Abstract
The composition of dietary macronutrients (proteins, carbohydrates, and fibers) and micronutrients (vitamins, phytochemicals) can markedly influence the development of immune responses to enteric infection. This has important implications for livestock production, where a significant challenge exists to ensure healthy and productive animals in an era of increasing drug resistance and concerns about the sector’s environmental footprint. Nutritional intervention may ultimately be a sustainable method to prevent disease and improve efficiency of livestock enterprises, and it is now well established that certain phytonutrients can significantly improve animal performance during challenge with infectious pathogens. However, many questions remain unanswered concerning the complex interplay between diet, immunity, and infection. In this review, we examine the role of phytonutrients in regulating immune and inflammatory responses during enteric bacterial and parasitic infections in livestock, with a specific focus on some increasingly well-studied phytochemical classes—polyphenols (especially proanthocyanidins), essential oil components (cinnamaldehyde, eugenol, and carvacrol), and curcumin. Despite the contrasting chemical structures of these molecules, they appear to induce a number of similar immunological responses. These include promotion of mucosal antibody and antimicrobial peptide production, coupled with a strong suppression of inflammatory cytokines and reactive oxygen species. Although there have been some recent advances in our understanding of the mechanisms underlying their bioactivity, how these phytonutrients modulate immune responses in the intestine remains mostly unknown. We discuss the complex inter-relationships between metabolism of dietary phytonutrients, the gut microbiota, and the mucosal immune system, and propose that an increased understanding of the basic immunological mechanisms involved will allow the rational development of novel dietary additives to promote intestinal health in farmed animals.
Keywords: curcumin, essential oils, immunity, infection, polyphenols, proanthocyanidins
Introduction
The livestock production sector is under increasing pressure to improve efficiency, to meet the demand of feeding a growing population from a shrinking resource base. There are major concerns about the contribution of livestock to greenhouse gas emissions, and also the use of antibiotics that may contribute to antibiotic resistance in humans. Thus, it is critical to improve farming methods to ensure the development of robust and resilient animals that can maintain production in the face of numerous environmental challenges (Eisler et al., 2014). Infectious diseases of the gastrointestinal tract are a major constraint on efficient and profitable production. Infection may directly reduce animal growth and performance through damage to the absorptive capacity of the gut, as well as more indirectly through modulatory effects to the gut microbiome and systemic metabolism (Xiong et al., 2019). In addition, resistance to many classes of antibiotics and antiparasiticides has necessitated the urgent need for novel solutions.
Manipulation of immune function during pathogen infection, whereby damaging inflammatory responses are minimized and protective and/or tolerogenic mechanisms are induced, is potentially a sustainable solution to the above issues. In this respect, it has long been recognized that nutrition plays a major role in resistance to infectious diseases, with appropriate dietary macronutrients (proteins, carbohydrates, and fibers) being important for effective immune responses. More recently, the contribution of micronutrients (e.g., vitamins) or other dietary components such as phytochemicals to the expression of specific immune mechanisms has been increasingly appreciated. As a result, the use of defined dietary supplements with immunostimulatory effects to prevent and/or control disease has recently been investigated (Ballou et al., 2019).
In this review, we will discuss how phytonutrients may modulate immune function during gut pathogen (bacterial and parasitic) infections, and highlight some examples of how this knowledge may be harnessed to contribute to sustainable livestock production. Our objective is not to provide an exhaustive overview of the many studies that demonstrated health benefits of phytonutrients in farm animals, which have been reviewed extensively (Lillehoj et al., 2018). Instead, we focus on the putative mechanisms underlying the activity of some selected phytonutrients, highlighting experiments not only in both livestock but also in basic cellular and rodent models. Finally, we suggest some pertinent areas for future investigation.
Gastrointestinal Infections in Livestock: A Significant Challenge
Bacterial or parasitic infections of the intestinal tract are highly prevalent in all farmed animals (Charlier et al., 2018; VanderWaal and Deen, 2018). In intensively farmed swine and poultry, large challenges exist due to enterotoxigenic Escherichia coli (ETEC), Brachyspira hyodysenteriae (swine dysentery), and Clostridium perfringens (Moore, 2016; Kongsted et al., 2018; Luise et al., 2019). Intestinal parasites are a major threat to the profitability of pasture-based livestock enterprises, due to helminths such as Haemonchus contortus or Teladorsagia circumcincta and coccidia (Eimeria spp.), causing severe production losses and ill-health in young sheep and goats (Charlier et al., 2018). Helminths such as Ascaris suum or Oesophagostomum spp. are a large problem in outdoor pig production, while A. suum continues to infect large numbers of indoor pigs despite attempts to eradicate it with increased hygiene (Vlaminck et al., 2015). A similar situation exists with the poultry roundworm Ascaridia galli and the protozoan coccidia Eimeria maxima and Eimeria tenella (Chapman, 2014). In addition, many zoonotic pathogens such as Campylobacter or enterohaemorrhagic E. coli can be present in the food chain, which represents a substantial threat to public health (Chlebicz and Śliżewska, 2018). Treatment of all these pathogens has routinely been based on herd-level administration of treatments such as zinc oxide and antimicrobials, which has led to major concerns over the impact on the environment, the risk of antibiotic-resistant bacterial infections in humans, and rapid increases in drug-resistant pathogen populations (Kruse et al., 2018; Burow et al., 2019; Morgan et al., 2019).
Infections of the intestinal tract may impose a severe burden on animal performance. In extreme cases, mortality may occur due to ileitis (caused by ETEC or Lawsonia infection in pigs), or haemonchosis in young ruminants. However, subclinical infections may also significantly impair production due to intestinal damage and increased partitioning of nutrients toward immune and inflammatory processes rather than growth (Colditz, 2008; Huntley et al., 2018). Even asymptomatic infections may have subtle effects on host metabolism, for example, by altering the composition of the host gut microbiome, which may reduce performance (Xiong et al., 2019). Thus, in the absence of continual pathogen suppression through prophylactic drug use, effective immune function is vital for efficient production. In this respect, the immune system plays a wide-ranging role beyond the obvious effect of clearing pathogens from the gut. Recent evidence suggests that many immune molecules such as cytokines and antimicrobial peptides also regulate wound healing, tissue repair, and general maintenance of intestinal homeostasis (Brestoff and Artis, 2015). Thus, defining feeding and management strategies that optimize balanced immune function, and thereby promote gut health and avoid excessive inflammation, are now key goals for the animal health and nutrition research communities.
The Role of Diet in Gastrointestinal Infection and Immunity
The gut mucosal immune system consists of a dense array of gut epithelial and innate immune cells, overlaying the adaptive compartment of the immune system in the gut-associated lymphoid tissue (GALT). The first line of defense is intestinal epithelial cells (IEC), which form a physical barrier to exclude microbes in the lumen from entering the host tissue. Moreover, IEC can sense different microbe-derived products and secrete various alarmins and antimicrobial peptides (Peterson and Artis, 2014). Pathogen antigens are sampled by macrophages and dendritic cells (DCs), which are capable of transporting antigen to the lymphatic system. Upon presentation of antigen, naive lymphocytes develop into effector or memory T-cells or plasma cells capable of secreting antibodies (Agace, 2008). A key facet of this system is appropriate immunoregulation. The continual stimulation of the mucosal barrier by both autochthonous and allochthonous microbes means that the system is normally highly controlled, with an array of sophisticated mechanisms existing to avoid over-reactions to relatively benign stimuli, while allowing expression of appropriate immune effector mechanisms against harmful pathogens (Blander et al., 2017). Unrestrained reactions to stimuli may result in chronic inflammation, which is a particularly costly phenomenon for livestock production. Activation of the immune system during pathogen infection incurs a considerable nutritional cost. This is due to, among others, increased metabolic activity, reprioritization of essential nutrients to immune processes rather than growth, and rapid cellular turnover to replenish damaged and necrotic tissues and support the increased proliferation of immune cells (Colditz, 2008). Thus, a balance exists between the need to clear pathogens, which can potentially cause mortality and/or morbidity, and the need to prevent continual inflammatory responses to the myriad of antigens faced by the animal gastrointestinal tract.
The influence of diet on pathogenic infections encompasses both direct antimicrobial effects of certain dietary compounds and immunomodulatory effects induced by both macronutrients and micronutrients. Many bioactive plant compounds have been speculated to directly kill pathogens in the gut, and some feed additives based on plant extracts can potentially reduce ETEC infection through direct antibacterial effects (Verhelst et al., 2014). However, the concentrations required in situ to have a marked bactericidal or anthelmintic effect are likely to be high. Thus, they may be relatively difficult to incorporate into defined diets without compromising nutritional quality. Indeed, this has been demonstrated by reduced growth rates in animals despite apparent antimicrobial effects (Verhelst et al., 2014;Desrues et al., 2016). In extensive, forage-based systems, certain pastures can be sown that balance a good nutritive value with high concentrations of natural antimicrobial compounds. A good example of this is chicory (Cichorium intybus), which is a highly palatable feed for grazing ruminants that is also rich in sesquiterpene lactones, which are known antiparasitic compounds that can kill pathogenic helminths such as Ostertagia ostertagi (Peña-Espinoza et al., 2018). However, in some systems, it may not always be practical to incorporate the required amounts of antimicrobial extracts to exert direct bactericidal effects.
In contrast, relatively modest changes in the nutrient composition of the diet can markedly affect the development of immune mechanisms. Immune function during pathogen infection may be improved either by 1) improved overall nutrition, allowing a quantitative increase in the production of immune effector molecules or 2) by a specific, qualitative augmentation of a particular immune reaction in response to the addition of a defined dietary component (Coop and Kyriazakis, 2001; Smith et al., 2018). With regard to overall nutrition, as many immune components (antibody production, lymphocyte proliferation) are nutritionally costly, provision of extra protein may allow more effective expression of immunity where dietary protein is limiting. This has been especially well-demonstrated in small ruminants, where improving both the quantity and quality of dietary protein results in enhanced immunological responsiveness to gastrointestinal parasites (Coop and Kyriazakis, 2001). The situation in monogastric animals is more complex. Higher levels of dietary protein can improve the resistance of pigs to intestinal helminth infection (Pedersen et al., 2002), but it is also well established that higher protein levels are a risk factor for increased severity of postweaning diarrhea and E. coli infection (Wellock et al., 2008; Heo et al., 2009). There is better evidence that manipulation of specific amino acids can enhance immune function. During acute immune and inflammatory responses, tryptophan (Trp) is often considered rate-limiting as it is rapidly catabolized by the upregulation of indoleamine 2,3-dioxygenase (IDO1), which accompanies lymphocyte activation. Increasing supply of Trp can potentially enhance immune function in pigs and poultry, although results have been inconsistent (Koopmans et al., 2012; Capozzalo et al., 2015). Similarly, glutamine or threonine supplementation may have the potential to improve innate immunity and maintenance of the gut barrier during coccidiosis or necrotizing enterocolitis in broilers (Wils-Plotz et al., 2013; Xue et al., 2018).
The role of micronutrients (e.g., trace elements and vitamins) is also becoming increasingly well established (reviewed by Smith et al., 2018). A multitude of elements must be supplied in sufficient quantities by the diet to allow effective expression of immunity. For example, the role of selenium and zinc in promoting cellular immune responses is clear. Similarly, vitamin deficiency is well known to impair immune function, and maintaining optimal levels of Vitamin A or Vitamin E, or supplementation above maintenance levels, may improve immune function and resistance to pathogens such as E. coli (Kim et al., 2016; Dalia et al., 2018).
In human health, the role of phytonutrients on immune function has been increasingly well studied (Martinez-Micaelo et al., 2012a). The positive effects of fruit and vegetable intake on gut health are self-evident, largely due to their fiber and vitamin content. Moreover, evidence now points also to the role of phytochemicals such as flavanols, carotenoids, and spice components (e.g., from cinnamon, garlic, or ginger) in reducing the risk of cancer and autoimmune disease due to their strong antioxidant effects and regulation of inflammatory responses (Ahn-Jarvis et al., 2019). Many of these phytochemicals are notably missing from livestock diets. However, the need to find sustainable alternatives to synthetic drug treatments (or zinc oxide) to maintain healthy gut function has led to renewed interest in how plant-derived phytonutrients may be used as dietary supplements in animals. Below, we will consider how some phytonutrients that have been recently promoted as livestock feed additives may modulate immune and inflammatory responses during gut pathogen infections. Such modulatory effects may reduce harmful immunopathology during infections and restore intestinal homeostasis or, in some cases, enhance or stimulate protective immune mechanisms that may help animals clear infections.
Examples of Phytonutrients With Immunomodulatory Potential
Polyphenols
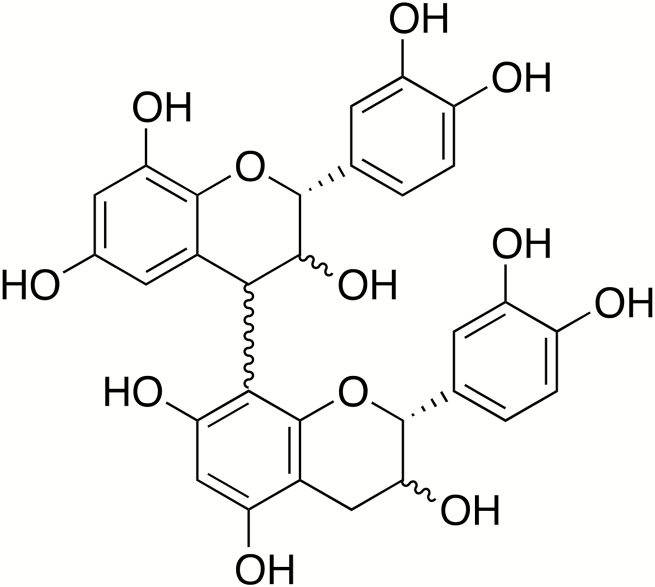
Polyphenols consist of a diverse group of plant secondary compounds featuring multiple phenol groups that are generally synthesized from precursors derived from the phenyl propanoid or shikimate pathways (Sharma et al., 2019). A number of isolated flavones and flavonols (e.g., quercetin) have been investigated for their antioxidant and anti-inflammatory properties in vitro or in rodents (Yi, 2018), but studies on the effects of purified flavonols on immune function and inflammation in livestock have not been performed in detail. In contrast, tannins (polymeric polyphenols) have received much attention as livestock feed-supplements. The tannin class of polyphenols includes both hydrolyzable tannins and condensed tannins (also known as proanthocyandins [PAC]). Hydrolyzable tannins consist of a glucose core surrounded by galloyl groups and are found in plants such as chestnut and acorns (Mueller-Harvey, 2006). Some types of hydrolyzable tannins yield ellagic acid after hydrolyzation, which can be toxic to ruminants, but has putative health benefits in monogastric animals (Marín et al., 2013). Moreover, chestnut tannins have been recently investigated as a potential therapeutic for ETEC infections in piglets (Girard et al., 2018). However, most research has focused on PAC, which have been of particular interest to animal nutritionists. Proanthocyandins consist of a diverse range of polymers based on monomeric units of the flavan-3-ols (epi)catechin and (epi)gallocatechin (Fig. 1) and are widely found in different foods and forages. Particularly rich sources are grapes, berries, and nuts, many of the Fabaceae family of legumes, and some varieties of sorghum. They have sometimes been considered to be antinutritional factors, particularly in monogastric animals, due to their ability to bind digestive enzymes and reduce digestibility of certain amino acids (Jansman, 1993). However, it is apparent that this situation may be more complex because low levels of PAC (often in the form of extracts from food ingredients such as grape or hops) may improve feed conversion and efficiency due to reduced levels of reactive oxidative species and inflammation in the gut (Gessner et al., 2013; Fiesel et al., 2014; Kafantaris et al., 2018). In vitro studies have also clearly shown that PAC possess antimicrobial properties, and for this reason, PAC-rich supplements have been investigated with some success as novel therapeutics against ETEC infection in piglets, perhaps due to enterotoxin neutralization (Coddens et al., 2017). Moreover, grazing on PAC-rich forages has been reported to be effective in reducing infection with coccidia and helminths in small ruminants (Heckendorn et al., 2006; Kommuru et al., 2014).
Figure 1.
Structure of a proanthocyandin dimer, consisting of 2 epicatechin molecules linked by an interflavanol bond.
The notion of polyphenols as immunomodulators in animals has been driven mainly by human health research. A plethora of studies have investigated (mainly using preclinical rodent models) the ability of various polyphenol-rich functional foods to restore balanced immune function during settings of acute and chronic inflammation, for instance, obesity and diabetes (Anhê et al., 2015). A consistent feature of this research is a dampening of excessive T-helper (Th)-1 and Th17 immune responses that may drive the immunopathology associated with conditions such as colitis (Yoshioka et al., 2008), while enhancing the production of anti-inflammatory cytokines and mucosal antibodies, and improving IEC barrier function (Pierre et al., 2013; Denis et al., 2015). Similar results have been observed in livestock. Grape seed PAC can reduce diarrhea in piglets post-weaning, which was associated with an increase in serum antibodies and antioxidant capacity, and a modulation of cytokine production (Hao et al., 2015). In ruminants, antibody production and cellular immune responses in intestinal tissue may also be significantly enhanced during parasite infection by dietary PAC (Rios-De Álvarez et al., 2008; Pathak et al., 2016). Thus, PAC may enhance the acquisition of specific immune responses and reduce pathogen-induced inflammation.
The mechanisms of how PAC may modulate host immune and inflammatory reactions remain unresolved. In vitro, PAC can effectively neutralize enterotoxins produced by pathogenic bacteria such as E. coli, which may lead to decreased production of proinflammatory cytokines from host IEC and thus alleviate the pathogen-induced immunopathology (Nunes et al., 2019). Moreover, dietary PAC have been reported to have prebiotic effects, whereby the growth of certain beneficial bacteria taxa such as Lactobacillus or Bifidobacteria is selectively promoted (Choy et al., 2014; Kafantaris et al., 2018; Solano-Aguilar et al., 2018). Perhaps consistent with this propagation of short-chain fatty acid (SCFA)-producing bacteria, fecal propionate concentrations are higher in pigs containing PAC-rich grape pomace (Williams et al., 2017c; Wu et al., 2019). A consequence of this altered bacterial metabolism may be a more tolerogenic response in immune cells residing in the GALT, as microbiota-derived SCFA may suppress the production of inflammatory cytokines and acute-phase proteins, and instead promote the production of protective IgA antibodies (Seifert and Watzl, 2007; Smith et al., 2013). Indeed, administration of pure PAC to mice can promote IgA production in the ileum (Pierre et al., 2014). As well as SCFA production, PAC have been shown to be extensively metabolized in the large intestine of monogastric animals, producing a number of small metabolites such as catechins and phenolic acids (Déprez et al., 2000; Choy et al., 2014; Dudonné et al., 2016). Some of these been speculated to modulate the activity of immune cells in the gut mucosa, as well as potentially having systemic health-promoting effects following absorption (Selma et al., 2009).
In addition to prebiotic effects and activity of phenolic metabolites, increasing evidence is emerging that the PAC themselves may directly act on immune cells to change their innate responses to pathogen signaling. In cultured macrophages or DCs, exposure to PAC results in less production of proinflammatory cytokines such as IL-12 following stimulation with bacterial lipopolysaccharide (LPS), and higher production of cytokines such as IL-10 that promote regulatory responses (Terra et al., 2007; Williams et al., 2017b). How PAC alters the production of these cytokines is not clear, but experimental data suggest either direct interference with cyclooxygenase signaling and NF-κB translocation or a modulation of intracellular nutrient-sensing pathways that affect signal transduction following LPS receptor-binding (Martinez-Micaelo et al., 2012b; Midttun et al., 2018). Interestingly, in these studies, immunomodulatory activity is consistently associated with PAC that possess a high degree of polymerization (pentamers or higher). As PAC that are bigger than dimers are not efficiently absorbed across the gut barrier into circulation (Ou and Gu, 2014), these results suggest that a major immunomodulatory mechanism is the activation and/or modulation of immune cells residing at the mucosal surface. In the in vivo situation, the biological effects of dietary PAC are probably manifested by a combination of direct interactions between PAC polymers and mucosal immune cells, and also through the microbiota-mediated metabolism of PAC and subsequent action of low-molecular-weight metabolites. The relative contribution of these 2 mechanisms will probably vary according to the prevailing intestinal milieu and will need to be tested empirically in different models of infection and inflammation.
How may these anti-inflammatory effects of PAC benefit animals during pathogen challenge? Clearly, suppression of overt inflammatory responses resulting from exposure to various gut microbes (e.g., after weaning) can reduce energy expenditure and allow nutrients to be partitioned more effectively toward growth (Huntley et al., 2018). However, generalized immunosuppression may be of little long-term benefit when animals need strong immune responses to protect themselves against pathogen infection. In this respect, some recent studies offer an insight into how dietary PAC may effectively augment appropriate pathogen-specific immune responses, while preventing harmful immunopathology. A key finding is that PAC-rich diets appear to selectively inhibit some aspects of inflammation, while augmenting other immune functions. In mouse models of obesity-induced inflammation, PAC-rich diets can suppress production of inflammatory markers in the intestinal mucosa, but promote expression of the antimicrobial peptide Regenerating islet-derived protein 3 gamma (Reg3γ), which is secreted by Paneth cells. Moreover, genes encoding the tight junction proteins Occludin and Claudin-3 are also upregulated (Hul et al., 2018). There is also evidence that expression of MUC2, encoding the goblet cell–specific mucin 2 (Muc2), is also increased by dietary PAC (Pierre et al., 2013). Paneth and goblet cell activities, accompanied by the production of mucins and the Reg3 family of proteins, are key components in maintaining barrier integrity and regulating intestinal homeostasis through interactions with both exogenous microbes and the commensal gut microbiota (Shan et al., 2013). Moreover, IFNγ directly inhibits Reg3γ production, whereas IL-12 may suppress Muc2 production by inhibiting IL-4 and IL-13 (Eriguchi et al., 2018). PAC can modulate the activity of DCs and T-helper cells, resulting in lower IL-12 and IFNγ production (Williams et al., 2017b), suggesting a mechanism whereby PAC directly drive innate immune function and maintain intestinal homoeostasis. Thus, PAC do not appear to indiscriminately suppress antimicrobial immune responses, but instead promote balanced immune function, which acts to protect the gut barrier and exclude potentially harmful pathogens, without causing overt and excessive inflammation.
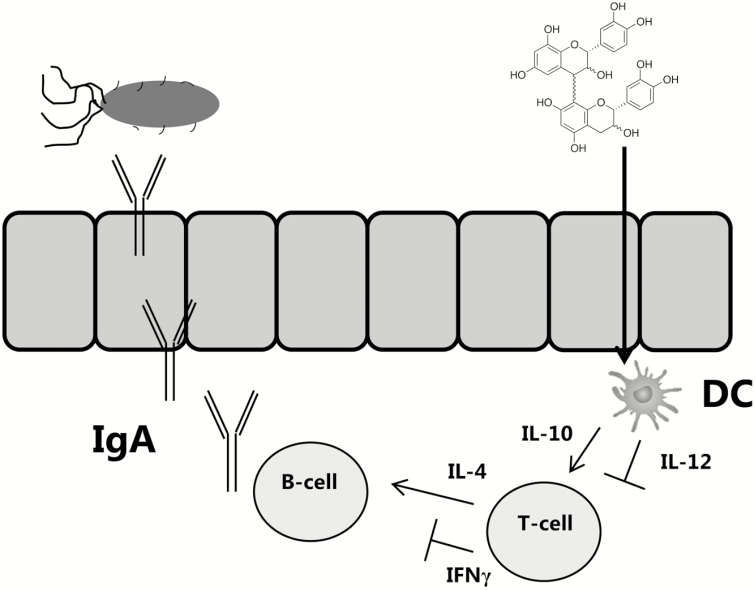
This hypothesis may help explain the observed benefits of PAC-rich supplements during pathogenic intestinal infections in pigs. In the case of ETEC infection, production of inflammatory cytokines in the mucosal barrier can be decoupled from the acquisition of protective immune mechanisms. Watery diarrhea and tissue damage results from the massive secretory actions of IEC following exposure to enterotoxin, and production of inflammatory mediators such as TNFα and IFNγ may contribute to accompanying fever and inappetence (Bosi et al., 2004; Nesta and Pizza, 2018). In humans, production of IFNγ appears to be associated with reduced clearance of ETEC infection, supporting the idea that the protective immune mechanisms are distinct from the proinflammatory cytokine response (Long et al., 2010). Instead, immunity is thought to derive mainly from mucosal antibodies, particularly IgG and IgA (Roy et al., 2008; Norton et al., 2015; Nesta and Pizza, 2018; Virdi et al., 2019). PAC may manifest protective effects in ETEC infection by direct inhibition of enterotoxins interacting with IEC, but also because of their intrinsic capacity to suppress the production of inflammatory cytokines from innate immune cells (Fig. 2). A consequence of reduced proinflammatory cytokine production may be the enhanced production of antibodies from B-cells in the GALT, as IFNγ and IL-12 can antagonize IgA production (Satorres et al., 2009; Lecocq et al., 2013). Thus, downregulation of the proinflammatory arm of the immune system may reciprocally activate effector mechanisms that are involved in protective immunity. This may also be particularly relevant for other mucosal pathogens such as helminths, where parasite clearance is normally dependent on Th2-type effector mechanisms and immunity is actively inhibited by Th1 cytokine production (Anthony et al., 2007). Indeed, in a pig model of helminth (A. suum) infection, a PAC-enriched diet enhanced the acquisition of A. suum-specific antibodies and Th2-related intestinal eosinophils (Williams et al., 2017c).
Figure 2.
One possible mechanism of how proanthocyanidins (PAC) may protect from infection in the gut by modulating innate immunity. PAC are sampled by dendritic cells (DCs) either from the lumen or after translocation of PAC by M-cells or goblet cells to the lamina propria. Production of IL-12 is then inhibited, whereas IL-10 is promoted, resulting in suppression of IFNγ by T-cells. Consequently, production of secretory IgA, which can neutralize pathogens such as Escherichia coli, is enhanced.
Overall, strong evidence exists now for the potential of dietary additives based on PAC-rich sources such as grape pomace to be used in both monogastric and ruminant production systems. Encouragingly for livestock producers, low levels of inclusion (<500 mg/kg) have been demonstrated to produce beneficial effects (Kafantaris et al., 2018). Furthermore, the cost-effectiveness of many of these additives is increased by ready availability as byproducts of other agricultural industries, such as wine and cider production (Sehm et al., 2007).
Essential Oil Components: Cinnamaldehyde, Eugenol, and Carvacrol
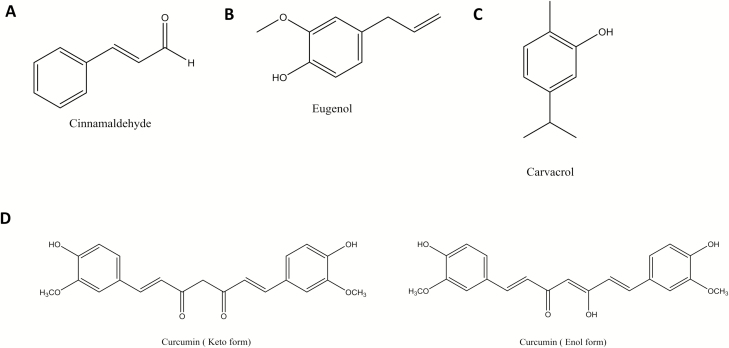
Essential oils are aromatic, hydrophobic solutions derived from many plants that are often used as flavoring agents in foods. Cinnamaldehyde, eugenol, and carvacrol are small, volatile compounds derived mainly from the essential oil of cinnamon, cloves, and oregano or thyme, respectively. All these compounds consist of an aromatic ring, with cinnamaldehyde also containing an aldehyde group, whereas eugenol and carvacrol have a hydroxyl group bound to the ring, making them part of the phenol family (Fig. 3A–C). They have been used for centuries as part of traditional folk medicine to treat ailments such as arthritis, and a number of recent studies have confirmed antioxidant and anti-inflammatory effects in in vitro cell models and in vivo rodent models (Ho et al., 2018; Lee et al., 2018; Mateen et al., 2019). Moreover, they are well-known antimicrobials. For example, CA has been used as both a preservative and flavoring agent in meat to prevent bacterial growth and spoilage (Zhang et al., 2014).
Figure 3.
Chemical structures of cinnamaldehyde (A), eugenol (B), carvacrol (C), and the keto and enol forms of curcumin (D).
All 3 compounds have been shown in vitro to reduce the production of inflammatory cytokines such as TNFα from macrophages (Chao et al., 2008; Liu et al., 2012). Thus, their potential to reduce inflammation in the gut of farm animals when used as a feed additive has recently been investigated. Cinnamaldehyde and carvacrol can modulate the expression of a number of gene networks related to immune defense and inflammatory responses in the chicken intestine, whereas cinnamaldehyde in combination with thymol lowers numbers of mucosal macrophages in the piglet gut post-weaning (Lillehoj et al., 2011; Jiang et al., 2015). Moreover, cinnamaldehyde and carvacrol (together with capsicum oleoresin) significantly suppressed expression of inflammatory cytokines such as IL-12 while improving growth rates in broilers (Pirgozliev et al., 2019). In a more detailed investigation of the effects of these compounds on gut health, Wlodarska et al. (2015) showed that administration of cinnamaldehyde, eugenol, or carvacrol in drinking water of mice resulted in a diverse modulation of gene expression profiles including upregulation of genes encoding Reg3γ, and the goblet cell protein trefoil factor-3. Notably, eugenol also thickened the mucus layer during Citrobacter rodentium infection, resulting in lower pathogen colonization and resulted a marked shift in the commensal microbiota with an increase in the Clostridiaceae family. Thus, the positive effects seen in livestock feeding trials may be related to increased mucosal barrier integrity and expression of antimicrobial peptides, preventing inflammatory responses and pathogen translocation.
In addition to suppressing inflammatory responses and changing the host microbiome, it has also been suggested that these compounds possess immunostimulatory properties that include the boosting of antibody production. In pigs, dietary addition of pure cinnamaldehyde or euegnol increases lymphocyte concentrations in blood (Yan and Kim, 2012), while feeding piglets a cinnamaldehyde and thymol additive results in higher amounts of IgA and IgM in serum, higher leukocyte phagocytosis rates, and lower E. coli excretion (Li et al., 2012a; Zeng et al., 2015). In addition, a combination of cinnamaldehyde and eugenol, or carvacrol combined with thymol, can increase vaccine-induced antibody titers in cattle and chickens, respectively (Du et al., 2016; de Souza et al., 2018). Moreover, a number of studies have investigated the effect of feeding cinnamaldehyde on immune function in defined infection models and demonstrated a boosting of production of specific antibodies against the parasites E. maxima in chickens (Lee et al., 2011b) and A. suum in pigs (Williams et al., 2017a).
The potential mechanisms underlying the increased humoral immune responses following supplementation with these essential oil components require further investigation, although some recent studies with cinnamaldehyde may offer an insight. Lee et al. (2011b) reported that in vitro exposure of chicken splenocytes to cinnamaldehyde resulted in increased proliferation; however, in vitro experiments with porcine blood lymphocytes have shown that cinnamaldehyde does not increase proliferation, nor induce selective maturation of antibody-secreting B-cells. In fact, in these experiments, cinnamaldehyde tended to inhibit mitogen-induced lymphocyte proliferation (Williams et al., 2017a). Thus, despite evidence of increase lymphocyte activity in vivo, direct contact between cinnamaldehyde and immune cells appears to be insufficient to drive cellular responses. It is more likely that the metabolic effects of cinnamaldehyde in vivo give rise to immunomodulatory effects. Cinnamaldehyde is capable of activating a number of intestinal ion channels such as TRP1A, and interactions with this receptor appear to partially govern the ability of cinnamaldehyde to reduce LPS-induced inflammation or induce glucose absorption in mouse intestinal tissue (Fothergill et al., 2016; Mendes et al., 2016). Notably, pigs fed cinnamaldehyde during A. suum infection have increased transcription of the genes encoding sodium/glucose cotransporter 1 and glucose transporter 2 in the jejunum (Williams et al., 2017a), suggesting that cinnamaldehyde activates certain receptors involved in nutrient uptake. The mechanistic link between cinnamaldehyde-mediated nutrient metabolism and immune function is not yet clear. However, as a small volatile compound, cinnamaldehyde or its metabolites probably activate xenobiotic-metabolizing circuits in host intestinal tissue. There is increasing evidence that sensing of both exogenous and endogenous xenobiotics regulates the development of immune cell populations at the gut mucosal surface (Chen and Sundrud, 2018). Such pathways include the aryl hydrocarbon receptor, a transcription factor active in many innate immune cells, and the cytochrome P450 pathway. Activation of these pathways can have a downstream effect on immune function, with activity of innate lymphoid cells and T-cells markedly influenced by metabolite-sensing through these pathways (Li et al., 2011; Chen and Sundrud, 2018). Further research is clearly required to understand how cinnamaldehyde and other compounds derived from essential oils exert their beneficial effects on immune function during pathogen infection. Despite this, amenability to large-scale synthesis and/or purification, together with promising results on animal performance, already support their inclusion in livestock diets, with reports of increased growth rates and feed conversion efficiency in animals fed low levels (<20 ppm) blends of essential oil components including cinnamaldehyde and eugenol (Li et al., 2012b; Zeng et al., 2015).
Curcumin
Curcumin is the yellow-colored spice that gives its turmeric its characteristic flavor. Chemically, curcumin is a type of phenol, specially a linear diarylheptanoid containing 2 aromatic rings (Fig. 3D). Curcumin has received much attention due to its putative anticancer effects, which have shown some promise in preclinical models; however, convincing clinical evidence does not yet exist (Lin et al., 2019a). What is clear is that, similar to the compounds described earlier, curcumin possesses strong natural antioxidant and anti-inflammatory properties (Karimi et al., 2019).
In livestock feeding trials, the potential of curcumin’s anti-inflammatory properties to improve performance has been demonstrated in poultry fed either pure curcumin or curcumin combined with CA and capsicum oleoresin (Bravo et al., 2014; Zhang et al., 2018). Moreover, inclusion of curcumin at relatively low levels (300 mg/kg) has been shown to alleviate E. coli-induced production of inflammatory mediators such as IL-1β, while promoting the secretion of antibodies (Xun et al., 2015; Gan et al., 2019). Mechanistically, this appears to be related to the ability of curcumin (in the form of turmeric oleoresin) to suppress macrophage numbers and functional activity in the ileal mucosa during pathogen challenge (Liu et al., 2014a,b). Interestingly, another study has shown that immune function was not altered by curcumin in healthy piglets, suggesting that perhaps pathogen challenge to the immune system may be necessary for measurable benefits of curcumin supplementation (Ilsley et al., 2005). In vitro experiments have shown that curcumin can alter macrophage activity by activating the Nrf2 pathway, thereby promoting antioxidant responses and inducing expression of glutathione peroxidases (Lin et al., 2019b). In addition, similar to some of the other phytonutrients discussed previously, curcumin can stimulate vaccine-induced antibody production, notably against the Eimeria antigens in poultry. Chickens supplemented with curcumin, together with capsicum oleoresin, had increased antigen-specific antibody responses, together with more T-helper cells and MHC-II-positive cells following parasite challenge (Lee et al., 2011a). Overall, although more research is needed to assess curcumin more critically under production conditions, its biological activity appears to align with other phytonutrients that have been studied, with a marked immunomodulatory effect during pathogen challenge that can regulate inflammation and expression of protective immune mechanisms.
Conclusions and Perspectives
The use of selected phytonutrients as feed additives that may improve immune function during gut infections has great potential to aid sustainable livestock production, in an era of less antimicrobial use and increased efficiency demands. Despite their diverse chemical structures, the specific phytonutrients detailed above have a number of similarities in their observed biological activities. These include clear anti-inflammatory effects in vitro and in vivo, which are coupled with a marked boosting of antibody production and mucosal barrier integrity, along with the stimulation of the growth of beneficial commensal bacterial taxa. Crucially, many of these phytonutrients appear to be able to maintain or restore protective and/or regulatory immune mechanisms while suppressing serious inflammation and immunopathology.
Despite these advances, a number of questions remained unresolved. What is the relative contribution of the host gut microbiota, and to what extent are these effects mediated by direct interactions with the host tissues and cells? Do some phytonutrients directly inhibit pathogen establishment, for example, by preventing adhesion to epithelial cells, or are the observed beneficial effects purely mediated by effects on host responses? Moreover, the practical application of bioactive phytonutrients may vary considerably according to the production system and prevailing pathogen challenges. Different immune mechanisms may be more or less relevant for different pathogens. In some cases, priming of the immune system to be better able to respond more robustly to antigen challenge may be the most appropriate goal. In other cases, actively modulating naturally induced immune mechanisms to polarize them toward different phenotypes (Th1 or Th2, or pro- or anti-inflammatory responses) may be desirable.
Furthermore, the precise mechanisms underlying many of these immunomodulatory effects remained unclear. The role of defined immune cell subsets such as DCs and innate lymphoid cells needs to be addressed, to understand how the metabolism of these dietary components effects inflammation and generation of pathogen-specific immune responses in the gut. Indeed, models of dietary phytonutrients in pathogen infection may be a useful system to address fundamental questions such as how xenobiotic-sensing pathways are coupled to the regulation of mucosal immune function. Such questions are not just of academic interest, as a detailed understanding of the mechanism-of-action of these phytonutrients will be crucial for optimization of this approach as a long-term solution to ensure gut health in farmed animals. Increasing our mechanistic understanding may help to resolve issues such as the most appropriate timing of dietary supplementation in relation to pathogen challenge (i.e., prophylactic or therapeutic administration), and whether there are different immunomodulatory effects of dietary additives in either healthy or diseased animals.
Finally, perhaps the biggest question of all is why should the mammalian intestinal tract, an organ separated by millions of years of evolution from plants, sense a number of these plant-derived compounds in a seemingly conserved way? A holistic understanding of the interactions between the animal, its diet, and the multitude of microorganisms that live within it may offer solutions to the substantial challenges facing modern animal production.
Acknowledgments
Work in A.R.W.’s group is funded mainly by the Independent Research Fund Denmark (Danmarks Frie Forskningsfond) (grants 12-126630; 4184-00377; 6111-00394; 7026-00094B). We thank all group members for productive discussions. L.Z. is a recipient of a scholarship from the China Scholarship Council (grant 201806910065). We are grateful to Iqbal Imran for preparing Fig. 3.
Glossary
Abbreviations
- DCs
dendritic cells
- ETEC
enterotoxigenic Escherichia coli
- GALT
gut-associated lymphoid tissue
- IEC
intestinal epithelial cells
- LPS
lipopolysaccharide
- Muc2
mucin 2
- PAC
proanthocyandins
- SCFA
short-chain fatty acid
- Reg3γ
regenerating islet-derived protein 3 gamma
- Th
T-helper
- Trp
tryptophan
Literature cited
- Agace W. W. 2008. T-cell recruitment to the intestinal mucosa. Trends Immunol. 29:514–522. doi: 10.1016/j.it.2008.08.003 [DOI] [PubMed] [Google Scholar]
- Ahn-Jarvis H. J., Parihar A., and Doseff I. A.. . 2019. Dietary flavonoids for immunoregulation and cancer: Food design for targeting disease. Antioxidants 8: E202. doi: 10.3390/antiox8070202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anhê F. F., Varin T. V., Le Barz M., Desjardins Y., Levy E., Roy D., and Marette A.. . 2015. Gut microbiota dysbiosis in obesity-linked metabolic diseases and prebiotic potential of polyphenol-rich extracts. Curr. Obes. Rep. 4:389–400. doi: 10.1007/s13679-015-0172-9 [DOI] [PubMed] [Google Scholar]
- Anthony R. M., Rutitzky L. I., Urban J. F. Jr., Stadecker M. J., and Gause W. C.. . 2007. Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 7:975–987. doi: 10.1038/nri2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou M. A., Davis E. M., and Kasl B. A.. . 2019. Nutraceuticals: An alternative strategy for the use of antimicrobials. Vet. Clin. North Am. Food Anim. Pract. 35:507–534. doi: 10.1016/j.cvfa.2019.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander J. M., Longman R. S., Iliev I. D., Sonnenberg G. F., and Artis D.. . 2017. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 18:851–860. doi: 10.1038/ni.3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosi P., Casini L., Finamore A., Cremokolini C., Merialdi G., Trevisi P., Nobili F., and Mengheri E.. . 2004. Spray-dried plasma improves growth performance and reduces inflammatory status of weaned pigs challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 82:1764–1772. doi: 10.2527/2004.8261764x [DOI] [PubMed] [Google Scholar]
- Bravo D., Pirgozliev V., and Rose S. P.. . 2014. A mixture of carvacrol, cinnamaldehyde, and capsicum oleoresin improves energy utilization and growth performance of broiler chickens fed maize-based diet. J. Anim. Sci. 92:1531–1536. doi: 10.2527/jas.2013-6244 [DOI] [PubMed] [Google Scholar]
- Brestoff J. R., and Artis D.. . 2015. Immune regulation of metabolic homeostasis in health and disease. Cell 161:146–160. doi: 10.1016/j.cell.2015.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burow E., Rostalski A., Harlizius J., Gangl A., Simoneit C., Grobbel M., Kollas C., Tenhagen B. A., and Käsbohrer A.. . 2019. Antibiotic resistance in Escherichia coli from pigs from birth to slaughter and its association with antibiotic treatment. Prev. Vet. Med. 165:52–62. doi: 10.1016/j.prevetmed.2019.02.008 [DOI] [PubMed] [Google Scholar]
- Capozzalo M. M., Kim J. C., Htoo J. K., de Lange C. F., Mullan B. P., Hansen C. F., Resink J. W., Stumbles P. A., Hampson D. J., and Pluske J. R.. . 2015. Effect of increasing the dietary tryptophan to lysine ratio on plasma levels of tryptophan, kynurenine and urea and on production traits in weaner pigs experimentally infected with an enterotoxigenic strain of Escherichia coli. Arch. Anim. Nutr. 69:17–29. doi: 10.1080/1745039X.2014.995972 [DOI] [PubMed] [Google Scholar]
- Chao L. K., Hua K. F., Hsu H. Y., Cheng S. S., Lin I. F., Chen C. J., Chen S. T., and Chang S. T.. . 2008. Cinnamaldehyde inhibits pro-inflammatory cytokines secretion from monocytes/macrophages through suppression of intracellular signaling. Food Chem. Toxicol. 46:220–231. doi: 10.1016/j.fct.2007.07.016 [DOI] [PubMed] [Google Scholar]
- Chapman H. D. 2014. Milestones in avian coccidiosis research: A review. Poult. Sci. 93:501–511. doi: 10.3382/ps.2013-03634 [DOI] [PubMed] [Google Scholar]
- Charlier J., Thamsborg S. M., Bartley D. J., Skuce P. J., Kenyon F., Geurden T., Hoste H., Williams A. R., Sotiraki S., Höglund J., . et al. 2018. Mind the gaps in research on the control of gastrointestinal nematodes of farmed ruminants and pigs. Transbound. Emerg. Dis. 65(Suppl. 1):217–234. doi: 10.1111/tbed.12707 [DOI] [PubMed] [Google Scholar]
- Chen M. L., and Sundrud M. S.. . 2018. Xenobiotic and endobiotic handling by the mucosal immune system. Curr. Opin. Gastroenterol. 34:404–412. doi: 10.1097/MOG.0000000000000478 [DOI] [PubMed] [Google Scholar]
- Chlebicz A., and Śliżewska K.. . 2018. Campylobacteriosis, salmonellosis, yersiniosis, and listeriosis as zoonotic foodborne diseases: A review. Int. J. Environ. Res. Pub Health 15:863. doi: 10.3390/ijerph15050863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy Y. Y., Quifer-Rada P., Holstege D. M., Frese S. A., Calvert C. C., Mills D. A., Lamuela-Raventos R. M., and Waterhouse A. L.. . 2014. Phenolic metabolites and substantial microbiome changes in pig feces by ingesting grape seed proanthocyanidins. Food Funct. 5:2298–2308. doi: 10.1039/c4fo00325j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddens A., Loos M., Vanrompay D., Remon J. P., and Cox E.. . 2017. Cranberry extract inhibits in vitro adhesion of F4 and F18+Escherichia coli to pig intestinal epithelium and reduces in vivo excretion of pigs orally challenged with F18+ verotoxigenic E. coli. Vet. Microbiol. 202:64–71. doi: 10.1016/j.vetmic.2017.01.019 [DOI] [PubMed] [Google Scholar]
- Colditz I. G. 2008. Six costs of immunity to gastrointestinal nematode infections. Parasite Immunol. 30:63–70. doi: 10.1111/j.1365-3024.2007.00964.x [DOI] [PubMed] [Google Scholar]
- Coop R. L., and Kyriazakis I.. . 2001. Influence of host nutrition on the development and consequences of nematode parasitism in ruminants. Trends Parasitol. 17:325–330. doi: 10.1016/s1471-4922(01)01900-6 [DOI] [PubMed] [Google Scholar]
- Dalia A. M., Loh T. C., Sazili A. Q., Jahromi M. F., and Samsudin A. A.. . 2018. Effects of vitamin E, inorganic selenium, bacterial organic selenium, and their combinations on immunity response in broiler chickens. BMC Vet. Res. 14:249. doi: 10.1186/s12917-018-1578-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza K. A., Cooke R. F., Schubach K. M., Brandão A. P., Schumaher T. F., Prado I. N., Marques R. S., and Bohnert D. W.. . 2018. Performance, health and physiological responses of newly weaned feedlot cattle supplemented with feed-grade antibiotics or alternative feed ingredients. Animal 12:2521–2528. doi: 10.1017/S1751731118000551 [DOI] [PubMed] [Google Scholar]
- Denis M. C., Desjardins Y., Furtos A., Marcil V., Dudonné S., Montoudis A., Garofalo C., Delvin E., Marette A., and Levy E.. . 2015. Prevention of oxidative stress, inflammation and mitochondrial dysfunction in the intestine by different cranberry phenolic fractions. Clin. Sci. (Lond). 128:197–212. doi: 10.1042/CS20140210 [DOI] [PubMed] [Google Scholar]
- Déprez S., Brezillon C., Rabot S., Philippe C., Mila I., Lapierre C., and Scalbert A.. . 2000. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. J. Nutr. 130:2733–2738. doi: 10.1093/jn/130.11.2733 [DOI] [PubMed] [Google Scholar]
- Desrues O., Pena-Espinoza M., Hansen T. V., Enemark H. L., Thamsborg S. M., . 2016. Anti-parasitic activity of pelleted sainfoin (Onobrychis viciifolia) against Ostertagia ostertagi and Cooperia oncophora in calves. Parasit. Vectors 9:319. doi: 10.1186/s13071-016-1617-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du E., Wang W., Gan L., Li Z., Guo S., and Guo Y.. . 2016. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 7:19. doi: 10.1186/s40104-016-0079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudonné S., Dal-Pan A., Dubé P., Varin T. V., Calon F., and Desjardins Y.. . 2016. Potentiation of the bioavailability of blueberry phenolic compounds by co-ingested grape phenolic compounds in mice, revealed by targeted metabolomic profiling in plasma and feces. Food Funct. 7:3421–3430. doi: 10.1039/c6fo00902f [DOI] [PubMed] [Google Scholar]
- Eisler M. C., Lee M. R., Tarlton J. F., Martin G. B., Beddington J., Dungait J. A., Greathead H., Liu J., Mathew S., Miller H., . et al. 2014. Agriculture: Steps to sustainable livestock. Nature 507:32–34. doi: 10.1038/507032a [DOI] [PubMed] [Google Scholar]
- Eriguchi Y., Nakamura K., Yokoi Y., Sugimoto R., Takahashi S., Hashimoto D., Teshima T., Ayabe T., Selsted M., Ouellette A. J... 2018. Essential role of IFN-γ in T cell–associated intestinal inflammation. JCI Insight 3:121886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiesel A., Gessner D. K., Most E., and Eder K.. . 2014. Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet. Res. 10:196. doi: 10.1186/s12917-014-0196-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fothergill L., Callaghan J. B., Rivera L. R., Lieu T., Poole D. P., Cho H. J., Bravo D. M., Furness J. B... 2016. Effects of food components that activate TRPA1 receptors on mucosal ion transport in the mouse intestine. Nutrients 8:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Z., Wei W., Li Y., Wu J., Zhao Y., Zhang L., Wang T., Zhong X... 2019. Curcumin and resveratrol regulate intestinal bacteria and alleviate intestinal inflammation in weaned piglets. Molecules 24:1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner D. K., Fiesel A., Most E., Dinges J., Wen G., Ringseis R., and Eder K.. . 2013. Supplementation of a grape seed and grape marc meal extract decreases activities of the oxidative stress-responsive transcription factors NF-κB and Nrf2 in the duodenal mucosa of pigs. Acta Vet. Scand. 55:18. doi: 10.1186/1751-0147-55-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M., Thanner S., Pradervand N., Hu D., Ollagnier C., and Bee G.. . 2018. Hydrolysable chestnut tannins for reduction of postweaning diarrhea: Efficacy on an experimental ETEC F4 model. PLoS One 13:e0197878. doi: 10.1371/journal.pone.0197878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao R., Li Q., Zhao J., Li H., Wang W., and Gao J... 2015. Effects of grape seed procyanidins on growth performance, immune function and antioxidant capacity in weaned piglets. Livest. Sci. 178:237–242. [Google Scholar]
- Heckendorn F., Häring D. A., Maurer V., Zinsstag J., Langhans W., and Hertzberg H.. . 2006. Effect of sainfoin (Onobrychis viciifolia) silage and hay on established populations of Haemonchus contortus and Cooperia curticei in lambs. Vet. Parasitol. 142:293–300. doi: 10.1016/j.vetpar.2006.07.014 [DOI] [PubMed] [Google Scholar]
- Heo J. M., Kim J. C., Hansen C. F., Mullan B. P., Hampson D. J., and Pluske J. R.. . 2009. Feeding a diet with decreased protein content reduces indices of protein fermentation and the incidence of postweaning diarrhea in weaned pigs challenged with an enterotoxigenic strain of Escherichia coli. J. Anim. Sci. 87:2833–2843. doi: 10.2527/jas.2008-1274 [DOI] [PubMed] [Google Scholar]
- Ho S.-C., Chang Y.-H., and Chang K.-S.. . 2018. Structural moieties required for cinnamaldehyde-related compounds to inhibit canonical IL-1β secretion. Molecules 23:3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hul M. L., Geurts H., Plovier C., Druart A., Everard M., Stahlman M., Rhimi K., Chira P. L., Teissedre N. M., Delzenne, et al. 2018. Reduced obesity, diabetes, and steatosis upon cinnamon and grape pomace are associated with changes in gut microbiota and markers of gut barrier. Am. J. Physiol.-Endocrinol. Metab. 314:E334–E352. [DOI] [PubMed] [Google Scholar]
- Huntley N. F., Nyachoti C. M., and Patience J. F.. . 2018. Lipopolysaccharide immune stimulation but not β-mannanase supplementation affects maintenance energy requirements in young weaned pigs. J. Anim. Sci. Biotechnol. 9:47. doi: 10.1186/s40104-018-0264-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilsley S. E., Miller H. M., and Kamel C.. . 2005. Effects of dietary Quillaja saponin and curcumin on the performance and immune status of weaned piglets. J. Anim. Sci. 83:82–88. doi: 10.2527/2005.83182x [DOI] [PubMed] [Google Scholar]
- Jansman A. J. 1993. Tannins in feedstuffs for simple-stomached animals. Nutr. Res. Rev. 6:209–236. doi: 10.1079/NRR19930013 [DOI] [PubMed] [Google Scholar]
- Jiang X. R., Awati A., Agazzi A., Vitari F., Ferrari A., Bento H., Crestani M., Domeneghini C., and Bontempo V.. . 2015. Effects of a blend of essential oils and an enzyme combination on nutrient digestibility, ileum histology and expression of inflammatory mediators in weaned piglets. Animal 9:417–426. doi: 10.1017/S1751731114002444 [DOI] [PubMed] [Google Scholar]
- Kafantaris I., Stagos D., Kotsampasi B., Hatzis A., Kypriotakis A., Gerasopoulos K., Makri S., Goutzourelas N., Mitsagga C., Giavasis I., . et al. 2018. Grape pomace improves performance, antioxidant status, fecal microbiota and meat quality of piglets. Animal 12:246–255. doi: 10.1017/S1751731117001604 [DOI] [PubMed] [Google Scholar]
- Karimi A., Ghodsi R., Kooshki F., Karimi M., Asghariazar V., Tarighat-Esfanjani A... 2019. Therapeutic effects of curcumin on sepsis and mechanisms of action: A systematic review of preclinical studies. Phytother. Res. 33:2798–2820. doi: 10.1002/ptr.6467 [DOI] [PubMed] [Google Scholar]
- Kim J. C., Mullan B. P., Black J. L., Hewitt R. J., van Barneveld R. J., and Pluske J. R.. . 2016. Acetylsalicylic acid supplementation improves protein utilization efficiency while vitamin E supplementation reduces markers of the inflammatory response in weaned pigs challenged with enterotoxigenic E. coli. J. Anim. Sci. Biotechnol. 7:58. doi: 10.1186/s40104-016-0118-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommuru D. S., Barker T., Desai S., Burke J. M., Ramsay A., Mueller-Harvey I., Miller J. E., Mosjidis J. A., Kamisetti N., and Terrill T. H.. . 2014. Use of pelleted sericea lespedeza (Lespedeza cuneata) for natural control of coccidia and gastrointestinal nematodes in weaned goats. Vet. Parasitol. 204:191–198. doi: 10.1016/j.vetpar.2014.04.017 [DOI] [PubMed] [Google Scholar]
- Kongsted H., Pedersen K., Hjulsager C. K., Larsen L. E., Pedersen K. S., Jorsal S. E., and Bækbo P.. . 2018. Diarrhoea in neonatal piglets: A case control study on microbiological findings. Porcine Health Manag. 4:17. doi: 10.1186/s40813-018-0094-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans S. J., van der Staay F. J., Le Floc’h N., Dekker R., van Diepen J. T., and Jansman A. J.. . 2012. Effects of surplus dietary L-tryptophan on stress, immunology, behavior, and nitrogen retention in endotoxemic pigs. J. Anim. Sci. 90:241–251. doi: 10.2527/jas.2010-3372 [DOI] [PubMed] [Google Scholar]
- Kruse A. B., Nielsen L. R., and Alban L.. . 2018. Herd typologies based on multivariate analysis of biosecurity, productivity, antimicrobial and vaccine use data from Danish sow herds. Prev. Vet. Med. (in press). doi: 10.1016/j.prevetmed.2018.06.008 [DOI] [PubMed] [Google Scholar]
- Lecocq M., Detry B., Guisset A., and Pilette C.. . 2013. FcαRI-mediated inhibition of IL-12 production and priming by IFN-γ of human monocytes and dendritic cells. J. Immunol. 190:2362–2371. doi: 10.4049/jimmunol.1201128 [DOI] [PubMed] [Google Scholar]
- Lee S. H., Lillehoj H. S., Jang S. I., Lee K. W., Bravo D., and Lillehoj E. P.. . 2011a. Effects of dietary supplementation with phytonutrients on vaccine-stimulated immunity against infection with Eimeria tenella. Vet. Parasitol. 181:97–105. doi: 10.1016/j.vetpar.2011.05.003 [DOI] [PubMed] [Google Scholar]
- Lee S. H., Lillehoj H. S., Jang S. I., Lee K. W., Park M. S., Bravo D., and Lillehoj E. P.. . 2011b. Cinnamaldehyde enhances in vitro parameters of immunity and reduces in vivo infection against avian coccidiosis. Br. J. Nutr. 106:862–869. doi: 10.1017/S0007114511001073 [DOI] [PubMed] [Google Scholar]
- Lee S. C., Wang S. Y., Li C. C., and Liu C. T.. . 2018. Anti-inflammatory effect of cinnamaldehyde and linalool from the leaf essential oil of Cinnamomum osmophloeum Kanehira in endotoxin-induced mice. J. Food Drug Anal. 26:211–220. doi: 10.1016/j.jfda.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Innocentin S., Withers D. R., Roberts N. A., Gallagher A. R., Grigorieva E. F., Wilhelm C., and Veldhoen M.. . 2011. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147:629–640. doi: 10.1016/j.cell.2011.09.025 [DOI] [PubMed] [Google Scholar]
- Li P., Piao X., Ru Y., Han X., Xue L., and Zhang H.. . 2012a. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian-Australas. J. Anim. Sci. 25:1617–1626. doi: 10.5713/ajas.2012.12292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. Y., Ru Y. J., Liu M., Xu B., Peron A., Shi X. G... 2012b. The effect of essential oils on performance, immunity and gut microbial population in weaner pigs. Livest. Sci. 145:119–123. [Google Scholar]
- Lillehoj H. S., Kim D. K., Bravo D. M., and Lee S. H.. . 2011. Effects of dietary plant-derived phytonutrients on the genome-wide profiles and coccidiosis resistance in the broiler chickens. BMC Proc. 5(Suppl. 4):S34. doi: 10.1186/1753-6561-5-S4-S34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj H., Liu Y., Calsamiglia S., Fernandez-Miyakawa M. E., Chi F., Cravens R. L., Oh S., and Gay C. G.. . 2018. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 49:76. doi: 10.1186/s13567-018-0562-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Bai D., Wei Z., Zhang Y., Huang Y., Deng H., and Huang X.. . 2019b. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS One 14:e0216711. doi: 10.1371/journal.pone.0216711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. R., Chang C. H., Hsu C. F., Tsai M. J., Cheng H., Leong M. K., Sung P. J., Chen J. C., Weng C. F... 2019a. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. (in press). doi: 10.1111/bph.14816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Song M., Che T. M., Bravo D., Maddox C. W., and Pettigrew J. E.. . 2014a. Effects of capsicum oleoresin, garlic botanical, and turmeric oleoresin on gene expression profile of ileal mucosa in weaned pigs. J. Anim. Sci. 92:3426–3440. doi: 10.2527/jas.2013-6496 [DOI] [PubMed] [Google Scholar]
- Liu Y., Song M., Che T. M., Bravo D., and Pettigrew J. E.. . 2012. Anti-inflammatory effects of several plant extracts on porcine alveolar macrophages in vitro. J. Anim. Sci. 90:2774–2783. doi: 10.2527/jas.2011-4304 [DOI] [PubMed] [Google Scholar]
- Liu Y., Song M., Che T. M., Lee J. J., Bravo D., Maddox C. W., and Pettigrew J. E.. . 2014b. Dietary plant extracts modulate gene expression profiles in ileal mucosa of weaned pigs after an Escherichia coli infection. J. Anim. Sci. 92:2050–2062. doi: 10.2527/jas.2013-6422 [DOI] [PubMed] [Google Scholar]
- Long K. Z., Rosado J. L., Santos J. I., Haas M., Al Mamun A., DuPont H. L., Nanthakumar N. N., and Estrada-Garcia T.. . 2010. Associations between mucosal innate and adaptive immune responses and resolution of diarrheal pathogen infections. Infect. Immun. 78:1221–1228. doi: 10.1128/IAI.00767-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luise D., Lauridsen C., Bosi P., and Trevisi P.. . 2019. Methodology and application of Escherichia coli F4 and F18 encoding infection models in post-weaning pigs. J. Anim. Sci. Biotechnol. 10:53. doi: 10.1186/s40104-019-0352-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín M., María Giner R., Ríos J. L., and Recio M. C.. . 2013. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J. Ethnopharmacol. 150:925–934. doi: 10.1016/j.jep.2013.09.030 [DOI] [PubMed] [Google Scholar]
- Martinez-Micaelo N., González-Abuín N., Ardèvol A., Pinent M., and Blay M. T.. . 2012a. Procyanidins and inflammation: Molecular targets and health implications. Biofactors 38:257–265. doi: 10.1002/biof.1019 [DOI] [PubMed] [Google Scholar]
- Martinez-Micaelo N., González-Abuín N., Terra X., Richart C., Ardèvol A., Pinent M., and Blay M.. . 2012b. Omega-3 docosahexaenoic acid and procyanidins inhibit cyclo-oxygenase activity and attenuate NF-κB activation through a p105/p50 regulatory mechanism in macrophage inflammation. Biochem. J. 441:653–663. doi: 10.1042/BJ20110967 [DOI] [PubMed] [Google Scholar]
- Mateen S., Rehman M. T., Shahzad S., Naeem S. S., Faizy A. F., Khan A. Q., Khan M. S., Husain F. M., and Moin S.. . 2019. Anti-oxidant and anti-inflammatory effects of cinnamaldehyde and eugenol on mononuclear cells of rheumatoid arthritis patients. Eur. J. Pharmacol. 852:14–24. doi: 10.1016/j.ejphar.2019.02.031 [DOI] [PubMed] [Google Scholar]
- Mendes S. J. F., Sousa F. I. A. B., Pereira D. M. S., Ferro T. A. F., Pereira I. C. P., Silva B. L. R., Pinheiro A. J. M. C. R., Mouchrek A. Q. S., Monteiro-Neto V., Costa S. K. P., . et al. 2016. Cinnamaldehyde modulates LPS-induced systemic inflammatory response syndrome through TRPA1-dependent and independent mechanisms. Int. Immunopharmacol. 34: 60–70. doi: 10.1016/j.intimp.2016.02.012 [DOI] [PubMed] [Google Scholar]
- Midttun H. L. E., Ramsay A., Mueller-Harvey I., and Williams A. R.. . 2018. Cocoa procyanidins modulate transcriptional pathways linked to inflammation and metabolism in human dendritic cells. Food Funct. 9:2883–2890. doi: 10.1039/c8fo00387d [DOI] [PubMed] [Google Scholar]
- Moore R. J. 2016. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 45:275–281. doi: 10.1080/03079457.2016.1150587 [DOI] [PubMed] [Google Scholar]
- Morgan E. R., Aziz N. A., Blanchard A., Charlier J., Charvet C., Claerebout E., Geldhof P., Greer A. W., Hertzberg H., Hodgkinson J., . et al. 2019. 100 Questions in livestock helminthology research. Trends Parasitol. 35:52–71. doi: 10.1016/j.pt.2018.10.006 [DOI] [PubMed] [Google Scholar]
- Mueller-Harvey I. 2006. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 86:2010–2037. [Google Scholar]
- Nesta B., and Pizza M.. . 2018. Vaccines against Escherichia coli. In: Frankel G. and Ron E. Z., editors, Escherichia coli, a versatile pathogen. Springer International Publishing, Cham, Switzerland: P. 213–242. [Google Scholar]
- Norton E. B., Branco L. M., and Clements J. D.. . 2015. Evaluating the A-subunit of the heat-labile toxin (LT) as an immunogen and a protective antigen against enterotoxigenic Escherichia coli (ETEC). PLoS One 10:e0136302. doi: 10.1371/journal.pone.0136302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes C., Figueiredo R., Laranjinha J., and da Silva G. J.. . 2019. Intestinal cytotoxicity induced by Escherichia coli is fully prevented by red wine polyphenol extract: Mechanistic insights in epithelial cells. Chem. Biol. Interact. 310:108711. doi: 10.1016/j.cbi.2019.06.024 [DOI] [PubMed] [Google Scholar]
- Ou K., and Gu L.. . 2014. Absorption and metabolism of proanthocyanidins. J. Funct. Foods 7:43–53. [Google Scholar]
- Pathak A. K., Dutta N., Banerjee P. S., Goswami T. K., and Sharma K.. . 2016. Effect of condensed tannins supplementation through leaf meal mixture on voluntary feed intake, immune response and worm burden in Haemonchus contortus infected sheep. J. Parasit. Dis. 40:100–105. doi: 10.1007/s12639-014-0455-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S., Saeed I., Michaelsen K. F., Friis H., and Murrell K. D.. . 2002. Impact of protein energy malnutrition on Trichuris suis infection in pigs concomitantly infected with Ascaris suum. Parasitology 124(Pt 5):561–568. doi: 10.1017/s0031182002001592 [DOI] [PubMed] [Google Scholar]
- Peña-Espinoza M., Valente A. H., Thamsborg S. M., Simonsen H. T., Boas U., Enemark H. L., López-Muñoz R., and Williams A. R.. . 2018. Antiparasitic activity of chicory (Cichorium intybus) and its natural bioactive compounds in livestock: A review. Parasit. Vectors 11:475. doi: 10.1186/s13071-018-3012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. W., and Artis D.. . 2014. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 14:141–153. doi: 10.1038/nri3608 [DOI] [PubMed] [Google Scholar]
- Pierre J. F., Heneghan A. F., Feliciano R. P., Shanmuganayagam D., Krueger C. G., Reed J. D., and Kudsk K. A.. . 2014. Cranberry proanthocyanidins improve intestinal sIgA during elemental enteral nutrition. JPEN. J. Parenter. Enteral Nutr. 38:107–114. doi: 10.1177/0148607112473654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre J. F., Heneghan A. F., Feliciano R. P., Shanmuganayagam D., Roenneburg D. A., Krueger C. G., Reed J. D., and Kudsk K. A.. . 2013. Cranberry proanthocyanidins improve the gut mucous layer morphology and function in mice receiving elemental enteral nutrition. JPEN. J. Parenter. Enteral Nutr. 37:401–409. doi: 10.1177/0148607112463076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirgozliev V., Mansbridge S. C., Rose S. P., Lillehoj H. S., and Bravo D.. . 2019. Immune modulation, growth performance, and nutrient retention in broiler chickens fed a blend of phytogenic feed additives. Poult. Sci. 98:3443–3449. doi: 10.3382/ps/pey472 [DOI] [PubMed] [Google Scholar]
- Rios-De Álvarez L., et al. 2008. The effect of dietary sainfoin (Onobrychis viciifolia) on local cellular responses to Trichostrongylus colubriformis in sheep. Parasitology 135:1117–1124. [DOI] [PubMed] [Google Scholar]
- Roy K., Hamilton D., Allen K. P., Randolph M. P., and Fleckenstein J. M.. . 2008. The EtpA exoprotein of enterotoxigenic Escherichia coli promotes intestinal colonization and is a protective antigen in an experimental model of murine infection. Infect. Immun. 76:2106–2112. doi: 10.1128/IAI.01304-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satorres S. E., Alcaráz L. E., Cargnelutti E., and Di Genaro M. S.. . 2009. IFN-gamma plays a detrimental role in murine defense against nasal colonization of Staphylococcus aureus. Immunol. Lett. 123:185–188. doi: 10.1016/j.imlet.2009.03.003 [DOI] [PubMed] [Google Scholar]
- Sehm J., Lindermayer H., Dummer C., Treutter D., and Pfaffl M. W.. . 2007. The influence of polyphenol rich apple pomace or red-wine pomace diet on the gut morphology in weaning piglets. J. Anim. Physiol. Anim. Nutr. (Berl). 91:289–296. doi: 10.1111/j.1439-0396.2006.00650.x [DOI] [PubMed] [Google Scholar]
- Seifert S., and Watzl B.. . 2007. Inulin and oligofructose: Review of experimental data on immune modulation. J. Nutr. 137(11 Suppl):2563S–2567S. doi: 10.1093/jn/137.11.2563S [DOI] [PubMed] [Google Scholar]
- Selma M. V., Espín J. C., and Tomás-Barberán F. A.. . 2009. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 57:6485–6501. doi: 10.1021/jf902107d [DOI] [PubMed] [Google Scholar]
- Shan M., Gentile M., Yeiser J. R., Walland A. C., Bornstein V. U., Chen K., He B., Cassis L., Bigas A., Cols M., . et al. 2013. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 342:447–453. doi: 10.1126/science.1237910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Shahzad B., Rehman A., Bhardwaj R., Landi M., Zheng B... 2019. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 24:2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. M., Howitt M. R., Panikov N., Michaud M., Gallini C. A., Bohlooly-Y M., Glickman J. N., and Garrett W. S.. . 2013. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341:569–573. doi: 10.1126/science.1241165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. D., Panickar K. S., Urban J. F. Jr., and Dawson H. D.. . 2018. Impact of micronutrients on the immune response of animals. Annu. Rev. Anim. Biosci. 6:227–254. doi: 10.1146/annurev-animal-022516-022914 [DOI] [PubMed] [Google Scholar]
- Solano-Aguilar G. I., Lakshman S., Jang S., Beshah E., Xie Y., Sikaroodi M., Gupta R., Vinyard B., Molokin A., Urban J. F. Jr., et al. . 2018. The effect of feeding cocoa powder and Lactobacillus rhamnosus on the composition and function of pig intestinal microbiome. Curr. Dev. Nutr. 2:nzy011. doi: 10.1093/cdn/nzy011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terra X., Valls J., Vitrac X., Mérrillon J. M., Arola L., Ardèvol A., Bladé C., Fernandez-Larrea J., Pujadas G., Salvadó J., . et al. 2007. Grape-seed procyanidins act as antiinflammatory agents in endotoxin-stimulated RAW 264.7 macrophages by inhibiting NFkB signaling pathway. J. Agric. Food Chem. 55:4357–4365. doi: 10.1021/jf0633185 [DOI] [PubMed] [Google Scholar]
- VanderWaal K., and Deen J.. . 2018. Global trends in infectious diseases of swine. Proc. Natl. Acad. Sci. USA 115:11495–11500. doi: 10.1073/pnas.1806068115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhelst R., Schroyen M., Buys N., and Niewold T.. . 2014. Dietary polyphenols reduce diarrhea in enterotoxigenic Escherichia coli (ETEC) infected post-weaning piglets. Livest. Sci. 160:138–140. [Google Scholar]
- Virdi V., Palaci J., Laukens B., Ryckaert S., Cox E., Vanderbeke E., Depicker A., and Callewaert N.. . 2019. Yeast-secreted, dried and food-admixed monomeric IgA prevents gastrointestinal infection in a piglet model. Nat. Biotechnol. 37:527–530. doi: 10.1038/s41587-019-0070-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaminck J., Düsseldorf S., Heres L., and Geldhof P.. . 2015. Serological examination of fattening pigs reveals associations between Ascaris suum, lung pathogens and technical performance parameters. Vet. Parasitol. 210:151–158. doi: 10.1016/j.vetpar.2015.04.012 [DOI] [PubMed] [Google Scholar]
- Wellock I. J., Fortomaris P. D., Houdijk J. G., and Kyriazakis I.. . 2008. Effects of dietary protein supply, weaning age and experimental enterotoxigenic Escherichia coli infection on newly weaned pigs: Performance. Animal 2:825–833. doi: 10.1017/S1751731108001559 [DOI] [PubMed] [Google Scholar]
- Williams A. R., Hansen T. V. A., Krych L., Ahmad H. F. B., Nielsen D. S., Skovgaard K., Thamsborg S. M... 2017a. Dietary cinnamaldehyde enhances acquisition of specific antibodies following helminth infection in pigs. Vet. Immunol. Immunopathol. 189:43–52. [DOI] [PubMed] [Google Scholar]
- Williams A. R., Klaver E. J., Laan L. C., Ramsay A., Fryganas C., Difborg R., Kringel H., Reed J. D., Mueller-Harvey I., Skov S., . et al. 2017b. Co-operative suppression of inflammatory responses in human dendritic cells by plant proanthocyanidins and products from the parasitic nematode Trichuris suis. Immunology 150:312–328. doi: 10.1111/imm.12687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. R., Krych L., Fauzan Ahmad H., Nejsum P., Skovgaard K., Nielsen D. S., and Thamsborg S. M.. . 2017c. A polyphenol-enriched diet and Ascaris suum infection modulate mucosal immune responses and gut microbiota composition in pigs. PLoS One 12:e0186546. doi: 10.1371/journal.pone.0186546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wils-Plotz E. L., Jenkins M. C., and Dilger R. N.. . 2013. Modulation of the intestinal environment, innate immune response, and barrier function by dietary threonine and purified fiber during a coccidiosis challenge in broiler chicks. Poult. Sci. 92:735–745. doi: 10.3382/ps.2012-02755 [DOI] [PubMed] [Google Scholar]
- Wlodarska M., Willing B. P., Bravo D. M., and Finlay B. B.. . 2015. Phytonutrient diet supplementation promotes beneficial Clostridia species and intestinal mucus secretion resulting in protection against enteric infection. Sci. Rep. 5:9253. doi: 10.1038/srep09253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Ma N., Song P., He T., Levesque C., Bai Y., Zhang A., and Ma X.. . 2019. Grap seed proanthocyanidin affects lipid metabolism via changing gut microflora and enhancing propionate production in weaned pigs. J. Nutr. 149:1523–1532. doi: 10.1093/jn/nxz102 [DOI] [PubMed] [Google Scholar]
- Xiong X., Tan B., Song M., Ji P., Kim K., Yin Y., and Liu Y.. . 2019. Nutritional intervention for the intestinal development and health of weaned pigs. Front. Vet. Sci. 6:46. doi: 10.3389/fvets.2019.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G. D., Barekatain R., Wu S. B., Choct M., and Swick R. A.. . 2018. Dietary l-glutamine supplementation improves growth performance, gut morphology, and serum biochemical indices of broiler chickens during necrotic enteritis challenge. Poult. Sci. 97:1334–1341. doi: 10.3382/ps/pex444 [DOI] [PubMed] [Google Scholar]
- Xun W., Shi L., Zhou H., Hou G., Cao T., and Zhao C.. . 2015. Effects of curcumin on growth performance, jejunal mucosal membrane integrity, morphology and immune status in weaned piglets challenged with enterotoxigenic Escherichia coli. Int. Immunopharmacology 27:46–52. doi: 10.1016/j.intimp.2015.04.038 [DOI] [PubMed] [Google Scholar]
- Yan L., and Kim I. H.. . 2012. Effect of eugenol and cinnamaldehyde on the growth performance, nutrient digestibility, blood characteristics, fecal microbial shedding and fecal noxious gas content in growing pigs. Asian-Australas. J. Anim. Sci. 25:1178–1183. doi: 10.5713/ajas.2012.12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Y. S. 2018. Regulatory roles of flavonoids on inflammasome activation during inflammatory responses. Mol. Nutr. Food Res. 62:e1800147. doi: 10.1002/mnfr.201800147 [DOI] [PubMed] [Google Scholar]
- Yoshioka Y., Akiyama H., Nakano M., Shoji T., Kanda T., Ohtake Y., Takita T., Matsuda R., and Maitani T.. . 2008. Orally administered apple procyanidins protect against experimental inflammatory bowel disease in mice. Int. Immunopharmacol. 8:1802–1807. doi: 10.1016/j.intimp.2008.08.021 [DOI] [PubMed] [Google Scholar]
- Zeng Z., Xu X., Zhang Q., Li P., Zhao P., Li Q., Liu J., and Piao X.. . 2015. Effects of essential oil supplementation of a low-energy diet on performance, intestinal morphology and microflora, immune properties and antioxidant activities in weaned pigs. Anim. Sci. J. 86:279–285. doi: 10.1111/asj.12277 [DOI] [PubMed] [Google Scholar]
- Zhang J. F., Bai K. W., Su W. P., Wang A. A., Zhang L. L., Huang K. H., and Wang T.. . 2018. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult. Sci. 97:1209–1219. doi: 10.3382/ps/pex408 [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhou W., Zhang W., Yang A., Liu Y., Jiang Y., Huang S., and Su J.. . 2014. Inhibitory effects of citral, cinnamaldehyde, and tea polyphenols on mixed biofilm formation by foodborne Staphylococcus aureus and Salmonella enteritidis. J. Food Prot. 77:927–933. doi: 10.4315/0362-028X.JFP-13-497 [DOI] [PubMed] [Google Scholar]