Abstract
Background
End‐stage renal disease (ESRD) patients often require either the formation of an arteriovenous (AV) fistula or an AV interposition prosthetic shunt for haemodialysis. These access sites should ideally have a long life and a low rate of complications (for example thrombosis, infection, stenosis, aneurysm formation and distal limb ischaemia). Although some of the complications may be unavoidable, any adjuvant technique or medical treatment aimed at decreasing complications would be welcome. This is the second update of the review first published in 2004.
Objectives
To assess the effects of adjuvant drug treatment in ESRD patients on haemodialysis via autologous AV fistulae or prosthetic interposition AV shunts.
Search methods
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator searched the Specialised Register (last searched March 2015) and CENTRAL (2015, Issue 2).
Selection criteria
Randomised controlled trials (RCTs) of active drug versus placebo in people with ESRD undergoing haemodialysis via an AV fistula or prosthetic interposition AV graft.
Data collection and analysis
For this update, the two review authors (NCT, ADS) independently assessed trial quality and one review author (NCT) extracted data. Information on adverse events was collected from the trials. The primary outcome was the long‐term fistula or graft patency rate. Secondary outcomes included duration of hospital stay, complications and number of related surgical interventions.
Main results
For this update, an additional six studies were deemed suitable for inclusion, making a total of 15 trials with 2230 participants. Overall the quality of the evidence was low due to short follow‐up periods, heterogeneity between trials and moderate methodological quality of the studies due to incomplete reporting. Medical adjuvant treatments used in the trials were aspirin, ticlopidine, dipyridamole, dipyridamole plus aspirin, warfarin, fish oil, clopidogrel, sulphinpyrazone, and human type I pancreatic elastase (PRT‐201). Where possible, the included studies were pooled into similar medical adjuvant groups for meta‐analyses.
All included studies reported on graft patency by measuring graft thrombosis. There was insufficient evidence to determine if there was a difference in graft patency in studies comparing aspirin versus placebo (three RCTs, 175 participants) (odds ratio (OR) 0.40, 95% confidence interval (CI) 0.07 to 2.25; P = 0.30). The meta‐analysis for graft patency comparing ticlopidine versus placebo (three RCTs, 339 participants) favoured ticlopidine (OR 0.45, 95% CI 0.25 to 0.82; P = 0.009). There was insufficient evidence to determine if there was a difference in graft patency in studies comparing fish oil versus placebo (two RCTs, 220 participants; OR 0.24, 95% CI 0.03 to 1.95; P = 0.18); and studies comparing clopidogrel and placebo (two RCTs, 959 participants; OR 0.40, 95% CI 0.13 to 1.19; P = 0.10). Similarly, there was insufficient evidence to determine if there was a difference in graft patency in three studies (306 participants) comparing PRT‐201 versus placebo (OR 0.75, 95% CI 0.42 to 1.32; P = 0.31); in one trial comparing the effect of dipyridamole versus placebo (42 participants; OR 0.46, 95% CI 0.11 to 1.94, P = 0.29) and dipyridamole plus aspirin versus placebo (41 participants; OR 0.64, CI 0.16 to 2.56, P = 0.52); in one trial comparing low‐dose warfarin with placebo (107 participants; OR 1.76, 95% CI 0.78 to 3.99, P = 0.17); and one trial (16 participants) comparing sulphinpyrazone versus placebo (OR 0.43, 95% CI 0.03 to 5.98, P = 0.53). The single trial evaluating warfarin was terminated early because of major bleeding events in the warfarin group. Only two studies published data on the secondary outcome of related interventions (surgical or radiological); there was insufficient evidence to determine if there was a difference in related interventions between placebo and treatment groups. No studies reported on the length of hospital stay and data reporting on complications was limited and varied between studies.
Authors' conclusions
The meta‐analyses of three studies for ticlopidine (an anti‐platelet treatment), which all used the same dose of treatment but with a short follow‐up of only one month, suggest ticlopidine may have a beneficial effect as an adjuvant treatment to increase the patency of AV fistulae and grafts in the short term. There was insufficient evidence to determine if there was a difference in graft patency between placebo and other treatments such as aspirin, fish oil, clopidogrel, PRT‐201, dipyridamole, dipyridamole plus aspirin, warfarin, and sulphinpyrazone. However, the quality of the evidence was low due to short follow‐up periods, the small number of studies for each comparison, heterogeneity between trials and moderate methodological quality of the studies due to incomplete reporting. It, therefore, appears reasonable to suggest further prospective studies be undertaken to assess the use of these anti‐platelet drugs in renal patients with an arteriovenous fistula or graft.
Plain language summary
Medical adjuvant treatment to increase the patency of arteriovenous fistulae and grafts used for renal dialysis
Background
People with advanced kidney disease (end‐stage renal disease) need dialysis to perform kidney functions. In haemodialysis, blood is filtered through a machine. To allow a large enough passage for blood to flow between the person and the machine, an artery and a vein can be surgically joined (to form an arteriovenous fistula) or an artificial graft (a substitute for a vein) is used to join the artery to the vein. These access points might last for years but can become blocked or infected. This review investigates if additional medical therapy can keep these dialysis access points functioning.
Key results
The review authors found 15 randomised controlled trials (evidence current to March 2015) with a total of 2230 participants, of anti‐platelet drugs (such as ticlopidine, aspirin, dipyridamole and clopidogrel) or anti‐thrombotic and other drug treatments used to prevent blockages in the artery and vein access points for dialysis. Where possible, similar studies were pooled. Pooled data from three trials (339 participants) comparing ticlopidine (a platelet aggregation inhibitor) with placebo, showed improved blood flow at one month. There was insufficient evidence of an effect on blood flow from pooled data from three trials comparing aspirin with placebo (175 participants) or from two trials using fish oil for 12 months (220 participants). Three studies assessed the effect of human type I pancreatic elastase (PRT‐201) with placebo in 306 participants. Overall the trials showed there was insufficient evidence of an effect on blood flow between active treatment (PRT‐201) and placebo. Two trials compared clopidogrel with placebo in 959 participants and again showed there was insufficient evidence of an effect between the treatments. Single trials involving 16 to 36 participants compared dipyridamole, dipyridamole plus aspirin or sulphinpyrazone (a uricosuric drug) with placebo. The estimated effects were compatible with both benefits and harm. One trial comparing warfarin with placebo (107 participants) was terminated early because of major bleeding events in the warfarin group. Only two studies reported on related interventions (surgical or radiological); there was insufficient evidence of an effect on related interventions between placebo and treatment. No studies reported on the length of hospital stay and information on complications of treatment was limited and, if reported, varied from study to study. Most had a short follow‐up period so that any benefits in the longer term are not clear.
Quality of the evidence
Overall the quality of the evidence was low due to short follow‐up periods, small number of studies for each comparison, and differences between the studies (in follow‐up time and dosages used). In addition the methodological quality of the studies was moderate due to incomplete reporting.
Background
Description of the condition
End‐stage renal disease (ESRD) patients continue to die prematurely despite continuing advances in our knowledge on dialysis. Renal patients on long‐term haemodialysis require either an autologous (using the individual's own tissue) arteriovenous (AV) fistula (when an artery is connected to a vein) or an AV interposition prosthetic shunt (when an artery is connected to a vein through an artificial graft) for access. These access sites should ideally have a long life and a low rate of complications (for example thrombosis, infection, stenosis, aneurysm formation and distal limb ischaemia). Currently, there is no access type that fulfils all these criteria. However, long‐term (up to four or five years) patency rates demonstrate that autologous AV fistulae have the best outcomes with fewer surgical interventions (Churchill 1992; Metha 1991; Pisoni 2002). Complications from haemodialysis access result in frequent hospitalisation of patients with ESRD and often require further intervention.
Why it is important to do this review
Although some of the complications may be unavoidable, any adjuvant technique or medical treatment aimed at decreasing complications would be welcome. It is recognised that up to 85% of cases of vascular access failure may be due to thrombosis caused by myointimal hyperplasia (proliferation of the cells lining the blood vessel), particularly in prosthetic grafts (Kanterman 1995). There is already some evidence suggesting that the formation of an autologous AV fistula may be preferable to prosthetic material and that graft surveillance programmes lead to improvements in long‐term graft patency rates (National Kidney Foundation Dialysis Outcome Quality Initiative) (DOQI 1997). However, there is also some accumulating evidence that drug manipulation for patients with ESRD and autologous AV fistula (Dember 2008; Ghorbani 2009), and those with prosthetic interposition AV shunts (Lok 2012; Schmitz 2002), may confer additional benefits in terms of long‐term patency and fewer complications.
Objectives
To assess the effects of adjuvant drug treatment in ESRD patients on haemodialysis via autologous AV fistulae or prosthetic interposition AV shunts.
The primary outcome measure was long‐term graft patency rate, as outlined by life tables or Kaplan‐Meier survival curves (Kaplan 1958). Secondary outcome measures included complications such as infection, aneurysm formation, distal limb ischaemia and need for surgical or radiological intervention.
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials of active drug versus placebo in people with ESRD who were undergoing haemodialysis via an AV fistula or prosthetic interposition AV shunt. Any method of randomisation was accepted and differences in quality were taken into account in the analysis. Trials that were not analysed on an intention‐to‐treat basis were included provided that all randomised participants were accounted for.
Types of participants
People with ESRD and receiving haemodialysis through an AV fistula or a prosthetic AV shunt. All types of AV fistulae or AV prosthetic shunts positioned in the upper or lower limb, or at special sites (for example axillary artery to axillary vein), were considered. People on peritoneal dialysis, although they may have a patent AV fistula or interposition prosthetic AV shunt, were not included.
Types of interventions
Any drug therapy given to people with ESRD with the aim of improving the patency of AV fistulae or prosthetic grafts, compared with placebo. Examples included aspirin in AV fistulae (Andrassy 1974); and dipyridamole in AV prosthetic grafts (Sreedhara 1994).
We excluded studies investigating medical adjuvant therapy versus no treatment or intervention.
Types of outcome measures
Primary outcomes
The primary outcome measure considered was graft patency. It was intended to measure graft patency rates using life tables or Kaplan‐Meier survival curves but the trials did not provide sufficient data to be pooled (Kaplan 1958). Future updates of this review will incorporate these measures if sufficient data become available.
Secondary outcomes
Secondary outcome measures included duration of hospital stay; complications such as infection, aneurysm formation, stenosis and distal limb ischaemia; and the number of related surgical or radiological interventions.
Search methods for identification of studies
Electronic searches
For this update the Cochrane Peripheral Vascular Diseases (PVD) Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched March 2015) and the Cochrane Central Register of Controlled Trials (CENTRAL) 2015, Issue 2, part of the Cochrane Library, (www.cochranelibrary.com). See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Peripheral Vascular Diseases Group module in the Cochrane Library (www.cochranelibrary.com).
Searching other resources
The reference lists of all included and excluded studies were cross‐checked for additional publications which may have been suitable.
Data collection and analysis
Selection of studies
For this update, one of the review authors (NCT) identified possible trials and the other review author (ADS) independently assessed the unblinded reports to confirm eligibility for inclusion in the review. Any disagreement was resolved by discussion.
Data extraction and management
Data were extracted independently by NCT using proformas designed by the Cochrane PVD Group. Discrepancies were discussed and reviewed by ADS. Data were extracted from the published reference papers directly. No attempt was made to obtain additional unpublished data. All analyses were based on endpoint data from the individual clinical trials. If several trials assessed the effect of the same adjuvant therapy (even if dosage was different) then results were amalgamated.
Assessment of risk of bias in included studies
The methodological quality of included trials was assessed independently by two review authors (NCT, ADS). Discrepancies were resolved by discussion. Quality was assessed using the 'Risk of bias' tool as described in section 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This assesses various domains (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and ‘other bias’) and determines the level of bias for each domain, namely 'low', 'high' or 'unclear' risk of bias. These assessments were reported for each individual study in the Risk of bias in included studies tables.
Measures of treatment effect
Statistical analysis was performed according to the statistical guidelines for authors in the Cochrane PVD Group information. Odds ratio (OR) with 95% confidence intervals (CI) were used as the measure of effect for each dichotomous outcome.
Unit of analysis issues
The participant was the unit of analysis.
Three trials had unit of analysis issues which required further evaluation by the review authors.
The Grontoft 1998 study had a non‐standard design as it allowed people who had an initial failed AV fistula formation to re‐enter the study after a three week 'washout period'. In addition, the published results had to be carefully interpreted as there was confusion between the number of participants within the study compared to the number of operations (i.e. AV fistulae formed), and participants who died were excluded from the published analysis. Overall analysis for this review was undertaken using the number of operations performed on an 'intention‐to‐treat' basis.
The results of the Ghorbani 2009 study had to be carefully assessed by the review authors as the original data were not analysed on an 'intention‐to‐treat' basis, but analysed by the number of participants who completed the trial.
Grontoft 1985 also published their analysed results based on the number of participants who completed the trial, not an 'intention‐to‐treat' basis. It was not possible to use the intention‐to‐treat numbers because insufficient data regarding the original randomisation groups were provided. We therefore used the numbers of participants who completed the trial.
Dealing with missing data
Analysis was performed on a complete case basis and no attempt was made to contact study authors for further follow‐up data. It was not necessary to contact authors for additional data.
Assessment of heterogeneity
Chi² tests were used to assess for heterogeneity between grouped trials, with P values lower than 0.2 being used as an indication of the possibility of the presence of significant heterogeneity. Some trials contained low participant numbers and therefore the power of this test is likely to be low if a small P value was used (Higgins 2011).
Assessment of reporting biases
There were insufficient studies identified to create funnel plots to assess reporting bias.
Data synthesis
Where there were sufficient data from more than one trial, a meta‐analysis using a Mantel‐Haenszel fixed‐effect model was used. Where heterogeneity was identified (Chi² tests with P values lower than 0.2) we used a random‐effects model. Review Manager 5 software was used for data synthesis (Review Manager 5).
Subgroup analysis and investigation of heterogeneity
No subgroup analysis was performed as insufficient data were available to perform additional subgroup analyses on infection, aneurysm formation and distal limb ischaemia.
Sensitivity analysis
Sensitivity analysis was not performed due to insufficient data.
Results
Description of studies
Results of the search
See Figure 1.
1.
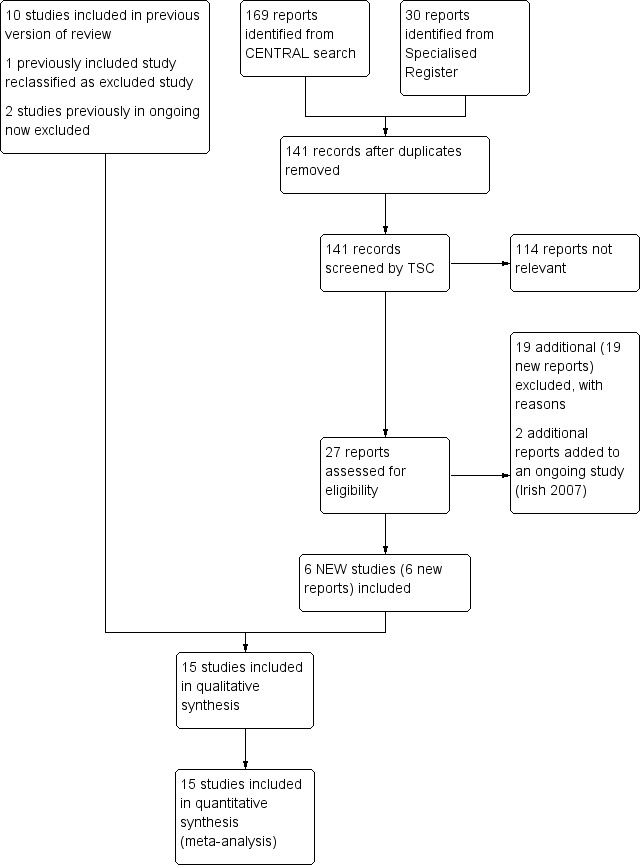
Study flow diagram.
Fifteen randomised controlled trials fulfilled the criteria for consideration in the review (Andrassy 1974; Crowther 2002; Dember 2008; Dwivedi 2014; Fiskerstrand 1984; Ghorbani 2009; Grontoft 1985; Grontoft 1998; Harter 1979; Hye 2014; Lok 2012; Michie 1977; Peden 2013; Schmitz 2002; Sreedhara 1994). Twenty‐six studies were excluded (Albert 1978; Alexopoulos 2011; Bhomi 2008; Chen 2013; D'Ayala 2008; De Fijter 1995; Dixon 2009; Dulude 2001; Fuster 2001; Janicki 2003; Kaegi 1975; Kaufman 2003; Kooistra 1994; Krasinski 2004; Lee 2009; Lin 2007; Lin 2013; Malovrh 2009; Mileti 1995; Radmilovic 1998; Rouzrokh 2009; Taber 1992; Trimarchi 2006; Wang 2010; Wasse 2014; Yuto 2012). One ongoing study was identified (Irish 2007).
Included studies
For this update an additional six studies were included (Dember 2008; Dwivedi 2014; Ghorbani 2009; Hye 2014; Lok 2012; Peden 2013), making a total of 15 included studies (Andrassy 1974; Crowther 2002; Dember 2008; Dwivedi 2014; Fiskerstrand 1984; Ghorbani 2009; Grontoft 1985; Grontoft 1998; Harter 1979; Hye 2014; Lok 2012; Michie 1977; Peden 2013; Schmitz 2002; Sreedhara 1994).
Three of the trials compared the effect of aspirin versus placebo and included a total of 175 participants (Andrassy 1974; Harter 1979; Sreedhara 1994). The follow‐up time and the dosage of aspirin given were different in each of the trials. In Sreedhara 1994, 39 participants undergoing insertion of a new arteriovenous (AV) polytetrafluoroethylene (PTFE) graft were followed up for 18 months over a period of six years. Twenty participants received aspirin 325 mg once daily and the remainder received placebo. Andrassy 1974 included 92 participants undergoing Brescia‐Cimino fistula formation. Of these, 45 participants received aspirin 500 mg once daily whereas the other 47 participants were given placebo. The total follow‐up period was 28 days. Finally, the Harter trial included 44 participants undergoing AV shunt formation between the radial artery and the cephalic vein using silastic (silicone rubber) material with a Teflon adapter (Harter 1979). Nineteen participants received a single dose of 160 mg once‐daily aspirin and 25 participants received placebo. The follow‐up period was approximately five months.
Sreedhara 1994 also examined the effects of dipyridamole versus placebo with a total number of 42 participants in a parallel group trial over a six‐year period. Twenty‐three participants received dipyridamole at a dose of 75 mg three times a day; the other 19 received the equivalent placebo. All participants were followed up for 18 months.
In a separate arm of the trial, Sreedhara 1994 studied the effect a combination of dipyridamole and aspirin had versus placebo. Forty‐one participants were involved (22 receiving dipyridamole 75 mg with 325 mg aspirin daily; 19 participants taking placebo).
Only type I participants (participants requiring a new AV expanded polytetrafluoroethylene (ePTFE) graft) were included in all analyses of this review. Additional data on type II participants (participants with an ePTFE graft which had thrombosed requiring thrombectomy or additional PTFE jump graft) was provided but not included for analysis as it was decided this cohort was high risk for thrombosis and did not meet our inclusion criteria.
Three trials compared ticlopidine with placebo, with a total number of 339 participants undergoing AV fistula formation or graft interposition (Fiskerstrand 1984; Grontoft 1985; Grontoft 1998). There were no separate data for participants undergoing either fistula formation or graft interposition. Each participant was given either 250 mg of ticlopidine twice daily or the equivalent placebo. All participants were followed up for one month in all three trials.
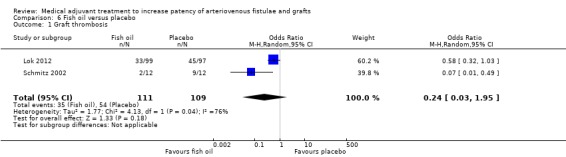
Two trials examined the effects of fish oil (4 g once daily) versus placebo, and included a total of 220 participants undergoing graft formation with a follow‐up time of 12 months (Lok 2012; Schmitz 2002). In the Schmitz 2002 trial 24 participants were enrolled (12 participants received fish oil and 12 received an equivalent dose of a control oil). The primary outcome was graft thrombosis, but the authors did not state how patency was assessed. The Lok 2012 trial included 196 participants (99 participants received fish oil and 97 received a placebo oil capsule). The primary outcome was loss of native patency, which was defined as the graft having a primary event of thrombosis or requiring radiological or surgical intervention to maintain patency or promote maturation. However, they did provide separate data on graft thrombosis and this was included in the meta‐analysis.
Crowther 2002 compared the effects of low intensity warfarin treatment (variable dose but a target international normalised ratio (INR) for prothrombin time of 1.4 to 1.9) with placebo. A total of 107 participants were randomised (56 to warfarin and 51 to placebo); all had newly placed PTFE grafts. How the authors assessed patency was not clear in that graft thrombosis was described as the "inability to dialyse or the need for immediate intervention to allow dialysis". This study was terminated early due a high rate of serious adverse events in the treatment arm.
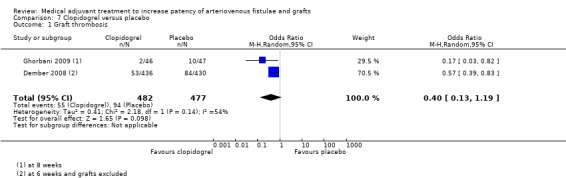
Two trials compared the effects of clopidogrel versus placebo and included a total of 959 participants (Dember 2008; Ghorbani 2009). Dember 2008 assessed 866 participants for patency failure six weeks after native arteriovenous fistula formation. A total of 436 participants received a loading dose of 300 mg clopidogrel on postoperative day one followed by 75 mg daily; and 430 received matching placebo daily. Ghorbani 2009 compared 46 participants who received 75 mg clopidogrel daily with 47 who received placebo. Treatment was initiated 7 to 10 days prior to formation of a native arteriovenous fistula and continued for six weeks postoperatively. The primary outcome was fistula failure at eight weeks.
Michie 1977 compared the uricosuric drug sulphinpyrazone (200 mg four times daily) with placebo. Sixteen participants were randomised (eight to each group). There was heterogeneity across the treatment groups (six participants had AV fistulae and two had bovine interposition grafts in both groups). Participants were followed up for approximately three months or until 33 dialysis sessions had been undertaken.
Two trials from the same investigational group assessed the effect of a recombinant type I pancreatic elastase (PRT‐201) applied directly to the adventitia of the inflow and outflow blood vessels of newly‐created radiocephalic or brachiocephalic fistulae (Hye 2014; Peden 2013). The phase 1 trial compared 45 participants who received a variety of different doses of PRT‐201 to 21 participants who received placebo (Peden 2013). The phase 2 trial compared 51 participants receiving placebo to 51 participants who received 10 μg, and 49 participants who received 30 μg, of PRT‐201 (Hye 2014). All participants in both trials were followed up for 12 months. Dwivedi 2014 also investigated the effect of PRT‐201 on AV grafts. This compared 28 participants who received placebo compared to 61 participants who received varying doses of PRT‐201 applied directly to the vein‐graft anastomosis and the adjacent outflow vein at the time of AV graft construction. All participants were followed up for 12 months.
In most studies, patency of AV shunts and grafts was assessed by detecting the presence of fistula or graft thrombosis. In the Andrassy 1974, Dember 2008 and Ghorbani 2009 studies, fistula clotting was detected by palpation (touch) and auscultation (listening with a stethoscope). In addition to those parameters, Sreedhara 1994 also included the presence of thrombus that was noticed during introduction of the dialysis needle into the graft, whereas the Dwivedi 2014, Grontoft 1998, Peden 2013 and Hye 2014 studies added the use of Doppler technique to confirm the presence or absence of blood flow. The Harter study documented thrombosis by physical removal of thrombi from either the venous or arterial limbs of the shunt when dialysis was initiated (Harter 1979). The Crowther 2002 study defined graft thrombosis as an inability to dialyze or a need for immediate intervention to allow dialysis; and the earlier Grontoft 1985 study documented patency by simply stating that each fistula was assessed at regular intervals. The Lok 2012 study monitored participants biweekly and performed an angiogram if decreasing dialysis flow rates indicated stenosis. Michie 1977 used Doppler, ease of cannulation, palpation and auscultation to assess patency; there was no documentation of how patency was assessed in the Fiskerstrand or Schmitz studies (Fiskerstrand 1984; Schmitz 2002).
See Characteristics of included studies for further details.
Excluded studies
See Characteristics of excluded studies
For this update an additional 19 studies were excluded (Alexopoulos 2011; Bhomi 2008; Chen 2013; D'Ayala 2008; De Fijter 1995; Dixon 2009; Fuster 2001; Kooistra 1994; Lee 2009; Lin 2007; Lin 2013; Malovrh 2009; Mileti 1995; Rouzrokh 2009; Taber 1992; Trimarchi 2006; Wang 2010; Wasse 2014; Yuto 2012). In total 26 studies were excluded (Albert 1978; Alexopoulos 2011; Bhomi 2008; Chen 2013; D'Ayala 2008; De Fijter 1995; Dixon 2009; Dulude 2001; Fuster 2001; Janicki 2003; Kaegi 1975; Kaufman 2003; Kooistra 1994; Krasinski 2004; Lee 2009; Lin 2007; Lin 2013; Malovrh 2009; Mileti 1995; Radmilovic 1998; Rouzrokh 2009; Taber 1992; Trimarchi 2006; Wang 2010; Wasse 2014; Yuto 2012).
Thirteen published studies were reviewed as they appeared to meet the review inclusion criteria, but the study designs did not incorporate a placebo‐controlled group (Albert 1978; Bhomi 2008; Chen 2013; D'Ayala 2008; Dixon 2009; Fuster 2001; Lee 2009; Lin 2007; Lin 2013; Malovrh 2009; Trimarchi 2006; Wang 2010; Yuto 2012); or were not randomised (Janicki 2003). These were therefore not deemed suitable for inclusion in the review. Trimarchi 2006 had been included in previous editions of this Cochrane review but on repeat evaluation it was concluded that the study did not meet inclusion criteria as there was no placebo‐controlled arm to the trial. Malovrh 2009, evaluating the effect of blood volume expansion during surgery, did not use a suitable randomisation method or have placebo‐controlled groups, and was deemed not to meet review criteria. Rouzrokh 2009 presented unclear results which included an assessment of fistula patency, but the timeframe of treatment or assessment was not documented. These data could not therefore be included for analysis. Wasse 2014 provided data on the percentage of participants who were successfully using their AV fistula or AV graft at six months but did not provide data to calculate the number of thromboses and therefore patency. There are multiple reasons why a fistula may not be used after creation besides loss of patency, therefore this study was excluded. A Dutch randomised placebo‐controlled trial examined the effect of fish oil and placebo (De Fijter 1995). However the trial outcomes did not include fistula patency and participants included both those on haemodialysis and peritoneal dialysis. A single study examining the effect of sulphinpyrazone versus placebo was also considered (Kaegi 1975). Some of the participants also received warfarin either at the start or during the trial and, as the method for randomisation was not clearly concealed, this study was not included in this review. We excluded the Kooistra 1994 trial because long‐standing (more than 6 weeks old) AV fistulae were assessed, and concurrent initiation of recombinant human erythropoietin with antiplatelet agent was studied, which we deemed a confounding factor. Another considered study analysed the effects of clopidogrel and aspirin versus placebo on rates of thrombosis in PTFE grafts (Kaufman 2003). However, the total number of thrombotic episodes in each group could not be determined from the results and so the study was excluded. A further trial examined the effects of pentoxifylline versus placebo on rates of thrombosis in external AV fistulae (Radmilovic 1998). As this method of fistula formation is now obsolete, the authors felt that including this study would be inappropriate. One trial sought to assess the effects of CJC‐1004 (a locally acting anti‐thrombotic agent) on graft occlusion (Dulude 2001). However, this was published as an abstract and, despite a literature search, no follow‐up paper could be found. Similarly, no relevant data were published by Alexopoulos 2011, Krasinski 2004 and Taber 1992. One further reference to a study that appeared to meet the inclusion criteria was retrieved (Mileti 1995). The reference described the rationale and design of an ongoing study. No results have been published.
Risk of bias in included studies
The summarised data are included in the Characteristics of included studies table and Figure 2; Figure 3
2.
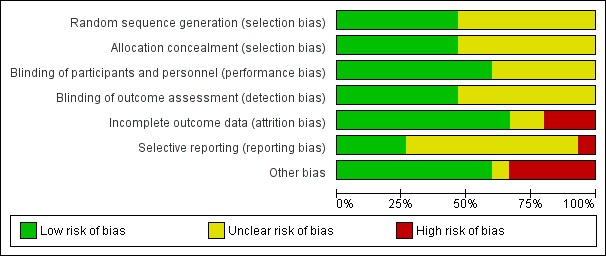
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
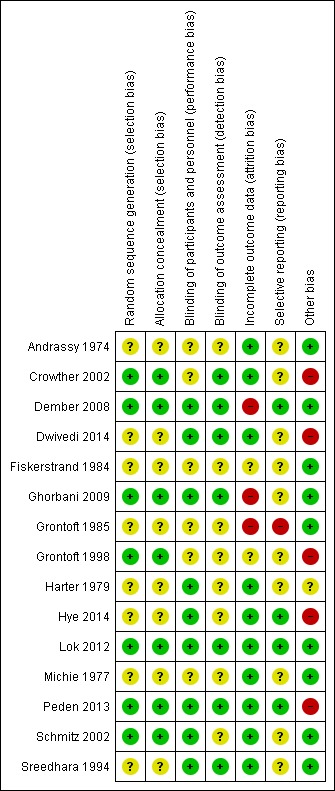
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Crowther 2002, Dember 2008, Ghorbani 2009, Lok 2012, Peden 2013, and Schmitz 2002 all utilised a permuted block randomisation schedule performed via an independent operator and so were deemed to have low risk of allocation bias. The Grontoft 1998 study used an independent statistician and a minimisation scheme based on four weighted stratification variables, implying that allocation concealment was acceptable.
Andrassy 1974 used an independent statistician but the precise method of randomisation was not stated, and Sreedhara 1994 stated a pre‐determined schedule was used for randomisation but exact details were not provided. These were classed as having an unclear risk of selection bias.
Dwivedi 2014, Fiskerstrand 1984, Grontoft 1985, Harter 1979, Hye 2014, and Michie 1977 did not provide sufficient data to allow a judgement of bias.
Blinding
Dember 2008, Dwivedi 2014, Ghorbani 2009, Lok 2012, Peden 2013, and Sreedhara 1994 had thorough blinding of participants and assessors, providing a low risk of performance and detection bias.
Participants who developed a short‐term indication for warfarin therapy were not excluded from analysis in the Crowther 2002 study and it is therefore unclear if there may have been a element of performance bias. However it is clear that detection bias was low risk as the clinical staff who assessed graft thrombosis were blinded to allocation assignment.
Harter 1979, Hye 2014, and Schmitz 2002 clearly stated that participants were blinded to treatment allocation, indicating a low risk of performance bias, but did not provide sufficient data on outcome assessment to allow a judgement of detection bias.
The Grontoft 1998 study allowed re‐entry of participants into the study after secondary randomisation, and this may have lead to unblinding of the participants.
Andrassy 1974, Fiskerstrand 1984, Grontoft 1985, and Michie 1977 did not provide sufficient data to allow a judgement of bias.
Incomplete outcome data
Andrassy 1974, Harter 1979, Hye 2014, Lok 2012, Michie 1977, and Peden 2013 had no missing data and a low risk of attrition bias.
In the Schmitz 2002 study only one participant (out of 24) was lost to follow‐up; and 12 (out of 107 randomised) within the Sreedhara 1994 study were lost to follow‐up or did not complete the study. Both of these were deemed to have a low risk of attrition bias.
Crowther 2002 followed up all participants and ascertained the outcome for all participants, indicating a low risk of attrition bias. When data were analysed, participants who had a renal transplant, developed a clinical indication for the active treatment in this study (i.e. warfarin) or had their AV graft removed were censored.
Dember 2008 did not assess patency in 11 participants at six weeks and Ghorbani 2009 required re‐analysis of the published data as the study authors did not perform analysis on an intention‐to‐treat basis but no participants were lost to follow‐up. Eighteen participants did not complete the study protocol.
Dwivedi 2014 analysed all participants on an 'as‐treated' basis although 50% of participants did not complete the study (45 out of 89). Reasons for non‐completion included losses to follow‐up, participant death, AV graft abandonment and recovery of renal function.
Grontoft 1985 performed analysis on the number of participants who completed the trial. As data were not provided within the publication of the initial randomisation groups it was therefore not possible to determine if, and how many, participants did not complete the trial.
In the Grontoft 1998 study, data were missing on one participant but an additional 17 participants could not have the AV fistulae or grafts evaluated. These were included in the data analysis though.
Fiskerstrand 1984 did not provide sufficient data to allow a judgement of bias.
Selective reporting
A pre‐study protocol was not available in 10 studies and therefore it is not possible to determine if outcome reporting was selective (Andrassy 1974; Crowther 2002; Dwivedi 2014; Fiskerstrand 1984; Ghorbani 2009; Grontoft 1998; Harter 1979; Michie 1977; Schmitz 2002; Sreedhara 1994).
Dember 2008, Hye 2014, and Lok 2012 did not deviate from pre‐study protocol‐determined outcomes and have a low risk of reporting bias.
The authors of the Grontoft 1985 study provide an 'as treated' analysis, and additional data to permit an 'intention‐to‐treat' analysis was not provided.
Other potential sources of bias
Dwivedi 2014, Hye 2014 and Peden 2013 had a high risk of other bias as they were financially supported by Proteon Therapeutics who presumably supplied the recombinant human pancreatic elastase used.
The Crowther 2002 study protocol was changed after an interim analysis with regards to the dosing of warfarin, and 40% of participants also received additional antiplatelet therapy during the study period. This could have potentially influenced the study outcomes, including the high rate of bleeding reported in this study, and so was it judged to be at high risk of bias.
Other potential bias within studies include the re‐entry of participants into randomisation after a washout period of three weeks if their first operation failed, which was permitted in the Grontoft 1998 study protocol. Harter 1979 did not have a pre‐specified enrolment value, and protocol stated that enrolment would continue until 24 thrombi were observed so was judged as having an unclear risk of bias.
Andrassy 1974, Dember 2008, Fiskerstrand 1984, Ghorbani 2009, Grontoft 1985, Lok 2012, Michie 1977, Schmitz 2002, and Sreedhara 1994 had no obvious other source of bias and so were at low risk of bias.
Effects of interventions
Primary outcomes
Graft patency
For the primary outcome 'graft patency' the numbers recorded for analyses were the number of grafts that occluded during the specified time period as reported by the study authors. This should be kept in mind when interpreting the presented data.
Graft patency rates as outlined by life tables or Kaplan‐Meier survival curves were not carried out as the description of the trials did not provide sufficient data (Kaplan 1958).
Aspirin versus placebo ‐ Analysis 1.1
Andrassy 1974 reported that 2 out of 45 in the aspirin group developed a graft thrombosis compared with 11 out of 47 who received placebo (odds ratio (OR) 0.15, 95% confidence interval (CI) 0.03 to 0.73). In the Harter 1979 study, 6 out of 19 in the aspirin group developed a graft thrombosis compared with 18 out of 25 in the placebo group (OR 0.18, 95% CI 0.05 to 0.66). In the Sreedhara 1994 study, 10 out of 20 who received aspirin developed a graft thrombosis compared with 6 out of 19 on placebo (OR 2.17, 95% CI 0.59 to 7.99).
Due to evidence of heterogeneity (Chi2 9.31, P = 0.01) we used a random‐effects model meta‐analysis. The overall results showed no differences between the treatments (OR 0.40, 95% CI 0.07 to 2.25). The overall P value was 0.30.
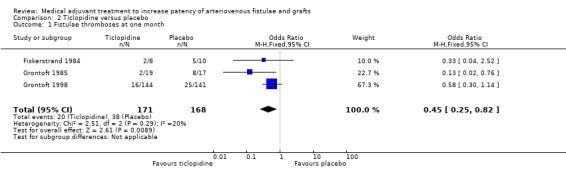
Ticlopidine versus placebo ‐ Analysis 2.1
All three trials comparing ticlopidine with placebo favoured treatment (Fiskerstrand 1984; Grontoft 1985; Grontoft 1998). In the Fiskerstrand 1984 study, 2 out of 8 participants in the ticlopidine group compared with 5 out of 10 in the placebo group developed fistulae thromboses at one month (OR 0.33, 95% CI 0.04 to 2.52). In the earlier Grontoft study, only 2 out of 19 who received treatment developed fistulae thromboses compared to 8 out of 17 on placebo (OR 0.13, 95% CI 0.02 to 0.76) (Grontoft 1985). In Grontoft 1998, 16 out of 144 participants who received ticlopidine developed thromboses in their fistulae compared with 25 out of 141 in the placebo group (OR 0.58, 95% CI 0.30 to 1.14).
The overall result of the meta‐analysis also favoured treatment (OR 0.45, 95% CI 0.25 to 0.82). The overall P value was 0.009.
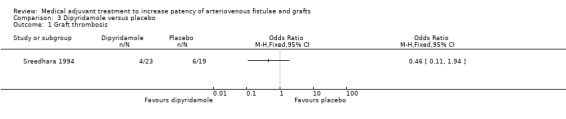
Dipyridamole versus placebo ‐ Analysis 3.1
The single trial that examined the effect of dipyridamole versus placebo followed participants for up to 18 months (Sreedhara 1994). Four out of 23 participants taking dipyridamole developed thrombosis compared to six out of 19 taking placebo. The overall result also favoured treatment (OR 0.46, 95% CI 0.11 to 1.94).
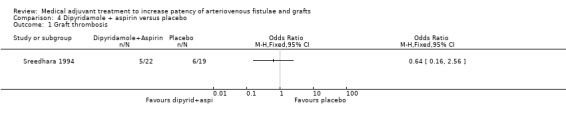
Dipyridamole + Aspirin versus placebo ‐ Analysis 4.1
The Sreedhara trial also used a parallel group design to examine the effect of dipyridamole plus aspirin versus placebo (Sreedhara 1994). Five out of 22 participants who received a combination of dipyridamole and aspirin developed a graft thrombosis compared to 6 out of 19 who received placebo (OR 0.64, CI 0.16 to 2.56).
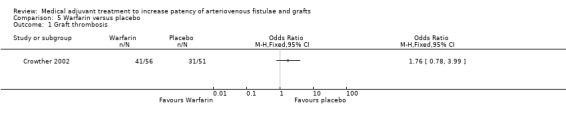
Warfarin versus placebo ‐ Analysis 5.1
The single trial that examined the effect of low‐intensity warfarin treatment versus placebo followed up participants for 37 months (before the trial was terminated prematurely due to a significant increase in bleeding seen in the treatment group) (Crowther 2002). The overall result favoured placebo (OR 1.76, CI 0.78 to 3.99) but this was not statistically significant (P = 0.17).
Fish oil versus placebo ‐ Analysis 6.1
The Schmitz 2002 trial examined the effects of fish oil versus placebo on graft thrombosis. Two out of 12 participants taking fish oil developed a thrombosis compared with 9 out of 12 who received placebo. The overall result favoured fish oil (OR 0.07, 95% CI 0.01 to 0.49). In the Lok 2012 study 33 out of 99 participants taking fish oil had graft thrombosis at 12 months compared with 45 out of 97 who received placebo. (OR 0.58, 95% CI 0.32 to 1.03).
Due to evidence of heterogeneity (Chi2 4.13, P = 0.04) we used a random‐effects model meta‐analysis. The overall results showed no differences between the treatments (OR 0.24, 95% CI 0.03 to 1.95). The overall P value was 0.18.
Clopidogrel versus placebo ‐ Analysis 7.1
Dember 2008 reported that 53 out of 436 participants in the treatment group and 84 out of 430 in the placebo arm developed fistula thrombosis at six weeks (OR 0.57, 95% CI 0.39 to 0.83). In the Ghorbani study 2 out of the 46 participants in the clopidogrel group compared with 10 out of 47 in the placebo group had AVF failure at eight weeks (OR 0.17, 95% CI 0.03 to 0.82) (Ghorbani 2009).
Due to evidence of heterogeneity (Chi2 2.18, P = 0.14) we used a random‐effects model meta‐analysis. The overall results showed no differences between the treatments (OR 0.40, 95% CI 0.13 to 1.19). The overall P value was 0.10.
Sulphinpyrazone versus placebo ‐ Analysis 8.1
The Michie 1977 trial examined the effects of sulphinpyrazone versus placebo on native fistulae or bovine graft thrombosis. One out of eight participants taking sulphinpyrazone developed a graft thrombosis compared with two of eight taking placebo (although two participants in the placebo group did not complete the study protocol). The overall result favoured sulphinpyrazone (OR 0.43, 95% CI 0.03 to 5.98).
PRT‐201 versus placebo – Analysis 9.1
Peden 2013 reported that 8 out of 45 participants in the treatment group (all doses of PRT‐201 combined) and 3 out of 21 in the placebo arm developed fistula thrombosis at 12 months (OR 1.30, 95% CI 0.31 to 5.48). In the Hye 2014 study 14 out of the 100 participants in the treatment group (all doses of PRT‐201 combined) compared with 12 out of 51 in the placebo group had AVF thrombosis within 12 months (OR 0.53, 95% CI 0.22 to 1.25). In the Dwivedi 2014 study looking at AV grafts, 26 out of 61 participants in the treatment group (all doses of PRT‐201 combined) and 13 out of 28 in the placebo arm developed AV graft thrombosis at 12 months (OR 0.86, 95% CI 0.35 to 2.11).
The overall results of the meta‐analysis favoured treatment (OR 0.75, 95% CI 0.42 to 1.32) but this was not statistically significant (P = 0.31).
Secondary outcomes
Data on the secondary outcome measures outlined in the 'Criteria for considering studies for this review' section were not specific enough to allow pooled analysis.
Duration of hospital stay
None of the studies included data on length of hospital stay.
Complications
The later Grontoft study reported data on a total of 45 complications, including hepatic, haematological, haemostatic, cutaneous, gastrointestinal, cardiovascular, and other miscellaneous complications affecting 30 participants on ticlopidine (Grontoft 1998). A total number of 39 complications were recorded in participants taking placebo. There were four deaths in the placebo group and two in the ticlopidine group.
The Crowther study also included information on bleeding complications for participants receiving warfarin (Crowther 2002). Six major bleeds occurred in five participants: three upper gastrointestinal (GI) bleeds, one cerebral haematoma after a road traffic accident, and one femoral artery injury after coronary angiography. All five of the participants were also taking anti‐platelet therapy at the time of the bleeding event. A further 18 participants (7 allocated to placebo) experienced a minor bleeding event, however according to the study authors there was no significant difference between the two groups (P = 0.30). There were five deaths in the treatment group and seven in the placebo group.
Dember 2008 also reported data on bleeding complications and mortality occurring within 30 days of completion of study medications for participants taking clopidogrel and placebo. Overall, bleeding events were similar for both groups (2.9% versus 2.8% respectively, P = 0.84) as was all‐cause mortality (0.9 in both groups) as reported by the study authors.
The Ghorbani study evaluating the effects of clopidogrel included information on bleeding and mortality (Ghorbani 2009). GI bleeding was reported in 2.1% of the treatment group and 3.2% of the placebo group (P = 0.31), and non‐GI tract bleeding in 5.3% and 4.3% respectively (P = 0.63). There were two mortalities in both groups. There was no severe bleeding during the active treatment period and no deaths were attributable to bleeding.
Sreedhara 1994 stated that 34 participants experienced adverse events which resulted in discontinuation of study medication (combination of dipyridamole, aspirin or placebo). These participants were considered to have completed the study protocol however. The most common adverse events were GI bleeding and nausea across all study groups. There were also seven mortalities during the study.
Lok 2012 did include data on mortality, reporting that 16 participants died (8 in each group) after starting the study medication but before 12 months of follow‐up were completed.
The only adverse event reported by Fiskerstrand 1984 was the development of a rash in a single participant treated with ticlopidine. No data on mortality were stated.
The Andrassy study comparing aspirin and placebo reported a greater risk of gastric pain and epistaxis (11% aspirin: 4% placebo) for participants but the risk of GI bleeding or wound hematoma was the same in both groups (4% in both arms) (Andrassy 1974).
Dwivedi 2014, Peden 2013 and Hye 2014 listed several adverse events including local symptoms secondary to the creation of a new AV fistula but according to the study authors there were no significant differences between placebo and PRT‐201‐treated participants.
Number of related radiological or surgical interventions
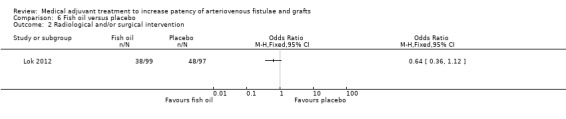
The Lok study comparing fish oil and placebo reported data on several complications, including a reduced number of radiological/surgical interventions in the intervention group but this was not significant (38 out of 99 versus 48 out of 97) (OR 0.64, 95% CI 0.36 to 1.12; P = 0.12) (Lok 2012).
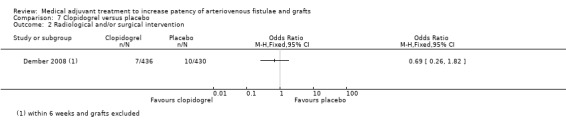
Also, Dember 2008 studied clopidogrel versus placebo and reported the frequency of additional interventions required to maintain patency in AV fistulae but this was not statistically significant (7 out of 436 participants taking clopidogrel and 10 out of 430 taking placebo) (OR 0.69, 95% CI 0.26 to 1.82; P = 0.45).
The Dwivedi 2014 study comparing various doses of PRT‐201 versus placebo calculated the rate of all procedures required to restore or maintain patency as a mean number per participant per year. There was no statistically significant difference between groups according to the study authors (4.4 for the placebo group versus 3.3 for all doses of PRT‐201).
Subgroup and sensitivity analyses
No subgroup or sensitivity analyses were undertaken due to insufficient data.
Discussion
Summary of main results
All but three (Crowther 2002; Peden 2013; Sreedhara 1994) of the 14 included studies revealed a beneficial effect with the active drug. However, there may be an element of bias due to clinical heterogeneity with the dose and length of follow‐up in the aspirin trials. In addition, one of the aspirin trials was conducted over 40 years ago with a relatively high dose of aspirin (500 mg/once daily) (Andrassy 1974). It should be noted that there was a marked discrepancy between results in the aspirin versus placebo trials, with Sreedhara 1994 favouring placebo and the Andrassy 1974 and Harter 1979 studies favouring treatment. The authors in the Sreedhara study postulate several possible biochemical and pharmacological actions of aspirin that may have worsened its results.
The results in the ticlopidine trials clearly favoured anti‐platelet treatment, using a dose of 250 mg twice daily, although the follow‐up time was only one month (Fiskerstrand 1984; Grontoft 1985; Grontoft 1998).
The Crowther study, comparing warfarin against placebo, had the longest follow‐up at 37 months (Crowther 2002). Despite this, there was no beneficial effect on the group of participants treated with warfarin. This study was stopped prematurely due to serious side effects. Therefore, the risk‐benefit does not favour anticoagulation unless there is another established indication.
Schmitz 2002 and Lok 2012 studied the effect of fish oil on graft patency and both trials favoured treatment. However, there was statistically significant heterogeneity between the trials (despite the same dose and length of follow‐up) and the random‐effects meta‐analysis showed no difference between the treatments.
Michie 1977 appears to show a benefit in terms of reducing graft thrombosis in participants treated with sulphinpyrazone. However, this study was conducted over 30 years ago with relatively small participant numbers and over a short follow‐up period (three months). Again, there is insufficient evidence from a single trial to recommend its use.
The clopidogrel versus placebo trials showed a beneficial effect in favour of 75 mg/day clopidogrel but the random‐effects meta‐analysis showed no difference between treatment (Dember 2008; Ghorbani 2009). There was statistical heterogeneity and the weighting of the meta‐analysis was biased towards the much larger Dember trial (Dember 2008).
Finally, Peden 2013, Hye 2014 and Dwivedi 2014 studied the effect of PRT‐201 versus placebo. The Peden 2013 and Hye 2014 studies were phase 1 and phase 2 trials using a variety of active treatment doses (range 3.3 μg to 9000 μg) on AV fistulae. The follow‐up for both trials was 12 months. Peden 2013 and Hye 2014 did not find a difference between treatment and placebo. Dwivedi 2014 looked at AV grafts but again used a variety of treatment doses (0.01 mg to 9.0 mg). Outcomes were reported after 12 months, and showed a non‐significant benefit in favour of treatment. The overall meta‐analysis of the three studies showed there was insufficient evidence of, and effect on, graft patency between PRT‐201 and placebo.
It appears, therefore, that at least in the short term anti‐platelet drugs such as ticlopidine may have some clinical advantages compared with placebo. No trials with follow‐up longer than 36 months demonstrated a beneficial effect of anti‐thrombotic or anti‐platelet treatment to increase patency of arteriovenous fistulae and grafts, thus their long‐term effect remains unclear.
Overall completeness and applicability of evidence
Many of the comparisons included only one or two studies while data on the secondary outcome measures outlined in the 'Criteria for considering studies for this review' section were not specific enough to allow pooled analysis.
As mentioned above, no trials with follow‐up longer than 36 months demonstrated a beneficial effect of anti‐thrombotic or anti‐platelet treatment to increase patency of arteriovenous fistulae and grafts, thus their long‐term effect remains unclear.
Quality of the evidence
The limitations of this meta‐analysis deserve to be mentioned. Firstly, some of the trials are not recent (the most recent was from 2014 but three are 30 or more years old). Additionally, most of the trials had a short period of follow‐up and many of the comparisons included only one or two studies. The methodological quality of the trials was moderate mainly due to the variation in detail provided in the study reports. Finally, there is evidence of heterogeneity in the aspirin, fish oil and clopidogrel trials. Therefore the overall quality of the evidence within this review is classed as low.
Potential biases in the review process
A thorough search for potential studies was performed.
For three trials unit of analysis issues were identified (Ghorbani 2009; Grontoft 1985; Grontoft 1998.
The Grontoft 1998 study had a non‐standard design as it allowed participants who had an initial failed AV fistula formation to re‐enter the study after a three week 'washout period'. According to the study authors 25 participants re‐entered the trial (12 in placebo and 13 in ticlopidine group). In addition the published results had to be carefully interpreted as there was confusion between the number of participants within the study compared to the number of operations (i.e. AV fistulae formed), and participants who died were excluded from the published analysis. Overall analysis for this review was undertaken using the number of operations performed on an 'intention‐to‐treat' basis. In total 258 participants were randomised with a total of 285 operations.
The results of the Ghorbani 2009 study had to be carefully assessed by the review authors as the original data was not analysed on an 'intention‐to‐treat' basis, but analysed by the number of participants who completed the trial.
Grontoft 1985 also published their analysed results based on the number of participants who completed the trial, not on an 'intention‐to‐treat' basis. As review authors it was not possible to use the intention‐to‐treat numbers because insufficient data regarding the original randomisation groups were provided. We therefore used the numbers of participants who completed the trial.
For the primary outcome 'graft patency', the numbers recorded for analyses were the number of grafts that occluded during the specified time period as reported by the study authors. This should be kept in mind when interpreting the presented data.
Agreements and disagreements with other studies or reviews
A meta‐analysis published by Coleman 2010 found a beneficial effect of antiplatelet agents in reducing thrombosis in arteriovenous fistulae but not with grafts. There is significant cross‐over with our review however, as Coleman 2010 included 10 studies in total in their meta‐analysis, and 8 of these studies are also included within our review. For our review Dixon 2009 was deemed not suitable for inclusion as there was cross‐over between participants who were prescribed aspirin prior to the trial; and Kooistra 1994 assessed participants who had concurrent administration of recombinant human erythropoietin and aspirin, so this was deemed to be confounding.
Authors' conclusions
Implications for practice.
The meta‐analyses of three studies for ticlopidine (an anti‐platelet treatment) which all used the same dose of treatment but with a short follow‐up of only one month, suggest ticlopidine may have a beneficial effect as an adjuvant treatment to increase the patency of AV fistulae and grafts in the short term. There was insufficient evidence to determine if there was a difference in graft patency between placebo and other treatments such as aspirin, fish oil, clopidogrel, PRT‐201, dipyridamole, dipyridamole plus aspirin, warfarin, and sulphinpyrazone. However, the quality of the evidence was low due to short follow‐up periods, the small number of studies for each comparison, heterogeneity between trials, and moderate methodological quality of the studies due to incomplete reporting. It therefore appears reasonable to suggest further prospective studies be undertaken to assess the use of these anti‐platelet drugs in renal patients with an arteriovenous fistula or graft.
Implications for research.
Clearly there is a lack of evidence confirming the long‐term effects of anti‐platelet and anti‐thrombotic drugs. Further randomised controlled trials with at least one or two years' follow‐up are required to address these clinical issues.
What's new
| Date | Event | Description |
|---|---|---|
| 13 June 2015 | New citation required but conclusions have not changed | Searches re‐run. Six new trials included and 19 new studies excluded, one study previously included reclassified as excluded study. New author added to review team. Risk of bias tables completed in keeping with current Cochrane policy. More evidence offered to support conclusions, conclusions not changed |
| 13 June 2015 | New search has been performed | Searches re‐run. Six new trials included and 19 new studies excluded, one study previously included reclassified as excluded study |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 19 May 2008 | New citation required but conclusions have not changed | New citation: conclusions not changed. Review updated with the addition of an additional author. |
| 19 May 2008 | New search has been performed | Four new trials added to included studies, six trials added to excluded studies and five trials added to ongoing studies. More evidence offered to support conclusions. |
| 19 May 2008 | Amended | Converted to new review format. |
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor: [Renal Dialysis] this term only | 3343 |
| #2 | MeSH descriptor: [Hemodiafiltration] this term only | 172 |
| #3 | MeSH descriptor: [Hemofiltration] this term only | 318 |
| #4 | MeSH descriptor: [Hemodialysis, Home] this term only | 51 |
| #5 | (hemodialysis or haemodialysis) | 5378 |
| #6 | (hemofiltration or haemofiltration) | 613 |
| #7 | (hemodiafiltration or haemodiafiltration) | 371 |
| #8 | dialysis | 9041 |
| #9 | (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8) | 10441 |
| #10 | MeSH descriptor: [Arteriovenous Fistula] this term only | 32 |
| #11 | MeSH descriptor: [Arteriovenous Shunt, Surgical] this term only | 222 |
| #12 | ((vascular next access) or (venous next access)) | 806 |
| #13 | (fistula* or avf* or graft or shunt) | 17642 |
| #14 | MeSH descriptor: [Blood Vessel Prosthesis] this term only | 452 |
| #15 | #10 or #11 or #12 or #13 or #14 | 18366 |
| #16 | MeSH descriptor: [Graft Occlusion, Vascular] explode all trees | 524 |
| #17 | (occlud* or occlusion or obstruct*):ti,ab,kw in Trials | 18531 |
| #18 | stenos*:ti,ab,kw in Trials | 4525 |
| #19 | restenos*:ti,ab,kw in Trials | 1879 |
| #20 | #16 or #17 or #18 or #19 | 23080 |
| #21 | #9 and #15 and #20 in Trials | 169 |
Data and analyses
Comparison 1. Aspirin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Graft thrombosis | 3 | 175 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.07, 2.25] |
1.1. Analysis.
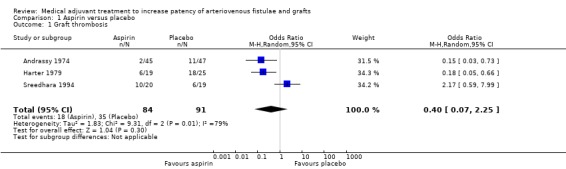
Comparison 1 Aspirin versus placebo, Outcome 1 Graft thrombosis.
Comparison 2. Ticlopidine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fistulae thromboses at one month | 3 | 339 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.25, 0.82] |
2.1. Analysis.
Comparison 2 Ticlopidine versus placebo, Outcome 1 Fistulae thromboses at one month.
Comparison 3. Dipyridamole versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Graft thrombosis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.
Comparison 3 Dipyridamole versus placebo, Outcome 1 Graft thrombosis.
Comparison 4. Dipyridamole + aspirin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Graft thrombosis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
4.1. Analysis.
Comparison 4 Dipyridamole + aspirin versus placebo, Outcome 1 Graft thrombosis.
Comparison 5. Warfarin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Graft thrombosis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
5.1. Analysis.
Comparison 5 Warfarin versus placebo, Outcome 1 Graft thrombosis.
Comparison 6. Fish oil versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Graft thrombosis | 2 | 220 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.03, 1.95] |
| 2 Radiological and/or surgical intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
6.1. Analysis.
Comparison 6 Fish oil versus placebo, Outcome 1 Graft thrombosis.
6.2. Analysis.
Comparison 6 Fish oil versus placebo, Outcome 2 Radiological and/or surgical intervention.
Comparison 7. Clopidogrel versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Graft thrombosis | 2 | 959 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.13, 1.19] |
| 2 Radiological and/or surgical intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
7.1. Analysis.
Comparison 7 Clopidogrel versus placebo, Outcome 1 Graft thrombosis.
7.2. Analysis.
Comparison 7 Clopidogrel versus placebo, Outcome 2 Radiological and/or surgical intervention.
Comparison 8. Sulphinpyrazone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Graft thrombosis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
8.1. Analysis.
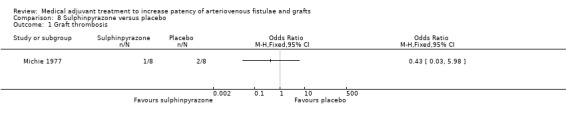
Comparison 8 Sulphinpyrazone versus placebo, Outcome 1 Graft thrombosis.
Comparison 9. PRT‐201 versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Graft thrombosis | 3 | 306 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.42, 1.32] |
9.1. Analysis.
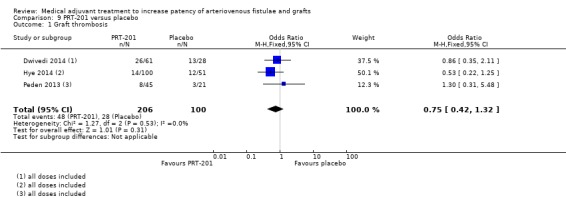
Comparison 9 PRT‐201 versus placebo, Outcome 1 Graft thrombosis.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andrassy 1974.
| Methods | Study design: randomised, double‐blind clinical trial | |
| Participants | Country: Germany No. of participants: 92 Age: not stated Gender: M = 50, F = 42 Inclusion criteria: end‐stage renal failure Exclusion criteria: not stated |
|
| Interventions | Treatment: aspirin 500 mg daily (n = 45) Control: placebo (n = 47) Duration: started 1 day pre‐operatively and continued for 28 days |
|
| Outcomes | Primary: fistula thrombosis, 28 days follow‐up | |
| Notes | Brescia‐Cimino fistula formation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Independent statistician used but exact method not stated |
| Allocation concealment (selection bias) | Unclear risk | No pre‐allocation bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to assess |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Pre‐study protocol not available |
| Other bias | Low risk | Nil obvious |
Crowther 2002.
| Methods | Study design: randomised, double‐blind clinical trial | |
| Participants | Country: Canada (2 university‐based teaching hospitals & community dialysis unit) No. of participants: 107 Number of participants prematurely discontinuing: placebo: 11; warfarin: 12 Age: 20 to 85 years Gender: M = 61, F = 46 Inclusion criteria: haemodialysis‐dependent or planned haemodialysis in near end‐stage renal disease patients Exclusion criteria: recent major haemorrhage (within 6 months), allergy to warfarin, persistent thrombocytopenia, inability to take oral medications, already taking warfarin for other reasons at start of trial, expectation of recovery of renal function or life expectancy less than two months, lack of informed consent or inability to give informed consent |
|
| Interventions | Treatment: warfarin variable dose with target INR 1.4 to 1.9 Control: placebo Duration: 37 months. Initiation within 7 days of surgery |
|
| Outcomes | Primary: graft thrombosis | |
| Notes | PTFE grafts Trial terminated November 2000 due to increased number of major bleeding events in treatment group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed computer‐generated randomisation scheme in random permuted blocks of 4, with stratification by clinical centre, use of antiplatelet therapy and whether the graft was placed in a participant currently on haemodialysis or in anticipation of the need for haemodialysis |
| Allocation concealment (selection bias) | Low risk | No pre‐allocation bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants who developed a short‐term indication for warfarin therapy (e.g. acute venous thrombosis) were not excluded from analysis |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Graft thrombosis was determined by clinical staff blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants who had a renal transplant or developed an indication for long‐term warfarin therapy or if the graft was removed were censored. Complete follow‐up and outcome ascertainment was achieved in all participants |
| Selective reporting (reporting bias) | Unclear risk | Pre‐study protocol not available |
| Other bias | High risk | Protocol for warfarin dosing changed after interim analysis 40% of participants received antiplatelet therapy (ASA) during the study period |
Dember 2008.
| Methods | Study design: multicenter, randomised, double‐blind placebo‐controlled clinical trial | |
| Participants | Country: United States No. of participants randomised: 877 No. of participants who completed the trial: 799 (8 did not receive intervention as randomised, 70 discontinued study drug early) Age: mean age Treatment = 52.7 years; Placebo = 54.5 years Gender: M = 548 males, F = 329 females Inclusion criteria: individuals undergoing creation of a new upper extremity arteriovenous fistula Exclusion criteria: active bleeding or bleeding events requiring red blood cell transfusion within the previous 12 weeks, platelet count less than 75 x 103 μL, known coagulopathy, acute ulcer disease, systolic blood pressure > 200 mm Hg or diastolic blood pressure > 115 mm Hg, advanced liver disease, inability to discontinue antiplatelet or anticoagulant therapy including aspirin during the study drug period, pregnancy, and current substance abuse |
|
| Interventions | Treatment: loading dose of clopidogrel 300 mg on day 1 post‐operation followed by 75 mg daily Control: placebo od Duration: 42 days total |
|
| Outcomes | Primary: thrombosis (patency failure) 6 weeks after fistula creation Secondary: failure to attain suitability for dialysis |
|
| Notes | Early termination of enrolment after fourth interim analysis, when study information fraction had reached 0.813 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated permuted block randomisation with stratification by location of fistula and by centre |
| Allocation concealment (selection bias) | Low risk | No pre‐allocation bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated that participants and members of the study team were blinded to treatment assignment. Placebo and the study drug were provided by the same company |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was performed by trained study personnel who were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Results re‐analysed by review authors |
| Selective reporting (reporting bias) | Low risk | Pre‐study protocol available |
| Other bias | Low risk | Nil obvious |
Dwivedi 2014.
| Methods | Study design: randomised, double‐blind, placebo‐controlled, phase 1‐2, single‐dose escalation study | |
| Participants | Country: United States No. of participants randomised: 115 No. of participants who received allocated intervention: 89 Age: placebo 60 ± 13, all PRT‐201 56 ± 12 Gender (%): placebo: 61% M, all PRT‐201 48% M Inclusion criteria: at least 18 years of age with chronic kidney disease either receiving maintenance haemodialysis or expected to commence within 3 months Exclusion criteria: alpha 1‐antitrypsin deficiency and suspected ipsilateral outflow vein or central vein lumen stenosis or occlusion |
|
| Interventions | Treatment: single application of: Low dose [0.01, 0.03 mg] PRT‐201 (n = 24) Medium dose [0.1, 0.3, 1.0 mg] PRT‐201 (n = 12) High dose [3.0, 6.0, 9.0 mg] PRT‐201 (n = 25) Control: placebo (n = 28) Duration: 2.5 mL of solution or placebo were administered as a series of drops over a period of 10 minutes to the exposed graft‐vein anastomosis and the adjacent outflow vein at the time of AVG creation. The wound was then lavaged with saline for one minute |
|
| Outcomes | Primary: proportion with > 25% intraoperative increase in diameter of AVG outflow vein Others: change and percentage change in diameter of AVG outflow vein, change and percentage change in the intraoperative blood flow volume, primary unassisted patency and secondary patency loss |
|
| Notes | Only arteriovenous grafts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | No pre‐allocation bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States that investigator, clinical staff, participants and central readers of digital photographs were blinded. The research pharmacist who mixed the treatment solutions was the only unblinded personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assumed based on above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 45 participants did not complete the study, but analysis completed on all participants 'as treated' |
| Selective reporting (reporting bias) | Unclear risk | Pre‐study protocol not available |
| Other bias | High risk | 1) There was a participant who crossed‐over after being randomised from the placebo group to the treatment group due to pharmacist error 2) The study was supported financially by Proteon Therapeutics |
Fiskerstrand 1984.
| Methods | Study design: randomised, double‐blind, clinical trial | |
| Participants | Country: United Kingdom No. of participants recruited: 18 No. of participants who completed the trial: 15 Age: not stated Gender: not stated Inclusion criteria: patients requiring access surgery for chronic haemodialysis Exclusion criteria: patients requiring anti‐platelet or anticoagulant therapy; history of peptic ulcer; known bleeding disorder; platelet count < 100,000/mm3 |
|
| Interventions | Treatment: ticlopidine 250 mg bid Control: placebo bid Duration: medication started two days pre‐operatively and continued for one month postoperation |
|
| Outcomes | Primary: fistula thrombosis | |
| Notes | Brescia‐Cimino AVF | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient data provided to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient data provided to assess |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient data provided to assess |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient data provided to assess |
| Selective reporting (reporting bias) | Unclear risk | Pre‐study protocol not available |
| Other bias | Low risk | Nil obvious |
Ghorbani 2009.
| Methods | Study design: randomised, double‐blind clinical trial | |
| Participants | Country: Iran No. of participants randomised: 93 No. of participants who completed the trial: 75 (6 withdrew consent, 11 participants developed complications, 1 participant died) Age: mean age in years 45.03 ± 1.2 Gender: M = 48, F = 45 Inclusion criteria: individuals ≥ 18 years undergoing haemodialysis requiring a primary AVF or requiring a new AVF at a different site Exclusion criteria: history of gastrointestinal bleeding or previous bleeding episodes within six months prior to the initiation of the study; patients already receiving chronic anticoagulation therapy (antiplatelet agents or warfarin); terminal or life‐threatening disease; pregnancy; malignant hypertension; platelet count of < 100,000/mm3; and other demonstrated medical condition that would make antiplatelet therapy dangerous |
|
| Interventions | Treatment: clopidogrel 75 mg daily Control: matching placebo od Duration: initiated 7 to 10 days prior to scheduled surgery and continued for up to six weeks postoperatively Total follow‐up: six months |
|
| Outcomes | Primary outcome: incidence of primary AVF failure eight weeks after fistula formation Secondary outcome: incidence of successful haemodialysis within six months of fistula formation |
|
| Notes | Autologous AV fistulae | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block randomisation schedule by central coordinating centre with stratification according to medical centre |
| Allocation concealment (selection bias) | Low risk | No pre‐allocation bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated that participants and medical personnel were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Fistula failure was determined by either the study coordinator or site principal investigator who was blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Re‐analysis of results performed as authors did not analyse on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Unclear risk | Pre‐study protocol not available |
| Other bias | Low risk | Nil obvious |
Grontoft 1985.
| Methods | Study design: randomised, double‐blind clinical trial | |
| Participants | Country: Sweden No. of participants recruited: 42 No. of participants who completed the trial: 36 Losses to follow‐up: 3 received artificial grafts, 1 received other form of anticoagulant and 2 serious side effects suspected Age: 24 to 72 years Gender: M = 28, F = 14 Inclusion criteria: patients undergoing fistula operation Exclusion criteria: patients with bleeding tendency; reduced platelet counts; receiving anticoagulants, anti‐inflammatories or platelet aggregation inhibitors |
|
| Interventions | Treatment: ticlopidine 250 mg bid Control: placebo bid Duration: 2 days pre‐ and 4 weeks postoperative |
|
| Outcomes | Fistula thrombosis Platelet aggregation |
|
| Notes | Native AV fistulae | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient data provided to assess |
| Allocation concealment (selection bias) | Unclear risk | Insufficient data provided to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient data provided to assess |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient data provided to assess |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Initial randomisation groups not stated prior to exclusions, therefore analysis completed on the number who completed the trial |
| Selective reporting (reporting bias) | High risk | Authors provide 'as treated' analysis, not on an 'intention‐to‐treat' basis |
| Other bias | Low risk | Nil obvious |
Grontoft 1998.
| Methods | Study design: randomised, double‐blind, multicentre, clinical trial | |
| Participants | Country: Sweden and Finland (8 Swedish, 1 Finnish centre) No. of participants recruited: 258 (285 operations) Non‐evaluability amongst randomised operations: consent withdrawn = 2, no medication taken = 4, no new anastomosis = 11, no data recovered = 1 Age: placebo 58 ± 15, ticlopidine 56 ± 15 Gender: placebo M = 77, F = 47; ticlopidine M = 75, F = 43 Inclusion criteria: patients with chronic renal failure predialysis or on dialysis; patients requiring AVF Patients in whom first operation failed could be re‐entered after washout period Exclusion criteria: patients requiring anti‐platelet or anticoagulant therapy; history of hepatic disease; severe or malignant hypertension; gastrointestinal ulcer; bleeding disorder; reduced white blood cells or platelets |
|
| Interventions | Treatment: ticlopidine 250 mg bid Control: placebo bid Duration: aim to start 7 days (minimum 3 days) pre‐ and 28 days post‐surgery |
|
| Outcomes | Primary: fistula thrombosis Secondary: Biochemical markers: urea, haemoglobin and cholesterol levels |
|
| Notes | Native AV fistulae, transposition grafts and synthetic grafts Recruitment interrupted for 4 months due to serious adverse event |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation scheme based on the 4 weighted stratification variables of the participating centre |
| Allocation concealment (selection bias) | Low risk | No pre‐allocation bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Potential risk due to the re‐entry of certain participants into randomisation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Potential risk due to the re‐entry of certain participants into randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data missing on 267 operations |
| Selective reporting (reporting bias) | Unclear risk | Pre‐study protocol not available |
| Other bias | High risk | Participants in whom the first operation failed could be re‐entered after a 3‐week washout period |
Harter 1979.
| Methods | Study design: randomised, double‐blind, clinical trial | |
| Participants | Country: United States No. of participants: 44 Age: aspirin 53 ± 14 years, placebo 46 ± 16 years Gender: M = 20, F = 24 Inclusion criteria: patients undergoing chronic haemodialysis Exclusion criteria: recent gastrointestinal bleeding |
|
| Interventions | Treatment: aspirin 160 mg/day Control: placebo Duration: aspirin or placebo started 1 month postoperation and continued for approximately 5 months |
|
| Outcomes | Primary: graft thrombosis | |
| Notes | AV shunts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated Unequal group sizes |
| Allocation concealment (selection bias) | Unclear risk | Insufficient data to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated that participants and members of the study team were blinded to treatment assignment. Placebo and the study drug were provided by the same company |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient data to assess |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 31 participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | Pre‐study protocol not available |
| Other bias | Unclear risk | No pre‐specified enrolment value. Enrolment would continue until 24 thrombi were observed. |
Hye 2014.
| Methods | Study design: randomised, double‐blind, placebo‐controlled phase 2 clinical trial | |
| Participants | Country: United States No. of participants randomised: 169 No. of participants who received allocated intervention: 151 Age: placebo 59 ± 15, 10 μg PRT‐201 59 ± 18, 30 μg PRT‐201 59 ± 15 Gender: placebo 63% M, 10 μg PRT‐201 55% M, 30 μg PRT‐201 55% M Inclusion criteria: at least 18 years of age with chronic kidney failure either receiving maintenance haemodialysis or expected to commence within 6 months and required creation of an AVF Exclusion criteria: not stated |
|
| Interventions | Treatment: single application of: 10 μg PRT‐201 (n = 59) 30 μg PRT‐201 (n = 53) Control: placebo (n = 57) Duration: 2.5 mL of solution or placebo were administered over a period of 10 minutes to the exposed inflow artery, anastomosis and outflow vein at the time of AVF creation. The wound was then lavaged with saline for one minute |
|
| Outcomes | Primary: primary patency Secondary: secondary patency, unassisted maturation, use for haemodialysis, lumen stenosis |
|
| Notes | Only radiocephalic or brachiocephalic autologous fistulae | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | No pre‐allocation bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded to treatment received. Not stated if personnel were blinded but as study is reported as double blind, we assume a low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessor of fistula patency not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24 participants did not complete the study. 'Intention‐to‐treat' analysis performed |
| Selective reporting (reporting bias) | Low risk | Pre‐study protocol available |
| Other bias | High risk | The study was supported financially by Proteon Therapeutics |
Lok 2012.
| Methods | Study design: multicenter, randomised, placebo‐controlled clinical trial | |
| Participants | Country: 12 Canadian and 3 United States sites No. of participants randomised: 201 No. of participants who started the study intervention: 196 No. of participants who completed the trial: 164 (5 excluded prior to receiving treatment, 32 did not complete 12 months follow‐up) Age: 27 to 88 years Gender: M = 98, F = 98 Inclusion criteria: adults (≥ 18 years) who required a synthetic arteriovenous graft for chronic haemodialysis. The graft could be either a ‘first access’ or a ‘subsequent access’ after a previously failed access Exclusion criteria: reversible renal failure; active malignancy; pregnancy; malignant hypertension; active major bleed in the prior month; receiving more than 2 antiplatelet agents or anticoagulants; life expectancy less than 6 months; surgical revision of a previous access; arteriovenous graft that failed prior to and including postoperative day 7; ingestion of any form of fish oil at time of randomisation; allergy to fish or fish products; enrolment in another interventional study of arteriovenous grafts |
|
| Interventions | Treatment: four 1 gram fish oil capsules per day. Each capsule was MEG‐3 (Ocean Nutrition Canada Ltd), which contained 48% (400 mg/capsule) eicosapentaenoic acid (EPA) and 25% (200 mg/capsule) docosahexaenoic acid (DHA) Control: 4 placebo capsules per day containing 1% peppermint‐flavoured corn oil Duration: 12 months |
|
| Outcomes | Primary: proportion with loss of native patency within 12 months Secondary: rate and proportion of graft thrombosis, proportion with radiological or surgical intervention, cumulative graft patency, cardiovascular outcomes |
|
| Notes | Synthetic arteriovenous grafts Premature termination due to slow recruitment and lack of additional funds decreased study power from 80% to 74% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed allocation by central independent randomisation facility with stratification by site and first access or subsequent access |
| Allocation concealment (selection bias) | Low risk | No pre‐allocation bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated that participants, study coordinators, care‐givers and site pharmacists were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment undertaken by an independent assessor who was blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Five participants excluded after randomisation, but before treatment initiated. All participants were included in the primary analysis, even if they didn't complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Pre‐study protocol available |
| Other bias | Low risk | Nil obvious |
Michie 1977.
| Methods | Study design: randomised, double‐blind, parallel‐group trial | |
| Participants | Country: United States No. of participants: 16 No. of participants who completed the protocol = 14 (1 lost to FU, 1 died) Age: 31 to 64 years Gender: M = 10, F = 8 Race: Negro = 15, Caucasian = 1 Inclusion criteria: chronic renal failure scheduled to begin haemodialysis Exclusion criteria: not stated Paticipants were not allowed to take acetylsalicylic acid or products containing this drug or other drugs known to modify platelet functions during the experiment period |
|
| Interventions | Treatment: sulphinpyrazone 200 mg qds Control: placebo qds Duration: initiated 2 days prior to surgery and continued for nearly 3 months (33 dialysis sessions) |
|
| Outcomes | Primary: graft thrombosis Secondary: haematological variables |
|
| Notes | AV fistulae and bovine grafts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient data provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient data provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient data provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient data provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14 participants completed the study. All randomised participants received the appropriate treatment |
| Selective reporting (reporting bias) | Unclear risk | Pre‐study protocol not available |
| Other bias | Low risk | Nil obvious |
Peden 2013.
| Methods | Study design: randomised, double‐blind, placebo‐controlled, phase 1‐2, dose‐escalation study | |
| Participants | Country: United States No. of participants randomised: 66 Age: placebo 52 ± 17, all PRT‐201 57 ± 15 Gender: placebo 71% M, all PRT‐201 73% M Inclusion criteria: at least 18 years of age with chronic kidney failure either receiving maintenance haemodialysis or expected to commence within 6 months and required creation of an AVF Exclusion criteria: alpha 1‐antitrypsin deficiency; cephalic vein lumen diameter by ultrasound < 2.0 mm with a tourniquet; lack of continuity of the cephalic vein with the subclavian vein; central venous stenosis or occlusion; and treatment with any investigational agent within 30 days or investigational antibody therapy within 90 days of signing the informed consent document |
|
| Interventions | Treatment: single application of: Low dose [0.0033, 0.01, 0.033 mg] PRT‐201 (n = 16) Medium dose [0.1, 0.33, 1.0 mg] PRT‐201 (n = 17) High dose [3.0, 6.0, 9.0 mg] PRT‐201 (n = 12) Control: placebo (n = 21) Duration: 2.5 mL of solution or placebo were administered over a period of 10 minutes to the exposed inflow artery, anastomosis and outflow vein at the time of AVF creation. The wound was then lavaged with saline for one minute |
|
| Outcomes | Unassisted primary and secondary patency Others include: intra‐operative increase in AVF outflow vein diameter or blood flow, AVF maturation, incidence of stenosis and time to use |
|
| Notes | Only radiocephalic or brachiocephalic autologous fistulae | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation sequence in blocks of 6 without stratification |
| Allocation concealment (selection bias) | Low risk | No pre‐allocation bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated that participants, investigators, clinical staff and the central reader of photographs and ultrasound were all blinded. The research pharmacist who mixed the treatment solutions was the only unblinded person |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants did not complete the study, but were included in the analysis |
| Selective reporting (reporting bias) | Low risk | An ad hoc subgroup analysis for early surgical failures was undertaken |
| Other bias | High risk | 1) There was a participant who crossed‐over after being randomised from the placebo group to the treatment group due to pharmacist error 2) The study was supported financially by Proteon Therapeutics |
Schmitz 2002.
| Methods | Study design: randomised, double‐blind clinical trial | |
| Participants | Country: United States No. of participants: 24 Age: 46 to 58 years Gender: M = 11, F = 13 Inclusion criteria: newly placed PTFE graft Exclusion criteria: patients needing fistula revision; history of gastro‐intestinal bleeding; already taking warfarin or an anti‐platelet agent; terminal or life‐threatening disease; pregnancy; or malignant hypertension |
|
| Interventions | Treatment: 4000 mg fish oil once daily Control: placebo od Duration: 12 months (medication started within 2 weeks of graft placement) |
|
| Outcomes | Primary: graft thrombosis | |
| Notes | PTFE grafts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block randomisation scale |
| Allocation concealment (selection bias) | Low risk | No pre‐allocation bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo and treatment capsules manufactured by the same company |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to assess |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants received the appropriate treatment. Only one was lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Pre‐study protocol not available |
| Other bias | Low risk | Nil obvious |
Sreedhara 1994.
| Methods | Study design: randomised, double‐blind, parallel group study | |
| Participants | Country: United States No. of participants recruited: 108 No. of participants randomised: 107 (1 participant excluded after receiving Brescia‐Cimino fistula) Type I patients = 84 (patients who required a new AV ePTFE graft for chronic HD) Type II patients = 23 (patients on chronic HD with AV ePTFE with graft thrombosis requiring new graft) No. of participants who completed the protocol: 96 (2 lost to FU, 9 withdrew consent or had transplant ‐ all Type I patients) Age: mean age 54.4 years, range 19 to 84 Gender: M = 45, F = 63 Inclusion criteria: type I patients: patients who required a new AV ePTFE graft for chronic HD; type II patients: patients on chronic HD with AV ePTFE with graft thrombosis requiring new graft Exclusion criteria: uncontrolled hypertension; history of peptic ulcer disease; haemophilia; von Willebrand's disease; other bleeding disorders; malignancy; hypersensitivity to the study drugs |
|
| Interventions | 1. Dipyridamole 75 mg tid with aspirin placebo od
2. Dipyridamole placebo tid with aspirin 325 mg od
3. Dipyridamole 75 mg tid with aspirin 325 mg od
4. Dipyridamole placebo tid with aspirin placebo od Duration: aim to start 2 days pre‐op and continue for 18 months or until graft thrombosis |
|
| Outcomes | Primary: graft thrombosis | |
| Notes | PTFE grafts Type II patients not included in review meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Pre‐determined schedule (not detailed) |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated that neither participants nor investigator could determine the specific treatment protocol |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Graft thrombosis assessed by an investigator who was blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 participants who were enrolled did not complete the study or were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Pre‐study protocol not available |
| Other bias | Low risk | Nil obvious |
AV: arteriovenous AVF: arteriovenous fistula AVG: arteriovenous graft ePTFE: expanded polytetrafluoroethylene F: females FU: follow‐up HD: haemodialysis M: males od: once a day bid: twice a day tid: three times a day qds: four times a day
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albert 1978 | Study compares acetylsalicylic acid with sulphinpyrazone, no placebo group included so did not meet inclusion criteria |
| Alexopoulos 2011 | No data on patency rates or secondary outcomes published |
| Bhomi 2008 | Not a placebo‐controlled trial |
| Chen 2013 | This is a comparison of two different treatments versus no treatment, and no placebo was used for the trial. Therefore, did not meet inclusion criteria |
| D'Ayala 2008 | Not a placebo‐controlled trial |
| De Fijter 1995 | No data published on patency rates or secondary outcomes. The study mixed participants on haemodialysis and peritoneal dialysis |
| Dixon 2009 | Dipyridamole + aspirin combination was the treatment arm versus placebo; however if the participants were taking aspirin prior to the study initiation this was not stopped. Therefore, no true placebo was used for all participants |
| Dulude 2001 | Only abstract available; not enough information presented to allow adequate data extraction of primary or secondary outcomes |
| Fuster 2001 | Not a placebo‐controlled trial. The paper described the rationale and design of an ongoing study. No results have been published |
| Janicki 2003 | It was not a randomised trial as there is no mention of any randomisation process. There is no data on date of the study, thus the control group could have been a historical group. There is no information on how patency or thrombosis was assessed |
| Kaegi 1975 | Some participants were on warfarin at the start of the study. Other participants were started on this drug during the trial |
| Kaufman 2003 | Not able to elicit absolute number of thrombotic episodes from results, as only a table of cumulative incidence, hazard ratios and risk reduction figures. The average age of the dialysis access was 565 days (placebo) and 587 days (treatment) |
| Kooistra 1994 | Long‐standing (> 6 weeks old) AV fistulae assessed, and concurrent initiation of recombinant human erythropoietin with antiplatelet agent was studied, so was a confounding factor |
| Krasinski 2004 | Abstract only available: not able to elicit absolute numbers of thrombotic episodes from results or secondary outcomes |
| Lee 2009 | This randomised trial looked at the effect of different routes of administration of the study drug. It did not therefore include a true placebo |
| Lin 2007 | Not placebo controlled. Study undertaken on AV fistulae which had been functional for at least 3 months |
| Lin 2013 | Not placebo controlled. Study undertaken on AV fistulae which had been functional for at least 6 months |
| Malovrh 2009 | Not placebo controlled. Not truly randomised (groups based on vessel quality, and if fistula was non‐functional at the end of surgery) |
| Mileti 1995 | The paper described the rationale and design of an ongoing study. No results have been published |
| Radmilovic 1998 | The trial looked at external fistulae, a technique now not practised and numbers of thrombotic episodes could not be elicited from the results |
| Rouzrokh 2009 | Unclear results with the time frame of assessment of patency not clear |
| Taber 1992 | Abstract only available: no data published on patency rates or secondary outcomes |
| Trimarchi 2006 | This is a comparison of treatment versus no treatment, and no placebo was used for trial. Therefore, excluded from review. *Note: this study was mistakenly included in previous versions of this review |
| Wang 2010 | Not a placebo‐controlled trial |
| Wasse 2014 | Patency defined as AVF or AVG being successfully used at six months, therefore no data provided on actual thrombotic events as other factors could have precluded the AVF or AVG use |
| Yuto 2012 | Not a placebo‐controlled trial |
AV: arteriovenous AVF: arteriovenous fistula AVG: arteriovenous graft
Characteristics of ongoing studies [ordered by study ID]
Irish 2007.
| Trial name or title | FAVOURED (Fish oil and aspirin in vascular access outcomes in renal disease) |
| Methods | Factorial‐trial design. Medication commenced pre‐operatively and continued for 3 months post surgery |
| Participants | Stage 4 or 5 chronic kidney disease patients with arteriovenous fistulae |
| Interventions | Low‐dose aspirin and fish oil either alone or in combination |
| Outcomes | Primary outcome: fistula patency at 3 months Secondary outcomes: patency at 6 months, primary patency time, secondary (assisted) patency time, adverse events (e.g. bleeding) |
| Starting date | 2007 |
| Contact information | |
| Notes |
Differences between protocol and review
In the previous published version of this review (Osborn 2008), the quality of trials was investigated using the methods of Jadad (Jadad 1996); and Schulz (Schultz 1995). In keeping with updated requirements of Cochrane, methodological quality has now been assessed using the 'Risk of bias' tool as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Contributions of authors
NCT: assessed trials to confirm eligibility, quality and extracted data, updated the text of the review ADS: assessed trials to confirm eligibility and quality, updated the text of the review
Sources of support
Internal sources
No sources of support supplied
External sources
-
The Chief Scientist Office, Scottish Executive Health Directorates, the Scottish Government, UK.
The PVD Group editorial base is supported by the Chief Scientist Office.
Declarations of interest
NCT: none known ADS: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Andrassy 1974 {published data only}
- Andrassy K, Malluche H, Bornefeld H, Comberg M, Ritz E, Jesdinsky H, et al. Prevention of p.o. clotting of av. cimino fistulae with acetylsalicyl acid: results of a prospective double blind study. Klinische Wochenschrift 1974;52(7):348‐9. [DOI] [PubMed] [Google Scholar]
Crowther 2002 {published data only}
- Crowther MA, Clase CM, Margetts PJ, Julian J, Lambert K, Sneath D, et al. Low‐intensity warfarin is ineffective for the prevention of PTFE graft failure in patients on hemodialysis: a randomized controlled trial. Journal of the American Society of Nephrology 2002;13(9):2331‐7. [DOI] [PubMed] [Google Scholar]
Dember 2008 {published data only}
- Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, et al for the Dialysis Access Consortium Study Group. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA 2008;299(18):2164‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwivedi 2014 {published data only}
- Dwivedi AJ, Roy‐Chaudhury P, Peden EK, Browne BJ, Ladenheim ED, Scavo VA, et al. Application of human type I pancreatic elastase (PRT‐201) to the venous anastomosis of arteriovenous grafts in patients with chronic kidney disease. Journal of Vascular Surgery 2014;15(5):376‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiskerstrand 1984 {published data only}
- Fiskerstrand CE, Thompson IW, Burnet ME, Williams P, Anderton JL. Double‐blind randomized trial of the effect of ticlopidine in arteriovenous fistulas for haemodialysis. Artificial Organs 1985;9(1):61‐3. [DOI] [PubMed] [Google Scholar]
Ghorbani 2009 {published data only}
- Ghorbani A, Aalamshah M, Shahbazian H, Ehsanpour A, Aref A. Randomized controlled trial of clopidogrel to prevent primary arteriovenous fistula failure in hemodialysis patients. Indian Journal of Nephrology April 2009;19(2):57‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grontoft 1985 {published data only}
- Grontoft KC, Mulec H, Gutierrez A, Olander R. Thromboprophylactic effect of ticlopidine in arteriovenous fistulas for haemodialysis. Scandinavian Journal of Urology and Nephrology 1985;19(1):55‐7. [DOI] [PubMed] [Google Scholar]
Grontoft 1998 {published data only}
- Grontoft KC, Larsson R, Mulec H, Weiss LG, Dickinson JP. Effects of ticlopidine in A‐V fistula surgery in uremia. Fistual Study Group. Scandinavian Journal of Urology and Nephrology 1998;32(4):276‐83. [DOI] [PubMed] [Google Scholar]
Harter 1979 {published data only}
- Harter HR, Burch JW, Majerus PW, Stanford N, Delmez JA, Anderson CB, et al. Prevention of thrombosis in patients on haemodialysis by low‐dose aspirin. New England Journal of Medicine 1979;301(11):577‐9. [DOI] [PubMed] [Google Scholar]
Hye 2014 {published data only}
- Hye RJ, Peden EK, O'Connor TP, Browne BJ, Dixon BS, Schanzer AS, et al. Human type I pancreatic elastase treatment of arteriovenous fistulas in patients with chronic kidney disease. Journal of Vascular Surgery 2014;60(2):454‐61. [DOI] [PubMed] [Google Scholar]
Lok 2012 {published data only}
- Anon. Fish oil inhibition of stenosis in haemodialysis grafts study. http://www.isrctn.com/ISRCTN15838383 2007.
- Lok CE, Allon M, Donnelly S, Dorval M, Hemmelgarn B, Moist L, et al. Design of the fish oil inhibition of stenosis in hemodialysis grafts (FISH) study. Clinical Trials 2007;4(4):357‐67. [DOI] [PubMed] [Google Scholar]
- Lok CE, Moist L, Hemmelgarn BR, Tonelli M, Vazquez MA, Dorval M, et al for the Fish Oil Inhibition of Stenosis in Hemodialysis Grafts (FISH) Study Group. Effect of fish oil supplementation on graft patency and cardiovascular events among patients with new synthetic arteriovenous hemodialysis grafts. JAMA 2012;307(17):1809‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Michie 1977 {published data only}
- Michie DD, Wombolt DG. Use of sulfinpyrazone to prevent thrombus formation in arteriovenous fistulas and bovine grafts of patients on chronic hemodialysis. Current Therapeutic Research, Clinical and Experimental 1977;22(1):196‐204. [Google Scholar]
Peden 2013 {published data only}
- Peden EK, Leeser DB, Dixon BS, El‐Klatib MT, Roy‐Chaudhury P, Lawson JH, et al. A multi‐center, dose‐escalation study of human type I pancreatic elastase (PRT‐201) administered after arteriovenous fistula creation. Journal of Vascular Access 2013;14(2):143‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmitz 2002 {published data only}
- Schmitz PG, McCloud LK, Reikes ST, Leonard CL, Gellens ME. Prophylaxis of hemodialysis graft thrombosis with fish oil: double‐blind, randomized, prospective trial. Journal of the American Society of Nephrology 2002;13:184‐90. [DOI] [PubMed] [Google Scholar]
Sreedhara 1994 {published data only}
- Sreedhara R, Himmelfarb J, Lazarus JM, Hakim RM. Anti‐platelet therapy in graft thrombosis: results of a prospective, randomized double blind study. Kidney International 1994;45(5):1477‐83. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Albert 1978 {published data only}
- Albert FW, Schmidt U, Harzer R. Postoperative thromboprophylaxis for cimino‐fistulae with sulfinpyrazone in comparison to acetylsalicylic acid. Krankenhausarzt 1978;51:712‐8. [Google Scholar]
Alexopoulos 2011 {published data only}
- Alexopoulos D, Panagiotou A, Xanthopoulou I, Komninakis D, Kassimis G, Davlouros P, et al. Antiplatelet effects of prasugrel vs. double clopidogrel in patients on hemodialysis and with high on‐treatment platelet reactivity. Journal of Thrombosis and Haemostasis 2011;9(12):2379‐85. [DOI] [PubMed] [Google Scholar]
Bhomi 2008 {published data only}
- Bhomi KK, Shrestha S, Bhattachan CL. Role of systemic anticoagulation in patients undergoing vascular access surgery. Nepal Medical College Journal : NMCJ 2008;10(4):222‐4. [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen L, Ling YS, Lin CH, Guan TJ. Combined use of heparin and anisodamine reduces the risk of early thrombosis in native arteriovenous fistula. Vascular 2013;21(6):369‐74. [DOI] [PubMed] [Google Scholar]
D'Ayala 2008 {published data only}
- D'Ayala M, Smith RM, Martone C, Briggs W, Deitch JS, Wise L. The effect of systemic anticoagulation in patients undergoing angioaccess surgery. Annals of Vascular Surgery 2008;22:11‐5. [DOI] [PubMed] [Google Scholar]
De Fijter 1995 {published data only}
- Fijter CWH, Popp‐Snijders C, Oe LP, Tran DD, Meulen J, Donker AJM. Does additional treatment with fish oil mitigate the side effects of recombinant human erythropoietin in dialysis patients?. Haematologia 1995;80:332‐4. [PubMed] [Google Scholar]
Dixon 2009 {published data only}
- Dixon BS, Beck GJ, Dember LM, Vazquez MA, Greenberg A, Delmez JA, et al. Use of aspirin associates with longer primary patency of hemodialysis grafts. Journal of the American Society of Nephrology : JASN. 2011;22(4):773‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon BS, Beck GJ, Vazquez MA, Greenberg A, Delmez JA, Allon M, et al. Effect of dipyridamole plus aspirin on hemodialysis graft patency. New England Journal of Medicine 2009;360:2191‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dulude 2001 {published data only}
- Dulude H, Kaplan M, Odland M, Marbury T, Hoggard JG, Ploth D, et al. Escalating single doses of CJC‐1004, a locally acting antithrombotic agent in hemodialysis patients with vascular access occlusion. Journal of the American Society of Nephrology 2001;12:287A. [Google Scholar]
Fuster 2001 {published data only}
- Fuster V, Charlton P, Boyd A. Clinical protocol. A phase IIb, randomized, multicenter, double‐blind study of the efficacy and safety of Trinam (EG004) in stenosis prevention at the graft‐vein anastomosis site in dialysis patients. Human Gene Therapy 2001;12(16):2025‐7. [PubMed] [Google Scholar]
Janicki 2003 {published data only}
- Janicki K, Bojarska SA, Pietura R, Janicka L. Preventive effects of ticlopidine on the incidence of late A‐V fistula thrombosis complications in haemodialyses patients. Annales Universitatis Mariae Curie Sklodowska Sectio D: Medicina 2003;58:215‐8. [PubMed] [Google Scholar]
Kaegi 1975 {published data only}
- Kaegi A, Pineo GF, Shimizu A, Trivedi H, Hirsh J, Gent M. The role of sulfinpyrazone in the prevention of arterio‐venous shunt thrombosis. Circulation 1975;52(3):497‐9. [DOI] [PubMed] [Google Scholar]
Kaufman 2003 {published data only}
- Kaufman JS, O'Connor TZ, Zhang JH, Cronin RE, Fiore LD, Ganz MB, et al. Veterans Affairs Cooperative Study Group on Hemodialysis Access Graft Thrombosis. Randomized controlled trial of clopidogrel plus aspirin to prevent hemodialysis access graft thrombosis. Journal of the American Society of Nephrology 2003;14(9):2313‐21. [DOI] [PubMed] [Google Scholar]
Kooistra 1994 {published data only}
- Kooistra MP, Es A, Marx JJM, Hertsig MLA, Struyvenberg A. Low‐dose aspirin does not prevent thrombovascular accidents in low‐risk hemodialysis patients during treatment with recombinant human erythropoietin. Nephrology, Dialysis, Transplantation 1994;9:1115‐20. [DOI] [PubMed] [Google Scholar]
Krasinski 2004 {published data only}
- Krasinski Z, Dzieciuchowicz L, Kulesza J, Pietrzak I, Gabriel M, Krasinka B, et al. Influence of long‐term therapy with low molecular weight heparin on patency of arterio‐venous fistula for hemodialysis. ERA ‐ EDTA ELI Congress Abstracts. 2004:360.
Lee 2009 {published data only}
- Lee YK, Koo JR, Kim JK, Park II, Joo MH, Yoon JW, et al. Effect of route of EPO administration on hemodialysis arteriovenous vascular access failure: a randomized controlled trial. American Journal of Kidney Diseases 2009;53(5):815‐22. [DOI] [PubMed] [Google Scholar]
Lin 2007 {published data only}
- Lin CC, Chang CF, Lai MY, Chen TW, Lee PC, Yang WC. Far‐infrared therapy: a novel treatment to improve access blood flow and unassisted patency of arteriovenous fistula in hemodialysis patients. Journal of the American Society of Nephrology 2007;18(3):985‐92. [DOI] [PubMed] [Google Scholar]
Lin 2013 {published data only}
- Lin CC, Yang WC. Far infrared therapy improves arteriovenous fistula maturation. Nephrology Dialysis Transplantation 2013;28:i226‐7. [DOI] [PubMed] [Google Scholar]
- Lin CC, Yang WC, Chen MC, Liu WS, Yang CY, Lee PC. Effect of far infrared therapy on arteriovenous fistula maturation: an open‐label randomized controlled trial. American Journal of Kidney Diseases 2013;62(2):304‐11. [DOI] [PubMed] [Google Scholar]
Malovrh 2009 {published data only}
- Malovrh M. Expansion of blood volume increases the primary patency rate of arteriovenous fistulas for hemodialysis in patients with critical arterial quality. Therapeutic Apheresis and Dialysis 2009;13(4):345‐9. [DOI] [PubMed] [Google Scholar]
Mileti 1995 {published data only}
- Mileti M, Petri G, Bacchi M, Ogliari V, Pecchini F, Bufano G, et al. A trial to evaluate the efficacy of picotamide in preventing thrombotic occlusion of the vascular access in hemodialysis patients. Journal of Nephrology 1995;8(3):167‐72. [Google Scholar]
Radmilovic 1998 {published data only}
- Radmilovic A, Boric Z, Naumovic T, Stamenkovic M, Musikic P. Shunt thrombosis prevention in hemodialysis patients ‐ A double‐blind, randomized study: pentoxifylline vs placebo. Angiology 1998;38(7):499‐506. [DOI] [PubMed] [Google Scholar]
Rouzrokh 2009 {published data only}
- Rouzrokh M, Reza Abbasi M, Reza Mirshemirani A, Reza Sobhiyeh M. The effect of antiplatelet drugs on the patency rate of arterio‐venous fistulae in hemodialysis patients. Iranian Journal of Pharmaceutical Research 2010;9(4):451‐7. [PMC free article] [PubMed] [Google Scholar]
Taber 1992 {published data only}
- Taber T, Maikranz P, Haag B, Dilley R, Gaylord G. Hemodialysis vascular graft stenosis may be altered by low‐molecular weight dextran (lmd), but not by aspirin (asa) [abstract]. Journal of the American Society of Nephrology 1992; Vol. 3, issue 3:397.
Trimarchi 2006 {published data only}
- Trimarchi H, Young P, Forrester M, Schropp J, Pereyra H, Freixas E. Clopidogrel diminishes hemodialysis acces graft thrombosis. Nephron. Clinical Practice 2006;102:c128‐32. [DOI] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang BR, Rowe VL, Wan Ham S, Han S, Patel K, Weaver FA, et al. A prospective clinical evaluation of the effects of intraoperative systemic anticoagulation in patients undergoing arteriovenous fistula surgery. The American Surgeon 2010;76:1112‐4. [DOI] [PubMed] [Google Scholar]
Wasse 2014 {published data only}
- Wasse H, Huang R, Long Q, Zhao Y, Singapuri S, McKinnon W, et al. Very high‐dose cholecalciferol and arteriovenous fistula maturation in ESRD: a randomized, double‐blind, placebo‐controlled pilot study. Journal of Vascular Access 2014;15(2):88‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yuto 2012 {published data only}
- Yuto J, Ehara Y, Shibata K, Iwamoto T, Yasuda G, Yatsu K, et al. Effect of sarpogrelate on fistula patency of forearm arteriovenous anastomosis in uremic patients. Kidney Research and Clinical Practice 2012;31:A86. [Google Scholar]
References to ongoing studies
Irish 2007 {published data only}
- Irish A. A randomised, double‐blind, placebo‐controlled, factorial‐design trial to assess the effect of aspirin and fish oil in the prevention of early thrombosis in arterio‐venous fistulae in patients with Stage IV or V chronic kidney disease requiring haemodialysis ‐ FAVOURED (Fish oil and Aspirin in Vascular access OUtcomes in REnal Disease). Cochrane Renal Group Prospective Trials Register [http://www.cochrane renal.org./dbsearch.php.] 2007.
- Irish A. Baseline characteristics of the patients participating in the favoured trial. Nephrology 2010;15:35. [Google Scholar]
- Irish A. Fish oil and aspirin in vascular access outcomes in renal disease. Australasian Kidney Trials Network [http://www.uq.edu.au./aktn./index.html.] 2007.
- Irish A, Dogra G, Mori T, Beller E, Heritier S, Hawley C, et al. Preventing AVF thrombosis: The rationale and design of the Omega‐3 fatty acids (Fish Oils) and Aspirin in Vascular access OUtcomes in REnal Disease (FAVOURED) study. http://www.biomedcentral.com/1471‐2369/10/1 (accessed 23 March 2015). [DOI: 10.1186/1471-2369-10-1] [DOI] [PMC free article] [PubMed]
Additional references
Churchill 1992
- Churchill DN, Taylor DW, Cook RJ, Laplante P, Barre P, Cartier P, et al. Canadian hemodialysis morbidity study. American Journal of Kidney Disease 1992;19(3):214‐34. [DOI] [PubMed] [Google Scholar]
Coleman 2010
- Coleman CI, Tuttle LA, Teevan C, Baker WL, White CM, Reinhart KM. Antiplatelet agents for the prevention of arteriovenous fistula and graft thrombosis: a meta analysis. International Journal of Clinical Practice August 2010;9:1239‐44. [DOI] [PubMed] [Google Scholar]
DOQI 1997
- Anonymous. NKF‐DOQI clinical practice guidelines for vascular access. National Kidney Foundation ‐ Dialysis Outcomes Quality Initiative. American Journal of Kidney Diseases 1997;30 Suppl 3:150‐91. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kanterman 1995
- Kanterman RY, Vesely TM, Pilgram TK, Guy BW, Windus DW, Picus D. Dialysis access grafts: anatomic localisation of venous stenosis and results of angioplasty. Radiology 1995;195(1):135‐9. [DOI] [PubMed] [Google Scholar]
Kaplan 1958
- Kaplan EL, Meier P. Non‐parametric estimation from incomplete observations. Journal of the American Statistical Association 1958;53:457‐81. [Google Scholar]
Metha 1991
- Metha S. Statistical summary of clinical results of vascular access procedures for haemodialysis. In: Sommer BG, Henry ML editor(s). Vascular Access for Hemodialysis‐II (Chapter 11). W L Gore, 1991:145‐57. [Google Scholar]
Pisoni 2002
- Pisoni RL, Young EW, Dykstra DM, Greenwood RN, Hecking E, Gillespie B, et al. Vascular access use in Europe and the United States: results from the DOPPS. Kidney International 2002;61(1):305‐16. [DOI] [PubMed] [Google Scholar]
Review Manager 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
References to other published versions of this review
Da Silva 2003
- Silva AF, Escofet X, Rutherford PA. Medical adjuvant treatment to increase patency of arteriovenous fistulae and grafts. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD002786] [DOI] [PubMed] [Google Scholar]
Osborn 2008
- Osborn G, Escofet X, Silva A. Medical adjuvant treatment to increase patency of arteriovenous fistulae and grafts. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD002786.pub2] [DOI] [PubMed] [Google Scholar]


