Abstract
Background
Approximately one‐fifth of all subfertile couples seeking fertility treatment show clinically relevant levels of anxiety, depression, or distress. Psychological and educational interventions are frequently offered to subfertile couples, but their effectiveness, both in improving mental health and pregnancy rates, is unclear.
Objectives
To assess the effectiveness of psychological and educational interventions for subfertile couples on psychological and fertility treatment outcomes.
Search methods
We searched (from inception to 2 April 2015) the Cochrane Gynaecology and Fertility Group Specialised Register of Controlled Trials, the Cochrane Central Register of Controlled Trials (CENTRAL; Issue 2, 2015), MEDLINE, EMBASE, PsycINFO, EBSCO CINAHL, DARE, Web of Science, OpenGrey, LILACS, PubMed, and ongoing trials registers. We handsearched reference lists and contacted experts in the field.
Selection criteria
We included published and unpublished randomised controlled trials (RCTs), cluster randomised trials, and cross‐over trials (first phase) evaluating the effectiveness of psychological and educational interventions on psychological and fertility treatment outcomes in subfertile couples.
Data collection and analysis
Two review authors independently assessed trial risk of bias and extracted data. We contacted study authors for additional information. Our primary outcomes were psychological measures (anxiety and depression) and fertility rates (live birth or ongoing pregnancy). We assessed the overall quality of the evidence using GRADE criteria.
As we did not consider the included studies to be sufficiently similar to permit meaningful pooling, we summarised the results of the individual studies by presenting the median and interquartile range (IQR) of effects as well as the minimum and maximum values. We calculated standardised mean differences (SMDs) for continuous variables and odds ratios (ORs) for dichotomous outcomes.
Main results
We included 39 studies involving 4925 participants undergoing assisted reproductive technology. Studies were heterogeneous with respect to a number of factors, including nature and duration of interventions, participants, and comparator groups. As a result, we judged that pooling results would not result in a clinically meaningful estimate of a treatment effect. There were substantial methodological weaknesses in the studies, all of which were judged to be at high risk of bias for one or more quality assessment domains. There was concern about attrition bias (24 studies), performance bias for psychological outcomes (27 studies) and fertility outcomes (18 studies), and detection bias for psychological outcomes (26 studies). We therefore considered study‐specific estimates of intervention effects to be unreliable. Thirty‐three studies reported the outcome mental health. Only two studies reported the outcome live birth, and both of these had substantial attrition. One study reported ongoing pregnancy, again with substantial attrition. We have combined live birth and ongoing pregnancy in one outcome.
Psychological outcomes
Studies utilised a variety of measures of anxiety and depression. In all cases a low score denoted benefit from the intervention.
SMDs for anxiety were as follows: psychological interventions versus attentional control or usual care: median (IQR) = ‐0.30 (‐0.84 to 0.00), minimum value ‐5.13; maximum value 0.84, 17 RCTs, 2042 participants; educational interventions versus attentional control or usual care: median = 0.03, minimum value ‐0.38; maximum value 0.23, 4 RCTs, 330 participants.
SMDs for depression were as follows: psychological interventions versus attentional control or usual care: median (IQR) = ‐0.45 (‐0.68 to ‐0.08), minimum value ‐3.01; maximum value 1.23, 12 RCTs, 1160 participants; educational interventions versus attentional control or usual care: median = ‐0.33, minimum value ‐0.46; maximum value 0.17, 3 RCTs, 304 participants.
Fertility outcomes
When psychological interventions were compared with attentional control or usual care, ORs for live birth or ongoing pregnancy ranged from minimum value 1.13 to maximum value 10.05. No studies of educational interventions reported this outcome.
Authors' conclusions
The effects of psychological and educational interventions on mental health including distress, and live birth or ongoing pregnancy rates is uncertain due to the very low quality of the evidence. Existing trials of psychological and educational interventions for subfertility were generally poorly designed and executed, resulting in very serious risk of bias and serious inconsistency in study findings. There is a need for studies employing appropriate methodological techniques to investigate the benefits of these treatments for this population. In particular, attentional control groups should be employed, that is groups receiving a treatment that mimics the amount of time and attention received by the treatment group but is not thought to have a specific effect upon the participants, in order to distinguish between therapeutic and non‐specific effects of interventions. Where attrition cannot be minimised, appropriate statistical techniques for handling drop‐out must be applied. Failure to address these issues in study design has resulted in studies that do not provide a valid basis for answering questions about the effectiveness of these interventions.
Plain language summary
Psychological and educational interventions for subfertile men and women
Background: Approximately one‐fifth of all subfertile couples seeking fertility treatment show clinically relevant levels of anxiety, depression, or distress. Psychological and educational interventions are frequently offered to subfertile couples, but their effectiveness, both in improving mental health and pregnancy rates, is unclear. Objective: To assess the effectiveness of psychological and educational interventions for subfertile couples on psychological and fertility treatment outcomes. Study characteristics: We included 39 studies involving 4925 participants undergoing assisted reproductive technology. Studies varied widely with respect to a number of factors, including nature and duration of interventions, participants, and comparator groups. The evidence is current to April 2015. Key results: There were substantial methodological weaknesses in the studies, all of which were judged to be at high risk of bias for one or more quality assessment domains. We therefore determined that pooling results would not result in a clinically meaningful estimate of a treatment effect and that we could not present a pooled analysis in the 'Summary of findings' table. There was concern about bias because of differences in care and amount of attention given to participants for psychological outcomes (27 studies) and fertility outcomes (18 studies), the amount of withdrawal (24 studies), and the manner in which outcome measurements were taken (26 studies). We therefore considered the results from each study to be unreliable. Thirty‐three studies reported the outcome mental health. Only two studies reported the outcome live birth, and one study reported ongoing pregnancy; all of these studies had substantial attrition. It was not possible to answer the review question for any of the primary outcomes. Quality of the evidence: We judged the overall quality of the evidence to be very low, the main reasons being very serious risk of bias and serious inconsistency in study findings.
Summary of findings
Summary of findings for the main comparison. Psychological and educational interventions versus attentional control or usual care.
| Psychological and educational interventions for subfertile men and women | |||||
|
Patient or population: Subfertile men and women Settings: Secondary healthcare setting Intervention: Psychological or educational intervention Comparison: Control (attentional control or usual care) | |||||
| Outcomes | Comparison | Median (IQR) and minimum and maximum values | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Psychological or educational intervention versus attentional control or usual care | |||||
|
Anxiety Different scales for anxiety (low score indicates benefit from intervention) |
12 studies revealed no evidence of a difference. 8 studies suggested an advantage from the intervention, and 1 study suggested a disadvantage from the intervention | Psychological interventions: median (IQR) = ‐0.30 (‐0.84 to 0.00), 17 RCTs, 2042 participants.
Minimum value ‐5.13; maximum value 0.84. Educational interventions: median = 0.03, 4 RCTs, 330 participants. Minimum value ‐0.38; maximum value 0.23 |
2372 (21 RCTs) | ⊕⊝⊝⊝ very low1,2,3 | Illustrative comparative risks not calculable due to clinical heterogeneity |
|
Depression Different scales for depression (low score indicates benefit from intervention) |
11 studies revealed no evidence of a difference. 3 studies suggested an advantage from the intervention, and 1 study suggested a disadvantage from the intervention. | Psychological interventions: (median (IQR) = ‐0.45 (‐0.68 to ‐0.08), 12 RCTs, 1160 participants. Minimum value ‐3.01; maximum value 1.23. Educational interventions: median = ‐0.33, 3 RCTs, 304 participants. Minimum value ‐0.46; maximum value 0.17 |
1464 (15 RCTs) |
⊕⊝⊝⊝ very low1,2,3 | |
| Live birth or ongoing pregnancy (complete‐case analysis) | 2 studies revealed no evidence of a difference. 1 study suggested an advantage from the intervention4 | Psychological interventions: odds ratio minimum value 1.13, maximum value 10.05. Educational interventions: no data available |
387 (3 RCTs) | ⊕⊝⊝⊝ very low1,2,3 | |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Very serious risk of bias particularly relating to attrition and inadequate control for non‐specific benefits. 2Inconsistency between the studies in clinical characteristics as well as in study findings. 3Serious imprecision. Most studies had small sample sizes, and effects estimates crossed the line of no effect. Very low event rates for fertility outcomes. 4Domar et al. 2000 revealed very large odds ratios, but these are likely to be overestimates due to high control group attrition.
IQR: interquartile range RCT: randomised controlled trial
Background
Description of the condition
Subfertility is a condition of the reproductive system and is defined by the failure to get pregnant within 12 months, when couples have regular unprotected intercourse with the aim of getting pregnant (Zegers‐Hochschild 2009). Primary subfertility is defined as occurring where a couple has never been pregnant, and secondary subfertility is where a couple is unable to bear a child following a previous pregnancy or previously carrying a pregnancy to a live birth (WHO 2013). Worldwide, an estimated 48.5 million couples are subfertile, of which 19.2 million couples have primary subfertility and 29.3 million couples have secondary subfertility (Mascarenhas 2012). Age affects subfertility rates. The prevalence of primary subfertility is higher among women aged 20 to 24 years (2.7%, 95% confidence interval (CI) 2.4% to 3.0%) in comparison with women aged 25 to 29 years (2.0%, 95% CI 1.8% to 2.2%). On the other hand, the prevalence of secondary subfertility is much higher among older women: 27.1% (95% CI 24.7% to 29.9%) in women aged 40 to 44 years in comparison with 2.6% (95% CI 2.3% to 3.0%) in women aged 20 to 24 years (Mascarenhas 2012).
Fertility treatment has been widely used since the introduction of in vitro fertilisation (IVF) technology in 1978 and new developments in IVF in the late 1980s such as stimulated IVF cycles with human menopausal gonadotropin (hMG) and pituitary desensitisation in order to decrease the incidence of premature ovulation (Wang 2006). More than one million cycles of assisted reproductive technology (ART) were initiated in 2006 (Mansour 2014). However, fertility treatment may result in a significant psychological burden, especially when the treatment does not result in a clinical pregnancy or a live birth (Beaurepaire 1994; Dhaliwal 2004; Gameiro 2012; Musa 2014; Terzioglu 2007; Verhaak 2005). In a recent study, the incidence of depressive or anxious symptoms, measured between the first visit to a fertility clinic and the start of treatment, was 18.5% higher in women and 7.4% higher in men seeking fertility treatment (Chiaffarino 2011) than before the initiation of the treatment cycle. Moreover, women who had undergone their first fertility treatment were found to have significantly more anxiety and depression after their first failed treatment cycle than before the initiation of the treatment cycle (Verhaak 2005). Couples who had undergone fertility treatment also more often showed marital dissatisfaction compared to couples that had conceived (Slade 1997). Couples undergoing IVF who had more physical and/or emotional problems had more IVF‐related absence from work compared to couples undergoing IVF who had fewer physical and/or emotional problems (Bouwmans 2008). At a certain point, about one‐fifth of all infertile couples experience reproductive medicine as so stressful that they could need psychological counselling, according to several studies (Boivin 1999; Gameiro 2015; Verhaak 2007), and, according to Gameiro (Gameiro 2012), even discontinue fertility treatment due to psychological burden (19%) or relational and personal problems (17%) across any stage of fertility treatment.
Supporting couples who experience a significant psychological burden from fertility treatment could potentially lead to better functioning in daily life and reduce discontinuation of fertility treatment (Boivin 2003; De Liz 2005; Gameiro 2012). In addition, psychological and educational interventions may improve their chances of conceiving (De Liz 2005; Hämmerli 2009). If pregnancy rates were found to be increased due to psychological and educational interventions, the duration of fertility treatment may be shortened and in turn psychological problems would be fewer.
Description of the intervention
For this review, we have classified the interventions into the following two categories.
Firstly, psychological interventions are interventions of a named therapy, in general or a specific kind of therapy, or interventions aimed to change behaviour or cognition, or both, regarding subfertility and its treatment, as well as changing the emotional impact of it. Mind‐and‐body interventions are behavioural treatment interventions including, for example, meditation, hypnotherapy, and yoga (Domar 1990), and are considered to be psychological interventions.
Secondly, educational interventions are interventions that may include information on subfertility, its causes, treatment instructions (medical or procedural information), and information to improve self management and self efficacy (such as skills training, psycho‐education). These interventions are aimed to alleviate distress. Self help interventions and decision aids are also considered to be educational interventions.
How the intervention might work
Psychological interventions aim to provide support for the impact of subfertility and fertility treatment on mental health, which could include ways to manage negative emotions. The interventions are aimed at improving mental health or facilitating adjustment to an important life event. There are several types of psychological intervention; frequently used examples are cognitive, behavioural, and psychodynamic therapies.
Cognitive therapeutic interventions are aimed at changing dysfunctional cognitions and beliefs about subfertility and its consequences. Research has shown that dysfunctional cognitions are related to negative appraisal of stressful situations resulting in negative emotions such as depression and anxiety. Learning to recognise and alter such cognitions is an effective way to reduce emotional stress (Cuijpers 2013). Behavioural therapeutic interventions are aimed at changing behaviour regarding coping with subfertility and its consequences. Behavioural interventions are frequently combined with cognitive interventions. Psychodynamic interventions are aimed at alleviating internal conflicts that are believed to be hampering actual emotional processing and having a negative impact on pregnancy, and have been found to originate from an incident in childhood in some women (Boivin 2003). Mind‐and‐body interventions integrate aspects of these therapeutic interventions with body‐focused interventions, such as meditation, yoga, or meaning‐based interventions, and are linked to complementary medicine.
Educational interventions could increase the knowledge of subfertile men and women regarding subfertility and its consequences. Subfertile men and women could also develop better skills to deal with their condition and the psychological burden that comes with it. With more knowledge and skills, they could experience a reduced psychological burden during fertility treatment. Self help interventions and decision aids also increase knowledge and support the decision‐making process (van Peperstraten 2010), which alleviates distress.
Why it is important to do this review
Subfertility affects many people around the world and has a considerable impact on both families and individuals. Although treatment options are available, couples experience a heavy burden, and the emotional impact is shown to be considerable. These psychological problems can have a negative influence on many facets of an individual's life, for example in relationships, at work, and in the social environment (Bouwmans 2008; Slade 1997). Psychological and educational interventions are frequently offered to subfertile couples, but the efficacy of these interventions, both in improving mental health and pregnancy rates, is unclear.
Three reviews have previously evaluated the effectiveness of psychological and educational interventions on mental health in subfertility. One review was a narrative review (Boivin 2003), and two consisted of meta‐analyses (De Liz 2005; Hämmerli 2009). The reviews included different studies and also considered studies without a comparison group. As a result, the reviews reached conflicting conclusions on the impact of therapy. See Agreements and disagreements with other studies or reviews for additional information.
This systematic review considers the role of psychological and educational interventions in improving mental health, quality of life, and pregnancy rates, as well as which patient groups they are most effective for, and in which phase of treatment the intervention should be given.
Objectives
To assess the effectiveness of psychological and educational interventions for subfertile couples on psychological and fertility treatment outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised controlled trials (RCTs) and cluster randomised trials were eligible for inclusion. We included cross‐over trials, but included only data from the first phase in meta‐analyses, as the cross‐over is not a valid design in this context. We excluded non‐randomised studies, as they are associated with a high risk of bias. We also excluded quasi‐randomised studies.
Types of participants
Study participants were men or women, or both, with a diagnosis of subfertility. We included subfertile men and women in the following three phases of treatment:
Pre: from the diagnosis of subfertility until the initiation of fertility treatment.
Duringa: from the initiation of fertility treatment (medically assisted reproduction (MAR) or assisted reproductive technology (ART)) until the end of fertility treatment.
Post: in the case of failure or therapy resistance, when the decision is made not to continue fertility treatment, or in the case of ongoing psychological distress after successful fertility treatment (e.g. a live birth).
aWe included couples during fertility treatment if they were treated with MAR, or more specifically with ART. MAR comprised ovulation induction, controlled ovarian stimulation, ovulation triggering, ART procedures, and intrauterine, intracervical, and intravaginal (artificial) insemination with the semen of the husband or partner or a donor. ART comprised all treatments or procedures that include the in vitro handling of both human oocytes and sperm or of embryos for the purpose of establishing a pregnancy. ART did not include artificial insemination (Zegers‐Hochschild 2009).
Types of interventions
Trials evaluating the effects of psychological and educational interventions on subfertility were eligible for inclusion. We included interventions as long as the aim of the intervention was to alleviate distress and the intervention reported on our outcomes. The psychological and educational interventions had to be specifically named as 'extra' or 'in addition to the usual treatment'.
The psychological interventions could be delivered by specifically skilled personnel in psychosocial care (therapists) such as psychologists, counsellors, and psychotherapists.
Educational interventions could be delivered by medical personnel such as nurses, midwives, and doctors. Besides medical personnel, psycho‐education could have been delivered by psychologists or counsellors. Self help interventions and decision aids did not require a facilitating professional but were sometimes also delivered by therapists or medical personnel.
Therapy setting: the psychological and educational interventions could be provided in individual, couple, or group therapy.
Mode of delivery: the psychological and educational interventions could be provided through:
communication via face‐to‐face, Internet, or telephone contact;
written information in leaflets, booklets, decision aids, and on the Internet;
self help.
Duration: the duration of the psychological and educational interventions could vary widely, for example from one session to 64 sessions or more (or from one week to 64 weeks or more).
Comparison groups consisted of attentional control groups (intervention groups where participants were blinded and were not aware if they were in the intervention group or control group) or routine care (usual care, this may include tender love and care (TLC), an educational intervention without the aim of alleviating distress, or waiting lists for psychological interventions).
We excluded the following interventions: alternative invasive interventions such as acupuncture, phytotherapy, Chinese herbs, lifestyle interventions, and medication only.
Types of outcome measures
Outcomes had to be measured during face‐to‐face contact or through self report questionnaires. The measures had to report whether a person had improved, changed, or deteriorated, or they had to quantify the extent to which a person has improved, changed, or deteriorated.
Primary outcomes
Psychological outcomes
1. Anxiety and depression. If studies reported more than one scale measuring anxiety and depression, we gave preference to the Spielberger State‐Trait Anxiety Inventory (STAI), Spielberger 1989, and the Beck Depression Inventory (BDI), Beck 1961.
Fertility treatment outcomes
2. Live birth or ongoing pregnancy rates, defined as the percentage of women who gave birth to a live fetus after 20 completed weeks of gestation or the percentage of women in whom evidence of a gestational sac with fetal heart motion was found at 12 weeks, confirmed with ultrasound.
Secondary outcomes
Psychological outcomes
3. Mental health, including distress, worries, negative mood, positive mood, anger, and happiness, as measured by validated scales.
4a. General quality of life. If studies reported more than one scale, we gave preference to the 36‐Item Short Form Health Survey (SF‐36) (Tarlov 1989), then to other generic scales such as the European Quality of Life instrument (EuroQol 1990).
4b. Fertility‐specific quality of life. Any measure that quantified the extent to which a person experiences a lower quality of life specifically because of being subfertile and its consequences, for example the Fertility Quality of Life Tool (FertiQoL) (Boivin 2011).
5. Social support, including general social support, perceived social support, marital satisfaction, sexual satisfaction, and partner relationship satisfaction, as measured by validated scales.
Fertility treatment outcomes
6. Clinical pregnancy rates, defined as the percentage of women with definitive clinical signs of pregnancy or in whom one or more gestational sacs were found by ultrasonographic visualisation, or both.
7. Discontinuation of fertility treatment, measured as the percentage of couples who had quit fertility treatment before the achievement of a pregnancy and during the psychological intervention.
Search methods for identification of studies
We searched for all published and unpublished studies of psychological and educational interventions, without language restrictions and in consultation with the Cochrane Gynaecology and Fertility Group (CGF) Trials Search Co‐ordinator.
Electronic searches
We searched the following electronic databases, trial registers, and websites:
the CGF Specialised Register of Controlled Trials (from inception to 2 April 2015) (Appendix 1);
in Ovid the Cochrane Central Register of Controlled Trials (CENTRAL) (from inception to Issue 2, 2015) (Appendix 2);
Ovid MEDLINE (from inception to 2 April 2015) (Appendix 3);
Ovid EMBASE (from inception to 2 April 2015) (Appendix 4);
Ovid PsycINFO (from inception to 2 April 2015) (Appendix 5);
EBSCO CINAHL (from inception to 2 April 2015) (Appendix 6);
AMED (from inception to 2 April 2015) (Appendix 7).
The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying randomised trials, which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.0.2, Chapter 6, 6.4.11) (Higgins 2011). The EMBASE and CINAHL searches were combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) http://www.sign.ac.uk/methodology/filters.html#random.
Other electronic sources of trials included the following.
Trial registers for ongoing and registered trials:
http://www.clinicaltrials.gov (a service of the US National Institutes of Health);
the World Health Organization International Clinical Trials Registry Platform search portal at http://www.who.int/trialsearch/Default.aspx.
Database of Abstracts of Reviews of Effects (DARE) in the Cochrane Library at http://onlinelibrary.wiley.com/o/cochrane/cochrane_cldare_articles_fs.html (for reference lists from relevant non‐Cochrane reviews;
Web of Science at http://wokinfo.com/ (source of trials and conference abstracts);
OpenGrey at http://www.opengrey.eu/ (for unpublished literature from Europe);
LILACS database at http://regional.bvsalud.org/php/index.php?lang=en (for trials from the Portuguese‐ and Spanish‐speaking world);
PubMed (for recent trials not yet indexed in MEDLINE).
The search output was managed with EndNote® (Endnote), which listed all studies and removed duplicates.
Searching other resources
We handsearched reference lists of articles retrieved by the search and contacted experts in the field to obtain additional data. We also handsearched relevant journals and conference abstracts that were not covered in the CGF Specialised Register, in liaison with the Trials Search Co‐ordinator.
Data collection and analysis
Selection of studies
Two review authors (JV, CV, or WN) independently scanned the titles and abstracts of the studies found in the literature search to identify potentially eligible studies. We retrieved the full texts of all potentially eligible studies, and two review authors independently examined these full‐text articles, selecting those studies eligible for inclusion in the review. We corresponded with study investigators as required to clarify study eligibility. The two review authors discussed and resolved any disagreements about whether to include or exclude a study. We documented the selection process with a PRISMA flow chart (see Figure 1).
1.
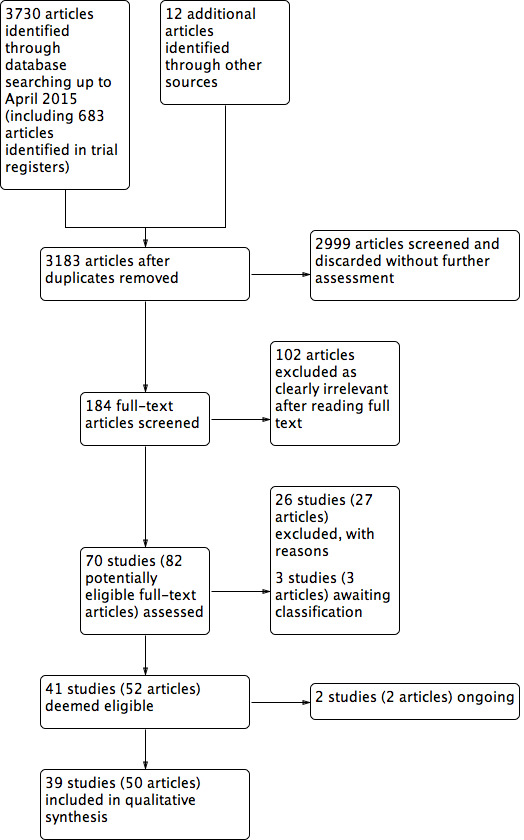
Study flow diagram.
We produced a 'Characteristics of included studies' table for each study considered suitable for inclusion. We listed all characteristics of each individual study in this table. Studies that did not meet the inclusion criteria after examination of the full text were excluded, if appropriate. We produced a 'Characteristics of excluded studies' table for each study that was excluded after reading of the full text, which included the reason for exclusion.
Data extraction and management
Two review authors extracted the data from all included studies using a data extraction form. The two review authors discussed and resolved minor disagreements. The data extraction forms included study characteristics and outcome data. Where studies had multiple publications, the main trial report was used as the reference paper, and additional details were derived from secondary papers. We corresponded with study investigators for further data on methods and results, as required.
We extracted the following information.
Trial characteristics:
Trial design (randomised controlled trial, including cross‐over trial, cluster randomised trial);
Means of funding (e.g. charities and trusts, pharmaceutical companies, hospital funding);
'Risk of bias' assessment (allocation, blinding, incomplete outcome data, selective reporting, other sources of bias);
Power calculation (yes or no);
Intention‐to‐treat analysis (yes or no);
Number of participants included and excluded;
Length of follow‐up (in months), lost to follow‐up (number of participants).
Participant characteristics:
Gender;
Age (years);
Type of subfertility (primary or secondary subfertile) and diagnosis;
Duration of subfertility (years);
Number of in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatments;
Phase in fertility process (pre‐fertility treatment, during fertility treatment, post‐fertility treatment);
Participants’ psychological history;
Comparison group.
Intervention characteristics:
Type of psychological or educational intervention (e.g. cognitive behavioural therapy, counselling);
Therapy setting (individual, couple, or group intervention; and face‐to‐face, telephone, Internet, or written information);
Initiation of psychological or educational intervention (pre‐, during, or post‐fertility treatment);
Duration of intervention (in weeks, intensity per week, and total time expressed in hours. In the case of delivering information once through leaflets, booklets, or Internet, no duration in weeks was recorded);
Personnel delivering the intervention (nurse, counsellor, psychologist, psychotherapist, doctor or psychiatrist).
Types of outcome measures:
As described above (see the Criteria for considering studies for this review).
Assessment of risk of bias in included studies
Two review authors (JV, JW, CV, or WN) independently assessed and reported the risk of bias using a standardised form ('Risk of bias' tool) from the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 8 (Higgins 2011).
We reported the following domains:
selection (random sequence generation and allocation concealment);
performance (blinding of participants and personnel);
detection (blinding of outcome assessors);
attrition (incomplete outcome data);
reporting (selective reporting);
other bias.
The two review authors resolved disagreements through discussion.
The different primary outcomes could have been differentially affected. For example, performance bias could have affected psychological and fertility outcomes differently, as psychological outcomes were subjective and more prone to be affected, whereas fertility treatment outcomes were more objective. We described all judgements fully and presented the conclusions in the 'Risk of bias' table, which was incorporated into the interpretation of review findings by means of sensitivity analyses.
We took care to search for within‐trial selective reporting, such as trials failing to report obvious outcomes, or reporting them in insufficient detail to allow inclusion. We sought published protocols and compared the outcomes between the published protocol and the final published study.
Measures of treatment effect
We extracted data when standardised and validated questionnaires and interviews were used to assess the outcomes of the interventions. If non‐standardised or non‐validated questionnaires and interviews were used, we took this into account in the 'Risk of bias' assessment. For each outcome, we extracted the means and standard deviations at the start and at the end of the intervention plus at all follow‐up time points. We recorded sample size at each follow‐up assessment.
Dichotomous measures
We used the numbers of events in the intervention and control groups of each study to calculate Mantel‐Haenszel odds ratios (ORs).
Live birth rates.
Ongoing pregnancy rates.
Clinical pregnancy rates.
Discontinuation of fertility treatment.
Continuous measures
We treated ordinal data, for example quality of life, as continuous data. We listed our preferred scales for the outcomes. If all trials had reported the preferred scale, we would have assessed the mean difference (MD). In order of preference, and according to availability after seeking additional details from the corresponding authors, we planned to use the estimated difference at outcome adjusting for baseline, the change from baseline to outcome, or the outcome score. If our preferred scale was not available but trials included comparison on other scales measuring the same outcome, then we would have analysed the standardised mean difference (SMD) of the outcome scores without consideration of baseline values (Section 9.4.5.2 of Higgins 2011). As not all trials reported the preferred scale, we used the SMD of the outcome scores without consideration of baseline values. We calculated SMDs in the usual manner, by dividing the difference in means by the pooled standard deviation for that study.
Mental health, divided into category. Preferred scales regarding anxiety and depression were the Spielberger State‐Trait Anxiety Inventory (STAI), Spielberger 1989, and the Beck Depression Inventory (BDI), Beck 1961.
General quality of life, the preferred scale was the 36‐Item Short Form Health Survey (SF‐36) (Tarlov 1989).
Fertility‐specific quality of life, the preferred scale was the Fertility Quality of Life Tool (FertiQoL) (Boivin 2011).
Social support, as measured by validated scales.
We compared the final values between treatment groups. In the case of change scores, to differentiate between effect sizes, a relevant improvement was a pre‐post effect size of at least 0.2 standard deviations (SDs) in the score of questionnaires or an effect size of 0.2 SD between the intervention group and the control group in the scoring of questionnaires. Where a study reported change scores (and SDs) only, we sought statistical advice regarding appropriate strategies for pooling. We planned to pool results where studies reported change and end scores using the same scale, but there were insufficient studies in any comparison for this to be possible.
We presented 95% confidence intervals (CI) for all outcomes. Where data to calculate ORs or MDs were not available, we utilised the most detailed numerical data available that facilitated similar analyses of included studies (for example test statistics, P values). We compared the magnitude and direction of effect reported by studies with how they were presented in the review, taking into account legitimate differences.
Unit of analysis issues
We analysed data per man, woman, or couple. We counted multiple live births (for example twins or triplets) as one live birth event.
Cross‐over trials
We included only first‐phase data from cross‐over trials.
Cluster randomised trials
'Unit of analysis error' may occur if cluster randomised trials are incorrectly analysed. We therefore planned to report the methods used in the analysis of cluster randomised trials. We planned to consider the data from cluster randomised trials for meta‐analysis when the analyses were carried out appropriately. We then would have used the effect estimates and their standard errors for meta‐analysis employing the generic inverse‐variance method in Review Manager 2014 (RevMan). If the analysis of cluster randomised trials had been inappropriate, we planned to adjust the standard errors if an appropriate estimate of the intracluster correlation coefficient (ICC) could be obtained from a reliable external source. Otherwise, we planned to analyse the data, if possible, at the cluster level using the generic inverse‐variance method. We would subsequently also have carried out a sensitivity analysis. Due to clinical heterogeneity, we did not perform meta‐analysis and therefore did not use the generic inverse‐variance method to analyse 'unit of analysis error'.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible. We contacted the trial authors by email, post, or telephone to request relevant missing data. Where we were unable to obtain these missing data, we undertook imputation of individual values for the primary outcomes only. Pregnancy was assumed not to have occurred in participants without a reported outcome. For other outcomes, we analysed only the available data. If studies reported sufficient detail to calculate MDs but provided no information on associated SDs, we assumed the outcome to have an SD equal to the highest SD from other studies within the same analysis.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We planned to assess heterogeneity by visual inspection of the forest plots. We would also have assessed statistical heterogeneity by using the Chi2 test and evaluating the P value (Higgins 2011). We would have assessed the I2 statistic to quantify heterogeneity. An I2 value greater than 50% would have been taken to indicate substantial heterogeneity (Higgins 2003; Higgins 2011). We would have explored reasons for heterogeneity by using subgroup analyses. However, due to the large amount of clinical heterogeneity assessed by consideration of the features of the included studies, we did not perform meta‐analysis and did not utilise the Chi2 test and the I2 statistic.
Assessment of reporting biases
In view of the difficulty of detecting publication bias and other biases, the review authors aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If there had been 10 or more studies in an analysis, we would have created a funnel plot to assess the potential for publication bias, but there were insufficient studies in any comparison for this to be possible.
Data synthesis
We planned to carry out statistical analyses with Review Manager 5 (Review Manager 2014) using a fixed‐effect model. If separate data from women and men had been available, we would have included them separately in the meta‐analysis. If we had found substantial heterogeneity between studies, sufficient to suggest that treatment effects may differ between trials, we planned to explore this heterogeneity by sensitivity analysis followed by random‐effects model meta‐analysis if required.
If the studies were sufficiently similar, we planned to combine the data using a fixed‐effect model in the following comparisons:
Psychological interventions versus usual care or attentional control.
Educational interventions versus usual care or attentional control.
Psychological interventions versus educational interventions.
Psychological and educational interventions versus usual care or attentional control.
An increase in the odds of a particular outcome, which may be beneficial (for example live birth) or detrimental (for example adverse effects), is displayed graphically in the forest plots to the right of the centre‐line, and a decrease in the odds of an outcome to the left of the centre‐line.
As a matter of fact, after assessing the included studies, we did not consider them to be sufficiently similar to permit meaningful pooling. We instead summarised the results of the individual studies by presenting the median and interquartile range of effects, including standardised mean differences for continuous variables.
Subgroup analysis and investigation of heterogeneity
We planned to investigate heterogeneity using subgroup analysis of potential confounding factors. This would have consisted of presenting and possibly pooling results within strata defined by the factors outlined below.
Duration of psychological or educational intervention: to analyse longer interventions (three weeks or longer), shorter interventions (shorter than three weeks), and interventions where the duration is not stated. It has been suggested that longer interventions are more effective than shorter interventions (Boivin 2003; Hämmerli 2009).
Therapy setting: to analyse individual interventions, couple interventions and group interventions, and interventions where the therapy setting is not stated. It has been suggested that group interventions are more effective than individual interventions (Boivin 2003; Hämmerli 2009).
Personnel delivering the psychological or educational intervention: to assess if outcomes differed depending on which personnel delivered the interventions: psychologists, counsellors, and psychotherapists versus medical and nursing personnel.
Upon commencement of data extraction, it became apparent that a distinction had to be made between studies using 'usual care' as a control and studies using attentional control groups, because studies in the former category do not account for non‐specific placebo‐type effects. At this point, we decided that control group (usual care or attentional) was an important stratification variable that we had omitted at the protocol stage, and that this should be added to the list of prespecified analyses presented above. If we detected substantial heterogeneity, we planned to explore possible explanations in sensitivity analyses. We would have taken any statistical heterogeneity into account when interpreting the results, especially if there was any variation in the direction of effect. As we did not perform meta‐analysis, we did not explore further by sensitivity analyses.
Sensitivity analysis
During the process of undertaking this review the authors made many decisions. Some of these decisions were necessarily somewhat arbitrary or subjective. We planned sensitivity analysis to show that the findings of the review did not depend on those arbitrary decisions. We planned to perform sensitivity analyses to explore the influence of:
Randomisation: we planned to perform a sensitivity analysis without cross‐over trials and cluster randomised trials;
'Risk of bias' assessment: we planned to perform a sensitivity analysis without studies with a major risk of bias (defined as two or more domains assessed as high risk of bias);
Odds ratio: we planned to perform a sensitivity analysis in case the summary effect measure was risk ratio rather than odds ratio;
Pooling of the ongoing pregnancy rates: we planned to perform a sensitivity analysis in which the ongoing pregnancy rate was not pooled with the live birth rate;
Dealing with missing data: we planned to perform a sensitivity analysis utilising alternative imputation strategies;
Random‐effects model: we planned to perform a sensitivity analysis using a random‐effects model.
However, we did not conduct these sensitivity analyses because we did not perform meta‐analysis and no data were pooled.
Overall body of evidence: Summary of findings table
We planned to make a 'Summary of findings' table using Guideline Development Tool software (GRADEpro). We made a 'Summary of findings' table of the primary outcomes for overview without using Guideline Development Tool software, as pooling the data was not possible due to heterogeneity.
Results
Description of studies
We included randomised controlled trials and cluster‐randomised trials of psychological and educational interventions for improving anxiety, depression, and fertility outcomes for subfertile men and women.
Results of the search
The search strategy identified 3730 articles, and contacting authors of relevant published studies resulted in a further 12 articles. Removal of duplicates left 3183 articles. After title and abstract screening, we discarded 2999 articles without further assessment and further assessed 184 articles. We discarded 102 articles as clearly irrelevant, leaving 82 potentially relevant articles, which comprised 70 individual studies (as 12 articles consisted of duplicate publications or preliminary results). We excluded 26 studies (27 articles) after checking the full text, for reasons stated in the Excluded studies table. Three studies are awaiting assessment because results are not reported in the abstract and a full text article is not available (see Characteristics of studies awaiting classification), and two are ongoing (see Characteristics of ongoing studies). We have included the remaining 39 studies (50 articles). See Figure 1: Study flow diagram.
Included studies
See: Characteristics of included studies
Design
We included 39 randomised controlled trials (RCTs) (38 individually randomised and one cluster randomised) with a total of 4925 participants. Of these 4925 participants, 4312 are included from studies employing psychological interventions, and 613 are included from studies employing educational interventions. Of the 39 included studies, five were solely published as an abstract (Conrad 2013; Czamanski‐Cohen 2012; Moragianni 2009; Rasoulzadeh 2013; Wiener‐Megnazi 2006), and 34 were published as full‐text papers. The studies were conducted in many countries in Africa, Asia, Europe, and North America.
In the cluster randomised trial (Mori 2008), seven clusters were randomised into two groups. Analysis was done for all participants individually, but no adjustment for clustering was made.
The number of randomised participants per study ranged from n = 377, in Ockhuijsen 2014, to n = 12, in Soltani 2014.
Participants
The review included both men and women. Two studies focused on men (Conrad 2013; Pook 2005), 26 trials focused on women, and nine studies focused on both men and women, that is couples. Two studies did not report details about participants (Moragianni 2009; van Zyl 2005). Eighteen studies reported on participants with both primary and secondary subfertility. Nine studies reported on primary subfertile participants. Twelve studies did not report the type of subfertility of their participants. Duration of subfertility varied from 12 months, in Choobforoushzade 2011, Cousineau 2008, and Shahrestani 2012, to 18 years, in Domar 2000. Exclusion criteria varied considerably between studies. Most studies excluded participants with psychiatric disorders and participants who had previously received the same psychological intervention. One study specifically included depressed women and excluded participants without a diagnosis of depression (Faramarzi 2008).
The age of included participants varied from 18 to 55 years old. Most of the participants were in their early 30s. We included studies if participants were pre‐, during, or post‐fertility treatment. Seven studies included participants who were pre‐fertility treatment. Six studies included participants who were both pre‐ and during fertility treatment. Twenty‐two studies included participants who were currently receiving fertility treatment (during). Three studies did not report on this feature (Choobforoushzade 2011; Rasoulzadeh 2013; Soltani 2014).
Interventions
The studies in this review investigated psychological and educational interventions. Thirty‐four studies investigated psychological interventions, and five studies investigated educational interventions.
A variety of psychological interventions were used in the included studies: hypnosis (Catoire 2013), body‐mind(‐spirit) and mindfulness interventions (Chan 2006; Chan 2012; Domar 2011; Shahrestani 2012), a nursing intervention (Arslan‐Ozkan 2013), a health promotion model on the Internet with the goal of relieving distress (Cousineau 2008), cognitive (behavioural) therapy (Choobforoushzade 2011; Czamanski‐Cohen 2012; Domar 2000; Faramarzi 2008; Gorayeb 2012; Mosalanejad 2012; Sexton 2010; Shu‐Hsin 2003; Soltani 2014), expressive writing (Conrad 2013; Matthiesen 2012; Panagopoulou 2009), counselling (de Klerk 2005; Emery 2003; Kharde 2012; La Fianza 2014; Rasoulzadeh 2013; Skiadas 2011; Vizheh 2013), an interview with positive‐statement reading (van Zyl 2005), interpersonal therapy (Koszycki 2012), music therapy (Moragianni 2009; Murphy 2014), positive reappraisal coping intervention (PRCI) (Ockhuijsen 2014), relaxation therapy (Valiani 2010; Wiener‐Megnazi 2006), and psychotherapy (Zhu 2010).
The five educational interventions used in the included studies were: an interactive self help guide on the Internet (Haemmerli 2010), a booklet, homework, and stress management (Mori 2008), a leaflet outlining contents of a fertility workup (Pook 2005), face‐to‐face information about coping with medical investigations, a videotape, a sex information booklet, and a phone call every month (Takefman 1990), and interviews providing information to participants (Terzioglu 2001). The educational interventions were not delivered by personnel, except for the face‐to‐face information about coping and a phone call, in Takefman 1990, and the interviews, in Terzioglu 2001.
The type of personnel who delivered the psychological interventions was not stated in six studies. In 10 studies, no personnel were needed to deliver an intervention because it took place on the Internet, by writing therapy, or by a booklet. Twenty‐one studies reported on the type of personnel used: hypnotists, counsellors, researchers themselves, social workers, nurses, psychologists, therapists, gynaecologists, and an embryologist. All of these personnel types used face‐to‐face contact to deliver the intervention. Some of them also used telephone calls. By contrast educational interventions used videotapes, the Internet, face‐to‐face information provision, and written information.
Eighteen psychological interventions consisted of individual therapy. Four psychological interventions consisted of couple therapy. Nine psychological interventions consisted of group therapy (7 to 12 members per group). Two studies did not report on therapy setting (Czamanski‐Cohen 2012; Rasoulzadeh 2013). One study assessed both individual and group therapy (Kharde 2012); as 75% of the counselling sessions consisted of individual therapy, we included this study in the 'individual therapy' subgroup. The educational interventions consisted of individual and couple therapy. The Haemmerli study provided an Internet intervention for both individuals and couples (Haemmerli 2010); we included this study in the 'individual therapy' group since more than half of the participants were individuals and the analysis was performed using data of all individuals rather than of couples.
The duration of interventions varied widely: from 20 minutes (once), in Murphy 2014, to 28.5 hours (divided over many weeks) in Domar 2011. Unfortunately, duration of the intervention was not clearly stated in 11 studies. We included the three studies measuring anxiety or depression or both in the 'unknown duration' subgroup (Kharde 2012; Takefman 1990; van Zyl 2005).
Control interventions consisted of: participants receiving usual care; on a waiting list; and in an 'attentional control group'.
In the Haemmerli study (Haemmerli 2010), waiting list participants were used as a comparison group (instead of participants receiving usual care). According to the protocol, participants on a waiting list were a legitimate comparison group to include in the review. However, we have included this study in the usual care subgroup in the results of this review since it was unclear whether or not a waiting list would adequately control for non‐specific attentional effects. It should be noted that being placed on a waiting list could influence participants in a different way from receiving usual care; participants on a waiting list may experience more feelings of anxiety and depression because of uncertainty during the waiting period and the absence of medical or psychological personnel to help them cope with their feelings. However, as this was the only study that used a waiting‐list control group, we decided to include it with the studies controlled by usual care and to highlight concerns over the choice of control group in this study in the 'Risk of bias' assessment.
Eight studies used attentional control groups. Anxiety and depression data were presented in four of these and are included in the forest plots (Catoire 2013; Koszycki 2012; Mori 2008; Takefman 1990). Using an attentional control group is a way to minimise performance bias in psychological interventions in which participants cannot be blinded to the intervention they receive. Participants in an attentional control group receive a similar (control) intervention that mimics the amount of time and attention the intervention group receives, in order to confer the general advantages of attention and participation without providing the specific postulated benefits of the experimental treatment. In this way participants can be partially blinded and therapy effects can be distinguished from non‐specific placebo effects. As noted above in Subgroup analysis and investigation of heterogeneity, results were grouped in the forest plots according to whether or not they had used attentional or usual care control groups for this reason. Even though this subgroup was not prespecified, making a clear distinction between therapy effects and non‐specific placebo effects could add relevant information to this review. We made this decision after starting data extraction.
In the study of Ockhuijsen (Ockhuijsen 2014), a three‐arm RCT consisting of an intervention group, a monitoring group, and a routine care group was performed. The monitoring group was taken as the comparison group, as it more closely resembled the intervention group. Both groups completed a Daily Record Keeping form, which was not completed by the routine care control group. In the study of Arslan‐Ozkan (Arslan‐Ozkan 2013), the attentional control group received usual nursing care including interviews, therefore participants in the control group may have assumed they received the actual intervention of interest.
The mean age of participants in the comparison groups was slightly higher in some of the studies. However, 11 studies did not report the mean age of the comparison groups.
Outcomes
Primary outcomes
Twenty‐two studies measured anxiety. The majority used the Spielberger State‐Trait Anxiety Inventory (STAI) to measure anxiety amongst participants (Catoire 2013; Chan 2012; Domar 2000; Emery 2003; Haemmerli 2010; Moragianni 2009; Murphy 2014; Panagopoulou 2009; Shu‐Hsin 2003; Takefman 1990; Terzioglu 2001; Wiener‐Megnazi 2006; Zhu 2010). Other questionnaires used to measure anxiety were: Beck Anxiety Inventory (BAI) (van Zyl 2005), Cattell Anxiety Inventory (CAI) (Faramarzi 2008), Depression Anxiety Stress Scale (DASS‐21) (Mosalanejad 2012; Soltani 2014), Hamilton Anxiety and Depression Scale (HADS) (de Klerk 2005; Mori 2008; Ockhuijsen 2014), Hamilton Anxiety Scale (HAM‐A) (Kharde 2012), Zung Self‐Rating Anxiety Scale (Z‐SAS) (La Fianza 2014), and the subscale anxiety of the Hamilton Depression Scale (HAM‐D) (Koszycki 2012).
Fifteen studies measured depression. The majority used Beck Depression Inventory (II) (BDI‐II) to measure depression amongst participants (Domar 2000; Emery 2003; Faramarzi 2008; Koszycki 2012; Terzioglu 2001; van Zyl 2005). Other questionnaires used to measure depression were: Center for Epidemiologic Studies Depression Scale (CES‐D) (Haemmerli 2010), Depression Anxiety Stress Scale (DASS‐21) (Mosalanejad 2012; Soltani 2014), Hamilton Anxiety and Depression Scale (HADS) (de Klerk 2005; Mori 2008; Ockhuijsen 2014), Hamilton Depression Scale (HAM‐D) (Kharde 2012), and the Zung Self‐Rating Depression Scale (Z‐SDS) (La Fianza 2014; Shu‐Hsin 2003; Zhu 2010).
Anxiety and depression subscales were also measured by the Symptom Checklist‐90 (SCL‐90) (Sexton 2010). Unfortunately, two different sets of data were presented, and it was not clear which was correct. We sent emails to the author, but have received no response, therefore no data could be presented. The Wiener‐Megnazi study measured state anxiety but no data were presented (Wiener‐Megnazi 2006), therefore no data could be presented in this review. The La Fianza study measured anxiety and depression by the Zung Self‐Rating scales, but as no postintervention data were presented, no data could be presented in this review (La Fianza 2014).
Two studies reported live birth rates after 20 weeks of gestation. One study reported live birth rate within 12 months after fertilisation (Domar 2000). One study reported live birth rate within 10 months after embryo transfer (Catoire 2013). Two studies reported ongoing pregnancy rates. One study reported ongoing pregnancy rates at 12 weeks of gestation (Chan 2006). The Chan 2012 study reported ongoing pregnancy rates after 8 to 10 weeks instead of 12 weeks of gestation, and therefore could not be included in any analysis (Chan 2012).
Domar 2000 reported change scores instead of end scores. The difference in mean change scores are presented in the forest plots for this study. Emery 2003 presented means and standard deviations (SDs) for men and women separately. We calculated the means and SDs of men and women combined by using a pooled variance formula. This was also necessary for measures of marital and sexual satisfaction in Vizheh 2013. Terzioglu 2001 presented SDs for pregnant and non‐pregnant women separately, and no SDs for men in the study. We obtained the SDs for women by pooling the pregnant and non‐pregnant SDs. We estimated the SDs for men using those reported in Emery 2003, which was the only study reporting anxiety and depression data for men only. We then pooled the men and women SDs by using a pooled variance formula to obtain the overall combined estimates.
Secondary outcomes
Nineteen studies measured additional mental health outcomes. If studies reported two questionnaires regarding secondary mental health outcomes, we chose the questionnaire with the best validity. Distress and well‐being were measured by: Body‐Mind‐Spirit Well‐Being Inventory (BMSWBI) (Chan 2012), Copenhagen Multi‐centre Psychosocial Infertility (COMPI) (Matthiesen 2012), Daily Record Keeping (DRK) (de Klerk 2005; Ockhuijsen 2014), Fertility Problem Inventory (FPI) (Cousineau 2008; Koszycki 2012; Sexton 2010; Shahrestani 2012; Valiani 2010), Infertility Distress Scale (IDS) (Arslan‐Ozkan 2013; Conrad 2013; Haemmerli 2010; Pook 2005), Positive and Negative Affect Schedule (PANAS) negative subscale (Panagopoulou 2009), Perceived Stress Scale (PSS) (Czamanski‐Cohen 2012; Skiadas 2011), and stress subscale from DASS‐21 (Mosalanejad 2012; Soltani 2014). Mood was measured by Profile of Mood States (POMS) (Domar 2000). Unfortunately, we have not presented the Fertility Problem Inventory (FPI) data of the Sexton study because of the use of two different (and discrepant) sets of data (Sexton 2010). There are no Infertility Distress Scale (IDS) data of the Conrad study because these were not presented in the abstract (Conrad 2013).
Three studies measured general quality of life using three different questionnaires: General Health Questionnaire (GHQ) (Faramarzi 2008), 36‐Item Short Form Health Survey (SF‐36) (Mori 2008), and World Health Organization Quality of Life ‐ short version (WHOQOL‐BREF) (Choobforoushzade 2011). The included studies did not report fertility‐specific quality of life. Seven studies measured social support. Relationship satisfaction was measured by Revised Dyadic Adjustment Scale (RDAS), in Cousineau 2008, and the subscale interpersonal support of the Health‐Promoting Lifestyle Profile (HPLP), in Domar 2000. Marital satisfaction was measured by Kansas Marital Satisfaction (KMS) (Chan 2012), Marital Satisfaction Questionnaire (MSQ) (Vizheh 2013), Marital Disaffection Scale (MDS) (Domar 2000), and Marital Adjustment Inventory (MAI) (Kharde 2012). Sexual satisfaction was measured by Sexual Satisfaction Questionnaire (SSQ), in Vizheh 2013, and Temperament and Character Inventory (TCI), in Conrad 2013. Unfortunately, no data were presented in the Conrad study. One study measured social support in total (Rasoulzadeh 2013), but unfortunately no data were presented.
Sixteen studies measured additional fertility treatment outcomes. Eight studies measured clinical pregnancy rates (Domar 2011; Haemmerli 2010; Koszycki 2012; Mori 2008; Murphy 2014; Ockhuijsen 2014; Takefman 1990; Zhu 2010); however, one study did not report the measuring method (Mori 2008), one study did not report the total number of participants at the time point of measuring (Haemmerli 2010), and one study did not report the time point of measuring (Zhu 2010).
Other studies reported biochemical pregnancy rates (de Klerk 2005), or pregnancy rates measured two or four weeks after embryo transfer (Emery 2003; Gorayeb 2012; Matthiesen 2012; Panagopoulou 2009). Three studies reported pregnancy rates without further definition (Czamanski‐Cohen 2012; Skiadas 2011; Vizheh 2013); we could not include these studies in any analyses because they did not meet the outcome criteria.
The final secondary outcome was discontinuation of fertility treatment. One‐third (11 of the 31 studies) reported numbers of couples who quit fertility treatment before the achievement of a pregnancy and during the psychological or educational intervention.
Questionnaires used
The included studies used 30 different questionnaires. For an explanation and details of each questionnaire, see Table 2.
1. Questionnaires used.
| Questionnaire | Additional information | Studies using this questionnaire |
| Anxiety | ||
| STAI (Spielberger State‐Trait Anxiety Inventory) |
Range 20 to 80 (state) and 20 to 80 (trait) where 20 is no anxiety and 80 is most severe anxiety | Catoire 2013; Chan 2006; Chan 2012; Domar 2000; Emery 2003; Haemmerli 2010; Moragianni 2009; Murphy 2014; Panagopoulou 2009; Shu‐Hsin 2003; Takefman 1990; Terzioglu 2001; Wiener‐Megnazi 2006; Zhu 2010 |
| BAI (Beck Anxiety Inventory) |
Range 0 to 63 where 0 is no anxiety and 63 is most severe anxiety | van Zyl 2005 |
| CAI (Catell Anxiety Inventory) |
Range 0 to 80 where 0 is no anxiety and 80 is most severe anxiety | Faramarzi 2008 |
| DASS‐21 (Depression Anxiety Stress Scale) |
Range 0 to 21 where 0 is no anxiety and 21 is most severe anxiety | Mosalanejad 2012; Soltani 2014 |
| HADS (Hamilton Anxiety and Depression Scale) |
Range 0 to 21 where 0 is no anxiety and 21 is most severe anxiety | de Klerk 2005; Mori 2008; Ockhuijsen 2014 |
| HAM‐A (Hamilton Anxiety Scale) |
Range 0 to 56 where 0 is no anxiety and 56 is most severe anxiety | Kharde 2012 |
| Subscale anxiety of HAM‐D (Hamilton Depression Scale) | Range 0 to 15 where 0 is no anxiety and 15 is most severe anxiety | Koszycki 2012 |
| Z‐SAS (Zung Self‐Rating Anxiety Scale) | Range 20 to 80 where 20 is no anxiety and 80 is most severe anxiety | La Fianza 2014 |
| Depression | ||
| BDI‐(II) (Beck Depression Inventory (II)) |
Range 0 to 63 where 0 is no depression and 63 is most severe depression | Domar 2000; Emery 2003; Faramarzi 2008; Koszycki 2012; Terzioglu 2001; van Zyl 2005 |
| CES‐D (Center for Epidemiologic Studies Depression Scale) |
Range 0 to 60 where 0 is no depression and 60 is most severe depression | Haemmerli 2010 |
| DASS‐21 (Depression Anxiety Stress Scale) |
Range 0 to 21 where 0 is no depression and 21 is most severe depression | Mosalanejad 2012 |
| HADS (Hamilton Anxiety and Depression Scale) |
Range 0 to 21 where 0 is no depression and 21 is most severe depression | de Klerk 2005; Mori 2008; Ockhuijsen 2014 |
| HAM‐D (Hamilton Depression Scale) |
Range 0 to 52 where 0 is no depression and 52 is most severe depression | Kharde 2012 |
| Z‐SDS (Zung Self‐Rating Depression Scale) |
Range 20 to 80 where 20 is no depression and 80 is most severe depression | La Fianza 2014; Shu‐Hsin 2003; Zhu 2010 |
| Distress and well‐being | ||
| BMSWBI (Body‐Mind‐Spirit Well‐Being Inventory) |
Range 0 to 560 where 0 is no distress and 560 is most severe distress | Chan 2012 |
| COMPI (Copenhagen Multi‐centre Psychosocial Infertility) |
Range 0 to 54 where 0 is no distress and 54 is most severe distress | Matthiesen 2012 |
| DRK (Daily Record Keeping) |
Range unknown. 21 items with 4‐point Likert scale. Low score is good | de Klerk 2005; Ockhuijsen 2014 |
| FPI (Fertility Problem Inventory) |
Range 46 to 276 where 46 is no distress and 276 is most severe distress | Cousineau 2008; Koszycki 2012; Shahrestani 2012; Valiani 2010 |
| IDS (Infertility Distress Scale) |
Range 0 to 32 where 0 is no distress and 32 is most severe distress | Arslan‐Ozkan 2013; Haemmerli 2010; Pook 2005 |
| Negative subscale of PANAS (Positive and Negative Affect Schedule) |
Range 10 to 50 where 10 is no or slightly distressed and 50 is most severe distress | Panagopoulou 2009 |
| PSS (Perceived Stress Scale) |
Range 0 to 56 where 0 is no distress and 56 is most severe distress | Czamanski‐Cohen 2012; Skiadas 2011 |
| Stress subscale from DASS‐21 (Depression Anxiety Stress Scale) |
Range 0 to 21 where 0 is no distress and 21 is most severe distress | Mosalanejad 2012 |
| POMS (Profile of Mood States) |
Range 0 to 65 where 0 is no distress and 65 is most severe distress | Domar 2000 |
| General QoL | ||
| GHQ (General Health Questionnaire) |
Range 0 to 84 where 0 is low QoL and 84 is high QoL | Faramarzi 2008 |
| SF‐36 (subscale MCS) (36‐Item Short Form Health Survey, subscale mental component summary) |
Range of MCS (mental component summary) is 17 to 62 where 17 is low QoL and 62 is high QoL | Mori 2008 |
| WHOQOL‐BREF (World Health Organization Quality of Life ‐ short version) |
Range 16 to 80 where 16 is low QoL and 80 is high QoL | Choobforoushzade 2011 |
| Social support | ||
| RDAS (Revised Dyadic Adjustment Scale) |
Range 0 to 69 where 0 is low relationship satisfaction and 69 is high relationship satisfaction | Cousineau 2008 |
| Subscale interpersonal support of HPLP (Health‐Promoting Lifestyle Profile) |
Range unknown. Only change scores presented | Domar 2000 |
| KMS (Kansas Marital Satisfaction) |
Range 3 to 21 where 3 is low marital satisfaction and 21 is high marital satisfaction | Chan 2012 |
| MSQ (Marital Satisfaction Questionnaire) |
Range 18 to 90 where 18 is low marital satisfaction and 90 is high marital satisfaction | Vizheh 2013 |
| MDS (Marital Disaffection Scale) |
Range unknown. 9 items. Only change scores presented | Domar 2000 |
| MAI (Marital Adjustment Inventory) |
Range 10 to 100 where 10 is low marital adjustment and 100 is high marital adjustment | Kharde 2012 |
| SSQ (Sexual Satisfaction Questionnaire) |
Range 11 to 55 where 11 is low sexual satisfaction and 55 is high sexual satisfaction | Vizheh 2013 |
| Folkman and Lazarus' Ways of Coping Questionnaire | Range unknown | Rasoulzadeh 2013 |
| Temperament and Character Inventory | Range unknown | Conrad 2013 |
QoL: quality of life
Excluded studies
See: Characteristics of excluded studies
We excluded 26 studies from the review for the following reasons:
Thirteen were not RCTs.
One did not select the participants of interest for this review.
Two did not provide a psychological or an educational intervention.
Four did not select the right comparison for this review.
Six did not provide relevant outcomes after sufficient contact with the authors.
Risk of bias in included studies
See 'Risk of bias' summary in Figure 2 and 'Risk of bias' graph in Figure 3 for an overview on risk of bias.
2.
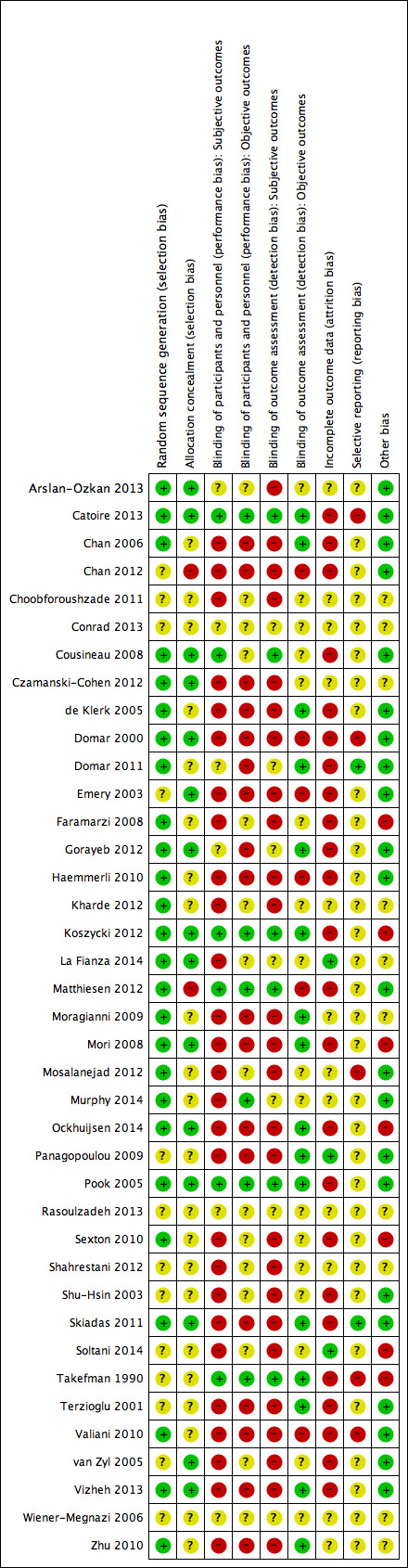
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
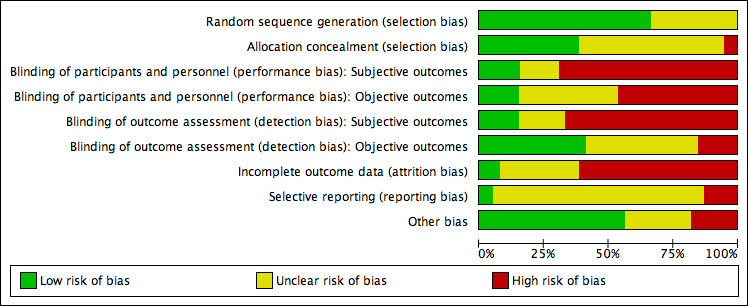
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
Twenty‐six studies were at low risk of bias related to sequence generation, as these studies used computer randomisation or a random numbers table. The other 13 studies did not describe the method used and were at unclear risk of bias.
Allocation concealment
Fifteen studies were at low risk of bias related to allocation concealment, as these studies used opaque, sealed envelopes or a research co‐ordinator who did the allocation, but did not decide which participants were included. Twenty‐two studies failed to describe methods of allocation concealment and were at unclear risk of bias for this domain. Two studies were at high risk of bias related to allocation concealment, as these studies used methods of high risk related to allocation concealment (that is drawing lots by the researcher and allocation by incoming order of informed consent).
Blinding
Six studies were at low risk of performance bias regarding subjective outcomes, as participants (and personnel) were adequately blinded. Six studies were at low risk of performance bias regarding objective outcomes, as participants (and personnel) were adequately blinded. Six studies regarding subjective outcomes and 15 studies regarding objective outcomes scored unclear risk with the annotation 'not applicable', as these studies did not examine a subjective or an objective outcome. Twenty‐seven studies regarding subjective outcomes and 18 studies regarding objective outcomes were at high risk of performance bias, as participants (and personnel) were not (adequately) blinded.
Six studies were at low risk of detection bias regarding subjective outcomes, as participants became their own outcome assessors by completing questionnaires, and these participants were adequately blinded. Sixteen studies were at low risk of detection bias regarding objective outcomes. Seven studies regarding subjective outcomes scored unclear risk of bias as blinding was not reported, and 17 studies regarding objective outcomes scored unclear risk with the annotation 'not applicable' for the same reason as stated above. Twenty‐six studies regarding subjective outcomes and six studies regarding objective outcomes were at high risk of detection bias, as the outcome assessors were not adequately blinded, and there were concerns over differential attrition between the groups (objective outcomes).
Incomplete outcome data
Three studies were at low risk of bias related to attrition (La Fianza 2014; Panagopoulou 2009; Soltani 2014), as these studies appeared to have no or just one participant who was either lost to follow‐up or withdrawn (this was not stated, but could be inferred from the degrees of freedom in a reported F statistic in the Panagopoulou 2009 study), and were therefore able to realise an analysis that adhered to intention‐to‐treat principles, without trial group changes. Twelve studies scored unclear risk of attrition bias, as the use of intention‐to‐treat analysis was not (clearly) reported, possible attrition was not reported, and reasons for withdrawal were not reported. Twenty‐four studies were at high risk of attrition bias, as more than 20% of the participants were excluded, withdrew, or were lost to follow‐up; these numbers of loss were imbalanced between the intervention and control groups; or the reasons for exclusion, withdrawal, or loss to follow‐up could be considered to directly contribute to risk of bias (for example pregnant women were excluded from the analysis).
Selective reporting
Two studies were at low risk of reporting bias, as these reported all outcomes named in the protocol or the World Health Organization trials register (Domar 2011; Skiadas 2011). Thirty‐two studies scored unclear risk of reporting bias, as these did not publish a study protocol, and therefore reporting bias could not be judged. Five studies were at high risk of reporting bias, as these did not report all outcomes named in the protocol or reported more outcomes than named in the protocol. The first could lead to not reporting non‐significant outcomes, and the latter could be a symptom of fishing the data for significant outcomes, both of which tend to lead to overestimation of the effect. Two studies did not report pregnancy rates, while planning to in the protocol (Mosalanejad 2012; Valiani 2010). Another study explicitly solely reported significant outcomes at 12 months (Domar 2000). Two studies selected one questionnaire to report from several but did not describe the selection procedure (Catoire 2013; Takefman 1990). These arbitrary decisions will affect the inferences of the studies.
Other potential sources of bias
Seven studies were at high risk of other potential sources of bias. Two studies used a translated version of a questionnaire that was not validated (Faramarzi 2008; Koszycki 2012). Using a non‐validated questionnaire could lead to underestimation of both beneficial and harmful effects (Higgins 2011), and therefore could be a source of bias. Other studies reported baseline imbalances despite apparently appropriate randomisation and concealment (Mori 2008; Takefman 1990). One study reported an additional effect of part of the intervention (Daily Record Keeping questionnaire) that was given both to the intervention group and the monitoring control group (Ockhuijsen 2014). One study presented two inconsistent sets of results (Sexton 2010). The cluster randomised trial used cluster randomisation but did not account for this in the analysis (Mori 2008). Another randomised trial randomised by couples but analysed individuals and did not account for this in the analysis (Soltani 2014). We deemed the risk of bias to be unclear in 10 studies. Three of these studies were translated, and we therefore could have missed other sources of bias (Choobforoushzade 2011; Shahrestani 2012; Zhu 2010). One study did not present a baseline summary (Kharde 2012). In one study the baseline scores of the used questionnaire were imbalanced (La Fianza 2014), and we therefore could not judge baseline imbalances. Five studies consisted of an abstract only (Conrad 2013; Czamanski‐Cohen 2012; Moragianni 2009; Rasoulzadeh 2013; Wiener‐Megnazi 2006). We found no potential sources of other within‐study bias in the other 22 studies.
Effects of interventions
See: Table 1
We did not conduct meta‐analysis for the following reasons:
We judged there to be substantial clinical heterogeneity in participant characteristics, nature of interventions, intervention delivery, duration of intervention, and outcome measures, such that the pooled estimate would not have represented a clinically meaningful summary (see Description of studies). We deemed pooling not to be appropriate even after studies had been stratified according to the factors listed in the Methods section, because even within these subgroups, trials remained heterogeneous in relation to other factors.
In addition to this, we considered the overall bias in this collection of studies to be substantial, so that even if the studies were deemed to be broadly commensurable, the pooled estimate could have been highly misleading.
In light of these concerns, we considered a narrative review format to be more suitable for the presentation of results, illustrated with forest plots for summary purposes. In order to create these forest plots, we used the standardised mean difference because outcome measures differed between the studies. As we decided not to perform meta‐analysis, we have presented the study‐specific estimates without pooling. We have presented full control group sizes for those studies with multiple intervention arms. However, we would urge the reader to interpret these in the context of conclusions relating to the generally low methodological quality of the studies, rather than to take these estimates at face value. We have presented all estimates so that positive values correspond to intervention effects.
For details of the questionnaires used in the included studies, see Table 2. For the Summary of Findings table, see Table 1.
1 Psychological interventions versus usual care or attentional control
Thirty‐four studies compared these interventions and reported outcomes included in this review. There was a lack of data in six studies (Conrad 2013; Czamanski‐Cohen 2012; La Fianza 2014; Rasoulzadeh 2013; Sexton 2010; Wiener‐Megnazi 2006). Twenty‐eight studies reported usable outcome data and are presented in forest plots for summary purposes.
Primary outcomes
We have presented an overview of the primary outcomes anxiety and depression in forest plots in Analysis 1.1 to Analysis 1.6 for summary purposes. To be consistent with the protocol, studies are stratified according to length of treatment (short duration, long duration, unknown duration) and therapy setting (individual, couple, group therapy). For reasons described in the methods, we have also presented results stratified by type of control (usual care, attentional control). We did not perform stratification by personnel because type of personnel appeared to coincide with the type of intervention (psychological personnel provided a psychological intervention and medical personnel provided an educational intervention).
1.1. Analysis.
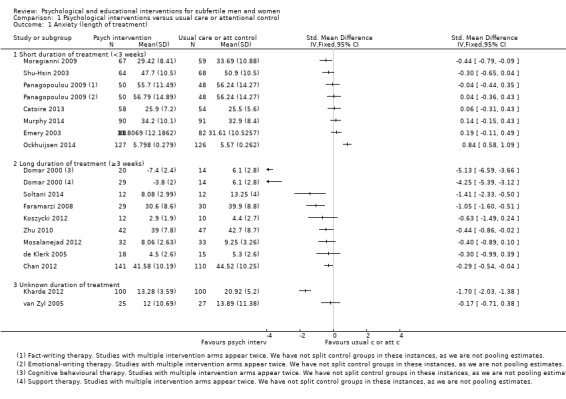
Comparison 1 Psychological interventions versus usual care or attentional control, Outcome 1 Anxiety (length of treatment).
1.6. Analysis.
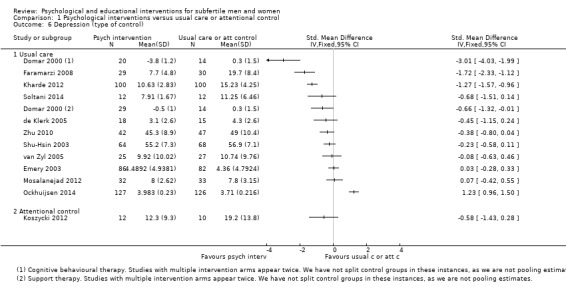
Comparison 1 Psychological interventions versus usual care or attentional control, Outcome 6 Depression (type of control).
Anxiety (Analyses 1.1 to 1.3)
We have calculated 19 standardised mean differences (SMDs) from 17 studies assessing psychological interventions and reporting measures of anxiety. Five estimates were positive, and 14 were negative, corresponding to a beneficial effect of treatment (median (interquartile range (IQR)) = ‐0.30 (‐0.84 to 0.00) with a range of ‐5.13 to 0.84, 17 RCTs, 2042 participants, very low‐quality evidence). The 95% confidence intervals (CIs) of eight studies with negative estimates excluded zero. One 95% CI from a study with a positive estimate excluded zero (Ockhuijsen 2014). Studies of longer duration generally had negative estimates that were larger than those arising from studies of shorter duration (Analysis 1.1). While this would be consistent with greater effects of treatments with longer trials, it might also be explained by increased performance and attrition biases manifesting in these studies. Estimates of trials of group therapy were all negative (Analysis 1.2), although this stratum largely coincided with the long duration stratum, so that similar concerns apply. This is most apparent from two large estimates of ‐4.3 and ‐5.1 arising from one study of a group therapy of long duration (Domar 2000), in which over 60% of control group participants dropped out.
1.2. Analysis.
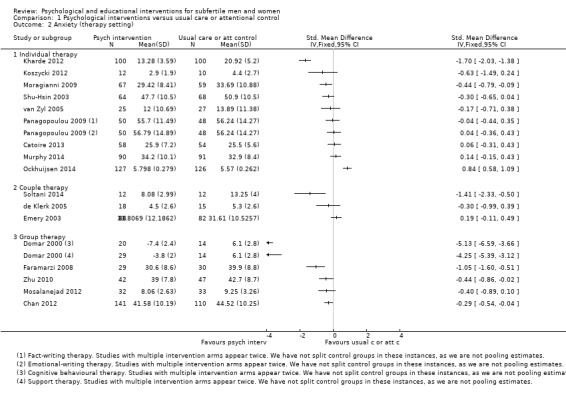
Comparison 1 Psychological interventions versus usual care or attentional control, Outcome 2 Anxiety (therapy setting).
Depression (Analyses 1.4 to 1.6)
We calculated 13 SMDs from 12 studies assessing psychological interventions reporting depression outcomes. Ten estimates were negative, and three were positive (median (IQR) = ‐0.45 (‐0.68 to ‐0.08) with a range of ‐3.01 to 1.23, 12 RCTs, 1160 participants, very low‐quality evidence). The 95% CIs of four studies with negative estimates excluded zero. One 95% CI from a study with a positive estimate excluded zero (Ockhuijsen 2014). Analogous comments can be made about the estimates from studies of long duration (Analysis 1.4) and group therapy (Analysis 1.5) as were made in relation to anxiety above: larger, negative estimates appearing in these strata may be explicable by susceptibility to biases.
1.4. Analysis.
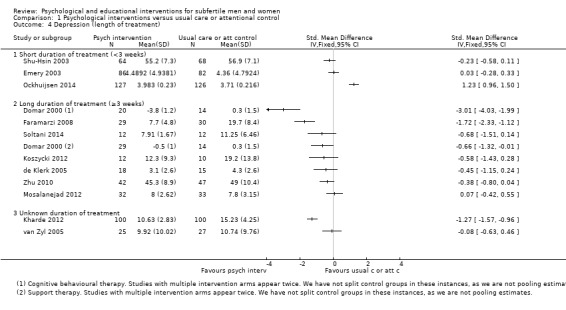
Comparison 1 Psychological interventions versus usual care or attentional control, Outcome 4 Depression (length of treatment).
1.5. Analysis.
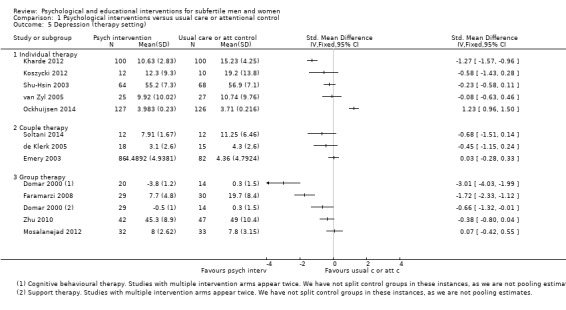
Comparison 1 Psychological interventions versus usual care or attentional control, Outcome 5 Depression (therapy setting).
Live birth or ongoing pregnancy (Analyses 1.7 and 1.8)
Two studies reported on live birth, and one reported ongoing pregnancy rates. Live birth and ongoing pregnancy have been displayed on the same forest plot. We have presented two sets of estimates: one assuming that participants with missing outcome data had failed outcomes (Analysis 1.7), and one showing the results of complete‐case analyses (Analysis 1.8). We have explained in the Discussion why neither set of estimates can be considered reliable. All four odds ratios (ORs) from these three studies were positive under both analyses.
1.7. Analysis.
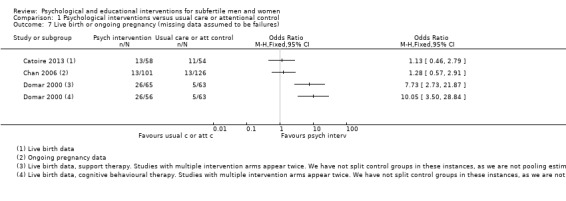
Comparison 1 Psychological interventions versus usual care or attentional control, Outcome 7 Live birth or ongoing pregnancy (missing data assumed to be failures).
1.8. Analysis.
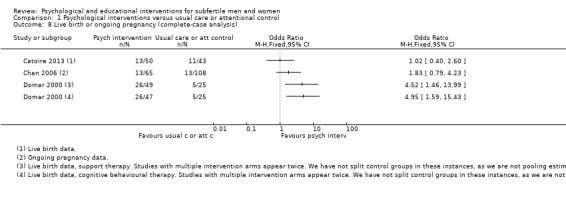
Comparison 1 Psychological interventions versus usual care or attentional control, Outcome 8 Live birth or ongoing pregnancy (complete‐case analysis).
Under the assumption of failed outcomes for those studies with missing data, two studies had small to moderate ORs of 1.13, in Catoire 2013, and 1.28, in Chan 2006, and both had 95% CIs that were consistent with substantial effects in either direction. Two estimates from one study were incredibly large (10.1 and 7.7) with definitive 95% CIs (3 RCTs, 456 participants, very low‐quality evidence) (Domar 2000). These overestimates are a product of high control group attrition combined with the assumption of failure for unobserved outcomes.
Under the complete‐case analysis, one OR becomes negligible at 1.02 (Catoire 2013), while one is increased to 1.83 (Chan 2006). The intervals for both of these remain consistent with substantial effects in either direction. The two estimates from Domar 2000 remain large and positive, but are attenuated to 4.52 and 4.95 (3 RCTs, 387 participants, very low‐quality evidence).
Secondary outcomes
Mental health ‐ distress and well‐being (Analysis 1.9)
We calculated 19 SMDs from 14 studies assessing psychological interventions and reporting measures of distress and well‐being (Analysis 1.9). Fourteen estimates were negative, and four were positive (median (IQR) = ‐0.51 (‐1.50 to ‐0.06) with a range of ‐4.22 to 0.27, 14 RCTs, 1547 participants, very low‐quality evidence). The 95% CIs of 11 studies with negative estimates excluded zero. One 95% CI from a study with a positive estimate excluded zero (Ockhuijsen 2014). Again, the estimates from one study were very large and negative (‐3.19 and ‐4.22) due to unbalanced attrition, which was already explained above (Domar 2000).
1.9. Analysis.
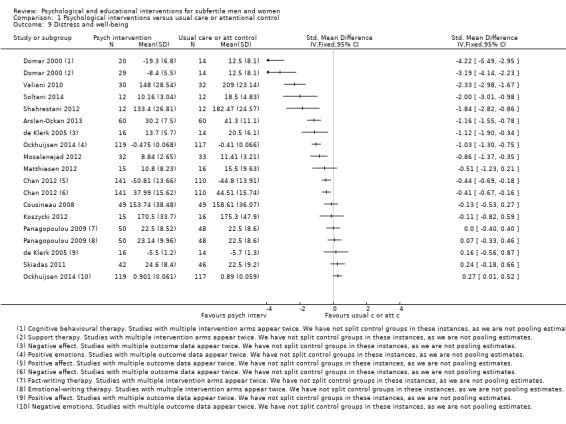
Comparison 1 Psychological interventions versus usual care or attentional control, Outcome 9 Distress and well‐being.
Quality of life ‐ general quality of life (Analysis 1.10) and fertility‐specific quality of life
Two studies assessing psychological interventions reported general quality of life (Analysis 1.10). One estimate was positive, and one was negative (2.04 and ‐1.23, 2 RCTs, 83 participants, very low‐quality evidence). The 95% CI of the study with a positive estimate excluded zero (Choobforoushzade 2011). The 95% CI from the study with a negative estimate excluded zero (Faramarzi 2008).
1.10. Analysis.
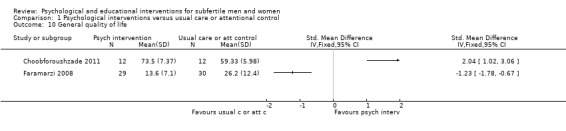
Comparison 1 Psychological interventions versus usual care or attentional control, Outcome 10 General quality of life.
No studies assessed psychological interventions and reported fertility‐specific quality of life.
Social support (Analysis 1.11)
Five studies assessing psychological interventions reported social support using several questionnaires consisting of three main groups: relationship satisfaction, marital satisfaction, and sexual satisfaction (Analysis 1.11). Footnotes were used to indicate which outcome the studies reported.
1.11. Analysis.
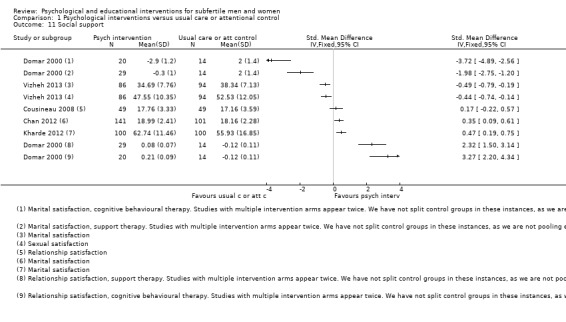
Comparison 1 Psychological interventions versus usual care or attentional control, Outcome 11 Social support.
Social support ‐ Relationship satisfaction
Two studies assessing psychological interventions reported relationship satisfaction. All three estimates were positive (2 RCTs, 161 participants, very low‐quality evidence). The 95% CIs of one study with positive estimates excluded zero (Domar 2000).
Social support ‐ Marital satisfaction
Four studies assessing psychological interventions reported marital satisfaction. Three estimates were negative, and two were positive (4 RCTs, 685 participants, very low‐quality evidence).
Social support ‐ Sexual satisfaction
One study assessing psychological interventions reported sexual satisfaction (Vizheh 2013). The estimate was negative, and the 95% CI of the study excluded zero (1 RCT, 180 participants, very low‐quality evidence).
Clinical pregnancy (Analysis 1.12)
Five studies assessing psychological interventions reported clinical pregnancy rates. We have presented estimates based on complete‐case analyses (Analysis 1.12).
1.12. Analysis.
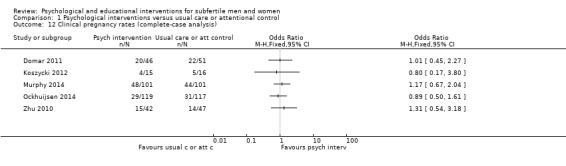
Comparison 1 Psychological interventions versus usual care or attentional control, Outcome 12 Clinical pregnancy rates (complete‐case analysis).
In the Domar study (Domar 2011), clinical pregnancy was confirmed by a foetal heartbeat at seven weeks' gestation. In the Ockhuijsen study (Ockhuijsen 2014), clinical pregnancy was confirmed by one or more gestational sacs on ultrasonography or definitive clinical signs of pregnancy (Zegers‐Hochschild 2009). Zhu 2010 and Murphy 2014 did not report any additional information on clinical pregnancy rates. In the Koszycki study (Koszycki 2012), pregnancy rates were measured after six months of follow‐up.
One estimate was positive but negligible, two were positive, and two were negative (5 RCTs, 555 participants, very low‐quality evidence). The 95% CIs of all five were consistent with substantial effects in either direction.
Discontinuation of fertility treatment (Analysis 1.13)
Eight studies assessing psychological interventions reported discontinuation rates (Analysis 1.13). Three studies had ORs below 1, and five studies had ORs above 1 (median (IQR) = 1.20 (0.60 to 1.47) with a range of 0.34 to 2.06, 8 RCTs, 1053 participants, very low‐quality evidence). The 95% CI of Chan 2006 was suggestive of a disadvantage of the intervention of indeterminate size. The intervals of the remaining studies were consistent with substantial effects in either direction .
1.13. Analysis.
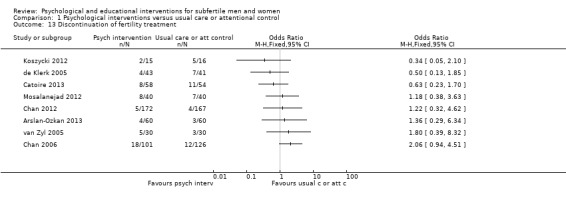
Comparison 1 Psychological interventions versus usual care or attentional control, Outcome 13 Discontinuation of fertility treatment.
2 Educational interventions versus usual care or attentional control
Five studies compared these interventions and reported outcomes included in this review.
Primary outcomes
We have presented an overview of the primary outcomes anxiety and depression in forest plots in Analysis 2.1 to Analysis 2.6 for summary purposes. To be consistent with the protocol, studies are stratified by each of length of treatment (short duration, long duration, unknown duration) and therapy setting (individual, couple, group therapy). For reasons described in the methods, we have also presented results stratified by type of control (usual care, attentional control). We did not perform stratification by personnel because type of personnel appeared to coincide with the type of intervention (psychological personnel provided a psychological intervention and medical personnel provided an educational intervention).
2.1. Analysis.
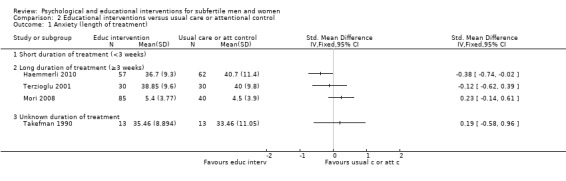
Comparison 2 Educational interventions versus usual care or attentional control, Outcome 1 Anxiety (length of treatment).
2.6. Analysis.
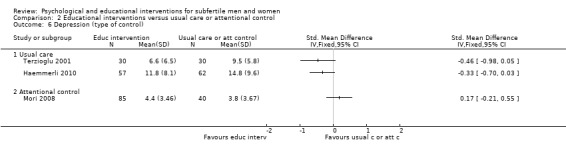
Comparison 2 Educational interventions versus usual care or attentional control, Outcome 6 Depression (type of control).
Anxiety (Analyses 2.1 to 2.3)
We calculated four SMDs from four studies assessing educational interventions and reporting measures of anxiety. Two estimates were positive, and two were negative (median = 0.03, with a range of ‐0.38 to 0.23, 4 RCTs, 330 participants, very low‐quality evidence). The 95% CI of one study with a negative estimate excluded zero, corresponding to an advantage of intervention of indeterminate size (Haemmerli 2010). Notably, the two negative estimates were of studies using a usual care control group, and the two positive estimates were of studies using an attentional control group (Analysis 2.3). This might be explained by the fact that an attentional control group minimises the possibility of performance bias, as explained in the Discussion.
2.3. Analysis.
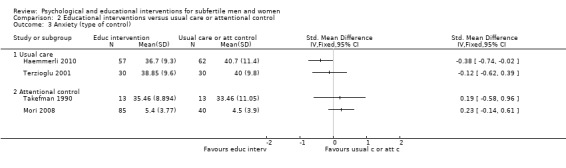
Comparison 2 Educational interventions versus usual care or attentional control, Outcome 3 Anxiety (type of control).
Depression (Analyses 2.4 to 2.6)
We calculated three SMDs from three studies assessing educational interventions and reporting measures of depression. Two estimates were negative, and one was positive (median = ‐0.33 with a range of ‐0.46 to 0.17, 3 RCTs, 304 participants, very low‐quality evidence). None of the 95% CIs of the three studies excluded zero. Analogous comments can be made about the estimates from studies using a usual care control group and an attentional control group as were made in relation to anxiety above: the direction of the estimate coincided with type of control group. Larger, negative estimates may be explicable by susceptibility to performance bias (Analysis 2.6).
Live birth or ongoing pregnancy
None of the included studies comparing educational interventions with usual care or attentional control assessed live birth rates or ongoing pregnancy rates.
Secondary outcomes
Mental health ‐ distress and well‐being (Analysis 2.7)
Two studies assessing educational interventions reported distress and well‐being (Analysis 2.7). One estimate was positive, and one was negative (2 RCTs, 323 participants, very low‐quality evidence). The 95% CIs of the studies did not exclude zero, although the interval for Pook 2005 was suggestive of an advantage of intervention of indeterminate magnitude.
2.7. Analysis.
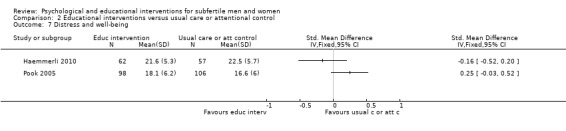
Comparison 2 Educational interventions versus usual care or attentional control, Outcome 7 Distress and well‐being.
Quality of life ‐ general quality of life and fertility‐specific quality of life
One study assessing an educational intervention reported general quality of life. In the cluster randomised Mori study (Mori 2008), the intervention group scored 46.9 (5.86), and the control group scored 49.1 (6.12) (mean (SD)). This was suggestive of no difference in general quality of life between the intervention group and the control group, as the 95% CI excluded clinically notable effects (mean difference (MD) ‐0.37, 95% CI ‐0.75 to 0.01, n = 125 (7 clusters)). The actual level of certainty in this conclusion will be overstated due to the fact that no allowance for intracluster correlation of outcomes was made.
No studies assessed educational interventions and reported fertility‐specific quality of life.
Social support
No studies comparing educational interventions and usual care or attentional control reported social support.
Clinical pregnancy (Analysis 2.8)
Three studies assessing educational interventions reported clinical pregnancy rates. We have presented estimates based on complete‐case analyses (Analysis 2.8).
2.8. Analysis.
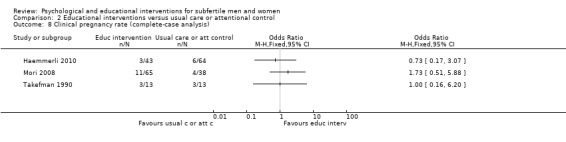
Comparison 2 Educational interventions versus usual care or attentional control, Outcome 8 Clinical pregnancy rate (complete‐case analysis).
In the Haemmerli study (Haemmerli 2010), clinical pregnancy was measured according to clinical or ultrasound visualisation of a gestational sac at two months. The denominators used for these outcome data were not reported, but were calculated as 43 for the treatment group and 64 for the control group. This means that while the full initial control group was included in these rates, only a subset of the treatment group was considered in Analysis 2.8. This may have resulted in the disadvantage of the treatment group compared to the control group being understated.
In the Mori study (Mori 2008), no further definition of clinical pregnancy was given. In the Takefman study (Takefman 1990), pregnancy rates within six months after the intervention were measured. Full outcome data were presented. One point estimate was negative, one was positive, and one suggested no difference (3 RCTs, 236 participants, very low‐quality evidence).
Discontinuation of fertility treatment (Analysis 2.9)
Four studies assessing educational interventions reported discontinuation rates. One had no discontinuations in either arm, and could not be displayed on a forest plot (Takefman 1990). Of the three studies included in the forest plot (Analysis 2.9), one study had an OR above 1, and two studies had ORs below 1 (3 RCTs, 514 participants, very low‐quality evidence). The interval for Pook 2005 suggested an advantage of the intervention of indeterminate size. The intervals for the remaining studies were consistent with substantial effects in either direction (Haemmerli 2010; Pook 2005). In the Haemmerli study (Haemmerli 2010), more participants in the control group dropped out. This may be because of the use of a waiting‐list control group.
2.9. Analysis.
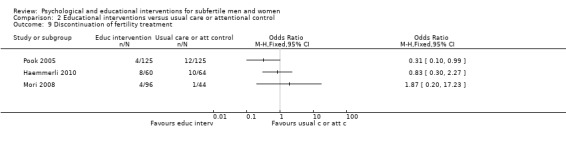
Comparison 2 Educational interventions versus usual care or attentional control, Outcome 9 Discontinuation of fertility treatment.
3 Psychological interventions versus educational interventions
None of the included studies compared psychological interventions with educational interventions.
4 Psychological and educational interventions versus usual care or attentional control
This overall comparison was moot due to the fact that pooling was not done. Different scales were used.
Primary outcomes
Anxiety
Twelve studies revealed no evidence of a difference. Eight studies suggested an advantage from the intervention, and one study suggested a disadvantage from the intervention.
Depression
Eleven studies revealed no evidence of a difference. Three studies suggested an advantage from the intervention, and one study suggested a disadvantage from the intervention.
Live birth or ongoing pregnancy (complete‐case analysis)
Two studies revealed no evidence of a difference. One study suggested an advantage from the intervention.
Findings were similarly uncertain for secondary outcomes, as noted under comparisons one and two.
Discussion
Summary of main results
The overall conclusion of the review is that the effectiveness of psychological and educational interventions for subfertile men and women is uncertain due to heterogeneity and biases in the existing studies.
Performing methodologically sound randomised controlled trials of psychological and educational interventions in subfertile men and women is challenging. Consequently, there is a lack of good‐ or even moderate‐quality evidence on which to base an assessment of the effectiveness of psychological and educational interventions for subfertile men and women. The included studies were diverse with respect to a number of factors, including duration and nature of intervention, choice of control group, and inclusion criteria. We determined that pooling estimates of treatment effect across such clinically heterogeneous studies would not result in a clinically meaningful summary. We have instead adopted a narrative approach and have presented study‐specific estimates of treatment effect in forest plots stratified by relevant study characteristics where appropriate.
Although we have presented the results of each study in this manner, it should be stressed that we do not endorse these as estimates of the effect of the interventions, owing to methodological weaknesses in the trials. We judged all of the included studies with a full text available to be at high risk of bias for at least one domain. Consequently, the results of the studies are likely to misrepresent treatment effects to an unknown degree. We judged three studies to have high risk of bias in just one domain (Cousineau 2008; La Fianza 2014; Pook 2005), owing to concerns about differential loss to follow‐up. However, this is not to say that these studies are ‘less biased’ than the others or that their results are more reliable. Indeed, the results from these studies should be treated with the same degree of caution as studies with more numerous methodological weaknesses. The particular methodological flaws evident in this body of trials are discussed below.
By examining the forest plots relating to the psychological outcomes anxiety and depression, we can note, tentatively, that point estimates in the comparison of psychological interventions versus usual care or attentional control for the outcome anxiety appear to be larger in studies with longer treatment duration (Analysis 1.1). This might be because performance and attrition biases manifest to a greater degree in these studies, exaggerating treatment effects. In support of this conjecture, it can be noted that the studies in the long‐duration subgroup have smaller numbers of included participants in general. In particular, the two large estimates from Domar 2000 were obtained from quite small numbers of participants following substantial attrition. Similar comments can be made in relation to duration and the outcome depression (Analysis 1.4). A visual comparison of the estimated effect sizes in studies with and without an attentional control group would be a way to investigate the impact of performance bias in this review (Analysis 1.3; Analysis 1.6), but in practice there are too few studies of psychological interventions with attentional control groups reporting on the outcomes anxiety and depression for this to be possible. There do not appear to be systematic differences in intervention effects once we stratify according to setting (individual, group, or couple therapy) (Analysis 1.2; Analysis 1.5). The small number of studies of educational interventions included in the forest plots precludes even a tentative description of the results along these lines.
1.3. Analysis.
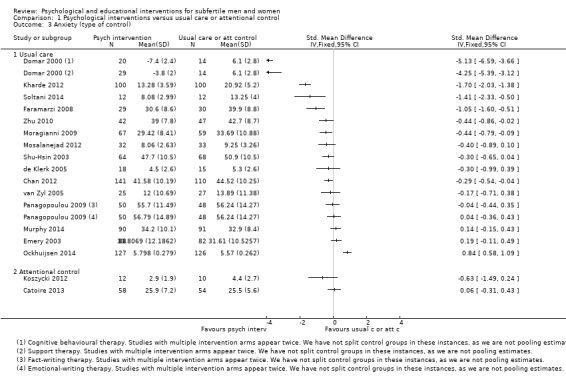
Comparison 1 Psychological interventions versus usual care or attentional control, Outcome 3 Anxiety (type of control).
We are unable to comment on the primary outcome live birth with any confidence, as only two studies of psychological interventions reported this (Catoire 2013; Domar 2000), and both were subject to considerable and unbalanced attrition (particularly Domar 2000). We have presented forest plots of live birth and ongoing pregnancy both under the assumption that participants with missing outcome data were failures (Analysis 1.7) and with a complete‐case analysis (Analysis 1.8). However, neither of these analyses are likely to represent sensible estimates of the treatment effects. Loss to follow‐up in this context is very likely to be informative, which means that the likelihood that a participant exits the study is related to their success probability. In the presence of informative attrition, complete‐case analyses do not preserve the balance over prognostic factors achieved by randomisation. Consequently, such analyses constitute comparisons of improper subgroups of participants rather than genuine randomised comparisons. This is a particular concern when attrition rates are high or imbalanced between treatment arms or both. Percentages of missing data for fertility outcomes are displayed in the following tables, showing both of these characteristics to be present in most of the studies reporting these outcomes.
Missing data for fertility outcomes in trials of psychological interventions
| Intervention rate | Control group rate | |
| Live birth | ||
| Domar 2000 | 13% and 28%* | 60%* |
| Catoire 2013 | 14% | 20% |
| Ongoing pregnancy | ||
| Chan 2006 | 32% | 9% |
| Clinical pregnancy | ||
| Domar 2011 | 34% overall | 34% overall |
| Ockhuijsen 2014 | 28% | 19% |
| Zhu 2010 | Not reported | Not reported |
| Koszycki 2012 | 13% | 38% |
| Murphy 2014 | 10% | 10% |
*Calculated from reported event rates and percentages.
Missing data for fertility outcomes in trials of educational interventions
| Intervention rate | Control group rate | |
| Clinical pregnancy | ||
| Haemmerli 2010 | 28%* | 0%* |
| Mori 2008 | 20% | 4% |
| Takefman 1990 | 0% | 0% |
*Calculated from reported event rates and percentages.
It is possible to present estimates including all randomised participants by assuming that all those for whom outcome data are missing were unsuccessful (Analysis 1.7). This should not be seen as a remedy for high or uneven attrition or both in this case. Participants may drop out of courses of psychological treatment for a variety of reasons. Some participants may abandon treatment because they feel it is hopeless to continue. Others may abandon treatment because they believe they do not need it. Some may even be lost to follow‐up because they have achieved pregnancy. Assuming no chance of pregnancy for any participant who drops out is clearly inappropriate. These problems are compounded in Domar 2000, where a relatively small proportion of participants were undergoing assisted reproductive technology, and live birth was reported by telephone. Moreover, 60% of the control group outcomes were unobserved in this study, with the implication that we must apply the assumption to the majority of these participants. Even a more nuanced method of imputation would be considered suspect in the presence of such a high rate of attrition. Missing data rates for the intervention arms were 13% and 28%, respectively. In combination, these factors result in exaggerated and implausible odds ratios of 10.1 and 7.7 for the interventions in this study. Performing analyses under alternative assumptions for live birth rates in dropouts would be possible. However, while we could concoct any number of just‐so stories to justify a particular assumption, we do not actually know how live birth rates for dropouts would compare to those who were retained in the studies, or whether live birth rates would differ between those who dropped out of an intervention group compared to those who dropped out of a control group. Given high and uneven attrition rates, there would be a risk that we would obtain estimates reflecting our prior expectations rather than the results of the included studies.
It is tempting to compare the live birth estimates from Domar 2000 with those from Catoire 2013, where problems due to level and imbalance of attrition are not so severe. However, the disparity between the trial protocols precludes a meaningful comparison of this sort. The two trials differ in relation to interventions (hypnosis 90 minutes before embryo transfer in one study compared to 2 hours per week for 10 weeks of either cognitive behavioural therapy or meeting in a support group in the other), control groups (diazepam prior to embryo transfer compared to ongoing usual care), and the proportion of participants undergoing assisted reproductive technology (all participants in one study compared to group‐specific rates of between 13% to 20% in the other). This is a clear example of the clinical heterogeneity in this body of trials that we have described.
Similar concerns over attrition undermine estimates of clinical pregnancy, for which we have presented complete‐case analyses (Analysis 1.12; Analysis 2.8). The exception to this is Takefman 1990, which appears to report on clinical pregnancy rates in all randomised participants. This study provides limited evidence: clinical pregnancy rates in both intervention arms were identical, but the small sample size in this study (13 per arm) means that these results are consistent with large effects in either direction.
Discontinuation of fertility treatment was not relevant to all studies: it was both relevant to and reported in eight studies of psychological interventions (Analysis 1.13) and four studies of educational interventions, one of which could not be displayed due to having zero events in either arm (Analysis 2.9). No study of a psychological intervention showed a definitive effect on discontinuation, and with the exception of Chan 2006, all 95% confidence intervals were consistent with substantial effects in either direction. The interval of Chan 2006 differed by suggesting a disadvantage of treatment in this regard, or at most a small advantage. Of the four trials of educational interventions, two were consistent with substantial effects in either direction, and one suggested an advantage of indeterminable magnitude (Pook 2005). This latter study found that male participants were more likely to attend a fertility workup appointment at an andrology clinic if they were sent an information leaflet. The authors noted that the mechanism of action was unclear; although the intention was to alleviate participant concerns about the workup process, the leaflet may actually have worked by reminding participants that the appointment was taking place. The trial with no discontinuations in either arm contributes little to our understanding due to its small sample size (Takefman 1990).
Overall completeness and applicability of evidence
We included 39 studies, all of which sought to address questions relevant to this review. Due to the relatively broad nature of the review topic, trials with disparate study designs were deemed to be applicable. A consequence of this was that the trials were deemed to be too heterogeneous to permit the pooling of results in meta‐analysis. Furthermore, although the study‐specific objectives generally coincided with the (broad) review question, methodological weaknesses cast significant doubt on the reliability of the results.
Notably, only two studies of psychological interventions reported the primary outcome live birth (Catoire 2013; Domar 2000). High and differential rates of attrition in both studies meant that these results could not be taken as valid estimates of intervention effects, leaving no reliable evidence of effects of psychological interventions on live birth. No studies of educational interventions reported on the outcome live birth. Only one study reported the outcome ongoing pregnancy (Chan 2006). In the absence of access to study protocols, it is unclear whether or not the low number of studies reporting this outcome is an indication of within‐study selective reporting.
In light of these considerations, the studies cannot be seen to provide a reliable answer to the review question.
Quality of the evidence
GRADE assessment
We created a 'Summary of findings' table for summary purposes. The different aspects of study quality could not be evaluated using the standard GRADE criteria. We have presented one narrative GRADE assessment for all of our primary outcomes, as we believe that the final assessment is applicable to them all.
Limitations in the design and implementation: We judged most of the included studies to be at high risk of bias for several domains, and every study to be at high risk of bias for at least one domain. The results of the studies are likely to misrepresent treatment effects to an unknown degree, particularly due to performance and attrition biases. We have therefore downgraded the quality of evidence of all outcomes by two levels, from 'high' to 'low'.
Indirectness of evidence: Our review question was broad, with the result that studies with a variety of interventions, comparators, participants, and outcomes were compatible with the inclusion criteria. These studies could all be seen as relevant to our review question, although some studies focused on particular populations, such as people suffering from depression (Faramarzi 2008). We therefore did not downgrade for indirectness, although the lack of studies reporting on live birth or ongoing pregnancy can be noted here.
Heterogeneity and inconsistency of results: Our concerns regarding heterogeneity related to the substantial variation in the protocols of the studies included in the review, so that pooling of data was not judged to be meaningful. This heterogeneity can be seen, in part, as a symptom of the generality of the review question, but it is also indicative of the wide range of different types of psychological interventions that are studied. It is one of the merits of this review that the differences in content of the interventions is taken into account, an aspect that is frequently lacking in other reviews. However, the incommensurability of the studies does prevent us from drawing a unified conclusion. Accordingly, we downgraded from 'low' to 'very low' on this basis.
Imprecision of results: As pooling did not occur, this domain is less applicable to the present case. However, most of the studies had relatively small sample sizes that were probably insufficient for the purposes of demonstrating clinically meaningful effects. We would downgrade on this basis if our assessment was not already that the quality of evidence was 'very low'.
Publication bias: We were able to create funnel plots for the primary outcomes anxiety and depression for the comparison of psychological interventions versus attentional control or usual care (Figure 4; Figure 5). There was some suggestion of publication bias in both of these, although this is obfuscated by the relatively small number of studies shown in the plot. The plot of anxiety scores (Figure 4) is further obfuscated by two SMDs exceeding 4 arising from one study (Domar 2000). These might be explained by biases in this study; the results are based on small numbers of participants because of particularly high rates of attrition. Another large study had a comparatively large SMD (Kharde 2012), although attrition rates in this case are unknown. The impression of publication bias is slightly stronger for the outcome depression, although not definitive (Figure 5). Due to this uncertainty, we would refrain from downgrading further on this basis, although we note that the point is immaterial because the grading has already been dropped to 'very low' on the basis of the previous criteria.
4.
Funnel plot of comparison: 1 Psychological interventions versus usual care or attentional control, outcome: 1.3 Anxiety (type of control).
5.
Funnel plot of comparison: 1 Psychological interventions versus usual care or attentional control, outcome: 1.6 Depression (type of control).
Explanation
We judged all of the included studies with full text to be at high risk of bias in some aspect. Figure 3 shows that the most common concerns were in relation to performance bias for both subjective and objective outcomes, detection bias for subjective outcomes, and attrition bias, each of which were present in over 50% of studies. Accordingly, we downgraded the overall quality of evidence to 'low' on this basis.
Performance bias may occur when participants and personnel are not blinded to the treatment allocation, leading to differences in behaviour both by and towards participants in different treatment arms, obfuscating effects attributable to treatment. In studies of psychological interventions, there may be particular concern that apparent effects of treatment may in fact be a consequence of non‐specific attentional effects. This may be compounded by the difficulty in instantiating blinding for interventions of this nature. The use of an attentional control group may alleviate some of the concern related to performance bias in participants, as this allows for the effects of the intervention over and above these placebo‐like effects to be considered. Five studies used an attentional control group. The use of usual care control groups (24 studies) does not allow for the separation of specific benefits of treatment from attentional effects. We would expect the observed differences in studies using usual care comparator groups to exceed those in studies using attentional control groups, although the small number of studies in the latter category reporting on the review outcomes precluded investigation of this point. It must be recognised that the former type of study is answering a different research question to the latter, and is not appropriate if the interest is in delineating treatment effects as distinct from the general benefits of receiving attention. However, there is likely to be performance bias even when attentional control groups are employed, due to the impossibility of blinding study personnel to the matter of which group is the active treatment and which is the control. We considered both psychological and fertility outcomes to be at risk of performance bias. Indeed, a premise of many of the included studies is that these interventions might lead to improved fertility outcomes; it would appear to follow that non‐specific attentional effects could influence these endpoints. Accordingly, we judged fertility outcomes to be susceptible to performance bias. We therefore consider performance bias to be prevalent in these studies. The impact of this on estimates of treatment effect is unclear, although we would expect it to manifest by exaggerating apparent treatment effects.
The difficulty in blinding studies of psychological interventions is also likely to have resulted in detection bias in most of the trials, whereby the measurement of the outcome is influenced by participant knowledge of the treatment received. As for performance bias, concerns about detection bias might be exacerbated for studies that do not employ an attentional control, but are not eliminated for those that do. We considered psychological and fertility outcomes to be at differential risk of detection bias. Psychological outcomes are subjective, and it is clear that awareness of treatment allocation may influence how these are reported and recorded. Fertility outcomes are somewhat more objective. However, there would still be scope for bias to occur if investigators showed a preference to ascertain pregnancy or live birth outcomes in the treatment group compared to the control group. We therefore ranked those studies with lower rates of outcome reporting in the control group to be at high risk of detection bias for objective outcomes.
Missing outcome data was a major concern in this body of trials, due to high rates of attrition and the fact that the reasons for dropping out of the study were likely to be related to the outcomes. Estimates of treatment effect based only on those participants retained in the study do not reflect randomised comparisons and are likely to be misleading. We have presented forest plots for live birth and ongoing pregnancy both with a complete‐case analysis and with participants for whom outcome data were missing assumed to be failures. As we have explained above, neither of these strategies can be seen as appropriate remedies to the problem of attrition here. In general, we did not find evidence to suggest that the implications of drop‐out for intention‐to‐treat principles were appreciated by the study authors, with an absence of statistical techniques for handling informative missing data. For example, Domar 2000 stated that their Kaplan‐Meier analysis of live birth was valid because they had censored those who were lost to follow‐up. This would only be appropriate if the reasons for loss to follow‐up were completely independent of the probability of success, which is unrealistic (Daya 2005). The direction and size of the bias arising from this is unclear, although clearly the strategy of treating dropouts as failures has resulted in particularly exaggerated effect estimates for Domar 2000, where fertility outcomes could be ascertained for 60% of the control group compared to 13% and 28% of the interventions groups.
Descriptions of methods for concealing the allocation schedule were inadequate in over half of the studies, so that it was unclear if the allocation was genuinely randomised. We judged that these biases could affect the estimates of treatment effect to an unspecified degree.
We further downgraded the assessment to 'very low' on the basis of the heterogeneity of trials, which prevented us from arriving at a clear answer to the review question.
Potential biases in the review process
The search resulted in a collection of disparate trials judged to be eligible for inclusion in the review. We were consequently faced with decisions about how best to summarise this heterogeneous body of studies. This included decisions relating to whether or not choices made prospectively at the protocol stage remained appropriate in light of the studies that were actually identified as eligible for inclusion. We judged that the most appropriate way to summarise the evidence would be to adopt a narrative approach to data synthesis rather than performing meta‐analysis. We have presented study‐specific estimates of treatment effect in forest plots. For the primary outcomes anxiety and depression, these have been grouped (stratified) by relevant study characteristics, three of which were discussed in the protocol under Subgroup Analysis and Investigation of Heterogeneity. It was not possible to carry out grouping by the fourth criterion listed in the protocol, personnel delivering the intervention, for two reasons: few studies reported this and where it was reported, personnel appeared to be rather consistent within our defined comparisons. We additionally made the post‐hoc decision to group studies according to whether they used an attentional control group or a usual care control group. In light of concerns relating to performance and detection bias with interventions of this nature, we considered the failure to distinguish studies with different types of comparator group to represent a serious omission from the review protocol. Despite selecting this as the best way to present the evidence, we found that all studies were at high risk of bias for at least one domain, so that the individual estimates of treatment effect are likely to be misleading. We chose to deal with this point by presenting the study‐specific treatment effects while stressing the high risk of bias throughout the review. This allows for the results of the included studies to be shown while emphasising the fact these results are unreliable.
We acknowledge that the decisions described above are to some extent arbitrary; an alternative team of review authors could have reasonably arrived at different conclusions about how best to summarise the results (specifically regarding decisions about whether or not pooling should be performed and how studies should be grouped). However, we have adopted the approach of carefully explaining the decisions made during the review process and the motivation for these decisions. Most importantly, we believe that the overall conclusion of the review, that there is insufficient evidence to comment on the effectiveness of psychological and educational interventions for subfertile men and women due to heterogeneity and biases in the existing studies, is not affected by these ambiguities.
Agreements and disagreements with other studies or reviews
Three previously published reviews examined the effectiveness of psychological (and educational) interventions for subfertile patients on mental health (and pregnancy rates).
Boivin 2003
Boivin conducted a narrative review on psychological interventions (including psychosocial interventions and educational interventions) in infertility (Boivin 2003). Boivin included 25 studies in their review (of which two studies are included in this review). Half of the interventions showed a positive effect regarding psychosocial and educational interventions on anxiety and depression of infertile patients. Results showed positive effects of psychotherapy on emotional well‐being of infertile patients. Boivin reported that in general, psychological interventions are more successful in reducing negative affect than in influencing interpersonal functioning. Infertility‐specific distress was reduced in all studies (six out of six analyses). The review suggested that educational interventions were more effective than counselling interventions. Pregnancy rates were unlikely to be affected by psychosocial interventions. However, the review included non‐randomised studies and studies with no comparator group. In addition, the authors noted recurring methodological weaknesses in the studies, including concerns over substantial rates of attrition (studies retained 59% of the initially recruited cohort, on average). The authors identified a subset of 11 studies that they considered to be of a higher methodological standard, which they defined as having random allocation (nine studies) or having a controlled pre‐post design (two studies). The authors noted other substantive and systematic methodological flaws, and it is unclear why these were not also used as criteria for defining this subset of supposedly superior studies. The authors marked the studies in this subset so that they could be distinguished in results tables, but decided that pooling of results would not be reasonable. In spite of the differences in inclusion criteria and in the handling of biases, the findings of the review can be seen as broadly consistent with our own. Indeed, Boivin 2003 urge for their findings to be considered in the context of serious concerns regarding study quality and conclude that higher‐quality research is needed. This coincides with our own conclusions.
With regard to the outcomes anxiety and depression, we could say that psychological interventions might reduce feelings of anxiety and depression. Twelve out of 15 studies found a positive effect estimate, and seven of these studies found evidence of an effect of a psychological intervention on anxiety. Eight out of 11 studies found a positive effect estimate, and four of these studies found evidence of an effect of a psychological intervention on depression. However, we have tried to stress that these apparent effects might be attributable to systematic biases in this body of studies.
We examined mental health, including well‐being, distress, and positive and negative affect. While Boivin reported a reduction in distress in all six studies, we have not seen such a convincing result in our review. There was evidence of a reduction in distress in four studies (Domar 2000; Mosalanejad 2012; Shahrestani 2012; Valiani 2010), and no evidence of a reduction in distress in seven studies (Cousineau 2008; Haemmerli 2010; Koszycki 2012; Matthiesen 2012; Panagopoulou 2009; Pook 2005; Skiadas 2011). Again, in those studies that did present an apparent effect, this may be an artefact of bias. We therefore do not share the conclusion that psychological and educational interventions reduce distress. A possible further explanation for the different results could be the generality of our review question, including not only counselling, psychotherapeutic, and cognitive behavioural interventions as psychological interventions, but also body‐mind‐spirit, writing, and online interventions. The same applies to educational interventions. Moveover, the division of interventions into a psychological and an educational group is somewhat arbitrary; Boivin placed Domar 2000 in the educational group, while we have placed this study in the psychological group because of the cognitive behavioural therapy the participants received.
In relation to negative affect, we can draw identical conclusions. In our review there was evidence of a reduction in negative affect in three studies examining a psychological intervention (Chan 2012; de Klerk 2005; Ockhuijsen 2014), while no studies showed no evidence of an effect. However, we emphasise that these results are unreliable due to biases in the studies.
Interpersonal functioning was also covered in our secondary outcomes as part of the outcome social support ‐ relationship satisfaction. Due to different inclusion and exclusion criteria, we included one study examining interpersonal support (Domar 2000), while Boivin included nine studies examining interpersonal support, amongst others Domar 2000. According to Boivin, psychological interventions failed to demonstrate consistent positive effects on interpersonal relationships. In our review we could draw no conclusions regarding interpersonal functioning because of the inclusion of only one study.
Boivin found that pregnancy rates were unlikely to be influenced by psychosocial interventions. In our review ongoing pregnancy rates were examined by only one study (Chan 2006), and this study showed no evidence of an effect of a psychological intervention on ongoing pregnancy. Regarding clinical pregnancy rates, seven included studies in our review found no evidence of an effect of a psychological or an educational intervention on clinical pregnancy. Therefore there seems to be no effect of a psychological or an educational intervention on clinical pregnancy.
De Liz and Strauss 2005
De Liz and Strauss conducted a meta‐analysis of the efficacy of group versus individual and couple psychotherapy in infertile patients (De Liz 2005). Unfortunately, many of the included studies did not supply a comparison group design, and therefore results must be viewed with caution. Results suggest that psychotherapy reduces anxiety and depression in infertile patients similarly in individuals, couples, and groups. Furthermore, psychotherapy possibly enhanced conception success. In our review, we found the same result regarding anxiety and depression: there do not appear to be systematic differences in intervention effects according to anxiety and depression once we stratify according to setting (individual, couple, or group therapy) (Analysis 1.2; Analysis 1.5), but results must be viewed with caution, because trials within each stratum possess quite different features.
While De Liz and Strauss reported a possible effect on conception success, there seems to be no evidence of an effect of a psychological or an educational intervention on clinical pregnancy in our review. Furthermore, we are unable to comment on the primary outcome live birth, as only two studies reported this, and both were subject to considerable attrition.
Haemmerli 2009
Haemmerli conducted a meta‐analysis examining mental health and pregnancy rates in infertile patients undergoing psychological interventions (Hämmerli 2009). The authors did not discuss the fact that pooling studies with such varied designs might not be clinically meaningful, a point we have elected to emphasise. The findings indicated no evidence of an effect of psychological interventions regarding mental health, that is depression and anxiety; interpersonal functioning; and infertility‐specific stress. Haemmerli did report a positive effect of psychological interventions on pregnancy rate (risk ratio 1.42, 99% confidence interval 1.02 to 1.96).
Although the authors did assess the quality of the included studies, presenting relevant design features in a table, they did not incorporate these findings into their interpretation of their analysis, beyond a brief comment that methodological flaws were present in some studies.
We would argue that due to heterogeneity and biases, there is insufficient evidence to state that psychological interventions have a positive effect on mental health including distress. We cannot support the finding of a positive effect of psychological interventions on pregnancy rate because of insufficient methodological quality of studies.
Authors' conclusions
Implications for practice.
The effect of psychological and educational interventions on mental health including distress, and live birth or ongoing pregnancy rates is uncertain due to the very low quality of the evidence.
Implications for research.
This review has highlighted the fact that key methodological principles underlying the design and conduct of randomised controlled trials are not well understood or are otherwise not well implemented in this area, leading us to conclude that the review question cannot be answered using existing research. We would draw attention to particular problematic features and would urge researchers to avoid these deficiencies in future trials through appropriate design and analysis.
High rates of attrition in these trials presented a particular concern. Attrition in this scenario is likely to be informative, in the sense that predictors of drop‐out are prognostic of outcome (Daya 2005; Luke 2013; Troude 2014). Particularly concerning cases of this were those where participants who achieved pregnancy were excluded from psychological outcome assessment. In general, the included studies failed to demonstrate an appreciation of this point, and included only those participants for whom outcome data were available (such analyses were sometimes described as 'intention to treat', even when substantial numbers of participants were excluded). Where the issue of missing data was acknowledged, the methods employed were not adequate to compensate for the fact that censoring was informative (for example baseline values carried forward, use of Kaplan‐Meier analysis). By treating drop‐out as ignorable, researchers are effectively presenting comparisons of improper subgroups of participants, which do not preserve the balance of confounding factors achieved by random allocation to treatment groups. Many of the studies included in this review therefore do not constitute genuine randomised trial evidence. We recommend that in future studies, attempts should be made to minimise missing outcome data by employing end‐of‐study assessments. However, loss to follow‐up and withdrawal are inevitable features of trials in subfertility and of psychological interventions. Where missing outcome data do occur, we urge that appropriate techniques, such as multiple imputation, should be employed (Sterne 2009). Researchers should endeavour to collect psychological outcome assessments even from those participants who become pregnant. Although statistical techniques exist for the estimation of causal effects in the presence of censoring due to a critical event (Rubin 2006; Seuc 2013), their relative complexity will place them beyond the reach of most researchers.
Blinding of investigators and personnel delivering treatment is generally impracticable in this context. However, researchers should employ appropriate attentional control groups in order to reduce performance biases arising from participant knowledge of assignment and to distinguish therapeutic benefit from non‐specific attentional effects. The question of whether or not placebo‐type effects result in improved psychological and fertility outcomes may be valid, but is probably not the question that studies of specific psychological and educational interventions, some of which may be costly, are generally attempting to address. Where there is genuine interest in non‐specific effects of interventions, this might be investigated by including both attentional and usual care comparator groups in a study. The use of a waiting list might constitute an adequate attentional control because the anticipation of future treatment may confer the kind of non‐specific benefits discussed here.
Another point, which has not been discussed in the main text of this review, is the fact that statistical analysis in this context is complicated by the fact that participants may receive treatment in groups, as couples, or from one of several therapists. These features induce clustering effects that must be accounted for at the analysis stage in order to obtain valid confidence intervals and P values (Roberts 2005). Clustering arising due to randomisation at the cluster level (as in a cluster randomised controlled trial) must be similarly taken into account using appropriate statistical methods.
While we appreciate that the techniques referred to here can be challenging to implement, failure to do so will lead to inappropriate conclusions. This in turn may lead to treatment decisions based on spurious findings. It is therefore imperative that future trials of psychological or educational interventions are conducted to a higher methodological standard.
Finally, although few trials reported on the outcome live birth, it is not clear that further trials investigating the impact of interventions on live birth are warranted at this stage. More knowledge would be useful concerning possible working mechanisms explaining the relationship between mental health and the outcome live birth.
Acknowledgements
We would like to thank Jürgen Barth, Katja Hämmerli, and Hansjorg Znoj for their earlier work in developing the protocol and Marian Showell (the Trials Search Co‐ordinator of Cochrane Gynaecology and Fertility Group) for developing a search strategy and running the search. We are also grateful to Helen Nagels (Managing Editor of Cochrane Gynaecology and Fertility Group) and Vanessa Jordan (New Zealand Cochrane fellow) for answering our questions. We would like to thank Vahid Seyfoddin, Mandana Ghodratipour, Qi Chen, Rana Taghipouran, and Mahyar Osanlouy for translating the Persian and Chinese articles. We would like to thank Jane Marjoribanks for her help updating the review.
Appendices
Appendix 1. CGF Specialised Register search strategy
Keywords CONTAINS "IVF" or "in vitro fertilization" or "in‐vitro fertilisation" or "ICSI" or"intracytoplasmic sperm injection" or "Embryo" or "in‐vitro fertilization" or "ART" or "assisted conception" or "assisted reproduction" or "artificial insemination" or "IUI" or "IVF‐ET" or "subfertility" or "Infertility" or Title CONTAINS "IVF" or "in vitro fertilization" or "in‐vitro fertilisation" or "ICSI" or"intracytoplasmic sperm injection" or "Embryo" or "in‐vitro fertilization" or "ART" or "assisted conception" or "assisted reproduction" or "artificial insemination" or "IUI" or "IVF‐ET" or "subfertility" or "Infertility"
AND
Keywords CONTAINS "cognitive behavioral therapy" or "cognitive coping strategies" or "cognitive approaches" or "behavioral coping strategies" or "behavioral therapy" or "therapy group" or "counseling" or "counselling" or "psycho‐educational intervention" or "Psychological" or "Psychological therapies" or "psychological therapy" or "psychosocial therapy" or "Psychotherapy" or "coping strategies" or Title CONTAINS"cognitive behavioral therapy" or "cognitive coping strategies" or "cognitive approaches" or "behavioral coping strategies" or "behavioral therapy" or "therapy group" or "counseling" or "counselling" or "psycho‐educational intervention" or "Psychological" or "Psychological therapies" or "psychological therapy" or "psychosocial therapy" or "Psychotherapy" or "coping strategies" or "education" or "educational intervention" or "decision aid" or "decision making" or "Decision‐making aid" or "pamphlet" or "internet" or "Web‐based decision support" or "website" or "written information" or "booklet"
Appendix 2. CENTRAL search strategy
1 exp decision support techniques/ (1733) 2 (decision$ adj3 support$).tw. (654) 3 (decision$ adj3 aid$).tw. (437) 4 decision board$.tw. (10) 5 (pamphlet$ and decision$).tw. (41) 6 (decision adj2 tool$).tw. (116) 7 written information.tw. (313) 8 (pamphlet$ and decision$).tw. (41) 9 (workbook$ and decision$).tw. (10) 10 education$ booklet$.tw. (140) 11 (video$ and decision$).tw. (216) 12 (compact disc$ and decision$).tw. (1) 13 (cd‐rom and decision$).tw. (12) 14 (web based and decision$).tw. (131) 15 (dvd and decision$).tw. (18) 16 audiocassette.tw. (19) 17 (audiocassette and decision$).tw. (1) 18 (multimedia and decision$).tw. (29) 19 decision$ analysis.tw. (116) 20 (web site$ and decision$).tw. (19) 21 (website$ and decision$).tw. (59) 22 web‐based module.tw. (9) 23 web‐based tool.tw. (29) 24 (interactive and decision$).tw. (147) 25 (audiotape$ and decision$).tw. (19) 26 (decision$ adj2 card$).tw. (24) 27 (booklet$ and decision$).tw. (80) 28 (leaflet$ and decision$).tw. (34) 29 (computer$ and decision$).tw. (518) 30 (software and decision$).tw. (86) 31 (multifaceted and decision$).tw. (28) 32 (smartphone$ and decision$).tw. (4) 33 ((apps or app) and decision$).tw. (4) 34 (multimodal and decision$).tw. (13) 35 (internet and decision$).tw. (82) 36 (pictur$ and decision$).tw. (50) 37 (counse?ling and decision$).tw. (220) 38 (worksheet$ and decision$).tw. (10) 39 (face to face and decision$).tw. (44) 40 (instruction$ and decision$).tw. (120) 41 (interview$ and decision$).tw. (406) 42 exp Psychotherapy/ (13811) 43 Psychotherap$.tw. (3014) 44 Psycho‐therap$.tw. (9) 45 psychosocial.tw. (4592) 46 psychological.tw. (10103) 47 psychodynamic.tw. (347) 48 psychoanaly$.tw. (104) 49 psychiatr$.tw. (7386) 50 exp Counseling/ (2891) 51 counse?ling.tw. (6331) 52 exp Behavior Therapy/ (9352) 53 cognitive.tw. (22427) 54 psychoeducation$.tw. (887) 55 psycho‐education$.tw. (285) 56 education$.tw. (18986) 57 (sex$ adj3 therap$).tw. (380) 58 (cogniti$ adj3 therap$).tw. (5252) 59 problem solving.tw. (1463) 60 (mind? adj3 body program$).tw. (6) 61 mind‐body program$.tw. (6) 62 exp Social Support/ (2139) 63 exp Adaptation, Psychological/ (3716) 64 (coping adj3 strateg$).tw. (606) 65 exp Patient Education as Topic/ (5804) 66 Heidelberg.tw. (870) 67 exp Relaxation Therapy/ (1339) 68 Relax$ Therap$.tw. (214) 69 (internet adj3 support$).tw. (116) 70 self help.tw. (1146) 71 hypnosis.tw. (660) 72 Behavio?r$ Therap$.tw. (4560) 73 cognitive restructuring.tw. (269) 74 emotion$ therap$.tw. (5) 75 emotion$ focus$.tw. (94) 76 exp Stress, Psychological/ (3494) 77 couple$ therap$.tw. (98) 78 Relaxation technique$.tw. (245) 79 exp Yoga/ (276) 80 yoga.tw. (704) 81 (emotion$ adj3 express$).tw. (482) 82 focal counse?ling.tw. (0) 83 exp Psychotherapy, Group/ (2240) 84 Group$ intervention$.tw. (3534) 85 Group therap$.tw. (1276) 86 autogen$ training.tw. (112) 87 telephone.tw. (5798) 88 ((internet adj5 therap$) or (internet adj5 program$)).tw. (630) 89 ((web‐based adj5 therap$) or (web‐based adj5 program$)).tw. (322) 90 (web‐based adj5 support).tw. (83) 91 (online adj5 support).tw. (106) 92 ((online adj5 therap$) or (online adj5 program$)).tw. (306) 93 ((computer adj5 therap$) or (computer adj5 program$)).tw. (991) 94 (computer adj5 support).tw. (155) 95 (distance adj3 therap$).tw. (35) 96 E‐therap$.tw. (78) 97 or/1‐96 (85177) 98 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (1753) 99 embryo transfer$.tw. (1122) 100 vitro fertili?ation.tw. (1557) 101 ivf‐et.tw. (301) 102 ivf.tw. (2356) 103 icsi.tw. (902) 104 intracytoplasmic sperm injection$.tw. (511) 105 (blastocyst adj2 transfer$).tw. (118) 106 exp reproductive techniques, assisted/ or exp insemination, artificial/ or exp ovulation induction/ (2457) 107 assisted reproduct$.tw. (504) 108 artificial insemination.tw. (96) 109 iui.tw. (375) 110 intrauterine insemination$.tw. (482) 111 ovulation induc$.tw. (559) 112 (ovari$ adj2 stimulat$).tw. (953) 113 superovulat$.tw. (153) 114 ovarian hyperstimulation.tw. (649) 115 COH.tw. (152) 116 infertil$.tw. (2283) 117 subfertil$.tw. (185) 118 (ovari$ adj2 induction).tw. (32) 119 (asthenozoospermia or oligospermia or azoospermia).tw. (215) 120 Asthenospermia.tw. (33) 121 Teratospermia.tw. (2) 122 exp Spermatozoa/ (366) 123 Sperm$.tw. (2203) 124 semen.tw. (750) 125 oligoasthenoteratozoospermi$.tw. (16) 126 exp infertility/ or exp infertility, male/ (1663) 127 Infertility, Female/ (929) 128 fertility.tw. (910) 129 childlessness.tw. (6) 130 (desire adj3 child$).tw. (21) 131 child$ wish.tw. (2) 132 or/98‐131 (8122) 133 97 and 132 (336)
Appendix 3. MEDLINE search strategy
1 exp decision support techniques/ (62205) 2 (decision$ adj3 support$).tw. (12632) 3 (decision$ adj3 aid$).tw. (3964) 4 decision board$.tw. (39) 5 (pamphlet$ and decision$).tw. (101) 6 (decision adj2 tool$).tw. (2460) 7 written information.tw. (1359) 8 (pamphlet$ and decision$).tw. (101) 9 (workbook$ and decision$).tw. (23) 10 education$ booklet$.tw. (238) 11 (video$ and decision$).tw. (1888) 12 (compact disc$ and decision$).tw. (12) 13 (cd‐rom and decision$).tw. (67) 14 (web based and decision$).tw. (1332) 15 (dvd and decision$).tw. (38) 16 audiocassette.tw. (36) 17 (audiocassette and decision$).tw. (2) 18 (multimedia and decision$).tw. (233) 19 decision$ analysis.tw. (3651) 20 (web site$ and decision$).tw. (347) 21 (website$ and decision$).tw. (812) 22 web‐based module.tw. (34) 23 web‐based tool.tw. (809) 24 (interactive and decision$).tw. (1656) 25 (audiotape$ and decision$).tw. (345) 26 (decision$ adj2 card$).tw. (239) 27 (booklet$ and decision$).tw. (200) 28 (leaflet$ and decision$).tw. (278) 29 (computer$ and decision$).tw. (8639) 30 (software and decision$).tw. (3183) 31 (multifaceted and decision$).tw. (487) 32 (smartphone$ and decision$).tw. (98) 33 ((apps or app) and decision$).tw. (98) 34 (multimodal and decision$).tw. (415) 35 (internet and decision$).tw. (1874) 36 (pictur$ and decision$).tw. (1685) 37 (counse?ling and decision$).tw. (4011) 38 (worksheet$ and decision$).tw. (51) 39 (face to face and decision$).tw. (797) 40 (instruction$ and decision$).tw. (1655) 41 (interview$ and decision$).tw. (13851) 42 exp Psychotherapy/ (153017) 43 Psychotherap$.tw. (32747) 44 Psycho‐therap$.tw. (89) 45 psychosocial.tw. (62832) 46 psychological.tw. (137973) 47 psychodynamic.tw. (4252) 48 psychoanaly$.tw. (12180) 49 psychiatr$.tw. (181308) 50 exp Counseling/ (33891) 51 counse?ling.tw. (63088) 52 exp Behavior Therapy/ (54114) 53 cognitive.tw. (210601) 54 psychoeducation$.tw. (2788) 55 psycho‐education$.tw. (844) 56 education$.tw. (363002) 57 (sex$ adj3 therap$).tw. (2541) 58 (cogniti$ adj3 therap$).tw. (12667) 59 problem solving.tw. (12686) 60 (mind? adj3 body program$).tw. (12) 61 mind‐body program$.tw. (12) 62 exp Social Support/ (52979) 63 exp Adaptation, Psychological/ (103435) 64 (coping adj3 strateg$).tw. (9250) 65 exp Patient Education as Topic/ (71541) 66 Heidelberg.tw. (4071) 67 exp Relaxation Therapy/ (7296) 68 Relax$ Therap$.tw. (556) 69 (internet adj3 support$).tw. (486) 70 self help.tw. (4787) 71 hypnosis.tw. (6225) 72 Behavio?r$ Therap$.tw. (13261) 73 cognitive restructuring.tw. (590) 74 emotion$ therap$.tw. (21) 75 emotion$ focus$.tw. (1002) 76 exp Stress, Psychological/ (95281) 77 couple$ therap$.tw. (465) 78 Relaxation technique$.tw. (1080) 79 exp Yoga/ (1627) 80 yoga.tw. (2248) 81 (emotion$ adj3 express$).tw. (6418) 82 focal counse?ling.tw. (1) 83 exp Psychotherapy, Group/ (23037) 84 Group$ intervention$.tw. (2151) 85 Group therap$.tw. (3833) 86 autogen$ training.tw. (627) 87 telephone.tw. (41778) 88 ((internet adj5 therap$) or (internet adj5 program$)).tw. (1497) 89 ((web‐based adj5 therap$) or (web‐based adj5 program$)).tw. (1194) 90 (web‐based adj5 support).tw. (402) 91 (online adj5 support).tw. (855) 92 ((online adj5 therap$) or (online adj5 program$)).tw. (1458) 93 ((computer adj5 therap$) or (computer adj5 program$)).tw. (16337) 94 (computer adj5 support).tw. (1397) 95 (distance adj3 therap$).tw. (133) 96 E‐therap$.tw. (340) 97 or/1‐96 (1374846) 98 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (33363) 99 embryo transfer$.tw. (8609) 100 vitro fertili?ation.tw. (17570) 101 ivf‐et.tw. (1890) 102 ivf.tw. (17231) 103 icsi.tw. (5818) 104 intracytoplasmic sperm injection$.tw. (5194) 105 (blastocyst adj2 transfer$).tw. (590) 106 exp reproductive techniques, assisted/ or exp insemination, artificial/ or exp ovulation induction/ (54494) 107 assisted reproduct$.tw. (9726) 108 artificial insemination.tw. (5126) 109 iui.tw. (1272) 110 intrauterine insemination$.tw. (1886) 111 ovulation induc$.tw. (3499) 112 (ovari$ adj2 stimulat$).tw. (5130) 113 superovulat$.tw. (2968) 114 ovarian hyperstimulation.tw. (3992) 115 COH.tw. (1187) 116 infertil$.tw. (44444) 117 subfertil$.tw. (3721) 118 (ovari$ adj2 induction).tw. (232) 119 (asthenozoospermia or oligospermia or azoospermia).tw. (5846) 120 Asthenospermia.tw. (279) 121 Teratospermia.tw. (143) 122 exp Spermatozoa/ (56531) 123 Sperm$.tw. (109350) 124 semen.tw. (23020) 125 oligoasthenoteratozoospermi$.tw. (300) 126 exp infertility/ or exp infertility, male/ (54158) 127 Infertility, Female/ (24040) 128 fertility.tw. (56069) 129 childlessness.tw. (547) 130 (desire adj3 child$).tw. (993) 131 child$ wish.tw. (41) 132 or/98‐131 (249050) 133 randomized controlled trial.pt. (388473) 134 controlled clinical trial.pt. (88931) 135 randomized.ab. (313272) 136 randomised.ab. (62198) 137 placebo.tw. (163881) 138 clinical trials as topic.sh. (171664) 139 randomly.ab. (226562) 140 trial.ti. (134997) 141 (crossover or cross‐over or cross over).tw. (63172) 142 or/133‐141 (987675) 143 exp animals/ not humans.sh. (4007251) 144 142 not 143 (910515) 145 97 and 132 and 144 (535)
Appendix 4. EMBASE search strategy
1 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (55063) 2 embryo$ transfer$.tw. (13430) 3 in vitro fertili?ation.tw. (21463) 4 icsi.tw. (10190) 5 intracytoplasmic sperm injection$.tw. (6619) 6 (blastocyst adj2 transfer$).tw. (1175) 7 ivf.tw. (26121) 8 exp infertility therapy/ or exp artificial insemination/ or exp intrauterine insemination/ or exp ovulation induction/ (80649) 9 assisted reproduct$.tw. (14029) 10 artificial insemination.tw. (4837) 11 iui.tw. (2082) 12 intrauterine insemination$.tw. (2606) 13 ovulation induc$.tw. (4455) 14 (ovari$ adj2 stimulat$).tw. (7371) 15 superovulat$.tw. (3120) 16 ovarian hyperstimulation.tw. (5466) 17 COH.tw. (1586) 18 infertil$.tw. (57356) 19 subfertil$.tw. (4588) 20 (ovari$ adj2 induction).tw. (281) 21 infertility/ or female infertility/ or male infertility/ (72646) 22 childlessness.tw. (620) 23 (desir$ adj3 child$).tw. (1766) 24 child wish.tw. (63) 25 or/1‐24 (158980) 26 exp psychotherapy/ (187167) 27 Psychotherap$.tw. (45484) 28 Psycho‐therap$.tw. (146) 29 psychosocial.tw. (81376) 30 psychological.tw. (199956) 31 exp "psychological and psychiatric procedures"/ or exp psychological well being/ (1153830) 32 exp psychodynamics/ (155291) 33 psychodynamic$.tw. (7390) 34 psychoanaly$.tw. (17196) 35 exp psychoanalysis/ (34223) 36 exp social psychiatry/ or exp psychiatry/ (106838) 37 psychiatr$.tw. (241466) 38 exp counseling/ or exp sexual counseling/ (111518) 39 counse?ling.tw. (81338) 40 exp behavior therapy/ or exp cognitive therapy/ (62131) 41 behavio?r$ therap$.tw. (18967) 42 cognitive therap$.tw. (3347) 43 exp psychoeducation/ or exp coping behavior/ (43494) 44 psychoeducation.tw. (2203) 45 coping behavio?r$.tw. (1697) 46 psycho‐education.tw. (670) 47 education$.tw. (450367) 48 exp patient education/ (89218) 49 (sex$ adj3 therap$).tw. (3681) 50 (mind adj2 body program$).tw. (26) 51 (coping adj3 strateg$).tw. (12261) 52 Heidelberg.tw. (34457) 53 exp relaxation training/ (8655) 54 relaxation therap$.tw. (698) 55 relaxation training.tw. (1451) 56 (internet adj3 support).tw. (486) 57 (internet adj3 program$).tw. (907) 58 (web adj3 support).tw. (449) 59 (web adj3 program$).tw. (1361) 60 (online adj3 program$).tw. (1230) 61 (online adj3 support).tw. (874) 62 self help.tw. (5973) 63 hypnosis.tw. (7350) 64 cognitive restructuring.tw. (942) 65 emotion$ therap$.tw. (35) 66 emotion$ focus$.tw. (1247) 67 couples therap$.tw. (289) 68 relaxation techniques.tw. (1153) 69 exp yoga/ (4318) 70 yoga.tw. (3135) 71 focal counse?ling.tw. (1) 72 exp group therapy/ (16478) 73 Group therap$.tw. (5003) 74 autogen$ training.tw. (769) 75 telephone.tw. (53285) 76 group intervention$.tw. (3364) 77 or/26‐76 (2003675) 78 Clinical Trial/ (840051) 79 Randomized Controlled Trial/ (364025) 80 exp randomization/ (65421) 81 Single Blind Procedure/ (19752) 82 Double Blind Procedure/ (118675) 83 Crossover Procedure/ (41969) 84 Placebo/ (253071) 85 Randomi?ed controlled trial$.tw. (112094) 86 Rct.tw. (16332) 87 random allocation.tw. (1383) 88 randomly allocated.tw. (21824) 89 allocated randomly.tw. (1999) 90 (allocated adj2 random).tw. (719) 91 Single blind$.tw. (15402) 92 Double blind$.tw. (148182) 93 ((treble or triple) adj blind$).tw. (430) 94 placebo$.tw. (210158) 95 prospective study/ (281006) 96 or/78‐95 (1434583) 97 case study/ (30835) 98 case report.tw. (276505) 99 abstract report/ or letter/ (917017) 100 or/97‐99 (1218220) 101 96 not 100 (1395798) 102 25 and 77 and 101 (1087)
Appendix 5. PsycINFO search strategy
1 exp Infertility/ or exp Reproductive Technology/ (2743) 2 (infertil$ or subfertil$).tw. (2673) 3 vitro fertili?ation.tw. (569) 4 (IVF or ICSI).tw. (428) 5 intracytoplasmic sperm injection$.tw. (42) 6 artificial insemination.tw. (227) 7 intrauterine insemination.tw. (18) 8 childlessness.tw. (505) 9 fertility.tw. (5403) 10 or/1‐9 (8879) 11 exp Analytical Psychotherapy/ or exp Individual Psychotherapy/ or exp Interpersonal Psychotherapy/ or exp Psychodynamic Psychotherapy/ or exp Expressive Psychotherapy/ or exp Supportive Psychotherapy/ or exp Psychotherapy/ or exp Group Psychotherapy/ (182486) 12 Psychotherap$.tw. (103298) 13 psychodynamic$.tw. (20036) 14 psychoeducation.tw. (2941) 15 Psycho‐therap$.tw. (210) 16 sex$ therap$.tw. (1652) 17 exp Sex Therapy/ (1811) 18 counse?ling.tw. (73261) 19 exp Counseling/ or exp Group Counseling/ or exp Psychotherapeutic Counseling/ or exp Educational Counseling/ (68075) 20 exp Behavior Therapy/ (17343) 21 behavio?r$ therap$.tw. (26189) 22 (coping adj3 strateg$).tw. (16744) 23 coping behavio?r$.tw. (8092) 24 exp Coping Behavior/ (39233) 25 educational program$.tw. (9829) 26 exp Relaxation Therapy/ (3348) 27 relaxation technique$.tw. (998) 28 relaxation therap$.tw. (689) 29 internet support.tw. (99) 30 internet based.tw. (3395) 31 exp Emotion Focused Therapy/ or exp Marriage Counseling/ or exp Couples Therapy/ (8045) 32 couple$ therap$.tw. (3505) 33 exp Cognitive Therapy/ or exp Cognitive Behavior Therapy/ (24661) 34 CBT.tw. (8627) 35 emotion$ therap$.tw. (66) 36 (emotion$ adj3 express$).tw. (13819) 37 Group$ intervention$.tw. (3470) 38 Group therap$.tw. (12459) 39 autogen$ training.tw. (880) 40 telephone.tw. (18885) 41 (decision$ adj3 support$).tw. (4847) 42 (decision$ adj3 aid$).tw. (1576) 43 decision board$.tw. (14) 44 (pamphlet$ and decision$).tw. (64) 45 (decision adj2 tool$).tw. (653) 46 written information.tw. (488) 47 (pamphlet$ and decision$).tw. (64) 48 (workbook$ and decision$).tw. (95) 49 education$ booklet$.tw. (79) 50 (video$ and decision$).tw. (1943) 51 (compact disc$ and decision$).tw. (8) 52 (cd‐rom and decision$).tw. (48) 53 (web based and decision$).tw. (758) 54 (dvd and decision$).tw. (63) 55 audiocassette.tw. (42) 56 (audiocassette and decision$).tw. (1) 57 (multimedia and decision$).tw. (195) 58 decision$ analysis.tw. (730) 59 (web site$ and decision$).tw. (278) 60 (website$ and decision$).tw. (603) 61 web‐based module.tw. (27) 62 web‐based tool.tw. (115) 63 (interactive and decision$).tw. (1817) 64 (audiotape$ and decision$).tw. (279) 65 (decision$ adj2 card$).tw. (51) 66 (booklet$ and decision$).tw. (126) 67 (leaflet$ and decision$).tw. (54) 68 (computer$ and decision$).tw. (5179) 69 (software and decision$).tw. (1381) 70 (multifaceted and decision$).tw. (403) 71 (smartphone$ and decision$).tw. (34) 72 ((apps or app) and decision$).tw. (212) 73 (multimodal and decision$).tw. (224) 74 (internet and decision$).tw. (1724) 75 (pictur$ and decision$).tw. (1764) 76 (counse?ling and decision$).tw. (3843) 77 (worksheet$ and decision$).tw. (126) 78 (face to face and decision$).tw. (934) 79 (instruction$ and decision$).tw. (4783) 80 (interview$ and decision$).tw. (15457) 81 exp Decision Making/ or exp Decision Support Systems/ (72354) 82 (internet adj5 therap$).tw. (546) 83 (internet adj5 support$).tw. (897) 84 (internet adj5 program$).tw. (884) 85 (web‐based adj5 support).tw. (292) 86 (web‐based adj5 therap$).tw. (107) 87 (web‐based adj5 program$).tw. (614) 88 (online adj5 support).tw. (1311) 89 (online adj5 therap$).tw. (419) 90 (online adj5 program$).tw. (1444) 91 (computer based adj3 mediated).tw. (5) 92 (distance adj3 therap$).tw. (136) 93 E‐therap$.tw. (141) 94 support therap$.tw. (160) 95 or/11‐94 (494510) 96 10 and 95 (1540) 97 random.tw. (43148) 98 control.tw. (335052) 99 double‐blind.tw. (18704) 100 clinical trials/ (8494) 101 placebo/ (4024) 102 exp Treatment/ (609826) 103 or/97‐102 (934789) 104 96 and 103 (665)
Appendix 6. CINAHL search strategy
| # | Query | Results |
| S51 | S35 AND S49 | 97 |
| S50 | S35 AND S49 | 722 |
| S49 | S36 OR S37 or S38 or S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 | 951,591 |
| S48 | TX allocat* random* | 4,230 |
| S47 | (MH "Quantitative Studies") | 13,240 |
| S46 | (MH "Placebos") | 9,158 |
| S45 | TX placebo* | 33,549 |
| S44 | TX random* allocat* | 4,230 |
| S43 | (MH "Random Assignment") | 38,924 |
| S42 | TX randomi* control* trial* | 85,379 |
| S41 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 761,293 |
| S40 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 114 |
| S39 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 0 |
| S38 | TX clinic* n1 trial* | 170,610 |
| S37 | PT Clinical trial | 77,615 |
| S36 | (MH "Clinical Trials+") | 185,604 |
| S35 | S11 AND S34 | 2,775 |
| S34 | S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 | 794,413 |
| S33 | TX online support | 730 |
| S32 | TX e‐therap* | 1,211 |
| S31 | TX decision aid* | 1,369 |
| S30 | TX distance therap* | 99 |
| S29 | TX web‐based instruction | 94 |
| S28 | (MH "Decision Support Systems, Clinical") OR (MH "Support, Psychosocial") | 47,699 |
| S27 | TX education | 548,880 |
| S26 | (MH "Stress Management") OR "stress management" OR (MM "Stress, Psychological") | 21,803 |
| S25 | TX relaxation techniques | 2,988 |
| S24 | TX CBT | 2,163 |
| S23 | TX psychoeducation* | 3,182 |
| S22 | TX hypnosis | 2,790 |
| S21 | TX psychologic* | 161,794 |
| S20 | (MH "Adaptation, Psychological") OR (MH "Psychology, Educational") OR (MH "Psychology, Clinical") | 21,758 |
| S19 | TX emotion* expression | 808 |
| S18 | TX internet based support | 141 |
| S17 | TX Counselling | 11,768 |
| S16 | (MH "Counseling") OR "counseling" OR (MH "Couples Counseling") OR (MH "Sexual Counseling") | 34,808 |
| S15 | TX psychotherap* | 29,766 |
| S14 | TX cognitive behavior* therap* | 2,722 |
| S13 | TX Cognitive behavioural Therap* | 1,306 |
| S12 | (MH "Cognitive Therapy") OR (MH "Psychotherapy+") OR "psychotherapy" | 121,172 |
| S11 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 | 13,958 |
| S10 | (MM "Fertility") OR "fertility" | 5,794 |
| S9 | TX IUI | 79 |
| S8 | "intrauterine insemination" | 139 |
| S7 | (MM "Reproduction Techniques") OR "assisted reproductive techniques" | 1,793 |
| S6 | TX ICSI | 249 |
| S5 | TX intracytoplasmic sperm injection* | 232 |
| S4 | (MH "Fertilization in Vitro") OR "Ivf" | 2,811 |
| S3 | TX Subfertil* | 403 |
| S2 | TX infertil* | 7,356 |
| S1 | (MM "Infertility") | 3,688 |
Appendix 7. AMED search strategy
1 exp Infertility female/ or exp Infertility male/ (248) 2 (infertil$ or subfertil$).tw. (332) 3 vitro fertili?ation.tw. (33) 4 (ivf or ICSI).tw. (39) 5 intracytoplasmic sperm injection$.tw. (11) 6 fertility.tw. (204) 7 or/1‐6 (499) 8 exp Stress psychological/ or exp Psychometrics/ or exp Psychology/ (22033) 9 psychological.tw. (11019) 10 cognitive behavio?r therapy.tw. (179) 11 CBT.tw. (205) 12 exp Psychotherapy/ (8713) 13 Psychotherap$.tw. (2309) 14 counse?ling.tw. (2530) 15 exp Counseling/ (1756) 16 exp Patient education/ (1718) 17 education.tw. (17808) 18 mind body program$.tw. (3) 19 relaxation technique$.tw. (140) 20 relaxation therap$.tw. (83) 21 exp Relaxation/ or exp Psychosomatic therapies/ (6611) 22 sex$ therap$.tw. (17) 23 couple$ therap$.tw. (14) 24 self help.tw. (483) 25 (emotion$ adj3 express$).tw. (211) 26 emotion$ therap$.tw. (6) 27 psychological.tw. (11019) 28 or/8‐27 (53483) 29 7 and 28 (60)
Data and analyses
Comparison 1. Psychological interventions versus usual care or attentional control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anxiety (length of treatment) | 17 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Short duration of treatment (<3 weeks) | 7 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Long duration of treatment (≥3 weeks) | 8 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Unknown duration of treatment | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Anxiety (therapy setting) | 17 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Individual therapy | 9 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Couple therapy | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Group therapy | 5 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Anxiety (type of control) | 17 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Usual care | 15 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Attentional control | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Depression (length of treatment) | 12 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Short duration of treatment (<3 weeks) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Long duration of treatment (≥3 weeks) | 7 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Unknown duration of treatment | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Depression (therapy setting) | 12 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Individual therapy | 5 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Couple therapy | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Group therapy | 4 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Depression (type of control) | 12 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Usual care | 11 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Attentional control | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Live birth or ongoing pregnancy (missing data assumed to be failures) | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Live birth or ongoing pregnancy (complete‐case analysis) | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Distress and well‐being | 14 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 General quality of life | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Social support | 5 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Clinical pregnancy rates (complete‐case analysis) | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13 Discontinuation of fertility treatment | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Educational interventions versus usual care or attentional control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anxiety (length of treatment) | 4 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Short duration of treatment (<3 weeks) | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Long duration of treatment (≥3 weeks) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Unknown duration of treatment | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Anxiety (therapy setting) | 4 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Individual therapy | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Couple therapy | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Group therapy | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Anxiety (type of control) | 4 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Usual care | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Attentional control | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Depression (length of treatment) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Short duration of treatment (<3 weeks) | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Long duration of treatment (≥3 weeks) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Unknown duration of treatment | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Depression (therapy setting) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Individual therapy | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Couple therapy | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Group therapy | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Depression (type of control) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Usual care | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Attentional control | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Distress and well‐being | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Clinical pregnancy rate (complete‐case analysis) | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Discontinuation of fertility treatment | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
2.2. Analysis.
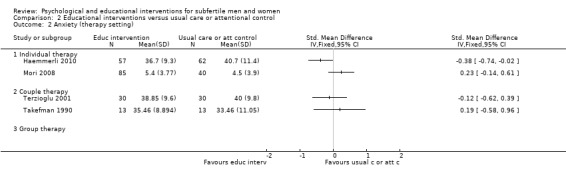
Comparison 2 Educational interventions versus usual care or attentional control, Outcome 2 Anxiety (therapy setting).
2.4. Analysis.
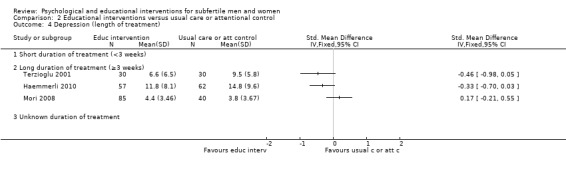
Comparison 2 Educational interventions versus usual care or attentional control, Outcome 4 Depression (length of treatment).
2.5. Analysis.
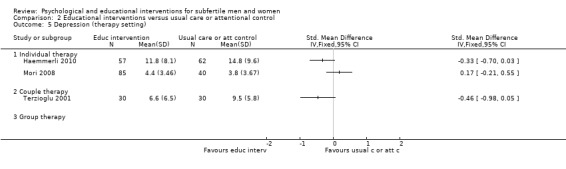
Comparison 2 Educational interventions versus usual care or attentional control, Outcome 5 Depression (therapy setting).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arslan‐Ozkan 2013.
| Methods | Randomised controlled trial | |
| Participants | Married infertile women at the University hospital, infertility centre in Antalya, Turkey. The women were between 18 and 45 years old, were diagnosed with primary infertility, and were able to speak, read, and write in Turkish. Exclusion criteria were: secondary infertility, diagnosed with a chronic disease, under the age of 18 or above 45. 120 women were randomised into the intervention group (n = 60) and the control group (n = 60) |
|
| Interventions | The women in the intervention group received 45 to 90 minutes of Watson's nursing care programme including counselling, relaxation exercises, a music CD, a booklet, and a back massage. The attentional control group received usual care, including regular interviews. Following the final test, the control group received relaxation exercises, a music CD, and a booklet. The interventions were delivered during fertility treatment, individually, face to face, by a nurse | |
| Outcomes | Distress (measured by Infertility Distress Scale) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation was performed by a statistician using SAS version 8.2 (SAS Institute 2001) |
| Allocation concealment (selection bias) | Low risk | A sealed envelope method was used. Women were blinded to treatment allocation |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Participants were blinded, attentional control group. Personnel not blinded. Personnel could have influenced the participants |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | The investigator was not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rate was 12.5% (15/120 were lost to follow‐up). Last observation carried forward method was used, but hard to see how this would apply to pretest and post‐test data There was no significant difference between the intervention and control group in terms of attrition |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | Low risk | No other obvious sources of bias |
Catoire 2013.
| Methods | Randomised controlled trial | |
| Participants | Women included in an IVF procedure in a French medically assisted procreation center, aged between 18 and 38 years and having their first embryo transfer. Excluded were women with a psychiatric disorder, who used sedatives or tranquilisers, who had uterus malformation, who had a contraindication to pregnancy, who had donor oocytes, or who had frozen oocytes. Mean (SD) age was 30.3 (3.6) years in the intervention group and 32.0 (3.5) years in the control group. 112 women were randomised into the intervention group (n = 58) or the control group (n = 54) | |
| Interventions | The intervention group received 20 to 30 minutes of hypnosis plus lactose (placebo). The attentional control group received 20 to 30 minutes of muscle relaxation therapy (placebo) plus diazepam (usual care). These interventions were delivered during fertility treatment, individually, face to face, by a hypnotist | |
| Outcomes | Pregnancy and live birth rate measured 10 months post‐embryo transfer, state anxiety (measured pre‐ and post‐embryo transfer by STAI) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Used a randomisation table to separate the patients into two groups" |
| Allocation concealment (selection bias) | Low risk | "The patients, biologists, gynaecologists in charge of the patients were unaware of the randomization" |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Investigators have done a good job of blinding women, biologists, and gynaecologists, using a double‐dummy design. Only the hypnotist was unblinded |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Investigators have done a good job of blinding women, biologists, and gynaecologists, using a double‐dummy design. Only the hypnotist was unblinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Self reported anxiety score; participants and personnel were adequately blinded. Biologist who collected the information was blind to the allocation |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Participants and personnel were adequately blinded. Biologist who collected the information was blind to the allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Numbers of women excluded from final analysis were high; 20% in diazepam group and 14% in hypnosis group. No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Anxiety measured with a numeric scale; pain, relaxation, and satisfaction were measured, but are not presented in the study. Authors only report state anxiety (and not trait anxiety) from STAI. No reasons are given, and there is no indication as to whether this decision was made in advance or was post‐hoc |
| Other bias | Low risk | No other obvious sources of bias. A validated questionnaire was used for the primary outcome |
Chan 2006.
| Methods | Randomised controlled trial | |
| Participants | Women who attended the Assisted Reproduction unit in Hong Kong for the first cycle of IVF. The women were primary or secondary subfertile. Mean (SD) age of the subfertile women was 36.0 (3.28) years in the intervention group and 35.0 (3.49) years in the control group. The duration of subfertility was 5.0 (2.0 to 11.0) years in the intervention group and 5.0 (1.0 to 15.0) years in the control group. 227 women were randomised into the intervention group (n = 101) and into the control group (n = 126) | |
| Interventions | The intervention group received the EBMS (Eastern Body‐Mind‐Spirit) approach, including mini‐lectures on traditional Chinese medicine, stress‐reduction training, activities, and reading materials for 12 hours. The intervention consisted of group therapy, delivered before fertility treatment, both face to face and written information, delivered by Chan, an experienced practitioner of EMBS. The control group received no intervention |
|
| Outcomes | Clinical pregnancy, ongoing pregnancy (measured at 8 to 10 weeks gestation), anxiety (measured by C‐STAI), childbearing importance (self and marriage, measured by Childbearing Importance Index). Measured during recruitment (T1), on the first day of ovarian simulation (T2), and on the day of ET (T3) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization process was performed according to well‐established guidelines." "Drawing lots achieved randomization." |
| Allocation concealment (selection bias) | Unclear risk | “Participants were notified of their group assignment individually.” However, the researchers could have known the allocation sequence |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding of participants and personnel. Subjective outcomes are likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No blinding of participants and personnel. Pregnancy rates could be influenced |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Outcome assessor not blinded, may affect reporting of subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Assessor not blinded, but fewer women missing from control group. Greater attempts of intervention group follow‐up than of control group follow up, which could lead to bias, will be unlikely in this case |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal > 20% and not balanced between groups. No intention‐to‐treat analysis, only reported on those who did not drop out of the intervention |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to protocol |
| Other bias | Low risk | No other obvious sources of bias. A validated questionnaire was used for the primary outcome |
Chan 2012.
| Methods | Randomised controlled trial | |
| Participants | Women who were subfertile and beginning their first IVF cycle were included. Exclusion criteria were: having a psychiatric disorder, receiving psychiatric medication, receiving psychosocial treatment, serious marital discord, psychotic features or suicidal ideation. 339 women were randomised into the intervention group (n = 172) or the control group (n = 167). In the intervention group, 108 women were primary subfertile and 33 women were secondary subfertile. In the control group, 80 women were primary subfertile and 30 women were secondary subfertile. Mean (SD) age was 34.51 (3.42) years in the intervention group and 34.32 (3.09) years in the control group. Mean duration of subfertility was 5 (range 2 to 15) years in the intervention group and 5 (range 1 to 16) years in the control group | |
| Interventions | The intervention group received I‐BMS: psycho‐educational group counselling addressing physical health, psychosocial well‐being, and spiritual well‐being. The face‐to‐face counselling was delivered by the authors (counsellors) before fertility treatment and lasted 12 hours total. The control group received no intervention | |
| Outcomes | State and trait anxiety (measured by C‐STAI), marital satisfaction (measured by Chinese Kansas Marital Satisfaction Scale), importance of childbearing (measured by Childbearing Importance Index), and physical distress, daily functioning, positive and negative affect, and spirituality (measured by Body‐Mind‐Spirit Well‐Being Inventory). Ongoing pregnancy rates were measured (at 8 to 10 weeks gestation). The psychological outcomes were measured at baseline (T0), on the day of starting ovarian stimulation (T1), and on the day undertaking embryo transfer (T2) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Study participants were then randomly assigned into one of two groups” but method unclear |
| Allocation concealment (selection bias) | High risk | “by drawing lots performed by the researcher” |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding of participants and personnel, subjective outcomes likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No blinding of participants and personnel, objective outcomes could be influenced |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | High risk for blinding of outcome assessor because the woman is the outcome assessor and is not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | There is more loss to follow‐up in the control group, possibly because of less enthusiasm for following up women in the control group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 31 women of the intervention group (18%), 57 women of the control group (34.1%) withdrew (total 26%). There was a significantly higher drop‐out rate in the control group (P < 0.001). They investigated why: dropouts had a shorter duration of marriage, reported lower child importance and higher baseline marital satisfaction |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | Low risk | No other obvious sources of bias. A validated questionnaire was used for the primary outcome |
Choobforoushzade 2011.
| Methods | Randomised controlled trial | |
| Participants | 24 women were selected through an interview by a specialist from 214 women in the gynaecology clinic in Yazd city. The women were 25 to 35 years old, had finished at least high school, and were 1 to 6 years subfertile. Women were excluded if they had a psychological condition. 24 women were randomised into the experimental group (n = 12) and into the control group (n = 12) |
|
| Interventions | The experimental group received individual cognitive therapy and stress control therapy (face to face) for 20 hours, delivered by the researcher. The control group did not receive any additional intervention |
|
| Outcomes | Quality of life (measured by WHOQOL questionnaire). Measured at baseline, at 10 weeks (after treatment), and at 14 weeks (follow‐up) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding of participants and personnel. Subjective outcomes are likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | High risk for blinding of outcome assessor because the woman is the outcome assessor and is not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There seem to be no withdrawals, no exclusions. However, not stated if there was any attrition |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to protocol |
| Other bias | Unclear risk | We used a translator for this Persian article and therefore could have missed other sources of bias |
Conrad 2013.
| Methods | Randomised controlled trial | |
| Participants | Subfertile men between 18 and 55 years of age with a pathological spermiogram were included. 56 men were randomised | |
| Interventions | The intervention group wrote on 3 days for 20 minutes about highly emotional topics. The control group wrote about neutral topics (attentional control) | |
| Outcomes | Distress (measured by Infertility Distress Scale), infertility‐related thoughts of helplessness, sexual satisfaction (measured by Temperament and Character Inventory). Measured at 3 months |
|
| Notes | Only abstract is available. Results expressed as "d" ‐ presumably Cohen's d, whereby 0.2 = small effect, 0.5 = medium effect, 0.8 = large effect | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "Patients were randomly allocated to two treatment conditions". Methods not further described |
| Allocation concealment (selection bias) | Unclear risk | States "Patients were randomly allocated to two treatment conditions". Methods not further described |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | No mention of blinding. Control group had a placebo intervention |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No mention of blinding. Control group had a placebo intervention |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated whether there were any dropouts |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | Unclear risk | Very few details about methods, as only abstract is available. No data suitable for analysis |
Cousineau 2008.
| Methods | Randomised controlled trial | |
| Participants | Female fertility patients from 3 fertility centers in west and mid USA who were both primary and secondary subfertile, had access to a computer with Internet, were married or in cohabitation, and were not currently involved in a professionally led informative support group or workshop. The mean age was 34 years | |
| Interventions | Solomon four group design: group 1 and 3 viewed an Internet program that seeks to expand the positive potential of individuals toward health. Group 2 and 4 did not view the program. Group 1 was the intervention group, and group 2 was control group. Women viewed 63 minutes (mean), during fertility treatment and individually | |
| Outcomes | Infertility distress (measured by Fertility Problem Inventory), adjustment in relationships (measured by Revised Dyadic Adjustment Scale), negative support (measured by Perceived Negative Support Scale). All measured at pre‐ and post assessment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “were randomised”, ”randomly permutated blocks of size four were used within 2 strata” |
| Allocation concealment (selection bias) | Low risk | “Study research coordinator did allocation, but did not decide which participants were included” |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | “participants were not aware of...” Solomon four group design used |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Women filled in the questionnaires and were blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis not performed, and withdrawals were all from the intervention groups, none from the control groups |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | Low risk | No other obvious sources of bias. Validated questionnaires were used |
Czamanski‐Cohen 2012.
| Methods | Randomised controlled trial | |
| Participants | Primary infertile women undergoing IVF (n = 50). Excluded if: biological children at home, no Hebrew speaking, axis I diagnosis. | |
| Interventions | The intervention group (n = 25) received 5 to 6 cognitive behavioural intervention sessions during fertility treatment. The control group (n = 25) received usual care | |
| Outcomes | Plasma cortisol levels, Perceived Stress Scale, pregnancy rate. Perceived Stress Scale measured before ovarian stimulation, at ovum pick up, and at pregnancy test |
|
| Notes | Only the abstract is available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Those who agreed to participate were provided with a ID number blindly by the nurse according to when they were accepted for treatment at the IVF clinic. We used Research Randomizer software (Urbaniak & Plous, 2009) to randomise the 50 numbers to control and intervention groups |
| Allocation concealment (selection bias) | Low risk | Those who agreed to participate were provided with a ID number blindly by the nurse according to when they were accepted for treatment at the IVF clinic. We used Research Randomizer software (Urbaniak & Plous, 2009) to randomise the 50 numbers to control and intervention groups |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No mention of blinding. Subjective outcomes could be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No mention of blinding. Objective outcomes could be influenced |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No mention of blinding. Subjective outcomes could be influenced |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No mention of blinding, but objective outcomes unlikely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated whether there were any dropouts |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available, pregnancy rates not clearly reported |
| Other bias | Unclear risk | Very few details about methods, as only abstract is available. No data suitable for analysis |
de Klerk 2005.
| Methods | Randomised controlled trial | |
| Participants | Couples admitted to an infertility treatment programme for the first time were included. Inclusion criteria were: indication for IVF treatment, women aged < 41 years, a stable relationship, no severe psychological problems as assessed by a physician. Couples were excluded if they were not able to complete the questionnaires in Dutch. 84 couples with primary or secondary subfertility were randomised into an intervention group (n = 43) or a control group (n = 41). Mean (SD) age of the women in the intervention and control group was 33.4 (4.7) and 33.3 (5.2), respectively. Mean (SD) duration of subfertility in the intervention and control group was 4.0 (1.7) years and 4.3 (3.6) years, respectively | |
| Interventions | The intervention group received 3 face‐to‐face counselling sessions of 1 hour (total 3 hours of couple therapy): 1 week before the first day of ovarian stimulation, 6 to 9 days after embryo transfer, and 2 weeks after the day of the pregnancy test. During fertility treatment, delivered by a social worker who had been trained in experiential psychosocial therapy. The control group received routine care |
|
| Outcomes | Positive affect and negative affect (measured by the Daily Record Keeping (DRK) chart), depression and anxiety (measured by the HADS), and biochemical pregnancy rate. Measured at baseline and 2 weeks after the intervention. DRK was measured at 7 time points (baseline, stimulation, oocyte retrieval, fertilisation, embryo transfer, waiting days, pregnancy test) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The couples were randomised according to a computer‐generated random numbers table into 1 of 2 groups |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding of participants and personnel. Subjective outcomes are likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No blinding of participants and personnel. Objective outcomes could possibly be influenced |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | High risk for blinding of outcome assessor because the participant is the outcome assessor and is not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Assessor not blinded, but fewer participants missing from control group. Greater attempts of intervention group follow‐up than of control group follow up, which could lead to bias, will be unlikely in this case |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Approximately 50% withdrawals, and the analysis included only the remaining participants |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to protocol |
| Other bias | Low risk | No other obvious sources of bias. Validated questionnaires were used for primary outcomes |
Domar 2000.
| Methods | Randomised controlled trial | |
| Participants | Women who had been trying to conceive for 1 to 2 years, both primary and secondary subfertile. Exclusion criteria were: not English speaking, practicing any relaxation technique, participating in any support group or psychotherapy, taking psychotropic medication, being clinically depressed (BDI score above 15, HRSD score above 11, or clinical depression on the clinical interview). 184 women were randomised into the intervention group (n = 56), the support group (n = 65), and the control group (n = 63). Mean (SD) age was 33.96 (4.32) years in the intervention group and 35.19 (4.84) in the control group. Duration of subfertility was 18.68 (3.66) years in the intervention group and 17.44 (3.36) years in the control group |
|
| Interventions | The intervention group received face‐to‐face cognitive behavioural group (8 to 12 participants) therapy for 10 weeks, 2 hours per week pre‐ and during fertility treatment, delivered by experienced group leaders. The support group received a face‐to‐face group session in which the therapist talked about a different topic each week for 10 weeks, 2 hours per week. The control group received usual care | |
| Outcomes | Depression (measured by BDI), anxiety (measured by STAI), distress (measured by POMS), marital distress (measured by MDS), self esteem (measured by RSES), and live birth rate (measured from start until the end of year 1) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to a computer‐generated random numbers table, but according to 2 randomisation procedures: into 1 of 3 groups or into intervention vs control. When recruitment was down, patients were recruited to treatment vs control, with alternation between treatment allocations. Participants were switched between the two intervention groups (but stayed in the original group assignment with analysis (intention to treat)) |
| Allocation concealment (selection bias) | Low risk | During the second randomisation procedure, investigators would know which treatment the participant would receive were they allocated to a treatment group. However, comparisons between each treatment arm and the control arm would still be valid |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Questionnaires: no blinding of participants and personnel, outcomes likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Pregnancy rates: no blinding of participants and personnel, outcomes could be influenced |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Questionnaires are participant‐reported outcomes, so without blinding high risk of bias |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Pregnancy rates: obtained from participant report, outcome could be influenced |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis, but many withdrawals (35% total: 60% of the control group women, 26% of the support group women, and 16% of the intervention group women discontinued). Withdrawal not balanced between groups. Reasons for withdrawal not balanced |
| Selective reporting (reporting bias) | High risk | 5 questionnaires are reported on that were not named in the protocol. Furthermore, although psychological scales were measured at both 6 and 12 months, only those that were significant were reported at 12 |
| Other bias | Low risk | No other obvious sources of bias. Validated questionnaires were used for primary outcomes |
Domar 2011.
| Methods | Randomised controlled trial | |
| Participants | Women of a private practice in Boston who were scheduled to begin their first IVF cycle, who were 40 years old or younger, who had not participated in a mind‐body group before, who had daily access to the Internet, and spoke fluent English. 97 women were randomised into the intervention group (n = 46) and the control group (n = 56). Mean age was 34 years. Duration of subfertility was 2.0 (1.2) years in the intervention group and 2.5 (2.2) in the control group |
|
| Interventions | The intervention group received group mind‐body therapy (face to face) for 10 sessions in 10 weeks (total 28.5 hours) during fertility treatment, delivered by a PhD psychologist. Mind‐body is a stress management program including cognitive behaviour therapy, relaxation training, negative health behaviour modification, and social support components. The control group did not receive therapy during fertility treatment. They did receive a spa gift for each 3‐month period of staying in the study and a bonus 100‐dollar gift if they stayed in for a year | |
| Outcomes | Clinical pregnancy rate (defined as confirmation of foetal heartbeat at 7 weeks' gestation with appropriate crown‐rump length) | |
| Notes | Inclusion criteria (English speaking) and exclusion criteria (lower social economic state) could lead to a different intervention effect | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved through the use of a computer‐generated random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Each participant received a phone call or an email notifying them of their assignment. Not clear if researcher was aware of allocation |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Not applicable |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No blinding of participants, objective outcomes could be influenced. Personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Personnel is the outcome assessor and is blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | > 20% withdrawals (33%), withdrawals were balanced across groups |
| Selective reporting (reporting bias) | Low risk | The outcomes that are named in the WHO trials register are reported |
| Other bias | Low risk | No other obvious sources of bias |
Emery 2003.
| Methods | Randomised controlled trial | |
| Participants | All couples recruited in the IVF programme of the University hospital in Lausanne, Switzerland were eligible if this was their first IVF treatment for their first child (primary subfertility), and if both partners spoke French and lived in Switzerland. Mean (SD) age was 34.4 (4.9) years for men and 32.1 (3.9) years for women. Mean duration of subfertility was 3.8 (2.1) years. 200 participants (100 couples) were randomised into the intervention group A (n = 100) and into the control group B (n = 100) |
|
| Interventions | The intervention group A received 60 to 90 minutes of routine pre‐IVF counselling per couple (face‐to‐face interview) that focused on the narrative capacities of couples. The control group B did not receive counselling | |
| Outcomes | Anxiety (measured by STAI), depression (measured by BDI), and pregnancy rate (measured 14 days after IVF treatment). Anxiety and depression were measured before counselling and 6 weeks after IVF | |
| Notes | Patients who wanted to participate had higher education level than patients who did not want to be in the randomised trial. This could lead to a different intervention effect | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was carried out by a secretary who numbered 50 questionnaires (101 to 125 and 301 to 325 for groups A and B) and put the questionnaires in sealed envelopes and mixed them randomly |
| Allocation concealment (selection bias) | Low risk | The research investigator received the sealed envelopes and handed 1 envelope to every couple who was willing to participate |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding of participants and personnel, subjective outcomes likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Participants and personnel not blinded, objective outcomes could be influenced |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | High risk for blinding of outcome assessor because the participant is the outcome assessor and is not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Assessor not blinded, may affect reporting of objective outcomes because more participants missing from control group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals are less than 20% and balanced between groups. However, there is no proper intention‐to‐treat analysis, as participants moved to other groups and were analysed in the new groups |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to protocol |
| Other bias | Low risk | No other obvious sources of bias. Validated questionnaires were used for primary outcomes |
Faramarzi 2008.
| Methods | Randomised controlled trial | |
| Participants | Depressed women (BDI score between 10 and 47) who had been trying to conceive for more than 2 years. Women were included if they were less than 45 years old, were not currently participating any therapy, had more than 5 years education, and had decided not to undergo fertility treatment until 3 months afterward 124 women were randomised into the intervention group (n = 42) or the fluoxetine group (n = 42) or the control group (n = 40). Mean (SD) age was 28.3 (3.8) years in the intervention group and 28.4 (5.3) years in the control group. The intervention group was 5.4 (3.9) years subfertile, and the control group was 5.7 (4.4) years subfertile | |
| Interventions | The intervention group received 20 hours of cognitive behavioural therapy. They received face‐to‐face group therapy before starting fertility treatment delivered by a gynaecologist and an expert psychologist who had trained for the CBT program. The control group did not receive any intervention | |
| Outcomes | Depression (measured by Beck Depression Inventory), anxiety (measured by Cattell Anxiety Inventory), general health (measured by General Health Questionnaire), and infertility distress (measured by Fertility Problem Inventory), measured at baseline and post‐treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “124 participants were divided randomly to three groups according to a computer generated randomization list: CBT, antidepressant drugs and a control group. Participants were labelled randomly to numbers 1‐124 in a computer list. Numbers 1,4,7... for CBT, numbers 2,5,8 ... for fluoxetine and numbers 3,6,9 ... for control group." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding of participants and personnel, subjective outcomes likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Outcome assessor is participant and is not blinded, may affect reporting of subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 89 out of 124 women finished the study (28% withdrawals). Withdrawals were equally divided between intervention and control group, but the reasons for withdrawal were not equally divided |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | High risk | The Persian version of the CAI was not validated. Using a possible insensitive questionnaire could lead to underestimation of both beneficial and harmful effects (Higgins 2011) |
Gorayeb 2012.
| Methods | Randomised controlled trial | |
| Participants | Couples to be submitted to AR techniques (IVF and ICSI) were included. Inclusion criteria were: at least 2 years subfertile, the couple had a stable union, the couple had to pay their treatment, the couple lived at a maximum distance of 150 km from the reproduction centre. Exclusion criteria were: FSH level lower than 12.0 mIU/ml, repetitive abortion, and women older than 40 years. 285 couples were randomised into the intervention group (n = 161) and the control group (n = 124). Participants were both primary and secondary subfertile. Mean (SD) age was 32.04 (3.94) years in the intervention group and 32.42 (3.72) years in the control group | |
| Interventions | The intervention group received cognitive behavioural group therapy, consisting of cognitive restructuring, muscle relaxation techniques, social support, and information about AR techniques. The therapy was face‐to‐face, pre‐fertility treatment and took 10 hours in total. The therapy was delivered by the researcher (psychologist) and a gynaecologist. The control group received medical treatment only | |
| Outcomes | Pregnancy rate (measured by beta hCG blood test 15 days after embryo transfer) | |
| Notes | Couples had to pay for their IVF/ICSI treatment. Poor couples were excluded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Were selected randomly distributed throughout drawing lots in each group." |
| Allocation concealment (selection bias) | Low risk | Names were placed in envelopes and distributed blindly into 2 groups |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Not applicable |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No blinding of participants and personnel, objective outcomes could be influenced |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcome assessors (gynaecology professionals) were blinded, did not know who was in which group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More participants withdrew in the intervention group due to not attending the psychological sessions. Reasons for withdrawal were not balanced between intervention and control group. Participants who did not transfer embryos or did not undergo IVF/ICSI were excluded, because pregnancy could not occur. But these participants could have had no impact or a negative impact of the psychological intervention and therefore could have been excluded because of no intervention effect |
| Selective reporting (reporting bias) | Unclear risk | Unclear without a published protocol |
| Other bias | Low risk | No other obvious sources of bias |
Haemmerli 2010.
| Methods | Randomised controlled trial | |
| Participants | Swiss and German subfertile women and men, suffering from primary or secondary subfertility for at least 1 year, not undergoing any other psychological treatment, having access to a computer with Internet connection, and at least 18 years of age. 124 individuals and couples were randomised into the intervention group (n = 60) and the waiting‐list control group (n = 64). Mean age was 33.50 (range 22 to 45) years and participants were 3.10 (range 1 to 10) years subfertile | |
| Interventions | The intervention group received an Internet intervention: an interactive self help guide containing a module for participants to establish regular text‐based contact with a therapist, a monitoring and feedback system and collaborative elements and forums in 13 sessions, 108 web pages. Participants were free to decide how much time to spend on the intervention. Pre‐ and during fertility treatment, individual/couple intervention, delivered by therapists (master's degree in clinical psychology and 2 postgraduate students of psychology) through the Internet. The waiting‐list control group received the Internet intervention after 8 weeks | |
| Outcomes | Depression (measured by Center for Epidemiologic Studies Depression Scale), anxiety (measured by STAI), infertility distress (measured by Infertility Distress Scale), and pregnancy rates. Measured at baseline, 2 months, and 5 months follow‐up | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "One hundred and forty‐four participants met all the inclusion criteria and were randomized to the treatment group (n = 60) or waiting‐list control group (n = 64) using an online randomization program (Randomization.com 2008)." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding of participants and personnel, subjective outcomes likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No blinding of participants and personnel, objective outcomes could be influenced |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Outcome assessor is participant and is not blinded, may affect reporting of subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Assessor not blinded, may affect reporting of objective outcomes because substantially more participants were missing from control group at follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out less than 20% at post‐treatment outcome assessment; however, drop‐out was differential between groups and informative (including 9% of control group withdrawing due to pregnancy). Inappropriate methods for missing data used (baseline carried forward) |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | Low risk | No other obvious sources of bias. Validated questionnaires were used for primary outcomes |
Kharde 2012.
| Methods | Randomised controlled trial | |
| Participants | Subfertile women who were attending an assisted reproduction centre during treatment cycles. 200 women were randomised into an intervention group (n = 100) or a control group (n = 100) | |
| Interventions | The intervention group received 3 individual counselling sessions and 3 to 4 group counselling sessions (face to face) about coping strategies during fertility treatment. They watched a video demonstration. The control group received routine fertility treatment | |
| Outcomes | Depression (measured by Hamilton Rating Scale for Depression), anxiety (measured by Hamilton Rating Scale for Anxiety), self esteem (measured by Rosenberg Self‐Esteem Scale), and marital adjustment (measured by Marital Adjustment Inventory) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “computer generated random number table was used for sampling” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding of participants and personnel, subjective outcomes likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Outcome assessor is participant and is not blinded, may affect reporting of subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals, no exclusions. However, not stated if there was any attrition |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | Unclear risk | Baseline summaries are not reported. Validated questionnaires were used for primary outcomes |
Koszycki 2012.
| Methods | Randomised controlled trial | |
| Participants | Women 18 to 45 years old who were subfertile for more than 1 year and undergoing assessment or treatment for subfertility. Women were excluded if they had a lifetime history of psychosis or bipolar disorder, had a history of substance use disorders in the last 6 months, had a high suicide risk, or had concomitant treatment with any psychotherapy, psychotropic medications, or natural products intended to treat psychiatric symptoms. 31 women were randomised into an Interpersonal Psychotherapy group (IPT, n = 15) or a Brief Supportive Psychotherapy group (BSP, n = 16). 80% in the IPT group and 75% in the BSP group was primary subfertile, and the mean (SD) age was 35.5 (4.5) years. Mean duration of subfertility was 3.6 (3.2) years | |
| Interventions | IPT consisted of 3 phases of interpersonal therapy. BSP (attentional control) consisted of the Rogerian client‐centred therapy with psycho‐education about depression. Duration of both interventions was 10 hours total. The interventions were individually delivered during fertility treatment, face to face by doctoral‐level female clinicians with previous supervised training in IPT/BSP | |
| Outcomes | Depression (measured by Montgomery‐Asberg Depression Rating Scale), symptom severity (measured by Clinical Global Impression ‐ Severity scale), anxiety (measured by HAM‐D subscale), social adjustment (measured by Social Adjustment Scale ‐ Self‐Report), depression (measured by BDI‐II), infertility specific distress (measured by FPI), and pregnancy rates after 6 months of follow‐up. The outcomes were measured at baseline, in weeks 4, 8, and 12 (or endpoint), and at 6 months (follow‐up) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "were randomized" Email author: “Participants were randomized in a 1:1 ratio using a computer based random number generation program prepared in advance by a RA.” |
| Allocation concealment (selection bias) | Low risk | Email author: “After verification of eligibility participants were assigned a unique study number ‐ participants were assigned to the next available allocation by the onsite research coordinator.” |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Participants were not aware of which treatment was the intervention and which treatment was the control, and therefore were blinded |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Participants were not aware of which treatment was the intervention and which treatment was the control, and therefore were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Participants were the outcome assessors and were blinded. “The data were entered by a research assistant ‐ participant's research files only include information with their study ID numbers ‐ no information about treatment is included in the research file.” |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | “The data were entered by a research assistant ‐ participant's research files only include information with their study ID numbers ‐ no information about treatment is included in the research file.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 26% of participants withdrew |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | High risk | No other obvious sources of bias. The HAM‐D subscale used to measure anxiety was not validated. Using a possibly insensitive instrument could lead to underestimation of both beneficial and harmful effects (Higgins 2011) |
La Fianza 2014.
| Methods | Randomised controlled trial | |
| Participants | 217 women requiring HSG for infertility workup (109 intervention/108 control). The diagnosis of infertility was done by a senior gynaecologist. Both primary and secondary subfertility. Mean age was 34 to 35 years |
|
| Interventions | Counselling (n = 109): 45‐minute individualised session 48h before HSG. Therapists were specifically trained to provide a health education component consisting of information about HSG procedure and its potential painfulness. The therapist provided a method for stress management focusing on improvement of family support, effective problem solving, and personal coping. Control (n = 108): no intervention |
|
| Outcomes | Zung Self‐Rating Anxiety Scale (Z‐SAS) and Zung Self‐Rating Depression Scale (Z‐SDS) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. Numbers were placed in opaque envelopes |
| Allocation concealment (selection bias) | Low risk | Group allocation was managed by an independent administrator |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Control group did not receive any intervention. Not blinded |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Unclear whether outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 20% withdrawal (1 withdrawal in intervention group). Only reported on those who did not drop out of the intervention |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | Unclear risk | Z‐SAS baseline score, Z‐SDS baseline score were imbalanced (intervention group scored higher). Statistical data were not reported in a form suitable for meta‐analysis |
Matthiesen 2012.
| Methods | Randomised controlled trial | |
| Participants | Patients enrolling in their first ART treatment (IVF/ICSI) in a fertility clinic in Denmark who were able to read and understand Danish, were primary or secondary subfertile, and were not undergoing treatment with PGD. 82 participants (37 couples and 8 individual women) were randomised into an intervention group (n = 42) and a control group (n = 40). Mean age was 33.17 (±4.15) years and mean duration of subfertility was 2.0 (±1.2) years |
|
| Interventions | The intervention group received an EWI (expressive writing intervention) where they had to write about their deepest feelings and thoughts for 3 days, 20 minutes. The attentional control group had to write in an emotionally neutral manner about their daily activities for 3 days, 20 minutes. These interventions were during fertility treatment, individually done | |
| Outcomes | Infertility‐related stress (measured by COMPI), mood (measured by POMS‐R), and pregnancy rates (measured 5 days after ET, HCG blood test) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation was done using a computer program. A randomisation list (blocks of 20) was generated by the computer program with a total number of permutations of the numbers 1 and 2.” |
| Allocation concealment (selection bias) | High risk | “Condition allocation, according to the current condition code on the rank ordered list of permutated conditions, was made to couples in the incoming order of their informed consent.” |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Participants were blinded. Personnel were not blinded |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Participants were blinded. Personnel were not blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Participants were the outcome assessors (filling in questionnaires) and they were blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Personnel were the outcome assessor and were not blinded, outcome could be influenced |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Many withdrawals, more than 50% after follow‐up. Participants who stayed in the study were significantly more anxious, depressed, and stressed at baseline |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | Low risk | Validated questionnaires were used |
Moragianni 2009.
| Methods | Prospective randomised trial | |
| Participants | People undergoing IVF. 126 participants were randomised into the intervention group (n = 67) and the control group (n = 59) | |
| Interventions | The intervention group received 20 minutes of live harp music during embryo transfer. The control group received standard care | |
| Outcomes | Anxiety (measured by STAI) and clinical pregnancy rate | |
| Notes | Only the abstract is available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table used |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding of participants and personnel, subjective outcomes likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No blinding of participants and personnel, objective outcomes could be influenced |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | High risk for blinding of outcome assessor because the participant is the outcome assessor and is not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcome assessor not blinded but no more participants missing from the control group. Greater attempts of intervention group follow‐up than of control group follow up, which could lead to bias, will be unlikely in this case |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not enough information from abstract |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | Unclear risk | Other sources of bias could have been missed because only the abstract was found. A validated questionnaire was used for the primary outcome |
Mori 2008.
| Methods | Cluster randomised trial | |
| Participants | Japanese women aged below 35 years with primary subfertility who had been undergoing general fertility treatment for less than 2 years in hospitals and clinics across Japan. Women were excluded if they were candidates for any artificial treatment or if they had an adopted child, foster child, or stepchild. 140 women were randomised into the intervention group (n = 96) or the control group (n = 44). In the intervention group, mean (SD) age was 30.4 (2.87) years and duration of subfertility was 2.3 (1.43) years. In the control group, mean (SD) age was 31.3 (2.49) years and duration of subfertility was 2.2 (1.5) years | |
| Interventions | The intervention group received booklet A during fertility treatment. They read booklet A, did homework assignments related to stress management, used a stress diary, relaxation diary, social support network, and a stress calendar. Once per month, they received feedback by email from the investigator (nurse‐midwife). The control group received booklet B with a short description of stress management. The control group did not do any homework assignments and did not receive feedback. The intervention lasted 14 weeks, individual, and by written information | |
| Outcomes | Depression and anxiety (measured by Hospital Anxiety and Depression Scale), general quality of life (measured by SF‐36) and pregnancy rates | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We randomly assigned 7 institutions into an experimental group (4 institutions) and a control group (3 institutions) by using uniform random numbers in Excel |
| Allocation concealment (selection bias) | Low risk | Nurses and nurse‐midwives in charge of Recruit and participants were not informed if A and B was experimental group |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and personnel were not blinded and were likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No blinding of participants and personnel, objective outcomes could be influenced |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | High risk for blinding of outcome assessor because the participant is the outcome assessor and is not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Assessor not blinded, but fewer participants missing from control group. Greater attempts of intervention group follow‐up than of control group follow up, which could lead to bias, will be unlikely in this case |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal rate is 31 (> 20% withdrawals) in the intervention group (11 pregnant, 16 did not do their homework, and 4 stopped treatment) versus 6 in the control group (4 pregnant, 1 did not submit questionnaire, 1 was transferred to another clinic). The withdrawal of 16 participants who did not do their homework (the intervention was likely to not have been successful for them) gives an overestimation of the effect in the intervention group |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | High risk | There was baseline imbalance in treatment methods between the intervention and control group despite apparently suitable procedures for randomisation and concealment. Validated questionnaires were used for the primary outcomes |
Mosalanejad 2012.
| Methods | Randomised controlled trial | |
| Participants | Primary subfertile women who were residents of Jahrom, Iran. Inclusion criteria were: 20 to 40 years of age, valid cell phone numbers, able to read and write, and no somatic or psychiatric problems. 80 women were randomised into the intervention group (n = 40) and the control group (n = 40). Duration of subfertility was 12 years in the intervention group and 16 years in the control group |
|
| Interventions | The intervention group received E‐cognitive‐emotional self‐disclosure group CBT for 24 hours total, during fertility treatment, face to face, delivered by specialists. The control group received no intervention | |
| Outcomes | Depression, stress, and anxiety (measured by the Depression Anxiety Stress Scale), worry (measured by the Penn State Worry Questionnaire) The outcomes were measured 1 week prior to the first CBT meeting and at the last CBT meeting | |
| Notes | Only women included with a mobile phone, ability to read and write. This could mean a selection on the higher socioeconomic status | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Table of random numbers: random into group A or group B” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding of participants and personnel, subjective outcomes likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | High risk for blinding of outcome assessor because the woman is the outcome assessor and is not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 19% of women (15) were excluded during the study. Exclusion was even between groups, reasons for exclusion were not reported |
| Selective reporting (reporting bias) | High risk | Pregnancy rates and follow‐up (named in protocol) not reported |
| Other bias | Low risk | No other obvious sources of bias. A validated questionnaire was used for the primary outcomes |
Murphy 2014.
| Methods | Randomised controlled trial | |
| Participants | Subfertile women aged 21 to 44, all under the care of Abington Reproductive Medicine and Genetics and requiring in vitro fertilisation–embryo transfer. Women were excluded if they were already enrolled in other in vitro fertilisation–embryo transfer clinical trials or undergoing pre‐implantation genetic diagnosis. 202 women were randomised into the intervention group (n = 101) and the control group (n = 101). Mean (SD) age was 33.6 (4.7) years in the intervention group and 34.1 (4.3) years in the control group |
|
| Interventions | The intervention group received harp music therapy during embryo transfer for 20 minutes, face to face, delivered by a certified music practitioner. The control group received usual treatment |
|
| Outcomes | Anxiety (measured by STAI) and clinical pregnancy rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised per a random numbers table to the harp therapy group or the standard treatment group |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and personnel not blinded |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | < 10% withdrawals and exclusions, women were excluded postintervention from the analysis, reasons given. No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | Low risk | No other obvious sources of bias. A validated questionnaire was used for the primary outcomes |
Ockhuijsen 2014.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing a stimulated or cryopreserved IVF/ICSI treatment cycle at a university hospital in the Netherlands. Women were excluded if they could not speak Dutch. 377 women were randomised into the PRCI group (n = 127), the monitoring group (n = 117), and the control group (n = 124). Mean (SD) age was 34.9 (4.7) years in PRCI group, 34.6 (4.7) in monitoring group, and 34.8 (5.0) in control group. They were both primary and secondary subfertile. Duration of subfertility was 3.4 (2.2) years in PRCI group, 3.1 (2.2) in monitoring group, and 3.1 (2.3) in control group |
|
| Interventions | The PRCI group received a small card with 10 positive reappraisal statements and a leaflet with explanation as a self administered coping intervention. They read the card twice a day for 14 days during fertility treatment and filled in the DRK for 14 days. The monitoring group only filled in the DRK for 14 days. The control group received routine care | |
| Outcomes | Anxiety and depression (measured by HADS), positive and negative emotions daily (measured 14 days by DRK), clinical pregnancy (with foetal heartbeat) (measured 6 weeks post ET) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A computer‐generated table of random numbers was used to achieve the stratified randomization of the 372 women who met the eligibility criteria” |
| Allocation concealment (selection bias) | Low risk | Patient records were concealed to the independent researcher who performed allocation |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Although the women did not know what the intervention was, those in the PRCI group had "something extra" given to them, probably resulting in non‐specific placebo effects. Personnel appear to be adequately blinded. Those performing embryo transfer blind to allocation |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Although the women did not know what the intervention was, those in the PRCI group had "something extra" given to them. The premise of this review is that psychological states may influence pregnancy/birth outcomes |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Self reported questionnaires are used. Women were not adequately blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | “An independent research assistant verified random data input for accuracy of the database.” Pregnancy outcome was ascertained for all randomised women |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High levels (> 20%) of drop‐out in all groups. Mixed‐effects models used to include women with incomplete sets of observations, but no imputation of missing data |
| Selective reporting (reporting bias) | Unclear risk | Protocol: quality of life and coping will be measured. Not reported. Primary outcome was stated |
| Other bias | High risk | As noted by authors, possible effects in 2 of the arms due to daily monitoring by DRK. A validated questionnaire was used for the primary outcomes |
Panagopoulou 2009.
| Methods | Randomised controlled trial | |
| Participants | Participants were recruited from women who were undergoing IVF in an assisted reproduction clinic in the North of Greece. Women were excluded if they had a history of psychiatric disorder, were unable to understand Greek or English, or if they were undergoing procedures including donor eggs, uteri, and sperm. Finally, women were excluded if they already had participated in the study in a previous IVF cycle. The mean (SD) age of the women was 33.8 (4.6) years and they were primary and secondary subfertile. The mean (SD) duration of subfertility was 18 months (14 months). 148 women were randomised into the emotional‐writing condition (n = 50), the fact‐writing condition (n = 50) and the control condition (n = 48). | |
| Interventions | In the "emotional writing condition", women were asked to write "about your deepest thoughts and feelings regarding the subfertility and its treatment. The important thing is that in your writing you really let go and explore your very deepest emotions. Do not worry about grammar or about using correct Greek: the only rule is that once you start writing, you go on writing until the end of the time period". In the "fact‐writing condition", women were asked to write about the "facts concerning the subfertility and its treatment", and the "control condition" solely received the standard medical instructions and went home. All interventions were carried out every day for a week for 20 minutes per day (2 hours and 20 minutes in total) during fertility treatment and were individual (written instructions and telephone calls) | |
| Outcomes | Non‐specific distress (measured by the STAI‐state and the Negative Subscale from the PANAS), fertility‐specific distress (measured by the ISS and the PSRS), and pregnancy rates (positive pregnancy rate, negative pregnancy rate, biochemical pregnancy rate). Measured at embryo transfer (baseline, T1) and 2 weeks later (after the end of writing, T2) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding of participants and personnel. Subjective outcomes are likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No blinding of participants and personnel. Objective outcomes could have been influenced |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | "Experimenters who conducted the psychological assessments and data analysis were blind to the group allocation of the women. Information on group allocation and writing instructions were given to participants after the first psychological assessment by an experimenter different to the one who conducted the assessments." But women are the real outcome assessors and they are not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Pregnancy outcomes were obtained from medical records. Pregnancy outcome data were complete |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There appears to be 1 woman missing from the analysis of psychological outcomes (the F statistic has been calculated on 2 and 144 degrees of freedom instead of 2 and 145, suggesting 1 missing), but too low to pose risk. Complete pregnancy outcome data |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | Low risk | No other obvious sources of bias. Validated questionnaires were used |
Pook 2005.
| Methods | Randomised controlled trial | |
| Participants | Men applying for fertility workup in an andrology clinic in Marburg, Germany. Inclusion criteria were: appointments made on behalf of the man's initiative, men who were applying for fertility diagnostics, and they had to be first‐time visitors to the andrology clinic. 250 men were randomised into the treatment group (125 men) or the control group (125 men). Men in the treatment group were 33.2 (±6.2) years old and were 2.6 (±2.25) years subfertile. Men in the control group were 34.0 (±6.1) years old and were 2.6 (±2.14) years subfertile | |
| Interventions | The treatment group received a leaflet outlining the contents of the fertility workup prior to the workup (before fertility treatment). The control group did not receive any additional care | |
| Outcomes | Infertility distress (measured by the Infertility Distress Scale), measured before fertility workup | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “based on a computer‐generated randomization list” |
| Allocation concealment (selection bias) | Low risk | “Directly after the call, the reception staff allocated the next available number for entry into the study to the patient. Once each day, the reception staff received information about the assignment of the numbers” |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Men were blinded to allocation (since they were not informed that they were in a trial). There is little scope for additional performance bias on behalf of personnel in this trial |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Men were blinded to allocation (since they were not informed that they were in a trial). There is little scope for additional performance bias on behalf of personnel in this trial |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Men filled out the questionnaire before the medical exam, and they were blinded. There may be limited scope for some bias at the point of data extraction, as this is not discussed |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | As for subjective outcomes: little scope for bias here |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis, large number of exclusions (19%), not equally distributed |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | Low risk | No other obvious sources of bias. A validated questionnaire was used |
Rasoulzadeh 2013.
| Methods | Randomised controlled trial | |
| Participants | Primary infertile women (n = 60) | |
| Interventions | The intervention group received collaborative counselling during 5 sessions, face to face by midwife, gynaecologist, and a classified psychologist. The control group received routine counselling | |
| Outcomes | Problem‐focused coping strategies, including "seeking social support" (measured by Folkman and Lazarus' Ways of Coping Questionnaire) measured at the beginning and at the embryo transfer | |
| Notes | Only the abstract is available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “were randomly allocated” but not stated how |
| Allocation concealment (selection bias) | Unclear risk | Allocation not stated in abstract |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Blinding not stated in abstract |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Blinding of outcome assessor not stated in abstract |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Follow‐up not mentioned in abstract |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | Unclear risk | Too few details in the abstract to be sure of the methodology |
Sexton 2010.
| Methods | Randomised controlled trial | |
| Participants | Female participants from multiple fertility clinics throughout the USA who were at least 18 years of age, who were receiving infertility‐related medical assessments or treatments or both, who had a BDI‐II score of < 20, who were not reporting any current suicidal ideation or intent, who were not receiving any psychological care, and who had Internet access. 43 women were randomised into an experimental condition (n = 21) or a control condition (n = 22). Women suffered from primary or secondary subfertility, had a mean age of 32.6 (±4.8) years and were subfertile for 2.5 (±2.1) years | |
| Interventions | The experimental condition received WCWI (web‐based coping with infertility) during fertility treatment. Both CBT and psycho‐education were incorporated into the intervention. Women were allowed to devote as much time as they wanted (0 minutes, maximum 2 weeks) to the intervention. The Internet intervention was individual therapy. The control condition was placed on a waiting list | |
| Outcomes | General stress (measured by Symptom Checklist‐90‐Revised) and infertility stress (measured by Fertility Problem Inventory). Measured at baseline and postintervention (2 weeks) | |
| Notes | It should be noted that women in both intervention and control group became eligible for a lottery drawing for 50‐dollar gift cards as an honorarium for their assistance | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Random assignment was completed with the use of a random sequence generator computer program.” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. The random.org website used for randomisation provides a random list but does not conceal it |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and personnel were not blinded. Outcomes are likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Outcome assessor not blinded, may affect reporting of subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Many withdrawals (9 out of 43, 21%), and a further 3 did not provide measurements for the outcome assessment |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | High risk | Authors present 2 sets of results, which are inconsistent. Unclear which set is correct, or if inference was based on the correct set. Baseline summaries of the groups were not presented, so it is unclear if the groups were adequately balanced (confounding). Authors conducted and reported hypothesis tests of baseline demographics, but these are both uninformative and inappropriate. One woman in control group returned questionnaire 4 months after the end of the study and was excluded. Although this might be reasonable, it is not clear that any ‘cut‐off’ for receiving responses was defined in advance, and this increases researcher degrees of freedom |
Shahrestani 2012.
| Methods | Randomised controlled trial | |
| Participants | Subfertile women referred to Vali‐e‐Asr Reproductive Health Research Center undergoing IVF treatment in 2011 with high scores in the Irrational Parenthood Cognitions questionnaire and Fertility Problem Inventory. Duration of infertility was > 1 year. 24 women were randomised into the experimental group (12 women) and the control group (12 women) | |
| Interventions | The experimental group received MBCT training (face to face, group therapy) for 16 hours during their fertility treatment. The control group did not receive any mental health services | |
| Outcomes | Quality‐of‐life scores (measured by Irrational Parenthood Cognitions questionnaire and Fertility Problem Inventory), measured before and after the intervention | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "were randomly assigned". Method of assignment not stated |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not stated |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and personnel not blinded, likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Outcome assessor is participant and is not blinded, may affect reporting of subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals, no exclusions. However, not stated if there was any attrition |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to protocol |
| Other bias | Unclear risk | We used a translator for this Persian article and therefore could have missed other sources of bias |
Shu‐Hsin 2003.
| Methods | Randomised controlled trial | |
| Participants | Subfertile women who participated in the IVF‐ET program in an infertility treatment center in Taiwan. Inclusion and exclusion criteria not reported. Primary or secondary subfertility not reported. 132 women were randomised into the intervention group (n = 64) and the control group (n = 68). Mean (SD) age was 31.8 (4.2) years for women in the intervention group and 32.3 (4.1) years in the control group. Mean (SD) duration of subfertility was 4.3 (2.5) years in the intervention group and 4.4 (3.6) years in the control group | |
| Interventions | The intervention group received a nursing crisis intervention pre‐ and during fertility treatment. Firstly education by viewing a videotape about the therapeutic process. They received self instructional materials including another videotape on self hypnosis and muscle relaxation and had 1 practice session. They received cognitive behavioural counseling individually at the end through the telephone. On average, participants performed the skills twice a week and had CBT once or twice a week. The control group did not receive an additional nursing crisis intervention |
|
| Outcomes | Anxiety (measured by C‐STAI), depression (measured by Zung Self‐Rating Depression Scale), sexual problems, and interpersonal relationship (measured by infertility questionnaire) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported how they randomised |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and personnel not blinded, subjective outcomes likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Self reported outcomes, and participants are not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 69% of initially included participants were assessed in the intervention group. Withdrawals more than 20%. Withdrawal rate of control group is not stated |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | Low risk | No other obvious sources of bias. Validated questionnaires were used for the primary outcomes |
Skiadas 2011.
| Methods | Randomised controlled trial | |
| Participants | All women aged 18 to 45 years undergoing their first IVF treatment. Excluded were women using donor eggs or a gestational carrier, cryopreserved embryos or having day 5 transfers, women with a self reported history of anxiety or depression or already seeing a mental health professional, or unable to speak English. 131 women were randomised into the intervention group (n = 65) and the control group (n = 66). The women were primary and secondary subfertile. Mean (SD) age was 35.0 (4.2) in the intervention group and 34.1 (4.9) in the control group |
|
| Interventions | The intervention group received 2 phone calls between day 2 and 4 after ET and day 5 and 9 after ET (during fertility treatment). Phone calls were 5 to 15 minutes, maximum of 30 minutes total. The phone calls were individual therapy delivered by a social worker. The control group received usual care (no phone calls) | |
| Outcomes | Distress (measured by Perceived Stress Scale) and pregnancy rates | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization numbers were generated using block sizes of 6, 8, and 10 to ensure relatively equal numbers of participants per group (Block Stratified Randomization Windows version 5.0, Johns Hopkins Oncology Center)." |
| Allocation concealment (selection bias) | Low risk | "Two identical envelopes containing the group allocation were sealed inside of a larger external envelope. Once the external envelope was opened, the patient was considered randomized. Patients received one of the sealed, opaque, coin‐sized envelopes." |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding of participants. Personnel blinded, but outcomes likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No blinding of participants. Personnel blinded, but outcomes could be influenced |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were outcome assessors, as they filled in the questionnaires. Not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Personnel who took pregnancy rates blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Many withdrawals (more than 33%) |
| Selective reporting (reporting bias) | Low risk | They did report all outcomes named in the protocol |
| Other bias | Low risk | No other obvious sources of bias. A validated questionnaire was used |
Soltani 2014.
| Methods | Randomised trial | |
| Participants | Infertile couples (6 couples, 12 participants) from Tehran, Iran were included if they had a high score on depression, anxiety, and/or stress according to the DASS. Individuals with a history of alcohol and substance abuse, brain damage, and any other psychiatric disorders, as measured by DSM‐IV‐TR, were excluded from the study | |
| Interventions | The intervention group received emotionally focused couple therapy face to face during 10 meetings in 10 weeks. The control group did not receive counselling but received usual care | |
| Outcomes | Depression, anxiety, and stress measured by DASS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The selected couples were randomly divided”. Unknown how the couples were selected |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants were not blinded, neither was the personnel |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were outcome assessors and were not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals, no exclusions |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | High risk | Couples were randomised together and then analysed as individuals. Although no baseline imbalances, with this small number of participants this may induce bias. |
Takefman 1990.
| Methods | Randomised controlled trial | |
| Participants | Married couples who were commencing an infertility medical investigation. Inclusion criteria were: females had to experience primary subfertility and had to be unaware of the reason for their failure to get pregnant. 39 couples were randomised into the Emotional and Sexual Information Group (IG) (n = 13), the Emotional IG (n = 13), and the Procedural IG (n = 13). Mean (SD) age of the males was 32.3 (5.2) years and mean (SD) age of the females was 29.8 (4.1) years. Duration of subfertility was 2.3 (1.7) years for all couples | |
| Interventions | The Emotional and Sexual IG received information about how to cope better with medical investigations. Each couple then viewed a 15‐min videotape and read a 15‐page sex information booklet. The Emotional IG (attentional control) received the information and viewed the videotape. The Procedural IG (attentional control) received the information and viewed a 12‐min videotape that described only procedural aspects. The intervention took 1 day (pre‐fertility treatment, face to face), and couples received a phone call every month | |
| Outcomes | Pregnancy, anxiety (measured by STAI‐state). Pregnancy was measured 6 months post‐testing. Anxiety was measured pre‐ and post‐testing | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “each couple was then randomly assigned to one of three groups” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Participants were blinded: “in order to equate for expectations of benefit across groups”. Personnel not stated |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Participants were blinded: “in order to equate for expectations of benefit across groups”. Personnel not stated |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Participants were blinded and were the outcome assessors |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding, but the follow‐up appears to be complete. Greater attempts of intervention group follow‐up than of control group follow up, which could lead to bias, will be unlikely in this case |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High bias due to removal of 4 couples from pre‐post analyses owing to the fact that that they fell pregnant, i.e. the participants with the desired outcome were removed. It is unclear which intervention group these participants came from. Given that there were 9 pregnancies in total, it is also unclear why only 4 excluded |
| Selective reporting (reporting bias) | High risk | Numerous questionnaires administered. Of those assessing psychological adjustment, only STAI‐state reported as the measurements “were highly correlated” and STAI‐state “was selected for analysis because it was considered the best measure of an individual’s current stress level.” Other measurements were selected for inclusion in the analysis on the basis of model selection procedures, which were not described and must be to some extent arbitrary. No primary outcome is named. Only the values of those measurements for which significant change was observed are reported in Table 2. There is great scope for ‘researcher degrees of freedom’ as a result of these features ‐ arbitrary decisions which may affect the inference of the study |
| Other bias | High risk | Baseline imbalances between the groups in education and body image. A validated questionnaire was used for the primary outcome |
Terzioglu 2001.
| Methods | Randomised controlled trial | |
| Participants | Couples who applied to the ART unit of Hacettepe University hospital in Ankara, Turkey. Inclusion criteria were: no previous ART experience, at least elementary education, stable marital relationship, no previous history of live birth (primary subfertile). Exclusion criteria were: failure in ovulation induction or fertilisation, ovarian hyperstimulation syndrome, non‐existence of sperm, and questionnaires not handed in after ET. 90 couples were randomised into an intervention group (n = 45) and a control group (n = 45). Age ranged from 20 years to above 40 years. Duration of subfertility ranged from 2 to 8 years |
|
| Interventions | The intervention group received detailed information and a written general treatment procedure to take home. Additionally, they could ask many questions, there was daily telephone contact with nurse practitioner, and the nurse practitioner was present at time of oocyte pickup and ET. The couples received 5 information sessions (15 to 30 minutes) from the investigator. During fertility treatment, face to face, telephone, and written information. The control group received usual care, including detailed information and a written general treatment procedure to take home |
|
| Outcomes | Anxiety (measured by STAI), depression (measured by BDI), and pregnancy rates | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random permuted blocks. Unclear, because account given in the manuscript of randomisation of 90 participants does not appear to coincide with randomisation list of 60 participants sent by author |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and personnel not blinded, subjective outcomes likely to be influenced. In addition to the intervention, the experimental group appears to have received additional attention throughout, with daily contact with the nurse practitioner. There is huge scope for attentional bias here |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Participants and personnel not blinded, which could influence objective outcomes. In addition to the intervention, the experimental group appears to have received additional attention throughout, with daily contact with the nurse practitioner. There is huge scope for attentional bias here |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | High risk for blinding of outcome assessor because the participant is the outcome assessor and is not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Complete outcome data for pregnancy, so no evidence of detection bias here |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15 couples were removed from the control group postallocation, and it is unclear if these removal criteria were prespecified. 15 couples removed from experimental group also |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to protocol |
| Other bias | Low risk | Validated questionnaires were used for the primary outcomes |
Valiani 2010.
| Methods | Randomised controlled trial | |
| Participants | Subfertile women aged between 18 and 35 years old, under treatment of IVF or ICSI or both in Isfahan Infertility Clinic in Iran. Women were included if they were diagnosed with primary subfertility, were undergoing IVF/ICSI treatment, and had Iranian nationality. Women were excluded if they had a psychological illness, were using drugs or psychiatric medications, had more than 2 IVF/ICSI treatments previously, had an adopted child, or if a relative of first degree had died during the past 2 months. Mean (SD) age of the women was 29.4 (4.1) years. Mean (SD) duration of subfertility was 5.6 (3.9) years. 62 women were randomised into the intervention group (n = 32) or the control group (n = 30) |
|
| Interventions | The intervention group received 12 individual sessions (6 before embryo transfer and 6 after embryo transfer) of 30 minutes of relaxation therapy, by muscle contraction and relaxation by the researcher who was present in the room during the first 6 sessions playing a CD. Final 6 sessions were at home. The control group did not receive additional treatment | |
| Outcomes | Infertility stress (measured by Newton's infertility stress questionnaire at baseline and 15 days post‐embryo transfer) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “were randomly divided using simple random sampling” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding of participants and personnel, subjective outcomes likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Participants and personnel were not blinded, there was no attentional control |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | ”All the questionnaires were completed under supervision of the researcher...” The researcher was unblinded. Participants filled in the questionnaire and were not blinded either. |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | The outcome assessor in this case was unblinded, and there was more drop‐out in the treatment group (16%) versus in the control group (11%) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16% and 11% of participants missing from treatment and control groups respectively (differential loss to follow‐up). No intention‐to‐treat analysis was used |
| Selective reporting (reporting bias) | High risk | Authors state in methods that the result of the pregnancy test was recorded by the researcher. Pregnancy rates are not reported. Unclear whether or not other outcomes were measured. No outcome specified as primary |
| Other bias | Low risk | No other obvious sources of bias. A validated questionnaire was used |
van Zyl 2005.
| Methods | Randomised controlled trial | |
| Participants | Patients aged 20 to 40 years qualifying for IVF in Pretoria, South Africa, were included. 60 participants were randomised into a support counselling group (n = 30) or a control group (n = 30). The mean duration of subfertility was 4.40 years in the intervention group and 5.07 in the control group (SDs not mentioned). Participants were both primary and secondary subfertile | |
| Interventions | The support counselling group received an individual (face‐to‐face) structured interview by the embryologist and a copy of positive self statements in coping suggested by Donald Meichenbaum (Sue 1994) before fertility treatment started. The embryologist called the participants early in the mornings after oocyte aspiration (pre‐ and during fertility treatment). The control group met the embryologist briefly before the AR programme. They received routine care and were asked to phone the specialist's rooms every day to find out about the embryo development and the next step in the programme | |
| Outcomes | Anxiety (measured by Beck Anxiety Inventory), depression (measured by Beck Depression Inventory), escapism, self blame, minimisation, seeking of meaning, instrumental action, exercised caution, negotiation, and support‐mobilisation (measured by coping scale based on the study of Folkman and Lazarus (Folkman 1986)). These outcomes were measured pre‐ and post‐treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “were randomly assigned” |
| Allocation concealment (selection bias) | Low risk | “the names of relevant patients in both the support counselling and control groups were communicated on a daily basis to the embryologist” |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding of participants and personnel, subjective outcomes likely to be influenced. The study does not appear to have been appropriately controlled. Control group participants were asked to telephone the embryologist every day for information. It is not obvious that this provides suitable control for non‐specific placebo effects, and may potentially be burdensome |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Outcome assessor is participant and is not blinded, may affect reporting of subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The report explicitly states that some participants were withdrawn due to poor treatment response (5 from the treatment and 3 from the control group). This will induce bias |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | Low risk | Validated questionnaires were used for the primary outcomes |
Vizheh 2013.
| Methods | Randomised controlled trial | |
| Participants | Monogamous married couples in Vali‐e‐Asr Reproductive Health Research Center with subfertility duration between 1 and 10 years, with no children and no history of ART. No drug abuse and no mental or physical ailments. 100 couples were randomised into an intervention group (n = 50) or a control group (n = 50). Mean (SD) age in the intervention group was 26.88 (4.23) for women and 32.14 (4.49) for men. Mean (SD) age in the control group was 27.44 (4.65) for women and 32.46 (7.31) for men. The couples were both primary and secondary subfertile. Duration of subfertility was 5.55 (3.19) in the intervention group and 5.93 (3.14) in the control group |
|
| Interventions | The couples in the intervention group received 3 to 4.5 hours of face‐to‐face couple counselling delivered by a counsellor during 3 weeks of fertility treatment. The control group only received routine therapies and services, no counselling | |
| Outcomes | Marital satisfaction (measured by Marital Satisfaction Questionnaire), sexual satisfaction (measured by Sexual Satisfaction Questionnaire), and pregnancy rates | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of random number table |
| Allocation concealment (selection bias) | Low risk | Allocation performed by personnel who did not know participants |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding of participants and personnel, subjective outcomes likely to be influenced. |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No blinding of participants and personnel, objective outcomes could be influenced |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participant‐reported outcome, participants were not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Assessor not blinded, but fewer participants missing from control group. Greater attempts of intervention group follow‐up than of control group follow up, which could lead to bias, will be unlikely in this case |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14% and 6% loss to follow‐up in treatment and control arms, respectively (differential loss to follow‐up). Participants excluded for reasons pertaining to fertility outcome (excluded if pregnancy achieved during treatment) |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to protocol |
| Other bias | Low risk | No other obvious sources of bias. Validated questionnaires were used |
Wiener‐Megnazi 2006.
| Methods | Randomised controlled trial | |
| Participants | Women undergoing standard IVF‐ET treatment who agreed to participate and could read and understand Hebrew | |
| Interventions | The intervention group received a modified PMR (progressive muscular relaxation) technique for 15 to 20 minutes before ET and daily during the luteal phase. The 2 attentional control groups listened to music or short stories under the same conditions | |
| Outcomes | State anxiety was measured after ET and during the luteal phase | |
| Notes | Only the abstract is available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "One hundred and twenty three women undergoing standard IVF‐ ET treatment were randomly allocated into three groups". No further details provided |
| Allocation concealment (selection bias) | Unclear risk | States "One hundred and twenty three women undergoing standard IVF‐ ET treatment were randomly allocated into three groups". No further details provided |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Blinding not stated |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Blinding not stated |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 121/123 women included in analysis. Intention to treat not clear |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to the protocol |
| Other bias | Unclear risk | Very few details about methods, as only abstract is available. No data suitable for analysis |
Zhu 2010.
| Methods | Randomised controlled trial | |
| Participants | Participants were women who visited Peking University Third Hospital, Department of Gynecology and Obstetrics for their IVF treatment. They had to meet the IVF criteria, were between 20 and 40 years of age, had more than secondary education, and lived in Beijing. Women were excluded if they had language problems, were unable to complete 6 sessions of group therapy, had mental health problems themselves or in their family, had a low IQ, or had cancer or other major diseases. Women were primary or secondary subfertile. 100 women were allocated into the intervention group (n = 50) or the control group (n = 50). The mean (SD) age of the women was 33 (5) years in both the intervention and control groups. Duration of subfertility was 5.1 (2.9) years in the intervention group and 4.6 (3.2) years in the control group | |
| Interventions | The intervention group received group psychotherapy (face to face) during fertility treatment for 3 weeks (2 sessions a week, 6 sessions in total). The control group did not receive any additional intervention |
|
| Outcomes | Clinical pregnancy rate, state and trait anxiety (measured by STAI), and depression (measured by SDS). Measured at baseline and after treatment (3 weeks from baseline) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The participants were numbered 1‐100 when they entered the trial. They used excel RAND software on the computer to allocate the participants into the treatment group and the control group." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not stated |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding of participants and personnel. Subjective outcomes are likely to be influenced |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No blinding of participants and personnel. Objective outcomes could be influenced |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Outcome assessor is participant and is not blinded, may affect reporting of subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Assessor not blinded, but fewer participants missing from control group. Greater attempts of intervention group follow‐up than of control group follow up, which could lead to bias, will be unlikely in this case |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Less than 20% withdrawals, reason for withdrawals not reported. Unclear if intention‐to‐treat analysis was used. Unclear if attrition was reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear without access to protocol |
| Other bias | Unclear risk | We used a translator for this Chinese article and therefore could have missed other sources of bias. Validated questionnaires were used for the primary outcomes |
AR: assisted reproduction ART: assisted reproductive technology BDI: Beck Depression Inventory CAI: Cattell Anxiety Inventory CBT: cognitive behavioural therapy COMPI: Copenhagen Multi‐centre Psychosocial Infertility C‐STAI: Chinese version of the State‐Trait Anxiety Inventory DASS: Depression Anxiety Stress Scale DRK: Daily Record Keeping DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders 4th Edition Text Revision ET: embryo transfer FPI: Fertility Problem Inventory FSH: Follicle Stimulating Hormone HADS: Hamilton Anxiety and Depression Scale HAM‐D: Hamilton Depression Scale HRSD: Hamilton Rating Scale for Depression HSG: Hysterosalpingography I‐BMS: Intervention of Body Mind Spirit ICSI: intracytoplasmic sperm injection ISS: Infertility Specific Stress scale ITT: intention to treat IVF‐ET: in vitro fertilisation‐embryo transfer IVF: in vitro fertilisation MBCT: mindfulness based cognitive therapy MDS: marital distress scale PANAS: Positive and Negative Affect Schedule PGD: Pre‐implantation Genetic Diagnosis POMS: Profile of Mood States PRCI: positive reappraisal coping intervention PSRS: physical stress reactions scale PSS: Perceived Stress Scale RSES: Rosenberg self‐esteem scale SD: standard deviation SDS: Self‐Rating Depression Scale SF‐36: 36‐Item Short Form Health Survey STAI: State‐Trait Anxiety Inventory WHO: World Health Organization WHOQOL: World Health Organization Quality of Life Assessment
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abedinia 2009 | Inappropriate comparison |
| Connolly 1993 | Includes couples attempting to conceive for less than 1 year. No separate data reported and attempts to obtain this from study authors were unsuccessful (no reply) |
| Feili 2012 | No outcomes of interest |
| Garcia 2003 | No randomisation |
| Heidari 2002 | Not properly randomised (quasi‐randomised) |
| Hope 2010 | No outcomes of interest after asking the author |
| Hosaka 2002 | Not properly randomised |
| Khalatbari 2011 | States that sample was selected randomly from women with high levels of depression, but no indication that allocation to intervention or control group was randomised. Attempts to contact author unsuccessful (no reply) |
| Kheirkhah 2014 | Not properly randomised (quasi‐randomised) |
| Lancastle 2008 | Not properly randomised (quasi‐randomised) |
| Mosalanejad 2013 | Not properly randomised |
| Mousavinik 2012 | Randomisation unclear. Attempts to contact author unsuccessful (no reply) |
| Nagaoka 2012 | No outcomes of interest reported in abstract. Attempts to contact author unsuccessful |
| Nelen 2013 | No outcomes of interest |
| Nieschlag 1998 | Not a psychological or an educational intervention |
| Nilforooshan 2006 | Quasi‐randomised |
| Pakgohar 2008 | Not properly randomised |
| Ramezanzadeh 2007 | Inappropriate comparison |
| Ramezanzadeh 2011 | Inappropriate comparison |
| Rossi 2013 | No outcomes of interest |
| Sarrel 1985 | Not a psychological or educational intervention |
| Strauss 1997 | Not randomised |
| Strauss 2000 | Not properly randomised |
| Tang 2013 | No outcomes of interest |
| Tuschen‐Caffier 1999 | Not randomised |
| Zhou 2012 | Not a psychological or an educational intervention |
Characteristics of studies awaiting assessment [ordered by study ID]
Jacobs 2003.
| Methods | Not clear |
| Participants | Female participants self identifying with infertility‐related emotional distress |
| Interventions | The intervention group received a bibliotherapy approach (book on coping with infertility). The control group was on a waiting list |
| Outcomes | Amongst others, depression and anxiety, measured pre‐treatment, post‐treatment, and at 3 months' follow‐up |
| Notes | No results, no full text available |
Liswood 1995.
| Methods | Randomised controlled trial |
| Participants | Couples whose infertility was of at least 2 years' duration, selected by physicians or from adoption agencies |
| Interventions | The intervention group received six 1‐hour sessions of cognitive behavioural therapy within a marital context. The control group received no treatment |
| Outcomes | Marital intimacy, psychological distress, and social functioning |
| Notes | No results, no full text available |
Vause 2011.
| Methods | Randomised controlled trial |
| Participants | 50 women undergoing their first cycle of IVF |
| Interventions | The intervention group received an interactive web‐based teaching tool. The control group received nurse‐led traditional didactic teaching. Administered before starting IVF cycle |
| Outcomes | Postintervention stress survey: end scores. Stress scores are a secondary outcome; no data yet published on this outcome |
| Notes | Only the abstract is available. Funded by Merck. We emailed first author at her place of work (October 2015) to inquire if more results have been published |
Characteristics of ongoing studies [ordered by study ID]
Huppelschoten 2012.
| Trial name or title | Improving patient‐centeredness of fertility care using a multifaceted approach: study protocol for a randomised controlled trial |
| Methods | Cluster randomised trial |
| Participants | Infertile patients from 32 Dutch infertility clinics who underwent at least 1 cycle of medically assisted reproduction |
| Interventions | A multifaceted approach, including audit and feedback, educational outreach visits, and patient‐mediated interventions |
| Outcomes | Patient‐centredness, patients' quality of life and levels of distress |
| Starting date | April 2012 |
| Contact information | w.nelen@obgyn.umcn.nl |
| Notes |
Patel 2014.
| Trial name or title | Effectiveness of modified mindfulness based cognitive therapy in reducing emotional distress in infertility couples |
| Methods | Randomised controlled trial |
| Participants | Female partner of couple with primary infertility, age 20 to 35 years, with 5‐ to 10‐year history of infertility. Polycystic ovarian syndrome in female partner and/or oligospermia and varicocele in male partner. Suitable for intrauterine insemination. Significant infertility stress score on Fertility Problem Inventory. Excludes women having in vitro fertilisation. Target sample size = 30 |
| Interventions | Modified mindfulness based cognitive therapy: 6 daily 1.5‐hour sessions, along with intrauterine insemination. Unclear what control interventions is |
| Outcomes | Infertility‐specific stress, measured on Fertility Problem Inventory. Anxiety and depression (Hamilton Anxiety and Depression Scale), fertility‐related quality of life (Fertility Quality of Life Tool (FertiQoL)); measured at 1 month |
| Starting date | 30 May 2015 |
| Contact information | psvn.sharma@manipal.edu |
| Notes |
Differences between protocol and review
We changed the objective of the review from "to assess the efficacy and safety..." to "to assess the effectiveness...", as the objective was to assess effectiveness of psychological and educational interventions.
We adapted the definitions of "pre‐fertility treatment" and "during fertility treatment", as the former definition of "pre‐fertility treatment" (diagnostic phase) was contrary to men and women diagnosed with subfertility as one of our inclusion criteria.
We grouped results in the forest plots according to whether or not they had used attentional or usual care control groups. We made this decision after starting data extraction.
We rated attrition bias as 'high risk' if more than 20% of participants dropped out from at least one arm or there was substantial imbalance in attrition rates between arms or both. This was not specified in the protocol.
We combined the outcomes live birth and ongoing pregnancy in one composite outcome.
We stated in the protocol that we would not pool studies unless they were sufficiently similar. However, we did not state what we would do in the event that they were not. We have added details of our approach (presenting median and interquartile ranges for effect sizes) to the section Data synthesis. We have added a statement regarding how to calculate standardised mean differences into the section Measures of treatment effect.
Contributions of authors
JV drafted the protocol and participated in devising the search, selected the studies, extracted and analysed the data, and drafted the systematic review.
CV acted as a clinical expert, commented on the protocol, acted as a second assessor of the literature, and commented on the systematic review.
WN acted as a clinical expert, commented on the protocol, acted as a second assessor of the literature, and commented on the systematic review.
JW commented on the protocol, acted as a second assessor of the literature, extracted and analysed the data, commented on the systematic review, and drafted the Discussion section of the systematic review.
CF commented on the protocol, helped to resolve differences in opinion as a third review author, and commented on the systematic review.
Sources of support
Internal sources
Radboudumc, Nijmegen, Netherlands.
External sources
None known, Other.
Declarations of interest
JV: None known.
CV: None known.
WN: None known.
JW: None known.
CF: None known.
New
References
References to studies included in this review
Arslan‐Ozkan 2013 {published data only}
- Arslan‐Özkan İ, Okumuş H, Buldukoğlu K. A randomized controlled trial of the effects of nursing care based on Watson’s Theory of Human Caring on distress, self‐efficacy and adjustment in infertile women. Journal of Advanced Nursing 70;8(1801‐12). [DOI] [PubMed] [Google Scholar]
Catoire 2013 {published data only}
- Catoire P, Delaunay L, Dannappel T, Baracchini D, Marcadet‐Fredet S, Moreau O, et al. Hypnosis versus diazepam for embryo transfer: a randomized controlled study. American Journal of Clinical Hypnosis 2013;55:378‐86. [DOI] [PubMed] [Google Scholar]
Chan 2006 {published data only}
- Chan C, Ng E, Chan C, Ho, Chan T. Effectiveness of psychosocial group intervention for reducing anxiety in women undergoing in vitro fertilization: a randomized controlled study. Fertility and Sterility 2006;85:339‐46. [DOI] [PubMed] [Google Scholar]
Chan 2012 {published data only}
- Chan C, Chan C, Ng E, Ho P, Chan T, Lee G, et al. Incorporating spirituality in psychosocial group intervention for women undergoing in vitro fertilization: a prospective randomized controlled study. Psychology & Psychotherapy: Theory, Research & Practice 2012;85:356‐73. [DOI] [PubMed] [Google Scholar]
Choobforoushzade 2011 {published data only}
- Choobforoushzade A, Kalantari M, Molavi H. The effectiveness of cognitive behavioral stress management therapy on quality of life in infertile women. Iranian Journal of Obstetrics, Gynecology and Infertility 2011;14. [Google Scholar]
Conrad 2013 {published data only}
- Conrad R, Karpawitz‐Godt A, Allam J, Geiser F, Ven H, Haidl G. Expressive writing in male infertility ‐ a randomized controlled trial. Psychotherapy and Psychosomatics 2013;82:21. [Google Scholar]
Cousineau 2008 {published data only}
- Cousineau T, Green T, Corsini E, Seibring A, Showstack M, Applegarth L, et al. Online psychoeducational support for infertile women: a randomized controlled trial. Human Reproduction 2008;23:554‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Czamanski‐Cohen 2012 {published data only}
- Czamanski‐Cohen J, Sarid O, Cwikel J, Zeadna A, Levitas E, Har‐Vardi I. Practicing cognitive behavioral interventions (CBI) increases pregnancy rates in women undergoing IVF. Fertility and Sterility 2012;98 Suppl 1:S45 Abstract no:O‐147. [Google Scholar]
de Klerk 2005 {published data only}
- Klerk C, Hunfeld J, Duivenvoorden H, Outer M, Fauser B, Passchier J, et al. Effectiveness of a psychosocial counselling intervention for first‐time IVF couples: a randomized controlled trial. Human Reproduction 2005;20:1333‐8. [DOI] [PubMed] [Google Scholar]
- Klerk C, Hunfeld J, Macklon N, Passchier J. Little effect of one psychosocial counselling session on emotional distress experienced by couples undergoing infertility treatment. Human Reproduction 2003;18:47‐8, Abstract no: O‐136. [Google Scholar]
Domar 2000 {published data only}
- Domar A, Clapp D, Slawsby B, Kessel B, Orav J, Freizinger M. The efficacy of group psychological interventions on reducing distress in infertile women. Fertility and Sterility 1999;72:S44‐5. [Google Scholar]
- Domar A, Clapp D, Slawsby E, Dusek J, Kessel B, Freizinger M. Impact of group psychological interventions on pregnancy rates in infertile women. [Erratum appears in Fertil Steril 2000 Jul;74(1):190]. Fertility and Sterility 2000;73:805‐11. [DOI] [PubMed] [Google Scholar]
- Domar A, Clapp D, Slawsby E, Kessel B, Orav J, Freizinger M. The impact of group psychological interventions on distress in infertile women. Health Psychology 2000;19:568‐75. [DOI] [PubMed] [Google Scholar]
Domar 2011 {published data only}
- Domar A, Backman K, Alper M, Berger B, Wiegand B, Nikolovski J. The impact of group mind/body participation on pregnancy rates in IVF patients: A randomized controlled trial. Fertility and Sterility 2009;92:S2. [Google Scholar]
- Domar A, Rooney K, Wiegand B, Orav E, Alper M, Berger B, et al. Impact of a group mind/body intervention on pregnancy rates in IVF patients. Fertility and Sterility 2011;95:2269‐73. [DOI] [PubMed] [Google Scholar]
Emery 2003 {published data only}
- Emery M, Beran M, Darwiche J, Oppizzi L, Joris V, Capel R, et al. Results from a prospective, randomized, controlled study evaluating the acceptability and effects of routine pre‐IVF counselling. Human Reproduction 2003;18:2647‐53. [DOI] [PubMed] [Google Scholar]
- Emery M, Beran M, Darwiche J, Oppizzi L, Joris V, Capel R, et al. What do couples expect and what do they receive through systematic pre‐IVF counselling? Results from a randomized controlled study. Human Reproduction 2002;17:192. [DOI] [PubMed] [Google Scholar]
- Emery M, Beran M, Darwish J, Oppizzi L, Joris V, Capel R, et al. Does counselling prior to IVF affect anxiety and depression scores? Preliminary results of a randomized clinical trial. Human Reproduction 2001;16:201. [Google Scholar]
Faramarzi 2008 {published data only}
- Faramarzi M, Alipor A, Esmaelzadeh S, Kheirkhah F, Poladi K, Pash H. Treatment of depression and anxiety in infertile women: cognitive behavioral therapy versus fluoxetine. Journal of Affective Disorders 2008;108:159‐64. [DOI] [PubMed] [Google Scholar]
- Faramarzi M, Kheirkhah F, Esmaelzadeh S, Alipour A, Hjiahmadi M, Rahnama J. Is psychotherapy a reliable alternative to pharmacotherapy to promote the mental health of infertile women? A randomized clinical trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2008;141:49‐53. [DOI] [PubMed] [Google Scholar]
- Faramarzi M, Pasha H, Esmailzadeh S, Kheirkhah F, Heidary S, Afshar Z. The effect of the cognitive behavioral therapy and pharmacotherapy on infertility stress: A randomized controlled trial. International Journal of Fertility and Sterility 2013;7:199‐206. [PMC free article] [PubMed] [Google Scholar]
Gorayeb 2012 {published data only}
- Gorayeb R, Borsari A, Rosa‐e‐Silva A, Ferriani R. Brief cognitive behavioral intervention in groups in a Brazilian assisted reproduction program. Behavioral Medicine 2012;38:29‐35. [DOI] [PubMed] [Google Scholar]
Haemmerli 2010 {published data only}
- Haemmerli K, Znoj H, Berger T. Internet‐based support for infertile patients: a randomized controlled study. Journal of Behavioral Medicine 2010;33:135‐46. [DOI] [PubMed] [Google Scholar]
Kharde 2012 {published data only}
- Kharde S, Pattad S, Bhogale G. Effectiveness of a therapeutic counseling intervention for depression, anxiety, self esteem and marital adjustment among infertile women. International Journal of Nursing Education 2012;4:151‐4. [Google Scholar]
Koszycki 2012 {published data only}
- Koszycki D, Bisserbe J, Blier P, Bradwejn J, Markowitz J. Interpersonal psychotherapy versus brief supportive therapy for depressed infertile women: First pilot randomized controlled trial. Archives of Women's Mental Health 2012;15:193‐201. [DOI] [PubMed] [Google Scholar]
La Fianza 2014 {published data only}
- Fianza A, Dellafiore C, Travaini D, Broglia D, Gambini F, Scudeller L, et al. Effectiveness of a single education and counseling intervention in reducing anxiety in women undergoing hysterosalpingography: a randomized controlled trial. The Scientific World Journal 2014;ID 598293:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthiesen 2012 {published data only}
- Frederiksen Y, Zachariae R, Schmidt L, Ingerslev H. Effects of expressive writing intervention on infertility‐related symptoms in couples undergoing assisted reproductive technology treatment ‐ a feasibility study. Human Reproduction 2011;26 Suppl 1:i262 Abstract no: P‐364. [Google Scholar]
- Matthiesen S, Klonoff‐Cohen H, Zachariae R, Jensen‐Johansen M, Nielsen B, Frederiksen Y, et al. The effect of an expressive writing intervention (EWI) on stress in infertile couples undergoing assisted reproductive technology (ART) treatment: a randomized controlled pilot study. British Journal of Health Psychology 2012;17:362‐78. [DOI] [PubMed] [Google Scholar]
Moragianni 2009 {published data only}
- Moragianni V, Hopkins J, Somkuti S, Lee A, Schinfeld J, Barmat L. Randomized trial of Harp Music Therapy in IVF‐ET. Fertility and Sterility 2009;92 Suppl 1:S147‐8. [Google Scholar]
Mori 2008 {published data only}
- Mori A, Shimizu K, Kawamoto M, Momoi M, Nagamori K. Partnerships between fertility nurses and self‐help groups in Britain to support coping with infertility [Japanese]. Bulletin of St Luke's College of Nursing 2008;9. [Google Scholar]
Mosalanejad 2012 {published data only}
- Mosalanejad L, Koolaee A, Behbahani B. Looking out for the secret wound: The effect of E‐cognitive group therapy with emotional disclosure on the status of mental health in infertile women. International Journal of Fertility and Sterility 2012;6:87‐94. [PMC free article] [PubMed] [Google Scholar]
Murphy 2014 {published data only}
- Murphy EM, Nichols J, Somkuti SG, Sobel M, Braverman A, Barmat LI. Randomized trial of harp therapy during in vitro fertilization‐embryo transfer. Journal of Evidence‐Based Complementary & Alternative Medicine 2014;19(2):93‐8. [DOI] [PubMed] [Google Scholar]
Ockhuijsen 2014 {published data only}
- Ockhuijsen H, Hoogen A, Eijkemans M, Macklon N, Boivin J. The impact of a self‐administered coping intervention on emotional well‐being in women awaiting the outcome of IVF treatment: a randomized controlled trial. Human Reproduction May 2014. [DOI: 10.1093/humrep/deu093] [DOI] [PubMed] [Google Scholar]
Panagopoulou 2009 {published data only}
- Panagopoulou E, Montgomery A, Tarlatzis B. Experimental emotional disclosure in women undergoing infertility treatment: Are drop outs better off?. Social Science & Medicine 2009;69:678‐81. [DOI] [PubMed] [Google Scholar]
Pook 2005 {published data only}
- Pook M, Krause W. Stress reduction in male infertility patients: a randomized, controlled trial. Fertility and Sterility 2005;83:68‐73. [DOI] [PubMed] [Google Scholar]
Rasoulzadeh 2013 {published data only}
- Rasoulzadeh BM, Latifinejad RR. Adopting problem‐focused coping strategies following implementation of a collaborative counseling program in infertile women undergoing IVF. Conference: 14th Royan Congress on Reproductive Biomedicine and 8th Royan Nursing and Midwifery Seminar Tehran Islamic Republic of Iran. International Journal of Fertility and Sterility: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=emed13&AN=71791030. 2013; Vol. 7:46.
Sexton 2010 {published data only}
- Sexton M, Byrd M, O'Donohue W, Jacobs N. Web‐based treatment for infertility‐related psychological distress. Archives of Women's Mental Health 2010;13:347‐58. [DOI] [PubMed] [Google Scholar]
Shahrestani 2012 {published data only}
- Shahrestani M, Qanbari B, Nemati S, Rahbardar H. The effectiveness of mindfulness based cognitive group therapy (MBCT) on improving perceived infertility‐related stress and irrational parenthood cognitions among infertile women undergoing IVF treatment. Iranian Journal of Obstetrics, Gynecology and Infertility 2012;15:28‐38. [Google Scholar]
Shu‐Hsin 2003 {published data only}
- Shu‐Hsin L. Effects of using a nursing crisis intervention program on psychosocial responses and coping strategies of infertile women during in vitro fertilization. Journal of Nursing Research 2003;11:197‐208. [DOI] [PubMed] [Google Scholar]
Skiadas 2011 {published data only}
- Skiadas C, Terry K, Pari M, Geoghegan A, Lubetsky L, Levy S, et al. Does emotional support during the luteal phase decrease the stress of in vitro fertilization?. Fertility and Sterility 2011;96:1467‐72. [DOI] [PubMed] [Google Scholar]
Soltani 2014 {published data only}
- Soltani M, Shairi MR, Roshan R, Rahimi C. The impact of emotionally focused therapy on emotional distress in infertile couples. International Journal of Fertility and Sterility 2014;7(4):337‐44. [PMC free article] [PubMed] [Google Scholar]
Takefman 1990 {published data only}
- Takefman J, Brender W, Boivin J, Tulandi T. Sexual and emotional adjustment of couples undergoing infertility investigation and the effectiveness of preparatory information. Journal of Psychosomatic Obstetrics & Gynecology 1990;11:275‐90. [Google Scholar]
Terzioglu 2001 {published data only}
- Terzioglu F. Investigation into effectiveness of counseling on assisted reproductive techniques in Turkey. Journal of Psychosomatic Obstetrics & Gynecology 2001;22:133‐41. [DOI] [PubMed] [Google Scholar]
Valiani 2010 {published data only}
- Valiani M, Abediyan S, Ahmadi S, Pahlavanzadeh S, Hassanzadeh A. The effect of relaxation techniques to ease the stress in infertile women. Iranian Journal of Nursing and Midwifery Research 2010;15:259‐64. [PMC free article] [PubMed] [Google Scholar]
van Zyl 2005 {published data only}
- Zyl C, Niemandt C, Dyk C, Rooyen L, Lourens J, Coetzee C. The embryologist as counsellor during ART. Human Reproduction 2003;18:84, Abstract no: O‐244. [Google Scholar]
- Zyl C, Dyk A, Niemandt C. The embryologist as counsellor during assisted reproduction procedures. Reproductive Biomedicine Online 2005;11:545‐51. [DOI] [PubMed] [Google Scholar]
Vizheh 2013 {published data only}
- Vizheh M, Pakgohar M, Babaei G, Ramezanzadeh F. Effect of counseling on quality of marital relationship of infertile couples: a randomized, controlled trial (RCT) study. Archives of Gynecology & Obstetrics 2013;287:583‐9. [DOI] [PubMed] [Google Scholar]
Wiener‐Megnazi 2006 {published data only}
- Wiener‐Megnazi Z, Adin Zinman M, Zeidner M, Dirnfeld M. Study of a novel progressive muscular relaxation technique and state‐anxiety in IVF patients during ET and luteal phase. Human Reproduction 2006;21:i64. [Google Scholar]
Zhu 2010 {published data only}
- Zhu H, Hu P, Qiao J. Effects of group psychotherapy on mood in patients undergoing in vitro fertilization and embryo transfer. Chinese Mental Health Journal 2010;24:912‐6. [Google Scholar]
References to studies excluded from this review
Abedinia 2009 {published data only}
- Abedinia N, Ramezanzadeh F, Noorbala A. Effects of a psychological intervention on quality of life in infertile couples. Journal of Family and Reproductive Health 2009;3:87‐93. [Google Scholar]
Connolly 1993 {published data only}
- Connolly K, Edelmann R, Bartlett H, Cooke I, Lenton E, Pike S. An evaluation of counselling for couples undergoing treatment for in‐vitro fertilization. Human Reproduction 1993;8:1332‐8. [DOI] [PubMed] [Google Scholar]
Feili 2012 {published data only}
- Feili A, Borjali A, Sohrabi F, Farrokhi N. The comparative efficacy of cognitive‐behavior therapy and Teasdale mindfulness‐based cognitive therapy of infertile depressed women's rumination. Armaghane Danesh 2012;17(1):14‐21. [Google Scholar]
Garcia 2003 {published data only}
- Garcia R, Sala M, Lafuente R, Brassesco A, Cairo O, Villanueva D, et al. Role of the relaxation therapies in IVF treatments. Human Reproduction 2003;18:206. [Google Scholar]
Heidari 2002 {published data only}
- Heidari P, Latif N, Sahebi A, Jahaniyan M, Mazloum S. Impact of cognitive behaviour therapy on anxiety level of primary infertile women undergoing IUI. Medical Journal of Reproduction & Infertility 2002. [Google Scholar]
Hope 2010 {published data only}
- Hope N, Rombauts L. Can an educational DVD improve the acceptability of elective single embryo transfer? A randomized controlled study. Fertility and Sterility 2010;94:489‐95. [DOI] [PubMed] [Google Scholar]
Hosaka 2002 {published data only}
- Hosaka T, Matsubayashi H, Sugiyama Y, Izumi S, Makino T. Effect of psychiatric group intervention on natural‐killer cell activity and pregnancy rate. General Hospital Psychiatry 2002;24:353‐6. [DOI] [PubMed] [Google Scholar]
Khalatbari 2011 {published data only}
- Khalatbari J, Ghorbanshirodi S, Akhshabi M, Hamzehpour T, Esmaeilpour M. The effectiveness of the behavioral‐cognitive therapy on the reduction of the rate of the depression and anxiety of the infertile women of the Rasht city. Indian Journal of Science and Technology 2011;4:1578‐82. [Google Scholar]
Kheirkhah 2014 {published data only}
- Kheirkhah M, Vahedi M, Jenani P. The effect of group counseling on infertility adjustment of infertile women in Tabriz Al‐Zahra clinic. IJOGI: http://pesquisa.bvsalud.org/ses/resource/pt/CN‐01022290 2014;17(113):7‐14. [Google Scholar]
Lancastle 2008 {published data only}
- Boivin J, Lancastle D. The medical waiting period and IVF: characteristics, challenges and interventions. Human Reproduction. 2008;23 Suppl 1:i4 Abstract No:O‐009 Oral. [DOI] [PubMed] [Google Scholar]
- Lancastle D, Boivin J. A feasibility study of a brief coping intervention (PRCI) for the waiting period before a pregnancy test during fertility treatment. Human Reproduction 2008;23:2299‐307. [DOI] [PubMed] [Google Scholar]
Mosalanejad 2013 {published data only}
- Mosalanejad L, Koolee A. Looking at infertility treatment through the lens of the meaning of life: The effect of group logotherapy on psychological distress in infertile women. International Journal of Fertility and Sterility 2013;6:224‐31. [PMC free article] [PubMed] [Google Scholar]
Mousavinik 2012 {published data only}
- Mousavinik M. Effect of rational emotive behavior therapy on depression in infertile women. ZENITH International Journal of Multidisciplinary Research 2012;2:77‐84. [Google Scholar]
Nagaoka 2012 {published data only}
- Nagaoka Y. The effect of guided imagery to reduce pain at the time of in vitro fertilization egg retrieval: A randomized controlled trial. Fertility and Sterility 2012;1:S114. [Google Scholar]
Nelen 2013 {published data only}
- Nelen W, Huppelschoten A, Verkerk E, Adang E, Kremer J. Towards more patient‐centred fertility care: What does it cost. Human reproduction: 29th Annual Meeting of the European Society of Human Reproduction and Embryology. July 2013; Vol. 28:i284‐5.
Nieschlag 1998 {published data only}
- Nieschlag E, Behre H, Fischedick A, Hertle L. Treatment of varicocele in the age of "evidence‐based medicine". Medical counseling is as successful as interventional treatment (ligation or embolization). Urologe (Ausg. A) 1998;37:265‐9. [DOI] [PubMed] [Google Scholar]
Nilforooshan 2006 {published data only}
- Nilforooshan P, Ahmadi A, Abedi M, Ahmadi M. Counseling based on interacting cognitive subsystems and its effect on anxiety of infertile couples. Pakistan Journal of Psychological Research 2006;21:95‐103. [Google Scholar]
- Nilforooshan P, Ahmadi A, Abedi M, Ahmadi M. Studying the effect of cognitive‐behavioral counseling based on interacting cognitive subsystems on depression of infertile couples. Middle East Fertility Society Journal 2006;11:43‐7. [Google Scholar]
Pakgohar 2008 {published data only}
- Pakgohar M, Vizheh M, Babaee G, Ramezanzadeh F, Abedininia N. Effect of counseling on sexual satisfaction among infertile women referred to Tehran Fertility Center [Farsi]. Hayat 2008;14:79. [Google Scholar]
Ramezanzadeh 2007 {published data only}
- Ramezanzadeh F, Noorbala A, Malak Afzali H, Abedinia N, Rahimi A, Shariet M, et al. Effectiveness of psychiatric and counseling interventions on fertility rate in infertile couples. Tehran University Medical Journal 2007;65:57‐63. [Google Scholar]
Ramezanzadeh 2011 {published data only}
- Ramezanzadeh F, Noorbala A, Abedinia N, Rahimi A, Naghizadeh M. Psychiatric intervention improved pregnancy rates in infertile couples. The Malaysian Journal of Medical Science 2011;18:16‐24. [PMC free article] [PubMed] [Google Scholar]
Rossi 2013 {published data only}
- Rossi BV, Chang C, Berry KF, Hornstein MD, Missmer SA. In vitro fertilization outcomes and alcohol consumption in at‐risk drinkers: the effects of a randomized intervention. American Journal on Addictions 2013;22(5):481‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarrel 1985 {published data only}
- Sarrel P, DeCherney A. Psychotherapeutic intervention for treatment of couples with secondary infertility. Fertility and Sterility 1985;43:897‐900. [DOI] [PubMed] [Google Scholar]
Strauss 1997 {published data only}
- Strauss B, Hepp L, Mettler L, Stading G. Development and evaluation of a counselling programme for involuntary childlessness for women and couples. Verhaltenstherapie 1997;7:A2. [Google Scholar]
Strauss 2000 {published data only}
- Strauss B, Städing G. Fokale Beratungskonzepte in der Fertilitätsmedizin. 17. Göttingen: Hogrefe, 2000. [Google Scholar]
Tang 2013 {published data only}
- Tang WH, Jiang H, Ma LL, Hong K, Zhao LM, Liu DF, et al. Tadalafil combined with behavior therapy for semen collection from infertile males in whom masturbation fails. Zhonghua Nan Ke Xue 2013;19(5):439‐42. [PubMed] [Google Scholar]
Tuschen‐Caffier 1999 {published data only}
- Tuschen‐Caffier B, Florin I, Krause W, Pook M. Cognitive‐behavioral therapy for idiopathic infertile couples. Psychotherapy & Psychosomatics 1999;68:15‐21. [DOI] [PubMed] [Google Scholar]
Zhou 2012 {published data only}
- Zhou Y. The role of the synchronous involvement of spouse in therapy for male infertility. Chinese Journal of Andrology 2012;26:42‐4. [Google Scholar]
References to studies awaiting assessment
Jacobs 2003 {published data only}
- Jacobs N. A bibliotherapy approach for treating the psychological sequelae of infertility. University of Nevada, Reno 2003;Abstract only. [Google Scholar]
Liswood 1995 {published data only}
- Liswood M. Treating the crisis of infertility: A cognitive‐behavioral approach. Dissertation Abstracts International Section A: Humanities and Social Sciences 1995;55:4006. [Google Scholar]
Vause 2011 {published data only}
- Vause T, Evans M, Vause T, Min J. Comparison of an interactive web‐based teaching tool and traditional didactic learning for IVF patients: A randomized controlled trial. Fertility and Sterility 2011;1:S179. [Google Scholar]
References to ongoing studies
Huppelschoten 2012 {published data only}
- Huppelschoten A, Duijnhoven N, Hermens R, Verhaak C, Kremer J, Nelen W. Improving patient‐centeredness of fertility care using a multifaceted approach: study protocol for a randomized controlled trial. Trials [Electronic Resource] 2012;13:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppelschoten D, Aarts J, Empel I, Nelen W, Kremer J. Effects of a multifaceted approach on improvement of patient centredness in fertility care, a study protocol. Human Reproduction 2011;26 Suppl 1:i270 Abstract no: P‐383. [Google Scholar]
Patel 2014 {unpublished data only}
- Patel A. Effectiveness of modified mindfulness based cognitive therapy in distressed couples with infertility, undergoing intra‐uterine insemination. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2015/07/005973 2015.
Additional references
Beaurepaire 1994
- Beaurepaire J, Jones M, Thiering P, Saunders D, Tennant C. Psychosocial adjustment to infertility and its treatment: male and female responses at different stages of IVF/ET treatment. Journal of Psychosomatic Research 1994;38:229‐40. [DOI] [PubMed] [Google Scholar]
Beck 1961
- Beck A, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561‐71. [DOI] [PubMed] [Google Scholar]
Boivin 1999
- Boivin J, Scanlan L, Walker S. Why are infertile patients not using psychosocial counselling?. Human Reproduction 1999;14(5):1384‐91. [DOI] [PubMed] [Google Scholar]
Boivin 2003
- Boivin J. A review of psychosocial interventions in infertility. Social Science and Medicine 2003;57:2325–41. [DOI] [PubMed] [Google Scholar]
Boivin 2011
- Boivin J, Takefman J, Braverman A. The fertility quality of life (FertiQoL) tool: development and general psychometric properties. Human Reproduction 2011;26:2084‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bouwmans 2008
- Bouwmans C, Lintsen B, Al M, Verhaak C, Eijkemans R, Habbema J, et al. Absence from work and emotional stress in women undergoing IVF or ICSI: an analysis of IVF‐related absence from work in women and the contribution of general and emotional factors. Acta Obstetricia et Gynecologica Scandinavica 2008;87:1169‐75. [DOI] [PubMed] [Google Scholar]
Chiaffarino 2011
- Chiaffarino F, Baldini M, Scarduelli C, Bommarito F, Ambrosio S, D'Orsi C. Prevalence and incidence of depressive and anxious symptoms in couples undergoing assisted reproductive treatment in an Italian infertility department. European Journal of Obstetrics & Gynecology and Reproductive Biology 2011;158(2):235‐41. [DOI] [PubMed] [Google Scholar]
Cuijpers 2013
- Cuijpers P, Berking M, Andersson G, Quigley L, Kleiboer A, Dobson K. A meta‐analysis of cognitive‐behavioural therapy for adult depression, alone and in comparison with other treatments. Canadian Journal of Psychiatry 2013;58(7):376‐85. [DOI] [PubMed] [Google Scholar]
Daya 2005
- Daya S. Life table (survival) analysis to generate cumulative pregnancy rates in assisted reproduction: are we overestimating our success rates?. Human Reproduction 2005;20(5):1135‐43. [DOI] [PubMed] [Google Scholar]
De Liz 2005
- Liz T, Strauss B. Differential efficacy of group and individual/couple psychotherapy with infertile patients. Human Reproduction 2005;20:1324–32. [DOI] [PubMed] [Google Scholar]
Dhaliwal 2004
- Dhaliwal L, Gupta K, Gopalan S, Kulhara P. Psychological aspects of infertility due to various causes ‐ prospective study. International Journal of Fertility and Women's Medicine 2004;49:44‐8. [PubMed] [Google Scholar]
Domar 1990
- Domar A, Seibel M, Benson H. The mind/body program for infertility: a new behavioral treatment approach for women with infertility. Fertility and Sterility 1990;53:246‐9. [DOI] [PubMed] [Google Scholar]
Endnote [Computer program]
- Thomson Reuters. EndNote [Computer program on www.endnote.com]. Thomson Reuters, November 2013.
EuroQol 1990
- EuroQol Group. EuroQol ‐ a new facility for the measurement of health‐related quality of life. Health Policy 1990;16:199‐208. [DOI] [PubMed] [Google Scholar]
Folkman 1986
- Folkman S, Lazarus R, Dunkel‐Schetter C. Dynamics of a stressful encounter: cognitive appraisal, coping, and encounter outcomes. Journal of Personality and Social Psychology 1986;50:992‐1003. [DOI] [PubMed] [Google Scholar]
Gameiro 2012
- Gameiro S, Boivin J, Peronace L, Verhaak C. Why do patients discontinue fertility treatment? A systematic review of reasons and predictors of discontinuation in fertility treatment. Human Reproduction update 2012;18:652‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gameiro 2015
- Gameiro S, Boivin J, Dancet E, Klerk C, Emery M, Lewis‐Jones C, et al. ESHRE guideline: routine psychosocial care in infertility and medically assisted reproduction ‐ a guide for fertility staff. Human Reproduction 2015;30(11):2476‐85. [DOI] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. McMaster University, November 2014.
Higgins 2003
- Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins J, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org:. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011].. [Google Scholar]
Hämmerli 2009
- Hämmerli K, Znoj H, Barth J. The efficacy of psychological interventions for infertile patients: a meta‐analysis examining mental health and pregnancy rate. Human Reproduction Update 2009;15:279‐95. [DOI] [PubMed] [Google Scholar]
Luke 2013
- Luke B, Brown M, Wantman E, Lederman A, Gibbons W, Schattman G, et al. Cumulative birth rates with linked assisted reproductive technology cycles. The New England Journal of Medicine 2013;366:2483‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mansour 2014
- Mansour R, Ishihara O, Adamson G, Dyer S, Mouzon J, Nygren K, et al. International Committee for Monitoring Assisted Reproductive Technologies world report: Assisted Reproductive Technology 2006. Human Reproduction 2014;29(7):1536‐51. [DOI] [PubMed] [Google Scholar]
Mascarenhas 2012
- Mascarenhas M, Flaxman S, Boerma T, Vanderpoel S, Stevens G. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Medicine 2012;9:e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Musa 2014
- Musa R, Ramli R, Yazmie A, Khadijah M, Hayati M, Midin M, et al. A preliminary study of the psychological differences in infertile couples and their relation to the coping styles. Comprehensive Psychiatry 2014;55 (Supp 1):S65‐9. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2005
- Roberts C, Roberts S. Design and analysis of clinical trials with clustering effects due to treatment. Clinical Trials 2005;2(2):152‐62. [DOI] [PubMed] [Google Scholar]
Rubin 2006
- Rubin D. Causal inference through potential outcomes and principal stratification: application to studies with “censoring” due to death. Statistical Science 2006;21(3):299‐309. [Google Scholar]
Seuc 2013
- Seuc A, Peregoudov A, Pilar Betran A, Metin Gulmezoglu A. Intermediate outcomes in randomized clinical trials: an introduction. Trials March 2013;14(78). [DOI] [PMC free article] [PubMed] [Google Scholar]
Slade 1997
- Slade P, Emery J, Lieberman B. A prospective, longitudinal study of emotions and relationships in in‐vitro fertilization treatment. Human Reproduction 1997;12:183‐90. [DOI] [PubMed] [Google Scholar]
Spielberger 1989
- Spielberger C. State‐trait Anxiety Inventory: Bibliography. 2nd Edition. Palo Alto, CA: Consulting Psychologists Press, 1989. [Google Scholar]
Sterne 2009
- Sterne J, White I, Carlin J, Spratt M, Royston P, Kenward M, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ (Clinical research ed.) 2009;338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sue 1994
- Sue D, Sue D, Sue S. Understanding abnormal behaviour (4th edition). Houghton Mifflin, Boston 1994. [Google Scholar]
Tarlov 1989
- Tarlov A, Ware J Jr, Greenfield S, Nelson E, Perrin E, Zubkoff M. The Medical Outcomes Study. An application of methods for monitoring the results of medical care. JAMA 1989;262:925‐30. [DOI] [PubMed] [Google Scholar]
Terzioglu 2007
- Terzioglu F. Anxiety of infertile men who undergo genetic testing for assisted reproductive treatment. Journal of Psychosomatic Obstetrics and Gynaecology 2007;28:147‐53. [DOI] [PubMed] [Google Scholar]
Troude 2014
- Troude P, Guibert J, Bouyer J, Rochebrochard E. Medical factors associated with early IVF discontinuation. Reproductive Biomedicine Online 2014;28(3):321‐9. [DOI] [PubMed] [Google Scholar]
van Peperstraten 2010
- Peperstraten A, Hermens R, Nelen W, Stalmeier P, Wetzels A, Maas P, et al. Deciding how many embryos to transfer after in vitro fertilisation: development and pilot test of a decision aid. Patient Education and Counseling 2010;78(1):124‐9. [DOI] [PubMed] [Google Scholar]
Verhaak 2005
- Verhaak C, Smeenk J, Evers A, Minnen A, Kremer J, Kraaimaat F. Predicting emotional response to unsuccessful fertility treatment: a prospective study. Journal of Behavioral Medicine 2005;28:181‐90. [DOI] [PubMed] [Google Scholar]
Verhaak 2007
- Verhaak C, Smeenk J, Nahuis M, Kremer J, Braat D. Long‐term psychological adjustment to IVF/ICSI treatment in women. Human Reproduction 2007;22(1):305‐8. [DOI] [PubMed] [Google Scholar]
Wang 2006
- Wang J, Sauer M. In vitro fertilization (IVF): a review of 3 decades of clinical innovation and technological advancement. Therapeutics and Clinical Risk Management 2006;2(4):355‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2013
- World Health Organization. Infertility definitions and terminology. http://www.who.int/reproductivehealth/topics/infertility/definitions/en/index.html (accessed 9 October 2013).
Zegers‐Hochschild 2009
- Zegers‐Hochschild F, Adamson G, Mouzon J, Ishihara O, Mansour R, Nygren K, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertility and Sterility 2009;92:1520‐4. [DOI] [PubMed] [Google Scholar]


