Abstract
Background
The health of school-aged children (SAC) is often compromised by malaria parasitaemia (MP), soil-transmitted helminths (STH), and malnutrition in the tropics. The aim of this study was to determine the prevalence and influence of MP, STH and malnutrition on haemoglobin (Hb) levels as well as identify its predictors.
Methods
This cross-sectional study was carried out in SAC (4–14 years) in Owe, Mpundu and Meanja villages in Muyuka, Southwest Cameroon. Hb concentration was measured using a URIT-12 Hb meter while MP and STH were determined by Giemsa staining of blood films and Kato-Katz technique respectively. Anthropometric measures (weight, height and mid upper arm circumference (MUAC)) of malnutrition (z-scores of <−2 standard deviations below mean) were obtained by standard methods. Categorical and continuous variables were compared appropriately, and multiple linear regression model was used to determine predictors of Hb level.
Results
The prevalence of MP, STH, anaemia and malnutrition in the 401 SAC examined were 33.9%, 2.2%, 75.3% and 24.4% respectively. The prevalence of MP varied significantly with locality (P = 0.031). Stunting occurred commonly (23.7%) and was significantly higher in males (28.6%), children 11–14 years old (38.3%) and those of Meanja locality (47.4%) than their counterparts. Significantly higher prevalence of anaemia was observed in children of Meanja (89.5%) and those both MP positive and malnourished (86.2%). Moderate anaemia occurred commonly (60.6%) and children ≤6 years old had significantly (P = 0.034) higher prevalence (75.0%). Mean Hb level varied significantly (P = 0.004) with age and those ≤6 years old infected with MP had significantly (P = 0.022) lower values. Significant predictors of Hb levels were the MUAC (P <0.001) and the MP status (P = 0.035). Based on the Hb level (>11g/dL) and the absence of MP, STH and malnutrition, 13.7% of the SAC were considered as healthy.
Conclusions
The health of a majority of SAC is compromised by malaria, helminthiasis, malnutrition and other conditions not investigated. Anaemia is of major public health concern hence, intervention programmes that integrate malaria control with improvement of educational levels especially on proper nutrition and health care practices are desirable.
Background
Health, as defined by the World Health Organization (WHO), is a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity [1]. The relative level of wellness and illness of an individual which considers the presence of biological or physiological dysfunction, symptoms and functional impairment defines the health status of an individual. The health of school-age children (SAC) is usually compromised by common diseases such as malaria (which remains a considerable public health problem in much of the tropics and subtropics) and helminthiasis [2–4]. In Cameroon, malaria and soil-transmitted helminth (STH) infections are both widespread and are accountable for increased morbidities and associated consequences in vulnerable populations, including SAC [5–8].
Plasmodium falciparum is the most prevalent malaria parasite in the WHO African Region, accounting for 99.7% of estimated malaria cases in 2017 and Cameroon is amongst the 11 high—burden countries that account for more than 70% of the global malaria cases and deaths [2]. P. falciparum is also the most pathogenic species and remains a major cause of morbidity and mortality, with children less than five years of age and pregnant women severely affected [9]. In 2017, children aged under 5 years accounted for 61% (266 000) of all malaria deaths worldwide [2]. Consequently, control measures in endemic regions have focused on the protection of these two groups at highest risk of malaria disease. On the other hand, older children who are less often symptomatic, and may play an important role in transmission, have not traditionally been the focus of intensive detection and control strategies [10].
School age children rather than preschool children or adults, are most at risk of Plasmodium helminth co-infection and thereby at greatest risk of the consequences of co-infection [3]. Co-infections with helminth and malaria parasites have negative impact upon host and synergisms between multiple parasite species infections and infection intensity are known to exacerbate anaemia [3, 8, 11]. STH infections can accelerate or exacerbate malnutrition hence infections with STH and malaria parasite could singly or combined be contributing factors of malnutrition and/or anaemia as shown by several studies [12–15].
Malnutrition is said to be the underlying cause of deaths in 48% of children below 5 years in Cameroon [16] while the burden of malnutrition in SAC is infrequently determined especially in rural areas. Common nutritional indicators of subclinical undernutrition such as underweight, wasting and stunting in children are proxy indicators of overall well-being and reflect, the burden of infectious diseases in the community [17]. The nutritional status of SAC impacts their health, cognition, and subsequently their educational achievement [18]. Manifestation of malnutrition is often observed in terms of anaemia, micronutrient deficiencies (iron, folic acid, riboflavin, vitamin A and B12) and anthropometric measurements. Hence, the assessment of nutritional status of this population segment is essential for making progress towards improving the overall health of SAC [19].
Hb concentration is the most reliable indicator of anaemia at the population level, as opposed to clinical measures which are subjective and therefore have more room for error [20]. Anaemia is an indicator of both poor nutrition (micronutrient deficiency) and poor health (chronic infections, predominantly malaria, hereditary haemoglobinopathies). In most developing countries, anaemia is a public health problem and SAC are more vulnerable due to their rapid physical and physiological development [20, 21]. The prevalence of anaemia among SAC in Africa ranges from 64.3% to 71% [20] and in the Mount Cameroon area it ranges from 19.8% to 44.2% [22, 23]. Anaemia is considered as a public health problem (prevalence ≥ 5%) when the Hb value is below the population specific Hb threshold. The prevalence of anaemia is objective and quantifiable and can be measured in the most remote areas where access to health care is a challenge. In addition, anaemia is a major complication of several neglected tropical diseases (NTD). Moreover, it changes in predictable fashions with alterations in disease burden [24].
While studies may have been carried out on single infections with malaria parasite or co-infections with STHs in different populations, there is a dearth of knowledge on the health status of SAC in Cameroon especially in rural settings. Thus, the main objectives of this study were to determine the prevalence of MP, STH and malnutrition, assess their influence on Hb levels as well as identify the predictors of Hb levels. We hypothesised that the level of haemoglobin is a valid indicator in predicting the health status of SAC in rural areas endemic for malaria and NTDs such as soil-transmitted helminths.
Methods
Study sites
The study sites comprised of Owe, Mpundu and Meanja villages of Muyuka Sub-division located at the foot of Mount Cameroon. Owe is located at an altitude of 59m, latitude 4°17ʹ23ʺ N, longitude 9° 22ʹ50ʺ E to 84m a.s.l., latitude 4°18ʹ00ʺ N and longitude 9° 22ʹ32ʺ E. Mpundu is located at an altitude of 54m, latitude 4°14ʹ14ʺ N, longitude 9°24ʹ44ʺ E, to 68m a.s.l, latitude, 4°14ʹ42ʺ N and longitude 9°23ʹ40ʺ E. Meanja is located at 62m above sea level (a.s.l.), latitude 4° 14ʹ53ʺN, longitude 9°23ʹ48ʺ E to 69m a.s.l., latitude 4° 15ʹ53ʺN and longitude 9°24ʹ48ʺ E as shown in Fig 1. Owe, Mpundu and Meanja villages are 7km, 12km and 5.6 km away from Muyuka town respectively. The rainy season in the area, runs from March to October while the dry season runs from November to mid-March. Annual temperature ranges from 18–35°C. The natives of these villages are known as the Balongs [25]. Subsistence and small-scale cash crop farming constitute the mainstay of the villages. Other nearby landmarks include: River Mungo, a Cameroon Development Corporation (CDC) workers’ camp and CDC palm and rubber plantations. Owe has no closed potable water source but has streams which serve as a source of drinking water and other household activities while Meanja and Mpundu have closed potable water source. They lack health facilities such as clinics/hospitals and residents tend to visit pro-pharmacies more often when sick than hospitals for drugs.
Fig 1. Map showing the location of study areas in Muyuka, Southwest Cameroon.
Study design
The cross-sectional study was carried out simultaneously in three localities between the months of March and June 2015.
Study population
The study population constituted SAC (4–14 years old) of both sexes whose parents or legal representatives had signed a written consent/assent form. Primary schools were selected at random from a list of schools operating in these villages and children selected at random from each class.
Sampling size
The sample size was determined using the formula n = Z2pq/d2 [26], where n represented the sample size required; Z was 1.96, which is the standard normal deviate (for a 95% confidence interval, CI); p was 35.5%, the prevalence of P. falciparum [8] or 14.0%, the proportion of helminth infection [27]; q was 1-p, the proportion of malaria parasite negatives or helminth negative; and d was 0.05, the acceptable error willing to be committed. A minimum sample size of 268 was obtained from the average of the calculated sample size for the prevalence of P. falciparum (351) and that of helminth (185).
Sampling procedure
Five (05) schools (Government school (GS) Owe, Catholic school (CS) Owe, GS Mpundu, CS Mpundu and CS Meanja) selected by random sampling from a list of nine schools operating in these villages agreed to take part in the study. A representative number of children were selected at random by balloting from each class. First, the children, head teachers, and village chiefs were sensitized on the protocol and benefits of the exercise to the children and to the community. Informed consent forms were sent to parents/guardians through the pupils explaining the purpose and benefits of the study as well as the precautions taken to minimize risk. Children who returned with signed consent forms were enrolled in the study.
The samples collected comprised of finger prick blood for MP detection and speciation, and Hb measurement for assessment of anaemia as well as stool sample for the detection of egg or larva of STHs. The investigative methods included the use of questionnaires, clinical evaluation and laboratory methods such as Giemsa-stained microscopy and Kato-Katz technique.
Questionnaire
A simple pre-tested structured questionnaire (S1 File) was administered to each pupil with the assistance of schoolteachers and investigators. Information on important demographic data such as age, sex, area of residence was obtained as well as information relating to malaria and STH infection epidemiology. In brief, the questionnaire sought to obtain information on insecticide treated net (ITN) possession and use, house type, bushes around the residence, pre-fever history, history of anti-malarials, toilet availability and type, hand washing, shoe wearing, and farming frequency.
Clinical evaluation
The axial temperature of each child was measured using a mercury thermometer to determine the presence of fever. Children who had temperatures ≥ 37.5°C were reported to have fever.
The age, height, weight and mid upper arm circumference (MUAC) of participants were measured in order to determine anthropometric indices. Height and weight measurements were recorded to the nearest 0.1cm and 0.5kg respectively. Weight was measured using a mechanical weighing scale and heights and MUAC were measured using a measuring tape. Ages of children were obtained from school records with the permission of the head teacher. The z scores height-for-age, weight-for-age, weight-for-height were computed based on the WHO 2006 growth reference curves [28]. Stunting was defined as height-for-age z (HAZ) score of < -2; underweight as weight-for-age z (WAZ) score of < -2 and wasting as weight-for-height z (WHZ) score < -2. A child was considered malnourished if he or she scored < -2 in one of the z scores (HAZ, WAZ, WHZ). Z score of < -3 indicated severe stunting, underweight and wasting.
Collection of blood and stool samples
Finger prick blood was collected from each pupil with signed assent form. The first drop of blood was immediately wiped off and the next drop was placed on the Hb test strip already inserted into the URIT-12 haemoglobin meter (URIT Medical Electronic co., Ltd, London, United Kingdom) for Hb determination. The Hb value displayed was recorded to the nearest 0.1 g/dL. A participant was considered anaemic when Hb concentration fell below the WHO reference values for age or gender [29]. Anaemia was further classified as either mild (between 10.0 g/dL and the level in the WHO reference values for age), moderate (7.0–9.9 g/dL based on WHO reference for age) or severe (<7 g/dL and WHO reference for age) [29]. Two drops of whole blood were placed on a labelled, grease-free glass slide 1mm apart for the preparation of thin and thick films for the detection and speciation of malaria parasites, as described by Cheesbrough [30].
Each child was provided with a labelled screw-capped plastic container to return with a fresh midday stool sample. A single stool sample was collected from each participant. The fresh samples were preserved with 10% formalin to maintain the morphology of the egg. Samples were transported in a specimen box to the Malaria Research Laboratory of the University of Buea for diagnosis by the Kato-Katz technique.
Laboratory procedures
Both thin and thick blood films prepared in the field were air-dried. The thin blood film was fixed in 75% methanol, and both thick and thin blood films were stained using 10% Giemsa solution for 15 minutes [30]. Slides were then microscopically examined for the presence of malaria parasites by two independent parasitologists, and in the case of any disparity they were read by a third parasitologist. Slides were considered positive when asexual forms and/or gametocytes of any Plasmodium species were observed on the blood film. Parasite density per μL of blood was determined based on the number of parasites per 200 leukocytes on thick blood film assuming a white blood cell count (WBC) of 8000 leucocytes/uL of blood [30]. Asymptomatic malaria parasitaemia was defined as the presence of Plasmodium parasites with an axillary temperature of < 37.5°C, while clinical malaria parasitaemia was defined as the presence of any species of Plasmodium together with an axillary temperature of ≥ 37.5°C or reported fever in the previous 48 hours, or headache or joint pain. Parasitaemia was classified as low (≤ 500 parasite /μL of blood), moderate (501–5000 parasites/μL of blood) and high (>5000 parasites/μL of blood).
Stool smears were prepared and examined using the Kato-Katz thick smear method, as described by Cheesbrough [30]. Duplicate smears were prepared for each specimen. Each slide was allowed to clear for 30 min, and then examined at 100× total magnification within one hour of preparation to avoid missing hookworm eggs. Morphological identification of eggs of A. lumbricoides, T. trichiura and hookworm were based on identification aids [30]. All the eggs in the 41.7 mg of stool were counted and multiplied by 24 to compute the number of eggs per gram of faeces (epg). As a quality control measure, all positive slides and 20% of randomly selected negative smears were re-examined within 24 hours by a third experienced parasitologist who had no knowledge of the previous results. An average of the counts was utilised.
Statistical analysis
All data collected was entered into Microsoft Excel and cleaned for entry errors (MS Excel 2016). Data was further analysed with the IBM-statistical package for the social sciences (IBM-SPSS) version 19 (IBM—SPSS, Inc, Chicago, IL, USA). Data was summarized into means and standard deviations (SD), and proportions used in the evaluation of descriptive statistics. Prevalence of malaria, STH infections, anaemia and malnutrition were determined and compared using the Chi-square (χ2) test. The mean (SD) Hb levels were compared using non-parametric tests [(Mann Whitney U and Kruskal Wallis tests) and parametric tests (t-test and analysis of variance (ANOVA)] where appropriate. Malaria parasite counts were log transformed before analysis. The Pearson correlation coefficient (r) was used to evaluate the linear correlation between age, sex, MUAC, nutritional status, MP status, level of education, type of school, locality and Hb level. The attributable risk (AR%) of anaemia caused by malaria and malnutrition was calculated accordingly [31]: [(𝑛1𝑚0 − 𝑛0𝑚1)/𝑛(𝑛0+𝑚0)] ×100, where 𝑛0 = anaemic children without malaria and 𝑛1 = anaemic children with malaria, whereby 𝑛0 + 𝑛1 = 𝑛, 𝑚0 = non anaemic children without malaria, and 𝑚1 = non anaemic children with malaria, whereby 𝑚0 + 𝑚1 = 𝑚. The multiple linear regression (MLR) model (enter) with Hb as the dependent variable was run to examine the influence of the following independent variables; age, sex, MUAC, nutritional status, MP status, level of education, type of school and locality. All 401 participants were included in the model. Significant levels were measured at 95% confidence interval (CI) with significant differences set at P < 0.05.
Ethics statement
Administrative clearances were obtained from the Regional Delegation of Basic Education as well as from the Catholic Education Board. The institutional review board hosted by the Faculty of Health Sciences, University of Buea issued the ethical clearance document after reviewing the study protocol, participant’s information sheet and assent forms. Authorization to proceed with the study in these villages within the selected school was obtained from village chiefs and school head teachers. Children participated in the study if a parent or guardian signed the informed consent form. The parents or guardian and their children were informed that their participation in the study was voluntary and they could withdraw at any time without any explanation.
Results
Socio-demographic characteristics of the study participants
A total of 401 pupils with a mean (SD) age of 8.94 (2.9) years (range = 4–14) of both sexes (212 females and 189 males) were examined. Majority of the participants were pupils from Owe locality (47.6%), Catholic schools (58.1%) and between the age range of 7–10 years (58.4%). A greater proportion of the participants used bed nets (75.1%) and lived in plank houses (85%) as shown in Table 1. The proportion of children whose house floors were cemented (93.0%) was higher compared to their counterparts with earthen house floors (0.7%).
Table 1. Characteristics of the study participants.
| Characteristic | Number examined | % | |
|---|---|---|---|
| Sex | Male | 189 | 47.1 |
| Female | 212 | 52.9 | |
| Age group (years) | ≤ 6 | 60 | 15.0 |
| 7–10 | 234 | 58.3 | |
| 11–14 | 107 | 26.7 | |
| Locality | Owe | 191 | 47.6 |
| Meanja | 38 | 9.5 | |
| Mpundu | 172 | 42.9 | |
| Schools | Government school | 168 | 41.9 |
| Catholic school | 233 | 58.1 | |
| ITN use | Yes | 301 | 75.1 |
| No | 100 | 24.9 | |
| House type | Plank | 341 | 85.0 |
| Block | 60 | 15.0 | |
Clinical profile of participants
The mean (SD) HAZ, WAZ, and WHZ scores were -1.17 (1.2), -0.34 (1.2) and 2.91 (4.0) respectively. The prevalence of fever, MP, STH infections, anaemia and malnutrition were 15% (95% CI = 11.7–18.7%), 33.9% (95% CI = 29.5–38.7%), 2.2% (95% CI = 1.8–2.7%), 75.3% (95% CI = 70.9–79.3%) and 24.4% (95% CI = 20.5–28.9%) respectively (Table 2).
Table 2. Clinical characteristics of the 401 SAC.
| Characteristics | Number | % |
|---|---|---|
| Prevalence | ||
| Prevalence of fever (≥ 37.5°C) | 60 | 15.0 |
| Overall prevalence of MP | 136 | 33.9 |
| Overall prevalence of STH | 9 | 2.2 |
| Prevalence of anaemia | 302 | 75.3 |
| Prevalence of malnutrition | 98 | 24.4 |
| Means | Mean (SD) | Range |
| Mean age in years | 8.9 (0.1) | 4–14 |
| Mean weight in kg | 124.6 (0.6) | 98–157 |
| Mean height in cm | 27.29 (0.3) | 15–53 |
| Mean MUAC in cm | 18.6 (2.08) | 13.6–33.0 |
| Mean HAZ | -1.20 (1.2) | -5.50–2.40 |
| Mean WAZ | - 0.35 (1.2) | -2.76–5.51 |
| Mean WHZ | 0.93 (1.6) | -2.79–8.28 |
| Mean temperature in°C | 36.85 (0.1) | 33–39.5 |
| Mean haemoglobin in g/dL | 10.6 (1.4) | 4.4–14.3 |
| Mean MP density | 1326.2 (3939) | 40–34000 |
HAZ = Height-for-age z score; MP = Malaria parasite; MUAC = Mid upper arm circumference; STH = Soil-transmitted helminths; WAZ = Weight-for-age z score; WHZ = Weight-for-height z score.
All MP detected were Plasmodium falciparum. Out of the 9 children with STH infection, 5 (55.6%) were infected with Trichuris and 4 (44.4%) with Ascaris, no mixed infections, hookworm, and Strongyloides were observed. Out of the 60 (15%, 95% CI = 11.7–18.7%) pupils with fever, 22 (36.7%, 95% CI = 25.2–49.4%) of them were positive for MP. ITN usage was highest in children ≤ 6 years old (72.1%), followed by 7–10 years (64.8%) and least in 11–14 years old (53.3%).
MP, STH and malnutrition prevalence
Out of the 136 children with malaria parasitaemia 114 (83.8%, 95% CI = 76.7–89.1%) of the infections were asymptomatic. MP prevalence varied significantly (P = 0.031) among the localities only. The children of Meanja had the highest prevalence of MP (50.0%) when compared with their Owe and Mpundu equivalent (Table 3).
Table 3. Prevalence of MP, STH and malnutrition by sex, age, locality and type of school.
| Parameter | Category | N | MP % (n) | STH % (n) | Stunting % (n) | Underweight % (n) | Wasting % (n) | Malnutrition % (n) |
|---|---|---|---|---|---|---|---|---|
| Sex | Male | 189 | 34.9 (66) | 2.6 (5) | 28.6 (54) | 4.8 (9) | 0.0 (0) | 29.6 (56) |
| Female | 212 | 33.0 (70) | 1.9 (4) | 19.3 (41) | 4.2 (9) | 0.7 (1) | 19.8 (42) | |
| P- value | 0.688 | 0.619 | 0.03 | 0.803 | 0.304 | 0.022 | ||
| Age group in years | ≤ 6 | 60 | 36.7 (22) | 1.6 (1) | 6.7 (4) | 1.7 (1) | 1.7 (1) | 10.0 (6) |
| 7–10 | 234 | 29.5 (69) | 1.3 (3) | 21.4 (234) | 3.4 (8) | 0.0 (0) | 21.8 (51) | |
| 11–14 | 107 | 42.1 (45) | 4.7 (5) | 38.3 (41) | 8.4 (9) | 0.0 (0) | 38.3 (41) | |
| P–value | 0.067 | 0.139 | < 0.001 | 0.061 | 0.117 | < 0.001 | ||
| Locality of School | Owe | 191 | 28.8 (55) | 2.1 (4) | 25.1 (48) | 6.8 (13) | 0.7 (1) | 26.7 (51) |
| Meanja | 38 | 50.0 (19) | 5.1 (2) | 47.4 (18) | 2.6 (1) | 0.0 (0) | 47.4 (18) | |
| Mpundu | 172 | 36.0 (62) | 1.8 (3) | 16.9 (29) | 2.3 (4) | 0.0 (0) | 16.9 (29) | |
| P–value | 0.031 | 0.431 | <0.001 | 0.101 | 0.560 | <0.001 | ||
| School Type | Government | 168 | 30.4 (51) | 3.0 (5) | 21.4 (36) | 6.0 (10) | 0 0 (0) | 22.0 (37) |
| Catholic | 233 | 36.5 (85) | 1.7 (4) | 25.3 (59) | 3.4 (8) | 0.6 (1) | 26.2 (61) | |
| P- value | 0.201 | 0.500 | 0.366 | 0.229 | 0.367 | 0.339 |
P- value obtained using χ2. P -values in bold are statistically significant.
STH was prevalent in 2.2% (95% CI = 1.8–2.7%) of the children with no significant differences observed with respect to gender (P = 0.619), age (P = 0.139), locality (P = 0.431), and the type of school (P = 0.500) as shown in Table 3.
Of the 98 children with malnutrition, stunting the most common form occurred in 95 (96.9%), while underweight and wasting occurred in 18 (18.4%) and 1 (1.0%) of them, respectively. Overall, the prevalence of stunting was 23.7% (95% CI = 19.8–28.1%), underweight was 4.5% (95% CI = 2.9–7.0%) while wasting was 0.3% (95% CI = (0.06–1.8%). The prevalence of malnutrition, more specifically stunting was significantly higher in males (28.6%), children of the 11–14 years age group (38.3%) and those of Meanja locality (47.4%) than their respective counterparts as shown in Table 3.
Anaemia prevalence and its severity
Overall, anaemia occurred in 75.3% (95% CI = 90.9–79.3%) of the pupils with similar prevalence in males (74.6%) and females (75.9%). Although not statistically significant, the prevalence of anaemia was higher in the ≤ 6 years age group (81.7%), children from GS (78.6%) and those positive for MP (80.9%) than their contemporaries. However, significantly higher (P = 0.035) prevalence of anaemia was observed in children of the Meanja locality (89.5%) when compared with those of Owe (70.7%) and Mpundu (77.3%) as revealed in Table 4.
Table 4. Prevalence and severity of anaemia with respect to sex, age, locality, type of school and malaria parasite.
| Characteristic | N | Anaemia prevalence % (n) | χ2 P value | Mild anaemia prevalence % (n) | Moderate anaemia prevalence % (n) | Severe anaemia prevalence % (n) | χ2 P value | |
|---|---|---|---|---|---|---|---|---|
| Sex | Male | 189 | 74.6 (141) | 13.7 (29) | 61.3 (130) | 0.9 (2) | ||
| Female | 212 | 75.9 (161) | 0.756 | 12.2 (23) | 59.8 (113) | 2.6 (5) | 0.584 | |
| Age group in years | ≤ 6 | 60 | 81.7 (49) | 6.7 (4) | 75.0 (45) | 0.0 (0) | ||
| 7–10 | 234 | 74.8 (175) | 0.433 | 11.1 (26) | 61.5 (144) | 2.1 (5) | 0.034 | |
| 11–14 | 107 | 72.9 (78) | 20.6 (22) | 50.5 (54) | 1.9 (2) | |||
| Locality | Owe | 191 | 70.7 (135) | 12.6 (24) | 55.5 (106) | 2.6 (5) | ||
| Meanja | 38 | 89.5 (34) | 0.035 | 13.2 (5) | 76.3 (29) | 0.0 (0) | 0.132 | |
| Mpundu | 172 | 77.3 (133) | 13.4 (23) | 62.8 (108) | 1.2 (2) | |||
| School Type | Catholic | 233 | 73.0 (170) | 0.199 | 14.2 (33) | 57.5 (134) | 1.3 (3) | 0.334 |
| Government | 168 | 78.6 (131) | 11.3 (19) | 64.9 (109) | 2.4 (4) | |||
| MP status | Positive | 136 | 80.9 (110) | 0.064 | 13.2 (18) | 66.2 (90) | 1.5 (2) | 0.290 |
| Negative | 265 | 72.5 (192) | 12.8 (34) | 57.7 (153) | 1.9 (5) | |||
| MP density category | Low | 85 | 81.6 (71) | 17.2 (15) | 63.2 (55) | 1.1 (1) | ||
| Moderate | 40 | 80.0 (40) | 0.948 | 5.0 (2) | 72.5 (29) | 2.5 (1) | 0.669 | |
| High | 9 | 77.8 (9) | 11.1 (1) | 66.7 (6) | 0.0 (0) | |||
P—values in bold are statistically significant.
Mild anaemia = Hb between 10.0 g/dL and the level in the WHO reference values for age.
Moderate anaemia = Hb between 7.0–9.9 g/dL based on WHO reference for age.
Severe anaemia = Hb < 7 g/dL and WHO reference for age.
Low MP density = ≤ 500 parasite /μL of blood.
Moderate malaria parasite density = 501–5000 parasites/μL of blood.
High malaria parasite density = >5000 parasites/μL of blood.
Moderate anaemia (Hb = 7.0–9.9 g/dL) occurred commonly (60.6%, 95% CI = 55.7–65.3%) in the participants than mild (13.0%, 95% CI = 10.3–16.6%) and severe anaemia (1.8%, 95% CI = 0.9–3.6%). While no significant differences in anaemia severity were observed by sex, locality, type of school, MP status and density, the prevalence of moderate anaemia was highest in children of the ≤ 6 years age group (75.0%) and the difference was statistically significant at P = 0.034 (Table 4).
Infection status, anaemia prevalence and haemoglobin levels
Of the 401 participants, 48.6% (195) had no MP, STH or malnutrition. Correspondingly, 25.7% (103) had MP only, 0.7% (3) had STH only,16.2% (65) were malnourished only, 7.2% (29) had both MP and were malnourished, 0.5% (2) were infected with STH and malnourished and 1.0% (4) were co-infected with MP and STH. The prevalence of anaemia and mean (SD) Hb levels in g/dL as influenced by infection category and nutritional status is shown in Table 5. Children who were MP positive and malnourished as well, had the highest prevalence of anaemia (86.2%) followed by those infected with MP only (79.6%) and malnutrition only (79.6%). However, the AR of anaemia caused by MP + malnutrition, MP only and malnutrition only were 0.96%, 3.8%, and 1.1% respectively.
Table 5. Anaemia prevalence and mean (SD) Hb (g/dL) levels by sex and age as influenced by infection category and nutritional status.
| Parameter | n | Prevalence of anaemia % (n) | Overall mean (SD) Hb (g/dL) level | Mean (SD) Hb (g/dL) level by sex | P valuea | Mean (SD) Hb (g/dL) level by age group in years | P- Valueb | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | ≤ 6 | 7–10 | 11–14 | ||||||
| STH Only | 3 | 66.7 (2) | 10.3 (1.8) | 10.0 (2.4) | 11.0 (-) | - | 8.3 | 11.4 (0.5) | - | - |
| MP Only | 103 | 79.6 (82) | 10.5 (1.2) | 10.4 (1.2) | 10.5 (1.1) | 0.706 | 10.0 (0.9)c | 10.4 (1.2) | 10.9 (1.3)c | 0.022 |
| Malnutrition only | 65 | 73.8 (48) | 10.5 (1.6) | 10.2 (1.7) | 10.8 (1.4) | 0.296 | 10.3 (0.7) | 10.4 (1.4) | 10.6 (20) | 0.753 |
| MP + Malnutrition | 29 | 86.2 (25) | 10.4 (1.2) | 10.5 (1.3) | 10.2 (1.1) | 0.326 | 9.0 (0.3) | 10.3 (1.1) | 10.6 (1.2) | 0.143 |
| Negative | 195 | 71.8 (140) | 10.8 (1.3) | 10.9 (1.3) | 10.7 (1.4) | 0.338 | 10.4 (1.3) | 10.8 (1.3) | 11.2 (1.4) | 0.058 |
| Total | 401 | 75.3 (302) | 10.6 (1.4) | 10.6 (1.3) | 10.6 (1.4) | 0.889 | 10.2 (1.2)d | 10.6 (1.3) | 10.9 (1.5)d | 0.004 |
Abbreviations: STH Soil transmitted helminth, MP Malaria parasite
MP Only = Infection with malaria parasite only.
Malnutrition only = Participants with malnutrition only (Absence of MP and STH).
MP + Malnutrition = Participants with malaria parasite and malnutrition.
Negative = Negative for MP and STH as well as absence of malnutrition.
P–valuea obtained by t- test.
P–valueb obtained by ANOVA.
Figures in bold are statistically significant.
c Mean (SD) Hb (g/dL) significantly different (Post Hoc Turkey HSD test: P = 0.017).
d Mean (SD) Hb (g/dL) significantly different (Post Hoc Turkey HSD test: P = 0.003).
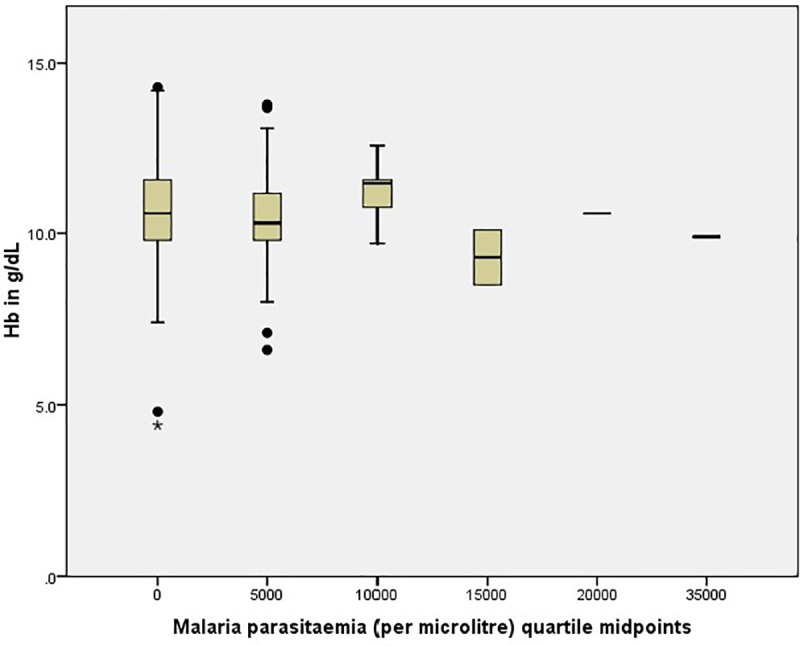
Overall, the mean (SD) Hb varied significantly (P = 0.004) with age with the highest level occurring in children 11–14 years old [10.9 (1.5) g/dL] and the lowest in the ≤ 6 years old [10.2 (1.2) g/dL]. As shown in Table 5, the mean (SD) Hb (g/dL) levels in all conditions were lower than the normal Hb levels for age and sex. Statistically significant (P = 0.022) only, was the difference in mean Hb levels with age in children infected with MP where, children ≤ 6 years old had the lowest mean Hb [10.0(0.9) g/dL] when compared with the 7–10 years [10.4 (1.2) g/dL] and the 11–14 years [10.9 (1.2) g/dL] age groups. The mean Hb level was lowest in those with parasitaemia of 15000 parasites/μL when compared with the aparasitaemic and the other quartiles of malaria parasite densities as shown in Fig 2.
Fig 2. Haemoglobin level by malaria parasitaemia in the study population.
Predictors of haemoglobin levels
The tolerance statistics of the multiple linear regression (MLR) model were all below 1 and all the variance inflation factors (VIF) were less than 2. Bivariate correlations with Hb level revealed a significant positive relationship with age (r = 0.150, P = 0.001), MUAC (r = 0.263, P < 0.001) and level of education (r = 0.145, P = 0.002) while a significant negative relationship was observed with MP status (r = -0.085, P = 0.045), type of school (r = -0.099, P = 0.024) and locality (r = -0.089, P = 0.038). In the MLR model, the only factors identified as significant predictors of Hb levels were the MUAC (P < 0.001) and the MP status (P = 0.035) as shown in Table 6.
Table 6. MLR model showing influence of independent variables on haemoglobin level.
| Independent variable | B | Standard error | 95% CI | P- value |
|---|---|---|---|---|
| Age | -0.007 | 0.041 | -0.088–0.075 | 0.873 |
| Sex | 0.032 | 0.134 | -0.231–0.295 | 0.813 |
| MUAC | 0.157 | 0.042 | -0.074–0.240 | <0.001*** |
| Nutritional status | -0.113 | 0.166 | -0.440–0.214 | 0.498 |
| MP status | -0.294 | 0.139 | -0.567 - -0.021 | 0.035* |
| Level of education | 0.148 | 0.086 | -0.021–0.316 | 0.085 |
| Type of School | -0.204 | 0.135 | -0.468–0.061 | 0.131 |
| Locality | -0.066 | 0.070 | -0.206–0.072 | 0.347 |
Abbreviations: MUAC Mid upper arm circumference, MP Malaria parasite.
Dependent variable: Hb (g/dl)
* P is significant at the 0.05 level.
*** P is significant at the 0.001 level. Model summary: R = 0.311, R2 = 0. 097, Adjusted R2 = 0.078, F = 5.251, P < 0.01, N = 401.
Health status in the population
Based on the Hb level (Hb > 11g/dL) and the absence of fever, MP, STH and malnutrition, 13.7% (95% CI = 10.7–17.4%) of the SAC were considered as healthy in the study population. The proportion of healthy children was comparable among the age groups (≤ 6 years = 13.3%, 7–10 years = 15% and 11–14 years = 11.2%; P = 0.645) and sex (female = 13.7%, male = 13.8%; P = 0.982). Among the 195 negative for MP, STH, and malnutrition, 55 (28.2%, 95% CI = 22.4–34.9%) were classified as healthy. Even though more males (32.1%) and children of the 11–14 years age group (33.3%) were healthier than females (25.4%) and those ≤ 6 years (24.2%) and 7–10 years (27.8%) old the differences were not statistically significant (P = 0.308, P = 0.693), respectively.
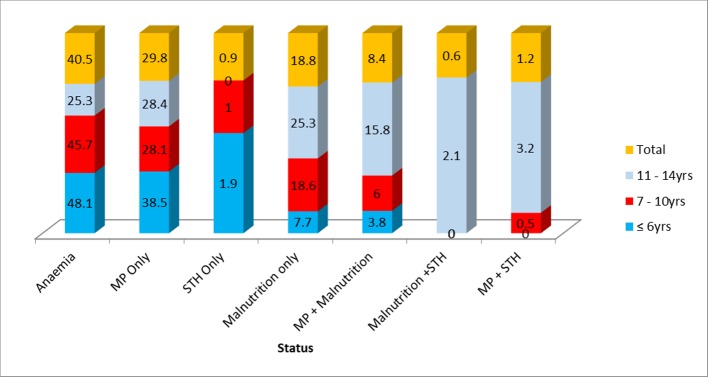
Among the 346 (86.3%) unhealthy children, anaemia occurred most frequently (40.5%), followed by MP (29.8%) and malnutrition (18.8%) as shown in Fig 3. The occurrence of the various conditions varied significantly (χ2 = 34.88, P < 0.001) with the age group. Children in the ≤ 6 years group had the highest prevalence of anaemia (48.1%) and MP (38.5%) while those of the 11–14 years age group had the highest prevalence of malnutrition (25.3%), MP + malnutrition (15.8%), malnutrition + STH (2.1%) and MP + STH (3.2%) as revealed in Fig 3.
Fig 3. Prevalence (%) of the different conditions in unhealthy participants as influenced by age.
Discussion
Understanding the burden of malaria, malnutrition and helminth infection in SAC is essential to finding delivery mechanisms to help implement control measures in this at-risk population. This study assessed the health status of SAC with respect to malaria, malnutrition and STH infections using the level of Hb as an indicator.
The prevalence of STH infections was very low (2.2%) when compared with that (33.7%) of a similar study carried out in the same region two years earlier [32]. However, it is comparable to the 1% obtained in SAC in Tiko Health District [33] and 2.5% in selected rural, semi-urban and urban communities [22] all in the Mount Cameroon area. While a high burden of infection is not very common in regions targeted for elimination, the low prevalence could be as a result of the mass chemotherapy with mebendazole in school children initiated in Cameroon through the Ministry of Public Health since 2004 [5]. Furthermore, a combination of auto-medication, history of chemotherapy, changes in environmental and behavioural factors by individuals may have led to the sustained low prevalence of STH infection in the area.
The low occurrence of STH infection in SAC in the area limits the expression of its influence on the Hb level. However, out of the 3 children who were infected with STH only, 2 (66.7%) were anaemic. Moreover, those with STH infection had lower mean Hb levels when compared with their negative counterparts. Although this observation is limiting, it corroborates the negative effect of STH infection on the Hb values reported in abundant STH (Ascaris lumbricoides, Trichuris trichiura) infections among school children of Kashmir Valley India [34].
The overall MP prevalence (33.9%) is comparable to the 33.0% obtained in school children in Bomaka and Molyko in the Mount Cameroon Area [35] while, it is lower when compared with the 44.26% obtained by Kimbi et al. [36] in SAC children in Muea in the Mount Cameroon area and the 50.7% by Makoge et al. [37] in primary school pupils in Mbonge Sub-Division, Cameroon. Plasmodium falciparum infection prevalence as high as 60% in school children has been reported in Malawi [38] and a comparable prevalence of 30% in similar transmission settings in Uganda [39]. It is worth noting that majority (83.8%) of the MP infections were asymptomatic as most SAC do not have any symptoms because they have acquired some immunity. While acknowledging the decline in malaria prevalence due to sustained malaria control interventions implemented by the Cameroon Government through the National Malaria Control Programme [40] this burden highlights the need to embark upon malaria control in SAC in Cameroon.
Although all three localities are rural, children of Meanja had the highest significant prevalence of MP. More specifically the prevalence was highest in SAC of the 11–14 years age group when compared with the other age groups. As expected, the heterogeneity in prevalence of malaria parasite is demonstrated in the area of study. Even though the distribution of malaria cases with age is influenced by the transmission intensity [41], it is likely that other ecological factors and behaviours favoured the occurrence and age-related distribution of the infection in the area. Inhabitants of Meanja live near the CDC rubber and palm plantations which together with a lot of bushes around, creates an ecosystem appropriate for Anopheles mosquito development in the locality. In addition, older children in rural areas are more involved in outdoor activities such as farming and household activities like fetching water from far water sources early in the morning exposing them to the mosquito vector.
The high prevalence of anaemia (75.3%) in SAC in this rural area with a significant majority (60.6%) being moderate anaemia is an indication that anaemia is a major public health problem. This prevalence was higher than those obtained by other authors in the region and elsewhere [22, 37, 38, 42]. This probably reflects the poor state of health of children in the area. The aetiology of anaemia in SAC is multifactorial and the relative importance of each cause varies from place to place. Albeit it is difficult to differentiate the influence of malaria parasite on anaemia in SAC, the attributable risk of anaemia due to malaria parasite (3.8%), malnutrition only (1.1%) and MP and malnutrition (0.96%) is very low. However, it is very evident from the findings that SAC with these conditions especially those with MP and malnutrition had the highest prevalence of anaemia and lowest Hb levels when compared with their negative counterparts suggesting their contributions to the burden of anaemia in the area. The highest occurrence of anaemia in SAC of Meanja locality (89.5%) that also had the highest prevalence of malaria parasite lends more support to this assertion.
The observed prevalence of malnutrition (24.4%) in SAC in this area shows it is a public health issue. The very low mean HA (-1.17) and WA (-0.33) z scores culminating in the presence of stunting (23.7%) and underweight (4.5%) observed in the children highlights the degree of growth failure in height and weight in SAC in this rural area. The prevalence of stunting is similar to that observed in primary school children in Nairobi-Kenya [43] but higher than the 11.3% observed in SAC in North-Eastern Ethiopia [44]. Male children like their counterparts in Kenya and Ethiopia [44, 45] had a significant tendency of being stunted than females while a study in Southern Ethiopia [46] showed no major differences in prevalence of stunting in males and females. The growth and development of male children is influenced by environmental and nutritional stress more than the female [47]. Hence, it is likely that the mean energy intake for boys in this rural area did not meet the energy requirements as boys are more hyperactive in this age range than females who spend more time in food preparation and may thus have increasingly access to excess food.
In line with the other studies stunting was found to be significantly higher in the older age group [44, 46]. Stunting which is a marker of chronic malnutrition is more likely to be apparent with increase in age. Hence, its increased presence in older children which in addition are in a transition to the adolescent stage in life that has its own nutritional needs [48] which may not be adequate in this rural environment.
Findings from the study revealed the mean Hb level of SAC in these rural settings are comparatively lower than the WHO level for age and sex even though an ideal Hb level is not yet established. While the mean Hb level in all conditions were lower, that of children in the 6 years age group and below with malaria parasite infection was statistically significant. Furthermore, the model identified MP status as a significant predictor of Hb level with a negative relationship. Several other studies have associated Plasmodium infection to lower Hb level [42, 49, 50]. This highlights the insidious effect asymptomatic malaria parasitaemia may have on the Hb level of younger children who have not developed anti disease immunity.
On the other hand, the MUAC was identified as a significant predictor of Hb level with a positive association as shown in the MLR model. While further investigation is necessary to assert this association, the MUAC which measures only acute malnutrition could be an alternative of great value especially in resource poor settings were Hb measurement is unaffordable. Blood Hb measurement, a common indicator for diagnosing anaemia, requires trained personnel, expensive equipment or well-developed laboratory facilities which is often unavailable in rural areas. Measuring the MUAC is much cheaper and easier than measuring weight and height. In addition, it is less affected by acute dehydration than weight-based indices [51].
Furthermore, keeping an update information on the level of education of parent or caregiver is of utmost value as findings in a bivariate analysis demonstrated a significant positive association of parent/caregiver level of education with the Hb level. While acknowledging the limitations of self-reported levels of education and its potential of introducing bias, this finding is not surprising as low educational level can lead to low income and socio-economic status and thus inability to provide for proper feeding and affordable health care. Basic causes of anaemia reported in other studies include, maternal level of education and household wealth rank [52].
The magnitude of unhealthy SAC (86.3%) in the rural setting surpasses the occurrence of healthy children and that is a cause for concern. Although the presence of these conditions contributed to the unhealthy status of SAC, the fact that anaemia occurred in 71.8% of the negatives demonstrates their health is compromised by other ailments that were not investigated in the study. One of the limitations of this study is that the number of infections investigated are fewer albeit those evaluated are reported to be of common occurrence in the Mount Cameroon area [8, 37]. More research involving a wider range of morbidities (micro-nutrient deficiency, bacterial and viral infections) other than the conditions investigated that may be responsible for the unhealthy status of the children needs to be carried out. However, the lowered haemoglobin level observed in measurable and unmeasurable ailments lends support to the fact that haemoglobin level could be used as an indicator of the health status of children.
Conclusions
It is evident from this study that the health of majority of SAC in these rural settings in the Mount Cameroon area is compromised by malaria, helminthiasis, malnutrition and other conditions not investigated. Anaemia is of major public health concern and the Hb level could serve as a prognostic marker of the health status of SAC. Children with MP and malnutrition had the highest prevalence of anaemia and lowest Hb levels indicating their contributions to the burden of anaemia even though the attributable risk to it was insignificant. Malaria parasite is a significant negative predictor of Hb level hence, there is a need for intervention programmes targeting SAC in rural areas that integrates proper malaria control measures with improvement of educational level of parent/caregiver especially on proper nutrition and health care practices that will ensure health and well-being of the children.
Supporting information
(PDF)
Acknowledgments
The authors are thankful to the village chiefs of Owe, Mpundu and Meanja, head teachers of the various primary schools, parents/guardians as well as the children who participated in the study. We acknowledge the support of IIE-SRF (Institute of International Education- Scholar Rescue Fund) and MPH programme, College of Veterinary Medicine, Cornell University, Ithaca, New York in providing the fellowship and right academic environment respectively for the drafting of this manuscript.
Data Availability
All relevant data are within the manuscript.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.World Health Organization. Constitution of the World Health Organization–Basic documents, Forty-fifth edition, Supplement, October 2006. https://www.who.int/governance/eb/who_constitution_en.pdf.
- 2.WHO. World malaria report 2018. Geneva. World Health Organization 2018. https://www.who.int/malaria/publications/world-malaria-report-2018/en/.pdf.
- 3.Brooker S, Akhwale W, Pullan R, Estambale B, Clarke SE, Snow RW. et al. Epidemiology of Plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anemia, and prospects for combining control. Am J Trop Med Hyg. 2007; 77: 88–98. [PMC free article] [PubMed] [Google Scholar]
- 4.Hurlimann E, Houngbedji CA, Yapi RB, Ndri PB, Silue KD, Soro G, et al. Health-related quality of life among school children with parasitic infections: findings from a national cross-sectional survey in Cote d’Ivoire PLoS Negl Trop Dis. 2014; 8: e3287 10.1371/journal.pntd.0003287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tchuem-Tchuente LA, N’Goran EK. Schistosomiasis and soil-transmitted helminthiasis control in Cameroon and Côte d’Ivoire: Implementing control on a limited budget. Parasitol. 2009; 136: 1–7. [DOI] [PubMed] [Google Scholar]
- 6.Nkuo-Akenji TK, Chi PC, Cho JF, Ndamukong KK, Sumbele I. Malaria and helminth co-infection in children living in a malaria endemic setting of mount Cameroon and predictors of anemia. J Parasitol. 2006; 92: 1191–1195. 10.1645/GE-895R.1 [DOI] [PubMed] [Google Scholar]
- 7.Kimbi HK, Lum E, Wanji S, Mbuh JV, Nyanga JLN, Eyong ECJ, et al. Coinfections of asymptomatic malaria and soil-transmitted helminths in school children in localities with different levels of urbanization in the Mount Cameroon Region. J Bacteriol Parositol. 2012; 3: 134. [Google Scholar]
- 8.Sumbele IUN, Nkemnji GB and Kimbi HK. Soil-transmitted helminths and Plasmodium falciparum malaria among individuals living in different agroecosystems in two rural communities in the Mount Cameroon area: a cross-sectional study. Infect Dis Poverty. 2017; 6: 67 10.1186/s40249-017-0266-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. World malaria report 2012. Geneva. World Health Organization 2012. https://www.who.int/malaria/publications/world_malaria_report_2012.
- 10.Walldorf JA, Cohee LM, Coalson JE, Bauleni A, Nkanaunena K, Tembo AK. et al. School-age children are a reservoir of malaria infection in Malawi. PLoS One. 2015; 10: e0134061 10.1371/journal.pone.0134061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh K. Pathogenesis of anaemia in malaria: a concise review. Parasitol Resource. 2007; 101: 1463–1469. [DOI] [PubMed] [Google Scholar]
- 12.Nkuo-Akenji TN, Sumbele I, Mankah EN, Njunda AL, Samje M, Kamga L. The burden of malaria and malnutrition among children less than 14 years of age in a rural village of Cameroon. AJFAND. 2008; 8: 252–264. [Google Scholar]
- 13.Tchinda VHM, Ponka R, Ndzi SE, Madocgne AK, Amédée A, Grâce TM, et al. Prevalence of malaria and soil-transmitted helminth infections and their association with under-nutrition in schoolchildren residing in Mfou health district in Cameroon. JPHE. 2012; 4: 253–260. [Google Scholar]
- 14.Mbuh JV, Nembu NE. Malnutrition and intestinal helminth infections in school children from Dibanda, Cameroon. J Helminthol. 2013; 87: 46–51. 10.1017/S0022149X12000016 [DOI] [PubMed] [Google Scholar]
- 15.Sumbele IUN, Bopda OSM, Kimbi HK, Ning TR, Nkuo-Akenji T. Nutritional status in a malaria meso endemic area: cross sectional study on the prevalence, intensity, predictors, influence on malaria parasitaemia and anaemia severity. BMC Public Health. 2015; 15: 1099 10.1186/s12889-015-2462-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MINSANTE. Midterm health expenditure framework 2011–2013. www.minsante.cm 2010. www.minsante.gov.cm.
- 17.Schelp FP. Nutrition and infection in Tropical countries-implications of public intervention- a personal perspective. Nutrition. 1998; 14: 217–222. 10.1016/s0899-9007(97)00436-x [DOI] [PubMed] [Google Scholar]
- 18.Best C, Neufingerl N, van Geel L, van den Briel T, Osendarp S. The nutritional status of school-aged children: why should we care? Food Nutr Bull. 2010; 31: 400–417. 10.1177/156482651003100303 [DOI] [PubMed] [Google Scholar]
- 19.Fazili A, Mir A, Pandit IM, Bhat IA, Rohul J, Shamila H. Nutritional status of school age children (5‑14 years) in a Rural Health Block of North India (Kashmir) Using WHO Z‑Score System. Online J Health Allied Scs. 2012; 11: 2. [Google Scholar]
- 20.Benoist BD, McLean E, Egll I, Cogswell M. Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia. Geneva: WHO global database on anaemia; 2008. [Google Scholar]
- 21.Pasricha SR, Drakesmith H, Black J, Hipgrave D, Biggs BA. Control of iron deficiency anaemia in low- and middle- income countries. Blood J. 2013; 121: 2607–2617. [DOI] [PubMed] [Google Scholar]
- 22.Ndamukong-Nyanga JL, Kimbi HK, Sumbele IUN, Nana Y, Bertek S, Ndamukong KJN. A Cross-sectional study on the influence of altitude and urbanisation on co-infection of malaria and soil-transmitted helminths in Fako Division, South West Cameroon. IJTDH. 2015; 8: 150–164. [Google Scholar]
- 23.Sumbele IUN, Kimbi HK, Ndamukong-Nyanga JL, Nweboh M, Anchang-Kimbi JK, Lum E. et al. Malarial anaemia and anaemia severity in apparently healthy primary school children in urban and rural settings in the mount Cameroon Area: Cross sectional survey. PLoS One. 2015;10: e0123549 10.1371/journal.pone.0123549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bates I, Mckew S. Sarkinfada F. Anaemia. A useful indicator of neglected disease burden and control. PLoS Medicine. 2007; 4: e231 10.1371/journal.pmed.0040231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United Councils and Cities of Cameroon (UCCC), 2014, http://cvuc.cm/national/index.php/fr/carte-communale/regiondu-sud/142.
- 26.Bryan FJ. The design and analysis of research studies. UK: University of Otago, Cambridge University Press; 1992. [Google Scholar]
- 27.Ebai CB, Kimbi HK, Sumbele IUN, Yunga JB and Lehman LG. Epidemiology of Plasmodium falciparum malaria in the Ikata-Likoko Area of Mount Cameroon: A cross sectional study. IJTDH. 2016; 16: 1–12. [Google Scholar]
- 28.WHO. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. Geneva: World Health Organization; 2006. [Google Scholar]
- 29.WHO. Iron deficiency anaemia: assessment, prevention and control, a guide for programme managers. Geneva, Switzerland: World Health Organization Publication; 2001. http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/index.html. [Google Scholar]
- 30.Cheesbrough M. District laboratory practice in tropical countries. Part1& 2. New York: Cambridge University Press; 2009. [Google Scholar]
- 31.Benichou J. A review of adjusted estimators of attributable risk,” Stat Meth Med Res, 2001; 10: 195–216. [DOI] [PubMed] [Google Scholar]
- 32.Ntonifor HN, Green AE, Bopda MOS, Taboth JT. Epidemiology of urinary schistosomiasis and soil-transmitted helminthiasis in a recently established focus behind Mount Cameroon. Int J Curr Microbiol Appl Sci. 2015; 4: 1056–1066. [Google Scholar]
- 33.Tabi ESB, Eyong EM, Akum EA, Löve J, Cumber SN. Soil-transmitted helminth infection in the Tiko Health District, South West Region of Cameroon: a post-intervention survey on prevalence and intensity of infection among primary school children. PAMJ. 2018; 30: 74 10.11604/pamj.2018.30.74.15676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wani SA, Ahmad F, Zargar SA, Dar ZA, Dar PA, Tak H. et al. Soil-transmitted helminths in relation to haemoglobin status among school children of the Kashmir Valley. J Parasitol. 2008; 94: 591–593. 10.1645/GE-1400.1 [DOI] [PubMed] [Google Scholar]
- 35.Kimbi HK, Nana Y, Sumbele IN, Anchang-Kimbi JK, Lum E, Tonga C, et al. Environmental factors and preventive methods against malaria parasite prevalence in rural Bomaka and urban Molyko, Southwest Cameroon. J Bacteriol Parasitol. 2013; 4: 1. [Google Scholar]
- 36.Kimbi HK, Keka FC, Nyabeyeu HN, Ajeagah HU, Tonga CF, Lum E, et al. An update of asymptomatic falciparum malaria in school children in Muea, Southwest Cameroon. J Bacteriol Parasitol. 2012; 3: 8. [Google Scholar]
- 37.Makoge VD, Mbah GA, Nkengazong L, Sahfe NE, Moyou RS. Falciparum malaria, helminth infection, and anaemia in asymptomatic pupils in four villages in Cameroon. Eur J Zoological Research. 2012; 1: 54–59. [Google Scholar]
- 38.Mathanga DP, Halliday KE, Jawati M, Verney A, Bauleni A, Sande J, et al. The high burden of malaria in primary school children in Southern Malawi. Am J Trop Med Hyg. 2015; 93: 779–789. 10.4269/ajtmh.14-0618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nankabirwa J, Wandera B, Kiwanuka N, Staedke SG, Kamya MR, Brooker SJ. Asymptomatic Plasmodium infection and cognition among primary school children in a high malaria transmission setting in Uganda. Am J Trop Med Hyg. 2013; 88: 1102–1108. 10.4269/ajtmh.12-0633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sumbele IUN, Ning TR, Bopda OSM, Nkuo-Akenji T. Variation in malariometric and red cell indices in children in the Mount Cameroon area following enhanced malaria control measures: evidence from a repeated cross-sectional study. Malar J. 2014; 13: 334 10.1186/1475-2875-13-334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nankabirwa J, Brooker SJ, Clarke SE, Fernando D, Gitonga CW, Schellenberg D et al. Malaria in school-age children in Africa: an increasingly important challenge. Trop Med Int Health. 2014; 19: 1294–1309. 10.1111/tmi.12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sumbele IUN, Samje M, Nkuo-Akenji T. A longitudinal study on anaemia in children with Plasmodium falciparum infection in the Mount Cameroon region: prevalence, risk factors and perceptions by caregivers. BMC Infect Dis. 2013; 13: 123 10.1186/1471-2334-13-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mwaniki EW, Makokha AN. Nutrition status and associated factors among children in public primary schools in Dagoretti, Nairobi, Kenya. Afri Health Sc. 2013; 13: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menber Y, Tsegaye D, Woday A, Cherie H, Kebede S. Prevalence of stunting and associated factors among school age children in primary schools of Haik Town, South Wollo Zone, North-Eastern Ethiopia, 2017. J Clin Cell Immunol. 2018; 9:1. [Google Scholar]
- 45.Tariku EZ, Abebe GA, Melketsedik ZA, Gutema BT. Prevalence and factors associated with stunting and thinness among school-age children in Arba Minch Health and Demographic Surveillance Site, Southern Ethiopia. PLoS One. 2018; 13: e0206659 10.1371/journal.pone.0206659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bogale TY, Bala ET, Tadesse M, Asamoah BO. Prevalence and associated factors for stunting among 6–12 years old school age children from rural community of Humbo district, Southern Ethiopia. BMC Public Health. 2018; 18: 653 10.1186/s12889-018-5561-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stinson S. Sex differences in environmental sensitivity during growth and development. Am J Physical Anthropol. 1985; 28 (S6):123–147. [Google Scholar]
- 48.Akseer N, Al-Gashm S, Mehta S, Mokdad A, Bhutta ZA. Global and regional trends in the nutritional status of young people: a critical and neglected age group. Ann NY Acad Sci. 2017;1393: 3–20. 10.1111/nyas.13336 [DOI] [PubMed] [Google Scholar]
- 49.Nzobo BJ, Ngasala BE, Kihamia CM. Prevalence of asymptomatic malaria infection and use of different malaria control measures among primary school children in Morogoro Municipality, Tanzania. Malar J. 2015; 14: 491 10.1186/s12936-015-1009-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akiyama T, Pongvongsa T, Phrommala S, Taniguchi T, Inamine Y, Takeuchi R, et al. Asymptomatic malaria, growth status, and anaemia among children in Lao People’s Democratic Republic: a cross‑sectional study. Malar J. 2016; 15: 499 10.1186/s12936-016-1548-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mwangome MK, Fegan G, Prentice AM, Berkley JA. Are diagnostic criteria for acute malnutrition affected by hydration status in hospitalized children? A repeated measures study. Nutr J. 2011; 10: 92 10.1186/1475-2891-10-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siekmans K, Receveur O, Haddad S. Can an integrated approach reduce child vulnerability to anaemia? Evidence from three African Countries. PLoS One. 2014;9(2): e90108. [DOI] [PMC free article] [PubMed] [Google Scholar]