Abstract
Background
Asthma is the most common chronic disease in childhood. Breathing exercise techniques have been widely used by researchers and professionals in the search for complementary therapies for the treatment of asthma.
Objectives
To assess the effects of breathing exercises in children with asthma.
Search methods
We searched for trials in the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PsycINFO, CINAHL and AMED and handsearched respiratory journals and meeting abstracts. We also consulted trial registers and reference lists of included articles.
The literature search was run up to September 2015.
Selection criteria
We included randomised controlled trials of breathing exercises alone versus control or breathing exercises as part of a more complex intervention versus control in children with asthma.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. The primary outcomes were quality of life, asthma symptoms and serious adverse events. The secondary outcomes were reduction in medication usage, number of acute exacerbations, physiological measures (lung function (especially low flow rates) and functional capacity), days off school and adverse events.
Main results
The review included three studies involving 112 participants. All the included studies performed the comparison breathing exercises as part of a more complex intervention versus control. There were no trials comparing breathing exercises alone with control. Asthma severity of participants from the included studies varied. The studies measured: quality of life, asthma symptoms, reduction in medication usage, number of acute exacerbations and lung function. Breathing exercise techniques used by the included studies consisted of lateral costal breathing, diaphragmatic breathing, inspiratory patterns and pursed lips. One study included in the review did not specify the type of breathing exercise used. The control groups received different interventions: one received placebo treatment, one an educational programme and doctor appointments, and one was not described. There were no reported between‐group comparisons for any of the primary outcomes. We judged the included studies as having an unclear risk of bias.
Authors' conclusions
We could draw no reliable conclusions concerning the use of breathing exercises for children with asthma in clinical practice. The breathing exercises were part of a more comprehensive package of care, and could not be assessed on their own. Moreover, there were methodological differences among the three small included studies and poor reporting of methodological aspects and results in most of the included studies.
Plain language summary
Breathing exercises for children with asthma
Background
Asthma is a chronic (persistent) inflammatory disease of the lungs that can lead to airflow obstruction (blockage) causing difficulty in breathing. The worldwide high prevalence of asthma has become a public health problem due to the great healthcare costs resulting from hospitalisation and medicines. Moreover, asthma is the most common chronic disease in childhood. Breathing exercises are a non‐drug treatment that have been routinely used in the treatment of people with asthma. Breathing exercises aim to control the hyperventilation (overbreathing) symptoms of asthma and can include the Papworth method, Buteyko breathing technique, yoga or any other similar method that focusses on changing the breathing pattern.
Review question
We wanted to look at the evidence for the effects of breathing exercises in children with asthma.
Key results
We found three studies involving 112 children with mild to severe asthma. All the included studies compared breathing exercises as part of a more complex treatment (inspiratory muscle training, relaxation exercises, endurance exercises, rhythmic mobilization exercises, vibrations, percussion, forced expiration technique) versus control. The studies varied in size from 28 to 60 children. Samples consisted of inpatients and outpatients. The control groups received different treatments: one received placebo (pretend) treatment, one an educational programme and doctor appointments, and one was not described. We found no primary outcomes (measures of quality of life, asthma symptoms and side effects of treatment) that were reported as comparisons between the treatment and control groups.
Quality of the evidence
The included studies had an overall small number of participants and sessions. No included study compared breathing exercises alone versus a control. Instead, breathing exercises were part of a package of treatments and were compared to a control. The methods used to conduct the studies were not as well reported as we would like and so were unclear about the quality of the trials. Overall, we judged the included studies as being at an unclear risk of bias and the quality of the evidence included in the review was low.
Conclusion
We could draw no reliable conclusions concerning the use of breathing exercises for children with asthma in clinical practice.
Summary of findings
Summary of findings for the main comparison. Breathing exercises as part of a package of interventions compared with control for asthma in children.
| Breathing exercises as part of a package of interventions compared with control for asthma in children | ||||||
|
Patient or population: children with asthma Settings: inpatient and outpatient Intervention: breathing exercises as part of a package of interventions Comparison: control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Breathing exercises as part of a package of interventions | |||||
|
Quality of life Follow‐up: 1 month |
See comment | See comment | See comment | 28 (one study) | ⊕⊕⊝⊝ low1,2 |
No between‐group comparisons reported |
|
Symptoms of asthma Follow‐up: 1 and 3 months |
See comment | See comment | See comment | 78 (two studies) | ⊕⊕⊝⊝ low1,3 | No between‐group comparisons reported |
| Serious adverse events | See comment | See comment | See comment | See comment | See comment | No studies reported serious adverse events |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
- One point deducted because methods of randomisation, allocation concealment and any attempts to blind outcome assessors were not described in one study assessing this outcome.
- One point deducted to reflect selective reporting as data provided by only one trial.
- One point deducted because one study had a high risk of bias for 'methods of randomisation' and unclear risk of bias for allocation concealment and any attempts to blind outcome assessors, participants and personnel.
Background
Description of the condition
Asthma is a chronic inflammatory disorder of the lungs that can lead to structural and functional changes resulting from bronchial hyper‐responsiveness and airflow obstruction (Allen 2012; Brightling 2012; Holgate 2009; Taylor 2008; Zhang 2010). Symptoms of asthma include recurrent episodes of wheeze, cough, breathlessness and chest tightness, together with episodes of marked worsening of symptoms known as exacerbations (Bateman 2008; Brightling 2012; Zhang 2010). The diagnosis of asthma is based on the person's medical history, physical examination findings, and lung function and laboratory test results (Sveum 2010).
Asthma is a serious public health problem and a major cause of disability and health resource utilisation among those affected (Bateman 2008; Eisner 2012; To 2012). Around 300 million people of all ages worldwide are affected by asthma (Bateman 2008; Bousquet 2010; Brightling 2012). Asthma is the most common chronic disease in childhood (Solé 2006). Increased morbidity, mortality and economic costs are associated with people with severe or difficult‐to‐treat asthma, particularly in industrialised countries (Eisner 2012; Zhang 2010). In addition, psychological symptoms may interfere with the severity of respiratory symptoms and may influence quality of life (Juniper 2004; Rimington 2001). Such consequences affect not only the person but the whole family universe (Nogueira 2009), especially when it comes to children.
Asthma is sometimes associated with symptomatic hyperventilation, which decreases carbon dioxide (CO2) levels, causing hypocapnia (Bruton 2005a; Laffey 2002; Thomas 2001). Hypocapnia resulting from hyperventilation may perpetuate the bronchospasm, culminating in a cycle of progressive hypocapnia and increasing bronchospasm (Laffey 2002). Thus, hypocapnia may contribute to increased airway resistance in people with asthma (Laffey 2002; van den Elshout 1991). This fact has led to increasing interest in strategies that can be used to reduce hyperventilation.
Description of the intervention
The main objective of asthma treatment is to achieve and maintain its clinical control (GINA 2015). Although no cure for asthma is known, its symptoms are controllable in most people (Taylor 2008). Asthma treatment can be pharmacological or non‐pharmacological or a combination of these approaches; it can include strategies of symptom control (information on environmental triggers and asthma education) (CTS 2012), which improve health‐related quality of life (BTS 2014; Burgess 2011; Rimington 2001; Welsh 2011). Pharmacological treatment of asthma consists of maintaining control of the disease with the least medication, thereby minimising risks of adverse effects (Sveum 2010).
Non‐pharmacological treatments have been used widely by researchers and professionals in the search for complementary therapies for the treatment of asthma; their use is reported in approximately 42% of people in some populations (Blanc 2001). Some people are interested in non‐pharmacological therapies because they may feel or hope that they will lead to improvement in overall health (Bishop 2008), and because they are keen to try to reduce the need for pharmacological treatment (Brien 2011). Non‐pharmacological interventions include breathing exercises, homeopathy, acupuncture, aromatherapy, reflexology, massage, inspiratory muscle training (IMT) and the Alexander technique (Blanc 2001; Bruton 2005b; Cooper 2003; Dennis 2012; Grammatopoulou 2011; Holloway 2007; McCarney 2003; McHugh 2003).
Breathing exercises have been used routinely by physiotherapists and other professionals to control the hyperventilation symptoms of asthma (Bruton 2005b), and can be provided in the form of the Papworth method, the Buteyko breathing technique, yoga or any similar intervention that manipulates the breathing pattern (Ram 2003). Even though breathing exercises are commonly used, there is no consensus regarding the effectiveness of breathing exercises (Ernst 2000; Freitas 2013; Ram 2003). It was previously reported that groups of people with the same baseline characteristics may show different responses to different breathing exercise techniques (Prem 2013). In addition, the duration of the intervention may interfere with the response to treatment, as has been suggested previously (Grammatopoulou 2011). One previous systematic review on breathing exercises for asthma included studies performed in participants with mild to severe asthma (Ernst 2000). However, there was no meta‐analysis assessing the impact of breathing exercises at different levels of asthma severity.
How the intervention might work
Breathing exercise techniques focus on the use of an appropriate breathing pattern to reduce hyperventilation and hyperinflation, thereby normalising CO2 levels, which may reduce bronchospasm and breathlessness (Bruton 2005b; Burgess 2011). Such techniques may also be used to help reduce anxiety associated with asthma symptoms (Singh 1990). Therefore, breathing exercises in people with asthma may also provide psychological benefits by increasing people's sense of control over their condition (Ram 2003).
Why it is important to do this review
The worldwide high prevalence of asthma has become a public health problem because of the high healthcare costs resulting from hospitalisation and medication (Giavina‐Bianchi 2010). Asthma promotes changes in the whole family context, not only because of the costs associated with health care, but also because of the negative impact of this condition on daily living, including people's quality of life (Ferreira 2010).
Asthma control is promoted by the correct use of medication and may be associated with other therapies, such as breathing exercises. Such techniques have been widely used as adjunct therapy in the treatment of people with asthma, generating considerable interest among researchers to develop studies that aim to provide evidence of this intervention. We published a Cochrane systematic review looking at the use of breathing exercises in adults with asthma (Freitas 2013). This review included studies that differed significantly in terms of intervention characteristics, such as types of breathing exercises, numbers of participants, numbers and duration of sessions, reported outcomes and statistical presentation of data. Such differences limited meta‐analysis and attainment of conclusive results. However, the review indicated that breathing exercises are a safe and well‐tolerated intervention for people with asthma. Similarly, two previous systematic reviews provided no conclusive evidence (Ernst 2000; Ram 2003), even though outcomes reported from individual trials showed that breathing exercises may have a role in the treatment and management of asthma.
It is important to synthesise the evidence obtained on such techniques, taking into account their effects in the paediatric population. To our knowledge, no systematic review on this topic has been published previously. Thus, within this review, we aimed to summarise and assess evidence from randomised controlled trials (RCTs) regarding the effects of breathing exercises in children with asthma.
Objectives
To assess the effects of breathing exercises in children with asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs.
Types of participants
We included trials involving children (younger than 18 years of age) with a diagnosis of asthma excluding other associated respiratory diseases.
Types of interventions
We included trials comparing breathing exercises alone versus a control or breathing exercises as part of a more complex intervention versus a control.
Types of outcome measures
Primary outcomes
Quality of life (measured by any respiratory disease‐specific or generic instrument).
Asthma symptoms (measured by any respiratory disease‐specific or generic instrument).
Serious adverse events (any undesired outcomes due to the intervention).
Secondary outcomes
Reduction in medication usage (e.g. inhaled or oral corticosteroids or rescue bronchodilator).
Number of acute exacerbations (mean number and number of participants experiencing one or more exacerbations).
Physiological measures, such as lung function (especially low flow rates) and functional capacity.
Days off school.
Adverse events.
It was not a criteria for inclusion that a study reported the outcomes.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Trials Search Co‐ordinator for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and by handsearching of respiratory journals and meeting abstracts (see Appendix 1 for further details). We searched all records in the CAGR using the search strategy provided in Appendix 2.
We searched trials registers such as ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/). The searches for all databases were from their inception to September 2015, and there was no restriction on language of publication imposed.
Searching other resources
We consulted reference lists of all primary studies and review articles for additional studies. We searched relevant manufacturers' websites for trial information.
We also searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
Two review authors (TMFM and DAF) independently screened titles and abstracts for inclusion of all potential studies identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study report/publication, and two review authors (TMFM and DAF) independently screened the full text, identified studies for inclusion, and identified and recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion, or, when required, we consulted a third review author (KMPPM). We identified and excluded duplicates. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and a 'Characteristics of included studies' table.
Data extraction and management
To record study characteristics and outcome data, we used a data collection form that had been piloted on at least one study in the review. One review author extracted the following study characteristics from included studies.
Methods: study design, total duration of study, method of randomisation, method of allocation concealment, outcome assessor blinding, number of study centres and locations, study setting, withdrawals and drop‐outs, and dates of the study.
Participants: number, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
Interventions: types of breathing exercises, methods (including numbers and duration of sessions and methods used in control group comparisons).
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial and notable conflicts of interest of trial authors.
Two review authors (TMFM and DAF) independently extracted outcome data from included studies. We noted in the 'Characteristics of included studies' table whether outcome data were reported in a usable way. We resolved disagreements by consensus or by involving a third review author (KMPPM). One review author (TMFM) transferred data into Review Manager 5 (RevMan 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review against the study reports. A second review author (DAF) spot‐checked study characteristics against the trial report to confirm accuracy.
Assessment of risk of bias in included studies
Two review authors (TMFM and DAF) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We resolved disagreements by discussion or by involving another review author (KMPPM). We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low or unclear and provided a quote from the study report, together with justification for our judgement, in the 'Risk of bias' table. We summarised the risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for an unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a participant‐reported pain scale).
When considering treatment effects, we took into account the risk of bias for studies that contributed to that outcome.
Measures of treatment effect
We planned to analyse dichotomous data as odds ratios, and continuous data as mean differences or standardised mean differences with 95% confidence intervals (CIs). We entered data presented on a scale with a consistent direction of effect. We undertook meta‐analyses only when this was meaningful (i.e. if treatments, participants and the underlying clinical question were similar enough for pooling to make sense).
When multiple trial arms were reported in a single trial, we included only the relevant arms. If two comparisons (e.g. breathing exercise A versus control and breathing exercise B versus control) had been combined in the same meta‐analysis, we would have halved the control group to avoid double‐counting.
Unit of analysis issues
Cross‐over trials
We did not include cross‐over studies, as the design was not appropriate for this intervention.
Cluster‐randomised trials
We planned to include data from cluster‐randomised trials if the information was available, but we identified no cluster‐randomised trials. For cluster‐randomised trials, we planned to adjust results when the unit of analysis in the trial was presented as the total number of individual participants instead of as the number of clusters. We planned to adjust the results using mean cluster size and the intracluster correlation co‐efficient (ICC) (Higgins 2011b). For meta‐analysis, we planned to combine data from individually randomised trials using the generic inverse‐variance method, as described in Chapter 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Dealing with missing data
We contacted investigators or study sponsors when possible to verify key study characteristics and to obtain missing numerical outcome data (e.g. when a study was identified as an abstract only).
Assessment of heterogeneity
We assessed heterogeneity in trial results by inspecting the forest plots to detect non‐overlapping CIs and by applying the Chi2 test (with P value = 0.10 indicating statistical significance). We used the I2 statistic to measure heterogeneity among trials in each analysis. We did not find substantial heterogeneity among the studies included in the meta‐analyses. For future updates, if we identify substantial heterogeneity (greater than 50%) we will report this and will explore possible causes by pre‐specified subgroup analysis (Higgins 2011c).
Assessment of reporting biases
If we were able to pool more than 10 trials, we would create and examine a funnel plot to explore possible small‐study and publication biases (Higgins 2011d).
Data synthesis
We used Review Manager 5 to combine outcomes when possible (RevMan 2014). We used a fixed‐effect model and had planned to use a random‐effects model if we observed substantial heterogeneity (I2 greater than 50%).
'Summary of findings' table
We planned to create a 'Summary of findings' table using the following outcomes: quality of life, asthma symptoms, serious adverse events, reduction in medication usage, number of acute exacerbations, physiological measures and days off school. In the current version of this review, the 'Summary of findings' table included the outcomes: quality of life, asthma symptoms and serious adverse events. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies contributing data to the meta‐analyses for pre‐specified outcomes. We applied methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011e) using GRADEpro software. We justified all decisions to downgrade or upgrade the quality of studies by using footnotes, and we made comments when necessary to aid readers' understanding of the review.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses if we had identified substantial heterogeneity.
Degree of asthma severity.
Duration of treatment.
Types of breathing exercises.
We planned to use the following outcomes in subgroup analyses.
Quality of life.
Reduction in medication usage.
We planned to use the formal test for subgroup interactions in Review Manager 5 (RevMan 2014).
Sensitivity analysis
If the authors were able to include sufficient data, we planned to carry out the following sensitivity analyses.
Trial quality (studies with overall high risk of bias versus overall low risk of bias).
Results
Description of studies
Results of the search
The search of the CAGR returned 129 references. Of these, we identified seven as potentially relevant, and we retrieved the full‐text articles for closer inspection, of which we included three in the review (Asher 1990; Karakoç 2000; Lima 2008). See Figure 1 for full details on the results of the search.
1.
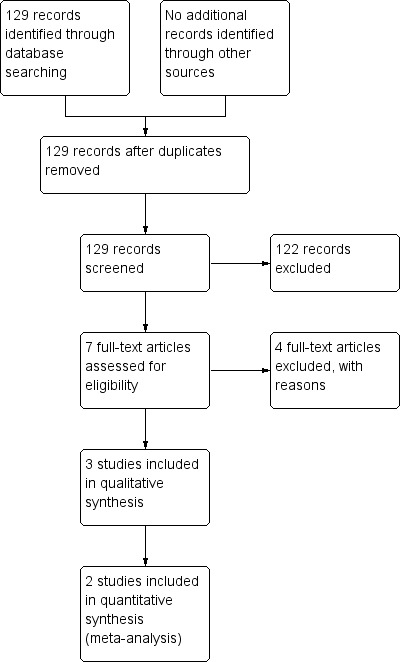
Study flow diagram
Included studies
The review included three studies (Asher 1990; Karakoç 2000; Lima 2008), involving 112 participants. See the 'Characteristics of included studies' table for full details on each study.
Setting and populations
One trial was conducted in Brazil (Lima 2008), one in New Zealand (Asher 1990), and one in Turkey (Karakoç 2000). Two trials were published in English (Asher 1990; Karakoç 2000), and one in Portuguese (Lima 2008). The studies varied in size from 28 to 60 participants. The age of the participants ranged from six to 13 years in two studies (Asher 1990; Lima 2008). However, one study did not describe the age range of the children included (Karakoç 2000). The samples consisted of outpatients (Karakoç 2000; Lima 2008), and inpatients (Asher 1990).
Interventions and control groups
All the included studies performed the comparison breathing exercises as part of a more complex intervention versus control. There were no trials comparing breathing exercises alone with control.
The study of Asher 1990 used breathing exercise techniques that consisted of lateral costal breathing and diaphragmatic breathing. The study performed vibrations, percussions and forced expiration technique. Each child received four treatments of one hour over a two‐day period (two each day). The control group received a placebo treatment that consisted of a visit for 20 minutes by a volunteer who provided emotional support to the children in hospital.
In Karakoç 2000, the pulmonary rehabilitation programme consisted of relaxation exercises, endurance exercises, breathing exercises and rhythmic mobilisation exercises. Children and their parents had visited the Physical Medicine and Rehabilitation Department at the first visit and they were thought to perform this programme at home for 30 days. The control group was not described.
In the study of Lima 2008, participants undertook IMT and breathing exercises that consisted of diaphragmatic breathing, inspiratory patterns and pursed lips and these were performed with the children in prone and seated positions. The intervention group received 14 sessions (performed twice a week). Each session lasted 50 minutes of which 25 minutes consisted of breathing exercises. Breathing exercises were performed before IMT. Children from this group also had doctor appointments and received an asthma education programme. The control group received an educational programme and doctor appointments.
Outcomes
The three included studies did not specify their primary and secondary outcomes.
Excluded studies
After we had retrieved the full text of potentially eligible trials, we excluded four studies from the review. Reasons for exclusion are described in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
Full details of risk of bias judgements are in the 'Characteristics of included studies' table and in Figure 2.
2.
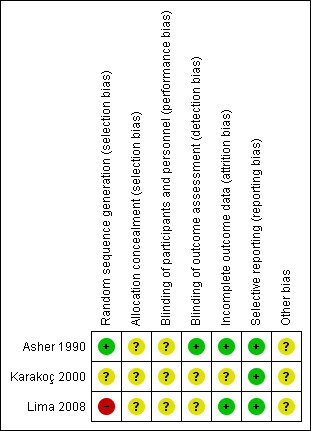
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
One included study performed adequate sequence generation and was at low risk of bias (Asher 1990). In this study, participants were assigned using a table of random numbers. One study was reported as randomised but gave no description of the method used and we therefore judged it at unclear risk of bias (Karakoç 2000). One included study reported that randomisation was undertaken by simple drawing (Lima 2008). As this method may not be considered as an adequate random component, we therefore judged it to be at a high risk of bias.
None of the included studies described the method used for allocation concealment, and therefore we judged them as having an unclear risk of bias (Asher 1990; Karakoç 2000; Lima 2008).
Blinding
Double‐blinding is not possible or practical in studies involving breathing exercises. Participants in these trials must know whether or not they are undertaking breathing training or asthma education due to the characteristics of the intervention. However, it is possible to blind the assessor who is analysing the results of the trial.
None of the included studies described blinding of participants or personnel, so we judged them as being at an unclear risk of bias (Asher 1990; Karakoç 2000; Lima 2008).
One study described blinding of outcome assessors (Asher 1990). In this study, a technician blinded to the treatment assessed lung function. Thus, the study was at low risk of bias. Two studies did not describe blinding of outcome assessment, so we judged them as having an unclear risk of bias (Karakoç 2000; Lima 2008).
Incomplete outcome data
One study did not describe the occurrence of withdrawals and drop‐outs and we judged this as having an unclear risk of bias (Karakoç 2000). Two studies described withdrawals and drop‐outs and we judged them at low risk of bias because the reasons for missing outcome data were unlikely to be related to true outcomes (Asher 1990; Lima 2008). The study of Asher 1990 affirmed that four children completed the initial treatment, but not the final treatment: one of each group due to early discharge, and one child of the intervention group withdrew twice due to headache and vomiting. Lima 2008 affirmed that two children were out of the age range proposed by the study and eight children did not complete the final treatment.
Selective reporting
The three included studies adequately reported outcome data as listed in the methods, although the protocol for each study was not available (Asher 1990; Karakoç 2000; Lima 2008). We judged that there was a low risk of reporting bias.
Other potential sources of bias
All the studies had an unclear risk of bias, as they did not provide sufficient information to allow assessment of whether an important risk of bias was present (Asher 1990; Karakoç 2000; Lima 2008).
Effects of interventions
See: Table 1
Primary outcomes
Quality of life
Only one study reported quality of life, but the study did not provide between‐group analysis (Karakoç 2000). The study specified that the quality of life questionnaire used was developed by Juniper 1993.
Karakoç 2000 reported data at baseline and at one month after baseline (post‐treatment). The group receiving the intervention had an improvement in quality of life after the end of the treatment (P value = 0.009). However, the study did not find a difference in quality of life scores when comparing baseline and post‐treatment in the control group (P value = 0.16).
Asthma symptoms
Two studies involving 78 participants assessed asthma symptoms, but provided no between‐group analysis (Karakoç 2000; Lima 2008).
The study of Karakoç 2000 reported data at baseline and at one month after baseline (post‐treatment). Lima 2008 reported data at baseline and at three months after baseline. In Karakoç 2000, the intervention group showed an improvement in symptom scores when comparing values before and after treatment (P value = 0.01). In Lima 2008, there was a significant improvement in symptom scores after the intervention group received physiotherapy (P value < 0.0001). The study did not provide a description of the type of score or questionnaire used. However, this study assessed symptom variables such as: diurnal symptoms, nocturnal symptoms and impaired daily activity.
Serious adverse events
None of the included studies reported serious adverse events (Asher 1990; Karakoç 2000; Lima 2008).
Secondary outcomes
Reduction in medication usage
Two studies assessed reduction in medication use, but provided no between‐group analysis (Karakoç 2000;Lima 2008).
In the study of Karakoç 2000, there was a statistically significant decrease in medication score in the rehabilitation group (P value < 0.05) when comparing baseline and post‐treatment. The study of Lima 2008 affirmed there was a reduction in medication use (rescue bronchodilator) when comparing baseline with post‐treatment in the intervention group (P value < 0.0001). However, these studies did not state how the assessments were undertaken.
Number of acute exacerbations
One included study assessed the number of acute exacerbations and found that there was no significant reduction in the number of visits to the emergency department and hospitalisations between groups (P value = 0.17) (Lima 2008). This study affirmed there was a reduction in the number of acute exacerbations (described as "frequency of asthma attacks" in the study) after treatment in the intervention group (P value < 0.0001). However, this study did not state how the assessment was undertaken.
Physiological measures ‐ lung function and functional capacity
All included studies assessed lung function (Asher 1990; Karakoç 2000; Lima 2008).
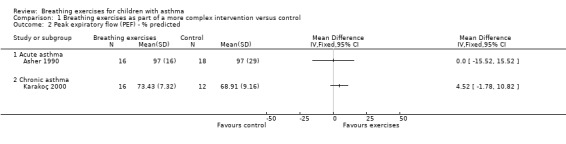
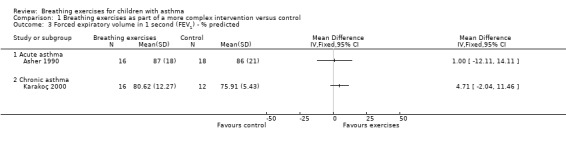
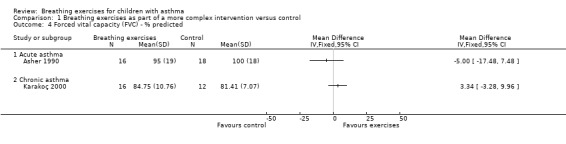
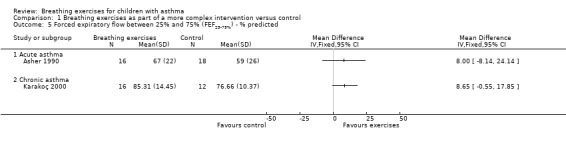
Asher 1990 assessed forced vital capacity (FVC), functional residual capacity (FRC), residual volume (RV), total lung capacity (TLC), peak expiratory flow (PEF), forced expiratory volume in one second (FEV1) and forced expiratory flow between 25% and 75% (FEF25‐75%). Values were expressed as percent predicted compared to reference standards. Taking into consideration the baseline, lung function at the end of the study was not statistically different between groups.
Karakoç 2000 found that pulmonary function measures (vital capacity (VC), FVC, FEV1, PEF and FEF25‐75%) expressed as percent predicted) significantly improved in the treatment group after following the intervention (P value < 0.05), whereas there was no difference in the control group. Assessments were undertaken before and after the study (Karakoç 2000). There was no between‐group analysis.
Lima 2008 assessed PEF in three time points: baseline (T0), 49 days (T1) and 90 days (T2) after treatment. Values were expressed as absolute values. There was no difference in the control group in all the assessments. In the intervention group, there was a statistical improvement between T0 and T1, and T0 and T2. There was also a significant difference between groups at T1 and T2. However, the P value was not provided. We included data in a forest plot considering T1 as post‐baseline (Figure 3). Data were shown as separate subgroups according to post‐baseline (T1) and three months' post‐baseline (T3).
3.
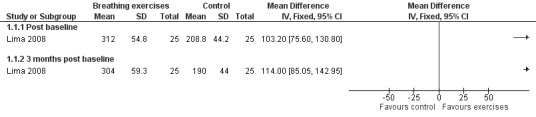
Forest plot of comparison: 1 Physiological measures, outcome: 1.2 Peak expiratory flow (PEF) ‐ absolute values
Forest plots show the results for the studies of Asher 1990 and Karakoç 2000 (Figure 4; Figure 5; Figure 6; Figure 7). The Asher 1990 study looked at acute asthma and Karakoç 2000 looked at chronic asthma, so these are shown as separate subgroups and we have not combined the results.
4.
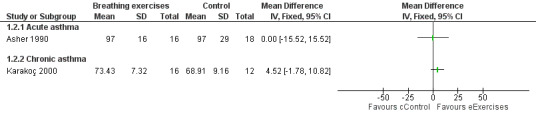
Forest plot of comparison: 1 Physiological measures, outcome: 1.1 Peak expiratory flow (PEF) ‐ % predicted.
5.
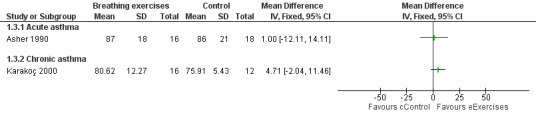
Forest plot of comparison: 1 Physiological measures, outcome: 1.2 Forced expiratory volume in one second (FEV1) ‐ % predicted.
6.
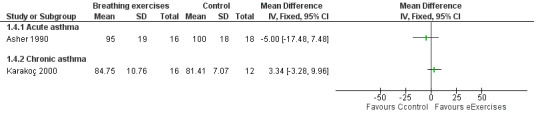
Forest plot of comparison: 1 Physiological measures, outcome: 1.3 Forced vital capacity (FVC) ‐ % predicted.
7.
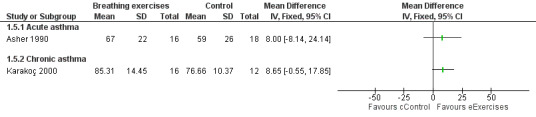
Forest plot of comparison: 1 Physiological measures, outcome: 1.4 Forced expiratory flow between 25% and 75% (FEF25‐75%) ‐ % predicted.
Days off school
None of the included studies reported days off school (Asher 1990; Karakoç 2000; Lima 2008).
Adverse events
None of the included studies reported adverse events (Asher 1990; Karakoç 2000; Lima 2008).
Discussion
Summary of main results
We assessed the effects of breathing exercises in the treatment of children with asthma. The age range of participants varied from six to 13 years old. A total of 124 children satisfied the inclusion criteria in the three studies. Twelve children did not complete the treatment, leaving 112 participants. However, one study did not report if there were withdrawals and drop‐outs. The studies differed significantly regarding some characteristics of the intervention such as: type of breathing exercise, number of participants, number and duration of sessions, and severity of asthma. Moreover, the included studies only performed the comparison breathing exercises as part of a more complex intervention versus control. None of the studies described between‐group analysis well. Overall, we assessed the risk of bias of the included studies as uncertain and we graded the overall quality of the evidence as low.
None of the included studies reported between‐group comparisons of the results of our primary outcomes.
Overall completeness and applicability of evidence
The outcomes assessed by the included studies did not address all the outcomes proposed by the review. From the eight outcomes proposed, two were not assessed: days off school and adverse effects. Moreover, the breathing exercises used in the included studies did not comprise all the breathing exercises techniques. In one study, children performed lateral costal breathing and diaphragmatic breathing (Asher 1990), and in another study, children performed diaphragmatic breathing and inspiratory patterns with pursed lips (Lima 2008). One study did not describe the breathing exercise technique used (Karakoç 2000). One previous Cochrane review on breathing exercises for adults with asthma included studies that performed breathing exercise techniques that were not performed in the present review such as the Buteyko method, the Papworth method and yoga (Freitas 2013). However, we did include both comparisons proposed in the protocol of this review (breathing exercises alone versus control or breathing exercises as part of a more complex intervention versus control). One study compared IMT plus breathing exercises versus asthma education (Lima 2008). Asher 1990 compared breathing exercises, vibrations, percussions and forced expiration technique versus a placebo treatment. One study did not describe the control group and the intervention consisted of relaxation exercises, endurance exercises, breathing exercises and rhythmic mobilisation exercises (Karakoç 2000). The included studies in this review did not isolate the breathing exercises component of the intervention and, thus, it was not possible to be sure what was the 'active component' of the intervention.
The studies included children with different levels of asthma severity when compared to the Cochrane review performed with adults (Freitas 2013): uncontrolled (Lima 2008), mild persistent or moderate (Karakoç 2000), and severe acute asthma (Asher 1990). Furthermore, participants in the included studies consisted of both inpatients (Asher 1990) and outpatients (Karakoç 2000; Lima 2008). In the study of Karakoç 2000, children and their parents were taught to perform the exercises at home for 30 days, whereas in the study of Lima 2008, children performed the intervention supervised by a professional.
The age range of two included studies was six to 13 years old (Asher 1990; Lima 2008), while this was not described in one included study (Karakoç 2000); however, the mean age of the children was approximately 10 years old. It is known that several changes occur in the respiratory system during childhood (Merkus 1996; Prasad 2008). Thus, the intervention to be used, as well as the results found, may be different between children and adolescents.
Quality of the evidence
We downgraded our assessment of the quality of the evidence presented in this review due to concerns about small sample size, a small number of sessions in some studies, and limitations in the design and reporting of studies leading to risk of bias.
No included studies compared breathing exercises alone versus control. All the included studies compared breathing exercises as part of a more complex intervention (such as IMT and endurance training) versus control. This fact limited the interpretation of the benefits of breathing exercises alone.
The included studies had an overall small number of participants. The impact of a small sample size on a trial's result was reported by Moher 1994. Moher 1994 reviewed 383 RCTs and concluded that most trials with negative results did not have large enough sample sizes to detect relative difference.
The CONSORT (Consolidated Standards of Reporting Trials) statement recommends a description on how sample size was determined (CONSORT 2010). Only one study from the included studies performed sample size and power calculations (Lima 2008, based on maximal inspiratory pressure). Moreover, the number of sessions among studies was small with a larger duration of seven weeks.
From the three studies included in the review, we classified only one overall to have a low risk of bias (Asher 1990), we classified Karakoç 2000 as having an unclear risk of bias, whereas Lima 2008 was classified as having a high risk of bias. In addition, not all included studies described allocation concealment and we classified this as an unclear risk of bias. Inadequate reporting of trial methods can severely impede the assessment of trial quality and the risk of bias in trial results (Savović 2012). Moreover, this study also affirmed that this is a particular problem for the assessment of sequence generation and allocation concealment, which are often not described in trial publications (Savović 2012). In addition, inadequately reported randomisation has been associated with bias in estimating the effectiveness of interventions (Moher 2001).
When conducting an RCT that involves breathing exercises it is not possible for participants and the personnel delivering the intervention to be blinded (Holloway 2007); this is known as performance bias. However, it is possible for the personnel collecting and analysing data to be blinded (detection bias). Only one study was at low risk of detection bias and the other two studies were at unclear risk of bias. According to Savović 2012, the lack of or unclear double‐blinding (participants and personnel) can be associated with marked exaggeration of intervention effect estimates.
The studies did not describe the units for some of the outcomes well. For example, asthma symptoms and reduction in medication usage were reported as a score; however, there was no description of the type of score or questionnaire.
Potential biases in the review process
We made an effort to apply robust methods in the process of analysing the search, collecting data, performing meta‐analysis and assessing risk of bias. Nevertheless, some points must be taken into consideration.
There were some insufficient methodological details as well as missing quantitative outcome data in the included studies. Incomplete outcome data limited analysis once data from these studies could not be entered into a meta‐analysis. Moreover, the subgroup and the sensitivity analyses proposed by the review were not possible due to the impossibility of obtaining sufficient data.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first systematic review designed to assess the effects of breathing exercises in children with asthma. Three previous systematic reviews were performed with the same objective, but not with the same population (Ernst 2000; Freitas 2013; Ram 2003). All the previous systematic reviews included studies that were performed with adults with asthma. However, the review of Ernst 2000 included one study that was also included in the present review. Ernst 2000 assessed asthma symptoms and lung function. The outcomes assessed by Ram 2003 were quality of life, asthma symptoms, number of exacerbations and lung function; and Freitas 2013 assessed quality of life, asthma symptoms, number of acute exacerbations, capnography and pulmonary function. The study of Ernst 2000 included six studies that performed yoga, diaphragmatic breathing and slow deep breathing. Ram 2003 included six studies and evaluated the Buteyko method, yoga and diaphragmatic breathing. Freitas 2013 selected 13 studies that performed diaphragmatic breathing, the Buteyko method, yoga, the Papworth method and short breathing retraining.
Breathing exercises are used by many people with asthma worldwide as an adjunctive treatment with the aim of developing a more efficient pattern of respiration, decreasing the respiratory rate (Bruton 2005b; Thomas 2009), and, thus, achieving a control of the disease that may also improve quality of life (Grammatopoulou 2011).
Ernst 2000 stated that it was not possible to make firm judgements in their review, and recommended that further rigorous trials should be carried out in order to make data available to answer this question. Similarly, the systematic review performed by Ram 2003 concluded that it was not possible to draw any firm conclusions regarding the effectiveness of breathing exercises in the management of asthma. Freitas 2013 concluded that there was not enough evidence supporting the efficacy of breathing exercises in adults with asthma.
It is important to emphasise that there are some methodological differences between the current systematic review and the previous reviews (Ernst 2000; Freitas 2013; Ram 2003). The review of Ernst 2000 and Ram 2003 were published in the early 2000s. Moreover, Ernst 2000 included two crossover studies, which we excluded as we did not believe this was a suitable trial design for this intervention due to carry‐over effects. Besides that, the current review compared breathing exercises as part of a more complex intervention versus control.
Authors' conclusions
Implications for practice.
Due to the small number of participants, methodological differences, and heterogeneity in the populations and interventions in the included studies we found no conclusive evidence for the benefits or risks of breathing exercises in children with asthma and, thus, we could infer no clinically meaningful implications in this review.
Implications for research.
There is a need for well‐conducted RCTs to assess if breathing exercises in addition to conventional care can improve outcomes for children with asthma. Ideally, the breathing exercises should be the sole additional intervention and should be compared to an inactive control or usual care alone, in order that the added benefits and risks of breathing exercises can be isolated. Breathing exercises are much simpler and more accessible than many of the other interventions in the management of children with asthma. Any new studies must include the full description of the outcome assessments and a more detailed description of the interventions used. Furthermore, much more attention needs to be paid to good reporting and high‐quality study design in any future studies, including items such as: adequate random sequence generation and allocation concealment, blinding of outcome assessors, determination of the trial sample size before the beginning of the study and between‐group analysis.
Acknowledgements
The review authors would like to thank Emma Welsh (the Managing Editor of the Cochrane Airways Group) for assistance provided at the start of this review, Elizabeth Stovold (the Information Specialist of the Cochrane Airways Group) for the search strategy used in the review and Emma Jackson (Editorial Assistant of the Cochrane Airways Group) for assistance provided.
Anne Holland was the Editor for this review and commented critically on the review.
The background and methods sections of this review were based on a standard template used by the Cochrane Airways Group.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly4 |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify randomised controlled trials (RCTs)
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and the RCT filter were adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to identify relevant trials from the Cochrane Airways Group Specialised Register (CAGR)
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 MeSH DESCRIPTOR Breathing Exercises
#6 (breath*) NEAR5 (technique* or exercise* or re‐train* or train* or re‐educat* or educat* or physiotherap* or "physical therapy" or "respiratory therapy")
#7 buteyko or "qigong yangsheng" or pranayama* OR yoga*
#8 "breathing control"
#9 #5 or #6 or #7 or #8
#10 #4 and #9
#11 child* or paediat* or pediat* or adolesc* or infan* or toddler* or bab* or young* or preschool* or "pre school*" or pre‐school* or newborn* or "new born*" or new‐born* or neo‐nat* or neonat*
#12 MeSH DESCRIPTOR Child Explode All
#13 MeSH DESCRIPTOR Pediatrics Explode All
#14 MeSH DESCRIPTOR Infant Explode All
#15 MeSH DESCRIPTOR Adolescent Explode All
#16 #11 or #12 or #13 or #14 or #15
#17 #10 and #16
[Note: in search line #1, MISC1 refers to the field in the record where the reference has been coded for condition, in this case, asthma]
Data and analyses
Comparison 1. Breathing exercises as part of a more complex intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peak expiratory flow (PEF) ‐ absolute values | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Post baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 3 months post baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Peak expiratory flow (PEF) ‐ % predicted | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Acute asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Chronic asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Forced expiratory volume in 1 second (FEV1) ‐ % predicted | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Acute asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Chronic asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Forced vital capacity (FVC) ‐ % predicted | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Acute asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Chronic asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Forced expiratory flow between 25% and 75% (FEF25‐75%) ‐ % predicted | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Acute asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Chronic asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
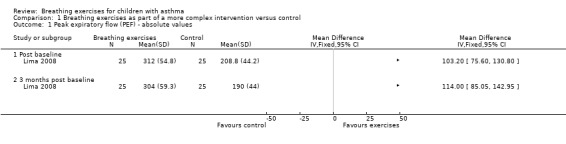
Comparison 1 Breathing exercises as part of a more complex intervention versus control, Outcome 1 Peak expiratory flow (PEF) ‐ absolute values.
1.2. Analysis.
Comparison 1 Breathing exercises as part of a more complex intervention versus control, Outcome 2 Peak expiratory flow (PEF) ‐ % predicted.
1.3. Analysis.
Comparison 1 Breathing exercises as part of a more complex intervention versus control, Outcome 3 Forced expiratory volume in 1 second (FEV1) ‐ % predicted.
1.4. Analysis.
Comparison 1 Breathing exercises as part of a more complex intervention versus control, Outcome 4 Forced vital capacity (FVC) ‐ % predicted.
1.5. Analysis.
Comparison 1 Breathing exercises as part of a more complex intervention versus control, Outcome 5 Forced expiratory flow between 25% and 75% (FEF25‐75%) ‐ % predicted.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asher 1990.
| Methods | Design: randomised controlled trial Total duration of study: six months Country: New Zealand Study setting: inpatient Method of randomisation: participants were assigned using a table of random numbers to either a placebo treatment group or a chest physiotherapy (PT) group Method of allocation concealment: not described Outcome assessor blinding: assessor was blinded to the treatment received Withdrawals/drop‐outs: four withdrawals |
|
| Participants | Severity of condition: acute severe asthma Diagnostic criteria: acute asthma was defined as "acute asthma poorly responsive to inhaled bronchodilator on admission to hospital" Total sample: 38 children (19 in each group). Results available for 34 children (16 in chest PT group and 18 in placebo group) Mean age: 9.5 ± 2.7 years in chest PT group and 10.0 ± 2.6 years in placebo group Gender: 11 boys and eight girls in chest PT group and nine boys and 10 girls in placebo group Age range: 6‐13 years Exclusion criteria: participants who were critically ill or who had complications identified on a chest radiograph such as lobar atelectasis or pneumonia |
|
| Interventions | Treatments were started in the study between six and 24 hours after admission to hospital. Each child then received four treatments over a two‐day period (two each day). First treatment began in the morning and the subsequent treatments were separated by approximately 4 hours. Each treatment period lasted about 1 hour. Before every treatment, participants received inhaled salbutamol via a nebuliser over 10 minutes, followed by a rest period of 20 minutes. This was then followed by either a placebo treatment or chest PT lasting 20‐30 minutes Intervention: during PT sessions appropriate techniques were used according to the participant's presentation. The breathing exercise techniques used were lateral costal breathing and diaphragmatic breathing. The study also performed vibrations, percussions and the forced expiration technique. Education and psychological support were also provided as appropriate Control: a 20‐minute visit by a volunteer who provided emotional support to children in hospital. They were instructed to provide no form of chest PT and to ignore coughing |
|
| Outcomes | Lung function | |
| Notes | Financial support from the Auckland Medical Research Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned using a table of random numbers to either a placebo treatment group or a chest PT group |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available, but the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
Karakoç 2000.
| Methods | Design: double‐blind randomised study Total duration of study: not described Country: Turkey Study setting: outpatient Method of randomisation: not described Method of allocation concealment: not described Outcome assessor blinding: not described Withdrawals/drop‐outs: not described |
|
| Participants | Severity of condition: mild persistent or moderate asthma Diagnostic criteria: not described Total sample: 28 children Mean age: 10.8 ± 2.3 years in intervention group and 10.2 ± 2.4 years in control group Gender: seven boys and nine girls in intervention group, six boys and six girls in control group Age range: not described Inclusion criteria: children had to be using the same medications at least for 6 months |
|
| Interventions | Intervention (active treatment): pulmonary rehabilitation programme consisted of relaxation exercises, endurance exercises, breathing exercises and rhythmic mobilisation exercises. Children and their parents had visited the Physical Medicine and Rehabilitation Department for the first visit and they were thought to perform this programme at home for 30 days. The study did not describe the type of breathing exercises used Control group: not described |
|
| Outcomes | Quality of life index Symptoms scores Medication scores Pulmonary function |
|
| Notes | No financial support described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available, but the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
Lima 2008.
| Methods | Design: randomised controlled trial Total duration of study: seven months Country: Brazil Study setting: outpatient Method of randomisation: simple drawing at the moment when each child was admitted to the programme Method of allocation concealment: not described Outcome assessor blinding: not described Withdrawals/drop‐outs: two withdrawals and eight drop‐outs |
|
| Participants | Severity of condition: uncontrolled asthma Diagnostic criteria: medical diagnosis according to the 1st Brazilian Consensus of Asthma Management Total sample: 60 children. Results available for 50 children (25 in each group) Mean age: 9.6 ± 1.2 years in intervention group and 9.76 ± 1.2 years in control group Gender: nine boys and 16 girls in intervention group, seven boys and 18 girls in control group Age range: 8‐12 years Inclusion criteria: children aged 8‐12 years, not attending a prior Physiotherapy/Medical programme, uncontrolled asthma, be part of the "Assistance Programme for the Asthmatic Patient" (Programa de Assistência ao Paciente Asmático) of the Federal University of Maranhão (Brazil) |
|
| Interventions | Intervention (active treatment): received 14 sessions (performed twice a week) of two treatments (inspiratory muscle training and breathing exercises). Each session lasted 50 minutes of which 25 minutes consisted of breathing exercises. Breathing exercises consisted of diaphragmatic breathing, inspiratory patterns and pursed lips and were performed with the children in prone and seated positions. Each breathing exercise was repeated 10 times. Breathing exercises were performed before inspiratory muscle training. Children also had doctor appointments and received an asthma education programme (described below in the control group) Control group: children of the control group attended an asthma education programme that was performed once a month with 60‐minute duration. They received information regarding asthma, such as: symptoms, environmental 'triggers' and basic information related to medication. Children also had doctor appointments |
|
| Outcomes | Peak expiratory flow Severity variables (frequency of asthma attacks, symptoms, daily living activities, medication, hospitalisations, visits to the emergency department) |
|
| Notes | No financial support described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation undertaken by simple drawing of lots |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available, but the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chiang 2009 | Breathing exercise was not a major component of the intervention |
| DiDario 2010 | Physiotherapy sessions did not involve breathing exercises |
| Flapper 2008 | Intervention consisted of physical exercises |
| Laurino 2012 | Mean age of participants was > 18 years |
Differences between protocol and review
There was a change to the outcomes included in the 'Summary of findings' table.
Contributions of authors
Thalita Macêdo: selected the studies, extracted data, entered data into Review Manager 5, carried out the analysis, interpreted data and drafted the final review.
Diana Freitas: selected the studies, extracted data, entered data into Review Manager 5, carried out the analysis, interpreted data and drafted the final review.
Gabriela Chaves: drafted the final review.
Elizabeth Holloway: drafted the final review and contributed with clinical expertise.
Karla Mendonça: co‐ordinated the review, made an intellectual contribution, interpreted data and drafted the final review.
Sources of support
Internal sources
Federal University of Rio Grande do Norte, Brazil.
External sources
The authors declare that no external funding was received for this systematic review, Other.
Declarations of interest
None known.
New
References
References to studies included in this review
Asher 1990 {published data only}
- Asher MI, Douglas C, Airy M, Andrews D, Trenholme A. Effects of chest physical therapy on lung function in children recovering from acute severe asthma. Pediatric Pulmonology 1990;9(3):146‐51. [DOI] [PubMed] [Google Scholar]
Karakoç 2000 {published data only}
- Karakoç GB, Yilmaz M, Sur S, Altintas DU, Sarpel T, Kendirli SG. The effects of daily pulmonary rehabilitation program at home on childhood asthma. Allergologia et Immunopathologia 2000;28(1):12‐4. [PubMed] [Google Scholar]
Lima 2008 {published data only}
- Lima EVNCL, Lima WL, Nobre A, Santos AM, Brito LMO, Costa MRSE. Inspiratory muscle training and respiratory exercises in children with asthma [Treinamento muscular inspiratório e exercícios respiratórios em crianças asmáticas]. Jornal Brasileiro de Pneumologia 2008;34(8):552‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chiang 2009 {published data only}
- Chiang LC, Ma WF, Huang JL, Tseng LF, Hsueh KC. Effect of relaxation‐breathing training on anxiety and asthma signs/symptoms of children with moderate‐to‐severe asthma: a randomized controlled trial. International Journal of Nursing Studies 2009;46(8):1061‐70. [DOI] [PubMed] [Google Scholar]
DiDario 2010 {published data only}
- DiDario AG, Whelan MA, Hwan WH, Yousef E, Cox TJ, Oldham HM, et al. Efficacy of chest physiotherapy in pediatric patients with acute asthma exacerbations. Pediatric Asthma, Allergy and Immunology 2010;22(2):69‐74. [Google Scholar]
Flapper 2008 {published data only}
- Flapper BC, Duiverman EJ, Gerritsen J, Postema K, Schans CP. Happiness to be gained in paediatric asthma care. European Respiratory Journal 2008;32(6):1555‐62. [DOI] [PubMed] [Google Scholar]
Laurino 2012 {published data only}
- Laurino RA, Barnabé V, Saraiva‐Romanholo BM, Stelmach R, Cukier A, Nunes Mdo P. Respiratory rehabilitation: a physiotherapy approach to the control of asthma symptoms and anxiety. Revista do Hospital das Clínicas 2012;67(11):1291‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Allen 2012
- Allen JC, Seidel P, Schlosser T, Ramsay EE, Ge Q, Ammit AJ. Cyclin D1 in ASM cells from asthmatics is insensitive to corticosteroid inhibition. Journal of Allergy 2012:307838. [DOI: 10.1155/2012/307838] [DOI] [PMC free article] [PubMed]
Bateman 2008
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M. Global strategy for asthma management and prevention: GINA executive summary. European Respiratory Journal 2008;31(1):143‐78. [DOI] [PubMed] [Google Scholar]
Bishop 2008
- Bishop FL, Yardley L, Lewith GT. Treat or treatment: a qualitative study analyzing patients' use of complementary and alternative medicine. American Journal of Public Health 2008;98(9):1700‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blanc 2001
- Blanc PD, Trupin L, Earnest G, Katz PP, Yelin EH, Eisner MD. Alternative therapies among adults with a reported diagnosis of asthma or rhinosinusitis: data from a population‐based survey. Chest 2001;120(5):1461‐7. [DOI] [PubMed] [Google Scholar]
Bousquet 2010
- Bousquet J, Mantzouranis E, Cruz AA, Aït‐Khaled N, Baena‐Cagnani CE, Bleecker ER, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. Journal of Allergy and Clinical Immunology 2010;126(5):926‐38. [DOI] [PubMed] [Google Scholar]
Brien 2011
- Brien SB, Bishop FL, Riggs K, Stevenson D, Freire V, Lewith G. Integrated medicine in the management of chronic illness: a qualitative study. British Journal of General Practice 2011;61(583):e89‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brightling 2012
- Brightling CE, Gupta S, Gonem S, Siddiqui S. Lung damage and airway remodelling in severe asthma. Clinical and Experimental Allergy 2012;42(5):638‐49. [DOI] [PubMed] [Google Scholar]
Bruton 2005a
- Bruton A, Holgate ST. Hypocapnia and asthma: a mechanism for breathing retraining?. Chest 2005;127(5):1808‐11. [DOI] [PubMed] [Google Scholar]
Bruton 2005b
- Bruton A, Lewith GT. The Buteyko breathing technique for asthma: a review. Complementary Therapies in Medicine 2005;13(1):41‐6. [DOI] [PubMed] [Google Scholar]
BTS 2014
- British Thoracic Society. British guideline on the management of asthma, 2014. www.brit‐thoracic.org.uk/document‐library/clinical‐information/asthma/btssign‐asthma‐guideline‐2014/ (accessed 26 January 2016).
Burgess 2011
- Burgess J, Ekanayake B, Lowe A, Dunt D, Thien F, Dharmage SC. Systematic review of the effectiveness of breathing retraining in asthma management. Expert Review of Respiratory Medicine 2011;5(6):789‐807. [DOI] [PubMed] [Google Scholar]
CONSORT 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine 2010;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2003
- Cooper S, Oborne J, Newton S, Harrison V, Thompson Coon J, Lewis S, et al. Effect of two breathing exercises (Buteyko and pranayama) in asthma: a randomised controlled trial. Thorax 2003;58(8):674‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
CTS 2012
- Lougheed MD, Lemiere C, Ducharme FM, Licskai C, Dell SD, Rowe BH, et al. Canadian Thoracic Society Asthma Clinical Assembly. Canadian Thoracic Society 2012 guideline update: diagnosis and management of asthma in preschoolers, children and adults. Canadian Respiratory Journal 2012;19(2):127‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dennis 2012
- Dennis JA, Cates CJ. Alexander technique for chronic asthma. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD000995.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisner 2012
- Eisner MD, Yegin A, Trzaskoma B. Severity of asthma score predicts clinical outcomes in patients with moderate to severe persistent asthma. Chest 2012;141(1):58‐65. [DOI] [PubMed] [Google Scholar]
Ernst 2000
- Ernst E. Breathing techniques ‐ adjunctive treatment modalities for asthma? A systematic review. European Respiratory Journal 2000;15(5):969‐72. [DOI] [PubMed] [Google Scholar]
Ferreira 2010
- Ferreira LN, Brito U, Ferreira PL. Quality of life in asthma patients. Revista Portuguesa de Pneumologia 2010;16(1):23‐55. [PubMed] [Google Scholar]
Freitas 2013
- Freitas DA, Holloway EA, Bruno SS, Chaves GSS, Fregonezi GAF, Mendonça KMPP. Breathing exercises for adults with asthma. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD001277.pub3] [DOI] [PubMed] [Google Scholar]
Giavina‐Bianchi 2010
- Giavina‐Bianchi P, Aun MV, Bisaccioni C, Agondi R, Kalil J. Difficult‐to‐control asthma management through the use of a specific protocol. Clinics 2010;65(9):905‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
GINA 2015
- Global Initiative for Asthma. Global strategy for asthma management and prevention 2015. www.ginasthma.com (accessed 26 January 2016).
Grammatopoulou 2011
- Grammatopoulou EP, Skordilis EK, Stavrou N, Myrianthefs P, Karteroliotis K, Baltopoulos G, et al. The effect of physiotherapy‐based breathing retraining on asthma control. Journal of Asthma 2011;48(6):593‐601. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochranehandbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochranehandbook.org.
Higgins 2011c
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochranehandbook.org.
Higgins 2011d
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochranehandbook.org.
Higgins 2011e
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochranehandbook.org.
Holgate 2009
- Holgate ST, Arshad HS, Roberts GC, Howarth PH, Thurner P, Davies DE. A new look at the pathogenesis of asthma. Clinical Science 2009;118(7):439‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holloway 2007
- Holloway EA, West RJ. Integrated breathing and relaxation training (the Papworth method) for adults with asthma in primary care: a randomised controlled trial. Thorax 2007;62(12):1039‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juniper 1993
- Juniper EF, Guyatt GH, Ferrie PJ, Griffith LE. Measuring quality of life in asthma. American Review Respiratory Disease 1993;147:832‐8. [DOI] [PubMed] [Google Scholar]
Juniper 2004
- Juniper EF, Wisniewski ME, Cox FM, Emmett AH, Nielsen KE, O'Byrne PM. Relationship between quality of life and clinical status in asthma: a factor analysis. European Respiratory Journal 2004;23(2):287‐91. [DOI] [PubMed] [Google Scholar]
Laffey 2002
- Laffey JG, Kavanagh BP. Hypocapnia. New England Journal of Medicine 2002;347(1):43‐53. [DOI] [PubMed] [Google Scholar]
McCarney 2003
- McCarney RW, Brinkhaus B, Lasserson TJ, Linde K. Acupuncture for chronic asthma. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD000008.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McHugh 2003
- McHugh P, Aitcheson F, Duncan B, Houghton F. Buteyko breathing technique for asthma: an effective intervention. New Zealand Medical Journal 2003;116(1187):U710. [PubMed] [Google Scholar]
Merkus 1996
- Merkus PJ, Have‐Opbroek AA, Quanjer PH. Human lung growth: a review. Pediatric Pulmonology 1996;21(6):383‐97. [DOI] [PubMed] [Google Scholar]
Moher 1994
- Moher D, Dulberg CS, Wells GA. Statistical power, sample size, and their reporting in randomized controlled trials. JAMA: the Journal of the American Medical Association 1994;272(2):122‐4. [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357(9263):1191‐4. [PubMed] [Google Scholar]
Nogueira 2009
- Nogueira KT, Silva JRL, Lopes CS. Quality of life of asthmatic adolescents: assessment of asthma severity, comorbidity, and life style [in Portuguese]. Jornal de Pediatria 2009;85(6):523‐30. [DOI] [PubMed] [Google Scholar]
Prasad 2008
- Prasad SA, Main E, Dodd ME. Finding consensus on the physiotherapy management of asymptomatic infants with cystic fibrosis. Pediatric Pulmonology 2008;43(3):236‐44. [DOI] [PubMed] [Google Scholar]
Prem 2013
- Prem V, Sahoo RC, Adhikari P. Comparison of the effects of Buteyko and pranayama breathing techniques on quality of life in patients with asthma: a randomized controlled trial. Clinical Rehabilitation 2013;27(2):133‐41. [DOI] [PubMed] [Google Scholar]
Ram 2003
- Ram FS, Holloway EA, Jones PW. Breathing retraining for asthma. Respiratory Medicine 2003;97(5):501‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rimington 2001
- Rimington LD, Davies DH, Lowe D, Pearson MG. Relationship between anxiety, depression, and morbidity in adult asthma patients. Thorax 2001;56(4):266‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Savović 2012
- Savović J, Jones H, Altman D, Harris R, Jűni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Singh 1990
- Singh V, Wisniewski A, Britton J, Tattersfield A. Effect of yoga breathing exercises (pranayama) on airway reactivity in subjects with asthma. Lancet 1990;335(8702):1381‐3. [DOI] [PubMed] [Google Scholar]
Solé 2006
- Solé D, Wandalsen GF, Camelo‐Nunes IC, Naspitz CK. Prevalence of symptoms of asthma, rhinitis, and atopic eczema among Brazilian children and adolescents identified by the International Study of Asthma and Allergies in Childhood (ISAAC) ‐ Phase 3. Jornal de Pediatria 2006;82(5):341‐6. [DOI] [PubMed] [Google Scholar]
Sveum 2010
- Sveum R, Bergstrom J, Brottman G, Hanson M, Heiman M, Johns K, et al. Institute for Clinical Systems Improvement. Diagnosis and management of asthma. Updated 2012. www.icsi.org/_asset/rsjvnd/Asthma.pdf (accessed 27 January 2016).
Taylor 2008
- Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, et al. A new perspective on concepts of asthma severity and control. European Respiratory Journal 2008;32(3):545‐54. [DOI] [PubMed] [Google Scholar]
Thomas 2001
- Thomas M, McKinley RK, Freeman E, Foy C. Prevalence of dysfunctional breathing in patients treated for asthma in primary care: cross sectional survey. BMJ 2001;322(7294):1098‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2009
- Thomas M, McKinley RK, Mellor S, Watkin G, Holloway E, Scullion J, et al. Breathing exercises for asthma: a randomised controlled trial. Thorax 2009;64:55‐61. [DOI] [PubMed] [Google Scholar]
To 2012
- To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, et al. Global asthma prevalence in adults: findings from the cross‐sectional world health survey. BMC Public Health 2012;12:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
van den Elshout 1991
- Elshout FJ, Herwaarden CL, Folgering HT. Effects of hypercapnia and hypocapnia on respiratory resistance in normal and asthmatic subjects. Thorax 1991;46(1):28‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Welsh 2011
- Welsh EJ, Hasan M, Li P. Home‐based educational interventions for children with asthma. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD008469.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2010
- Zhang X, Köhl J. A complex role for complement in allergic asthma. Expert Review of Clinical Immunology 2010;6(2):269‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]


