Abstract
Background
Fatigue is a common symptom in patients with brain tumors, but comprehensive studies on fatigue in patients with meningioma specifically are lacking. This study examined the prevalence and correlates of fatigue in meningioma patients.
Methods
Patients with grade I meningioma completed the Multidimensional Fatigue Inventory (MFI-20) before and 1 year after neurosurgery. The MFI consists of 5 subscales: General Fatigue, Physical Fatigue, Mental Fatigue, Reduced Motivation, and Reduced Activity. Patients’ scores were compared with normative data. Preoperative fatigue was compared with postoperative fatigue. Correlations with sex, age, education, tumor hemisphere, preoperative tumor volume, antiepileptic drugs (AEDs), symptoms of anxiety/depression, and self-reported cognitive complaints were explored.
Results
Questionnaires were completed by 65 patients preoperatively, and 53 patients postoperatively. Of 34 patients, data from both time points were available. Patients had significantly higher fatigue levels on all subscales compared to normative values at both time points. Mean scores on General Fatigue, Physical Fatigue, and Mental Fatigue remained stable over time and improvements were observed on Reduced Motivation and Reduced Activity. Preoperatively, the prevalence of high fatigue (Z-score ≥ 1.3) varied between 34% for Reduced Motivation and 43% for General Fatigue/Mental Fatigue. The postoperative prevalence ranged from 19% for Reduced Activity to 49% on Mental Fatigue. Fatigue was associated with cognitive complaints, anxiety and depression, but not with education, tumor lateralization, tumor volume, or AEDs.
Conclusion
Fatigue is a common and persistent symptom in patients with meningioma undergoing neurosurgery. Findings emphasize the need for more research and appropriate care targeting fatigue for meningioma patients.
Keywords: brain neoplasms, fatigue, meningioma, neurosurgery, patient-reported outcome measures
Meningiomas are for the most part slow-growing tumors that compress the surrounding, healthy brain and eventually may cause symptoms. They account for approximately one-third of all diagnosed primary CNS tumors.1 Most meningiomas will remain asymptomatic and undetected during a person’s lifetime, but a subset receives medical attention because of related symptoms (eg, seizures or neurological deficits) or because they are coincidentally detected on a brain scan.2 Observation (wait-and-scan), neurosurgical resection, and (stereotactic) radiation therapy are the most common treatment options. The majority of meningiomas are benign (ie, >90% WHO grade I) and have a favorable long-term prognosis.1,3 A distinctly worse prognosis is generally observed in patients with atypical (WHO grade II) or anaplastic (WHO grade III) meningiomas. These tumors grow faster, are more likely to recur, and may invade the brain.3 It is a common clinical presumption that patients with grade I meningioma have the most favorable recovery in terms of quality of life and return to normal socioprofessional life. However, accumulating evidence indicates that a significant number of these patients experience cognitive deficits and lower quality of life, even long after treatment has ended.4,5
Fatigue is a very common symptom in patients with primary brain tumors, with prevalence estimates varying between 39% and 96%.6–8 Fatigue is described as a subjective feeling of tiredness and a lack of energy.9 It is a multidimensional construct, wherein a distinction can be made between physical and mental fatigue.10,11 In healthy individuals, fatigue is a normal and adaptive response to physical or mental activities that can be alleviated by periods of sleep or rest. However, in neurological and oncological patients, fatigue can be a persisting and/or relapsing symptom, which is not in proportion to recent activities and not adequately alleviated by rest.10,11 Importantly, fatigue can substantially interfere with patients’ personal and professional activities, and it can significantly lower patients’ quality of life.6,12
Most of the research on fatigue in brain tumor patients has been conducted in patients with glioma, often malignant tumors that grow from glial or precursor cells in the brain.1 These studies indicate that symptoms of fatigue are quite common already prior to treatment, and that they can persist several years thereafter.7,8,13,14 Fatigue in glioma patients has been associated with various factors, including higher age, female sex, left-hemispheric location, radiotherapy, chemotherapy, the use of antiepileptic drugs (AEDs) and opioids, psychological distress, sleep disturbances, and cognitive complaints.7,15,16
Little research has been conducted on fatigue in patients with meningioma. Several studies on quality of life in patients with meningioma made use of instruments including a few items on fatigue (eg, Konglund et al17 and Bunevicius and colleagues18). In addition, a handful of studies evaluated the (side) effects of (stereotactic) radiotherapy in which fatigue was one of the outcome measures.19–22 These studies suggest fatigue is present in patients with meningioma, but firm conclusions cannot be drawn because mostly heterogeneous samples or small samples of meningioma patients were included. Moreover, fatigue has never been included as a primary outcome and, consequently, results regarding fatigue have not always fully been described, have not been described separately for patients with meningioma, or not described at all. Also, a comparison with a control group has often been lacking, which may have distorted findings in patient samples, since fatigue is also a common complaint in the general population. Additionally, all previous studies assessed fatigue with single-item measures (yes/no), or with very brief unidimensional questionnaires or subscales whereas it is a multidimensional construct. As a consequence, there is insufficient understanding of the severity and type of fatigue in patients with meningioma.
This study evaluates fatigue, using a validated multidimensional questionnaire, in a select sample of patients with WHO grade I meningioma, before surgery and 1 year after surgery. Patients’ mean levels of fatigue were compared with normative data from a large sample of the general population. Furthermore, proportions of patients with (very) high fatigue scores were examined. Additionally, relationships of fatigue with sociodemographic, clinical, and psychological variables were explored.
Materials and Methods
Participants
Patients with histologically proven intracranial meningioma (WHO grade I), who underwent surgery between June 2014 and July 2017 at the Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands, were included in this study. Patients were excluded if they had multiple meningiomas; a history of intracranial neurosurgery or whole-brain radiation therapy; a history of severe psychiatric or neurological disease; a KPS less than 70; a lack of basic proficiency in Dutch; or severe motor, language, or visual problems, limiting the ability to complete the assessments. Patients with severe surgery-related complications (eg, stroke or meningitis) were excluded from the 12-month postoperative analyses.
Procedure
Data for this study were prospectively collected as part of a larger follow-up study in patients with intracranial tumors who undergo resective surgery at the Elisabeth-TweeSteden Hospital. The study was approved by the Medical Ethics Committee Brabant (project number NL41351.008.12). Informed consent was obtained from all individual patients included in this study.
Neuropsychological assessments are administered 1 day before surgery (T0) and 3 months after surgery (T3; not used in the present analyses). These assessments have been embedded in standard clinical care for patients with intracranial tumors and information from these assessments is also used in the multidisciplinary consultation that takes place every month. Three months after surgery, patients are invited by nurse practitioners to participate in a follow-up assessment (T12) for research purposes. Neuropsychological assessments consist of a standardized interview, questionnaires on anxiety, depression and cognitive complaints, and standardized neuropsychological tests (not included in the present analyses). Questionnaires on work, community integration, and fatigue are administered at T0 and T12, but not at T3. All assessments were conducted in the hospital by well-trained test technicians.
This study focused on self-reported fatigue, which was examined 1 day before surgery (T0) and 1 year after surgery (T12). Because the pre- and postoperative questionnaires on fatigue were added to the existing test protocol simultaneously in June 2015, patients who participated in the 1-year postsurgery measurement between June 2015 and June 2016 (and had their preoperative assessment between June 2014 and June 2015) completed the MFI at T12, but did not fill out the preoperative questionnaire on fatigue.
Study Measures
Fatigue
Symptoms of fatigue were assessed using the Multidimensional Fatigue Inventory (MFI). Participants were asked to report their fatigue experiences over “the last few days.” This 20-item questionnaire takes about 5 minutes to administer and covers the following 5 dimensions of fatigue: General Fatigue, Physical Fatigue, Mental Fatigue, Reduced Motivation, and Reduced Activity. These scales are based on ways in which fatigue can be expressed, as indicated in the literature and resulting from patient interviews.23 Reliability of the 5 different scales are sufficient, with Cronbach α ranging from 0.72 to 0.87.23 Questionnaires were not included if there were >4 missing answers. In case of 1-4 missing items in less than 5% of the cases (missing at random or missing completely at random), use was made of data imputation (using the mean of a patient’s filled-out items on that particular scale). Representative normative data from the general population of Germany (n = 2037) were available for comparison.24 We used these norms to convert patients’ raw scores into sex- and age-corrected Z-scores per subscale for both time points. Higher Z-scores indicate greater fatigue severity. “High” and “very high” fatigue scores were determined by using widely used cut-offs of, respectively, 1.3 (90th percentile) and 2.0 (97.5th percentile).25,26
Anxiety and depressive symptoms
The Hospital Anxiety and Depression Scale (HADS),27 originally developed for somatic outpatients, was used to assess symptoms of anxiety and depression. This widely used screening instrument consists of 14 items, referring to symptoms within the last week, from which an anxiety scale score (HADS-A) and a depression scale score (HADS-D) can be derived. Higher scores indicate more psychological distress. Reliability of the Dutch version of the HADS is satisfactory to good, with test-retest reliability coefficients between 0.86 and 0.90 and Cronbach α ranging from 0.71 to 0.90.28
Cognitive complaints
The Cognitive Failures Questionnaire (CFQ)29 was used to measure subjective cognitive functioning. Frequency of everyday cognitive failures in motor function, perception, and memory was assessed with 25 items, with response options from 0 (never) to 4 (very often). Psychometric properties of the Dutch version of the CFQ were sufficient, with test-retest reliability of 0.83 and Cronbach α of 0.75 and 0.81.30
Sociodemographic and clinical variables
Number of years of education and completed level of education were self-reported by the patients during a standardized interview. Education was classified using the Dutch coding system of Verhage,31 which ranges from 1 (only primary school) to 7 (university degree). Its 7 categories were subdivided into 3 levels, namely low (Verhage 1 to 4), middle (Verhage 5), and high educational level (Verhage 6 and 7). Relevant clinical information was extracted from electronic medical charts. The location of the tumor was classified by the neurosurgeon. Tumor volume was semiautomatically segmented by trained researchers using the software application ITK-SNAP.32
Statistical Analysis
Data are presented as means ± SDs or frequencies and percentages. Preoperative and postoperative fatigue scores of the patient sample were compared with the normative sample using 2-tailed 1-sample z-tests. Two-tailed one-sample z-tests are conducted, since the means and SDs of the general population (ie, normative sample) are known (M = 0, SD = 1). The standardized mean differences between patients and controls can be interpreted as effect sizes, with 0.20-0.49 indicating small effects, 0.50-0.79 medium effects, and ≥0.80 reflecting large effects.33 Changes from preoperative to postoperative mean scores were examined using 2-tailed paired-sample t-tests. Effect sizes were calculated by dividing the mean difference by its SD (Cohen d = Mdiff/SDdiff), again with 0.20-0.49 small, 0.50-0.79 medium, and ≥0.80 large effects.34 Automatically, correlation coefficients between preoperative and postoperative levels of fatigue were calculated. Correlation coefficients of 0.10 to 0.29 were considered as small, 0.30 to 0.49 were considered as medium, and 0.50 to 1.0 reflected large correlation coefficients.34
The prevalence of high and very high fatigue levels was determined by counting individual patients who scored above the cut-offs of Z ≥ 1.3 (90th percentile) and Z ≥ 2.0 (97.5th percentile), respectively,25,26 for each of the MFI subscales at each time point.
To investigate clinical and demographic factors associated with dimensions of fatigue, correlation coefficients were calculated between the subscales of the MFI and sex, age, level of education, tumor hemisphere, preoperative tumor volume, use of AEDs, self-reported symptoms of anxiety/depression, and self-reported cognitive complaints. Selected variables were mainly based on previous studies in neuro-oncological patients.7,15 Sex- and age-corrected fatigue scores were used,24 but these variables were included in the correlation analysis as well to check whether there was any additional effect of sex and age in this patient sample. Pearson product-moment correlations (r) were calculated for the continuous variables, Spearman rank-order correlations (ρ) were applied to the ordinal variable (ie, level of education), and point-biserial correlations (rpb) were used for the dichotomous variables. Interpretation of the correlation coefficients is described above.
Statistical analyses were conducted using SPSS Statistics (version 24.0), with an α level of 0.05.
Results
Patient Characteristics
Data from preoperative assessments of 65 patients were included in this study (Table 1). Their mean age was 56.2 ± 12.1 years and 74% were female. The majority of tumors were located in the frontal lobe (63%) and mean tumor volume was 42.7 ± 26.0 cm3. At 1-year post surgery, data were available from 53 patients, of whom 34 also participated in the preoperative assessment. Data imputation was used in 4 cases with single missing values. Table 1 presents sociodemographic and clinical characteristics of the different groups.
Table 1.
Sociodemographic, Clinical and Psychological Characteristics of the Different Groups
| Characteristic | Patients at T0 | Patients at T12 | Subgroup With Both Assessments | |
|---|---|---|---|---|
| Sample size (n) | 65 | 53 | 34 | |
| Age at T0 (mean; SD) | 56.2; 12.1 | 54.8; 11.3 | 54.2; 11.4 | |
| Sex (n female; %) | 48; 74% | 40; 76% | 25; 74% | |
| Years of education (mean; SD) | 14.4; 3.8 | 14.9; 3.5 | 14.9; 3.5 | |
| Level of education (n; %)a | ||||
| Low | 17; 26% | 12; 23% | 8; 24% | |
| Middle | 24; 37% | 10; 38% | 15; 44% | |
| High | 24; 37% | 21; 40% | 11; 32% | |
| Tumor hemisphere (n; %) | ||||
| Right | 29; 45% | 25; 47% | 14; 41% | |
| Left | 26; 40% | 20; 38% | 15; 44% | |
| Bilateral | 10; 15% | 8; 15% | 5; 15% | |
| Tumor location (n; %) | ||||
| Frontal | 41; 63% | 28; 53% | 19; 56% | |
| Nonfrontal | 16; 25% | 18; 34% | 10; 29% | |
| Posterior fossa | 8; 12% | 7; 13% | 5; 15% | |
| Presenting neurological symptom (n; %)a | ||||
| Visual deficit | 16; 25% | 12; 23% | 9; 26% | |
| Headache, dizziness | 14; 22% | 11; 21% | 8; 24% | |
| Cognitive or language deficits | 12; 18% | 9; 17% | 4; 12% | |
| Seizure | 11; 17% | 9; 17% | 6; 18% | |
| Focal weakness | 6; 9% | 7; 13% | 3; 9% | |
| Accidental finding | 3; 5% | 2; 4% | 2; 6% | |
| Other | 3; 5% | 3; 6% | 2; 6% | |
| Preoperative tumor volume (cm3; mean; SD) b | 42.7; 26.0 | 41.7; 27.0 | 42.4; 25.7 | |
| Use of antiepileptic drugs (n; %) | 10; 15% | 8; 15% | 5; 15% | |
| Symptoms of anxiety (mean; SD) | 7.1; 4.5 | 4.0; 3.2 | 6.5; 4.0c | 3.6; 3.0d |
| Symptoms of depression (mean; SD) | 6.1; 4.3 | 3.7; 3.6 | 5.3; 4.0c | 3.4; 3.7d |
| Cognitive complaints (mean; SD) | 27.7; 13.0 | 33.3; 16.0 | 25.3; 12.5c | 32.4; 16.4d |
aPercentages may not add up because of rounding.
bData were available for 58 patients at T0 and 49 patients at T12.
cAt T0.
dAt T12.
Mean Levels of Fatigue in Patients With Meningioma
Results of the group-level analyses are listed in Table 2. Patients’ mean scores were significantly higher on each subscale of the MFI, both pre- and postoperatively, compared with norms from the general population (all P values < .01). The largest effects were observed on the subscales of General Fatigue and Mental Fatigue, with effect sizes ranging from 0.89 to 1.07.
Table 2.
Preoperative and Postoperative Mean Patients’ Levels of Fatigue Compared With Normative Values
| MFI Subscale | N | Meana | SD | z Value | P Value | Effect Sizeb |
|---|---|---|---|---|---|---|
| Preoperative Fatigue (T0) | ||||||
| General Fatigue | 65 | 1.07 | 1.46 | 8.59 | <.001c | 1.07 |
| Physical Fatigue | 65 | 0.76 | 1.40 | 6.13 | <.001c | 0.76 |
| Mental Fatigue | 65 | 1.02 | 1.46 | 8.20 | <.001c | 1.02 |
| Reduced Activity | 65 | 0.88 | 1.24 | 7.12 | <.001c | 0.88 |
| Reduced Motivation | 65 | 0.77 | 1.40 | 6.18 | <.001c | 0.77 |
| Postoperative Fatigue (T12) | ||||||
| General Fatigue | 53 | 0.89 | 1.34 | 6.47 | <.001c | 0.89 |
| Physical Fatigue | 53 | 0.44 | 1.03 | 3.17 | .002c | 0.44 |
| Mental Fatigue | 53 | 1.07 | 1.38 | 7.82 | <.001c | 1.07 |
| Reduced Activity | 53 | 0.38 | 1.11 | 2.74 | .006c | 0.38 |
| Reduced Motivation | 53 | 0.36 | 1.16 | 2.64 | .008c | 0.36 |
Abbreviation: MFI, Multidimensional Fatigue Inventory.
aHigher scores indicate higher levels of fatigue. Test values (based on norms of Schwarz et al,24): μ = 0; σ = 1.
bStandardized mean differences can be interpreted as effect sizes, with 0.20-0.49 indicating small effects, 0.50-0.79 medium effects, and ≥0.80 reflecting large effects.33
c P < .05.
In the subset of patients who underwent both assessments (n = 34), improvements over time were observed for Reduced Activity and Reduced Motivation. No significant differences were observed between pre- and postoperative mean levels of General Fatigue, Physical Fatigue, and Mental Fatigue (Table 3).
Table 3.
Preoperative Levels of Fatigue Compared With Postoperative Levels of Fatigue in Patients With Meningioma
| T0-T12 Pairs | N | Mean Difference | SDdiff | t Value | P Value | Effect Sizea | r b |
|---|---|---|---|---|---|---|---|
| General Fatigue | 34 | 0.09 | 1.67 | 0.31 | .759 | 0.05 | 0.37c |
| Physical Fatigue | 34 | 0.40 | 1.40 | 1.68 | .102 | 0.29 | 0.39c |
| Mental Fatigue | 34 | 0.23 | 1.44 | 0.94 | .355 | 0.16 | 0.48c |
| Reduced Activity | 34 | 0.63 | 1.43 | 2.57 | .015c | 0.44 | 0.25 |
| Reduced Motivation | 34 | 0.64 | 1.29 | 2.91 | .006c | 0.50 | 0.45c |
aCohen d = Mdiff/SDdiff, with 0.20-0.49 indicating small effects, 0.50-0.79 medium effects, and ≥0.80 reflecting large effects34
bCoefficients for correlations between pre- and postsurgery fatigue; coefficients of 0.10 to 0.29 were considered as small, 0.30 to 0.49 as medium, and 0.50 to 1.0 reflected large correlation coefficients.34
c P < .05
Prevalence and Severity of Fatigue in Patients With Meningioma
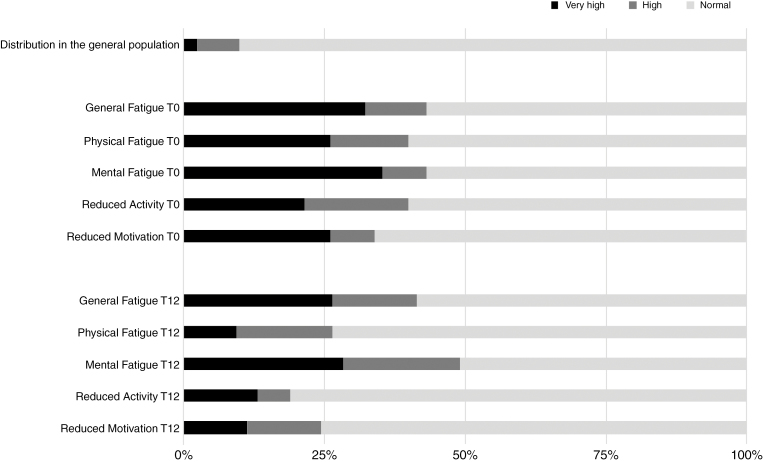
Figure 1 illustrates the proportions of patients scoring normal, high, and very high per subscale of the MFI. Preoperatively, the prevalence of high fatigue (Z-score ≥ 1.3) varied between 34% for Reduced Motivation and 43% for General Fatigue and Mental Fatigue. Postoperative prevalence of high fatigue ranged from 19% for Reduced Activity to 49% for Mental Fatigue.
Fig. 1.
Prevalence of Fatigue in Patients With Meningioma at T0 (n = 65) and T12 (n = 53).
In total, 44/65 patients (68%) scored high (Z-score ≥ 1.3) on 1 or more subscales of the MFI before surgery. Of these 44 patients, 35 scored very high (Z-score ≥ 2.0) on 1 or more subscales. Postoperatively, 30/53 patients (57%) scored high on 1 or more subscales and 21 of these patients scored very high on 1 or more subscales.
Correlates of Fatigue in Patients With Meningioma
As shown in Table 3, the preoperative fatigue scores were weakly to moderately correlated with fatigue scores at T12 (rs between 0.25 and 0.48). Furthermore, correlation analyses showed medium to large associations between fatigue and self-reported symptoms of depression and cognitive complaints pre- and postsurgery, and with anxiety postsurgery (Table 4). We found no clear correlations between standardized scores on the subscales of the MFI and sex, age, education, tumor hemisphere, preoperative tumor volume, and use of AEDs.
Table 4.
Correlates of Fatigue in Meningioma Patients
| Preoperative Assessment (T0) (n = 65) | Postoperative Assessment (T12) (n = 53) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| General Fatigue | Physical Fatigue | Mental Fatigue | Reduced Activity | Reduced Motivation | General Fatigue | Physical Fatigue | Mental Fatigue | Reduced Activity | Reduced Motivation | |
| Sex | 0.02 | 0.09 | 0.04 | –0.02 | 0.03 | 0.20 | 0.13 | 0.07 | 0.08 | –0.05 |
| Age | –0.24 | –0.23 | –0.15 | –0.11 | 0.14 | –0.14 | 0.08 | –0.19 | 0.06 | 0.10 |
| Level of education | –0.06 | 0.03 | –0.12 | –0.11 | –0.15 | 0.12 | 0.08 | 0.06 | 0.15 | 0.01 |
| Tumor hemispherea | 0.03 | –0.08 | 0.02 | 0.09 | –0.06 | –0.14 | –0.06 | –0.07 | –0.16 | –0.06 |
| Preoperative tumor volume | 0.18 | 0.20 | 0.10 | 0.20 | 0.11 | –0.23 | –0.15 | 0.06 | –0.29b | –0.14 |
| Use of AEDs | –0.10 | –0.10 | 0.00 | 0.01 | –0.26b | 0.21 | 0.27 | 0.13 | 0.26 | 0.04 |
| Anxiety | 0.17 | 0.14 | 0.16 | 0.09 | 0.36 b | 0.39 b | 0.37 b | 0.49 b | 0.31 b | 0.44 b |
| Depression | 0.44 b | 0.47 b | 0.38 b | 0.49 b | 0.65 b | 0.58 b | 0.61 b | 0.59 b | 0.59 b | 0.56 b |
| Cognitive complaints | 0.38 b | 0.40 b | 0.47 b | 0.41 b | 0.30 b | 0.54 b | 0.43 b | 0.71 b | 0.38 b | 0.39 b |
Abbreviation: AEDs: anticonvulsant drugs.
Correlations of 0.10 to 0.29 were considered as small, 0.30 to 0.49 as medium, and 0.50 to 1.0 reflected large correlation coefficients.34 Correlations >.29 in bold.
aPatients with bilateral tumors were not included in these analyses.
b P < .05.
Discussion
In this study we comprehensively examined pre- and postsurgical prevalence, severity, and correlates of fatigue in patients with meningioma using a multidimensional fatigue instrument. Symptoms of fatigue were assessed in patients with WHO grade I meningioma prior to surgery (n = 65) and 1 year after surgery (n = 53). On all subscales of the MFI, patients reported more fatigue compared with norms of the general population, both before and 1 year after surgery. In total, 68% and 57% of the patients scored (very) high on 1 or more subscales of the MFI before and after surgery, respectively. In general, proportions of patients scoring very high were larger than proportions of patients scoring high, indicating that the reported symptoms were rather severe than mild. Furthermore, mean levels of General Fatigue, Physical Fatigue, and Mental Fatigue did not decrease significantly over the 1-year follow-up period in a subgroup of patients (n = 34). These findings indicate that fatigue is a substantial and persistent clinical problem in meningioma patients up to 1 year after surgery.
The prevalence rates found in this study roughly correspond with those found in patients with glioma.7,8,14 This may seem remarkable given the differences in etiology and oncological prognosis between meningioma and glioma. Gliomas infiltrate the brain and are the leading cause of death in patients because of disease progression. Meningiomas, on the other hand, grow extraaxially and are mostly benign.1 However, previous studies have also demonstrated long-term impairments in cognitive functioning and quality of life in patients with meningioma.4,5 Although it is often assumed that meningioma patients recover well after surgery, this research contributes to the finding that a substantial number of patients are left with various problems, even long after medical treatment has ended.
Our results indicate that patients’ motivation and activity were significantly increased 1 year after surgery, but serious fatigue remained present in their daily functioning. Persistent symptoms of fatigue can lead to several problems, including difficulties in social participation, mental health issues, or inability to return to (previous) work.35 Fatigue may affect not only patients’ lives, but also the lives of their families.35 However, results of the within-group analyses must be interpreted with some caution, since only some of the participants completed both the preoperative and postoperative questionnaires (n = 34). Furthermore, although stability is observed at the group level on 3 subscales of the MFI, it is possible that different patterns of change occur at the individual level.36 An interesting next step would be to look at individual-level change in fatigue scores (compared with change scores of an appropriate control group) and predictors of improvement or decline using a longitudinal study design with more patients.
In the present sample, fatigue was associated with self-reported symptoms of depression, anxiety, and cognitive complaints. These findings correspond with previous observations in patients with glioma,7,8,17,37 as well as with findings in other patient populations.38–40 Owing to interconnectedness and overlap of symptoms, it is difficult to distinguish between, for example, a major depressive disorder and serious fatigue. Depression can cause fatigue and vice versa, and a third factor can cause both depression and fatigue. It is possible that these symptoms are an expression of shared neurobiological mechanisms (eg, inflammation or brain abnormalities), but these mechanisms have not yet been extensively studied in patients with meningioma. Furthermore, sleep-wake disturbances are common in brain tumor patients,41,42 and often co-occur with symptoms of fatigue, depression, and anxiety, but for this study, we did not collect data on sleep quality. More extensive research is necessary to gain insight into causal relationships between fatigue and its multifactorial determinants in patients with meningioma.
In this study, the highest prevalence rates were found for Mental Fatigue. Short, unidimensional questionnaires or subscales used in previous research often contain floor and ceiling effects due to the narrow range of possible scores and, moreover, tend to measure mainly symptoms of physical fatigue.43 To prevent problems with (mental) fatigue being underdiagnosed and thus undertreated, we recommend the use of a short, validated, multidimensional screening tool, such as the MFI, for patients with surgically treated meningioma during aftercare. Ideally, for each patient with increased scores, contributing and perpetuating factors should be identified using a comprehensive examination. By addressing these specific factors, treatment can be better tailored to the individual patient.44 Although only a few intervention studies have been conducted on fatigue in patients with brain tumors, there is some evidence that patients who experience fatigue may benefit from exercise interventions or psychological interventions (eg, cognitive behavioral therapy or educational programs) to help patients manage symptoms of fatigue.41,45–48 Treatment with psychostimulants have been shown to have insufficient effect on symptoms of fatigue in patients with brain tumors.49,50
This study has some limitations that should be addressed in further studies. The sample sizes were relatively small, and only a subset of patients completed both the preoperative questionnaire and the 1-year follow-up assessment. There are other factors that may have affected generalizability. For example, we included participants who underwent surgery and who had relatively favorable clinical characteristics (eg, patients without a history of neurological/psychiatric disorders, with a KPS above 70, and without surgery-related complications). This could have resulted in an underestimation of fatigue in patients with meningioma in general. It is also possible that the timing of the first assessment (ie, 1 day before surgery) may have influenced the results, since psychological distress appeared to be related to self-reported fatigue.
The current study is a necessary first step in investigating fatigue in patients with meningioma, but clearly more work has to be undertaken in this area. The relationship among fatigue, sleep quality, medication use, and objective measures of cognitive functioning should be further clarified. At the same time, research on treatment options for fatigue in patients with brain tumors should be expanded.45
The findings of the current study indicate that fatigue is a serious, common, and persistent symptom in patients with meningioma undergoing neurosurgery. Health care providers and researchers should be aware of this, pay attention to this debilitating symptom, and provide appropriate care.
Funding
This work was supported by ZonMw, the Dutch organization for health research and innovation [grant numbers 842003007, 842003009].
Conflict of interest statement. None declared.
Acknowledgment
The authors would like to thank the research assistants for their contribution to the data collection.
References
- 1. Ostrom QT, Gittleman H, Xu J, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol. 2016;18(suppl 5):v1–v75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821–1828. [DOI] [PubMed] [Google Scholar]
- 3. Whittle IR, Smith C, Navoo P, Collie D.. Meningiomas. Lancet. 2004;363(9420):1535–1543. [DOI] [PubMed] [Google Scholar]
- 4. Meskal I, Gehring K, Rutten GJ, Sitskoorn MM.. Cognitive functioning in meningioma patients: a systematic review. J Neurooncol. 2016;128(2):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zamanipoor Najafabadi AH, Peeters MCM, Dirven L, et al. Impaired health-related quality of life in meningioma patients—a systematic review. Neuro Oncol. 2017;19(7):897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fox SW, Lyon D, Farace E. Symptom clusters in patients with high-grade glioma. J Nurs Scholarsh. 2007;39(1):61–67. [DOI] [PubMed] [Google Scholar]
- 7. Valko PO, Siddique A, Linsenmeier C, Zaugg K, Held U, Hofer S.. Prevalence and predictors of fatigue in glioblastoma: a prospective study. Neuro Oncol. 2015;17(2):274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Coevorden-van Loon EMP, Coomans MB, Heijenbrok-Kal MH, Ribbers GM, van den Bent MJ.. Fatigue in patients with low grade glioma: systematic evaluation of assessment and prevalence. J Neurooncol. 2017;133(2):237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barsevick AM, Cleeland CS, Manning DC, et al. ASCPRO recommendations for the assessment of fatigue as an outcome in clinical trials. J Pain Symptom Manage. 2010;39(6):1086–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Finsterer J, Mahjoub SZ. Fatigue in healthy and diseased individuals. Am J Hosp Palliat Care. 2014;31(5):562–575. [DOI] [PubMed] [Google Scholar]
- 11. Karshikoff B, Sundelin T, Lasselin J. Role of inflammation in human fatigue: relevance of multidimensional assessments and potential neuronal mechanisms. Front Immunol. 2017;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lovely MP, Miaskowski C, Dodd M. Relationship between fatigue and quality of life in patients with glioblastoma multiforme. Oncol Nurs Forum. 1999;26(5):921–925. [PubMed] [Google Scholar]
- 13. Aprile I, Chiesa S, Padua L, et al. Occurrence and predictors of the fatigue in high-grade glioma patients. Neurol Sci. 2015;36(8):1363–1369. [DOI] [PubMed] [Google Scholar]
- 14. Struik K, Klein M, Heimans JJ, et al. Fatigue in low-grade glioma. J Neurooncol. 2009;92(1):73–78. [DOI] [PubMed] [Google Scholar]
- 15. Armstrong TS, Cron SG, Bolanos EV, Gilbert MR, Kang DH.. Risk factors for fatigue severity in primary brain tumor patients. Cancer. 2010;116(11):2707–2715. [DOI] [PubMed] [Google Scholar]
- 16. Gehring K, Taphoorn MJ, Sitskoorn MM, Aaronson NK.. Predictors of subjective versus objective cognitive functioning in patients with stable grades II and III glioma. Neurooncol Pract. 2015;2(1):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Konglund A, Rogne SG, Lund-Johansen M, Scheie D, Helseth E, Meling TR.. Outcome following surgery for intracranial meningiomas in the aging. Acta Neurol Scand. 2013;127(3):161–169. [DOI] [PubMed] [Google Scholar]
- 18. Bunevicius A, Tamasauskas S, Deltuva V, Tamasauskas A, Radziunas A, Bunevicius R. Predictors of health-related quality of life in neurosurgical brain tumor patients: focus on patient-centered perspective. Acta Neurochir (Wien). 2014;156(2):367–374. [DOI] [PubMed] [Google Scholar]
- 19. Combs SE, Adeberg S, Dittmar JO, et al. Skull base meningiomas: long-term results and patient self-reported outcome in 507 patients treated with fractionated stereotactic radiotherapy (FSRT) or intensity modulated radiotherapy (IMRT). Radiother Oncol. 2013;106(2):186–191. [DOI] [PubMed] [Google Scholar]
- 20. Chao ST, Thakkar VV, Barnett GH, et al. Prospective study of the short-term adverse effects of Gamma Knife radiosurgery. Technol Cancer Res Treat. 2012;11(2):117–122. [DOI] [PubMed] [Google Scholar]
- 21. Kaul D, Budach V, Misch M, Wiener E, Exner S, Badakhshi H.. Meningioma of the skull base: long-term outcome after image-guided stereotactic radiotherapy. Cancer Radiother. 2014;18(8):730–735. [DOI] [PubMed] [Google Scholar]
- 22. Maquilan G, Grover S, Alonso-Basanta M, Lustig RA.. Acute toxicity profile of patients with low-grade gliomas and meningiomas receiving proton therapy. Am J Clin Oncol. 2014;37(5):438–443. [DOI] [PubMed] [Google Scholar]
- 23. Smets EMA, Garssen B, Bonke B, De Haes JCJM.. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325. [DOI] [PubMed] [Google Scholar]
- 24. Schwarz R, Krauss O, Hinz A. Fatigue in the general population. Onkologie. 2003;26(2):140–144. [DOI] [PubMed] [Google Scholar]
- 25. Lezak MD, Howieson DB, Bigler ED, Tranel D.. Neuropsychological Assessment. 5th ed New York, NY: Oxford University Press; 2012. [Google Scholar]
- 26. Bouma A, Mulder J, Lindeboom J, Schmand B, eds.. Handboek Neuropsychologische Diagnostiek [Handbook Neuropsychological Assessment]. 2e herz. dr. Amsterdam, Netherlands: Pearson; 2012. [Google Scholar]
- 27. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 28. Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, Van Hemert AM.. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27(2):363–370. [DOI] [PubMed] [Google Scholar]
- 29. Broadbent DE, Cooper PF, FitzGerald P, Parkes KR.. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21(pt 1):1–16. [DOI] [PubMed] [Google Scholar]
- 30. Merckelbach H, Muris P, Nijman H, De Jong PJ. Self-reported cognitive failures and neurotic symptomatology. Pers Individ Dif. 1996;20(6):715–724. [Google Scholar]
- 31. Verhage F. Intelligentie en Leeftijd Onderzoek bij Nederlanders van twaalf Tot Zevenenzeventig Jaar [Intelligence and Age: Research Study in Dutch Individuals Aged Twelve to Seventy-Seven]. Assen, Netherlands: Van Gorcum; 1964. [Google Scholar]
- 32. Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128. [DOI] [PubMed] [Google Scholar]
- 33. Glass GV, McGaw B, Smith ML.. Meta-Analysis in Social Research. Beverly Hills, CA: Sage;1981. [Google Scholar]
- 34. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 35. Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR.. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(suppl 1):4–10. [DOI] [PubMed] [Google Scholar]
- 36. van Loenen IS, Rijnen SJM, Bruijn J, Rutten GM, Gehring K, Sitskoorn MM.. Group changes in cognitive performance after surgery mask changes in individual patients with glioblastoma. World Neurosurg. 2018;117:e172–e179. [DOI] [PubMed] [Google Scholar]
- 37. Pelletier G, Verhoef MJ, Khatri N, Hagen N.. Quality of life in brain tumor patients: the relative contributions of depression, fatigue, emotional distress, and existential issues. J Neurooncol. 2002;57(1):41–49. [DOI] [PubMed] [Google Scholar]
- 38. Millikin CP, Rourke SB, Halman MH, Power C.. Fatigue in HIV/AIDS is associated with depression and subjective neurocognitive complaints but not neuropsychological functioning. J Clin Exp Neuropsychol. 2003;25(2):201–215. [DOI] [PubMed] [Google Scholar]
- 39. Stulemeijer M, Vos PE, Bleijenberg G, van der Werf SP.. Cognitive complaints after mild traumatic brain injury: things are not always what they seem. J Psychosom Res. 2007;63(6):637–645. [DOI] [PubMed] [Google Scholar]
- 40. Passier PE, Post MW, van Zandvoort MJ, Rinkel GJ, Lindeman E, Visser-Meily JM.. Predicting fatigue 1 year after aneurysmal subarachnoid hemorrhage. J Neurol. 2011;258(6):1091–1097. [DOI] [PubMed] [Google Scholar]
- 41. Armstrong TS, Shade MY, Breton G, et al. Sleep-wake disturbance in patients with brain tumors. Neuro Oncol. 2017;19(3):323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jeon MS, Dhillon HM, Agar MR. Sleep disturbance of adults with a brain tumor and their family caregivers: a systematic review. Neuro Oncol. 2017;19(8):1035–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Knobel H, Loge JH, Brenne E, Fayers P, Hjermstad MJ, Kaasa S.. The validity of EORTC QLQ-C30 fatigue scale in advanced cancer patients and cancer survivors. Palliat Med. 2003;17(8):664–672. [DOI] [PubMed] [Google Scholar]
- 44. Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Canc Netw. 2015;13(8):1012–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Day J, Yust-Katz S, Cachia D, et al. Interventions for the management of fatigue in adults with a primary brain tumour. Cochrane Database Syst Rev. 2016;4:CD011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Colledge F, Brand S, Pühse U, et al. A twelve-week moderate exercise programme improved symptoms of depression, insomnia, and verbal learning in post-aneurysmal subarachnoid haemorrhage patients: a comparison with meningioma patients and healthy controls. Neuropsychobiology. 2017;76(2):59–71. [DOI] [PubMed] [Google Scholar]
- 47. Armstrong TS, Gilbert MR. Practical strategies for management of fatigue and sleep disorders in people with brain tumors. Neuro Oncol. 2012;14(suppl 4):iv65–iv72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Amidei C. Symptom-based interventions to promote quality survivorship. Neuro Oncol. 2018;20(suppl 7):vii 27–vii 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Butler JM Jr, Case LD, Atkins J, et al. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys. 2007;69(5):1496–1501. [DOI] [PubMed] [Google Scholar]
- 50. Boele FW, Douw L, de Groot M, et al. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: a multicenter randomized controlled trial. Neuro Oncol. 2013;15(10):1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]