Abstract
Background
Serratia marcescens is an important nosocomial pathogen and the characteristic property of resistance conferred by extended-spectrum beta-lactamase or a novel AmpC cephalosporinase was not unusual in Taiwan. This study investigated the trends in antimicrobial resistance in S. marcescens from a nationwide surveillance in Taiwan.
Materials and methods
S. marcescens isolates were collected biennially between 2002 and 2010 from medical centers and regional hospitals throughout Taiwan, as part of the Taiwan Surveillance of Antimicrobial Resistance program. Minimal inhibitory concentrations were determined by the Clinical and Laboratory Standards Institute reference broth microdilution method.
Results
A total of 403 nonduplicate S. marcescens isolates were collected, mostly from respiratory samples (157, 39.0%), followed by the urinary tract samples (90, 22.3%). Overall, 99.3% isolates were susceptible to imipenem, 93.8% to ceftazidime, 89.2% to minocycline, 87.8% to amikacin, 86.8% to cefepime, 82.9% to aztreonam, 73.2% to ceftriaxone, 72.7% to levofloxacin, 63.8% to ciprofloxacin, 60.8% to trimethoprim/sulfamethoxazole (TMP/SMX), and 59.6% to gentamicin. A significantly increased susceptibility rate after 2004 was observed for the following antibiotics: amikacin (73.8% vs. 97.1%), gentamicin (40.0% vs. 72.4%), ciprofloxacin (53.8% vs. 70.4%), ceftriaxone (53.8% vs. 86.0%), cefepime (74.4% vs. 95.1%), aztreonam (72.5% vs. 89.7%), and TMP/SMX (41.3% vs. 73.7%).
Conclusion
In this 8-year study, the susceptibility of S. marcescens to ceftazidime and imipenem remained consistently high in Taiwan. S. marcescens isolates demonstrated relatively higher resistance to ciprofloxacin and levofloxacin, and therefore continued surveillance of antimicrobial resistance, especially for fluoroquinolone, is warranted.
Keywords: Antimicrobial resistance, Serratia marcescens, Taiwan Surveillance of Antimicrobial Resistance
Introduction
Serratia species are identified as aerobic, motile Gram-negative rods, which occupy various habitats (mainly water, plants, and soil). Human infections by members of the genus Serratia were not well recognized until the latter half of the 20th century.1 Serratia marcescens accounts for the majority of isolates and appears to be a pathogen capable of causing a wide spectrum of clinical diseases, including wound infections, urinary tract infections, pneumonia, central nervous system infections such as meningitis, and bloodstream infections.1, 2, 3, 4, 5, 6 Although S. marcescens is a rare cause of community-acquired infections, it has emerged as an important nosocomial pathogen that has been cultured from a variety of sources, including disinfectants,7, 8, 9 pressure transducers,10 bronchoscopes,11 and multidose medication vials.12 Factors such as debilitating clinical condition, lengthy ward stay, exposure to medical interventions, and increased frequency and intensity of direct contact with staff hands predispose patients to S. marcescens infection. S. marcescens isolates account for 12.6% of nosocomial urinary tract infection and resulted in 4.86% fatality in a single institute in Taiwan.13 Although S. marcescens has a relatively low virulence, it often causes nosocomial infections in severely immunocompromised or critically ill patients.1, 2 The mortality rate of S. marcescens bacteremia was approximately 40–50% in previous studies.14, 15
S. marcescens is usually resistant to ampicillin, amoxicillin, amoxicillin/clavulanate, ampicillin/sulbactam, narrow-spectrum cephalosporins, cefuroxime, cephamycins, nitrofurantoin, and colistin.1, 16, 17, 18 S. marcescens also harbors a chromosomal ampC gene that can extend resistance to several more β-lactam antibiotics.1 In a nationwide surveillance of antimicrobial resistance from Taiwan in 2000, over half (52%) of S. marcescens were resistant to ciprofloxacin, 48%, 24%, and 23% were resistant to cefotaxime, aztreonam, and cefepime, respectively.19 However, many studies in recent years suggested that the occurrence of extended-spectrum beta-lactamase (ESBL)-producing isolates of S. marcescens was not unusual in Taiwan.17, 18, 20, 21, 22 In addition, an institutional prolonged spread of clonally related S. marcescens isolates with a novel AmpC cephalosporinase (S4) that confers a phenotype of resistance to cefotaxime was identified.23 Therefore, continuous and extensive surveillance of the antimicrobial resistance among S. marcescens isolates is necessary in Taiwan. The aim of this study was to investigate the trends in antimicrobial resistance in S. marcescens from a nationwide surveillance in Taiwan.
Materials and methods
Isolate collection and identification
S. marcescens isolates were collected biennially between 2002 and 2010, corresponding to periods III–VII of the Taiwan Surveillance of Antimicrobial Resistance (TSAR) program, from medical centers and regional hospitals throughout Taiwan. In 2002, 2004, 2008, and 2010, isolates were collected between July and September from the same 26 hospitals, except that isolates in 2006 were from 25 hospitals.24, 25 These hospitals comprised 11 medical centers and 15 regional hospitals, which are located in all four regions of Taiwan, namely seven, eight, eight, and three hospitals in the north, central, south, and east regions, respectively. Details of the collection process of the TSAR program have been described previously.19, 24, 25 Each isolate was subcultured onto the appropriate agar plates (BBL; Becton Dickinson Microbiology Systems, Cockeysville, MD, USA) to check for purity. S. marcescens was identified by standard conventional biochemical tests followed by confirmation with the VITEK Gram-negative Identification plus cards (bioMérieux VITEK, Hazelwood, MO, USA), and analytical profile index (API) 20E or API 32GN (bioMérieux, Marcy-l'Etoile, France).
Antimicrobial susceptibility testing
Antimicrobial susceptibility was determined by the reference broth microdilution method using freshly prepared cation-adjusted Müeller–Hinton broth and following the criteria proposed by the Clinical and Laboratory Standards Institute (CLSI).26 Minimum inhibitory concentration (MIC) interpretive criteria were defined by the CLSI guidelines for all drugs except tigecycline, for which the U.S. Food and Drug Administration breakpoints were used.
Statistical analyses
All analyses were performed with SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Significance of differences in frequencies and proportions were tested by Pearson χ2 test, and p ≤ 0.05 was considered to be statically significant.
Results
A total of 403 nonduplicate S. marcescens isolates were identified and tested for susceptibility during the 8-year study period of 2002–2010. Table 1 summarizes the source breakdown of the 403 isolates from each run of TSAR. Isolates were mostly recovered from respiratory samples (157 isolates, 39.0%), followed by the urinary tract (90, 22.3%), and blood (77, 19.1%). Most isolates (345, 85.6%) were from inpatients, including 93 (23.1%) from the intensive care unit (ICU) and 252 (62.5%) from non-ICU patients. Isolates from northern Taiwan comprised the largest proportion (154, 38.2%). Among the 390 isolates whose patient age was known, the mean age was 65.4 ± 20.5 years and 60.25% were from those aged ≥65 years.
Table 1.
Source breakdown of 403 Serratia marcescens isolates by year, hospital type, geographic region, specimen type, and patient location
| Source category |
Number (% of source category) of isolates from each year |
|||||
|---|---|---|---|---|---|---|
| TSAR | III (2002) | IV (2004) | V (2006) | VI (2008) | VII (2010) | Total |
| Total | 95 | 65 | 71 | 88 | 84 | 403 |
| Hospital type | ||||||
| Medical center | 33 (34.7) | 35 (53.8) | 32 (45.1) | 39 (44.3) | 38 (45.2) | 177 (43.9) |
| Regional hospital | 62 (65.3) | 30 (46.2) | 39 (54.9) | 49 (55.7) | 46 (54.8) | 226 (56.1) |
| Region of Taiwan | ||||||
| Northern | 34 (35.8) | 17 (26.2) | 24 (33.8) | 41 (46.6) | 38 (45.2) | 154 (38.2) |
| Central | 28 (29.5) | 29 (44.6) | 21 (29.6) | 31 (35.2) | 23 (27.4) | 132 (32.8) |
| Southern | 18 (18.9) | 16 (24.6) | 22 (31.0) | 14 (15.9) | 20 (23.8) | 90 (22.3) |
| Eastern | 15 (15.8) | 3 (4.6) | 4 (5.6) | 2 (2.3) | 3 (3.6) | 27 (6.7) |
| Specimen type | ||||||
| Blood | 20 (21.1) | 10 (15.4) | 8 (11.3) | 19 (21.6) | 20 (23.8) | 77 (19.1) |
| Respiratory tract | 29 (30.5) | 19 (29.2) | 32 (45.1) | 39 (44.3) | 38 (45.2) | 157 (39.0) |
| Urine | 31 (32.6) | 21 (32.3) | 19 (26.8) | 10 (11.4) | 9 (10.7) | 90 (22.3) |
| Pus/abscess/wound | 12 (12.6) | 15 (23.1) | 7 (9.9) | 12 (13.6) | 12 (14.3) | 58 (14.4) |
| Othersa | 3 (3.2) | 0 (0.0) | 5 (7.0) | 8 (9.1) | 5 (6.0) | 21 (5.2) |
| Patient location | ||||||
| ICU inpatient | 18 (18.9) | 13 (20.0) | 22 (31.0) | 19 (21.6) | 21 (25.0) | 93 (23.1) |
| Non-ICU inpatientb | 68 (71.6) | 38 (58.5) | 34 (47.9) | 57 (64.8) | 55 (65.5) | 252 (62.5) |
| OPD/ER | 9 (9.5) | 14 (21.5) | 15 (21.1) | 12 (13.6) | 8 (9.5) | 58 (14.4) |
ER = emergency room; ICU = intensive care unit; OPD = outpatient department; TSAR = Taiwan Surveillance of Antimicrobial Resistance program.
Data are presented as n (%).
Others: one from ascites, one from joints, two from bile, four from ears, seven from catheter tips, four from eyes/conjunctiva/corneas, one from urethra, and one from discharge.
Included six isolates from respiratory care center/respiratory care ward.
The in vitro susceptibilities, MIC50, MIC90 (MIC at which 50% and 90% of isolates were inhibited), and MIC range of the isolates to various antimicrobial agents are shown in Table 2 . Overall, 99.3% of isolates were susceptible to imipenem, 93.8% to ceftazidime, 86.0% to piperacillin/tazobactam, 72.7% to levofloxacin, 63.8% to ciprofloxacin, 60.8% to trimethoprim/sulfamethoxazole (TMP/SMX), and 59.6% to gentamicin according to the CLSI criteria. By contrast, susceptibility of ≤10% for each round of TSAR was observed for ampicillin, amoxicillin/clavulanate, cefazolin, cefoxitin, cefuroxime, and tetracycline. The MIC50 and MIC90 of tigecycline were 0.5 μg/mL and 1 μg/mL, respectively. The susceptibility rate of S. marcescens from different patient locations was compared (Table 3 ). Isolates from the ICU had higher rates of nonsusceptibility than isolates from other locations for amikacin, gentamicin, ciprofloxacin, levofloxacin, ceftazidime, cefepime, and TMP/SMX, but the differences were not significant. The susceptibility rate to ciprofloxacin/levofloxacin was lower in northern Taiwan than other regions (p = 0.025 and p = 0.002, respectively).
Table 2.
MICs and susceptible percentage of Serratia marcescens isolates
| Antimicrobial agent | TSAR | MIC (mcg/mL) |
% of isolates |
||
|---|---|---|---|---|---|
| MIC50 | MIC90 | Range | Susceptible | ||
| Amikacin | III–VII | ≤4 | >32 | ≤2–>32 | 87.8 |
| Gentamicin | III–VII | 1 | >8 | ≤0.5–>8 | 59.6 |
| Ciprofloxacin | III–VII | 0.5 | >2 | ≤0.03–6 | 63.8 |
| Levofloxacina | VI–VII | ≤1 | 4 | ≤0.5–>8 | 72.7 |
| Ceftazidime | III–VII | ≤1 | 4 | ≤0.5–>16 | 93.8 |
| Ceftriaxone | III–VII | 2 | >32 | ≤0.5–>32 | 73.2 |
| Cefepime | III–VII | ≤1 | >16 | ≤0.5–>16 | 86.8 |
| Aztreonam | III–VII | ≤2 | >16 | ≤1–>16 | 82.9 |
| Imipenem | III–VII | 1 | 2 | ≤0.25–>8 | 99.3 |
| TMP/SMX | III–VII | ≤0.5 | 4 | ≤0.5–>4 | 60.8 |
| Piperacillin/Tazobactamb | V–VII | 8 | 32 | ≤4–>64 | 86.0 |
| Tigecyclinea | VI–VII | 0.5 | 1 | ≤0.25–2 | 98.8 |
MIC = minimum inhibitory concentration; MIC50 and MIC90 = MIC at which 50% and 90% of isolates were inhibited; TMP/SMX = trimethoprim/sulfamethoxazole; TSAR = Taiwan Surveillance of Antimicrobial Resistance program.
Isolates during 2008–2010 = 172.
Isolates during 2006–2010 = 243.
Table 3.
Comparison of Serratia marcescens susceptibility among isolates from different patient locations and specimen types
| Antimicrobial agent | Patient location |
p | ||
|---|---|---|---|---|
| Non-ICU (n = 252) |
ICU (n = 93) |
OPD/ER (n = 58) |
||
| S | S | S | ||
| Amikacin | 88.5 | 83.9 | 91.4 | 0.334 |
| Gentamicin | 60.3 | 54.8 | 63.8 | 0.531 |
| Ciprofloxacin | 64.3 | 61.3 | 65.5 | 0.849 |
| Levofloxacin | 74.1 | 70.7 | 72.7 | 0.841 |
| Ceftazidime | 94.0 | 92.5 | 94.8 | 0.803 |
| Ceftriaxone | 75.0 | 73.1 | 65.5 | 0.343 |
| Cefepime | 87.7 | 82.8 | 89.7 | 0.395 |
| Aztreonam | 85.3 | 75.3 | 84.5 | 0.086 |
| Imipenem | 98.8 | 100 | 100 | 0.433 |
| TMP/SMX | 60.3 | 59.1 | 65.5 | 0.727 |
| Piperacillin/Tazobactam | 89.7 | 82.3 | 86.0 | 0.082 |
Data are presented as %.
ER = emergency room; ICU = intensive care unit; OPD = outpatient department; S = susceptible; TMP/SMX = trimethoprim/sulfamethoxazole.
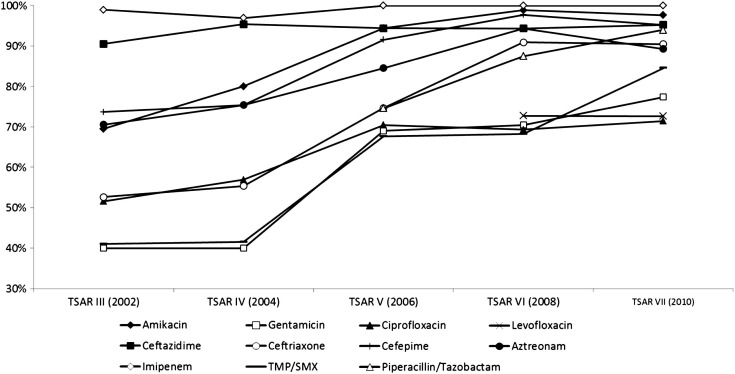
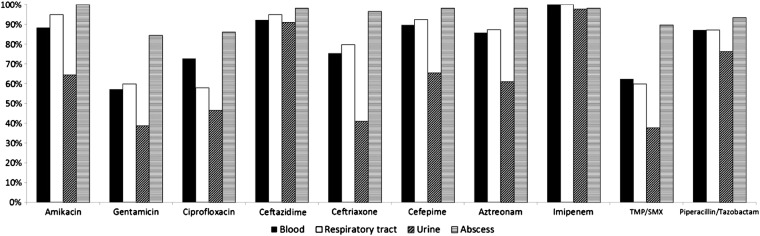
Fig. 1 illustrates the comparison of susceptibility rate for selected antibiotics from different specimen sources. We found that isolates from urine had the lowest susceptibility rate for the various antibiotics. Isolates from urine were significantly less susceptible than those from nonurine sources for amikacin (64.4% vs. 94.6%, p < 0.001), gentamicin (38.9% vs. 65.5%, p < 0.001), ciprofloxacin (46.7% vs. 68.7%, p < 0.001), ceftriaxone (41.1% vs. 82.4%, p < 0.001), cefepime (65.6% vs. 93.0%, p < 0.001), aztreonam (61.1% vs. 89.1%, p < 0.001), and TMP/SMX (37.8% vs. 67.4%, p < 0.001). Trends in susceptibility of S. marcescens to various antibiotics over 8 years are demonstrated in Fig. 2 . S. marcescens remained consistently highly susceptible to imipenem and ceftazidime throughout the study period. A significantly increased susceptibility rate after 2004 was observed for the following antibiotics: amikacin (73.8% vs. 97.1%, p < 0.001), gentamicin (40.0% vs. 72.4%, p < 0.001), ciprofloxacin (53.8% vs. 70.4%, p = 0.001), ceftriaxone (53.8% vs. 86.0%, p < 0.001), cefepime (74.4% vs. 95.1%, p < 0.001), aztreonam (72.5% vs. 89.7%, p < 0.001), and TMP/SMX (41.3% vs. 73.7%, p < 0.001).
Figure 1.
Comparison of susceptibility of Serratia marcescens to various antibiotics in different specimens. TSAR = Taiwan Surveillance of Antimicrobial Resistance program.
Figure 2.
Trends in susceptibility of Serratia marcescens to various antibiotics over 10 years.
Discussion
In the present study, we examined trends in susceptibility to multiple antibiotics for 403 nonduplicate clinical S. marcescens isolates in Taiwan between 2002 and 2010. The results show that the activities of ceftazidime and imipenem have remained consistently high over the years. Susceptibilities of ≤10% for each round of TSAR were observed for ampicillin, amoxicillin/clavulanate, cefazolin, cefoxitin, cefuroxime, and tetracycline. Fluoroquinolones showed less activity against S. marcescens than other β-lactams.
In Taiwan, the occurrence of CTX-M-3 ESBL-producing isolates of S. marcescens was not unusual,17, 18, 20, 21, 22 and the novel AmpC cephalosporinase (S4) was reported previously.23 The distinctive property of resistance to cefotaxime but not ceftazidime was due to CTX-M-3 ESBL and SRT-like AmpC such as S4 conferring the same phenotype of resistance to cefotaxime. The antibiogram-based method to simplify the screening of potential ESBL-producing populations among S. marcescens isolates has also been reported recently.22 Although the molecular characteristics of isolates were lacking in the current study, the higher susceptibility of ceftazidime than ceftriaxone corresponded to the previous studies,17, 18, 20, 21, 22, 23 and further underlined the need to establish practical guidelines for ESBL screening, confirmation, and reporting for chromosomal AmpC producers in Taiwan.21
In the Tigecycline Evaluation and Surveillance Trial study, 4857 isolates of S. marcescens collected globally between 2004 and 2007 with susceptibilities ≥ 90% were observed for amikacin, levofloxacin, ceftazidime, cefepime, imipenem, meropenem, piperacillin/tazobactam, and tigecycline.27 A similar study conducted by Hawser et al collected 753 clinical isolates of S. marcescens throughout the Asia–Pacific region between 2004 and 2010 and demonstrated constantly high susceptibility to levofloxacin (84–97%).28 A characteristic finding in the present study was the much lower susceptibility to levofloxacin and ceftriaxone compared with the global data. Susceptibility to levofloxacin in the current study was only tested between 2008 and 2010, but susceptibility to ciprofloxacin was tested throughout the study period. The much lower susceptibility to ciprofloxacin suggests that resistance to fluoroquinolones is the major concern surrounding drug-resistant S. marcescens in Taiwan. The susceptibility rate to ceftriaxone was 55–85% between 2004 and 2010 in the Asia–Pacific region, which is consistent with our findings. Hawser et al also demonstrated a decline in susceptibility to minocycline (decreased to 41–60% in 2008–2010) and tigecycline in S. marcescens. 28 By contrast, susceptibility to minocycline and tigecycline tested in 2010 in the present study showed higher susceptibility rates of 89.2% and 98.8%, respectively.
Shih et al found that 99% of S. marcescens blood isolates in 1999–2003 were sensitive to ceftazidime, but only 19% were sensitive to ciprofloxacin, 32% to levofloxacin, and 18% to TMP/SMX in a single institute in Taiwan.15 The high susceptibility rate of ceftazidime is consistent with the current study. A previous nationwide surveillance of antimicrobial resistance among Enterobacteriaceae at the ICUs of 10 major teaching hospitals in Taiwan in 2005 reported low susceptibility of S. marcescens to ciprofloxacin (43%) and levofloxacin (66%).29 Despite_ENREF_23 the present study showing increased susceptibilities to ciprofloxacin (64.3%) and levofloxacin (74.1%) compared with previous results in Taiwan, the resistance to fluoroquinolones continues to be a problem in Taiwan.
Examination of trends in antimicrobial susceptibility has revealed higher resistant rates for amikacin, gentamicin, ciprofloxacin, ceftriaxone, cefepime, aztreonam, and TMP/SMX prior to 2004 than those after that date. The higher proportion of isolates recovered from urine between 2002 and 2004 may have influenced the results. The significantly higher proportion of isolates recovered from urine between 2002 and 2004 (32.5%) than that after 2004 (15.6%, p < 0.001) may have influenced the results. The reasons why isolates from urine had the lowest susceptibility rate for the various antibiotics cannot be clearly defined. It might imply that we should put more focus on the resistance pattern in the urinary isolates. In addition, after the emergence of severe acute respiratory syndrome in 2003, the reinforcement of prevention of health care-associated infection in hospitals resulted in a more cautious approach to intervention with antimicrobials as a part of the infection control policy.30 The trends in total consumption of aminoglycosides, extended-spectrum cephalosporins, and fluoroquinolones significantly increased prior to 2004 and remained stable after 2004 in the hospital-wide investigation.30, 31 The reduced consumption of antibiotics in Taiwan may explain the observation of greater susceptibility after 2004 in the present study.
We recognize a number of potential biases in the present study. The first limitation concerns the specimen types. Isolates from the urinary tract showed higher resistance rates to many antibiotics than those from other sites; therefore, the higher percentage of urine specimen prior to 2004 may result in lower overall susceptibility in this period. Second, <10% of isolates were collected from eastern Taiwan and the data are likely to have underestimated the events occurring in this region. Third, we did not analyze the resistant mechanism due to the lack of molecular method such as multiplex polymerase chain reaction screening of ampC genes in these S. marcescens isolates. Finally, given the limited clinical information from this surveillance, we cannot exactly determine the clinical significance of these isolates.
In conclusion, the results of this study suggest that the susceptibility of S. marcescens to ceftazidime and imipenem in Taiwan remained consistently high over the study period. S. marcescens isolates from Taiwan demonstrated relatively higher resistance to ciprofloxacin and levofloxacin than other β-lactams, and continued surveillance of antimicrobial resistance in S. marcescens, especially for fluoroquinolones, is warranted. TSAR is ongoing in Taiwan involving clinically important bacteria. The longitudinal surveillance study will continue to provide key information related to antimicrobial resistance over time.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
We express our sincere appreciation to the hospitals that participated in the TSAR program. This project was supported by an intramural grant from the National Health Research Institutes (NHRI) (grant nos. ID-099-PP-01 and ID-100-PP-01) and Yen Tjing Ling Medical Foundation (grant no. CI-100-30). We thank the technical staff at NHRI for their assistance in identification and antimicrobial susceptibility testing.
References
- 1.Mahlen S.D. Serratia infections: from military experiments to current practice. Clin Microbiol Rev. 2011;24:755–791. doi: 10.1128/CMR.00017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu V.L. Serratia marcescens: historical perspective and clinical review. N Engl J Med. 1979;300:887–893. doi: 10.1056/NEJM197904193001604. [DOI] [PubMed] [Google Scholar]
- 3.Laupland K.B., Parkins M.D., Gregson D.B., Church D.L., Ross T., Pitout J.D. Population-based laboratory surveillance for Serratia species isolates in a large Canadian health region. Eur J Clin Microbiol Infect Dis. 2008;27:89–95. doi: 10.1007/s10096-007-0400-7. [DOI] [PubMed] [Google Scholar]
- 4.Lu P.L., Liu Y.C., Toh H.S., Lee Y.L., Liu Y.M., Ho C.M. Epidemiology and antimicrobial susceptibility profiles of Gram-negative bacteria causing urinary tract infections in the Asia-Pacific region: 2009–2010 results from the Study for Monitoring Antimicrobial Resistance Trends (SMART) Int J Antimicrob Agents. 2012;40:S37–S43. doi: 10.1016/S0924-8579(12)70008-0. [DOI] [PubMed] [Google Scholar]
- 5.Maki D.G., Hennekens C.G., Phillips C.W., Shaw W.V., Bennett J.V. Nosocomial urinary tract infection with Serratia marcescens: an epidemiologic study. J Infect Dis. 1973;128:579–587. doi: 10.1093/infdis/128.5.579. [DOI] [PubMed] [Google Scholar]
- 6.Wu Y.M., Hsu P.C., Yang C.C., Chang H.J., Ye J.J., Huang C.T. Serratia marcescens meningitis: epidemiology, prognostic factors and treatment outcomes. J Microbiol Immunol Infect. 2013;46(4):259–265. doi: 10.1016/j.jmii.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Vigeant P., Loo V.G., Bertrand C., Dixon C., Hollis R., Pfaller M.A. An outbreak of Serratia marcescens infections related to contaminated chlorhexidine. Infect Control Hosp Epidemiol. 1998;19:791–794. doi: 10.1086/647728. [DOI] [PubMed] [Google Scholar]
- 8.Archibald L.K., Corl A., Shah B., Schulte M., Arduino M.J., Aguero S. Serratia marcescens outbreak associated with extrinsic contamination of 1% chlorxylenol soap. Infect Control Hosp Epidemiol. 1997;18:704–709. doi: 10.1086/647516. [DOI] [PubMed] [Google Scholar]
- 9.Rabier V., Bataillon S., Jolivet-Gougeon A., Chapplain J.M., Beuchée A., Bétrémieux P. Hand washing soap as a source of neonatal Serratia marcescens outbreak. Acta Paediatr. 2008;97:1381–1385. doi: 10.1111/j.1651-2227.2008.00953.x. [DOI] [PubMed] [Google Scholar]
- 10.Beck-Sague C.M., Jarvis W.R. Epidemic bloodstream infections associated with pressure transducers: a persistent problem. Infect Control Hosp Epidemiol. 1989;10:54–59. doi: 10.1086/645961. [DOI] [PubMed] [Google Scholar]
- 11.Vandenbroucke-Grauls C.M., Baars A.C., Visser M.R., Hulstaert P.F., Verhoef J. An outbreak of Serratia marcescens traced to a contaminated bronchoscope. J Hosp Infect. 1993;23:263–270. doi: 10.1016/0195-6701(93)90143-n. [DOI] [PubMed] [Google Scholar]
- 12.Grohskopf L.A., Roth V.R., Feikin D.R., Arduino M.J., Carson L.A., Tokars J.I. Serratia liquefaciens bloodstream infections from contamination of epoetin alfa at a hemodialysis center. N Engl J Med. 2001;344:1491–1497. doi: 10.1056/NEJM200105173442001. [DOI] [PubMed] [Google Scholar]
- 13.Liu J.W., Hsu Y.M., Huang Y.F. Independent prognostic factors for fatality in patients with urinary tract infection caused by Serratia marcescens. Infect Control Hosp Epidemiol. 2004;25:80–82. doi: 10.1086/502297. [DOI] [PubMed] [Google Scholar]
- 14.Yu W.L., Lin C.W., Wang D.Y. Serratia marcescens bacteremia: clinical features and antimicrobial susceptibilities of the isolates. J Microbiol Immunol Infect. 1998;31:171–179. [PubMed] [Google Scholar]
- 15.Shih H.I., Lee H.C., Lee N.Y., Chang C.M., Wu C.J., Wang L.R. Serratia marcescens bacteremia at a medical center in southern Taiwan: high prevalence of cefotaxime resistance. J Microbiol Immunol Infect. 2005;38:350–357. [PubMed] [Google Scholar]
- 16.Stock I., Grueger T., Wiedemann B. Natural antibiotic susceptibility of strains of Serratia marcescens and the S. liquefaciens complex: S. liquefaciens sensu stricto, S. proteamaculans and S. grimesii. Int J Antimicrob Agents. 2003;22:35–47. doi: 10.1016/s0924-8579(02)00163-2. [DOI] [PubMed] [Google Scholar]
- 17.Yu W.L., Wu L.T., Pfaller M.A., Winokur P.L., Jones R.N. Confirmation of extended-spectrum beta-lactamase-producing Serratia marcescens: preliminary report from Taiwan. Diagn Microbiol Infect Dis. 2003;45:221–224. doi: 10.1016/s0732-8893(02)00539-4. [DOI] [PubMed] [Google Scholar]
- 18.Wu L.T., Tsou M.F., Wu H.J., Chen H.E., Chuang Y.C., Yu W.L. Survey of CTX-M-3 extended-spectrum beta-lactamase (ESBL) among cefotaxime-resistant Serratia marcescens at a medical center in middle Taiwan. Diagn Microbiol Infect Dis. 2004;49:125–129. doi: 10.1016/j.diagmicrobio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Lauderdale T.L., Clifford McDonald L., Shiau Y.R., Chen P.C., Wang H.Y., Lai J.F. The status of antimicrobial resistance in Taiwan among gram-negative pathogens: the Taiwan Surveillance of Antimicrobial Resistance (TSAR) program. Diagn Microbiol Infect Dis. 2000;2004(48):211–219. doi: 10.1016/j.diagmicrobio.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Cheng K.C., Chuang Y.C., Wu L.T., Huang G.C., Yu W.L. Clinical experiences of the infections caused by extended-spectrum beta-lactamase-producing Serratia marcescens at a medical center in Taiwan. Jpn J Infect Dis. 2006;59:147–152. [PubMed] [Google Scholar]
- 21.Yu W.L., Chuang Y.C., Walther-Rasmussen J. Extended-spectrum beta-lactamases in Taiwan: epidemiology, detection, treatment and infection control. J Microbiol Immunol Infect. 2006;39:264–277. [PubMed] [Google Scholar]
- 22.Su P.A., Wu L.T., Cheng K.C., Ko W.C., Chuang Y.C., Yu W.L. Screening extended-spectrum beta-lactamase production in Enterobacter cloacae and Serratia marcescens using antibiogram-based methods. J Microbiol Immunol Infect. 2010;43:26–34. doi: 10.1016/S1684-1182(10)60004-7. [DOI] [PubMed] [Google Scholar]
- 23.Yu W.L., Ko W.C., Cheng K.C., Chen H.E., Lee C.C., Chuang Y.C. Institutional spread of clonally related Serratia marcescens isolates with a novel AmpC cephalosporinase (S4): a 4-year experience in Taiwan. Diagn Microbiol Infect Dis. 2008;61:460–467. doi: 10.1016/j.diagmicrobio.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Kuo S.C., Chang S.C., Wang H.Y., Lai J.F., Chen P.C., Shiau Y.R. Emergence of extensively drug-resistant Acinetobacter baumannii complex over 10 years: nationwide data from the Taiwan Surveillance of Antimicrobial Resistance (TSAR) program. BMC Infect Dis. 2012;12:200. doi: 10.1186/1471-2334-12-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen F.J., Huang I.W., Wang C.H., Chen P.C., Wang H.Y., Lai J.F. mecA-Positive Staphylococcus aureus with low-level oxacillin MIC in Taiwan. J Clin Microbiol. 2012;50:1679–1683. doi: 10.1128/JCM.06711-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CLSI . Clinical and Laboratory Standards Institute; Wayne: 2010. Performance standards for antimicrobial susceptibility testing; twentieth informational supplement. CLSI document M100-S20. [Google Scholar]
- 27.Garrison M.W., Mutters R., Dowzicky M.J. In vitro activity of tigecycline and comparator agents against a global collection of Gram-negative and Gram-positive organisms: Tigecycline Evaluation and Surveillance Trial 2004 to 2007. Diagn Microbiol Infect Dis. 2009;65:288–299. doi: 10.1016/j.diagmicrobio.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Hawser S.P., Bouchillon S.K., Hackel M., Chen M., Kim E.C. Trending 7 years of in vitro activity of tigecycline and comparators against Gram-positive and Gram-negative pathogens from the Asia-Pacific region: Tigecycline Evaluation Surveillance Trial (TEST) 2004–2010. Int J Antimicrob Agents. 2012;39:490–495. doi: 10.1016/j.ijantimicag.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Jean S.S., Hsueh P.R., Lee W.S., Chang H.T., Chou M.Y., Chen I.S. Nationwide surveillance of antimicrobial resistance among Enterobacteriaceae in intensive care units in Taiwan. Eur J Clin Microbiol Infect Dis. 2009;28:215–220. doi: 10.1007/s10096-008-0610-7. [DOI] [PubMed] [Google Scholar]
- 30.Lai C.C., Wang C.Y., Chu C.C., Tan C.K., Lu C.L., Lee Y.C. Correlation between antibiotic consumption and resistance of Gram-negative bacteria causing healthcare-associated infections at a university hospital in Taiwan from 2000 to 2009. J Antimicrob Chemother. 2011;66:1374–1382. doi: 10.1093/jac/dkr103. [DOI] [PubMed] [Google Scholar]
- 31.Chan Y.Y., Lin T.Y., Huang C.T., Deng S.T., Wu T.L., Leu H.S. Implementation and outcomes of a hospital-wide computerised antimicrobial stewardship programme in a large medical centre in Taiwan. Int J Antimicrob Agents. 2011;38:486–492. doi: 10.1016/j.ijantimicag.2011.08.011. [DOI] [PubMed] [Google Scholar]