Abstract
Sites of infection and inflammation can be misleading in oncology PET/CT imaging because these areas commonly show 18F-FDG activity. Caution in the interpretation must be taken to avoid the misdiagnosis of malignancy. Utilization of both CT findings as well as patient history can help differentiate benign infectious and inflammatory processes from malignancy, although occasionally additional work-up may be required. This article discusses the mechanism of 18F-FDG uptake in infection and inflammation with illustrative examples.
Keywords: 18F-FDG PET/CT, Infection, Inflammation, Imaging pitfalls
1. Introduction
18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) is commonly performed in oncology patients for staging neoplasms and evaluating treatment response. However, uptake of FDG is not entirely specific for neoplasm, and in addition to treatment-induced inflammatory changes, a variety of benign infectious and inflammatory processes can result in focal radiopharmaceutical uptake. These foci of uptake are often considered diagnostic pitfalls when interpreting oncologic 18F-FDG PET/CT’s as they may lead to false-positive results [1]. Clinicians must be vigilant for unusual patterns of radiopharmaceutical uptake due to infectious and inflammatory etiologies in order to avoid misdiagnosis of malignancy. Patient history, knowledge of typical patterns of metastatic spread of the malignancy being evaluated, as well as the co-acquired CT findings can guide interpretation to the correct cause(s) of the abnormal FDG uptake. At times, additional diagnostic steps will need to be performed to confirm the etiology of abnormal patterns of FDG distribution. This review article discusses mechanisms of 18F-FDG uptake in tumors in contrast to infection and inflammation with examples of infectious and inflammatory pitfalls in oncologic 18F-FDG PET/CT imaging and interpretation.
2. Mechanism of uptake and metabolism of 18F-FDG
Throughout the last several decades, 18F-FDG, a glucose analogue, has been used for the detection and evaluation of a wide range of solid and hematological malignancies. Generally, cancer cells demonstrate increased rates of glucose utilization [2]. It is this principle of a relative increased glucose metabolism of many neoplasms that allows for the increased uptake and the depiction of malignancies using 18F-FDG.
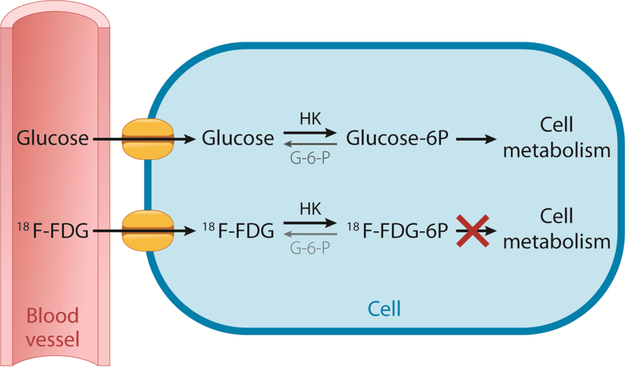
After intravenous injection, 18F-FDG enters cells via glucose transporter membrane proteins. Once in the cell, 18F-FDG undergoes phosphorylation by the enzyme, hexokinase. Phosphorylated glucose undergoes further metabolism in the cell through glycolysis or glycogen formation. However, unlike glucose after phosphorylation, 18F-FDG cannot undergo further metabolism (Fig. 1) [3]. Although, either substrate may be dephosphorylated by glucose-6-phosphatase (G6P) if present; molecular factors leading to relative increased accumulation of 18F-FDG in cancers compared to normal tissues include over-expression of glucose transport membrane proteins, increased hexokinase enzyme activity, as well as relative diminished G6P activity [4]. Cancer cells have relatively low G6P activity, hence reducing further cellular metabolism. Consequently, phosphorylated 18F-FDG is often described as being “trapped” after hexokinase-mediated phosphorylation in cancer cells.
Fig. 1.
Glucose and 18F-FDG Cellular Uptake and Metabolism. Both glucose and 18F-FDG enter the cells by glucose transporter membrane proteins. Once in the cell, both are phosphorylated by hexokinase (HK), which can be reversed by glucose-6-phosphatase (G-6-P) if present. Phosphorylated glucose (Glucose-6 P) is available to be further metabolized by the cell, whereas phosphorylated 18F-FDG (18F-FDG-6 P) cannot be further metabolized and is therefore considered “trapped”.
At the site of inflammation/infection, inflammatory cells (neutrophils, activated macrophages, and lymphocytes) show increased 18F-FDG accumulation through a common mechanism [5,6]. Mochizuki et al. demonstrated elevated FDG uptake and glucose membrane transporter expression in rats inoculated with malignant cells (allogenic hepatoma cells) as well as rats infected with Staphylococcus aureus [7]. Furthermore, increased 18F-FDG uptake has also been noted within inflammatory cells associated with tumors, and can be part of the cellular response to treatment [8].
3. Infectious and inflammatory pitfalls in oncologic imaging
Unexpected and incidental foci of uptake in oncologic 18F-FDG PET/CT’s is relatively common with diagnostic considerations including unusual sites of metastases, a second synchronous neoplasm, surgical or procedural interventions, as well as infection and inflammation [9]. Given their increased uptake on 18F-FDG PET/CT, infectious and inflammatory responses have the potential to be misinterpreted as metastatic disease. Following review of greater than 1000 patients 18F-FDG PET/CT’s, Metser et al. reported greater than one quarter of these examinations contained incidental foci of benign radiopharmaceutical uptake [10]. The majority of these foci of FDG uptake were attributed to infectious and non-infectious inflammation. Pneumonia, upper respiratory tract infections, and wound infections were among the most common etiologies identified incidentally 18F-FDG PET/CT done for oncologic indications [11]. The presence of longstanding, indwelling, catheters, lines and tubes, often generate a foreign body-like inflammatory responses, characterized by formation of granulation tissues with intense FDG uptake. However, numerous additional common and uncommon benign causes of uptake have been described (Table 1) [12].
Table 1.
Common and uncommon benign inflammatory and infectious causes of FDG uptake in oncology patients.
| Location | Etiology |
|---|---|
| Brain / CNS | Brain abscess |
| Toxoplasmosis | |
| Viral encephalitis | |
| Septic emboli | |
| Nocardia | |
| Meningitis | |
| Head and Neck | Lymphoid tissue, Waldeyer’s ring, palatine and lingual tonsils |
| Thornwaldt cyst | |
| Sinusitis (maxillary, ethmoid, nasal, frontal) | |
| Inflammatory jugulo-digastric lymph nodes | |
| Dental infections | |
| Parotitis | |
| Thyroiditis | |
| Mucositis after chemoradiation therapy | |
| Otitis media | |
| Otitis externa | |
| Mastoiditis | |
| Tracheostomy | |
| Thorax | |
| Lungs | Pneumonia |
| Atelectasis / collapse | |
| Opportunistic infections (pneumocystis, nocardia, fungal) | |
| Sarcoidosis | |
| Tuberculosis | |
| Rheumatoid nodules | |
| Bleomycin toxicity | |
| Septic emboli | |
| Post-radiation inflammation | |
| Pleura | Talc pleurodesis |
| Exudative pleural effusions | |
| Mediastinum | Chronic granulomatous disease |
| Sarcoidosis | |
| Tuberculosis | |
| Heart and large vessels | Endocarditis (abscess) |
| Myocarditis | |
| Sarcoidosis | |
| Infected prosthetic valves | |
| Iatrogenic (LVAD, surgical clips, pacemakers wires, AICD wires) | |
| Infected central lines | |
| Mycotic aneurysms | |
| Large-vessel vasculitis | |
| Miscellaneous | Esophagitis |
| Mastitis | |
| Breast abscess | |
| Fat necrosis | |
| Inflammatory axillary lymph nodes | |
| Abdomen | |
| Liver | Liver abscess |
| Hepatitis | |
| Cholangitis | |
| Gallbladder | Cholecystitis |
| Pancreas | Pancreatitis, pancreatic pseudocyst |
| Spleen | Granulomatous disease |
| Large and small bowel | Ulcerative colitis |
| Pseudomembranous colitis immunotherapy related colitis | |
| Bowel perforation | |
| Appendicitis | |
| Diverticulitis | |
| Small bowel ischemia | |
| Duodenitis | |
| Mesenteric adenitis | |
| Percutaneous drains and feeding tubes | |
| Stomach | Gastritis |
| Kidneys | Pyelonephritis |
| Renal abscess | |
| Retroperitoneal fibrosis | |
| Pelvis | Inflammatory inguinal lymph nodes |
| Salpingo-oophoritis | |
| Endometritis | |
| Prostatitis | |
| Epididymo-orchitis | |
| Musculoskeletal | |
| Bone | Discitis |
| Osteomyelitis | |
| Inflammatory degenerative disease | |
| Pressure sores | |
| Infected diabetic foot ulcers | |
| Orthopedic prosthesis infections and surgical hardware | |
| Chronic migratory recurrent osteomyelitis | |
| Muscles and joints | Dermatomyositis |
| Polymyositis | |
| Rheumatoid arthritis | |
| Rhabdomyositis | |
| Intramuscular abscess | |
| Vessels | Giant cell arteritis |
| Vasculitis | |
| Infected aortic-iliac-femoral grafts | |
| Infected A-V fistula | |
| Skin / subcutaneous soft tissues | Hidradenitis suppurativa |
| Wound infections | |
| Sebaceous cyst | |
| Acne | |
| Eczema | |
| Fournier’s gangrene | |
| Iatrogenic injection granuloma |
3.1. Differentiating infectious/inflammatory uptake from malignancy
The clinical challenge remains to reliably differentiate infectious and inflammatory FDG uptake from malignancy. Ideally, an easily obtained and reproducible quantifiable measurement would guide interpretation to the correct diagnosis. However, at this time no such measurement exists. Unfortunately, although an easily obtained parameter, the standard uptake value (SUV), cannot differentiate malignancy from infectious and inflammatory uptake and no reliable, absolute SUV threshold has been found that can be used clinically differentiate benign from malignant uptake due to the similar mechanism of FDG accumulation between tumor cells and white blood cells [13].
There has been interest in using relative or comparative SUV measurements to differentiate etiologies of abnormal 18F-FDG uptake. Dual time point imaging, in which a second delayed acquisition is obtained, has been proposed as an alternative to increase the overall sensitivity and specificity for the detection of malignancy by 18F-FDG PET/CT. With this protocol malignancies generally exhibit increased FDG uptake on the delayed acquisition, whereas infectious and inflammatory foci typically demonstrate decreased uptake on delayed imaging [14,15]. However, the performance, overall reliability and usefulness of dual time point imaging has had mixed results, limiting its routine clinical use for evaluating incidental foci of uptake on 18F-FDG PET/CT [16]. Published in 2013, the EANM/SNMMI Guidelines for the use of PET/CT in infection and inflammation does not support use of dual time point imaging specifically citing poor dependability in differentiating neoplasm from infection [17].
Glucose update and utilization rates can be leveraged to distinguish metastasis from inflammation. If the intensity of 18F-FDG uptake at an indeterminate site is different to that of a known malignancy, a separate process may be considered. This concept of the metabolic signature is based upon the presumption of similar rates of glucose utilization between the primary neoplasm and sites of metastasis. A subjective assessment is easily performed using the whole-body maximum intensity projection images. A quantified comparison can also be performed, but caution is advised as there is no relative or absolute SUV difference that can be used in clinical practice for evaluating different metabolic signatures. This concept of metabolic “signature” has demonstrated to be useful in suggesting an incidental focus of uptake on PET/CT to represent a different process other than the primary malignancy that might include a second, synchronous primary neoplasm and/or an inflammatory/infectious process [18,19].
Although metabolic signatures are useful, there are several potential pitfalls to their use. Concluding a separate process based upon different metabolic signatures in small (< 8 mm) lesions should be avoided as processes that are below the limits of the spatial resolution of PET (< 1 cm) may lead to inaccurate SUV’s. Similarly, when a lesion demonstrates a large cystic and/or necrotic component, SUV measurements may be deceptively low. Additionally, it is prudent for clinicians to be aware of neoplasms that are prone to varying degrees of differentiation including leukemias, lymphomas, thyroid cancers, and neuroendocrine tumors. In these cases, contrasting intensities of 18F-FDG uptake may reflect a neoplasm with varying degrees of de-differentiation with more aggressive foci of disease generally having greater dependence upon glucose metabolism and thus higher FDG uptake [20].
Relevant patient history and symptoms may suggest the underlying etiology for the abnormal FDG uptake. Obtaining a detailed history specifically inquiring about patient oncologic history, treatment, procedures, infections, sites of pain, and other acute medical conditions can be performed in-person or by written questionnaire. The PET scan should then be interpreted in the context of the oncologic history. A knowledge of the typical pattern of metastases for the malignancy under investigation can be a very useful guide. For example, nodal metastatic disease has a tendency towards asymmetric distribution, whereas a symmetric pattern of FDG uptake in lymph nodes in the neck or in the mediastinum, often points to an inflammatory etiology. Also, certain lymph node stations are more commonly related to inflammatory or infectious processes, such as the jugulodigastric, axillary, hilar, and inguinal nodes. If possible, the images can be reviewed before the patient leaves the imaging center and more specific queries can be made about current symptoms and history to clarify the imaging findings.
In addition to patient history, corresponding CT images may guide the interpreting physician to the correct cause(s) of the focus of uptake. The interpreting physician must be familiar with common CT imaging presentations of acute inflammatory conditions such as lobar airspace disease indicating pneumonia, presence of gas locules in infections fluid collections and peri-visceral fat stranding often encountered in acute infectious/inflammatory processes in the abdomen and pelvis.
Despite these strategies, there will be indeterminate foci of uptake of 18F-FDG PET/CT in oncology patients. In such cases, clinical guidance can be made to the referring physician to determine optimal post PET/CT follow up imaging strategies. Tools available include clinical correlation, immediate or short-term follow-up diagnostic imaging, or endoscopy. The choice will largely depend upon symptoms and treatment management.
4. Common incidental infectious and inflammatory findings in oncologic 18F-FDG PET/CT
In addition to the general principles and strategies listed above, a familiarity of common infectious and inflammatory processes encountered in oncologic 18F-FDG PET/CT is beneficial and when recognized should raise the possibility of non-malignant disease.
4.1. Head and neck
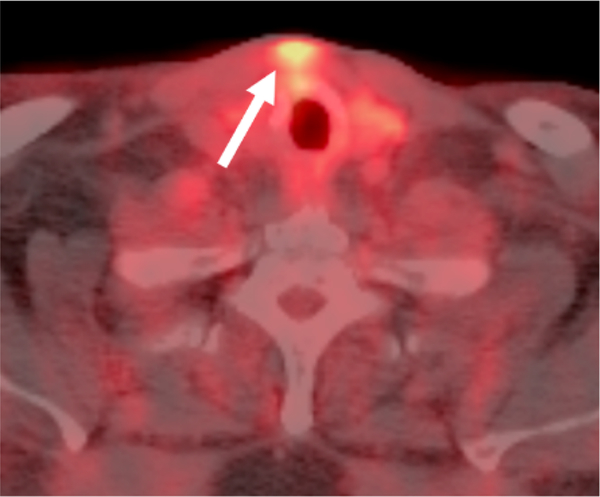
Incidental uptake in the head and neck is a relatively common. In a retrospective study evaluating incidental FDG uptake in 293 patients with head and neck cancer, 45 of the 134 incidental findings were located in the extra-thyroidal head and neck [21]. A variety of nonneoplastic processes can result in incidental 18F-FDG uptake. Focal uptake in the maxilla or mandible is often seen in dental related infections and periodontal disease [22]. Variable degrees of symmetric and asymmetric uptake often are encountered in Waldeyer’s ring in infection and inflammation [23]. These processes may also result in uptake in reactive lymph nodes in the head and neck. Following chemotherapy for oropharyngeal cancer mucositis may be encountered in the hypopharynx. Inflammatory and/or infectious 18F-FDG uptake has also been noted in lacrimal, salivary, and thyroid glands as well the paranasal sinuses [24–27]. Classically, diffuse thyroid uptake suggests an underlying inflammatory process whereas as focal uptake requires further evaluation for a possible underlying nodule. A common procedure in patients treated for head and neck cancer, focal uptake can be seen at tracheostomy sites (Fig. 2). Brain infections including abscesses have also been described as being 18F-FDG-avid [28,29].
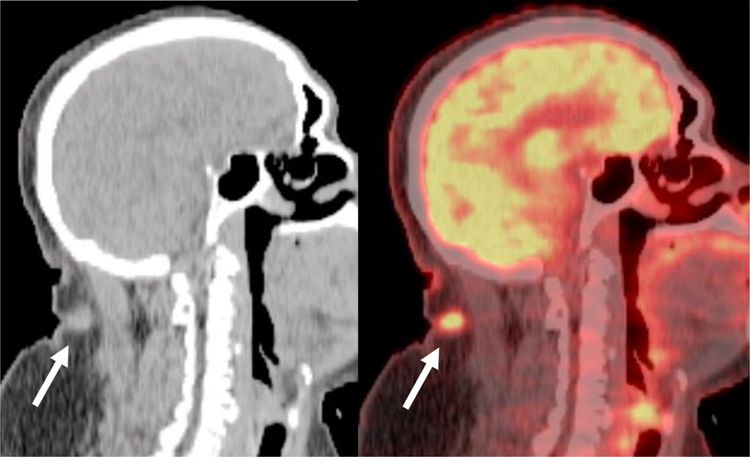
Fig. 2.
Focal Inflammatory 18F-FDG uptake at Site of a Previous Tracheostomy. Axial fused PET/CT demonstrates focal uptake in the soft tissues (arrow) in the anterior neck superficial to the thyroid gland that correlated to the site of postsurgical inflammation at the site of a previous tracheostomy.
4.2. Thorax
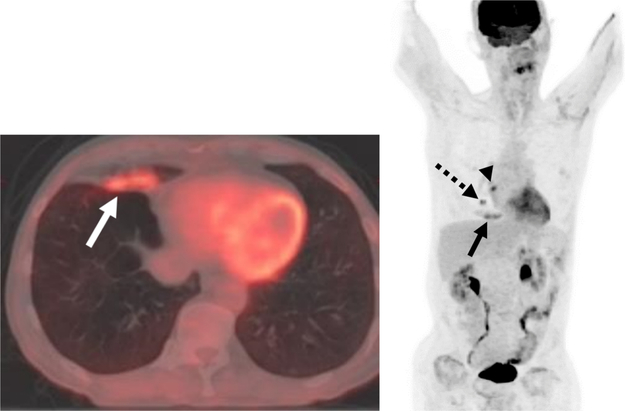
There are numerous causes of infectious and inflammatory uptake in the chest. A wide array of pulmonary infections can be 18F-FDG-avid including typical and atypical organisms [12,28,30,31]. In lung cancer patients, particular awareness for post-obstructive pneumonias should be made as the lung neoplasm may narrow or invade the airway (Fig. 3). In this setting the central lung cancer often has a more intense metabolic signature to the diffuse uptake in the collapsed lung parenchyma. Newer immunotherapy agents can result in a pneumonitis, organizing pneumonia, and/or sarcoid-like reactions, which may demonstrate 18F-FDG-avidity [32–34]. Focal organizing pneumonias can be particularly problematic as their mass-like hypermetabolic presentation is easily mistaken for malignancy [35]. The sequelae of radiation therapy in pleura and lung parenchyma will often have a geographical and diffuse or linear configuration, distinguishing it from more focal uptake from malignancy. Talc pleurodesis performed for management of malignant and benign pleural effusions with intense inflammatory foreign body response characteristically is depicted with high FDG uptake.
Fig. 3.
Postobstructive 18F-FDG-avid Airspace Disease in a Patient with Lung Malignancy.
A, B Axial fused PET/CT and maximum intensity projection image demonstrates focal uptake in right middle lobe airspace disease (solid arrows) in a patient with a hypermetabolic central right lung nodule (dashed arrow) and an adjacent right hilar lymph node (arrowhead).
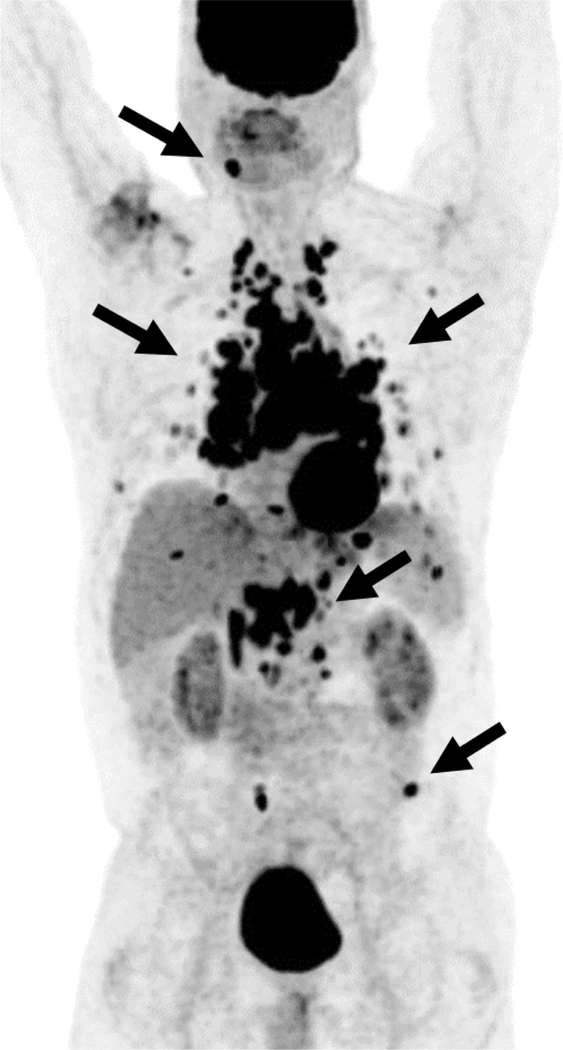
Sarcoidosis is a non-caseating granulomatous inflammatory disease often with manifestations in the thorax including adenopathy and interstitial lung disease including nodularity that can be hypermetabolic on 18F-FDG PET/CT [36]. This can lead to the erroneous interpretation of malignancy in hypermetabolic thoracic foci as sarcoidosis is an excellent mimicker of malignancy (Fig. 4) [37]. In addition to thoracic nodal uptake, pulmonary and extra-thoracic manifestations of sarcoid can also demonstrate increased FDG uptake suggesting a more aggressive malignant process including focal uptake in abdominal viscera and in the skeleton [38,39]. Great caution should be taken when interpreting oncologic 18F-FDG PET/CT in patients with sarcoidosis given the high rate of non-malignant 18F-FDG-uptake.
Fig. 4.
Multiple Foci of Abnormal Uptake in a 56-year-old male with 18F-FDG-avid Sarcoidosis.
Maximum intensity projection image from an 18F-FDG PET/CT multiple hypermetabolic foci (arrows) in lymph nodes, pulmonary nodules, as well as several osseous foci that were initially viewed with concern for a malignancy such as lymphoma. However, subsequent tissue sampling revealed sarcoidosis as the underlying cause.
Extra-pulmonic infectious and inflammatory foci are also routinely encountered. Present on many oncologic 18F-FDG PET/CT’s, mild uptake is often seen in hilar and mediastinal lymph nodes, related to chronic granulomatous disease, often associated with nodal calcifications and granulomata in the lungs. Mild increased linear uptake in the esophagus and gastroesophageal junction is often related to inflammation including reflux esophagitis. However, when esophageal uptake is more focal and/or intense, it warrants endoscopic correlation to exclude malignant or pre-malignant conditions [40].
Other abnormal foci of infectious and inflammatory uptake in the chest associated with FDG uptake include fibrosing mediastinitis and a variety of infectious and inflammatory processes of the breasts [41,42]. In oncologic 18F-FDG PET/CT imaging, myocardial uptake is variable and a variety of normal patterns have been described [43]. This is due to myocardial metabolism of both glucose and fatty acids. Despite variable physiologic uptake in the myocardium, pathologic inflammatory and/or infectious uptake in or adjacent to the heart has been described including pericarditis, myocarditis, sarcoidosis, as well as uptake in the adjacent great vessels due to inflammation/infections including vasculitis (Fig. 5) [44,45].
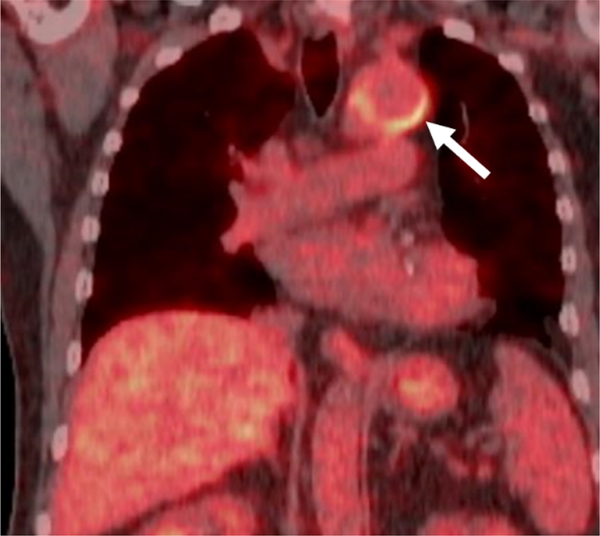
Fig. 5.
Incidental Non-infectious Inflammatory Uptake in a Recently Placed Thoracic Aortic Graft in a 59-year-old male.
Fused coronal 18F-FDG PET/CT demonstrates mild intensity curvilinear uptake (arrow) along portions of a recently placed thoracic aortic graft in an oncology patient without signs/symptoms of infection.
4.3. Abdomen and pelvis
In the abdomen and pelvis, the bowel is a common site for infectious and inflammatory processes. Abnormal focal uptake correlating to abscesses, appendicitis, infectious and inflammatory colitis, and diverticulitis have been reported (Figs. 6 and 7) [46–51]. Correlation with CT signs of inflammation is recommended as there are other causes of increased FDG uptake in the bowel including metformin as well ureteral diversion procedures in which physiologic FDG uptake in urine can be seen in the bowel lumen [52,53].
Fig. 6.
42-year-old Woman Receiving Immunotherapy for Metastatic Melanoma.
A, Maximum intensity projection image from an 18F-FDG PET/CT demonstrates diffuse hypermetabolic activity in the colon (arrows) and diffuse uptake involving the pancreas (arrowhead).
B, Coronal PET images show diffuse uptake throughout the entire colon consistent with colitis (arrows), an adverse effect related to immunotherapy. </p/> C, Axial fused PET/CT show diffuse uptake throughout the pancreas (arrowheads) consistent with pancreatitis related to immunotherapy.
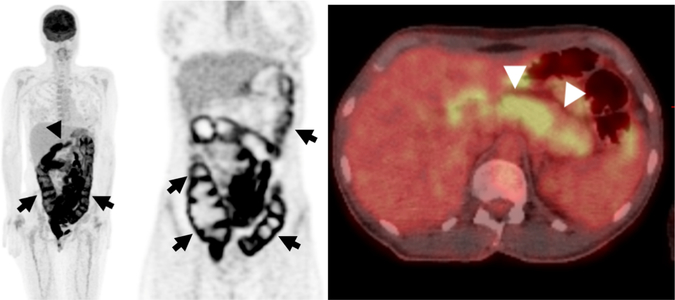
Fig. 7.
Hypermetabolic Right-sided Abdominal Abscess 64 year-old Man.
A, B Axial and C, D coronal PET and CT images from an 18F-FDG PET/CT demonstrates a hypermetabolic fluid collection with peripheral uptake and central photopenia in the right abdomen (arrows). The patient had recent colonoscopy and biopsy of a polyp at the cecum with suspicion for perforation after the procedure. The abscess required to surgical drainage and antibiotic treatment.
In addition to the gastrointestinal tract, other viscera in the abdomen and pelvis may demonstrate infectious and inflammatory uptake on 18F-FDG PET/CT. Increased FDG uptake in the pancreas from pancreatitis may been mistaken for malignancy (Fig. 6) [54,55]. Although classically thought of resulting in increased uptake throughout the pancreas, focal uptake in the pancreas may also represent pancreatitis [56]. To make diagnostic interpretation even more challenging, a case of a hypermetabolic pancreatic mass with superimposed diffuse 18F-FDG uptake throughout the pancreas from pancreatitis has been reported [57].
Additional abdominal visceral infection and inflammatory processes may be encountered when interpreting 18F-FDG PET/CT. Although differentiation of from neoplasm is difficult, a ring-like distribution of abnormal radiopharmaceutical uptake in the gallbladder has been observed in cases of acute and chronic cholecystitis [58,59]. Hepatic and splenic infections including atypical etiologies from fungal and parasitic infection, i.e., amebiasis, should be considered when encountering abnormal uptake in these organs [60–63]. Non-infectious inflammatory uptake from processes such as sarcoidosis has also been described in abdominal viscera including the liver and the spleen [64]. Similarly, inflammatory 18F-FDG uptake in retroperitoneal fibrosis may be mistaken for malignancy [65]. Knowledge of the radiation treatment field is valuable as inflammatory uptake in adjacent viscera has been described including in the liver in a patient with esophageal carcinoma [66].
Although the genitourinary system is a common site for infection, differentiation of abnormal 18F-FDG uptake in the kidney from physiologic uptake can be challenging. Strategies to detect abnormal uptake include appropriate windowing of the PET images as well as review of the CT images looking for focal anatomic lesions. Renal tuberculosis, acute pyelonephritis, xanthogranulomatous pyelonephritis, and renal abscesses have all been encountered on oncologic 18F-FDG PET/CT and may be mistaken for a neoplastic process [67–70]. Gonadal infection has been mistaken for neoplasm on 18F-FDG PET/CT including tubo-ovarian abscesses and orchitis [71–73].
4.4. Miscellaneous
Musculoskeletal processes may lead to focal uptake on 18F-FDG PET/CT. Incidental inflammatory uptake from degenerative osteoarthritis is ubiquitous in oncologic 18F-FDG PET imaging. However, more acute infectious processes requiring management such as discitis, osteomyelitis and septic arthritis can also demonstrate focal radiopharmaceutical uptake and may be mistaken for metastases (Figs. 8–10) [74,75]. Focal 18F-FDG uptake in the muscles related to myositis may also be mistaken for sites of malignancy [76,77].
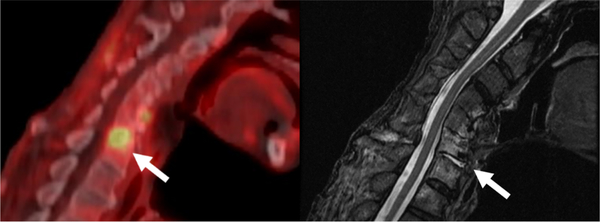
Fig. 8.
67-year-old Male with Focal 18F-FDG uptake correlating to Cervical Discitis/Osteomyelitis.
A, Fused sagittal 18F-FDG PET/CT demonstrates focal uptake at C6-C7 (arrow) concerning for infection.
B, Sagittal T2 weighted images demonstrates focal edema/fluid in the C6-C7 disc space and adjacent vertebral bodies (arrows) concerning for discitis/osteomyelitis. Patient’s spinal infection was determined to be a complication of his treatment for his head and neck cancer.
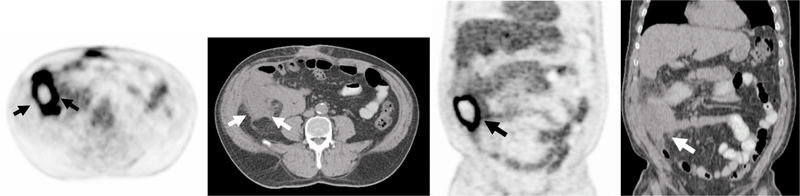
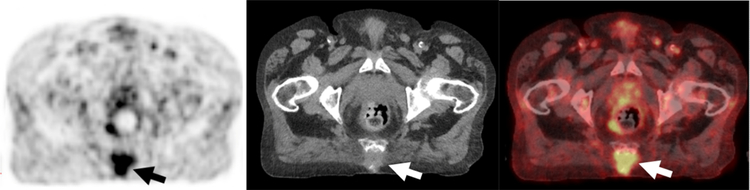
Fig. 10.
Hypermetabolic Septic Arthritis Incidentally Detected in on 18F-FDG PET/CT performed for a Solitary Pulmonary Nodule Evaluation.
A, B, Axial 18F-FDG PET and fused PET/CT demonstrates curvilinear uptake in the right sacroiliac joint and surrounding soft tissues (arrows) corresponding to a septic joint with a sinus tract. The patient had a history of multiple pelvic surgeries due to prior trauma.
Cutaneous and subcutaneous 18F-FDG uptake can be encountered in a variety of infectious and inflammatory processes including common processes such as sebaceous cysts, acne vulgaris and insect bites to less common entities such as hidradenitis suppurativa (Fig. 11) [78,79]. Granulomas in the subcutaneous soft tissues and muscles are commonly seen with iatrogenic injections. Fortunately, direct inspection of the skin is easily done clinically and can assist to determining the etiology of abnormal unexpected uptake. Similarly, knowledge of recent procedures is useful as postoperative inflammation, infections, and/or other complications may be encountered on subsequent 18F-FDG PET/CT and may result in abnormal uptake (Fig. 12) [80].
Fig. 11.
72-year-old male with Lung Cancer with Incidental Focal Uptake in the Neck.
A, B, Sagittal CT and fused 18F-FDG PET/CT of the neck demonstrates a small subcutaneous hypermetabolic nodule in the posterior soft tissues of the neck (arrow). Clinically this site corresponded to a furuncle (boil) with adjacent skin erythema.
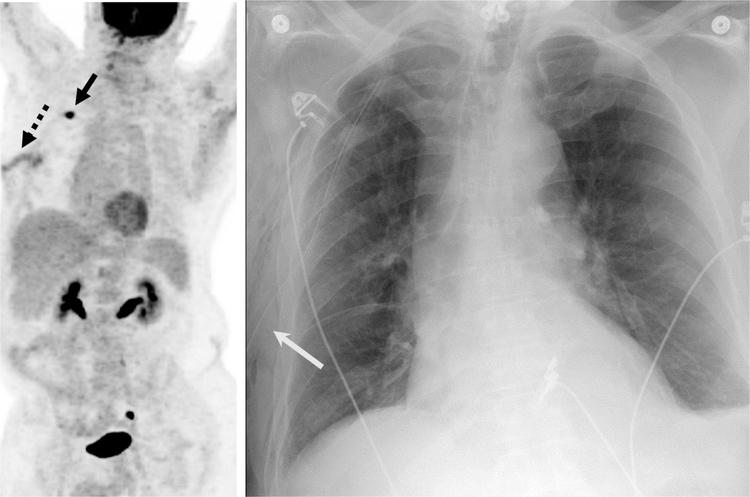
Fig. 12.
67-year-old man with a Recent Diagnosis of Lung Carcinoma.
A, Maximum intensity projection image from an 18F-FDG PET/CT demonstrates a hypermetabolic right upper lobe mass (solid black arrow) with an additional linear focus of uptake in the subcutaneous tissues of the right chest wall (dashed arrow) that was inflammatory due to a right thoracotomy tube placed due to a post-biopsy pneumothorax.
B, Frontal chest radiograph demonstrates the location of the right thoracotomy tube (solid white arrow) confirming the inflammatory etiology of the FDG uptake.
5. F-FDG for infection localization
Given this often-encountered incidental infectious and inflammatory uptake in 18F-FDG PET/CT, there is a potential clinical use for 18F-FDG for infection imaging as an alternative to traditional radiolabeled leukocyte imaging with either 111In or 99mTc. Potential advantages of 18F-FDG for localization of infection include high sensitivity, relatively shorter imaging times and higher spatial resolution compared to traditional leukocyte imaging [5]. Modern PET acquisition is typically accompanied with whole-body CT correlation, which can assist in interpretation. Furthermore, 18F-FDG imaging does not require radiolabeling white blood cells, which alleviates potential risks that accompany the handling blood products [81]. Limitations of 18F-FDG PET/CT include clinical availability (lack of reimbursement in the U.S.), imperfect specificity of FDG uptake, as well as a wide variety of variants of physiologic FDG uptake that might confound interpretation. Similar to radiolabeled leukocytes, 18F-FDG PET/CT cannot distinguish infection from inflammation.
Although infection localization is not an FDA approved indication for 18F-FDG PET/CT in the United States, national societies have provided practice guidelines. In 2013, the Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the European Association of Nuclear Medicine (EANM) published a joint practice guideline regarding the utilization of 18F-FDG PET/CT for infection and inflammation detection including clinical indications and technical protocols [17]. These societies identified the major clinical indications for 18F-FDG infection localization including peripheral osteomyelitis (non-diabetic and non-postoperative foot), spinal infection, fever of unknown origin, metastatic infection, and bacteremia in high-risk patients.
6. Conclusion
18F-FDG PET/CT continues to be a frequently performed imaging study in oncologic imaging. Accurate staging requires the interpreting physician to be familiar with non-oncologic causes of focal uptake, which may be mistaken of foci of malignancy and strategies to avoid such errors. As 18F-FDG appears to be an excellent detector of infection, the use of 18F-FDG PET/CT to detect and localize sites of infection will likely increase in the future.
Fig. 9.
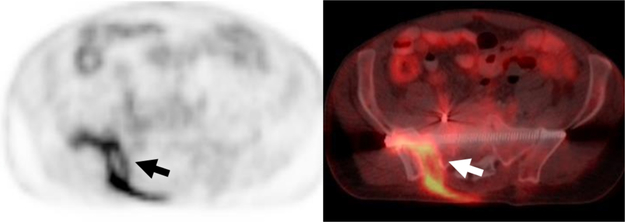
72-year-old Man with Sacral Decubitus Ulcer.
A-C, Axial PET, CT and fused from an 18F-FDG PET/CT demonstrates focal uptake within an ulcer with extension to the involve the sacrum consistent with infected sacral decubitus ulcer and sacral osteomyelitis.
Acknowledgement
Original artwork for Fig. 1 is by Danielle Dobbs, Medical Illustrator, Department of Radiology, University of Michigan
Funding
This article did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. DMT is supported by the COBRE-Redox Signaling at MUSC.
References
- [1].Safaie E, Matthews R, Bergamaschi R, PET scan findings can be false positive, Tech. Coloproctol 19 (6) (2015) 329–330. [DOI] [PubMed] [Google Scholar]
- [2].Annibaldi A, Widmann C, Glucose metabolism in cancer cells, Curr. Opin. Clin. Nutr. Metab. Care 13 (4) (2010) 466–470. [DOI] [PubMed] [Google Scholar]
- [3].Gallagher BM, Fowler JS, Gutterson NI, MacGregor RR, Wan CN, Wolf AP, Metabolic trapping as a principle of oradiopharmaceutical design: some factors resposible for the biodistribution of [18F] 2-deoxy-2-fluoro-D-glucose, J. Nucl. Med 19 (10) (1978) 1154–1161. [PubMed] [Google Scholar]
- [4].Smith TA, FDG uptake, tumour characteristics and response to therapy: a review, Nucl. Med. Commun 19 (2) (1998) 97–105. [DOI] [PubMed] [Google Scholar]
- [5].Vaidyanathan S, Patel CN, Scarsbrook AF, Chowdhury FU, FDG PET/CT in infection and inflammation–current and emerging clinical applications, Clin. Radiol 70 (7) (2015) 787–800. [DOI] [PubMed] [Google Scholar]
- [6].Love C, Tomas MB, Tronco GG, Palestro CJ, FDG PET of infection and inflammation, Radiographics 25 (5) (2005) 1357–1368. [DOI] [PubMed] [Google Scholar]
- [7].Mochizuki T, Tsukamoto E, Kuge Y, Kanegae K, Zhao S, Hikosaka K, Hosokawa M, Kohanawa M, Tamaki N, FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models, J. Nucl. Med 42 (10) (2001) 1551–1555. [PubMed] [Google Scholar]
- [8].Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T, Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography, J. Nucl. Med 33 (11) (1992) 1972–1980. [PubMed] [Google Scholar]
- [9].Wang G, Lau EW, Shakher R, Rischin D, Ware RE, Hong E, Binns DS, Hogg A, Drummond E, Hicks RJ, How do oncologists deal with incidental abnormalities on whole-body fluorine-18 fluorodeoxyglucose PET/CT? Cancer 109 (1) (2007) 117–124. [DOI] [PubMed] [Google Scholar]
- [10].Metser U, Miller E, Lerman H, Even-Sapir E, Benign nonphysiologic lesions with increased 18F-FDG uptake on PET/CT: characterization and incidence, AJR Am. J. Roentgenol 189 (5) (2007) 1203–1210. [DOI] [PubMed] [Google Scholar]
- [11].Wong PS, Lau WF, Worth LJ, Thursky KA, Drummond E, Slavin MA, Hicks RJ, Clinically important detection of infection as an’ incidental’ finding during cancer staging using FDG-PET/CT, Intern. Med. J 42 (2) (2012) 176–183. [DOI] [PubMed] [Google Scholar]
- [12].Bakheet SM, Powe J, Benign causes of 18-FDG uptake on whole body imaging, Semin. Nucl. Med 28 (4) (1998) 352–358. [DOI] [PubMed] [Google Scholar]
- [13].Keyes JW Jr, SUV: standard uptake or silly useless value? J. Nucl. Med 36 (10) (1995) 1836–1839. [PubMed] [Google Scholar]
- [14].Zhuang H, Pourdehnad M, Lambright ES, Yamamoto AJ, Lanuti M, Li P, Mozley PD, Rossman MD, Albelda SM, Alavi A, Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes, J. Nucl. Med 42 (9) (2001) 1412–1417. [PubMed] [Google Scholar]
- [15].Schillaci O, Use of dual-point fluorodeoxyglucose imaging to enhance sensitivity and specificity, Semin. Nucl. Med 42 (4) (2012) 267–280. [DOI] [PubMed] [Google Scholar]
- [16].Lee ST, Scott AM, Are we ready for dual-time point FDG PET imaging? J. Med. Imaging Radiat. Oncol 55 (4) (2011) 351–352. [DOI] [PubMed] [Google Scholar]
- [17].Jamar F, Buscombe J, Chiti A, Christian PE, Delbeke D, Donohoe KJ, Israel O, Martin-Comin J, Signore A, EANM/SNMMI guideline for 18F-FDG use in inflammation and infection, J. Nucl. Med 54 (4) (2013) 647–658. [DOI] [PubMed] [Google Scholar]
- [18].Kashyap R, Chakravarthy R, Reddy K, Ali MA, Buddharaju LN, Muntimadugu B, Metabolic signature” of the lesions on F18-fluoro-deoxyglucose positron emission tomography: a clue to the underlying pathology, Indian J. Nucl. Med 29 (3) (2014) 193–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Manoharan P, Cronin PP, Brown RK, Metastatic head and neck Cancer Conflated with pulmonary embolism and infarct on PET/CT, Radiol. Case Rep 2 (3) (2007) 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hofman MS, Hicks RJ, How we read oncologic FDG PET/CT, Cancer Imaging 16 (1) (2016) 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Britt CJ, Maas AM, Kennedy TA, Hartig GK, Incidental findings on FDG PET/CT in head and neck Cancer, Otolaryngol. Head. Neck Surg 158 (3) (2018) 484–488. [DOI] [PubMed] [Google Scholar]
- [22].Purohit BS, Ailianou A, Dulguerov N, Becker CD, Ratib O, Becker M, FDG-PET/CT pitfalls in oncological head and neck imaging, Insights Imaging 5 (5) (2014) 585–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Blodgett TM, Fukui MB, Snyderman CH, Ft B. Branstetter, McCook BM, Townsend DW, Meltzer CC, Combined PET-CT in the head and neck: part 1. Physiologic, altered physiologic, and artifactual FDG uptake, Radiographics 25 (4) (2005) 897–912. [DOI] [PubMed] [Google Scholar]
- [24].Liu Y, Chronic sialadenitis with marked lymphadenopathy mimicking lymphoma on FDG PET/CT, Clin. Nucl. Med 39 (8) (2014) 738–739. [DOI] [PubMed] [Google Scholar]
- [25].Jadvar H, Bonyadlou S, Iagaru A, Colletti PM, FDG PET-CT demonstration of Sjogren’s sialoadenitis, Clin. Nucl. Med 30 (10) (2005) 698–699. [DOI] [PubMed] [Google Scholar]
- [26].Yasuda S, Shohtsu A, Ide M, Takagi S, Takahashi W, Suzuki Y, Horiuchi M, Chronic thyroiditis: diffuse uptake of FDG at PET, Radiology 207 (3) (1998) 775–778. [DOI] [PubMed] [Google Scholar]
- [27].Yasuda S, Shohtsu A, Ide M, Takagi S, Kijima H, Horiuchi M, Elevated F-18 FDG uptake in plasmacyte-rich chronic maxillary sinusitis, Clin. Nucl. Med 23 (3) (1998) 176–178. [DOI] [PubMed] [Google Scholar]
- [28].Mascarenhas NB, Lam D, Lynch GR, Fisher RE, PET imaging of cerebral and pulmonary Nocardia infection, Clin. Nucl. Med 31 (3) (2006) 131–133. [DOI] [PubMed] [Google Scholar]
- [29].Meyer MA, Hubner KF, Raja S, Hunter K, Paulsen WA, Sequential positron emission tomographic evaluations of brain metabolism in acute herpes encephalitis, J. Neuroimaging 4 (2) (1994) 104–105. [DOI] [PubMed] [Google Scholar]
- [30].Kapucu LO, Meltzer CC, Townsend DW, Keenan RJ, Luketich JD, Fluorine-18-fluorodeoxyglucose uptake in pneumonia, J. Nucl. Med 39 (7) (1998) 1267–1269. [PubMed] [Google Scholar]
- [31].Kono M, Yamashita H, Kubota K, Kano T, Mimori A, FDG PET imaging in pneumocystis pneumonia, Clin. Nucl. Med 40 (8) (2015) 679–681. [DOI] [PubMed] [Google Scholar]
- [32].Widmann G, Nguyen VA, Plaickner J, Jaschke W, Imaging features of toxicities by immune checkpoint inhibitors in cancer therapy, Curr. Radiol. Rep 5 (11) (2016) 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Raad RA, Kannan R, Madden K, Pavlick A, Ipilimumab-induced organizing pneumonia on 18F-FDG PET/CT in a patient with malignant melanoma, Clin. Nucl. Med 42 (7) (2017) e345–e346. [DOI] [PubMed] [Google Scholar]
- [34].Kwak JJ, Tirumani SH, Van den Abbeele AD, Koo PJ, Jacene HA, Cancer immunotherapy: imaging assessment of novel treatment response patterns and immune-related adverse events, Radiographics 35 (2) (2015) 424–437. [DOI] [PubMed] [Google Scholar]
- [35].Baha A, Yildirim F, Kokturk N, Akdemir UO, Demircan S, Turktas H, 18 F-FDG uptake in focal organising pneumonia mimicking bronchial carcinoma, Clin. Respir. J 10 (6) (2016) 740–745. [DOI] [PubMed] [Google Scholar]
- [36].Lewis PJ, Salama A, Uptake of fluorine-18-fluorodeoxyglucose in sarcoidosis, J. Nucl. Med 35 (10) (1994) 1647–1649. [PubMed] [Google Scholar]
- [37].Zivin S, David O, Lu Y, Sarcoidosis mimicking metastatic breast cancer on FDG PET/CT, Intern. Med 53 (21) (2014) 2555–2556. [DOI] [PubMed] [Google Scholar]
- [38].Prabhakar HB, Rabinowitz CB, Gibbons FK, O’Donnell WJ, Shepard JA, Aquino SL, Imaging features of sarcoidosis on MDCT, FDG PET, and PET/CT, AJR Am. J. Roentgenol 190 (3 Suppl) (2008) S1–6. [DOI] [PubMed] [Google Scholar]
- [39].Makis W, Palayew M, Rush C, Probst S, Disseminated multi-system sarcoidosis mimicking metastases on (18)F-FDG PET/CT, Mol. Imaging Radionucl. Ther 27 (2) (2018) 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Prabhakar HB, Sahani DV, Fischman AJ, Mueller PR, Blake MA, Bowel hot spots at PET-CT, Radiographics 27 (1) (2007) 145–159. [DOI] [PubMed] [Google Scholar]
- [41].Takalkar AM, Bruno GL, Makanjoula AJ, El-Haddad G, Lilien DL, Payne DK, A Potential Role for F-18 FDG PET/CT in Evaluation and Management of Fibrosing Mediastinitis, Clin. Nucl. Med 32 (9) (2007) 703–706. [DOI] [PubMed] [Google Scholar]
- [42].Adejolu M, Huo L, Rohren E, Santiago L, Yang WT, False-positive lesions mimicking breast cancer on FDG PET and PET/CT, AJR Am. J. Roentgenol 198 (3) (2012) W304–14. [DOI] [PubMed] [Google Scholar]
- [43].Maurer AH, Burshteyn M, Adler LP, Steiner RM, How to differentiate benign versus malignant cardiac and paracardiac 18F FDG uptake at oncologic PET/CT, Radiographics 31 (5) (2011) 1287–1305. [DOI] [PubMed] [Google Scholar]
- [44].Betancourt Cuellar SL, Palacio D, Benveniste MF, Carter BW, Gladish G, Pitfalls and misinterpretations of cardiac findings on PET/CT imaging: a careful look at the heart in oncology patients, Curr. Probl. Diagn. Radiol (2018). [DOI] [PubMed] [Google Scholar]
- [45].Lobert P, Brown RK, Dvorak RA, Corbett JR, Kazerooni EA, Wong KK, Spectrum of physiological and pathological cardiac and pericardial uptake of FDG in oncology PET-CT, Clin. Radiol 68 (1) (2013) e59–71. [DOI] [PubMed] [Google Scholar]
- [46].Bachmeyer C, Kerrou K, Chosidow O, Frances C, Montravers F, 18-F fluorodeoxyglucose positron emission tomography indicating unsuspected infections in two patients with dermatomyositis, Clin. Exp. Dermatol 34 (8) (2009) e769–71. [DOI] [PubMed] [Google Scholar]
- [47].Hannah A, Scott AM, Akhurst T, Berlangieri S, Bishop J, McKay WJ, Abnormal colonic accumulation of fluorine-18-FDG in pseudomembranous colitis, J. Nucl. Med 37 (10) (1996) 1683–1685. [PubMed] [Google Scholar]
- [48].Kim BW, Kim HW, Won KS, Kang YN, Choi BW, Colonic parasitic infection mimicking peritoneal seeding on 18F-FDG PET/CT, Clin. Nucl. Med 42 (8) (2017) e365–e366. [DOI] [PubMed] [Google Scholar]
- [49].Koff SG, Sterbis JR, Davison JM, Montilla-Soler JL, A unique presentation of appendicitis: F-18 FDG PET/CT, Clin. Nucl. Med 31 (11) (2006) 704–706. [DOI] [PubMed] [Google Scholar]
- [50].Rayamajhi SJ, Gorla AKR, Basher RK, Sood A, Mittal BR, Unsuspected active ulcerative colitis in a patient with dermatomyositis: a rare association detected on (18)F-FDG PET/CT during the search for an occult malignancy, Indian J. Nucl. Med 32 (2) (2017) 130–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Vaz SC, Oliveira C, Castanheira JC, Silva AF, Costa DC, 18F-FDG uptake in ischemic colitis during follow-up of a patient with lung cancer, Clin. Nucl. Med 42 (8) (2017) e367–e370. [DOI] [PubMed] [Google Scholar]
- [52].Gontier E, Fourme E, Wartski M, Blondet C, Bonardel G, Le Stanc E, Mantzarides M, Foehrenbach H, Pecking AP, Alberini JL, High and typical 18F-FDG bowel uptake in patients treated with metformin, Eur. J. Nucl. Med. Mol. Imaging 35 (1) (2008) 95–99. [DOI] [PubMed] [Google Scholar]
- [53].Viglianti BL, Wong KK, Gross MD, Wale DJ, Common pitfalls in oncologic FDG PET/CT imaging, J. Am. Osteopath. Coll. Radiol 7 (1) (2018) 5–17. [Google Scholar]
- [54].Hsu WL, Chang SM, Wu PY, Chang CC, Localized autoimmune pancreatitis mimicking pancreatic cancer: case report and literature review, J. Int. Med. Res 46 (4) (2018) 1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zheng L, Xing H, Li F, Huo L, Focal autoimmune pancreatitis mimicking pancreatic cancer on FDG PET/CT imaging, Clin. Nucl. Med 43 (1) (2018) 57–59. [DOI] [PubMed] [Google Scholar]
- [56].Dong A, Dong H, Zhang L, Zuo C, Hypermetabolic lesions of the pancreas on FDG PET/CT, Clin. Nucl. Med 38 (9) (2013) e354–66. [DOI] [PubMed] [Google Scholar]
- [57].Hennessy G, Vamadevan S, Loh H, Bui C, Le K, Mansberg R, Pancreatic cancer complicated by pancreatitis demonstrated on FDG PET/CT, Clin. Nucl. Med 42 (3) (2017) 239–240. [DOI] [PubMed] [Google Scholar]
- [58].Kitazono MT, Colletti PM, FDG PET imaging of acute cholecystitis, Clin. Nucl. Med 31 (1) (2006) 23–24. [DOI] [PubMed] [Google Scholar]
- [59].Yu JQ, Kung JW, Potenta S, Xiu Y, Alavi A, Zhuang H, Chronic cholecystitis detected by FDG-PET, Clin. Nucl. Med 29 (8) (2004) 496–497. [DOI] [PubMed] [Google Scholar]
- [60].Albano D, Bosio G, Bertoli M, Petrilli G, Bertagna F, Hepatosplenic Candidiasis Detected by (18)F-FDG-PET/CT, Asia Ocean. J. Nucl. Med. Biol 4 (2) (2016) 106–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cavailloles FA, Mure A, Nasser H, Lecapitaine AL, Granier F, Multiple liver amoebic abscesses detected on FDG PET/CT, Clin. Nucl. Med 39 (1) (2014) 79–80. [DOI] [PubMed] [Google Scholar]
- [62].Gibson GM, Arnold C, Ravi Kumar AS, 18F-FDG uptake in multiple splenic foci on PET/CT: an unusual case of visceral leishmaniasis, Clin. Nucl. Med 39 (9) (2014) 828–830. [DOI] [PubMed] [Google Scholar]
- [63].Zhou W, Zhao J, Xing Y, Chen X, Song J, Diffuse hepatic amebiasis detected by FDG PET/CT, Clin. Nucl. Med 40 (2) (2015) e167–70. [DOI] [PubMed] [Google Scholar]
- [64].Sobic-Saranovic D, Artiko V, Obradovic V, FDG PET imaging in sarcoidosis, Semin. Nucl. Med 43 (6) (2013) 404–411. [DOI] [PubMed] [Google Scholar]
- [65].Agrawal A, Nair N, Baghel N, F-18 FDG PET in Ormond disease in a patient with renal cell carcinoma, Clin. Nucl. Med 32 (4) (2007) 320–322. [DOI] [PubMed] [Google Scholar]
- [66].Demey K, Van Veer H, Nafteux P, Deroose CM, Haustermans K, Coolen J, Vandecaveye V, Coosemans W, Van Cutsem E, Hepatic radiation injury mimicking metastasis in distal esophageal cancer, Acta Chir. Belg 117 (4) (2017) 250–255. [DOI] [PubMed] [Google Scholar]
- [67].Cheng G, Torigian DA, Alavi A, FDG PET/CT and MRI findings in a patient with focal xanthogranulomatous pyelonephritis mimicking cystic renal malignancy, Clin. Nephrol 76 (6) (2011) 484–486. [DOI] [PubMed] [Google Scholar]
- [68].Jung JS, Lee SM, Kim HJ, Jang SH, Lee JW, A case of septic pulmonary embolism associated with renal abscess mimicking pulmonary metastases of renal malignancy, Ann. Nucl. Med 28 (4) (2014) 381–385. [DOI] [PubMed] [Google Scholar]
- [69].McCammack KC, Hawkes NC, Silverman ED, Paz DA, PET/CT appearance of acute pyelonephritis, Clin. Nucl. Med 38 (7) (2013) e299–301. [DOI] [PubMed] [Google Scholar]
- [70].Subramanyam P, Palaniswamy SS, Dual Time Point (18)F-FDG PET/CT Imaging Identifies Bilateral Renal Tuberculosis in an Immunocompromised Patient with an Unknown Primary Malignancy, Infect. Chemother 47 (2) (2015) 117–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kim YI, Kim SK, Lee JW, Lee SM, Kim TS, Ovarian mass mimicking malignancy: a case report, Nucl. Med. Mol. Imaging 44 (4) (2010) 290–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mansberg R, Ho B, Bui C, False Positive FDG PET/CT of Recurrent Testicular Tumour Due to Orchitis, Mol. Imaging Radionucl. Ther 23 (1) (2014) 28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Rakheja R, Makis W, Hickeson M, Bilateral Tubo-Ovarian Abscess Mimics Ovarian Cancer on MRI and (18)F-FDG PET/CT, Nucl. Med. Mol. Imaging 45 (3) (2011) 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Saad Aldin E, Sekar P, Saad Eddin Z, Keller J, Pollard J, Incidental diagnosis of sternoclavicular septic arthritis with Moraxella nonliquefaciens, IDCases 12 (2018) 44–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zaha H, Onomura M, Nishikuramori Y, Pyogenic vertebral osteomyelitis in a breast cancer patient: report of a case, Surg. Today 42 (10) (2012) 1022–1025. [DOI] [PubMed] [Google Scholar]
- [76].Chen SJ, Wang XY, Hua FC, Guan YH, Detection of multiple muscle involvement in eosinophilic myositis with (1)(8)F-FDG PET/CT, Eur. J. Nucl. Med. Mol. Imaging 40 (8) (2013) 1297. [DOI] [PubMed] [Google Scholar]
- [77].Bennett O, Ravi Kumar AS, Agnew J, Focal inflammatory myositis on 18F-FDG PET/CT, Clin. Nucl. Med 41 (6) (2016) 469–471. [DOI] [PubMed] [Google Scholar]
- [78].Metser U, Tau N, Benign cutaneous and subcutaneous lesions on FDG-PET/CT, Semin. Nucl. Med 47 (4) (2017) 352–361. [DOI] [PubMed] [Google Scholar]
- [79].Asamoah P, Wale DJ, Viglianti BL, Wong KK, Gross M, Multiple Hypermetabolic Subcutaneous Lesions From Hidradenitis Suppurativa Mimicking Metastases on 18F-FDG PET/CT, Clin. Nucl. Med 43 (1) (2018) 73–74. [DOI] [PubMed] [Google Scholar]
- [80].Garg G, Benchekroun MT, Abraham T, FDG-PET/CT in the postoperative period: utility, expected findings, complications, and pitfalls, Semin. Nucl. Med 47 (6) (2017) 579–594. [DOI] [PubMed] [Google Scholar]
- [81].Rojas-Burke J, Health officials reacting to infection mishaps, J. Nucl. Med 33 (4) (1992) 13N–14N 27N. [PubMed] [Google Scholar]