Abstract
Background
Oral daily tenofovir (TFV) disoproxil fumarate/emtricitabine (TDF/FTC) for human immunodeficiency virus (HIV) pre-exposure prophylaxis (PrEP) is highly effective for HIVprevention, yet long-term effects are not fully understood. We investigated the effects of PrEP on the rectal microbiome in a cohort of men who have sex with men (MSM).
Methods
This cross-sectional analysis included HIV-negative MSM either on PrEP (n = 37) or not (n = 37) selected from an ongoing cohort using propensity score matching. Rectal swabs were used to examine microbiome composition using 16S ribosomal ribonucleic acid gene sequencing, and associations between PrEP use and microbiota abundance were examined. Hair specimens were used to quantify TFV and FTC exposure over the past 6 weeks on a subset of participants (n = 15).
Results
Pre-exposure prophylaxis use was associated with a significant increase in Streptococcus abundance (adjusted P = .015). Similar associations were identified using least absolute shrinkage and selection operator (LASSO) regression, confirming the increase in Streptococcus and also showing increased Mitsuokella, Fusobacterium, and decreased Escherichia/Shigella. Increased Fusobacterium was significantly associated with increasing TFV exposure.
Conclusions
Oral TDF/FTC for PrEP is associated with rectal microbiome changes compared to well matched controls, specifically increased Streptococcus and Fusobacterium abundance. This study highlights the need for future investigations of the role of microbiome changes on HIV susceptibility and effectiveness of PrEP.
Keywords: men who have sex with men (MSM), pre-exposure prophylaxis (PrEP), rectal microbiome
Human immunodeficiency virus (HIV) transmission remains a global public health concern, with 1.8 million new HIV diagnoses globally and 38 739 new diagnoses in the United States in 2017 [1, 2]. Oral daily tenofovir (TFV) disoproxil fumarate/emtricitabine (TDF/FTC) is highly effective for the prevention of HIV-1 infection. This has been demonstrated in multiple placebo-controlled randomized clinical trials in men who have sex with men (MSM) [3], serodiscordant or other high-risk heterosexual men and women [4–7], and people who inject drugs [8]. Post hoc analyses of these and other studies have shown that efficacy is highly dependent upon adherence [9, 10], with minimum of 4 doses per week needed for protection among MSM [9]. Given its efficacy and general tolerability, uptake of oral PrEP has increased significantly since it was approved by the US Food and Drug Administration in 2012. Currently, there are an estimated 270 000 PrEP users in the United States [11].
One of the reasons PrEP has had such large uptake is that oral TDF/FTC has relatively few side effects and adverse events. However, it is not completely without risk. Known long-term risks associated with TDF/FTC for PrEP are nephrotoxicity and loss of bone mineral density, similar to those observed in HIV-positive persons using TDF/FTC as part of a combination antiretroviral treatment regimen [12–14]. Side effects, which are most commonly observed during the initial month of treatment and abate with continued use, include gastrointestinal upset, nausea, vomiting, or diarrhea; this is most common during the initial month of treatment and generally abates with continued use. Given the relatively recent approval of PrEP, other risks associated with long-term use of TDF/FTC alone are still under evaluation.
Mucosal tissue concentrations of PrEP drugs after oral TDF/FTC are 100-fold higher in the rectal mucosa compared with cervicovaginal tissues and can remain detectable for 14 days [15]. Although this likely allows for its efficacy in preventing mucosal HIV transmission, it also raises the question of how the presence of TDF/FTC may interact with commensal intestinal bacteria at that site. The trillions of bacteria residing in the gastrointestinal tract, commonly termed the microbiome, have an increasingly appreciated role in health and disease [16, 17], including in HIV acquisition and progression [18–23]. Dysbiosis, or changes in microbiome composition, has been associated with HIV infection and may not fully resolve with antiretroviral therapy (ART) [20, 24, 25], suggesting that ART itself could affect dysbiosis. At least 2 studies have also shown microbiome differences by ART regimen among treated HIV-infected individuals [25, 26]. Dubé et al [27] recently found microbiome changes in a small longitudinal study of MSM initiating PrEP over 48 weeks, although this analysis was unable to account for sexual behavior.
In this study, we investigate rectal microbiome composition in a cohort of young MSM on PrEP compared to well matched controls, and we use hair analyses to examine TFV and FTC exposure in relationship to the microbiome. More important, we matched controls using multiple clinical and behavioral factors, including sexual activity and substance use, which have been shown to independently affect dysbiosis in HIV [28, 29].
METHODS
Study Population
Participants were selected from an ongoing cohort study examining substance use and HIV in young HIV-positive and HIV-negative MSM (The mSTUDY [National Institute on Drug Abuse]). The mSTUDY was approved by the UCLA Office of the Human Research Protection Program Institutional Review Board, and all subjects provided written informed consent at study entry. All participants in The mSTUDY complete biannual visits consisting of history and examination, clinical laboratory tests, sexually transmitted infection screening, urine toxicology, specimen collection for biorepository, and detailed behavioral questionnaire. This cross-sectional study used specimens and data from HIV-negative mSTUDY participants on PrEP or not on PrEP collected at the baseline study visit. There were 200 available specimens from HIV-negative participants enrolled between August 2014 and July 2017; of these, 74 were included in this study. Inclusion criteria for the PrEP group (n = 37) was based on clinician review of medications and confirmed by self-report using a series of PrEP-related questions. Inclusion criteria for the control group was no self-report of current PrEP use and no history of PrEP or PEP in the last 6 months (n = 27 were excluded based on these criteria). From the remaining 136 eligible non-PrEP users, we selected a control group (n = 37) based on matching characteristics (see Statistical Analysis).
Rectal Specimen Collection and Processing
Rectal swabs (FLOQSwabs; Copan Diagnostics, Murrieta, CA) were collected under direct mucosal visualization via anoscopy without preparatory enema then immediately frozen neat at −80°C until processing in bulk. Samples were lysed by transferring to Lysing Matrix E tubes (MP Biomedicals, Burlingame, CA) containing RLT lysis buffer (QIAGEN, Hilden, Germany) and bead-beated on a TissueLyser (QIAGEN). Deoxyribonucleic acid (DNA) was then extracted using the AllPrep DNA/RNA/Protein kit (QIAGEN) per the manufacturer’s protocol.
16S Ribosomal Ribonucleic Acid Gene Sequencing and Microbiome Data Processing
Microbiome profiling was performed by sequencing the V4 region of the 16S ribosomal ribonucleic acid (rRNA) gene as previously described [30, 31]. In brief, the V4 region was amplified using Golay-barcoded primers 515F/806R in triplicate reactions. Negative controls from the DNA extraction and polymerase chain reaction (PCR) steps, as well as independent aliquots of a bacterial mock community, were processed in parallel to identify contaminant sequences and ensure data reproducibility. The PCR products were pooled and sequenced on the Illumina MiSeq platform using 2 × 150 base pair v2 chemistry. Sequences were demultiplexed with Golay error correction using QIIME v1.9.1 [32]. Divisive Amplicon Denoising Algorithm (DADA2) version 1.8 was used for error correction, exact sequence inference, read merging, and chimera removal [33]. The resultant amplicon sequence variant (ASV) table comprised 3 576 558 total merged read pairs (mean per sample = 48 332; range, 10 906 to 96 109) after removal of contaminant ASVs, defined as those with at least 10% of their total abundance derived from negative control samples. Rarefaction was performed at a depth of 10 906 reads for the corresponding analyses (zero-inflated negative binomial, alpha diversity). For all other analyses, counts were transformed to relative abundances. Taxonomic assignment was done using RDP trainset 16 [34]. All sequence data has been deposited into BioProject with the accession number PRNJA422134.
Hair Analysis for Drug Concentration
Scalp hair samples collected as part of the standard mSTUDY protocol were available from this study visit for n = 15 of the participants on PrEP. The TFV and FTC concentrations were quantified by validated liquid chromatography-tandem mass spectrometry methods with multiple reaction monitoring mode in the UCSF Hair Analytical Laboratory (HAL) using the proximal 1.5 cm of hair (representing approximately 6 weeks of drug exposure) [35].
Statistical Analyses
Control participants were selected using 1:1 propensity score (PS) matching of PrEP users to nonusers. Propensity scores were estimated using logistic regression; covariates included in the PS model are detailed in Table 1. The PrEP users were then individually matched to nonusers using the “optimal” matching algorithm, which minimizes the average absolute distance across all matched pairs (R package “MatchIt”) [36]. To assess the quality of matches, we calculated the standardized mean difference in each covariate after matching and tested for significant differences in any covariate between groups (Table 1).
Table 1.
Study Participant Characteristics
| Participant Characteristics | Control (n = 37) | PrEP (n = 37) | P Valuea | SMD |
|---|---|---|---|---|
| Age [mean (sd), median] | 30.1 (6.7), 29 | 29.8 (6.5), 28 | .85 | .05 |
| Race/Ethnicity (n, %) | .90 | .12 | ||
| Black Non-Hispanic | 19 (51.4) | 21 (56.8) | ||
| Hispanic | 13 (35.1) | 12 (32.4) | ||
| Other Non-Hispanic | 5 (13.5) | 4 (10.8) | ||
| Obese (BMI >30 or waist >40 inches) (n, %) | 17 (45.9) | 13 (35.1) | .88 | .22 |
| Duration of PrEP use (weeks) [mean (sd), median] | NA | 54.4 (56), 40.9 | NA | NA |
| Recent RAI (7 days) (n, %) | 16 (43.2) | 17 (45.9) | .99 | .05 |
| Number of RAI acts (30 days) [mean (sd), median] | 2.1 (5.5), 0 | 2.2 (4.5), 0 | .91 | .03 |
| Methamphetamine useb (n, %) | .99 | .07 | ||
| Daily | 1 (2.7) | 1 (2.7) | ||
| Weekly | 0 (0.0) | 0 (0.0) | ||
| Monthly/less | 7 (18.9) | 6 (16.2) | ||
| Never | 29 (78.4) | 30 (81.1) | ||
| Marijuana use (n, %) | .77 | .26 | ||
| Daily | 11 (29.7) | 9 (24.3) | ||
| Weekly | 6 (16.2) | 5 (13.5) | ||
| Monthly/less | 13 (35.1) | 12 (32.4) | ||
| Never | 7 (18.9) | 11 (29.7) | ||
| Smoking (n, %) | .70 | .20 | ||
| >1 pack/day | 2 (5.4) | 1 (2.7) | ||
| <1 pack/day | 11 (29.7) | 9 (24.3) | ||
| Nonsmoker | 24 (64.9) | 27 (73.0) | ||
| Binge alcohol use (n, %) | .99 | .06 | ||
| Weekly | 1 (2.7) | 1 (2.7) | ||
| Monthly/less | 26 (70.3) | 27 (73.0) | ||
| Never | 10 (27.0) | 9 (24.3) | ||
| Antibiotic use in past monthc (n, %) | 4 (10.8) | 4 (10.8) | .99 | 0 |
Abbreviations: BMI, body mass index; NA, not applicable; PrEP, pre-exposure prophylaxis; RAI, receptive anal intercourse; sd, standard deviation; SMD, standardized mean difference.
a t tests were used for continuous variables, and Fisher’s exact tests were used for categorical variables.
bSubstance use data refers to past 6 month and was collected by self-report. Binge alcohol refers to >6 drinks at a time on more than 1 occasion.
cAntibiotics included single-dose azithromycin, ceftriaxone, or doxycycline only.
Only genera with at least 1% relative abundance in 10% of samples were included for differential testing, resulting in a total of 29 genera. ZINB models were used to test for differential abundance in bacterial genera between groups with multinomial least absolute shrinkage and selection operator (LASSO) models used as a confirmatory analysis (R packages “pscl” and “glmnet”). Correction for multiple hypothesis testing was performed using the Benjamini-Hochberg false discovery rate method. All statistical analyses were performed using R v3.4.3.
RESULTS
Participant Characteristics and Matching
A total of n = 74 HIV-negative participants were included in this cross-sectional study, with 37 participants on PrEP and 37 matched control participants not on PrEP. The average age was 30.3 years with 32.4% Hispanic and 52.7% non-Hispanic black. Given the significant influence of clinical and behavioral confounders on microbiome composition [28, 29], control participants were selected using 1:1 matching on age, race/ethnicity, obesity, substance use including methamphetamine, marijuana, alcohol, and tobacco, and receptive anal intercourse frequency. Participant characteristics are described in detail in Table 1.
Rectal Microbiome Composition in Pre-Exposure Prophylaxis (PrEP) Users Compared to Non-PrEP Users
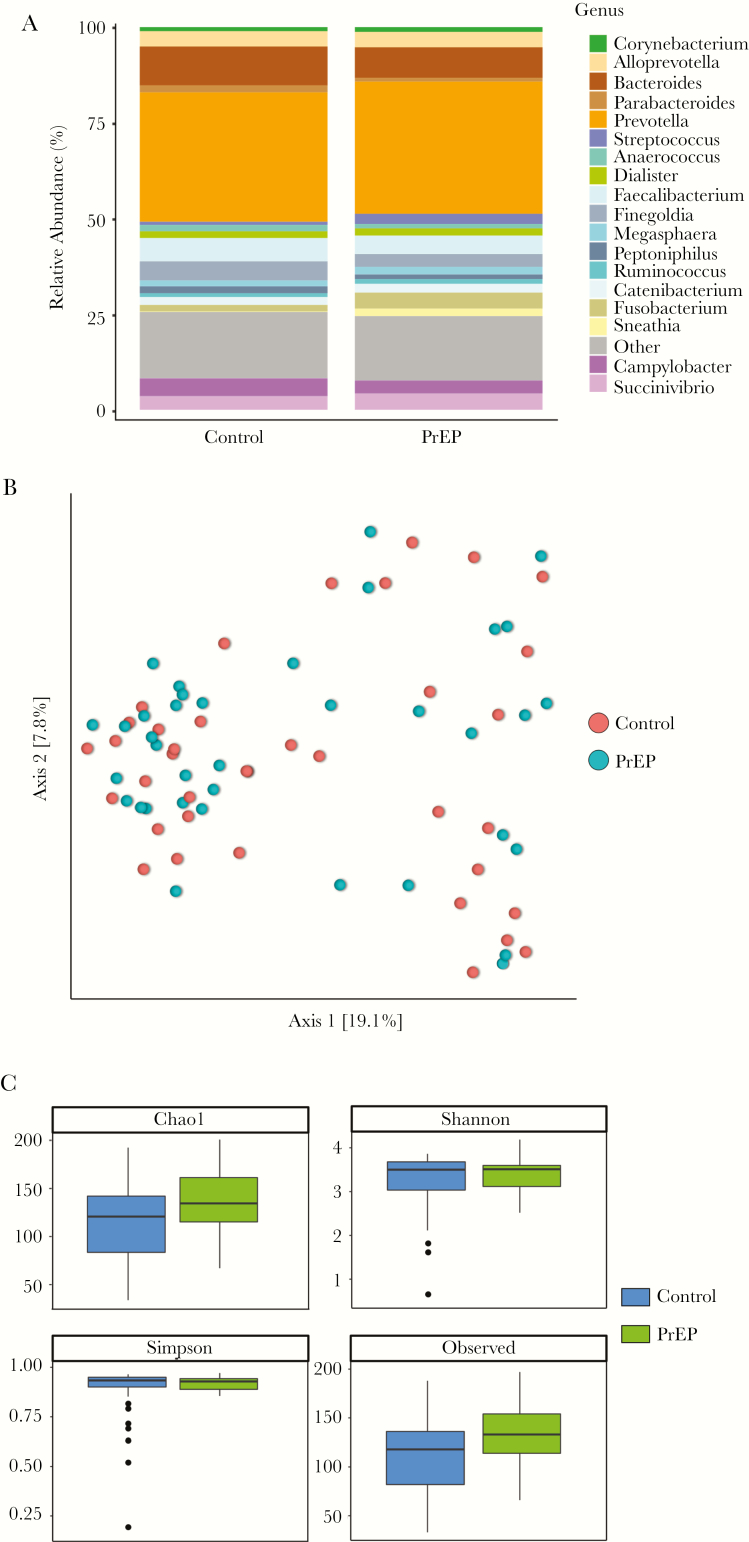
The median duration of PrEP use in our study population was 41 weeks (interquartile range, 14–59 weeks). Adherence was assessed in all participants using self-report and clinician review of medication records. We further examined adherence to PrEP by quantifying TFV and FTC levels in hair specimens in a subset of PrEP users (Table 2). Hair analysis in this case records cumulative exposure to PrEP drugs over a 6-week time period [35]. The average TFV level was 0.027 ng/mg hair, and FTC level was 0.42 ng/mg hair among the 15 participants with available hair specimens. Based on prior studies [35], these concentrations are associated with adherence of at least 4 doses per week in 73% of sampled participants. This is in agreement with the self-report adherence measures in this study, which showed 78% participants reporting very good or excellent adherence. We examined rectal microbiome composition between participants on PrEP compared to matched participants not on oral PrEP using targeted 16S rRNA gene sequencing. Averaged relative abundances of bacterial genera between the 2 groups are shown in Figure 1A. No significant differences were seen in overall composition (Figure 1B) or alpha diversity (Figure 1C) between PrEP users and nonusers.
Table 2.
TFV and FTC Concentrations in Hair Samplesa
| Hair Sample No. | Duration of PrEP (Weeks) | TFV Concentrationb (ng/mg Hair) | FTC Concentration (ng/mg Hair) |
|---|---|---|---|
| 1 | 32 | 0.026 | 0.582 |
| 2 | 10 | 0.026 | 0.477 |
| 3 | 57 | 0.014 | 0.121 |
| 4 | 25 | 0.050 | 0.619 |
| 5 | NA | BLQ | BLQ |
| 6 | 142 | 0.027 | 0.265 |
| 7 | 11 | 0.037 | 0.705 |
| 8 | 28 | 0.040 | 1.167 |
| 9 | 11 | 0.009 | 0.213 |
| 10 | 11 | 0.031 | 0.761 |
| 11 | 59 | 0.028 | 0.537 |
| 12 | 156 | 0.005 | 0.040 |
| 13 | 11 | 0.027 | 0.092 |
| 14 | 47 | 0.030 | 0.093 |
| 15 | 48 | 0.020 | 0.194 |
Abbreviations: BLQ, below lower limit of quantification; FTC, emtricitabine; NA, not available; PrEP, pre-exposure prophylaxis; TFV, tenofovir.
aHair samples were analyzed by high-performance liquid chromatography-tandem mass spectrometry.
bOn average, a TFV value of 0.038 ng/mg hair is consistent with dosing 7 days per week, and 0.023 ng/mg hair is consistent with dosing 4 days per week [35]. In our small subset, 73% (11 of 15) had TFV values indicating at least 4 days per week adherence to PrEP.
Figure 1.
Microbiome composition between pre-exposure prophylaxis (PrEP) and control participants. (A) Taxonomic distribution between PrEP and control participants. Stacked columns show averaged relative abundance as a percentage of total bacterial sequences at the genus level. (B) Principal coordinate analysis showed no difference in overall composition. (C) Alpha diversity measures were not different between PrEP and control participants.
Pre-Exposure Prophylaxis Use Associates With Differential Abundance of Specific Bacterial Genera
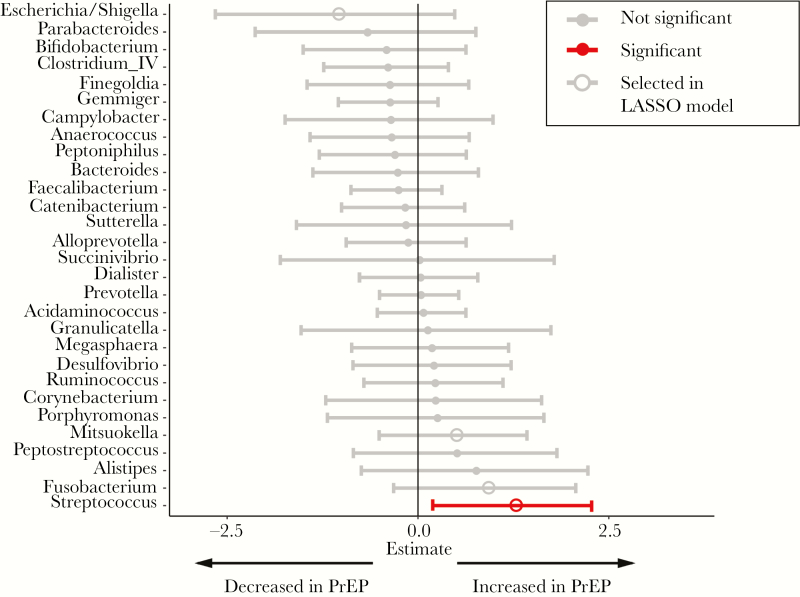
To examine whether specific bacterial genera were associated with PrEP use, we used zero-inflated negative binomial models (Figure 2). After P value adjustment, only increased relative abundance of Streptococcus (Padj = .02) was associated with PrEP use. To further examine these findings, we used binomial LASSO regression analyses. Using this model, we also found that PrEP use was associated with increased Streptococcus, Fusobacterium, Mitsuokella, and decreased Escherichia/Shigella abundance (Figure 2). Similar analyses performed at the species level showed no significant associations (data not shown).
Figure 2.
Associations between microbiome composition and pre-exposure prophylaxis (PrEP) use. Forest plot showing associations of specific bacterial genera with PrEP use using ZINB regression analysis of genera with 1% relative abundance in at least 10% of samples. Red indicates associations that remained statistically significant (P < .1) after false discovery rate adjustment. Open circles indicate bacterial genera that were also selected using least absolute shrinkage and selection operator (LASSO) regression.
Correlations Between Tenofovir and Emtricitabine Levels and Specific Bacterial Genera
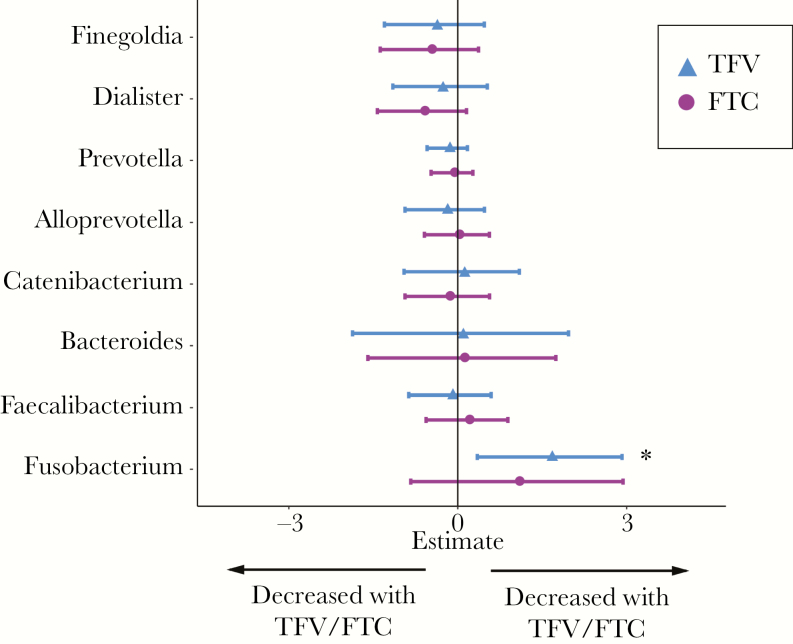
Finally, we examined correlations between specific bacterial genera and measured TFV and FTC levels in our subset of PrEP users with hair specimens. Despite the small sample size in this analysis, increasing TFV levels was significantly associated with an increased relative abundance of Fusobacterium (Padj = .008), suggesting that there may be a dose-response relationship between PrEP exposure and increased Fusobacterium abundance (Figure 3). A similar association was seen with FTC levels, although this finding did not reach statistical significance.
Figure 3.
Associations between microbiome composition and tenofovir (TFV) or emtricitabine (FTC) exposure levels. Forest plots showing associations of specific bacterial genera with TFV (blue) or FTC (purple) levels using ZINB regression analysis. * indicates associations that remained statistically significant (P < .1) after false discovery rate adjustment. Analysis was based on a limited subset of participants with available hair samples (n = 15).
DISCUSSION
The clinical consequences of PrEP in young healthy adults is an area of particular interest because this method of HIV prevention is highly effective and is a major focus of the new plan to End the HIV Epidemic in the United States [37]. In this study, we examined rectal microbiome changes between well matched MSM with and without PrEP use. No significant alterations were seen in overall microbiome composition or diversity; however, some specific taxa changes were noted in those on PrEP including increased Streptococcus, Fusobacterium, and Mitsuokella and decreased Escherichia/Shigella abundance. Moreover, Fusobacterium abundance correlated with long-term TFV exposure levels quantified by hair analysis.
Our study found significant increased abundance of Streptococcus in PrEP users compared with MSM not on PrEP (Figure 2). A recent study similarly identified significant associations between PrEP and Streptococcus abundance in the rectal microbiome of MSM [27], although it observed decreased Streptococcus after initiation of PrEP in 4 of 8 participants. Differences in our results and this study could be explained by study design (cross-sectional vs longitudinal), microbiome analysis method, and potential differences in confounders between study populations. Nonetheless, the similar identification of Streptococcus abundance with PrEP use in both studies suggests this may be an interaction worthy of further study.
We identified an increase in Fusobacterium abundance associated with PrEP use, which correlated with TFV levels in hair analysis (Figure 3). Among treated HIV-infected individuals, increased Fusobacterium has been associated with poor immune recovery even after adjustment for MSM status [38]. Fusobacterium has similarly been associated with other intestinal inflammatory conditions, including inflammatory bowel disease and proinflammatory gene expression in colorectal cancer [39, 40]. The mucosal immune consequence of these changes in the rectal microbiome in HIV-negative individuals on PrEP is not known. However, given the clinical associations between inflammation and HIV acquisition risk despite the use of TFV products [41], this may be an area of important further investigation.
The mechanism by which oral TDF/FTC for PrEP may alter the rectal microbiome composition is not clear. Multiple studies have recently highlighted the effects that multiple drug classes can have on gut microbiome variation, including commonly used medications such as metformin and nonsteroidal anti-inflammatory drugs [42–46]. One plausible explanation is off-target antibacterial activity of TDF/FTC. A recent high-throughput screen of drugs for anticommensal activity with 40 representative bacterial strains found that 24% of human-targeted drugs have antibacterial activity including 40% of antiviral drugs [42]. Another recent in vitro study specifically examined unintended antibacterial effects of commonly used antiretroviral drugs. This study did not show activity with TFV or FTC; however, drugs were only tested against Escherichia coli and Bacillus subtilis [47]. In the vaginal compartment, microbiota have been associated with decreased concentrations of oral antiretroviral drugs [48], and direct interactions between commensal bacteria and topical TFV have been demonstrated [49, 50]. Even in the absence of direct antibiotic effects, these drugs may alter local cellular or bacterial metabolic activity or gene expression such that certain bacterial communities are favored.
Interactions with the microbiome can also alter systemic drug function, including response to cancer therapeutics [51], digoxin [52], metformin [46], and topical TFV gel [49, 50]. In the case of metformin, changes in the microbiome appear necessary for drug activity in a study using germ-free mice [46]. Klatt et al [49] showed that specific vaginal bacteria such as Gardnerella vaginalis metabolize TFV leading to decreased efficacy of TFV gel microbicide for HIV prevention in women. Taneva et al [50] similarly found that Lactobacillus crispatus and G vaginalis decreased TFV bioavailability and anti-HIV activity, respectively, in women using TFV gel. Because our study was cross-sectional and modest in size, we could not examine the relationship between the microbiome and PrEP effectiveness. However, defining the role of the microbiome in PrEP efficacy may be an important consideration in future clinical trials of PrEP therapeutics. Microbiome interactions may also contribute to drug side effects and even toxicities. Oral TDF/FTC for PrEP is associated with varying gastrointestinal symptoms during the first month of use, often termed PrEP “start-up syndrome”. It is possible that these symptoms occur due to PrEP-associated microbiome alterations and abate as the gastrointestinal tract acclimates to these changes.
Our study has limitations that should be considered while interpreting the results. The overall study size is modest (n = 74); however, this study is the largest study to date to examine microbiome changes in HIV-negative MSM on oral PrEP. More important, we used 1:1 matching on a multitude of important clinical and behavioral confounders, including sexual behavior, which greatly strengthens the study results. However, we cannot fully exclude the possibility of residual confounding from additional unidentified factors. Furthermore, we did not have dietary data available, and we were unable to account for this potentially important confounder. Finally, the cross-sectional design limits our ability to precisely attribute the observed microbiome differences solely to PrEP use. Our ability to detect dose-response relationships between increasing TFV or FTC drug levels and the abundance of specific bacterial taxa does provide further evidence to support the relationship between PrEP use and these microbiome changes.
CONCLUSIONS
This study adds to the growing literature highlighting xenobiotic-microbiome interactions. It is the largest such study to specifically examine the effects of oral TDF/FTC for PrEP on the gut microbiome. We identified several specific bacterial taxa associated with PrEP, including Streptococcus, which was similarly identified in a prior study [27], and also showed a dose-dependent association between TFV exposure and Fusobacterium abundance. With increasing efforts focused on expanding PrEP use for HIV prevention, it will be important to consider these potential interactions with the microbiome and continue research to better understand their clinical consequences.
Acknowledgments
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was funded by the National Institutes of Health (NIH) (Grants U01 DA036267 [to P. M. G. and S. S.] and R36 DA046310 [to R. R. C.]), National Institute of Mental Health (NIMH) (Grant P30 MH058107; to S. S.), and National Institute of Allergy and Infectious Disease (NIAID) (Grants K08 AI124979 [to J. A. F.] and 2R01AI098472 [to M. G.]). Additional support was provided by the UCLA AIDS Institute and UCLA CFAR Microbiome and Mucosal Immunology Core (Grant P30 AI028697). Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by NIAID of the NIH under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the NIMH.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: CROI 2019: 26th Conference on Retroviruses and Opportunistic Infections, March 4–7, 2019, Seattle, WA.
References
- 1. Centers for Disease Control and Prevention. HIV Surveillance Report Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 30 January 2019. [Google Scholar]
- 2. UNAIDS. UNAIDS Data 2018. Available at: http://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf. Accessed 30 January 2019.
- 3. Grant RM, Lama JR, Anderson PL, et al. ; iPrEx Study Team Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baeten JM, Donnell D, Ndase P, et al. ; Partners PrEP Study Team Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thigpen MC, Kebaabetswe PM, Paxton LA, et al. ; TDF2 Study Group Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 6. Marrazzo JM, Ramjee G, Richardson BA, et al. ; VOICE Study Team Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Damme L, Corneli A, Ahmed K, et al. ; FEM-PrEP Study Group Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choopanya K, Martin M, Suntharasamai P, et al. ; Bangkok Tenofovir Study Group Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381:2083–90. [DOI] [PubMed] [Google Scholar]
- 9. Anderson PL, Glidden DV, Liu A, et al. ; iPrEx Study Team Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koss CA, Bacchetti P, Hillier SL, et al. Differences in cumulative exposure and adherence to tenofovir in the VOICE, iPrEx OLE, and PrEP demo studies as determined via hair concentrations. AIDS Res Hum Retroviruses 2017; 33:778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. AVAC. PrEPWatch Available at: https://www.prepwatch.org. Accessed 2 July 2019.
- 12. Tang EC, Vittinghoff E, Anderson PL, et al. Changes in kidney function associated with daily tenofovir disoproxil fumarate/emtricitabine for HIV preexposure prophylaxis use in the United States demonstration project. J Acquir Immune Defic Syndr 2018; 77:193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gandhi M, Glidden DV, Mayer K, et al. Association of age, baseline kidney function, and medication exposure with declines in creatinine clearance on pre-exposure prophylaxis: an observational cohort study. Lancet HIV 2016; 3:e521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mulligan K, Glidden DV, Anderson PL, et al. ; Preexposure Prophylaxis Initiative Study Team Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2015; 61:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med 2011; 3:112re4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med 2016; 375:2369–79. [DOI] [PubMed] [Google Scholar]
- 17. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell 2014; 157:121–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dillon SM, Lee EJ, Kotter CV, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol 2014; 7:983–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lozupone CA, Li M, Campbell TB, et al. Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe 2013; 14:329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cook RR, Fulcher JA, Tobin NH, et al. Effects of HIV viremia on the gastrointestinal microbiome of young MSM. AIDS 2019; 33:793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mutlu EA, Keshavarzian A, Losurdo J, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog 2014; 10:e1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McHardy IH, Li X, Tong M, et al. HIV Infection is associated with compositional and functional shifts in the rectal mucosal microbiota. Microbiome 2013; 1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vujkovic-Cvijin I, Dunham RM, Iwai S, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med 2013; 5:193ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lozupone CA, Rhodes ME, Neff CP, et al. HIV-induced alteration in gut microbiota: driving factors, consequences, and effects of antiretroviral therapy. Gut Microbes 2014; 5:562–70. [DOI] [PubMed] [Google Scholar]
- 25. Pinto-Cardoso S, Lozupone C, Briceño O, et al. Fecal bacterial communities in treated HIV infected individuals on two antiretroviral regimens. Sci Rep 2017; 7:43741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Villanueva-Millán MJ, Pérez-Matute P, Recio-Fernández E, et al. Differential effects of antiretrovirals on microbial translocation and gut microbiota composition of HIV-infected patients. J Int AIDS Soc 2017; 20:21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dubé MP, Park SY, Ross H, et al. Daily HIV pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate-emtricitabine reduced Streptococcus and increased Erysipelotrichaceae in rectal microbiota. Sci Rep 2018; 8:15212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Noguera-Julian M, Rocafort M, Guillén Y, et al. Gut microbiota linked to sexual preference and HIV infection. EBioMedicine 2016; 5:135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fulcher JA, Hussain SK, Cook R, et al. Effects of substance use and sex practices on the intestinal microbiome during HIV-1 infection. J Infect Dis 2018; 218:1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bender JM, Li F, Martelly S, et al. Maternal HIV infection influences the microbiome of HIV-uninfected infants. Sci Transl Med 2016; 8:349ra100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pannaraj PS, Li F, Cerini C, et al. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr 2017; 171:647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010; 7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Callahan BJ, McMurdie PJ, Rosen MJ, et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 2016; 13:581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 2007; 73:5261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu AY, Yang Q, Huang Y, et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP). PLoS One 2014; 9:e83736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ho D, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. Journal of Statistical Software 2011; 42:28. [Google Scholar]
- 37. Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–5. [DOI] [PubMed] [Google Scholar]
- 38. Lee SC, Chua LL, Yap SH, et al. Enrichment of gut-derived Fusobacterium is associated with suboptimal immune recovery in HIV-infected individuals. Sci Rep 2018; 8:14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013; 14:207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tahara T, Hirata I, Nakano N, et al. Potential link between Fusobacterium enrichment and DNA methylation accumulation in the inflammatory colonic mucosa in ulcerative colitis. Oncotarget 2017; 8:61917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Naranbhai V, Abdool Karim SS, Altfeld M, et al. ; CAPRISA004 TRAPS team Innate immune activation enhances HIV acquisition in women, diminishing the effectiveness of tenofovir microbicide gel. J Infect Dis 2012; 206:993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018; 555:623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Forslund K, Hildebrand F, Nielsen T, et al. ; MetaHIT consortium Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015; 528:262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Falony G, Joossens M, Vieira-Silva S, et al. Population-level analysis of gut microbiome variation. Science 2016; 352:560–4. [DOI] [PubMed] [Google Scholar]
- 45. Rogers MAM, Aronoff DM. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin Microbiol Infect 2016; 22:178.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med 2017; 23:850–8. [DOI] [PubMed] [Google Scholar]
- 47. Shilaih M, Angst DC, Marzel A, et al. Antibacterial effects of antiretrovirals, potential implications for microbiome studies in HIV. Antivir Ther 2018; 23:91–4. [DOI] [PubMed] [Google Scholar]
- 48. Donahue Carlson R, Sheth AN, Read TD, et al. The female genital tract microbiome is associated with vaginal antiretroviral drug concentrations in human immunodeficiency virus-infected women on antiretroviral therapy. J Infect Dis 2017; 216:990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Klatt NR, Cheu R, Birse K, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 2017; 356:938–45. [DOI] [PubMed] [Google Scholar]
- 50. Taneva E, Sinclair S, Mesquita PM, et al. Vaginal microbiome modulates topical antiretroviral drug pharmacokinetics. JCI Insight 2018; 3:e99545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Helmink BA, Khan MAW, Hermann A, et al. The microbiome, cancer, and cancer therapy. Nat Med 2019; 25:377–88. [DOI] [PubMed] [Google Scholar]
- 52. Koppel N, Bisanz JE, Pandelia ME, Turnbaugh PJ, Balskus EP. Discovery and characterization of a prevalent human gut bacterial enzyme sufficient for the inactivation of a family of plant toxins. eLife 2018; 7:e33953. [DOI] [PMC free article] [PubMed] [Google Scholar]