Abstract
This article reviews the common infectious causes of diarrhea in cats and discusses selected diseases in greater depth. A systematic approach to a cat with possible infectious diarrhea is presented, along with a detailed description of the best way to perform an in-house fecal examination. The goal here is to provide the reader with a logical list of differentials and the information necessary to pursue a diagnosis in an efficient manner.
Keywords: diarrhea, fecal examination, parvovirus, Campylobacter, Clostridium, Giardia, Tritrichomonas, Cryptosporidium, Toxocara
Diarrhea due to infectious causes is a common problem in feline practice, particularly in young cats and those with access to the outside. Clinical signs may be minimal and intermittent (eg, ascarid infection) or life threatening (eg, feline panleukopenia), and a definitive diagnosis may require a simple stool examination or elaborate testing. For any patient presenting with diarrhea, a careful consideration of infectious causes is always warranted, and sound clinical judgment is necessary to decide which possibilities should be pursued.
This article will review the broad categories of infectious diarrhea in cats and will discuss the more challenging causes in greater depth.
Categorization
It is easiest to categorize the causes of infectious diarrhea by organism type, because this facilitates a logical consideration of likely diagnoses. See Table 1 for a list of the common infectious agents.
Table 1.
Common Causes of Infectious Diarrhea in the Cat
| Organism Type | Specific Disease | Diagnostic Method(s) |
|---|---|---|
| Viral | Feline parvovirus* | Fecal antigen |
| ELISA serology | ||
| Feline coronovirus | Serology (supportive) | |
| Bacterial | Campylobacter species* | Fecal cytology |
| Fecal culture | ||
| Clostridium perfringens* | Fecal cytology | |
| Fecal enterotoxin ELISA | ||
| Fecal PCR | ||
| Salmonella species | Fecal culture | |
| Fungal | Histoplasma capsulatum | Rectal wall cytology |
| Serum/urine antigen assay | ||
| Protozoal | Giardia duodenalis* | Fecal saline smear |
| Fecal flotation (ZnSO4) | ||
| Fecal antigen ELISA | ||
| Tritrichomonas foetus* | Fecal saline smear | |
| Fecal culture | ||
| Fecal PCR | ||
| Cryptosporidium species | Fecal saline smear | |
| Fecal ELISA | ||
| Isospora species | Fecal flotation | |
| Helminth | Toxocara cati* | Fecal flotation |
| Toxascaris leonina | Fecal flotation | |
| Trichuris vulpis | Fecal flotation | |
| Ancylostoma tubaeforme | Fecal flotation |
Diseases discussed in the text are marked with an asterisk (*).
Viral
Viral diarrheas are most often seen in kittens or cats recently introduced to multi-cat environments, and should be considered in any susceptible individual presenting with acute gastroenterocolitis accompanied by fever.
Feline Parvovirus/Panleukopenia
This is a highly contagious disease, with high morbidity and mortality in unvaccinated kittens.1 The virus is able to survive in the environment for long periods, and most infections are thought to occur by indirect contact with a contaminated environment.
Initial signs include fever and vomiting. Affected cats are depressed and become rapidly dehydrated. Diarrhea is not a consistent finding and may occur later in the course of the illness.
Diagnosis is usually made on the basis of the appropriate clinical signs accompanied by leukopenia (total white cell count below 3000 per microliter). Commercial enzyme-linked immunosorbent assay (ELISA) tests for the detection of fecal canine parvoviral (CPV) antigens are able to detect feline parvovirus (FPV) infection; however, viral shedding may have waned by the time clinical signs appear. A negative CPV ELISA test cannot therefore reliably exclude a diagnosis of FPV. It is important to remember that recent immunization for feline panleukopenia can result in positive results on fecal ELISA tests for up to 2 weeks, so results should be interpreted with caution in a recently vaccinated patient.2 Serology, using virus neutralizing antibodies, is regarded as the most reliable way to confirm FPV infection; a 4-fold increase in titers taken 2 weeks apart is diagnostic.1
Aggressive supportive care, along with broad-spectrum antibiotic coverage, is the only therapy for affected cats. Those that survive the first week will usually recover, but a full return to health may take several weeks. Life-long immunity occurs after natural infection.
Enteric Coronavirus/Feline Enteric Coronavirus
Feline enteric coronaviruses (FECVs) are epitheliotrophic organisms, spread by the fecal-oral route. The incidence of infection in catteries is high, and many kittens are seropositive by 12 weeks of age.3 However, clinical signs are usually minimal and self-limiting. In a small number of individuals, the virus mutates and infects macrophages. This triggers an immune-mediated response by the host, and feline infectious peritonitis (FIP) ensues. It is important to remember that FECVs are endemic and infections are generally subclinical; serologic testing for this virus in cats with diarrhea is therefore inappropriate.
Bacterial
Although several bacterial species have been associated with diarrhea in cats, identifying a causal relationship is difficult because these species can be present in clinically normal individuals. A bacterial etiology should be considered if disease occurs in young kittens, is triggered by antibiotic therapy, or seems to be contagious.4 Fecal culture can be complicated, and samples often require special handling for optimum results.
In general, acute bacterial enteritis or enterocolitis in cats is likely to be self-limiting, and the administration of antibiotics may not be beneficial. However, therapy should certainly be considered if a zoonotic agent is identified, or if signs of systemic illness (eg, fever, leukocytosis) are noted.
Campylobacter
Campylobacter organisms are Gram-negative motile rods. Although several species have been identified in both asymptomatic and diarrheic cats, C. jejuni is the organism most often associated with clinical disease.5 The severity of diarrhea in infected cats seems to be dependent on several factors, including the infective dose, the level of protective antibodies, and the presence of other intestinal pathogens.6
Clinical signs are most likely in animals less than 6 months of age, and usually consist of mucoid diarrhea, often with fresh blood. Diagnosis in the hospital setting is difficult, because phase-contrast microscopy is needed to identify the motile, curved bacteria. Routine fecal cytology with Gram staining may reveal the gull wing–shaped rods, but many other organisms (eg, Helicobacter) have a similar appearance. Culture of fresh feces remains the most reliable way to establish a diagnosis. The organism is quite hardy both at room temperatures and when refrigerated, so transportation to a laboratory is certainly feasible. Anaerobic swabs should be used, because the organism is microaerophilic. Also, special culture methods enhance the likelihood of successful growth, so the laboratory should be informed ahead of time if Campylobacter is suspected.
It has not been shown if antibiotic therapy actually affects the natural course of the disease, but it may help prevent infection of other animals and people in the household. Erythromycin is the recommended drug in humans and has been shown to be effective (based on fecal culture studies) in cats.7 The dose is 10 mg/kg by mouth every 8 hours for 5 days.
Clostridium perfringens
This is an anaerobic Gram-positive bacillus. It is a normal inhabitant of the intestinal tract of many animals, and its role as a pathogen in cats is not well understood. A trigger of some kind is generally necessary before Clostridium perfringens causes clinical illness; this trigger (eg, a change in diet, anorexia, or antibiotic administration) causes the vegetative form of the organism to sporulate and release enterotoxins.8
Clinical signs associated with Clostridial enterotoxin release include diarrhea, often with blood and mucus; signs of systemic disease are not expected. Fecal cytology may reveal numerous spores, which are certainly supportive of the diagnosis, but it is important to remember that large numbers of endospores have been reported in the stools of healthy cats (Fig 1). In dogs, no association has been found between fecal endospore counts and the presence of Clostridial enterotoxins.9
Figure 1.
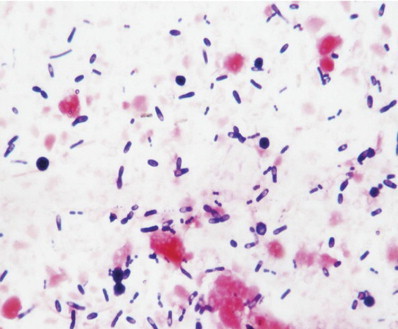
Stained fecal smear (modified Wright's stain) from a healthy, nondiarrheic cat showing numerous endospores of Clostridium perfringens (magnification ×1000). Reproduced with permission.3
Various assays for detection of enterotoxins are presently available, but some questions have arisen about their specificity.10 Most commercial laboratories offer a human-based ELISA test; a positive test result certainly supports the diagnosis, but a negative result does not exclude it.
More recently, polymerase chain reaction (PCR) testing has been used to identify the gene that encodes for enterotoxin production. This test is performed on feces and is highly sensitive. The Gastrointestinal Laboratory at Texas A&M University offers this test; further details are available at http://www.cvm.tamu.edu/gilab.
Most affected cats will show clinical improvement 2 days after starting amoxicillin (20 mg/kg by mouth every 12 hours) or tylosin (30 mg/kg by mouth every 12 hours).8 A 14-day course is recommended even if the stools return to normal sooner. Increasing the fermentable fiber in the diet (eg, with canned pumpkin: 1 tablespoon twice daily) may prevent relapse.
Antibiotic-Responsive Diarrhea
There is tremendous controversy regarding the role of intestinal bacteria in the development and perpetuation of chronic enteropathies. It has been suggested that the absolute number of bacteria within the small intestine may cause disease (so called “small intestinal bacterial overgrowth”), or that aberrant host responses to “normal” bacterial populations result in intestinal inflammation and dysfunction in susceptible individuals. The situation in cats is certainly unclear, and no firm evidence has been presented in support of either theory. However, some cats with chronic, undefined diarrhea do show clinical improvement with antibiotic therapy. This may be due to the eradication of occult/unknown pathogens or due to an alteration in the host response to endogenous flora. If empirical antibiotics are considered for a cat with chronic diarrhea, suitable choices would include metronidazole (15 mg/kg by mouth twice daily) or tylosin (10 mg/kg by mouth 3 times daily).11 Treatment should be administered for at least 4 weeks and may need to be repeated if clinical signs recur.
Protozoal
The importance of protozoal infections in feline patients has become more apparent in recent years, with the identification of unfamiliar pathogens (eg, Tritrichomonas foetus) and the advances in our ability to detect familiar organisms (eg, fecal ELISA testing for Giardia).
Giardia duodenalis
This is a protozoal organism capable of infecting most mammalian species. Several genotypes have been identified, which may have differing host species specificities.12 Giardia duodenalis exists in 2 forms—the trophozoite and the cyst. The trophozoite is motile and is found in the intestinal lumen, whereas the cyst is the transmissible form and is capable of prolonged survival in the environment.13 Transmission occurs through ingestion of the cyst; once in the duodenum, each cyst releases 2 trophozoites, which then attach to the intestinal epithelium.
Subclinical infections are common and contribute to environmental contamination. Clinical illness usually occurs in kittens and catteries; administration of immunosuppressive doses of steroids may precipitate diarrhea in carrier individuals. The diarrhea associated with Giardiaisis is usually fatty and malodorous; blood and mucus are uncommon and fever and vomiting are not expected.
Diagnosis can be made by identification of motile trophozoites on a fecal saline smear; a drop of iodine makes it easier to identify the parasite, but does inhibit motility. Giardia trophozoites are pear-shaped and symmetrical, with 2 distinct nuclei in the cranial portion (Fig 2). The cystic form (Fig 3) is most readily detected by the zinc sulfate flotation method, but it is important to remember that clinical signs may occur 1 to 2 days before cysts appear in the feces. Fecal Giardia antigens can be detected with commercially available ELISA kits (eg, SNAP Giardia test, Idexx Laboratories Inc., Westbrook, MA, USA; and ProSpecT Giardia Rapid Assay, Alexon Inc., Sunnyvale, CA, USA). These have not been extensively validated in cats, but seem to have similar sensitivity and specificity to zinc sulfate flotation methods.14 Some commercial laboratories use direct immunofluorescence assays to detect Giardia cysts; feces should be preserved in formalin before shipping to maximize accuracy. PCR methods are probably the most sensitive way to detect infection, but are not widely available.15
Figure 2.
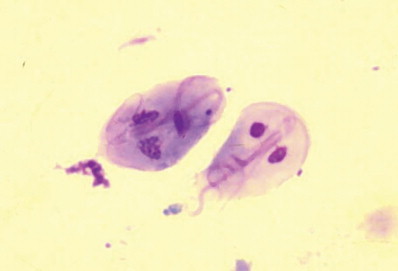
Giemsa-stained fecal smear showing two Giardia trophozoites exhibiting the characteristic pear, or teardrop, shape with bilateral symmetry when viewed from the top, two nuclei, and fibrils running the length of the parasite. Reproduced with permission.3
Figure 3.

Zinc sulfate fecal flotation showing Giardia cysts with distinctive fibrils (axonemes) coursing the length of the cyst (magnification ×400). Reproduced with permission.3
Several drugs have shown efficacy against Giardia, but none are currently approved for this use in cats. Although metronidazole has been widely used in this context, recent studies have indicated poor efficacy, likely because of parasitic resistance.12 The current treatment of choice is fenbendazole, at 25 mg/kg by mouth every 12 hours for 5 days. Albendazole is equally efficacious, but is associated with an idiosyncratic myelotoxicity in cats and should be avoided. A recent study of experimentally infected kittens reported that a combination of febantel, pyrantel, and praziquantel (Drontal Plus, Bayer Animal Health, Shawnee Mission, KS, USA) decreased fecal shedding (based on immunofluorescence assay testing). However, few cats in the treatment group had diarrhea, so the clinical usefulness of this protocol needs further investigation.16
Treatment must be accompanied by thorough efforts to control environmental contamination, because re-infection is otherwise likely. Steam cleaning or disinfection with quaternary ammonium products is recommended, followed by a drying out period of several days. Animals should be bathed before re-entering the environment, because cysts may be carried in on fur.
Giardia duodenalis is potentially zoonotic, and owners should be warned of the risk of transmission to other pets and family members. Although a vaccine using inactive trophozoites is licensed for use in cats in the United States, it appears to limit rather than prevent infection and does not prevent cyst shedding in previously infected individuals.17
Tritrichomonas foetus
This protozoa is a well-recognized cause of abortion in cattle, but has recently been identified as a cause of colitis in cats. In contrast to Giardia duodenalis, Tritrichomonas foetus exists only in the trophozoite form. Transmission occurs by direct ingestion of fecal material, because the organism cannot survive for long in the environment.
The epidemiology of Tritrichomonas foetus in cats is not well understood, but cats kept in high-density housing are more likely to carry the organism. It is not thought to be a component of the normal intestinal flora, although infection does not necessarily result in clinical signs.18
When illness occurs, it is generally reported in young cats living in crowded environments. The organism inhabits the distal ileum, cecum, and colon, and can result in a chronic waxing and waning large bowel diarrhea. In severely affected cats, the anal area becomes markedly reddened and painful, and feces may be passed involuntarily while the patient is sleeping.
The diagnosis can sometimes be made by direct observation of the trophozoites on a direct fecal smear. It is easy to mistake tritrichomonads for Giardia trophozoites, but closer inspection reveals a distinctive undulating membrane along the entire body (Fig 4). Their motility patterns are also different, with a jerkier, axial motion noted with Tritrichomonas foetus. If a direct fecal smear is negative, the organism may be cultured with a commercially prepared medium and container (InPouch TF-Feline, Biomed Diagnostics, White City, OR, USA). This can be easily performed in the clinic with a peppercorn-sized stool sample. The pouch is kept at room temperature and is examined under the microscope every day for evidence of the motile trophozoites (Fig 5). It can take up to a week for the organism to be evident. A more expensive, but more sensitive, method for confirming infection is fecal PCR. This test is performed at North Carolina State University; details on sample handling and submission are provided at http://www.cvm.ncsu.edu/docs/documents/tritrichomonas_PCR_submission.pdf.
Figure 4.
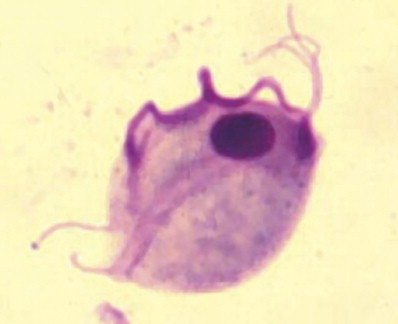
Giemsa-stained fecal smear showing characteristic appearance of Tritrichomonas foetus with its 3 anterior flagellae and long, undulating membrane. Reproduced with permission.3
Figure 5.
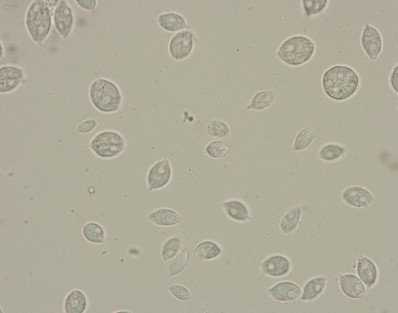
Tritrichomonas foetus trophozoites in culture medium (InPouch TF; Biomed Diagnostics) isolated form a diarrheic cat (magnification ×400). Reproduced with permission.3
Treatment of Tritrichomonas foetus has historically been problematic. Many cats will transiently improve with a range of different antibiotics, but then relapse soon afterward. Consistent and sustained clinical improvements have been documented with oral ranidazole (30-50 mg/kg by mouth every 12 hours), and negative posttreatment fecal PCR tests have been reported.19 This drug is presently the treatment of choice, but is not currently licensed for this use in cats and must be obtained from a compounding pharmacy. Ronidazole has been associated with a reversible neurotoxicity, similar to that reported with metronidazole, and characterized by cerebellar signs.20
Many cats seem to improve without treatment as they get older, and spontaneous resolution of diarrhea often occurs within 2 years. However, many of these cats remain positive on fecal PCR testing, suggesting that a chronic, asymptomatic carrier state occurs.21
Cryptosporidium parvum
Cryptosporidium parvum is a coccidial protozoan. Several genotypes of Cryptosporidium have been identified, but C. parvum is most often associated with clinical disease in cats. Infection occurs by ingestion of the highly resistant spores, usually via contaminated food or water; in susceptible individuals, the infective dose can be very low.
The pathophysiology of Cryptosporidium parvum infection is poorly understood. It may be a primary pathogen, but most clinical cases are noted in association with immunosuppression or environmental stress.22 The organism primarily colonizes the small intestinal epithelium, and causes a malabsorptive and secretory diarrhea.23 Thick-walled sporulated oocysts are then shed in the stools, while thin-walled oocysts remain in the lumen and result in autogenous reinfection.
Clinical signs reflect small intestinal dysfunction, and include weight loss and high-volume, low-frequency stools. Chronically infected cats may also have tenesmus and other signs of secondary colonic inflammation. Diagnosis can be made by direct fecal examination, but flotation techniques (using sugar solutions) are generally more sensitive. The cysts are small and tend to cling to the coverslip, so careful focusing may be needed to optimize visualization. Infection does not always produce disease, and cysts may be identified in apparently healthy kittens.24 The prevalence of this asymptomatic carrier state seems to decrease with age, suggesting some kind of acquired immunity results in clearance of the organism.
Commercial laboratories may use special staining and sedimentation techniques to improve the reliability of the fecal examination, so it may be necessary to specifically request a search for Cryptosporidia if this a concern. In addition, fecal ELISA and fluorescent antibody tests are routinely available, and do appear to improve the chances of identifying infected animals.25 PCR-based tests appear to be the most sensitive way to identify the organism, but are rarely indicated in the clinical setting.26
Most infections appear to be self-limiting, and most cats will quickly improve with simple supportive care. Provision of supplemental glutamine may be helpful, because this may promote enterocyte recovery. At the present time, trying to treat cryptosporidiosis infection is a challenge, because there are no licensed therapies for this disorder. Paromomycin has been suggested, but is associated with acute renal failure and is therefore not recommended.21 Azithromycin and tylosin may be beneficial and could be considered in debilitated or immunocompromised patients.
Helminths
Intestinal parasitism is often subclinical, except in kittens or immunocompromised adult cats. The shedding of eggs in the stools can be intermittent, so it is always appropriate to administer a broad-spectrum dewormer (eg, fenbendazole; 50 mg/kg by mouth daily for 3 days) to any patient with diarrhea, even if a fecal flotation is negative.
Toxocara cati
This is one of the most commonly identified feline parasites, with infection rates ranging from 10% to 85% in the United States. This organism is zoonotic, so recognition of infected individuals is particularly important. The organisms of Toxocara cati and Toxascaris leonina are indistinguishable on fecal flotation (Fig 6). However, because both are treated with the same agents, differentiating the two is rarely necessary.
Figure 6.
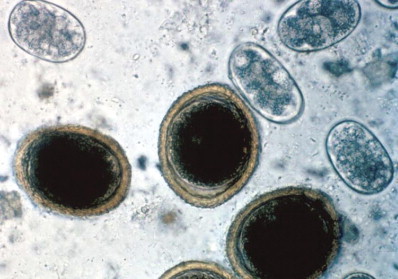
Fecal flotation showing large, thick-walled ova of Toxocara cati and Ancylostoma caninum ova (magnification ×400). Reproduced with permission.3
General Approach to the Feline Patient with Suspected Infectious Diarrhea
After obtaining a detailed history and performing a physical examination, a thorough evaluation of the feces is the next logical step. A saline smear (also called a wet prep), fecal cytological examination, and fecal flotation should be performed. Details of sample preparation and examination are provided below. In many cases, a diagnosis can be obtained by these simple steps.
If the patient is systemically ill, with fever, dehydration, or vomiting, viral and bacterial causes of the diarrhea should be considered. Supportive care should be provided, and additional diagnostics (eg, laboratory work, imaging studies) should be considered. If the diarrhea persists, fecal culture may be helpful.
Additional diagnostics are warranted if the standard fecal evaluations do not establish a diagnosis. Deciding which tests to perform next will require some thought, and factors such as access to the outdoors (consider Giardia), a recent change in diet (consider Clostridium perfringens), or signalment (consider Tritrichomonas foetus) should be carefully weighed.
It can also be helpful to determine if diarrhea is primarily due to small intestinal disease or if large bowel signs predominate, because some pathogens selectively impact one section of the intestinal tract (see Fig 7). This differentiation can help highlight or eliminate particular diseases and guide diagnostic and therapeutic decisions.
Figure 7.
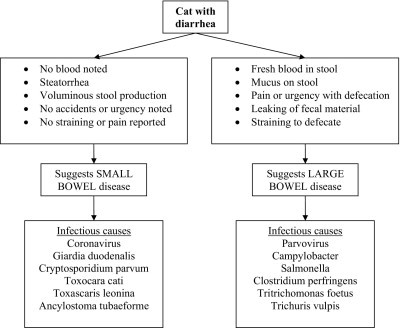
Differentiation of small and large bowel diarrhea and likely infectious causes.
Because intestinal parasitism is a common cause of diarrhea, it is certainly acceptable to administer a broad-spectrum anthelminthic even if fecal tests are negative. Fenbendazole, given at 50 mg/kg by mouth for 3 days, will eradicate ascarids, hookworms, and whipworms.
Figure 8 provides an algorithm for a logical diagnostic plan.
Figure 8.
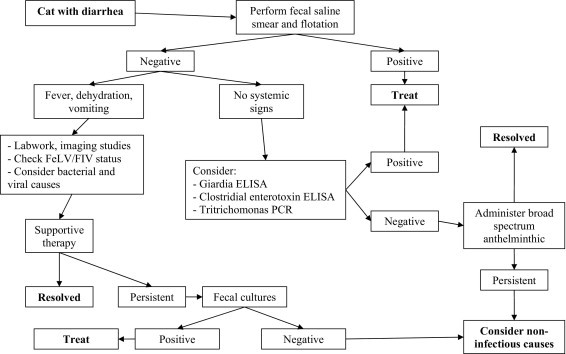
Algorithm for the diagnostic approach to a cat with suspected infectious diarrhea.
Fecal Evaluation
Saline Smear
Mix feces with saline solution until it forms a thin slurry (do not use water because this will cause lysis of the cells). Put a drop of this on a slide, and place a coverslip on top. If prepared correctly, you should be able to read newsprint through the slide. Feces should be examined within 30 minutes of collection, and not refrigerated before examination. Examine the smear at 10× initially, and move to 40× to confirm your findings.
Look for: Giardia duodenalis trophozoites
Tritrichomonas foetus trophozoites
Tip: Lugol's iodine may improve visualization of the internal structures of trophozoites and cysts, but will kill the organisms and stop motility.
Fecal Cytology
Smear feces across a slide, trying to keep the layer as thin as possible, but without exerting excessive pressure. Let the slide air dry and then stain with a modified Wright's stain.
Look for: Inflammatory cells
Clostridial spores (interpret with caution)
Campylobacter-like organisms (interpret with caution)
Fecal Flotation
Flotation methods are designed to improve the recovery of nematode ova and coccidial oocysts. Centrifugation flotation methods substantially improve the sensitivity of the process, and should be routinely performed. Mix 1/2 of a teaspoon of fresh feces with 5 to 10 mL of flotation solution. Sheather's sugar solution is an appropriate choice for most purposes, but zinc sulfate should be used if Giardia is suspected. Strain the mixture through a tea strainer or cheesecloth, using more flotation solution, into a centrifuge tube. If a centrifuge with swinging buckets is available, fill the tube with enough solution to create a meniscus, and place a coverslip on top. Centrifuge for 10 minutes at 1500 rpm. If a fixed bucket centrifuge is used, add enough flotation solution to create a meniscus after spinning, and then place the coverslip on top. Let the sample stand for 3 to 4 minutes, and then transfer the meniscus to a slide for examination at 10×.
Look for: Toxocara cati
Toxascaris leonina
Trichuris vulpis
Ancylostoma tubaeforme
Cryptosporidia oocysts
Giardia cysts (use zinc sulfate-specific gravity 1.18-1.2)
Preserving Samples
A saline smear is only useful if performed on body temperature feces less than 30 minutes after manual collection or defecation. Trophozoites lose their motility and become unrecognizable after short periods outside the host, so this examination really needs to be performed in the clinic. If a flotation study cannot be performed immediately, it is acceptable to refrigerate the sample. Cysts, oocysts, and helminth ova are generally hardy, and can survive several days of careful storage. If samples are shipped elsewhere for microscopic examination, a preservative should be used; formalin or commercially available products such as Proto-Fix (AlphaTec Systems, Inc., Vancouver, WA, USA) and Ecofix (Meridian Diagnostics, Inc., Cincinnati, OH, USA) are appropriate choices. If an infectious zoonosis such as Cryptosporidium parvum is suspected, the outside of the container should be disinfected and the laboratory should be informed of possible risk.
References
- 1.Greene C.E., Addie D. Feline parvoviral infections. In: Greene C.E., editor. Infectious Diseases of the Dog and Cat. ed 3. Saunders Elsevier; St Louis: 2006. pp. 78–86. [Google Scholar]
- 2.Patterson E.V., Reese M.J., Tucker S.J. Effect of vaccination on parvovirus antigen testing in kittens. J Am Vet Med Assoc. 2007;230:359–363. doi: 10.2460/javma.230.3.359. [DOI] [PubMed] [Google Scholar]
- 3.Foley J.E., Poland A., Carlson J. Patterns of feline coronavirus infection and fecal shedding from cats in multiple-cat environments. J Am Vet Med Assoc. 1997;210:1307–1312. [PubMed] [Google Scholar]
- 4.Marks S.L., Willard M.D. Diarrhea in kittens. In: August J.R., editor. Consultations in Feline Internal Medicine. ed 5. Saunders Elsevier; St Louis: 2006. pp. 133–143. [Google Scholar]
- 5.Bender J.B., Shulman S.A., Averbeck G.A. Epidemiologic features of Campylobacter infection among cats in the upper Midwestern United States. J Am Vet Med Assoc. 2005;226:544–547. doi: 10.2460/javma.2005.226.544. [DOI] [PubMed] [Google Scholar]
- 6.Sandberg M., Bergsjø B., Hofshagen M. Risk factors for Campylobacter infection in Norwegian cats and dogs. Prev Vet Med. 2002;55:241–253. doi: 10.1016/s0167-5877(02)00095-8. [DOI] [PubMed] [Google Scholar]
- 7.Fox J.G. Enteric bacterial infections. In: Greene C.E., editor. Infectious Diseases of the Dog and Cat. ed 3. Saunders Elsevier; St Louis: 2006. pp. 339–343. [Google Scholar]
- 8.Marks S.L., Kather E.J. Enteric bacterial infections. In: Greene C.E., editor. Infectious Diseases of the Dog and Cat. ed 3. Saunders Elsevier; St. Louis: 2006. pp. 363–369. [Google Scholar]
- 9.Weese J.S., Staempfli H.R., Prescott J.F. The roles of Clostridium difficile and enterotoxigenic Clostridium perfringens in diarrhea in dogs. J Vet Int Med. 2001;15:374–378. [PubMed] [Google Scholar]
- 10.Cave N.J., Marks S.L., Kass P.H. Evaluation of a routine diagnostic fecal panel for dogs with diarrhea. J Am Vet Med Assoc. 2002;221:52–59. doi: 10.2460/javma.2002.221.52. [DOI] [PubMed] [Google Scholar]
- 11.Simpson KW: Chronic enteropathies: how should I treat them? In: Proceedings of the 21st Annual Forum of the American College of Veterinary Internal Medicine. Lakewood, CO, American College of Veterinary Internal Medicine, 2003
- 12.Vasilopulos R.J., Rickard L.G., Mackin A.J. Genotypic analysis of Giardia duodenalis in domestic cats. J Vet Intern Med. 2007;21 doi: 10.1892/0891-6640(2007)21[352:gaogdi]2.0.co;2. 352-35. [DOI] [PubMed] [Google Scholar]
- 13.Barr S.C. Enteric protozoal infections. In: Greene C.E., editor. Infectious Diseases of the Dog and Cat. ed 3. Saunders Elsevier; St Louis: 2006. pp. 736–742. [Google Scholar]
- 14.Mekaru S.R., Marks S.L., Felley A.J. Comparison of direct immunofluorescence, immunoassays, and fecal flotation for detection of Cryptosporidium spp. and Giardia spp. in naturally exposed cats in 4 Northern California animal shelters. J Vet Int Med. 2007;21:959–965. doi: 10.1892/0891-6640(2007)21[959:codiia]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.McGlade T.R., Robertson I.D., Elliot A.D. High prevalence of Giardia detected in cats by PCR. Vet Parasitol. 2003;110:197–205. doi: 10.1016/s0304-4017(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 16.Scorza A.V., Radecki S.V., Lappin M.R. Efficacy of a combination of febantel, pyrantel, and praziquantel for the treatment of kittens experimentally infected with Giardia species. J Feline Med Surg. 2006;8:7–13. doi: 10.1016/j.jfms.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein J.E., Radecki S.V., Lappin M.R. Efficacy of Giardia vaccination in the treatment of giardiasis in cats. J Am Vet Med Assoc. 2003;222:1548–1551. doi: 10.2460/javma.2003.222.1548. [DOI] [PubMed] [Google Scholar]
- 18.Gookin J.L. Enteric protozoal infections. In: Greene C.E., editor. Infectious Diseases of the Dog and Cat. ed 3. Saunders Elsevier; St Louis: 2006. pp. 745–750. [Google Scholar]
- 19.Gookin J.L., Copple C.N., Papich M.G. Efficacy of ronidazole for treatment of feline Tritrichomonas foetus infection. J Vet Intern Med. 2006;20:536–543. doi: 10.1892/0891-6640(2006)20[536:eorfto]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Rosadeo T.W., Specht A., Marks S.L. Neurotoxicosis in 4 cats receiving ronidazole. J Vet Intern Med. 2007;21:328–331. doi: 10.1111/j.1939-1676.2007.tb02968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster D.M., Gookin J.L., Poore M.F. Outcome of cats with diarrhea and Tritrichomonas foetus infection. J Am Vet Med Assoc. 2004;225:888–892. doi: 10.2460/javma.2004.225.888. [DOI] [PubMed] [Google Scholar]
- 22.Barr S.C. Cryptosporidiosis and cyclosporiasis. In: Greene C.E., editor. Infectious Diseases of the Dog and Cat. ed 3. Saunders Elsevier; St Louis: 2006. pp. 785–791. [Google Scholar]
- 23.Gookin J.L., Nordone S.K., Argenzio R.A. Host responses to Cryptosporidium infection. J Vet Intern Med. 2002;16:12–21. doi: 10.1892/0891-6640(2002)016<0012:hrtci>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Shukla R., Giraldo P., Kraliz A. Cryptosporidium spp. and other zoonotic enteric parasites in a sample of domestic dogs and cats in the Niagara region of Ontario. Can Vet J. 2006;47:1179–1184. [PMC free article] [PubMed] [Google Scholar]
- 25.Marks S.L., Hanson T.E., Melli A.C. Comparison of direct immunofluorescence, modified acid-fast staining, and enzyme immunoassay techniques for detection of Cryptosporidium spp in naturally exposed kittens. J Am Vet Med Assoc. 2004;225:1549–1553. doi: 10.2460/javma.2004.225.1549. [DOI] [PubMed] [Google Scholar]
- 26.Scorza A.V., Brewer M.M., Lappin M.R. Polymerase chain reaction for the detection of Cryptosporidium spp. in cat feces. J Parasitol. 2003;89:423–426. doi: 10.1645/0022-3395(2003)089[0423:PCRFTD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]


