Abstract
Background
Neurotoxicity is a frequent side effect of cytotoxic chemotherapy and affects a large number of patients. Despite the high medical need, few research efforts have addressed the impact of cytotoxic agents on cognition (ie, postchemotherapy cognitive impairment; PCCI). One unsolved question is whether individual cytotoxic drugs have differential effects on cognition. We thus examine the current state of research regarding PCCI. Neurological symptoms after targeted therapies and immunotherapies are not part of this review.
Methods
A literature search was conducted in the PubMed database, and 1215 articles were reviewed for predefined inclusion and exclusion criteria. Thirty articles were included in the systematic review.
Results
Twenty-five of the included studies report significant cognitive impairment. Of these, 21 studies investigated patients with breast cancer. Patients mainly received combinations of 5-fluorouracil, epirubicin, cyclophosphamide, doxorubicin, and taxanes (FEC/FEC-T). Five studies found no significant cognitive impairment in chemotherapy patients. Of these, 2 studies investigated patients with colon cancer receiving 5-fluorouracil and oxaliplatin (FOLFOX). Independent risk factors for PCCI were patient age, mood alterations, cognitive reserve, and the presence of apolipoprotein E e4 alleles.
Conclusions
There is evidence that certain chemotherapy regimens cause PCCI more frequently than others as evidenced by 21 out of 23 studies in breast cancer patients (mainly FEC-T), whereas 2 out of 3 studies with colon cancer patients (FOLFOX) did not observe significant changes. Further studies are needed defining patient cohorts by treatment protocol in addition to cancer type to elucidate the effects of individual cytotoxic drugs on cognitive functions.
Keywords: chemotherapy, cognition, cognitive decline, neurotoxicity
In Germany, 482 500 patients were diagnosed with a malignant disease in 2016. The number of new cancer cases per year has increased in 10 years (2002-2012) by 13% for men and 10% for women, and the prediction for 2020 is that 519 000 people in Germany will be newly diagnosed with cancer. Incidence rates for cancer have been steadily increasing since the 1970s, making cancer one of the most frequent causes of death in women (22%) and men (28%).1 In 2012 the incidence of malignant diseases was 14.1 million worldwide, and 8.2 million people died from cancer.2
Owing to the frequency of malignant tumor diseases and the large number of deaths caused by such diseases, curative therapy is of great importance to patients and society. For most tumor entities, therapy consists of a combination of surgical resection, cytotoxic chemotherapy, radiation therapy, and, more recently, targeted therapies. Thanks to these multimodal treatment strategies, cancer death rates have been declining by 15% to 20% in women and 20% to 30% in men since the 1990s,1 putting a larger focus on long-term side effects of cancer treatment.
Efficacy and Neurotoxicity of Cytotoxic Chemotherapy
In this review, the term cytotoxic chemotherapy is used to describe the “classical” chemotherapeutic drugs: ie, vinca alkaloids, folic acid antagonists, pyrimidine and purine analogues, anthracyclines, platinum compounds, taxanes, topoisomerase inhibitors, and cytotoxic antibiotics such as bleomycin and mitomycin. With these compounds, the 5-year survival rate of adult tumor patients can be improved by 2.2% on average. However, these values are an average of 22 tumor types with significant variations. For example, the effect is significantly greater for some tumor diseases: rectal cancer (3.4% to 5.4%), ovarian cancer (8.7% to 8.9%), non-Hodgkin lymphoma (10.5%), cervical cancer (12%), Hodgkin disease (35.8% to 40.3%), and testicular cancer (37.7% to 41.8%).3 Cytotoxic chemotherapy comes with significant side effects, mainly bone marrow suppression, peripheral polyneuropathy, and nausea.4 Many tumor patients treated with chemotherapy also report permanent cognitive impairment.5 Changes in cognition have a great influence on the patients’ quality of life and are often perceived as frustrating.6 The frequency of this phenomenon, which is also often called “chemobrain,” “chemofog,” or “chemodementia” in nonscientific literature, is estimated at 17% to 33% across all chemotherapy patients.7 However, some studies show discrepancies between the cognitive limitations perceived by patients and objectively measurable cognitive deficits: In fact, the perceived cognitive limitations are usually much greater than the objectively measured ones.4,8 At the same time, many tumor patients exhibit concomitant diseases such as depression, anemia, and fatigue, but also infections, which in turn can cause cognitive dysfunctions. These make a careful systematic investigation of the phenomenon demanding.
In 2012 a meta-analysis by Jim et al showed that the cognitive deficits in patients treated with chemotherapy are limited to the domains of verbal ability and visual-spatial ability when compared with a control group or a baseline examination prior to chemotherapy.9 In a meta-analysis from the same year, Hodgson and colleagues also demonstrated limitations in the cognitive domains of executive function and memory.10 A more recent meta-analysis by Ono et al showed that patients with breast cancer may also develop cognitive deficits completely independent of chemotherapy, hence suggesting a direct effect by the tumor on cognition.11 Owing to the heterogeneity of the clinical data, the existence of postchemotherapy cognitive impairment (PCCI)—which is defined as measurable decline of learning and memory after chemotherapy treatment—remains controversial. To make matters worse, only a few studies have investigated the effect of a specific chemotherapy regimen on the cognitive abilities of treated patients. Given the diverse biological effector mechanisms of cytotoxic chemotherapy, it is, however, highly likely that individual substances mediate distinct effects in the CNS and may differ from each other in this respect. In the present review we thus summarize data published between 2006 and 2016 with respect to PCCI.
Materials and Methods
Literature Search in PubMed
A search query with the following combinations of search terms was carried out in the electronic literature database PubMed: “chemotherapy-related cognitive impairment”; “chemotherapy AND cognitive dysfunction AND cancer”; “cancer-survivors AND cognitive impairment AND chemotherapy”; “cognitive function AND chemotherapy AND cancer treatment AND cognition assessment”; “cognition AND cancer AND chemotherapy”; “chemobrain AND cancer AND cognition”; “cognitive disorders AND chemotherapy AND cancer AND cognition assessment”; and “chemobrain AND cancer AND cognition.”
Selection of Studies
The next step was to define quality criteria to evaluate the studies. We followed the guidelines of good clinical practice and included only studies that fulfilled the following inclusion criteria:
1) Studies with adult patients (age ≥18 years)
2) Publication 2006 to 2016
3) Longitudinal prospective studies, ie, patients needed to be examined at least twice, whereby 1 measurement had to be prior to chemotherapy (“baseline”) and at least 1 after chemotherapy.
Furthermore, the following exclusion criteria were defined:
1) Patients must have received chemotherapy with cytotoxic substances. Studies that concerned only immunomodulatory or hormonal drugs, pure radiation therapy, or pure surgical therapy were excluded.
2) Studies examining patients with primary CNS tumors or adult survivors of childhood cancers were excluded.
3) Studies that included patients with brain metastases and/or brain irradiation in their analysis were excluded.
4) Cognitive abilities must have been examined objectively using standardized measurement procedures. Studies that had assessed only patients’ subjective cognitive impairment (SCI) or included only imaging data were excluded.
5) Original data had to be presented in the study. Reviews, meta-analyses, and case reports were excluded.
6) Interventional studies aimed at avoiding cognitive impairment were excluded because the effect of chemotherapy could no longer be clearly detected.
Results
Study Selection and Data Quality
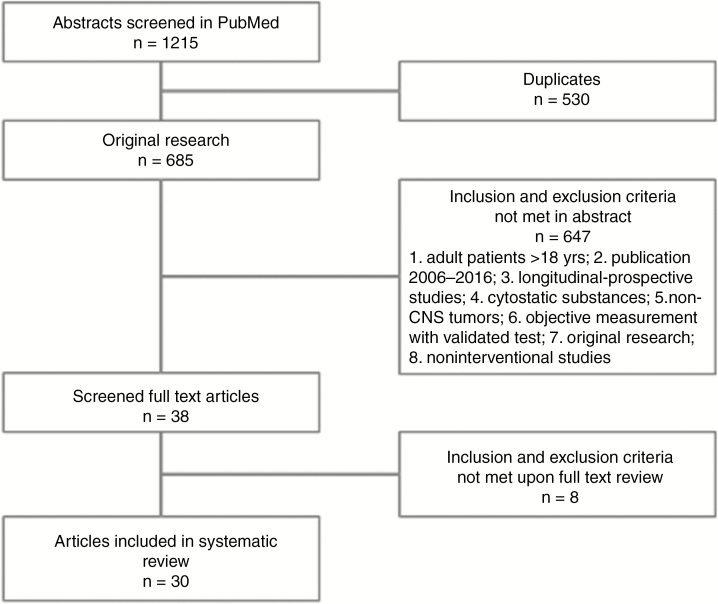
A search of PubMed with the above-mentioned search terms identified 1215 abstracts. Of these, 530 were duplicates. The remaining 685 original abstracts were filtered according to the above-described quality criteria, and 38 studies remained that fulfilled the inclusion and exclusion criteria. Upon review of the full text, an additional 8 studies were excluded for quality reasons (eg, no baseline testing, only patient-reported data), leaving 30 studies to be included for final evaluation. The study selection process is summarized in Fig. 1.
Fig. 1.
Overview of Screening and Elimination Process
Of the remaining 30 studies, 13 studies had investigated only a group of tumor patients treated with chemotherapy. Four studies included a chemotherapy-treated group and a control group of cancer patients who were treated not with chemotherapy but exclusively with antihormonal substances or by surgical resection or targeted radiation. Two studies had investigated chemotherapy-treated tumor patients and a control group of healthy patients. Ten studies had examined chemotherapy patients, a control group of locally or antihormonally treated tumor patients as well as a second control group of healthy patients. One study had included a group of cardiologic patients and a group of healthy volunteers as controls. The characteristics of the included studies are summarized in Table 1. Most studies used standardized and validated neuropsychological tests to assess cognitive impairment.
Table 1.
Summary of Reviewed Studies
| First Author, y | Tumor | CTx Patients, No. | Cytotoxic Drugs | Control Group No. and Treatment | Second Control Group No. and Treatment | Timepoints of Assessment | Examined Cognitive Domains | Cognitive Deficits Observed (Yes or No) |
|---|---|---|---|---|---|---|---|---|
| Ahles, 2010, 2014 | Breast cancer | 60 | DXR+CP (+PTX, 5-FU or DTX) 5-FU+EPR+CP CP+MTX+5-FU |
72 Local treatment | 45 Healthy women | Prior to CTx; 1, 6, and 18 mos after CTx | Verbal and visuospatial memory, working memory, processing speed, concentration, reaction time, premorbid intelligence level | Yes |
| Ando-Tanabe, 2014 | Breast cancer | 18 | DXR+CP/EPR+CP followed by PTX/DTX | 20 Healthy women | – | Prior to CTx; 1 and 6 mos after CTx | Verbal and visuospatial memory, concentration, information processing, executive function | No |
| Andreis, 2013 | Colon cancer | 57 | 5-FU+OX+LV (FOLFOX4) | – | – | Prior to CTx; directly and 6 mos after CTx | Global cognitive function, visuospatial memory, processing speed, verbal memory | No |
| Biglia, 2012 | Breast cancer | 40 | CP+EPR+5-FU (+DTX) (FEC/FEC-T) DXR+PTX | – | – | Prior to CTx; 1, 3 and 6 mos after baseline | Global cognitive function, visual attention, processing speed, short-term memory, verbal learning, intelligence level | Yes |
| Chen, 2014 | Breast cancer | 42 | Not described | 37 Local treatment | 37 Healthy women | Prior to CTx; after CTx | Not described | Yes |
| Cheung, 2015 | Breast cancer | 99 | DXR+CP DTX+CP | – | – | Prior to CTx; 6 wks after baseline and at end of CTx | Processing speed, reaction time, memory, attention | Yes |
| Collins, 2013 | Breast cancer | 28 | Not described | 28 Antihormonal treatment | 28 Healthy volunteers | Prior to CTx; 1 mo after CTx | Executive function, speech, processing speed, verbal and visuospatial memory and function, working memory | Yes |
| Collins, 2013 | Breast cancer | 60 | CP+EPR+5-FU +DTX (FEC-T) (and more) | 60 Healthy women | – | Prior to CTx; after every CTx cycle | Processing speed, working memory, verbal and visual memory | Yes |
| Collins, 2009 | Breast cancer | 53 | CP+EPR+5-FU (FEC) DXR+CP (and more) | 40 Antihormonal therapy | – | Prior to CTx; 1 and 12 mos after CTx | Executive function, speech, processing speed, verbal learning and memory, visuospatial function, working memory, motor function | Yes |
| Cruzado, 2014 | Colon cancer | 81 | 5-FU+OX +LV (FOLFOX4) | – | – | Prior to CTx; prior to last CTx cycle and 6 mos after CTx | Attention, visuomotor function, executive function, verbal learning and memory | Yes |
| Debess, 2010 | Breast cancer | 75 | CP+EPR+5-FU (FEC) | 45 No treatment or antihormonal | 208 Healthy women | Prior to CTx; 1 mo after CTx | Concentration, episodic memory, attention, awareness and flexibility, visual scanning, executive function | Yes |
| Deprez, 2010 | Breast cancer | 34 | CP+EPR+5-FU (+PTX) (FEC/FEC-T) | 16 Local treatment | 19 Healthy women | Prior to CTx; 3-5 mos after CTx | Attention, concentration, memory, executive function, motor processing speed | Yes |
| Hedayati, 2012 | Breast cancer | 18 | CP+EPR+5-FU (FEC) DTX+DXR +CP | 59 No treatment or antihormonal | 69 Healthy women | Prior to surgery; prior to CTx; 1 and 3 mos after CTx | Processing speed, reaction time, memory, attention | Yes |
| Hermelink, 2007, 2008, 2010 | Breast cancer | 101 | EPR+CP+PTX/EPR+PTX+CP+MTX+5-FU | – | – | Prior to CTx; prior to last CT cycle | Verbal memory, attention, working memory, psychomotor function, processing speed, cognitive flexibility, executive function, intelligence | Yes |
| Hess, 2010 | Ovarian cancer | 27 | Not specified | – | – | Prior to CTx; after 3 and 6 cycles of CTx | Attention, processing speed, reaction time | Yes |
| Hess, 2015 | Cancer of ovaries, tubes or peritoneum | 231 | Not specified | – | – | Prior to CTx; after 3 and 6 cycles of CTx; 6 mos after CTx | Processing speed, reaction time, memory, attention | Yes |
| Jansen, 2011 | Breast cancer | 71 | DXR+CP (+DTX) | – | – | Prior to CTx; after 4 cycles, 1 wk and 6 mos after CTx | Short-term and long-term memory, visuospatial function, speech, attention, global cognitive function | Yes |
| Koleck, 2014 | Breast cancer | 37 | Not specified | 41 Antihormonal treatment | 50 Healthy women | Prior to CTx; 6 and 12 mos after baseline | Attention, learning and memory, motor speed, cognitive flexibility, executive function, visuospatial function | Yes |
| Mehlsen, 2009 | Breast cancer | 36 | CP+EPR +5-FU (FEC) | 14 Cardiologic patients | 17 Healthy volunteers | CT group: prior to CTx; 4-6 wks after CTxControls: upon hospitalization; 3 mos after baseline | Processing speed, working memory, visuospatial function, verbal and visual memory, semantic fluidity, reaction inhibition | Yes |
| Quesnel, 2009 | Breast cancer | 41 | DXR+CP(+DTX) CP+EPR+5-FU (FEC) | 40 Radiation | 45 Healthy women | Prior to CTx; directly and 3 mos after CTx (controls only once) | Verbal and visual memory, attention, concentration, executive function, processing speed, semantic fluidity | Yes |
| Stewart, 2008 | Breast cancer | 61 | CP+EPR+5-FU (FEC) DXR+CP (and more) | 51 Antihormonal treatment | – | Prior to CTx; directly after CTx | Executive function, speech, motor speed, processing speed, verbal and visual learning and memory, visuospatial function, working memory | Yes |
| Tager, 2010 | Breast cancer | 30 | DXR+CP (+DTX/PTX) CP+MTX+5-FU | 31 Other adjuvant therapies | – | Prior to CTx; 1 and 7 mos after CTx | Motor speed, speech, attention, concentration, working memory, visuospatial function, verbal and visual memory | Yes |
| Vardy, 2015 | Colorectal cancer | 173 | 5-FU+OX | 116 Surgery only | 72 Healthy volunteers | Prior to CTx; 6, 12, and 24 mos after baseline | Memory, attention, executive function, decision making | No |
| Vearncombe, 2009 | Breast cancer | 136 | Not specified | – | – | Prior to CTx; 1 mo after CTx | Verbal learning and memory, abstraction, motor coordination | Yes |
| Wefel, 2010 | Breast cancer | 42 | CP+EPR+5-FU (+PTX) (FEC/FEC-T) | – | – | Prior to CTx; 3, 7, and 13 mos after baseline | Attention, processing speed, learning and memory | Yes |
| Wefel, 2014 | Germ cell tumors | 55 | Several treatment protocols | 12 Surgery only | – | Prior to CTx; 1 week after CTx; 1 y after baseline | Attention, motor speed, learning and memory, speech, executive function, motor function | Yes |
| Whitney, 2008 | Non–small cell lung cancer | 14 | Not specified | – | – | Prior to CTx; 1 and 7 mos after CTx | Not specified | Yes |
Abbreviations: CP, cyclophosphamide; CTx, chemotherapy; DTX, docetaxel; DXR, doxorubicin; EPR, epirubicin; FEC/FEC-T, 5-fluorouracil, epirubicin, cyclophosphamide, doxorubicin, and taxanes; 5-FU, 5-fluorouracil; LV, leucovorin; MTX, methotrexate; OX, oxaliplatin; PTX, paclitaxel.
Of the studies examined, 8 did not explicitly list which chemotherapy protocols their study patients had received during the investigation of cognitive deficits. The breast cancer patients in most studies were treated with various combinations of cyclophosphamide, epirubicin or doxorubicin, docetaxel or paclitaxel, doxorubicin, and 5-fluorouracil (FEC or FEC-T regimen). Three studies explicitly investigated patients with colorectal cancer or colon carcinoma who were treated with the FOLFOX regimen (folinic acid, 5-fluorouracil, oxaliplatin) or with 5-fluorouracil and oxaliplatin only.
Cognitive Impairment During Chemotherapy
A few studies reported changes in cognition during chemotherapy: Collins et al examined 60 breast cancer patients after each chemotherapy cycle and compared them with a healthy control group. The researchers observed that cognitive performance deteriorated after each chemotherapy cycle, with the domain of memory predominantly being affected and apparent already after the first chemotherapy cycle. Patients received 3 cycles of 5-fluorouracil, epirubicin, and cyclophosphamide followed by 3 cycles of docetaxel (FEC-T regimen).12 This is consistent with the results of Cheung and colleagues, who found that out of 99 breast cancer patients receiving anthracycline-based chemotherapy (doxorubicin and cyclophosphamide), 13% experienced memory loss during chemotherapy compared with baseline. Less-affected domains were attention (7% of patients) and reaction and processing speed (4% of patients).13 Hess et al found deficits in 2 or more cognitive domains after the third chemotherapy cycle compared with baseline in 48% of patients with stage III-IV ovarian carcinoma or primary peritoneal carcinoma in 27 patients who received at least 6 cycles of chemotherapy.14 In conclusion, a significant number of patients experience cognitive decline by as early as the first chemotherapy cycle.
Cognitive Deficits After Completion of Chemotherapy
Far more common (25 out of 30 studies) than the above-mentioned early changes in cognition were reports of significant cognitive deficits in patients when tested immediately or up to 1 month after completion of chemotherapy, compared with baseline and compared with 1 or more control groups.12–36 The frequency of cognitive deterioration after completion of chemotherapy and compared with the baseline varied greatly between 17% and 86% in these studies.
The cognitive domains most affected were (in descending frequency of citation): processing speed (17%),13,20,23,29,30 verbal learning and/or memory (17%),12,16,22,23,35 attention (13%),13,15,21,33 motor function and coordination (13%),20–22,28 executive function (13%),21,23,31,32 memory (10%),13,21,34 reaction speed (7%),13,34 visual memory (7%),25,36 psychomotor speed (7%),27,33 semantic fluidity (7%),19,35 concentration (3%),33 working memory (3%),25 and visuospatial ability (3%).21 The proportion of patients with significant PCCI in 2 or more domains ranged from 3% to 62%.14,20,21,23
Four studies found no significant difference between baseline and postchemotherapy assessment and/or compared with 1 or more control groups.37–40 Vardy et al observed cognitive impairment in colorectal cancer patients compared with healthy controls, although there was no difference between the chemotherapy group and the cancer group without chemotherapy.39 Andreis and colleagues also investigated patients with colon carcinomas treated with a combination of 5-fluorouracil and oxaliplatin and found no changes in cognition due to chemotherapy.37,Hermelink et al reported in 2007 a significant general improvement in cognitive function in 101 breast cancer patients after adjustment for exercise effects in 28% of the patients, whereas 27% of the patients showed significant deterioration.19 In conclusion, most studies measured PCCI immediately or up to 1 month after completion of chemotherapy and found significant changes compared with premorbid values and/or control patients.
Long-Term Cognitive Impairment After Chemotherapy
Quesnel and colleagues reported that breast cancer patients were still impaired in the domain of verbal learning and memory 3 months after completion of chemotherapy.35 According to another study, some breast cancer patients had improvement in their memory function 3 months after chemotherapy compared with effects seen immediately after completion of chemotherapy, but results were not statistically significant.34 Six months after the completion of chemotherapy, the outcome was even more diverse: Some studies reported an improvement in cognition, whereas others measured a further deterioration of cognitive performance. Ahles et al showed that patients had improvement in their verbal ability compared with the performances immediately after chemotherapy.30 This is in line with data from non–small cell lung cancer patients who also showed improvement of the pronounced impairment seen directly after chemotherapy.24 However, in their 2010 study of 42 breast cancer patients Wefel et al found cognitive deficits in 61% of patients 6 months after chemotherapy with 5-fluorouracil, doxorubicin, and cyclophosphamide (FAC regimen). Of these patients, 71% had already shown signs of cognitive deterioration at an acute interval, but 29% had newly developed cognitive dysfunction.23 Wefel and colleagues were able to replicate their results in another trial showing that 52% to 67% of chemotherapy patients had worsened significantly compared with the control group between the examination immediately after chemotherapy and the examination 6 months later in 2 or more cognitive domains.28 Jansen et al reported similar results, with 20% of chemotherapy patients showing further deterioration in 2 or more tests at 6 months after chemotherapy compared with immediate effects. Most affected were visuospatial skills (39%), motor function (21%), short-term memory (18%), attention (14%), and speech (7%).21 However, in most studies, cognitive function remained at the same level compared to immediately after completion of chemotherapy treatment and was still significantly impaired compared with pretreatment levels and/or control.16,20,27 Further long-term evaluation at 1 year after chemotherapy in 2 studies revealed no difference in cognitive functions in the chemotherapy group compared to controls.25,30 In conclusion, in most patients cognitive performance is still affected 6 months after completion of chemotherapy, but seems to regenerate afterward. Only a few patients exhibit a worsening of symptoms after completing chemotherapy.
Clinical Features of Postchemotherapy Cognitive Impairment and Lack of Standardized Testing
Independent of the time point of assessment, the cognitive domains that seem to be most affected in PCCI patients are verbal learning and memory, attention and processing speed as well as visuospatial skills. PCCI patients typically face greater difficulties when presented with a more complex task or multiple tasks, which should be taken into account when choosing a neuropsychological test. Additionally, 1 large issue remains: Present PCCI research lacks standardization in terms of the neuropsychological tests used to assess cognition,41 which hampers comparability of results. Although most of the studies reviewed used validated neuropsychological tests, often the reason for choosing these tests remains unclear and most studies did not state the sensitivity and specificity of the neuropsychological tests with regard to their results.42
Factors Influencing the Development of Postchemotherapy Cognitive Impairment
Koleck et al found that possession of 1 or more apolipoprotein E e4 alleles contributes to poorer verbal learning and memory performance.43 In parallel, Ahles and colleagues also showed that apolipoprotein E e4, especially in combination with smoking, had a negative effect on cognitive function, that was stronger in the chemotherapy group than in the 2 control groups.29
With respect to age, it was demonstrated that older patients were more affected by cognitive impairment after chemotherapy than younger patients.30 Another contributing factor seems to be “cognitive reserve,” which describes the premorbid cognitive ability—in this case before the chemotherapy treatment—compared with age-matched normal values. Older patients with low cognitive reserve were mainly affected by the chemotherapy with regard to processing speed.30 Even though this has not been systematically investigated, one can argue that patients experiencing SCI, mild cognitive impairment, or even dementia prior to the start of chemotherapy could also be strongly affected by PCCI because these patients have a low cognitive reserve as well. To date, premorbid cognitive deficits are a common exclusion criterion for most PCCI studies. In addition, decreased educational status plays an important role in the development of PCCI.20 Not surprisingly a link was demonstrated between cognitive deterioration and decreased mood and depressive symptoms in chemotherapy patients.26,38 Interestingly, elevated levels of interleukin-1 and interleukin-6 were measured in PCCI patients,13 which might offer some insights into underlying pathophysiological processes.
Clinical Treatment Approaches
Different neurostimulants have been assessed in cancer patients suffering mainly from cancer-related fatigue. Several smaller prospective trials indicate that modafinil, which increases catecholaminergic signaling, can enhance speed of memory, episodic memory, and attention skills. It is unclear whether these effects are the result of improved fatigue symptoms or an actual improvement in memory (reviewed by Davis et al44). Methylphenidate, which acts in a similar fashion to amphetamines, has no effects on cognitive dysfunction in adults suffering from cancer,45,46 but can improve hypoactive delirium.47 Rodent research has pointed to an improvement in PCCI by administration of donepezil,48 a centrally acting anticholinergic drug. Smaller studies indicated that children with brain irradiation and adults with primary CNS tumors also benefitted in terms of cognitive function,49,50 but no systematic data are available on PCCI. The major side effects of donepezil include vomiting, diarrhea, and weight loss, limiting its administration in cancer patients. The effects of memantine, an NMDA (N-methyl-d-aspartate) antagonist, have clinically been studied only in patients with whole-brain irradiation. Here it was shown that memantine could prolong the cognitive decline associated with irradiation.51 To our knowledge, at the time of writing this review, no systematic clinical data exist regarding the treatment of PCCI.
Discussion
A major limitation of this review and the overall research in the field is the fact that most studies (23 out of 30 studies in this review alone) have focused on breast cancer patients, which leads to an inherent bias. This issue seems to be hard to overcome given that breast cancer patients represent the ideal cohort for clinical research on this topic for a multitude of reasons: a) breast cancer is a common tumor entity; b) breast cancer patients usually have limited comorbidities and are in good general health, limiting confounders; c) breast cancer survival rates allow for longitudinal assessment; and d) there are a variety of treatment options allowing for proper control groups. Vardy et al noted that more studies are emerging focusing on PCCI in other tumor cohorts, but to date the overwhelming evidence still remains from breast cancer patients.41
Another major limitation of the clinical trials on PCCI and also the most plausible explanation for the heterogeneity of the data is that the vast majority of studies (73% in this review alone) do not specify which chemotherapy the patients received and, more important, included patients with different chemotherapy protocols in their trials. Because it can be assumed that not all cytotoxic drugs exhibit the same effect on different cells of the CNS, it will be important in future research to explicitly distinguish between different chemotherapy protocols and to investigate their respective effects on cognition. It is noteworthy, however, that out of 3 studies that investigated patients with colorectal carcinomas treated with FOLFOX,16,37,39 2 studies found no significant changes in cognitive performance after chemotherapy. In contrast, 21 out of 23 studies investigating breast cancer patients with different chemotherapy protocols found significant cognitive impairment in their patients after chemotherapy. In most trials, breast cancer patients were treated with a combination of the following cytotoxic drugs: cyclophosphamide, epirubicin or doxorubicin, docetaxel or paclitaxel, doxorubicin, and 5-fluorouracil (FAC or FEC [+T]). This is perhaps a first indication that different cytotoxic drugs also have different toxic effects on the CNS.
Adding to the heterogeneity of the data is the lack of standardization of neuropsychological tests in studies investigating PCCI,8 the often absent reports on sensitivity and specificity of neuropsychological tests with regard to PCCI,42 and a missing consensus as to how the data should be analyzed and interpreted.52 Furthermore, future clinical trials should correct for learning or practice effects if repeated neuropsychological testing is conducted, particularly if fewer than 6 months apart.53 Additionally, one can argue that the available neuropsychological tests to date may not be sensitive enough to detect subtle changes in cognition such as PCCI, which is a problem that has previously emerged when investigating SCI. Therefore, more research efforts are needed to develop new testing methods and validate these first in healthy patients to establish age-dependent values of the norm and then, in a second step, verify their sensitivity in larger cohorts of patients with various subtypes of cognitive decline.
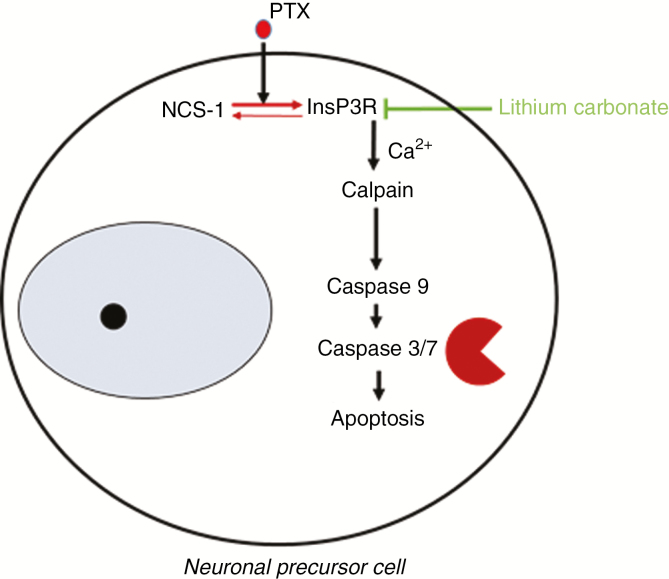
Another major limitation of clinical PCCI research is rooted in the very nature of chemotherapy treatment, which consists mostly of a combination therapy of 2 or more cytotoxic substances, making specific changes in cognition difficult to trace back to 1 substance. Here, preclinical experimental research provides a clear advantage: Individual (dose-dependent) effects of cytotoxic drugs can be studied more directly on different cells of the nervous system as well as in healthy rodents, thereby excluding cancer-associated alterations and limiting confounding factors. Furthermore, it allows the tracking of abnormalities induced by cytotoxic drugs on multiple levels ranging from molecular and cellular alterations to functional and histological changes. For example, using cell-culture techniques, we were previously able to demonstrate that the microtubule-stabilizing agent paclitaxel enhances the interaction of the neuronal calcium sensor 1-protein (NCS-1) with the inositol-1,4,5-trisphosphate-receptor (InsP3R), which results in a calcium (Ca2+) efflux from the endoplasmic reticulum into the cytosol. Cytoplasmic Ca2+ spikes trigger the Ca2+-dependent protease calpain,54,55 activating caspase-mediated cell death mechanisms56 (Fig. 2). In adult mice, clinically relevant doses of paclitaxel not only induced a sensory axonal polyneuropathy, but also led to the development of distinct visuospatial learning and memory deficits much like in PCCI patients. We traced these changes back to an impaired hippocampal neurogenesis: Interestingly, paclitaxel concentrations increased by 7-fold in the hippocampus compared with the neocortex.56 The NCS-1/InsP3R interaction can be successfully inhibited with lithium ions (Li+). In fact, we were able to demonstrate that cells are protected from paclitaxel-induced cell death by (sub-)therapeutic levels of Li+, and mice preemptively treated with Li+ did not develop paclitaxel-induced cognitive changes.56 Upstream of the NCS-1/InsP3R pathway, another potential molecular target could be the pathway by which paclitaxel enters neuronal cells: Various groups have shown that paclitaxel enters dorsal-root ganglia via an organic anion-transporting polypeptide (OATP) and genetic or pharmacologic knockout of OATP1B2 inhibits the development of paclitaxel-induced neuropathies.57 Further downstream of the NCS-1/InsP3R interaction, the above-mentioned activation of calpain leads to the transduction of nuclear factor–κB into the nucleus and consecutive release of interleukin-6 by dorsal root ganglia neurons, which in turn initiates a secondary neuroimmune interaction (Huehnchen et al, unpublished). Whether these findings can be extrapolated to paclitaxel-induced cognitive impairment is currently under investigation.
Fig. 2.
Summary of Molecular Effects Induced in Neuronal Precursor Cells by the Cytotoxic Drug Paclitaxel
Binding of paclitaxel to NCS-1 stabilizes the Ca2+-bound conformation of this protein and thus causes an increased binding of NCS-1 to the InsP3R. As a result, the InsP3R is positively modulated, and an increased release of Ca2+ from the endoplasmic reticulum to the cytoplasm occurs. Ca2+ in turn, among other cellular processes, activates the Ca2+-dependent protease µ-calpain, which not only degrades NCS-1 in a negative feedback loop, but is also able to trigger a number of molecular pathways, including apoptosis. Interaction of NCS-1 with the InsP3R can be inhibited with lithium ions. Ca2+ indicates calcium; InsP3R, inositol-1,4,5-trisphosphate-receptor; NCS-1, neuronal calcium sensor 1-protein; PTX, paclitaxel. Figure adapted from Huehnchen et al.56
These findings open the perspective to preventive neuroprotective treatments whose cognitive side effects may be prevented by a substance-specific neuroprotective comedication. Thus far, preclinical studies have focused only on de novo changes. However, it would also be of clinical relevance to test whether symptoms of PCCI would be enhanced in mice with premorbid cognitive dysfunctions such as Alzheimer models.
Conclusions
Despite the heterogeneity of the clinical data with regard to the incidence of PCCI, the overwhelming amount of longitudinal prospective trials, meta-analyses, and imaging studies all point to a neurobiological basis of PCCI. This notion is supported by preclinical research. The growing concern of cancer patients with regard to this phenomenon should be directly addressed by physicians. Whereas some patients show a stagnant clinical remission, most patients’ cognitive function improved within 1 year after completing chemotherapy, depending on age and cognitive reserve. Further prospective trials are urgently needed that examine the effects of specific drugs and drug combinations on patients’ cognition and stratifying for known risk factors as well as controlling for age, education, and premorbid cognitive function. Promising novel neuroprotective strategies should be translated to clinical trials.
Funding
This work was been supported by the Federal Ministry of Education and Research via a grant from the Center for Stroke Research Berlin [01 EO 0801 to M.E.]; the Deutsche Forschungsgemeinschaft [EXC 257 NeuroCure]; and the Rahel Hirsch fellowship program of the Charité Universitätsmedizin Berlin [to P.H.].
Conflict of interest statement. None declared.
References
- 1. Barnes B, Kraywinkel K, Nowossadeck E, et al. Bericht zum Krebsgeschehen in Deutschland 2016. Berlin: Robert Koch-Institut; 2016. [Google Scholar]
- 2. International Agency for Research on Cancer. World cancer factsheet. 2014. https://www.cancerresearchuk.org/sites/default/files/cs_report_world.pdf [Google Scholar]
- 3. Morgan G, Ward R, Barton M. The contribution of cytotoxic chemotherapy to 5-year survival in adult malignancies. Clin Oncol (R Coll Radiol). 2004;16(8):549–560. [DOI] [PubMed] [Google Scholar]
- 4. Vardy J, Tannock I. Cognitive function after chemotherapy in adults with solid tumours. Crit Rev Oncol Hematol. 2007;63(3):183–202. [DOI] [PubMed] [Google Scholar]
- 5. Ahles TA, Saykin A. Cognitive effects of standard-dose chemotherapy in patients with cancer. Cancer Invest. 2001;19(8):812–820. [DOI] [PubMed] [Google Scholar]
- 6. Boykoff N, Moieni M, Subramanian SK. Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surviv. 2009;3(4):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boehmerle W, Huehnchen P, Endres M. Neurologische Nebenwirkungen von Zytostatika. Aktuelle Neurologie. 2014;41(1):21–34. [Google Scholar]
- 8. Tannock IF, Ahles TA, Ganz PA, Van Dam FS. Cognitive impairment associated with chemotherapy for cancer: report of a workshop. J Clin Oncol. 2004;22(11):2233–2239. [DOI] [PubMed] [Google Scholar]
- 9. Jim HS, Phillips KM, Chait S, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol. 2012;30(29):3578–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hodgson KD, Hutchinson AD, Wilson CJ, Nettelbeck T. A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev. 2013;39(3):297–304. [DOI] [PubMed] [Google Scholar]
- 11. Ono M, Ogilvie JM, Wilson JS, et al. A meta-analysis of cognitive impairment and decline associated with adjuvant chemotherapy in women with breast cancer. Front Oncol. 2015;5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Collins B, MacKenzie J, Tasca GA, Scherling C, Smith A. Cognitive effects of chemotherapy in breast cancer patients: a dose-response study. Psychooncology. 2013;22(7):1517–1527. [DOI] [PubMed] [Google Scholar]
- 13. Cheung YT, Ng T, Shwe M, et al. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann Oncol. 2015;26(7):1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hess LM, Chambers SK, Hatch K, et al. Pilot study of the prospective identification of changes in cognitive function during chemotherapy treatment for advanced ovarian cancer. J Support Oncol. 2010;8(6):252–258. [DOI] [PubMed] [Google Scholar]
- 15. Biglia N, Bounous VE, Malabaila A, et al. Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: a prospective study. Eur J Cancer Care (Engl). 2012;21(4):485–492. [DOI] [PubMed] [Google Scholar]
- 16. Cruzado JA, López-Santiago S, Martínez-Marín V, José-Moreno G, Custodio AB, Feliu J. Longitudinal study of cognitive dysfunctions induced by adjuvant chemotherapy in colon cancer patients. Support Care Cancer. 2014;22(7):1815–1823. [DOI] [PubMed] [Google Scholar]
- 17. Hermelink K, Henschel V, Untch M, Bauerfeind I, Lux MP, Munzel K. Short-term effects of treatment-induced hormonal changes on cognitive function in breast cancer patients: results of a multicenter, prospective, longitudinal study. Cancer. 2008;113(9):2431–2439. [DOI] [PubMed] [Google Scholar]
- 18. Hermelink K, Küchenhoff H, Untch M, et al. Two different sides of ‘chemobrain’: determinants and nondeterminants of self-perceived cognitive dysfunction in a prospective, randomized, multicenter study. Psychooncology. 2010;19(12):1321–1328. [DOI] [PubMed] [Google Scholar]
- 19. Hermelink K, Untch M, Lux MP, et al. Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer. 2007;109(9):1905–1913. [DOI] [PubMed] [Google Scholar]
- 20. Hess LM, Huang HQ, Hanlon AL, et al. Cognitive function during and six months following chemotherapy for front-line treatment of ovarian, primary peritoneal or fallopian tube cancer: an NRG oncology/gynecologic oncology group study. Gynecol Oncol. 2015;139(3):541–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19(10):1647–1656. [DOI] [PubMed] [Google Scholar]
- 22. Vearncombe KJ, Rolfe M, Wright M, Pachana NA, Andrew B, Beadle G. Predictors of cognitive decline after chemotherapy in breast cancer patients. J Int Neuropsychol Soc. 2009;15(6):951–962. [DOI] [PubMed] [Google Scholar]
- 23. Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116(14):3348–3356. [DOI] [PubMed] [Google Scholar]
- 24. Whitney KA, Lysaker PH, Steiner AR, Hook JN, Estes DD, Hanna NH. Is “chemobrain” a transient state? A prospective pilot study among persons with non–small cell lung cancer. J Support Oncol. 2008;6(7):313–321. [PubMed] [Google Scholar]
- 25. Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psychooncology. 2009;18(2):134–143. [DOI] [PubMed] [Google Scholar]
- 26. Stewart A, Collins B, Mackenzie J, Tomiak E, Verma S, Bielajew C. The cognitive effects of adjuvant chemotherapy in early stage breast cancer: a prospective study. Psychooncology. 2008;17(2):122–130. [DOI] [PubMed] [Google Scholar]
- 27. Tager FA, McKinley PS, Schnabel FR, et al. The cognitive effects of chemotherapy in post-menopausal breast cancer patients: a controlled longitudinal study. Breast Cancer Res Treat. 2010;123(1):25–34. [DOI] [PubMed] [Google Scholar]
- 28. Wefel JS, Vidrine DJ, Marani SK, et al. A prospective study of cognitive function in men with non-seminomatous germ cell tumors. Psychooncology. 2014;23(6):626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ahles TA, Li Y, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: the impact of APOE and smoking. Psychooncology. 2014;23(12):1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28(29):4434–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen X, Li J, Zhu C, Li D, Zhang J, Wang K. Cognitive function in breast cancer patients on chemotherapy: a longitudinal study [article in Chinese]. Zhonghua Yi Xue Za Zhi. 2014;94(1):27–30. [PubMed] [Google Scholar]
- 32. Debess J, Riis JØ, Engebjerg MC, Ewertz M. Cognitive function after adjuvant treatment for early breast cancer: a population-based longitudinal study. Breast Cancer Res Treat. 2010;121(1):91–100. [DOI] [PubMed] [Google Scholar]
- 33. Deprez S, Amant F, Smeets A, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30(3):274–281. [DOI] [PubMed] [Google Scholar]
- 34. Hedayati E, Alinaghizadeh H, Schedin A, Nyman H, Albertsson M. Effects of adjuvant treatment on cognitive function in women with early breast cancer. Eur J Oncol Nurs. 2012;16(3):315–322. [DOI] [PubMed] [Google Scholar]
- 35. Quesnel C, Savard J, Ivers H. Cognitive impairments associated with breast cancer treatments: results from a longitudinal study. Breast Cancer Res Treat. 2009;116(1):113–123. [DOI] [PubMed] [Google Scholar]
- 36. Collins B, Mackenzie J, Kyeremanteng C. Study of the cognitive effects of chemotherapy: considerations in selection of a control group. J Clin Exp Neuropsychol. 2013;35(4):435–444. [DOI] [PubMed] [Google Scholar]
- 37. Andreis F, Ferri M, Mazzocchi M, et al. Lack of a chemobrain effect for adjuvant FOLFOX chemotherapy in colon cancer patients. A pilot study. Support Care Cancer. 2013;21(2):583–590. [DOI] [PubMed] [Google Scholar]
- 38. Ando-Tanabe N, Iwamitsu Y, Kuranami M, et al. Cognitive function in women with breast cancer receiving adjuvant chemotherapy and healthy controls. Breast Cancer. 2014;21(4):453–462. [DOI] [PubMed] [Google Scholar]
- 39. Vardy JL, Dhillon HM, Pond GR, et al. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: a prospective, longitudinal, controlled study. J Clin Oncol. 2015;33(34):4085–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mehlsen M, Pedersen AD, Jensen AB, Zachariae R. No indications of cognitive side-effects in a prospective study of breast cancer patients receiving adjuvant chemotherapy. Psychooncology. 2009;18(3):248–257. [DOI] [PubMed] [Google Scholar]
- 41. Vardy J, Wefel JS, Ahles T, Tannock IF, Schagen SB. Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Ann Oncol. 2008;19(4):623–629. [DOI] [PubMed] [Google Scholar]
- 42. Jansen CE, Miaskowski CA, Dodd MJ, Dowling GA. A meta-analysis of the sensitivity of various neuropsychological tests used to detect chemotherapy-induced cognitive impairment in patients with breast cancer. Oncol Nurs Forum. 2007;34(5):997–1005. [DOI] [PubMed] [Google Scholar]
- 43. Koleck TA, Bender CM, Sereika SM, et al. Apolipoprotein E genotype and cognitive function in postmenopausal women with early-stage breast cancer. Oncol Nurs Forum. 2014;41(6):E313–E325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Davis J, Ahlberg FM, Berk M, Ashley DM, Khasraw M. Emerging pharmacotherapy for cancer patients with cognitive dysfunction. BMC Neurol. 2013;13:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mar Fan HG, Clemons M, Xu W, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer. 2008;16(6):577–583. [DOI] [PubMed] [Google Scholar]
- 46. Lower EE, Fleishman S, Cooper A, et al. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. J Pain Symptom Manage. 2009;38(5):650–662. [DOI] [PubMed] [Google Scholar]
- 47. Gagnon B, Low G, Schreier G. Methylphenidate hydrochloride improves cognitive function in patients with advanced cancer and hypoactive delirium: a prospective clinical study. J Psychiatry Neurosci. 2005;30(2):100–107. [PMC free article] [PubMed] [Google Scholar]
- 48. Winocur G, Binns MA, Tannock I. Donepezil reduces cognitive impairment associated with anti-cancer drugs in a mouse model. Neuropharmacology. 2011;61(8):1222–1228. [DOI] [PubMed] [Google Scholar]
- 49. Castellino SM, Tooze JA, Flowers L, et al. Toxicity and efficacy of the acetylcholinesterase (AChe) inhibitor donepezil in childhood brain tumor survivors: a pilot study. Pediatr Blood Cancer. 2012;59(3):540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shaw EG, Rosdhal R, D’Agostino RB Jr, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24(9):1415–1420. [DOI] [PubMed] [Google Scholar]
- 51. Brown PD, Shook S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiation therapy (WBRT): first report of RTOG 0614, a placebo-controlled, double-blind, randomized trial. Int J Radiat Oncol Biol Phys. 2012;84(3 suppl):S1–S2. [Google Scholar]
- 52. Shilling V, Jenkins V, Trapala IS. The (mis)classification of chemo-fog—methodological inconsistencies in the investigation of cognitive impairment after chemotherapy. Breast Cancer Res Treat. 2006;95(2):125–129. [DOI] [PubMed] [Google Scholar]
- 53. Cerulla N, Arcusa À, Navarro JB, et al. Cognitive impairment following chemotherapy for breast cancer: the impact of practice effect on results. J Clin Exp Neuropsychol. 2019;41(3):290–299. [DOI] [PubMed] [Google Scholar]
- 54. Boehmerle W, Splittgerber U, Lazarus MB, et al. Paclitaxel induces calcium oscillations via an inositol 1,4,5-trisphosphate receptor and neuronal calcium sensor 1-dependent mechanism. Proc Natl Acad Sci U S A. 2006;103(48):18356–18361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boehmerle W, Zhang K, Sivula M, et al. Chronic exposure to paclitaxel diminishes phosphoinositide signaling by calpain-mediated neuronal calcium sensor-1 degradation. Proc Natl Acad Sci U S A. 2007;104(26):11103–11108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huehnchen P, Boehmerle W, Springer A, Freyer D, Endres M. A novel preventive therapy for paclitaxel-induced cognitive deficits: preclinical evidence from C57BL/6 mice. Transl Psychiatry. 2017;7(8):e1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leblanc AF, Sprowl JA, Alberti P, et al. OATP1B2 deficiency protects against paclitaxel-induced neurotoxicity. J Clin Invest. 2018;128(2):816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]