Highlights
► EGCG was esterified for improved lipophilicity and bioefficiency. ► The lipophilic EGCG esters exhibited better antioxidant activity than EGCG. ► The lipophilic EGCG esters, not EGCG, were effective antivirals against HCV and HIV.
Keywords: Epigallocatechin gallate (EGCG), Ester, Lipophilic, Antioxidant, Antiviral
Abstract
The water soluble green tea polyphenol epigallocatechin gallate (EGCG) was lipophilised by esterification with different fatty acids for expanded applications. Four lipophilic ester derivatives of EGCG, namely EGCG-O-tetrastearate, EGCG-O-tetraeicosapentaenoate, EGCG-O-tetradocosahexaenoate, and EGCG-O-octabutyrate, were prepared and evaluated for their antioxidant and antiviral activities in vitro. Incorporation of fatty acids, especially the long chain polyunsaturated fatty acids (PUFA), into EGCG resulted in increased peroxyl radical scavenging activity, as measured by ORAC (oxygen radical absorbance capacity) assay, and metal chelation capacity. However, the esters exhibited decreased reducing power. Antiviral activities of EGCG derivatives were remarkably higher than the parent EGCG molecule, which showed relatively weak effects. The EGCG–PUFA esters were 1700-fold more effective in inhibiting hepatitis C virus (HCV) protease than the positive control embelin. The derivatives also acted as α-glucosidase inhibitors, suggesting their potential in anti-HIV (human immunodeficiency virus) treatment. The results suggest that ester derivatives of EGCG with improved bioactivities may serve as excellent functional food ingredients and natural health products. Moreover, the omega-3 PUFA in the derivatives may also render additional or synergistic health benefits.
1. Introduction
Tea and tea products contain substantial amounts of bioactive flavonoids and have proven to render multiple functional and physiological effects (Cabrera, Artacho, & Giménez, 2006). Many of the health effects related to tea consumption have been attributed to its catechin (simple or condensed) content, which is also responsible for the characteristic colour, flavour and aroma of tea. Among catechins found in tea, epigallocatechin gallate (EGCG) is the predominant one (McKay & Blumberg, 2002) and has been extensively studied as a potential health promoting agent. EGCG has a four ring structure with 8 hydroxyl groups, and is readily dissolved in water while sparingly soluble in hydrophobic media. The hydrophilic nature of EGCG restricts its bioefficiency in lipophilic environments due to limited solubility and hence hindered approach to the sites of action. Moreover, the hydrophilicity of EGCG may account for its poor cellular absorption in vivo and thus low bioavailability when ingested orally. In order to take better advantage of this multifunctional compound, the water-soluble EGCG was structurally modified by esterification with different fatty acids, including stearic (SA), eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids as described elsewhere (Zhong & Shahidi, 2011). The tetraesters produced showed enhanced lipophilicity and improved radical scavenging capacity compared to EGCG. Lipophilisation of EGCG through esterification may be useful for improving its bioefficiency in lipophilic media or even introducing novel bioactivities due to the fatty acid moieties incorporated, especially when the health beneficial long-chain omega-3 polyunsaturated fatty acids (PUFA) are involved. This study was designed to investigate the lipophilised EGCG derivatives as potential alternatives of EGCG for incorporation in foods and as natural health products. Four EGCG-fatty acid esters (Fig. 1 ), namely EGCG-3′,5′,3″,5″-O-tetrastearate (compound 1), EGCG-3′,5′,3″,5″-O-tetraeicosapentaenoate (compound 2), EGCG-3′,5′,3″,5″-O-tetradocosahexaenoate (compound 3), and EGCG-octabutyrate (compound 4) were evaluated for their antioxidant and antiviral activities in vitro.
Fig. 1.
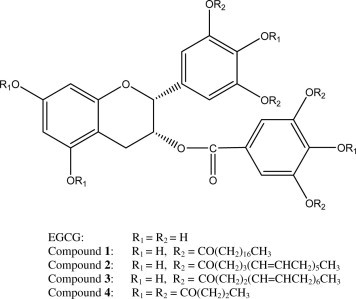
Chemical structures of EGCG and its derivatives.
EGCG is known as a powerful antioxidant which protects against free radical-mediated oxidative changes in both food and living organisms. EGCG acts as a scavenger of many reactive oxygen/nitrogen species (ROS/RNS) such as superoxide radical anion, peroxyl and hydroxyl radicals, singlet oxygen, nitric oxide and peroxynitrite, among others. These reactive species are implicated in human pathogenesis including inflammation and carcinogenesis. EGCG may trap peroxyl radicals and thus break the chain reaction of free radicals and terminate lipid oxidation. An electron paramagnetic resonance (EPR) study on tea catechins indicated that EGCG trapped 6 superoxide anion or hydroxyl radicals, whereas epicatechin (EC) trapped only 2 free radicals (Yang, Sheng, Hou, Zhao, & Xin, 1994). EGCG can also inhibit oxidation by chelating metal ions, such as those of iron and copper, which are catalysts in free radical generation. Antioxidant activity of EGCG depends on many factors, including reducing potential, chelating behaviour, pH, solubility characteristics, bioavailability, and stability in the environment (Luczaj & Skrzydlewska, 2005), which are in turn determined by the structural features of the molecule. Thus, the effect of structure modification on antioxidant activity of EGCG was investigated in this work.
Antiviral activity of EGCG has also been reported. EGCG has been shown to inhibit the maturation, replication, infectivity and function of numerous viruses, including adenovirus, coronavirus, influenza virus, rotavirus, herpes simplex virus (HSV), enterovirus (EV, e.g. coxsackievirus, poliovirus, hepatitis A virus), and human immunodeficiency virus (HIV) (Clark et al., 1998, Friedman, 2007, Ho et al., 2009, Nance et al., 2009, Weber et al., 2003), among others, via multiple mechanisms. Antiviral activities of EGCG are thought to be attributed to its ability to act as an antioxidant, to inhibit enzymes, to suppress viral RNA synthesis, to disrupt cell membranes, to bind to virulent proteins, thus preventing their penetration into the receptor cells, and to trigger the host cell self-defense mechanisms (Friedman, 2007). In this study, the antiviral potential of EGCG and its lipophilic derivatives was evaluated in vitro as inhibitory activity against NS3/4A protease, an important enzyme for the maturation of hepatitis C virus (HCV), and α-glucosidase, the enzyme essential for HIV infectivity.
2. Materials and methods
2.1. Materials
EGCG was supplied by GlaxoSmithKline Consumer Healthcare (Parsippany, NJ, USA). Butyroyl and stearoyl chlorides were purchased from Nu-chek Prep Inc. (Elysian, MN, USA). EPA and DHA were prepared, according to a procedure described by Zhong and Shahidi (2011), from oils provided by Fuso Pharmaceutical Industries Ltd. (Osaka, Japan) and Martek Bioscience Corporation (Columbia, MD, USA), respectively. Randomly methylated cyclodextrin (RMCD) was purchased from Cyclodextrin Technologies Inc. (High Springs, FL, USA). Trolox was purchased from Acros Organics (Fair Lawn, NJ, USA). SensoLyte 520 HCV Protease Assay Kit was purchased from AnaSpec (San Jose, CA, USA). Emelin, acarbose, α-glucosidase (from Bacillus stearothermophilus) and other chemicals were purchased from Sigma–Aldrich Canada Ltd. (Oakville, ON, Canada). All solvents used were obtained from Fisher Scientific Ltd. (Ottawa, ON, Canada). The solvents employed were of HPLC and reagent grade.
2.2. Preparation of EGCG esters
EGCG esters were prepared by acylation of EGCG with different fatty acids, namely SA, EPA and DHA, through their corresponding acyl chlorides, as described elsewhere (Zhong & Shahidi, 2011). Perbutyrated EGCG was prepared using excess butyroyl chloride. The composition of the crude products containing a mixture of EGCG polyesters was determined by HPLC–MS and the predominant EGCG esters were isolated by flash column chromatography. The purified EGCG esters in the purified form (compounds 1–4) were identified by HPLC–MS, 1H NMR and 13C NMR, and subjected to antioxidant and antiviral activity assessment by using in vitro assays.
2.3. ORAC (oxygen radical absorbance capacity)
The ORAC assay for EGCG and its derivatives (compounds 1–3) was carried out using a Fluostar Optima plate reader (BMG Labtech, Durham, NC, USA) equipped with an incubator and two injector pumps. A modified method for lipophilic antioxidants was followed, in which RMCD was used as water solubility enhancer, as described by Huang, Ou, Hampsch-Woodill, Flanagan, and Deemer (2002). Trolox standards (6.25–50 μM) and samples (0.25 μM) were dissolved in acetone/water (1:1, v/v) containing 7% (w/v) RMCD. Fluorescein and AAPH [2,2′-azobis (2-aminopropane) dihydrochloride] were used as the probe and radical generator, respectively. Trolox standard or test compound solutions (20 μl, 7% RMCD used as a control) were added to each well of a 96-well black microplate (Corning Life Sciences, Kennebunk, Maine, USA), followed by 200 μl of fluorescein in phosphate buffer solution (0.11 μM). The plate was incubated at 37 °C for 15 min and the machine was programmed to inject 75 μl of AAPH (17.2 mg/ml in buffer) into each well. The conditions used were as follows: 0.3 s position delay, 8 s orbital shaking before each cycle with 4 mm width, 210 s cycle time, and 25 cycles. Fluorescence was measured at an excitation wavelength of 485 nm and emission of 520 nm. A standard curve was plotted and ORAC values for test compounds were obtained as trolox equivalents (TE).
2.4. Reducing power
The reducing power of EGCG and its derivatives (compounds 1–3) was determined according to Oyaizu (1986). One millilitre of test compounds (1 mM in 95% ethanol) was mixed with 2.5 ml of phosphate buffer solution (PBS, 0.2 M, pH 6.6) and 2.5 ml of a 1% solution of potassium ferricyanide, K3Fe(CN)6. The mixture was incubated at 50 °C for 20 min. Trichloroacetic acid (TCA, 10%, 2.5 ml) was added to the mixture, and the content was centrifuged at 770 × g for 10 min. An aliquot (2.5 ml) of the supernatant was transferred to a tube containing 2.5 ml of distilled water and 0.5 ml of ferric chloride FeCl3 (0.1%). The content was mixed well and the absorbance was recorded at 700 nm. Ascorbic acid (1–6 mM in 95% ethanol) was used as a standard.
2.5. Metal chelation
The metal chelation capacity of EGCG and its derivatives (compounds 1–3) was measured according to Decker and Welch (1990) with some modifications. Ethanolic solutions of test compounds (0.2 ml, 1 mM) were mixed well with 1.74 ml of ethanol and 0.02 ml of ferrous chloride (FeCl2, 2 mM). To the mixture, 0.04 ml of ferrozine (5 mM) was added, and the reaction mixture allowed to stand for 10 min for colour development. The absorbance was then read at 562 nm. A blank without ferrozine was used for each compound, since the antioxidant-Fe2+ complex gives a colour that might interfere with the absorbance readings. Metal chelation capacity was calculated using the following equation:
where, the control is devoid of test compounds, while the blank contained no ferrozine.
2.6. HCV protease inhibitory activity
Inhibitory activity of EGCG and its derivatives (compounds 1–4) against HCV protease was evaluated as an indicator for their antiviral activity. The assay was conducted following the method described by Ma, Wei, Wang, and Hattori (2009) using a SensoLyte 520 HCV Protease Assay Kit. An aliquot (2 μl) of test compounds dissolved in dimethyl sulphoxide (DMSO) was added to each well of a 384-well black assay plate, followed by addition of 8 μl of HCV NS3/4A protease solution (0.5 μg/ml). The reaction was initiated by adding 10 μl of freshly diluted substrate (100 × dilution of a DMSO stocking solution). The mixture was incubated at room temperature for 30 min, and the fluorescence intensity was measured at 485 nm for excitation and 535 nm for emission by a TECAN GENios plate reader. Inhibitory activity of test compounds was calculated as inhibition percentage according to the following equation:
where, F control and F sample represent the fluorescence value of the control without test compounds and of those with added test compounds. All fluorescence values were corrected with a substrate blank. Samples were assayed at different concentrations to plot a concentration versus inhibition percentage curve, and IC50 values were obtained from the curve for each sample. A known HCV protease inhibitor, embelin, was used as a reference.
2.7. α-Glucosidase inhibitory activity
The α-glucosidase inhibitory activity of EGCG and its derivatives (compounds 1–4) was determined following the procedure described by Ma, Hattori, Daneshtalab, and Wang (2008). To each well of a 96-well plate 40 μl of 4-nitrophenyl α-d-glucopyranoside (2 mM, dissolved in 100 mM potassium phosphate buffer, pH 7.0) and 5 μl of sample solution (in DMSO) were added. The reaction was initiated by adding 5 μl of the enzyme solution (0.3 μU/ml α-glucosidase from B. stearothermophilus). The plate was incubated at 37 °C for 20 min, and the absorbance was read, before and after incubation, at 405 nm with an InterMed ImmunoReader (Nippon InterMed K.K., Tokyo, Japan). The absorbance change (ΔA) was recorded and compared between control and samples. The inhibitory activity of test compounds against α-glucosidase was calculated as follow:
where, ΔA control and ΔA sample represent the absorbance change after incubation of the control (DMSO only) and samples, respectively. Acarbose was used as a reference inhibitor for α-glucosidase.
2.8. Statistical analysis
One-way analysis of variance (ANOVA) with pairwise comparisons (Tukey’s HSD) was performed at a P < 0.05 level using Sigmastat for Windows version 2.0 (Jandel Corp., San Rafael, CA, USA) to determine the significant differences.
3. Results and discussion
3.1. Radical scavenging capacity
EGCG derivatives (compounds 1–3) were effective in scavenging DPPH (1,1-diphenyl-2-picrylhydrazyl) radicals (Zhong & Shahidi, 2011). The radical scavenging capacity of EGCG was significantly enhanced by the esterification process and incorporation of the fatty acids, which was attributed to the increased lipophilicity and hence greater accessibility/affinity of the derivatives to the lipophilic DPPH radical than the parent EGCG molecule. In this work, the EGCG derivatives were evaluated for their ability to scavenge the more biologically relevant peroxyl radical, which was measured as oxygen radical absorbance capacity (ORAC).
The ORAC assay utilises a biologically relevant radical source and has been established as a standard method for assessing the activity of hydrophilic antioxidants. In this study, a modified ORAC assay procedure for lipophilic antioxidants, proposed by Huang et al. (2002), was employed; this procedure uses acetone/water as the solvent and RMCD as a solubility enhancer. The results showed that peroxyl radicals generated by AAPH were scavenged by EGCG and its derivatives (compounds 1, 2 and 3) to a greater extent (15–85 folds) than by the reference antioxidant trolox (Fig. 2 ). Compound 4 was not examined for its antioxidant activity as all hydroxyl groups in EGCG were esterified with butyric acid leaving no hydrogen donors available.
Fig. 2.
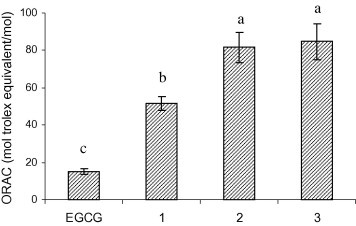
ORAC values of EGCG and its derivatives as trolox equivalents (compounds 1, 2 and 3 are EGCG tetraesters of SA, EPA and DHA, respectively).
The ORAC values of the derivatives were higher than that of the parent EGCG molecule, indicating their greater hydrogen atom donating ability under the test conditions. The decreased number of hydroxyl groups in EGCG derivatives did not appear to negatively affect the antioxidant activity of the test compounds. Wright, Johnson, and Dilabio (2001) stated that the number of hydroxyl groups in EGCG is largely irrelevant to its antioxidant activity, and it is the strategic placing of such groups that does matter. The substituents on the phenyl ring play an essential role in the efficacy of phenolic antioxidant via both electronic and steric effects. Electron donating groups at the ortho and para positions are able to lower the activation energy for hydrogen abstraction and thus enhancing the hydrogen donating ability of the antioxidant (Singh, O’Malley, & Popelier, 2005). In the EGCG derivatives, the O-acylation replaced the strong electron donating hydroxyl group (a combination of inductive and resonance effects) with a weaker electron donating ester group, which would presumably lead to compromised hydrogen donating ability of the molecule. However, the substitution also hinders the formation of the moderately strong hydrogen bonding of the adjacent hydroxyl groups in the EGCG molecule. These intramolecular hydrogen bonds contribute to the stabilisation of the molecule and increased bond dissociation enthalpy (BDE) of the O–H bond, i.e. resistance of the hydrogen atom to dissociation (Wright et al., 2001). Ortho-substitution of EGCG might result in enhanced hydrogen atom donating capacity by reducing intramolecular hydrogen bonds and BDE.
Sterically, the acylation might have altered the three-dimensional conformation of EGCG, which might facilitate hydrogen atom donation, hence increasing its radical scavenging capacity. Conformational characteristics are also important factors that affect the activity of antioxidants. Hakamata et al. (2006) reported that planar catechin (the catechol and chroman structure are constrained to be planar) showed a hydrogen transfer rate that was 5-fold faster than that of the native (+)-catechin. The scavenging activities of catechin against DPPH and AAPH radicals were increased by switching to the planar conformation. In this study, the acylation of hydroxyl groups on the phenyl ring might have led to planar conformation formation or other steric changes that enhance the π-conjugation of the substituents with the phenyl ring and hence the hydrogen donating ability of the molecule. In addition to the conformational attribute, the steric crowding caused by incorporation of high-molecular-weight bulky moieties might contribute to the greater hydrogen donating ability of the EGCG derivatives. Lucarini, Pedrielli, and Pedulli (1996) demonstrated that ortho-substituted phenols had lowered BDE due to the steric repulsion between the substituents and the hydroxyl group, especially when the substituents are bulky.
In summary, the combined electronic and steric factors discussed above might explain the overall improved radical scavenging activity of the EGCG derivatives. Among the derivatives, compounds 2 and 3 containing PUFA showed higher ORAC values than compound 1 which had a saturated alkyl chain. The superior hydrogen atom donating capacity of compounds 2 and 3 may be due to the presence of electron-rich double bonds in the PUFA that may alter the electron distribution on the phenyl rings as well as the bent structures of PUFA chains that may help form more sterically-favoured conformations for facilitating hydrogen atom donation.
3.2. Reducing power
In addition to hydrogen atom donation, antioxidants may also inhibit oxidation through single electron transfer. The antioxidant can deactivate a free radical or reduce an oxidant by donating an electron and forming an antioxidant radical cation, followed by rapid and reversible deprotonation (Wright et al., 2001). The antioxidant radical formed is then stabilised by electron delocalisation (resonance), as in hydrogen atom donation mechanism. Although the net result of electron transfer is the same as the hydrogen atom transfer route, the ability of an antioxidant to donate an electron or a hydrogen atom may vary depending on both intrinsic and extrinsic factors. The ability of an antioxidant to act as an electron donor, or its reducing power, is determined by the ionisation potential (IP) of the compound and is strongly solvent-dependant (Wright et al., 2001).
The reducing power of EGCG and its derivatives was measured as Fe3+–Fe2+ transformation mediated by the test compounds and expressed as ascorbic acid (a known reducing agent) equivalents. The results showed that both EGCG and its derivatives (compounds 1–3) exhibited 2–13 folds greater reducing power than ascorbic acid (Fig. 3 ). EGCG had the highest reducing power among all. Acylation with long chain fatty acids led to a dramatic decrease of reducing power, as found for compounds 1–3. This is not in agreement with the free radical scavenging capacity of the tested compounds (Fig. 2). The poor correlation observed may arise from different mechanisms as well as the reaction environments involved in the two assays. As already mentioned, the electron donating ability of antioxidants is dependent on their IP, which may be altered by structure modification. Substituents on the phenyl ring may change not only the BDE, but also the IP. The presence of electron-donating substituent groups can stabilise the phenoxyl radical cation and hence lowering the IP and enhancing the electron donating capability. Therefore, replacement of the strong electron-donating hydroxyl group with a weaker ester group results in increased IP and decreased reducing power of the antioxidant. As observed in this case, acylated EGCG showed lower reducing power than the original molecule. The opposite trend was observed for ORAC, although phenyl substitution influences the O–H BDE in a similar manner, possibly because the electronic effect of the substituents on hydrogen atom donation was counteracted by other factors, such as hydrogen bonding and steric changes. Moreover, the BDE of a phenolic antioxidant seems to be less sensitive to substitution than the IP. For example, BDE decreases by 1 kcal/mol, while IP decreases by over 8 kcal/mol, when an aminophenol is methylated (Wright et al., 2001).
Fig. 3.
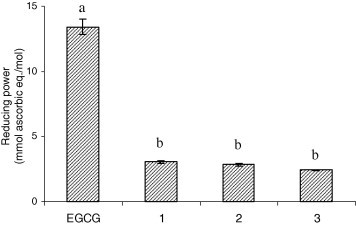
Reducing power of EGCG and its derivatives as ascorbic acid equivalents (compounds 1, 2 and 3 are EGCG tetraesters of SA, EPA and DHA, respectively).
Solubility also affects the effectiveness of antioxidants, especially their reducing power since electron transfer mechanism is strongly solvent dependent due to solvent stabilisation of charged species (Wright et al., 2001). EGCG derivatives with enhanced lipophilicity (Zhong & Shahidi, 2011) have poor solubility in aqueous media and hence compromised activity under the hydrophilic test environment. Moreover, they might form micelles in the assay solution with the B and D rings bearing long chain fatty acid moieties which may be buried in the hydrophobic core of the micelles, leaving only the A ring as the site available for antioxidant action. Similar changes in reducing power caused by acylation have been found for rutin esters (Lue et al., 2010).
3.3. Metal chelation
Metals play important roles in health. For example, iron is essential in oxygen transport, respiration, and activity of many enzymes. However, transition metals are extremely reactive and act as catalysts for lipid oxidation by generating free radicals. They can react with hydrogen or lipid peroxides and produce hydroxyl or peroxyl radicals, as in Fenton reaction. Iron or copper-induced LDL cholesterol oxidation has been implicated in atherosclerosis and other cardiovascular diseases. Metal chelators such as many polyphenols can bind to metal ions and form a stable complex with reduced redox potential, thus suppressing the pro-oxidant effect of metal ions. EGCG is a known metal chelator due to its vicinal trihydroxy structure, in which oxygen atoms act as electron donors to form bonds with the electrophilic metal ions.
In this study, chelation capacity of EGCG and its ester derivatives for ferrous ion was evaluated. All test compounds exhibited ferrous ion chelation activity, ranging from 8% to 35% (Fig. 4 ). The highest chelation activity was found for compound 2, followed by compound 3, indicating that the PUFA esters of EGCG were more potent metal chelators than the parent EGCG and its saturated ester (compound 1) which did not show any significant difference (P > 0.05) from that of EGCG. Metal chelation ability has been related to geometric feature of the chelator-metal complex (e.g. ionic diameter, ring size, conformation, etc.) (Hassan, 1992), and can therefore be positively or negatively influenced by any structural modifications that may lead to geometric alterations of the complex. The greater metal chelation capability found for EGCG–PUFA esters is possibly due to steric changes resulting from the bent structure of the PUFA chain that favours the stability of the antioxidant-Fe2+ complex. The electron-rich double bonds in the PUFA may also contribute to stabilising the metal ion complex.
Fig. 4.
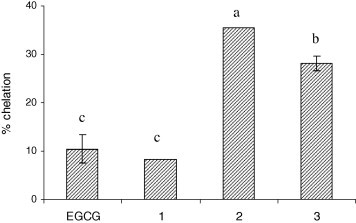
Metal chelation capacity (%) of EGCG and its derivatives (compounds 1, 2 and 3 are EGCG tetraesters of SA, EPA and DHA, respectively).
3.4. HCV protease inhibitory activity
Hepatitis C virus (HCV) infection is a serious health threat globally. More than 170 million people worldwide are chronically infected with HCV (Qiu et al., 2009). Chronic infection with HCV is associated with liver cirrhosis that often leads to hepatic failure and hepatocellular carcinoma (HCC) (Chen & Tan, 2005). Currently approved therapies using interferon and pegylated interferon in combination with ribavirin can achieve only limited virologic response rates in a significant patient population (e.g. those infected with HCV genotype 1) and have been shown to have side effects (Chen & Tan, 2005), necessitating development of new antiviral drugs. The NS3/4A protease, one of the most thoroughly characterised HCV enzymes, has become an important target for anti-HCV treatment. The viral NS3/4A protease mediates the cleavage of the HCV polyprotein to release the functional proteins that are essential for viral propagation. Inhibitors of NS3/4A protease bind to the enzyme and inhibit activation of viral proteins, thus blocking HCV replication in infected host cells (Chen & Njoroge, 2009).
The inhibitory effect of EGCG and its derivatives against HCV NS3/4A protease was examined using an in vitro assay and compared with embelin, a known HCV protease inhibitor. EGCG did not show any significant effect in inhibiting the protease, having a high IC50 value of >200 μM compared to that of the positive control embelin (10.19 μM), as shown in Table 1 . However, distinctive inhibition was observed for its ester derivatives, and compounds 2 and 3 containing omega-3 PUFA displayed a protease inhibitory activity that was 1700-fold stronger than that of embelin. Although less effective than compounds 2 and 3, compound 1 inhibited the NS3/4A protease to approximately 80-fold higher than embelin. The perbutyrated EGCG (compound 4) with all 8 hydroxyl groups occupied with short chain acyl moieties was not expected to possess any antioxidant activity due to absence of the functional OH groups. However, it exhibited a protease inhibitory activity 10-fold stronger than that of embelin. The remarkable improvement of EGCG derivatives in inhibiting HCV protease might arise from the changes in steric features and hydrophilic–lipophilic balance (HLB) of the molecules leading to their superior binding affinity to the enzyme. A study on antiviral activities of theaflavins by molecular modelling revealed that steric and conformational effects govern the infectivity of the virus (Clark et al., 1998). In this study, the ester derivatives of EGCG with fatty acids, especially long chain PUFA with a bent chain structure, may be more sterically favoured than EGCG in binding to the protease and thus inhibiting its function in virus replication.
Table 1.
Inhibitory effect (IC50) of EGCG and its derivatives against HCV protease.a
| Compounds | EGCG | 1 | 2 | 3 | 4 | Embelin |
|---|---|---|---|---|---|---|
| IC50 (μM) | >200 | 0.13 | 0.006 | 0.006 | 0.98 | 10.19 |
| RSD (%) | 5.5 | 1.8 | 3.4 | 3.0 | 4.4 | 9.0 |
RSD: relative standard deviation.
Compounds 1, 2 and 3 are EGCG tetraesters of SA, EPA and DHA, respectively; compound 4 is EGCG-octabutyrate.
3.5. α-Glucosidase inhibitory activity
α-Glucosidases are important hydrolytic enzymes in carbohydrate digestion and vital for biosynthesis of viral envelope glycoproteins. Glycosylation of the viral envelope glycoproteins is essential for infectivity of HIV, and inhibition of α-glucosidase provides a promising strategy for developing novel anti-HIV drugs. Moreover, inhibition of α-glucosidase may also have a therapeutic effect against type II diabetes by interfering with digestion of carbohydrates and delaying glucose absorption.
Catechin and its planar analogues have been investigated for α-glucosidase inhibitory activity (Hakamata et al., 2006). Only a weak inhibitory effect (IC50 >500 μM) was found for (+)-catechin, while the lipophilic planar analogues (alkyl chains incorporated in a heterocycle ring) exhibited strong inhibition (IC50 = 0.7–47.5 μM) against α-glucosidase. Another study on chlorogenic acid derivatives suggested that hydrophobic interactions were involved in α-glucosidase binding and inhibiting activity of these compounds (Ma et al., 2008). In this study, the ester derivatives of EGCG were tested for their α-glucosidase inhibitory activity and compared with the parent EGCG molecule and a positive control acarbose, a known α-glucosidase inhibitor used to reduce postprandial hyperglycaemia. Table 2 presents the IC50 values of all test compounds, among which acarbose was found to be the most potent inhibitor of α-glucosidase. EGCG showed the highest IC50 value (>200 μM) among all tested compounds. The derivatives with enhanced lipophilicity had significantly improved effectiveness in inhibiting α-glucosidase. Compound 1 had the lowest IC50 value, i.e. highest potency as α-glucosidase inhibitor among all derivatives, followed by compound 4. The less lipophilic derivatives, compounds 2 and 3, were less effective. The results suggest that the binding affinity of EGCG and its derivatives to α-glucosidase may be dependent on a combined factor of hydrophobic interaction and steric features, since compound 1 showed a higher inhibitory activity than compound 4 while being less hydrophobic.
Table 2.
Inhibitory effect (IC50) of EGCG and its derivatives against α-glucosidase.a
| Compounds | EGCG | 1 | 2 | 3 | 4 | Acarbose |
|---|---|---|---|---|---|---|
| IC50 (μM) | >200 | 2.62 | 15.66 | 15.87 | 6.87 | 0.15 |
| RSD (%) | 8.0 | 1.9 | 7.0 | 2.7 | 4.7 | 5.3 |
RSD: relative standard deviation.
Compounds 1, 2 and 3 are EGCG tetraesters of SA, EPA and DHA, respectively; compound 4 is EGCG-octabutyrate.
4. Conclusions
Ester derivatives of EGCG with enhanced lipophilicities exhibited higher antioxidant activities than the parent EGCG molecule in scavenging peroxyl radicals and chelation of pro-oxidant metal ions, possibly due to a combined electronic and steric effects. However, reducing power of the derivatives was lower than EGCG, which may be explained by their poor solubility in the aqueous test medium or formation of micelles. The derivatives also showed excellent antiviral activities in inhibiting HCV protease and α-glucosidase, which were not significant for EGCG. The steric features and lipophilicity of the derivatives may be responsible for the increased binding affinity to the enzymes, leading to greater inhibition. These results suggest that lipophilic EGCG derivatives may be used as potential functional ingredients of food, cosmetics, drugs and natural health products for health promoting purposes. However, these findings are based on in vitro chemical assays, and more research on their bioactivities in real food and biological systems is needed for better understanding and utilisation of these derivatives as functional compounds.
Acknowledgements
One of us (F.S.) thanks the Natural Sciences and Engineering Research Council (NSERC) of Canada for financial support and AFMNet (Advanced Foods and Materials Network) for partial funding.
References
- Cabrera C., Artacho R., Giménez R. Beneficial effects of green tea – A review. Journal of the American College Nutrition. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- Chen K.X., Njoroge F.G. A review of HCV protease inhibitors. Current Opinion in Investigational Drugs. 2009;10:821–837. [PubMed] [Google Scholar]
- Chen S.H., Tan S.L. Discovery of small-molecule inhibitors of HCV NS3-4A protease as potential therapeutic agents against HCV infection. Current Medicinal Chemistry. 2005;12:2317–2342. doi: 10.2174/0929867054864769. [DOI] [PubMed] [Google Scholar]
- Clark K.J., Grant P.G., Sarr A.B., Belakere J.R., Swaggerty C.L., Phillips T.D. An in vitro study of theaflavins extracted from black tea to neutralize bovine rotavirus and bovine coronavirus infections. Veterinary Microbiology. 1998;63:147–157. doi: 10.1016/S0378-1135(98)00242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker E.A., Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. Journal of Agricultural and Food Chemistry. 1990;38:674–677. [Google Scholar]
- Friedman M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Molecular Nutrition and Food Research. 2007;51:116–134. doi: 10.1002/mnfr.200600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakamata W., Nakanishi I., Masuda Y., Shimizu T., Higuchi H., Nakamura Y. Planar catechin analogues with alkyl side chains: A potent antioxidant and α-glucosidase inhibitor. Journal of the American Chemical Society. 2006;128:6524–6525. doi: 10.1021/ja057763c. [DOI] [PubMed] [Google Scholar]
- Hassan R.M. Alginate polyelectrolyte lonotropic gels. XIII geometrical aspects for chelation in metal alginate complexes related to their physico-chemical properties. Polymer International. 1992;31:81–86. [Google Scholar]
- Ho H.Y., Cheng M.L., Weng S.F., Leu Y.L., Chiu D.T.Y. Antiviral effect of epigallocatechin gallate on enterovirus 71. Journal of Agricultural and Food Chemistry. 2009;57:6140–6147. doi: 10.1021/jf901128u. [DOI] [PubMed] [Google Scholar]
- Huang D., Ou B., Hampsch-Woodill M., Flanagan J.A., Deemer E.K. Development and validation of oxygen radical absorbance capacity assay for lipophilic antioxidants using randomly methylated β-cyclodextrin as the solubility enhancer. Journal of Agricultural and Food Chemistry. 2002;50:1815–1821. doi: 10.1021/jf0113732. [DOI] [PubMed] [Google Scholar]
- Lucarini M., Pedrielli P., Pedulli G.F. Bond dissociation energies of O–H bonds in substituted phenols from equilibration studies. Journal of Organic Chemistry. 1996;61:9259–9263. [Google Scholar]
- Luczaj W., Skrzydlewska E. Antioxidative properties of black tea. Preventive Medicine. 2005;40:910–918. doi: 10.1016/j.ypmed.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Lue B.M., Nielsen N.S., Jacobsen C., Hellgren L., Guo Z., Xu X. Antioxidant properties of modified rutin esters by DPPH, reducing power, iron chelation and human low density lipoprotein assays. Food Chemistry. 2010;123:221–230. [Google Scholar]
- Ma C.M., Hattori M., Daneshtalab M., Wang L. Chlorogenic acid derivatives with alkyl chains of different lengths and orientations: Potent α-glucosidase inhibitors. Journal of Medicinal Chemistry. 2008;51:6188–6194. doi: 10.1021/jm800621x. [DOI] [PubMed] [Google Scholar]
- Ma C.M., Wei Y., Wang Z.G., Hattori M. Triterpenes from Cynomorium songaricium—Analysis of HCV protease inhibitory activity, quantification, and content change under the influence of heating. Journal of Natural Medicines. 2009;63:9–14. doi: 10.1007/s11418-008-0267-7. [DOI] [PubMed] [Google Scholar]
- McKay D.L., Blumberg J.B. The role of tea in human health: An update. Journal of the American College Nutrition. 2002;21:1–13. doi: 10.1080/07315724.2002.10719187. [DOI] [PubMed] [Google Scholar]
- Nance C.L., Siwak E.B., Shearer W.T. Preclinical development of the green tea catechin, epigallocatechin gallate, as an HIV-1 therapy. Journal of Allergy and Clinical Immunology. 2009;123:459–465. doi: 10.1016/j.jaci.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyaizu M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. Japanese Journal of Nutrition. 1986;44:307–315. [Google Scholar]
- Qiu P., Sanfiorenzo V., Curry S., Guo Z., Liu S., Skelton A. Identification of HCV protease inhibitor resistance mutations by selection pressure-based method. Nucleic Acids Research. 2009;37 doi: 10.1093/nar/gkp251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., O’Malley P.J., Popelier P.L. Mechanistic aspects of hydrogen abstraction for phenolic antioxidants. Electronic structure and topological electron density analysis. Physical Chemistry Chemical Physics. 2005;7:614–619. doi: 10.1039/b415075a. [DOI] [PubMed] [Google Scholar]
- Weber J.M., Ruzindana-Umunyana A., Imbeault L., Sircar S. Inhibition of adenovirus infection and adenain by green tea catechins. Antiviral Research. 2003;58:167–173. doi: 10.1016/s0166-3542(02)00212-7. [DOI] [PubMed] [Google Scholar]
- Wright J.S., Johnson E.R., Dilabio G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. Journal of American Chemical Society. 2001;123:1173–1183. doi: 10.1021/ja002455u. [DOI] [PubMed] [Google Scholar]
- Yang X., Sheng S., Hou J., Zhao B., Xin W. Mechanism of scavenging effects of (−)-epigallocatechin gallate on active oxygen free radicals. Acta Pharmacologica Sinica. 1994;15:350–353. (in Chinese) [PubMed] [Google Scholar]
- Zhong Y., Shahidi F. Lipophilized epigallocatechin gallate (EGCG) derivatives as novel antioxidants. Journal of Agricultural and Food Chemistry. 2011;59:6526–6533. doi: 10.1021/jf201050j. [DOI] [PubMed] [Google Scholar]


