Abstract
Background
The incidence of symptomatic radiation necrosis (RN) has risen as radiotherapy is increasingly used to control brain tumor progression. Traditionally managed with steroids, symptomatic RN can remain refractory to medical treatment, requiring surgical intervention for control. The purpose of our study was to assess a single institution’s experience with craniotomy for steroid-refractory pure RN.
Methods
The medical records of all tumor patients who underwent craniotomies at our institution from 2011 to 2016 were retrospectively reviewed for a history of preoperative radiotherapy or radiosurgery. RN was confirmed histopathologically and patients with active tumor were excluded. Preoperative, intraoperative, and outcome information was collected. Primary outcomes measured were postoperative KPS and time to steroid freedom.
Results
Twenty-four patients with symptomatic RN were identified. Gross total resection was achieved for all patients. Patients with metastases experienced an increase in KPS (80 vs 100, P < .001) and required a shortened course of dexamethasone vs patients with high-grade gliomas (3.4 vs 22.2 weeks, P = .003). RN control and neurological improvement at 13.3 months’ follow-up were 100% and 66.7%, respectively. Adrenal insufficiency after rapidly tapering dexamethasone was the only morbidity (n = 1). Overall survival was 93.3% (14/15) at 1 year.
Conclusion
In cases of treatment-refractory symptomatic RN, resection can lead to an overall improvement in postoperative health status and neurological outcomes with minimal RN recurrence. Craniotomy for surgically accessible RN can safely manage symptomatic patients, and future studies assessing the efficacy of resection vs bevacizumab may be warranted.
Keywords: metastatic brain tumor, outcomes, primary brain tumor, radiation necrosis, salvage craniotomy
With the increasing use of radiation therapy (RT) in neuro-oncology for metastatic and primary brain tumors and longer patient outcomes, the incidence of radiation-induced necrosis (RN) has increased significantly in the last decade.1–3 RN classically demonstrates histological changes including inflammatory infiltrate, coagulative, and liquefactive necrosis and vascular damage that may produce significant edema and symptomatology.4–6 Depending on the location, RN manifests with mass effect symptoms such as personality changes, seizures, headaches or focal deficits.4,7 The diagnosis of suspected RN classically relies on a combination of clinic-radiologic criteria, timing of radiation (>6 months), and metabolic/perfusion imaging; however, tissue diagnosis is the only definitive confirmatory test.8,9
When symptomatic, suspected RN is first managed medically with a short course of steroids; however, many patients may remain steroid dependent or refractory to high-dose steroids. Additionally, steroids have both long and short-term toxicity and may affect systemic treatment. In these select patients, salvage medical treatment options such as VEGF inhibitors (bevacizumab), hyperbaric oxygen, and antiplatelet and anticoagulation medications have been offered with varying degrees of success.5,10–12 In cases of treatment-refractory or “malignant radiation necrosis,” more invasive treatment options such as laser interstitial thermal therapy or surgical resection may be required. However, the current literature surrounding the safety and efficacy of surgical resection is limited to smaller case series and case studies, and direct comparison is difficult because of a lack of consistency in histopathology and reporting outcomes.13–18 The goal of this study is therefore to assess a single institution’s experience with craniotomy for confirmed treatment-refractory symptomatic radiation. To our knowledge, this remains the largest study to date assessing the utility of craniotomies for this indication.
Methods
Patient Selection
After institutional review board approval, the medical records of 1400 brain tumor patients who underwent a craniotomy at our institution between 2011 to 2016 were retrospectively reviewed for a history of preoperative RT. Patients with either metastatic or primary intracranial brain tumors were included. WHO grade III and IV astrocytomas were grouped as high-grade gliomas (HGGs). A total of 136 patients were identified with preoperative RT and were further assessed for the presence or absence of radiation necrosis. Patients received whole-brain radiotherapy (WBRT), external beam radiotherapy (EBRT), or stereotactic radiosurgery (SRS). RN was confirmed by our institution’s pathology department using standard histopathological criteria, and patients with any fraction of active tumor were excluded. Because each patient received a craniotomy with maximal tumor resection, a large mass of gross tumor tissue could be sent from each procedure to our pathologists. Each patient had between 2 to 6 gross tumor samples sent as either 1 or multiple sections for pathological analysis, depending on lesion size, and if any single sample was positive for active tumor cells, the patient was excluded. Active tumor was determined by Ki-67 staining for nuclear proliferation and phosphohistone H3 staining for mitotic figures. Histopathological criteria for radiation necrosis were defined as the following: coagulative and fibrinoid necrosis, weakly active inflammatory areas, hyalinized vasculature, and focal areas of perivascular lymphocytes.6 Only patients who failed conservative trial of steroid treatment (less than 1 month) were included in our analysis. Treatment-refractory RN was defined as radiographic or symptomatic progression of disease despite high-dose steroids, or prolonged (greater than 4 weeks) steroid dependence.
Data Gathering
Data related to demographics, radiation history, tumor diagnosis, surgical procedure, and outcomes were collected from patient charts. The presence of hypermethylated O-6-methylguanine-DNA methyltransferase and mutated isocitrate dehydrogenase (IDH) genetic mutations was reported when present. Radiological interpretation of cerebral blood volume (CBV) changes on preoperative MRI perfusion scans was also included when present. Craniotomies were either gross total or subtotal resections as determined by postoperative MR imaging. The primary outcomes were changes in baseline KPS (measured at 2 weeks postprocedure) and steroid freedom. Preoperatively, steroid-dependent patients typically remained on 2 to 4 mg of dexamethasone every 6 hours. Local control was defined as the length of time after surgery without evidence of radiographic disease in the surgical cavity. Progression-free survival (PFS) was defined as time after craniotomy until the patient experienced a worsening of neurological symptoms or the development of new neurological symptoms. Neurological improvement was defined as an improvement in the neurological physical exam at the latest follow-up time point as conducted by a single neurologist at our institution and included focal deficits, headache, vertigo, and seizure activity. Information related to neurological improvement, PFS, and overall survival was collected from a retrospective review of medical records. Overall improvement in the long-term was defined as an improvement in KPS at last follow-up.
Statistical Analysis
Mean differences in time to surgery by tumor type were analyzed using one-way ANOVA. Preoperative, postoperative, and long-term KPS scores were compared using a paired 2-tailed t-test. PFS and steroid-freedom survival curves were modeled using Kaplan-Meier curves and analyzed using log-rank analysis. Patients who were lost to follow-up were censored after their longest follow-up time point. Missing data points were excluded when computing results. P values <.05 were indicative of statistical significance. Statistical analysis and graph creation was performed using MATLAB R2017B (MathWorks) and JMP Pro Version 14 (SAS Institute).
Results
Patient Cohort
Twenty-four patients with histologically confirmed radiation necrosis were identified (Table 1). Mean age at surgery was 60.3 ± 10.0 years and 14 patients were female. The original pathology was metastases (58.3%, n = 14), HGG (25%,n = 6), meningioma (12.5%,n = 3), and esthesioneuroblastoma (4.2%, n = 1) (Table 2). The primary origin of metastatic disease was lung (42.8%, n = 6), breast (35.7%, n = 5), dermatologic including squamous cell and melanoma (14.3%, n = 2), and renal (7.1%, n = 1). Less than half of the patients had a history of a previous craniotomy (45.8%, n = 11). Patients received an average of 41.5 ± 17.1 Gy at 23.4 ± 24.6 months prior to surgery. Average time to discharge after surgery was 1.4 days, and 100% (n = 24) of patients underwent gross total resection of their lesion. Mean PFS was 11 months (range, 0.4-42.4 months) at a mean follow-up of 13.3 months (range, 0.42-42.4 months) after surgery. Overall, the majority of patients experienced immediate improvement in symptomatology (66.7%, n = 16), with 33.3% (n = 8) remaining at their neurologic baseline. No patients experienced clinical deterioration after surgery. Neurological improvement at last follow-up was noted in 66.7% of patients (n = 16), and 8.3% (n = 2) remained at their neurological baseline. Local control was 91.7% (n = 22) in our series. Median time to steroid freedom after surgery was 2.4 weeks. Overall survival rate was 93.3% (14/15) at 1 year and 66.7% (4/6) at 2 years after craniotomy.
Table 1.
Baseline Characteristics for 24 Patients With Radiation Necrosis
| Patient Characteristicsa | (N = 24) |
|---|---|
| Age (years) | 60.3 (57-67) |
| Gender | |
| Male, n (%) | 10 (41.7%) |
| Female, n (%) | 14 (58.3%) |
| Tumor | |
| HGG, n (%) | 6 (25%) |
| Metastasis, n (%) | 14 (58.3%) |
| Meningioma, n (%) | 3 (12.5%) |
| Esthesioneuroblastoma, n (%) | 1 (4.2%) |
| PFS (months) | 3.8 (.55-16) |
| Follow-up (months) | 7.8 (.79-18.7) |
| Off steroid (weeks) | 2.4 (2-6.5) |
Abbreviations: HGG, high-grade glioma; PFS, progression-free survival; Q1, first quartile; Q3: third quartile.
aMedian value (Q1, Q3).
Table 2.
Case Series of Surgical Resection for Radiation Necrosis
| Case | Tumor | RT/Gy (IDL %) | Age/Sex | RT to Surgery Time (mo) | Preop KPS | Postop KPS | Off-Steroids Time (wks) | Latest FU Time (mo) | Latest KPS | PFSa (mo) | Worsening/New Symptoms at Progressionc | Neurological Improvement on Latest Exam | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Meningioma | SRS/– | 70/F | 26.5 | 80 | 100 | 2.29 | .53 | 100 | .53 | – | Y | – |
| Case 2 | Otherb | EBRT/60 | 48/M | 7.6 | 80 | 90 | 2.29 | 13.65 | 90 | 6.55 | Impulsiveness; difficulty concentrating | Y | – |
| Case 3 | HGG | –/– | 60/F | 22.6 | 80 | 90 | 14.43 | 5.25 | 70 | 2.5 | L-sided weakness | Same | TTR 2.5 mo; fell at long-term FU |
| Case 4 | HGG | EBRT/62.5; SRS/– | 34/M | 11.74 | 100 | 100 | 75.7 | 20.61 | 90 | 15.81 | Fall requiring hospitalization | Y | IDH, MGMT + |
| Case 5 | Metastasis (lung) | SRS/20 (72) | 67/F | 6.55 | 90 | 100 | 5 | 16.48 | 0 | 16.48 | – | N | Adrenal insufficiency postop; expired at 16.48 mo |
| Case 6 | Meningioma | SRS/– | 72/F | 66.42 | 20 | 100 | 1 | .5 | 100 | .5 | – | Y | – |
| Case 7 | HGG | EBRT/59.4 | 60/M | 20.81 | 100 | 100 | 7.86 | 6.81 | 70 | 1.32 | L-arm/hand numbness and tingling | N | TTR 1.29 mo; MGMT + |
| Case 8 | HGG | EBRT/60 | 62/F | 21.84 | 80 | 80 | 2.14 | .55 | 80 | .55 | – | Same | Placed on continuous low-dose dexamethasone |
| Case 9 | Metastasis (lung) | SRS/18; EBRT/30 | 70/F | 18.81 | 90 | 90 | 6 | 18.13 | 80 | 8.77 | R-hand progressive numbness | N | Bevacizumab after surgery |
| Case 10 | Meningioma | SRS/20 (87) | 54/F | 119.68 | 80 | 90 | 8.42 | 62 | 90 | 57 | L-leg weakness; focal seizures | Y | – |
| Case 11 | Metastasis (skin) | SRS/20 (50); WBRT/30 | 62/M | 15.77 | 70 | 100 | 2.42 | 6.35 | 100 | 6.35 | – | Y | – |
| Case 12 | Metastasis (lung) | SRS/21 (50) | 66/M | 33.29 | 90 | 100 | 2 | 6.94 | 90 | 4.87 | Sharp, radiating headaches | Y | – |
| Case 13 | HGG | EBRT/– | 76/F | 7.79 | 90 | 90 | 9.85 | 21.42 | 80 | 21.42 | – | Y | – |
| Case 14 | Metastasis (breast) | SRS/20 (77) | 59/F | 41.5 | 100 | 100 | 4.43 | 12.29 | 90 | 12.29 | – | Y | – |
| Case 15 | Metastasis (lung) | WBRT/37.5; SRS/18 | 57/F | 8.93 | 90 | 100 | 9.42 | 14 | 20 | 2.8 | L homonymous hemianopsia; constant headaches | N | Hospital for brain herniation at long-term FU |
| Case 16 | Metastasis (breast) | SRS/25 | 64/F | 12.77 | 80 | 100 | 2.3 | .5 | 100 | .5 | – | Y | – |
| Case 17 | HGG | EBRT/54.9 | 52/M | 4.48 | 80 | 100 | 3 | 42.39 | 100 | 42.39 | – | Y | |
| Case 18 | Metastasis (breast) | WBRT/30; SRS/20 (50) | 38/F | 30 | 70 | 70 | 1 | 8.6 | 0 | 0 | – | N | Immediate symptom progression; expired at 8.6 mo |
| Case 19 | Metastasis (kidney) | SRS/24 (100) | 65/M | 19.9 | 100 | 100 | 1 | 20.52 | 100 | 20.52 | – | Y | – |
| Case 20 | Metastasis (lung) | WBRT/35 | 55/M | 12.94 | 70 | 90 | 5.85 | 1.32 | 90 | 1.32 | – | Y | – |
| Case 21 | Metastasis (lung) | SRS/– | 69/M | 8.8 | 80 | 90 | 1.71 | .42 | 90 | .42 | – | Y | – |
| Case 22 | Metastasis (breast) | WBRT/32.3; SRS/25 | 68/F | 8 | 80 | 100 | 2 | 39.74 | 90 | 39.74 | – | Y | – |
| Case 23 | Metastasis (breast) | SRS/24 | 62/F | 15.13 | 80 | 90 | 2 | .87 | 80 | .87 | Mental status changes; urinary incontinence | N | Progression happened at latest FU |
| Case 24 | Metastasis (skin) | SRS/– | 69/M | 19 | 80 | 90 | 2 | .42 | 30 | .42 | Syncope, inability to move | Y | Progression happened at latest FU |
Abbreviations: EBRT, external beam radiotherapy; F, female; FU, follow-up; HGG, high-grade glioma; Gy, Gray; IDH, isocitrate dehydrogenase; IDL, isodose line %; L, left; M, male; MGMT, O-6-methylguanine-DNA methyltransferase; mo, months; N, no; NP, no progression to date; PFS, progression-free survival; R, right; RN, radiation necrosis; RT, radiotherapy; SRS, stereotactic radiosurgery; TTR, time to recurrence from surgery; WBRT, whole-brain radiotherapy; wks, weeks; Y, yes.
aDefined as recurrence or worsening of neurological symptoms present at baseline.
bEsthesioneuroblastoma.
cAt first time point of progression.
Radiation History
HGG patients received an average of 58.1 ± 2.78 Gy delivered by EBRT at 13.8 months prior to surgery (range, 4.5-21.8 months). Patients with brain metastases predominantly received SRS although 5 patients received a combination of SRS and WBRT or EBRT. SRS was administered to metastatic patients at an average of 21.4 ± 2.7 Gy at a median 15.1 months prior to surgery (range, 6.2-41.5 months). Patients with a history of WBRT received an average of 33 ± 3.3 Gy at 8.9 months prior to craniotomy (range, 5.4-30 months), whereas patients who received EBRT received an average of 30 Gy at 10.3 months prior. Median time from initial radiotherapy to surgery for metastatic patients was 15.5 months (range, 6.6-41.5 months). Median percentage isodose line for patients administered SRS was 72% (range, 50%-100%). Meningioma patients received 20 Gy at a median of 66.4 months prior (range, 26.5-119.6 months) through SRS. One-way ANOVA demonstrated a significant difference between time to surgery by tumor type (P < .001), with meningioma patients having a significantly longer time elapsed between radiation and surgery compared with HGG (P = .001) and metastases (P < .001). The esthesioneuroblastoma received 60 Gy at 7.6 months prior via EBRT.
Tumor Recurrence and PFS
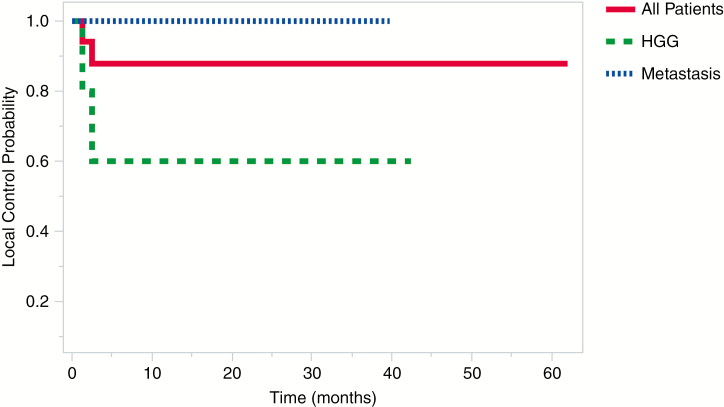
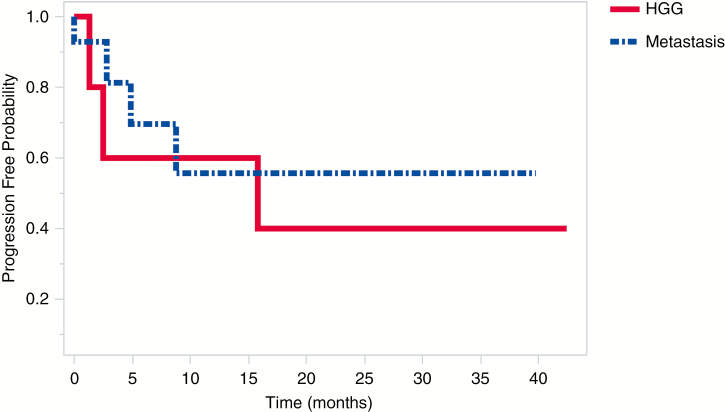
Out of the total cohort, 8.3% (n = 2, both HGG) of patients experienced local recurrence as seen on postoperative MRI at an average of 1.9 months (range, 1.3-2.5 months) postcraniotomy (Fig. 1). Average PFS for HGG patients was 14 ± 16.4 months compared with an average follow-up time point of 16.2 ± 15.4 months. PFS for metastatic patients, on the other hand, was 8.2 ± 11.1 months vs 10.5 ± 11 months follow-up; however, log-rank analysis indicated no significant difference in PFS between these 2 groups (P = .65; Fig. 2).
Fig. 1.
Comparison of Local Control Between Patients With Brain Metastases, High-Grade Glioma (HGG), and the Total Cohort. Local Control Refers to Control of Recurrence Within the Tumor Bed
Fig. 2.
Progression-Free Survival for the Predominant Tumor Types. Patients With Brain Metastases and High-Grade Glioma (HGG) Did Not Differ Significantly in Terms of Progression-Free Survival (P = .65)
KPS and Neurologic Outcomes
Patients experienced a significant increase in median KPS postcraniotomy (80 vs 100, P = .001) but not at latest follow-up (80 vs 90, P = .83). HGG patients did experience a slight improvement (but not significant) from pre- vs postoperative KPS (85 vs 95, P = .20) or pre- vs last follow-up (80 vs 80, P = .36). At latest follow-up neurological improvement was noted in 50% (3/6) in this group whereas 33.3% (2/6) remained at their neurologic baseline and 16.7% (1/6) deteriorated. Patients with metastatic disease experienced a significant increase in KPS after surgery (80 vs 100, P < .001) but not at last follow-up (80 vs 90, P = .63), and 64.3% (9/14) of these patients experienced neurological improvement at latest follow-up. There was no significant difference in outcome between patients with HGG vs metastatic disease at last follow-up. Meningioma patients did experience an increase (but not significantly) in pre- and postoperative KPS and pre- vs last follow-up at (80 vs 100, P = .23) and (80 vs 90, P = .23), respectively.
Steroid Freedom
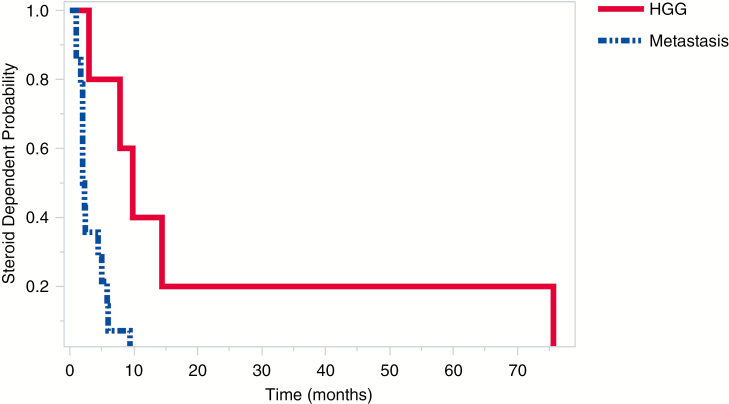
Postoperative patients were typically tapered from dexamethasone 10 mg every 6 hours to off over 1 week. Patients with metastatic disease needed a shorter mean duration of postoperative dexamethasone compared with patients with HGG (3.4 vs 22.2 weeks, log-rank, P = .003, Fig. 3). A total of 16.7% (1/6) of HGG patients were placed on continuous 2 mg twice daily dexamethasone. With the exception of these 2, median time to steroid freedom was 2.29 weeks (2.4 weeks for all combined). Meningioma patients averaged 3.9 weeks of steroid use, and the esthesioneuroblastoma patient needed 2.3 weeks to cease dexamethasone use.
Fig. 3.
Time to Steroid Freedom for the Predominant Tumor Types. Time to Steroid Freedom Is Significantly Reduced for Patients With Brain Metastases vs High-Grade Glioma (HGG) (P = .003)
MRI Perfusion Imaging
Preoperative MR perfusion imaging was available for 7 patients prior to surgery, and suggested RN (decreased CBV, compared with contralateral hemisphere) in 57.1% of patients (n = 4), but was equivocal or nonindicative in the rest of the cohort (42.9%, n = 3). Perfusion scans and functional imaging were preferred for only a handful of patients in the final cohort because of the lower yield that such modalities have for brain metastases vs primary brain tumors.19,20
Complications
Surgical morbidity was noted in 4.2% of patients (n = 1) due to adrenal insufficiency from a rapid dexamethasone taper. Two patients expired of their metastatic disease at 8.6 months and 16.5 months postcraniotomy, respectively. No complications were associated with the actual surgical procedure.
Discussion
In this study, we retrospectively reviewed the surgical outcomes of 24 patients with pure histopathologically confirmed RN. The majority of patients (66.7%) in our series experienced postoperative KPS improvement, and at latest follow-up 72.3% demonstrated neurological improvement. Salvage craniotomy for treatment-refractory RN was able to provide significant local control (91.7%), with significant functional improvement. To our knowledge, this is the largest study undertaken to analyze the safety and efficacy of surgical resection for symptomatic RN.
RN is reported to occur in between 3% to 24% of patients with a history of cerebral radiation although the specific rate depends on the type of tumor, disease progression, and modality of radiation.13,21 Time to onset of RN also varies widely and can range from 6 months to several years after radiation treatment.21–23 Factors that increase the risk for RN have been extensively covered in the literature and include tumor diameter on pre-SRS MRI, prior history of radiation, and the number and total dosage of the radiation fractions administered to the lesion.2,6,21,23–25 Specifically, minimizing the volume of irradiated healthy brain by optimizing the isodose line has been found to decrease the risk of RN.24,26,27 Although histology is the gold standard for diagnosing RN, many centers use a combination of radiological and clinical criteria to confirm RN in suspected cases. Preoperative imaging modalities such as MR perfusion, MR spectroscopy, and diffusion-weighted–MRI are typically used.2,21 In our series, MR perfusion predicted RN (low relative CBV) in 57.1% of cases, which is supported by the current literature indicating that elevated relative CBV can help distinguish recurrent tumor vs radiation necrosis.28–30
Medical Management
Typical primary treatment options for symptomatic RN include a trial of steroids for symptomatic control because they reduce the edema associated with mass effect. However, prolonged use of steroids can lead to typical signs of excess steroid consumption and immunosuppression.11 Given the morbidity of prolonged steroid use, in cases of steroid dependence or persistent symptomatology, salvage medical treatments are available. Perhaps the best known of these is bevacizumab, an inhibitor of VEGF, which studies have shown is upregulated in RN.31–33 Although bevacizumab has been used successfully as a salvage therapy, there are a few considerations that precluded it from being widely used at our institution.34 Patients in the literature who were treated with bevacizumab displayed only a moderate decrease in RN volumetry on MRI.31 Furthermore, its administration time period can last longer than 8 weeks of intravenous treatment, and almost 4 weeks must elapse before surgery can be reconsidered for any reason.31,35 The lack of sufficient information around long-term PFS and its adverse effects such as delayed wound healing, hemorrhage, and pulmonary emboli also have limited its use.34,35 Bevacizumab was therefore considered in our patients for whom surgical resection was precluded rather than a primary option for steroid-freedom. Another nonsurgical therapy that has been explored to variable degrees is combination oral vitamin E and pentoxifylline, which was shown to decrease edema volume (in 10/11 patients) in a small pilot study before having to be discontinued in 3 patients.36 Hyperbaric therapy, which is thought to mitigate the production of free radicals after cerebral radiation, has also been attempted in the past but the body of literature does not support its widespread use.2,31–33
Surgical Management
Given the limitations and uncertainty around salvage medical treatments, for large lesions that are surgically accessible, our institution opted to use salvage craniotomy as a potentially definitive treatment approach. The literature on surgical treatment for RN is limited to smaller case series and case reports consisting of heterogeneous tumor types and displays a wide range of outcomes.13–18 As our study has shown, surgical resection can be performed with minimal complications and lead both symptomatic control and functional improvement with high long-term survival (93.3% [14/15] and 66.7% [4/6] at 1 year and 2 years, respectively). The majority of patients in our study (~70%) demonstrated an increase in postoperative KPS, with metastatic patients demonstrating the most benefit.
Our study noted that HGG patients did not receive significant improvements in KPS compared with patients with metastatic disease (likely explained by high baseline KPS scores [median: 85]). Aside from the known propensity for glioblastoma (GBM) patients to have local tumor recurrence, studies suggest that the histopathology of GBM itself may predispose these patients to worse long-term outcomes after radiotherapy.37,38 In addition, HGG patients received a greater dosage of radiation and developed RN at a shorter mean time compared with metastatic patients. The combination of increased RT and increased sensitivity to adverse effects of RT in HGG patients may explain poor outcomes in this cohort, including the 2 patients who were unable to be weaned from steroids. Metastatic patients, however, were all weaned off steroids and required a shorter mean course (3.4 weeks) compared with HGG (22.2 weeks), which is consistent with previous studies.13–15 A 159-patient case series by Grossman et al that contained 18 GBM patients with symptomatic RN (defined as <20% residual/recurrent viable tumor on resection) noted 58.8% of patients needed further intervention after resection of the mass lesion and 2 needed continued steroid therapy.15 Our results suggest that while metastatic patients may benefit the most from lesionectomy, patients with primary high-grade brain tumors continue to fare poorly after surgery. Although conclusions from our series for other pathologies (meningioma, ependymoma, etc) cannot be inferred, literature suggests surgery remains a viable option for these pathologies as well.17,39,40
At long-term follow-up, there was a trend in improvement in KPS (median 90 KPS); however, this was not significant. This was most likely attributed to increased exposure to general health risks (falls, etc) and systemic disease progression (metastatic patients). Neurological improvement, however, from preoperative baseline was maintained in the majority of patients (~70%) at mean follow-up (13.3 months). This confirmed our view that the surgical benefit could be enduring in these patients for an extended period of time since patients with pure RN and even mixed RN (≤20% active tumor) are generally found to survive longer than those with active tumor recurrence.25 This highlights the importance of early identification of patients with treatment-refractory RN as these patients may benefit the most from surgical intervention. Instead of subjecting patients to long-term medical management such as high-dose steroids or a course of bevacizumab, early craniotomy for refractory lesions may reduce functional and neurological deterioration.16
Limitations
Our study is limited inherently by the retrospective nature of chart review and the uncertainty surrounding the accuracy and completeness of human data entry. In our analysis, we were also constrained to focusing on patients who were operated on at our institution by a single surgeon and who were deemed fit to benefit the most from craniotomy. This may expose our analysis both to selection and institutional bias and affect the generalizability of our results. The RTs that many of our patients were subject to for their tumors were also sometimes performed outside our institution at independent centers in South Florida. The planning and delivery of radiation for each tumor type can therefore vary in approach by practice location. However, we believe that these physician-dependent differences are minimal thanks to standardization and that our series offers valuable insight into surgical management of treatment-refractory radiation necrosis.
Conclusion
Craniotomy for surgically accessible radiation necrosis is a safe tool for neurosurgeons who are managing patients with previously radiated lesions that have failed conservative approaches. When patients are symptomatic or steroid dependent, resection of radiation necrosis, especially for patients with brain metastasis, can lead to an overall improvement in health status and neurological outcomes. Finally, resection provides complete control of this disease process with no recurrence of necrosis. This remains the largest study to date assessing the utility of craniotomies for resecting symptomatic treatment-refractory radiation necrosis.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement. None declared.
References
- 1. Kohutek ZA, Yamada Y, Chan TA, et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol. 2015;125(1):149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Loganadane G, Dhermain F, Louvel G, et al. Brain radiation necrosis: current management with a focus on non-small cell lung cancer patients. Front Oncol. 2018;8:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taal W, Brandsma D, de Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113(2):405–410. [DOI] [PubMed] [Google Scholar]
- 4. Chao ST, Ahluwalia MS, Barnett GH, et al. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys. 2013;87(3):449–457. [DOI] [PubMed] [Google Scholar]
- 5. Parvez K, Parvez A, Zadeh G. The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int J Mol Sci. 2014;15(7):11832–11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miyatake S, Nonoguchi N, Furuse M, et al. Pathophysiology, diagnosis, and treatment of radiation necrosis in the brain. Neurol Med Chir (Tokyo). 2015;55(Suppl 1):50–59. [PubMed] [Google Scholar]
- 7. Kim YZ, Kim DY, Yoo H, et al. Radiation-induced necrosis deteriorating neurological symptoms and mimicking progression of brain metastasis after stereotactic-guided radiotherapy. Cancer Res Treat. 2007;39(1):16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah AH, Snelling B, Bregy A, et al. Discriminating radiation necrosis from tumor progression in gliomas: a systematic review what is the best imaging modality? J Neurooncol. 2013;112(2):141–152. [DOI] [PubMed] [Google Scholar]
- 9. Shah AH, Kuchakulla M, Ibrahim GM, et al. Utility of magnetic resonance perfusion imaging in quantifying active tumor fraction and radiation necrosis in recurrent intracranial tumors. World Neurosurg. 2019;121:e836–e842. [DOI] [PubMed] [Google Scholar]
- 10. Boothe D, Young R, Yamada Y, Prager A, Chan T, Beal K.. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol. 2013;15(9):1257–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel U, Patel A, Cobb C, Benkers T, Vermeulen S. The management of brain necrosis as a result of SRS treatment for intra-cranial tumors. Translational Cancer Research. 2014;3(4):373–382. [Google Scholar]
- 12. Hu Q, Zhao J, Xu J, et al. Long-term relief of cerebral radiation necrosis treated with low-dose bevacizumab—a report of 2 cases. Oncol Res Treat. 2017;40(3):133–137. [DOI] [PubMed] [Google Scholar]
- 13. Telera S, Fabi A, Pace A, et al. Radionecrosis induced by stereotactic radiosurgery of brain metastases: results of surgery and outcome of disease. J Neurooncol. 2013;113(2):313–325. [DOI] [PubMed] [Google Scholar]
- 14. McPherson CM, Warnick RE. Results of contemporary surgical management of radiation necrosis using frameless stereotaxis and intraoperative magnetic resonance imaging. J Neurooncol. 2004;68(1):41–47. [DOI] [PubMed] [Google Scholar]
- 15. Grossman R, Shimony N, Hadelsberg U, et al. Impact of resecting radiation necrosis and pseudoprogression on survival of patients with glioblastoma. World Neurosurg. 2016;89:37–41. [DOI] [PubMed] [Google Scholar]
- 16. Kimura T, Sako K, Tohyama Y, et al. Diagnosis and treatment of progressive space-occupying radiation necrosis following stereotactic radiosurgery for brain metastasis: value of proton magnetic resonance spectroscopy. Acta Neurochir (Wien). 2003;145(7):557–564; discussion 564. [DOI] [PubMed] [Google Scholar]
- 17. Ikeda H, Kanai N, Kamikawa K. Delayed radiation necrosis of the brain simulating a brain tumor—report of two cases (author’s transl) [article in Japanese]. No Shinkei Geka. 1976;4(12):1205–1211. [PubMed] [Google Scholar]
- 18. Wong ST, Loo KT, Yam KY, et al. Results of excision of cerebral radionecrosis: experience in patients treated with radiation therapy for nasopharyngeal carcinoma. J Neurosurg. 2010;113(2):293–300. [DOI] [PubMed] [Google Scholar]
- 19. Fink KR, Fink JR. Imaging of brain metastases. Surg Neurol Int. 2013;4(suppl 4):S209–S219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kerkhof M, Ganeff I, Wiggenraad RGJ, et al. Clinical applicability of and changes in perfusion MR imaging in brain metastases after stereotactic radiotherapy. J Neurooncol. 2018;138(1):133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Siu A, Wind JJ, Iorgulescu JB, Chan TA, Yamada Y, Sherman JH.. Radiation necrosis following treatment of high grade glioma—a review of the literature and current understanding. Acta Neurochir (Wien). 2012;154(2):191–201; discussion 201. [DOI] [PubMed] [Google Scholar]
- 22. Safdari H, Fuentes JM, Dubois JB, Alirezai M, Castan P, Vlahovitch B.. Radiation necrosis of the brain: time of onset and incidence related to total dose and fractionation of radiation. Neuroradiology. 1985;27(1):44–47. [DOI] [PubMed] [Google Scholar]
- 23. Sneed PK, Mendez J, Vemer-van den Hoek JG, et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg. 2015;123(2):373–386. [DOI] [PubMed] [Google Scholar]
- 24. Korytko T, Radivoyevitch T, Colussi V, et al. 12 Gy Gamma Knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys. 2006;64(2):419–424. [DOI] [PubMed] [Google Scholar]
- 25. Rusthoven KE, Olsen C, Franklin W, et al. Favorable prognosis in patients with high-grade glioma with radiation necrosis: the University of Colorado reoperation series. Int J Radiat Oncol Biol Phys. 2011;81(1):211–217. [DOI] [PubMed] [Google Scholar]
- 26. Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC.. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77(4):996–1001. [DOI] [PubMed] [Google Scholar]
- 27. Zhao B, Jin JY, Wen N, et al. Prescription to 50-75% isodose line may be optimum for linear accelerator based radiosurgery of cranial lesions. J Radiosurg SBRT. 2014;3(2):139–147. [PMC free article] [PubMed] [Google Scholar]
- 28. Shah R, Vattoth S, Jacob R, et al. Radiation necrosis in the brain: imaging features and differentiation from tumor recurrence. Radiographics. 2012;32(5):1343–1359. [DOI] [PubMed] [Google Scholar]
- 29. Chuang MT, Liu YS, Tsai YS, Chen YC, Wang CK.. Differentiating radiation-induced necrosis from recurrent brain tumor using MR perfusion and spectroscopy: a meta-analysis. PLoS One. 2016;11(1):e0141438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prager AJ, Martinez N, Beal K, Omuro A, Zhang Z, Young RJ.. Diffusion and perfusion MRI to differentiate treatment-related changes including pseudoprogression from recurrent tumors in high-grade gliomas with histopathologic evidence. AJNR Am J Neuroradiol. 2015;36(5):877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Delishaj D, Ursino S, Pasqualetti F, et al. Bevacizumab for the treatment of radiation-induced cerebral necrosis: a systematic review of the literature. J Clin Med Res. 2017;9(4):273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nonoguchi N, Miyatake S, Fukumoto M, et al. The distribution of vascular endothelial growth factor-producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neurooncol. 2011;105(2):423–431. [DOI] [PubMed] [Google Scholar]
- 33. Nordal RA, Nagy A, Pintilie M, Wong CS.. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res. 2004;10(10):3342–3353. [DOI] [PubMed] [Google Scholar]
- 34. Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abrams DA, Hanson JA, Brown JM, Hsu FP, Delashaw JB Jr, Bota DA.. Timing of surgery and bevacizumab therapy in neurosurgical patients with recurrent high grade glioma. J Clin Neurosci. 2015;22(1):35–39. [DOI] [PubMed] [Google Scholar]
- 36. Williamson R, Kondziolka D, Kanaan H, Lunsford LD, Flickinger JC.. Adverse radiation effects after radiosurgery may benefit from oral vitamin E and pentoxifylline therapy: a pilot study. Stereotact Funct Neurosurg. 2008;86(6):359–366. [DOI] [PubMed] [Google Scholar]
- 37. Cheng L, Huang Z, Zhou W, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153(1):139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee ST, Seo Y, Bae JY, et al. Loss of pericytes in radiation necrosis after glioblastoma treatments. Mol Neurobiol. 2018;55(6):4918–4926. [DOI] [PubMed] [Google Scholar]
- 39. Wong ST, Loo KT, Yam KY, et al. Results of excision of cerebral radionecrosis: experience in patients treated with radiation therapy for nasopharyngeal carcinoma. J Neurosurg. 2010;113(2):293–300. [DOI] [PubMed] [Google Scholar]
- 40. Mou YG, Sai K, Wang ZN, et al. Surgical management of radiation-induced temporal lobe necrosis in patients with nasopharyngeal carcinoma: report of 14 cases. Head Neck. 2011;33(10):1493–1500. [DOI] [PubMed] [Google Scholar]