Abstract
Characterization of childhood and adolescent functional gastrointestinal disorders (FGIDs) has evolved during the 2−decade long Rome process now culminating in Rome IV. The era of diagnosing an FGID only when organic disease has been excluded is waning, as we now have evidence to support symptom-based diagnosis. In child/adolescent Rome IV, we extend this concept by removing the dictum that there was “no evidence for organic disease” in all definitions and replacing it with “after appropriate medical evaluation the symptoms cannot be attributed to another medical condition.” This change allows the clinician to perform selective or no testing to support a positive diagnosis of an FGID. We also point out that FGIDs can coexist with other medical conditions that themselves result in GI symptoms (eg, inflammatory bowel disease). In Rome IV, functional nausea and functional vomiting are now described. Rome III’s “abdominal pain related functional gastrointestinal disorders” has been changed to “functional abdominal pain disorders” and we have derived a new term, functional abdominal pain—not otherwise specified, to describe children who do not fit a specific disorder, such as irritable bowel, functional dyspepsia, or abdominal migraine. Rome IV FGID definitions should enhance clarity for both clinicians and researchers.
Keywords: Children, Adolescents, Abdominal Pain, Nausea, Vomiting, Functional Disorders
The Rome criteria provide symptom-based guidelines by which child and adolescent functional gastrointestinal disorders (FGID) can be diagnosed. Previous Rome III criteria were based mostly on consensus, as research in child/adolescent FGIDs was still largely lacking. An expanded evidence base from the last 10 years provides the basis for many of the recommendations of the child/adolescent committee for Rome IV. For disorders still lacking scientific data, the committee used clinical experience and consensus among the committee members.
The Rome IV functional gastrointestinal disorders (FGID) for children and adolescents are shown in Table 1. Rome III criteria emphasized that there should be “no evidence” for organic disease, which may have prompted a focus on testing.1 In Rome IV, the phrase “no evidence of an inflammatory, anatomic, metabolic, or neoplastic process that explain the subject’s symptoms” has been removed from diagnostic criteria. Instead, we include “after appropriate medical evaluation, the symptoms cannot be attributed to another medical condition.” This change permits selective or no testing to support a positive diagnosis of an FGID. We also point out that FGIDs can coexist with other medical conditions.2,3 Similarly, different FGIDs frequently coexist in the same patient. We have described 2 new disorders, functional nausea and functional vomiting. We changed “abdominal pain related functional gastrointestinal disorders” to “functional abdominal pain disorders” (FAPD) and have derived a new term, functional abdominal pain—not otherwise specified (FAP-NOS) to describe children who do not fit a specific disorder, such as irritable bowel, functional dyspepsia, or abdominal migraine. Minor modifications have been made to several other FGID.
Table 1.
Functional Gastrointestinal Disorders: Children and Adolescents
| H1. Functional nausea and vomiting disorders |
| H1a. Cyclic vomiting syndrome |
| H1b. Functional nausea and functional vomiting |
| H1c. Rumination syndrome |
| H1d. Aerophagia |
| H2. Functional abdominal pain disorders |
| H2a. Functional dyspepsia |
| H2b. Irritable bowel syndrome |
| H2c. Abdominal migraine |
| H2d. Functional abdominal pain-not otherwise specified |
| H3. Functional defecation disorders |
| H3a. Functional constipation |
| H3b. Nonretentive fecal incontinence |
H1. Functional Nausea and Vomiting Disorders
H1a. Cyclic Vomiting Syndrome
Epidemiology.
Data suggest a community prevalence of 0.2%–1.0% for cyclic vomiting syndrome (CVS) using Rome III criteria.4 Median age of symptom onset varies from 3.5 to 7 years, but CVS occurs from infancy to adulthood, with 46% having symptom start at 3 years of age or before.5
If abdominal pain and vomiting are present, the predominant or more consistent symptom should be considered for the primary diagnosis. If the predominant feature is abdominal pain, then abdominal migraine should be considered.
Rationale for changes in diagnostic criteria.
Rome IV criteria require that the attacks be stereotypical for the individual patient, occur within a 6-month period, that criteria for another FGID not be fulfilled, and that the primary and most severe symptom be vomiting rather than abdominal pain. The committee has changed the statement “return to usual state of health lasting weeks to months” to “episodes are separated by weeks to months with return to baseline health between episodes.” This change was made because “usual state of health” could have been misinterpreted as being asymptomatic between episodes and did not allow the coexistence of mild GI symptoms at baseline.
Clinical evaluation.
The committee endorses the clinical evaluation proposed in the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition CVS guidelines for children 2 to18 years of age.6 There is a higher likelihood of underlying neurometabolic diseases in children with early onset of symptoms and metabolic testing should be carried out during the vomiting episode and before administration of intravenous fluids to maximize detection of abnormalities. Chronic use of cannabis can be associated with repeated episodes of severe vomiting, nausea, and abdominal pain (cannabinoid hyperemesis syndrome) and should be considered in adolescent patients. Compulsive long hot water bath or shower (frequently lasting several hours) resulting in temporary symptom relief is common in cannabinoid hyperemesis syndrome.
Treatment.
The committee endorses the therapeutic approach recommended in the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition CVS guidelines.6 The guidelines recommend cyproheptadine in children <5 years of age and amitriptyline in children >5 years. Second-line treatment includes prophylaxis with propranolol for children of all ages. Some patients with CVS may require combinations of drugs or complementary treatments, such as acupuncture and/or cognitive-behavioral therapy to help control their symptoms.7 Mito-chondrial cofactors co-enzyme Q10 and L-carnitine have been used as adjunctive therapy in some patients.8 Abortive treatment is based on a combination of hydration and drug administration.
H1b. Functional Nausea and Functional Vomiting
Epidemiology.
There are no pediatric data on the prevalence of isolated nausea, isolated vomiting, or a combination of both in the literature.
Rationale for adoption of diagnostic entities.
Based on clinical experience, especially in children with anxiety or depression, functional nausea and functional vomiting are now included in Rome IV. Some patients have nausea alone, some have vomiting alone, and some have nausea and vomiting. We believe that the absence of concomitant pain in these disorders suggests they should not be included as part of functional dyspepsia.
Pathophysiologic considerations.
Some patients with these disorders also experience autonomic symptoms, such as sweating, dizziness, pallor, and tachycardia. Postural orthostatic tachycardia syndrome may include nausea and vomiting as part of its symptom complex,9 and should be distinguished from functional nausea and functional vomiting. Some children only experience nausea early in the morning, and observe that when they “sleep late,” that is, past the usual time when they would experience nausea, the nausea does not occur.
Clinical evaluation.
We have established functional nausea and functional vomiting as separate entities, but patients with chronic nausea commonly report mild vomiting with varying frequency. The presence of severe vomiting in addition to nausea presents a different situation in which central nervous system disease, GI anatomic abnormalities (eg, malrotation), gastroparesis, and intestinal pseudo-obstruction should be excluded. Biochemical testing may include measurement of serum electrolytes, calcium, cortisol, and thyroid hormone levels. Intestinal obstruction and motility disorders (eg, gastroparesis, intestinal pseudo-obstruction) should be considered and excluded in the presence of recurrent vomiting. A relationship between functional nausea or vomiting and delayed gastric emptying in children is not clearly established. We did not consider a normal upper GI endoscopy a requirement for a diagnosis of functional nausea without vomiting. Psychological evaluation is important in children with functional nausea or functional vomiting.
Treatment.
There are no published data on the treatment of isolated functional nausea and isolated functional vomiting (with or without nausea) in children. Mental health intervention should be offered first in those patients with obvious psychological comorbidities. Cognitive behavioral therapy and hypnotherapy have been used in patients with severe nausea due to chemotherapy10 and may be helpful in this condition as well. Cyproheptadine has been used in children with functional dyspepsia with nausea.11 Gastric electrical stimulation has been used to treat intractable dyspepsia (including nausea) in children and may be effective even in the absence of gastroparesis.12
H1c. Rumination Syndrome
Epidemiology.
Prevalence in adolescents and children is largely unknown. A limitation is that regurgitation and rumination often occur in secret without parents being aware. This may explain why rumination defined by Rome III could not be identified based on parental report in a large community-based study in the United States.13 Rumination can occur at any age, but some patient groups, such as adolescent girls, seem to be at higher risk.
Rationale for changes in criteria.
The name “adolescent rumination syndrome” is now “rumination syndrome” because children who are younger may also suffer from this condition. The elimination of “painless” from the description of the regurgitation is justified by the fact that often patients have another FAPD and by the fact that the act of regurgitation is often triggered by a sensation of discomfort (pressure, pain, burning) in the abdomen that is relieved by regurgitation. We eliminated the requirement that symptoms do not respond to gastroesophageal reflux disease treatment, as it is not compulsory to treat for gastroesophageal reflux disease before diagnosis of rumination. We added the need to rule out an eating disorder. Finally, we have eliminated the need for the act of rumination to “occur at least once per week” because patients with this condition usually have symptoms after each meal.
Pathophysiologic features.
The act of rumination is caused by an increase in intragastric pressure due to the contraction of the abdominal muscles and is associated with opening of the lower esophageal sphincter, leading to the return of gastric content into the esophagus. When gastro-jejunal manometry is used to aid in the diagnosis, the increase in intragastric or intra-abdominal pressure can be recognized as a simultaneous increase in pressure (“r” waves) across multiple areas of the upper gut. These pressure waves are thought to be the result of the contraction of the skeletal abdominal muscles. Fasting and postprandial motility is usually normal. It also has been suggested that when abdominal pressure increases, the gastroesophageal junction is displaced into the thorax, and it is this anatomic change rather than relaxation of the lower esophageal sphincter that explains the mechanism of voluntary regurgitation occurring during rumination syndrome.14
Psychological features.
There is often a triggering event before the onset of the symptoms of rumination. An intercurrent infectious process may cause vomiting and nausea, which do not disappear once the infection has resolved. On other occasions, a traumatic psychosocial event may be recognized at the onset of symptoms. Psychiatric disturbances can include depression, anxiety disorder, obsessive compulsive disorder, post-traumatic stress disorder, adjustment disorder, developmental delays, and attention deficit-hyperactivity disorder.15
Clinical evaluation.
Effortless repetitive regurgitation, reswallowing, and/or spitting within minutes of starting a meal define rumination. Other common complaints include abdominal pain, bloating, nausea, heartburn, and several somatic symptoms, such as headaches, dizziness, and sleeping difficulties. Differential diagnosis includes gastroesophageal reflux, gastroparesis, achalasia, bulimia nervosa, and other functional or anatomical gastric and small intestinal diseases, but in none of these entities does the regurgitation occur immediately after eating. One study suggested that high-resolution esophageal manometry can identify subgroups with distinct mechanisms of disease that respond to specific management strategies.16
Treatment.
A thorough understanding of rumination syndrome and a clear motivation to overcome it are critical in achieving successful treatment. Because rumination syndrome can be conceptualized in terms of a learned habit, treatment often has used strategies successful in the management of habit disorders. A successful novel inpatient interdisciplinary approach that involved pediatric psychology, pediatric gastroenterology, clinical nutrition, child life, therapeutic recreation, and massage therapy has been reported in adolescents with this condition.17
H1d. Aerophagia
Epidemiology.
In a large US population study using Rome III criteria, aerophagia was found in 4.2% of children by parental report of symptoms.13 A large school-based, cross-sectional study conducted in Sri Lanka used Rome III criteria and reported a prevalence of 7.5%.18 Aerophagia seems to be particularly common in patients with neuro-cognitive disabilities.
Rationale for changes in diagnostic criteria.
We have added “excessive” to the air swallowing and “increases during the day” to abdominal distention. All 3 criteria are now required to meet the diagnosis. Aerophagia has been deleted as a diagnosis in the adult Rome IV classification as it was believed to largely represent a mechanism for supragastric or gastric belching, as well as abdominal bloating or distention rather than a specific disorder. In contrast, the pediatric committee believes that aerophagia is a well-recognized condition in pediatrics.
Pathophysiologic features.
When air swallowing is excessive, gas fills the GI lumen, resulting in excessive belching, abdominal distention, flatus, and pain, presumably as a consequence of luminal distention. A subgroup of children seems unable to belch and, in those patients, symptoms of distention and pain may be more severe. A higher percentage of children with aerophagia were found to be exposed to stressful events compared with controls,18 and anxiety can be a cause for excessive air swallowing.
Clinical evaluation
Aerophagia may be confused with gastroparesis or other motility disorders, such as chronic intestinal pseudo obstruction. Bacterial overgrowth and malabsorption (particularly celiac disease and disaccharidase deficiency) are other etiologies of abdominal distention and excessive flatus. In older children, large amounts of air can be swallowed while chewing gum or drinking very quickly. Intestinal-related (abdominal pain, nausea, and early satiety) and extraintestinal symptoms (headache, sleeping difficulty, and lightheadedness) were found to be common among affected children.18
Treatment.
There are no controlled studies in children to guide therapy, which remains largely supportive and may include behavioral therapy, psychotherapy, and benzodiazepines.
H2. Functional Abdominal Pain Disorders
We have now changed “abdominal pain related functional gastrointestinal disorders” to “functional abdominal pain disorders.” We found that the term functional abdominal pain often was used to refer to any of the abdominal pain—related FGIDs (eg, FAP, irritable bowel syndrome [IBS], and functional dyspepsia [FD]).19 This inconsistent use of the term functional abdominal pain was considered a major problem by the Rome IV committee. The committee believes it is important to distinguish between different types of FAPD for clinical and research purposes. For those children not meeting criteria for IBS, FD, or abdominal migraine, we now use the term functional abdominal pain—not otherwise specified (NOS). Studies demonstrate that there can be overlap of more than 1 FAPD in an individual patient.13 For clinical purposes, we recognize that FAP will still be used; however, for research purposes, FAP—NOS, although potentially cumbersome, will hopefully improve specificity in identifying different disorders.
H2a. Functional Dyspepsia
Epidemiology.
A US nationwide survey of 949 mothers revealed that 1.4% of their children had pain or discomfort in the upper abdomen at least once weekly, but only 0.2% met the Rome III pediatric criteria for FD.13 In a community-based study in the northeast United States, 5%—10% of otherwise healthy adolescents reported dyspeptic symptoms.20
Rationale for changes in diagnostic criteria.
With recognition of dyspepsia subtypes, we have eliminated the requirement of pain to fulfill the criteria for FD. There is now evidence for dyspepsia subtypes in children. A study of 100 children identified by Rome II pediatric criteria as having FD were questioned for evidence of adult Rome III features of FD and 29% met criteria for postprandial distress syndrome, 24% for epigastric pain syndrome, 26% met criteria for both, and 21% fit neither.21 These adult Rome III subtypes related better to differences in mast cell densities and scores on psychological subscales than found with Rome II subtypes (ulcer-like, dysmotility-like).21 Nocturnal pain that awakens the individual from sleep was associated with higher duodenal mast cell density, whereas bloating was associated with lower levels of antral inflammation and higher self-reports of anxiety and somatization. Early satiation and postprandial fullness were associated with higher levels of depression and self-reported anxiety. Postprandial distress syndrome phenotype differs from functional nausea as nausea in the latter disorder can occur at any time and is often not related to meals.
Pathophysiologic features.
FD is a heterogeneous disorder likely associated with different underlying pathophysiologic disturbances associated with specific symptom patterns. Hypotheses include abnormalities of gastric motor function, visceral hypersensitivity due to central or peripheral sensitization, low-grade inflammation, and genetic predisposition.22 Impaired gastric accommodation, as determined by a decreased ability of the stomach to relax in response to a meal, has been demonstrated.23 Using electrogastrogram and gastric emptying studies, 50% of pediatric patients with FD had abnormal electrogastrogram and 47% slow gastric emptying.24 FD developed in 24% of children as a sequela of an acute bacterial, but not viral, gastroenteritis.25,26 Eosinophils and mast cells within the gastric lamina propria were increased in number in children with atopy and FD and degranulated rapidly after cow’s milk challenge.27 Using a barostat, several investigators have demonstrated that FD patients have lower sensory thresholds to balloon distention of the proximal stomach than healthy volunteers.28 There is no evidence in children that Helicobacter pylori gastritis causes dyspeptic symptoms in the absence of duodenal ulcer.
Clinical evaluation.
The role of esophagogastroduodenoscopy (EGD) in pediatric FD is unclear. One pediatric study suggested that duration of symptoms of <1 year and vomiting were risk factors for mucosal inflammation.29 A prospective study evaluated Rome III criteria and alarm features in 290 children (aged 4—18 years) undergoing EGD for chronic abdominal pain. EGD was thought to be diagnostic in 109 (38%), with gastroesophageal reflux and eosinophilic esophagitis being the most common findings.30 A report from an expert panel asked to evaluate the need for EGD in different case scenarios of children with dyspeptic symptoms suggested EGD was indicated in dyspeptic children with a family history of peptic ulcer disease or H pylori infection, children older than 10 years of age, when symptoms persist for >6 months, and if symptoms are severe enough to affect activities of daily living, including sleep.31 The Rome IV pediatric committee does not believe there is compelling evidence to require an EGD in order to make a diagnosis of FD, but recognizes that local practice patterns and social considerations may influence the decision. See Table 2 for alarm features suggesting further diagnostic testing.
Table 2.
Potential Alarm Features in Children With Chronic Ahdominal Paina
| Family history of inflammatory bowel disease, celiac disease, or peptic ulcer disease |
| Persistent right upper or right lower quadrant pain |
| Dysphagia |
| Odynophagia |
| Persistent vomiting |
| Gastrointestinal blood loss |
| Nocturnal diarrhea |
| Arthritis |
| Perirectal disease |
| Involuntary weight loss |
| Deceleration of linear growth |
| Delayed puberty |
| Unexplained fever |
Clinical judgment should he exercised, putting what might he considered an alarm sign into the whole context of the history and physical examination.
Treatment.
There are no adequately sized, double-blind, placebo-controlled pediatric studies of FD treatment. Foods aggravating symptoms (eg, caffeine containing, spicy, fatty) and nonsteroidal anti-inflammatory agents should be avoided. Psychological factors that can contribute to the severity of the problem should be addressed. Acid blockade with histamine receptor antagonists and proton pump inhibitors can be offered for pain predominant symptoms.32 If cure of FD is defined as complete symptomatic relief after 4 weeks of treatment, omeprazole is superior to ranitidine, famotidine, and cimetidine.33 Although convincing data are lacking, low-dose tricyclic antidepressant therapy with agents such as amitriptyline and imipramine is often considered in difficult cases. Nausea, bloating, and early satiety are more difficult to treat, and prokinetics such as cisapride and domperidone can be offered where available. A retrospective, open-label study suggested that cyproheptadine is safe and effective for treating dyspeptic symptoms in children.11 Gastric electrical stimulation seems to be a promising option for pediatric patients with FD refractory to medical treatment.34
H2b. Irritable Bowel Syndrome
Epidemiology.
School-based studies in Colombia and Sri Lanka found a prevalence of IBS of 4.9% and 5.4%, respectively.4,35 IBS prevalence in children across the United States based on parental report ranges from 1.2% to 2.9%.13,36
Pediatric IBS can be divided into subtypes analogous to adults reflecting the predominant stool pattern (IBS with constipation, IBS with diarrhea, IBS with constipation and diarrhea, and unspecified IBS).37
Rationale for changes in diagnostic criteria.
The term discomfort was removed from Rome III criteria, as it is not clear whether the distinction between pain and discomfort is quantitative or qualitative. The difference between functional constipation and IBS with constipation has been clarified. As many as 75% of children with constipation report pain,38 and studies have shown IBS patients often receive a diagnosis of functional constipation.39 The committee recommends that patients with constipation and abdominal pain initially be treated for constipation only. If abdominal pain resolves with constipation treatment, the patient has functional constipation. If pain does not resolve with appropriate constipation treatment alone, the patient likely has IBS with constipation. IBS subtypes, analogous to those described in adults, are now included in Rome IV. While the evidence base for IBS subtypes in children is limited, the committee thought that establishing the concept of subtypes in children might be useful for research purposes.
Pathophysiologic features.
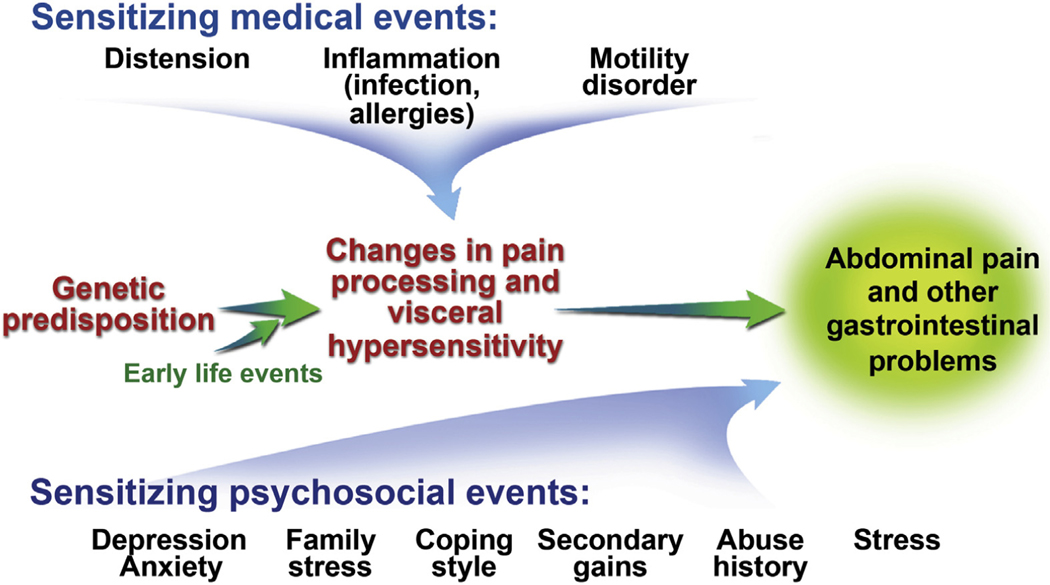
IBS is considered a disorder of the brain—gut axis (Figure 1). Symptoms (eg, diarrhea vs constipation, pain severity, psychosocial distress) in an individual patient reflect which components of the brain—gut axis are affected and to what degree. Some children with IBS have rectal but not gastric hyperalgesia, with the opposite present in some children with FAP-NOS.40,41 Visceral hypersensitivity may relate to the child’s psychological distress (anxiety, depression, impulsiveness, anger).42 Increased mucosal proinflammatory cytokines have been demonstrated and may be induced as a consequence of an acute infectious gastroenteritis (postinfectious IBS).25 Alterations in gut microbiome have been demonstrated, although it is not clear if these changes are the cause or result of IBS and its symptoms.43,44 Increased self-reported stress, anxiety, depression, and emotional problems may be seen in children with IBS.45,46 Noxious early life events (eg, surgery) have been associated with a higher risk for developing FAPDs in childhood, including IBS.47
Figure 1.
Pathophysiology of functional abdominal pain disorders. Visceral hyperalgesia leading to disability is shown as the final outcome of sensitizing medical factors that are superimposed on a background of genetic predisposition and early life events.
Clinical evaluation.
A careful history and physical examination may suggest functional constipation rather than IBS. Similarly, in the case of possible IBS with diarrhea, infection, celiac disease, carbohydrate malabsorption, and, less commonly, inflammatory bowel disease, warrant particular focus. Celiac disease can rarely present with constipation as well and warrants evaluation in children with IBS with constipation. The greater the number of alarm symptoms present, the greater the likelihood of an organic disease (Table 2). Determination of fecal calprotectin is increasingly being utilized as a noninvasive screen for intestinal mucosal inflammation and appears to be superior to standard testing such as C-reactive protein.48
Treatment.
There are very few double-blind, randomized treatment trials in pediatric patients with IBS. Most randomized pediatric studies have lumped all FAPDs together. There are data supporting the utility of pro-biotics.49,50 One small prospective, double-blind trial in children reported efficacy of peppermint oil in reducing pain severity.51 A recent, double-blind cross-over trial in children with IBS (all subtypes) suggested efficacy of an elimination diet reducing intake of fermentable oligosaccharides, disaccharides, monosaccharides, and polyols.52 Behavioral treatments, as described in the section on FAP-NOS, also can be recommended for pediatric IBS. The primary focus of behavioral treatments should be on optimizing symptom coping skills.
H2c. Abdominal Migraine
Epidemiology.
The frequency of abdominal migraine varies between 1% and 23%, depending on diagnostic criteria used for diagnosis.4,13,36,53 Since the Rome II criteria were replaced by Rome III, its frequency of diagnosis in children greatly increased. Rome III diagnostic criteria were more inclusive and less specific than Rome II criteria. Rome III criteria had a high positive predictive value (100%), but a low negative predictive value (7.7%), which might have led to other FAPDs being incorrectly diagnosed as abdominal migraine.54
Rationale for changes in diagnostic criteria.
The committee believes that the prevalence data based on Rome II criteria55 better represent the actual prevalence of abdominal migraine. To be consistent with diagnostic criteria for CVS, the committee decided to use the same frequency and number of episodes, that is, 2 within 6 months. The committee modified the following components of the major criteria: “periumbilical pain” is substituted for “midline pain, periumbilical or diffuse abdominal pain” as described in various publications56,57 and “Episodes are separated by weeks to months” is substituted for “return to baseline health,” as the latter phrase may not account for baseline GI symptoms and could be confusing to parents. To increase the specificity of diagnosis, the committee decided to add “stereotypical pattern and symptoms in the individual patient.” The diagnosis does not exclude the presence of other FAPDs for symptoms outside of the episodes. The committee stressed that the primary symptom should be abdominal pain.
Pathophysiologic features.
Abdominal migraine, CVS, and migraine headache likely share pathophysiologic mechanisms as well as being episodic, self-limited, and stereotypical, and with symptom-free intervals between attacks. Children with abdominal migraine and classic migraine report similar triggers (eg, stress, fatigue, and travel), associated symptoms (eg, anorexia, nausea, and vomiting), and relieving factors (eg, rest and sleep).58 Both abdominal migraine and CVS can evolve into migraine headaches in adulthood. Increased activity of excitatory amino acids has been found in patients with classic migraines, possibly explaining the efficacy of certain medications that increase γ-aminobutyric acid.59
Clinical evaluation.
The association of nonspecific prodromal symptoms, such as behavior or mood changes (14%), photophobia and vasomotor symptoms similar to those experienced by children with migraine headaches, and a history of symptom relief with antimigraine therapy, supports the diagnosis.57 Evaluation might require excluding processes associated with severe episodic symptoms, such as intermittent small bowel or urologic obstruction, recurrent pancreatitis, biliary tract disease, familial Mediterranean fever, metabolic disorders such as porphyria, and psychiatric disorders.
Treatment.
The treatment plan is determined by the frequency, severity, and impact of the abdominal migraine episodes on the child and family daily life. A double-blind, placebo-controlled, crossover trial in 14 children found a prophylactic benefit of oral pizotifen, a drug with antiserotonin and antihistamine effects.60 Prophylaxis with drugs such as amitriptyline,61 propranolol, and cyproheptadine62 has been successful.
H2d. Functional Abdominal Pain-Not Otherwise Specified Epidemiology
The term functional abdominal pain-not otherwise specified in Rome IV substitutes for the Rome III terms functional abdominal pain and FAPS. A mean of 35% to 38% of elementary school children report abdominal pain weekly.4,35 Only about one-third of these children meet Rome criteria for diagnosis of any FAPD. The prevalence of FAP-NOS is 2.7% in Colombian4 and 4.4% in Sri Lankan school-aged children according to Rome III criteria.35 Studies using parental report found a 1.2% prevalence of FAP-NOS in the US community36,63 and 2% in German school children.45
Justification for Changes in Diagnostic Criteria
The frequency of abdominal pain required for a diagnosis is changed from weekly to 4 times/month to align with the other FAPD criteria and allow inclusion of children who would otherwise not qualify for a FAPD, but have been found to be at risk for long-term negative consequences.36 The wording “that does not occur solely during physiologic events (eg, eating, menses)” has been added to harmonize with the adult criteria and to reflect the observation that patients with FAPDs may have worsening symptoms during physiologic events, such as eating and menses, and also have pain at other times. The committee is also dropping the FAPS category, given that loss of function can accompany other Rome diagnoses, such as IBS.
Pathophysiology
Studies separating FAP-NOS from IBS suggest that children with FAP-NOS in general do not have rectal hypersensitivity, in contrast to children with IBS.40,41 It has been reported that children with FAP-NOS have lower antral contractions and slower emptying rates of a liquid meal compared with healthy controls, but the clinical significance of this finding is unclear.64 There is evidence for the association between psychological distress and chronic abdominal pain in children and adolescents.45,65,66 Chronic abdominal pain is associated with stressful life events, such as parental divorce, hospitalization, bullying, and childhood abuse.35,67,68 How a child and his/her family copes with pain influences outcomes of FAPDs (Figure 2).
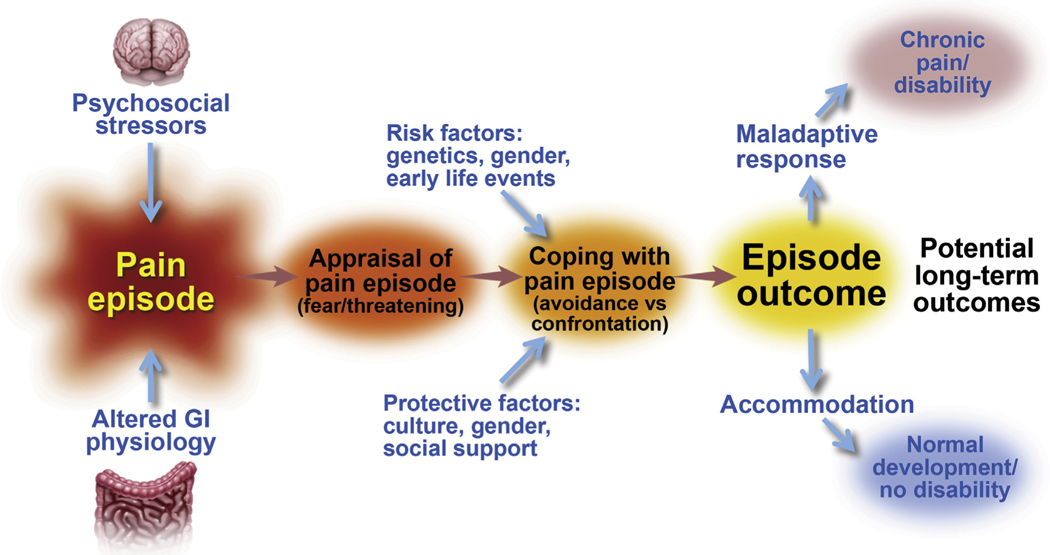
Figure 2.
The appraisal of any pain episode experienced by a child may have significant impact on the child’s ability to cope effectively and accommodate to the pain, and consequently his or her normal function and development. In the presence of risk factors or when protective factors are less effective, the child may develop a maladaptive response leading to a state of chronic pain. From Walker et al,90 adapted with permission.
Clinical evaluation.
Children with FAP-NOS frequently report nonspecific and extraintestinal somatic symptoms that do not necessarily require laboratory or radiologic investigation. Often for parental reassurance, limited diagnostic workup is performed. Special consideration should be given to the presence of autonomic symptoms, in particular in children with postural orthostatic tachycardia syndrome. See Table 2 for alarm features suggesting additional diagnostic testing.
Treatment.
Most treatment trials for FAPDs have lumped all disorders together limiting generalizability. Although adult trials have shown the efficacy of antispasmodics, mebeverine was not significantly better than placebo in children.69 A small trial of amitriptyline found benefits, while a large multicenter study did not.70,71 A recent large trial of citalopram found a trend toward effectiveness of citalopram compared to placebo in the treatment of children with FAP.72 Clinicians, patients, and parents should be aware of a black box warning issued by the US Food and Drug Administration for an increased risk of suicidal ideation in adolescents. Hypnotherapy73 and cognitive behavioral therapy74 have provided short- and long-term benefit in these patients.
H3. Functional Defecation Disorders
H3a. Functional Constipation
Epidemiology.
A systematic review reported a mean and median prevalence in children of 14% and 12%, respectively.75 The wide range in reported prevalence may be due to the use of different FC criteria and cultural influences. Peak incidence of constipation occurs at the time of toilet training with no sex differences.76 Childhood FC is distributed equally among different social classes with no relationship to family size, ordinal position of the child in the family, or parental age. Boys with constipation had higher rates of fecal incontinence than girls.
Justification for change in diagnostic criteria.
The only change is the decrease from 2 months to 1 month in the duration of symptoms needed to fulfill the criteria in order to harmonize with the European and the North American Societies for Pediatric Gastroenterology, Hepatology and Nutrition constipation guidelines, which suggested that the 2-month interval listed in the Rome III criteria for older children may unduly delay treatment in some children. The shorter interval is now similar to the time needed to fulfill the definition of FC in the neonate/toddler group.
Pathophysiology.
Because FC is equally common in both sexes and children with diverse socioeconomic backgrounds, dietary practices, and cultural influences,75 the triggering event is most likely the universal instinct to avoid defecation because of pain or social reasons (eg, school, travel). As a consequence of withholding, the colonic mucosa absorbs water from the feces and the retained stools become progressively more difficult to evacuate. This process leads to a vicious cycle of stool retention in which the rectum is increasingly distended, resulting in overflow fecal incontinence, loss of rectal sensation, and ultimately, loss of the normal urge to defecate. Increasing fecal accumulation in the rectum also causes decreased motility in the foregut, leading to anorexia, abdominal distention and pain.
Clinical evaluation.
We endorse the consensus guideline for the evaluation and treatment of the child with FC published by the European and the North American Societies for Pediatric Gastroenterology, Hepatology and Nutrition.77 Some of the recommendations from the guidelines are listed here:
ROME criteria are recommended for the definition of FC for all age groups
The diagnosis of FC is based on history and physical examination
Alarm signs and symptoms and diagnostic clues should be used to identify an underlying disease responsible for the constipation (Table 3)
If only one Rome criterion is present and the diagnosis of FC is uncertain, a digital examination of the anorectum is recommended to confirm the diagnosis and exclude underlying medical conditions.
There is no role for the routine use of an abdominal x-ray to diagnose FC
A plain abdominal radiograph may be used in a child if fecal impaction is suspected but in whom physical examination is unreliable/not possible
Routine allergy testing for cow’s milk allergy is not recommended in children with constipation in the absence of alarm symptoms
Laboratory testing to screen for hypothyroidism, celiac disease, and hypercalcemia is not recommended in children with constipation in the absence of alarm symptoms
The main indication to perform anorectal manometry in the evaluation of intractable constipation is to assess the presence of the rectoanal inhibitory reflex
Rectal biopsy is the gold standard for diagnosing Hirschsprung’s disease
A barium enema should not be used as an initial diagnostic tool for the evaluation of FC
Table 3.
Potential Alarm Features in Constipation
| Passage of meconium >48 h in a term newborn |
| Constipation starting in the first month of life |
| Family history of Hirschsprung’s disease |
| Ribbon stools |
| Blood in the stools in the absence of anal fissures |
| Failure to thrive |
| Bilious vomiting |
| Severe abdominal distension |
| Abnormal thyroid gland |
| Abnormal position of the anus |
| Absent anal or cremasteric reflex |
| Decreased lower extremity strength/tone/reflex |
| Sacral dimple |
| Tuft of hair on spine |
| Gluteal cleft deviation |
| Anal scars |
Treatment.
A systematic review showed that only 50% of children referred to a tertiary care center and followed for 6 to 12 months recovered and were taken off laxatives successfully.78,79 Education is as important as medical therapy and should include counseling families to recognize withholding behaviors and to use behavioral interventions, such as regular toileting, use of diaries to track stooling, and reward systems for successful evacuations.80 A normal fiber and fluid intake is recommended, while the addition of prebiotics and probiotics to the regimen currently does not seem to be supported by adequate evidence.
The pharmacologic approach comprises 2 steps: rectal or oral disimpaction for children who present with fecal impaction81 and maintenance therapy to prevent reaccumulation of feces using a variety of agents. Polyethylene glycol is first-line therapy for constipated children.77 In 3 recent Cochrane Reviews, polyethylene glycol was found superior to lactulose, although the quality of the evidence was poor due to sparse data, heterogeneity, and high risk of bias in the studies analyzed.82−84
H3b. Nonretentive Fecal Incontinence
Epidemiology.
Fecal incontinence is estimated to affect 0.8% to 4.1% of children in Western societies.
Justification for changes in diagnostic criteria.
To maintain consistency with FC, we have changed the duration of symptoms required for diagnosis from 2 to 1 month.
Pathophysiology.
Patients with nonretentive fecal incontinence (NFI) have normal defecation frequencies and colonic and anorectal motility parameters, differentiating this condition from FC. Total and segmental colonic transit times are significantly prolonged in constipated children compared with children with NFI.85 The diagnosis of NFI should be based on clinical symptoms, such as normal defecation frequency and absence of abdominal or rectal palpable mass, in combination with normal transit marker studies.86 NFI might be a manifestation of an emotional disturbance in a school-aged child and represent impulsive action triggered by unconscious anger. NFI has been described as a result of sexual abuse in childhood.87
Clinical evaluation.
In general, children with this condition have complete evacuation of colonic contents, not just staining of the underwear, in contrast to FC. Inquiry should be made whether there is a coexisting history of constipation, noting stool pattern (size and consistency of stools, withholding, straining), age of onset, type and amount of material evacuated, diet history, medications, coexisting urinary symptoms, psychosocial comorbidity, and family or personal stressors. Physical examination should focus on growth parameters, abdominal examination (distention, palpable stools), rectal examination (sacral dimple, position of anus, sphincter tone, rectal vault size, presence or absence of stool in rectum), and a thorough neurologic examination.
Treatment.
Parents need to understand that psychological disturbances, learning difficulties, and behavioral problems are usually significant contributors to the defecatory symptoms. Victims of sexual abuse must be identified and referred for appropriate counseling. The most successful approach to management of NFI involves behavioral therapy. Regular toilet training use with rewards and diminishing toilet phobia contribute to lower distress, restore normal bowel habits, and re-establish self-respect. It has been observed that in NFI, biofeedback therapy does not provide additional benefit compared with conventional therapy, even when improvement of defecation dynamics is obtained.88 A long-term follow-up study showed that after 2 years of intensive medical and behavioral treatment, only 29% of the children were completely free of fecal incontinence. At 18 years of age, 15% of adolescents with NFI still had the disorder.89 No prognostic factors for success were identified.
Recommendations for Future Research
Common research needs that apply to all pediatric FGIDs include:
Cross-cultural epidemiological studies
Natural history
Studies of pathophysiology, eg, the microbiome—brain— gut axis
-
Earlier access for children into clinical treatment trials of emerging medications
Specific needs that the committee recognizes include:
- CVS and abdominal migraine
- Comparative effectiveness treatment trials
- Long-term studies to provide guidelines on optimal timing of stopping prophylactic therapy.
- Functional nausea and functional vomiting
- Validation of the Rome IV criteria
- Rumination syndrome
- Establish effective treatment strategies for children too young or not cognitively mature to successfully engage in behavioral interventions.
- Aerophagia
- Define effective therapeutic strategies in children with and without neurodevelopmental deficits
- IBS and FAP-NOS
- Uncover pathophysiologic differences between IBS and FAP-NOS
- Define IBS subgroups by pathophysiology
- Elucidate the role of dietary factors and diet modification
- FD
- Define FD subgroups in children
- Define the role of upper GI endoscopy
- FC
- Assess safety of long-term osmotic and stimulant laxative use
- Determine the role of surgery in children who fail aggressive medical treatment
- Functional NFI
- Clarify the role of medical and behavioral treatments
Supplementary Material
H1a. Diagnostic Criteria for Cyclic Vomiting Syndrome Must include all of the following:
The occurrence of 2 or more periods of intense, unremitting nausea and paroxysmal vomiting, lasting hours to days within a 6-month period.
Episodes are stereotypical in each patient
Episodes are separated by weeks to months with return to baseline health between episodes.
After appropriate medical evaluation, the symp- toms cannot be attributed to another condition.
H1b. Diagnostic Criteriaa for Functional Nausea and Functional Vomiting
H1b1. Functional Nausea
Must include all of the following fulfilled for the last 2 months:
Bothersome nausea as the predominant symptom, occurring at least twice per week, and generally not related to meals
Not consistently associated with vomiting
After appropriate evaluation, the nausea cannot be fully explained by another medical condition
H1b2. Functional Vomiting
Must include all of the following:
On average, 1 or more episodes of vomiting per week
Absence of self-induced vomiting or criteria for an eating disorder or rumination
After appropriate evaluation, the vomiting cannot be fully explained by another medical condition
aCriteria fulfilled for at least 2 months before diagnosis.
H1c. Diagnostic Criteriaa for Rumination Syndrome Must include all of the following:
- Repeated regurgitation and rechewing or expul- sion of food that:
- Begins soon after ingestion of a meal
- Does not occur during sleep
Not preceded by retching
After appropriate evaluation, the symptoms cannot be fully explained by another medical condition. An eating disorder must be ruled out
aCriteria fulfilled for at least 2 months before diagnosis.
H1d. Diagnostic Criteriaa for Aerophagia Must include all of the following:
Excessive air swallowing
Abdominal distention due to intraluminal air which increases during the day
Repetitive belching and/or increased flatus
After appropriate evaluation, the symptoms cannot be fully explained by another medical condition.
aCriteria must be fulfilled for at least 2 months before diagnosis
H2a. Diagnostic Criteriaa for Functional Dyspepsia.
Must include 1 or more of the following bothersome symptoms at least 4 days per month:
Postprandial fullness
Early satiation
Epigastric pain or burning not associated with defecation
-
After appropriate evaluation, the symptoms cannot be fully explained by another medical condition.
aCriteria fulfilled for at least 2 months before diagnosis. Within FD, the following subtypes are now adopted:
Postprandial distress syndrome includes bother- some postprandial fullness or early satiation that prevents finishing a regular meal. Supportive fea- tures include upper abdominal bloating, post- prandial nausea, or excessive belching
Epigastric pain syndrome, which includes all of the following: bothersome (severe enough to interfere with normal activities) pain or burning localized to the epigastrium. The pain is not generalized or localized to other abdominal or chest regions and is not relieved by defecation or passage of flatus. Supportive criteria can include (a)burning quality of the pain but without a ret- rosternal component and (b) the pain commonly induced or relieved by ingestion of a meal but may occur while fasting.
H2b. Diagnostic Criteriaa for Irritable Bowel Syndrome Must include all of the following:
- Abdominal pain at least 4 days per month asso- ciated with one or more of the following:
- Related to defecation
- A change in frequency of stool
- A change in form (appearance) of stool
In children with constipation, the pain does not resolve with resolution of the constipation (chil- dren in whom the pain resolves have functional constipation, not irritable bowel syndrome)
After appropriate evaluation, the symptoms cannot be fully explained by another medical condition
aCriteria fulfilled for at least 2 months before diagnosis.
H2c. Diagnostic Criteriaa for Abdominal Migraine.
Must include all of the following occurring at least twice:
Paroxysmal episodes of intense, acute peri- umbilical, midline or diffuse abdominal pain last- ing 1 hour or more (should be the most severe and distressing symptom)
Episodes are separated by weeks to months.
The pain is incapacitating and interferes with normal activities
Stereotypical pattern and symptoms in the indi- vidual patient
- The pain is associated with 2 or more of the following:
- Anorexia
- Nausea
- Vomiting
- Headache
- Photophobia
- Pallor
After appropriate evaluation, the symptoms cannot be fully explained by another medical condition.
aCriteria fulfilled for at least 6 months before diagnosis.
H2d. Diagnostic Criteriaa for Functional Abdominal Pain—NOS.
Must be fulfilled at least 4 times per month and include all of the following:
Episodic or continuous abdominal pain that does not occur solely during physiologic events (eg, eating, menses)
Insufficient criteria for irritable bowel syndrome, functional dyspepsia, or abdominal migraine
After appropriate evaluation, the abdominal pain cannot be fully explained by another medical condition
aCriteria fulfilled for at least 2 months before diagnosis.
H3a. Diagnostic Criteria for Functional Constipation.
Must include 2 or more of the following occurring at least once per week for a minimum of 1 month with insufficient criteria for a diagnosis of irritable bowel syndrome:
2 or fewer defecations in the toilet per week in a child of a developmental age of at least 4 years
At least 1 episode of fecal incontinence per week
History of retentive posturing or excessive voli-tional stool retention
History of painful or hard bowel movements
Presence of a large fecal mass in the rectum
History of large diameter stools that can obstruct the toilet
H3b. Diagnostic Criteria for Nonretentive Fecal Incontinence.
At least a 1-month history of the following symptoms in a child with a developmental age older than 4 years:
Defecation into places inappropriate to the socio-cultural context
No evidence of fecal retention
After appropriate medical evaluation, the fecal incontinence cannot be explained by another medical condition
Abbreviations used in this paper:
- CVS
cyclic vomiting syndrome
- EGD
esophagogastroduodenoscopy
- FAPD
functional abdominal pain disorder
- FD
functional dyspepsia
- FGID
functional gastrointestinal disorder
- IBS
irritable bowel syndrome
- NFI
nonretentive fecal incontinence
- NOS
not otherwise specified
Footnotes
Conflicts of interest
The authors disclose the following: Carlo Di Lorenzo (QOL Medical, IM HealthScience, and Merck: consultant), Miguel Saps (QOL Medical, Nutricia, Ardelyx, Quintiles, Forest, and IM HealthScience: consultant), Robert J. Shulman (Gerson-Lehrman and Nutrinia: consultant; Mead Johnson: research support), Annamaria Staiano (Aboca and Nestec: clinical support; Aboca, D. M.G. Italy, and Sucampo AG: consultant; Angelini, Milté, Menarini, and Valeas: speaker), Miranda van Tilburg (Takeda: research support). The remaining authors disclose no conflicts.
Supplementary Material
Note: The first 50 references associated with this article are available below in print. The remaining references accompanying this article are available online only with the electronic version of the article. Visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2016.02.015.
References
- 1.Dhroove G, Chogle A, Saps M. A million-dollar work-up for ahdominal pain: is it worth it? J Pediatr Gastroenterol Nutr 2010;51:579–583. [DOI] [PubMed] [Google Scholar]
- 2.Faure C, Giguere L. Functional gastrointestinal disorders and visceral hypersensitivity in children and adolescents suffering from Crohn’s disease. Inflamm Bowel Dis 2008; 14:1569–1574. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman LA, Srinath AI, Goyal A, et al. The overlap of functional abdominal pain in pediatric Crohn’s disease. Inflamm Bowel Dis 2013;19:826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saps M, Nichols-Vinueza DX, Rosen JM, et al. Prevalence of functional gastrointestinal disorders in Colombian School children. J Pediatr 2014;164:542–545.e1. [DOI] [PubMed] [Google Scholar]
- 5.Fitzpatrick E, Bourke B, Drumm B, et al. The incidence of cyclic vomiting syndrome in children: population-based study. Am J Gastroenterol 2008;103:991–995; quiz 996. [DOI] [PubMed] [Google Scholar]
- 6.Li BU, Lefevre F, Chelimsky GG, et al. North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition consensus statement on the diagnosis and management of cyclic vomiting syndrome. J Pediatr Gastroenterol Nutr 2008;47:379–393. [DOI] [PubMed] [Google Scholar]
- 7.Slutsker B, Konichezky A, Gothelf D. Breaking the cycle: cognitive behavioral therapy and biofeedback training in a case of cyclic vomiting syndrome. Psychol Health Med 2010;15:625–631. [DOI] [PubMed] [Google Scholar]
- 8.Boles RG, Lovett-Barr MR, Preston A, et al. Treatment of cyclic vomiting syndrome with co-enzyme Q10 and amitriptyline, a retrospective study. BMC Neurol 2010; 10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ojha A, Chelimsky TC, Chelimsky G. Comorbidities in pediatric patients with postural orthostatic tachycardia syndrome. J Pediatr 2011;158:20–23. [DOI] [PubMed] [Google Scholar]
- 10.Richardson J, Smith JE, McCall G, et al. Hypnosis for nausea and vomiting in cancer chemotherapy: a systematic review of the research evidence. Eur J Cancer Care (Engl) 2007;16:402–412. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez L, Diaz J, Nurko S. Safety and efficacy of cyproheptadine for treating dyspeptic symptoms in children. J Pediatr 2013;163:261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teich S, Mousa HM, Punati J, et al. Efficacy of permanent gastric electrical stimulation for the treatment of gastroparesis and functional dyspepsia in children and adolescents. J Pediatr Surg 2013;48:178–183. [DOI] [PubMed] [Google Scholar]
- 13.Van Tilburg MAL, Walker L, Palsson O, et al. Prevalence of child/adolescent functional gastrointestinal disorders in a national U.S. community sample. Gastroenterology 2014;144(Suppl 1):S143–S144. [Google Scholar]
- 14.Gourcerol G, Dechelotte P, Ducrotte P, et al. Rumination syndrome: when the lower oesophageal sphincter rises. Dig Liver Dis 2011;43:571–574. [DOI] [PubMed] [Google Scholar]
- 15.Schroedl RL, Di Lorenzo C, Alioto A. Adolescent rumination syndrome. Pediatr Ann 2014;43:e95–e100. [DOI] [PubMed] [Google Scholar]
- 16.Aprile LR, Sifrim D, Dantas RO, et al. Rumination syndrome: characterization by esophageal manometry and multichannel intraluminal impedance. Gastroenterol Clin Biol 2008;32:976. [DOI] [PubMed] [Google Scholar]
- 17.Green AD, Alioto A, Mousa H, et al. Severe pediatric rumination syndrome: successful interdisciplinary inpatient management. J Pediatr Gastroenterol Nutr 2011; 52:414–418. [DOI] [PubMed] [Google Scholar]
- 18.Devanarayana NM, Rajindrajith S. Aerophagia among Sri Lankan schoolchildren: epidemiological patterns and symptom characteristics. J Pediatr Gastroenterol Nutr 2012;54:516–520. [DOI] [PubMed] [Google Scholar]
- 19.Di Lorenzo C, Colletti RB, Lehmann HP, et al. Chronic abdominal pain in children: a technical report of the American Academy of Pediatrics and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2005;40:249–261. [DOI] [PubMed] [Google Scholar]
- 20.Hyams JS, Burke G, Davis PM, et al. Abdominal pain and irritable bowel syndrome in adolescents: a community-based study. J Pediatr 1996;129:220–226. [DOI] [PubMed] [Google Scholar]
- 21.Schurman JV, Singh M, Singh V, et al. Symptoms and subtypes in pediatric functional dyspepsia: relation to mucosal inflammation and psychological functioning. J Pediatr Gastroenterol Nutr 2010;51:298–303. [DOI] [PubMed] [Google Scholar]
- 22.Tack J, Masaoka T, Janssen P. Functional dyspepsia. Curr Opin Gastroenterol 2011;27:549–557. [DOI] [PubMed] [Google Scholar]
- 23.Chitkara DK, Camilleri M, Zinsmeister AR, et al. Gastric sensory and motor dysfunction in adolescents with functional dyspepsia. J Pediatr 2005;146:500–505. [DOI] [PubMed] [Google Scholar]
- 24.Friesen CA, Lin Z, Hyman PE, et al. Electrogastrography in pediatric functional dyspepsia: relationship to gastric emptying and symptom severity. J Pediatr Gastroenterol Nutr 2006;42:265–269. [DOI] [PubMed] [Google Scholar]
- 25.Saps M, Pensabene L, Turco R, et al. Rotavirus gastro-enteritis: precursor of functional gastrointestinal disorders? J Pediatr Gastroenterol Nutr 2009;49:580–583. [DOI] [PubMed] [Google Scholar]
- 26.Saps M, Pensabene L, Di Martino L, et al. Post-infectious functional gastrointestinal disorders in children. J Pediatr 2008;152:812–816; 816 e1. [DOI] [PubMed] [Google Scholar]
- 27.Schappi MG, Borrelli O, Knafelz D, et al. Mast cell-nerve interactions in children with functional dyspepsia. J Pediatr Gastroenterol Nutr 2008;47:472–480. [DOI] [PubMed] [Google Scholar]
- 28.Simren M, Tack J. Functional dyspepsia: evaluation and treatment. Gastroenterol Clin North Am 2003; 32:577–599. [DOI] [PubMed] [Google Scholar]
- 29.Hyams JS, Davis P, Sylvester FA, et al. Dyspepsia in children and adolescents: a prospective study. J Pediatr Gastroenterol Nutr 2000;30:413–418. [DOI] [PubMed] [Google Scholar]
- 30.Thakkar K, Chen L, Tessier ME, et al. Outcomes of children after esophagogastroduodenoscopy for chronic abdominal pain. Clin Gastroenterol Hepatol 2014; 12:963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guariso G, Meneghel A, Dalla Pozza LV, et al. Indications to upper gastrointestinal endoscopy in children with dyspepsia. J Pediatr Gastroenterol Nutr 2010; 50:493–499. [DOI] [PubMed] [Google Scholar]
- 32.Camilleri M, Stanghellini V. Current management strategies and emerging treatments for functional dyspepsia. Nat Rev Gastroenterol Hepatol 2013;10:187–194. [DOI] [PubMed] [Google Scholar]
- 33.Dehghani SM, Imanieh MH, Oboodi R, et al. The comparative study of the effectiveness of cimetidine, ranitidine, famotidine, and omeprazole in treatment of children with dyspepsia. ISRN Pediatr 2011; 2011:219–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu PL, Teich S, Di Lorenzo C, et al. Improvement of quality of life and symptoms after gastric electrical stimulation in children with functional dyspepsia. Neurogastroenterol Motil 2013;25 567–e456. [DOI] [PubMed] [Google Scholar]
- 35.Devanarayana NM, Mettananda S, Liyanarachchi C, et al. Abdominal pain-predominant functional gastrointestinal diseases in children and adolescents: prevalence, symptomatology, and association with emotional stress. J Pediatr Gastroenterol Nutr 2011;53:659–665. [DOI] [PubMed] [Google Scholar]
- 36.Saps M, Adams P, Bonilla S, et al. Parental report of abdominal pain and abdominal pain-related functional gastrointestinal disorders from a community survey. J Pediatr Gastroenterol Nutr 2012;55:707–710. [DOI] [PubMed] [Google Scholar]
- 37.Rajindrajith S, Devanarayana NM. Subtypes and symptomatology of irritable bowel syndrome in children and adolescents: a school-based survey using Rome III criteria. J Neurogastroenterol Motil 2012;18:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgers R, Levin AD, Di Lorenzo C, et al. Functional defecation disorders in children: comparing the Rome II with the Rome III criteria. J Pediatr 2012;161:615–620 e1. [DOI] [PubMed] [Google Scholar]
- 39.van Tilburg MA, Squires M, Blois-Martin N, et al. Test of the child/adolescent Rome III criteria: agreement with physician diagnosis and daily symptoms. Neurogastroenterol Motil 2013;25 302–e246. [DOI] [PubMed] [Google Scholar]
- 40.Di Lorenzo C, Youssef NN, Sigurdsson L, et al. Visceral hyperalgesia in children with functional abdominal pain. J Pediatr 2001;139:838–843. [DOI] [PubMed] [Google Scholar]
- 41.Ginkel RV, Voskuijl WP, Benninga MA, et al. Alterations in rectal sensitivity and motility in childhood irritable bowel syndrome. Gastroenterology 2001;120:31–38. [DOI] [PubMed] [Google Scholar]
- 42.Iovino P, Tremolaterra F, Boccia G, et al. Irritable bowel syndrome in childhood: visceral hypersensitivity and psychosocial aspects. Neurogastroenterol Motil 2009; 21 940–e74. [DOI] [PubMed] [Google Scholar]
- 43.Saulnier DM, Riehle K, Mistretta TA, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology 2011; 141:1782–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajilic-Stojanovic M, Biagi E, Heilig HG, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011;141:1792–1801. [DOI] [PubMed] [Google Scholar]
- 45.Gulewitsch MD, Enck P, Schwille-Kiuntke J, et al. Rome III criteria in parents’ hands: pain-related functional gastrointestinal disorders in community children and associations with somatic complaints and mental health. Eur J Gastroenterol Hepatol 2013;25:1223–1229. [DOI] [PubMed] [Google Scholar]
- 46.Waters AM, Schilpzand E, Bell C, et al. Functional gastrointestinal symptoms in children with anxiety disorders. J Abnorm Child Psychol 2013;41:151–163. [DOI] [PubMed] [Google Scholar]
- 47.Bonilla S, Saps M. Early life events predispose the onset of childhood functional gastrointestinal disorders. Rev Gastroenterol Mex 2013;78:82–91. [DOI] [PubMed] [Google Scholar]
- 48.Henderson P, Casey A, Lawrence SJ, et al. The diagnostic accuracy of fecal calprotectin during the investigation of suspected pediatric inflammatory bowel disease. Am J Gastroenterol 2012;107:941–949. [DOI] [PubMed] [Google Scholar]
- 49.Horvath A, Dziechciarz P, Szajewska H. Meta-analysis: Lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment Pharmacol Ther 2011;33:1302–1310. [DOI] [PubMed] [Google Scholar]
- 50.Guandalini S, Magazzu G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr 2010;51:24–130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.