Abstract
The panel of laboratory tests available for diagnosis of gastrointestinal (GI) diseases in dogs and cats is wide, and, recently, several new tests have been developed. This article will focus on advances in laboratory tests that are available for the general practitioner for diagnosis of GI diseases. Laboratory tests for diagnosis of gastric and intestinal infectious diseases include fecal parasite screening tests, enzyme-linked immunosorbent assays for parvoviral enteritis, and some specific bacterial tests like fluorescent in situ hybridization for identification of specific bacteria attached to the intestinal epithelial cells. Serum concentrations of folate and cobalamin are markers of intestinal absorption, but are also changed in exocrine pancreatic insufficiency and intestinal bacterial overgrowth. Hypocobalaminemia is common in GI and pancreatic disease. Decreased serum trypsin-like immunoreactivity is a very sensitive and specific test for the diagnosis of exocrine pancreatic insufficiency in dogs and cats. Serum pancreatic lipase is currently the most sensitive and specific test to identify pancreatic cell damage and acute pancreatitis. However, serum canine pancreas-specific lipase is less sensitive in canine chronic pancreatitis. Increased serum trypsin-like immunoreactivity is also specific for pancreatic damage but is less sensitive. It is very likely that further studies will help to better specify the role of these new tests in the diagnosis of canine and feline pancreatic diseases.
Keywords: laboratory tests, intestinal diseases, pancreas, folate, cobalamin, pancreatic lipase, bacteria
Gastrointestinal (GI) and pancreatic diseases are frequently encountered in canine and feline medicine. The panel of laboratory tests available is wide, and recently several new tests have been developed to help veterinarians in their workup of such cases. These tests can help characterize infectious agents, functional changes, or organ damage, and are often the first step before identification of a lesion with a biopsy.
This review is limited to tests that can be performed on blood or fecal samples. Moreover, the reader is referred to a review on fecal cytology in a previous issue of this journal for further information.1
Tests for Gastrointestinal Infectious Diseases
Fecal Parasite Panel
GI parasites are a common cause of chronic GI disorders in dogs and cats. A routine fecal flotation should always be included in the early diagnostic workup of an animal with GI signs to screen for worms and common protozoans such as giardia and coccidia. A detailed review on the diagnostic methods of intestinal protozoans in dogs and cat is beyond the scope of this article, but the reader is referred to a recent issue of this journal devoted to protozoal diseases in small animals (2010, Vol 25, issue 3).
Fecal shedding of GI parasites can be intermittent and some clinically normal animals can shed parasites without showing clinical signs. Therefore, a negative test should always be confirmed, ideally by 2 additional fecal panels. A positive diagnostic test does not prove that the parasite is causing disease, but it is usually advised to treat before embarking on invasive and/or expensive tests (unless some other findings warrant immediate diagnostic workup).
Bacteria
Characterization of bacterial diarrhea is difficult in dogs and cats, mostly because healthy pets are usually carriers of important pathogenic bacteria such as Clostridium perfringens, Clostridium difficile, Escherichia coli, and Campylobacter spp.2, 3, 4 However, it might be important to screen for possible pathogenic bacteria in cases with acute diarrhea, especially because some agents such as Campylobacter, Clostridium, or enteropathogenic E. coli have contagious or zoonotic potential.
Clostridium perfringens may induce diarrhea via its cytotoxic entorotoxin (CPE). This toxin is produced during sporulation and released during lysis of vegetative cells. However, detection of endospores on a fecal smear does not correlate with presence of CPE nor is it associated with clinical signs of diarrhea in dogs.4, 5 Therefore, the only useful tests to identify C. perfringens–associated diarrhea are fecal CPE enzyme-linked immunosorbent assay (ELISA; reverse passive latex agglutination tests are not reliable) or identification of toxinogenic strains via polymerase chain reaction (PCR).4, 6 Fecal culture is not helpful because the bacteria is also isolated from healthy dogs.6
Clostridium difficile is another pathogen associated with diarrhea. It is especially prevalent with hospital-acquired diarrhea and secondary to antibiotic treatment.7 Both CPE and C. difficile toxin A measured by ELISA were strongly associated with acute hemorrhagic diarrheal syndrome in dogs.8 Five commercially available ELISAs for toxins A and B of C. difficile were reported to have low sensitivity (up to two thirds of false negatives) on fecal samples but very high sensitivity when performed on bacterial isolates.9 However, the specificities were high (i.e., there are limited numbers of false-positive results).
Campylobacter spp. have been identified in dogs and cats with acute diarrhea,10 but they are also found with similar prevalence in feces of clinically normal animals.2, 11, 12 Therefore, a positive culture result for Campylobacter does not prove disease association with the infectious agent. However, the potential risk for infectious or zoonotic disease may warrant treatment when the pet is exhibiting clinical signs. Identification of spiral- or seagull wing–shaped bacteria in a fecal smear might suggest the presence of Campylobacter, but this is not a sensitive nor a specific test. Only half of the dogs with positive culture had characteristic organisms on the fecal smear in one study, and other spiral-shaped bacteria such as Helicobacter spp. can be observed on the fecal smear.8 Recently, PCR tests for identification of C. jejuni and C. coli were made available (http://www.vetmed.tamu.edu/gilab); however, other Campylobacter species have been recovered from feline or canine feces.3, 11, 13
Escherichia coli can be associated with acute diarrhea in dogs either as the sole pathogen or in association with intestinal viruses or parasites.14 However, because E. coli is a major component of the normal intestinal flora,15 fecal cultures are unhelpful in incriminating E. coli as the cause of the diarrhea. Therefore, when E. coli–associated diarrhea is suspected, screening for specific toxins or genes (e.g., Shiga toxin,16 attaching and effacing A gene,14, 17 etc.) is mandatory.15 It was recently reported that granulomatous colitis in dogs is associated with the presence of an invasive and adherent strain of E. coli in the colonic mucosa.18 A diagnosis can be made by specific labeling of the bacteria in colonic biopsy samples by fluorescence in situ hybridization (FISH); the recommended treatment is fluorinated quinolones. Accurate diagnosis is important in these cases because inappropriate use of antibiotics may induce severe antibiotic resistance (including to fluorinated quinolones).19 The FISH test is commercially available at the Simpson Laboratory at Cornell University (http://www.vet.cornell.edu/labs/simpson/).
Salmonellosis seems extremely rare in dogs.10 The isolation rate of Salmonella spp. in feces of normal dogs and dogs with diarrhea is not different.2, 8 However, recently S. enterica was isolated in 93% of the fecal samples tested in a breeding facility where 3 puppies developed fatal clinical salmonellosis.20 The endemic circulation of the same strain of Salmonella in this breeding facility was attributed to the raw meat (classified as unfit for human consumption) that was used to feed the dogs.20
In conclusion, unless specific conditions that warrant a search for bacterial diarrhea are present (outbreak of diarrhea in a shelter or veterinary hospital or risk of zoonosis), routine fecal culture is not recommended in dogs and cats with GI signs. Identification of Clostridium toxins may help to diagnose hospital-acquired diarrhea due to C. difficile.
Finally, gastric Helicobacter spp. are normally present in the stomach of dogs and cats and there is no clear association between their presence and disease. Therefore, diagnostic tests such as rapid urease tests on gastric biopsy are not very useful in the clinical management of chronic GI cases. Histopathological identification of massive colonization of the gastric mucosa by spiral-shaped bacteria in association with inflammatory cells is usually enough to decide whether to treat or not for Helicobacter infection.
Viruses
The main viruses that a clinician may want to identify in a dog or cat with acute diarrhea are canine and feline parvoviruses because they are very resistant and highly contagious. Several point-of-care tests are available for the identification of canine parvovirus antigen in the feces (SNAP Parvo, IDEXX, Witness Parvo, Synbiotics). Their specificity is very high, but a false positive can occur with recent vaccination (less than 3 weeks). Their sensitivity is variable.21 Therefore, if canine parvovirus enteritis is suspected, a negative test should not be used to rule out the disease. Recently, these tests have been validated for the diagnosis of feline parvovirus. The reported positive and negative predictive values are 100% and 97%, respectively, for the 2 above-mentioned tests.22 However, a recent modified live parvovirus vaccination may induce a positive test for at least 2 weeks.23 Most of the time, serology is difficult to interpret unless the titers are very high or increasing titers can be proved in paired samples. Conventional or quantitative PCR tests for parvovirus and coronavirus are available in several commercial laboratories and can be used to confirm negative results with a rapid test and confirm strong clinical suspicions.
Fungal agents can be characterized with rectal scrapes or fine-needle aspiration of digestive masses. A urinary antigen test is available to screen for histoplasmosis (http://www.miravistalabs.com), but its diagnostic performances have not been evaluated yet.
Test for Gastric Function
Gastric Permeability
Increased permeability of the gastric wall can be assessed with the sucrose test. However, because this test is not available routinely in commercial laboratories, it will not be detailed in this review. The test has been used experimentally to screen for gastric damage associated with nonsteroid antiinflammatory drugs,24, 25 gastric surgery,26 or strenuous exercise27 in dogs.
Gastric Emptying Rate
The gold standard method to study gastric emptying is scintigraphy. Recently, a 13C-octanoic acid breath test was proposed in dogs.28 This test is not commercially available.
Test for Intestinal Function
Water-soluble Vitamins: Folate and Cobalamin Serum Concentrations
Folate and cobalamin (vitamin B12) are water-soluble vitamins present in large amounts in canine and feline diets. Therefore, nutritional deficiency is very unlikely, and even a strictly vegetarian diet with low cobalamin content does not cause a decrease in serum cobalamin in cats.29 Folate and cobalamin have specific GI metabolism and uptake. Dietary folates are absorbed in the jejunum after hydrolysis of the alimentary folate polyglutamates to folate monoglutamates by a jejunal conjugase. The folate monoglutamate is absorbed by a specific jejunal carrier. Because the concentration of folate is high in red blood cells, hemolysis can falsely increase serum folate concentration, but lipemia or hyperbilirubinemia have no effect on serum folate concentration.30 Dietary cobalamin is absorbed in the ileum by a specific carrier after receptor-mediated endocytosis. Briefly, dietary cobalamin is bound to proteins from which it is released by pepsinogen and hydrochloric acid in the stomach. Then, cobalamin binds to gastric and salivary R protein and is subsequently released in the proximal small intestine by pancreatic proteases. Cobalamin then forms a complex with intrinsic factor (which is excreted from the pancreas), and the complex is absorbed by a specific ileal carrier. Therefore, cobalamin intestinal metabolism is very dependent on normal exocrine pancreatic and intestinal functions. Serum cobalamin measurements are not affected by hemolysis, lipemia, or hyperbilirubinemia.30 Because folate and cobalamine are abundant in commercial diets, their serum concentrations should only be measured in fasted animals.
Serum folate and cobalamin concentrations have been studied in small intestinal diseases, exocrine pancreatic insufficiency (EPI), and small intestinal bacterial overgrowth (SIBO) (Table 1). Intestinal bacteria can synthesize folate and use cobalamin. In SIBO, serum folate can be increased and cobalamin decreased. In EPI, decreased excretion of intrinsic factor (which is necessary for cobalamin absorption) and secondary SIBO (a common complication of EPI) often leads to decreased serum concentrations of cobalamin. Bacteria of the distal small intestine and large intestine produce a lot of folate that is excreted in the feces30 and can be absorbed in coprophagic animals. Serum folate concentrations are often increased in EPI because of secondary SIBO and also coprophagia. In dogs with EPI, the prevalences of increased serum folate, decreased serum cobalamin, or the combination of both are 60%, 82%, and 47%, respectively.31 Severe hypocobalaminemia is reported in 36% of dogs with EPI and decreased folate in only 2% of dogs with EPI.31 In a recent study of cats with EPI, 10/10 cats were hypocobalaminemic and 4/10 had increased serum folate concentrations. None had decreased serum folate concentrations.32 Serum cobalamin concentrations should be documented in dogs and cats on diagnosis of EPI and should be monitored during treatment because cobalamin intestinal absorption does not normalize after oral supplementation of pancreatic extracts in dogs with experimentally induced EPI.33 Treatment with vitamin B12 injections is recommended for animals with EPI, as well as monitoring of its serum concentrations (especially if the clinical condition worsens or in the case of treatment failure).
Table 1.
Interpretation of Changes of Serum Cobalamin and Folate Concentrations in Dogs and Cats
| Increased | Decreased | |
|---|---|---|
| Serum folate | SIBO | Proximal small intestinal disease |
| Coprophagy | Long-term antibiotic treatment | |
| Serum cobalamin | EPI | |
| SIBO | ||
| Distal small intestinal disease | ||
| Congenital cobalamin malabsorption | ||
| Ileal resection |
Abbreviations: SIBO, Small intestinal bacterial overgrowth; EPI, exocrine pancreatic insufficiency.
Theoretically, in chronic small intestinal disease, serum concentrations of folate and/or cobalamin could decrease when the jejunum or the ileum are respectively affected. However, during chronic intestinal disease, SIBO is sometimes present and it may cause an increase in folate synthesis and an increase in cobalamin use by intestinal bacteria as described above. Hypocobalaminemia is reported in 6% to 73% of dogs with chronic enteropathies.34, 35, 36, 37 Moreover, low serum cobalamin has been associated with hypoalbuminemia and a negative outcome in canine chronic enteropathies.34 The effect of chronic intestinal disease on serum folate concentrations is less consistent in dogs, but increased serum folate suggesting secondary SIBO seems more prevalent than decreased folate concentration.35, 36 Hypocobalaminemia is prevalent (61%) in cats with chronic intestinal diseases (e.g., GI lymphoma, inflammatory bowel disease [IBD]) and also in cats with pancreatitis and liver diseases.38, 39 Changes in serum folate concentrations are inconsistently reported in feline chronic intestinal disease, but decreased folate is more frequent than increased folate in feline GI and pancreatic and liver diseases.38
The main purpose of cobalamin measurement is to identify deficiency. Cobalamin is involved in metabolism of sulfur-containing amino acids as well as in lipid and DNA synthesis. Therefore, cobalamin deficiency affects tissues with rapidly dividing cells, especially bone marrow and enterocytes.39 Cobalamin deficiency can lead to severe life-threatening metabolic disturbances in dogs40 and cats.39, 41, 42, 43, 44, 45 However, hypocobalaminemia is generally very responsive to parenteral supplementation and justifies rapid identification and early intervention.39, 43 In a recent study, healthy cats over 8 years of age had lower serum cobalamin concentrations compared with those of younger cats, suggesting that serum cobalamin should also be monitored in aging, healthy cats.46 In cats with hyperthyroidism, the prevalence of hypocobalaminemia was 40.8%, compared with 25% in a control group of euthyroid geriatric cats.47 Finally, breed-associated hypocobalaminemia is reported in several dog breeds including Border Collies, Giant Schnauzers, Beagles, Australian Shepherds, Shar Peis, Staffordshire Bull Terriers, and German Shepherds.48, 49 Therefore, close monitoring of serum cobalamin concentrations is suggested in sick dogs of these breeds.
Sugar Absorption Tests for Assessment of Permeability and Absorption
Assessment of intestinal absorption and permeability has been performed with sugar absorption tests in dogs and cats. However, these tests are not commercially available and their clinical applications are limited.30, 50, 51, 52, 53 They will not be covered in this review.
Fecal α1-Proteinase Inhibitor for Intestinal Protein Loss
α1-Proteinase inhibitor (α1-PI) is a protein that has a molecular mass similar to albumin and can leak through the intestinal wall in cases of protein-losing enteropathy (PLE). Because α1-PI is a protease inhibitor, it is resistant to hydrolysis and can be recovered in feces. In normal animals, only trace amounts of α1-PI are present in the feces.54 ELISAs have been developed and validated to measure α1-PI in canine serum and feces54 and in feline serum.55 Fecal α1-PI is not affected by long-term nonsteroidal antiinflammatory drug treatment with carprofen or meloxican in dogs.56 Fecal α1-PI has been suggested as an early marker of GI protein loss in dogs that are prone to PLE such as Soft-coated Wheaten Terriers57, 58, 59 and also in dogs with IBD before the development of hypoalbuminemia.60 Increased fecal α1-PI has been reported in cats with chronic GI disease and concurrent hypocobalaminemia.61 The test is available exclusively at the Gastrointestinal Laboratory at Texas A&M University (http://www.vetmed.tamu.edu/gilab/). The web site should be consulted for specific information about sampling before submission because specific sample preparation is necessary. This test is helpful in monitoring cases before they develop overt hypoalbuminemia in situations where PLE is strongly suspected such as in Soft-coated Wheaten Terriers. In other cases, the suspicion of PLE is mostly based on identifying hypoalbuminemia and ruling out proteinuria and hepatic insufficiency. The test can be helpful, however, in patients with renal and/or liver disease if concurrent PLE is suspected. It might confirm the presence of PLE and help in making a decision to perform intestinal biopsies.
Fecal Occult Blood Tests
Fecal occult blood tests are indicated in conditions where GI blood loss is suspected (e.g., unexplained microcytic anemia), but overt melena or hematochezia are not seen. The available tests are based on guaiac or orthotolodine reactions. They are sensitive to amounts of blood that are 20 to 50 times less than the amounts required to cause melena.62 Immunologic tests for humans should not be used because their cross-reactivities with canine or feline hemoglobin are not documented. The sensitivity of fecal occult blood tests is very high, but false-negative results are possible when only very small amounts of blood are leaking in the GI tract. False-positive results can occur when meat and/or raw fish are a component of the diet.63, 64 Therefore, in the face of a positive test, it is recommended to change the diet to a commercial fish-based (not raw fish) or soy-protein-based diet and to repeat the test after 5 days on the new food.
Tests of Bacterial Overgrowth
Dysbiosis is a general term that covers quantitative and/or qualitative changes in intestinal microbes. Dysbiosis might cause GI disease such as IBD, but it can also be a consequence of GI diseases such as EPI. SIBO, that is, an increase in the quantity of small intestinal bacteria, has been considered as a possible cause of chronic GI signs in dogs and cats. More recently, the term antibiotic responsive diarrhea (ARD) has been proposed to replace SIBO, which describes a syndrome in which animals with chronic diarrhea improve with antibiotic treatment, relapse after cessation of treatment, and have no other possible cause to explain the clinical disease.36 However, there is no complete overlap with SIBO. Some dogs may have a diagnosis of SIBO associated with other GI conditions that are not ARD.36
Direct Bacterial Counts
Direct bacterial counts of duodenal juice are considered the gold standard for diagnosis of SIBO. Historically, SIBO in dogs and cats was defined as bacterial counts above 105 colony-forming units (cfu)/mL for total bacteria or above 104 cfu/mL for anaerobes.65 Based on this definition, SIBO was incriminated as a cause of GI disease in dogs. However, recent studies have shown that the total number of bacteria is very variable and can exceed those numbers from time to time even in healthy dogs or cats.66, 67, 68 Therefore, to make a diagnosis of bacterial overgrowth, bacterial counts of duodenal juice should be above 2.7 × 109 cfu/mL or 1.1 × 109 cfu/mL in dogs and cats, respectively.66 Moreover, aside from quantification of the total number of bacteria, imbalances between the different species of the intestinal bacterial populations are probably also important. It is likely that the use of advanced molecular techniques such as massive parallel pyrosequencing or FISH will change the analyses of bacterial populations in chronic intestinal diseases such as IBD.69, 70, 71 Therefore, except in situations where a specific pathogen is targeted, it is probably of very low diagnostic yield to submit duodenal juice for culture in the workup of an animal with chronic GI disease.
Folates and Cobalamin for Bacterial Overgrowth
As mentioned above, increased serum folate concentrations, decreased serum cobalamin concentrations, or the combination of both, may be observed in SIBO. The validation of serum cobalamin and folate concentrations as a test for SIBO is difficult because there is currently no gold standard for diagnosis of SIBO. In one seminal study that included 41 dogs with SIBO (diagnosed by duodenal juice culture and total bacterial counts above 105 cfu/mL or anaerobic bacterial counts above 104 cfu/mL), high serum folate concentrations and low serum cobalamin concentrations had good specificity (79% and 87%, respectively) but low sensitivity (51% and 24%, respectively).65 The combination of high folate and low cobalamin had a sensitivity of 5% and specificity of 100% for SIBO.65 However, in a recent study, 7/9, 5/9, and 3/9 dogs with ARD had increased serum folate concentrations, decreased serum cobalamin concentrations, or a combination of both, respectively, but similar proportions of changes were observed in dogs with IBD.36
Unconjugated Bile Acids
Total unconjugated bile acids were proposed 10 years ago as a sensitive test for SIBO in dogs.72 However, the use of this test has been challenged by a recent study showing that increased total unconjugated bile acids was not correlated with ARD in dogs.36 This test is not commercially available.
Tests for Pancreatic Disease
The tests for pancreatic diseases can be divided into tests for exocrine function and tests for pancreatic damage or cytolysis.
Test for Exocrine Pancreatic Insufficiency
The TLI test measures trypsinogen and trypsin-like immunoreactivity in the blood. In a normal pancreas, the physiological turnover of pancreatic acinar cells allows leakage of small amounts of trypsinogen into the blood. In an animal with EPI, the amount of trypsinogen synthesized in pancreatic acinar cells is drastically reduced and because trypsinogen is pancreas specific, the concentration of trypsinogen in the blood is also severely reduced. The test is species specific and has been validated for use in dogs73 and cats.74 The canine TLI test is available in several laboratories, but the feline TLI test (fTLI) is only available at the Gastrointestinal Laboratory at Texas A&M. Because the test recognizes either canine or feline trypsinogen, it is not affected by pancreatic enzyme extracts used for the treatment of EPI. Serum TLI is increased after feeding in dogs75 and cats,76 and measurement should be made only after 12 hours of fasting. The test is affected by lipemia but not hemolysis or hyperbilirubinemia.30 The sensitivity and specificity of the decrease serum TLI for the diagnosis of exocrine pancreatic insufficiency are almost 100% in dogs73 and are probably also very high in cats.32 Other than EPI, the only cause for a decreased TLI is extreme protein malnutrition. This is because there is an adaptative response of the pancreatic enzyme synthesis to low protein diet in dogs with a direct relationship between serum TLI and the protein content of the diet.77 As for many laboratory tests, there is a gray zone for TLI in the diagnosis of EPI (2.5-5.7 μg/L in dogs and 8-12.4 μg/L in cats for the reference range provided by the GI laboratory at Texas A&M University). Animals with a TLI measurement in this gray zone may or may not progress to having TLI results diagnostic of EPI, and it is recommended to retest them within 1 to 3 months. In one study that followed up on 44 dogs with inconclusive TLI results, 20/44 dogs had a normal TLI on the second sample.78 TLI became diagnostic of EPI in 11/44 dogs and remained in the gray zone for 13/44 dogs. These latter dogs underwent gross examination and biopsy of the pancreas, which showed a remarkable reduction in the amount of normal exocrine pancreatic tissue, but 8 dogs had no GI signs and 5 dogs had intermittent GI signs atypical for EPI.78 These dogs were diagnosed with subclinical exocrine pancreatic insufficiency. It is recommended to retest these dogs on a regular basis because they may develop overt EPI that requires enzyme supplementation.
Increased serum TLI is discussed in the pancreatic cytolysis section.
The only other test that has been recently studied in canine EPI is fecal elastase-1 measurement. Elastase-1 is a pancreas-specific protease that is resistant to hydrolysis and stable during intestinal passage. A canine-specific ELISA has been developed and validated for the determination of elastase-1 in the stool.79 However, despite a high sensitivity of 100%, the test has a specificity of only 56.5% for the diagnosis of EPI.80 Therefore, it is probably not useful for the diagnosis of EPI in dogs.
Tests of Pancreatic Acinar Cell Damage
The use of diagnostic tests for the diagnosis of pancreatic disease and especially pancreatitis has been a matter of controversy over the last 10 years. The main tests available are measurement of serum amylase and lipase activity, and serum pancreatic lipase concentration. The validation of these tests is complex because it requires assessment of the organ specificity of the marker as a first step and subsequent field validation in clinical conditions. The main problem with the clinical validation is that there is no gold standard for the diagnosis of pancreatitis in dogs and cats. Histopathology of the pancreas might be considered a gold standard, but it is not always feasible and its interpretation is problematic. Histopathological lesions consistent with pancreatitis can be very localized and easily missed if systematic assessment of the entire organ is not performed.81 This is obviously impossible in field conditions except for postmortem studies. Furthermore, histopathological evidence of acute and chronic pancreatic inflammation does not always correlate with clinical signs of pancreatitis.81, 82 Also, other, less common pancreatic diseases, such as cysts or neoplasms, may induce pancreatic acinar cell damage and an increase in blood concentration of pancreatic enzymes. Because of lack of a practical gold standard, clinical research often relies on the combination of clinical presentation and results of a myriad of diagnostic tests, with or without histopathology, for the ultimate diagnosis of pancreatitis in dogs and cats.
Amylase and Lipase
Amylase and lipase are not specific markers of pancreatic disease. For instance, in pancreatectomized dogs, a significant amount of serum lipase and amylase activities are conserved.83 Moreover, dogs with EPI have serum lipase activity within the reference range that is not different from that of control dogs.84 Nonpancreatic diseases, such as renal failure, hepatic diseases, intestinal diseases, and lymphoma, may induce an increase in serum amylase and/or lipase activities in dogs.85, 86 To further complicate the picture, there is a daily rhythm of serum lipase and amylase activities in the dog (with a difference between trough and peak of 120 U/L and 220 U/L for lipase and amylase, respectively87) that makes the selection of a diagnostic threshold difficult.
In canine pancreatitis, plasma amylase activity is not increased unless lipase is increased. Therefore, measurement of amylase activity is not considered helpful.88 The sensitivity and specificity of amylase and lipase in the diagnostic of pancreatitis in dogs were 40% and 70%, and 66.7% and 60%, respectively, in one study.89 In a case series of 70 dogs with fatal acute pancreatitis confirmed in necropsy, 30.8% and 47.6% of the dogs had normal amylase or lipase, respectively.90 Moreover, in a recent report in which the diagnosis of acute pancreatitis was based on 5 different experts' opinions, amylase and lipase did not differ between dogs with and without pancreatitis.91 In a cohort of 14 dogs with histologically confirmed chronic pancreatitis, the sensitivity of amylase and lipase (3 times above the upper limit of the reference range) were 14% and 28%, respectively.92 The use of amylase and lipase for the diagnosis of pancreatitis in cats is seldom reported. However, in feline experimental pancreatitis, serum lipase was increased, whereas serum amylase was decreased.93
These findings suggest than serum amylase is of little clinical use in the diagnosis of pancreatitis in dogs and cats. Lipase might be considered useful as a screening test for pancreatitis in dogs, but it should not be used alone to make a definitive diagnosis of pancreatitis. Only elevations above 3 to 5 times the upper limit of the reference range of lipase might be considered suggestive of pancreatic damage86 and only in an animal with normal serum creatinine.
Pancreatic Lipase
Pancreatic lipase immunoreactivity (PLI) measurement has been made available after the isolation and purification of pancreas-specific lipase (PL) in dogs94 and cats.95 This enzyme is very specific to the pancreas in dogs96 (no immunoreactivity in any of the 37 organ tissues tested except the pancreas), but its distribution in feline tissues has not been reported. Several tests have been made available sequentially for the PLI measurement in dogs and cats: the original radioimmunoassay (RIA) based on polyclonal antibodies to native purified protein, Spec cPL and Spec fPL, which are commercial quantitative ELISAs based on monoclonal antibodies and recombinant antigen, and finally, SNAP cPL (IDEXX), a semiquantitative point-of-care ELISA based on monoclonal antibodies for the measurement of canine PL (cPL). The PLI RIA is not commercially available anymore. Analytical validation of the Spec cPL test has shown good precision and reproducibility and no interference of bilirubin, lipid, and hemoglobin on results.97 The SNAP cPL has 96% to 100% agreement with the Spec cPL in samples with normal PLI and 88% to 92% agreement in samples with elevated PLI.98 This means that in a case with a low to moderate index of suspicion of pancreatic damage, a positive SNAP cPL result should be confirmed with quantitative Spec cPL. As for TLI, PLI tests are species specific. PL is stable for 21 days at temperatures ranging from –80°C to 24°C and is not affected by long-term prednisolone treatment in dogs99 or by the fat content of the diet.75 However, there is a slight increase in canine PLI (cPLI)75 after eating, and therefore cPLI and feline PLI (fPLI) should be evaluated only in fasted animals. In normal dogs, pancreatic fine-needle aspiration or surgical biopsy had no effect on cPLI.100 Similarly, pancreatic laparoscopic biopsies had no effect on fPLI in healthy cats.101 However, because these studies were performed in healthy animals and the effect of fine-needle aspiration or biopsy in an abnormal pancreas is unknown, it is recommended to perform blood sampling for PLI before sampling of pancreatic tissue.
The effect of renal failure on cPL has been reported in only 1 abstract.102 In this study, 3/17 dogs with experimentally induced renal failure had cPLI values above the reference range and the median cPLI was significantly higher than the median cPLI measured in control dogs. However, the median cPLI in dogs with induced renal failure was not above the reference range.102 Therefore, the effect of renal failure is still questionable and further studies on spontaneous cases are necessary. In a recent study in cats, experimentally induced renal failure had no effect on both Spec fPL or fPLI measured by the original radioimmunoasay.103 cPLI may be increased in epileptic dogs treated with phenobarbital, potassium bromide, or a combination of both.104 The interpretation of these findings is open because no histologic evaluation of the pancreas was performed in this study.
In a recent study among 22 dogs with macroscopic evidence of acute or chronic pancreatitis, serum PL measured by RIA or Spec cPL was above the cut-off values suggested for pancreatitis in 14/22 dogs and above the upper limit of the reference ranges in 17/22 and 16/22 dogs in RIA and Spec cPL, respectively.105 Amylase and lipase activities were above the reference range in 9/22 and 7/22 dogs, respectively, and 3 times above the upper limit of the reference range (suggested cutoff for pancreatitis) in 4/22 and 3/22 dogs, respectively. TLI was above the reference range in 8/22 dogs.105 This study was limited to cases with mild or moderate pancreatitis based on histological evaluation, and it is likely that results could be different in more severe cases. A recent multi-institutional study yielded a sensitivity of 93% and specificity of 78% of Spec cPL for the diagnosis of canine acute pancreatitis. In this study, the diagnosis of acute pancreatitis was based on information from medical records and imaging findings that were evaluated by 5 different experts blinded to the results of Spec cPL.91 The negative and positive likelihood ratios were 0.029 and 1.3, i.e., Spec cPL within the reference range is better at rejecting the diagnosis than the opposite.91 This means that Spec cPL is currently the best diagnostic test for canine acute pancreatitis. However, as for any diagnostic test, there are false-positive and false-negative results and it is always advisable to make the diagnosis based on the combination of clinical signs, laboratory test results, and imaging studies.
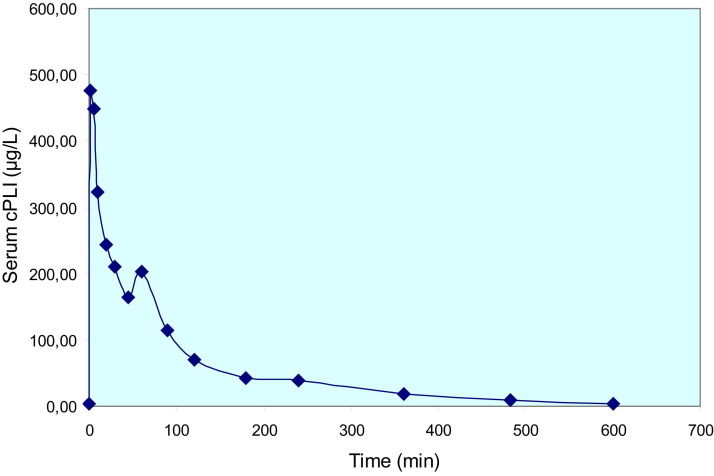
cPL has been evaluated for the diagnosis of histologically confirmed canine chronic pancreatitis with a sensitivity of 26% or 58% depending on the cutoff.92 This relatively modest sensitivity is probably explained by a combination of the rapid elimination of PL from the blood (Fig 1) and the high intra-individual variability of serum PL measurement.106 fPLI has been evaluated in the diagnosis of pancreatitis in 18 cats with biopsy-confirmed acute and/or chronic pancreatitis. The sensitivity and specificity of fPLI were 67% and 91%, respectively.107 When the analysis was restricted to cats with moderate to severe pancreatitis, the sensitivity and specificity were 100%. Overall, fPLI was the best-performing test compared with fTLI and ultrasonography for the diagnosis of pancreatitis in cats.107
Figure 1.
Elimination of canine pancreatic lipase after intravenous administration in a healthy dog. Serum cPLI was measured after intravenous injection of purified canine pancreatic lipase. This graph shows that elimination of lipase is very rapid in a healthy dog.
The use of PLI for the follow-up of pancreatitis has been challenged recently because in some dogs with clinical presentation compatible with pancreatitis and increased Spec cPL, the Spec cPL may remain increased despite clinical improvement.108 Moreover, because of high intra-individual variability in healthy dogs (193.8%), the difference in measured Spec cPL between serial measurements has to be above 281 μg/L to be clinically significant.106 It is very likely that this variability would be similar or even greater in dogs with pancreatitis. However, despite this very high variability, values measured in healthy dogs remained in the current reference range for Spec cPL. When using Spec PL for the diagnosis of pancreatitis, a cutoff has been fixed (> 5.4 μg/L in cats and > 400 μg/L in dogs) and there is a gray zone between the cutoff and the upper limit of the reference range (3.5 μg/L in cats and 200 μg/L in dogs). For gray-zone results, it is currently recommended to repeat the test after 2 to 3 weeks or earlier if the clinical condition suddenly worsens.
In a recent study, increased Spec cPL was the only factor predicting euthanasia or poor response to steroid therapy in a cohort of dogs with IBD.37 This might suggest that IBD-associated pancreatic damage is a poor prognostic indicator, but no pancreatic histopathology was performed in the study. In the same type of study performed in cats with IBD, 9/23 cats had fPLI (measured by RIA) above the diagnostic cutoff for pancreatitis. These cats had lower cobalamin and albumin than cats with normal fPLI, but no correlation was found between increased fPLI and outcome.109
TLI as a Test for Pancreatic Damage
The TLI test has been proposed as a test of pancreatic damage because the enzyme is also specific to the pancreas. Experimentally induced renal failure in cats was associated with an increase in fTLI above the reference range in 13/20 cats,110 suggesting that a false diagnosis of pancreatitis could be made in a patient with renal failure, and also that a cat with renal failure and EPI may have an fTLI within the normal range and therefore give a false-negative result in the diagnosis of EPI. Three independent clinical studies, which included cats with histologically confirmed acute and chronic pancreatitis, reported sensitivities and specificities of TLI ranging from 28% to 44.4% and from 70% to 90%, respectively, depending on the cutoff applied for the diagnosis of pancreatitis107, 111, 112 In dogs, sensitivities of canine TLI (cTLI) for the diagnosis of pancreatitis (acute or chronic) ranged from 38% to 45.5%.89, 105 Two dogs with renal failure, among a control group of 28 dogs with nonpancreatic diseases, had increased cTLI (specificity of 92%).89 In a recent study on a group of 14 dogs with chronic pancreatitis, the sensitivity of cTLI was 17%.92 Therefore, TLI is not sensitive but fairly specific (if renal failure is not present) for the diagnosis of pancreatitis in dogs or cats. This lack of sensitivity is probably related to the very rapid elimination of trypsinogen from the blood (at least in dogs). However, it is unlikely that a dog with acute pancreatitis and increased cTLI will not have concurrently increased cPLI.105
Other Tests for Pancreatitis
Trypsinogen activation peptide (TAP) is the cleavage peptide produced by the action of enterokinase on trypsinogen. Normally, TAP is produced in the intestinal lumen and not absorbed. However, when there is abnormal activation of trypsinogen in the pancreas, such as in pancreatitis, TAP is released into the circulation and then eliminated by the kidney. Only 7/15 dogs with pancreatitis had an increased plasma TAP concentration, and plasma TAP concentrations were also increased in 3/7 dogs with renal disease and 1 dog with diabetes mellitus.89 Therefore, TAP concentration is not a sensitive or specific test for the diagnosis of pancreatitis in dogs and is not better than serum lipase activity.89 Recently, TAP was evaluated in a group of cats with acute pancreatitis and did not show any benefit over fTLI for the diagnosis of the condition.113 Moreover, this test is not available in commercial laboratories.
Measurement of serum trypsin-α1PI complexes has been proposed for the diagnosis of pancreatitis in dogs. In a normal animal, trypsin should not be present in the blood, and, when present, it is rapidly complexed with the protease inhibitor α1PI. Therefore, serum trypsin-α1PI complexes should be a good marker of activation of trypsinogen to trypsin in the pancreas, and its leakage into the blood, with secondary neutralization by α1 PI. A serum trypsin-α1PI test has been validated in dogs, but only 7/22 dogs with pancreatitis had trypsin α1PI complexes above the reference range.105
Amylase and lipase activities have been measured in abdominal fluid in dogs with and without pancreatitis.114 When free abdominal fluid is present, high lipase activity (especially when compared with blood lipase activity) may help differentiate pancreatitis from other causes (e.g., abdominal trauma, neoplasia, intestinal perforation, etc.), despite some overlap between these different conditions.114
In conclusion, PLI, trypsinogen, and TLI are pancreas specific and, when elevated in the blood, are indicative of pancreatic damage. They should be envisioned as cytolytic enzymes, mirroring the approach that has been used for decades with liver-specific enzymes. They should be considered markers of acinar cell damage in conditions ranging from transient increase in plasma membrane permeability to complete necrosis (e.g., acute-necrotizing pancreatitis). They cannot be considered as markers of a specific pancreatic disease, namely pancreatitis. The diagnosis of pancreatitis is largely a diagnosis of exclusion. It should rely on a combination of a strong clinical suspicion, results of laboratory tests, imaging studies, and, whenever possible, cytological (Fig 2) or histopathological evidence of pancreatic inflammation. The clinical significance of mild pancreatic lesions observed in dogs and cats that have no clinical signs of pancreatitis is questionable. Considering the high prevalence of lesions suggestive of chronic pancreatitis in necropsy of dogs (34%)115 and cats (67%) presenting for other diseases, and in apparently healthy cats (45%),82 it is very important to keep in mind that the positive predictive value of any test improves substantially when performed in an animal that is likely to have the disease. This means that the performance of the diagnostic tests for pancreatitis will be better when applied to selected cases with a high index of suspicion of the disease.
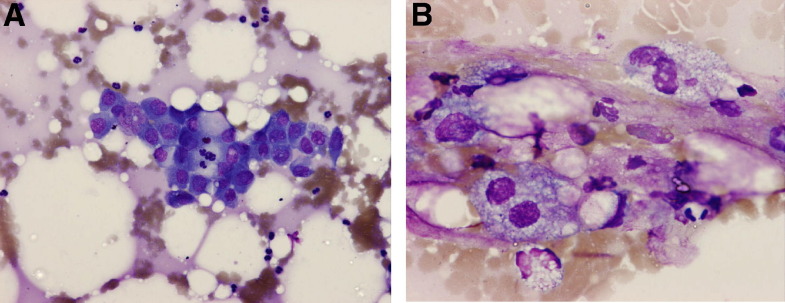
Figure. 2.
Pancreatic fine-needle aspiration of a dog with acute pancreatitis. (A) Cluster of cohesive cells with basophilic cytoplasm. Central nucleolated nucleus is suggestive of activated pancreatic cells. Moderate numbers of neutrophils surround the pancreatic epithelium. May-Grünwald-Giemsa, ×200. (B) Peritoneal lesions secondary to a pancreatitis. Granulomatous steatitis. Several macrophages with fine vacuolation around droplets of fat associated with neutrophils. May-Grünwald-Giemsa, ×400.
(Courtesy of Dr. C. Trumel.)
References
- 1.Broussard J.D. Optimal fecal assessment. Clin Tech Small Anim Pract. 2003;18:218–230. doi: 10.1016/S1096-2867(03)00076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marks S.L., Kather E.J. Bacterial-associated diarrhea in the dog: a critical appraisal. Vet Clin North Am Small Anim Pract. 2003;33:1029–1060. doi: 10.1016/s0195-5616(03)00091-3. [DOI] [PubMed] [Google Scholar]
- 3.Rossi M., Hanninen M.L., Revez J. Occurrence and species level diagnostics of Campylobacter spp., enteric Helicobacter spp. and Anaerobiospirillum spp. in healthy and diarrheic dogs and cats. Vet Microbiol. 2008;129:304–314. doi: 10.1016/j.vetmic.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Weese J.S., Staempfli H.R., Prescott J.F. The roles of Clostridium difficile and enterotoxigenic Clostridium perfringens in diarrhea in dogs. J Vet Intern Med. 2001;15:374–378. [PubMed] [Google Scholar]
- 5.Marks S.L., Melli A., Kass P.H. Evaluation of methods to diagnose Clostridium perfringens-associated diarrhea in dogs. J Am Vet Med Assoc. 1999;214:357–360. [PubMed] [Google Scholar]
- 6.Marks S.L., Kather E.J., Kass P.H. Genotypic and phenotypic characterization of Clostridium perfringens and Clostridium difficile in diarrheic and healthy dogs. J Vet Intern Med. 2002;16:533–540. doi: 10.1892/0891-6640(2002)016<0533:gapcop>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Clooten J., Kruth S., Arroyo L. Prevalence and risk factors for Clostridium difficile colonization in dogs and cats hospitalized in an intensive care unit. Vet Microbiol. 2008;129:209–214. doi: 10.1016/j.vetmic.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Cave N.J., Marks S.L., Kass P.H. Evaluation of a routine diagnostic fecal panel for dogs with diarrhea. J Am Vet Med Assoc. 2002;221:52–59. doi: 10.2460/javma.2002.221.52. [DOI] [PubMed] [Google Scholar]
- 9.Chouicha N., Marks S.L. Evaluation of five enzyme immunoassays compared with the cytotoxicity assay for diagnosis of Clostridium difficile-associated diarrhea in dogs. J Vet Diagn Invest. 2006;18:182–188. doi: 10.1177/104063870601800207. [DOI] [PubMed] [Google Scholar]
- 10.Sokolow S.H., Rand C., Marks S.L. Epidemiologic evaluation of diarrhea in dogs in an animal shelter. Am J Vet Res. 2005;66:1018–1024. doi: 10.2460/ajvr.2005.66.1018. [DOI] [PubMed] [Google Scholar]
- 11.Sandberg M., Bergsjo B., Hofshagen M. Risk factors for Campylobacter infection in Norwegian cats and dogs. Prev Vet Med. 2002;55:241–253. doi: 10.1016/s0167-5877(02)00095-8. [DOI] [PubMed] [Google Scholar]
- 12.Spain C.V., Scarlett J.M., Wade S.E. Prevalence of enteric zoonotic agents in cats less than 1 year old in central New York State. J Vet Intern Med. 2001;15:33–38. doi: 10.1892/0891-6640(2001)015<0033:poezai>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Chaban B., Ngeleka M., Hill J.E. Detection and quantification of 14 Campylobacter species in pet dogs reveals an increase in species richness in feces of diarrheic animals. BMC Microbiol. 2010;10:73. doi: 10.1186/1471-2180-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turk J., Maddox C., Fales W. Examination for heat-labile, heat-stable, and Shiga-like toxins and for the eaeA gene in Escherichia coli isolates obtained from dogs dying with diarrhea: 122 cases (1992-1996) J Am Vet Med Assoc. 1998;212:1735–1736. [PubMed] [Google Scholar]
- 15.DebRoy C., Maddox C.W. Identification of virulence attributes of gastrointestinal Escherichia coli isolates of veterinary significance. Anim Health Res Rev. 2001;2:129–140. [PubMed] [Google Scholar]
- 16.Staats J.J., Chengappa M.M., DeBey M.C. Detection of Escherichia coli Shiga toxin (stx) and enterotoxin (estA and elt) genes in fecal samples from non-diarrheic and diarrheic greyhounds. Vet Microbiol. 2003;94:303–312. doi: 10.1016/s0378-1135(03)00134-2. [DOI] [PubMed] [Google Scholar]
- 17.Sancak A.A., Rutgers H.C., Hart C.A. Prevalence of enteropathic Escherichia coli in dogs with acute and chronic diarrhoea. Vet Rec. 2004;154:101–106. doi: 10.1136/vr.154.4.101. [DOI] [PubMed] [Google Scholar]
- 18.Simpson K.W., Dogan B., Rishniw M. Adherent and invasive Escherichia coli is associated with granulomatous colitis in boxer dogs. Infect Immun. 2006;74:4778–4792. doi: 10.1128/IAI.00067-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craven M., Dogan B., Schukken A. Antimicrobial resistance impacts clinical outcome of granulomatous colitis in boxer dogs. J Vet Intern Med. 2010;24:819–824. doi: 10.1111/j.1939-1676.2010.0527.x. [DOI] [PubMed] [Google Scholar]
- 20.Morley P.S., Strohmeyer R.A., Tankson J.D. Evaluation of the association between feeding raw meat and Salmonella enterica infections at a Greyhound breeding facility. J Am Vet Med Assoc. 2006;228:1524–1532. doi: 10.2460/javma.228.10.1524. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz S., Coenen C., Konig M. Comparison of three rapid commercial Canine parvovirus antigen detection tests with electron microscopy and polymerase chain reaction. J Vet Diagn Invest. 2009;21:344–345. doi: 10.1177/104063870902100306. [DOI] [PubMed] [Google Scholar]
- 22.Neuerer F.F., Horlacher K., Truyen U. Comparison of different in-house test systems to detect parvovirus in faeces of cats. J Feline Med Surg. 2008;10:247–251. doi: 10.1016/j.jfms.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson E.V., Reese M.J., Tucker S.J. Effect of vaccination on parvovirus antigen testing in kittens. J Am Vet Med Assoc. 2007;230:359–363. doi: 10.2460/javma.230.3.359. [DOI] [PubMed] [Google Scholar]
- 24.Craven M., Chandler M.L., Steiner J.M. Acute effects of carprofen and meloxicam on canine gastrointestinal permeability and mucosal absorptive capacity. J Vet Intern Med. 2007;21:917–923. doi: 10.1892/0891-6640(2007)21[917:aeocam]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Meddings J.B., Kirk D., Olson M.E. Noninvasive detection of nonsteroidal anti-inflammatory drug-induced gastropathy in dogs. Am J Vet Res. 1995;56:977–981. [PubMed] [Google Scholar]
- 26.Mathon D.H., Dossin O., Palierne S. A laparoscopic-sutured gastropexy technique in dogs: mechanical and functional evaluation. Vet Surg. 2009;38:967–974. doi: 10.1111/j.1532-950X.2009.00592.x. [DOI] [PubMed] [Google Scholar]
- 27.Davis M., Willard M., Williamson K. Temporal relationship between gastrointestinal protein loss, gastric ulceration or erosion, and strenuous exercise in racing Alaskan sled dogs. J Vet Intern Med. 2006;20:835–839. doi: 10.1892/0891-6640(2006)20[835:trbgpl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.McLellan J., Wyse C.A., Dickie A. Comparison of the carbon 13-labeled octanoic acid breath test and ultrasonography for assessment of gastric emptying of a semisolid meal in dogs. Am J Vet Res. 2004;65:1557–1562. doi: 10.2460/ajvr.2004.65.1557. [DOI] [PubMed] [Google Scholar]
- 29.Wakefield L.A., Shofer F.S., Michel K.E. Evaluation of cats fed vegetarian diets and attitudes of their caregivers. J Am Vet Med Assoc. 2006;229:70–73. doi: 10.2460/javma.229.1.70. [DOI] [PubMed] [Google Scholar]
- 30.Suchodolski J.S., Steiner J.M. Laboratory assessment of gastrointestinal function. Clin Tech Small Anim Pract. 2003;18:203–210. doi: 10.1016/S1096-2867(03)00075-6. [DOI] [PubMed] [Google Scholar]
- 31.Batchelor D.J., Noble P.J., Taylor R.H. Prognostic factors in canine exocrine pancreatic insufficiency: prolonged survival is likely if clinical remission is achieved. J Vet Intern Med. 2007;21:54–60. doi: 10.1892/0891-6640(2007)21[54:pficep]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Thompson K.A., Parnell N.K., Hohenhaus A.E. Feline exocrine pancreatic insufficiency: 16 cases (1992-2007) J Feline Med Surg. 2009;11:935–940. doi: 10.1016/j.jfms.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson K.W., Morton D.B., Batt R.M. Effect of exocrine pancreatic insufficiency on cobalamin absorption in dogs. Am J Vet Res. 1989;50:1233–1236. [PubMed] [Google Scholar]
- 34.Allenspach K., Wieland B., Grone A. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med. 2007;21:700–708. doi: 10.1892/0891-6640(2007)21[700:ceideo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 35.Craven M., Simpson J.W., Ridyard A.E. Canine inflammatory bowel disease: retrospective analysis of diagnosis and outcome in 80 cases (1995-2002) J Small Anim Pract. 2004;45:336–342. doi: 10.1111/j.1748-5827.2004.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 36.German A.J., Day M.J., Ruaux C.G. Comparison of direct and indirect tests for small intestinal bacterial overgrowth and antibiotic-responsive diarrhea in dogs. J Vet Intern Med. 2003;17:33–43. doi: 10.1892/0891-6640(2003)017<0033:codait>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 37.Kathrani A., Steiner J.M., Suchodolski J. Elevated canine pancreatic lipase immunoreactivity concentration in dogs with inflammatory bowel disease is associated with a negative outcome. J Small Anim Pract. 2009;50:126–132. doi: 10.1111/j.1748-5827.2008.00693.x. [DOI] [PubMed] [Google Scholar]
- 38.Reed N., Gunn-Moore D., Simpson K. Cobalamin, folate and inorganic phosphate abnormalities in ill cats. J Feline Med Surg. 2007;9:278–288. doi: 10.1016/j.jfms.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpson K.W., Fyfe J., Cornetta A. Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease. J Vet Intern Med. 2001;15:26–32. doi: 10.1892/0891-6640(2001)015<0026:scoscv>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 40.Battersby I.A., Giger U., Hall E.J. Hyperammonaemic encephalopathy secondary to selective cobalamin deficiency in a juvenile Border collie. J Small Anim Pract. 2005;46:339–344. doi: 10.1111/j.1748-5827.2005.tb00330.x. [DOI] [PubMed] [Google Scholar]
- 41.Packer R.A., Cohn L.A., Wohlstadter D.R. D-lactic acidosis secondary to exocrine pancreatic insufficiency in a cat. J Vet Intern Med. 2005;19:106–110. doi: 10.1892/0891-6640(2005)19<106:dastep>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 42.Ruaux C.G., Steiner J.M., Williams D.A. Metabolism of amino acids in cats with severe cobalamin deficiency. Am J Vet Res. 2001;62:1852–1858. doi: 10.2460/ajvr.2001.62.1852. [DOI] [PubMed] [Google Scholar]
- 43.Ruaux C.G., Steiner J.M., Williams D.A. Early biochemical and clinical responses to cobalamin supplementation in cats with signs of gastrointestinal disease and severe hypocobalaminemia. J Vet Intern Med. 2005;19:155–160. doi: 10.1892/0891-6640(2005)19<155:ebacrt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 44.Ruaux C.G., Steiner J.M., Williams D.A. Relationships between low serum cobalamin concentrations and methlymalonic acidemia in cats. J Vet Intern Med. 2009;23:472–475. doi: 10.1111/j.1939-1676.2009.0308.x. [DOI] [PubMed] [Google Scholar]
- 45.Salvadori C., Cantile C., De Ambrogi G. Degenerative myelopathy associated with cobalamin deficiency in a cat. J Vet Med A Physiol Pathol Clin Med. 2003;50:292–296. doi: 10.1046/j.1439-0442.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- 46.Parnell N.K., Moore G.E., Suchodolski J.S. Influence of age on serum cobalamin and folate concentrations in healthy cats [abstract] J Vet Intern Med. 2008;22:809. [Google Scholar]
- 47.Cook A.K., Suchodolski J.S., Steiner J.M., Robertson J.E. The prevalence of hypocobalaminaemia in cats with spontaneous hyperthyroidism. J Small Anim Pract. 2011;52:101–106. doi: 10.1111/j.1748-5827.2010.01027.x. [DOI] [PubMed] [Google Scholar]
- 48.Grutzner N., Bishop M.A., Suchodolski J.S. Association study of cobalamin deficiency in the Chinese Shar Pei. J Hered. 2010;101:211–217. doi: 10.1093/jhered/esp100. [DOI] [PubMed] [Google Scholar]
- 49.Dandrieux J.R.S., Noble P.J., Halladay L.J. Breed predisposition for severe hypocobalaminemia and relation to folate concentration in dogs with gastrointestinal disease [abstract] J Vet Intern Med. 2010;24:722. [Google Scholar]
- 50.Allenspach K., Steiner J.M., Shah B.N. Evaluation of gastrointestinal permeability and mucosal absorptive capacity in dogs with chronic enteropathy. Am J Vet Res. 2006;67:479–483. doi: 10.2460/ajvr.67.3.479. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi S., Ohno K., Uetsuka K. Measurement of intestinal mucosal permeability in dogs with lymphocytic-plasmacytic enteritis. J Vet Med Sci. 2007;69:745–749. doi: 10.1292/jvms.69.745. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez H., Berghoff N., Suchodolski J.S. Kinetic analysis of 5 sugar probes in dog serum after orogastric administration. Can J Vet Res. 2009;73:217–223. [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez H., Suchodolski J.S., Berghoff N. Development and analytic validation of a gas chromatography-mass spectrometry method for the measurement of sugar probes in canine serum. Am J Vet Res. 2009;70:320–329. doi: 10.2460/ajvr.70.3.320. [DOI] [PubMed] [Google Scholar]
- 54.Melgarejo T., Williams D.A., Asem E.K. Enzyme-linked immunosorbent assay for canine alpha 1-protease inhibitor. Am J Vet Res. 1998;59:127–130. [PubMed] [Google Scholar]
- 55.Fetz K., Ruaux C.G., Steiner J.M. Purification and partial characterization of feline alpha1-proteinase inhibitor (falpha1-PI) and the development and validation of a radioimmunoassay for the measurement of falpha1-PI in serum. Biochimie. 2004;86:67–75. doi: 10.1016/j.biochi.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Murphy K.F., German A.J., Ruaux C.G. Fecal alpha1-proteinase inhibitor concentration in dogs receiving long-term nonsteroidal anti-inflammatory drug therapy. Vet Clin Pathol. 2003;32:136–139. doi: 10.1111/j.1939-165x.2003.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 57.Littman M.P., Dambach D.M., Vaden S.L. Familial protein-losing enteropathy and protein-losing nephropathy in Soft Coated Wheaten Terriers: 222 cases (1983-1997) J Vet Intern Med. 2000;14:68–80. doi: 10.1892/0891-6640(2000)014<0068:fpleap>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 58.Vaden S.L., Hammerberg B., Davenport D.J. Food hypersensitivity reactions in Soft Coated Wheaten Terriers with protein-losing enteropathy or protein-losing nephropathy or both: gastroscopic food sensitivity testing, dietary provocation, and fecal immunoglobulin E. J Vet Intern Med. 2000;14:60–67. doi: 10.1892/0891-6640(2000)014<0060:fhrisc>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 59.Vaden S.L., Sellon R.K., Melgarejo L.T. Evaluation of intestinal permeability and gluten sensitivity in Soft-Coated Wheaten Terriers with familial protein-losing enteropathy, protein-losing nephropathy, or both. Am J Vet Res. 2000;61:518–524. doi: 10.2460/ajvr.2000.61.518. [DOI] [PubMed] [Google Scholar]
- 60.Murphy K.F., German A.J., Ruaux C.G. Fecal alpha1-proteinase inhibitor concentration in dogs with chronic gastrointestinal disease. Vet Clin Pathol. 2003;32:67–72. doi: 10.1111/j.1939-165x.2003.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 61.Fetz K., Steiner J.M., Ruaux C. Increased fecal α1-proteinase inhibitor concentrations in cats with gastrointestinal disease [abstract] J Vet Intern Med. 2005;19:474. [Google Scholar]
- 62.Gilson S.D., Parker B.B., Twedt D.C. Evaluation of two commercial test kits for detection of occult blood in feces of dogs. Am J Vet Res. 1990;51:1385–1387. [PubMed] [Google Scholar]
- 63.Cook A.K., Gilson S.D., Fischer W.D. Effect of diet on results obtained by use of two commercial test kits for detection of occult blood in feces of dogs. Am J Vet Res. 1992;53:1749–1751. [PubMed] [Google Scholar]
- 64.Tuffli S.P., Gaschen F., Neiger R. Effect of dietary factors on the detection of fecal occult blood in cats. J Vet Diagn Invest. 2001;13:177–179. doi: 10.1177/104063870101300218. [DOI] [PubMed] [Google Scholar]
- 65.Rutgers H.C., Batt R.M., Elwood C.M. Small intestinal bacterial overgrowth in dogs with chronic intestinal disease. J Am Vet Med Assoc. 1995;206:187–193. [PubMed] [Google Scholar]
- 66.Johnston K.L. Small intestinal bacterial overgrowth. Vet Clin North Am Small Anim Pract. 1999;29:523–550. [PubMed] [Google Scholar]
- 67.Johnston K.L., Lamport A.I., Ballevre O.P. Effects of oral administration of metronidazole on small intestinal bacteria and nutrients of cats. Am J Vet Res. 2000;61:1106–1112. doi: 10.2460/ajvr.2000.61.1106. [DOI] [PubMed] [Google Scholar]
- 68.Johnston K.L., Swift N.C., Forster-van Hijfte M. Comparison of the bacterial flora of the duodenum in healthy cats and cats with signs of gastrointestinal tract disease. J Am Vet Med Assoc. 2001;218:48–51. doi: 10.2460/javma.2001.218.48. [DOI] [PubMed] [Google Scholar]
- 69.Suchodolski J.S. Microbes and gastrointestinal health of dogs and cats. J Anim Sci. Nov 12, 2010 doi: 10.2527/jas.2010-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suchodolski J.S., Xenoulis P.G., Paddock C.G. Molecular analysis of the bacterial microbiota in duodenal biopsies from dogs with idiopathic inflammatory bowel disease. Vet Microbiol. 2010;142:394–400. doi: 10.1016/j.vetmic.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 71.Xenoulis P.G., Palculict B., Allenspach K. Molecular-phylogenetic characterization of microbial communities imbalances in the small intestine of dogs with inflammatory bowel disease. FEMS Microbiol Ecol. 2008;66:579–589. doi: 10.1111/j.1574-6941.2008.00556.x. [DOI] [PubMed] [Google Scholar]
- 72.Melgarejo T., Williams D.A., O'Connell N.C. Serum unconjugated bile acids as a test for intestinal bacterial overgrowth in dogs. Dig Dis Sci. 2000;45:407–414. doi: 10.1023/a:1005493416946. [DOI] [PubMed] [Google Scholar]
- 73.Williams D.A., Batt R.M. Sensitivity and specificity of radioimmunoassay of serum trypsin-like immunoreactivity for the diagnosis of canine exocrine pancreatic insufficiency. J Am Vet Med Assoc. 1988;192:195–201. [PubMed] [Google Scholar]
- 74.Steiner J.M., Medinger T.L., Williams D.A. Development and validation of a radioimmunoassay for feline trypsin-like immunoreactivity. Am J Vet Res. 1996;57:1417–1420. [PubMed] [Google Scholar]
- 75.James F.E., Mansfield C.S., Steiner J.M. Pancreatic response in healthy dogs fed diets of various fat compositions. Am J Vet Res. 2009;70:614–618. doi: 10.2460/ajvr.70.5.614. [DOI] [PubMed] [Google Scholar]
- 76.Steiner J.M., Williams D.A. Influence of feeding on serum feline trypsin-like immunoreactivity. Am J Vet Res. 1999;60:895–897. [PubMed] [Google Scholar]
- 77.Carro T., Williams D.A. Relationship between dietary protein concentration and serum trypsin-like immunoreactivity in dogs. Am J Vet Res. 1989;50:2105–2107. [PubMed] [Google Scholar]
- 78.Wiberg M.E., Nurmi A.K., Westermarck E. Serum trypsinlike immunoreactivity measurement for the diagnosis of subclinical exocrine pancreatic insufficiency. J Vet Intern Med. 1999;13:426–432. doi: 10.1892/0891-6640(1999)013<0426:stimft>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 79.Spillmann T., Wittker A., Teigelkamp S. An immunoassay for canine pancreatic elastase 1 as an indicator for exocrine pancreatic insufficiency in dogs. J Vet Diagn Invest. 2001;13:468–474. doi: 10.1177/104063870101300603. [DOI] [PubMed] [Google Scholar]
- 80.Spillmann T., Eigenbrodt E., Sziegoleit A. [Determination and clinical relevance of fecal pancreatic elastase in dogs] Tierarztl Prax Ausg K Klientiere Heimtiere. 1998;26:364–368. [PubMed] [Google Scholar]
- 81.Newman S., Steiner J., Woosley K. Localization of pancreatic inflammation and necrosis in dogs. J Vet Intern Med. 2004;18:488–493. doi: 10.1892/0891-6640(2004)18<488:lopian>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 82.De Cock H.E., Forman M.A., Farver T.B. Prevalence and histopathologic characteristics of pancreatitis in cats. Vet Pathol. 2007;44:39–49. doi: 10.1354/vp.44-1-39. [DOI] [PubMed] [Google Scholar]
- 83.Simpson K.W., Simpson J.W., Lake S. Effect of pancreatectomy on plasma activities of amylase, isoamylase, lipase and trypsin-like immunoreactivity in dogs. Res Vet Sci. 1991;51:78–82. doi: 10.1016/0034-5288(91)90035-m. [DOI] [PubMed] [Google Scholar]
- 84.Steiner J.M., Rutz G.M., Williams D.A. Serum lipase activities and pancreatic lipase immunoreactivity concentrations in dogs with exocrine pancreatic insufficiency. Am J Vet Res. 2006;67:84–87. doi: 10.2460/ajvr.67.1.84. [DOI] [PubMed] [Google Scholar]
- 85.Polzin D.J., Osborne C.A., Stevens J.B. Serum amylase and lipase activities in dogs with chronic primary renal failure. Am J Vet Res. 1983;44:404–410. [PubMed] [Google Scholar]
- 86.Steiner J.M. Diagnosis of pancreatitis. Vet Clin North Am Small Anim Pract. 2003;33:1181–1195. doi: 10.1016/s0195-5616(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 87.Piccione G., Giannetto C., Fazio F. Daily rhythm of serum lipase and alpha-amylase activity in fed and fasted dogs. J Vet Diagn Invest. 2008;20:795–799. doi: 10.1177/104063870802000614. [DOI] [PubMed] [Google Scholar]
- 88.Strombeck D.R., Farver T., Kaneko J.J. Serum amylase and lipase activities in the diagnosis of pancreatitis in dogs. Am J Vet Res. 1981;42:1966–1970. [PubMed] [Google Scholar]
- 89.Mansfield C.S., Jones B.R. Plasma and urinary trypsinogen activation peptide in healthy dogs, dogs with pancreatitis and dogs with other systemic diseases. Aust Vet J. 2000;78:416–422. doi: 10.1111/j.1751-0813.2000.tb11833.x. [DOI] [PubMed] [Google Scholar]
- 90.Hess R.S., Kass P.H., Shofer F.S. Evaluation of risk factors for fatal acute pancreatitis in dogs. J Am Vet Med Assoc. 1999;214:46–51. [PubMed] [Google Scholar]
- 91.McCord K., Davis J., Leyva F. A multi-institutional study evaluating diagnostic utility of Spec cPL in the diagnosis of acute pancreatitis in dogs [abstract] J Vet Intern Med. 2009;23:734. [Google Scholar]
- 92.Watson P.J., Archer J., Roulois A.J. Observational study of 14 cases of chronic pancreatitis in dogs. Vet Rec. 2010;167:968–976. doi: 10.1136/vr.c4912. [DOI] [PubMed] [Google Scholar]
- 93.Kitchell B.E., Strombeck D.R., Cullen J. Clinical and pathologic changes in experimentally induced acute pancreatitis in cats. Am J Vet Res. 1986;47:1170–1173. [PubMed] [Google Scholar]
- 94.Steiner J.M., Williams D.A. Purification of classical pancreatic lipase from dog pancreas. Biochimie. 2002;84:1245–1253. doi: 10.1016/s0300-9084(02)00016-0. [DOI] [PubMed] [Google Scholar]
- 95.Steiner J.M., Wilson B.G., Williams D.A. Purification and partial characterization of feline classical pancreatic lipase. Comp Biochem Physiol B Biochem Mol Biol. 2003;134:151–159. doi: 10.1016/s1096-4959(02)00222-1. [DOI] [PubMed] [Google Scholar]
- 96.Steiner J.M., Berridge B.R., Wojcieszyn J. Cellular immunolocalization of gastric and pancreatic lipase in various tissues obtained from dogs. Am J Vet Res. 2002;63:722–727. doi: 10.2460/ajvr.2002.63.722. [DOI] [PubMed] [Google Scholar]
- 97.Huth S.P., Relford R., Steiner J.M. Analytical validation of an ELISA for measurement of canine pancreas-specific lipase. Vet Clin Pathol. 2010;39:346–353. doi: 10.1111/j.1939-165X.2010.00245.x. [DOI] [PubMed] [Google Scholar]
- 98.Beall M.J., Cahill R., Pigeon K. Performance validation and method comparison of an in-clinic enzyme-linked immunosorbent assay for the detection of canine pancreatic lipase. J Vet Diagn Invest. 2011;23:115–119. doi: 10.1177/104063871102300120. [DOI] [PubMed] [Google Scholar]
- 99.Steiner J.M., Teague S.R., Lees G.E. Stability of canine pancreatic lipase immunoreactivity concentration in serum samples and effects of long-term administration of prednisone to dogs on serum canine pancreatic lipase immunoreactivity concentrations. Am J Vet Res. 2009;70:1001–1005. doi: 10.2460/ajvr.70.8.1001. [DOI] [PubMed] [Google Scholar]
- 100.Cordner A.P., Armstrong P.J., Newman S.J. Effect of pancreatic tissue sampling on serum pancreatic enzyme levels in clinically healthy dogs. J Vet Diagn Invest. 2010;22:702–707. doi: 10.1177/104063871002200505. [DOI] [PubMed] [Google Scholar]
- 101.Cosford K.L., Shmon C.L., Myers S.L. Prospective evaluation of laparoscopic pancreatic biopsies in 11 healthy cats. J Vet Intern Med. 2010;24:104–113. doi: 10.1111/j.1939-1676.2009.0420.x. [DOI] [PubMed] [Google Scholar]
- 102.Steiner J.M., Finco D.R., Gumminger S.R. Serum canine pancreatic lipase immunoreactivity (cPLI) in dogs with experimentally induced renal failure. J Vet Intern Med. 2001;15:311. [abstract] [Google Scholar]
- 103.Xenoulis P., Finco D.R., Suchodolski J.S. Serum fPLI and SPEC fPL concentrations in cats with experimentally induced chronic renal failure. J Vet Intern Med. 2009;23:758. doi: 10.1111/jvim.16296. [abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Steiner J.M., Xenoulis P.G., Anderson J.A. Serum pancreatic lipase immunoreactivity concentrations in dogs treated with potassium bromide and/or phenobarbital. Vet Ther. 2008;9:37–44. [PubMed] [Google Scholar]
- 105.Steiner J.M., Newman S., Xenoulis P. Sensitivity of serum markers for pancreatitis in dogs with macroscopic evidence of pancreatitis. Vet Ther. 2008;9:263–273. [PubMed] [Google Scholar]
- 106.Carney PC, Ruaux GC, Suchodolski J, et al: Biological variability of canine pancreatic lipase immunoreactivity and C-reactive protein concentrations in clinically healthy dogs. Paper presented at: 2010 ACVIM Forum, Anaheim, CA
- 107.Forman M.A., Marks S.L., De Cock H.E. Evaluation of serum feline pancreatic lipase immunoreactivity and helical computed tomography versus conventional testing for the diagnosis of feline pancreatitis. J Vet Intern Med. 2004;18:807–815. doi: 10.1892/0891-6640(2004)18<807:eosfpl>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 108.Prior L.M., Forman M.A., Shiroma J. Serial evaluation of canine pancreatic lipase (SPEC cPL) in dogs with clincal signs of pancreatitis [abstract] J Vet Intern Med. 2009;23:733. [Google Scholar]
- 109.Bailey S., Benigni L., Eastwood J. Comparisons between cats with normal and increased fPLI concentrations in cats diagnosed with inflammatory bowel disease. J Small Anim Pract. 2010;51:484–489. doi: 10.1111/j.1748-5827.2010.00973.x. [DOI] [PubMed] [Google Scholar]
- 110.Steiner J.M., Finco D.R., Williams D.A. Serum trypsin-like immunoreactivity (fTLI) in cats with experimentally induced chronic renal failure. J Vet Intern Med. 2002;16:819. [abstract] [Google Scholar]
- 111.Gerhardt A., Steiner J.M., Williams D.A. Comparison of the sensitivity of different diagnostic tests for pancreatitis in cats. J Vet Intern Med. 2001;15:329–333. [PubMed] [Google Scholar]
- 112.Swift N.C., Marks S.L., MacLachlan N.J. Evaluation of serum feline trypsin-like immunoreactivity for the diagnosis of pancreatitis in cats. J Am Vet Med Assoc. 2000;217:37–42. doi: 10.2460/javma.2000.217.37. [DOI] [PubMed] [Google Scholar]
- 113.Allen H.S., Steiner J., Broussard J. Serum and urine concentrations of trypsinogen-activation peptide as markers for acute pancreatitis in cats. Can J Vet Res. 2006;70:313–316. [PMC free article] [PubMed] [Google Scholar]
- 114.Guija de Arespacochaga A., Hittmair K.M., Schwendenwein I. Comparison of lipase activity in peritoneal fluid of dogs with different pathologies—a complementary diagnostic tool in acute pancreatitis? J Vet Med A Physiol Pathol Clin Med. 2006;53:119–122. doi: 10.1111/j.1439-0442.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 115.Watson P., Herrtage M. Chronic pancreatitis in dogs. Vet Rec. 2003;152:340. [PubMed] [Google Scholar]