Abstract
Human infections with influenza H7 subtypes, such as H7N9, have raised concerns worldwide. Here, we report a human infection with a novel influenza A(H7N4) virus. A 68 years-old woman with cardiovascular and cholecystic comorbidities developed rapidly progressed pneumonia with influenza-like-illness as initial symptom, recovered after 23 days-hospitalization including 8 days in ICU. Laboratory indicators for liver and blood coagulation dysfunction were observed. Oseltamivir phosphate, glucocorticoids and antibiotics were jointly implemented, with nasal catheterization of oxygen inhalation for this patient. We obtained the medical records and collected serial respiratory and blood specimens from her. We collected throat, cloacal and/or feces samples of poultry and wild birds from the patient’s backyard, neighborhood, local live poultry markets (LPMs) and the nearest lake. All close contacts of the patient were followed up and sampled with throat swabs and sera. Influenza viruses and other respiratory pathogens were tested by real-time RT-PCR, viral culturing and/or sequencing for human respiratory and bird samples. Micro-neutralizing assay was performed for sera. A novel reassortant wild bird-origin H7N4 virus is identified from the patient and her backyard poultry (chickens and ducks) by sequencing, which is distinct from previously-reported avian H7N4 and H7N9 viruses. At least four folds increase of neutralizing antibodies to H7N4 was detected in her convalescent sera. No samples from close contacts, wild birds or other poultry were tested positive for H7N4 by real-time RT-PCR.
Keywords: Avian influenza virus (AIV), Human infection, H7N4, Epidemiology, Pneumonia
1. Introduction
Avian influenza virus (AIV) H7N9 has raised global concerns since human infections of low pathogenic (LP) and highly pathogenic (HP) H7N9 viruses emerged in 2013 and 2017, respectively [1], [2]. The virus has caused five waves of virulent human infections in China Mainland [3], and cases have been exported to other districts in Asia and North America [4], [5], [6], with a huge economic loss [7], [8]. The H7 subtype HA gene has been found in combination with all nine NA subtype genes [9]. Other influenza A subtype H7 viruses, such as H7N2, H7N3 and H7N7, were documented being capable of infecting human as well [10], [11], [12], [13], but usually self-limiting.
Both HP and LP strains of influenza A (H7N4) virus have been identified from domestic poultry and wild birds in Australia, Korea, Europe and North America [14], [15], [16], [17]. Here, we report a human case with severe pneumonia infected by a novel wild bird-origin H7N4 virus.
2. Methods and materials
2.1. Case identification
A surveillance network on hospitalized pneumonia patients was established in Jiangsu Province, Eastern China since 2012, as a part of the China National Unknown Cause Pneumonia Surveillance Network. Patients admitted to a local hospital with diagnosis of pneumonia, who have rapid progress but lack of identified pathogenic causes, are required to be sampled and sent to laboratory networks for pathogenic analyses, including influenza viruses. As a practice in China, empirical antiviral treatment (neuraminidase inhibitor) is recommended for suspected influenza patients before laboratory confirmation, in order to acquire the most health benefits.
2.2. Epidemiological investigation and specimen collection
We acquired patient’s epidemiological information, such as demographic characteristics, recent direct or indirect exposures to poultry or other animals, recent travel history and history of seasonal influenza vaccination, by a face-to-face interview with the patient and her family members. Patient’s clinical data, including underlying medical conditions, clinical symptoms and signs, radiographic examinations, clinical laboratory testing results, clinical treatment, clinical complications and outcome, were derived from her medical records. Serial respiratory and blood samples were taken at an interval of 1–3 days until the patient’s recovery.
Close contacts were defined as individuals who had provided care to, had been living with, or had potentially been directly exposed to respiratory secretions or body fluids of the patient during the time span of one day before the illness onset to the isolation of the patient. We took respiratory and blood samples from all close contacts, and followed them up daily for any signs or symptoms of respiratory illness till the 28th day after their last unprotected contact with the patient. No antiviral chemoprophylaxis was implemented. Those who shared a similar exposure history with the patient, such as had been exposed to the same animals or environment during the 14 days before illness onset of the patient, were defined as co-exposers.
We collected throat, cloacal and/or feces samples of poultry and wild birds from the patient’s backyard, neighborhood, local live poultry markets (LPMs) and the nearest lake, for tracing source and spread range of the virus.
2.3. Laboratory analyses
2.3.1. Nucleic acid extraction and detection
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.scib.2018.07.003.
Nucleic acids were extracted using Viral DN&RNA Kit (Tianlong Bio-Technology Co., Ltd, Suzhou, China), according to the manufacturer’s instructions. Commercial real-time RT-PCR kits for detecting H1 to H16 and N1 to N9 subtypes were performed to detect the influenza subtypes (Jiangsu Bioperfectus Technologies Co., LTD, Taizhou, China). In addition, the patient’s throat swabs were tested for 28 other respiratory pathogens (Table S1 online) using laboratory-developed specific real-time PCR assays (conditions and primers are available on request).
Supplementary data 1.
2.3.2. Virus isolation
Throat swabs of the patient, cloacal swabs of chicken and ducks were collected and maintained in a viral transport medium. The swabs were propagated in the allantoic sac and amniotic cavity of 9-to-11-day-old specific pathogen-free (SPF) embryonated chicken eggs for 72 h at 36 °C. The virus titer was established with a haemagglutination (HA) test using turkey red blood cells and was recorded as the reciprocal of the highest dilution of the virus that induced hemagglutination.
2.3.3. Micro-neutralization assay
Micro-neutralization (MN) tests (from titers ≥40) were performed for patient’s serum samples collected during acute and convalescent stage of illness course, according to WHO manual [18]. The standard antigen used in the assay was produced from the A(H7N4) isolate from the chicken fed by the patient (A/Chicken/Jiangsu/1/2018 H7N4).
2.3.4. Genome sequencing and phylogenetic analysis
Virus RNA was reversely transcripted into cDNA and viral genomes were amplified [19], [20]. Sequencing was done with the Illumina MiSeq platform as previously described [21]. Maximum-likelihood phylogenetic analysis was conducted with RAxML-HPC2 on the Extreme Science and Engineering Discovery Environment (XSEDE) version 8.2.10 with GTR+Γ model and 1000 bootstraps for all the eight genes of the eight H7N4 influenza viruses by using the top 100 BLAST hits of A/Chicken/Jiangsu/103/2018(H7N4), which has the highest identity with A/Jiangsu/1/2018(H7N4), available from GenBank and Global Initiative on Sharing All Influenza Data (GISAID). Each tree was rooted with the earliest isolate among the hits and was visualized by FigTree v1.4.3. Of note, five human H7N9 viruses from 2013 to 2017 were added in the HA tree.
2.4. Ethic issue
The National Health and Family Planning Commission deemed the data collection for this case to be part of the continuing public health outbreak investigation and exempt from institutional review board assessment. The identity information of the case was unrevealed in this report. The institutional review board of Jiangsu Provincial Center for Disease Control and Prevention (JSCDC) approved the assessment of these close contacts. Written informed consent was obtained from the close contacts.
3. Results
3.1. Social-demographic and clinical characteristics of the patient
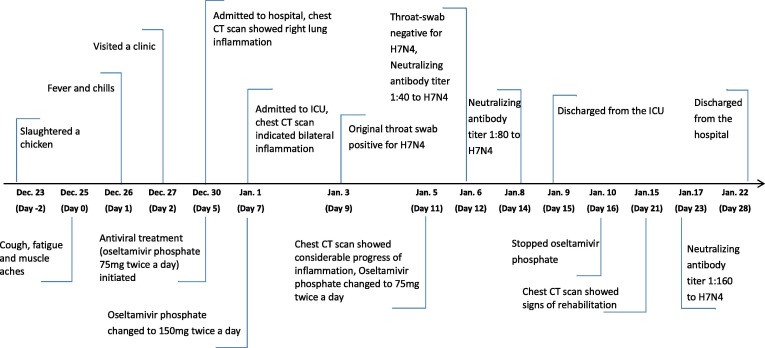
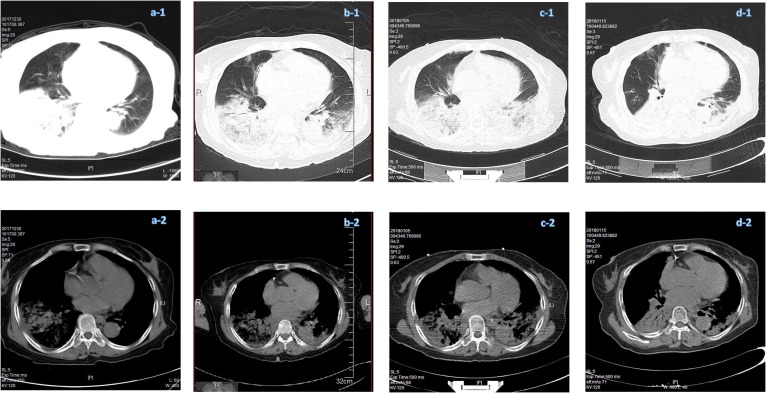
On January 3, 2018, a patient, female Han Chinese aged 68 years, with rapidly progressed pneumonia was detected through the Hospitalized Pneumonia Surveillance in Jiangsu Province. Her medical records retrieved from local Hospital Information System showed that she had comorbidities of coronary heart disease, hypertension, periapical periodontitis, chronic cholecystitis and cholecystolithiasis, and underwent cholecystectomy in 2016. She had no history of influenza vaccination. The timeline of her current disease course was shown in Fig. 1 . She developed initial symptoms of cough, fatigue and sore muscles on Dec. 25, 2017 (day 0), and fever and chills the next day (day 1). She visited a local clinic on day 2 and was treated with Shengmai (a traditional Chinese medicine for cardiovascular disorders) intravenously once. Her symptom progressed rapidly during the following days, with a peak body temperature of 39 °C. She was admitted to the hospital on day 5, and to ICU on day 7 with complications of respiratory failure, liver and blood coagulation dysfunction. Chest CT scan showed increased density in inferior lobe of right lung and right pleural effusion on day 5, bilateral pneumonia with consolidation of right lung on day 7, and a rapid progression of diffuse ground-glass opacities with consolidation in right lung on day 11 (Fig. 2 a–c).
Fig. 1.
(Color online) Timeline of the clinical course and key indicators of the H7N4 patient.
Fig. 2.
(Color online) Chest CT scans of the H7N4 patient. (a–d) Were obtained on day 5, day 7, day 11 and day 21 after illness onset, respectively. a-1, b-1, c-1, d-1: Lung window; a-2, b-2, c-2, d-2: Mediastinal window.
The number of white blood cell was within normal range on the day of hospital admission (day 5) and was decreased on the day of ICU admission (day 7). Lymphocytopenia was observed from day 5 to day 8. High concentration of aspartate aminotransferase (AST) was constantly recorded till day 16. Blood platelet count remained decreased until day 10, and D-dimer concentration was elevated on all tests. Blood concentrations of urea nitrogen and creatinine were normal through all tests, but urine protein was detected on day 6 and day 7. Reduced arterial partial pressure of oxygen was recorded the day of admission to hospital prior to the implementation of oxygen inhalation, and remained decreased till day 11 (Table 1 ). Antiviral treatment (Oseltamivir phosphate) was initiated empirically on day 5 (75 mg twice a day) and was terminated after her discharge from ICU. The dose was doubled during the first 4 days of her stay in ICU (Fig. 1). Nasal catheterization of oxygen inhalation was implemented from the day of admission till her discharge from ICU. Glucocorticoids, intravenous albumin and immune globulin therapy were jointly implemented, with combination of antibiotics for bacteria secondary infection. Methylprednisolone sodium succinate was administered from day 5 to day 10. The dose was 20 mg per day for 2 days prior to ICU admission, 40 mg twice a day for the first 2 days in ICU and 40 mg per day for the following 2 days. Cefoxitin Sodium (2 g per day) and Azithromycin Lactobionate (0.5 g per day) were jointly administered for 2 days before the patient’s ICU admission. Moxifloxacin hydrochloride (0.4 g per day) was implemented during her stay in ICU instead. After that, Cefoperazone Sodium and Sulbactam Sodium (3 g per day) were initiated on day 16 as a substitution, and Linezolid (0.6 g per day) was added the following day. Antibiotics treatment ended on day 24.
Table 1.
Clinical laboratory tests.
| Selected indicators | Days after disease onset |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | 10 | 12 | 14 | 16 | 19 | 21 | |
| Number of white blood cells (×109/L) | 3.61 | 3.56 | 2.82↓ | 2.44↓ | 6.14 | 7.92 | 8.22 | 12.36↑ | 7.26 | 6.52 |
| Number of neutrophils (×109/L) | 2.73 | 2.49 | 2.23 | 1.96 | 4.46 | 5.81 | 6.01 | 9.72↑ | 5.52 | 4.79 |
| Proportion of neutrophils (%) | 75.60↑ | 70.1 | 79.14↑ | 80.30↑ | 72.7 | 73.3 | 73 | 78.6↑ | 76↑ | 73.5 |
| Number of lymphocytes (×109/L) | 0.76↓ | 0.97↓ | 0.52↓ | 0.39↓ | 1.21 | 1.39 | 1.27 | 1.57 | 1.25 | 1.15 |
| Proportion of lymphocytes (%) | 21.1 | 27.2 | 18.44↓ | 16.00↓ | 19.7↓ | 17.6↓ | 15.5↓ | 12.7↓ | 17.2↓ | 17.6↓ |
| aC-reactive protein (mg/L) | <0.5 | 49.14↑ | 28.55↑ | 32.80↑ | 6.5↑ | 16.9↑ | 6.5↑ | 23.3↑ | – | 3.4 |
| Alanine transaminase, ALT (U/L) | – | – | 47.0↑ | 38 | 74.0↑ | 94.0↑ | 65↑ | 54↑ | – | 25 |
| Aspartate aminotransferase, AST (U/L) | 48.9↑ | – | 53.0↑ | 45.0↑ | 89.0↑ | 65.0↑ | 38↑ | 27 | – | 14 |
| Lactic dehydrogenase (U/L) | 508↑ | 657↑ | 614↑ | 547↑ | 496↑ | 389↑ | 305↑ | 210 | ||
| Alkaline phosphatase (U/L) | – | – | 48 | 47 | 44 | 52 | 64 | 81 | – | 94 |
| Creatine kinase (U/L) | 427↑ | – | 277↑ | – | 29 | – | – | – | – | |
| Creatinine (μmol/L) | 57.2 | – | 40.9 | 57.8 | 60.8 | 64.4 | 68.1 | 74 | – | 78.4 |
| Blood urea nitrogen (mmol/L) | 7.95 | – | 5.7 | 5.5 | 7.3 | 6.7 | 7.7 | 7.1 | – | 4.4 |
| Total protein (g/L) | – | – | 53.2 | 51.3↓ | 66.2 | 75.4 | 76.7 | 72.4 | – | 64.4↓ |
| Albumin (g/L) | – | – | 29.8↓ | 23.1↓ | 33.6↓ | 37.4↓ | 38.6↓ | 38.1↓ | – | 33.4↓ |
| Globin (g/L) | – | – | 23.4 | 28.2 | 32.6 | 38 | 38.1 | 34.3 | – | 31 |
| Platelet (×109 per L) | 87↓ | 58↓ | 58↓ | 64↓ | 137 | 205 | 335 | 344 | 315 | 220 |
| Antithrombin III (%) | 63↓ | 73↓ | 69↓ | 69↓ | 78 | 85 | – | – | – | – |
| D-dimer (μg/mL) | 3.06↑ | 4.82↑ | 4.14↑ | 4.11↑ | 2.58↑ | 1.96↑ | – | – | – | – |
| Arterial partial pressure of oxygen (mmHg) | 45.7↓ | 66.1↓ | 75.0↓ | 67↓ | 66↓ | 104 | 97 | – | – | – |
| Oxygenation Index (mmHg) | – | – | 188 | 163 | 165 | 254 | 237 | – | – | – |
| Procalcitonin (ng/mL) | – | – | – | 0.64↑ | – | – | – | 0.34 | – | – |
| Hemoglobin (g/L) | 123 | 129 | 127 | 131 | 117 | 113↓ | 115↓ | 112↓ | 101↓ | 99↓ |
| bUrine protein (g/L) | – | 2+ | 0.25↑ | – | – | – | – | – | – | – |
Normal ranges of selected indicators were presented in Table S4 online.
Hypersensitive C-reactive protein was tested on day 5 and day 6.
Urine protein was tested qualitatively on day 6 and quantitatively on day 7.
Mycoplasma pneumoniae (MP) was cultured from patient’s throat swab collected on the day of ICU admission (day 7), but the specific IgM antibody was only weakly positive in patient’s blood specimen obtained on day 8, indicated by indirect immunofluorescent assay (kits manufactured by VIRCELL, S.L.). During her stay in ICU, patient’s sputum was collected on day 8, 9, 12, 13 and 14. From them, Monilia albican was continuously cultured and Klebsiella pneumoniae was cultured from the sputum collected on day 12 only. The patient recovered and was discharged from hospital on day 28 (Fig. 1, Fig. 2d).
3.2. Laboratory analyses
Throat swab samples obtained from the patient on day 9 and day 11 were shown to be positive for H7N4, while samples obtained since day 12 showed negative, by real-time RT-PCR. All samples showed negative for other influenza subtypes and 28 respiratory pathogens, except for herpes simplex virus-1 (HSV-1, positive from day 9 to day 17) and human coronavirus type 229E (HCov 229E, positive from day 20 to day 27) (Table S1). Next generation sequencing (NGS) and genome assembly showed 8 consensus gene segments of H7N4 from the throat swab on day 9 (designated as A/Jiangsu/1/2018 (H7N4), henceforth, JS1). The results were confirmed subsequently by the Chinese National Influenza Center, the WHO Reference Laboratory. Micro-neutralization assay showed that patient’s antibody titer to H7N4 was less than 40 on day 9, when she was in ICU with considerable progress of bilateral pneumonia indicated by CT scan and throat swab positive for H7N4 virus by real-time RT-PCR. On day 12, a neutralizing antibody titer of 40 to H7N4 was detected from serum, while the patient’s throat swab became negative for H7N4 virus by real-time RT-PCR. The neutralizing antibody titer continued increasing to 80 on day 14 and to 160 on day 23, which coincided with the patient’s discharge from ICU and rehabilitation indicated by chest CT scans (Table S2 online). In contrast, HSV-1 was detected in patient’s throat swabs even two days after the patient’s discharge from ICU, and HCov 229E was only detected during the patient’s convalescent stage.
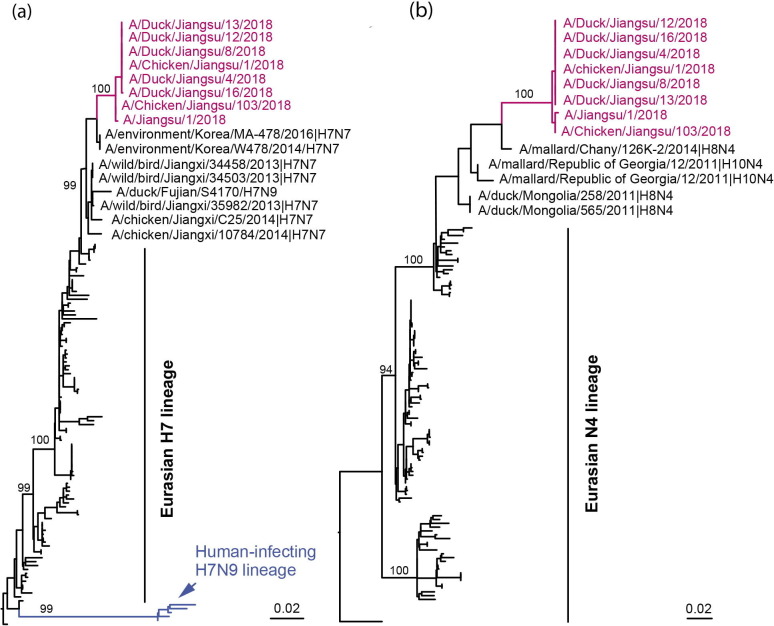
As we failed to isolate virus from throat swab of the patient collected on the 5th day post antiviral treatment, viral genome was sequenced following influenza virus specific amplification and was deposited in the GISAID (accession number: EPI1139727-EPI1139730, EPI1139738, EPI1139761, EPI1139780, EPI1139806). Meanwhile, we successfully isolated five and two H7N4 strains from the duck and chicken raised by the patient, respectively, and sequenced the genomes (Fig. 3 ). Theses viral genome sequences were also deposited in the GISAID (accession number: EPI1139887-EPI1139894, EPI1150038-EPI1150053, EPI1150055 -EPI1150086).
Fig. 3.
(Color online) Phylogenetics of HA and NA genes of the novel H7N4 viruses. (a) HA; (b) NA. Maximum likelihood trees were inferred by using RAxML with GTR-Γ model. The branch of H7N4 viruses were in magenta, and the lineage of human-infecting H7N9 virus was in blue. The bootstrap values of major branches were labeled.
Sequence comparison showed that all strains isolated from chicken and ducks shared high similarity with JS1 (99.5–100%). BLAST search against GenBank and Gisaid showed that all eight gene segments of JS1 were homologous to those of influenza viruses of the Eurasian waterfowl. The haemagglutinin gene shared the highest identity with A/environment/Korea/MA-478/2016 (H7N7) and A/environment/Korea/W478/2014 (H7N7) (98.5% identity), and the neuraminidase gene was most similar to that of A/mallard/Chany/126K-2/2014 (H8N4) (98.5% identity). All the internal genes were distinct from H7 and N4 subtypes previously reported (Table S3 online).
Phylogenetic analysis showed that the H7 genes belonged to the Euroasian lineage. They were closely related to the H7N7 viruses from wild birds in China and Korea, but separated from the human-infecting H7N9 (Fig. 3a). The NA and internal genes phylogenies showed a similar topology that all genes belonged to the Euroasian lineage and had been circulating within migratory birds (Fig. 3b). Of note, the JS1 had all eight genes most closely related to A/chicken/Jiangsu/103/2018 from the phylogenetic trees, the virus isolated from her fed chicken in her backyard.
Key functional loci analyses indicated that avian H7N4 strains were nearly identical to JS1. The HA possessed a low pathogenic pattern (ELPKGR|G) at the cleavage site, and the receptor binding site presented the QSG motif at residues 226–228 (H3 numbering), which was different from that of the H7N9 virus (LSG). No deletion was detected in the NA stalk. All viruses had H274 (N2 numbering) in NA and S31 in M2, indicative of susceptibility to oseltamivir and adamantane. Mutations of M30D and T215A in M1, and P42S in NS1 were observed in all viruses. Of special note, the JS1 possessed the substitution of E627K in PB2, whereas the poultry H7N4 viruses presented the consensus E627. Analysis of the sequencing reads revealed that JS1 had 98.4% of A (resulting in K627) and 1.6% of G (resulting in E627). The most closely related virus (A/chicken/Jiangsu/103/2018) had 0.8% of A and 99.2% of G at 627 in PB2 (Table 2 ).
Table 2.
Mutations in human and poultry H7N4 viruses, compared with H7N9 (A/Anhui/1/2013).
| Gene | Key sites | Patient’s strains | Poultry strains | H7N9 strain |
|---|---|---|---|---|
| HA | Cleavage site | PELPKGRGLF | PELPKGRGLF | PEIPKGRGLF |
| RBS positions (H3 numbering), altered receptor specificity | ||||
| Q226L | Q | Q | L | |
| S227N | S | S | S | |
| G228S | G | G | G | |
| NA | Stalk, 69-73 deletion | No | No | Yes |
| Antiviral resistance (oseltamivir) | ||||
| H274Y | H | H | H | |
| R292K | R | R | R | |
| PB2 | Enhanced polymerase activity and increased virulence in mice | |||
| L89V | V | V | V | |
| E627K | K | E | K | |
| PB1 | H5 virus transmissible among ferrets | |||
| H99Y | H | H | H | |
| I368V | I | I | V | |
| PB1-F2 | Full length | 90aa | 90aa | 90aa |
| M1 | Increased virulence in mice | |||
| M30D | D | D | D | |
| T215A | A | A | A | |
| M2 | Antiviral resistance (amantadine) S31N | S | S | N |
| NS1 | Increased virulence in mice P42S | S | S | S |
3.3. Epidemiological investigation
The patient lives in a rural area in southern Jiangsu Province of eastern China. She had no history of visiting LPMs, or travelling other area in the previous 4 weeks before her illness onset. She runs a crab farm and lives by the side of her crab pond with her husband. Her residence is at least 500 m away from the hamlets nearby. The patient purchased 26 chickens and 23 ducks from LPMs during August and September in 2017, and raised them in her backyard for self-consumption. The ducks were usually sent to the adjacent river for breeding where wild birds (predominantly, egret) were frequently observed. On Dec. 23, 2017 (2 days preceding her illness onset), the patient helped her husband slaughter one of the chicken raised in her backyard.
A total of 28 close contacts were identified, including four family members and 24 health care workers. During the 28-day following-up, two of them developed fever and cough, and recovered. However, throat swabs collected from them before empirical antiviral treatment were negative for influenza by real-time RT-PCR. Paired serum samples from ten of the close contacts including a co-exposer and nine health workers were collected at the beginning and the end of the medical observation, single serum sample was collected from each of other close contacts at the end of the following-up, but none showed detectable neutralizing antibodies to H7N4 virus.
After the patient was suspected infection with H7N4 virus, throat and/or cloacal swabs were sampled from each of the poultry raised by her (all 19 ducks and 16 chickens available when the investigation was implemented). None of the poultry showed signs of illness. Among them, 18 ducks and six chickens were tested positive for H7N4, negative for other subtypes by real-time RT-PCR. From Jan. 6 to Jan 11, 2018, a total of 46 cloacal swab or feces samples were collected from the chickens and ducks raised by other residents who live in the same region, and all were negative for H7N4 and other influenza subtypes. Moreover, 20 swab or feces samples were collected from the nearest local LPMs where the patient and her neighbors bought poultry. The results were also negative for H7N4, but nine samples were H9N2 positive. A total of 16 and 25 feces samples of wild birds (probable egrets) were obtained around the patient’s residence and the nearby lake, respectively, but all were H7N4 negative.
4. Discussion and conclusion
We report the infections of both human and poultry with a novel reassortant avian influenza A(H7N4) virus, and a poultry-to-human viral transmission route.
The patient was featured with initial symptoms of influenza-like-illness, followed by rapidly progressed pneumonia. No conjunctivitis presented. As many of the H7N9 patients, the H7N4 patient also had similar comorbidities, clinical abnormalities and complications, as well as bacterial secondary infection. Analogous treatment [22], such as the combination of antiviral, immunoglobulin and antibiotics therapy, showed effectiveness for treating H7N4 infection in this case as well. The patient’s recovery was associated with the negativity of H7N4 virus in throat swabs and the elevation of neutralizing antibody titers to H7N4 in sera. Both results of clinical blood biochemistry tests, such as normal or decreased number of white blood cells and lymphocytopenia during acute phase, and fast progressed diffuse ground-glass opacities with consolidation indicated by chest CT scans, are the typical characteristics of infection with avian influenza, rather than MP, which is featured with slowly developing syndrome and bibasilar streaky infiltrates presented on chest CT scan [23]. The detection of HSV-1 was probably due to the reactivation of the latent virus [24], and the detection of HCov 229E during the patient’s recovery phase was likely a mild secondary infection. The occasional identification of Klebsiella pneumoniae in patient’s sputum was probably due to a transient nosocomial infection or contamination [25], as the pathogen was consistently negative for PCR in the patient’s throat swabs collected from day 11 to day 27. Our PCR-testing platform for 28 respiratory pathogens, including viruses and bacteria, provided an efficient alternative for detecting infection or co-infection of pathogenic agents.
Specific antiviral treatment was initiated early in this H7N4 case (5 days after onset of disease), compared with the median time of H7N9 patients (7 days) [22]. High-dose corticosteroid therapy (>150 mg/d methylprednisolone or equivalent) was reported to significantly increase mortality and viral shedding time of H7N9 patients [26]. In this case, the dose implemented was low to moderate (20–80 mg/d).
HP and LP H7N4 viruses have been identified in avian from Australia, Korea, Europe and North America [14], [15], [16], [17], but not from China before. However, the genomic analysis indicated that the H7N4 viruses described here differed from those reported previously, especially for the six internal genes. In addition, the H7N4 viruses were in a different subclade from H7N9 viruses. Its apparently low pathogenicity could help it to circulate in poultry without noticing, which highlighted the importance of hospitalized pneumonia surveillance in early detection of novel pathogens.
Several features of the H7N4 virus might contribute to the disease severity and recovery of the patient. The binding preference of avian-like α2, 3-linked sialic acid receptor, which is dominant in human lower respiratory tract, makes the virus could result in lung damage [27]. K627 in PB2 could increase virulence in mammalian hosts [28]. Comparison of the NGS data of JS1 and chicken viruses suggests that virus adaptation caused by E627K conversion had occurred during virus replication in the patient. Besides, M30D and T215A mutations in M1, and P42S mutation in NS1 are associated with increased virulence in mice [29]. The existence of multiple comorbidities of the patient could also add to the severity of the H7N4 infection. In contrast, the susceptibility of the virus to antivirals (H274 in NA) suggested that the early administration of Oseltamivir phosphate probably contributed to the fully recovery of the patient.
Unlike the H7N2, H7N3 and H7N7 mainly caused only mild symptoms [10], [11], [12], [13], the H7N4 virus led to a severe human infection, as most of the reported H7N9 patients [22]. However, distinct from the H7N9, all gene segments of the H7N4 were from influenza viruses of wild birds, where reassortments might occur. And the human infection might have occurred during her butchering the chicken. The molecular data supported the assumption, as one of the chicken H7N4 viruses (A/Chicken/Jiangsu/103/2018) shared the most similarity to human-infecting H7N4 virus (A/Jiangsu/1/2018).
Some person-to-person transmission of influenza H7 subtype viruses, such as H7N9 and H7N7 [13], [30], has been reported. However, in this study, the medical observation and laboratory testing of close contacts showed no evidence of person-to-person transmission of this novel H7N4 virus, in the context of no close contacts receiving oseltamivir chemoprophylaxis. Absence of the novel H7N4 virus in poultry raised by other residents who lived in the same region, and in local LPMs also indicates that the virus has not spread out by poultry. However, considering the virus was very likely carried by migratory birds, similar cross-species transmission would happen elsewhere at the interface of wild and domestic birds, and thus infect humans.
The symptomatic surveillance system and pathogen detection platform, including NGS, established in Jiangsu Province play an important role in the early identification and early intervention on novel influenza viruses which could infect human. Our study added that a novel influenza H7 subtype virus could severely infect human. Unlike H7N9, the new wild bird-origin H7N4 virus can directly cause human infection, without reassortments with any other poultry influenza viruses. These results highlight a potential threat on human health that backyard poultry feeding might serve as the media in the human infection with AIVs carried by wild birds.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgement
This work was supported by National Science and Technology Major Project of China (2015ZX09101044), Science & Technology Demonstration Project for Emerging Infectious Diseases Control and Prevention of Jiangsu Province, China (BE2015714 & BE2017749) and Key Medical Discipline of Jiangsu Science & Technology Project of China (epidemiology, ZDXKA2016008). The authors would like to thank Dr. Di Liu (Wuhan Institute of Virology, Chinese Academy of Sciences, China) for his assistance in phylogenetic analysis and manuscript preparation.
Biographies

Xiang Huo received his Bachelor of Medicine and M.Sc. Degree from School of Public Health, Nanjing Medical University in 2004 and 2007. He works in Department of Acute Infectious Disease, Jiangsu Provincial Center for Disease Control and Prevention thereafter. His research interests focus on the epidemiology, control and evaluation of acute infectious diseases, especially respiratory diseases, including influenza and other respiratory pathogens.

Chang-Jun Bao received his M.Sc. degree from Southeast University. He serves as the chief of Department of Acute Infectious Diseases Control and Prevention in Jiangsu Province Center for Disease Control and Prevention. His research interests include epidemiology and control strategies of acute infectious disease and emerging infectious diseases.

George F. Gao is the Director-General of Chinese Center for Disease Control and Prevention; and a Professor at the Institute of Microbiology, Chinese Academy of Sciences. Dr. Gao’s research interests include enveloped viruses and molecular immunology, mainly focusing on the enveloped virus entry and release, esp. interspecies transmission (host jump) of influenza virus and coronaviruses. His research has recently expanded on public health policy and global health strategy. He has been elected as a member/ fellow of several academies, including Chinese Academy of Sciences, the Third World Academy of Sciences (TWAS), European Molecular Biology Organization (EMBO) and Royal Society of Edinburgh (RSE).

Feng-Cai Zhu is the deputy Director of Jiangsu Provincial Center for Disease Control and Prevention, a member of National Drug Review Expert and of the National Expert Advisory Committee on Immunization Program. His main research interests are infectious disease control and clinical trial of vaccines.
Contributor Information
Chang-jun Bao, Email: bao2000_cn@163.com.
George F. Gao, Email: gaof@im.ac.cn.
Feng-Cai Zhu, Email: jszfc@vip.sina.com.
References
- 1.Gao R., Cao B., Hu Y. Human infection with a novel avian–origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 2.Ke C., Mok C., Zhu W. Human Infection with Highly Pathogenic Avian Influenza A(H7N9) Virus, China. Emerg Infect Dis. 2017;23:1332–1340. doi: 10.3201/eid2308.170600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X., Jiang H., Wu P. Epidemiology of avian influenza A H7N9 virus in human beings across five epidemics in mainland China, 2013–17: an epidemiological study of laboratory–confirmed case series. Lancet Infect Dis. 2017;17:822–832. doi: 10.1016/S1473-3099(17)30323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.William T., Thevarajah B., Lee S.F. Avian influenza (H7N9) virus infection in Chinese tourist in Malaysia, 2014. Emerg Infect Dis. 2015;21:142–145. doi: 10.3201/eid2101.141092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skowronski D.M., Chambers C., Gustafson R. Avian influenza A(H7N9) virus infection in 2 travelers returning from China to Canada, January 2015. Emerg Infect Dis. 2016;22:71–74. doi: 10.3201/eid2201.151330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kile J.C., Ren R., Liu L. Update: increase in human infections with novel Asian lineage avian influenza A(H7N9) viruses during the fifth epidemic – China, October 1, 2016–August 7, 2017. MMWR Morb Mortal Wkly Rep. 2017;66:928–932. doi: 10.15585/mmwr.mm6635a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi X., Jiang D., Wang H. Calculating the burden of disease of avian–origin H7N9 infections in China. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2013-004189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huo X., Chen L.L., Hong L. Economic burden and its associated factors of hospitalized patients infected with A (H7N9) virus: a retrospective study in Eastern China, 2013–2014. Infect Dis Poverty. 2016;5:79. doi: 10.1186/s40249-016-0170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdelwhab E.M., Veits J., Mettenleiter T.C. Prevalence and control of H7 avian influenza viruses in birds and humans. Epidemiol Infect. 2014;142:896–920. doi: 10.1017/S0950268813003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marinova-Petkova A., Laplante J., Jang Y. Avian influenza A(H7N2) virus in human exposed to sick cats, New York, USA, 2016. Emerg Infect Dis. 2017;23:2046–2049. doi: 10.3201/eid2312.170798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tweed S.A., Skowronski D.M., David S.T. Human illness from avian influenza H7N3. British Columbia. Emerg Infect Dis. 2004;10:2196–2199. doi: 10.3201/eid1012.040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puzelli S., Rossini G., Facchini M. Human infection with highly pathogenic A(H7N7) avian influenza virus, Italy, 2013. Emerg Infect Dis. 2014;20:1745–1749. doi: 10.3201/eid2010.140512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Ry V.B.H.M., Meijer A., Koopmans M., de Jager C.M. Human–to–human transmission of avian influenza A/H7N7, The Netherlands, 2003. Euro Surveill. 2005;10:264–268. [PubMed] [Google Scholar]
- 14.Selleck P.W., Arzey G., Kirkland P.D. An outbreak of highly pathogenic avian influenza in Australia in 1997 caused by an H7N4 virus. Avian Dis. 2003;47:806–811. doi: 10.1637/0005-2086-47.s3.806. [DOI] [PubMed] [Google Scholar]
- 15.Terregino C., Cattoli G., De Nardi R. Isolation of influenza A viruses subtype H7N7 and H7N4 from waterfowl in Italy. Vet Rec. 2005;156:292. doi: 10.1136/vr.156.9.292-a. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y.I., Kim S.W., Si Y.J. Genetic diversity and pathogenic potential of low pathogenic H7 avian influenza viruses isolated from wild migratory birds in Korea. Infect Genet Evol. 2016;45:268–284. doi: 10.1016/j.meegid.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y., Bailey E., Spackman E. Limited antigenic diversity in contemporary H7 avian-origin influenza A viruses from North America. Sci Rep. 2016;6:20688. doi: 10.1038/srep20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Laboratory Procedures: Serological detection of avian influenza A(H7N9) infections by microneutralization assay; 2013. <http://www.who.int/influenza/gisrs_laboratory/cnic_serological_diagnosis_microneutralization_a_h7n9.pdf> [accessed March 12, 2018].
- 19.Hoffmann E., Stech J., Guan Y., Webster R.G., Perez D.R. Universal primer set for the full–length amplification of all influenza A viruses. Arch Virol. 2001;146:2275–2289. doi: 10.1007/s007050170002. [DOI] [PubMed] [Google Scholar]
- 20.Zhou B., Jerzak G., Scholes D.T., Donnelly M.E., Li Y., Wentworth D.E. Reverse genetics plasmid for cloning unstable influenza A virus gene segments. J Virol Methods. 2011;173:378–383. doi: 10.1016/j.jviromet.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui L., Liu D., Shi W. Dynamic reassortments and genetic heterogeneity of the human–infecting influenza A (H7N9) virus. Nat Commun. 2014;5:3142. doi: 10.1038/ncomms4142. [DOI] [PubMed] [Google Scholar]
- 22.Gao H.N., Lu H.Z., Cao B. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med. 2013;368:2277–2285. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- 23.Atkinson T.P., Balish M.F., Waites K.B. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev. 2008;32:956–973. doi: 10.1111/j.1574-6976.2008.00129.x. [DOI] [PubMed] [Google Scholar]
- 24.Saleh D., Herpes Bermudez R. StatPearls Publishing; Treasure Island (FL): 2018. Simplex, Type 1. StatPearls [Internet] [Google Scholar]
- 25.Zhang Y., Yao Z., Zhan S. Disease burden of intensive care unit–acquired pneumonia in China: a systematic review and meta–analysis. Int J Infect Dis. 2014;29:84–90. doi: 10.1016/j.ijid.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 26.Cao B., Gao H., Zhou B. Adjuvant corticosteroid treatment in adults with influenza A (H7N9) viral pneumonia. Crit Care Med. 2016;44:e318–e328. doi: 10.1097/CCM.0000000000001616. [DOI] [PubMed] [Google Scholar]
- 27.van Riel D., Munster V.J., de Wit E. H5N1 virus attachment to lower respiratory tract. Science. 2006;312:399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- 28.Hatta M., Gao P., Halfmann P., Kawaoka Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science. 2001;293:1840–1842. doi: 10.1126/science.1062882. [DOI] [PubMed] [Google Scholar]
- 29.To K.K., Tsang A.K., Chan J.F., Cheng V.C., Chen H., Yuen K.Y. Emergence in China of human disease due to avian influenza A(H10N8)––cause for concern? J Infect. 2014;68:205–215. doi: 10.1016/j.jinf.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Qi X., Qian Y.H., Bao C.J. Probable person to person transmission of novel avian influenza A (H7N9) virus in Eastern China, 2013: epidemiological investigation. BMJ. 2013;347 doi: 10.1136/bmj.f4752. [DOI] [PMC free article] [PubMed] [Google Scholar]