoli sprouts. The temperature during HPP was 30 ± 2 °C. Asterisks indicate significant differences between the HP-treated samples in comparison to the control sample on the same day (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; two-tailed, unpaired t test). Different letters within one treatment indicate significantly different results over time (p ≤ 0.05; ANOVA, followed by S–N–K).
For the HP-treated broccoli sprouts, a significant decrease of the lightness was observed during the storage time compared to the untreated sample (Figure 2a). From the fourth day of storage, the a* values significantly increased, indicating a turnover from green to red (Figure 2b). Together with the decreased lightness, this results in a brownish coloration. Interestingly, this effect during storage was less pronounced for the sprouts treated at 100 and 600 MPa.
The loss of the green color can be a result of the breakdown of chlorophylls. Directly after HPP (500 MPa for 1 min at ambient temperature), Krebbers et al.41 reported a more intense green color of green beans by decreased L*, a*, and b* values. Application of HP can increase permeability of plant cells, resulting in the leakage of chlorophyll into the intercellular space, likely the reason for the initial more intense bright green color.36,41 HPP in combination with elevated temperatures, even for a short time, showed a visible color change from green to olive green in green beans and basil.41,42 During storage, the green color of the HP-treated beans decreased and turned into a pale yellow/green color, as observed in the present experiments. These negative effects could be caused by residual activity of enzymes, such as chlorophyllase, peroxidase, or lipoxygenase.41 HPP at elevated temperatures, which resulted in enzyme inactivation, showed no further color change during storage.36,41
Polyphenol oxidase (PPO) activity is responsible for enzymatic browning reactions in damaged fruits and vegetables and was also found in broccoli.43 Because of the brown coloration and accompanying changes in appearance and organoleptic properties, inactivation is desirable. Studies showed that mushroom and potato PPO are very pressure-stable. Treatments at 800–900 MPa were necessary for activity reduction.44 Apricot, strawberry, and grape PPO were inactivated at lower pressures (100, 400, and 600 MPa, respectively).45
The less browning of the sprouts treated at 100 and 600 MPa is probably a result of different PPO activities. At 100 MPa, the limiting factor was most likely the cell disruption, which is necessary for contact between PPO and phenolic compounds. At 600 MPa, PPO could have been inactivated. However, in the present experiments, the activity of the PPO was not determined. A supposition can be made only on the basis of the color characteristics. Thus, results should be used with caution.
All in all, consuming raw broccoli sprouts may preserve nutrients and the myrosinase enzyme but comes with the trade-off of a potential safety concern. Thermal pasteurization of the broccoli sprouts needs about 70 °C to reach a 5 log reduction of target pathogens. HP pasteurization treatments use pressures in the range of 600 MPa at chilled or ambient temperatures for specified duration. Since 1997, HPP has been successfully applied to ready-to-eat (RTE) meats, seafood, marinated raw meats, juices, salads, and fruit and vegetable products. Critical parameters for the process include initial product temperature, process pressure, and treatment temperature. Factors such as product acidity, water activity, and composition need to be considered.46 Thus, more research is needed to optimize HP treatment conditions for raw broccoli sprouts to ensure its microbiological safety while preserving broccoli sprout nutrients. Because more glucosinolates were turned over into isothiocyanates after HPP at 400–600 MPa, these treatments may be interpreted to have a positive effect on the health potential of broccoli sprouts. This may degrade nutrients and product quality. Measuring the enzyme activity of myrosinase and the formation of sulforaphane nitrile should be included in further experiments to validate the results and extend the knowledge about HPP effects. As a result of the different extents of the browning in the HP-treated sprouts and consumer desire for bright green sprouts, the responsible enzymes should be focused on in further experiments.
Supplementary Material
Acknowledgments
Funding
This work was funded by the Center for Advanced Functional Foods Research and Entrepreneurship at The Ohio State University.
ABBREVIATIONS USED
- ACN
acetonitrile
- DCM
dichloromethane
- ESP
epithiospecifier protein
- HP
high pressure
- HPP
high-pressure processing
- PPO
polyphenol oxidase
- RP
reversed phase
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jafc.7b01380.
Schematic procedure of the broccoli sprout processing (Supporting Figure 1) (PDF)
REFERENCES
- (1).Bricker GV; Riedl KM; Ralston RA; Tober KL; Oberyszyn TM; Schwartz SJ Isothiocyanate metabolism, distribution, and interconversion in mice following consumption of thermally processed broccoli sprouts or purified sulforaphane. Mol. Nutr. Food Res 2014, 58, 1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Fahey JW; Zhang Y; Talalay P Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. U. S. A 1997, 94, 10367–10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Fenwick GR; Griffiths NM; Heaney RK Bitterness in Brussels sprouts (Brassica oleracea L. var gemmifera): The role of glucosinolates and their breakdown products. J. Sci. Food Agric 1983, 34, 73–80. [Google Scholar]
- (4).Bones AM; Rossiter JT The myrosinase–glucosinolate system, its organisation and biochemistry. Physiol. Plant 1996, 97, 194–208. [Google Scholar]
- (5).Burow M; Rice M; Hause B; Gershenzon J; Wittstock U Cell- and tissue-specific localization and regulation of the epithiospecifier protein in. Plant Mol. Biol 2007, 64, 173–185. [DOI] [PubMed] [Google Scholar]
- (6).Rungapamestry V; Duncan AJ; Fuller Z; Ratcliffe B Effect of cooking Brassica vegetables on the subsequent hydrolysis and metabolic fate of glucosinolates. Proc. Nutr. Soc 2007, 66, 69–81. [DOI] [PubMed] [Google Scholar]
- (7).Matusheski NV; Jeffery EH Comparison of the bioactivity of two glucoraphanin hydrolysis products found in broccoli, sulforaphane and sulforaphane nitrile. J. Agric. Food Chem 2001, 49, 5743–5749. [DOI] [PubMed] [Google Scholar]
- (8).Matusheski NV; Swarup R; Juvik JA; Mithen R; Bennett M; Jeffery EH Epithiospecifier protein from broccoli (Brassica oleracea L. ssp. italica) inhibits formation of the anticancer agent sulforaphane. J. Agric. Food Chem 2006, 54, 2069–2076. [DOI] [PubMed] [Google Scholar]
- (9).Drewnowski A; Gomez-Carneros C Bitter taste, phytonutrients, and the consumer: A review. Am. J. Clin. Nutr 2000, 72, 1424–1435. [DOI] [PubMed] [Google Scholar]
- (10).Okunade OA; Ghawi SK; Methven L; Niranjan K Thermal and pressure stability of myrosinase enzymes from black mustard (Brassica nigra L. W.D.J. Koch. var. nigra), brown mustard (Brassica juncea L. Czern. var. juncea) and yellow mustard (Sinapsis alba L. subsp. maire) seeds. Food Chem. 2015, 187, 485–490. [DOI] [PubMed] [Google Scholar]
- (11).Song L; Thornalley PJ Effect of storage, processing and cooking on glucosinolate content of Brassica vegetables. Food Chem. Toxicol 2007, 45, 216–224. [DOI] [PubMed] [Google Scholar]
- (12).Jones RB; Frisina CL; Winkler S; Imsic M; Tomkins RB Cooking method significantly effects glucosinolate content and sulforaphane production in broccoli florets. Food Chem. 2010, 123, 237–242. [Google Scholar]
- (13).Matusheski NV; Juvik JA; Jeffery EH Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry 2004, 65, 1273–1281. [DOI] [PubMed] [Google Scholar]
- (14).Martínez-Villaluenga C; Frías J; Gulewicz P; Gulewicz K; Vidal-Valverde C Food safety evaluation of broccoli and radish sprouts. Food Chem. Toxicol 2008, 46, 1635–1644. [DOI] [PubMed] [Google Scholar]
- (15).Balasubramaniam VM; Martínez-Monteagudo SI; Gupta R Principles and application of high pressure-based technologies in the food industry. Annu. Rev. Food Sci. Technol 2015, 6, 435–462. [DOI] [PubMed] [Google Scholar]
- (16).Yang Y; Meier F; Ann Lo J; Yuan W; Lee Pei Sze V; Chung H-J; Yuk H-G Overview of recent events in the microbiological safety of sprouts and new intervention technologies. Compr. Rev. Food Sci. Food Saf 2013, 12, 265–280. [Google Scholar]
- (17).National Advisory Committee on Microbiological Criteria for Foods. Microbiological safety evaluations and recommendations on sprouted seeds. Int. J. Food Microbiol 1999, 52, 123–153. [DOI] [PubMed] [Google Scholar]
- (18).Ariefdjohan MW; Nelson PE; Singh RK; Bhunia AK; Balasubramaniam VM; Singh N Efficacy of high hydrostatic pressure treatment in reducing Escherichia coli O157 and Listeria monocytogenes in alfalfa seeds. J. Food Sci 2004, 69, M117–M120. [Google Scholar]
- (19).Sikin AM; Zoellner C; Rizvi SS Current intervention strategies for the microbial safety of sprouts. J. Food Prot 2013, 76, 2099–2123. [DOI] [PubMed] [Google Scholar]
- (20).Wang C; Riedl KM; Somerville J; Balasubramaniam VM; Schwartz SJ Influence of high-pressure processing on the profile of polyglutamyl 5-methyltetrahydrofolate in selected vegetables. J. Agric. Food Chem 2011, 59, 8709–8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Thai Nguyen L; Rastogi NK; Balasubramaniam VM Evaluation of the instrumental quality of pressure-assisted thermally processed carrots. J. Food Sci 2007, 72, E264–E270. [DOI] [PubMed] [Google Scholar]
- (22).Rajan S; Ahn J; Balasubramaniam VM; Yousef AE Combined pressure–thermal inactivation kinetics of Bacillus amyloliquefaciens spores in egg patty mince. J. Food Prot 2006, 69, 853–860. [DOI] [PubMed] [Google Scholar]
- (23).Rasanayagam V; Balasubramaniam VM; Ting E; Sizer CE; Anderson C; Bush C Compression heating of selected fatty food substances during high pressure processing. J. Food Sci 2003, 68, 254–259. [Google Scholar]
- (24).Van Eylen D; Bellostas N; Strobel BW; Oey I; Hendrickx M; Van Loey A; Sørensen H; Sørensen JC Influence of pressure/temperature treatments on glucosinolate conversion in broccoli (Brassica oleraceae L. cv italica) heads. Food Chem. 2009, 112, 646–653. [Google Scholar]
- (25).Van Eylen D; Oey I; Hendrickx M; Van Loey A Kinetics of the stability of broccoli (Brassica oleracea cv. italica) myrosinase and isothiocyanates in broccoli juice during pressure/temperature treatments. J. Agric. Food Chem 2007, 55, 2163–2170. [DOI] [PubMed] [Google Scholar]
- (26).Van Eylen D; Indrawati; Hendrickx M; Van Loey A Temperature and pressure stability of mustard seed (Sinapis alba L.) myrosinase. Food Chem. 2006, 97, 263–271. [Google Scholar]
- (27).Ghawi SK; Methven L; Rastall RA; Niranjan K Thermal and high hydrostatic pressure inactivation of myrosinase from green cabbage: A kinetic study. Food Chem. 2012, 131, 1240–1247. [Google Scholar]
- (28).Ludikhuyze L; Ooms V; Weemaes C; Hendrickx M Kinetic study of the irreversible thermal and pressure inactivation of myrosinase from broccoli (Brassica oleracea L. cv. italica). J. Agric. Food Chem 1999, 47, 1794–1800. [DOI] [PubMed] [Google Scholar]
- (29).Van Eylen D; Oey I; Hendrickx M; Van Loey A Effects of pressure/temperature treatments on stability and activity of endogenous broccoli (Brassica oleracea L. cv. italica) myrosinase and on cell permeability. J. Food Eng 2008, 89, 178–186. [Google Scholar]
- (30).Frandsen HB; Markedal KE; Martín-Belloso O; Sánchez-Vega R; Soliva-Fortuny R; Sørensen H; Sørensen S; Sørensen JC Effects of novel processing on glucosinolates and membrane associated myrosinases in broccoli. Pol. J. Food Nutr. Sci 2014, 64, 17–25. [Google Scholar]
- (31).Van Eylen D; Oey I; Hendrickx M; Van Loey A Behavior of mustard seed (Sinapis alba L.) myrosinase during temperature/pressure treatments: A case study on enzyme activity and stability. Eur. Food Res. Technol 2008, 226, 545–553. [Google Scholar]
- (32).Clarke JD; Riedl K; Bella D; Schwartz SJ; Stevens JF; Ho E Comparison of isothiocyanate metabolite levels and histone deacetylase activity in human subjects consuming broccoli sprouts or broccoli supplement. J. Agric. Food Chem 2011, 59, 10955–10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Tian Q; Rosselot RA; Schwartz SJ Quantitative determination of intact glucosinolates in broccoli, broccoli sprouts, Brussels sprouts, and cauliflower by high-performance liquid chromatography–electrospray ionization–tandem mass spectrometry. Anal. Biochem 2005, 343, 93–99. [DOI] [PubMed] [Google Scholar]
- (34).Matusheski NV; Wallig MA; Juvik JA; Klein BP; Kushad MM; Jeffery EH Preparative HPLC method for the purification of sulforaphane and sulforaphane nitrile from Brassica oleracea. J. Agric. Food Chem 2001, 49, 1867–1872. [DOI] [PubMed] [Google Scholar]
- (35).Mithen R; Faulkner K; Magrath R; Rose P; Williamson G; Marquez J Development of isothiocyanate-enriched broccoli, and its enhanced ability to induce phase 2 detoxification enzymes in mammalian cells. Theor. Appl. Genet 2003, 106, 727–734. [DOI] [PubMed] [Google Scholar]
- (36).Oey I; Lille M; Van Loey A; Hendrickx M Effect of high-pressure processing on colour, texture and flavour of fruit- and vegetable-based food products: A review. Trends Food Sci. Technol 2008, 19, 320–328. [Google Scholar]
- (37).Butz P; Edenharder R; Fernández García A; Fister H; Merkel C; Tauscher B Changes in functional properties of vegetables induced by high pressure treatment. Food Res. Int 2002, 35, 295–300. [Google Scholar]
- (38).Van Loey A; Ooms V; Weemaes C; Van den Broeck I; Ludikhuyze L; Indrawati; Denys S; Hendrickx M Thermal and pressure–temperature degradation of chlorophyll in broccoli (Brassica oleracea L. italica) juice: A kinetic study. J. Agric. Food Chem 1998, 46, 5289–5294. [Google Scholar]
- (39).Hansen ME; Sørensen H; Cantwell M Changes in acetaldehyde, ethanol and amino acid concentrations in broccoli florets during air and controlled atmosphere storage. Postharvest Biol. Technol 2001, 22, 227–237. [Google Scholar]
- (40).Yamauchi N; Watada AE Chlorophyll and xanthophyll changes in broccoli florets stored under elevated CO2 or ethylene-containig atmosphere. HortScience 1998, 33, 114–117. [Google Scholar]
- (41).Krebbers B; Matser AM; Koets M; Van den Berg RW Quality and storage-stability of high-pressure preserved green beans. J. Food Eng 2002, 54, 27–33. [Google Scholar]
- (42).Krebbers B; Matser A; Koets M; Bartels P; Van Den Berg R High pressure-temperature processing as an alternative for preserving basil. High Pressure Res. 2002, 22, 711–714. [Google Scholar]
- (43).Gawlik-Dziki U; Szymanowska U; Baraniak B Characterization of polyphenol oxidase from broccoli (Brassica oleracea var. botrytis italica) florets. Food Chem. 2007, 105, 1047–1053. [Google Scholar]
- (44).Gomes MRA; Ledward DA Effect of high-pressure treatment on the activity of some polyphenoloxidases. Food Chem. 1996, 56, 1–5. [Google Scholar]
- (45).Hendrickx M; Ludikhuyze L; Van den Broeck I; Weemaes C Effects of high pressure on enzymes related to food quality. Trends Food Sci. Technol 1998, 9, 197–203. [Google Scholar]
- (46).National Advisory Committee on Microbiological Criteria for Foods. Requisite scientific parameters for establishing the equivalence of alternative methods of pasteurization. J. Food Prot 2006, 69, 1190–1216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.