Graphical abstract
Keywords: DNA vaccine, Immune response, Infectious disease, Plasmids, Vaccine
Abstract
Engineering vaccine-based therapeutics for infectious diseases is highly challenging, as trial formulations are often found to be nonspecific, ineffective, thermally or hydrolytically unstable, and/or toxic. Vaccines have greatly improved the therapeutic landscape for treating infectious diseases and have significantly reduced the threat by therapeutic and preventative approaches. Furthermore, the advent of recombinant technologies has greatly facilitated growth within the vaccine realm by mitigating risks such as virulence reversion despite making the production processes more cumbersome. In addition, seroconversion can also be enhanced by recombinant technology through kinetic and nonkinetic approaches, which are discussed herein. Recombinant technologies have greatly improved both amino acid-based vaccines and DNA-based vaccines. A plateau of interest has been reached between 2001 and 2010 for the scientific community with regard to DNA vaccine endeavors. The decrease in interest may likely be attributed to difficulties in improving immunogenic properties associated with DNA vaccines, although there has been research demonstrating improvement and optimization to this end. Despite improvement, to the extent of our knowledge, there are currently no regulatory body-approved DNA vaccines for human use (four vaccines approved for animal use). This article discusses engineering DNA vaccines against infectious diseases while discussing advantages and disadvantages of each, with an emphasis on applications of these DNA vaccines.
Statement of Significance
This review paper summarizes the state of the engineered/recombinant DNA vaccine field, with a scope entailing “Engineering DNA vaccines against infectious diseases”. We endeavor to emphasize recent advances, recapitulating the current state of the field. In addition to discussing DNA therapeutics that have already been clinically translated, this review also examines current research developments, and the challenges thwarting further progression. Our review covers: recombinant DNA-based subunit vaccines; internalization and processing; enhancing immune protection via adjuvants; manufacturing and engineering DNA; the safety, stability and delivery of DNA vaccines or plasmids; controlling gene expression using plasmid engineering and gene circuits; overcoming immunogenic issues; and commercial successes. We hope that this review will inspire further research in DNA vaccine development.
1. Introduction
Infectious diseases are caused by a variety of microoganisms including bacteria and viruses and can often lead to a decrease in the quality of life and increased (risks of) mortality. In 2016, 1.6 million people died due to human immunodeficiency virus (HIV) alone. The infectious disease burden can be delineated using disability-adjusted life years (DALYs). Infectious diseases were listed by the World Health Organization (WHO) as a number of the top contributing issues. With regard to what was infectious disease-related, the DALYs for lower respiratory infectious diseases, HIV/AIDS, and tuberculosis were 1939, 855, and 763 per 100,000 and were ranked as 2nd, 11th, and 12th, respectively, in 2015 [1]. Various therapeutic and preventative interventions have been used to combat infectious diseases. Vaccination in particular has played a critical role in preventing, treating, and even eradicating diseases (i.e., smallpox). Poliomyelitis or polio is another deadly infectious disease that mainly affects children. The inactivated polio vaccine and oral polio vaccine are two types of vaccines that helped reduce polio by 99% worldwide; and now 80% of the population worldwide live in polio-free regions [2], [3]. As of 2016, inactivated polio vaccine immunizations have helped to globally eradicate wild poliovirus type 2 serotype.
Polio vaccines remain effective despite the evolution of the virus because an antigen was selected which generates antibodies against a conserved region. However, for other pathogens with hypervariability, vaccine efficacy may be more limited. DNA vaccines can be used to overcome this challenge, as the engineered DNA can encode specific antigens. DNA recombinant-based subunit vaccines have been known to have enhanced stability, to a degree, for transportation and storage in comparison to traditional protein-based vaccines. There is greater control over the risks of virulence reversion when using DNA vaccines; therefore, DNA vaccines are considered in some aspects to be safer. Because of this, DNA vaccines can have potentially greater utility for immunocompromised patients. However, there are remaining issues that must be resolved for enabling DNA vaccines to be clinically translated for human use. The main remaining issue is the lack of immunogenicity associated with DNA vaccines. The current state of DNA vaccines is discussed within this review article.
More specifically, this review discusses: recombinant DNA subunit vaccines; mechanisms of internalization and processing; enhancing the duration of protection through nonkinetic approaches; manufacturing and engineering DNA; the safety and stability of DNA vaccines; plasmid delivery; controlling gene expression by plasmid engineering and gene circuits; overcoming immunogenic issues of DNA vaccines; examples of successful commercial DNA vaccine; and also reports our perspectives on future directions in the field.
2. Recombinant DNA subunit vaccines
A brief overview of vaccine classifications is found in Table 1 . Extensive reviews have been written containing information regarding vaccine classifications, namely: live attenuated vaccines [4]; polysaccharide vaccines [5], [6]; inactivated vaccines [7]; and DNA vaccines [4], [5], [6], [7], [8]. Fig. 1 shows the number of publications between 1931 and April 2018 for different types of vaccines, which can be an indicator of popularity. We report values based on PubMed results using EndNote between 1931 and 2020, where the projected values, as indicated by an asterisk ‘*’ for 2011–2020, were calculated by multiplying the current values by the ratio 120/87.5 (number of total months within the timeframe of interest divided by the number of months to date). DNA vaccines, in particular, show an increased number of PubMed results when compared to other vaccine types. However, the hype of DNA vaccines is dampened, thereby reaching a plateau, likely owing to difficulties in achieving sufficient immunogenic levels for protection. DNA vaccines, however, can be engineered to have effectively zero risk for virulence reversion.
Table 1.
Generalized qualitative comparisons of vaccine classifications. Please note that a few exceptions may contradict the generalized qualitative comparisons. Details on administration routes, advantages and disadvantages regarding production, immunogenicity, biosafety, transportation/storage, and examples of commercialized DNA- and amino acid-based vaccines for each vaccine classification are tabulated. The ‘+’ and ‘−’ denote general pros and cons.
| Live attenuated vaccines | Killed or Inactivated vaccines | Inactivated toxoid vaccines | Polysaccharide vaccines | Conjugate polysaccharide vaccines | Recombinant protein vaccines | DNA vaccines | |
|---|---|---|---|---|---|---|---|
| Further Classification | Whole-cell vaccines | Whole-cell vaccines | – | Subunit vaccines | Subunit vaccines | Subunit vaccines | Subunit vaccines |
| Administration | i.e., Subcutaneous, percutaneous, oral, intranasal | i.e., Intramuscular, intradermal, subcutaneous | i.e., Intramuscular | i.e., Intramuscular, subcutaneous | i.e., Intramuscular | i.e., Intramuscular, Mucosal |
|
| Production | − Bacteria applications + Virus culture + Large-scale production − Biocontainment |
+ Low-cost production + Large-scale production |
− Toxin purification procedures − High-cost production |
+ Bacterial capsule components − Biocontainment |
− Protein folding − Protein degradation + Encapsulation |
+ Molecular stability + Low-cost production + Encapsulation |
|
| Immunogenicity | + Natural infection + Immunization efficiency + Single or few doses + Life-long protection |
+ Natural infection − Multiple doses − Adjuvant use + Long-term protection |
− Multiple doses − Adjuvant use |
+ Molecular pathogen − Multiple doses − Adjuvant use |
+ Stimulus by Conjugation − Multiple doses − Adjuvant use |
+ Particle design − Multiple doses − Adjuvant use |
+ Animal applications − Human applications + Particle design |
| Biosafety | − Risk of infection − Risk of reversion − Risk for the immuno-incompetent |
+ Safer than LAVs | + Fewer side effects (i.e., local and systemic) |
+ No live pathogenic components + Fewer side effects |
+ No live components + Fewer side effects − Autoimmune response − Integration to genome − Indel mutations |
||
| Storage and Transportation | − Cold chain delivery | − Cold chain delivery | + Less need for cold chain delivery | + Less need for cold chain delivery | + Less need for cold chain delivery | + Less need for cold chain delivery | + Less need for cold chain delivery |
| Licensed vaccines (trade names) | M-M-R II, OPV, FluMist, Rotarix, ProQuad, Adenovirus, Zostavax | Pediarix, Ipol, BioThrax, Infanrix, Daptacel, Gardasil, Cervarix, Flublok | Decavac, Tenivac | Menomune, Pneumovax 23 | PedvaxHIB, Hiberix, Comvax, Prevnar 13 | Engerix-B, Recombivax HB | – |
Fig. 1.
Research trends of various vaccine types. Values are based on PubMed results (which could be an indicator for popularity) using EndNote between 1931 and 2020, where the projected values, as indicated by an asterisk (‘*’) for 2011–2020, were calculated by multiplying the current values by the ratio 120/87.5. The total number of months is 120, and the number of months to date within the time period of interest (Jan 2011–April 2018) is 87.5. The following keywords were entered into the “title” form in EndNote when searching for PubMed research articles: subunit vaccine, live attenuated vaccine, toxoid vaccine, inactivated vaccine, polysaccharide vaccine, recombinant vaccine, and DNA vaccine. The total number of results would be different, however, if the abstract or other keywords had been queried.
For recombinant DNA subunit vaccines, a replicating vector, typically yeast or bacteria, is transformed with an engineered DNA sequence for transcription and translation and can then be administered to the patient. For instance, the hepatitis B DNA vaccine is based on different domains of the hepatitis B virus’ surface antigen (HBsAg) and is often administered with an adjuvant to improve immunogenicity [9], [10]. DNA vaccines encode for antigens that then mainly elicit humoral immune response similar to that of natural infection [11]. Hepatitis B DNA vaccines are often administered using liposomal or cationic carriers.
DNA and protein vaccines share a major part of the production process but vary in the mechanisms elicited by the immune system. The first step consists of inserting the gene of interest (GOI) into the plasmid vector, followed by transformation into replicating bacteria or yeast. After the replication, the recombinant protein is extracted, purified, and prepared as a vaccine. In the case of DNA vaccines, the replicating vector is usually disrupted and the engineered DNA is extracted and purified to produce the final vaccine. After administration to the patient, recombinant protein vaccines are internalized within the host cells and processed to antigenic peptides, which elicit CD8+ T cell responses. The DNA vaccine must achieve being internalized by the nucleus to use the host molecular machinery to promote the transient expression of the GOI. After the transcription and translation of the GOI, it is processed to antigenic peptides, and these antigenic peptides elicit immune responses.
In the case of a DNA vaccine, the engineered DNA can be administered to the patient with or without (i.e., gene gun) a carrier. Generally, nonviral gene delivery results in transient gene expression (TGE) of the antigen of interest. DNA vaccines were introduced in the late 90s, when Wolff, et al. [12] intramuscularly injected plasmid DNA (pDNA) in mice. Subsequently, Jiao, et al. [13] proposed the delivery of DNA plasmid to nonhuman primates, and Ulmer, et al. [14] described a vaccine against influenza using pDNA in mice. The following sections provide in-depth information about DNA vaccines: manufacturing and engineering DNA vaccines; safety and stability of DNA vaccines; kinetic control for DNA vaccine release; barriers for the delivery of pDNA vaccines; active nuclear transport for the nuclear uptake of DNA; controlling gene expression through plasmid engineering and gene circuits; techniques to overcome immunogenic issues of DNA vaccines; and the physical methods for DNA vaccine delivery in preclinical and clinical trials.
Pros: DNA vaccines have low production cost when compared to protein vaccines and enhanced stability for transportation and storage and can be administered to immunocompromised patients. Cons: low immunogenicity and may require multiple booster doses.
3. Mechanisms of internalization and processing
Fig. 2, Fig. 3 delineate how the plasmid and the amino acid-based vaccine mechanisms of internalization and processing vary, in addition to the antigen presentation, respectively. Please note that in Fig. 2, certain cellular processes shown are generally for somatic cells (i.e., plasmid delivery), while other processes are for antigen presenting cells (APCs) (i.e., MHC I/II-peptide display). For example, the translated product within a somatic cell from a plasmid could transport extracellularly and then be uptaken by an APC. A vaccine is processed by three main pathways: direct somatic cell engulfment/APC display (Fig. 2(1, 5, 3), thus leading to MHC II-peptide display); phagocytosis of a transfected (nonviral) or transformed (viral) cell/cross-priming (Fig. 2(1, 4, 6), thus leading to MHC I-peptide display); and Toll-like receptor (TLR) activation (Fig. 2(2, 7)). A vaccine may be taken up intracellularly by endocytosis within APCs (Fig. 2(1)) [15] or interact extracellularly by the TLR2/4 pathway, thereby resulting in the expression of proinflammatory cytokines and chemokines (interleukin (IL)-8/10, TNFα) (Fig. 2(2)). When nonviral gene delivery systems are used to deliver DNA vaccines, the proton sponge effect plays an important role for the pDNA to escape the endolysosomal degradation pathway (Fig. 2(3)). pDNA can be either randomly encapsulated by the nuclear envelope reforming postmitosis or actively transported to the nucleus through nuclear localization signals (i.e., DNA-targeted sequences – SV40 [16]). DNA vaccines may contain unmethylated CpG sequences that function as a pathogen-associated molecular pattern (PAMP) and can activate TLR9 in the endosome, eventually releasing IL-4 and interferon (IFN)α (Fig. 2(4)).
Fig. 2.
DNA- and amino acid-based antigen internalization and processing. Please note that certain cellular processes shown are generally for somatic cells, while others are for antigen presenting cells (APCs). (1) endocytosis; (2) extracellular activation through TLR2/4 resulting in the expression of proinflammatory cytokines and chemokines (IL-8/10, TNFα); (3) proton sponge effect for DNA vaccines (4) CpG sequences activating TLR9 and resulting in IL-4 and IFNα release; (5) cytosolic degradation pathway resulting in (6) MHC II antigen presentation to CD4+ T cells; (7) proteasome degradation pathway and the eventual MHC I antigen presentation to CD8+ T cells. Direct somatic cell engulfment/APC display entails (1, 5, 3), leading to MHC II-peptide display. Steps for phagocytosis of a transfected (nonviral) or transformed (viral) cell/cross-priming entails (1, 4, 6), leading to MHC 1-peptide display. Steps for Toll-like receptor (TLR) activation entails (2, 7). (APC, antigen-presenting cell; CCR5, C–C chemokine receptor type 5; CpG DNA, DNA fragment containing cytosine nucleotide followed by guanine nucleotide; ER, endoplasmic reticulum; IFN, interferon; IL, interleukin; MHC, major histocompatibility complex; TNF, tumor necrosis factor; TLR, Toll-like receptor; VLP, virus-like particle).
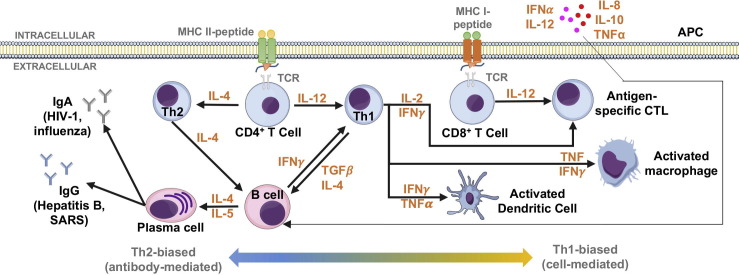
Fig. 3.
DNA- and amino acid-based antigen presentation. Th2-biased (antibody-mediated) and Th1-biased (cell-mediated) responses, depending on the context of antigen presentation. [197] (APC, antigen-presenting cell; CTL, cytotoxic T lymphocyte; IFN, interferon; Ig, immunoglobulin; IL, interleukin; MHC, major histocompatibility complex; TCR, T cell receptor; Th, T helper cell; TNF, tumor necrosis factor).
4. Enhancing the duration of immune protection using adjuvants
Enhancing the duration of immune protection by nonkinetic approaches can be achieved by (1) molecular immunostimulants (e.g., nucleic acids, polysaccharides, proteins, and polymers) and (2) advanced vaccine particles (e.g., nanoparticles, microparticles, and virus-like particles) (Table 2 ) [17], [18].
Table 2.
Examples of adjuvants [20], [19], [21], [22], [144], [145], [146], [162], [163], [164], [165], [166], [167], [168], [169], [170], [171], [172], [173], [174], [175], [176], [177], [178], [179], [180], [181], [182], [183], [184], [185], [186], [187], [188], [189], [190], [191], [192], [193], [194], [195], [196].
| Adjuvant | Composition | Disease | Vaccine Type (comercial name) | Immune response | Research stage | Year |
|---|---|---|---|---|---|---|
| Alum | Alum, 3-O-desacyl-4′-monophosphoryl lipid A (AS04®) (GSK MPL®) | Influenza A | Virus-like particle (M2eVLP) | Improved cross-protection | Murine | 2014 |
| Human Papillomavirus | Protein vaccine (HPV-16 L1/HPV-18 L1) [GSK Cervarix®] | Enhanced antibody response | Clinical study (girls 9–14 years) | 2015 | ||
| Toxoplasmosis | DNA vaccine (toxifilin gene) | Enhanced humoral response and switched from Th2 to Th1 | Murine | 2016 | ||
| Hepatitis B | Protein vaccine (HBsAg) [GSK FENDrix®] | Enhanced humoral and cell-mediated immune reponses | Clinical study (renal-transplanted patients) | 2017 | ||
| Squalene oil-in-water emulsions | Squalene, MONTANE 80, Eumulgin B1 PH (AF03®) | Influenza | Protein vaccine (haemagluttinin) | Enhanced humoral immune response | Murine | 2014 |
| Squalene, MPL®, Saponin (QS21®) (AS02®) | Pneumonia | Protein vaccine (PhtD) | Increased frequency of CD4+ T cells and memory B cells | Phase I | 2015 | |
| Squalene, Tween 80, sorbitan triolate (MF59®) | HIV-1C | Protein vaccine (gp140) | Enhanced humoral and cell-mediated immune reponses | Phase I | 2016 | |
| Squalene, glycerol, egg phosphatidylcholine, poloxamer, ammonium phosphate buffer (Stable Emulsion, SE) | Influenza A/H5 | Protein vaccine (rHA) [Panblock®] | Met criterion for seroconversion rate | Phase II | 2017 | |
| Squalene, Tween 80, α-tocopherol (AS03®) | Influenza A/H5N1 | Inactivated virus vaccine (SV) | Increased levels of IL-6 and IL-10 within 24 h after vaccination | Phase I | 2017 | |
| Liposomes | MPL®, saponin (QS21®) (AS01®) | Malaria | Protein vaccine (RTS,S) [Mosquirix®] | Immunoprotection for 3–4 year infants, enhanced efficiency with booster | Phase III | 2015 |
| Varicella zoster virus | Subunit vaccine (HZ/su) [GSK Shingrix®] | Significant immune protection in adults ≥50 years | Phase III | 2015 | ||
| DDA®, TDB® (CAF01®) | Tuberculosis | Subunit vaccine (H56/CAF01®) | Longlasting immunoprotection and enhanced CD4+ T cell response | Phase I | 2016 | |
| TLR ligand | GLA-AF (TRL4 ligand) | HIV clade C | DNA vaccine/Attenuated vaccine/Protein vaccine (HIV env/gag-pol-nef, MVA-C, HIV CN54dp140) | Enhanced T and B cell immune responses | Murine | 2014 |
| dsRNA analog Poly(I:C) (TRL3 ligand) | Seasonal influenza | Inactivated vaccine (TIV) | Enhanced humoral response | Murine | 2014 | |
| Human Papillomavirus | Subunit vaccine (HIV-1 gp140) | Longlasting IgG/IgA response | Murine | 2016 | ||
| CpG 1018 (TLR9 ligand) (Dynavax HEPLISAV-B®) | Hepatitis B | Protein vaccine (HBsAg) [Dynavax HEPLISAV-B®] | Enhanced superior seroprotection | Phase III | 2015 | |
| CpG 7909 (TLR9 ligand) | Meningococcal infection | Subunit vaccine (dLPS/OMP) | Early and increased IgG/IgM response | Phase I | 2015 | |
| Cationic antimicrobial polypeptide/IC31® (TLR9 ligand) | Tuberculosis | Protein vaccine (H4:IC31®) | High frequency CD4+ T cells and longlasting memory response | Phase I | 2015 | |
| Alum-absorbed GLA/SLA (TRL4 ligand) | Malaria | Protein vaccine (GMZ2.6C) | Enhanced parasite-specific antibody and induced Th1 response | Murine | 2016 | |
| Lipopolypeptide Pam2/Pam3 (TLR2/6, TLR2/1 ligand) | Parasitic helminths | Autoclaved vaccine (ALM + Pam2/Pam3) | Th2 polarization | Murine, parasite | 2016 | |
| dsRNA analog Poly(I:C) (TRL3 ligand) | Cancer | Protein vaccine (Db126 WT-1) | Infiltration of CD8+ T cells in tumor | Murine | 2016 | |
| Human Papillomavirus | Subunit vaccine (HIV-1 gp140/HSV-2 gD) | Longlasting IgG/IgA response | Murine | 2016 | ||
| Alum-absorbed GLA/SLA (TRL4 ligand) | Malaria | Protein vaccine (GMZ2.6C) | Enhanced parasite-specific antibody and induced Th1 response | Murine | 2016 | |
| Alum-absorbed SMIP7.10 (TRL7 ligand) | Meningococcal infection | Conjugated polysaccharide vaccine (MenC-CRM197) | Th1 polarization | Murine | 2016 | |
| Imidazoquinolines (TLR7/TLR8 ligand) | Various | Various | Th1 polarization | Clinical study (newborn) | 2016 | |
| Polysaccharide | Inulin (Advax®) | Hepatitis B | Protein vaccine (HBsAg) | Enhanced humoral and cell-mediated immune reponses | Phase I | 2014 |
| Seasonal influenza | Inactivated vaccine (TIV) | Low-dose TIV/Advax® induced efficient immune response | Phase I | 2016 | ||
| Chitosan | Tetanus | Toxoid vaccine (TT) | Enhanced mucosal immune response | – | 2014 | |
| Influenza | Subunit vaccine (HA-split) | Enhanced humoral and cell-mediated immune reponses | – | 2014 | ||
| Various | Various | Th1 polarization | – | 2015 | ||
| Genetic Adjuvant | IL-12/IL-15 plasmid | HIV-1 | DNA vaccine | No enhanced immune response, tolerable adjuvanted-vaccine | Phase I | 2012 |
| IL-12 plasmid | DNA vaccine | Induction of CD4+/CD8+ T cell response | Phase I | 2013 | ||
| NF-κB subunit p65/RelA, Type-1 transactivator T-bet | DNA vaccine (HIV pGag, pEnv) | Enhanced T, B cell, and antibody responses | Murine | 2014 | ||
| PPE44/pCI-OVA | Tuberculosis | Live attenuated vaccine/pDNA (BCG) | Enhanced T and B cell immune responses | Murine | 2014 | |
| Cholera Toxin Subunit A | Cholera | DNA vaccine (HIV-1 Tat-Rev-Vif-Integrase-Nef) | Upregulation of IL-6, IL-1β | Murine | 2014 | |
| C-terminal Hsp70 | Infectious bursal disease (chicken) | DNA vaccine (VP2 gene of IBDV) | Enhanced humoral and cell-mediated immune reponses | Chicken | 2015 | |
| GM-CSF | Cancer | DNA vaccine (MUC1-VEGFR2) | Inhibition of tumorigenic cell growth | Murine | 2016 | |
4.1. Biopolymers
Biopolymers (i.e., polysaccharides and chitosan) are widely used for medical applications and have been reported to have adjuvant action in specific applications. For example, polysaccharide-based adjuvants can also improve the immunogenicity of vaccines. Chitosan is a known mucoadhesive and may exhibit important roles in mucosal immunization [82]. Sawaengsak, et al. [19] reported nanoparticles of chitosan combined with tripolyphosphate and hemagglutinin (HA) induced a two-fold increase in the number of splenocytes that secrete IFNγ in mice infected with the influenza virus when compared with a nonadjuvanted HA vaccine [20]. Gordon, et al. [21], [22] reported that inulin-based adjuvants (Advax®) elicit efficient immune responses against hepatitis B (Phase I). At least a fourfold increase in seroprotection and CD4+ T-cell responsiveness was observed for subjects immunized with Advax® when compared to HBsAg alone. However, biopolymers have difficulties to control drug release owing to their solubility in water. For example, a report comparing pectin content demonstrated that higher pectin content resulted in increased erosion, thus leading to faster drug release; on the basis of the type of application, this concept can be an advantage or disadvantage depending on the length scale of drug release desired [23]. The reported pectin/amylose formulations released ∼50%–100% of the drug within a 4 h timeframe in a gastrointestinal context.
4.2. Polymers
Relatively nontoxic and degradable (i.e., through hydrolysis of esters or amidolysis of amides) polymers such as poly(anhydride) (PA), poly-N-isopropyl acrylamide (PNIPAAm), and PLGA have found applications in vaccines. Tamayo, et al. [24] reported the recognition of PA nanoparticles by TLR2/4/5, thereby eliciting CD8+ T cell responses and Th1 polarization (for further information regarding Th1 polarization, refer to Fig. 3) in mice. Wafa, et al. [25] showed that the polymeric structure affects the intensity of the CD8+ T cell response and increased the protective duration. PA nanoparticle-based vaccines were recently employed for immunizing against swine influenza within pigs [26] and against H5N1 influenza [27]. Shakya, et al. [28] discussed the use of PNIPAAm combined with collagen type II as a polymeric adjuvant to reduce the risk of triggering autoimmune responses.
Several studies describe the enhanced immunogenicity due to the adjuvant properties of PLGA in comparison to nonadjuvanted antigen priming for subunit vaccines (recommended review article [29]). The size of PLGA particles (i.e., nano- or microparticle), as well as the monomeric L:G ratio played a crucial role in modulating immunogenicity [30]. Li, et al. [31] described an increase in Th1 cytokine expression (IFNγ and IL-17) to their pertussis toxoid vaccine by employing dual-range-sized particles ranging from 200 to 300 nm and 5 µm with a monomeric ratio of 50:50. Another example is that of Cruz, et al. [32], who reported an increased T-cell response by employing PLGA nanoparticles (50:50 L:G) for encapsulating and delivering a protein vaccine.
4.3. Molecular adjuvants for DNA vaccines
Ligands against TLRs, namely, TLR-3 and TLR-9, have been shown to improve immune response [33]. Cytokines such as interleukin (IL)-2, IL-12, and IL-15 encoded in plasmids have also shown to enhance immunogenicity [34], [35]. Immune signaling molecules like TRIF and HMGB1 can also be encoded by the plasmid and have been successfully used as adjuvants along with DNA vaccines [36]. Genetic knockdown of genes, like PD-ligand 1 known to counteract DNA vaccines, using shRNA or siRNA has also shown to be effective adjuvants for DNA vaccines [37]. For further information with regard to adjuvants that are specifically used along with DNA vaccines, please refer to the following extensive review articles by Li et al: [38] and by Saade F. and Petrovsky N. [36].
(For sections 4.1, 4.2, 4.3) Pros: Similar to kinetic approaches, nonkinetic methods can also be used to achieve long-term and enhanced immune responses. Cons: adjuvants that are commonly used including alum and calcium salts can cause severe inflammation and are not compatible with, or advantageous for, certain antigens [39] .
5. Manufacturing and engineering DNA vaccines
With continual improvements in DNA sequencing technology, we can better understand key conserved sequences that can be transcribed and translated into antigens and discover or engineer sequences that may be used for protection (i.e., TSOL18 [40], [41], [42]). Nucleic acid code can be mass produced by DNA recombinant technology, and these codes can be directly delivered and subsequently processed and displayed by APCs for eventual seroconversion by the patient’s own transcription and translation machinery. Furthermore, peptides or proteins or subunits thereof can be mass produced in cells (i.e., E. coli and yeast) by DNA recombinant technology for manufacturing DNA vaccine.
Engineering and manufacturing DNA vaccines involve multidisciplinary fields such as molecular biology/biochemistry, separation techniques, and material science. A GOI can be inserted into a self-replicating organism (bacteria or yeast). A circular pDNA is usually used as the DNA cloning vector, which can then be extracted from the replicating organism and chemically purified [43]. The pDNA can be engineered to be expressed in bacteria (i.e., Escherichia coli (E. coli)) [44] or yeast (i.e., Pichia pastoris) [45], [46] for amplification purposes in bioreactors and can also be expressed in mammalian cells for a therapeutic purpose. The major components involved within an engineered DNA plasmid are the origin of replication, promoter, GOI, and commonly an antibiotic resistance gene for selection [47]. As for E. coli-based plasmid production, the composition of the culture media can vary from mineral-containing media to complex mixtures supplemented with yeast extracts. After replication in the bioreactor, bacteria can undergo a lysing process through alkaline or thermal treatments, followed by plasmid purification through precipitation/centrifugation [48], [49]. This large-scale pDNA production is cost-effective in comparison to LAV and IV manufacturing processes, possibly making the technology cheaper in the long run for consumers. The relatively recent development of minicircles (episomal DNA)/miniplasmids (i.e., P1, P7, and F)/ministrings [50], [51], [52], [53], [54], [55] can potentially be transported more easily through cellular barriers to improve bioavailability [51].
For the sake of brevity, a discussion of downstream processing was omitted. Please refer to the following articles for further downstream processing information: [56], [57], [58].
6. Safety and stability of DNA vaccines
To date, there is no licensed DNA vaccine for use in humans [59], [60]. The scientific community has high expectations for DNA vaccine use because of the following reasons: (1) the ability to control the transcribed and translated product, thereby controlling the ability of the antigen to not revert to a virulent pathogenic form; (2) the relatively low production cost of DNA vaccines; and (3) the innate stability of DNA vaccines for storage and transportation [61], [62] purposes.
6.1. Auto-immunity
A potential disadvantage of DNA vaccines is that they could potentially trigger autoimmune diseases by eliciting anti-DNA antibody production, owing to the use of prokaryotic DNA vectors. Such vectors may contain unmethylated CpG sequences and may be recognized as a PAMP by the immune system (i.e., TLR9) [13], [63] and can result in Th1-biased immune responses [64], [65]. In 1989, Gilkeson, et al. [66] associated prokaryotic double-stranded DNA (dsDNA) immunization with the development of anti-dsDNA antibodies in mice. In 1994, Lilic, et al. [67] described the induction of anti-DNA antibodies after the administration of a subunit vaccine against hepatitis B virus (HBV), and recently, Zafrir, et al. [68] reported that the HBV vaccine induced an autoimmune syndrome when used with the required adjuvants. On the other hand, other studies suggested no direct effect of vaccinations triggering autoimmune diseases [69].
6.2. Insertional mutagenesis of viral and nonviral delivery methods
DNA vaccines may cause indel mutations, the risks of which depend on the mechanism of delivery. Despite nonviral gene delivery systems being safer than viral vectors, risks of insertional mutagenesis are not zero, although the risks are believed to be lower than those of everyday random mutations. Unfortunately, often such nonviral risks are based on the insertion of a functional fluorescence-based marker where the entire functional sequence must be inserted not only within the genome but also into a region that is expressed. Nonviral risks of insertional mutagenesis may be higher than that currently believed. Such risks are also a function of the sequence and whether the DNA is supercoiled.
The administration of a DNA vaccine exposes the patient to foreign DNA or its fragments that could be inserted into the host’s chromosomal DNA [70]. In the case of incorporation into an exon, an insertional mutation or a frameshift mutation occurs. Such mutations can cause a gene to malfunction or inactivate (i.e., a tumor suppressor gene). The insertion of foreign genes into the host genome could also lead to constituent expression of previously silent bacterial/parasite genes that have been inserted. The insertion may also increase the probability of rearrangements or breaking. The guidelines for industrial production of DNA vaccines published by the FDA recommend that the pDNA is supercoiled to more than 80% to help prevent insertional mutagenesis. It has been reported that the FDA recommends integration studies, regardless of the delivery method, if there are greater than 10,000 copies of foreign DNA per microgram of host DNA [71]. Interestingly, minicircle DNA [51], [53], [54], [72], [73] and ministring DNA [74], [75] have been reported to have less risk of insertional mutagenesis because the major bacterial DNA (i.e., the unmethylated CpG repeats functioning as PAMPs) is removed.
6.3. Environmental stability (nonbiological)
DNA vaccines have an advantage of not requiring such rigorous temperature control as the protein-based vaccines require. However, the stability of nucleic acid-based vaccines under dry conditions (i.e., storage and transportation) or under aqueous conditions is highly dependent on stabilization techniques. Crine, et al. [76] reported the stability of apurinic sequence sites to remain undamaged for approximately 288–335 h at 37 °C, pH 7.21, and in a buffer solution that is similar to physiological media. Karni, et al. [77] investigated the thermal degradation rates of pDNA (0.75 µg of pUC19) and found that there was complete degradation after 9 min at 170 °C in an aqueous solution. When the pDNA was incubated at 200 °C under aqueous conditions, no intact pDNA could be found after 20 s. In the case of dried pDNA, the degradation temperature was reported to have a low threshold. They incubated pDNA and pUC19 at 10 °C increments for 5 min and found that pDNA started degrading at 130 °C and completely degraded at approximately 190 °C (in comparison to degradation at 95 °C). In addition, prokaryotic DNA was reported to be more thermally stable than eukaryotic DNA [78].
6.4. Biologically relevant stability
The stability of nucleic acids in the physiological environment depends on specific conditions at the intracellular, tissue, and systemic levels. In an in vivo environment, there is variation in pH (∼5–7.45), exposure to various enzymes (i.e., nucleases) and reactive oxygen species, as well as the possibility of being epigenetically modified or modified by host DNA repair mechanisms [79].
The development of delivery systems for nucleic acids (e.g., polymeric and lipid vectors), which control the microenvironment of the nucleic acid, helps to maintain the molecular stability, as there is a decrease in the degradation rate when present in a complexed state (i.e., polyplex and lipoplex) versus a free state [80]. For example, the in vivo half-life for a nonionically complexed DNA is approximately 10 min in the extracellular environment, whereas the intracellular half-life is longer, close to 1–4 h. Despite the advantage in being in a complexed state in terms of its structural stability, if the DNA is excessively complexed, then successful transcription will not be as efficient, as the plasmid will not be accessible to the transcription machinery [81], [82].
Evans, et al. [83] described the relevance of controlling the main mechanisms of DNA degradation (e.g., depurination/β-elimination and free radical oxidation by employing chelators and hydroxyl radical scavengers). The use of ethanol and EDTA (ethylenediaminetetraacetic acid) or DTPA (diethylenetriaminepentaacetic acid) in DNA-based formulations is associated with enhancing the remaining DNA amount from approximately 30% to 70% after 1 month incubation at 50 °C.
Peptide nucleic acids (PNAs) are uncharged molecules that demonstrate increased resistance against nucleases and RNases in comparison to endogenous nucleic acid, which can have benefits for specific gene delivery and DNA vaccine applications. The conjugation of PNAs with cell-penetrating peptides (CPPs) can enhance the molecular stability and facilitate transfection [84]. In addition to being highly stable, CPP-conjugated PNAs have improved DNA sequence recognition and a higher affinity with lipid membranes because of the peptide backbone [85].
Epigenetic modifications affect the molecular stability and the genetic expression of DNA. Modifications such as DNA methylation are more likely to occur in CpG-rich regions [86], and the methylated regions may subsequently recruit DNA methylated-binding proteins and histone deacetylases (HDACs), which downregulate gene expression [87], as the transcriptional machinery cannot access the DNA.
7. Plasmid delivery
At a systemic level, eliciting specific antibody subtype responses depends on the contexts and routes of the delivery methods. For instance, a vaccine containing a Lactobacillus vector against the influenza A virus induces a higher survival rate in a murine model when administered through the intranasal route than through the oral route [88]. IgA titers were measured for an anti-HIV DNA vaccine, and these titers were found to be increased for the intranasal route in comparison to the intramuscular (IM) route after DNA vaccination [89]. Vaccines administered through the IM route can induce greater IgG2a titers, whereas a gene gun method can elicit a greater IgG1 antibody response [90].
At a cellular level, the transportation of highly charged macromolecules such as DNA across a negatively charged phospholipid bilayer membrane and subsequently through the highly restrictive nuclear envelope is challenging. More specifically, the main barriers for the delivery of pDNA vaccine are as follows: stability (described in the previous section), cellular uptake, endolysosomal escape, decomplexation from the carrier, and nuclear envelope translocation. The cellular uptake of nonviral gene delivery carriers can be uptaken through clathrin- or caveolae-mediated endocytosis, or macropinocytosis (endocytic pathways) [91], [92]. Plasmids can be internalized within the nucleus through intranuclear injection, direct/indirect nuclear localization signals (i.e., DNA-targeted sequences), and encapsulation by the nuclear envelope upon reformation postmitosis [93].
7.1. Physical methods for DNA vaccine delivery
7.1.1. Electroporation
Electroporation (EP) causes transient pores in the plasma membrane of host cells to increase the uptake kinetics of pDNA under an electrical field. In a recent clinical trial, a DNA vaccine was delivered by IM-EP using Ichor Medical Systems TriGrid™ Delivery system (TDS-IM) [94]. The results demonstrated that the DNA vaccines delivered by EP are safe and effective for eliciting strong immune responses.
7.1.2. Gene gun
Another physical method developed to deliver plasmids epidermally is the gene gun [95]. A particle-mediated epidermal delivery (PMED) gene gun was used in preclinical trials to deliver a DNA vaccine against the dengue virus in nonhuman primates. Plasmids, including the oligonucleotide sequence of the vaccine antigen, were precipitated onto 1 µm-diameter gold beads. The DNA-adsorbed gold beads were then delivered using a gas-pressurized gene gun, which is a needle-free device [96]. The difference in PMED in terms of IM and intradermal injections using a needle and syringe is that PMED enables direct delivery of the vaccine into the intracellular environment more efficiently, thereby improving the cellular uptake and resulting in higher immune responses with substantially lower doses (100- to 1000-fold) of DNA [97]. Choi, et al. [98] compared three different delivery methods (IM, intradermal, and epidermal inoculation) using plasmid-coated gold beads through particle-pressurized bombardment (i.e., gene gun). The intradermal injection and gene gun resulted in specific IgG antibody responses but not IgA. Despite the induction of IgG responses, both the gene gun and intradermal administration methods failed to protect mice from a rotavirus infection in that particular study.
Other physical methods have included physically puncturing the cells by using microneedles (impalefection) [99], hydrostatic pressure, squeezing cells in a microfluidic chamber (in addition to EP) [100], sonoporation [101], and intranasal delivery [38]. For interested readers regarding physical methods for intracellular delivery, we would recommend the publications from Meacham, et al [102], [103].
(For sub-sections 7.1.1, 7.1.2) Pros: EP and gene gun are safe to use for delivering DNA and have shown immune responses in specific applications. EP requires less DNA without compromising efficacy. Cons: EP is often invasive and limited for in vivo applications and may lead to cell death. The gene gun method has limited or no control over the insertion sites of the DNA and is relatively expensive [104] .
7.2. Nonviral methods (lipid- and polymer-based)
Lipids or cationic, amine-containing polymers are often complexed with anionic plasmid macromolecules into nanosized particles, and this facilitates cellular uptake and inhibits the degradation of the plasmid during trafficking both extracellularly and intracellularly. In an uncomplexed state, half-lives of plasmids extracellularly in serum and intracellularly is in the order of a few minutes and hours, respectively. The mass of free polymer carrier in the solution is critical for the delivery efficiency and is typically administered in manyfold excess for transfection to occur. Owing to the need for free polymer to be in excess, despite taking advantage of the enhanced permeability and retention effect for the larger polyplexes, the free polymer is not able to sufficiently transport to the site of interest [105]. For this reason, nonviral gene delivery systems are often injected locally. Furthermore, many researchers have endeavored to improve the buffering capacity, but generally, the carriers are excessively toxic by doing so. Interested readers may refer to the following review for further information with regard to nonviral gene delivery barriers [82], [106], [107]. The following paragraphs discuss cell specificity, hydrophobicity, the proton sponge effect, and nuclear uptake for the readers’ convenience.
7.2.1. Cell specificity of nonviral gene delivery systems
With regard to cell specificity, surface modifications can be employed for enhancement of uptake and structures of the carriers can be screened by using high-throughput methods to discover cell specificity [108]. Guerrero-Cázares, et al. [109] demonstrated that biodegradable poly(beta-amino ester) nanoparticles present specific transfection efficacy to human brain tumor-initiating cells compared to noncancerous cells. Similar results were also reported in a previous work on hepatoma cells [110] and glioblastoma cells [111]. The exact mechanisms of chemical structure-related cell specificity remain unknown. However, it is important to note that the cell specificity of the nonviral gene delivery system was not due to factors such as cellular division rate, cellular uptake amounts of nanoparticles, or media composition. Although the exact mechanism remains elusive, it is hypothesized that different pathways of endocytosis, tuned by different polymer structures, may be the answer to the cell specificity differences. Understanding the Structure Activity Relationships (SARs) is an ongoing endeavor within the field. Enabling the ability to engineer optimized delivery systems is the objective in elucidating such SARs.
7.2.2. Hydrophobicity of nonviral gene delivery systems
An analysis of the interaction of the cell membrane with amphiphilic materials has demonstrated that adsorption of hydrophobic moieties (i.e., Lipofectamine2000®/3000®, lipid-based systems, or a greater number of carbons in the polymer backbone or side chain) can cause disruption or destabilization of the cell membrane, thereby facilitating endosomal uptake [81], [112], [113]. Therefore, promoting cellular uptake is often accomplished by using polymers or lipids designed to increase the interaction between nanoparticles and the cell membrane [82]. Hydrophobicity is one of the main variables that is varied to optimize a nonviral gene delivery system.
7.2.3. Proton sponge effect for nonviral gene delivery systems
After internalization through an endocytic pathway, the pDNA needs to efficiently escape from the endolysosomal degradation pathway into the cytoplasm to avoid degradation. Several authors have observed enhanced endosomal escape using polyethyleneimine (PEI) conjugated to the pDNA to further enhance the “proton sponge effect,” which promotes endosomal swelling and rupture [114]. This effect occurs because of the buffering capacity of the unprotonated amine groups on PEI. The PEI becomes increasingly protonated during the acidification process of the endosomes and thus imbalanced osmotic pressure differences form across the membrane. This is due to influx of Cl− ions and water molecules into the endosome [115]. Other escape mechanisms have involved fusogenic peptides. For example, Nakase, et al. suggested the association between PEI and the fusogenic peptide GALA on the cationic lipid-coated exosomes, which increased endosomal escape of exosomes that are useful for nucleic acid delivery [116]. Hu, et al. [117] developed a DNA vaccine against malignant melanomas employing TAT-based fusogenic peptides with PEI-mannose nanoparticles adsorbed onto microneedles [118]. The use of the TAT-based fusogenic peptide resulted in T-cell activation and enhanced expression of IFNγ IL-12 [119].
7.2.4. Nuclear uptake
A nuclear localization sequence can also be indirectly incorporated into the pDNA by using DNA targeting sequences (DTSs) [120]. A DTS is a DNA subsequence that can be incorporated into the pDNA and is a binding site for NLS-containing protein. Presumably, after the DNA is separated from the complex, karyopherins are able to help facilitate the nuclear uptake of the plasmid. This technology was derived from viruses, for example, the simian virus 40 (SV40), which has a DTS within its genome [80], [121], uses this mechanism to improve its ability to replicate. Thus, when the SV40 DTS sequence (5′-AACCAGCTGTGGAATGTGTGTCAGTTAGGGTGTGGAAAGTCCCCAGGCTCCCCAGCAGGCAGAAGTATGCAAAGCAT-3′) is incorporated into the pDNA of interest, improved gene transfection can similarly be observed (in some cell types) [122].
(For sub-sections 7.2.1, 7.2.2, 7.2.3, 7.2.4) Pros: Nonviral delivery systems are relatively safer to use than their viral counterparts and benefit from being more cost-effective and easier to chemically modify and optimize. Cons: Nonviral systems generally result in temporary expression and also relatively lower expression during the period of expression [123] .
8. Controlling gene expression through plasmid engineering and gene circuits
Designing a plasmid entails the selection of the DNA insert or the GOI, the DNA vector or backbone of the plasmid, the transformation protocol (which depends on the species of the host organism (i.e., competent cells are often used for transformation in E. coli)), and the host organism (i.e., bacteria (E. coli) or yeast (P. pastoris)).
8.1. Minicircle DNA and CpG repeats
Relatively recent technologies suggest that the use of minicircle DNA increases therapy biosafety because bacterial DNA is removed from the parental plasmid such that only the GOI and promoter/terminator sequences essentially remain [124]. Because parental bacteria sequences are absent to a greater extent, the lack of CpG sequences has resulted in longer half-lives of the plasmid. However, for specific applications, encoding CpG is desirable for enhancing the immunogenic response. Moreover, studies have demonstrated that gene transfer using minicircles induces a 10- to 1000-fold increase in the long-term transgene expression in vivo and in vitro when compared to pDNA for specific applications, which can potentially improve the immunogenicity of DNA vaccines [125].
To improve pDNA TGE efficiency, early studies investigated various combinations of promoters/enhancers to control gene expression, as well as the TGE efficiency for HIV-1 vaccination [126], [127]. Sun, et al. [128] and Li, et al. [129] reported the use of post-translational regulatory elements to improve the efficiency of DNA vaccines. Such approaches will likely benefit the field.
8.2. Codon optimization
Codon optimization adapts DNA sequences between species to augment expression. Amino acids are coded by synonymous genetic codons, and the frequency of the synonymous codon being used depends on the species. Gene expression can be optimized by utilizing the advantage of information of codon usage in specific organisms. During the plasmid design, low-frequency eukaryotic codons in the foreign DNA backbone are identified and replaced for high-frequency codons, while still maintaining their respective coded amino acids [130], [131], [132]. There has been recent progress in terms of enhancing the immunogenicity in mice and chicken primed with such codon-optimized DNA vaccines [133], [134], [135].
8.3. Synthetic biology
Synthetic gene circuits provide tools for accurately controlling gene expression [136]. The plasmid sequence itself can be engineered to control the expression (i.e., CRISPR/Cas9 technology) [137], [138], [139]. For example, Brophy and Voigt [140] constructed a gene circuit in E. coli with an antisense promoter placed next to the terminator to study the function of antisense promoters as regulators. The same group has also worked on designing computational tools such as the python language-based “DNAplotlib” library to visualize the gene circuit outline [141]. Another application using gene circuits and synthetic biology is the orthogonal polymerases, which independently control multiple pathways in the same cell [142]. Using multiple activator-chaperone pairs has also been observed to improve the orthogonality in gene circuits [143]. Moon, et al. [143] used a four-input AND gate that controlled the orthogonal gene expression of multiple pathways at the same time, independent of one another, employing E. coli.
Deans, et al. investigated a mechanism that enables control over the gene expression profile through engineered inducers within genetic circuits [136], thus demonstrating the potential to switch a GOI on/off through molecular controllers. Such applications could potentially enable the regulation of the immune response in terms of intensity and type (cell mediated/humoral) in the future for vaccine applications. Recently, synthetic biology has been widely used for genetic engineering applications to conduct developmental and functional studies.
(For sub-sections 8.1, 8.2, 8.3) Pros: Plasmid engineering is a potential method to control gene expression, but currently, technology is generally limited to nontherapeutic genes. As this nascent field continues to grow, there will likely be great improvement in terms of controlling expression as a function of the presence or absence of key entities. Synthetic biology provides a number of tools, such as CRISPR, to efficiently add or delete the GOI. Cons: Extraneous mutations (i.e., indels) can often occur in technologies, such as CRISPR, thereby leading to severe side effects. The complexity of certain gene circuits can lead to unreliable gene expression and safety concerns regarding specificity and off-target effects which will need to be thoroughly investigated.
9. Techniques to overcome immunogenic issues of DNA vaccines
Despite the positive outlook, DNA vaccines have low immunogenicity and hence require immunostimulant adjuvants and/or additional priming or boosters. The immunostimulants and adjuvants, depending on the type, may require more complex storage and transportation conditions. Methods involving the use of cytokines (i.e., IL-4) or the granulocyte-macrophage colony-stimulating factor (GM-CSF) have proven useful in enhancing the immune response. For example, Kalams, et al. [144] reported that IL-12 and/or IL-15 produced a safe and immunogenic response for their HIV-1 Gag DNA vaccine. Kalams, et al. also evaluated the positive impact of EP delivery methods for the HIV-1 Gag antigen/IL-12 adjuvant plasmid vector in a phase I clinical trial. Other studies have also assessed the immunogenicity of DNA vaccines containing chemokine-expressing sequences for HIV-1 immunization [144], [145], [146]. The CpG oligodeoxynucleotide (ODN) is another common genetic adjuvant used for DNA vaccines [9], [147], [148]. Preclinical studies have demonstrated that CpG ODN activates TLR9-bearing B cells and plasmacytoid dendritic cells and further support the induction of strong Th1-type responses [149]. In addition to engineering the ratio of CpG motifs in DNA vaccines to improve immunogenicity, administering additional synthetic CpG ODNs or co-administering a second plasmid cloned with CpG motifs has been investigated. Kojima, et al. delivered a CpG-encoding plasmid 2 days and 4 days both before and after an HIV DNA vaccine was delivered (as well as at the same time). Results showed that a significant boost of HIV-specific IgG and cytotoxic T lymphocyte activity can be obtained when the CpG plasmid was administered either 2 or 4 days after HIV DNA vaccination [150].
Consecutive immunization by using a prime/boost strategy is also applied in many clinical trials to overcome poor immunogenicity of DNA vaccines. The immunization involves priming by a DNA vaccine followed by boosting with (recombinant) antigenic protein [151]. For example, Dale, et al. investigated the use of pDNA and recombinant fowlpox virus (rFPV) vaccines as potential HIV-1 vaccine candidates; their group demonstrated this prime-boost vaccine system can induce stronger antigen-specific T-cell responses [152]. A large and immediate T-cell immune response was observed after administering the rFPV boost in macaques 12 days after priming with DNA vaccines. However, a similar boost in immunity may not be obtained while reboosting with a second dose of the same recombinant protein vaccine. This suggests that the doses and the intervals between doses for multiple boosting vaccinations need to be further evaluated.
10. Examples of DNA vaccine commercial successes
According to the DNA vaccine database, DNAVAxDB [153], there have been more than 20,000 articles related to DNA vaccine, up to and including the year 2017, indexed in PubMed and Google Scholar. However, to the extent of our knowledge, there has not yet been a licensed DNA vaccine for use in humans in the U.S., Europe, or Japan. Because the gene-based formulations require greater safety evaluation than conventional vaccines (e.g., nucleic acid integration into the host’s chromosomal genome), the timeline for approving DNA vaccines for humans is relatively lengthy. While many human DNA vaccines are still being evaluated in clinical trials, to the extent of our knowledge, there have been four DNA vaccines approved for veterinary applications. Overviews of DNA vaccines approved for animal models have been reported in several articles [154], [155], [156]. These approved DNA vaccines are for a number of species including equine, salmon, porcine, and canine. The earliest DNA vaccine approvals were obtained in 2005, namely, the West Nile–Innovator® (equine) and Apex-IHN® (salmon). These two vaccines are prophylactic vaccines for infectious diseases encoding subcomponents of the viral proteins for generating antibodies against the West Nile Virus and the infectious hematopoietic necrosis virus, respectively. In 2008, a single dose of vaccine expressing the growth hormone LifeTide® SW5 (i.e., porcine) was licensed for gene therapy. The latest approval was in 2010, namely, Oncept™ (canine), which is used for cancer immunotherapy against oral melanoma (Table 3 ). Although the noninfectious vaccines are not the main focus of this review, the two vaccines are included in Table 3 to provide a wholistic picture of the current progress of DNA animal vaccines.
Table 3.
Recent licensed protein and DNA vaccine therapies (data extracted from 2001 to 2017; some of which are not related to infectious diseases).
|
Human | |||||
|---|---|---|---|---|---|
| Vaccine Type | Vaccine Target | Product Name | Administration | Date of Approval | Company involved |
| Live attenuated vaccine | Cholera | Vaxchora® | Oral | 2016 | PaxVax |
| Influenza | FluMist® | Intranasal | 2003 | MedImmune | |
| Inactivated vaccine | Influenza virus subtypes A and type B | Flucelvax® | Intramuscular | 2012 | Novartis |
| Influenza | Fluzone® | Intradermal | 2002 | Sanofi Pasteur | |
| Inactivated hepatitis A and recombinant protein | Hepatitis A and B | Twinrix® | Intramuscular | 2001 | GlaxoSmithKline |
| Toxoid conjugated vaccine | Invasive meningococcal disease | Menveo® | Intramuscular | 2010 | Novartis |
| Haemophilus influenzae type b | Hiberix® | Intramuscular | 2009 | GlaxoSmithKline | |
| Diphtheria, Tetanus, Acellular Pertussis, Hepatitis B, Polio | Pediarix® | Intramuscular | 2002 | GlaxoSmithKline | |
| Polysaccharide vaccine | Streptococcus pneumoniae | Prevnar 13® | Intramuscular | 2010 | Wyeth |
| Recombinant proteins | Meningococcal Group B | Bexsero® | Intramuscular | 2015 | Novartis |
| Influenza virus subtypes A and type B | Flublok® | Intramuscular | 2013 | Protein Science | |
| Animal | |||||
| DNA vaccine | West Nile virus | West Nile-Innovator® | Horses | 2005 | Fort Dodge |
| Infectious haematopoietic necrosis virus (IHNV) | Apex-IHN® | Salmon | 2005 | Novartis | |
| Growth hormone releasing hormone (GHRH) | LifeTide® SW5 | Swine | 2008 | VGX™ Animal Health | |
| Melanoma | Oncept™ | Dogs | 2010 | Merial | |
To date, there have been 462 clinical trials (early phase to phase IV; search term: “DNA vaccine”) according to ClinicalTrial.gov. The U.S. was responsible for 248 of these clinical trials. Studies which have been suspended, terminated, withdrawn, or have an unknown status are excluded from these numbers. Details on patented and/or sponsored DNA vaccine technologies are summarized in Table 4 . The majority of these trials (13 of 18 selected trials) are investigating prophylactic vaccines for infectious diseases (i.e., HIV, influenza, and hepatitis B infections). A detailed analysis of clinical trials of gene-based prophylactic vaccines (including virus vaccines) was reported by Nakayama, et al. in 2015 [157].
Table 4.
Selected current DNA vaccines progressing through Phase I to III clinical trials.
| Human | Product name | Vector name | Vaccine formulation | Delivery method | Clinical status | Trial number | Sponsor |
|---|---|---|---|---|---|---|---|
| DNA vaccine priming only | |||||||
| HIV infection (Prophylactic vaccine) | PENNVAX®-B | – | DNA vaccine | CELLECTRA® intramuscular electroporation device | Phase I | NCT01082692 | Inovio Pharmaceuticals |
| PENNVAX®-GP | – | DNA vaccine + plasmid cytokine adjuvant (IL-12) | Intradermal injection/ intramuscular injection with CELLECTRA® electroporation | Phase I | NCT02431767 | Inovio Pharmaceuticals | |
| Malaria (Prophylactic vaccine) | EP-1300 | DNA vaccine | Intramuscular injection with electroporation via TriGrid™ Delivery System (TDS) | Phase I | NCT01169077 | KAEL-GemVax | |
| Hepatitis B (Therapeutic immunotherapy) | INO-1800® | – | DNA vaccine + plasmid cytokine adjuvant (IL-12) | CELLECTRA® electroporation device | Phase I | NCT02431312 | Inovio Pharmaceuticals |
| Zika virus infections (Prophylactic vaccine) | GLS-5700® | pGX7201 | DNA vaccine only | Intradermal injection with CELLECTRA® electroporation | Phase I | NCT02809443, NCT02887482 | GeneOne Life Science, Inovio Pharmaceuticals |
| Middle East respiratory syndrome coronavirus (MERS CoV) (Prophylactic vaccine) | GLS-5300® | – | DNA vaccine only | Intramuscular injection with CELLECTRA® electroporation | Phase I | NCT02670187 | GeneOne Life Science, Inovio Pharmaceuticals |
| Influenza A H5N1 and H1N1 subtypes (Prophylactic vaccine) | INO-3510® | – | DNA vaccine only | Intradermal injection with CELLECTRA® electroporation | Phase I | NCT01405885 | Inovio Pharmaceuticals |
| Viral hemorrhagic fever with renal syndrome (Prophylactic vaccine) | HTNV/PUUV DNA Vaccine | pWRG/HTN-M(co)/pWRG/PUUV-M(s2) | DNA vaccine only | Intramuscular injection with electroporation via TriGrid™ Delivery System (TDS) | Phase II | NCT02116205 | Ichor Medical Systems US Army Medical Research and Materiel Command |
| Cytomegalovirus infection in hematopoietic cell transplantation/solid organ (kidney) transplantation | ASP0113 | TransVax | DNA vaccine with poloxamer-based CRLL1005 delivery system | Intramuscular injection | Phase III | NCT01877655, NCT01974206 | Astellas, Pharma Vical |
| HSV-2 infection (Therapeutic vaccine) | herpes simplex bivalent DNA vaccine | VCL-HB01 | DNA vaccine only | Intramuscular injection | Phase II | NCT02837575 | Vical |
| DNA vaccine priming and other vaccine boosting | |||||||
| HIV infection (Prophylactic vaccine) | GOVX-B11 | pGA2/JS7 | DNA vaccine prime + MVA vaccine boost | Intramuscular injection | Phase II | NCT00820846 | GeoVax |
| HIV infection (Prophylactic vaccine) | PENNVAX®-G | – | DNA vaccine prime + MVA-CMDR vaccine boost | Intramuscular delivery by Biojector 2000® needleless device/CELLECTRA® intramuscular electroporation device | Phase I | NCT01260727 | Inovio Pharmaceuticals |
| Malaria (Prophylactic vaccine) | – | – | DNA vaccine prime + adenovirus type 5 vaccine (Ad) boost | Intramuscular delivery by Biojector 2000® needleless device | Phase I/IIa | NCT00870987 | Vical, U.S. Army Medical Research and Materiel Command |
To date, most clinical trials have demonstrated no significant evidence indicating DNA insertional mutagenesis into the chromosomes of hosts and immunologic tolerance in terms of developing anti-DNA antibodies. However, the transfection efficiency of DNA plasmids is generally poor resulting in an insufficient induction of an immune response, which is possibly one of the greatest challenges in current clinical trials. As a result, more trials have been utilizing hybridized formulations (i.e., CpG) and/or higher DNA doses for greater expression levels and immunogenicity [151]. A number of current clinical trials for HIV infections are an example of such endeavors. The GOVX-B11 vaccine, currently in phase II, constitutes two components: a DNA vaccine used to prime a person’s immune response and a recombinant MVA (modified vaccinia Ankara, attenuated vaccine) used to boost the primed response. Their studies were conducted in HIV-infected participants who were currently undergoing antiretroviral treatment. It has been demonstrated that GOVX-B11 is capable of eliciting antibodies and a T cell response against a heterologous challenge [158]. On the other hand, the PENNVAX DNA vaccines developed by Inovio Pharmaceuticals, currently in phase I, utilize the prime-boost strategy, similar to that of GOVX-B11, by combining a viral vector vaccine. Furthermore, Inovio demonstrated that the immune response can be enhanced when PENNVAX is delivered in vivo by intradermal EP compared to a syringe injection [52], [159]. These results reveal that the modification of DNA vaccine formulations or combinatorial delivery approaches can improve the potency and efficacy of DNA vaccines in human clinical trials.
In summary, as of 2017, a large number of second-generation vaccines have been licensed and released on the market. Some of these DNA vaccines have also been approved for sale for animal health applications (summarized in Table 2, Table 3). These reports provide evidence for the potential and growth of a market for new vaccine technologies. Regarding the feasibility of current trials of DNA vaccines, further advancement of plasmid formulations and delivery approaches will be necessary for the DNA vaccine platform to be realized within humans.
11. Conclusion and future directions
The optimization of vaccine scheduling has been generally limited to investigations where time was varied by month [160]. Optimizing vaccine schedules with smaller steps in time will likely result in enhanced seroconversion and protection. In terms of engineering the kinetics for vaccines by auto-boosting technologies, the practicality of receiving doses on short time scales is increasingly more reasonable. New technologies such as SEAL [161] are seriously needed and are likely to be highly impactful. Potential complications such as the junction of the seal must be mitigated to the extent possible, however. Removal of the need to seal would be advantageous but highly challenging. Such endeavors would be likely worthwhile (i.e., direct injection systems and microfluidic-based technologies).
Enhancing the ability to stabilize the cargo within is challenging. Having a depot environment that is optimized and catered to a given antigen is possible within a depot vaccine system in terms of excipients and cryoprotectant content, as well as ratios of excipients to antigen. However, if multiple antigen types are to be delivered from the vaccine depot, it is highly challenging to cater to multiple antigen types in the most optimal fashion within a single depot. Endeavors to deliver vaccine depots with environments that are most optimally catered to each specific antigen within a given injectable vaccine system would also be beneficial and challenging from an engineering standpoint.
To date, although clinical translation of gene-based systems has been limited, the U.S. has recently approved two gene therapies. As more gene-modulating technologies receive approval, obtaining 510(k) clearance will be increasingly common for this field. DNA vaccines, although promising, suffer limitations owing to their inability to be sufficiently immunogenic. Techniques to engineer DNA-based systems such as prime/booster methods, as well as the incorporation of adjuvants (transcribed/translated or codelivered), will continue to improve the immunogenicity for attaining protective seroconversion.
Currently, safety assessments for gene delivery systems undergoing approval processes are arduous to determine risks of insertional mutagenesis. Although gene sequencing is becoming increasingly cheaper over the years, conducting qRT-PCR or qPCR is time consuming and requires relatively more sophisticated instrumentation in comparison to fluorescence-based assays. Endeavoring to properly assess insertional mutagenesis risks for gene-modulating technologies would probably be highly useful if the assay is fluorescence-based and sufficiently reliable.
To further the development of vaccines with improved functionality, an interdisciplinary-focused research will be of great utility. In light of research discussed herein, the vaccine realm will be experiencing great improvements in the following areas in the coming years: further optimization of vaccine scheduling; duration of protection through nonkinetic approaches; enhancement of antigen stability; improved immunogenicity of DNA vaccines; manufacturing and engineering vaccines in general, and the ability to control gene circuits for gene-modulating technologies.
References
- 1.Disease Burden and Mortality Estimates, http://www.who.int/healthinfo/global_burden_disease/estimates/en/index2.html, 2016.
- 2.10 Facts on Polio Eradication, http://www.who.int/features/factfiles/polio/en/, 2017.
- 3.The Vaccines, http://polioeradication.org/polio-today/polio-prevention/the-vaccines/opv/, 2016.
- 4.Minor P.D. Live attenuated vaccines: historical successes and current challenges. Virology. 2015;479–480:379–392. doi: 10.1016/j.virol.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Watson L., Wilson B.J., Waugh N. Pneumococcal polysaccharide vaccine: a systematic review of clinical effectiveness in adults. Vaccine. 2002;20(17–18):2166–2173. doi: 10.1016/s0264-410x(02)00112-3. [DOI] [PubMed] [Google Scholar]
- 6.Daniels C.C., Rogers P.D., Shelton C.M. A review of pneumococcal vaccines: current polysaccharide vaccine recommendations and future protein antigens. J. Pediatr. Pharmacol. Ther. 2016;21(1):27–35. doi: 10.5863/1551-6776-21.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho H.W., Howard C.R., Lee H.W. Review of an inactivated vaccine against hantaviruses. Intervirology. 2002;45(4–6):328–333. doi: 10.1159/000067925. [DOI] [PubMed] [Google Scholar]
- 8.Liu M.A. DNA vaccines: a review. J. Intern. Med. 2003;253(4):402–410. doi: 10.1046/j.1365-2796.2003.01140.x. [DOI] [PubMed] [Google Scholar]
- 9.Qiu Y., Guo L., Zhang S., Xu B., Gao Y., Hu Y., Hou J., Bai B., Shen H., Mao P. DNA-based vaccination against hepatitis B virus using dissolving microneedle arrays adjuvanted by cationic liposomes and CpG ODN. Drug Deliv. 2016;23(7):2391–2398. doi: 10.3109/10717544.2014.992497. [DOI] [PubMed] [Google Scholar]
- 10.Endmann A., Klunder K., Kapp K., Riede O., Oswald D., Talman E.G., Schroff M., Kleuss C., Ruiters M.H., Juhls C. Cationic lipid-formulated DNA vaccine against hepatitis B virus: immunogenicity of MIDGE-Th1 vectors encoding small and large surface antigen in comparison to a licensed protein vaccine. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khatri K., Goyal A.K., Gupta P.N., Mishra N., Vyas S.P. Plasmid DNA loaded chitosan nanoparticles for nasal mucosal immunization against hepatitis B. Int. J. Pharm. 2008;354(1–2):235–241. doi: 10.1016/j.ijpharm.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Wolff J.A., Malone R.W., Williams P., Chong W., Acsadi G., Jani A., Felgner P.L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 13.Jiao S., Williams P., Berg R.K., Hodgeman B.A., Liu L., Repetto G., Wolff J.A. Direct gene transfer into nonhuman primate myofibers in vivo. Hum. Gene Ther. 1992;3(1):21–33. doi: 10.1089/hum.1992.3.1-21. [DOI] [PubMed] [Google Scholar]
- 14.Ulmer J.B., Donnelly J.J., Parker S.E., Rhodes G.H., Felgner P.L., Dwarki V.J., Gromkowski S.H., Deck R.R., DeWitt C.M., Friedman A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259(5102):1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 15.Dobrovolskaia M.A., McNeil S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007;2(8):469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y., Liang W., Qiu Y., Cespi M., Palmieri G.F., Mason A.J., Lam J.K. Incorporation of a nuclear localization signal in pH responsive LAH4-L1 peptide enhances transfection and nuclear uptake of plasmid DNA. Mol. Pharm. 2016;13(9):3141–3152. doi: 10.1021/acs.molpharmaceut.6b00338. [DOI] [PubMed] [Google Scholar]
- 17.Gilhodes J.C., Jule Y., Kreuz S., Stierstorfer B., Stiller D., Wollin L. Quantification of pulmonary fibrosis in a bleomycin mouse model using automated histological image analysis. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0170561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciabattini A., Pettini E., Fiorino F., Pastore G., Andersen P., Pozzi G., Medaglini D. Modulation of primary immune response by different vaccine adjuvants. Front. Immunol. 2016;7:427. doi: 10.3389/fimmu.2016.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawaengsak C., Mori Y., Yamanishi K., Mitrevej A., Sinchaipanid N. Chitosan nanoparticle encapsulated hemagglutinin-split influenza virus mucosal vaccine. AAPS PharmSciTech. 2014;15(2):317–325. doi: 10.1208/s12249-013-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barhate G., Gautam M., Gairola S., Jadhav S., Pokharkar V. Enhanced mucosal immune responses against tetanus toxoid using novel delivery system comprised of chitosan-functionalized gold nanoparticles and botanical adjuvant: characterization, immunogenicity, and stability assessment. J. Pharm. Sci. 2014;103(11):3448–3456. doi: 10.1002/jps.24161. [DOI] [PubMed] [Google Scholar]
- 21.Gordon D., Kelley P., Heinzel S., Cooper P., Petrovsky N. Immunogenicity and safety of Advax, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: a randomized controlled Phase 1 study. Vaccine. 2014;32(48):6469–6477. doi: 10.1016/j.vaccine.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon D.L., Sajkov D., Honda-Okubo Y., Wilks S.H., Aban M., Barr I.G., Petrovsky N. Human phase 1 trial of low-dose inactivated seasonal influenza vaccine formulated with Advax delta inulin adjuvant. Vaccine. 2016;34(33):3780–3786. doi: 10.1016/j.vaccine.2016.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carbinatto F.M., de Castro A.D., Evangelista R.C., Cury B.S.F. Insights into the swelling process and drug release mechanisms from cross-linked pectin/high amylose starch matrices. Asian J. Pharm. Sci. 2014;9(1):27–34. [Google Scholar]
- 24.Tamayo I., Irache J.M., Mansilla C., Ochoa-Reparaz J., Lasarte J.J., Gamazo C. Poly(anhydride) nanoparticles act as active Th1 adjuvants through Toll-like receptor exploitation. Clin. Vaccine Immunol. 2010;17(9):1356–1362. doi: 10.1128/CVI.00164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wafa E.I., Geary S.M., Goodman J.T., Narasimhan B., Salem A.K. The effect of polyanhydride chemistry in particle-based cancer vaccines on the magnitude of the anti-tumor immune response. Acta Biomater. 2017;50:417–427. doi: 10.1016/j.actbio.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhakal S., Goodman J., Bondra K., Lakshmanappa Y.S., Hiremath J., Shyu D.L., Ouyang K., Kang K.I., Krakowka S., Wannemuehler M.J., Won Lee C., Narasimhan B., Renukaradhya G.J. Polyanhydride nanovaccine against swine influenza virus in pigs. Vaccine. 2017;35(8):1124–1131. doi: 10.1016/j.vaccine.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Ross K.A., Loyd H., Wu W., Huntimer L., Ahmed S., Sambol A., Broderick S., Flickinger Z., Rajan K., Bronich T., Mallapragada S., Wannemuehler M.J., Carpenter S., Narasimhan B. Hemagglutinin-based polyanhydride nanovaccines against H5N1 influenza elicit protective virus neutralizing titers and cell-mediated immunity. Int. J. Nanomed. 2015;10:229–243. doi: 10.2147/IJN.S72264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shakya A.K., Kumar A., Holmdahl R., Nandakumar K.S. Macrophage-derived reactive oxygen species protects against autoimmune priming with a defined polymeric adjuvant. Immunology. 2016;147(1):125–132. doi: 10.1111/imm.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva A.L., Soema P.C., Slutter B., Ossendorp F., Jiskoot W. PLGA particulate delivery systems for subunit vaccines: linking particle properties to immunogenicity. Hum. Vaccine Immunother. 2016;12(4):1056–1069. doi: 10.1080/21645515.2015.1117714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mittal G., Sahana D.K., Bhardwaj V., Ravi Kumar M.N. Estradiol loaded PLGA nanoparticles for oral administration: effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J. Control. Release. 2007;119(1):77–85. doi: 10.1016/j.jconrel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Li P., Asokanathan C., Liu F., Khaing K.K., Kmiec D., Wei X., Song B., Xing D., Kong D. PLGA nano/micro particles encapsulated with pertussis toxoid (PTd) enhances Th1/Th17 immune response in a murine model. Int. J. Pharm. 2016;513(1–2):183–190. doi: 10.1016/j.ijpharm.2016.08.059. [DOI] [PubMed] [Google Scholar]
- 32.Cruz L.J., Tacken P.J., Eich C., Rueda F., Torensma R., Figdor C.G. Controlled release of antigen and Toll-like receptor ligands from PLGA nanoparticles enhances immunogenicity. Nanomedicine (London) 2017;12(5):491–510. doi: 10.2217/nnm-2016-0295. [DOI] [PubMed] [Google Scholar]
- 33.Ma J., Wang H., Zheng X., Xue X., Wang B., Wu H., Zhang K., Fan S., Wang T., Li N., Zhao Y., Gao Y., Yang S., Xia X. CpG/Poly (I:C) mixed adjuvant priming enhances the immunogenicity of a DNA vaccine against eastern equine encephalitis virus in mice. Int. Immunopharmacol. 2014;19(1):74–80. doi: 10.1016/j.intimp.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Bergamaschi C., Kulkarni V., Rosati M., Alicea C., Jalah R., Chen S., Bear J., Sardesai N.Y., Valentin A., Felber B.K., Pavlakis G.N. Intramuscular delivery of heterodimeric IL-15 DNA in macaques produces systemic levels of bioactive cytokine inducing proliferation of NK and T cells. Gene Ther. 2015;22(1):76–86. doi: 10.1038/gt.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naderi M., Saeedi A., Moradi A., Kleshadi M., Zolfaghari M.R., Gorji A., Ghaemi A. Interleukin-12 as a genetic adjuvant enhances hepatitis C virus NS3 DNA vaccine immunogenicity. Virol. Sin. 2013;28(3):167–173. doi: 10.1007/s12250-013-3291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saade F., Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Exp. Rev. Vaccines. 2012;11(2):189–209. doi: 10.1586/erv.11.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang W. Blockade of B7–H1 enhances dendritic cell-mediated T cell response and antiviral immunity in HBV transgenic mice. Vaccine. 2012;30(4):758–766. doi: 10.1016/j.vaccine.2011.11.076. [DOI] [PubMed] [Google Scholar]
- 38.Li L., Petrovsky N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Exp. Rev. Vaccines. 2016;15(3):313–329. doi: 10.1586/14760584.2016.1124762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sivakumar S.M., Safhi M.M., Kannadasan M., Sukumaran N. Vaccine adjuvants – current status and prospects on controlled release adjuvancity. Saudi Pharm. J. 2011;19(4):197–206. doi: 10.1016/j.jsps.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrison G.B.L., Heath D.D., Dempster R.P., Gauci C., Newton S.E., Cameron W.G., Robinson C.M., Lawrence S.B., Lightowlers M.W., Rickard M.D. Identification and cDNA cloning of two novel low molecular weight host-protective antigens from Taenia ovis oncospheres. Int. J. Parasitol. 1996;26(2):195–204. doi: 10.1016/0020-7519(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 41.Johnson K.S., Harrison G.B.L., Lightowlers M.W., Ohoy K.L., Cougle W.G., Dempster R.P., Lawrence S.B., Vinton J.G., Heath D.D., Rickard M.D. Vaccination against ovine cysticercosis using a defined recombinant antigen. Nature. 1989;338(6216):585–587. doi: 10.1038/338585a0. [DOI] [PubMed] [Google Scholar]
- 42.Lightowlers M.W., Rolfe R., Gauci C.G. Taenia saginata: Vaccination against cysticercosis in cattle with recombinant oncosphere antigens. Exp. Parasitol. 1996;84(3):330–338. doi: 10.1006/expr.1996.0121. [DOI] [PubMed] [Google Scholar]
- 43.Ghanem A., Healey R., Adly F.G. Current trends in separation of plasmid DNA vaccines: a review. Anal. Chim. Acta. 2013;760:1–15. doi: 10.1016/j.aca.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Zhu K., Jin H., He Z., Zhu Q., Wang B. A continuous method for the large-scale extraction of plasmid DNA by modified boiling lysis. Nat. Protoc. 2006;1(6):3088–3093. doi: 10.1038/nprot.2006.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogl T., Ahmad M., Krainer F.W., Schwab H., Glieder A. Restriction site free cloning (RSFC) plasmid family for seamless, sequence independent cloning in Pichia pastoris. Microb. Cell Fact. 2015;14:103. doi: 10.1186/s12934-015-0293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cregg J.M., Barringer K.J., Hessler A.Y., Madden K.R. Pichia pastoris as a host system for transformations. Mol. Cell. Biol. 1985;5(12):3376–3385. doi: 10.1128/mcb.5.12.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams P.D., Kingston P.A. Plasmid-mediated gene therapy for cardiovascular disease. Cardiovasc. Res. 2011;91(4):565–576. doi: 10.1093/cvr/cvr197. [DOI] [PubMed] [Google Scholar]
- 48.Borja G.M., Meza Mora E., Barron B., Gosset G., Ramirez O.T., Lara A.R. Engineering Escherichia coli to increase plasmid DNA production in high cell-density cultivations in batch mode. Microb. Cell Fact. 2012;11:132. doi: 10.1186/1475-2859-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Listner K., Bentley L.K., Chartrain M. A simple method for the production of plasmid DNA in bioreactors. Methods Mol. Med. 2006;127:295–309. doi: 10.1385/1-59745-168-1:295. [DOI] [PubMed] [Google Scholar]
- 50.Miura N., Shaheen S.M., Akita H., Nakamura T., Harashima H. A KALA-modified lipid nanoparticle containing CpG-free plasmid DNA as a potential DNA vaccine carrier for antigen presentation and as an immune-stimulative adjuvant. Nucl. Acids Res. 2015;43(3):1317–1331. doi: 10.1093/nar/gkv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munye M.M., Tagalakis A.D., Barnes J.L., Brown R.E., McAnulty R.J., Howe S.J., Hart S.L. Minicircle DNA provides enhanced and prolonged transgene expression following airway gene transfer. Sci. Rep. 2016;6:23125. doi: 10.1038/srep23125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Q., Jiang W., Chen Y., Liu P., Sheng C., Chen S., Zhang H., Pan C., Gao S., Huang W. In vivo electroporation of minicircle DNA as a novel method of vaccine delivery to enhance HIV-1-specific immune responses. J. Virol. 2014;88(4):1924–1934. doi: 10.1128/JVI.02757-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kay M.A., He C.Y., Chen Z.Y. A robust system for production of minicircle DNA vectors. Nat. Biotechnol. 2010;28(12):1287–1289. doi: 10.1038/nbt.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Z.Y., He C.Y., Ehrhardt A., Kay M.A. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol. Ther. 2003;8(3):495–500. doi: 10.1016/s1525-0016(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 55.Nafissi N., Slavcev R. Bacteriophage recombination systems and biotechnical applications. Appl. Microbiol. Biotechnol. 2014;98(7):2841–2851. doi: 10.1007/s00253-014-5512-2. [DOI] [PubMed] [Google Scholar]
- 56.Xenopoulos A., Pattnaik P. Production and purification of plasmid DNA vaccines: is there scope for further innovation? Exp. Rev. Vaccines. 2014;13(12):1537–1551. doi: 10.1586/14760584.2014.968556. [DOI] [PubMed] [Google Scholar]
- 57.Ferreira G.N., Monteiro G.A., Prazeres D.M., Cabral J.M. Downstream processing of plasmid DNA for gene therapy and DNA vaccine applications. Trends Biotechnol. 2000;18(9):380–388. doi: 10.1016/s0167-7799(00)01475-x. [DOI] [PubMed] [Google Scholar]
- 58.Kramberger P., Urbas L., Strancar A. Downstream processing and chromatography based analytical methods for production of vaccines, gene therapy vectors, and bacteriophages. Hum. Vaccine Immunother. 2015;11(4):1010–1021. doi: 10.1080/21645515.2015.1009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li L., Saade F., Petrovsky N. The future of human DNA vaccines. J. Biotechnol. 2012;162(2–3):171–182. doi: 10.1016/j.jbiotec.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin X., Morgan C., Yu X., DeRosa S., Tomaras G.D., Montefiori D.C., Kublin J., Corey L., Keefer M.C., Network N.H.V.T. Multiple factors affect immunogenicity of DNA plasmid HIV vaccines in human clinical trials. Vaccine. 2015;33(20):2347–2353. doi: 10.1016/j.vaccine.2015.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Heijden I., Beijnen J.H., Nuijen B. Long term stability of lyophilized plasmid DNA pDERMATT. Int. J. Pharm. 2013;453(2):648–650. doi: 10.1016/j.ijpharm.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 62.Pogocki D., Schoneich C. Chemical stability of nucleic acid-derived drugs. J. Pharm. Sci. 2000;89(4):443–456. doi: 10.1002/(SICI)1520-6017(200004)89:4<443::AID-JPS2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 63.Kronbichler A., Kerschbaum J., Mayer G. The influence and role of microbial factors in autoimmune kidney diseases: a systematic review. J. Immunol. Res. 2015;2015 doi: 10.1155/2015/858027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krieg A.M. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006;5(6):471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 65.Scheiermann J., Klinman D.M. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine. 2014;32(48):6377–6389. doi: 10.1016/j.vaccine.2014.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gilkeson G.S., Grudier J.P., Karounos D.G., Pisetsky D.S. Induction of anti-double stranded DNA antibodies in normal mice by immunization with bacterial DNA. J. Immunol. 1989;142(5):1482–1486. [PubMed] [Google Scholar]
- 67.Lilic D., Ghosh S.K. Liver dysfunction and DNA antibodies after hepatitis B vaccination. Lancet. 1994;344(8932):1292–1293. doi: 10.1016/s0140-6736(94)90776-5. [DOI] [PubMed] [Google Scholar]
- 68.Zafrir Y., Agmon-Levin N., Paz Z., Shilton T., Shoenfeld Y. Autoimmunity following hepatitis B vaccine as part of the spectrum of 'Autoimmune (Auto-inflammatory) Syndrome induced by Adjuvants' (ASIA): analysis of 93 cases. Lupus. 2012;21(2):146–152. doi: 10.1177/0961203311429318. [DOI] [PubMed] [Google Scholar]
- 69.Mor G., Singla M., Steinberg A.D., Hoffman S.L., Okuda K., Klinman D.M. Do DNA vaccines induce autoimmune disease? Hum. Gene Ther. 1997;8(3):293–300. doi: 10.1089/hum.1997.8.3-293. [DOI] [PubMed] [Google Scholar]
- 70.Wurtele H., Little K.C., Chartrand P. Illegitimate DNA integration in mammalian cells. Gene Ther. 2003;10(21):1791–1799. doi: 10.1038/sj.gt.3302074. [DOI] [PubMed] [Google Scholar]
- 71.Klinman D.M., Klaschik S., Tross D., Shirota H., Steinhagen F. FDA guidance on prophylactic DNA vaccines: analysis and recommendations. Vaccine. 2010;28(16):2801–2805. doi: 10.1016/j.vaccine.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Monjezi R., Miskey C., Gogishvili T., Schleef M., Schmeer M., Einsele H., Ivics Z., Hudecek M. Enhanced CAR T-cell engineering using non-viral sleeping beauty transposition from minicircle vectors. Leukemia. 2017;31(1):186–194. doi: 10.1038/leu.2016.180. [DOI] [PubMed] [Google Scholar]
- 73.Colluru V.T., Zahm C.D., McNeel D.G. Mini-intronic plasmid vaccination elicits tolerant LAG3+ CD8+ T cells and inferior antitumor responses. Oncoimmunology. 2016;5(10) doi: 10.1080/2162402X.2016.1223002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wong S., Lam P., Nafissi N., Denniss S., Slavcev R. Production of double-stranded DNA ministrings. J. Vis. Exp. 2016;(108):53177. doi: 10.3791/53177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sum C.H., Nafissi N., Slavcev R.A., Wettig S. Physical characterization of gemini surfactant-based synthetic vectors for the delivery of linear covalently closed (LCC) DNA ministrings. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0142875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crine P., Verly W.G. A study of DNA spontaneous degradation. Biochim. Biophys. Acta, Gen. Subj. 1976;442(1):50–57. doi: 10.1016/0005-2787(76)90174-x. [DOI] [PubMed] [Google Scholar]
- 77.Karni M., Zidon D., Polak P., Zalevsky Z., Shefi O. Thermal degradation of DNA. DNA Cell Biol. 2013;32(6):298–301. doi: 10.1089/dna.2013.2056. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen-Hieu T., Aboudharam G., Drancourt M. Heat degradation of eukaryotic and bacterial DNA: an experimental model for paleomicrobiology. BMC Res. Notes. 2012;5:528. doi: 10.1186/1756-0500-5-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362(6422):709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 80.Bishop C.J., Majewski R.L., Guiriba T.M., Wilson D.R., Bhise N.S., Quinones-Hinojosa A., Green J.J. Quantification of cellular and nuclear uptake rates of polymeric gene delivery nanoparticles and DNA plasmids via flow cytometry. Acta Biomater. 2016;37:120–130. doi: 10.1016/j.actbio.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bishop C.J., Abubaker-Sharif B., Guiriba T., Tzeng S.Y., Green J.J. Gene delivery polymer structure-function relationships elucidated via principal component analysis. Chem. Commun. 2015;51(60):12134–12137. doi: 10.1039/c5cc04417k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bishop C.J., Kozielski K.L., Green J.J. Exploring the role of polymer structure on intracellular nucleic acid delivery via polymeric nanoparticles. J. Control. Release. 2015;219:488–499. doi: 10.1016/j.jconrel.2015.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Evans R.K., Xu Z., Bohannon K.E., Wang B., Bruner M.W., Volkin D.B. Evaluation of degradation pathways for plasmid DNA in pharmaceutical formulations via accelerated stability studies. J. Pharm. Sci. 2000;89(1):76–87. doi: 10.1002/(SICI)1520-6017(200001)89:1<76::AID-JPS8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 84.Jain H.V., Beaucage S.L. An, amphipathic trans-acting phosphorothioate DNA element delivers uncharged PNA and PMO nucleic acid sequences in mammalian cells. Curr. Protoc. Nucl. Acid Chem. 2016;64 doi: 10.1002/0471142700.nc0469s64. 4 69 1–4 69 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siddiquee S., Rovina K., Azriah A. A review of peptide nucleic acid. Adv. Tech. Biol. Med. 2015:1–10. [Google Scholar]
- 86.Takai D., Jones P.A. Comprehensive analysis of CpG islands in human chromosomes 21 and 22. Proc. Natl. Acad. Sci. U.S.A. 2002;99(6):3740–3745. doi: 10.1073/pnas.052410099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jones P.A. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012;13(7):484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 88.Youn H.N., Lee D.H., Lee Y.N., Park J.K., Yuk S.S., Yang S.Y., Lee H.J., Woo S.H., Kim H.M., Lee J.B., Park S.Y., Choi I.S., Song C.S. Intranasal administration of live Lactobacillus species facilitates protection against influenza virus infection in mice. Antiviral Res. 2012;93(1):138–143. doi: 10.1016/j.antiviral.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 89.Sasaki S., Sumino K., Hamajima K., Fukushima J., Ishii N., Kawamoto S., Mohri H., Kensil C.R., Okuda K. Induction of systemic and mucosal immune responses to human immunodeficiency virus type 1 by a DNA vaccine formulated with QS-21 saponin adjuvant via intramuscular and intranasal routes. J. Virol. 1998;72(6):4931–4939. doi: 10.1128/jvi.72.6.4931-4939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pertmer T.M., Roberts T.R., Haynes J.R. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J. Virol. 1996;70(9):6119–6125. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mulcahy L.A., Pink R.C., Carter D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adkins C.E., Mittapalli R.K., Manda V.K., Nounou M.I., Mohammad A.S., Terrell T.B., Bohn K.A., Yasemin C., Grothe T.R., Lockman J.A., Lockman P.R. P-glycoprotein mediated efflux limits substrate and drug uptake in a preclinical brain metastases of breast cancer model. Front. Pharmacol. 2013;4:136. doi: 10.3389/fphar.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu V.B., Williams D.J., Won Y.J., Ikeda S.R. Intranuclear microinjection of DNA into dissociated adult mammalian neurons. J. Vis. Exp. 2009;(34) doi: 10.3791/1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hooper J.W., Moon J.E., Paolino K.M., Newcomer R., McLain D.E., Josleyn M., Hannaman D., Schmaljohn C. A phase 1 clinical trial of Hantaan virus and Puumala virus M-segment DNA vaccines for haemorrhagic fever with renal syndrome delivered by intramuscular electroporation. Clin. Microbiol. Infect. 2014;20(Suppl. 5):110–117. doi: 10.1111/1469-0691.12553. [DOI] [PubMed] [Google Scholar]
- 95.McBurney S.P., Sunshine J.E., Gabriel S., Huynh J.P., Sutton W.F., Fuller D.H., Haigwood N.L., Messer W.B. Evaluation of protection induced by a dengue virus serotype 2 envelope domain III protein scaffold/DNA vaccine in non-human primates. Vaccine. 2016;34(30):3500–3507. doi: 10.1016/j.vaccine.2016.03.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yager E.J., Stagnar C., Gopalakrishnan R., Fuller J.T., Fuller D.H. Biolistic DNA Delivery: Methods and Protocols. 2013. Optimizing particle-mediated epidermal delivery of an influenza DNA vaccine in ferrets; pp. 223–237. [DOI] [PubMed] [Google Scholar]
- 97.Fuller D.H., Loudon P., Schmaljohn C. Preclinical and clinical progress of particle-mediated DNA vaccines for infectious diseases. Methods. 2006;40(1):86–97. doi: 10.1016/j.ymeth.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 98.Choi A.H., Knowlton D.R., McNeal M.M., Ward R.L. Particle bombardment-mediated DNA vaccination with rotavirus VP6 induces high levels of serum rotavirus IgG but fails to protect mice against challenge. Virology. 1997;232(1):129–138. doi: 10.1006/viro.1997.8552. [DOI] [PubMed] [Google Scholar]
- 99.Duong H.T.T., Kim N.W., Thambi T., Phan V.G., Lee M.S., Yin Y., Jeong J.H., Lee D.S. Microneedle arrays coated with charge reversal pH-sensitive copolymers improve antigen presenting cells-homing DNA vaccine delivery and immune responses. J. Control. Release. 2018;269:225–234. doi: 10.1016/j.jconrel.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 100.Sharei A., Zoldan J., Adamo A., Sim W.Y., Cho N., Jackson E., Mao S., Schneider S., Han M.J., Lytton-Jean A., Basto P.A., Jhunjhunwala S., Lee J., Heller D.A., Kang J.W., Hartoularos G.C., Kim K.S., Anderson D.G., Langer R., Jensen K.F. A vector-free microfluidic platform for intracellular delivery. Proc. Natl. Acad. Sci. U.S.A. 2013;110(6):2082–2087. doi: 10.1073/pnas.1218705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Karshafian R., Bevan P.D., Williams R., Samac S., Burns P.N. Sonoporation by ultrasound-activated microbubble contrast agents: effect of acoustic exposure parameters on cell membrane permeability and cell viability. Ultrasound Med. Biol. 2009;35(5):847–860. doi: 10.1016/j.ultrasmedbio.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 102.Meacham J.M., Durvasula K., Degertekin F.L., Fedorov A.G. Physical methods for intracellular delivery: practical aspects from laboratory use to industrial-scale processing. J. Lab. Autom. 2014;19(1):1–18. doi: 10.1177/2211068213494388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meacham J.M., Durvasula K., Degertekin F.L., Fedorov A.G. Enhanced intracellular delivery via coordinated acoustically driven shear mechanoporation and electrophoretic insertion. Sci. Rep. 2018;8(1):3727. doi: 10.1038/s41598-018-22042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baltes N.J., Gil-Humanes J., Voytas D.F. Genome engineering and agriculture: opportunities and challenges. Prog. Mol. Biol. Transl. Sci. 2017;149:1–26. doi: 10.1016/bs.pmbts.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ketola T.-M., Hanzlíková M., Urtti A., Lemmetyinen H., Yliperttula M., Vuorimaa E. Role of polyplex intermediate species on gene transfer efficiency: polyethylenimine–DNA complexes and time-resolved fluorescence spectroscopy. J. Phys. Chem. B. 2011;115(8):1895–1902. doi: 10.1021/jp109984c. [DOI] [PubMed] [Google Scholar]
- 106.Sunshine J.C., Bishop C.J., Green J.J. Advances in polymeric and inorganic vectors for nonviral nucleic acid delivery. Ther. Deliv. 2011;2(4):493–521. doi: 10.4155/tde.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.J.C. Sunshine, C.J. Bishop, J.J. Green, Advances in polymeric and inorganic vectors for nonviral nucleic acid delivery, 2011. [DOI] [PMC free article] [PubMed]
- 108.Guerrero-Cazares H., Tzeng S.Y., Young N.P., Abutaleb A.O., Quinones-Hinojosa A., Green J.J. Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. ACS Nano. 2014;8(5):5141–5153. doi: 10.1021/nn501197v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guerrero-Cázares H., Tzeng S.Y., Young N.P., Abutaleb A.O., Quiñones-Hinojosa A., Green J.J. Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. ACS Nano. 2014;8(5):5141–5153. doi: 10.1021/nn501197v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tzeng S.Y., Higgins L.J., Pomper M.G., Green J.J. Biomaterial-mediated cancer-specific DNA delivery to liver cell cultures using synthetic poly (beta-amino esters) J. Biomed. Mater. Res. Part A. 2013;101(7):1837. doi: 10.1002/jbm.a.34616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tzeng S.Y., Guerrero-Cázares H., Martinez E.E., Sunshine J.C., Quiñones-Hinojosa A., Green J.J. Non-viral gene delivery nanoparticles based on poly (β-amino esters) for treatment of glioblastoma. Biomaterials. 2011;32(23):5402–5410. doi: 10.1016/j.biomaterials.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bishop C.J., Ketola T.M., Tzeng S.Y., Sunshine J.C., Urtti A., Lemmetyinen H., Vuorimaa-Laukkanen E., Yliperttula M., Green J.J. The effect and role of carbon atoms in poly(beta-amino ester)s for DNA binding and gene delivery. J. Am. Chem. Soc. 2013;135(18):6951–6957. doi: 10.1021/ja4002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sims L.B., Curtis L.T., Frieboes H.B., Steinbach-Rankins J.M. Enhanced uptake and transport of PLGA-modified nanoparticles in cervical cancer. J. Nanobiotechnol. 2016;14:33. doi: 10.1186/s12951-016-0185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Behr J.-P. The proton sponge: a trick to enter cells the viruses did not exploit. CHIMIA Int. J. Chem. 1997;51(1–2):34–36. [Google Scholar]
- 115.Benjaminsen R.V., Mattebjerg M.A., Henriksen J.R., Moghimi S.M., Andresen T.L. The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 2013;21(1):149–157. doi: 10.1038/mt.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nakase I., Kogure K., Harashima H., Futaki S. Application of a fusiogenic peptide GALA for intracellular delivery. Methods Mol Biol. 2011;683:525–533. doi: 10.1007/978-1-60761-919-2_37. [DOI] [PubMed] [Google Scholar]
- 117.Hu Y., Xu B., Xu J., Shou D., Liu E., Gao J., Liang W., Huang Y. Microneedle-assisted dendritic cell-targeted nanoparticles for transcutaneous DNA immunization. Polym. Chem. 2015;6(3):373–379. [Google Scholar]
- 118.Martens T.F., Remaut K., Demeester J., De Smedt S.C., Braeckmans K. Intracellular delivery of nanomaterials: how to catch endosomal escape in the act. Nano Today. 2014;9(3):344–364. [Google Scholar]
- 119.Nicoli F., Finessi V., Sicurella M., Rizzotto L., Gallerani E., Destro F., Cafaro A., Marconi P., Caputo A., Ensoli B., Gavioli R. The HIV-1 Tat protein induces the activation of CD8+ T cells and affects in vivo the magnitude and kinetics of antiviral responses. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0077746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu Q., Wang J., Shen J., Liu M., Jin X., Tang G., Chu P.K. Intracellular pathways and nuclear localization signal peptide-mediated gene transfection by cationic polymeric nanovectors. Biomaterials. 2012;33(4):1135–1145. doi: 10.1016/j.biomaterials.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 121.Badding M.A., Vaughan E.E., Dean D.A. Transcription factor plasmid binding modulates microtubule interactions and intracellular trafficking during gene transfer. Gene Ther. 2012;19(3):338–346. doi: 10.1038/gt.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sacramento C.B., Moraes J.Z.d., Denapolis P., Han S.W. Gene, expression promoted by the SV40 DNA targeting sequence and the hypoxia-responsive element under normoxia and hypoxia. Braz. J. Med. Biol. Res. 2010;43(8):722–727. doi: 10.1590/s0100-879x2010007500064. [DOI] [PubMed] [Google Scholar]
- 123.Nayerossadat N., Maedeh T., Ali P.A. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012;1:27. doi: 10.4103/2277-9175.98152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Darquet A.M., Cameron B., Wils P., Scherman D., Crouzet J. A new DNA vehicle for nonviral gene delivery: supercoiled minicircle. Gene Ther. 1997;4(12):1341–1349. doi: 10.1038/sj.gt.3300540. [DOI] [PubMed] [Google Scholar]
- 125.Huang M., Chen Z., Hu S., Jia F., Li Z., Hoyt G., Robbins R.C., Kay M.A., Wu J.C. Novel minicircle vector for gene therapy in murine myocardial infarction. Circulation. 2009;120(11 Suppl):S230–S237. doi: 10.1161/CIRCULATIONAHA.108.841155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shen X., Pitol A.K., Bachmann V., Hacker D.L., Baldi L., Wurm F.M. A simple plasmid-based transient gene expression method using high five cells. J. Biotechnol. 2015;216:67–75. doi: 10.1016/j.jbiotec.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 127.Cervera L., Gutierrez-Granados S., Martinez M., Blanco J., Godia F., Segura M.M. Generation of HIV-1 Gag VLPs by transient transfection of HEK 293 suspension cell cultures using an optimized animal-derived component free medium. J. Biotechnol. 2013;166(4):152–165. doi: 10.1016/j.jbiotec.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 128.Sun J., Li D., Hao Y., Zhang Y., Fan W., Fu J., Hu Y., Liu Y., Shao Y. Posttranscriptional regulatory elements enhance antigen expression and DNA vaccine efficacy. DNA Cell Biol. 2009;28(5):233–240. doi: 10.1089/dna.2009.0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li K., Gao L., Gao H., Qi X., Gao Y., Qin L., Wang Y., Wang X. Codon optimization and woodchuck hepatitis virus posttranscriptional regulatory element enhance the immune responses of DNA vaccines against infectious bursal disease virus in chickens. Virus Res. 2013;175(2):120–127. doi: 10.1016/j.virusres.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 130.Grantham R., Gautier C., Gouy M., Mercier R., Pave A. Codon catalog usage and the genome hypothesis. Nucl. Acids Res. 1980;8(1):r49–r62. doi: 10.1093/nar/8.1.197-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nagata T., Uchijima M., Yoshida A., Kawashima M., Koide Y. Codon optimization effect on translational efficiency of DNA vaccine in mammalian cells: analysis of plasmid DNA encoding a CTL epitope derived from microorganisms. Biochem. Biophys. Res. Commun. 1999;261(2):445–451. doi: 10.1006/bbrc.1999.1050. [DOI] [PubMed] [Google Scholar]
- 132.Jacobs T.M., Yumerefendi H., Kuhlman B., Leaver-Fay A. SwiftLib: rapid degenerate-codon-library optimization through dynamic programming. Nucl. Acids Res. 2015;43(5) doi: 10.1093/nar/gku1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hannaman D., Dupuy L.C., Ellefsen B., Schmaljohn C.S. A Phase 1 clinical trial of a DNA vaccine for Venezuelan equine encephalitis delivered by intramuscular or intradermal electroporation. Vaccine. 2016;34(31):3607–3612. doi: 10.1016/j.vaccine.2016.04.077. [DOI] [PubMed] [Google Scholar]
- 134.Datta D., Bansal G.P., Gerloff D.L., Ellefsen B., Hannaman D., Kumar N. Immunogenicity and malaria transmission reducing potency of Pfs48/45 and Pfs25 encoded by DNA vaccines administered by intramuscular electroporation. Vaccine. 2017;35(2):264–272. doi: 10.1016/j.vaccine.2016.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stachyra A., Redkiewicz P., Kosson P., Protasiuk A., Gora-Sochacka A., Kudla G., Sirko A. Codon optimization of antigen coding sequences improves the immune potential of DNA vaccines against avian influenza virus H5N1 in mice and chickens. Virol. J. 2016;13(1):143. doi: 10.1186/s12985-016-0599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Deans T.L., Singh A., Gibson M., Elisseeff J.H. Regulating synthetic gene networks in 3D materials. Proc. Natl. Acad. Sci. U.S.A. 2012;109(38):15217–15222. doi: 10.1073/pnas.1204705109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Deans T.L., Cantor C.R., Collins J.J. A tunable genetic switch based on RNAi and repressor proteins for regulating gene expression in mammalian cells. Cell. 2007;130(2):363–372. doi: 10.1016/j.cell.2007.05.045. [DOI] [PubMed] [Google Scholar]
- 138.Smanski M.J., Bhatia S., Zhao D., Park Y., Woodruff L B.A., Giannoukos G., Ciulla D., Busby M., Calderon J., Nicol R., Gordon D.B., Densmore D., Voigt C.A. Functional optimization of gene clusters by combinatorial design and assembly. Nat. Biotechnol. 2014;32(12):1241–1249. doi: 10.1038/nbt.3063. [DOI] [PubMed] [Google Scholar]
- 139.Brophy J.A.N., Voigt C.A. Principles of genetic circuit design. Nat. Methods. 2014;11(5):508–520. doi: 10.1038/nmeth.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brophy J.A., Voigt C.A. Antisense transcription as a tool to tune gene expression. Mol. Syst. Biol. 2016;12(1):854. doi: 10.15252/msb.20156540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Der B.S., Glassey E., Bartley B.A., Enghuus C., Goodman D.B., Gordon D.B., Voigt C.A., Gorochowski T.E. DNAplotlib: programmable visualization of genetic designs and associated data. ACS Synth. Biol. 2016 doi: 10.1021/acssynbio.6b00252. [DOI] [PubMed] [Google Scholar]
- 142.Temme K., Hill R., Segall-Shapiro T.H., Moser F., Voigt C.A. Modular control of multiple pathways using engineered orthogonal T7 polymerases. Nucl. Acids Res. 2012;40(17):8773–8781. doi: 10.1093/nar/gks597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Moon T.S., Lou C., Tamsir A., Stanton B.C., Voigt C.A. Genetic programs constructed from layered logic gates in single cells. Nature. 2012;491(7423):249–253. doi: 10.1038/nature11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kalams S.A., Parker S., Jin X., Elizaga M., Metch B., Wang M., Hural J., Lubeck M., Eldridge J., Cardinali M., Blattner W.A., Sobieszczyk M., Suriyanon V., Kalichman A., Weiner D.B., Baden L.R., Network N.H.V.T. Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0029231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kalams S.A., Parker S.D., Elizaga M., Metch B., Edupuganti S., Hural J., De Rosa S., Carter D.K., Rybczyk K., Frank I., Fuchs J., Koblin B., Kim D.H., Joseph P., Keefer M.C., Baden L.R., Eldridge J., Boyer J., Sherwat A., Cardinali M., Allen M., Pensiero M., Butler C., Khan A.S., Yan J., Sardesai N.Y., Kublin J.G., Weiner D.B., Network N.H.V.T. Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. J. Infect. Dis. 2013;208(5):818–829. doi: 10.1093/infdis/jit236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shedlock D.J., Tingey C., Mahadevan L., Hutnick N., Reuschel E.L., Kudchodkar S., Flingai S., Yan J., Kim J.J., Ugen K.E., Weiner D.B., Muthumani K. Co-administration of molecular adjuvants expressing NF-kappa B subunit p65/RelA or type-1 transactivator T-bet enhance antigen specific DNA vaccine-induced immunity. Vaccines (Basel) 2014;2(2):196–215. doi: 10.3390/vaccines2020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yu Y.Z., Ma Y., Xu W.H., Wang S., Sun Z.W. Combinations of various CpG motifs cloned into plasmid backbone modulate and enhance protective immunity of viral replicon DNA anthrax vaccines. Med. Microbiol. Immunol. 2015;204(4):481–491. doi: 10.1007/s00430-014-0359-9. [DOI] [PubMed] [Google Scholar]
- 148.He Z., Xu J., Tao W., Fu T., He F., Hu R., Jia L., Hong Y. A recombinant plasmid containing CpG motifs as a novel vaccine adjuvant for immune protection against herpes simplex virus 2. Mol. Med. Rep. 2016;14(2):1823–1828. doi: 10.3892/mmr.2016.5439. [DOI] [PubMed] [Google Scholar]
- 149.Verthelyi D. Springer; 2006. Adjuvant Properties of CpG Oligonucleotides in Primates, DNA Vaccines; pp. 139–158. [DOI] [PubMed] [Google Scholar]
- 150.Kojima Y., Xin K.-Q., Ooki T., Hamajima K., Oikawa T., Shinoda K., Ozaki T., Hoshino Y., Jounai N., Nakazawa M. Adjuvant effect of multi-CpG motifs on an HIV-1 DNA vaccine. Vaccine. 2002;20(23–24):2857–2865. doi: 10.1016/s0264-410x(02)00238-4. [DOI] [PubMed] [Google Scholar]
- 151.Liu M.A., Ulmer J.B. Human clinical trials of plasmid DNA vaccines. Adv. Genet. 2005;55:25–40. doi: 10.1016/S0065-2660(05)55002-8. [DOI] [PubMed] [Google Scholar]
- 152.Dale C.J., Thomson S., De Rose R., Ranasinghe C., Medveczky C.J., Pamungkas J., Boyle D.B., Ramshaw I.A., Kent S.J. Springer; 2006. Prime-boost Strategies in DNA Vaccines, DNA Vaccines; pp. 171–197. [DOI] [PubMed] [Google Scholar]
- 153.Racz R., Li X., Patel M., Xiang Z., He Y. DNAVaxDB: the first web-based DNA vaccine database and its data analysis. BMC Bioinf. 2014;15(4):S2. doi: 10.1186/1471-2105-15-S4-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pereira V.B., Zurita-Turk M., Saraiva T.D.L., De Castro C.P., Souza B.M., Agresti P.M., Lima F.A., Pfeiffer V.N., Azevedo M.S.P., Rocha C.S. DNA vaccines approach: from concepts to applications. World J. Vaccines. 2014;2014 [Google Scholar]
- 155.Redding L., Weiner D.B. DNA vaccines in veterinary use. Exp. Rev. Vaccines. 2009;8(9):1251–1276. doi: 10.1586/erv.09.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kutzler M.A., Weiner D.B. DNA vaccines: ready for prime time? Nat. Rev. Genet. 2008;9(10):776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nakayama Y., Aruga A. Comparison of current regulatory status for gene-based vaccines in the US, Europe and Japan. Vaccines. 2015;3(1):186–202. doi: 10.3390/vaccines3010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Thompson M., Heath S.L., Sweeton B., Williams K., Cunningham P., Keele B.F., Sen S., Palmer B.E., Chomont N., Xu Y. DNA/MVA vaccination of HIV-1 infected participants with viral suppression on antiretroviral therapy, followed by treatment interruption: elicitation of immune responses without control of re-emergent virus. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0163164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nilsson C., Hejdeman B., Godoy-Ramirez K., Tecleab T., Scarlatti G., Bråve A., Earl P.L., Stout R.R., Robb M.L., Shattock R.J. HIV-DNA given with or without intradermal electroporation is safe and highly immunogenic in healthy swedish HIV-1 DNA/MVA vaccinees: a phase I randomized trial. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0131748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.C.f.D. Control, Prevention, Recommended Immunization Schedule for Children and Adolescents 18 years or Younger, United States 2017, 2017.
- 161.McHugh K.J., Nguyen T.D., Linehan A.R., Yang D., Behrens A.M., Rose S., Tochka Z.L., Tzeng S.Y., Norman J.J., Anselmo A.C. Fabrication of fillable microparticles and other complex 3D microstructures. Science. 2017;357(6356):1138–1142. doi: 10.1126/science.aaf7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee Y.N., Kim M.C., Lee Y.T., Hwang H.S., Cho M.K., Lee J.S., Ko E.J., Kwon Y.M., Kang S.M. AS04-adjuvanted virus-like particles containing multiple M2 extracellular domains of influenza virus confer improved protection. Vaccine. 2014;32(35):4578–4585. doi: 10.1016/j.vaccine.2014.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Leung T.F., Liu A.P., Lim F.S., Thollot F., Oh H.M., Lee B.W., Rombo L., Tan N.C., Rouzier R., Friel D., De Muynck B., De Simoni S., Suryakiran P., Hezareh M., Folschweiller N., Thomas F., Struyf F. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine administered according to 2- and 3-dose schedules in girls aged 9–14 years: Results to month 12 from a randomized trial. Hum. Vaccine Immunother. 2015;11(7):1689–1702. doi: 10.1080/21645515.2015.1050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Apter D., Wheeler C.M., Paavonen J., Castellsague X., Garland S.M., Skinner S.R., Naud P., Salmeron J., Chow S.N., Kitchener H.C., Teixeira J.C., Jaisamrarn U., Limson G., Szarewski A., Romanowski B., Aoki F.Y., Schwarz T.F., Poppe W.A., Bosch F.X., Mindel A., de Sutter P., Hardt K., Zahaf T., Descamps D., Struyf F., Lehtinen M., Dubin G., Group H.P.S. Efficacy of human papillomavirus 16 and 18 (HPV-16/18) AS04-adjuvanted vaccine against cervical infection and precancer in young women: final event-driven analysis of the randomized, double-blind PATRICIA trial. Clin. Vaccine Immunol. 2015;22(4):361–373. doi: 10.1128/CVI.00591-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Song P., He S., Zhou A., Lv G., Guo J., Zhou J., Han Y., Zhou H., Hao Z., Cong H. Vaccination with toxofilin DNA in combination with an alum-monophosphoryl lipid A mixed adjuvant induces significant protective immunity against Toxoplasma gondii. BMC Infect. Dis. 2017;17(1):19. doi: 10.1186/s12879-016-2147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lindemann M., Zaslavskaya M., Fiedler M., Wilde B., Heinemann F.M., Heinold A., Horn P.A., Witzke O. Humoral and cellular responses to a single dose of fendrix in renal transplant recipients with non-response to previous hepatitis B vaccination. Scand. J. Immunol. 2017;85(1):51–57. doi: 10.1111/sji.12497. [DOI] [PubMed] [Google Scholar]
- 167.Pion C., Courtois V., Husson S., Bernard M.C., Nicolai M.C., Talaga P., Trannoy E., Moste C., Sodoyer R., Legastelois I. Characterization and immunogenicity in mice of recombinant influenza haemagglutinins produced in Leishmania tarentolae. Vaccine. 2014;32(43):5570–5576. doi: 10.1016/j.vaccine.2014.07.092. [DOI] [PubMed] [Google Scholar]
- 168.Leroux-Roels I., Devaster J.M., Leroux-Roels G., Verlant V., Henckaerts I., Moris P., Hermand P., Van Belle P., Poolman J.T., Vandepapeliere P., Horsmans Y. Adjuvant system AS02V enhances humoral and cellular immune responses to pneumococcal protein PhtD vaccine in healthy young and older adults: randomised, controlled trials. Vaccine. 2015;33(4):577–584. doi: 10.1016/j.vaccine.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 169.Gray G.E., Mayer K.H., Elizaga M.L., Bekker L.G., Allen M., Morris L., Montefiori D., De Rosa S.C., Sato A., Gu N., Tomaras G.D., Tucker T., Barnett S.W., Mkhize N.N., Shen X., Downing K., Williamson C., Pensiero M., Corey L., Williamson A.L. Subtype C gp140 vaccine boosts immune responses primed by the South African AIDS vaccine initiative DNA-C2 and MVA-C HIV vaccines after more than a 2-Year Gap. Clin. Vaccine Immunol. 2016;23(6):496–506. doi: 10.1128/CVI.00717-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Treanor J.J., Chu L., Essink B., Muse D., El Sahly H.M., Izikson R., Goldenthal K.L., Patriarca P., Dunkle L.M. Stable emulsion (SE) alone is an effective adjuvant for a recombinant, baculovirus-expressed H5 influenza vaccine in healthy adults: a phase 2 trial. Vaccine. 2017;35(6):923–928. doi: 10.1016/j.vaccine.2016.12.053. [DOI] [PubMed] [Google Scholar]
- 171.Howard L.M., Hoek K.L., Goll J.B., Samir P., Galassie A., Allos T.M., Niu X., Gordy L.E., Creech C.B., Prasad N., Jensen T.L., Hill H., Levy S.E., Joyce S., Link A.J., Edwards K.M. Cell-based systems biology analysis of human AS03-adjuvanted H5N1 avian influenza vaccine responses: a phase I randomized controlled Trial. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0167488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rts S.C.T.P. Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386(9988):31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Olotu A., Fegan G., Wambua J., Nyangweso G., Leach A., Lievens M., Kaslow D.C., Njuguna P., Marsh K., Bejon P. Seven-year efficacy of RTS, S/as01 malaria vaccine among young African children. N. Engl. J. Med. 2016;374(26):2519–2529. doi: 10.1056/NEJMoa1515257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lal H., Cunningham A.L., Godeaux O., Chlibek R., Diez-Domingo J., Hwang S.J., Levin M.J., McElhaney J.E., Poder A., Puig-Barbera J., Vesikari T., Watanabe D., Weckx L., Zahaf T., Heineman T.C., Group Z.O.E.S. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 2015;372(22):2087–2096. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 175.Woodworth J.S., Cohen S.B., Moguche A.O., Plumlee C.R., Agger E.M., Urdahl K.B., Andersen P. Subunit vaccine H56/CAF01 induces a population of circulating CD4 T cells that traffic into the Mycobacterium tuberculosis-infected lung. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hamborg M., Kramer R., Schante C.E., Agger E.M., Christensen D., Jorgensen L., Foged C., Middaugh C.R. The physical stability of the recombinant tuberculosis fusion antigens h1 and h56. J. Pharm. Sci. 2013;102(10):3567–3578. doi: 10.1002/jps.23669. [DOI] [PubMed] [Google Scholar]
- 177.McKay P.F., Cope A.V., Mann J.F., Joseph S., Esteban M., Tatoud R., Carter D., Reed S.G., Weber J., Shattock R.J. Glucopyranosyl lipid A adjuvant significantly enhances HIV specific T and B cell responses elicited by a DNA-MVA-protein vaccine regimen. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0084707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Oh J.Z., Ravindran R., Chassaing B., Carvalho F.A., Maddur M.S., Bower M., Hakimpour P., Gill K.P., Nakaya H.I., Yarovinsky F., Sartor R.B., Gewirtz A.T., Pulendran B. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity. 2014;41(3):478–492. doi: 10.1016/j.immuni.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bardel E., Doucet-Ladeveze R., Mathieu C., Harandi A.M., Dubois B., Kaiserlian D. Intradermal immunisation using the TLR3-ligand Poly (I:C) as adjuvant induces mucosal antibody responses and protects against genital HSV-2 infection. NPJ Vaccines. 2016;1:16010. doi: 10.1038/npjvaccines.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Azuma M., Takeda Y., Nakajima H., Sugiyama H., Ebihara T., Oshiumi H., Matsumoto M., Seya T. Biphasic function of TLR3 adjuvant on tumor and spleen dendritic cells promotes tumor T cell infiltration and regression in a vaccine therapy. Oncoimmunology. 2016;5(8) doi: 10.1080/2162402X.2016.1188244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Janssen J.M., Jackson S., Heyward W.L., Janssen R.S. Immunogenicity of an investigational hepatitis B vaccine with a toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared with a licensed hepatitis B vaccine in subpopulations of healthy adults 18–70 years of age. Vaccine. 2015;33(31):3614–3618. doi: 10.1016/j.vaccine.2015.05.070. [DOI] [PubMed] [Google Scholar]
- 182.Cross A.S., Greenberg N., Billington M., Zhang L., DeFilippi C., May R.C., Bajwa K.K. Phase 1 testing of detoxified LPS/group B meningococcal outer membrane protein vaccine with and without synthetic CPG 7909 adjuvant for the prevention and treatment of sepsis. Vaccine. 2015;33(48):6719–6726. doi: 10.1016/j.vaccine.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Geldenhuys H., Mearns H., Miles D.J., Tameris M., Hokey D., Shi Z., Bennett S., Andersen P., Kromann I., Hoff S.T., Hanekom W.A., Mahomed H., Hatherill M., Scriba T.J., Group H.I.T.S., van Rooyen M., Bruce McClain J., Ryall R., de Bruyn G., Group H.I.T.S. The tuberculosis vaccine H4:IC31 is safe and induces a persistent polyfunctional CD4 T cell response in South African adults: a randomized controlled trial. Vaccine. 2015;33(30):3592–3599. doi: 10.1016/j.vaccine.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 184.Baldwin S.L., Roeffen W., Singh S.K., Tiendrebeogo R.W., Christiansen M., Beebe E., Carter D., Fox C.B., Howard R.F., Reed S.G., Sauerwein R., Theisen M. Synthetic TLR4 agonists enhance functional antibodies and CD4+ T-cell responses against the Plasmodium falciparum GMZ2.6C multi-stage vaccine antigen. Vaccine. 2016;34(19):2207–2215. doi: 10.1016/j.vaccine.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 185.Halliday A., Turner J.D., Guimaraes A., Bates P.A., Taylor M.J. The TLR2/6 ligand PAM2CSK4 is a Th2 polarizing adjuvant in Leishmania major and Brugia malayi murine vaccine models. Parasit Vectors. 2016;9:96. doi: 10.1186/s13071-016-1381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Buonsanti C., Balocchi C., Harfouche C., Corrente F., Galli Stampino L., Mancini F., Tontini M., Malyala P., Bufali S., Baudner B., De Gregorio E., Valiante N.M., O'Hagan D.T., Rappuoli R., D'Oro U. Novel adjuvant Alum-TLR7 significantly potentiates immune response to glycoconjugate vaccines. Sci. Rep. 2016;6:29063. doi: 10.1038/srep29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.van Haren S.D., Dowling D.J., Foppen W., Christensen D., Andersen P., Reed S.G., Hershberg R.M., Baden L.R., Levy O. Age-specific adjuvant synergy: dual TLR7/8 and mincle activation of human newborn dendritic cells enables Th1 polarization. J. Immunol. 2016;197(11):4413–4424. doi: 10.4049/jimmunol.1600282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Smith A.J., Li Y., Bazin H.G., St-Jean J.R., Larocque D., Evans J.T., Baldridge J.R. Evaluation of novel synthetic TLR7/8 agonists as vaccine adjuvants. Vaccine. 2016;34(36):4304–4312. doi: 10.1016/j.vaccine.2016.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fox C.B., Orr M.T., Van Hoeven N., Parker S.C., Mikasa T.J., Phan T., Beebe E.A., Nana G.I., Joshi S.W., Tomai M.A., Elvecrog J., Fouts T.R., Reed S.G. Adsorption of a synthetic TLR7/8 ligand to aluminum oxyhydroxide for enhanced vaccine adjuvant activity: a formulation approach. J. Control. Release. 2016;244(Pt A):98–107. doi: 10.1016/j.jconrel.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Arthanari S., Mani G., Peng M.M., Jang H.T. Chitosan-HPMC-blended microspheres as a vaccine carrier for the delivery of tetanus toxoid. Artif. Cells Nanomed. Biotechnol. 2016;44(2):517–523. doi: 10.3109/21691401.2014.966193. [DOI] [PubMed] [Google Scholar]
- 191.Carroll E.C., Jin L., Mori A., Munoz-Wolf N., Oleszycka E., Moran H.B., Mansouri S., McEntee C.P., Lambe E., Agger E.M., Andersen P., Cunningham C., Hertzog P., Fitzgerald K.A., Bowie A.G., Lavelle E.C. The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity. 2016;44(3):597–608. doi: 10.1016/j.immuni.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bruffaerts N., Romano M., Denis O., Jurion F., Huygen K. Increasing the vaccine potential of live M. bovis BCG by coadministration with plasmid DNA encoding a tuberculosis prototype antigen. Vaccines (Basel) 2014;2(1):181–195. doi: 10.3390/vaccines2010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wan Y., Ren X., Ren Y., Wang J., Hu Z., Xie X., Xu J. As a genetic adjuvant, CTA improves the immunogenicity of DNA vaccines in an ADP-ribosyltransferase activity- and IL-6-dependent manner. Vaccine. 2014;32(19):2173–2180. doi: 10.1016/j.vaccine.2014.02.056. [DOI] [PubMed] [Google Scholar]
- 194.Qiu S., Ren X., Ben Y., Ren Y., Wang J., Zhang X., Wan Y., Xu J. Fusion-expressed CTB improves both systemic and mucosal T-cell responses elicited by an intranasal DNA priming/intramuscular recombinant vaccinia boosting regimen. J. Immunol. Res. 2014;2014 doi: 10.1155/2014/308732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Maity H.K., Dey S., Mohan C.M., Khulape S.A., Pathak D.C., Vakharia V.N. Protective efficacy of a DNA vaccine construct encoding the VP2 gene of infectious bursal disease and a truncated HSP70 of Mycobacterium tuberculosis in chickens. Vaccine. 2015;33(8):1033–1039. doi: 10.1016/j.vaccine.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 196.Ruan J., Duan Y., Li F., Wang Z. Enhanced synergistic anti-Lewis lung carcinoma effect of a DNA vaccine harboring a MUC1-VEGFR2 fusion gene used with GM-CSF as an adjuvant. Clin. Exp. Pharmacol. Physiol. 2017;44(1):71–78. doi: 10.1111/1440-1681.12654. [DOI] [PubMed] [Google Scholar]
- 197.Liu Y., Yin H., Zhao M., Lu Q. TLR2 and TLR4 in autoimmune diseases: a comprehensive review. Clin. Rev. Allergy Immunol. 2014;47(2):136–147. doi: 10.1007/s12016-013-8402-y. [DOI] [PubMed] [Google Scholar]