Abstract
Objective:
In selected rectal cancer patients with residual local disease following neoadjuvant chemoradiation (CRT) and the preference of an organ preservation pathway, additional treatment with dose escalation by endoluminal radiotherapy (RT) may ultimately result in a clinical complete response. To date, the widespread introduction of selective endoluminal radiation techniques is hampered by a lack of evidence-based guidelines that describe the radiation treatment volume in relation to the residual tumor mass. In order to convert an incomplete response into a complete one with additional treatment such as dose-escalation with endoluminal RT from a theoretical perspective, it seems important to treat all remaining microscopic tumor cells after CRT. In this setting, residual tumor extension beneath normal appearing mucosa (microscopic intramural spread – MIS) becomes relevant for accurate tumor volume and margin estimation. With the goal of providing evidence-based guidelines that define an appropriate treatment volume and patient selection, we present results from a meta-analysis based on individual patient data of studies that have assessed the extent or range of MIS of rectal cancers after neoadjuvant CRT. This meta-analysis should provide an estimate of the residual tumor volume/extension that needs to be targeted by any additional radiation therapy boost in order to achieve complete tumor eradication after initial incomplete or near-complete response following standard CRT.
Methods and Materials:
A PubMed search was performed. Additional articles were selected based on identification from reference lists. Papers were eligible when reporting MIS in patients who were treated by total mesorectal excision or local excision/transanal endoscopic microsurgery (TEM) after neo-adjuvant long-course CRT. The mean MIS was calculated for the entire group along with the 70th until 95th percentiles. Additional exploratory subgroup analyses were performed.
Results:
Individual patient data from 349 patients with residual disease from five studies were analyzed. 80% of tumors showed no MIS. In order to appropriately treat MIS in 95% of rectal cancer patients after CRT, a margin of 5.5 mm around the macroscopic tumor would suffice. An exploratory subgroup analysis showed that T-stage after CRT (ypT) and time interval between neoadjuvant CRT and surgery are significant factors predicting the extent of MIS (p<0.001.) The group of ypT1 had the smallest MIS, followed by the ypT3-4 group, while the ypT2 group had the largest MIS (p < 0.001). Regarding time interval between CRT and surgery, a statistically significant difference was seen when comparing the three time-interval groups (less than 8 weeks, 8-12 weeks, and more than 12 weeks), where waiting more than 12 weeks after CRT resulted in the largest MIS (p < 0.0001).
Conclusion:
Based on this meta-analysis, in order to treat the MIS for 95% of rectal cancer patients after CRT, a Clinical Target Volume (CTV) margin of 5.5 mm from the lateral most edge of the macroscopic tumor would suffice. 80% of tumors showed no MIS and would not require an extra CTV margin for treatment. These findings support the feasibility of localized radiotherapy boosts for dose-escalation to improve response among patients with incomplete response after standard CRT and can also be applied in the surgical setting.
Keywords: rectal cancer, response, chemoradiation, microscopic spread, margin concept, brachytherapy, contact therapy, boost
Introduction
The treatment and outcomes for rectal cancer patients have dramatically improved in the last decades. The implementation of total mesorectal excision (TME), which enables an R0 resection of the primary tumor and potentially involved mesorectal lymph nodes, has resulted in a decrease of locoregional recurrences1. The introduction of neoadjuvant therapy (radiotherapy or chemoradiation (CRT)) based on high-risk factors has led to a further decline in locoregional failure2,3. Despite these improvements, the combination of neoadjuvant CRT and a TME-based rectal cancer resection is associated with an increased risk of fecal incontinence, low anterior resection syndrome, as well as sexual and urinary dysfunction4–7. For elderly patients, significant peri-operative morbidity and mortality risk also exist8,9. Additionally, patients with distally located rectal tumors often face a permanent colostomy, which may have a significant impact on quality of life10,11.
Following long-course neoadjuvant CRT using standardized doses (usually 50 Gy or 50.4 Gy in 25 or 28 fractions, respectively), a pathologic complete response is seen in 8-20% of patients after surgery3,12. Phase I-II trials have shown that in highly selected patients with a complete clinical response after neoadjuvant treatment, a watch and wait protocol might be considered instead of surgery13,14. This could spare selected patients an extensive operation and, for patients with distal tumors, a permanent colostomy. The number of complete responses is likely to increase if higher radiation doses to the tumor could be used, as shown in a phase II trial using a boost dose given by brachytherapy15,16. The radiation boost can be given to the tumor using either external beam radiotherapy (EBRT) or an endoluminal technique such as brachytherapy or contact X-ray radiotherapy (CXT)15,17,18. This boost can be administered before or after CRT. Giving the boost dose following CRT has the advantage that (a) it could potentially be delivered to a smaller tumor volume resulting in less toxicity (as tumor volume generally shrinks during CRT) and (b) that it may even be completely avoided in case of complete clinical response.
Important advantages of endoluminal techniques include the possibility to apply a more selective/localized boost compared to EBRT. Selective irradiation allows tumor dose escalation to higher levels and limits the chance of radiation-induced toxicity19. Hence, CXT according to the Papillon method has been re-introduced in a limited number of clinics. Due to the sharply falling depth-dose characteristics of CXT, fractional doses up to 30 Gy and total doses up to 90 Gy can be applied without causing significant normal tissue toxicity17,20. As described above, a brachytherapy boost has also been used, showing an increase in the rate of pathological complete response15.
To date, the widespread introduction of selective endoluminal radiation techniques is hampered by the lack of evidence-based guidelines that describe the radiation treatment volume. In order to obtain a durable complete response, it would seem important from a theoretical perspective to treat all tumor cells remaining after CRT. This entails treating not only any visible mucosal lesion, in radiotherapy terms called the Gross Tumor Volume (GTV), but also potential microscopic intramural spread (MIS) or fragments of the tumor in the wall, called the Clinical Target Volume (CTV). Hence, the CTV should include the GTV as well as a margin for potential MIS. To provide evidence-based guidelines that define an appropriate treatment volume, we performed a meta-analysis based on individual patient data of studies that have assessed the extent or range of MIS of rectal tumors after neoadjuvant CRT.
The data generated by this meta-analysis can also be applied in the surgical setting. Local excision via transanal approaches including Transanal Endoscopic Microsurgery (TEM) or Transanal Minimally Invasive Surgery (TAMIS) of a residual (small) tumor after CRT are surgical organ-preserving alternatives to the selective radiation boost21,22. Here too, there is no clear consensus on the margin of “healthy” tissue surrounding the residual tumor containing potential microscopic disease that should be excised23. The results of this meta-analysis could therefore also be used to determine the surgical margin for local surgical techniques or the distal margin when a sphincter-sparing Low Anterior Resection with coloanal anastomosis is being considered in patients with an ultra-distal rectal cancer.
As certain tumor characteristics, such as tumor size, lymphatic, vascular or perineural invasion, may be predictive for the presence of MIS24, the secondary aim of this meta-analysis was to identify potential factors that may be predictive for the absence or presence and the extent of MIS. Such factors may be useful in the future to select patients who are suitable candidates for selective endoluminal boosting and omission of surgery or very localized surgery, or who are likely better off with non-organ preserving surgery.
Methods
Protocol and registration
This paper was written using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Checklist of items to include when reporting a systematic review and meta-analysis 200925.
Search strategy
A search was performed in November 2016 by the first and second-to-last authors and updated on May 9th, 2018 by the first author. The PubMed search strategy used is listed below:
“Rectal Neoplasms”[Mesh] AND ((“neoadjuvant therapy”[MeSH Terms] OR (“neoadjuvant”[All Fields] AND “therapy”[All Fields]) OR “neoadjuvant therapy”[All Fields] OR “neoadjuvant”[All Fields]) AND spread[All Fields])
“Rectal Neoplasms”[Mesh] AND (lateral[All Fields] AND spread[All Fields] AND (“CRT”[MeSH Terms]
“Rectal Neoplasms”[Mesh] AND (intramural[All Fields] AND spread[All Fields] AND (“CRT”[MeSH Terms] OR “CRT”[All Fields] OR “CRT”[All Fields]))
“Rectal Neoplasms”[Mesh] AND (intramural[All Fields] AND spread[All Fields])
“Rectal Neoplasms”[Mesh] AND spread[All Fields] AND (“CRT”[MeSH Terms]
“Rectal Neoplasms”[Mesh] AND microscopic[All Fields] AND (“CRT”[MeSH Terms]
Additional articles were selected based on identification from reference lists.
Study selection
Published articles were selected and evaluated by the first and second-to-last authors. First, eligibility was determined based on title and abstract screening. Remaining articles were selected based on full-text screening. Studies were eligible when reporting in English, experimental or observational studies, and reporting submucosal or otherwise MIS in patients who received a total mesorectal excision or local excision/TEM after neo-adjuvant long-course CRT. Studies only including patients who received surgery immediately after neo-adjuvant treatment were excluded, as little to no pathological response was expected. Publication dates between 1970 and 2018 were included. We determined 1970 as cut-off value due to differences in standard treatment for rectal cancer and advances in the technical aspects of radiation oncology in the recent decades. Conference abstracts were excluded. Authors of selected papers were approached by e-mail and asked whether they were willing to collaborate on this meta-analysis project. Authors who agreed were asked to fill in a data transfer agreement to ensure confidentiality from both parties, after which the anonymized individual patient data were transferred. Selected papers of which the authors eventually did not send their individual patient data or from which no response was received were excluded from analysis after several attempts of communication via mail and phone.
Data extraction and analysis
Data was extracted by full-text screening of the study as well as from the individual patient data using a self-made format reporting on (1) basic study demographics (country, study design, years of patient inclusion, number of patients and stages of disease); (2) treatment demographics (radiation dose, type of chemotherapy, median length of follow up and primary endpoints); (3) reporting of intramural spread; (4) risk of bias assessment. Patients with a pathological complete response were excluded. Descriptive and statistical analyses of the combined individual patient data were performed.
Statistics
The mean MIS was calculated for the entire group along with the percentiles between the 70th and the 95th by increments of 5. 95% Confidence intervals for these different percentiles were calculated using a bootstrap procedure with 10.000 samples.
An explorative analysis (percentiles with confidence intervals) was also performed on subgroups to test whether certain factors were predictive for MIS. Subgroups were made on the basis of ypT stage (ypT1 vs. 2 vs. 3-4), tumor size (median split), tumor diameter (median split), tumor grade of differentiation in the surgical specimen (1 vs 2 vs 3), vascular invasion in the surgical specimen (yes/no), lymphatic invasion in the surgical specimen (yes/no), perineural invasion in the surgical specimen (yes/no), and time between CRT and surgery (less than 8 weeks vs. more than 8 weeks, less than 12 weeks vs. more than 12 weeks, and less than 8 weeks vs. 8-12 weeks vs. more than 12 weeks). All subgroups were compared using a non-parametric test, the Mann-Whitney U test in case of two groups and the Kruskal-Wallis test (with post-hoc pairwise Mann-Whitney U tests if applicable) in case of 3 or more groups.
Results
Study Selection
For the study selection flow chart, we refer to Figure 1. The PubMed search resulted in 168 records. Two additional records were included on identification of reference lists. Based on title and abstract screening, 143 publications were excluded due to various reasons, including the absence of pathology assessment and absence of neo-adjuvant treatment. After full text screening, eleven studies met the inclusion criteria and were included in this systematic review. The search was last updated in May 2018. Nine out of eleven authors responded that they were willing to send us their individual patient data. Two authors were unable to retrieve their databases due to changes of workplace and their papers were thus excluded. Of the seven studies that were then included, we received the individual patient data of five papers23,24,26–28. Two of the papers reported on the same study and therefore we received one dataset for these two papers26,27.
Fig. 1.
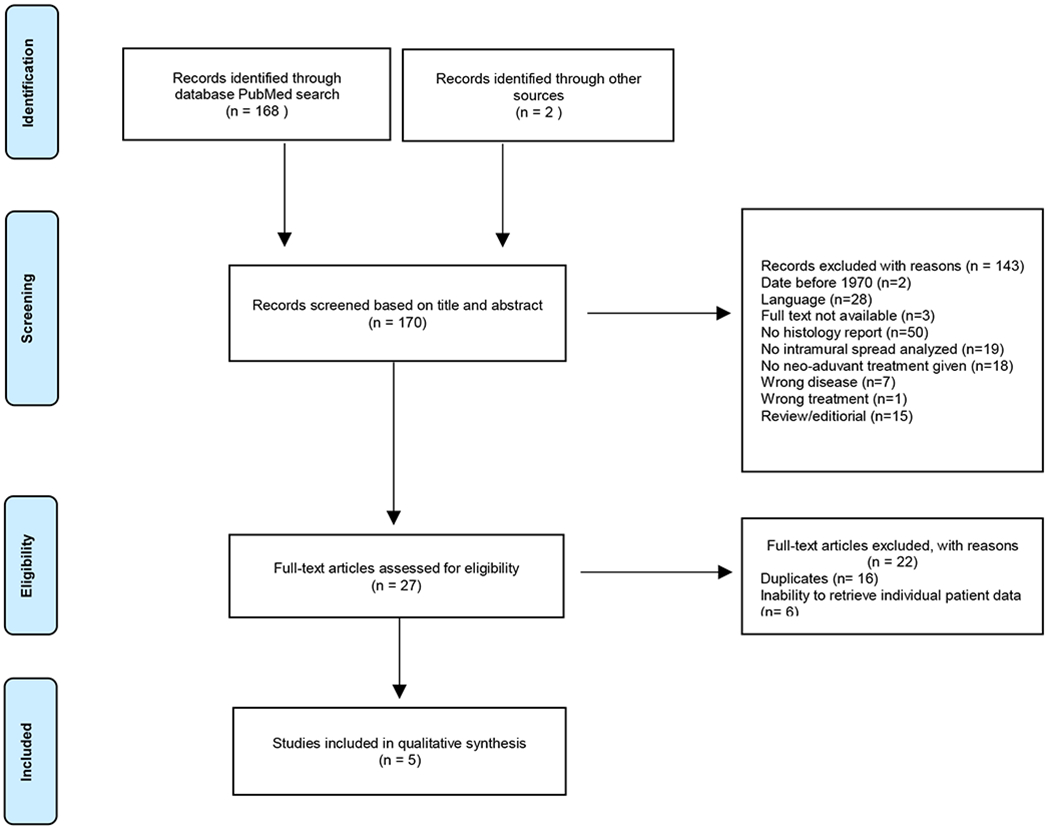
Study Selection Flowchart. This figure shows the selection process, as well as reasons for exclusion of papers.
Study characteristics
For a summary of the study demographics, we refer to Table 1. Five studies with individual patient data from 349 patients were included in this meta-analysis23,24,26–28. Two papers reported on the same prospective randomized trial comparing short-course radiotherapy (5x5 Gy) followed by immediate resection and CRT followed by delayed surgery. We excluded the patients in the short-course radiotherapy arm as for the purpose of this meta-analysis the response after CRT was of interest26,27. The remaining three studies included two prospective observational studies and one retrospective observational study 23,24,28.
Table 1.
Study Demographics
| Reference | Country | Study design | Years of patient inclusion | Total patients / patients with residual disease | Stage of disease with no. of patients (%) | End points |
|---|---|---|---|---|---|---|
| Chmielik et al. 27, Rutkowski et al.26 | Poland | Randomized trial | 1999-2002 | 85* / 79 (CRT) | cT3-cT4: 85 (100) | Comparison of long course CRT with short course RT in regard to sphincter preservation rate; compare distal intramural spread in 2 different RT groups |
| Guedj et al. 28 | France | Observational prospective | 2012-2014 | 124 / 102 | cT2: 9 (7.3) cT3: 94 (75.8) cT4: 9 (7.3) N+: 83 (66.9) N0: 25 (20.2) T- and/or N-stage missing: 28 (22.6) |
Intramural and mesorectal cancer spread |
| Guillem et al. 24 | USA | Observational prospective | 2000-2004 | 110** / 89 | cT2: 7 (6.4) cT3: 95 (86.4) cT4: 1 (0.9) N1: 79 (71.8) N0: 23 (20.9) T- and/or N-stage missing: 15 (13.6) |
Microscopic patterns of residual disease and circumferential and distal resection margins; identify clinicopathologic factors associated with residual disease |
| Perez et al.23 | Brazil | Observational retrospective | 2009-2011 | 30 / 30 | cT2: 12 (40) cT3: 18 (60) N1: 7 (23.3) N0: 23 (76.7) |
Patterns of tumor response |
| Total | 349 / 300 |
In the paper 86 patients are described as having received chemoradiation; however, one of these patients had only received 6 fractions of 1.8 Gy (total 7.2 Gy) and was thus excluded from our analysis.
In the paper 109 patients are included; however, the individual patient data that was delivered comprised of 110 patients.
Treatment characteristics
For a summary of treatment characteristics, we refer to Table 2. All included patients received long course CRT followed by delayed surgery. The most commonly used radiation scheme was 50.4 Gy delivered in fractions of 1.8 Gy to the primary tumor, pathological regional lymph nodes and elective lymph node areas. The most commonly used chemotherapy was 5-fluorouracil-based. The time from CRT to surgery varied from 4-6 weeks in the prospective randomized trial to a median of 16.5 weeks in the observational retrospective study23,26,27. All studies included patients who received TME surgery after neo-adjuvant CRT except for one study, in which all patients received TEM23.
Table 2.
Treatment Characteristics
| Reference | Treatment given | RT Dose | Chemotherapy | Median time between neoadjuvant therapy and surgery in weeks | Type of surgery |
|---|---|---|---|---|---|
| Chmielik et al. 27, Rutkowski et al.26 | Chemoradiation * | 50.4 Gy / 1.8 Gy fx/day; 5 fx per week | 5-Fluorouracil and leucovorin | 5.6 (range 3.1-18.6) | Abdominoperineal (n=35), Low anterior (n=48), Hartmann (n=3) |
| Guedj et al. 28 | Chemoradiation | 45-50 Gy; 5 fx per week over 5-6 weeks | 5-Fluorouracil -based | 9 (range 1.3-18) | Proctectomy with TME (n=118), Abdominoperineal (n=6) |
| Guillem et al. 24 | Chemoradiation | 48.6 Gy-54 Gy / 1.8 Gy fx/day, with 3.6 Gy boost to tumor; 5 fx per week | 5-Fluorouracil -based | 6.9 (range 2.7-22.1) | Abdominoperineal (n=22), Low anterior (n=87) |
| Perez et al.23 | Chemoradiation | 50.4-54 Gy / 1.8Gy fx/day | 5-Fluorouracil -based | 16.5 (range 6-160) | TEM |
Only the chemoradiation arm in Chmielik et al.27 was analyzed for the purpose of this meta-analysis.
Pathological analysis
In the prospective randomized trial, workshops for the participating pathologists were held before and during the trial to align the protocol and measurement methods of margins26,27. In the two observational prospective studies, pathological examination was done by one or two dedicated pathologists24,28. In all studies, pathological analysis was performed according to each institution’s standards.
We define MIS as the greatest distance between the microscopic tumor cells in the bowel wall and the nearest edge of the macroscopic ulcer/tumor residue parallel to and perpendicular to the bowel wall between the microscopic tumor cells in the bowel wall and the nearest superficial edge of the macroscopic ulcer/tumor residue. In the prospective randomized trial, as well as in the papers by Guillem et al. and Guedj et al., MIS parallel to the bowel wall in the distal direction of the tumor (closer to the anus) were analyzed and measured26,27. The study by Guedj et al. also examined the mesorectal spread of tumor 28. Perez et al. inspected MIS in all directions, parallel to the bowel wall23.
Results of individual studies
For the measured MIS as well as other results in each study, we refer to Supplementary table S1. Remarkably, there was quite a range of percentage of patients with MIS. Two studies showed MIS in 1.8% and 2.4% of patients with residual tumor, while the three other studies with smaller patient populations reported >50% of patients having MIS23,24,26–28. All cases of MIS were restricted to the bowel wall. However, one exception was made for a case in the study by Guedj et al., which included a tumor deposit in the mesorectal fat. As this pertained to a cT3 tumor, the possibility exists that this tumor deposit remained there due to tumor fragmentation. For this reason, we did not exclude this case from our analysis.
Syntheses of results
80% of patients showed no evidence of MIS. MIS ranged from 0 to 20mm, with a mean of 4.3 mm when only including patients with MIS. Figure 2a illustrates the total patient population included in this meta-analysis. Figure 2b shows a more detailed graph of only the patients with MIS. The CTV or local excision margin around the macroscopically visible tumor needed to treat all microscopic intramural disease in increasing percentages of patients are shown in Table 3. For example, the MIS for the 90th percentile was calculated to be 3 mm with a 95% confidence interval between 2 and 5 mm based on a bootstrap procedure of 10,000 samples. The analysis was performed including patients with a ypT0, as residual disease cannot be completely excluded when facing a ycT0 with a scar or other residual mucosal abnormality. However, results were also shown with exclusion of 48 patients who had a ypT0 in an attempt to assess robustness of our study data. This analysis showed very similar results (the 95th percentile became 6 mm instead of 5.5 mm), revealing that the impact of the ypT0 subgroup on the total cohort is negligible. When only including the patients with MIS in the analysis (n = 69), the 95th percentile becomes 10mm with a confidence interval of 9 – 19mm.
Figure 2a:
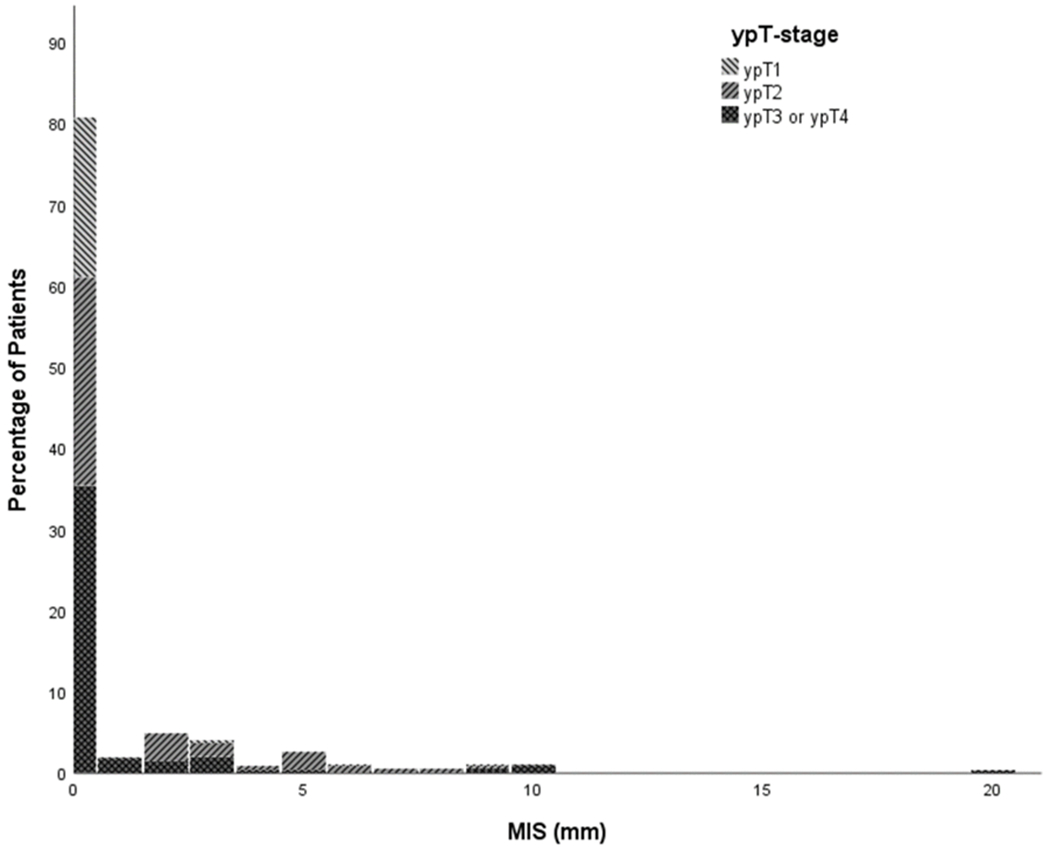
Percentage of MIS in total meta-analysis population. This figure shows the percentage of patients with respective MIS according to ypT stage.
Figure 2b:
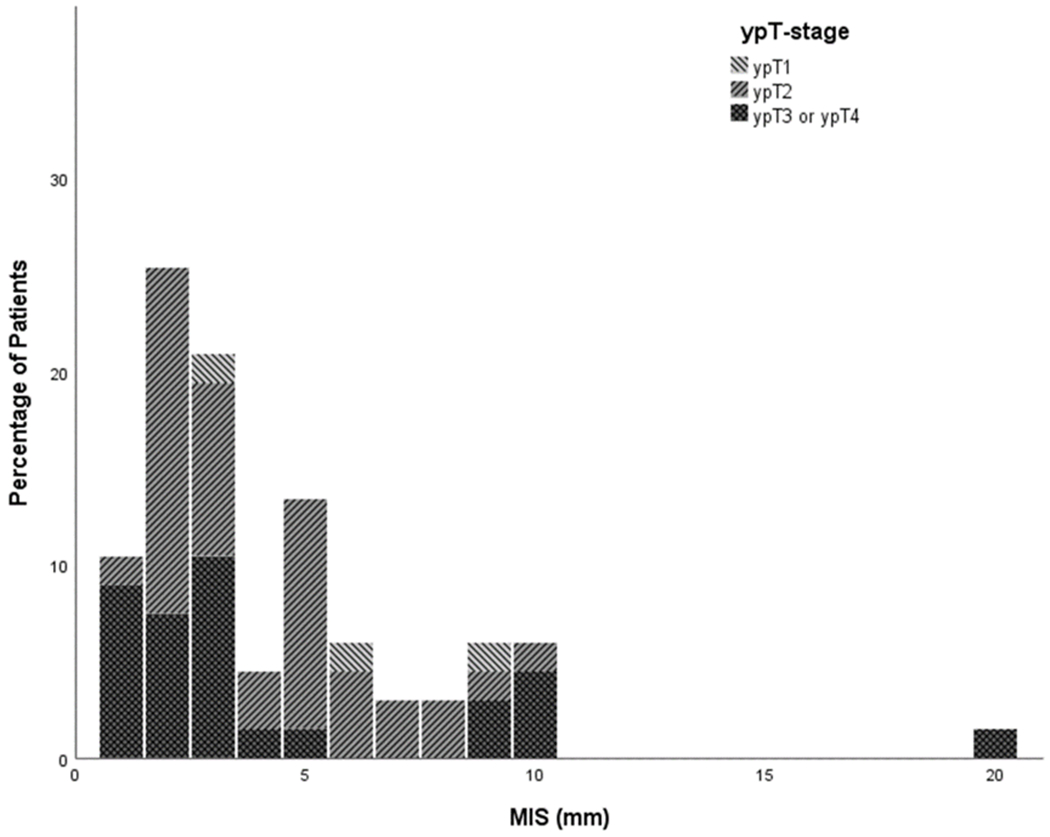
Percentage of MIS in group excluding tumors without MIS. This figure shows the percentage of patients with respective MIS according to ypT stage after exclusion of patients without MIS.
Table 3.
Margins needed to treat percentiles of patients and their confidence intervals. In this table, margins for the total group are shown, as well as after exclusion of 48 patients with a ypT0 after chemoradiation for comparison.
| Percentiles | Margin (mm) | 95% Confidence intervals | Margin after exclusion ypT0 (mm) | 95% Confidence intervals | Margin only patients with MIS (mm) | 95% Confidence intervals |
|---|---|---|---|---|---|---|
| 70 | 0 | 0 - 0 | 0 | 0 - 0 | 5 | 4 - 6.3 |
| 75 | 0 | 0 - 0 | 0 | 0 - 1 | 6 | 5 - 8 |
| 80 | 0 | 0 - 2 | 1.8 | 0 - 2 | 6.4 | 5 - 9 |
| 85 | 2 | 1 - 3 | 2 | 2 - 3 | 8 | 6 - 9.3 |
| 90 | 3 | 2 - 5 | 3 | 3 - 5 | 9 | 7 - 10 |
| 95 | 5.5 | 4.5 - 8 | 6 | 5 - 8.95 | 10 | 9 - 19 |
Additionally, the entire group was split in two based on the median tumor diameter after surgery (excluding one study for which the tumor diameters based on pathology were not provided24), being 24mm. No significant differences were seen in MIS percentiles when comparing tumors with diameters <24mm with those having diameters ≥24mm.
Additional exploratory analyses
Exploratory analyses were done to identify subgroups of patients who might have a higher risk of MIS, considering factors such as grade 3 tumors, lymphatic invasion, vascular invasion, perineural invasion, T-stage and time interval between CRT and surgery. The correlating mean MIS for these factors is shown in Table 4. Using post-hoc Mann-Whitney U test, significant differences were seen for all comparisons: ypT1 vs ypT2 (p < 0.001), ypT2 vs ypT3-4 (p=0.010) and ypT1 vs ypT3-4 (p=0.008). This means that the group of ypT1 has the smallest MIS, followed by the ypT3-4 group, while the ypT2 group had the largest MIS. Regarding time interval between CRT and surgery, a statistically significant difference was seen when comparing the three time-interval groups (less than 8 weeks, 8-12 weeks, and more than 12 weeks), where patients waiting for longer than 12 weeks after CRT had the largest MIS (p<0.0001). Due to the large group of tumors showing no MIS (80% in this meta-analysis) as well as missing information and skewed data, no other significant observations were made.
Table 4.
Predictive Factors
| Factor | Percentile | Microscopic intramural spread (mm) | 95% Confidence intervals | Comparison | Statistical significance *** | |
|---|---|---|---|---|---|---|
| Tumor grade* | 1 | 90 | 5 | 0 - 8 | 1 vs. 2 | no |
| 95 | 7.8 | 2 - 10 | 1 vs. 3 | no | ||
| 2 | 90 | 4.2 | 1 - 9 | 2 vs. 3 | no | |
| 95 | 9 | 3.2 - 11 | ||||
| 3 | 90 | 4.5 | 0 - 6 | |||
| 95 | NA | NA | ||||
| Vascular invasion** | no | 90 | 2 | 0 - 4 | no vs. yes | no |
| 95 | 5.3 | 2 - 8 | ||||
| yes | 90 | 0 | 0 - 2.4 | |||
| 95 | 1.2 | 0 - 20 | ||||
| Perineural invasion** | no | 90 | 0.5 | 0 - 3 | no vs. yes | no |
| 95 | 5 | 2 - 6.75 | ||||
| yes | 90 | 1.8 | 0 - 10 | |||
| 95 | 9.65 | 0 - 20 | ||||
| ypT stage | 1 | 90 | 0 | 0 - 2.1 | ypT1 vs. ypT2 | p < 0.001 |
| 95 | 1.05 | 0 - 7.05 | ||||
| 2 | 90 | 5 | 3 - 6 | |||
| 95 | 6.6 | 5 - 8 | ypT2 vs. ypT3-4 | p = 0.010 | ||
| 3-4 | 90 | 3 | 1 - 3 | |||
| 95 | 4.5 | 3 - 9.5 | ypT1 vs. ypT3-4 | p = 0.008 | ||
| Time interval CRT - surgery (weeks) **** | ≤ 8 | 90 | 3 | 2 - 5 | ≤ 8 vs. > 8 | no |
| 95 | 5 | 3 - 8.7 | ||||
| 8 - 12 | 90 | 0.6 | 0 - 5.3 | ≤ 8 vs. > 12 | p = 0.002 | |
| 95 | 5.15 | 0 - 11.5 | ||||
| > 12 | 90 | 7 | 4 - 9 | ≤ 12 vs. > 12 | p < 0.001 | |
| 95 | 8.5 | 6 - 9 | ≤ 8 vs. 8-12 vs. > 12 | p < 0.001 |
Discussion
This meta-analysis suggests that, to treat all microscopic intramural disease in 95% of patients with rectal cancer who achieve incomplete pathological response after standard CRT, a margin of 5.5 mm would be required around the macroscopically visible tumor. This is clinically relevant information when giving a radiation boost to these patients to improve primary tumor regression and achieve cCR. Additionally, this information can potentially be used when performing a local excision or a sphincter-sparing LAR after CRT in order to optimize chances of an R0 resection.
Because a true complete response is often difficult to discern after chemoradiation, we included the ypT0 patients in our meta-analysis. However, we performed the statistical analysis again on the group after excluding the ypT0 patients, and saw only minor changes in our results. For example, the 95th percentile increased by 0.5 mm and became 6mm, showing that our analysis was robust. The median tumor diameter after CRT was 24mm. Comparing the group <24mm and ≥24mm showed no significant differences in the MIS percentiles, suggesting that the required margins would be the same regardless of size of tumor.
Overall, 80% of the tumors showed no MIS. This suggests that there are two major groups of tumor response: those that retain MIS during tumor response and those that do not, the latter being by far the largest group. More research is needed to improve our ability to predict which tumors will display MIS, as these patients may need a larger margin for local radiation boost or surgical approaches.
Previous literature has shown that some rectal tumors show tumor fragmentation rather than concentric tumor shrinkage after CRT. This tumor fragmentation, or discontinuous spread of tumor, has been described in the studies by Perez et al., Chmielik et al., Rutkowski et al., and Guedj et al.23,26–28 A possible explanation for different patterns of tumor regression may be the presence of distinct degrees of intratumoral heterogeneity29. The coexistence of multiple subpopulations of radiosensitive and radioresistant cancer cells may have resulted in isolated foci of cancer cells, reflected by significant fragmentation of the cancer. This concept does pose some unexplained dilemmas, as it may mean that there may be residual disease in the entire area of original tumor, which would require that a radiation boost also be given on this original tumor volume23. However, given the reported small distances of MIS in the studies included in this meta-analysis, it seems that most tumors show a predominantly concentric shrinkage after CRT. This would mean that giving a radiation boost on a smaller volume should be feasible and safe for most patients. The same conclusion can be made for local excisions. In this meta-analysis, tumor fragmentation was reported for 36/349 (10.3%) patients and continuous intramural extension was reported for 39/349 (11.2%) patients. Patients without MIS, constituting the clear majority (80%) of patients in this meta-analysis, showed concentric shrinkage of tumor, as tumor fragments surrounding the central residual lesion would otherwise have been reported as MIS. The study by Guillem et al. only mentioned continuous intramural extension, thus it was assumed that there was no tumor fragmentation24. Conclusions could not be drawn about the distance of intramural spread related to the pattern of tumor response due to the small number of tumors showing MIS. Biomarkers that help predict type of tumor response are not yet known for this group of patients. MRI could potentially aid in differentiating between these two different types of regression. A few papers have described patterns of response using diffusion-weighted MRI’s during chemoradiation; however, data on accurate radiological detection of tumor fragmentation is still lacking30,31.
The potential effect of the time interval between neoadjuvant CRT and surgery also warrants additional research. Rectal tumor regression has been noted beyond 12 weeks following completion of CRT32,33. In a parallel to anal cancer where optimal assessment of response is considered to be 26 weeks, a significant proportion of patients with rectal cancer will only develop a complete clinical response after 16 weeks from CRT completion33,34. In our meta-analysis, tumor response was usually measured at an earlier time and in the prospective randomized trial, tumor response was already measured between four to six weeks after neo-adjuvant treatment26,27. The largest extension of MIS was seen at >12 weeks’ interval. For patients with a near complete response who prefer an organ-sparing pathway, often a longer waiting period is chosen in the hopes of gaining a complete response. The increased MIS after longer intervals could potentially be explained by the fact that delayed tumor evaluation may result in more MIS due to uneven shrinkage of the macroscopic tumor versus the microscopic tumor at this stage. The relative importance of the two processes may vary depending on the time interval after treatment. However, as there were variable time intervals between neo-adjuvant CRT and surgery across different institutions, this is clearly a potential source of bias.
As the use of contact therapy in the adjuvant setting is becoming more frequent35, parameters of the surgical specimen were considered potentially relevant and, therefore, reported in this study. In a recent study, MIS was seen as far as 4cm away from the visible tumor/ulcer and in up to half of patients36. It must be taken into account, however, that most of these patients would not be considered appropriate candidates for a local excision or a radiation boost as the residual cancers were large and advanced36. The maximum distance of MIS found in this meta-analysis was 20mm28. The possible correlation between ypT stage and extent of MIS is another factor requiring more research. In our analysis, ypT2 had the highest MIS as opposed to ypT1 or ypT3-4. Reasons for this outcome remain unclear, but perhaps the relatively low amount of MIS cases could contribute to this statistical outcome. This analysis was exploratory and must be confirmed by more studies.
Perez et al. describe in their paper that according to their measurements, a 1cm margin around the visible tumor (which is now generally used during local excision) would be inadequate and a 1.5cm margin would be safer36. In this series, a high number of tumors (70%) showed MIS. The time interval between neo-adjuvant chemoradiation and surgery in this series was also by far the highest, with a median of 16.5 weeks23. Curiously, this series also had a majority of patients with residual ypT2 tumors, consistent with the present analysis showing the largest extension for MIS. Altogether, restaging of patients with incomplete response showing residual ycT2 after more than 12 weeks from CRT may warrant additional CTV margin requirements. This analysis was exploratory and must be confirmed by further studies.
Limitations of our meta-analysis include the fact that individual patient data was only received from five out of eleven eligible studies, leaving out potentially useful data for 240 patients with residual disease after preoperative RT. The studies that had to be excluded due to unavailable data included a retrospective study that assessed distal MIS parallel to the bowel wall and found that 49.1% of 55 patients with residual cancer had MIS, with three patients showing a MIS of more than 2cm36. An Indian study including 41 patients with residual tumor of which 2 patients (5%) showed distal MIS37. A Japanese study compared two groups of patients with T2-T4 lower rectal cancer; one in which patients received preoperative radiation followed by surgery and one in which patients received immediate surgery. The goal was to analyze the effect of preoperative radiation on distal MIS among other endpoints. 47 patients were irradiated of which it is unclear how many of these patients had MIS. Interestingly, the mean extent of distal MIS was significantly lower in the irradiated group38. The same author published a second study comparing distal MIS in flattened- type and raised-type residual tumors after neoadjuvant chemoradiation in 34 patients. It is unclear how many patients showed distal MIS, yet the authors conclude that flattened-type tumors showed more diffusely distributed MIS compared to raised-type tumors39. Mezhir et al. published a study in which out of 18 patients with residual rectal cancer after neoadjuvant chemoradiation, 11 patients (61%) showed distal MIS, 91% of which was < 1cm (ref Mezhir). Another study analyzing 45 patients showed that 71% of patients had distal MIS40. Although the inclusion of these studies could have revealed some valuable insights, all possible attempts had been made to retrieve this data, leaving the authors convinced that no more actions could be undertaken. Another limitation of this analysis is that three out of the four patient populations only analyzed distal MIS parallel to the bowel wall (for surgical purposes), whereas we are interested in the spread parallel as well as perpendicular to the bowel wall, as this is relevant in the case of a radiation boost. Currently, a prospectively collected database of TME resections after neo-adjuvant chemoradiation is being analyzed for MIS in all directions parallel as well as perpendicular to the bowel wall, with the hopes of having more detailed information about the possible extent of MIS.
Additionally, little is known about the extent of shrinkage after formalin fixation of TME resections. Most papers report an enlargement/shrinkage of tumor diameter after fixation of around 5%41–43. However, Goldstein et al. have reported longitudinal shrinkages of up to 30%, after which he would suggest a correction factor of approximately 2x when interpreting margin lengths44. Eid et al. also observed histological processing variability of the lateral resection margins in rectal cancer, including increases and decreases in margins depending on the day the margin was measured45. Possibly the extent of tumor enlargement/shrinkage will be quite limited due to the status after CRT, after which at least partial fibrosis is expected which is less subject to deformation. Clearly, more research and standardization of pathological analysis needs to be carried out to clearly define the effect of pathological processing on tissue volume and MIS.
In conclusion, based on the meta-analysis of 5 studies including 349 patients, 80% of patients with rectal cancer will not have microscopic intramural spread (MIS) following CRT. In cases where MIS is detected, it is usually limited. Based on our calculations, it appears that in order to treat residual mural tumor and MIS successfully in 95% of rectal cancer patients with significant tumor response after CRT, a margin of 5.5 mm around the visible tumor would suffice. These data are of clinical importance, specifically when planning additional radiotherapy treatments or for surgical approaches, being local excisional approaches as well as sphincter sparing LAR.
Supplementary Material
Footnotes
Disclosure of potential conflicts of Interest:
Gabriel Paiva Fonseca, Murillo Bellezzo, Frank Verhaegen, Evert Jan Van Limbergen and Maaike Berbee have a patent EP16204735, rectal brachytherapy applicator with royalties paid.
The other authors have no potential conflicts of interest to disclose.
References
- 1.MacFarlane JK, Ryall RD & Heald RJ Mesorectal excision for rectal cancer. Lancet Lond. Engl 341, 457–460 (1993). [DOI] [PubMed] [Google Scholar]
- 2.van Gijn W et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 12, 575–582 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Sauer R et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med 351, 1731–1740 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Martens MH et al. Long-term Outcome of an Organ Preservation Program After Neoadjuvant Treatment for Rectal Cancer. J. Natl. Cancer Inst 108, (2016). [DOI] [PubMed] [Google Scholar]
- 5.Guren MG et al. Quality of life and functional outcome following anterior or abdominoperineal resection for rectal cancer. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol 31, 735–742 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Fazio VW et al. A randomized multicenter trial to compare long-term functional outcome, quality of life, and complications of surgical procedures for low rectal cancers. Ann. Surg 246, 481–8; discussion 488-490 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bregendahl S, Emmertsen KJ, Lous J & Laurberg S Bowel dysfunction after low anterior resection with and without neoadjuvant therapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis. Off. J. Assoc. Coloproctology G. B. Irel 15, 1130–1139 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Rabeneck L, Davila JA, Thompson M & El-Serag HB Outcomes in elderly patients following surgery for colorectal cancer in the veterans affairs health care system. Aliment. Pharmacol. Ther 20, 1115–1124 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Rutten H et al. Survival of elderly rectal cancer patients not improved: analysis of population based data on the impact of TME surgery. Eur. J. Cancer Oxf. Engl 1990 43, 2295–2300 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Dumont F, Ayadi M, Goere D, Honore C & Elias D Comparison of fecal continence and quality of life between intersphincteric resection and abdominoperineal resection plus perineal colostomy for ultra-low rectal cancer. J. Surg. Oncol 108, 225–229 (2013). [DOI] [PubMed] [Google Scholar]
- 11.McMullen CK et al. Greatest Challenges of Rectal Cancer Survivors: Results of a Population-Based Survey. Dis. Colon Rectum 59, 1019–1027 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maas M et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 11, 835–844 (2010). [DOI] [PubMed] [Google Scholar]
- 13.van der Valk MJM et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet Lond. Engl 391, 2537–2545 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Habr-Gama A et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann. Surg 240, 711–7; discussion 717-718 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appelt AL et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 16, 919–927 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Appelt AL, Ploen J, Vogelius IR, Bentzen SM & Jakobsen A Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int. J. Radiat. Oncol. Biol. Phys 85, 74–80 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerard J-P et al. Improved sphincter preservation in low rectal cancer with high-dose preoperative radiotherapy: the lyon R96–02 randomized trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 22, 2404–2409 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Burbach JPM et al. RandomizEd controlled trial for pre-operAtive dose-escaLation BOOST in locally advanced rectal cancer (RECTAL BOOST study): study protocol for a randomized controlled trial. Trials 16, 58 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fonteyne V et al. Rectal toxicity after intensity modulated radiotherapy for prostate cancer: which rectal dose volume constraints should we use? Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol 113, 398–403 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Sun Myint A et al. Combined modality treatment of early rectal cancer: the UK experience. Clin. Oncol. R. Coll. Radiol. G. B 19, 674–681 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Stijns RCH et al. Long-term Oncological and Functional Outcomes of Chemoradiotherapy Followed by Organ-Sparing Transanal Endoscopic Microsurgery for Distal Rectal Cancer: The CARTS Study. JAMA Surg. 154, 47–54 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van den Eynde F, Jaekers J, Fieuws S, D’Hoore AM & Wolthuis AM TAMIS is a valuable alternative to TEM for resection of intraluminal rectal tumors. Tech. Coloproctology (2019). doi: 10.1007/s10151-019-01954-7 [DOI] [PubMed] [Google Scholar]
- 23.Perez RO et al. Fragmented pattern of tumor regression and lateral intramural spread may influence margin appropriateness after TEM for rectal cancer following neoadjuvant CRT. J. Surg. Oncol 109, 853–858 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Guillem JG et al. A prospective pathologic analysis using whole-mount sections of rectal cancer following preoperative combined modality therapy: implications for sphincter preservation. Ann. Surg 245, 88–93 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberati A et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol 62, e1–34 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Rutkowski A et al. Distal bowel surgical margin shorter than 1 cm after preoperative radiation for rectal cancer: is it safe? Ann. Surg. Oncol 15, 3124–3131 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Chmielik E et al. Distal intramural spread of rectal cancer after preoperative radiotherapy: the results of a multicenter randomized clinical study. Int. J. Radiat. Oncol. Biol. Phys 65, 182–188 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Guedj N et al. Distal intramural and tumor spread in the mesorectum after neoadjuvant radiochemotherapy in rectal cancer: about 124 consecutive patients. Hum. Pathol 52, 164–172 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Bettoni F et al. Intratumoral Genetic Heterogeneity in Rectal Cancer: Are Single Biopsies representative of the entirety of the tumor? Ann. Surg 265, e4–e6 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Lambregts DMJ et al. Monitoring early changes in rectal tumor morphology and volume during 5weeks of preoperative chemoradiotherapy - An evaluation with sequential MRIs. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol 126, 431–436 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Lambregts DMJ et al. A Pattern-Based Approach Combining Tumor Morphology on MRI With Distinct Signal Patterns on Diffusion-Weighted Imaging to Assess Response of Rectal Tumors After Chemoradiotherapy. Dis. Colon Rectum 61, 328–337 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Macchia G et al. Time to surgery and pathologic complete response after neoadjuvant chemoradiation in rectal cancer: A population study on 2094 patients. Clin. Transl. Radiat. Oncol 4, 8–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Habr-Gama A et al. Achieving a Complete Clinical Response After Neoadjuvant Chemoradiation That Does Not Require Surgical Resection: It May Take Longer Than You Think! Dis. Colon Rectum (2019). doi: 10.1097/DCR.0000000000001338 [DOI] [PubMed] [Google Scholar]
- 34.Glynne-Jones R et al. Best time to assess complete clinical response after chemoradiotherapy in squamous cell carcinoma of the anus (ACT II): a post-hoc analysis of randomised controlled phase 3 trial. Lancet Oncol. 18, 347–356 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith FM et al. A cohort study of local excision followed by adjuvant therapy incorporating a contact X-ray brachytherapy boost instead of radical resection in 180 patients with rectal cancer. Colorectal Dis. Off. J. Assoc. Coloproctology G. B. Irel 21, 663–670 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Hayden DM et al. Tumor scatter after neoadjuvant therapy for rectal cancer: are we dealing with an invisible margin? Dis. Colon Rectum 55, 1206–1212 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Kapali AS, Chandramohan K & Jayasudha AV A Prospective Study of Distal Microscopic Spread in Rectal Cancer After Neoadjuvant Chemoradiation in Pinned and Unpinned Specimen. Indian J. Surg. Oncol 8, 469–473 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinoshita H et al. Pathological changes of advanced lower-rectal cancer by preoperative radiotherapy. Hepatogastroenterology. 51, 1362–1366 (2004). [PubMed] [Google Scholar]
- 39.Kinoshita O et al. Flattened tumor requires a more careful attention for residual distal cancer spread in locally advanced lower rectal carcinoma after chemoradiotherapy. Dig. Surg 32, 159–165 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Mezhir JJ et al. Presence of distal intramural spread after preoperative combined-modality therapy for adenocarcinoma of the rectum: what is now the appropriate distal resection margin? Surgery 138, 658–663; discussion 663-664 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Park HS, Lee S, Haam S & Lee GD Effect of formalin fixation and tumour size in small-sized non-small-cell lung cancer: a prospective, single-centre study. Histopathology 71, 437–445 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Tran T et al. Correcting the Shrinkage Effects of Formalin Fixation and Tissue Processing for Renal Tumors: toward Standardization of Pathological Reporting of Tumor Size. J. Cancer 6, 759–766 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pritt B, Tessitore JJ, Weaver DL & Blaszyk H The effect of tissue fixation and processing on breast cancer size. Hum. Pathol 36, 756–760 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Goldstein NS, Soman A & Sacksner J Disparate surgical margin lengths of colorectal resection specimens between in vivo and in vitro measurements. The effects of surgical resection and formalin fixation on organ shrinkage. Am. J. Clin. Pathol 111, 349–351 (1999). [DOI] [PubMed] [Google Scholar]
- 45.Eid I et al. Histological processing variability in the determination of lateral resection margins in rectal cancer. J. Clin. Pathol 60, 593–595 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


