Abstract
Milk-secreting epithelial cells of the mammary gland are functionally specialized for the synthesis and secretion of large quantities of neutral lipids, a major macronutrient in milk from most mammals. Milk lipid synthesis and secretion are hormonally regulated and secretion occurs by a unique apocrine mechanism. Neutral lipids are synthesized and packaged into perilipin-2 (PLIN2) coated cytoplasmic lipid droplets within specialized cisternal domains of rough endoplasmic reticulum (ER). Continued lipid synthesis by ER membrane enzymes and lipid droplet fusion contribute to the large size of these cytoplasmic lipid droplets (5–15 μm in diameter). Lipid droplets are directionally trafficked within the epithelial cell to the apical plasma membrane. Upon contact, a molecular docking complex assembles to tether the droplet to the plasma membrane and facilitate its membrane envelopment. This docking complex consists of the transmembrane protein, butyrophilin, the cytoplasmic housekeeping protein, xanthine dehydrogenase/oxidoreductase, the lipid droplet coat proteins, PLIN2, and cell death-inducing DFFA-like effector A. Interactions of mitochondria, Golgi, and secretory vesicles with docked lipid droplets have also been reported and may supply membrane phospholipids, energy, or scaffold cytoskeleton for apocrine secretion of the lipid droplet. Final secretion of lipid droplets into the milk occurs in response to oxytocin-stimulated contraction of myoepithelial cells that surround milk-secreting epithelial cells. The mechanistic details of lipid droplet release are unknown at this time. The final secreted milk fat globule consists of a triglyceride core coated with a phospholipid monolayer and various coat proteins, fully encased in a membrane bilayer.
Keywords: cell biology, contact, electron microscopy, endoplasmic reticulum, lipid droplet, membrane
Introduction
Lipids are key macronutrients of milk and a major source of calories and essential bioactive molecules required for neonatal growth and development in many mammalian species (Oftedal, 1984). Physiological conditions that reduce the quantity or alter the composition of lipids secreted into milk, such as maternal obesity, are linked to impaired lactation outcomes and altered neonatal metabolic function in humans and animal models (Wahlig et al., 2012; Rudolph et al., 2017). In mice, genetic deletion of key proteins regulating the synthesis or secretion of milk lipids have been shown to produce lactation deficiency or failure (Vorbach et al., 2002; Cases et al., 2004; Ogg et al., 2004; Beigneux et al., 2006; Russell et al., 2011; Monks et al., 2016).
Milk lipids are composed primarily of neutral lipids (98%–99%) in the form of triglycerides, diglycerides, and cholesteryl esters (Jensen et al., 1990; Jensen, 1999), which are packaged into cytoplasmic lipid droplets within specialized milk-secreting mammary epithelial cells. Unlike serum lipids, which are secreted as soluble lipoprotein particles by vesicle-mediated exocytosis, milk lipids are secreted by a unique apocrine mechanism in which cytoplasmic lipid droplets are secreted intact as membrane-coated structures, referred to as milk fat globules (McManaman, 2012). Evidence is emerging that milk lipid biogenesis and secretion involve specific membrane-and inter-organelle contacts, with novel molecular and structural features.
Lipid Droplet Synthesis in the Mammary Epithelial Cell Begins During Pregnancy
Milk-secreting mammary epithelial cells undergo functional differentiation and the capacity to synthesize milk substances during mid-pregnancy in most species. One of the earliest morphological features of this differentiation process, termed lactogenesis I, is the accumulation of cytoplasmic lipid droplets (Russell et al., 2007). These early droplets are synthesized primarily from fatty acids liberated from circulating lipoproteins by lipoprotein lipase located in the vascular bed of the mammary gland or by adipose-derived circulating fatty acids bound to albumin. However, after parturition and the onset of milk secretion, de novo synthesis of medium chain fatty acids by the milk-secreting epithelial cells from glucose occurs and synthesis of triglycerides increases precipitously, accompanying other changes in the gland such as closure of the tight junctions, milk protein and lactose synthesis and secretion, which define lactogenesis II (Rudolph et al., 2010; Lv et al., 2015).
The triglycerides that comprise the neutral lipid core of these cytoplasmic lipid droplets are synthesized by resident endoplasmic reticulum (ER) membrane enzymes, which catalyze sequential steps in the fatty acid esterification of glycerol-3-phosphate (Kennedy, 1957) or of sn2-monoacylglycerol (Coleman and Lee, 2004). Evidence from mouse knockout models has demonstrated the importance of triglyceride synthesis in the development and lipid secretion functions of mammary epithelial cells. Loss of glycerol-3-phosphate acyltransferase-4 (GPAT4), which catalyzes acylation of glycerol-3-phosphate, the first step in triglyceride synthesis, depletes milk-secreting mammary epithelial cells of cytoplasmic lipid droplets. Its loss also impairs mammary epithelium development and inhibits milk lipid secretion (Beigneux et al., 2006; Takeuchi and Reue, 2009). Similarly, loss of acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1), one of two DGAT enzymes that catalyze the final acylation step in TAG synthesis (Yen et al., 2008), in milk-secreting mammary epithelial cells impairs their functional differentiation and CLD accumulation, and inhibits milk secretion in mice (Cases et al., 2004). Interestingly, the observation that mammary gland expression of DGAT2, a major contributor to TAG synthesis from de novo synthesized fatty acids in many mammalian tissues (Yen et al., 2008), does not compensate for the effects of DGAT1 loss on mammary gland functional differentiation and CLD accumulation indicates that the two DGAT enzymes have different physiological functions in milk-secreting mammary epithelial cells (Cases et al., 2004).
Lipid Droplets Interact With ER
Cytoplasmic lipid droplets originate from the ER in eukaryotic cells, and connections between these structures are necessary for initial lipid droplet expansion (Robenek et al., 2006; Wilfling et al., 2013). It is generally thought that cytoplasmic lipid droplets form from specific tubular microdomains of the smooth ER, based on structural considerations and evidence that disrupting the function of proteins responsible for smooth ER structure interferes with lipid droplet formation/expansion (Kassan et al., 2013; Walther et al., 2017). However, unlike other highly lipogenic cells, such as hepatocytes and adrenal cortical cells (Baumann and Walz, 2001; Shibata et al., 2006), milk-secreting epithelial cells are highly enriched in rough ER (Jarasch et al., 1977; Wooding, 1977), which possess the enzymes required for neutral lipid synthesis (Bauman and Davis, 1974) and can form extensive connections with lipid droplets (Stemberger et al., 1984; Figure 1(a)).
Figure 1.
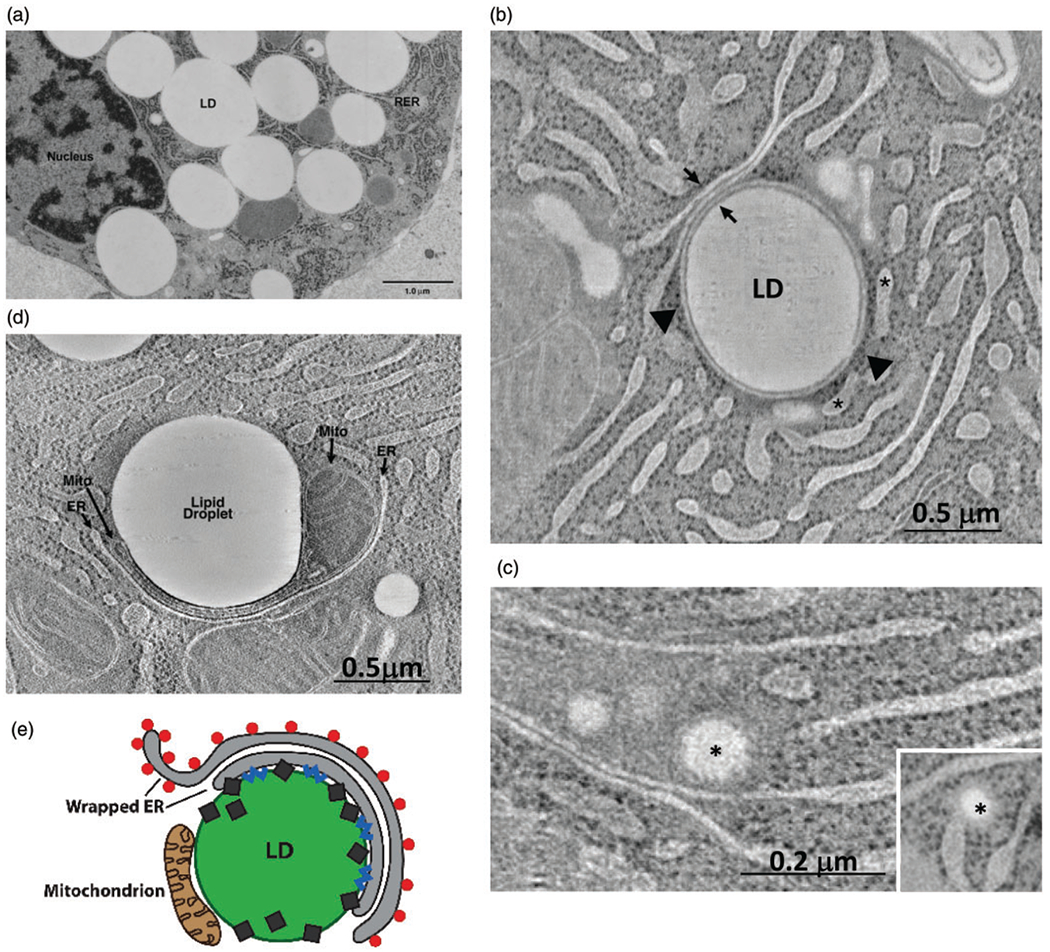
Cytoplasmic lipid droplet–organelle interactions. Representative images of cytoplasmic lipid droplet–organelle interactions in secretory mammary epithelial cells from lactating and pregnant rats, and cytoplasmic lipid droplets are labeled here as LD or lipid droplets. (a) Electron micrograph of a milk-secreting epithelial cell in a lactating animal showing cytoplasmic lipid droplets in contact with rough ER membranes. (b) Section of an electron tomogram of milk-secreting epithelial cell from a lactating animal showing a cytoplasmic lipid droplet completely wrapped by an ER cisterna that possesses ribosomes on its cytoplasmic facing membrane (arrowhead). This cisterna forms flattened contacts with two other cisternae (arrows) that also have ribosomes on membrane leaflets facing the cytoplasm. Rough ER cisternae that are nearby but do not contact the lipid droplet are indicated by asterisks. (c and inset) Tomographic slice from a milk-secreting epithelial cell of a day 10 pregnant animal showing lipid accumulation (asterisks) within the lumen of rough ER cisterna. (d) Tomographic slice from a lactating animal showing lipid droplet–mitochondria interactions. A mitochondrion (mito) is shown with a portion of its structure compressed between a lipid droplet and rough ER. Other mitochondria with normal structures are found in close proximity to the lipid droplet. (e) Model of lipid droplet–organelle interactions mediating milk-secreting epithelial cell lipid droplet expansion in lactating animals showing a cytoplasmic lipid droplet wrapped by concentric stacks of ER cisterna. Contact between the lipid droplet surface and inner cisternal membranes is mediated by PLIN2 (diamonds) and is characterized by the presence of neutral lipid synthesis enzymes (blue squiggles). The outer cisternal layer contains ribosomes (red circles) required for generating proteins needed for lipid droplet expansion. Lipid droplet-associated peridroplet mitochondria provide ATP required for lipid droplet expansion. LD = lipid droplet; ER = endoplasmic reticulum.
Evidence that lipid droplets originate from rough ER in milk-secreting epithelial cells, and that they are secreted into milk by a distinct mechanism, was obtained as early as 1967 by Stein and Stein. Using radioautography and electron microscopy (EM), these investigators showed that within 1 to 3 minutes after injecting radioactive palmitic or oleic acid into tail veins of lactating mice, labeled esters were first localized over rough ER cisternae in milk-secreting epithelial cells before being incorporated into rough ER-localized lipid droplets and then secreted into milk. Label was not observed in the Golgi or any secretory granules, indicating that unlike lipoproteins, milk lipids are not packaged into vesicles and secreted by an exocytotic mechanism. Subsequent proteomic analyses of membrane-free preparations of lipid droplets from lactating mice by Wu et al. (2000) identified ER lumenal and membrane proteins, which provided the first biochemical evidence of an ER origin of these structures.
Variable degrees of contact, ranging from discrete contacts at single or multiple sites to more extensive contacts that follow the contour of the lipid droplet surface, exist between lipid droplet and ER membranes (Salo and Ikonen, 2019). Using electron tomography and high-pressure freezing and freeze substitution approaches that preserve cellular structures in near native states (McIntosh, 2001), ER cisternae in milk-secreting epithelial cells from lactating rats have been shown to form unique, concentric, multilayered contacts with lipid droplets, which can cover large areas of the droplet surface (Ladinsky et al., 2019; Figure 1(b)). As originally proposed by Robenek et al. (2006) in the egg cup model of cytoplasmic lipid droplet expansion, this organization is ideally configured for transferring neutral lipids and proteins from their sites of synthesis on ER membranes to support production and growth of lipid droplets, which may be particularly important during lactation when demand for lipid formation is increased. Ribosomes on these cisternae are located on outer membrane leaflets facing the cytoplasm or on cisternal membranes distal to sites of contact. However, ribosomes are absent from cisternal membranes that contact the lipid droplet surface or other cisternae. Despite their lack of direct contact, sufficient numbers of ribosomes remain associated with the lipid droplet upon secretion that many groups now use this milk fraction to noninvasively sample the milk-secreting cell transcriptome (Maningat et al., 2007; Brenaut et al., 2012; Lemay et al., 2013). In addition, the abundance of ribosomal proteins in the proteome of milk fat globules is so great that they are routinely ignored in pathway analyses (Honvo-Houeto et al., 2016).
Cytoplasmic lipid droplets are hypothesized to originate by the accumulation of neutral lipids between leaflets of the ER membrane by a lensing mechanism (Walther et al., 2017). Some of the earliest evidence of this lensing concept was obtained from electron micrographs of milk-secreting epithelial cells in lactating mammary glands (Long and Patton, 1978; Keenan and Dylewski, 1985; Zaczek and Keenan, 1990), which showed lipids within distended ER membranes that were contiguous with ribosome-studded rough ER. More recently, Ladinsky et al. (2019) showed via EM tomography in milk-secreting epithelial cells from pregnant rats the presence of nascent lipid droplets within cisternal domains of rough ER, which are continuous with, and have the same density as, the ER lumen (Figure 1(c)). An ER luminal origin of lipid droplets is consistent with data from yeast indicating that neutral lipids accumulate in the ER lumen prior to be incorporated into droplets (Choudhary et al., 2011; Choudhary et al., 2015; Mishra et al., 2016). It is also supported by proteomic data that show selective enrichment of ER luminal proteins on cytoplasmic lipid droplets isolated from milk-secreting epithelial cells relative to those obtained from hepatocytes (Wu et al., 2000). Whether neutral lipid accumulation in the ER lumen ultimately leads to lipid droplet formation and how this transition occurs is not known. However, dynamic remodeling of ER-lipid droplet interacting domains involving fission and fusion of ER membranes (Walther et al., 2017) may provide a possible mechanism. Intriguingly, defects in lipid droplet formation in milk-secreting epithelial cells have been linked to abnormalities in ER membrane morphology associated with decreased levels of atlastin-2 (Le Guillou et al., 2019), a member of a family of GTPases previously shown to regulate ER fusion and lipid droplet size in Caenorhabditis elegans (Klemm et al., 2013).
Lipid Droplets in Mammary Epithelial Cells Are Coated With PLIN2
Lipid droplet–ER interactions are mediated by specific protein interactions (Walther et al., 2017). Lipid droplets in milk-secreting epithelial cells are coated by perilipin-2 (PLIN2, also known as adipophilin/ADPH and adipose differentiation-related protein/ADRP) (Wu et al., 2000), which is a prominent, constitutively associated lipid droplet coat protein whose actions have been shown to regulate droplet size and to promote neutral lipid accumulation in multiple cell types including milk-secreting epithelial cells during functional differentiation of the mammary gland (Listenberger et al., 2007; Russell et al., 2007, 2011; Orlicky et al., 2019). PLIN2 is detected at sites of contact between lipid droplets and ER membranes (Fujimoto and Parton, 2011; Ladinsky et al., 2019), and its depletion has been shown to increase their interaction (Ozeki et al., 2005). At ER and lipid droplet contact sites, PLIN2 is hypothesized to stabilize the forming lipid droplet at the ER membrane, drawing it into the cytoplasm, instead of into the lumen of the ER (Robenek et al., 2006). PLIN2 is also thought to stabilize lipid droplets by inhibiting lipolysis (Listenberger et al., 2007). Several lipases are known to be associated with the secreted milk fat globule, including bile salt-stimulated lipase (CEL), lipoprotein lipase, and patatin-like phospholipase domain containing 2 (PNPLA2, a.k.a. ATGL; Monks et al., 2016). Silencing PNPLA2/ATGL in milk-secreting epithelial cells increases cytoplasmic lipid droplet accumulation and cellular triglyceride levels (Li et al., 2015). In mice, PLIN2 loss is associated with increased PNPLA2/ATGL binding to lipid droplets in milk-secreting epithelial cells, an effect that is reversed by adenoviral expression of GFP-tagged PLIN2 (Russell et al., 2011). Although experiments with PLIN2-deficient mice show a major role for PLIN2 in stabilization of lipid droplets, details about mechanisms remain uncertain and compensation by other members of the perilipin family, such as Plin3, is likely (Sztalryd et al., 2006; Russell et al., 2011; Monks, unpublished). Experiments exploring the role of PLIN2 in lipid droplet growth, stabilization, transport, and secretion in the mammary gland are ongoing.
Lipid Droplets Associate With Other Organelles in Mammary Epithelial Cells
Lipid droplets in milk-secreting epithelial cells also interact with other organelles and cellular structures, including Golgi and mitochondria (Stemberger et al., 1984; Ladinsky et al., 2019). Large percentages of lipid droplets in the apical portion of milk-secreting epithelial cells are in contact with Golgi (21%), secretory vesicles (74%), and mitochondria (34%) (Stemberger et al., 1984). Benador et al. (2018) demonstrated that mitochondria bound to lipid droplets, termed peridroplet mitochondria (PDM) have functional and morphological properties that are distinct from cytoplasmic mitochondria. For example, PDM exhibit increased area contact with the lipid droplet surface and have metabolic properties that increase adenosine triphosphate (ATP) generation and pyruvate oxidation and promote triglyceride synthesis and lipid droplet expansion (Benador et al., 2018). Extensive contacts between mitochondria and lipid droplets have also been observed by electron tomography in milk-secreting epithelial cells from lactating rats (Ladinsky et al., 2019). Mitochondria that contact lipid droplets can be distant from sites of lipid droplet–ER contact (Ladinsky et al., 2019) or sandwiched between the lipid droplet surface and elements of rough ER (Figure 1(d)). Thus, lipid droplet expansion may be facilitated by distinct dynamic interactions with ER and mitochondrial domains that are specialized for integrating ATP production and neutral lipid synthesis.
Lipid Droplets Grow by CIDEA-Mediated Fusion
Although continued interaction of lipid droplets with ER allows direct synthesis to contribute to lipid droplet growth, fusion of lipid droplets in milk-secreting epithelial cells has been directly observed via intravital imaging, both within the cytoplasm and sites where lipid droplets are docked at the apical plasma membrane (Masedunskas et al., 2017; Mather et al., 2019). Cell death-inducing DFFA-like effector A (CIDEA) is a lipid droplet coat protein that has been identified as a key regulator of droplet fusion and lipid transfer in brown and white adipose (Christianson et al., 2010; Wu et al., 2014; Barneda et al., 2015). CIDEA is expressed in milk-secreting epithelial cells (Wang et al., 2012; Monks et al., 2016) and is seen to aggregate at points of contact between lipid droplets in these cells (Monks et al., 2016), where it is proposed to form channels that mediate neutral lipid flow from smaller to larger droplets (Gong et al., 2011; Jambunathan et al., 2011). This concept has been beautifully demonstrated in milk-secreting epithelial cells by intravital imaging (Mather et al., 2019), which showed numerous unidirectional fusion events in which small lipid droplets are subsumed into larger ones. The CIDEA appears to remain associated with the fused droplet as large droplets accumulate a CIDEA coat that is not seen on smaller, basally localized lipid droplets (Monks, unpublished). In the absence of CIDEA, lipid droplets in milk-secreting epithelial cells fail to grow larger than 5 μm, suggesting an upper limit for growth mediated solely by lipid synthesis in the absence of this fusion mechanism (Wang et al., 2012; Mather et al., 2019).
Lipid Droplets Traffic in Apical Direction During Milk Synthesis/Secretion
Directionally, specific lipid droplet movement has been observed to occur in multiple cell types (Welte, 2009), and several lines of evidence indicate that this movement is mediated by interactions with microtubules (Spandl et al., 2009; Welte, 2009; Orlicky et al., 2013). In milk-secreting epithelial cells, the secretion of lipid droplets as milk fat globules requires their transport from their site of synthesis on ER in the basolateral portion of the cell to the apical surface for release. Analysis of fixed specimens by EM showed that the majority of microtubules in milk-secreting epithelial cells of lactating rats are oriented perpendicular to the apical plasma membrane with minus ends directed toward the apically located centriole (Dylewski and Keenan, 1984), suggesting the possible involvement of microtubules for lipid droplet trafficking. Directed movement of lipid droplets in lactating mouse mammary gland was measured via intravital imaging and was shown to be slower than that reported for droplets moving on microtubules in cultured cells (Masedunskas et al., 2017). This motility difference may suggest that lipid droplets in milk-secreting epithelial cells hitchhike with other organelles on microtubules (Guimaraes et al., 2015). However, the microtubule motor proteins kinesin and dynein were both found in the proteomics data sets of mouse milk fat globules and lipid droplets isolated from milk-secreting epithelial cells of lactating mice (Wu et al., 2000; Monks et al., 2016). The association of secretory vesicles with lipid droplets has been observed by many labs (Figure 2(b)) and may be the source of these motor proteins. Definitive demonstration of lipid droplet trafficking on specific cytoskeletal elements and motor proteins is, unfortunately, still lacking.
Figure 2.
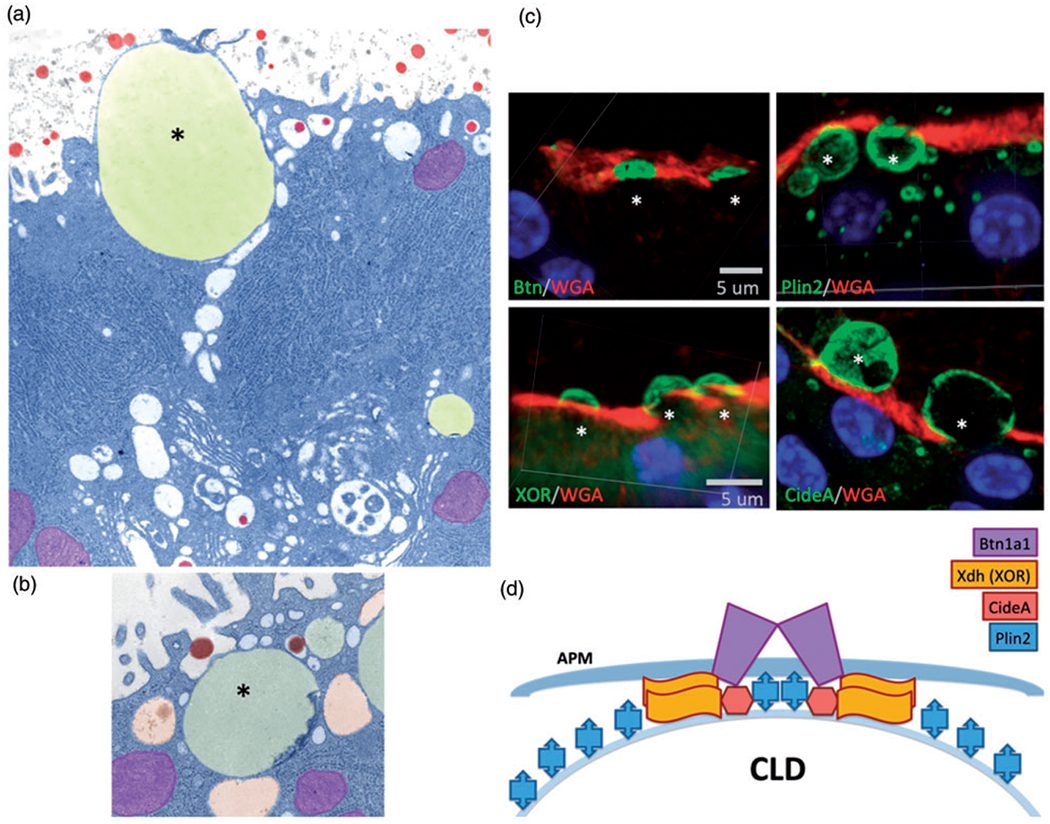
Cytoplasmic lipid droplet interaction with the apical plasma membrane. (a) Colorized electron micrograph showing a lipid droplet (yellow, asterisk) docked at the apical plasma membrane. Mitochondria are colored purple and casein micelles are colored red. (b) Colorized electron micrograph showing a lipid droplet (asterisk) surrounded by secretory vesicles (orange), and some containing casein micelles (red). (c) Immunofluorescence labeling of proteins in the docking complex that tethers the cytoplasmic lipid droplet to the membrane. BTN, PLIN2, XOR, and CIDEA are shown in green. The membrane is stained with WGA (red), and the nuclei are stained with DAPI (blue). (d) Diagram of the docking complex: BTN is a transmembrane protein (purple), and XDH/XOR is a cytoplasmic protein which binds directly to BTN. CIDEA is a lipid droplet-associated protein that concentrates in the dock, and PLIN2 is a lipid droplet coat protein which becomes covalently cross-linked to BTN and XOR. Stoichiometry was determined by proteomic analysis. WGA = wheat germ agglutinin; CLD = cytoplasmic lipid droplet; XOR = xanthine oxidoreductase; APM = apical plasma membrane.
Lipid Droplets Dock at the Apical Plasma Membrane
As the cytoplasmic lipid droplets come into contact with the apical plasma membrane, docking and progressive membrane envelopment occurs prior to release as milk fat globules by an apocrine mechanism of milk secretion (Figure 2(a); Mather and Keenan, 1998; Heid and Keenan, 2005). Ultrastructural analyses revealed that docked lipid droplets are connected to the cytoplasmic face of the apical membrane by a 10- to 20-nm wide hexagonally ordered electron dense layer (Mather and Keenan, 1998), and biochemical studies indicate that this material is composed of the cytoplasmic tail of the transmembrane protein butyrophilin (BTN), the cytosolic protein xanthine dehydrogenase/oxidoreductase (XDH/XOR), and the lipid droplet coat protein PLIN2, linked covalently by disulfide bonding (McManaman et al., 2002; Heid and Keenan, 2005). Several protein disulfide isomerases (P4hb, Pdia3, Pdia6, and Pdia4) are found associated with secreted milk fat globules (Monks et al., 2016). Although many details remain to be elucidated, direct binding of XDH/XOR to the B30.2 domain located in the cytoplasmic tail of BTN may form the basic structure (Jeong et al., 2009) and PLIN2, which contains distinct lipid droplet and phospholipid-binding domains (McManaman et al., 2003; Chong et al., 2011b), may act as a bridge between the lipid droplet and the membrane (Chong et al., 2011a). Our laboratory has also shown that CIDEA concentrates in the dock (Figure 2(c)), but it is unknown whether it interacts with BTN, XOR, or PLIN2, or perhaps self-associates, bridging between the apical plasma membrane and lipid droplet like PLIN2. Super-resolution microscopy (15 nm resolution) or cryoEM of the lattice would help with the elucidation of these structures.
Surprisingly, docking at the apical membrane is not absolutely required for milk lipid secretion. In mice in which XDH/XOR was specifically deleted from milk-secreting epithelial cells, lipid droplets fail to dock at the apical membrane, but they still undergo apocrine secretion and lactating dams are able to support their litters through weaning (Monks et al., 2016). Similarly in BTN and CIDEA knockout mice, which do exhibit lactation failure, lipid droplets appear to be secreted during the initial phase of lactation, although the process of secretion is impaired (Ogg et al., 2004; Wang et al., 2012). These observations suggest that lipid droplet-membrane docking may be an evolutionary adaptation of the apocrine mechanism to facilitate milk lipid secretion.
Lipid Droplets Are Secreted Into the Milk Surrounded by Membrane
Using intravital imaging, Masedunskas et al. (2017) showed that lipid droplets remain within the cytoplasm of milk-secreting epithelial cells in association with the apical plasma membrane until oxytocin-stimulated myoepithelial cell contraction induces their secretion. In the absence of oxytocin stimulation, membrane docked lipid droplets become progressively enveloped by the apical membrane but do not undergo release (Monks, unpublished). However, within 1 minute of exposure to oxytocin, myoepithelial cells rhythmically squeeze the entire alveolus and membrane-docked lipid droplets pinch off into the milk (Masedunskas et al., 2017). The mechanism of this release is unclear; however, the milk fat globule that is released is completely surrounded by membrane. It has been noted that the function of the membrane is “stabilization of milk fat in the dispersed form, prevention of flocculation and coalescence of globules, as well as protection against adverse effects of lipases” (Smoczynski, 2017, p. 120). Indeed, secretion of improperly docked droplets in BTN or XOR knockout mice produces structures that seem to have fragile membranes and greater tendency to aggregate, resulting in clogging of milk ducts (Ogg et al., 2004; Monks et al., 2016). Analysis of the complement of proteins associated with the milk fat globule by untargeted proteomics suggests that the origin of the surrounding membrane is likely a combination of apical plasma membrane, ER, and secretory vesicle membrane, with possible contribution by Golgi (Wu et al., 2000; Chat et al., 2011; Wooding and Sargeant, 2015; Honvo-Houeto et al., 2016). The membrane fusion and fission events mediating the apocrine secretion of milk fat globules are, as yet, unknown.
Lipid Droplets Are Degraded in Lysosomes During Mammary Gland Involution
Upon weaning of the neonate, the remaining lipid droplets and milk fat globules in the gland must be cleared away. Sargeant et al. (2014) have shown that cathepsin-D positive autophagic vacuoles containing LipidTox-stained droplets appear in the gland within 24 hours of removing litters from lactating dams, suggesting that lipid degradation occurs by lysosomal-mediated processes within milk-secreting epithelial cells. These investigators also obtained evidence that fatty acids released during lysosomal-mediated lipolysis of lipid droplets cause leakage of cathepsin D, which triggers apoptosis of milk-secreting epithelial cells during mammary gland involution (Sargeant et al., 2014). It is not clear whether the lipids seen within the lysosomes were cytoplasmic droplets undergoing lipophagy or milk fat globules which had undergone efferocytosis and fusion with lysosomes. Further studies are necessary to resolve this conundrum.
Summary
Lipid droplets begin to form in earnest in milk-secreting epithelial cells in mid pregnancy, as small droplets within the ER. PLIN2 associates with these droplets early in their formation and may be necessary for stabilization. Lipid droplets in the milk-secreting epithelial cells may stay closely associated with ER, but cytoskeletal elements may also be involved in their trafficking to the apical part of the cell. Upon contacting the apical plasma membrane, several proteins rearrange and become posttranslationally modified to stabilize a lipid droplet docking complex. In this tethered position, Golgi, mitochondria, and secretory vesicles are often seen in close contact with lipid droplets, although the functional significance of this association is unknown. Final secretion of the lipid droplet from the cell as a milk fat globule is stimulated by contraction of the surrounding myoepithelial cells. The final globule, transported to the neonate, is composed of a neutral lipid core completely surrounded by a membrane bilayer originating from the plasma membrane, with possible contributions of ER and secretory vesicle membranes as well.
Future Directions
Nearly, all the mechanisms presented here have been determined by (a) fixed tissue image analysis of the mammary gland, especially electron micrographs, (b) composition analyses of secreted milk fat globules, and (c) cell fractionation of whole mammary gland with some inferred from other eukaryotic systems. Major gaps exist in our knowledge including which cytoskeletal elements and motors are responsible for transport. Is there potential hitchhiking of lipid droplets for directional trafficking within the milk-secreting epithelial cells? What are the molecular details and energetics of dock formation? How does the cell regulate the phospholipid monolayer upon droplet fusion? How does the ultimate release of the final droplet occur, including signaling, membrane engulfment and fission? As so beautifully executed by Mather et al. (2019), the technology is finally available to use intravital imaging to probe the live, secreting, lactating mammary gland directly, and to begin to answer these questions.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grant R01HD093729.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Barneda D, Planas-Iglesias J, Gaspar ML, Mohammadyani D, Prasannan S, Dormann D, Han GS, Jesch SA, Carman GM, Kagan V, et al. (2015). The brown adipocyte protein CIDEA promotes lipid droplet fusion via a phosphatidic acid-binding amphipathic helix. eLife 4, e07485. doi: 10.7554/eLife.07485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman DE, Davis CL (1974). Biosynthesis of Milk Fat In: Lactation, ed. Larson BL, Smith VR, New York: Academic Press, 31–75. [Google Scholar]
- Baumann O, Walz B (2001). Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int Rev Cytol 205, 149–214. doi: 10.1016/s0074-7696(01)05004-5 [DOI] [PubMed] [Google Scholar]
- Beigneux AP, Vergnes L, Qiao X, Quatela S, Davis R, Watkins SM, Coleman RA, Walzem RL, Philips M, Reue K, Young SG (2006). Agpat6—a novel lipid biosynthetic gene required for triacylglycerol production in mammary epithelium. J Lipid Res 47, 734–744. doi: 10.1194/jlr.M500556-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benador IY, Veliova M, Mahdaviani K, Petcherski A, Wikstrom JD, Assali EA, Acin-Perez R, Shum M, Oliveira MF, Cinti S, et al. (2018). Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metab 27, 869–885.e6. doi: 10.1016/j.cmet.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenaut P, Bangera R, Bevilacqua C, Rebours E, Cebo C, Martin P (2012) Validation of RNA isolated from milk fat globules to profile mammary epithelial cell expression during lactation and transcriptional response to a bacterial infection. J Dairy Sci 95, 6130–6144. doi: 10.3168/jds.2012-5604 [DOI] [PubMed] [Google Scholar]
- Cases S, Zhou P, Shillingford JM, Wiseman BS, Fish JD, Angle CS, Hennighausen L, Werb Z, Farese RV Jr (2004). Development of the mammary gland requires DGAT1 expression in stromal and epithelial tissues. Development 131, 3047–3055. doi: 10.1242/dev.01158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chat S, Layani S, Mahaut C, Henry C, Chanat E, Truchet S (2011). Characterisation of the potential SNARE proteins relevant to milk product release by mouse mammary epithelial cells. Eur J Cell Biol 90, 401–413. doi: 10.1016/j.ejcb.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Chong BM, Reigan P, Mayle-Combs KD, Orlicky DJ, McManaman JL (2011a). Determinants of adipophilin function in milk lipid formation and secretion. Trends Endocrinol Metab 22, 211–217. doi: 10.1016/j.tem.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong BM, Russell TD, Schaack J, Orlicky DJ, Reigan P, Ladinsky M, McManaman JL (2011b). The adipophilin C-terminus is a self-folding membrane binding domain that is important for milk lipid secretion. J Biol Chem 286, 23254–23265. doi: 10.1074/jbc.M110.217091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary V, Jacquier N, Schneiter R (2011). The topology of the triacylglycerol synthesizing enzyme Lro1 indicates that neutral lipids can be produced within the luminal compartment of the endoplasmatic reticulum: Implications for the biogenesis of lipid droplets. Commun Integr Biol 4, 781–784. doi: 10.4161/cib.17830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary V, Ojha N, Golden A, Prinz WA (2015). A conserved family of proteins facilitates nascent lipid droplet budding from the ER. J Cell Biol 211, 261–271. doi: 10.1083/jcb.201505067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JL, Boutet E, Puri V, Chawla A, Czech MP (2010). Identification of the lipid droplet targeting domain of the Cidea protein. J Lipid Res 51, 3455–3462. doi: 10.1194/jlr.M009498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Lee DP (2004). Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res 43, 134–176. doi: 10.1016/s0163-7827(03)00051-1 [DOI] [PubMed] [Google Scholar]
- Dylewski DP, Keenan TW (1984). Centrioles in the mammary epithelium of the rat. J Cell Sci 72, 185–193. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Parton RG (2011). Not just fat: the structure and function of the lipid droplet. Cold Spring Harb Perspect Biol 3, a004838. doi: 10.1101/cshperspect.a004838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Sun Z, Wu L, Xu W, Schieber N, Xu D, Shui G, Yang H, Parton RG, Li P (2011). Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J Cell Biol 195, 953–963. doi: 10.1083/jcb.201104142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes SC, Schuster M, Bielska E, Dagdas G, Kilaru S, Meadows BR, Schrader M, Steinberg G (2015). Peroxisomes, lipid droplets, and endoplasmic reticulum “hitchhike” on motile early endosomes. J Cell Biol 211, 945–954. doi: 10.1083/jcb.201505086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heid HW, Keenan TW (2005). Intracellular origin and secretion of milk fat globules. Eur J Cell Biol 84, 245–258. doi: 10.1016/j.ejcb.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Honvo-Houeto E, Henry C, Chat S, Layani S, Truchet S (2016). The endoplasmic reticulum and casein-containing vesicles contribute to milk fat globule membrane. Mol Biol Cell 27, 2946–2964. doi: 10.1091/mbc.E16-06-0364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambunathan S, Yin J, Khan W, Tamori Y, Puri V (2011). FSP27 promotes lipid droplet clustering and then fusion to regulate triglyceride accumulation. PLoS One 6, e28614. doi: 10.1371/journal.pone.0028614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarasch ED, Bruder G, Keenan TW, Franke WW (1977). Redox constituents in milk fat globule membranes and rough endoplasmic reticulum from lactating mammary gland. J Cell Biol 73, 223–241. doi: 10.1083/jcb.73.1.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RG (1999). Lipids in human milk. Lipids 34, 1243–1271. doi: 10.1007/s11745-999-0477-2 [DOI] [PubMed] [Google Scholar]
- Jensen RG, Ferris AM, Lammi-Keefe CJ, Henderson RA (1990). Lipids of bovine and human milks: a comparison. J Dairy Sci 73, 223–240. doi: 10.3168/jds.S0022-0302(90)78666-3 [DOI] [PubMed] [Google Scholar]
- Jeong J, Rao AU, Xu J, Ogg SL, Hathout Y, Fenselau C, Mather IH (2009). The PRY/SPRY/B30.2 domain of butyrophilin 1A1 (BTN1A1) binds to xanthine oxidoreductase: implications for the function of BTN1A1 in the mammary gland and other tissues. J Biol Chem 284, 22444–22456. doi: 10.1074/jbc.M109.020446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassan A, Herms A, Fernandez-Vidal A, Bosch M, Schieber NL, Reddy BJ, Fajardo A, Gelabert-Baldrich M, Tebar F, Enrich C, et al. (2013). Acyl-CoA synthetase 3 promotes lipid droplet biogenesis in ER microdomains. J Cell Biol 203, 985–1001. doi: 10.1083/jcb.201305142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan TW, Dylewski DP (1985). Aspects of intracellular transit of serum and lipid phases of milk. J Dairy Sci 68, 1025–1040. doi: 10.3168/jds.S0022-0302(85)80925-5 [DOI] [PubMed] [Google Scholar]
- Kennedy EP (1957). Metabolism of lipides. Ann Rev Biochem 26, 119–148. doi: 10.1146/annurev.bi.26.070157.001003 [DOI] [PubMed] [Google Scholar]
- Klemm RW, Norton JP, Cole RA, Li CS, Park SH, Crane MM, Li L, Jin D, Boye-Doe A, Liu TY, et al. (2013). A conserved role for atlastin GTPases in regulating lipid droplet size. Cell Rep 3, 1465–1475. doi: 10.1016/j.celrep.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladinsky MS, Mardones GA, Orlicky DJ, Howell KE, McManaman JL (2019). Electron tomography revels that milk lipids originate from endoplasmic reticulum domains with novel structural features. J Mammary Gland Biol Neoplasia. In Press. doi: 10.1007/s10911-019-09438-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guillou S, Laubier J, Pechoux C, Aujean E, Castille J, Leroux C, Le Provost F (2019). Defects of the endoplasmic reticulum and changes to lipid droplet size in mammary epithelial cells due to miR-30b-5p overexpression are correlated to a reduction in Atlastin 2 expression. Biochem Biophys Res Commun 512, 283–288. doi: 10.1016/j.bbrc.2019.03.022 [DOI] [PubMed] [Google Scholar]
- Lemay DG, Ballard OA, Hughes MA, Morrow AL, Horseman ND, Nommsen-Rivers LA (2013). PLoS One 8(7): e67531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Luo J, Wang H, Shi H, Zhu J, Sun Y, Yu K, Yao D (2015). Adipose triglyceride lipase regulates lipid metabolism in dairy goat mammary epithelial cells. Gene 554, 125–130. doi: 10.1016/j.gene.2014.10.020 [DOI] [PubMed] [Google Scholar]
- Listenberger LL, Ostermeyer-Fay AG, Goldberg EB, Brown WJ, Brown DA (2007). Adipocyte differentiation-related protein reduces lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J Lipid Res 48, 2751–2761. doi: 10.1194/jlr.M700359-JLR200 [DOI] [PubMed] [Google Scholar]
- Long CA, Patton S (1978). Formation of intracellular fat droplets: interrelation of newly synthesized phosphatidylcholine and triglyceride in milk. J Dairy Sci 61, 1392–1399. doi: 10.3168/jds.S0022-0302(78)83740-0 [DOI] [PubMed] [Google Scholar]
- Lv Y, Guan W, Qiao H, Wang C, Chen F, Zhang Y, Liao Z (2015). Veterinary medicine and omics (veterinomics): metabolic transition of milk triacylglycerol synthesis in sows from late pregnancy to lactation. OMICS 19, 602–616. doi: 10.1089/omi.2015.0102 [DOI] [PubMed] [Google Scholar]
- Maningat PD, Sen P, Sunehag AL, Hadsell DL, Haymond MW (2007) Regulation of gene expression in human mammary epithelium: effect of breast pumping. J Endocrinol 195, 503–511. doi: 10.1677/JOE-07-0394 [DOI] [PubMed] [Google Scholar]
- Masedunskas A, Chen Y, Stussman R, Weigert R, Mather IH (2017). Kinetics of milk lipid droplet transport, growth, and secretion revealed by intravital imaging: lipid droplet release is intermittently stimulated by oxytocin. Mol Biol Cell 28, 935–946. doi: 10.1091/mbc.e16-11-0776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather IH, Keenan TW (1998). Origin and secretion of milk lipids. J Mammary Gland Biol Neoplasia 3, 259–273. doi: 10.1023/a:1018711410270 [DOI] [PubMed] [Google Scholar]
- Mather IH, Masedunskas A, Chen Y, Weigert R (2019). Symposium review: intravital imaging of the lactating mammary gland in live mice reveals novel aspects of milk-lipid secretion. J Dairy Sci 102, 2760–2782. doi: 10.3168/jds.2018-15459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JR (2001). Electron microscopy of cells: a new beginning for a new century. J Cell Biol 153, F25–32. doi: 10.1083/jcb.153.6.f25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManaman JL (2012). Milk lipid secretion: recent biomolecular aspects. Biomol Concepts 3, 581–591. doi: 10.1515/bmc-2012-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManaman JL, Palmer CA, Wright RM, Neville MC (2002). Functional regulation of xanthine oxidoreductase expression and localization in the mouse mammary gland: evidence of a role in lipid secretion. J Physiol 545, 567–579. doi: 10.1113/jphysiol.2002.027185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManaman JL, Zabaronick W, Schaack J, Orlicky DJ (2003). Lipid droplet targeting domains of adipophilin. J Lipid Res 44, 668–673. doi: 10.1194/jlr.C200021-JLR200 [DOI] [PubMed] [Google Scholar]
- Mishra S, Khaddaj R, Cottier S, Stradalova V, Jacob C, Schneiter R (2016). Mature lipid droplets are accessible to ER luminal proteins. J Cell Sci 129, 3803–3815. doi: 10.1242/jcs.189191 [DOI] [PubMed] [Google Scholar]
- Monks J, Dzieciatkowska M, Bales ES, Orlicky DJ, Wright RM, McManaman JL (2016). Xanthine oxidoreductase mediates membrane docking of milk-fat droplets but is not essential for apocrine lipid secretion. J Physiol 594, 5899–5921. doi: 10.1113/JP272390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oftedal OT (1984). Milk composition, milk yield and energy output at peak lactation: a comparative review. Symp Zool Soc Lond 51, 33–85. [Google Scholar]
- Ogg SL, Weldon AK, Dobbie L, Smith AJ, Mather IH (2004). Expression of butyrophilin (Btn1a1) in lactating mammary gland is essential for the regulated secretion of milk-lipid droplets. Proc Natl Acad Sci U S A 101, 10084–10089. doi: 10.1073/pnas.0402930101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlicky DJ, Libby AE, Bales ES, McMahan RH, Monks J, La Rosa FG, McManaman JL (2019). Perilipin-2 promotes obesity and progressive fatty liver disease in mice through mechanistically distinct hepatocyte and extra-hepatocyte actions. J Physiol 597, 1565–1584. doi: 10.1113/JP277140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlicky DJ, Monks J, Stefanski AL, McManaman JL (2013). Dynamics and molecular determinants of cytoplasmic lipid droplet clustering and dispersion. PLoS One 8, e66837. doi: 10.1371/journal.pone.0066837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeki S, Cheng J, Tauchi-Sato K, Hatano N, Taniguchi H, Fujimoto T (2005). Rab18 localizes to lipid droplets and induces their close apposition to the endoplasmic reticulum-derived membrane. J Cell Sci 118, 2601–2611. doi: 10.1242/jcs.02401 [DOI] [PubMed] [Google Scholar]
- Robenek H, Hofnagel O, Buers I, Robenek MJ, Troyer D, Severs NJ (2006). Adipophilin-enriched domains in the ER membrane are sites of lipid droplet biogenesis. J Cell Sci 119, 4215–4224. doi: 10.1242/jcs.03191 [DOI] [PubMed] [Google Scholar]
- Rudolph MC, Monks J, Burns V, Phistry M, Marians R, Foote MR, Bauman DE, Anderson SM, Neville MC (2010). Sterol regulatory element binding protein and dietary lipid regulation of fatty acid synthesis in the mammary epithelium. Am J Physiol Endocrinol Metab 299, E918–E927. doi: 10.1152/ajpendo.00376.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph MC, Young BE, Lemas DJ, Palmer CE, Hernandez TL, Barbour LA, Friedman JE, Krebs NF, MacLean PS (2017). Early infant adipose deposition is positively associated with the n-6 to n-3 fatty acid ratio in human milk independent of maternal BMI. Int J Obes (Lond) 41, 510–517. doi: 10.1038/ijo.2016.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell TD, Palmer CA, Orlicky DJ, Fischer A, Rudolph MC, Neville MC, McManaman JL (2007). Cytoplasmic lipid droplet accumulation in developing mammary epithelial cells: roles of adipophilin and lipid metabolism. J Lipid Res 48, 1463–1475. doi: 10.1194/jlr.M600474-JLR200 [DOI] [PubMed] [Google Scholar]
- Russell TD, Schaack J, Orlicky DJ, Palmer C, Chang BH, Chan L, McManaman JL (2011). Adipophilin regulates maturation of cytoplasmic lipid droplets and alveolae in differentiating mammary glands. J Cell Sci 124, 3247–3253. doi: 10.1242/jcs.082974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo VT, Ikonen E (2019). Moving out but keeping in touch: contacts between endoplasmic reticulum and lipid droplets. Curr Opin Cell Biol 57, 64–70. doi: 10.1016/j.ceb.2018.11.002 [DOI] [PubMed] [Google Scholar]
- Sargeant TJ, Lloyd-Lewis B, Resemann HK, Ramos-Montoya A, Skepper J, Watson CJ (2014). Stat3 controls cell death during mammary gland involution by regulating uptake of milk fat globules and lysosomal membrane permeabilization. Nat Cell Biol 16, 1057–1068. doi: 10.1038/ncb3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Voeltz GK, Rapoport TA (2006). Rough sheets and smooth tubules. Cell 126, 435–439. doi: 10.1016/j.cell.2006.07.019 [DOI] [PubMed] [Google Scholar]
- Smoczynski M (2017). Role of phospholipid flux during milk secretion in the mammary gland. J Mammary Gland Biol Neoplasia 22, 117–129. doi: 10.1007/s10911-017-9376-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandl J, White DJ, Peychl J, Thiele C (2009). Live cell multicolor imaging of lipid droplets with a new dye, LD540. Traffic 10, 1579–1584. doi: 10.1111/j.1600-0854.2009.00980.x [DOI] [PubMed] [Google Scholar]
- Stein O, Stein Y (1967). Lipid synthesis, intracellular transport, and secretion. II. Electron microscopic radioautographic study of the mouse lactating mammary gland. J Cell Biol 34, 251–263. doi: 10.1083/jcb.34.1.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemberger BH, Walsh RM, Patton S (1984). Morphometric evaluation of lipid droplet associations with secretory vesicles, mitochondria and other components in the lactating cell. Cell Tissue Res 236, 471–475. doi: 10.1007/bf00214252 [DOI] [PubMed] [Google Scholar]
- Sztalryd C, Bell M, Lu X, Mertz P, Hickenbottom S, Chang BH, Chan L, Kimmel AR, Londos C (2006). Functional compensation for adipose differentiation-related protein (ADFP) by TIP47 in an ADFP nullembryonic cell line. J Biol Chem 281, 34341–34348. doi: 10.1074/jbc.M602497200 [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Reue K (2009). Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol Endocrinol Metab 296, E1195–1209. doi: 10.1152/ajpendo.90958.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorbach C, Scriven A, Capecchi MR (2002). The housekeeping gene xanthine oxidoreductase is necessary for milk fat droplet enveloping and secretion: gene sharing in the lactating mammary gland. Genes Dev 16, 3223–3235. doi: 10.1101/gad.1032702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlig JL, Bales ES, Jackman MR, Johnson GC, McManaman JL, Maclean PS (2012). Impact of high-fat diet and obesity on energy balance and fuel utilization during the metabolic challenge of lactation. Obesity (Silver Spring) 20, 65–75. doi: 10.1038/oby.2011.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther TC, Chung J, Farese RV Jr (2017). Lipid droplet biogenesis. Annu Rev Cell Dev Biol 33, 491–510. doi: 10.1146/annurev-cellbio-100616-060608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Lv N, Zhang S, Shui G, Qian H, Zhang J, Chen Y, Ye J, Xie Y, Shen Y, et al. (2012). Cidea is an essential transcriptional coactivator regulating mammary gland secretion of milk lipids. Nat Med 18, 235–243. doi: 10.1038/nm.2614 [DOI] [PubMed] [Google Scholar]
- Welte MA (2009). Fat on the move: intracellular motion of lipid droplets. Biochem Soc Trans 37, 991–996. doi: 10.1042/BST0370991 [DOI] [PubMed] [Google Scholar]
- Wilfling F, Wang H, Haas JT, Krahmer N, Gould TJ, Uchida A, Cheng JX, Graham M, Christiano R, Frohlich F, et al. (2013). Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev Cell 24, 384–399. doi: 10.1016/j.devcel.2013.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooding FBP (1977). Comparative Mammary Fine Structure In: Comparative Aspects of Lactation, ed. Peaker M, London: Academic Press, 1–41. [Google Scholar]
- Wooding FB, Sargeant TJ (2015). Immunocytochemical evidence for Golgi vesicle involvement in milk fat globule secretion. J Histochem Cytochem 63, 943–951. doi: 10.1369/0022155415608918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Howell KE, Neville MC, Yates JR III, McManaman JL (2000). Proteomics reveal a link between the endoplasmic reticulum and lipid secretory mechanisms in mammary epithelial cells. Electrophoresis 21, 3470–3482. doi: [DOI] [PubMed] [Google Scholar]
- Wu L, Zhou L, Chen C, Gong J, Xu L, Ye J, Li D, Li P (2014). Cidea controls lipid droplet fusion and lipid storage in brown and white adipose tissue. Sci China Life Sci 57, 107–116. doi: 10.1007/s11427-013-4585-y [DOI] [PubMed] [Google Scholar]
- Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV Jr (2008). Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res 49, 2283–2301. doi: 10.1194/jlr.R800018-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaczek M, Keenan TS (1990). Morphological evidence for an endoplasmic reticulum origin of milk lipid globules obtained using lipid-selective staining procedures. Protoplasma 159, 179–182. doi: 10.1007/BF01322600 [DOI] [Google Scholar]


