Highlights
-
•
Ten new limonoids were obtained from Toona sinensis (A. Juss.) Roem.
-
•
Limonoid 4 display potent ABTS⋅+ scavenging activity.
-
•
Limonoids 5 and 7–10 possessed the high ABTS⋅+ and DPPH scavenging activities.
-
•
Limonoids 1–4 and 11 showed the elective inhibition of Cox-1.
-
•
Limonoids 1–4 and 11 exhibited the significant cytotoxicities.
Keywords: Toona sinensis, Meliaceae, Limonoids, Radical scavenging, Anti-inflammatory, Cytotoxic activities
Abstract
A phytochemical investigation of the ethanol extract of Toona sinensis (A. Juss.) Roem resulted in the isolation of ten new limonoids, toonasinenines A–J (1–10), together with two known compounds, toonafolin (11) and toonacilianin D (12). Their structures were determined by spectroscopic analyses. The isolated components were evaluated in vitro for radical scavenging potential using ABTS⋅+ and DPPH test, anti-inflammatory activities for Cox-1 and Cox-2, and cytotoxicies against nine tumour cell lines (A549, BGC-823, CHG-5, HCT15, HeLa, HepG2, MDA-MB-231, SHG-44 and SGC-7901 cells). As a result, 4, 5 and 7–10 showed potent radical scavenging activities, while limonoids 1–4 and 11 exhibited significant anti-inflammatory and cytotoxic potential.
1. Introduction
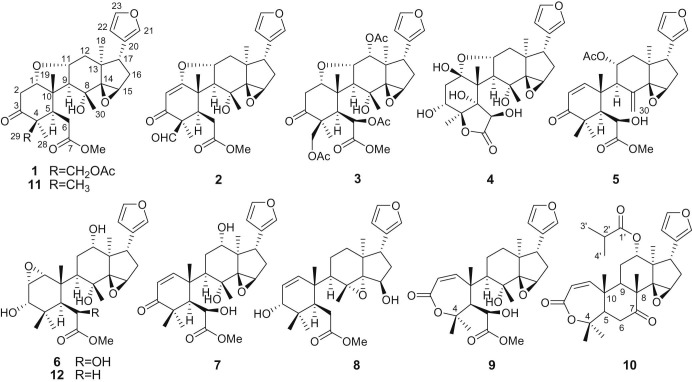
Toona sinensis (A. Juss.) Roem (Meliaceae), a perennial deciduous arbour, is widely distributed in eastern Asia (Edmonds & Staniforth, 1998). The leaves of T. sinensis, which contain a distinct flavour, are very popular in vegetarian cuisine and have long been used as a nutritious food in China and Malaysia and as animal fodder in India (Chen, Liang, Huang, & Huang, 2015). Almost every part of T. sinensis, including seeds, bark, root bark, petioles, and especially leaves, has a medicinal effect (Chang et al., 2006). The bark is used as astringent and depurative substance, the powdered root is used as a corrective, and the fruits are applied for the treatment of eye infection and used as an astringent. Leaves possess anti-inflammatory, antidoting, and worm-killing effects and are used in folk medicine to treat enteritis, dysentery, carbuncles, boils, dermatitis, scabies, tinea blanca, and especially abdominal tumours (Hsiang et al, 2013, Malairajan et al, 2007). Previous pharmacological and bioactivity studies on the extract of T. sinensis leaves have revealed anti-cancer (Chen, Chien, Huang, & Chia, 2014), anti-sepsis (Yang, Chen, Tsai, Huang, & Wang, 2014), antimicrobial (Wu et al., 2014), antinociceptive (Su et al., 2015), anti-inflammation (Hseu et al., 2011), anti-diabetes (Hsieh et al., 2012), antioxidant (Chen, Huang, Lin, Hsu, & Chung, 2013), and α-glucosidase inhibitory activities (Zhao, Zhou, Chen, & Wang, 2009). T. sinensis leaf extract inhibits microglia-mediated neuroinflammation (Wang, Tsai, Hsieh, Lin, & Lin, 2014), Leydig cell steroidogenesis (Poon, Leu, Hsu, Liu, & Huang, 2005) and severe acute respiratory syndromes (SARS) coronavirus replication (Chen et al., 2008). It also inhibits lipid accumulation and fatty acid oxidation in adipocytes (Liu, Tsai, & Chang, 2014). The safety profile and nonmutagenic characteristics of the extracts from T. sinensis have been evaluated using acute and sub-acute toxicity studies in mice and rat (Liao, Yeh, Lin, Wei, & Chung, 2009). Because of the pharmacological properties of T. sinensis leaves, the study of the chemical composition has been of particular interest. Currently, phytochemical studies have shown the presence of limonoids, flavonoids, phytols, coumarins and norcysteine derivative (Cheng et al., 2009). However, these studies mainly focus on the high polar extracts and volatile compounds. To find more pharmacologically and structurally interesting substances, the present investigation led to ten new limonoids, toonasinenines A–J (1–10), and 2 known compounds, toonafolin (11) and toonacilianin D (12) (Fig. 1 ). The structures of these compounds were elucidated mainly by nuclear magnetic resonance (NMR) spectroscopic and mass spectroscopic (MS) methods. Furthermore, all the limonoids were evaluated in vitro for their radical scavenging, anti-inflammatory and cytotoxic potential.
Fig. 1.
The structures of limonoids 1–12.
2. Materials and methods
2.1. General
Optical rotations were recorded on a Perkin-Elmer 341 polarimeter. IR spectra were taken on a Perkin-Elmer 577 spectrometer with KBr disks. NMR spectra were recorded on a Bruker AM-400 spectrometer. The coupling constants (J) were in Hz and chemical shift values (δ) were given in ppm with TMS as internal standard. ESI-MS and HR-ESI-MS spectra were obtained on an Esquire 3000plus (Bruker Daltonics) and a Finnigan LC QDECA instrument. Column chromatographic separations were performed with silica gel (200–300 mesh and H60, Qingdao Haiyang Chemical Group Corporation, China), MCI gel CHP20P (75–150 µm, Mitsubishi Chemical Industries, Tokyo, Japan), and Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden) as packing materials. TLC was carried out on precoated silica gel GF254 plates (Yantai Chemical Industries, Yantai, China). The TLC spots were visualised using 5% sulphuric acid in alcohol containing 10 mg/mL vanillin and viewed at 254 nm. Analytical HPLC was performed on a Waters 2690 instrument with a 996 PAD (photodiode array detector) with an Alltech ELSD 2000 detector. Semipreparative and preparative HPLC was carried out on a Varian SD1 instrument coupled with a 320 single-wave detector. Their chromatographic separations were taken on C-18 columns (250 × 10 mm, 5 µm, Waters, Massachusetts, USA; 220 × 25 mm, 10 µm, Merck, Darmstadt, Germany, respectively), with a gradient solvent system composed of MeOH and H2O, using a flow rate of 3.0 and 15.0 mL/min, respectively.
2.2. Plant material
The leaves of T. sinensis were collected in the suburb of Qujing, Yunan Province, China, in May 2014. A specimen (TS20140501), identified by one of the authors (X. Mao), was deposited in the Herbarium of the College of Biological Resources and Environment Science, Qujing Normal University, Qujing, China.
2.3. Extraction and isolation
The air-dried leaves of T. sinensis (5.0 kg) were ground into powder (80 mesh) and extracted with EtOH–H2O (95:5, v/v, 20 L) at ambient temperature three times (each for 7 days). After removal of solvent under reduced pressure, the crude extract (410 g) was partitioned between EtOAc and H2O to afford an EtOAc-soluble fraction (100 g) which was chromatographed on a silica gel column (1.5 kg) with CHCl3–MeOH mixtures (0:1, 100:1, 50:1, 20:1, 8:1, and 1:1, v/v) as elutant to obtain six fractions (F1–F6). F3 (5.9 g) was subjected to a MCI gel (MeOH–H2O, 50:50–90:10, v/v) to yield four subfractions (F3a–F3d). F3a (298 mg) was purified on reverse phase HPLC column eluting with methanol/water (65:35, v/v) to provide 7 (30 mg) and 12 (28 mg). F3b (226 mg) was separated on a column of C18 silica gel (MeOH–H2O, 45:55–60:40, v/v) followed by Sephadex LH-20 (CHCl3/MeOH, 1:1, v/v), furnishing 8 (37 mg). F3c (412 mg) was chromatographed on a preparative RP-HPLC column eluting with methanol/water (55:45, v/v) to afford 4 (43.7 mg) and 6 (37.1 mg). F4 (3.5 g) was applied to silica gel cc (petroleum ether–acetone; 4:1–1:1, v/v) to give five subfractions (F4a–F4e). F4b (698 mg) was separated on a SiO2 column with PE/Me2CO (2:1, v/v) as mobile phase and further purified on a preparative RP-HPLC column eluting with methanol/water (55:45, v/v) to afforded 1 (33.7 mg) and 3 (24.6 mg). F4c (963 mg) was subjected to silica gel CC with CHCl3–MeOH mixtures (50:1 and 30:1, v/v) as elutant to yield 5 (27 mg) and 10 (26.1 mg). F4d (897 mg) was applied to a column of reversed-phase silica gel (MeOH–H2O, 50:50, v/v) to yield four fractions F4d1–F4d4. Further chromatography of F4d2 (706 mg) on SiO2 column eluting with CHCl3/MeOH (95:5, v/v) and then purified by preparative HPLC, with CH3CN–H2O (1:1, v/v) as mobile phase, gave 2 (27 mg) and 9 (19 mg). F4d3 (249 mg) was separated on SiO2 column eluting with CHCl3/MeOH (95:5, v/v) and on Sephadex LH-20 column (CHCl3/MeOH, 1:1, v/v) to produce 11 (43 mg).
2.3.1. Toonasinenine A (1)
White amorphous powder, C29H38O9, −66.1 (c 0.25, MeOH), UV (MeOH) λmax (log ε): 210 (3.13) nm, IR (KBr) ν max: 3435, 2925, 1715, 1643, 1375, 1232, 797 cm−1, 1H and 13C NMR data see Table 1 , ESI-MS m/z: 553 ([M + Na]+), HR-ESI-MS: m/z 553.2408 ([M + Na]+, C29H38O9Na, calc. 553.2414).
Table 1.
1H NMR (400 MHz) and 13C NMR (100 MHz) data for compounds 1, 4–7 and 9: δ in ppm in CD3OD (multiplicities, J in Hz).
| No. | 1 | 4 | 5 | 6 | 7 | 9 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | δH | δC | δH | δC | δH | δC | |
| 1 | 3.80, dd (11.4, 5.0) | 85.5 | 107.5 | 7.30, d (10.7) | 155.7 | 3.23, d (4.3) | 57.9 | 6.80, d (10.0) | 152.6 | 6.28, d (11.8) | 150.1 | |
| 2α | 2.95, dd (15.8, 11.4) | 42.9 | 2.66, dd (14.2, 13.7) | 44.5 | 6.01, d (10.7) | 127.3 | 58.2 | 5.97, d (10.0) | 124.6 | 5.97, d (11.8) | 121.5 | |
| 2β | 2.66, overlapped | 2.01, dd (14.2, 4.4) | 3.11, m | |||||||||
| 3 | 208.8 | 4.34, dd (13.7, 4.4) | 70.8 | 207.1 | 3.69, d (2.8) | 74.0 | 204.8 | 166.7 | ||||
| 4 | 53.8 | 95.5 | 59.5 | 39.4 | 46.5 | 83.6 | ||||||
| 5 | 2.66, overlapped | 52.1 | 84.0 | 2.54, s | 48.5 | 2.34, s | 49.8 | 2.46, s | 48.8 | 2.48, s | 51.2 | |
| 6α | 2.63, dd (16.5, 1.8) | 32.9 | 3.89, s | 78.3 | 4.61, s | 72.5 | 4.42, s | 72.8 | 4.69, s | 71.2 | 4.69, s | 70.1 |
| 6β | 3.28, dd (16.5, 8.6) | |||||||||||
| 7 | 173.8 | 176.5 | 178.4 | 182.2 | 177.1 | 176.7 | ||||||
| 8 | 76.7 | 76.7 | 139.0 | 78.0 | 77.8 | 77.0 | ||||||
| 9 | 2.73, d (11.5) | 63.5 | 3.17, d (12.5) | 55.9 | 2.68, d (11.5) | 57.5 | 2.61, d (11.5) | 46.7 | 2.33, d (11.5) | 44.9 | 2.39, d (11.5) | 52.1 |
| 10 | 49.0 | 56.4 | 42.0 | 41.7 | 44.1 | 49.1 | ||||||
| 11α | 76.5 | 74.5 | 70.8 | 2.13, m | 35.3 | 2.21, m | 27.7 | 1.79, m | 24.7 | |||
| 11β | 3.95, m | 4.27, m | 5.16, m | 2.02, m | 2.07, m | 1.39, m | ||||||
| 12α | 1.62, dd (15.5, 5.6) | 41.0 | 1.55 dd (13.0, 6.6) | 46.7 | 1.86, dd (15.5, 11.6) | 42.8 | 77.2 | 62.1 | 1.55, dd (15.5, 11.2) | 35.3 | ||
| 12β | 2.44, dd (15.5, 11.6) | 2.36, dd (13.0, 10.6) | 1.92, dd (15.5, 5.6) | 4.07, dd (13.5, 10.8) | 4.08, dd (15.5, 11.6) | 1.94, dd (15.5, 5.4) | ||||||
| 13 | 41.6 | 42.9 | 45.4 | 47.8 | 45.9 | 41.0 | ||||||
| 14 | 74.3 | 73.8 | 73.3 | 76.5 | 74.6 | 74.6 | ||||||
| 15 | 3.46, s | 54.9 | 3.58, s | 57.2 | 4.06, s | 62.1 | 3.54, s | 56.9 | 3.47, s | 54.2 | 3.46, s | 54.4 |
| 16α | 1.81, dd (13.2, 6.6) | 31.3 | 1.87, dd (13.2, 6.6) | 32.5 | 2.00, dd (13.4, 11.0) | 32.1 | 1.89, dd (13.4, 11.2) | 33.4 | 1.79, m | 31.7 | 1.80, dd (13.2, 10.9) | 31.8 |
| 16β | 2.28, dd (13.2, 11.2) | 2.21, dd (13.2, 11.2) | 2.19, dd (13.4, 6.5) | 2.20, dd (13.4, 6.4) | 2.31, m | 2.25, dd (13.2, 6.4) | ||||||
| 17 | 2.76, dd (11.2, 6.6) | 41.2 | 2.74, dd (11.2, 6.6) | 43.6 | 2.63, dd (11.0, 6.5) | 41.4 | 2.73, dd (11.2, 6.4) | 44.0 | 2.77, dd (10.8, 6.4) | 41.3 | 2.74, dd (10.9, 6.4) | 41.3 |
| 18 | 1.05, s | 19.6 | 1.07, s | 23.9 | 0.71, s | 19.9 | 0.93, s | 16.6 | 0.84, s | 15.3 | 0.97, s | 22.7 |
| 19 | 1.08, s | 21.8 | 1.44, s | 13.5 | 1.22, s | 21.3 | 1.57, s | 19.4 | 1.76, s | 19.1 | 1.45, s | 21.0 |
| 20 | 122.7 | 124.9 | 124.8 | 125.4 | 123.3 | 123.0 | ||||||
| 21 | 7.15, br s | 139.3 | 7.32, s | 141.3 | 7.21, s | 141.4 | 7.24, s | 141.2 | 7.08, s | 139.0 | 7.13, s | 139.2 |
| 22 | 6.21, br s | 110.5 | 6.35, br s | 112.7 | 6.25, br s | 112.6 | 6.40, br s | 112.9 | 6.22, br s | 110.8 | 6.17, br s | 110.6 |
| 23 | 7.41, s | 143.1 | 7.42, br s | 144.4 | 7.44, br s | 144.9 | 7.39, br s | 144.3 | 7.35, br s | 142.7 | 7.36, br s | 142.9 |
| 28 | 1.02, s | 19.8 | 1.55, s | 15.8 | 1.07, s | 22. 4 | 0.85, s | 30.5 | 1.42, s | 24.8 | 1.46, s | 30.2 |
| 29a | 4.77, d (11.2) | 66.5 | 1.41, s | 26.2 | 1.12, s | 23.0 | 1.08, s | 21.9 | 1.70, s | 24.4 | ||
| 29b | 4.41, d (11.2) | |||||||||||
| 30a | 1.25, s | 20.3 | 1.29, s | 23.7 | 5.45, s | 121.6 | 1.25, s | 21.7 | 1.35, s | 21.7 | 1.34, s | 22.1 |
| 30b | 5.33, s | |||||||||||
| 7-OMe | 3.78, s | 52.2 | 3.67, s | 53.3 | 3.71, s | 53.4 | 3.78, s | 53.1 | 3.78, s | 52.8 | ||
| Ac | 2.23, s | 169.8 | 1.99, s | 171.7 | ||||||||
| 20.8 | 21.4 |
2.3.2. Toonasinenine B (2)
White amorphous powder, C27H32O8, −30.3 (c 0.24, MeOH), UV (MeOH) λmax (log ε): 210 (3.63), 259 (4.49) nm, IR (KBr) ν max: 3433, 2949, 1740, 1721, 1681, 1636, 1394, 1210, 1030 cm−1, ESI-MS m/z: 485 ([M + H]+), HR-ESI-MS: m/z 485.2179 ([M + H]+, C27H33O8, calc. 485.2175).
2.3.3. Toonasinenine C (3)
White amorphous powder, C33H42O13, +40.2 (c 0.15, MeOH), UV (MeOH) λmax (log ε): 207 (3.06) nm, IR (KBr) ν max: 3434, 2921, 1740, 1637, 1377 1232, 1072, 599 cm−1, ESI-MS m/z: 669 ([M + Na]+), HR-ESI-MS: m/z 669.2517 ([M + Na]+, C33H42O13Na, calc. 669.2523).
2.3.4. Toonasinenine D (4)
White amorphous powder, C25H32O10, +44.2 (c 0.10, MeOH), UV (MeOH) λmax (log ε): 207 (4.05) nm, IR (KBr) ν max: 3423, 2918, 2852, 1630, 1389, 1157, 1068, 1018 cm−1, 1H and 13C NMR data see Table 1, ESI-MS m/z: 515 ([M + Na]+), HR-ESI-MS: m/z 515.1887 ([M + Na]+, C25H32O10Na, calc. 515.1893).
2.3.5. Toonasinenine E (5)
White amorphous powder, C29H36O8, +70.6 (c 0.12, MeOH), UV (MeOH) λmax (log ε): 206 (3.86) nm, IR (KBr) ν max 3423, 2925, 1743, 1674, 1381, 1245, 1043 cm−1, 1H and 13C NMR data see Table 1, ESI-MS m/z: 535 ([M + Na]+), HR-ESI-MS: m/z 535.2303 ([M + Na]+, C29H36O8Na, calc. 535.2308).
2.3.6. Toonasinenine F (6)
White amorphous powder, C27H38O9, +70.6 (c 1, MeOH), UV (MeOH) λmax (log ε): 206 (3.86) nm, IR (KBr) ν max 3450, 2925, 1717, 1628, 1441, 1381, 1298, 1030, 600 cm−1, 1H and 13C NMR data see Table 1, ESI-MS m/z: 529 ([M + Na]+), HR-ESI-MS: m/z 529.2413 ([M + Na]+, C27H38O9Na, calc. 529.2414).
2.3.7. Toonasinenine G (7)
White amorphous powder, C27H36O8, −41.3 (c 0.09, MeOH), UV (MeOH) λmax (log ε): 238 (4.11) nm, IR (KBr) ν max 3602, 3449, 2924, 1725, 1622, 1247, 1157, 1024, 874 cm−1, 1H and 13C NMR data see Table 1, ESI-MS m/z: 489 ([M + H]+), HR-ESI-MS: m/z 489.2483 ([M + H]+, C27H37O8, calc. 489.2488).
2.3.8. Toonasinenine H (8)
White amorphous powder, C27H38O6, −8.1 (c 0.12, MeOH), UV (MeOH) λmax (log ε): 280 (3.16) nm, IR (KBr) ν max 3434, 2950, 1735, 1647, 1457, 1376, 1245, 1165, 1026, 874 cm−1, ESI-MS m/z: 481 ([M + Na]+), HR-ESI-MS: m/z 481.2560 ([M + Na]+, C27H38O6Na, calc. 481.2566).
2.3.9. Toonasinenine I (9)
White amorphous powder, C27H36O8, −53.9 (c 0.10, MeOH), UV (MeOH) λmax (log ε): 225 (3.55) nm, IR (KBr) ν max 3528, 2921, 1743, 1645, 1232, 602 cm−1, 1H and 13C NMR data see Table 1, ESI-MS m/z: 511 ([M + Na]+), HR-ESI-MS: m/z 511.2303 ([M + Na]+, C27H36O8Na, calc. 511.2308).
2.3.10. Toonasinenine J (10)
White amorphous powder, C30H38O7, −57.2 (c 0.15, MeOH), UV (MeOH) λmax (log ε): 225 (4.11) nm, IR (KBr) ν max 3563, 2934, 1730, 1700, 1615, 1385, 1157, 797 cm−1, ESI-MS m/z: 497 ([M + H]+), HR-ESI-MS: m/z 511.2693 ([M + H]+, C30H39O7, calc. 511.2696).
2.4. Microplate assay for radical scavenging activity DPPH
Microplate DPPH assay was performed as described according to Luo, Zhou, Ma, and Fu (2014). Briefly, successive sample dilutions (standard stocks of different samples 5 mM) in a 96-well plate afforded DPPH solution (40 µM in methanol) in a total volume of 0.2 mL. The absorbance value was recorded at 550 nm with a microplate read in triplicate. Results were determined each 5 min until 60 min in order to measure kinetic behaviour of the reaction. The percentage of remaining DPPH was calculated using the following: %DPPHrem = 100 × ([DPPH]sample/[DPPH]blank).
A calibrated Trolox (3.9 mM initial concentration) standard curve was also made. The percentage of remaining DPPH against the standard concentration was plotted in an exponential regression to obtain the amount of antioxidant necessary to decrease the initial DPPH concentration by 50% (IC50).
2.5. 2,2'-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical cation decolorisation assay
ABTS⋅+ scavenging activity was determined by the method of Gasca, Cabezas, Torras, Bastida, and Codina (2013). The radical cation was generated by the reaction between 7 mM ABTS in H2O and 2.45 mM potassium persulphate, stored at room temperature in the dark for 16 h. Before usage, the solution was diluted with phosphate buffer (pH 7.4, 0.05 M) to reach an absorbance of 0.800 ± 0.035 at 734 nm. Different concentrations of isolated compounds solution in methanol were added into 1 mL of ABTS⋅+ solution. The mixture was incubated in the dark at 37 °C. After 30 min of incubation, the percentage inhibition of absorbance at 734 nm was calculated for each concentration relative to a blank absorbance (methanol). All determinations were carried out in triplicate with Trolox as reference. The capability to scavenge the ABTS⋅+ was calculated with the following equation: ABTS⋅+ scavenging effect (%) = 100 − [(ASample/AControl) × 100], where ASample is absorbance of the remaining concentration of ABTS⋅+ in the presence of different compounds and AControl is the initial concentration of the ABTS⋅+. The stock concentrations of Trolox and tested compounds are the same as reported in DPPH assay.
2.6. Anti-inflammatory assay in vitro
The anti-inflammatory activities were measured according to the literature with slight modifications (Gao et al., 2015). The reaction system was incubated at 25 °C for 5 min, by sequential addition of the buffer, haem, pure compounds, and Cox-1 or Cox-2 into the system followed by mixing with arachidonic acid and TMPD. The optical density was recorded at 590 nm after another 15 min of incubation at 25 °C. SC-560 and NS-398 were used as positive controls which gave the inhibitions of Cox-1 (63.5%) and Cox-2 (97.0%) respectively (Table 2 ).
Table 2.
In vitro free radical scavenging and anti–inflammatory activities of compounds 1–12.
| Compounds | Free radical scavenging activity a | Anti-inflammatory activity b | ||
|---|---|---|---|---|
| DPPH IC50 | ABTS⋅+ IC50 | COX–1 | COX–2 | |
| 1 | – | – | 88.1 | 35.6 |
| 2 | – | – | 92.7 | 39.3 |
| 3 | – | – | 91.1 | 40.2 |
| 4 | 104.0 | 52.2 | 95.2 | 40.1 |
| 5 | 62.1 | 124.7 | 44.3 | 21.4 |
| 6 | 244.7 | 256.1 | 53.1 | 24.4 |
| 7 | 59.2 | 119.8 | <0 | <0 |
| 8 | 51.3 | 109.7 | <0 | <0 |
| 9 | 71.0 | 160.1 | <0 | <0 |
| 10 | 73.1 | 167.3 | 30.2 | 19.7 |
| 11 | – | – | 89.8 | 37.6 |
| 12 | – | – | 44.3 | 20.7 |
| SC–560 | 63.5 | |||
| NS–398 | 97.0 | |||
| Trolox | 42.8 | 80.1 | ||
All compounds and reference drug are expressed as IC50 values in µM. (–) No activity.
Percent inhibition (all compounds and reference drugs concentration: 100 µM).
2.7. Cytotoxicity assay in vitro
The cytotoxic potential of compounds 1–12 was evaluated by modified MTT method (Chen, Hung, Sung, Chen, & Kuo, 2011). Nine tumour cell lines (A549, BGC-823, CHG-5, HCT15, HeLa, HepG2, MDA-MB-231, SHG-44, and SGC-7901 cells) were cultured on RPMI-1640 medium supplemented with 10% foetal bovine serum, 100 µg/mL streptomycin and 100 U/mL penicillin in culture flasks in humidified atmosphere with 5% CO2 at 37 °C. For the cytotoxic tests, cells were harvested in exponential growth stage by trypsin digestion and centrifuging at 180 × g for 3 min, then resuspended in fresh medium at a cell density of 5 × 104 cells per mL. The cell suspension was dispensed into a 96-well microplate at 100 µL/well, and incubated at 37 °C in humidified atmosphere with 5% CO2 for 24 h, and then treated with test compounds at various concentrations (0, 1, 10, 100 µM). After 48 h of treatment, 50 µL of 1 mg/mL MTT solution was added into each well, and further incubated for 4 h. The cells in each well were solubilised with DMSO (100 µL for each well) and the optical density was recorded at 570 nm. All drug doses were examined at least three times with Adriamycin as the positive control. The IC50 values were derived from the mean OD values of the triplicate tests versus drug concentration curves and expressed as means ± standard deviation.
3. Results and discussion
3.1. Structure elucidation of the new limonoids
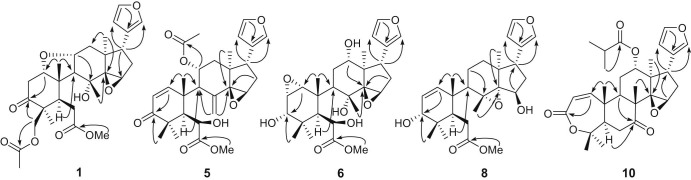
Compound 1 was obtained as a white amorphous powder. Its molecular formula was deduced as C29H38O9 by HR-ESI-MS spectrum, corresponding to 11 unsaturation degrees. The 1H NMR spectrum of 1 exhibited the presence of resonances for a methoxy proton at δ H 3.78 (s), four tertiary methyls [δ H 1.02, 1.05, 1.08, and 1.25 (each 3H, s)], one oxygenated methylene at δ H 4.77 and 4.41, three characteristic protons attached to a carbon adjacent to an oxygen atom [δ H 3.46 (s), 3.80 (dd, J = 11.4, 5.0 Hz) and 3.95 (m)], and a β-substituted furan ring group [δ H 6.21 (br s), 7.15 (br s), and 7.41 (s)]. Analyses of its 13C NMR spectrum displayed 29 carbon resonances due to six methyl (one oxygenated), five sp3 methylene (one oxygenated), nine methine (three oxygenated and three olefinic), seven quaternary (one ketone and one olefinic), and two ester carbonyl carbons. These functionalities (two double bonds and three cabonyl) accounted for 5 out of the 11 degrees of unsaturation. The remaining 6 degrees of unsaturation required 1 to be hexacyclic. Strong HMBC correlations of H-12 (δ H 2.44 and 1.62), H-16 (δ H 2.28 and 1.81), H-17 (δ H 2.76), and H-30 (δ H 1.25) with C-14 (δ C 74.3) and H-17 with C-15 (δ C 54.9) and C-21 (δ C 139.3) clearly indicated the five-membered ring D to have a 14,15-epoxy group. A ketone carbon resonance (δ C 208.8) was assigned to C-3 as judged from the HMBC correlations of Me-28/C-3 and Me-29/C-3. The oxygen bridge could then be located between C-1 and C-11 due to the HMBC correlation of H-1 (δ H 3.80) with C-3 and C-9 (δ C 63.5) and of H-11 (δ H 3.95) with C-10 (δ C 49.0) and C-13 (δ C 41.6), which was further confirmed by the correlations of H-1/H-2 and H-9/H-11/H-12 in 1H-1H COSY spectrum. These spectral evidences indicated that 1 might be a ring B-seco limonoid bearing the same C, D and E rings with a 14β,15β-epoxide identical with toonafolin (11) (Liu et al., 2012), obtained also from the same plant source. The only significant difference between 1 and toonafolin was the presence of an acetyl group at C-29 in 1, which was further confirmed by the HMBC correlation of the oxygenated methylene protons at δ H 4.77 and 4.41 (H-29) with the carbonyl carbon (δ C 169.8) of the acetyl group. On the basis of the observation of ROESY data similar to those of 11, the stereochemistry of 1 was expected to be the same. Ring A should be nearer to a chair conformation, in which H-1 and H3-19 are eclipsed which was supported by ROESY correlations of H-1β (δ H 3.80) with H-2β (δ H 2.66) and H3-19 (δ H 1.08). The ROESY correlations of H-1/H-2β, H-1/H3-19β, H-11/H3-19β, H-11/H-12β, and H-11/H3-30β suggested that H-1 and H-11 were β-orientated. Moreover, the existence of a correlation from H3-19β to H-11 (δ H 3.95) confirmed that H-11 is in the β- and the oxide ring in the α-configuration. The configuration of the 14,15-epoxide was deduced by NMR spectroscopic comparison with 1 and 11, which showed similar chemical shifts and proton coupling patterns. Consequently, compound 1 was unambiguously determined as toonasinenine A.
Compound 2 corresponded to molecular formula C27H32O8, which was determined by positive HR-ESI-MS data. The IR spectrum displayed absorption bands for saturated carbonyl (1740 cm−1), aldehyde (1721 cm−1), unsaturated carbonyl (1681 cm−1) and olefinic (1636 cm−1) groups. Comparison of NMR data of compounds 2 and 1 indicated that they were almost the same except for the ring A signals. In NMR experiments, one C C [δ C 185.2 (s), 98.2 (δ H 5.47, s, 1H)] was found and the signal of C-3 in 2 was upfield shifted to δ C 195.8. These data established that one α,β-unsaturated carbonyl group was located at C-1, C-2 and C-3. This deduction was further confirmed by the UV absorptions at λma 210 and 259 nm and by the HMBC correlations of H-2 to C-4 and C-10. Additionally, the chemical shifts of an aldehyde group [δ C 199.8 and δ H 9.82 (s)] in 2 taking the place of oxygenated methylene (C-29) in 1. Therefore, 2 was structurally elucidated to be toonasinenine B.
The molecular formula of the compound 3 was assigned as C33H42O13 on the basis of the quasimolecular ion peak m/z 669.2517 in positive HR-ESI-MS. The 1H and 13C NMR data (Table 1) of 3 were almost identical to those of 1 except that the C-atom signal assignable to C-12 was shifted downfield from δ C 41.0 in 1 to δ C 75.5 in compound 3, implying that there was one OAc group located at C-12. This was further confirmed by the cross-peaks between H-12 (δ H 5.49, dd, J = 16.0, 10.8 Hz) with C-9, C-14 and the carbonyl carbon (δ C 172.3) of the acetyl in the HMBC spectrum. The above evidences led to conclusion that the structure of compound 3 was determined as toonasinenine C.
Compound 4 possessed the molecular formula C25H32O10 as determined by analyses of NMR data and verified by the high-resolution mass spectrometry. The typical NMR patterns, especially the presence of the β-substituted furan ring and four tertiary methyl groups, implied that 4 was likely to be a norlimonoid (Liao et al., 2009). Comparison of its NMR spectroscopic data with those of toonaciliatin L (Liu et al., 2011) suggested that they are structural congeners. The mere 13C NMR difference was the NMR resonances corresponding to a methine (δ C 46.7; δ H 2.36 and 1.55, H-12) replaced those of the oxygenated methylene in toonaciliatin L. In the ROESY spectrum, the key cross-peak of H-3/H-2β elucidated that H-3 was axial towards the β-direction, which was also elucidated by the upfield shift for the carbon resonance of C-28 due to the γ-gauche effect of 3-OH. All available evidence suggested the structure of toonasinenine D.
Compound 5 exhibited a quasimolecular ion peak at m/z 535.2303 [M + Na]+ (calc. 535.2308) in positive HR-ESI-MS spectrum, which corresponded to the molecular formula C29H36O8. The 1H and 13C NMR data (Table 1) of compound 5 showed many similarities to those of 12-deacetoxytoonacilin, indicating that they are structural analogues with the only difference being the presence of OH-6 in 5 (Liu et al., 2012). This was confirmed by the HMBC correlations of H-6 (δ H 4.61) to C-4 (δ C 59.5) and C-10 (δ C 42.0), and of OMe (δ H 3.67) to C-7 (δ C 178.4). The β-oriented configuration of the OH at C-6 in 5 was deduced by comparing the chemical shifts of H-6 and C-6, and the coupling pattern with its analogues 12-deacetoxytoonacilin and substantiated by its ROESY spectrum. On the basis of these results, the structure of 5 can be represented as toonasinenine E.
Compound 6 was isolated as a white amorphous powder with molecular formula C27H38O9, as determined by HR-ESI-MS, showing an [M + Na]+ ion at m/z 529.2413. Analysis of the 1H and 13C NMR spectra suggested the presence of a disubstituted epoxy group (δ C 57.9, 58.2; δ H 3.23, 3.11), which was assigned to be α-oriented 1,2-epoxy on the basis of HMBC correlations of Me-19/C-1, H-2/C-10, and H-2/C-4, and the ROESY cross-peaks of H-1 with H-11α, H-11β and H3-30, and of H-2 with H-3 (Fig. 2 ). Comparison of NMR data of 6 and toonacilianin D (12) indicated that they were similar in structure, except that signal due to C-6 was shifted downfield to δ C 72.8 in 6, suggesting that there should be a hydroxy group located at C-6 (Liu et al., 2011). This deduction was further confirmed by the cross-peaks between H-6 (δ H 4.42), and C-4 and C-10 in the HMBC spectrum. The other three hydroxyl groups were placed on C-3 (δ C 74.0), C-8 (δ C 78.0), and C-12 (δ C 77.2), respectively, by the key HMBC correlations of H3-28 and H3-29 to C-3, of H-11 and H-15 to C-8, and of H-12 to C-9, C-14, and C-17, respectively. The ROESY correlations from both H3-28 and H3-29 to H-3 indicated gauche relationships of H3-28/H-3 and H3-29/H-3, and that H-3 was thus equatorial and towards the β-face. The strong ROESY correlations of H3-30/H-11β and H-12/H-17β unambiguously indicated the β-configurations of H-12 and H3-30. Thus, the structure of 6 was established as depicted.
Fig. 2.
Key HMBC ( ) correlations of compounds 1, 5, 6, 8 and 10.
) correlations of compounds 1, 5, 6, 8 and 10.
The positive HR-ESI-MS of compound 7 was consistent with the formula C27H36O8, corresponding to 10 degrees of unsaturation. The similarities between the 13C NMR of 7 and 6 suggested that they were structural congeners and that the major differences occurred in ring A. The resonances at δ C 204.8 (s), 152.6 (d), 124.6 (d), and 46.5 (s) of ring A were similar to those of ring A of 5, which indicated that an α,β-unsaturated ketone group was located at C-1, C-2 and C-3 in the ring A of 7. In the HMBC spectrum, correlations of H-6 to C-4, C-10, and C-7 suggested that a hydroxyl group was linked to C-6. The α-configuration of H-6 orientation was confirmed by the correlations between H-6/H-9 and H-9/H3-18 in the ROESY spectrum. Thus, compound 7, named toonasinenine G, was determined as shown in Fig. 1.
Compound 8 was isolated as a white amorphous powder. The molecular formula C27H38O6 was established by a positive ion HR-ESI-MS. The infrared spectrum exhibited the characteristic absorptions of OH group (3434 cm−1) and a C O double bond at 1647 cm−1. In 13C NMR spectrum, the characteristic signals for C-8 (δ C 69.4), C-14 (δ C 79.0) and C-15 (δ C 76.2) suggested the 8,14-epoxide ring and a hydroxyl at C-15 in 8, which was further confirmed by the presence of a spin system of O-H-15/H2 -16/H-17 in 1H-1H COSY and the HMBC correlation of H-11, H-15 with C-8 and H-16 and H-17 with C-14. The ROESY correlations of H3-19/H3-30 and H-15/H3-18 suggested that the 8,14-epoxide ring and the OH-15 were in α- and β-orientations respectively. Careful analysis of the NMR spectra suggested that 8 was also a ring B-seco limonoid bearing the same C, D and E rings with a 8,14-epoxy group as in toonaciliatin E (Liu et al., 2011). The hydroxyl group on C-3 (δ C 77.9) was elucidated by the key HMBC correlations of Me-28 and Me-29 to C-3. The ROESY cross-peaks from both Me-28 and Me-29 to H-3 indicated gauche relationships of Me-28 to H-3 and Me-29 to H-3, and that H-3 was thus β-orientations. Consequently, compound 8 was unambiguously determined as toonasinenine H.
Compound 9 was assigned a molecular formula C27H36O8 established by a positive HR-ESI-MS. The 1H NMR spectrum displayed six methyls (including one methoxy), one β-substituted furan ring (δ H 6.17, 7.13, and 7.36), and two olefinic methines [δ H 6.28 and 5.97 (each, d, J = 11.8 Hz)]. Additionally, an α,β-unsaturated lactone (δ C 166.7, 150.1, 121.5 and 83.6) was distinguished by analysis of its 13C NMR and HMBC data. The above evidence showed that compound 9 was a tetranortriterpenoid sharing some similarity to toonayunnanin E (Liu et al., 2012). The NMR difference indicated that the acetyl group at C-6 in toonayunnanin E was replaced by a hydroxyl group in 9, which was further confirmed by the upfield shift of C-6 to 70.1 in 9 and the HMBC correlations of H-6 (δ H 4.69) with C-4 (δ C 83.6) and C-10 (δ C 49.1). The 14,15-epoxide ring and a hydroxyl group at C-8 in 9 was elucidated by the HMBC correlations from H3-18, H-16, H-17 and H3-30 to C-14 and from H-17 to C-15. The ROESY correlation of H3-30/H3-19β suggested the α-configuration of 8-OH. The singlet of H-6 suggested that the dihedral angle between H-6 and H-5 is 90° in its preferred conformation, and the ROESY correlations of H-6/H-5, H-6/H-9, and H-6/H3-28 were consistent with a 6R* configuration. The configuration of the 14,15-epoxide ring was the same as 1 according to their similar ROESY correlations. Finally, the structure of 9 named toonayunnanin J was established.
Compound 10 gave a quasimolecular ion peak at m/z 511.2693 [M + H]+ (calc. 511.2696) in the HR-ESI-MS, accounting for a molecular formula C30H38O7. The IR absorptions at 1730 and 1615 cm−1 and the UV absorption at λmax 225 nm suggested an α,β-unsaturated ester. The presence of an isobutyryloxy group, five methyls, three methylenes, ten methines (five olefinic, three of which due to β-substituted furan ring), and seven quaternary carbons (one olefinic attributed to β-substituted furan ring, two oxygenated, and two carbonyl), were indicated by analysis of the 1H, 13C NMR, and DEPT data. The ketonic carbonyl group located at C-7 was confirmed by correlations of H3-30, H-9, and H-5 to C-7 in the HMBC experiment. These data showed that 10 was an evodulone-class limonoid possessing the same ring fusion as 9. The 13C NMR signals of 14,15-epoxide group [δ C 69.7 (s), and 55.4 (δ H 3.56, s, 1H)] were superimposable over those of 9. So the stereo-chemistry of the 14,15-epoxide was assigned to be in β-orientation. The HMBC correlations from H-12, H3-3′, H3-4′, H-2′ to C-1′ indicated that the isobutyryloxy group was attached to C-12. In the ROESY spectrum of 10, the correlations of H-12 with H3-19β and H-17β, and of H-7 with H3-30β and H-14, suggested that H-7 and H-12 were β-orientated. Thus, the structure of 10 was determined as toonayunnanin J.
The structural elucidations from the spectral data of the 2 known compounds, 11 and 12, are in agreement with those found in the literatures and characterised as toonafolin (11) and toonacilianin D (12) (Liu et al., 2011).
3.2. Radical scavenging activity
Antioxidant evaluation of 1–12 by both DPPH and ABTS assays (Table 2) showed that compounds 5 and 7–10 had significant antiradical activities to the tested radical of DPPH and ABTS⋅+. Comparing the IC50 values with that shown by Trolox in the two assays, compounds 4 seemed to possess stronger antiradical activities on radicals of ABTS⋅+ but lower scavenging activity on DPPH assays than other compounds.
3.3. Anti-inflammatory activity
The compounds 1–12 were tested in vitro for their anti-inflammatory activities (Table 2). Among the assayed compounds, 1–4 and 11 with 14β,15β-epoxide and 1α,11α-oxygen bridge displayed comparable selective inhibition of Cox-1 (>88%) with positive control SC–560, while alkaloids 5, 6 and 12 exhibited modest selective inhibition of Cox-1. The other compounds had no anti-inflammatory activities or selective inhibition of Cox-1 comparable to those of 1–4 and 12 although they possess the similar limonoid skeleton.
3.4. Cytotoxic activity on cancer cells
The cytotoxic activities of the isolated compounds were examined against A549 cells (human lung cancer), BGC-823 cells (human gastric carcinoma), CHG-5 (glioma), HCT15 cells (human colon cancer), HeLa cells (human cervical cancer), HepG2 cells (Human hepatocellular carcinoma), MDA-MB-231 cells (human breast cancer), SHG-44 (glioma), and SGC-7901 cells (human gastric adenocarcinoma) (Table 3 ). Among all compounds, B-seco limonoids 1–4 and 11 bearing 14β,15β-epoxide and 1α,11α-oxygen bridge possessed the higher cytotoxic potential (IC50 value less than 15 µM) than 5–7 and 12 with only 14β,15β-epoxide (IC50 value less than 25 µM) against all tested tumour cell lines except glioma cell lines. Additionally, limonoids 9 and 10 with a 14β,15β-epoxide in which A ring contain an α,β-unsaturated lactone displayed particular cytotoxicities against two glioma cell lines.
Table 3.
Cytotoxicities of compounds 1–12 against nine human tumour cell lines (IC50 ± SD, µM).
| Compounds | Cell lines | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A-549 | BGC-823 | CHG-5 | HCT15 | HeLa | HepG2 | MDA-MB-231 | SGC-7901 | SHG-44 | |
| 1 | 13.3 ± 3.0 | – | 14.6 ± 4.6 | 14.7 ± 4.3 | 14.0 ± 4.3 | 13.9 ± 3.7 | 14.2 ± 4.0 | 13.1 ± 4.9 | – |
| 2 | 5.7 ± 2.7 | 33.7 ± 7.8 | 5.0 ± 2.1 | 5.7 ± 2.3 | 6.2 ± 2.7 | 5.5 ± 1.6 | 6.0 ± 1.1 | 6.0 ± 2.1 | – |
| 3 | 9.7 ± 7.4 | – | 8.3 ± 5.7 | 10.1 ± 6.4 | 8.1 ± 5.1 | 9.1 ± 8.8 | 9.4 ± 7.3 | 9.4 ± 8.1 | – |
| 4 | 2.3 ± 0.6 | 27.9 ± 9.6 | 2.8 ± 0.7 | 2.6 ± 0.5 | 2.9 ± 0.8 | 3.0 ± 1.1 | 2.7 ± 0.9 | 2.1 ± 0.7 | 44.9 ± 10.3 |
| 5 | 20.4 ± 4.6 | – | 19.9 ± 5.3 | 21.5 ± 6.8 | 23.6 ± 5.0 | 23.4 ± 4.5 | 21.0 ± 3.5 | 21.1 ± 4.6 | – |
| 6 | 23.3 ± 5.0 | – | 23.9 ± 6.2 | 24.6 ± 6.1 | 24.7 ± 7.1 | 24.0 ± 5.3 | 22.4 ± 4.4 | 24.2 ± 5.0 | – |
| 7 | 18.4 ± 4.9 | – | 19.5 ± 5.3 | 18.4 ± 5.7 | 21.6 ± 5.3 | 21.7 ± 5.0 | 20.8 ± 5.3 | 19.9 ± 4.0 | – |
| 8 | 34.8 ± 7.2 | – | 31.2 ± 8.6 | 33.2 ± 8.7 | 31.4 ± 7.1 | 31.6 ± 6.1 | 33.2 ± 8.1 | 33.6 ± 7.9 | – |
| 9 | 44.3 ± 8.2 | 18.6 ± 4.0 | – | – | – | 43.2 ± 10.1 | – | 39.1 ± 9.7 | 28.0 ± 5.3 |
| 10 | – | 22.7 ± 4.1 | – | 49.7 ± 9.1 | – | 46.7 ± 9.8 | – | – | 31.4 ± 7.4 |
| 11 | 9.7 ± 2.7 | 47.1 ± 13.1 | 9.6 ± 1.3 | 11.2 ± 3.4 | 9.0 ± 2.1 | 10.7 ± 0.6 | 9.8 ± 2.4 | 10.5 ± 2.9 | – |
| 12 | 19.0 ± 3.0 | – | 20.0 ± 2.6 | 22.0 ± 2.9 | 22.4 ± 4.7 | 22.8 ± 5.1 | 23.8 ± 4.6 | 20.3 ± 4.8 | – |
| Doxorubicin | 0.01 | 0.02 | 0.03 | 0.05 | 0.04 | 0.03 | 0.02 | 0.04 | 0.04 |
The assay was done using three replicates and repeated four times. (–) IC50 > 50 µM.
The tender leaves of T. sinensis are used as a nutritious and functional food for treating enteritis, gastric ulcers, rheumatoid arthritis, cervicitis, urethritis, tympanitis, dysentery, itchiness, and cancer. In screening radical scavenging activities, compounds 5 and 7–10 exhibited significant antiradical activities to the tested radical of DPPH and ABTS⋅+. Due to their intrinsic structural variety, we were interested in establishing that α,β-unsaturated ketone moiety in the ring A, capable of donating an electron and hydrogen atom by resonance hybrid and keto-enol Keto-enol tautomerisation, may strengthen the actions of radical scavenging character. Compounds 4 exhibited more significant antiradical activities on radicals of ABTS⋅+ than on DPPH assays, which may be elucidated by the mechanism difference that the ABTS⋅+ reactions involve electron transfer and take place at a much faster rate compared to DPPH radicals. 4 possesses high amount of phoenols which can take part in electron transfer and hydrogen atom transfer reactions with many oxidising free radicals. In anti-inflammatory and cytotoxic bioassays, limonoids 1–4 and 11 bearing a 14β,15β-epoxide have higher activities relative to 5–7 and 12 with 8α,14α-epoxide. Among them, 4 exhibited the most significant anti-inflammatory and cytotoxic activities with the lowest IC50. These result suggested the 14β,15β-epoxide and 1α,11α-oxygen bridge may be important and the γ-lactone between C-4 and C-5 maybe strengthen for their anti-inflammatory and cytotoxic actions for this class of limonoids. In addition, limonoids 5 showed stronger cytotoxicities than 6, which indicated the 1,2-epoxy might be weaken the cytotoxic potential. Limonoids 9 and 10 displayed cytotoxic activities against gliom cell lines (IC50: 18.6 and 28.0 µM; 22.7 and 31.4 µM respectively). These result suggested that an α,β-unsaturated lactone in A ring should be important for their particular cytotoxicities against gliom cell lines.
4. Conclusions
Ten new limonoids, toonasinenines A-J (1–10), together with 2 known compounds, toonafolin (11) and toonacilianin D (12) were isolated from the stems of T. sinensis. All the limonoids were in vitro evaluated for their radical scavenging activities of ABTS⋅+ and DPPH, anti-inflammatory for Cox-1 or Cox-2, cytotoxicies against eight tumour cell lines. Compound 4 possess higher antiradical activities than other compounds on radicals of ABTS⋅+. Compounds 5 and 7–10 possessed strong scavenging activities of ABTS⋅+ and DPPH. Limonoids 1–4 and 11 with a 14β,15β-epoxide and a 1α,11α-oxygen bridge showed selective inhibition for COX-1 (>88%) and cytotoxicities against all tested tumour cell lines except glioma cell lines. Limonoids 9 and 10 with a 14β,15β-epoxide in which A ring contain an α,β-unsaturated lactone displayed particular cytotoxic activities against glioma cell lines. Taken together, the amounts of limonoids are an important index to evaluate the therapeutic effects for the medium polar extracts of T. sinensis.
Acknowledgments
The above research was made possible by the grant from the National Natural Science Foundation of China (31300370) and the Scientific Planning Project of the Applied Basic Research of Yunnan Province (S2012FZ0005).
Contributor Information
Jiang Hu, Email: hujiang@mail.kib.ac.cn.
Qin-Jie Zhao, Email: qjzhao@smmu.edu.cn.
References
- Chang H.L., Hsu H.K., Su J.H., Wang P.H., Chung Y.F., Chia Y.C., Tsai L.Y., Wu Y.C., Yuan S.S. The fractionated Toona sinensis leaf extract induces apoptosis of human varian cancer cells and inhibits tumor growth in a murine xenograft model. Gynecologic Oncology. 2006;102:309–314. doi: 10.1016/j.ygyno.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Chen C.J., Michaelis M., Hsu H.K., Tsai C.C., Yang K.D., Wu Y.C., Cinatl J., Jr., Doerr H.W. Toona sinensis Roem tender leaf extract inhibits SARS coronavirus replication. Journal of Ethnopharmacology. 2008;120(1):108–111. doi: 10.1016/j.jep.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.H., Huang F.S., Lin Y.C., Hsu C.K., Chung Y.C. Effects of water extract from anaerobic fermented Toona sinensis Roemor on the expression of antioxidant enzymes in the Sprague–Dawley Rats. Journal of Functional Foods. 2013;5:773–780. [Google Scholar]
- Chen J.J., Hung H.C., Sung P.J., Chen I.S., Kuo W.L. Aporphine alkaloids and cytotoxic lignans from the roots of Illigera luzonensis. Phytochemistry. 2011;72:523–532. doi: 10.1016/j.phytochem.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Chen Y.C., Chien L.H., Huang B.M., Chia Y.C. Toona sinensis (aqueous leaf extracts) induces apoptosis through the generation of ROS and activation of intrinsic apoptotic pathways in human renal carcinoma cells. Journal of Functional Foods. 2014;7:362–372. [Google Scholar]
- Chen Y.C., Liang Y.L., Huang Y.L., Huang B.M. Mechanism of Toona sinensis-stimulated adrenal steroidogenesis in primary rat adrenal cells. Journal of functional foods. 2015;14:318–323. [Google Scholar]
- Cheng K.W., Yang R.Y., Tsou S.C.S., Lo C.S.C., Ho C.T., Lee T.C., Wang M.F. Analysis of antioxidant activity and antioxidant constituents of Chinese toon. Journal of Functional Foods. 2009;1:253–259. [Google Scholar]
- Edmonds J.M., Staniforth M. Toona sinensis (Meliaceae) Curtis's Botanical Magazine. 1998;15:186–193. [Google Scholar]
- Gao C., Huang X.X., Bai M., Wu J., Li J.Y., Liu Q.B., Li L.Z., Song S.J. Anti-inflammatory sesquiterpene pyridine alkaloids from Tripterygium wilfordii. Fitoterapia. 2015;105:49–54. doi: 10.1016/j.fitote.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Gasca C.A., Cabezas F.A., Torras L., Bastida J., Codina C. Chemical composition and antioxidant activity of the ethanol extract and purified fractions of cadillo (Pavonia sepioides) Free Radicals and Antioxidants. 2013;3:S55–S61. [Google Scholar]
- Hseu Y.C., Chen S.C., Lin W.H., Hung D.Z., Lin M.K., Kuo Y.H., Cho H.J., Wang L., Yang H.L. Toona sinensis (leaf extracts) inhibit vascular endothelial growth factor (VEGF)-induced angiogenesis in vascular endothelial cells. Journal of Ethnopharmacology. 2011;134(1):111–121. doi: 10.1016/j.jep.2010.11.058. [DOI] [PubMed] [Google Scholar]
- Hsiang C.Y., Hseu Y.C., Chang Y.C., Kumar K.J., Ho T.Y., Yang H.L. Toona sinensis and its major bioactive compound gallic acid inhibit LPS-induced inflammation in nuclear factor-kappaB transgenic mice as evaluated by in vivo bioluminescence imaging. Food Chemistry. 2013;136:426–434. doi: 10.1016/j.foodchem.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Hsieh T.J., Tsai Y.H., Liao M.C., Du Y.C., Lien P.J., Sun C.C., Chang F.R., Wu Y.C. Anti-diabetic properties of non-polar Toona sinensis Roem extract prepared by supercritical-CO2 fluid. Food and Chemical Toxicology. 2012;50:779–789. doi: 10.1016/j.fct.2011.12.023. [DOI] [PubMed] [Google Scholar]
- Liao J.W., Yeh J.Y., Lin Y.C., Wei M.M., Chung Y.C. Mutagenicity and safety evaluation of water extract of fermented Toona sinensis roemor leaves. Journal of Food Science. 2009;74(1):T7–T13. doi: 10.1111/j.1750-3841.2008.01007.x. [DOI] [PubMed] [Google Scholar]
- Liu H.W., Tsai Y.T., Chang S.J. Toona sinensis leaf extract inhibits lipid accumulation through up-regulation of genes involved in lipolysis and fatty acid oxidation in adipocytes. Journal of Agricultural and Food Chemistry. 2014;62(25):5887–5896. doi: 10.1021/jf500714c. [DOI] [PubMed] [Google Scholar]
- Liu J.Q., Wang C.F., Li Y., Chen J.C., Zhou L., Qiu M.H. Limonoids from the leaves of Toona ciliata var. yunnanensis. Phytochemistry. 2012;76:141–149. doi: 10.1016/j.phytochem.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Liu J., Yang S.P., Su Z.S., Lin B.D., Wu Y., Yue J.M. Limonoids from the stems of Toona ciliata var. henryi (Meliaceae) Phytochemistry. 2011;72:2189–2196. doi: 10.1016/j.phytochem.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Luo Y.H., Zhou Z.Q., Ma S.C., Fu H.Z. Three new antioxidant furofuran lignans from Callicarpa nudiflora. Phytochemistry Letters. 2014;7:194–197. [Google Scholar]
- Malairajan P., Gopalakrishnan G., Narasimhan S., Veni K.J., Kavimani S. Anti-ulcer activity of crude alcoholic extract of Toona ciliata Roemer (heart wood) Journal of Ethnopharmacology. 2007;110:348–351. doi: 10.1016/j.jep.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Poon S.L., Leu S.F., Hsu H.K., Liu M.Y., Huang B.M. Regulatory mechanism of Toona sinensis on mouse Leydig cell steroidogenesis. Life Sciences. 2005;76(13):1473–1487. doi: 10.1016/j.lfs.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Su Y.F., Yang Y.C., Hsu H.K., Hwang S.L., Lee K.S., Lieu A.S., Chan T.F., Lin C.L. Toona sinensis leaf extract has antinociceptive effect comparable with non-steroidal anti-inflammatory agents in mouse writhing test. BMC Complementary and Alternative Medicine. 2015;15:70. doi: 10.1186/s12906-015-0599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.C., Tsai Y.J., Hsieh Y.C., Lin R.J., Lin C.L. The aqueous extract from Toona sinensis leaves inhibits microglia-mediated neuroinflammation. Kaohsiung Journal of Medical Sciences. 2014;30:73–81. doi: 10.1016/j.kjms.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.G., Peng W., Yi J., Wu Y.B., Chen T.Q., Wong K.H., Wu J.Z. Chemical composition, antimicrobial activity against Staphylococcus aureus and a pro-apoptotic effect in SGC-7901 of the essential oil from Toona sinensis (A. Juss.) Roem. leaves. Journal of Ethnopharmacology. 2014;154:198–205. doi: 10.1016/j.jep.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.J., Chen Y.C., Tsai Y.J., Huang M.S., Wang C.C. Toona sinensis leaf aqueous extract displays activity against sepsis in both in vitro and in vivo models. Kaohsiung Journal of Medical Sciences. 2014;30:279–285. doi: 10.1016/j.kjms.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Zhou X.W., Chen X.B., Wang Q.X. α-glucosidase inhibitory constituents from Toona sinensis. Chemistry of Natural Compounds. 2009;45:244–246. [Google Scholar]