Abstract
Objective:
Statins are hypothesized to have beneficial effects in asthma management through their pleiotropic anti-inflammatory effects. Several studies have examined this relationship, but have yielded conflicting results. This study investigates the effect of statin use on asthma-related hospitalizations and/or emergency department (ED) visits, and whether this relationship varies by concomitant inhaled corticosteroid (ICS) in a large cohort of asthma patients.
Methods:
Subjects with asthma, a recent history of asthma exacerbation, and who were 18 years or older were selected from the population-based Medco Health Solutions administrative database over a 1 year period. Prescription claims for statins and asthma medications, and asthma-related hospitalizations and/or ED visits were ascertained over a 12 month follow-up period. Subjects were stratified into two groups based on their ICS use.
Results:
A total of 3747 ICS users and 2905 non-ICS users were included in this study. Statin users represented 21% of ICS users and 11% of non-users. Among ICS users, statin use was significantly associated with decreased odds of asthma-related ED visits (OR = 0.77, 95% CI 0.64–0.94, p = 0.008), but not with asthma-related hospitalizations (OR = 1.09, 95% CI 0.92–1.30, p = 0.31). No significant associations were found among non-ICS users (for asthma-related ED visits: OR = 0.92, 95% CI 0.57–1.49, p = 0.73; asthma-related hospitalizations: OR = 1.10, 95% CI 0.85–1.41, p = 0.48). The statistical interactions between ICS and statin use on asthma-related hospitalizations and/or ED visits were not significant.
Conclusion:
Statin use is associated with fewer ED visits in asthma patients who are using ICS.
Keywords: Asthma, Exacerbation, Inhaled corticosteroids, Statins
Introduction
Statins, 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase inhibitors, are recommended to lower low-density lipoprotein cholesterol (LDL-C), thus reducing the risk of cardiovascular events1–5. Several studies have demonstrated the anti-inflammatory and immuno-modulatory properties of statins6–9, and statins have been postulated to play a role in the therapy of asthma10, a chronic inflammatory pulmonary condition affecting more than 25 million people in the United States11 and 300 million people worldwide12.
Randomized controlled trials of statin therapy in patients with asthma have shown mixed results, but most are limited by small samples sizes, short duration of intervention, and lack power to examine the effect of statins on asthma exacerbations13–17. In contrast, in murine models of allergic asthma, statins have been shown to increase lung compliance18, decrease pulmonary inflammation18,19, inhibit goblet cell hyperplasia20, and decrease antigen-induced bronchial smooth muscle hyperresponsiveness18,21,22.
The objective of this study is to examine the effect of statin use on asthma-related hospitalizations and/or emergency department (ED) visits, using a large, real-world, population-based cohort. In addition, we explored whether this effect varied with concomitant ICS use.
Patients and methods
Subjects
The Medco Health Solutions National Integrated Database is a large, de-identified medical and pharmacy claims database containing over 10 million patients throughout the US. This database includes demographic data for its members, pharmacy prescription data that includes information on retail and mail order medication claims, and medical claims data for inpatient, outpatient, and ED visits.
For the purpose of this retrospective cohort study, claims from January 1, 2009 to December 31, 2009 were analyzed (the accrual period). Subjects were considered to have asthma if they had an asthma International Classification of Diseases, Ninth revision (ICD-9) code (493.XX) on two or more medical claims on distinct dates at any point of service during the accrual period. The asthma index date was defined as the first medical claim for ICD-9 493.XX in the accrual period. Subjects were included if they were 18 years or older, had asthma, at least one asthma-related hospitalization or ED visit in the 12 months prior to the index date, and 24 months of continuous eligibility with medical and pharmacy benefits (12 months pre and post asthma index date). Clients who declined to have their members’ medical or pharmacy claims data for external use and publication were excluded from this study.
Statin therapy, asthma therapy, and asthma-related outcomes
Statin and asthma therapy were assessed through National Drug Codes for the respective medications. Statin therapy includes simvastatin, lovastatin, atorvastatin, pravastatin, rosuvastatin, fluvastatin, cerivastatin, and pitavastatin. In this study, subjects were considered ICS users if they had any pharmacy claim for an ICS inhaler or any ICS combination inhalers during the post-index period. Other asthma therapy was characterized using the following categories: anticholinergics, oral steroids, short-acting beta-agonists, long-acting beta-agonists, leukotriene modifiers, mast cell stabilizers, theophylline and derivatives, and anti-immunoglobulin E (IgE) agents.
Statin use was ascertained based on the statin exposure rate during the post-index period, which is defined as:
(# days of statin dispensed between asthma index date and the asthma-related outcome up to 12 months)
(# of days between asthma index date and the asthma-related outcome up to 12 months)
Subjects were considered to be statin users if their statin exposure rate was 0.5 or greater with a maximum of 1.0. The primary outcome of interest, asthma exacerbation post-asthma diagnosis index date, was defined three ways: first asthma-related hospitalization, first asthma-related ED visit, and a composite outcome consisting of first asthma-related hospitalization and/or ED visit. Asthma-related hospitalizations and ED visits were ascertained based on medical claims (ICD-9 493.XX) at point of care over the 12 months post-index date.
Statistical analysis
Descriptive analysis of baseline characteristics were calculated for the statin users and non-statin users. A sensitivity analysis was performed to examine the number of subjects considered to be statin users with different thresholds for the statin exposure rate. Subjects were stratified based on their post-index date ICS use, and multivariate logistic regression was performed within each stratum to assess the association between statin use and each of the three asthma-related outcomes. Sex was forced in as an obligatory covariate while other covariates were included into the final model if they had a p-value of<0.1 on univariate analysis with the outcome of interest. Covariates considered include: age, census region, two or more asthma-related hospitalizations and/or ED visits in the pre-index period, asthma therapy, and the number of oral corticosteroid (OCS) courses in the pre-index period. In order to account for indications for statin use, Deyo–Charlson conditions and obesity were assessed in the pre-index period and included in the list of covariates considered. Other baseline characteristics of interest included smoking status, total asthma medication co-pay, total asthma medication cost, types of provider (internal medicine, family medicine, pulmonology, allergy), and the number of days between the end of last statin claim and the asthma-related outcome. Overweight/obesity was defined by ICD-9 codes and/or claims for weight loss medication and smoking status was defined by ICD-9 codes and/or claims for smoking cessation therapy (see Table E1 in the online data supplement). In addition to stratification by ICS use, an interaction term between ICS and statin use was added to the model to explore potential effect modification by ICS use on the effect of statin on asthma-related outcomes. P-values are two-sided and considered statistically significant if <0.05. All analyses were performed using SAS (version 9.3; Cary, NC, USA: SAS Institute).
Results
Subject demographics
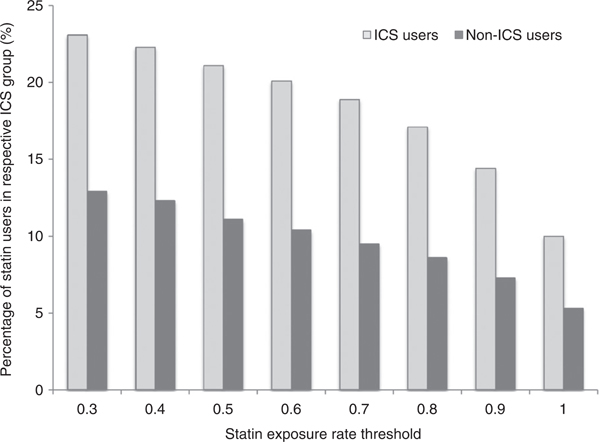
There were 137,924 subjects with asthma during the specified 12 month accrual period. Of these, 6652 subjects met the inclusion and exclusion criteria, of which 3747 (56%) used an ICS during post-index period and 2905 (44%) were not ICS users (Figure 1). Depending upon the statin exposure threshold the overall population statin exposure rate varied from 8% to 19% at a threshold of 1.0 and 0.3, respectively (Figure 2). Based on the significant statin exposure rate of ≥0.5, there were 792 (21%) and 323 (11%) statin users among the ICS-exposed and ICS-unexposed group, respectively.
Figure 1.
Selection of study patients from the Medco Health Solutions National Integrated Database.
Figure 2.
Percentage of statin users in each ICS group based on different statin exposure rate thresholds.
Statin users were older, with a mean age of 63.8 (SD 12.2) years, while non-statin users had a mean age of 49.3 (SD 17.3) years (Table 1). There were more male subjects among statin users (36% vs. 27% in non-statin users). Compared to non-users, a higher proportion of statin users were on asthma therapy, including the more frequent use of an ICS (59% vs. 44% in non-statin users, p<0.001). A higher percentage of non-statin users had two or more asthma-related hospitalizations and/or ED visits in the pre-index period (23% vs. 17% in statin users, p<0.001). A higher proportion of smokers was also observed among non-statin users. Overall, statin users had a higher prevalence of baseline cardiovascular disease, diabetes and other comorbid conditions and were more frequently dispensed other non-asthma chronic therapy such as beta-blockers or non-steroidal anti-inflammatory drugs (NSAIDs).
Table 1.
Baseline characteristics of statin users and non-statin users.
| Statin use | No statin use | p Value | |
|---|---|---|---|
| Total number of subjects | 1115 | 5537 | - |
| Age, mean (SD) | 63.8 (12.2) | 49.3 (17.3) | <0.001 |
| Female, % (n) | 64 (715) | 73 (4065) | <0.001 |
| Statin exposure rate over 12 months post-index | |||
| Mean (SD) | 0.90 (0.14) | 0.01 (0.07) | <0.001 |
| Asthma therapy in the 12 months pre-index date, % (n) | |||
| Inhaled corticosteroids | 59 (662) | 44 (2445) | <0.001 |
| Anticholinergics | 16 (182) | 7 (390) | <0.001 |
| Anticholinergics/short-acting beta-agonist combination | 12 (129) | 7 (377) | <0.001 |
| Oral corticosteroids | 49 (549) | 42 (2348) | <0.001 |
| Short-acting beta-agonists | 60 (663) | 55 (3059) | 0.010 |
| Long-acting beta-agonists | 5(51) | 2 (133) | <0.001 |
| Long-acting beta-agonists/ICS combination | 46 (513) | 33 (1809) | <0.001 |
| Leukotriene modifiers | 36 (405) | 24 (1310) | <0.001 |
| Mast cell stabilizers | 1 (12) | 1 (37) | 0.146 |
| Theophylline | 4 (44) | 2 (133) | 0.003 |
| Omalizumab | 0 (5) | 1 (43) | 0.238 |
| Total asthma medication co-pay, $, mean (SD) | 216.76 (19.4) | 135.01 (2.4) | <0.001 |
| ≥2 asthma-related ED/hospitalization events in the 12 months pre-index date, % (n) | 17 (185) | 23 (1296) | <0.001 |
| Number of bursts of oral corticosteroids in the 12 months pre-index date, mean (SD) | 0.73 (1.1) | 0.68 (1.2) | 0.177 |
| Tobacco use, % (n) | 23 (261) | 32 (1750) | <0.001 |
| Obesity, % (n) | 15 (164) | 12 (683) | 0.030 |
| Deyo-Charlson conditions, % (n) | |||
| Myocardial infarction | 7 (78) | 2 (101) | <0.001 |
| Congestive heart failure | 4 (161) | 6 (324) | <0.001 |
| Peripheral vascular disease | 8 (85) | 3 (161) | <0.001 |
| Cerebrovascular disease | 14 (158) | 5 (278) | <0.001 |
| Dementia | 1 (14) | 0 (24) | <0.001 |
| Chronic pulmonary disease† | 100 (1115) | 100 (5537) | <0.001 |
| Rheumatologic disease | 4 (47) | 4 (204) | 0.396 |
| Peptic ulcer disease | 2(17) | 1 (66) | 0.361 |
| Mild liver disease | 0 (5) | 1 (28) | 0.804 |
| Diabetes | 35 (395) | 15 (811) | <0.001 |
| Diabetes with chronic complications | 8 (84) | 3 (157) | <0.001 |
| Hemiplegia or paraplegia | 1 (7) | 0 (23) | 0.334 |
| Renal disease | 7 (83) | 3 (157) | <0.001 |
| Moderate or severe liver disease | 0 (2) | 0(12) | 1.000 |
| HIV/AIDS | 0 (0) | 0(16) | 0.072 |
| Any malignancy including leukemia and lymphoma | 11 (118) | 6 (348) | <0.001 |
| Metastatic solid tumor | 1 (11) | 1 (32) | 0.120 |
| Other non-asthma therapy, % (n) | |||
| Beta-blockers | 31 (346) | 10 (572) | <0.001 |
| Non-steroidal anti-inflammatory drugs (NSAID) | 33 (370) | 25 (1374) | <0.001 |
Includes asthma.
Statin use and asthma-related outcomes
Despite the smaller number of statin users in each ICS stratum, there were more asthma-related adverse events among statin users compared to non-users (Table 2). Common covariates included in all final models include age, sex, census region, ≥2 pre-index asthma-related hospitalizations or ED visits, and pre-index number of OCS bursts (Table 3). Among ICS users, statin use was significantly associated with decreased odds of asthma-related ED visits (OR = 0.77, 95% CI 0.64–0.94, p = 0.008), after adjustment for covariates (Table 4). Statin use among ICS users was not significantly associated with asthma-related hospitalizations (OR = 1.09, 95% CI 0.92–1.30, p = 0.31) or with the composite outcome of hospitalizations and/or ED visits (OR = 0.93, 95% CI 0.74–1.17, p = 0.54). Among non-ICS users, statin use was not associated with the asthma-related outcomes. The relationship between statin use and any of the asthma-related outcomes was not modified by concurrent ICS therapy (ICS ×statin interaction term p = 0.91 for hospitalizations, p = 0.69 for ED visits, p = 0.38 for hospitalizations and/or ED visits).
Table 2.
Number of asthma-related adverse outcomes among ICS users, non-ICS users, and the whole study cohort.
| Asthma-related hospitalizations, % (n) | Asthma-related ED visits, % (n) | Asthma-related hospitalization and/or ED visits, % (n) | |
|---|---|---|---|
| ICS use | |||
| Statin use | 76 (1433) | 86 (1222) | 79 (2478) |
| No statin use | 24 (462) | 14 (206) | 21 (653) |
| Total | 1895 | 1428 | 3131 |
| No ICS use | |||
| Statin use | 87 (1232) | 94 (976) | 89 (2107) |
| No statin use | 13 (187) | 6 (68) | 11 (249) |
| Total | 1419 | 1044 | 2356 |
| Whole cohort | |||
| Statin use | 80 (2665) | 89 (2198) | 84 (4585) |
| No statin use | 20 (649) | 11 (274) | 16(902) |
| Total | 3314 | 2472 | 5487 |
Table 3.
Covariates included in final models of statin use on asthma-related outcomes (p<0.1 on univariate analysis).
| ICS use | No ICS use | Statin × ICS interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Asthma-related hospitalizations | Asthma-related ED visits | Asthma-related hospitalizations and/or ED visits | Asthma-related hospitalizations | Asthma-related ED visits | Asthma-related hospitalizations and/or ED visits | Asthma-related hospitalizations | Asthma-related ED visits | Asthma-related hospitalizations and/or ED visits | |
| Age | x | X | X | X | X | X | X | X | X |
| Sex | X | X | X | X | X | X | X | X | X |
| Census region | X | X | X | X | X | X | X | X | X |
| ≥2 pre-index asthma-related hospitalizations or ED visits | X | X | X | X | X | X | X | X | X |
| Pre-index number of OCS bursts | X | X | X | X | X | X | X | X | X |
| Pre-index statin use | X | X | |||||||
| Pre-index NSAID use | X | X | X | ||||||
| Time from end of last statin claim to event | X | X | |||||||
| Rheumatologie disease | X | ||||||||
| Overweight/obesity | X | X | X | X | |||||
| Malignancy | X | X | X | X | |||||
| Diabetes | X | ||||||||
| Diabetes with chronic complications | X | X | |||||||
| Myocardial infarction | X | X | X | ||||||
| Peptic ulcer disease | X | X | X | ||||||
| Congestive heart failure | X | X | X | ||||||
| Renal disease | X | ||||||||
Table 4.
Effect of statin use on asthma-related adverse outcomes.
| Asthma-related hospitalizations | Asthma-related ED visits | Asthma-related hospitalizations and/or ED visits | ||||
|---|---|---|---|---|---|---|
| OR‡ (95% CI) | p Value‡ | OR‡ (95% CI) | p Value‡ | OR‡ (95% CI) | p Value‡ | |
| ICS use | ||||||
| Statin use vs. no statin use | 1.09(0.92,1.30) | 0.31 | 0.77 (0.64, 0.94) | 0.008 | 0.93 (0.74,1.17) | 0.54 |
| No ICS use | ||||||
| Statin use vs. no statin use | 1.10(0.85,1.41) | 0.48 | 0.92 (0.57, 1.49) | 0.73 | 0.83 (0.62, 1.13) | 0.24 |
| Statin × ICS | 0.91 | 0.69 | 0.38 | |||
Adjusted for covariates in Table 3.
Discussion
In this large population-based cohort study, we found a significant association between statin use and decreased odds of asthma-related ED visits among ICS users after adjusting for various potential confounders. This effect was not observed among subjects not on concurrent ICS therapy. Furthermore, our findings did not demonstrate a statistical interaction between ICS and statin use on adverse asthma outcomes.
Our findings are in accordance with recent observational studies. Using the Taiwan nationwide insurance database, after adjusting for a number of parameters, Huang et al. reported an association between any statin use and a lower incidence of hospitalization in patients with asthma23. Unlike this evaluation, the investigators defined statin use as anyone who received a statin; thus, exposure to a statin may be minimal prior to hospitalization for asthma. Additionally, in contrast to this study population, a minority of subjects in the Taiwanese cohort was on ICS therapy (12% among statin users vs. 59% in our study). As the authors pointed out, ICS are underused in Taiwan compared to other industrialized countries, and their results may not be generalizable to other populations. Another recent study found that statin therapy, after adjustment of age, gender, race, geographical region, and Charlson Comorbidity Index, was significantly associated with decreased odds of asthma-related ED visits and hospitalizations and/or ED visits among subjects with asthma on ICS therapy using the Mississippi Medicaid data24. Interestingly, the authors ascertained statin exposure over the 6 months prior to the index date and assessed the asthma outcomes over the 12 months following the index date. Although the authors had findings similar to ours, our study utilized patients throughout the US and was methodologically different as we assessed statin exposure up to the time of the asthma-related event. It is interesting to consider the temporal relationship between statin use and its potentially beneficial effects on asthma outcomes as well as the amount of exposure required for such benefits. Based on the cardiovascular literature, the effects of statins on C-reactive protein and low-density lipoprotein cholesterol may be seen within weeks25 while their effect on the reduction of plaque volume may take years26. While further studies are needed to clarify these temporal relationships between statins and asthma outcomes, we speculate that in asthma a reduction in sputum inflammatory cells may occur within weeks of starting statin therapy while it may take several months or even years before an effect on some spirometric parameters or airway remodeling may be detected. The optimal duration for future trials in this field will depend on the outcome of interest. For example, in addition to an increase in sample size from the previous trials and based on previous asthma trials27, we speculate that a trial of minimum 1 year duration may allow the detection of a difference in asthma exacerbations between individuals randomized to statins or placebo.
The finding of a beneficial statin effect in asthma contrasts with the results of several small randomized controlled trials (RCT), which demonstrated limited benefits across laboratory and clinical endpoints. A short-term RCT in atopic asthma subjects on ICS therapy compared 8 weeks of atorvastatin therapy (40 mg daily) to an 8 week placebo and found no difference in mean morning peak expiratory flow (PEF) between the two treatment periods13. However, a decrease in sputum macrophage count was noted with atorvastatin treatment. Cowan et al. showed no steroid-sparing effect of simvastatin (40 mg daily) in patients with eosinophilic asthma, with no difference in symptoms control, lung function and airway hyperresponsiveness between subjects on simvastatin and placebo14, despite a decrease in sputum eosinophils. Most of these trials have a short treatment period and small sample sizes, limiting their ability to examine the effects of statins on asthma exacerbation, a more infrequent outcome.
Despite a non-significant statistical interaction between ICS and statin use on asthma exacerbations, our findings are consistent with potential beneficial effects of statins among ICS users only, suggesting a biological interaction or a synergistic action between ICS and statins. Although often used together or even interchangeably, an absence of statistical interaction is not equivalent to an absence of biological interaction28. The literature on the synergistic effect of statins and ICS remains controversial. Corroborated by studies using murine models29, one clinical trial found that simvastatin enhances the anti-inflammatory properties of ICS through increased IL-10 secretion and activation of the indoleamine 2,3 dioxygenase pathway in subjects with mild asthma15 and another observed an improvement in asthma control in subjects with severe asthma on concomitant ICS and statin therapy30. However, Braganza et al. found no change in PEF with atorvastatin and ICS compared to ICS therapy alone16. Interestingly, while the study cited above by Cowan et al. showed no steroid-sparing effect of simvastatin in subjects with eosinophilic asthma, a significant improvement in Asthma Control Questionnaire score, forced expiratory volume in 1 second (FEV1), and sputum eosinophils was observed in the subjects weaned down to 0 μg of fluticasone and who were on simvastatin. These subjects generally had higher PEF and FEV1 compared to those who were unable to wean to no ICS. One possible explanation to these conflicting results is the different subtypes of asthma studied, which included mild asthma15, obesity-related severe asthma30, smokers with asthma16, and eosinophilic asthma14. Our population was heterogeneous and included a mixture of asthma sub-types. It is possible that among individuals with eosinophilic asthma, statin therapy benefits only those with mild asthma. While our findings add to the existing literature, further studies are needed to examine the potential synergistic action of ICS and statins on clinical asthma outcomes, particularly specific to distinct asthma subtypes and the mechanisms leading to these additive effects.
Our study has several strengths. First, the use of a large real-world and heterogeneous population allows for increased generalizability of our results. Our population is also different from previous large observational studies using administrative databases, one of which examined a Taiwanese population and another used a state Medicaid database. Second, we examined the effect modification by concurrent ICS therapy on the relationship between statin use and asthma-related hospitalization and/or ED visits. Our results add to the existing literature as no observational study has formally analyzed this effect previously. Lastly, we quantified a subject’s statin exposure by looking at their statin exposure rate prior to the adverse asthma-related event. This is similar to the medication possession ratio, but our definition allows for a better characterization of statin exposure leading up to the adverse event and disregards the statin exposure after the event.
Despite these strengths, several limitations are note-worthy. As in most observational studies examining the effect of a treatment, there may be residual confounding by indication. Subjects who were prescribed statin therapy may have more comorbidities, as is the case with our population, and may be inherently more at risk for asthma-related adverse outcomes. However, despite the observation that statin users had more prevalent co-morbid conditions at baseline, we found a beneficial effect of statins in this sicker group, reinforcing the conclusion that statins may be truly associated with a decreased risk of asthma-related ED visits. Given the administrative nature of the database, we did not have access to the specific indications for statin use. Thus, in order to address the issue of confounding by indication, we comprehensively adjusted for comorbid medical conditions that may be potential confounders. Several limitations are associated with the nature of administrative databases. These include potential for misclassification of exposures and outcomes, and lack of reliable data on adherence for certain medications, environmental conditions (e.g. exposure to allergens), and other lifestyle influences. Furthermore, it is unclear what level of statin exposure may be necessary to confer benefits in asthma. Choosing a too low or too high statin exposure rate threshold may lead to misclassification, therefore an a priori threshold of 0.5 was chosen. Our sensitivity analysis demonstrated that variations in this threshold shift the number of statin users only slightly in our population (3% less statin users for a threshold of 0.8 compared to 0.5), suggesting that a threshold of 0.5 may be acceptable. Lastly, we were unable to differentiate the different sub-groups of asthma using this claims-based database. Many previous studies have focused on patients with eosinophilic asthma and murine models of allergic asthma. It is possible that different subtypes of asthma, such as obesity-associated asthma, respond differently to statins given the presence of neutrophilic rather than eosinophilic inflammation31. We also did not exclude patients with concomitant asthma and chronic obstructive pulmonary disease, who may also respond differently to statins. Further studies are needed to explore the effects of statins on different asthma phenotypes.
Conclusion
Using a large population-based cohort of subjects with asthma, we found that statin use was associated with a decreased risk of asthma-related ED visits among ICS users. Despite a non-significant statistical interaction, our findings suggest a possible biological interaction between statins and ICS therapy, as the beneficial effects of statins were noted only among ICS users. Our study adds to the existing body of literature suggesting beneficial effects of statins on asthma exacerbations and larger clinical trials powered to examine asthma exacerbations are needed to confirm these effects.
Supplementary Material
Acknowledgments
Transparency
Declaration of funding
This work was funded by National Institutes of Health grants U01 HL65899 and R01 HL92197. This paper is subject to the NIH Public Access Policy (http://publicaccess.nih.gov).
Declaration of financial/other relationships
S.T.W. has served as an unpaid consultant for GlaxoSmithKline, Genetech, Novartis, Merck, Genome Network Sciences and Schering Plough. S.L.C. serves as a paid HE/OR advisor to Novo Nordisk, and has previously been employed by Medco Research Institute LLC, a wholly owned subsidiary of Express Scripts Holding Co. Inc. He is currently employed by Sanofi-Aventis. A.C.W. has received grant funding from the NIH. E.S. is an employee of Novartis Pharmaceuticals. S.M.T., V.H., S.G., A.A.L., and A.C.W. have disclosed that they have no significant relationships with or financial interests in any commercial companies related to this study or article.
CMRO peer reviewers may have received honoraria for their review work. The peer reviewers on this manuscript have disclosed that they have no relevant financial relationships.
Contributor Information
Sze Man Tse, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA; Massachusetts General Hospital, Boston, MA, USA.
Scott L. Charland, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of Colorado Anschutz Medical Campus, Aurora, CO, USA Outcomes Liaison, Evidence-Based Medicine, Sanofi-Aventis, Winter Park, CO, USA; Formally with Medco Research Institute, LLC, Bethesda, MD, USA (currently a wholly owned subsidiary of Express Scripts Holding Co. Inc.).
Eric Stanek, Formally with Medco Research Institute, LLC, Bethesda, MD, USA (currently a wholly owned subsidiary of Express Scripts Holding Co. Inc.).
Vivian Herrera, Formally with Medco Research Institute, LLC, Bethesda, MD, USA (currently a wholly owned subsidiary of Express Scripts Holding Co. Inc.).
Seth Goldfarb, Formally with Medco Research Institute, LLC, Bethesda, MD, USA (currently a wholly owned subsidiary of Express Scripts Holding Co. Inc.).
Augusto A. Litonjua, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA
Scott T. Weiss, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA.
Ann Chen Wu, Center for Child Health Care Studies, Department of Population Medicine, Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, MA, USA; Department of Pediatrics, Children’s Hospital, Boston, MA, USA.
References
- 1.Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) 2002. Available at: http://www.nhlbi.nih.gov/guidelines/cholesterol/index.htm [last accessed 8 March 2013]
- 2.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421 [PubMed] [Google Scholar]
- 3.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol 2004;44:720–32 [DOI] [PubMed] [Google Scholar]
- 4.Standards of medical care in diabetes – 2012. Diabetes Care 2012; 35(Suppl 1):S11–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol 2011;58:2432–46 [DOI] [PubMed] [Google Scholar]
- 6.Dinarello CA. Anti-inflammatory agents: present and future. Cell 2010;140:935–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hol J, Otterdal K, Breland UM, et al. Statins affect the presentation of endothelial chemokines by targeting to multivesicular bodies. PloS One 2012;7:e40673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwata A, Shirai R, Ishii H, et al. Inhibitory effect of statins on inflammatory cytokine production from human bronchial epithelial cells. Clin Exp Immunol 2012;168:234–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endres M Statins: potential new indications in inflammatory conditions. Atherosclerosis Suppl 2006;7:31–5 [DOI] [PubMed] [Google Scholar]
- 10.Zeki AA, Kenyon NJ, Goldkorn T. Statin drugs, metabolic pathways, and asthma: a therapeutic opportunity needing further research. Drug Metab Lett 2011;5:40–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief 2012:1–8 [PubMed] [Google Scholar]
- 12.Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004;59:469–78 [DOI] [PubMed] [Google Scholar]
- 13.Hothersall EJ, Chaudhuri R, McSharry C, et al. Effects of atorvastatin added to inhaled corticosteroids on lung function and sputum cell counts in atopic asthma. Thorax 2008;63:1070–5 [DOI] [PubMed] [Google Scholar]
- 14.Cowan DC, Cowan JO, Palmay R, et al. Simvastatin in the treatment of asthma: lack of steroid-sparing effect. Thorax 2010;65:891–6 [DOI] [PubMed] [Google Scholar]
- 15.Maneechotesuwan K, Ekjiratrakul W, Kasetsinsombat K, et al. Statins enhance the anti-inflammatory effects of inhaled corticosteroids in asthmatic patients through increased induction of indoleamine 2, 3-dioxygenase. J Allergy Clin Immunol 2010;126:754–62 e1 [DOI] [PubMed] [Google Scholar]
- 16.Braganza G, Chaudhuri R, McSharry C, et al. Effects of short-term treatment with atorvastatin in smokers with asthma – a randomized controlled trial. BMC Pulm Med 2011;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menzies D, Nair A, Meldrum KT, et al. Simvastatin does not exhibit therapeutic anti-inflammatory effects in asthma. J Allergy Clin Immunol 2007;119: 328–35 [DOI] [PubMed] [Google Scholar]
- 18.Zeki AA, Franzi L, Last J, et al. Simvastatin inhibits airway hyperreactivity: implications for the mevalonate pathway and beyond. Am J Respir Crit Care Med 2009;180:731–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imamura M, Okunishi K, Ohtsu H, et al. Pravastatin attenuates allergic airway inflammation by suppressing antigen sensitisation, interleukin 17 production and antigen presentation in the lung. Thorax 2009;64:44–9 [DOI] [PubMed] [Google Scholar]
- 20.Zeki AA, Bratt JM, Rabowsky M, et al. Simvastatin inhibits goblet cell hyperplasia and lung arginase in a mouse model of allergic asthma: a novel treatment for airway remodeling? Transl Res 2010;156:335–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiba Y, Arima J, Sakai H, et al. Lovastatin inhibits bronchial hyperresponsiveness by reducing RhoA signaling in rat allergic asthma. Am J Physiol Lung Cell Mol Physiol 2008;294:L705–13 [DOI] [PubMed] [Google Scholar]
- 22.Ahmad T, Mabalirajan U, Sharma A, et al. Simvastatin improves epithelial dysfunction and airway hyperresponsiveness: from asymmetric dimethylarginine to asthma. Am J Respir Cell Mol Biol 2011;44:531–9 [DOI] [PubMed] [Google Scholar]
- 23.Huang CC, Chan WL, Chen YC, et al. Statin use in patients with asthma: a nationwide population-based study. Eur J Clin Invest 2011;41:507–12 [DOI] [PubMed] [Google Scholar]
- 24.Lokhandwala T, West-Strum D, Banahan BF, et al. Do statins improve outcomes in patients with asthma on inhaled corticosteroid therapy? A retrospective cohort analysis. BMJ Open 2012;2:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonnet J, McPherson R, Tedgui A, et al. Comparative effects of 10-mg versus 80-mg atorvastatin on high-sensitivity C-reactive protein in patients with stable coronary artery disease: results of the CAP (Comparative Atorvastatin Pleiotropic effects) study. Clin Ther 2008;30:2298–313 [DOI] [PubMed] [Google Scholar]
- 26.Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006;295:1556–65 [DOI] [PubMed] [Google Scholar]
- 27.Szefler SJ, Chinchilli VM, Israel E, et al. Key observations from the NHLBI Asthma Clinical Research Network. Thorax 2012;67:450–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahlbom A, Alfredsson L. Interaction: a word with two meanings creates confusion. Eur J Epidemiol 2005;20:563–4 [DOI] [PubMed] [Google Scholar]
- 29.Jha A, Basu S, Ryu M, et al. Simvastatin significantly augments impact of fluticasone on allergic airway inflammation and hyperreactivity in mice. Am J Respir Crit Care Med 2013;187:A4010 [Google Scholar]
- 30.Zeki AA, Oldham J, Wilson M, et al. Statin use and asthma control in patients with severe asthma. BMJ Open 2013;3(8):1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Telenga ED, Tideman SW, Kerstjens HA, et al. Obesity in asthma: more neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy 2012;67:1060–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.