Abstract
Background
Translating outcomes achieved by clinical trials into routine care is crucial to improving outcomes of glioblastoma (GBM). This study examines the extent to which an advance in treatment for GBM has translated into meaningful, population-level survival benefits in New South Wales (NSW), Australia.
Methods
This retrospective cohort study used linked population-based cancer registry, admitted patient, and mortality datasets. The cohort (n = 2604) included NSW residents aged ≥18 years with a histologically confirmed GBM and a surgical resection between July 2001 and December 2012. The study outcome was all-cause survival, examined using multivariable proportional hazard models. The main study factor was period of surgery, categorized into 4 periods corresponding to different eras in temozolomide (TMZ) use. Survival was examined over time by age (≤70 and >70 years) and for a subcohort selected to approximate the seminal European Organisation for Research and Treatment of Cancer (Stupp) protocol trial cohort. TMZ use was estimated using aggregate prescription claims data.
Results
Median survival in 2001-2003, 2004-2006, 2007-2009, and 2010-2012 was 7.4, 9.0, 9.8, and 10.6 months, and risk-adjusted 2-year survival was 8.2%, 13.8%, 15.5%, and 18.3%, respectively. Survival improved for those aged ≤70 years and those aged >70 years. In the proxy trial subcohort, median and 2-year survival were 14.3 months and 27.3%, respectively. The volume of TMZ prescribed annually increased rapidly from 2005.
Conclusions
Introduction of TMZ into standard care in 2005 coincided with improvements in survival and a rapid increase in TMZ prescribing. Optimization of care has continued to improve survival of people with GBM in subsequent years.
Keywords: glioblastoma, population-based, survival, temozolomide
Primary malignant brain cancers are relatively rare but represent a serious health problem in terms of mortality and morbidity. Primary malignant brain cancers account for just more than 1% of newly diagnosed cancers and almost 3% of deaths due to cancer in New South Wales (NSW), Australia. Grade IV glioma or glioblastoma (GBM), the most common adult primary malignant brain cancer, has a particularly poor prognosis. Despite treatment advances for GBM in recent decades, population-level median survival is around 10 months1–3 and 5-year survival is around 5%.4–6
Temozolomide (TMZ), approved for use in the United States and internationally from 1999 onward, has been a major advance in the treatment of GBM.7,8 Initial trial evidence supported the use of TMZ to treat recurrent GBM and anaplastic astrocytoma.9,10 In 2005, a seminal randomized controlled trial (RCT) demonstrated clinically significant survival benefits from the addition of TMZ to standard treatment for newly diagnosed GBM.11 The RCT regimen, known as the “Stupp protocol” (also known as the European Organisation for Research and Treatment of Cancer-National Cancer Information Center [EORTC-NCIC] protocol), involved surgery followed by concomitant radiotherapy and TMZ followed by 6 cycles of TMZ over 6 months. In comparison to the standard care of surgery followed by radiotherapy, the addition of TMZ resulted in an increase in median survival from 12.1 months to 14.6 months for the patients in this trial and, more strikingly, an increase in 2-year survival from 10% to 26%.
Successful translation of the Stupp protocol into routine clinical care has been demonstrated to have meaningful survival benefits in institutional and general population studies.1,2,12–15 However, older adults (>70 years) were not included in the Stupp trial, and until a recent trial publication (which involved radiation hypofractionation),16 there was little evidence to support use of TMZ concomitant with radiotherapy in this group. With half of GBM cases diagnosed in older adults,6 optimizing treatment for this population group is important. Although delivery of comprehensive treatment, including treatment with the Stupp protocol, is much less common in older adults,1,17–20 some improvements in the survival of older adults have been observed since the introduction of TMZ.2,15
This study aimed to assess whether survival outcomes for people with GBM improved in NSW with the introduction and evolving use of TMZ, whether improvements in survival were maintained or extended in subsequent years, and whether improvements in survival were evident in older adults. These aims were assessed using linked, person-level data with population coverage for cancer diagnosis, hospital admissions, and mortality. Uptake of TMZ in NSW was assessed separately using aggregate prescription claims data.
Materials and Methods
Data Sources and Study Population
The study was a retrospective, consecutive cohort study using linked, statewide population-based datasets with excellent coverage of NSW, Australia. The person-level data sources used were the NSW Cancer Registry (CR), the NSW Admitted Patient Data Collection (APDC), and mortality data from the NSW Registry of Births, Deaths and Marriages. The CR is a statutory registry of mandatory notifications of all primary invasive cancer diagnoses in NSW. The APDC contains patient demographic details, procedures, and diagnoses for admissions in all NSW public and private hospitals. The APDC contains limited information on radiotherapy or chemotherapy, as these are mainly administered in outpatient settings in NSW. Data linkage was performed by the Centre for Health Record Linkage using probabilistic methods and best practice procedures for preserving privacy.21 The study was carried out under approval from the NSW Population and Health Services Research Ethics Committee (HREC/15/CIPHS/15), and individual consent for use of aggregate, deidentified data was not required.
The study cohort was defined as residents of NSW, aged 18 years and older, with a histologically confirmed diagnosis of GBM recorded in the CR between January 2001 and December 2012, and a biopsy or surgical resection performed at an NSW hospital between July 2001 and December 2012. GBM was identified by a combination of International Classification of Diseases for Oncology (third edition) topography (C71, C72.8, C72.9) and morphology codes (9440/3 glioblastoma, NOS; 9441/3 giant cell glioblastoma; or 9442/3 gliosarcoma). The CR confirmed that no changes were implemented to these codes over the study period. Biopsy or surgical resection was identified from linked hospital admission records in the APDC in the year following diagnosis.
Aggregate claims data for TMZ prescriptions subsidized through the Pharmaceutical Benefits Scheme (PBS) and the Department of Veterans’ Affairs (DVA) were accessed for the period 2000 to 2012.22 The PBS is a universal national Australian plan that provides affordable access to listed medicines dispensed at community pharmacies.23 The DVA is an equivalent scheme for veterans and their families. The PBS and DVA claims data together (hereafter referred to as PBS data) provide excellent coverage of TMZ prescribing in NSW over the study period. Chemotherapy in NSW is administered on an outpatient basis, with prescriptions issued to the patient and mostly dispensed at community pharmacies. Access to TMZ through the PBS was strictly controlled over the study period, with use limited to the specified indications and prescribers requiring prior approval for each script (usually obtained by a clinician phone call to a national PBS officer). Person-level pharmaceutical data were not available for inclusion in the data linkage at the time of this study.
Study Variables
The study outcome was all-cause postsurgical survival measured from the date of surgery until date of death or December 31, 2015 (last follow-up). Follow-up of mortality data ranged from 3 to 14 years. Mortality data provided complete follow-up for 1- and 2-year survival. The main study factor was the year of surgery (resection or biopsy), categorized into 4 periods corresponding to different eras in the evolution of TMZ use in NSW. In the period 2001-2003 TMZ was available (and listed on the PBS) to treat recurrent GBM (and recurrent anaplastic astrocytoma). During the period 2004-2006 use of TMZ to treat newly diagnosed GBM was being established internationally based on the EORTC-NCIC trial, with trial results presented at an international conference in June 2004, and published in mid-2005.11 By 2007-2009 use of TMZ to treat newly diagnosed GBM was expected to have been well established as standard care across the NSW healthcare system. The final period, 2010-2012, was examined to determine whether survival benefits were maintained or extended.
Potential risk factors, including age at surgery, comorbidity, urgency of admission (emergency, nonemergency), sex, and hospital type (public, private), were selected based on the literature, expert consultation, and availability. Comorbidity was measured by the Charlson Comorbidity Index24,25 based on diagnostic codes recorded in the index surgical admission. Conditions that may have been directly due to GBM (such as hemiplegia) were excluded.
The American Society of Anesthesiologists (ASA) physical status classification, a global score for the physical health of a patient immediately prior to surgery, was recorded in the APDC from mid-2002. Coverage was insufficient for inclusion of the ASA score in the main analysis. From 2007 onward it was complete for more than 70% of cases and was used to select a subcohort of people whose characteristics approximated those of participants included in the Stupp trial. This proxy RCT subcohort included cases treated from 2007 to 2012, aged 18 to 70 years, with a Charlson Comorbidity Index ≤1 and an ASA score of ≤2.
Statistical Analysis
Patient and tumor characteristics by age and treatment period were examined using the Fisher exact test. The relationship between period of treatment and patient survival at 1 and 2 years was examined using hierarchical Cox proportional-hazard models. Models were fitted for the whole cohort, separately for ages 18 to 70 years and the older than 70 years age groups, and for the proxy RCT subcohort. Models were adjusted for clustering within treating hospitals by specifying hospital as a second level in the model. Age at surgery (continuous variable), comorbidity, emergency admission, sex, and hospital type were retained in all models regardless of statistical significance. Violations of the proportional hazards assumption were checked by examining scaled Schöenfeld residuals, both graphically and analytically, and by fitting time-varying coefficients.26 No gross violations were observed.
Adjusted and unadjusted hazard ratios and estimates of 1- and 2-year survival using a direct estimation method were produced from each model.27 Median survival and interquartile range (IQR) were estimated using Kaplan–Meier survival curves.
Aggregate prescription data for TMZ use in NSW were examined separately. The total amount of TMZ prescribed annually was estimated using the dosage and maximum quantity information for each item specified in the PBS.
Statistical analyses were performed using STATA/MP 13 (Stata-Corp) and SAS version 9.4. (SAS Institute Inc).
Results
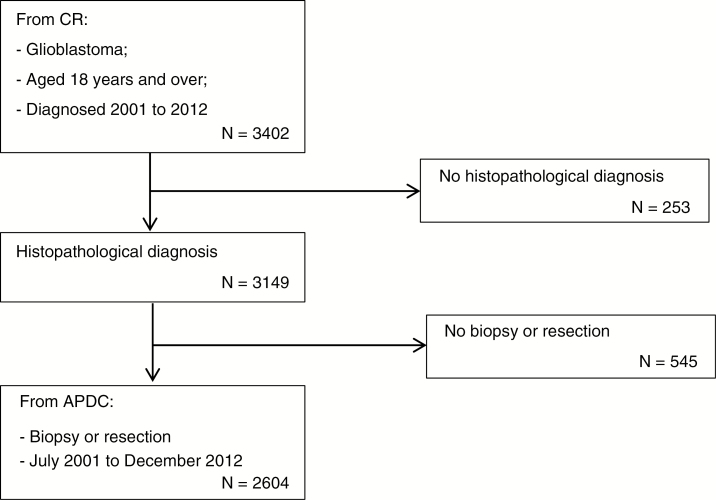
Between 2001 and 2012, a total of 3149 NSW residents aged 18 years and older had a histologically confirmed diagnosis of GBM (Fig. 1). A surgical resection or biopsy in the year following diagnosis in an NSW hospital between July 1, 2001 and December 31, 2012 was identified for 83% (n = 2604) of those diagnosed. Surgical procedures were not identified for some people since admission data from January to June 2001 and interstate hospital admissions for NSW residents traveling outside NSW for surgery were not available. People for whom a surgical resection or biopsy was not identified were excluded as the focus of the study was on postsurgical survival in NSW.
Fig. 1.
Selection of People Treated With Surgery for Glioblastoma in NSW July 2001 to December 2012.
APDC indicates New South Wales Admitted Patient Data Collection; CR, New South Wales Cancer Registry.
Cohort Characteristics
Sociodemographic and clinical characteristics of the study cohort are provided in Table 1. The median age at surgery was 65 years (range, 18-93 years). Comorbid conditions and biopsy without resection were less common among people aged 18 to 70 years compared with those older than 70 years (Fisher exact test P < .0001, and P < .0001, respectively). Fewer people treated in the later treatment periods were treated with a biopsy alone rather than a resection (22% in 2001-2003, 13% in 2010-2012, χ2 = 20.56, P < .0001) (not shown).
Table 1.
Sociodemographic and Clinical Characteristics of People Undergoing Biopsy or Resection for GBM in NSW, July 2001 to December 2012
| 18-70 Years (N = 1763) | >70 Years (N = 811) | All Ages (N = 2604) | |||||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | ||
| Age | 18-50 years | 404 | (22.5) | – | 404 | (15.5) | |
| 51-60 years | 601 | (33.5) | – | 601 | (23.1) | ||
| 61-70 years | 788 | (44.0) | – | 788 | (30.3) | ||
| 71-80 years | – | 654 | (80.6) | 654 | (25.1) | ||
| ≥81 years | – | 157 | (19.4) | 157 | (6.0) | ||
| Sex | Female | 685 | (38.2) | 337 | (41.6) | 1022 | (39.2) |
| Male | 1108 | (61.8) | 474 | (58.5) | 1582 | (60.8) | |
| Country of birth | Australia | 1213 | (67.7) | 530 | (65.4) | 1743 | (66.9) |
| Not Australia/unknown | 580 | (32.4) | 281 | (34.7) | 861 | (33.1) | |
| Major city resident | Major city | 1352 | (75.4) | 602 | (74.2) | 1954 | (75.0) |
| Regional and remote | 441 | (24.6) | 209 | (25.8) | 650 | (25.0) | |
| Area-based socioeconomic status | Quintile 1 (least disadvantaged) | 423 | (23.6) | 189 | (23.3) | 612 | (23.5) |
| Quintile 2 | 373 | (20.8) | 159 | (19.6) | 532 | (20.4) | |
| Quintile 3 | 342 | (19.1) | 164 | (20.2) | 506 | (19.4) | |
| Quintile 4 | 360 | (20.1) | 169 | (20.8) | 529 | (20.3) | |
| Quintile 5 (most disadvantaged) | 294 | (16.4) | 130 | (16.0) | 424 | (16.3) | |
| Urgency of admission | Emergency | 835 | (46.6) | 366 | (45.1) | 1201 | (46.1) |
| Other | 958 | (53.4) | 445 | (54.9) | 1403 | (53.9) | |
| Comorbidity | Comorbidity | 95 | (5.3) | 76 | (9.4) | 171 | (6.6) |
| No comorbidity | 1698 | (94.7) | 735 | (90.6) | 2433 | (93.4) | |
| Tumor location | Cerebrum | 105 | (5.9) | 54 | (6.7) | 159 | (6.1) |
| Frontal lobe | 520 | (29.0) | 218 | (26.9) | 738 | (28.3) | |
| Temporal lobe | 483 | (26.9) | 224 | (27.6) | 707 | (27.2) | |
| Parietal lobe | 298 | (16.6) | 139 | (17.1) | 437 | (16.8) | |
| Occipital lobe | 125 | (7.0) | 67 | (8.3) | 192 | (7.4) | |
| Cerebellum | 12 | (0.7) | 6 | (0.7) | 18 | (0.7) | |
| Overlapping lesion of brain | 157 | (8.8) | 63 | (7.8) | 220 | (8.4) | |
| Other | 93 | (5.3) | 40 | (4.9) | 133 | (5.1) | |
| Surgery type | Biopsy only | 271 | (15.1) | 174 | (21.5) | 445 | (17.1) |
| Resection | 1522 | (84.9) | 637 | (78.6) | 2159 | (82.9) | |
| Hospital type | Public | 1261 | (70.3) | 559 | (68.9) | 1820 | (69.9) |
| Private | 532 | (29.7) | 252 | (31.1) | 784 | (30.1) | |
| Period of treatment | 2001-2003a | 350 | (19.5) | 151 | (18.6) | 501 | (19.2) |
| 2004-2006 | 430 | (24.0) | 177 | (21.8) | 607 | (23.3) | |
| 2007-2009 | 506 | (28.2) | 227 | (28.0) | 733 | (28.1) | |
| 2010-2012 | 507 | (28.3) | 256 | (31.6) | 763 | (29.3) | |
Abbreviations: GBM, glioblastoma; NSW, New South Wales.
aData from July 2001.
Survival by Treatment Period
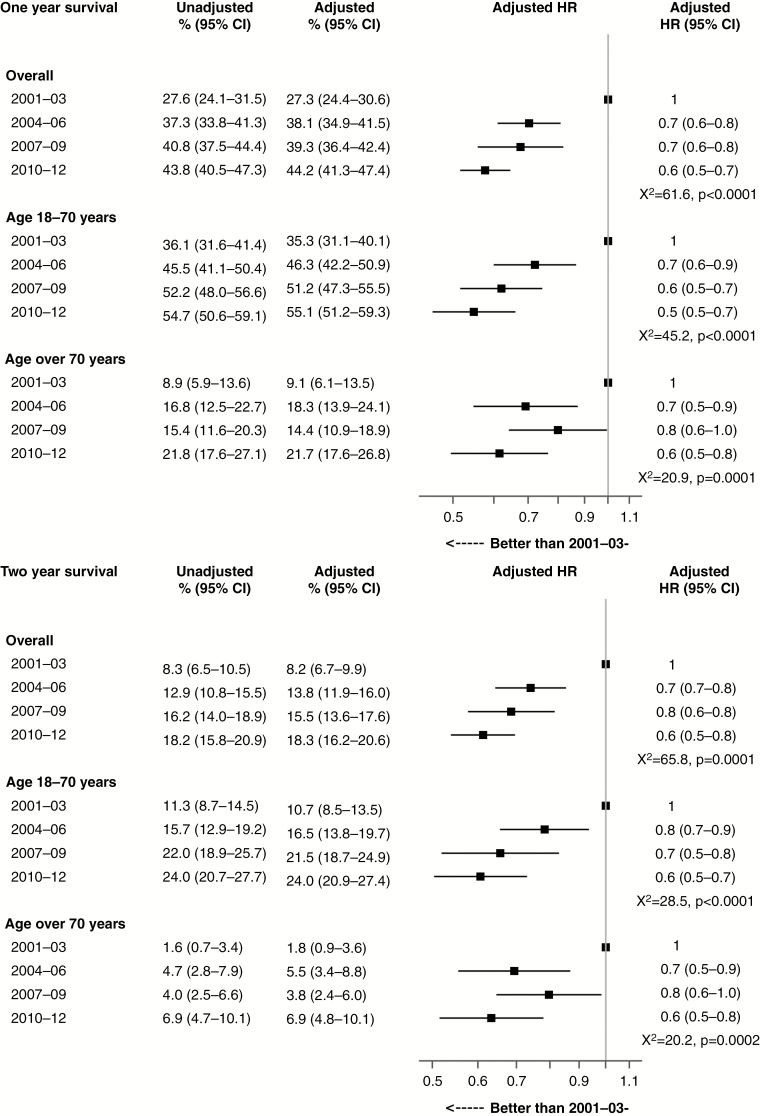
Overall, median survival increased by 1.6 months for people treated in 2004-2006 compared with those treated in 2001-2003, with smaller increases in survival in the subsequent periods (Table 2). Similar patterns were observed in estimates of 1- and 2-year survival for all ages (Fig. 2). Risk-adjusted 2-year survival was 8.2% (95% confidence interval (CI) 6.7-9.9) in 2001-2003 and 13.8% (95% CI 11.9-16.0) in 2004-2006, with minor increases in survival in the subsequent periods.
Table 2.
Median Postsurgical Survival for Glioblastoma, Overall and By Age Group, NSW, July 2001 to December 2012
| Median Survival In Months (IQR) | ||||||
|---|---|---|---|---|---|---|
| All Ages | 18 to 70 Years | >70 Years | ||||
| 2001-2003a | 7.4 | (2.9-13.1) | 9.1 | (4.1-15.8) | 4.6 | (2.1-7.7) |
| 2004-2006 | 9.0 | (3.6-15.4) | 10.8 | (5.0-17.6) | 5.2 | (2.3-10.2) |
| 2007-2009 | 9.8 | (3.8-17.5) | 12.4 | (6.6-22.1) | 4.8 | (2.1-9.5) |
| 2010-2012 | 10.6 | (4.4-19.0) | 13.3 | (6.7-22.8) | 6.3 | (2.1-12.8) |
| 2001-2012 | 9.2 | (3.8-16.5) | 11.6 | (5.6-20.4) | 5.1 | (2.3-10.0) |
Abbreviations: IQR, interquartile range; NSW, New South Wales.
aData from July 2001.
Fig. 2.
Risk-Adjusteda 1-Year and 2-Year Postsurgical Survival for Glioblastoma by Period of Treatment, New South Wales, July 2001 to December 2012.
HR indicates hazard ratio.
aAdjusted for age, sex, urgency of admission, comorbidity, and public or private hospital treatment.
For each treatment period, survival outcomes were substantially better among people aged 18 to 70 years compared with those older than 70 years. Median survival was about double for people aged 18 to 70 years compared with those older than 70 years for each treatment period (Table 2). Similarly, 1-year survival was 2 to 4 times greater and 2-year survival was 3 to 6 times greater for people aged 18 to 70 years compared with those older than 70 years (Fig. 2).
Improvements in all survival outcomes were observed over the study period among people aged 18 to 70 years and among people older than 70 years. The magnitude of gains in 1-year and 2-year survival from the first treatment period to the second were very similar for both age groups. For each subsequent treatment period, progressive incremental gains in survival were observed among people aged 18 to 70 years but not among people older than 70 years. Progress was mixed in this older age group with no gains in survival in 2007-2009 but further gains in 2010-2012.
Survival in an Approximate RCT Cohort
Median survival in the subcohort of people (N = 318) whose characteristics approximated the cohort in the Stupp trial11 (aged 18 to 70 years, Charlson Comorbidity Index ≤1, ASA ≤2) who were treated from 2007 to 2012 was 14.3 months (IQR 7.8-25.2). One- and 2-year survival were 57.8% (95% CI 52.8-63.2) and 27.3% (95% CI 23.0-32.2), respectively.
Prescribing of TMZ in NSW, 2000 to 2012
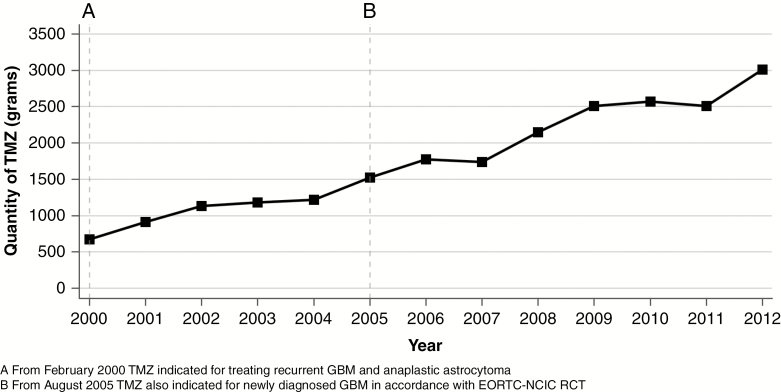
TMZ prescribing was first funded by the PBS in February 2000 for the treatment of recurrent GBM and anaplastic astrocytoma. Uptake of TMZ for the treatment of tumor recurrence was immediate, with annual prescribing increasing over 2001 and 2002. By 2004 the total amount of TMZ prescribed in NSW stabilized at 1200 grams annually (Fig. 3). In August 2005, funding of TMZ by the PBS expanded to include treatment of newly diagnosed GBM in combination with surgery and radiotherapy in accordance with the Stupp protocol.11 The total amount of TMZ prescribed in NSW increased immediately in 2005 and increased further in subsequent years. In 2009, 2500 grams of TMZ was prescribed in NSW and in 2012 this increased to 3000 grams.
Fig. 3.
Total Quantity of Temozolomide (TMZ) Reimbursed Annually by the Pharmaceutical Benefits Scheme in New South Wales, 2000 to 2012.
EORTC-NCIC indicates European Organisation for Research and Treatment of Cancer-National Cancer Information Center; GBM, glioblastoma; RCT, randomized controlled trial.
Discussion
This population-based study demonstrated clinically meaningful improvements in postsurgical survival among people treated for GBM in NSW from 2001 to 2012. The greatest improvement in survival was observed between 2001-2003 and 2004-2006, with median survival increasing from 7 months to 9 months and 1- and 2-year survival increasing from 27.3% to 38.1% and 8.2% to 13.8%, respectively. These improvements in survival coincided with the introduction of TMZ as a component of treatment for newly diagnosed GBM in NSW in 2005. An immediate increase in the quantity of TMZ prescribed in NSW in 2005 and 2006 was observed suggesting rapid uptake of the Stupp protocol. Smaller improvements in survival were observed over the subsequent periods, 2007-2009 and 2010-2012. Statistically significant and clinically meaningful improvements in survival were observed among younger (≤70 years) and older adults (>70 years) over the study period. Survival improvements were independent of changes over time in the characteristics of the patients undergoing surgery, such as age, comorbid conditions, and emergency admission.
The improvements in patient survival observed in NSW were similar to those observed in other health care systems where the Stupp protocol has been successfully implemented into routine clinical care. Prior to the Stupp protocol becoming standard care for people diagnosed with GBM, median survival was 7 to 8 months.1,2,17,28 Similar to this study, gains of around 2 months in median survival following uptake of the Stupp protocol have been reported by other population-based studies.1,2,17 Overall, patient outcomes in the current study were less favorable than those of the published RCT.11 This is not unexpected given the highly selected nature of the RCT cohort. As patient-level data on radiotherapy and chemotherapy treatment were not available for use in the current study, it is not known how many people received standard-care radiotherapy (60 Gy in 30 fractions) as opposed to shorter fractionation, or how many received TMZ concurrent with or following radiotherapy. Making use of available clinical data, a cohort was selected to approximate the characteristics of the Stupp study cohort.11 Given the characteristics of this cohort, the period of treatment, and the evidence of use of TMZ from claims data, it is reasonable to assume that most, if not all, were treated in accordance with the Stupp protocol. Median survival and 2-year survival in this proxy cohort (14.3 months and 27.3%, respectively) aligned very closely with the Stupp trial data (14.6 months and 26.0%).
Publication of the Stupp trial was a turning point for the treatment of GBM, invigorating clinical and research interest in this cancer. The consequent growth in research knowledge and clinical expertise has changed patterns of care and is reflected in sustained and progressive gains in survival following the change in standard treatment over a number of years.3,29 The prognostic value of gross total resection has been firmly established,30,31 and more aggressive surgical management is pursued supported by technological advances in imaging and neurosurgical techniques.32 Fewer biopsies and an increasing proportion of likely near-total resections have been observed over time in several studies.13,29 Similarly, in the current study fewer biopsies and an increase in the proportion of surgical resections were observed over time. One advance in neurosurgical technique, 5-aminolevulinic acid (5-ALA, Gliolan) fluorescence microscopy, facilitates greater extent of surgical resection and is associated with an increase in median overall survival. This advance gained approval for use in Australia in 2013, with limited use in 2012 through a Special Access Scheme, and so holds promise for further improvements in patient outcomes in subsequent years.32,33 Refinements in the delivery of radiotherapy have also occurred over the study period, including the use of more conformal intensity-modulated radiation therapy.34,35 With the intensification of treatment has come more structured multidisciplinary specialist care.36 In this context, people are benefiting from greater clinical expertise in recognizing and responding to treatment complications and tumor recurrences.37
Management of GBM among older adults is difficult given the greater frequency of frailty, coexisting comorbidity, and the greater susceptibility to treatment-related toxicity. Consistent with previous population studies, the survival of adults older than 70 years in the current study was substantially lower compared with those 70 years and younger.1–3,15 Nonetheless, survival in this age group did improve over time. Until the recent publication of a trial demonstrating a survival advantage for use of surgery followed by short-course radiotherapy with concomitant and subsequent cycles of TMZ,16 there was insufficient evidence to support a standard of care for older adults. A survey of the practices of Australian neuro-oncology services in 2009 revealed variation in approaches to treating older adults.38 Most clinicians favored using hypofractionated (ie, over 3 weeks compared with 6 weeks) radiotherapy alone following surgery. A few centers excluded adults from age 65 (or 70) years and older from Stupp protocol treatment. The gains in survival observed among older adults in NSW may be attributable to Stupp protocol treatment, hypofractionated radiotherapy treatment (with or without TMZ), more structured multidisciplinary, specialist care, or all of these factors in combination.
The major strength of this study lies in the statewide population-level data achieved through linkage of the CR with admitted patient data and mortality data. Use of these datasets provides excellent coverage of the general population, enables use of the gold standard in cancer diagnostic information, and enables use of standardized survival outcomes. These datasets use standardized classification systems both for diagnosis and surgical procedures across institutions and over time. Mortality data were available for a minimum of 3 years postdiagnosis. People with diagnosis of GBM were excluded from the study cohort when no relevant surgical hospital admission was identified. It is likely that many of these people were treated interstate. Additionally, admission data were not available for many people diagnosed in early 2001 as the hospital admission data were unavailable for people discharged prior to July 2001. A sensitivity analysis was performed to investigate potential bias introduced by this exclusion criterion. Changes in 1- and 2-year survival from the date of diagnosis were modeled for all cases diagnosed with GBM and for cases diagnosed with GBM with a surgical admission. The results for both sets of analyses were equivalent to the main study results in clinical and statistical significance. Aggregate prescription claims data were used to examine uptake of TMZ in accordance with the new standard of care. The data have excellent coverage of TMZ use for the study period. The subsidization of TMZ was universal when used in accordance with the Stupp protocol and for treatment of GBM and anaplastic astrocytoma at recurrence.
Collection, collation, and linkage of information from across the health care system are rapidly shifting in NSW. In the future, the availability of individual-level chemotherapy and radiotherapy treatment data at a population level, in addition to the currently available surgical information, will enable late-phase effectiveness studies to more comprehensively evaluate adoption of new treatment practices and the effects on patient outcomes. In the current study, the uptake and survival benefits of the Stupp protocol could not be directly examined, as detailed radiotherapy and chemotherapy treatment information was not available for linkage with other data sources. It should be noted, however, that over the study period no other treatment advances demonstrated the marked survival benefit observed for the addition of TMZ to standard care for GBM. For example, the addition of bevacizumab to standard care has demonstrated improvements in progression-free survival but overall survival benefits have not been demonstrated.39 Increasing access to MRI over the study period may have resulted in earlier tumor identification. This would potentially produce lead-time bias in terms of survival lengths. However, the survival benefits of Stupp protocol treatment in routine clinical care have been directly examined and confirmed in previous health system–wide studies.1,14 The effect of TMZ on improvements in survival of people with GBM in NSW may be underestimated in the current study as hospital admission data for the period prior to the introduction of TMZ for the treatment of tumor recurrence were not available. Data from the United States suggest improvements in survival outcomes for GBM first began when TMZ was used to treat tumors at recurrence.1,2
This study confirms that survival for people diagnosed with GBM improved in NSW following the addition to standard care of TMZ concomitant with radiotherapy followed by 6 cycles of TMZ over 6 months. Clinically significant gains in median survival and in the proportion of people surviving to 2 years coincided with a rapid increase in the prescribing of TMZ. Survival outcomes equivalent to the RCT have been achieved in routine clinical practice among people selected for good prognosis. Improvements in survival have also been achieved in routine clinical practice among older adults, a subgroup that was not included in the Stupp study. Growing clinical expertise and optimization of care in subsequent years have brought about progressive improvements in survival.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement. None declared.
References
- 1. Rønning PA, Helseth E, Meling TR, Johannesen TB.. A population-based study on the effect of temozolomide in the treatment of glioblastoma multiforme. Neuro Oncol. 2012;14(9):1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Darefsky AS, King JT Jr, Dubrow R. Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer. 2012;118(8):2163–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brandes AA, Franceschi E, Ermani M, et al. Pattern of care and effectiveness of treatment for glioblastoma patients in the real world: results from a prospective population-based registry. Could survival differ in a high-volume center? Neurooncol Pract. 2014;1(4):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16(7):896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Australian Institute of Health and Welfare. Brain and Other Central Nervous System Cancers. Canberra: AIHW;2017. [Google Scholar]
- 6. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017; 19(suppl 5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mutter N, Stupp R. Temozolomide: a milestone in neuro-oncology and beyond? Expert Rev Anticancer Ther. 2006;6(8):1187–1204. [DOI] [PubMed] [Google Scholar]
- 8. Stupp R, Gander M, Leyvraz S, Newlands E.. Current and future developments in the use of temozolomide for the treatment of brain tumours. Lancet Oncol. 2001;2(9):552–560. [DOI] [PubMed] [Google Scholar]
- 9. Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83(5):588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brada M, Hoang-Xuan K, Rampling R, et al. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol. 2001;12(2):259–266. [DOI] [PubMed] [Google Scholar]
- 11. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 12. van Genugten JA, Leffers P, Baumert BG, Tjon-A-Fat H, Twijnstra A.. Effectiveness of temozolomide for primary glioblastoma multiforme in routine clinical practice. J Neurooncol. 2010;96(2):249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Helseth R, Helseth E, Johannesen TB, et al. Overall survival, prognostic factors, and repeated surgery in a consecutive series of 516 patients with glioblastoma multiforme. Acta Neurol Scand. 2010;122(3):159–167. [DOI] [PubMed] [Google Scholar]
- 14. Dubrow R, Darefsky AS, Jacobs DI, et al. Time trends in glioblastoma multiforme survival: the role of temozolomide. Neuro Oncol. 2013;15(12):1750–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Korja M, Raj R, Seppä K, et al. Glioblastoma survival is improving despite increasing incidence rates: a nationwide study between 2000 and 2013 in Finland. Neuro Oncol. 2019;21(3):370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perry JR, Laperriere N, O’Callaghan CJ, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 17. Lawrence YR, Mishra MV, Werner-Wasik M, et al. Improving prognosis of glioblastoma in the 21st century: who has benefited most? Cancer. 2012;118(17):4228–4234. [DOI] [PubMed] [Google Scholar]
- 18. Rogne SG, Konglund A, Meling TR, et al. Intracranial tumor surgery in patients >70 years of age: is clinical practice worthwhile or futile? Acta Neurol Scand. 2009;120(5):288–294. [DOI] [PubMed] [Google Scholar]
- 19. Stark AM, Hedderich J, Held-Feindt J, Mehdorn HM.. Glioblastoma—the consequences of advanced patient age on treatment and survival. Neurosurg Rev. 2007;30(1):56–61; discussion 61–62. doi: 10.1007/s10143-006-0051-7. [DOI] [PubMed] [Google Scholar]
- 20. Hansen S, Rasmussen BK, Laursen RJ, et al. Treatment and survival of glioblastoma patients in Denmark: the Danish Neuro-Oncology Registry 2009-2014. J Neurooncol. 2018;139(2):479–489 [DOI] [PubMed] [Google Scholar]
- 21. Lawrence G, Dinh I, Taylor L. The Centre for Health Record Linkage: a new resource for health services research and evaluation. Health Inf Manag. 2008;37(2):60–62. [DOI] [PubMed] [Google Scholar]
- 22. Department of Human Services. Pharmaceutical Benefits Schedule Item Reports.http://medicarestatistics.humanservices.gov.au/statistics/pbs_ item.jsp. 2019. Accessed October 19, 2017.
- 23. Mellish L, Karanges EA, Litchfield MJ, et al. The Australian Pharmaceutical Benefits Scheme data collection: a practical guide for researchers. BMC Res Notes. 2015;8:634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. [DOI] [PubMed] [Google Scholar]
- 25. Sundararajan V, Quan H, Halfon P, et al. Cross-national comparative performance of three versions of the ICD-10 Charlson index. Med Care. 2007;45(12):1210–1215. [DOI] [PubMed] [Google Scholar]
- 26. Cleves M, Gutierrez R, Gould W, Marchenko Y.. An Introduction to Survival Analysis Using Stata. Texas: Stata Press;2010. [Google Scholar]
- 27. SAS Institute Inc. The PHREG Procedure. SAS/STAT 12.1 User’s Guide. Cary, NC: SAS Institute Inc;2012. [Google Scholar]
- 28. Rosenthal MA, Drummond KJ, Dally M, et al. Management of glioma in Victoria (1998-2000): retrospective cohort study. Med J Aust. 2006;184(6):270–273. [DOI] [PubMed] [Google Scholar]
- 29. Jayamanne D, Wheeler H, Cook R, et al. Survival improvements with adjuvant therapy in patients with glioblastoma. ANZ J Surg. 2018;88(3):196–201. [DOI] [PubMed] [Google Scholar]
- 30. Pan IW, Ferguson SD, Lam S. Patient and treatment factors associated with survival among adult glioblastoma patients: a USA population-based study from 2000-2010. J Clin Neurosci. 2015;22(10):1575–1581. [DOI] [PubMed] [Google Scholar]
- 31. Stummer W, van den Bent MJ, Westphal M. Cytoreductive surgery of glioblastoma as the key to successful adjuvant therapies: new arguments in an old discussion. Acta Neurochir (Wien). 2011;153(6):1211–1218. [DOI] [PubMed] [Google Scholar]
- 32. Zhao S, Wu J, Wang C, et al. Intraoperative fluorescence-guided resection of high-grade malignant gliomas using 5-aminolevulinic acid-induced porphyrins: a systematic review and meta-analysis of prospective studies. PLoS One. 2013;8(5):e63682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. [DOI] [PubMed] [Google Scholar]
- 34. Back M, Clifford S, Wheeler H, Eade T. Dosimetric improvements utilising intensity modulated radiation therapy for patients with glioblastoma multiforme. J Cancer Ther. 2013;4(11):18–24. [Google Scholar]
- 35. Cardinale R, Won M, Choucair A, et al. A phase II trial of accelerated radiotherapy using weekly stereotactic conformal boost for supratentorial glioblastoma multiforme: RTOG 0023. Int J Radiat Oncol Biol Phys. 2006;65(5):1422–1428. [DOI] [PubMed] [Google Scholar]
- 36. Australian Cancer Network Adult Brain Tumour Guidelines Working Party. Clinical Practice Guidelines for the Management of Adult Gliomas: Astrocytomas and Oligodendrogliomas. Sydney: Cancer Council Australia, Australian Cancer Network and Clinical Oncological Society of Australia Inc;2009. [Google Scholar]
- 37. Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ.. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. [DOI] [PubMed] [Google Scholar]
- 38. Chen JY, Hovey E, Rosenthal M, Livingstone A, Simes J.. Neuro-oncology practices in Australia: a cooperative group for neuro-oncology patterns of care study. Asia Pac J Clin Oncol. 2014;10(2):162–167. [DOI] [PubMed] [Google Scholar]
- 39. Norden AD, Drappatz J, Wen PY. Antiangiogenic therapies for high-grade glioma. Nat Rev Neurol. 2009;5(11):610–620. [DOI] [PubMed] [Google Scholar]