Abstract
Atherosclerosis develops at arterial sites where endothelial cells (ECs) are exposed to low time-averaged shear stress, in particular in regions of recirculating disturbed flow. To understand how hemodynamics contributes to EC dysfunction in atheroma development, an in vitro parallel plate flow chamber gasket was modified with protruding baffles to produce large recirculating flow regions. Computational fluid dynamics (CFD) predicted that more than 60% of the flow surface area was below the 12 dynes/cm2 atheroprotective threshold. Bovine aortic endothelial cells (BAECs) were then seeded in the parallel plate flow chamber with either the standard laminar or the new disturbed flow gasket (DFG) and exposed to flow for 36 h. Cell morphology, nitric oxide (NO), proliferation, permeability, and monocyte adhesion were assessed by phase contrast and confocal microscopy. BAEC exposed to 20 dynes/cm2 shear stress in the laminar flow device aligned and elongated in the flow direction while increasing nitric oxide, decreasing permeability, and maintaining low proliferation and monocyte adhesion. BAEC in the recirculating flow and low shear stress disturbed flow device regions did not elongate or align, produced less nitric oxide, and showed higher proliferation, permeability, and monocyte adhesion than cells in the laminar flow device. However, cells in disturbed flow device regions exposed to atheroprotective shear stress did not consistently align or decrease permeability, and these cells demonstrated low nitric oxide levels. The new parallel plate DFG provides a means to study recirculating flow, highlighting the complex relationship between hemodynamics and endothelial function.
Introduction
Cardiovascular diseases such as atherosclerosis are characterized by endothelial dysfunction. Atheroma initiation and growth occur at defined vascular locations, which correspond to low or oscillating shear stress exerted on the endothelial cells (ECs) by hemodynamic flow. In these “disturbed flow” regions, the time-averaged shear stress experienced by ECs may be below 4 dynes/cm2, significantly less than the 12–70 dynes/cm2 shear stress exerted in “atheroprotective” flow regions [1,2]. ECs sense and transduce these forces through mechanosensory mechanisms, including the platelet endothelial cell adhesion molecule-1 (PECAM-1)/vascular endothelial (VE)-cadherin/vascular endothelial growth factor receptor (VEGFR) complex found at cell–cell adherens junctions, to modulate their phenotype [3,4].
In steady laminar flow, ECs express an atheroprotective phenotype, maintaining vascular homeostasis through tight control of permeability, vascular tone, inflammation, and injury repair. ECs align and elongate actin stress fibers, associated focal adhesions, and the entire cell body with the flow direction [5–8]. Atheroprotective flow reinforces monolayer barrier integrity though enhanced homophilic VE-cadherin binding at cell–cell junctions, leading to decreased macromolecule permeability and immune cell invasion [9–11]. This flow further promotes nitric oxide (NO) production, reduces leukocyte adhesion molecule presentation, and induces cell cycle quiescence [1,12–14].
In contrast, ECs in disturbed flow regions express an atheroprone phenotype. Disturbed flow is a general term, which encompasses oscillatory/retrograde flow, impinging/stagnation point flow, and recirculating flow (vortices) following fluid separation. Collectively, disturbed flow inhibits cytoskeletal elongation in response to shear stress [1]; disrupts barrier integrity to enable atherogenic molecules and macrophages to infiltrate the vascular wall [1,12,13]; reduces NO availability either through decreasing its production by endothelial nitric oxide synthase or scavenging with reactive oxygen species [15–17]; and increases adhesion molecule expression leading to an activated, inflammatory EC state [18,19]. While EC dysfunction in disturbed flow has largely been attributed to shear stress magnitude, some studies have also shown that shear stress gradients can significantly alter EC morphological response and gene expression [20,21].
Several devices have been created to enable mechanistic study of disturbed flow on EC monolayers in vitro. Cone-and-plate devices [22–25] can precisely tune time-dependent shear stress over large cell monolayers for cell population analyses (e.g., Western blot) or imaging; however, these systems are generally limited to oscillatory disturbed flow patterns and the homogenous flow eliminates the ability to examine interactions among neighboring cell populations under different flow regimes [26]. Parallel plate flow chambers [6,27–29], including microfluidic devices, can be modified to produce a disturbed flow region using a vertical-step flow channel. These systems work well for imaging cells but primarily produce a small disturbed flow region that limits biochemical assays requiring larger cell numbers (e.g., immunoprecipitation). While capillary tubes [21] can be used to simulate impinging flow (such as at artery bifurcations), imaging is challenging within these three-dimensional (3D) devices. Therefore, new recirculating flow devices are needed that create larger disturbed flow areas, produce shear stress gradients, and facilitate both imaging and biochemical assays.
The objective of this study was to design, create, and characterize a parallel plate device that would create large EC areas exposed to disturbed flow. Since atherosclerotic lesions create a stenosis, resulting in downstream separated flow and low time-averaged shear stress recirculating vortices [30,31], we were particularly interested in recirculating flow. We first designed a parallel plate gasket with repeating baffle protrusions to induce tortuous flow by impinging fluid upstream and separating flow downstream of the baffles, respectively. We then assessed the morphological (F-actin, focal adhesions), functional (NO availability, proliferation, permeability), and inflammatory (monocyte adhesion) EC response in disturbed flow device regions corresponding to high shear stress, atheroprotective shear stress, recirculating flow, and low shear stress. This research demonstrates the potential of the new disturbed flow device to improve our understanding of complex interactions within the endothelium under disturbed flow conditions.
Methods
Cell Culture.
Primary bovine aortic endothelial cells (BAECs) were isolated by the collagenase dispersion method from adult bovine aortae obtained from a local abattoir. Cells were cultured in Dulbecco's Modified Eagle's medium (DMEM, Corning, NY) supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT), 1% glutamine (Gibco, Gaithersburg, MD), and 2% penicillin–streptomycin (Gibco). Cells were maintained in a 37 °C, 5% CO2 incubator, and culture medium was changed every 48 h. Cells were used up to passage 10. For experiments, BAEC were seeded at 6250 cells/cm2 onto 25 × 75 mm polystyrene microscope slides (Ted Pella, Redding, CA) coated with rat-tail type-I collagen (10 μg/mL for 3 h at 37 °C, Gibco), and allowed to attach for 60 min before additional supplemented DMEM was added. BAEC monolayers were cultured to confluency for 36–48 h before use.
THP-1 monocytes (ATCC) were provided courtesy of Dr. Kara Spiller (Drexel University). THP-1 s were maintained with RPMI-01640 medium (ThermoFisher, Waltham, MA) supplemented with 10% heat inactivated FBS (Gibco) and 1% penicillin–streptomycin in T75 tissue culture suspension flasks (ThermoFisher). Every 48 h, viable THP-1 s were counted using a Countess II FL (Invitrogen, Carlsbad, CA) with trypan blue (Corning). Cells were then centrifuged at 130 g for 10 min and resuspended in RPMI medium to maintain 2.0–8.0 × 105 viable cells/mL.
Computational Fluid Dynamics Modeling.
Steady, nonpulsatile fluid flow simulation was performed in a rectangular 10 × 58 × 0.127 mm3 3D space (x, y, z) with comsol multiphysics v4.0. Important simulation parameters are summarized in Table 1. Fluid flow was simulated with low Reynolds number k–ω (κ–ω) turbulent flow and wall function. The κ–ω model uses partial differential equations for the turbulent kinetic energy (κ) and the specific dissipation rate of turbulent kinetic energy into internal thermal energy (ω) to predict turbulence. This model was chosen as it more accurately approximates separated fluid flow in the viscous sublayer and buffer layer as the wall distance approaches 0. Flow medium was modeled as water-based culture medium (incompressible; density, ρ = 999.97 kg/m3; dynamic viscosity, μ = 0.78 × 10−3 Pa·s at 37 °C). Inlet and outlet velocities were 0.0543 m/s, corresponding to 20 dynes/cm2 shear stress in the atheroprotective laminar simulation. The converged solution was achieved using the Multifrontal Massively Parallel sparse direct Solver (MUMPS) with all-zero initial conditions; because κ–ω is sensitive to initial conditions, the first solution was used as initial conditions for the final approximation. Shear rate data were extracted from z = 0.127 mm, the bottom slice of the model corresponding to the endothelial plane surface in the experimental apparatus.
Table 1.
comsol v4.0 CFD parameters and settings for laminar and disturbed flow simulations
| Parameter setting (1) | Parameter setting (2) | |
|---|---|---|
| Mesh (tetrahedron) | Extra course preset | Refined thrice, “split longest side” |
| 93,400 total elements | Maximum element size: 0.0174 m | |
| Minimum element size: 5 × 10−6 m | ||
| Turbulence model | Low Reynolds number κ–ω | Incompressible single-phase flow |
| Zero-velocity wall function | — | |
| Fluid properties | Temperature: 310.15 K (37 °C) | — |
| Density: 999.97 kg/m3 | — | |
| Viscosity: 0.78 × 10−3 Pa·s | — | |
| Boundary conditions | Inflow/outflow velocity | Inlet: 0.0543 m/s (4.24 mL/min) |
| Outlet: 0.0543 m/s (4.24 mL/min) | ||
| Turbulence dimensions | Turbulent length scale: 6.35 × 10−5 m | |
| Turbulent intensity: 0.067 |
Note: “Parameter setting (1)” are the main selections while “parameter setting (2)” are additional selections or blanks required after choosing the corresponding “(1).”
Disturbed Gasket and Parallel Plate Flow Device.
The disturbed flow gasket (DFG) was designed to be the same size as the laminar flow gasket (LFG, 10 × 58 × 0.127 mm) with nine baffles, each 5 mm long (x) by 1 mm wide (y) with tips beveled to a 0.78 mm radius. The baffles were spaced 5 mm apart, with alternating orientation (Fig. 1(a)). Protruding baffles did not demonstrate noticeable deflection in this study. Remaining gasket corners were beveled at a 0.39 mm radius. The DFG was then manufactured by GlycoTech through the same proprietary procedure that they use to manufacture the LFG.
Fig. 1.

Disturbed flow parallel plate flow chamber gasket design and computational fluid dynamics analysis. (a) standard laminar flow (LFG, left) and new disturbed flow (DFG, middle and right) parallel plate flow chamber gaskets, (b) parallel plate chamber assembly, with gasket forming the flow region over endothelial cells cultured on a microscope slide. (c) Time-averaged computational fluid dynamics DFG gasket simulation using RAN κ–ω turbulence with no-slip walls in comsol multiphysics Software. Fluid was cell culture medium (μ = 0.78 × 10−3 Pa·s, 37 °C) and flow rate was 4.24 mL/min; (top) streamlines, (middle) shear stress heat map, (bottom) binary color map indicating shear stress above (red) and below (green) 12 dynes/cm2 (atheroprotective threshold); (d) selected DFG regions of interest, including: DFG-High (shear stress > 20 dynes/cm2); DFG-2 Pa (shear stress ∼20 dynes/cm2); DFG-Recirc (recirculating eddy); and DFG-Low (shear stress < 12 dynes/cm2).
All experiments were performed in a parallel plate flow chamber (GlycoTech, Gaithersburg, MD), which consists of a 0.127 mm thick silicone gasket sandwiched between an acrylic chamber and a polystyrene microscope slide seeded with endothelial cells (Fig. 1(b)). A REGLO Digital 8-roller peristaltic pump (Ismatec, Wertheim, Germany) drove fluid flow to the chamber, first passing through a Stoval Flow Cell bubble trap (Fisher Scientific, Hampton, NH), which both removed air bubbles and reduced flow pulsatility. Culture medium was recirculated through platinum cured silicone tubing (Cole Parmer, Vernon Hills, IL) and stored in an open medium reservoir. The entire apparatus was placed in a 37 °C, 5% CO2 incubator for the duration of each experiment, in which two chambers were run in parallel.
Confluent BAEC monolayers were exposed to flow for 36 h in the parallel plate flow chamber with either the LFG or DFG. Pump volume flow rate, which was maintained the same for both gaskets (4.24 mL/min), was selected so that endothelial cells in the LFG would be exposed to atheroprotective 20 dynes/cm2 (2 Pa) shear stress. Shear stress in the LFG was calculated using the Navier–Stokes equation for flow between two infinite stationary parallel plates
| (1) |
where b is chamber width (x-axis), h is chamber height (gasket thickness, z-axis), μ is fluid viscosity, Q is volume flow rate, and τ is shear stress magnitude. BAECs cultured on polystyrene microscope slides but not exposed to flow served as static controls.
Immunofluorescent Labeling.
After BAECs were exposed to fluid flow, samples were fixed with 4% paraformaldehyde at 4 °C for 15 min, permeabilized with 0.3% Triton X-100 at room temperature for 15 min, and blocked with 1% bovine serum albumin in phosphate-buffered saline (PBS) at room temperature for 60 min to reduce nonspecific binding. To quantify actin alignment, cells were labeled with rhodamine phalloidin (F-actin, 165 nM, Invitrogen) and bisbenzimide (nuclei, 0.2 μg/mL, Invitrogen) for 60 min at room temperature. Focal adhesion alignment was determined by labeling cells with a mouse anti-human vinculin primary antibody (V9131, 1:150, Sigma, St. Louis, MO ); cell proliferation with a mouse anti-human Ki-67 primary antibody (23,900, 1:100, Santa Cruz Biotechnology, Dallas, TX); and endothelial monolayer cell–cell junctions with a goat anti-human VE-cadherin primary antibody (sc-9989, 1:100, Santa Cruz) each for 18 h at 4 °C. Samples were then incubated with a species-appropriate Alexa Fluor® 488 secondary antibody and bisbenzimide for 60 min at room temperature. Slides were mounted on to 24 × 60 mm glass coverslips (VWR, Radnor, PA) using 1:1 PBS:glycerol. Samples were imaged at 63× (F-actin, vinculin, VE-cadherin) or 20× (Ki-67) on a Zeiss LSM 700 laser scanning confocal microscope, in Z-stacks with 0.74 μm intervals. Slices were compressed into maximum intensity projections prior to analysis. At least three images were taken per slide or per DFG zone, as appropriate.
Nitric Oxide Measurement.
Nitric oxide was measured using 4-amino-5-methylamino-2′,7′-difluoroflurescein diacetate (DAF-FM diacetate, Invitrogen). DAF-FM diacetate becomes fluorescent (ex/em: 495/515 nm) and can no longer permeate the cell membrane following oxidation by NO [32]. After flow conditioning, BAEC were removed from the parallel plate flow chamber and incubated with DAF-FM diacetate (5 μM) for 60 min at 37 °C, 5% CO2. Samples were then incubated with bisbenzimide for 30 min at 37 °C, 5% CO2 and fixed with 4% paraformaldehyde (15 min at 4 °C). Samples were imaged by confocal microscopy (20×) as previously described.
Endothelial Monolayer Permeability Measurement.
Bovine aortic endothelial cell monolayer permeability to macromolecules was quantified using a modified version of a previously described fluorescent molecule diffusion assay [33]. In brief, gelatin (Sigma) was biotinylated using an EZ-link NHS-LC-LC-Biotin kit (Invitrogen). BAECs were seeded on 10 μg/mL biotinylated gelatin-coated microscope slides, cultured to confluence, and exposed to flow as previously described. Static BAECs incubated with 25 μM H2O2 for 30 min at 37 °C served as the positive control. BAEC monolayers were then incubated with FITC–streptavidin (1:2000, Invitrogen) in DMEM, protected from light, for 1 h at 37 °C. Samples were then fixed with 4% paraformaldehyde and labeled for nuclei as previously described. Samples were imaged by confocal microscopy (63×) using 0.50 μm slice intervals. Slices were compressed into a maximum intensity projection for analysis.
Monocyte Adhesion.
Following flow exposure, 600,000 viable, undifferentiated THP-1 monocytes were added to BAEC in static conditions to avoid confounding effects of increased retention time and monocyte activation in specific DFG regions. BAEC in static conditions treated with 10 ng/mL bovine tumor necrosis factor-α (TNFα; ThermoFisher) for 24 h served as the positive control. Samples were then incubated at 37 °C for 20 min to allow THP-1 s to attach to the endothelial monolayer. After the incubation period, BAEC were gently washed with warm PBS.
Adhered monocytes were quantified using positive phase contrast microscopy by capitalizing on the Becke Line Effect. In the Becke Line Effect, a bright “halo” ring of light appears to move toward the center of an object in a medium with a lower refractive index (n), as the focal plane is elevated above the object. BAEC monolayers with adhered monocytes (n THP-1 > 1.35) [34] were imaged in PBS (n PBS = 1.33) [35] at 10× magnification. The focal plane was elevated until the Becke Line converged to the THP-1 cells center, and the adhered cells appeared as white, high-contrast objects. These high-contrast objects were manually counted and cross-referenced with endothelial monolayer images to exclude high-contrast artifacts in the endothelial layer.
Fluorescent Image Processing and Analysis.
F-actin fiber alignment and focal adhesion orientation were analyzed using custom matlab scripts previously developed in our lab. F-actin fiber alignment was quantified via edge detection, which uses image intensity changes to find the fiber edges and then determines the maximum intensity gradient direction to calculate fiber orientation angle [6]. Aligned fibers were defined as those that were oriented within ± 20 deg of the y-axis (0 deg, horizontal).
Focal adhesion orientation was quantified in vinculin confocal microscopy images using the built-in matlab function regionprops(), which measures white object geometric properties in binary images [36]. After vinculin was segmented and identified into focal adhesions, regionprops() was used to determine the major long axis and object orientation angle. Since focal adhesion angles ranged from −90 deg to 90 deg, the absolute value angle distribution (0–90 deg) mean was used to determine focal adhesion orientation.
DAF-FM diacetate-labeled images were iteratively thresholded to remove noise before quantifying total signal intensity to measure NO. Images from cells cultured in static conditions within each experiment were thresholded until a designated 100 × 100 pixel background region had fewer than 100 positive pixels (1% noise). Threshold values for static controls were then averaged to form an intra-experiment threshold which was applied to all flow images within that experiment. Total DAF-FM fluorescence intensity was calculated as the sum of all remaining pixel values after thresholding.
Ki-67, which localizes to the nucleus during cell proliferation [37], was quantified as the fraction of Ki-67-positive nuclei over total nuclei. Ki-67 positive and negative nuclei were identified with Otsu's thresholding method. This method assumes each image contains foreground and background pixels and calculates the optimal threshold to separate these two pixel classes while minimizing intraclass variance [38]. regionprops() was used to count contiguous objects and validated against hand-counted reference images to confirm accuracy.
Vascular endothelial-cadherin images were processed using edge-detection to segment foreground junction signal from background noise as described previously [6], followed by Watershed transform using matlab's built-in watershed function to identify discontinuities between signal pixels within the same cell–cell interface [39]. These techniques captured cell borders without retaining VE-cadherin signal within the cell body. VE-cadherin junction thickness was quantified by drawing a line perpendicular to a randomly selected junction at its thickest segment, recording the fluorescence intensity profile, and fitting the profile data with a one- or two-peak Gaussian curve. Junction width was defined as the curve width above 20% of the background intensity [40].
VE-cadherin junction integrity was quantified by determining the distance between labeled cell–cell junction segments. The two extrema along each VE-cadherin labeled object's long axis were determined using the matlab function regionprops. The nearest neighboring VE-cadherin positive pixel to each object's extrema was found using matlab's built in dnsearch function. The nearest neighboring pixel was required to be (a) less than 100 pixels away (∼10 μm at 63×), to remove connections across cell bodies; (b) unconnected to the other extrema for a given object, to prevent data point doubling; and (c) at less than ±90 deg from the object's long axis, assuming VE-cadherin elements within the same cell–cell boundary point in similar directions. A unique line was then drawn from that extrema to the nearest neighboring pixel, and this distance was defined as the connection distance. The average static sample histogram bin frequency was then subtracted from each sample's corresponding histogram frequency bin.
Statistical Analysis.
Statistics were performed in GraphPad Prism 5.01. All values are presented as mean ± standard deviation. Outliers were identified as being more than 1.5 interquartile ranges beyond the first or third data quartile and were subsequently removed. Comparisons were made with Kruskal–Wallis analyses with Dunn–Sidak multiple comparison post hoc as the data was significantly non-normal with reasonable variances, violating the parametric analysis of variance assumptions. Results used a significance threshold of α = 0.05, two-tailed. #, *, **, *** indicates p < 0.05, 0.01, 0.001, and 0.0001, respectively. All experiments were repeated at least three times.
Results
Disturbed Flow Simulation.
We first simulated flow through the parallel plate device fitted with the DFG to determine the fluid streamlines and shear stress profiles in different gasket regions. Time-averaged streamlines (Fig. 1(c), top) showed recirculating vortices (∼12 mm2) following each protruding baffle. Shear stress (Fig. 1(c), middle) was lowest in corners immediately downstream of baffles, where it approached a minimum of ∼0.1 dynes/cm2, and highest upstream of baffle tips, where it approached a maximum of ∼200 dynes/cm2. As flow progressed along the y-axis, overall shear stress increased with each progressive baffle obstruction. This gasket design fit our objective of creating a large EC area exposed to low shear stress, since over 60% of the gasket's flow surface area was below the 12 dynes/cm2 atheroprotective threshold (Fig. 1(c), bottom). Overall, the average shear stress across the entire flow surface area was 9.7 dynes/cm2, which was also below the atheroprotective shear stress threshold.
We identified four regions of interest for further study under experimental flow conditions (Fig. 1(d)): DFG-High, a unidirectional flow region with 20–60 dynes/cm2 shear stress, which is above the atheroprotective shear stress threshold; DFG-2 Pa, a unidirectional flow region with 10–30 dynes/cm2 shear stress, which is on average above the atheroprotective shear stress threshold; DFG-Recirc, a recirculating flow region with time-averaged shear stress below 2 dynes/cm2; and DFG-Low, a unidirectional flow region with shear stress below 12 dynes/cm2. To avoid potential entrance effects at the inlet and data variance due to increasing shear stress along the y-axis, images were taken at these defined regions of interest between the third and seventh baffles, where shear stress patterns and magnitudes were more homogeneous.
Endothelial Morphological Flow Response.
Since endothelial cells are known to align and elongate in atheroprotective flow, we imaged endothelial morphology after 36 h in the parallel plate flow chamber fitted with either the LFG or the DFG. BAECs cultured under static conditions retained a rounded, polygonal shape (Fig. 2(a)). Peripheral F-actin was prominent and thick. In contrast, BAECs exposed to 20 dynes/cm2 shear stress in the parallel plate flow chamber fitted with the LFG (LFG-2Pa) elongated and oriented their long axis parallel with the flow direction (y-axis). F-actin was primarily located in stress fibers crossing the cell body. Much like in LFG-2 Pa samples, DFG-High cells were elongated in the flow direction except for cells immediately adjacent to the flow chamber wall, which retained a rounded morphology likely due to edge effects. BAECs in DFG-2 Pa regions also retained a rounded morphology. Cells within the recirculating flow DFG-Recirc region were typically elongated and oriented toward the device center (DFG-2 Pa regions) but sometimes rounded like static cells. Cells in DFG-Low regions maintained a static-like morphology.
Fig. 2.

Endothelial cells aligned in the flow direction in high shear stress regions in both the laminar and disturbed flow devices. (a, top) BAEC phase contrast images (10×) after 36 h of flow in the parallel plate flow chamber fitted with either the LFG or DFG. BAECs in static conditions are shown as a control. (a, bottom) Confocal microscopy images (63×) showing actin fibers (rhodamine phalloidin, red) and nuclei (bisbenzimide, blue). (b, left) Representative F-actin fiber orientation histograms for BAEC adapted to various flow regimes. (b, right) Quantification of actin fiber alignment with the parallel plate flow chamber y-axis (295 images among 29 samples, across six independent experiments). (c, top) BAEC confocal microscopy images (63×) after 36 h of flow, with static control, labeled for vinculin (white). (c, bottom) Focal adhesion angle (red: aligned within ±20 deg of the y-axis; white: not aligned within ±20 deg of the y axis) analyzed via matlab image segmentation, superimposed onto processed vinculin (blue) confocal microscopy images. (d, left) Focal adhesion angle distribution representative rose plots for BAEC adapted to various flow regimes. (d, right) Quantification of focal adhesion angle (absolute value) with the parallel plate flow chamber y-axis (76 images among 24 samples, across three independent experiments). *, **, and *** indicate p < 0.01, 0.001, and 0.0001, respectively.
We next measured F-actin and focal adhesion orientation to quantitatively assess endothelial alignment. F-actin angle distribution demonstrated a clear orientation along the y-axis in both LFG-2 Pa and DFG-High region cells (Fig. 2(b), left). In contrast, cells in DFG-2 Pa and DFG-Low regions had random F-actin angle distributions, closely resembling static BAECs. Cells in DFG-Recirc regions aligned F-actin but not along the y-axis, mirroring qualitative cell morphology observations. F-actin alignment, represented as the percentage of F-actin fiber angles within ±20 deg of the y-axis (Fig. 2(b), right), showed that around 50% more actin fibers were aligned in the LFG-2 Pa- and DFG-High-conditioned cells compared to static and remaining DFG region cells (32% and 35% versus ∼22%; p < 0.0001).
Finally, we determined how vinculin-labeled focal adhesions aligned in the flow direction. Similar to F-actin, focal adhesions appeared randomly oriented in static culture cells. Focal adhesions aligned with the y-axis for BAEC in both LFG-2 Pa and DFG-High regions (Fig. 2(c)). Conversely, focal adhesions in BAEC in the remaining DFG regions appeared nearly randomly oriented. Quantitative focal adhesion angle distribution analysis showed that focal adhesion angles skewed toward 0 deg in the LFG-2 Pa and DFG-High samples, whereas all other samples showed a dispersed focal adhesion angle distribution (Fig. 2(d), left). Only cells in LFG-2 Pa and DFG-High regions showed statistically aligned focal adhesions as compared to cells in static culture (p < 0.0001) and most other disturbed flow regions (p < 0.01, except for DFG-Low). Static BAEC focal adhesion average angle was statistically comparable to DFG-2 Pa, DFG-Recirc, and DFG-Low (Fig. 2(d), right).
Endothelial Functional Response
Nitric Oxide.
We then determined endothelial functional response to the different flow regimes starting with NO, which represents the classic endothelial flow response. BAECs exposed to atheroprotective flow in the parallel plate flow chamber fitted with the LFG (LFG-2Pa) showed more NO, indicated by brighter and more widespread DAF-FM labeling compared to all other conditions (Fig. 3(a)). DAF-FM total fluorescence intensity in LFG-2 Pa BAECs was 7× higher than cells in static culture and more than 3× higher than cells in all DFG regions (Fig. 3(b), p < 0.0001). In contrast to the cell morphology results, BAECs in the DFG-High region did not display NO levels similar to the LFG-2 Pa atheroprotective flow regime despite sufficiently high shear stress.
Fig. 3.
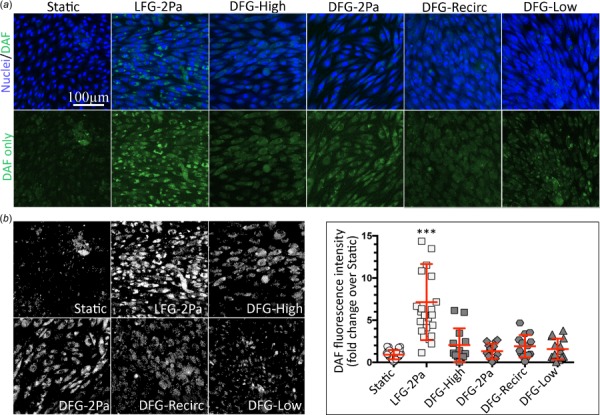
NO was elevated in BAEC after 36 h in the laminar but not disturbed flow parallel plate flow chamber. (a, top) BAEC confocal microscopy images (20×) in varied flow regimes for 36 h showing NO (DAF-FM, green) and nuclei (bisbenzimide, blue). (a, bottom) NO only (DAF-FM, green) confocal microscopy images (20×). (b, left) DAF signal after thresholding to remove noise. (b, right) Total DAF-FM fluorescence intensity quantification for BAEC exposed to static or flow conditions for 36 h (122 images among 18 samples, across 4 independent experiments). *** indicates p < 0.0001 (LFG compared to all other conditions).
Cell Proliferation.
Endothelial cells in atheroprotective flow regimes are quiescent, whereas cells in disturbed flow demonstrate increased proliferation [15]. We therefore examined cell proliferation through Ki-67 labeling (Fig. 4, representative images in A with analysis and quantification in B). The Ki-67 positive nuclei fraction was comparable between LFG-2 Pa conditioned and static culture BAECs. All disturbed flow conditions demonstrated statistically elevated Ki-67 positive nuclei when compared to BAECs in static culture (p < 0.05). Cells in the DFG-Recirc region were the most proliferative, with more than 6× the Ki-67 positive nuclei when compared to cells in static culture. The DFG-Recirc region began to approach the proliferation levels of subconfluent, static culture cells (positive control, data not shown), which demonstrated nearly 50% Ki-67-positive nuclei. Cell proliferation was only statistically higher for BAEC in the DFG-Recirc region than in the LFG-2 Pa region (p < 0.0001).
Fig. 4.
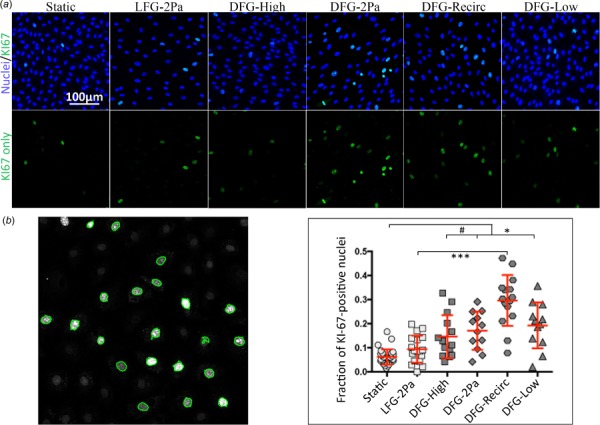
BAEC exposed to disturbed flow showed increased cell proliferation. (a, top) Confocal microscopy images (20×) of BAEC in varied flow regimes for 36 h showing proliferation (Ki-67, green) and nuclei (bisbenzimide, blue). (a, bottom) Ki-67 (green) only. (b, left) Ki-67-positive nuclei representative image counted by automated matlab code (green outline). (b, right) Quantification of KI67-positive nuclei fraction for BAEC exposed to varied flow regimes (113 images among 24 samples, across 3 independent experiments). #, *, **, and *** indicate p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively.
Permeability and Junction Integrity.
Since endothelial cells in disturbed flow regions are more permeable [15], we quantified BAEC monolayer permeability in the different flow regions by culturing cells on a biotin-conjugated gelatin substrate and adding soluble FITC-conjugated streptavidin. Endothelial cells cultured in static conditions or in the DFG-2 Pa, DFG-Recirc, and DFG-Low regions showed increased FITC–streptavidin bound to the subendothelial substrate (Fig. 5(a)) when compared to cells in the LFG-2 Pa and DFG-High flow regions. Total FITC signal intensity for static, DFG-Recirc, and DFG-Low samples was nearly twofold higher than the LFG-2 Pa or DFG-High samples (Fig. 5(b), p < 0.0001). Cell permeability in the DFG-2 Pa region was not statistically different from cell permeability in any of the other regions.
Fig. 5.
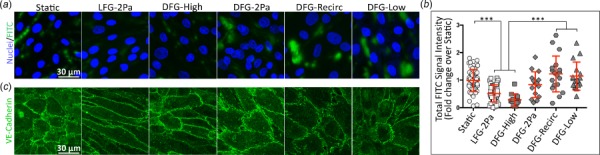
Endothelial monolayer permeability increased in lower shear stress disturbed flow regions. (a) Confocal microscopy images (63×) of FITC–streptavidin (green) permeation through an endothelial monolayer, with labeled nuclei (bisbenzimide, blue). Confluent BAEC monolayers grown on biotin-conjugated gelatin were exposed to various flow regimes for 36 h. Monolayers were then incubated with streptavidin-conjugated FITC, which diffused through leaky monolayers to bind to the culture surface via powerful biotin–streptavidin interactions. (b, left) FITC signal after thresholding to remove noise indicates pixels-of-interest surface area without brightness. (b, right) Total FITC fluorescence intensity quantification (161 images among 24 samples, across 4 independent experiments). *** indicates p < 0.0001.
We also quantitatively analyzed VE-cadherin to assess cell–cell junction integrity. BAECs adapted to recirculating disturbed flow (DFG-Recirc) and low shear stress (DFG-Low) had significantly thicker cell–cell junctions as compared to BAECs adapted to steady laminar flow (LFG-2 Pa; 6 μm and 5.5 μm versus 4 μm; p < 0.05 and 0.01, respectively; Figs. 6(a)–6(c)). Cell–cell junctions in the DFG-Low gasket region were thicker than those in the DFG-High region (5.5 μm versus 4 μm, p < 0.05). There was no significant difference between maximal junction thickness for BAEC in static culture or DFG-2 Pa region and any other sample, nor between LFG-2 Pa and DFG-High. Furthermore, the relative frequency of connection distances shorter than 1.5 μm, which indicates more continuous VE-cadherin at cell–cell borders, was higher in the LFG-2 Pa and DFG-High samples as compared to the DFG-2 Pa and DFG-Recirc samples (Figs. 6(e)–6(g)). Only LFG-2 Pa cell–cell junctions were statistically more continuous than static samples (p < 0.05).
Fig. 6.

Cell–cell junctions were thicker and less continuous in endothelial cells exposed to disturbed flow. (a) Confocal microscopy images (63×) of VE-cadherin (white) at cell–cell adherens junctions. (b) Eight randomly computer-chosen junctions per image. Magenta lines indicate measured signal intensity across cell borders. (c) Example junction width calculation. (d) Maximum junction width quantification (102 images among 15 samples, across 8 independent experiments). # and ** indicate p < 0.05 and 0.001, respectively. (e) VE-cadherin segmented with edge detection and Watershed algorithm. (f) Nearest-neighbor example calculation, with conversion from absolute frequency to relative-to-static frequency. (g) Maximum junction width quantification. 102 images among 15 samples, across eight independent experiments. #, *, **, and *** indicate p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively.
Monocyte Adhesion.
Finally, we assessed endothelial inflammation by quantifying THP-1 monocyte adhesion to monolayers exposed to the different flow regimes for 36 h. BAECs conditioned to static culture or in LFG-2 Pa, DFG-High, and DFG-2 Pa flow regions had statistically similar attached monocyte numbers. In contrast, BAECs in the DFG-Recirc and DFG-Low regions had approximately twice as many adhered monocytes compared to BAECs in the LFG-2 Pa region (Fig. 7; p < 0.001 and 0.01, respectively). For perspective, TNFα-treated static cultures had more than fivefold more adhered monocytes than untreated static cultures (data not shown, p ≪ 0.0001).
Fig. 7.

Monocyte adhesion increased to BAEC exposed to low shear stress disturbed flow. (a, top) Endothelial focal plane phase contrast images (10×) 20 min after THP-1 monocyte addition to 36 h flow-conditioned BAEC monolayers. (a, bottom) Phase contrast images of the same region but in the monocyte focal plane. (b, left) Representative analysis with monocytes highlighted in green. (b, right) Quantification of adhered monocytes to BAEC after 36 h of varied flow regimes. (114 images among 17 samples, across 3 independent experiments). * and ** indicate p < 0.01 and p < 0.001, respectively.
Discussion
Endothelial cells are known to be dysfunctional in recirculating disturbed flow regions in vivo. Yet in vitro devices that recreate recirculating disturbed flow generally have only small cell numbers exposed to the recirculating flow, making biochemical assays that require larger cell numbers difficult. The objective of this study was to design, build, and validate an in vitro device to create large recirculating disturbed flow areas. We showed that cells in the recirculating flow regions of the disturbed flow device did not elongate or align, produced less NO, and showed higher proliferation, permeability, and monocyte adhesion than cells in atheroprotective steady laminar flow. In addition, we found interesting differences between cells in regions of the disturbed flow device that should be atheroprotective (DFG-High, DFG-2Pa) and cells in steady laminar flow. While cells in the DFG-High region of the disturbed flow device did align with flow and were less permeable than cells in other disturbed flow device regions, their NO production, proliferation, and monocyte adhesion rates were statistically similar to cells in the recirculating flow and low shear stress regions. Cells in the DFG-2 Pa regions, which should have an atheroprotective phenotype based on shear stress level alone, were statistically similar to cells in the recirculating and low shear stress regions in all measurements. These results suggest interesting cell responses in varied flow regimes within the disturbed flow device.
The first design objective was to create a device that produced large physiologically relevant disturbed flow areas. in vivo, complex disturbed flow is associated with atherosclerotic plaque growth and progression [24,37]. Several existing in vitro devices create disturbed oscillatory, impinging, or recirculating flow; however, the EC area exposed to disturbed flow is usually small. Our device produced 425 mm2 of ECs exposed to shear stress less than 12 dynes/cm2, with approximately 108 mm2 of that found in recirculating flow. Compared to current device designs (summarized in Table 2), this is a 15-fold increase in disturbed flow-conditioned cells compared to the next largest parallel plate recirculating flow device. For gene expression or protein quantification assays that require cell lysates, cells in disturbed flow regions would need to be isolated prior to lysis to avoid obtaining a mixed population. A cloning ring or similar device could be used to select cells within a given region, and since the disturbed flow regions are relatively large, the cell number in each region would be adequate for assays such as real-time polymerase chain reaction and Western blot.
Table 2.
Current in vitro disturbed flow devices, with the approximate EC surface area (mm2) exposed to disturbed flow
| Flow device | Disturbed flow type | Recirculatory flow surface area | Total disturbed flow surface area | Reference |
|---|---|---|---|---|
| Cone and plate | Low shear stress (∼5 dynes/cm2); radial grooves in cone | Ø | 7850 mm2 | [41] |
| Oscillatory flow by reversing cone rotation (±5 dynes/cm2, 1 Hz) | Ø | 7850 mm2 | [19] | |
| Carotid artery bifurcation waveform by modulating cone rotation velocity | Ø | 14,000 mm2 | [25] | |
| Turbulent flow by increasing rotational velocity with 5 deg cone angle | Ø | 1200 mm2 | [42] | |
| Recirculatory flow via vertical step (V)a | 14.3 mm2 | 69 mm2 | [43] | |
| Parallel plate | Retrograde flow by removing one-way valve | Ø | 350 mm2 | [44] |
| Oscillatory (1±3 dynes/cm2, 1 Hz) | Ø | 1375 mm2 | [3] | |
| Recirculatory flow via vertical step (V)a | 8 mm2 | 12 mm2 | [13] | |
| Recirculatory flow via 63× 0.75 mm-wide vertical step microgrooves (V) | 50.25 mm2 | 50.25 mm2 | [45] | |
| Recirculatory flow via rectangular cutouts in flow gasket edges (P)a | 4 mm2 | Ø | [46] | |
| Recirculatory flow via gasket baffles (P) | 108 mm2 | 425 mm2 | ||
| Impinging flow | Recirculatory flow (V)a | 6.28 mm2 | Ø | [47] |
| Stagnation at impingement | Ø | 8.8 mm2 | [21] |
Note: EC area exposed to any form of disturbed flow and recirculating flow, as calculated from provided dimensions. Abbreviations: V—vertical or perpendicular recirculation to EC plane, P—parallel recirculation to EC plane, superscript a—flow geometry and properties were estimated from figure data, and Ø—flow area could not be measured due to applied flow nature or insufficient information. Uncited device in bold font refers to this study.
Our device also creates disturbed flow vortices that are parallel to the endothelial surface and perpendicular to the flow direction, which are more similar to the disturbed flow vortices around an obstruction such as an atherosclerotic plaque as opposed to the perpendicular vortices observed in flow over an atherosclerotic plaque, around a curved artery, or through an arterial bifurcation [31,48]. The cross flow from these disturbed flow vortices creates a transverse wall shear stress, which has been proposed to be an important component of multidimensional, complex, three-dimensional in vivo hemodynamics [49]. In addition, parallel disturbed flow vortices produce shear stress gradients in both the y- and x-directions, exposing EC to a more complex mix of neighboring stimuli. Despite the fluid dynamic differences between the parallel disturbed flow vortices in our device and the perpendicular disturbed flow vortices in other devices, EC in our system still showed typical responses to disturbed flow. This suggests that it may be shear stress magnitude, shear stress gradient, and/or oscillatory shear index that are important in EC dysfunction rather than vortex directionality.
Our device also produced shear stresses and shear stress gradients similar to those observed by in vivo study [8]. Magnetic resonance imaging of human carotid atherosclerotic lesions [30] reported shear stresses ranging from 137 dynes/cm2 at the obstruction apex to below 10 dynes/cm2 distal to the lesion, which is similar to maximum (DFG-High) and minimum (DFG-Recirc) shear stresses in our device. Shear stress values and the physiological distances over which they change suggests shear stress gradients of approximately −340 dynes/cm3 from the lesion apex to recirculating eddy. Similarly, our device generates gradients of −380 to −430 dynes/cm3 in corresponding DFG-High to DFG-Recirc regions.
We unexpectedly observed that ECs exposed to atheroprotective shear stress levels within the disturbed flow device did not exhibit a completely atheroprotective phenotype. NO in ECs in the DFG-High and DFG-2 Pa regions was statistically similar to cells in the recirculating and low shear stress regions. It is possible that disturbed flow, irrespective of shear stress magnitude, induces EC dysfunction. ECs in the DFG-High and DFG-2 Pa regions may also be affected by paracrine signaling from neighboring ECs in the disturbed flow or low shear stress regions, for example, from superoxide or peroxynitrite [50] or via micro-RNA (miRNA) transport among connected EC [51–53]. ECs in the disturbed flow device may further be affected by shear stress gradients in addition to shear stress magnitude, since shear stress gradients at high shear stresses impacted EC inflammatory molecule expression [54–56].
Interestingly, ECs exposed to recirculating flow (DFG-Recirc) and its associated low shear stress demonstrated frequent F-actin orientation bias toward the flow device center. In contrast, the focal adhesions in these ECs were randomly organized compared to the aligned focal adhesions in ECs exposed to steady laminar flow (LFG-2Pa) or high shear stress (DFG-High) [57]. Cells in these disturbed flow regions may have less stable focal adhesions due to their misalignment with F-actin, resulting in important changes to endothelial function and survival [7,58].
This study presents a new parallel plate device that exposes large EC areas to recirculating and low shear stress flow. However, it is not without limitations. Because the simulation was time-averaged steady flow, we could not calculate the oscillatory shear index, a useful metric in categorizing disturbed flow patterns. Furthermore, direct NO measurements in the media were not possible due to dilution, thus requiring indirect and semiquantitative labeling techniques. Additionally, NO-labeled DAF-FM signal intensity was reduced due to paraformaldehyde fixation after labeling, although fixed samples still distinguished among steady laminar flow and static or disturbed flow conditions. While fixation affected all samples similarly, the reduction in signal intensity may have decreased effect size between DFG-High and the remaining three DFG regions to nonsignificant levels. Finally, while the shear stress gradient magnitude in this device was similar to in vivo data, the obstruction geometry in this device and the scale over which the shear stress drops were smaller. in vivo lesions may experience a ∼120 dynes/cm2 shear stress drop across approximately 3.5 mm, compared to an average 36 dynes/cm2 shear stress drop in this device across 1 mm. Previous work examining gradients did so at much higher shear stress [20,21,59].
This paper presents the design for a disturbed flow parallel plate device to explore the effects of parallel disturbed flow vortices on endothelial function. We also identified atheroprotective endothelial functions that were not realized by endothelial cells in atheroprotective shear stress regions adjacent to disturbed flow or low shear stress regions. Additional research is needed to determine the molecular mechanisms through which this effect occurs, likely with a focus on reactive oxygen species and miRNA. Overall, the device is useful for studies in which large endothelial cell numbers exposed to disturbed flow are needed as well as for studies of EC interactions in complex flow.
Acknowledgment
We thank Dr. Kara Spiller for providing the monocytes and GlycoTech for manufacturing our custom disturbed flow gasket.
Contributor Information
Jason Matthew Sedlak, School of Biomedical Engineering, Science, and Health Systems, Drexel University, 3141 Chestnut Street, Philadelphia, PA 19104 e-mail: Jms526@drexel.edu .
Alisa Morss Clyne, Fellow ASME Department of Mechanical Engineering, Drexel University, 3141 Chestnut Street, Philadelphia, PA 19104 e-mail: asm67@drexel.edu .
Funding Data
National Institute of Health (NIH) grant DK102107-01 awarded to ASMC (Funder ID: 10.13039/100000009).
U.S. Department of Education (DOE) Graduate Assistance in Areas of National Need (GAANN) Interdisciplinary Collaboration and Research Enterprise (iCare) awarded to JS (Funder ID: 10.13039/100000138).
Nomenclature
- b =
parallel plate flow chamber channel width, m
- h =
parallel plate flow chamber channel height, m
- Pa =
pressure, 1 Pa = 10 dynes/cm2; N/m3
- Q =
volumetric flow rate, L/s or mL/min
- x, y, z =
coordinates, m
Greek Symbols
- μ =
dynamic viscosity, Pa·s
- τ =
shear stress, dynes/cm2
- ρ =
density, kg/m3
Acronyms and Abbreviations
- ATCC =
American Type Culture Collection
- BAEC =
bovine aortic endothelial cell
- CFD =
computational fluid dynamics
- Cx43 =
connexin 43
- DAF-FM =
4-amino-5-methylamino-2′,7′-difluoroflurescein diacetate
- DFG =
disturbed flow gasket
- DMEM =
Dulbecco's modified Eagle's medium
- EC =
endothelial cell
- eNOS =
endothelial nitric oxide synthase
- FBS =
fetal bovine serum
- LFG =
laminar flow gasket
- miRNA =
micro ribonucleic acid
- NO =
nitric oxide
- PBS =
phosphate buffered saline
- TGF-β =
transforming growth factor-beta
- TNFα =
tumor necrosis factor-alpha
References
- [1]. Davies, P. F. , Civelek, M. , Fang, Y. , and Fleming, I. , 2013, “The Atherosusceptible Endothelium: Endothelial Phenotypes in Complex Haemodynamic Shear Stress Regions In Vivo,” Cardiovasc. Res., 99(2), pp. 315–327. 10.1093/cvr/cvt101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Barbee, K. A. , Davies, P. F. , and Lal, R. , 1994, “Shear Stress-Induced Reorganization of the Surface Topography of Living Endothelial Cells Imaged by Atomic Force Microscopy,” Circ. Res., 74(1), pp. 163–171. 10.1161/01.RES.74.1.163 [DOI] [PubMed] [Google Scholar]
- [3]. Conway, D. E. , Coon, B. G. , Budatha, M. , Arsenovic, P. T. , Orsenigo, F. , Wessel, F. , Zhang, J. , Zhuang, Z. , Dejana, E. , Vestweber, D. , and Schwartz, M. A. , 2017, “VE-Cadherin Phosphorylation Regulates Endothelial Fluid Shear Stress Responses Through the Polarity Protein LGN,” Curr. Biol., 27(14), pp. 2219–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Coon, B. G. , Baeyens, N. , Han, J. , Budatha, M. , Ross, T. D. , Fang, J. S. , Yun, S. , Thomas, J. L. , and Schwartz, M. A. , 2015, “Intramembrane Binding of VE-Cadherin to VEGFR2 and VEGFR3 Assembles the Endothelial Mechanosensory Complex,” J. Cell Biol., 208(7), pp. 975–986. 10.1083/jcb.201408103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Kim, D. W. , Gotlieb, A. I. , and Langille, B. L. , 1989, “In Vivo Modulation of Endothelial F-Actin Microfilaments by Experimental Alterations in Shear Stress,” Aeteriosclerosis, 9(4), pp. 439–445. 10.1161/01.ATV.9.4.439 [DOI] [PubMed] [Google Scholar]
- [6]. Kemeny, S. F. , and Clyne, A. M. , 2011, “A Simplified Implementation of Edge Detection in MATLAB Is Faster and More Sensitive Than Fast Fourier Transform for Actin Fiber Alignment Quantification,” Microsc. Microanal., 17(2), pp. 156–166. 10.1017/S143192761100002X [DOI] [PubMed] [Google Scholar]
- [7]. Wozniak, M. A. , Modzelewska, K. , Kwong, L. , and Keely, P. J. , 2004, “Focal Adhesion Regulation of Cell Behavior,” Biochim. Biophys. Acta, Mol. Cell Res., 1692(2–3), pp. 103–119. [DOI] [PubMed] [Google Scholar]
- [8]. Galbraith, G. G. , Skalak, R. , and Chien, S. , 1998, “Shear Stress Induces Spatial Reorganization of the Endothelial Cell Cytoskeleton,” Cell Motil. Cytoskeleton, 40(4), p. 317. [DOI] [PubMed] [Google Scholar]
- [9]. Miao, H. , Hu, Y.-L. , Shiu, Y.-T. , Yuan, S. , Zhao, Y. , Kaunas, R. , Wang, Y. , Jin, G. , Usami, S. , and Chien, S. , 2005, “Effect of Flow Patterns on the Localization and Expression of VE-Cadherin at Vascular Endothelial Cell Junctions: In Vivo and In Vitro Investigations,” J. Vasc. Res., 42(1), p. 77. 10.1159/000083094 [DOI] [PubMed] [Google Scholar]
- [10]. Gavard, J. , and Gutkind, J. S. , 2006, “VEGF Controls Endothelial-Cell Permeability by Promoting the Beta-Arrestin-Dependent Endocytosis of VE-Cadherin,” Nat. Cell Biol., 8(11), pp. 1223–1234. 10.1038/ncb1486 [DOI] [PubMed] [Google Scholar]
- [11]. Schulte, D. , Kuppers, V. , Dartsch, N. , Broermann, A. , Li, H. , Zarbock, A. , Kamenyeva, O. , Kiefer, F. , Khandoga, A. , Massberg, S. , and Vestweber, D. , 2011, “Stabilizing the VE-Cadherin-Catenin Complex Blocks Leukocyte Extravasation and Vascular Permeability,” EMBO J., 30(20), pp. 4157–4170. 10.1038/emboj.2011.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Cerutti, C. , and Ridley, A. J. , 2017, “Endothelial Cell-Cell Adhesion and Signaling,” Exp. Cell Res., 358(1), pp. 31–38. 10.1016/j.yexcr.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Chiu, J. J. , Chen, C. N. , Lee, P. L. , Yang, C. T. , Chuang, H. S. , Chien, S. , and Usami, S. , 2003, “Analysis of the Effect of Disturbed Flow on Monocytic Adhesion to Endothelial Cells,” J. Biomech., 36(12), pp. 1883–1895. 10.1016/S0021-9290(03)00210-0 [DOI] [PubMed] [Google Scholar]
- [14]. Tardy, Y. , Resnick, N. , Nagel, T. , Gimbrone, M. A., Jr. , and Dewey, C. F. , 1997, “Shear Stress Gradients Remodel Endothelial Monolayers In Vitro Via a Cell-Proliferation-Migration-Loss Cycle,” Arterioscler. Thromb. Vasc. Biol., 17(11), pp. 3102–3106. 10.1161/01.ATV.17.11.3102 [DOI] [PubMed] [Google Scholar]
- [15]. Won, D. , Zhu, S. N. , Chen, M. , Teichert, A. M. , Fish, J. E. , Matouk, C. C. , Bonert, M. , Ojha, M. , Marsden, P. A. , and Cybulsky, M. I. , 2007, “Relative Reduction of Endothelial Nitric-Oxide Synthase Expression and Transcription in Atherosclerosis-Prone Regions of the Mouse Aorta and in an In Vitro Model of Disturbed Flow,” Am. J. Pathol., 171(5), pp. 1691–1704. 10.2353/ajpath.2007.060860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Heo, K.-S. , Fujiwara, K. , and Abe, J. , 2011, “Disturbed-Flow-Mediated Vascular Reactive Oxygen Species Induce Endothelial Dysfunction,” Circ. J., 75(12), pp. 2722–2730. 10.1253/circj.CJ-11-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Chiu, J. J. , and Chien, S. , 2011, “Effects of Disturbed Flow on Vascular Endothelium: Pathophysiological Basis and Clinical Perspectives,” Physiol. Rev., 91(1), pp. 327–387. 10.1152/physrev.00047.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Jenkins, N. T. , Padilla, J. , Boyle, L. J. , Credeur, D. P. , Laughlin, M. H. , and Fadel, P. J. , 2013, “Disturbed Blood Flow Acutely Induces Activation and Apoptosis of the Human Vascular Endothelium,” Hypertension, 61(3), pp. 615–621. 10.1161/HYPERTENSIONAHA.111.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Sorescu, G. P. , Sykes, M. , Weiss, D. , Platt, M. O. , Saha, A. , Hwang, J. , Boyd, N. , Boo, Y. C. , Vega, J. D. , Taylor, W. R. , and Jo, H. , 2003, “Bone Morphogenic Protein 4 Produced in Endothelial Cells by Oscillatory Shear Stress Stimulates an Inflammatory Response,” J. Biol. Chem., 278(33), pp. 31128–31135. 10.1074/jbc.M300703200 [DOI] [PubMed] [Google Scholar]
- [20]. Dolan, J. M. , Meng, H. , Sim, F. J. , and Kolega, J. , 2013, “Differential Gene Expression by Endothelial Cells Under Positive and Negative Streamwise Gradients of High Wall Shear Stress,” Am. J. Physiol. Cell Physiol., 305(8), pp. C854–C866. 10.1152/ajpcell.00315.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Szymanski, M. P. , Metaxa, E. , Meng, H. , and Kolega, J. , 2008, “Endothelial Cell Layer Subjected to Impinging Flow Mimicking the Apex of an Arterial Bifurcation,” Ann. Biomed. Eng., 36(10), pp. 1681–1689. 10.1007/s10439-008-9540-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Noris, M. , Morigi, M. , Donadelli, R. , Aiello, S. , Foppolo, M. , Todeschini, M. , Orisio, S. , Remuzzi, G. , Remuzzi, A. , Noris, M. , Todeschini, M. , Orisio, S. , Remuzzi, G. , and Remuzzi, A. , 1995, “Nitric Oxide Synthesis by Cultured Endothelial Cells Is Modulated by Flow Conditions,” Circ. Res., 76(4), pp. 536–543. 10.1161/01.RES.76.4.536 [DOI] [PubMed] [Google Scholar]
- [23]. Dewey, C. F. , Bussolari, S. R. , Gimbrone, M. A. , and Davies, P. F. , 1981, “The Dynamic Response of Vascular Endothelial Cells to Fluid Shear Stress,” ASME J. Biomech. Eng., 103(3), p. 177. 10.1115/1.3138276 [DOI] [PubMed] [Google Scholar]
- [24]. Buschmann, M. H. , Dieterich, P. , Adams, N. A. , and Schnittler, H. J. , 2005, “Analysis of Flow in a Cone-and-Plate Apparatus With Respect to Spatial and Temporal Effects on Endothelial Cells,” Biotechnol. Bioeng., 89(5), pp. 493–502. 10.1002/bit.20165 [DOI] [PubMed] [Google Scholar]
- [25]. Franzoni, M. , Cattaneo, I. , Ene-Iordache, B. , Oldani, A. , Righettini, P. , and Remuzzi, A. , 2016, “Design of a Cone-and-Plate Device for Controlled Realistic Shear Stress Stimulation on Endothelial Cell Monolayers,” Cytotechnology, 68(5), pp. 1885–1896. 10.1007/s10616-015-9941-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Brown, T. D. , 2000, “Techniques for Mechanical Stimulation of Cells In Vitro: A Review,” J. Biomech., 33(1), pp. 3–14. 10.1016/S0021-9290(99)00177-3 [DOI] [PubMed] [Google Scholar]
- [27]. Dardik, A. , Chen, L. , Frattini, J. , Asada, H. , Aziz, F. , Kudo, F. A. , and Sumpio, B. E. , 2005, “Differential Effects of Orbital and Laminar Shear Stress on Endothelial Cells,” J. Vasc. Surg., 41(5), pp. 869–880. 10.1016/j.jvs.2005.01.020 [DOI] [PubMed] [Google Scholar]
- [28]. Lu, Y. , Li, W. , Oraifige, I. , and Wang, W. , 2014, “Converging Parallel Plate Flow Chambers for Studies on the Effect of the Spatial Gradient of Wall Shear Stress on Endothelial Cells,” J. Biosci. Med., 2, pp. 50–56. 10.4236/jbm.2014.22008 [DOI] [Google Scholar]
- [29]. DePaola, N. , Davies, P. F. , Pritchard, W. F. , Florez, L. , Harbeck, N. , and Polacek, D. C. , 1999, “Spatial and Temporal Regulation of Gap Junction Connexin43 in Vascular Endothelial Cells Exposed to Controlled Disturbed Flows In Vitro,” Proc. Natl. Acad. Sci., 96(6), pp. 3154–3159. 10.1073/pnas.96.6.3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Kock, S. A. , Nygaard, J. V. , Eldrup, N. , Fründ, E. T. , Klærke, A. , Paaske, W. P. , Falk, E. , and Yong Kim, W. , 2008, “Mechanical Stresses in Carotid Plaques Using MRI-Based Fluid-Structure Interaction Models,” J. Biomech., 41(8), pp. 1651–1658. 10.1016/j.jbiomech.2008.03.019 [DOI] [PubMed] [Google Scholar]
- [31]. Slagger, C. J. , Wentzell, J. J. , Gijsen, F. J. H. , Thury, A. , van der Waal, A. C. , Schaar, J. A. , and Serruys, P. W. , 2005, “The Role of Shear Stress in the Destabilization of Vulnerable Plaques and Related Therapeutic Implications,” Nat. Clin. Pract. Cardiovasc. Med., 2(9), pp. 456–464. 10.1038/ncpcardio0298 [DOI] [PubMed] [Google Scholar]
- [32]. Itoh, Y. , Ma, F. H. , Hoshi, H. , Oka, M. , Noda, K. , Ukai, Y. , Kojima, H. , Nagano, T. , and Toda, N. , 2000, “Determination and Bioimaging Method for Nitric Oxide in Biological Specimens by Diaminofluorescein Fluorometry,” Anal. Biochem., 287(2), pp. 203–209. 10.1006/abio.2000.4859 [DOI] [PubMed] [Google Scholar]
- [33]. Dubrovskyi, O. , Birukova, A. A. , and Birukov, K. , 2013, “Measurement of Local Permeability at Subcellular Level in Cell Models of Agonist- and Ventilator-Induced Lung Injury,” Lab. Invest., 93(2), pp. 254–263. 10.1038/labinvest.2012.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Maltsev, V. P. , Hoekstra, A. G. , and Yurkin, M. A. , 2011, “Optics of White Blood Cells: Optical Models, Simulations, and Experiments,” Advanced Optical Flow Cytometry: Methods and Disease Diagnoses, Wiley, Hoboken, NJ, Chap. 4. [Google Scholar]
- [35]. Schoch, R. L. , Kapinos, L. E. , and Lim, R. Y. H. , 2012, “Supporting Information: Nuclear Transport Receptor Binding Avidity Triggers a Self-Healing Collapse Transition in FG-Nucleoporin Molecular Brushes,” PNAS, 109(42), pp. 16911–16916. 10.1073/pnas.1208440109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Canver, A. C. , and Clyne, A. M. , 2017, “Quantification of Multicellular Organization, Junction Integrity, and Substrate Features in Collective Cell Migration,” Microsc. Microanal., 23(1), pp. 22–33. 10.1017/S1431927617000071 [DOI] [PubMed] [Google Scholar]
- [37]. Scholzen, T. , and Gerdes, J. , 2000, “The Ki-67 Protein: From the Known and the Unknown,” J. Cell. Physiol., 182(3), pp. 311–322. 10.1002/(SICI)1097-4652(200003)182:33.0.CO;2-9 [DOI] [PubMed] [Google Scholar]
- [38]. Otsu, N. , 1979, “A Threshold Selection Method From Gray-Level Histograms,” IEEE Trans. Syst. Man. Cybern., 9(1), pp. 62–66. 10.1109/TSMC.1979.4310076 [DOI] [Google Scholar]
- [39]. Kornilov, A. , and Safonov, I. , 2018, “An Overview of Watershed Algorithm Implementations in Open Source Libraries,” J. Imaging, 4(10), p. 123. 10.3390/jimaging4100123 [DOI] [Google Scholar]
- [40]. Kohn, J. C. , Zhou, D. W. , Bordeleau, F. , Zhou, A. L. , Mason, B. N. , Mitchell, M. J. , King, M. R. , and Reinhart-King, C. A. , 2015, “Cooperative Effects of Matrix Stiffness and Fluid Shear Stress on Endothelial Cell Behavior,” Biophys. J., 108(3), pp. 471–478. 10.1016/j.bpj.2014.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Heo, K. S. , Lee, H. , Nigro, P. , Thomas, T. , Le, N. T. , Chang, E. , McClain, C. , Reinhart-King, C. A. , King, M. R. , Berk, B. C. , Fujiwara, K. , Woo, C. H. , and Abe, J. , 2011, “PKCzeta Mediates Disturbed Flow-Induced Endothelial Apoptosis Via P53 SUMOylation,” J. Cell Biol., 193(5), pp. 867–884. 10.1083/jcb.201010051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Davies, P. F. , Remuzzi, A. , Gordon, E. J. , Dewey, C. F. , and Gimbrone, M. A. , 1986, “Turbulent Fluid Shear Stress Induces Vascular Endothelial Cell Turnover In Vitro,” Proc. Natl. Acad. Sci. U. S. A., 83(7), pp. 2114–2117. 10.1073/pnas.83.7.2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. DePaola, N. , Gimbrone, M. A. , Davies, P. F. , and Dewey, C. F. , 1992, “Vascular Endothelium Responds to Fluid Shear Stress Gradients,” Arterioscler. Thromb. Vasc. Biol., 12(11), pp. 1254–1257. 10.1161/01.ATV.12.11.1254 [DOI] [PubMed] [Google Scholar]
- [44]. Estrada, R. , Giridharan, G. A. , Nguyen, M. D. , Prabhu, S. D. , and Sethu, P. , 2011, “Microfluidic Endothelial Cell Culture Model to Replicate Disturbed Flow Conditions Seen in Atherosclerosis Susceptible Regions,” Biomicrofluidics, 5(3), p. 032006. 10.1063/1.3608137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Patibandla, P. K. , Rogers, A. J. , Giridharan, G. A. , Pallero, M. A. , Murphy-Ullrich, J. E. , and Sethu, P. , 2014, “Hyperglycemic Arterial Disturbed Flow Niche as an In Vitro Model of Atherosclerosis,” Anal. Chem., 86(21), pp. 10948–10954. 10.1021/ac503294p [DOI] [PubMed] [Google Scholar]
- [46]. Wang, X. Q. , Nigro, P. , World, C. , Fujiwara, K. , Yan, C. , and Berk, B. C. , 2012, “Thioredoxin Interacting Protein Promotes Endothelial Cell Inflammation in Response to Disturbed Flow by Increasing Leukocyte Adhesion and Repressing Kruppel-Like Factor 2,” Circ. Res., 110(4), pp. 560–568. 10.1161/CIRCRESAHA.111.256362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Ostrowski, M. A. , Huang, N. F. , Walker, T. W. , Verwijlen, T. , Poplawski, C. , Khoo, A. S. , Cooke, J. P. , Fuller, G. G. , and Dunn, A. R. , 2014, “Microvascular Endothelial Cells Migrate Upstream and Align Against the Shear Stress Field Created by Impinging Flow,” Biophys. J., 106(2), pp. 366–374. 10.1016/j.bpj.2013.11.4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Launay, G. , Mignot, E. , Riviere, N. , and Perkins, R. , 2017, “An Experimental Investigation of the Laminar Horseshoe Vortex Around an Emerging Obstacle,” J. Fluid Mech., 830, p. 257. 10.1017/jfm.2017.582 [DOI] [Google Scholar]
- [49]. Mohamied, Y. , Sherwin, S. J. , and Weinberg, P. D. , 2017, “Understanding the Fluid Mechanics Behind Transverse Wall Shear Stress,” J. Biomech., 50, pp. 102–109. 10.1016/j.jbiomech.2016.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Balcerczyk, A. , Soszynski, M. , and Bartosz, G. , 2005, “On the Specificity of 4-Ammino-5-Methylamino-2′,7′-Diflurorofluorescein as a Probe for Nitric Oxide,” Free Radical Biol. Med., 39(3), pp. 327–335. 10.1016/j.freeradbiomed.2005.03.017 [DOI] [PubMed] [Google Scholar]
- [51]. Valiunas, V. , Polosina, Y. Y. , Miller, H. , Potapova, I. A. , Valiuniene, L. , Doronin, S. , Mathias, R. T. , Robinson, R. B. , Rosen, M. R. , Cohen, I. S. , and Brink, P. R. , 2005, “Connexin-Specific Cell-to-Cell Transfer of Short Interfering RNA by Gap Junctions,” J. Physiol., 568(2), pp. 459–468. 10.1113/jphysiol.2005.090985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Thuringer, D. , Jego, G. , Berthenet, K. , Hammann, A. , Solary, E. , and Garrido, C. , 2016, “Gap Junction-Mediated Transfer of MiR-145-5p From Microvascular Endothelial Cells to Colon Cancer Cells Inhibits Angiogenesis,” Oncotarget, 7(19), pp. 28160–28168. 10.18632/oncotarget.8583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Zong, L. , Zhu, Y. , Liang, R. , and Zhao, H. B. , 2016, “Gap Junction Mediated MiRNA Intercellular Transfer and Gene Regulation: A Novel Mechanism for Intercellular Genetic Communication,” Sci. Rep., 6, p. 19884. 10.1038/srep19884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. LaMack, J. A. , and Friedman, M. H. , 2007, “Individual and Combined Effects of Shear Stress Magnitude and Spatial Gradient on Endothelial Cell Gene Expression,” Am. J. Physiol.: Heart Circ. Physiol., 293(5), pp. H2853–H2859. 10.1152/ajpheart.00244.2007 [DOI] [PubMed] [Google Scholar]
- [55]. Balaguru, U. M. , Sundaresan, L. , Manivannan, J. , Majunathan, R. , Mani, K. , Swaminathan, A. , Venkatesan, S. , Kasiviswanathan, D. , and Chatterjee, S. , 2016, “Disturbed Flow Mediated Modulation of Shear Forces on Endothelial Plane: A Proposed Model for Studying Endothelium Around Atherosclerotic Plaques,” Sci. Rep., 6(1), p. 27304. 10.1038/srep27304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Deshmane, S. L. , Kremlev, S. , Amini, S. , and Sawaya, B. E. , 2009, “Monocyte Chemoattractant Protein-1 (MCP-1): An Overview,” J. Interferon Cytokine Res., 29(6), pp. 313–326. 10.1089/jir.2008.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Shyy, J. Y. J. , and Chien, S. , 2002, “Role of Integrins in Endothelial Mechanosensing of Shear Stress,” Circ. Res., 91(9), pp. 769–775. 10.1161/01.RES.0000038487.19924.18 [DOI] [PubMed] [Google Scholar]
- [58]. Huang, D. L. , Bax, N. A. , Buckley, C. D. , Weis, W. I. , and Dunn, A. R. , 2017, “Vinculin Forms a Directionally Asymmetric Catch Bond With F-Actin,” Science, 357(6352), p. 703. 10.1126/science.aan2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Yoshino, D. , Sakamoto, N. , and Sato, M. , 2017, “Fluid Shear Stress Combined With Shear Stress Spatial Gradients Regulates Vascular Endothelial Morphology,” Integr. Biol., 9(7), p. 584. 10.1039/C7IB00065K [DOI] [PubMed] [Google Scholar]


