Abstract
This study was conducted to investigate the effects of dietary supplementation with 2 sources of fiber, sugar beet pulp (SBP), and wheat bran (WB), on sow performance, milk quality, and intestinal health in piglets. Forty-five multiparous sows at day 85 of gestation were allocated to the following 3 treatments: 1) a corn-soybean meal basal diet (CON); 2) the CON diet supplemented with 20% SBP in gestation and 10% SBP in lactation (SBP); and 3) the CON diet supplemented with 30% WB in gestation and 15% WB in lactation (WB). The SBP diets increased (P < 0.05) sow ADFI during lactation, litter and piglet weaning weight, piglet ADG, immunoglobulin A (IgA), and interleukin-10 (IL-10) levels in the colostrum and IgA levels in the milk, while the WB diets only increased (P < 0.05) IL-10 levels in the milk when compared with the CON diets. Piglets from SBP-fed sows had greater (P < 0.05) serum growth hormone and insulin-like growth factor-1 levels than those from WB-fed or CON-fed sows, whereas piglets from WB-fed sows had greater (P < 0.05) serum GH levels than those from CON-fed sows. Serum diamine oxidase activity, endotoxin, IL-6, and tumor necrosis factor-α (TNF-α) levels were reduced (P < 0.05) in piglets from SBP-fed or WB-fed sows. Piglets from SBP-fed sows also had greater (P < 0.05) serum IL-10 levels than those from CON-fed sows. The ileal mRNA expression of TNF-α was reduced (P < 0.05) in piglets from SBP-fed or WB-fed sows. Piglets from SBP-fed sows had lower (P < 0.05) IL-6 expression, and greater (P < 0.05) IL-10 expression and secretory immunoglobulin A (SIgA) levels in the ileum than those from WB- or CON-fed sows. Piglets from WB-fed sows had greater (P < 0.05) IL-10 expression and SIgA levels compared with those from CON-fed sows. The ileal mRNA expression of occludin in the ileum was greater (P < 0.05) in piglets from SBP-fed sows than those from CON-fed sows. The ileal mRNA expression of ZO-1 was greater (P < 0.05) in piglets from WB-fed sows than those from CON-fed sows, but lower (P < 0.05) than those from SBP-fed sows. Piglets from SBP-fed sows had greater (P < 0.05) abundance of Christensenellaceae and butyrate levels in the colon, while piglets from WB-fed sows had greater (P < 0.05) abundance of Lactobacillaceae. Collectively, maternal SBP supplementation was more effective than WB in improving milk quality, enhancing growth performance and intestinal barrier function, and ameliorating intestinal inflammation in piglets.
Keywords: fiber source, intestinal health, milk quality, performance, piglet
Introduction
Prenatal and early postnatal life is a critical period for the development of the intestinal microbiota and immune system (Le Bourgot et al., 2014). During this period, maternal nutrition plays a vital role in offspring growth and immune system development (Macpherson et al., 2017). After birth, the offspring immunity mainly relies upon the maternal colostrum and milk because they contain a variety of immunoglobulins and cytokines that can stimulate the maturation of the immune system (Dzidic et al., 2018). In addition, maternal microbiota influences offspring gut colonization through direct contact with maternal microbiota during birth and through breast milk during lactation, which contributes to immune system development and other long-term health consequences in offspring (Thum et al., 2012; Gomez de Agüero et al., 2016). As maternal colostrum and milk quality and microbiota composition are influenced by diets, maternal dietary manipulation thus may be an effective way to improve offspring immunity and growth.
More recently, supplementation of probiotics and prebiotics to the maternal diet during gestation and lactation has been shown to improve the quality of colostrum and milk, modulate the gut microbiota, and stimulate the development of intestinal immunity in offspring (Le Bourgot et al., 2014; Laskowska et al., 2019). Dietary fiber (DF) is carbohydrate polymers that escape digestion in the small intestine and are partially or fully fermented in the large intestine (Rebello et al., 2016). It has been shown to have beneficial effects on gut microbiota, barrier function, and immunity in weaned and growing pigs (Wu et al., 2018; Jha et al., 2019). Previous studies focused largely on the effects of maternal DF diets on sow welfare, colostrum production, physiology, and performance (Quesnel et al., 2009; Jensen et al., 2012; Loisel et al., 2013). However, little information is available about the effects of maternal DF diets on the colostrum and milk quality and offspring immunity that are key to the growth and health of offspring. In addition, the physiological effects of DF vary greatly due to their different characteristics (Chen et al., 2013; Mudgil and Barak, 2013). Insoluble dietary fiber (IDF) is generally slowly fermented by gut bacteria, and increases gut transit rate and provides bulk to the diet, while soluble dietary fiber (SDF) is prone to bacterial fermentation resulting in lots of metabolites such as short chain fatty acids (SCFA) and increases digesta viscosity (Jarrett and Ashworth, 2018).
Wheat bran (WB) is a source of insoluble fiber, and rich in arabinoxylan and cellulose (Onipe et al., 2015). Previous studies have shown that WB altered intestinal microbiota composition, increased butyrate production, and improved intestinal barrier function in pigs (Chen et al., 2017; Zhao et al., 2018). Sugar beet pulp (SBP) contains high level of soluble fiber such as pectins and glucans, which is highly fermented in the hindgut (Wang et al., 2016). It also has been shown to have prebiotic potential to modulate the microbiota that can be beneficial for host health (Leijdekkers et al., 2014; Gómez et al., 2016). We hypothesized that WB and SBP could act as different prebiotics and have positive impact on milk quality, piglets’ immunity, microbiota, and growth. Therefore, the present study was conducted to evaluate the effects of WB and SBP in maternal diets on sow performance, milk quality, immunity, barrier function, and microbiota in piglets.
Materials and Methods
All animals used in this study were cared for strictly in accordance with the Chinese Guidelines for Animal Welfare and all procedures were approved by the China Agricultural University Institutional Animal Care and Use Committee (Beijing, China).
Animals, Diets, and Experimental Designs
A total of 45 multiparous sows (Yorkshire × Landrace; 3 to 6 of parity; average parity: 3.29; initial BW: 232.3 ± 16.5 kg) were randomly allocated to 3 treatment groups based on parity, backfat thickness, and body weight from day 85 of gestation until weaning. The 3 treatments included: 1) a corn-soybean meal basal diet with no additional fiber sources (CON, n = 15); 2) a corn-soybean meal basal diet supplemented with 20% sugar beet pulp in gestation and 10% sugar beet pulp in lactation (SBP, n = 15); and 3) a corn-soybean meal basal diet supplemented with 30% wheat bran in gestation and 15% wheat bran in lactation (WB, n = 15). The SBP and WB diets contained the same content of total dietary fiber. All diets were formulated to meet or exceed the nutrients requirements of gestating and lactating sows as recommended by the NRC (2012). The ingredients and compositions of the gestation and lactation diets are provided in Table 1.
Table 1.
Ingredients composition and nutrient levels of the experimental diets (%, as-fed basis)
| Item | Gestation | Lactation | ||||
|---|---|---|---|---|---|---|
| CON | SBP | WB | CON | SBP | WB | |
| Corn | 73.65 | 54.55 | 50.80 | 69.74 | 59.05 | 54.98 |
| Soybean meal | 22.00 | 21.50 | 15.00 | 26.00 | 26.00 | 23.00 |
| Wheat bran1 | 0.00 | 0.00 | 30.00 | 0.00 | 0.00 | 15.00 |
| Sugar beet pulp2 | 0.00 | 20.00 | 0.00 | 0.00 | 10.00 | 0.00 |
| Soybean oil | 0.85 | 0.85 | 0.85 | 0.65 | 1.53 | 3.50 |
| Dicalcium phosphate | 1.28 | 1.35 | 0.57 | 1.45 | 1.50 | 1.10 |
| Limestone | 1.07 | 0.60 | 1.48 | 0.78 | 0.54 | 0.97 |
| Salt | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| L-Lysine HCl | - | - | 0.15 | - | - | 0.06 |
| Valine | - | - | - | 0.23 | 0.23 | 0.24 |
| Premix3 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Chromium oxide | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Calculated composition | ||||||
| DE Kal/kg | 3353 | 3259 | 3037 | 3388 | 3388 | 3388 |
| Available P | 0.31 | 0.31 | 0.31 | 0.34 | 0.34 | 0.34 |
| SID lysine | 0.69 | 0.69 | 0.70 | 0.78 | 0.79 | 0.78 |
| SID methionine | 0.22 | 0.19 | 0.20 | 0.23 | 0.22 | 0.22 |
| SID threonine | 0.49 | 0.46 | 0.45 | 0.53 | 0.52 | 0.51 |
| SID tryptophan | 0.14 | 0.14 | 0.13 | 0.16 | 0.16 | 0.16 |
| SID valine | 1.05 | 1.04 | 1.02 | 0.85 | 0.85 | 0.85 |
| Analyzed composition | ||||||
| Calcium | 0.74 | 0.76 | 0.75 | 0.68 | 0.70 | 0.68 |
| Phosphorus | 0.56 | 0.55 | 0.58 | 0.60 | 0.59 | 0.62 |
| Crude protein | 15.29 | 15.41 | 15.48 | 17.21 | 17.25 | 17.21 |
| Total dietary fiber | 11.37 | 21.60 | 21.81 | 11.82 | 16.83 | 16.88 |
| Soluble dietary fiber | 1.39 | 4.06 | 1.86 | 1.43 | 2.72 | 1.70 |
| Insoluble dietary fiber | 9.98 | 17.54 | 19.95 | 10.39 | 14.11 | 15.18 |
1Analyzed composition of wheat bran (as-fed basis): DM, 89.37%; Ash, 5.82%; OM, 83.55%; CP, 17.12%; GE, 17.01 MJ/kg; NDF, 37.36%; ADF, 11.55%; TDF, 44.57%; SDF, 3.89%; IDF, 40.68%.
2Analyzed composition of sugar beet pulp (as-fed basis): DM, 91.42%; Ash, 6.79%; OM, 84.63%; CP, 10.29%; GE, 15.62 MJ/kg; NDF, 38.25%; ADF, 23.48%; TDF, 61.69%; SDF, 17.12%; IDF, 44.57%.
3Premix provided per kilogram of diet: Gestation: vitamin A, 11,000 IU; vitamin D3, 1,500 IU; vitamin E, 15 IU; vitamin K3, 1.6 mg; vitamin B1, 1.5 mg; vitamin B2, 3.0 mg; vitamin B6, 1.5 mg; vitamin B12, 0.015 mg; niacin, 22.5 mg; D-pantothenic acid, 15 mg; folic acid, 2.5 mg; biotic, 0.2 mg; Fe, 85 mg; Cu, 7.5 mg; Zn, 75 mg; Mn, 35 mg; I, 0.5 mg; Se, 0.3 mg; Lactation: vitamin A, 6,500 IU; vitamin D3, 1,550 IU; vitamin E, 15.5 IU; vitamin K3, 1.6 mg; vitamin B1, 1.6 mg; vitamin B2, 3.1 mg; vitamin B6, 1.5 mg; vitamin B12, 0.015 mg; niacin, 23 mg; D-pantothenic acid, 15.5 mg; folic acid, 2.5 mg; biotin, 0.2 mg; Fe, 85 mg; Cu, 10 mg; Zn, 100 mg; Mn, 50 mg; I, 0.5 mg; Se, 0.3 mg.
CON, control; SBP, sugar beet pulp; WB, wheat bran.
All sows were housed in individual gestation stalls (2.1 × 0.6 m) from day 85 until 106 of gestation. Sows were fed twice a day at 0800 and 1600 h. Because of the slightly lower DE in SBP and WB gestation diets, sows in CON, SBP, and WB groups received 3.00, 3.09, and 3.31 kg/d of diets, respectively to achieve the same DE intake. On day 107 of gestation, the sows were moved into the farrowing rooms with environment controlled systems and housed in individual farrowing crates (2.1 × 1.5 m). Room temperature was maintained at 21.6 °C. On the parturition day, sows were fed 0.5 kg and the ration was gradually increased by 1.0 kg/d until ad libitum feeding. Sows had free access to water throughout the entire experimental period. Within 24 h after farrowing, the litter size was standardized to approximately 11 piglets by cross-fostering within treatment. Piglets were weaned at 21 d of lactation and had no access to creep feed. During lactation, feed intake of each sow was recorded to calculate ADFI. At farrowing, the number of total born piglets, piglets born alive and stillborn piglets were recorded. And again at weaning, the number of live piglets was recorded and preweaning mortality was calculated. The individual piglet weight was recorded at birth and at weaning to calculate ADG.
Sample Collection
Immediately after parturition, approximately 30 mL of colostrum samples were collected manually from all functional teats (6 sows per treatment), and 21 d later approximately 30 mL of milk samples were also collected after injection of 2 mL oxytocin. The colostrum and milk samples were immediately frozen at −80 °C for further analysis.
On day 21 of lactation, blood samples were obtained from 6 piglets per treatment (the 6 piglets were from the same 6 sows for milk collection, 1 piglet from each litter close to the mean BW for that litter was selected). Blood for serum analysis were collected into evacuated tubes containing clot activator (Greiner Bio-One GmbH, Kremsmunster, Austria). Then, serum was isolated by centrifugation at 3,000 × g at 4 °C for 15 min, and frozen at −80 °C until subsequent analysis.
On day 21 of lactation, the same 6 piglets per treatment were euthanized humanely. The entire intestine was removed from the abdominal cavity, and ligated at the junction of each section. Then 3-cm segments were collected from the middle duodenum, jejunum, and ileum, and fixed in 10% phosphate-buffered formalin for morphological evaluation. Mucosal samples from the ileum were scraped, rapidly frozen in liquid nitrogen, and stored at −80 °C for further analysis. The digesta samples from the proximal colon were collected in sterile tubes, snap-frozen in liquid nitrogen, and stored at −80 °C for analysis of microbiota composition and SCFAs levels.
Chemical Analysis
Ingredients and diets were ground through a 1-mm screen, and then analyzed for DM (AOAC, 2007; method 930.15), CP (AOAC, 2007; method 976.05), and ash (AOAC, 2007; method 942.15). The NDF and ADF were determined using fiber analyzer (Ankom Technology, Macedon, NY) according to Van Soest et al. (1991). The GE was determined using an automatic adiabatic oxygen bomb calorimeter (Parr 6300 Calorimeter, Moline, IL). Total dietary fiber (TDF) and IDF were analyzed using AOAC (2007) methods 985.29 and 991.43, respectively. The SDF was calculated as the difference between TDF and IDF.
Milk and Serum Parameters Analysis
The levels of IgA, IgG, IgM, interleukin-6 (IL-6), IL-10, tumor necrosis factor-α (TNF-α) in colostrum and milk, and the levels of growth hormones, IGF-1, IgA, IgG, IgM, IL-6, IL-10, TNF-α, and diamine oxidase (DAO) activity in serum were measured by commercial ELISA kits according to the manufacturer’s instructions (Beijing Sino-uk Institute of Biological Technology, Beijing, China). Serum endotoxin levels were determined using a commercial chromogenic end point Tachypleus kit (Xiamen Limulus Amebocyte Lysate Company, Xiamen, China) according to the manufacturer’s instructions.
Secretory Immunoglobulin A Analysis
Secretory immunoglobulin A (sIgA) in ileal mucosa was measured using commercially ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Quantitative Real-Time PCR Analysis
The relative mRNA expression of key cytokines (TNF-α, IL-6, and IL-10) and tight junction proteins (occludin, claudin-1, and ZO-1) in the ileum were determined by quantitative real-time PCR (qRT-PCR). Total RNA was extracted from ileal mucosal samples using TRIZOL reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The quality and quantity of RNA was determined using a spectrophotometer (NanoDrop ND-1000; Thermo Fisher Scientific, DE). The integrity of RNA was determined by agarose gel electrophoresis. Then, the RNA was treated with DNase I (TaKaRa Biotechnology, Dalian, China) and used for reverse transcription and the polymerase chain reaction. First-strand cDNA was synthesized using reverse transcription kit (Invitrogen). Software (Oligo 6.0; Molecular Biology Insights, Cascade, CO) was used to design primers, which are listed in Table 2. Real-time PCR was performed with a volume of 10 μL containing 1 μL cDNA template, 5 μL SYBR Green mix, 0.2 μL ROX Reference Dye (50 times), and 0.2 μL each of forward and reverse primers. The thermal cycling conditions were as follows: predenaturation (10 s at 95 °C); 40 cycles of amplification (5 s at 95 °C and 20 s at 60 °C); melting curve construction (60 to 99 °C with heating rate of 0.1 °C/s and fluorescence measurements). Relative gene expression was expressed as a ratio of the target gene to the control genes using the 2–ΔΔCt method.
Table 2.
Primer sequences for real-time polymerase chain reaction1
| Gene | Primer sequence, 5′-3′ | Size, bp | Accession No. |
|---|---|---|---|
| ZO-1 | F: TCAAGGTCTGCCGAGACAAC | 140 | XM_003353439.2 |
| R: ATCACAGTGTGGTAAGCGCA | |||
| Claudin-1 | F: ACAGGAGGGAAGCCATTTTCA | 82 | NM_001244539.1 |
| R: TTTAAGGACCGCCCTCTCCC | |||
| Occludin | F: CAGGTGCACCCTCCAGATTG | 111 | NM_001163647.2 |
| R: TGGACTTTCAAGAGGCCTGG | |||
| TNF-α | F: CCAGACCAAGGTCAACCTCC | 103 | NM_214022.1 |
| R: TCCCAGGTAGATGGGTTCGT | |||
| IL-6 | F: GCTGCAGTCACAGAACGAGT | 118 | NM_001252429.1 |
| R: CAGGTGCCCCAGCTACATTA | |||
| IL-10 | F: GCATCCACTTCCCAACCA | 111 | L20001 |
| R: GCAACAAGTCGCCCATCT | |||
| GAPDH | F: GAAGGTCGGAGTGAACGGAT | 149 | AF017079 |
| R: CATGGGTAGAATCATACTGGAACA |
1ZO-1, Zonula occludens-1; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; IL-10, interleukin-10.
Small Intestinal Morphology Analysis
Intestinal samples were removed from 10% phosphate-buffered formalin, then dehydrated, cleared with xylene and embedded in paraffin wax. Serial sections (5 μm thickness) were cut with a LEICA RM2135 rotary microtome (Leica Microsystems GmbH, Wetzlar, Germany), and stained with hematoxylin and eosin. A minimum of 15 intact and well-oriented villi and their associated crypts from each segment were measured at 40× magnification with a light microscope (CK40, Olympus, Tokyo, Japan). Villus height was measured from the tip of the villi to the villus crypt junction, and crypt depth was defined as the depth of the invagination between adjacent villi (Shang et al., 2018).
Bacterial Microbiota by 16S rRNA Sequences Analysis
Bacterial DNA was isolated from colonic digesta using the DNA Kit (Omega Bio-tek, Norcross, GA) according to manufacturer’s instructions. The V3-V4 hypervariable regions of the bacterial 16S rRNA gene were amplified using primers F338 (5′-ACTCCTACGGGAGGCAGCAG-3′) and R806 (5′-GGACTACHVGGGTWTCTAAT-3′). The PCR conditions were predenaturation at 95 °C for 3 min, 27 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, elongation at 72 °C for 30 s, and final extension at 72 °C for 10 min. Amplicons were extracted from 2% agarose gels, and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA) and quantified using QuantiFluor-ST (Promega, USA). Purified amplicons were pooled in equimolar concentrations and paired-end sequenced (2 × 300) on an Illumina MiSeq platform according to the standard protocols. Raw fastq files were demultiplexed, and quality-filtered using QIIME (version 1.17). Operational taxonomic units (OTUs) were clustered with 97% similarity cutoff using UPARSE and chimeric sequences were identified and removed using UCHIME. The taxonomy of each 16S rRNA gene sequence was analyzed by RDP Classifier (http://rdp.cme.msu.edu/) against the silva (SSU128) 16S rRNA database using confidence threshold of 80%.
Short Chain Fatty Acids Analysis
Samples of colonic digesta were thawed on ice and thoroughly mixed immediately. Then, 1.0 g samples were suspended in 8 mL deionized water, and ultrasonic irradiation for 20 min, then centrifuged at 12,000 × g for 10 min. The supernatant was diluted 50 times and filtered through a 0.22-mm filter. Extracted sample solution was analyzed by a high-performance ion chromatography of ICS-3000 (Dionex, USA). The SCFAs levels were expressed as mg/g of digesta.
Statistical Analysis
All data except microbiota were analyzed with individual sow or piglet as an experimental unit using GLM procedures of SAS (version 9.2; SAS Inst. Inc., Cary, NC) followed by Tukey’s tests. Microbiota diversity metrics were performed from normalized OTU reads using R software (version 3.2.2). The relative abundances of microbiota composition at the phyla and family levels were analyzed by Kruskal–Wallis test. Significant difference was declared at P < 0.05, and tendency was declared at 0.05 ≤ P < 0.10.
Results
Sow Performance
The ADFI during lactation in sows fed SBP diets was greater (P < 0.05) than those fed CON diets, but not different from those fed WB diets (Table 3). There were no differences in the number of total born piglets, piglets born alive, still born piglets, piglets at weaning, preweaning mortality, litter birth weight, and piglet birth weight. However, sows fed SBP diets had greater (P < 0.05) litter weaning weight than those fed CON diets. The average weaning weight and average daily gain were increased (P < 0.05) in piglets from SBP-fed sows than those from CON-fed sows, but not different from those from WB-fed sows.
Table 3.
Effects of dietary fiber sources on sow performance1
| Item | CON | SBP | WB | SEM | P-value |
|---|---|---|---|---|---|
| Sow ADFI, kg/d | 4.80b | 5.48a | 5.16ab | 0.15 | 0.01 |
| Litter size | |||||
| Total born | 12.27 | 12.07 | 12.2 | 0.42 | 0.94 |
| Born alive | 11.33 | 11.4 | 11.67 | 0.19 | 0.79 |
| Still born | 0.93 | 0.67 | 0.53 | 0.36 | 0.31 |
| Weaned | 9.93 | 10.27 | 10.47 | 0.33 | 0.51 |
| Preweaning mortality, % | 12.09 | 9.64 | 10.12 | 1.83 | 0.61 |
| Litter weight | |||||
| At birth | 18.27 | 18.69 | 19.03 | 0.74 | 0.77 |
| At weaning | 56.94b | 64.39a | 62.71ab | 2.12 | 0.04 |
| Piglets | |||||
| Birth weight, kg | 1.63 | 1.65 | 1.65 | 0.06 | 0.96 |
| Weaning weight, kg | 5.74b | 6.26a | 5.97ab | 0.13 | 0.02 |
| ADG, g/d | 196b | 221a | 206ab | 5.39 | 0.01 |
1CON, control; SBP, sugar beet pulp; WB, wheat bran.
a,bMean values within a row with different letters differ at P < 0.05.
Immunoglobulin and Cytokine in the Colostrum and Milk
The levels of IgG, IgM, IL-6, and TNF-α in the colostrum were not affected by dietary treatments (Table 4). However, sows fed SBP diets had greater (P < 0.05) levels of IgA and IL-10 in the colostrum than those fed CON diets, but were not different from those fed WB diets. In addition, the levels of IgA in the milk were increased (P < 0.05) in sows fed SBP diets compared with those fed CON or WB diets. Sows fed SBP or WB diets had greater (P < 0.05) levels of IL-10 in the milk compared with sows fed CON diets. No differences were detected in the levels of IgG, IgM, IL-6, and TNF-α in the milk among treatments.
Table 4.
Effects of dietary fiber sources on immunoglobulins and cytokines levels in the colostrum and milk1
| Items | CON | SBP | WB | SEM | P-value |
|---|---|---|---|---|---|
| Colostrum | |||||
| IgA, g/L | 7.94b | 9.17a | 8.69ab | 0.28 | 0.03 |
| IgG, g/L | 63.79 | 67.66 | 65.32 | 2.67 | 0.60 |
| IgM, g/L | 2.52 | 2.70 | 2.68 | 0.08 | 0.22 |
| IL-6, pg/mL | 18.48 | 17.48 | 17.14 | 1.19 | 0.72 |
| IL-10, pg/mL | 6.05b | 9.79a | 7.42ab | 0.77 | 0.02 |
| TNF-α, pg/mL | 7.66 | 6.58 | 6.85 | 0.65 | 0.49 |
| Milk | |||||
| IgA, g/L | 3.26 | 3.39 | 3.21 | 0.10 | 0.48 |
| IgG, g/L | 24.75 | 24.98 | 24.78 | 0.30 | 0.84 |
| IgM, g/L | 1.15 | 1.18 | 1.15 | 0.02 | 0.38 |
| IL-6, pg/mL | 46.38 | 45.13 | 45.98 | 2.18 | 0.92 |
| IL-10, pg/mL | 2.62b | 3.57a | 3.42a | 0.19 | 0.02 |
| TNF-α, pg/mL | 20.47 | 19.68 | 19.11 | 1.01 | 0.65 |
1CON, control; SBP, sugar beet pulp; TNF-α, tumor necrosis factor-α; WB, wheat bran.
a,bMean values within a row with different letters differ at P < 0.05.
Serum Hormones
On day 21 of lactation, piglets from SBP-fed sows had greater (P < 0.05) levels of GH than those from WB-fed or CON-fed sows (Table 5). The IGF-1 levels were increased in piglets from SBP-fed or WB-fed sows compared with those from CON-fed sows. In addition, the levels of GH were greater (P < 0.05) in piglets from WB-fed sows than those from CON-fed sows.
Table 5.
Effects of maternal dietary fiber sources on serum hormones levels in piglets on day 21 of lactation1
| Items | CON | SBP | WB | SEM | P-value |
|---|---|---|---|---|---|
| GH | 3.37c | 4.23a | 3.78b | 0.10 | < 0.01 |
| IGF-1 | 156.09b | 187.86a | 167.02b | 4.40 | < 0.01 |
1CON, control; GH, growth hormones; SBP, sugar beet pulp; WB, wheat bran.
a–cMean values within a row with different letters differ at P < 0.05.
Serum Diamine Oxidase and Endotoxin
On day 21 of lactation, the diamine oxidase activity and endotoxin levels were decreased (P < 0.05) in piglets from SBP-fed or WB-fed sows when compared with those form CON-fed sows (Table 6).
Table 6.
Effects of maternal dietary fiber sources on serum diamine oxidase activity and endotoxin levels in piglets on day 21 of lactation1
| Items | CON | SBP | WB | SEM | P-value |
|---|---|---|---|---|---|
| DAO, U/L | 5.68a | 3.60b | 4.09b | 0.18 | < 0.01 |
| Endotoxin, EU/mL | 0.60a | 0.47b | 0.45b | 0.02 | < 0.01 |
1CON, control; DAO, diamine oxidase; SBP, sugar beet pulp; WB, wheat bran.
a,bMean values within a row with different letters differ at P < 0.05.
Serum Immunoglobulins and Cytokines
On day 21 of lactation, serum levels of IgA and IgG were not affected by dietary treatments (Table 7). But serum IgM levels tended to be increased (P = 0.08) in piglets from WB-fed sows compared with those from CON-fed sows. Compared with piglets from CON-fed sows, the serum levels of IL-6 and TNF-α were decreased (P < 0.05) in piglets from SBP-fed or WB-fed sows. The piglets from SBP-fed sows had greater (P < 0.05) serum IL-10 levels than those from CON-fed sows.
Table 7.
Effects of maternal dietary fiber sources on serum immunoglobulins and cytokines levels in piglets on day 21 of lactation1
| Items | CON | SBP | WB | SEM | P-value |
|---|---|---|---|---|---|
| IgA, g/L | 1.15 | 1.10 | 1.12 | 0.04 | 0.56 |
| IgG, g/L | 20.47 | 20.49 | 19.28 | 0.42 | 0.11 |
| IgM, g/L | 2.27 | 2.30 | 2.38 | 0.03 | 0.08 |
| IL-6, pg/mL | 178.49a | 154.30b | 161.80b | 3.08 | < 0.01 |
| IL-10, pg/mL | 4.55b | 5.13a | 4.91ab | 0.11 | 0.01 |
| TNF-α, pg/mL | 102.45a | 80.28b | 85.92b | 1.80 | < 0.01 |
1CON, control; SBP, sugar beet pulp; TNF-α, tumor necrosis factor-α; WB, wheat bran.
a,bMean values within a row with different letters differ at P < 0.05.
Ileal Mucosal Cytokines and Secretory IgA
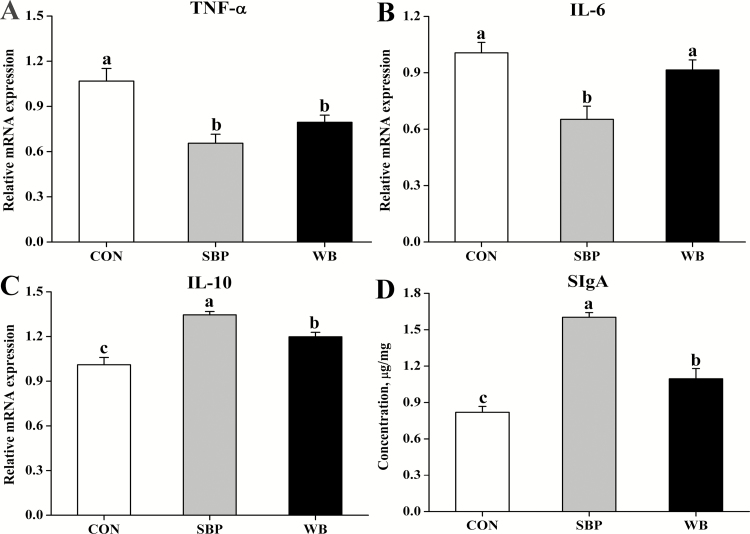
The mRNA expression of TNF-α in the ileum was reduced (P < 0.05) in piglets from SBP-fed or WB-fed sows than those from CON-fed sows (Fig. 1A). Piglets from SBP-fed sows had decreased (P < 0.05) mRNA expression of IL-6 compared with those from CON-fed or WB-fed sows (Fig. 1B). The mRNA expression of IL-10 was greater (P < 0.05) in piglets from WB-fed sows than those from CON-fed sows, but lower (P < 0.05) than those from SBP-fed sows (Fig. 1C). The levels of SIgA in the ileum were greater (P < 0.05) in piglets from SBP-fed sows compared with those from CON-fed or WB-fed sows (Fig. 1D). The piglets from WB-fed sows had greater (P < 0.05) ileal SIgA levels when compared with those from CON-fed sows.
Figure 1.
Effects of maternal dietary fiber sources on the relative mRNA expression of TNF-α (A), IL-6 (B) and IL-10 (C), and the levels of SIgA (D) in the ileum of piglets on day 21 of lactation. CON, control, SBP, sugar beet pulp, WB, wheat bran, TNF-α, tumor necrosis factor-α, IL-6, interleukin-6, IL-10, interleukin-10, SIgA, secretory immunoglobulin A. Values are presented as mean ± SEM, n = 6. a–cMeans without common letters differ at P < 0.05.
Ileal Mucosal Tight Junction Proteins
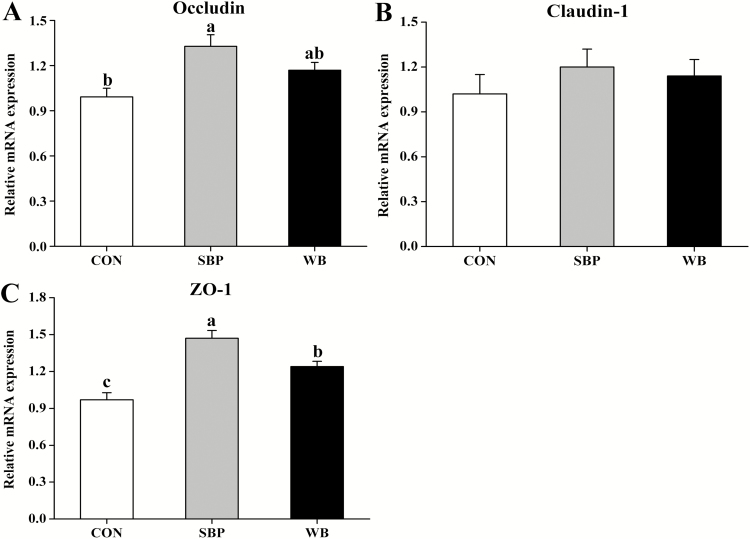
The mRNA expression of occludin in the ileum was up-regulated (P < 0.05) in piglets from SBP-fed sows than piglets from CON-fed sows, but not different from those from WB-fed sows (Fig. 2A). The mRNA expression of ZO-1 was greater (P < 0.05) in piglets from WB-fed sows than piglets from CON-fed sows, but lower (P < 0.05) than SBP-fed sows (Fig. 2B). There were no differences in the mRNA expression of claudin-1 among treatments (Fig. 2C).
Figure 2.
Effects of maternal dietary fiber sources on the relative mRNA expression of occludin (A), claudin-1 (B), and ZO-1 (C) in the ileum of piglets on day 21 of lactation. CON, control; SBP, sugar beet pulp; WB, wheat bran. Values are presented as mean ± SEM, n = 6. a–cMeans without common letters differ at P < 0.05.
Intestinal Morphology
There was an increasing tendency (P = 0.08) in the duodenal villus height to crypt depth ratio of piglets from SBP-fed sows compared with piglets from CON-fed sows (Table 8). Piglets from SBP-fed sows had greater (P < 0.05) jejunal villus height than those from CON-fed sows. The villus height, crypt depth, and villus height to crypt depth ratio in the ileum were not affected by dietary treatments.
Table 8.
Effects of maternal dietary fiber sources on intestinal morphology in piglets on day 21 of lactation1
| Items | CON | SBP | WB | SEM | P-value |
|---|---|---|---|---|---|
| Duodenum | |||||
| Villus height, μm | 491 | 531 | 516 | 26.24 | 0.56 |
| Crypt depth, μm | 318 | 308 | 310 | 10.45 | 0.78 |
| Villus height to crypt depth ratio | 1.54 | 1.72 | 1.66 | 0.05 | 0.08 |
| Jejunum | |||||
| Villus height, μm | 387b | 447a | 433ab | 15.63 | 0.04 |
| Crypt depth, μm | 240 | 259 | 249 | 7.86 | 0.27 |
| Villus height to crypt depth ratio | 1.61 | 1.73 | 1.74 | 0.05 | 0.20 |
| Ileum | |||||
| Villus height, μm | 385 | 429 | 414 | 14.53 | 0.12 |
| Crypt depth, μm | 251 | 256 | 250 | 11.64 | 0.94 |
| Villus height to crypt depth ratio | 1.54 | 1.69 | 1.67 | 0.05 | 0.13 |
1CON, control; SBP, sugar beet pulp; WB, wheat bran.
a,bMean values within a row with different letters differ at P < 0.05.
Colonic Microbiota
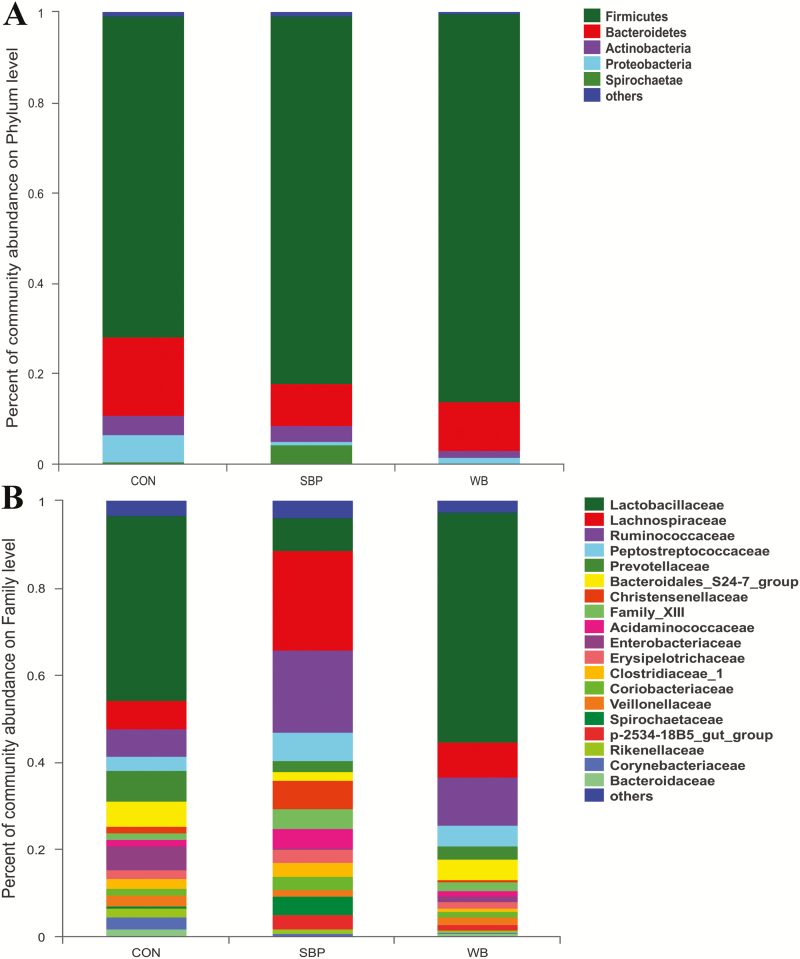
There were 454, 485, and 449 OTUs obtained from piglets in CON, SBP, and WB groups, respectively, of which 347 were common OTUs (Fig. 3A). Besides, a total of 85 unique OTUs were detected within the 3 treatments. The α-diversity indexes including Chao and Shannon indexes were not affected by dietary treatments (Fig. 3B and C). At the phylum level, Firmicutes and Bacteroidetes were the most abundant bacteria phyla representing approximate 90%, followed by Actinobacteria, Proteobacteria, and Spirochaetae (Fig. 4A). No differences were observed in the relative abundance of microbiota at the phylum level among treatments (Supplementary Table S1). At the family level, the dominant families within the Firmicutes phylum consisted of Lactobacillaceae, Lachnospiraceae, Ruminococcaceae, Peptostreptococcaceae, Christensenellaceae, Family XIII, and Acidaminococcaceae, while the main families within the Bacteridetes phylum were Prevotellaceae and Bacteroidales_S24-7_group (Fig. 4B). The relative abundance of Lachnospiraceae and Ruminococcaceae were increased but not significantly in piglets from SBP-fed sows (Supplementary Table S2). Moreover, the relative abundance of Christensenellaceae was increased significantly (P < 0.05) in piglets from SBP-fed sows, while the relative abundance of Lactobacillaceae was increased (P < 0.05) in piglets from WB-fed sows.
Figure 3.
Effects of maternal dietary fiber sources on the colonic microbiota richness in piglets on day 21 of lactation. (A) OTU Venn of 3 dietary treatments. (B) The Chao index of bacterial community. (C) Shannon index of bacterial community. The results were analyzed by Kruskal–Wallis H test and presented as mean values of different bacteria, n = 4. CON, control; SBP, sugar beet pulp; WB, wheat bran.
Figure 4.
Effects of maternal dietary fiber sources on the microbial community in piglets on day 21 of lactation. (A) Microbial community barplot at the phylum level. (B) Microbial community barplot at the family level. n = 4. CON, control; SBP, sugar beet pulp; WB, wheat bran.
Short Chain Fatty Acids
Piglets from SBP-fed sows tended to have greater (P = 0.09) acetate levels in the colon than those from WB-fed sows (Table 9). The butyrate levels were increased (P < 0.05) in piglets from SBP-fed sows when compared with those from CON-fed sows. No differences were observed in the levels of propionate, isobutyrate, valerate, isovalerate, and total SCFAs among treatments.
Table 9.
Effects of maternal dietary fiber sources on short chain fatty acids (SCFAs) levels in the colon of piglets on day 21 of lactation (mg/g)1
| Items | CON | SBP | WB | SEM | P-value |
|---|---|---|---|---|---|
| Acetate | 2.38 | 3.25 | 2.78 | 0.26 | 0.09 |
| Priopinate | 1.77 | 2.02 | 1.91 | 0.25 | 0.77 |
| Butyrate | 0.42b | 0.84a | 0.58ab | 0.11 | 0.04 |
| Isobutyrate | 0.20 | 0.17 | 0.17 | 0.06 | 0.91 |
| Valerate | 0.63 | 0.86 | 0.71 | 0.26 | 0.81 |
| Isovalerate | 0.43 | 0.34 | 0.47 | 0.13 | 0.77 |
| Total SCFAS | 5.82 | 7.49 | 6.60 | 0.64 | 0.22 |
1CON, control; SBP, sugar beet pulp; SCFAs, short chain fatty acids; WB, wheat bran.
a,bMean values within a row with different letters differ at P < 0.05.
Discussion
In the present study, maternal SBP diets improved growth performance of piglets compared with the CON diets, which was consistent with Cheng et al. (2018), who observed that the growth performance was increased in piglets from soluble fiber diets fed sows. In contrast, maternal WB diets did not affect the growth performance, suggesting that high soluble fiber such as SBP may be more effective than high insoluble fiber such as WB in improving the growth performance. However, contrary to our results, Danielsen and Vestergaard (2001) reported that piglet birth weight was reduced and weaning weight was not affected by fiber diets containing 50% SBP. The discrepancies may be due to the differences in additional doses of SBP. Dietary fiber has antinutritional effects, and thus excess fiber intake may have detrimental effects on sows.
Lactation feed intake is of greatest importance for litter growth performance and sow subsequent productivity (Sulabo et al., 2010). In this study, the lactation feed intake was increased in sows fed SBP diets compared with those fed CON diets. Indeed, previous studies also have shown positive effects of high fiber diets on lactation feed intake of sows (Quesnel et al., 2009; Tan et al., 2015). However, the current study also demonstrated that the SBP diets had more profound effects on the lactation feed intake than the WB diets. The most likely explanation may be that SBP is more fermentable than WB, and thereby can increase feed intake by improving insulin sensitivity (Tan et al., 2016). The increased lactation feed intake in SBP-fed sows indicated more energy and nutrient intake as all the diets were isoenergetic and isonitrogenous, which may, in turn, increase milk production, because at least half the amount of nitrogen (52%) and energy (50%) from the feed is transferred to milk at peak lactation (Theil et al., 2014). As sow milk production is the major determinant of a suckling piglet’s growth (Solà-Oriol and Gasa, 2017), therefore, the increased lactation feed intake was probably responsible for the greater growth rate of piglets from SBP-fed sows.
The neonatal piglet is completely reliant on the sow’s colostrum and milk for a range of antibodies and cytokines that impact its immunological and physiological development. Among these antibodies, IgA is not absorbed by the intestinal epithelium and usually provides local passive immunity in the gastrointestinal tract of piglets (Wagstrom et al., 2000). In this study, greater IgA levels in colostrum of sows fed SBP diets may help to improve the passive immunity, and then growth performance in piglets. IL-10, a cytokine with anti-inflammatory properties, has a central role in infection by limiting the immune response to pathogens and thereby preventing damage to the host (Saraiva and O’Garra, 2010). The greater levels of IL-10 observed in the colostrum and milk sows fed SBP diets suggested a reduction in the inflammatory and enhancement of the humoral immune response, which may also positively affect the immunity of piglets (Laskowska et al., 2019). Previous studies have shown that dietary probiotics supplementation in sows during pregnancy and lactation increased levels of cytokines IL-4, IL-10, and immunoglobulins in the colostrum and milk (Laskowska et al., 2019). Therefore, the increased IgA and IL-10 levels may be related to the change of microbiota composition induced by dietary fiber.
Postnatal growth is regulated by the somatotropic axis, in which GH can fulfil its function of growth promotion through regulating the synthesis of IGF-I (Butler and Le Roith, 2001). Hence, to investigate whether the effects of maternal dietary fiber on growth performance are related to the growth-related hormones, the serum levels of GH and IGF-1 were measured. The current study revealed that the piglets from the SBP-fed sows had the highest serum GH and IGF-1 levels, which may, in part, explain the improved growth performance. Similarly, Cheng et al. (2018) showed that the plasma levels of GH and IGF-1 were increased by a maternal soluble fiber diet. In addition, the piglets from the WB-fed sows also had greater serum levels of IGF-1 and GH compared with the CON. The results indicated that maternal WB had positive effects on growth to some extent, although no significant effects were observed in this study. Together, these results revealed that maternal dietary fiber had effects on growth hormones secretion in offspring, but the underlying mechanism need to be further investigated.
Cytokines, including proinflammatory cytokines and anti-inflammatory cytokines, are necessary mediators that direct inflammatory response, and their balance is important for protection against or susceptibility to infection (Xiong et al., 2015). Proinflammatory cytokines, such as TNF-α and IL-6, trigger inflammatory response and have negative effects on intestinal integrity and epithelial function (Al-Sadi et al., 2009). In contrast, anti-inflammatory cytokines, such as IL-10, are a series of immunoregulatory molecules that control the proinflammatory cytokine response (Opal and Depalo, 2000). The present study showed maternal SBP diets down-regulated the expression of proinflammatory cytokines TNF-α and IL-6, and up-regulated the expression of anti-inflammatory cytokine IL-10, while maternal WB diets down-regulated the expression of TNF-α and up-regulated the expression of IL-10 when compared with the CON, indicating that the inflammation was minimized in piglets of the 2 dietary treatments.
Secretory IgA (SIgA), which is produced by plasma cells, is the major antibody in local mucosal immunity that can protect the intestinal epithelium from enteric toxins and pathogenic microorganisms (Wu et al., 2016). In the present study, piglets from SBP-fed sows had highest SIgA concentration, followed by piglets from WB-fed sows, indicating the activation of the intestinal mucosal immunity in these piglets. Taken together, these findings suggested that maternal dietary fiber may induce an immune response in piglets by modulating the production of cytokines and antibodies.
The intestinal barrier integrity plays a vital role in maintaining intestinal homeostasis by preventing the entrance of pathogens, toxins, and antigens into the mucosal tissues (Turner, 2009). DAO is a highly active intracellular enzyme in the intestinal epithelium, and endotoxin is an important product of Gram-negative bacteria in the gut. They both can cross the epithelial mucosa into serum as intestinal permeability increases (Xiao et al., 2019). Therefore, the DAO activity and endotoxin concentration in serum are reliable markers of intestinal barrier integrity. In this study, the lower serum DAO activity and endotoxin concentration in piglets from SBP-fed or WB-fed sows indicated improved intestinal barrier integrity. The results were consistent with Cheng et al. (2018), who reported that an SF diet during pregnancy reduces the intestinal permeability in piglets, as evidenced by decreased plasma endotoxin, and diamine oxidase.
Tight junctions (TJs) exist in a planar network and function as barriers that seal the intercellular space between epithelial cells. Tight junctions contain protein complexes, are situated in the epidermal granular layer, and are composed primarily of claudins, occludin, and Zonula Occludens (ZO) proteins, which act in a systematic manner and regulate TJ function (Yin et al., 2015). In the present study, maternal SBP diet increased the ileal mRNA expressions of both occludin and ZO-1, while maternal WB diet only increased the ileal mRNA expressions of ZO-1, which were consistent with the reduced serum DAO activity and endotoxin levels observed in piglets from the 2 treatments. In similar to the present results, previous studies also demonstrated that dietary fiber had beneficial effects on gut barrier function (Chen et al., 2013), and, conversely, dietary fiber deprivation degraded the gut barrier function and enhanced pathogen susceptibility (Desai et al., 2016). It has been shown that intestinal TJ barrier is dynamically regulated by cytokines (Lee, 2015). Most proinflammatory cytokines, such as TNF-α, IFN-γ, IL-1β, and IL-6, induce a pathologic opening of the intestinal TJ barrier and increase intestinal epithelial permeability, while IL-10 has an important barrier protective effect (Al-Sadi et al., 2009). Therefore, the improved intestinal barrier function in piglets from the 2 fiber treatments may be due to the reduced expressions of TNF-α and IL-6 and the increased expression of IL-10. Taken together, these results demonstrated that maternal dietary fiber can improve intestinal barrier integrity by up-regulating the expressions of tight junction proteins and down-regulating the expressions of proinflammatory cytokines.
The small intestine is the major site of nutrient digestion and absorption, so a healthy morphology is crucial for digestive physiological function, and overall growth of the body (Cheng et al., 2017). The intestinal morphology including villus height, crypt depth, and villus height to crypt depth ratio is a useful criterion for estimating the digestive capacity of the small intestine (Jha et al., 2019). In the current study, the greater jejunal villus height in piglets from SBP-fed sows suggested better digestion and absorption capacity, which may, in turn, contribute to the better growth performance of piglets. It is well established that endotoxin induces a variety of morphologic alterations in the digestive tract, such as reduced villous height and increased crypt depth (Yang et al., 2014). Consequently, the lower endotoxin may be a reason for the improved morphology in piglets from SBP-fed sows.
Commensal microbiota in the gastrointestinal tract is crucial to the host growth and health by providing metabolic, immunologic, and protective functions (Yin et al., 2017; Duan et al., 2019). In this study, Firmicutes and Bacteroidetes were the predominant bacterial phyla with the relative abundance higher than approximately 90%, which was consistent with previous findings in suckling piglets (Chen et al., 2017). The Lactobacillaceae family, belonging to the Firmicutes phylum, is considered as a beneficial microbe to modulate gut health by improving intestinal barrier function and immunity (Yang et al., 2018). The current study showed that Lactobacillaceae was increased significantly in piglets from WB-fed sows, which could explain the increased intestinal barrier function and immunity. Lachnospiraceae and Rumininococcaceae are 2 of the most abundant families from the order Clostridiales, and have been associated with the maintenance of gut health (Biddle et al., 2013). In this study, piglets from SBP-fed sows had the highest abundances of Lachnospiraceae and Rumininococcaceae, indicating better gut health. The increased abundances of Lachnospiraceae and Rumininococcaceae may be attributed to the high content of fiber in sugar beet pulp, because they are regarded as fibrolytic specialists to degrade a wide variety of the complex plant substrates (Biddle et al., 2013). Christensenellaceae is considered as a butyrate producer, which play a key role in maintaining gastrointestinal tract structure and function by forming syntrophic partnerships with Methanobrevibacter (Jenkins et al., 2015). Another important finding in this study is that Christensenellaceae was increased significantly in piglets from SBP-fed sows, which may be an explanation for the improved intestinal function and immunity. Together, these findings indicated that the maternal dietary fiber sources could differently influence the composition of the intestinal microbiota in offspring.
Short chain fatty acids, mainly acetate, propionate and butyrate, are a major class of bacterial metabolites, which have been demonstrated to be key regulators of host metabolism and immunity (Yin et al., 2018; Zhang et al., 2018). Suzuki et al. (2008) demonstrated that SCFAs, especially acetate and propionate, enhance the intestinal barrier integrity in the colon and cultured intestinal cells. Furthermore, butyrate, as the major energy source for colonocytes, possesses the potential to suppress inflammatory reaction and promote epithelial cell proliferation and intestinal barrier function (Guilloteau et al., 2010; Grilli et al., 2016). The current study demonstrated that the piglets from SBP-fed sows tended to have greater acetate concentration and had greater butyrate concentration than those from CON-fed sows. The increased SCFAs may, in turn, result in the suppression of inflammatory immune activation, thereby protecting the intestinal barrier function.
In conclusion, maternal SBP supplementation was more effective than WB in improving milk quality, enhancing growth performance and intestinal barrier function, and ameliorating intestinal inflammation in piglets.
Supplementary Material
Footnotes
This study was financially supported by the National Natural Science Foundation of China (31772612) and CARS 35.
Literature Cited
- Al-Sadi R., Boivin M., and Ma T.. 2009. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. (Landmark Ed). 14:2765–2778. doi:10.2741/3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC 2007. Official methods of analysis, 18 th ed AOAC Int, Arlington, VA. [Google Scholar]
- Biddle A., Stewart L., Blanchard J., and Leschine S.. 2013. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity. 5: 627–640. doi:10.3390/d5030627 [Google Scholar]
- Butler A. A., and Le Roith D.. 2001. Control of growth by the somatropic axis: Growth hormone and the insulin-like growth factors have related and independent roles. Annu. Rev. Physiol. 63:141–164. doi: 10.1146/annurev.physiol.63.1.141 [DOI] [PubMed] [Google Scholar]
- Chen H., Chen D., Qin W., Liu Y., Che L., Huang Z., Luo Y., Zhang Q., Lin D., Liu Y., . et al. 2017. Wheat bran components modulate intestinal bacteria and gene expression of barrier function relevant proteins in a piglet model. Int. J. Food Sci. Nutr. 68:65–72. doi: 10.1080/09637486.2016.1212817 [DOI] [PubMed] [Google Scholar]
- Chen H., Mao X., He J., Yu B., Huang Z., Yu J., Zheng P., and Chen D.. 2013. Dietary fibre affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets. Br. J. Nutr. 110:1837–1848. doi: 10.1017/S0007114513001293 [DOI] [PubMed] [Google Scholar]
- Chen L., Xu Y., Chen X., Fang C., Zhao L., and Chen F.. 2017. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front. Microbiol. 8:1688. doi: 10.3389/fmicb.2017.01688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Wei H., Xu C., Xie X., Jiang S., and Peng J.. 2018. Maternal soluble fiber diet during pregnancy changes the intestinal microbiota, improves growth performance, and reduces intestinal permeability in piglets. Appl. Environ. Microb. 84: e01047–18. doi:10.1128/AEM.01047-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W., Lu J., Li B., Lin W., Zhang Z., Wei X., Sun C., Chi M., Bi W., Yang B., . et al. 2017. Effect of functional oligosaccharides and ordinary dietary fiber on intestinal microbiota diversity. Front. Microbiol. 8:1750. doi: 10.3389/fmicb.2017.01750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen V., and Vestergaard E.. 2001. Dietary fibre for pregnant sows: Effect on performance and behaviour. Anim. Feed Sci. Tech. 90: 71–80. doi:10.1016/s0377-8401(01)00197-3 [Google Scholar]
- Desai M. S., Seekatz A. M., Koropatkin N. M., Kamada N., Hickey C. A., Wolter M., Pudlo N. A., Kitamoto S., Terrapon N., Muller A., . et al. 2016. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167:1339–1353.e21. doi: 10.1016/j.cell.2016.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y., Zhong Y., Xiao H., Zheng C., Song B., Wang W., Guo Q., Li Y., Han H., and Gao J.. 2019. Gut microbiota mediates the protective effects of dietary β-hydroxy-β-methylbutyrate (hmb) against obesity induced by high-fat diets. FASEB. J. 3(9): 10019–10033. doi:10.1096/fj.201900665RR [DOI] [PubMed] [Google Scholar]
- Dzidic M., Boix-Amorós A., Selma-Royo M., Mira A., and Collado M.. 2018. Gut microbiota and mucosal immunity in the neonate. Med. Sci. 6: 56. doi:10.3390/medsci6030056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez de Agüero M., Ganal-Vonarburg S. C., Fuhrer T., Rupp S., Uchimura Y., Li H., Steinert A., Heikenwalder M., Hapfelmeier S., Sauer U., . et al. 2016. The maternal microbiota drives early postnatal innate immune development. Science 351:1296–1302. doi: 10.1126/science.aad2571 [DOI] [PubMed] [Google Scholar]
- Gómez B., Gullón B., Yáñez R., Schols H., and Alonso J. L.. 2016. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. J. Funct. Foods. 20: 108–121. doi: 10.1016/j.jff.2015.10.029 [Google Scholar]
- Grilli E., Tugnoli B., Foerster C. J., and Piva A.. 2016. Butyrate modulates inflammatory cytokines and tight junctions components along the gut of weaned pigs. J. Anim. Sci. 94: 433–436. doi:10.2527/jas2015-9787 [Google Scholar]
- Guilloteau P., Martin L., Eeckhaut V., Ducatelle R., Zabielski R., and Van Immerseel F.. 2010. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 23:366–384. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- Jarrett S., and Ashworth C. J.. 2018. The role of dietary fibre in pig production, with a particular emphasis on reproduction. J. Anim. Sci. Biotechnol. 9:59. doi: 10.1186/s40104-018-0270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins S. N., Waite I. S., Mansfield J., Kim J. C., and Pluske J. R.. 2015. Relationships between diets different in fibre type and content with growth, Escherichia coli shedding, and faecal microbial diversity after weaning. Anim. Prod. Sci. 55: 1451. doi:10.1071/ANv55n12Ab125 [Google Scholar]
- Jensen M. B., Pedersen L. J., Theil P. K., Yde C. C., and Bach Knudsen K. E.. 2012. Feeding motivation and plasma metabolites in pregnant sows fed diets rich in dietary fiber either once or twice daily. J. Anim. Sci. 90:1910–1919. doi: 10.2527/jas.2010-3289. [DOI] [PubMed] [Google Scholar]
- Jha R., Fouhse J. M., Tiwari U. P., Li L., and Willing B. P.. 2019. Dietary fiber and intestinal health of monogastric animals. Front. Vet. Sci. 6:48. doi: 10.3389/fvets.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowska E., Jarosz Ł., and Grądzki Z.. 2019. Effect of multi-microbial probiotic formulation bokashi on pro- and anti-inflammatory cytokines profile in the serum, colostrum and milk of sows, and in a culture of polymorphonuclear cells isolated from colostrum. Probiotics Antimicrob. Proteins 11:220–232. doi: 10.1007/s12602-017-9380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourgot C., Ferret-Bernard S., Le Normand L., Savary G., Menendez-Aparicio E., Blat S., Appert-Bossard E., Respondek F., and Le Huërou-Luron I.. 2014. Maternal short-chain fructooligosaccharide supplementation influences intestinal immune system maturation in piglets. Plos One 9:e107508. doi: 10.1371/journal.pone.0107508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H. 2015. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 13:11–18. doi: 10.5217/ir.2015.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijdekkers A. G. M., Aguirre M., Venema K., Bosch G., Gruppen H., and Schols H. A.. 2014. In vitro fermentability of sugar beet pulp derived oligosaccharides using human and pig fecal inocula. J. Agr. Food Chem. 62: 1079–1087. doi:10.1021/jf4049676 [DOI] [PubMed] [Google Scholar]
- Loisel F., Farmer C., Ramaekers P., and Quesnel H.. 2013. Effects of high fiber intake during late pregnancy on sow physiology, colostrum production, and piglet performance. J. Anim. Sci. 91:5269–5279. doi: 10.2527/jas.2013-6526. [DOI] [PubMed] [Google Scholar]
- Macpherson A. J., de Agüero M. G., and Ganal-Vonarburg S. C.. 2017. How nutrition and the maternal microbiota shape the neonatal immune system. Nat. Rev. Immunol. 17:508–517. doi: 10.1038/nri.2017.58. [DOI] [PubMed] [Google Scholar]
- Mudgil D., and Barak S.. 2013. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 61:1–6. doi: 10.1016/j.ijbiomac.2013.06.044. [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine, 11th revised edn. National Academy Press, Washington, DC. [Google Scholar]
- Onipe O. O., Jideani A. I., and Beswa D.. 2015. Composition and functionality of wheat bran and its application in some cereal food products. Int. J. Food Sci. Tech. 50: 2509–2518. doi:10.1111/ijfs.12935 [Google Scholar]
- Opal S. M., and DePalo V. A.. 2000. Anti-inflammatory cytokines. Chest 117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- Quesnel H., Meunier-Salaün M. C., Hamard A., Guillemet R., Etienne M., Farmer C., Dourmad J. Y., and Père M. C.. 2009. Dietary fiber for pregnant sows: Influence on sow physiology and performance during lactation. J. Anim. Sci. 87:532–543. doi: 10.2527/jas.2008-1231. [DOI] [PubMed] [Google Scholar]
- Rebello C. J., O’Neil C. E., and Greenway F. L.. 2016. Dietary fiber and satiety: The effects of oats on satiety. Nutr. Rev. 74:131–147. doi: 10.1093/nutrit/nuv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M., and O’Garra A.. 2010. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Shang Q. H., Ma X. K., Li M., Zhang L. H., Hu J. X., and Piao X. S.. 2018. Effects of α-galactosidase supplementation on nutrient digestibility, growth performance, intestinal morphology and digestive enzyme activities in weaned piglets. Anim. Feed Sci. Tech. 236: 48–56. doi:10.1016/j.anifeedsci.2017.11.008 [Google Scholar]
- Solà-Oriol D., and Gasa J.. 2017. Feeding strategies in pig production: Sows and their piglets. Anim. Feed Sci. Tech. 233: 34–52. doi:10.1016/j.anifeedsci.2016.07.018 [Google Scholar]
- Sulabo R. C., Jacela J. Y., Tokach M. D., Dritz S. S., Goodband R. D., DeRouchey J. M., and Nelssen J. L.. 2010. Effects of lactation feed intake and creep feeding on sow and piglet performance. J. Anim. Sci. 88:3145–3153. doi: 10.2527/jas.2009-2131. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Yoshida S., and Hara H.. 2008. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br. J. Nutr. 100:297–305. doi: 10.1017/S0007114508888733. [DOI] [PubMed] [Google Scholar]
- Tan C. Q., Wei H. K., Sun H. Q., Long G., Ao J. T., Jiang S. W., and Peng J.. 2015. Effects of supplementing sow diets during two gestations with konjac flour and saccharomyces boulardii on constipation in peripartal period, lactation feed intake and piglet performance. Anim. Feed Sci. Tech. 210: 254–262. doi:10.1016/j.anifeedsci.2015.10.013 [Google Scholar]
- Tan C., Wei H., Ao J., Long G., and Peng J.. 2016. Inclusion of Konjac flour in the gestation diet changes the gut microbiota, alleviates oxidative stress, and improves insulin sensitivity in sows. Appl. Environ. Microbiol. 82:5899–5909. doi: 10.1128/AEM.01374-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil P. K., Lauridsen C., and Quesnel H.. 2014. Neonatal piglet survival: Impact of sow nutrition around parturition on fetal glycogen deposition and production and composition of colostrum and transient milk. Animal 8:1021–1030. doi: 10.1017/S1751731114000950. [DOI] [PubMed] [Google Scholar]
- Thum C., Cookson A. L., Otter D. E., McNabb W. C., Hodgkinson A. J., Dyer J., and Roy N. C.. 2012. Can nutritional modulation of maternal intestinal microbiota influence the development of the infant gastrointestinal tract? J. Nutr. 142:1921–1928. doi: 10.3945/jn.112.166231. [DOI] [PubMed] [Google Scholar]
- Turner J. R. 2009. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Van Soest P. J., Robertson J. B., and Lewis B. A.. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Wagstrom E. A., Yoon K. J., and Zimmerman J. J.. 2000. Immune components in porcine mammary secretions. Viral Immunol. 13:383–397. doi: 10.1089/08828240050144699. [DOI] [PubMed] [Google Scholar]
- Wang L. F., Beltranena E., and Zijlstra R. T.. 2016. Diet nutrient digestibility and growth performance of weaned pigs fed sugar beet pulp. Anim. Feed Sci. Tech. 211: 145–152. doi:10.1016/j.anifeedsci.2015.11.005 [Google Scholar]
- Wu M., Xiao H., Liu G., Chen S., Tan B., Ren W., Bazer F. W., Wu G., and Yin Y.. 2016. Glutamine promotes intestinal SIgA secretion through intestinal microbiota and IL-13. Mol. Nutr. Food Res. 60:1637–1648. doi: 10.1002/mnfr.201600026. [DOI] [PubMed] [Google Scholar]
- Wu X., Chen D., Yu B., Luo Y., Zheng P., Mao X., Yu J., and He J.. 2018. Effect of different dietary non-starch fiber fractions on growth performance, nutrient digestibility, and intestinal development in weaned pigs. Nutrition 51-52:20–28. doi: 10.1016/j.nut.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Xiao L., Cui T., Liu S., Chen B., Wang Y., Yang T., Li T., and Chen J.. 2019. Vitamin A supplementation improves the intestinal mucosal barrier and facilitates the expression of tight junction proteins in rats with diarrhea. Nutrition 57:97–108. doi: 10.1016/j.nut.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Xiong X., Yang H. S., Wang X. C., Hu Q., Liu C. X., Wu X., Deng D., Hou Y. Q., Nyachoti C. M., Xiao D. F., . et al. 2015. Effect of low dosage of chito-oligosaccharide supplementation on intestinal morphology, immune response, antioxidant capacity, and barrier function in weaned piglets. J. Anim. Sci. 93:1089–1097. doi: 10.2527/jas.2014-7851. [DOI] [PubMed] [Google Scholar]
- Yang J., Qian K., Wang C., and Wu Y.. 2018. Roles of probiotic Lactobacilli inclusion in helping piglets establish healthy intestinal inter-environment for pathogen defense. Probiotics Antimicrob. Proteins 10:243–250. doi: 10.1007/s12602-017-9273-y. [DOI] [PubMed] [Google Scholar]
- Yang K. M., Jiang Z. Y., Zheng C. T., Wang L., and Yang X. F.. 2014. Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 92:1496–1503. doi: 10.2527/jas.2013-6619 [DOI] [PubMed] [Google Scholar]
- Yin J., Han H., Li Y., Liu Z., Zhao Y., Fang R., Huang X., Zheng J., Ren W., Wu F., . et al. 2017. Lysine restriction affects feed intake and amino acid metabolism via gut microbiome in piglets. Cell. Physiol. Biochem. 44:1749–1761. doi: 10.1159/000485782 [DOI] [PubMed] [Google Scholar]
- Yin J., Duan J. L., Cui Z. J., Cui Z., Ren W. K., Li T. J., and Yu Y. L.. 2015. Hydrogen peroxide-induced oxidative stress activates NF-κB and Nrf2/Keap1 signals and triggers autophagy in piglets. Rsc. Advances. 5: 15479–15486. doi:10.1039/C4RA13557A [Google Scholar]
- Yin J., Li Y., Han H., Chen S., Gao J., Liu G., Wu X., Deng J., Yu Q., Huang X., Fang R., Li T., Reiter R. J., Zhang D., Zhu C., Zhu G., Ren W., and Yin Y.. 2018. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high fat diet-fed mice. J. Pineal. Res. 65: e12524. doi:10.1111/jpi.12524 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Yu H., Xiao X., Hu L., Xin F., and Yu X.. 2018. Inulin-type fructan improves diabetic phenotype and gut microbiota profiles in rats. Peerj 6:e4446. doi: 10.7717/peerj.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. B., Liu P., Huang C. F., Liu L., Li E. K., Zhang G., and Zhang S.. 2018. Effect of wheat bran on apparent total tract digestibility, growth performance, fecal microbiota and their metabolites in growing pigs. Anim. Feed Sci. Tech. 239: 14–26. doi:10.1016/j.anifeedsci.2018.02.013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.