Abstract
Viral respiratory infections in CF patients are associated with an increase in morbidity at short and long term. Viral infections have a greater impact on CF patients compared to non-CF controls. They result in increased respiratory symptoms, deterioration of Shwachman and radiological scores, prolonged hospitalizations, a persistent decrease of pulmonary function, increased use of antibiotics and a higher frequency of exacerbations at follow-up. In addition, interaction between viruses and bacteria in CF is suggested. Some studies observe increased new bacterial colonization and raised antipseudomonal antibodies in episodes of viral respiratory infections. Experimental data suggest that increased virus replication, impaired specific anti-bacterial defense and increased adherence of bacteria play a role in the pathogenesis of viral respiratory infections in CF. Further knowledge about the role of viruses and interaction with bacteria in CF lung disease might result in new therapeutic strategies to improve prognosis of CF patients.
Keywords: CF, Virus, Clinical impact, Respiratory symptoms, Pulmonary exacerbation, Hospitalization, Pulmonary function testing, Antibiotics, Colonization, Pseudomonas aeruginosa, Virus replication, NOS-2 expression, Bacterial adherence
1. Introduction
CF is characterized by chronic inflammation, bacterial colonization and recurrent infections of the lung, resulting in irreversible deterioration of lung function and early death. Pseudomonas aeruginosa is one of the most important pathogens. Chronic colonization of the airways with P. aeruginosa is associated with progressive and irreversible lung disease. Besides a constitutive inflammatory state, CF typically has a course of exacerbations and remissions of pulmonary complaints, suggesting that external factors influence this course. Respiratory viruses are known to cause significant morbidity in infants, elderly and the immunocompromised. In asthma up to 80% of exacerbations in children are associated with viral infections [1].
In this review, we summarize current knowledge of epidemiology and clinical impact of viral infections in CF. Secondary, we describe experimental data concerning the pathogenetic role of respiratory viruses in CF pulmonary disease.
2. Clinical studies on viral infections in CF
2.1. Viral epidemiology in CF
Viral infections are often associated with worse respiratory symptoms in CF patients. In 13–52% of patients with an increase in respiratory lower tract symptoms a viral pathogen is detected [2], [3], [4], [5], [6], [7], [8], [9], [10], with higher percentages in younger than in older patients [7]. In 40% of cases with a pulmonary exacerbation, a virus was detected, compared to only 9% in those with a stable clinical condition [2].
Many different viruses are reported (Table 1 ). Frequencies are influenced by the detection method used by the individual investigator. Respiratory syncytial virus (RSV) represents 9–58% of all reported viruses, with the highest incidence in young children [2], [3]. Influenza A and B take 12–27%, or even up to 77% in one small study [5]. Parainfluenzavirus is found in lower frequencies, only one study showing a contribution of 43% [7]. Adenovirus is reported in 8–15% of all viruses. Most studies failed to detect rhinovirus, probably because the use of serology or culture. Serology is difficult due to the large number of rhinovirus subtypes and culture is relatively insensitive. Polymerase chain reaction (PCR) is up to eleven times more sensitive to detect rhinoviruses in children [11]. The only study using PCR demonstrated rhinoviruses in 58% of cases with respiratory complaints [10]. Other viral agents like enteroviruses, Epstein Barr virus and cytomegalovirus have been shown to play a role in CF respiratory symptoms in low frequencies. Coronavirus was reported only once with a frequency of 28% [8].
Table 1.
Distribution of respiratory viruses in case of an increase of respiratory symptoms in CF patients
| Author | Positive samples | Detection method | RSV (%) | Infl. virus AB (%) | Parainfl. virus (%) | Adenovirus (%) | Picornavirus (%) | Other |
|---|---|---|---|---|---|---|---|---|
| Hiatt PW ('99) | 26/150 | C,S | 21 | 26 | 15 | 15 | 23 | * |
| Armstrong D ('98) | 14/26 | I,C | 40 | 27 | 13 | * | 20 (13% RV) | * |
| Collinson J ('96) | 51/119 | P | * | * | * | * | 100 (41% RV) | * |
| Smyth AR ('95) | 44/157 | I,C,S,P | 9 | 12 | 12 | 9 | 58 RV | * |
| Pribble CG ('90) | 23/80 | C,S | 14 | 41 | 5 | * | 14 (9% RV) | 26% CV |
| Hordvik NL ('89) | 13/35 | C,S | 15 | 77 | 0 | * | 8 RV | * |
| Ong ELC ('89) | 11/92 | I,C,S,P | 0 | 27 | * | 9 | 55 (45% RV) | 9% CMV |
| Ramsey BW ('89) | 98/398 | C,S | 21 | 12 | 29 | 7 | 29 (26% RV) | 2% HSV |
| Abman SH ('88) | 12/30 | I,C | 58 | 0 | 18 | 8 | 8 EV | 8% CMV |
| Wang EL ('84) | 105 | C,S | 23 | 25 | 40 | 12 | * | * |
| Petersen NT ('81) | 63/332 | S | 19 | 24 | 43 | 14 | * | * |
I—immunofluorescence, C—culture, S—serology, P—PCR, *—Not mentioned, CMV—cytomegalovirus, EV—enterovirus, CV—coronavirus 229E, RV—rhinovirus, HSV—herpes simplex virus.
2.2. Clinical impact of viral infections
Viral respiratory infections occur in equal frequency in CF patients and healthy controls or sibs [4], [9], [12]. Also, the frequency of virus infections seems not to be associated with pre-existing clinical condition [12], although one study reported a possible association [4]. However, the clinical impact in CF patients is far beyond the virus-related morbidity in healthy controls [4], [9], [12].
In CF patients viral upper respiratory tract infections are associated with lower respiratory tract symptoms in 31%–76% [5], [13]. Respiratory viruses were detected in 40% of patients who needed hospitalization [2], [3]. CF patients were hospitalized for 10–22 days [2], [3], [12], [14], while none of a matched non-CF control group needed hospital care [4], [12].
Viral infections also cause increased long-term respiratory morbidity in CF patients. RSV infection resulted in a prolonged oxygen need (mean 2.9 months) in 5/7 hospitalized young CF children [2]. Several studies demonstrated a deterioration of Shwachman scores [10], [12], [13], [14] or radiological scores [2], [13], [14] at long-term follow up after viral respiratory infections. The severity of deterioration was related to the frequency of viral respiratory infections [12], [13] or hospitalizations [2]. Patients with virus associated lower respiratory tract infections had more frequently pulmonary exacerbations or hospitalizations [6], [12], [14] and a higher use of antibiotics [9], [10], [13] at long-term follow-up, compared to CF patients without virus associated lower respiratory tract infections.
2.3. Impact on pulmonary function
Viral respiratory infections in CF patients are associated with a decline in pulmonary function. Pulmonary function testing was performed in 9 of 12 clinical studies (Table 2 ).
Table 2.
Outcome data in clinical studies relating viral infections in CF patients
| Author | Pulmonary function testing | URTI | Shwachman score | Radiologic score | Hospitalization | Antibiotics |
|---|---|---|---|---|---|---|
| Hiatt PW | FRC, V'maxFRC | Yes | Yes | Brasfield | Yes | No |
| Armstrong D | No | No | No | No | Yes | Yes |
| Collinson J | FVC, FEV1 (> 6 yr) | Yes | Yes | Chrispin-Norman | Yes | Yes |
| Smyth AR | FVC, FEV1 | Yes | Yes | Chrispin-Norman | No | Yes |
| Winnie GB | FVC, FEV1 | No | Yes | Brasfield | Yes | No |
| Pribble CG | FVC, FEV1, FEF25, PEF | Yes | Yes | Brasfield | Yes | No |
| Hordvik NL | PEF (daily), FVC, FEV1, FEF25-75 | No | No | No | No | No |
| Ong ELC | No | Yes | No | No | No | No |
| Ramsey BW | FVC, FEV1, FEF25-75, TLC, RV, RV/TLC | No | Yes | No | No | Yes |
| Abman SH | No | Yes | No | Brasfield | Yes | No |
| Wang EL | FVC, FEV1, FEF25-75, TLC, RV, RV/TLC | Yes | Yes | Brasfield | Yes | Yes |
| Petersen NT | No | Yes | No | No | Yes | No |
URTI—upper respiratory tract infection.
*—Not mentioned.
FEV1 and FVC declined after virus associated lower respiratory tract infection in CF patients > 6 years [6], [10], [12], [13]. High frequency of viral upper respiratory tract infections was associated with a large decline of FEV1 and FVC [13]. Only one study showed no effect on FVC or RV/TLC [9].
A persistent decline of pulmonary function after viral infections was also found in CF patients < 6 years [4]. There was a decline in V'maxFRC and a rise in FRC, especially after RSV infection. V'maxFRC increased during follow-up in healthy controls and CF children with upper respiratory tract symptoms, but deteriorated in CF children with lower respiratory tract infections.
2.4. Conclusion clinical studies
Viruses are frequently isolated in CF patients with respiratory symptoms. The frequency and distribution of respiratory viruses are similar in CF patients and controls. RSV, influenza virus, parainfluenzavirus and adenovirus are the most frequently reported, but other viruses may be underreported for technical reasons. Viral respiratory infections in CF patients are associated with an increase of morbidity at short and long term. They result in increased respiratory symptoms, deterioration of Shwachman and radiological scores, prolonged hospitalizations, increased use of antibiotics and a higher frequency of exacerbations at follow-up. In contrast to healthy controls, viral infections often result in a persistent decrease of pulmonary function in CF patients.
3. Bacterial–viral interaction in CF
3.1. Clinical studies
The airways of most young children with CF are colonized with Haemophilus influenzae and Staphylococcus aureus. With increasing age pseudomonal colonization varies from 42% to 100% [4], [6], [8], [9], [10], [13], [14], with additional Burkholderia cepacia colonization in older patients [6], [8], [10]. In just a few patients no bacteria are detectable. Several data from clinical studies suggest interaction between viruses and bacteria in CF.
In 60–68% of cases new bacterial colonization is found during the viral season [15]. New bacterial colonization predominantly occurs within 3 weeks after a viral upper respiratory tract infection [13]. More specifically, 85% of new pseudomonal colonizations followed a viral upper respiratory tract infection within 3 weeks [13]. In 35% of patients who were hospitalized for a viral lower respiratory tract infection pseudomonal colonization was noticed within 12–60 months [3].
In 11–47% of patients with intermittent or chronic colonization a viral infection is followed by a rise in antipseudomonal antibodies [7]. The strongest association between a viral infection and rise in antipseudomonal antibodies was found after RSV infection. Twenty percent of all patients who developed chronic pseudomonal colonization had a previous RSV infection, compared to 7% of those who did not [7].
3.2. Experimental data on mechanisms
Experimental data on the effects of viral infections in CF are scarce.
Some studies suggest higher virus replication and impairment of the innate host defense in CF. Intrapulmonary influenza virus titres were significantly increased in mice with chronic P. aeruginosa infection, compared to control mice [16]. Increased virus replication was also found after parainfluenza virus infection of CF human airway epithelial cells, compared to controls [17]. Increased virus replication might explain the more severe and prolonged symptoms during viral infections in CF patients compared to healthy controls.
One of the possible causes of increased virus replication and of virus persistence might be a reduced production of respiratory nitric oxide (NO), which is an important part of the innate antiviral defense. Increased production of NO protects against viral infections [18]. In CF patients expression of the NO producing enzyme NO synthase type 2 (NOS2) is considerably decreased, while the IFN-γ-dependant antiviral host defense is intact.
An aberrant immune response to viral infections has also been found in other chronic respiratory illnesses, like asthma [19], [20]. It might be suggested that repeated viral infections lead, in the presence of an aberrant immune response, to an increased virus replication and hyperinflammation with increasing symptoms and pulmonary damage.
Viral infections might also facilitate infections with colonizing bacteria in patients with CF. Viral infections cause destruction of the epithelial barrier with loss of cilia and loss of tight junctions. This results in increased permeability and exposure of the respiratory basement membrane, leading to increased possibilities for bacteria to bind [21]. Bacterial adherence to virus infected cells is enhanced because bacteria can use viral glycoproteins and other virus induced receptors on the host cell membrane as bacterial receptors [22], [23], [24].
A few studies suggest that viral infection leads to modulation of the immune response, impairing specific anti-bacterial defense [25], [26], [27]. In a chronic P. aeruginosa infected mouse model fatal pneumonia was induced by pneumococcal infection following influenza virus infection. In the absence of the virus the mice survived the pneumococcal superinfection [16]. Influenza infection caused a significant increase in inflammatory cells and cytokine release, and suppressed neutrophil function.
Earlier data suggest interaction between specific viruses and bacteria in CF. Influenza virus seems to play a role in the adherence of P. aeruginosa to respiratory epithelial cells. Uninfected and influenza-infected murine tracheas were exposed to six different strains of P. aeruginosa. All of the strains adhered to desquamating cells of the infected trachea, but not to normal mucosa or regenerating epithelium [28].
In summary, experimental data suggest increased virus replication in CF which can result in severe pulmonary damage and which can facilitate infections with bacteria colonizing the CF lungs. More data, especially in the human model, are necessary to further elucidate the role of viruses in CF lung disease (Fig. 1 ).
Fig. 1.
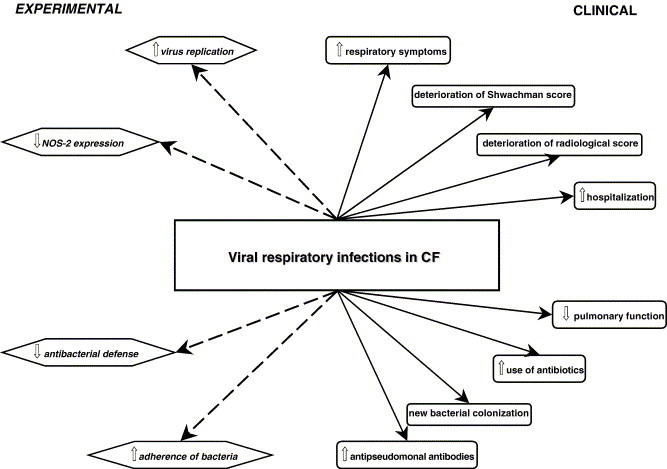
Overview of clinical and experimental data on viral respiratory infections in CF.
4. Implications
Further knowledge about the role of viruses in CF lung disease might result in new therapeutic strategies to improve prognosis of patients with CF.
Prevention of viral infections in patients with CF might be reached by active or passive immunization. At present, annual influenza vaccination is advised to all CF patients, although there are some doubts about its effectiveness [4], [9], [29], [30]. Passive immunization against RSV can be performed with paluvizumab, but data on effectiveness in CF patients are lacking. Active vaccination against RSV in CF patients is in development [31].
Another strategy might be inhibition of virus replication. Effective antiviral agents are available. Amantadine [32], zanamivir [33] and oseltamavir [34] can be used against influenza virus infection. Treatment with oseltamavir improved survival from 0% to 75% in a mouse model of secondary pneumococcal pneumonia after influenza virus infection, even when therapy was delayed for up to 5 days after influenza virus infection [35]. Ribavirine can possibly be used against RSV [36] and plecoranil against picornaviruses [37]. Beside these specific virus inhibitors, there is some indication that interferon [17] and statins [38] can interfere with virus replication.
Influencing the interaction between viruses and bacteria could be a next pathway to diminish respiratory morbidity in CF patients. Low-threshold use of antibiotics during viral infections in patients with CF might prevent secondary bacterial infections, but data are lacking. Further knowledge about specific interaction between viruses and bacteria might lead to development of new therapeutic options.
Up until now, there is no evidence for most suggested options in general, or more specifically in CF. Efforts should be made to design studies exploring these options.
References
- 1.Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Lampe F., Josephs L. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310(6989):1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abman S.H., Ogle J.W., Butler-Simon N., Rumack C.M., Accurso F.J. Role of respiratory syncytial virus in early hospitalizations for respiratory distress of young infants with cystic fibrosis. J Pediatr. 1988;113(5):826–830. doi: 10.1016/s0022-3476(88)80008-8. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong D., Grimwood K., Carlin J.B., Carzino R., Hull J., Olinsky A. Severe viral respiratory infections in infants with cystic fibrosis. Pediatr Pulmonol. 1998;26(6):371–379. doi: 10.1002/(sici)1099-0496(199812)26:6<371::aid-ppul1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Hiatt P.W., Grace S.C., Kozinetz C.A., Raboudi S.H., Treece D.G., Taber L.H. Effects of viral lower respiratory tract infection on lung function in infants with cystic fibrosis. Pediatrics. 1999;103(3):619–626. doi: 10.1542/peds.103.3.619. [DOI] [PubMed] [Google Scholar]
- 5.Hordvik N.L., Konig P., Hamory B., Cooperstock M., Kreutz C., Gayer D. Effects of acute viral respiratory tract infections in patients with cystic fibrosis. Pediatr Pulmonol. 1989;7(4):217–222. doi: 10.1002/ppul.1950070406. [DOI] [PubMed] [Google Scholar]
- 6.Ong E.L., Ellis M.E., Webb A.K., Neal K.R., Dodd M., Caul E.O. Infective respiratory exacerbations in young adults with cystic fibrosis: role of viruses and atypical microorganisms. Thorax. 1989;44(9):739–742. doi: 10.1136/thx.44.9.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen N.T., Hoiby N., Mordhorst C.H., Lind K., Flensborg E.W., Bruun B. Respiratory infections in cystic fibrosis patients caused by virus, chlamydia and mycoplasma—possible synergism with Pseudomonas aeruginosa. Acta Paediatr Scand. 1981;70(5):623–628. doi: 10.1111/j.1651-2227.1981.tb05757.x. [DOI] [PubMed] [Google Scholar]
- 8.Pribble C.G., Black P.G., Bosso J.A., Turner R.B. Clinical manifestations of exacerbations of cystic fibrosis associated with nonbacterial infections. J Pediatr. 1990;117(2 Pt. 1):200–204. doi: 10.1016/S0022-3476(05)80530-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsey B.W., Gore E.J., Smith A.L., Cooney M.K., Redding G.J., Foy H. The effect of respiratory viral infections on patients with cystic fibrosis. Am J Dis Child. 1989;143(6):662–668. doi: 10.1001/archpedi.1989.02150180040017. [DOI] [PubMed] [Google Scholar]
- 10.Smyth A.R., Smyth R.L., Tong C.Y., Hart C.A., Heaf D.P. Effect of respiratory virus infections including rhinovirus on clinical status in cystic fibrosis. Arch Dis Child. 1995;73(2):117–120. doi: 10.1136/adc.73.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jennings L.C., Anderson T.P., Werno A.M., Beynon K.A., Murdoch D.R. Viral etiology of acute respiratory tract infections in children presenting to hospital: role of polymerase chain reaction and demonstration of multiple infections. Pediatr Infect Dis J. 2004;23(11):1003–1007. doi: 10.1097/01.inf.0000143648.04673.6c. [DOI] [PubMed] [Google Scholar]
- 12.Wang E.E., Prober C.G., Manson B., Corey M., Levison H. Association of respiratory viral infections with pulmonary deterioration in patients with cystic fibrosis. N Engl J Med. 1984;311(26):1653–1658. doi: 10.1056/NEJM198412273112602. [DOI] [PubMed] [Google Scholar]
- 13.Collinson J., Nicholson K.G., Cancio E., Ashman J., Ireland D.C., Hammersley V. Effects of upper respiratory tract infections in patients with cystic fibrosis. Thorax. 1996;51(11):1115–1122. doi: 10.1136/thx.51.11.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winnie G.B., Cowan R.G. Association of Epstein–Barr virus infection and pulmonary exacerbations in patients with cystic fibrosis. Pediatr Infect Dis J. 1992;11(9):722–726. doi: 10.1097/00006454-199209000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Johansen H.K., Hoiby N. Seasonal onset of initial colonisation and chronic infection with Pseudomonas aeruginosa in patients with cystic fibrosis in Denmark. Thorax. 1992;47(2):109–111. doi: 10.1136/thx.47.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seki M., Higashiyama Y., Tomono K., Yanagihara K., Ohno H., Kaneko Y. Acute infection with influenza virus enhances susceptibility to fatal pneumonia following Streptococcus pneumoniae infection in mice with chronic pulmonary colonization with Pseudomonas aeruginosa. Clin Exp Immunol. 2004;137(1):35–40. doi: 10.1111/j.1365-2249.2004.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng S., De B.P., Choudhary S., Comhair S.A., Goggans T., Slee R. Impaired innate host defense causes susceptibility to respiratory virus infections in cystic fibrosis. Immunity. 2003;18(5):619–630. doi: 10.1016/s1074-7613(03)00114-6. [DOI] [PubMed] [Google Scholar]
- 18.Zheng S., Xu W., Bose S., Banerjee A.K., Haque S.J., Erzurum S.C. Impaired nitric oxide synthase-2 signaling pathway in cystic fibrosis airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2004;287(2):L374–L381. doi: 10.1152/ajplung.00039.2004. [DOI] [PubMed] [Google Scholar]
- 19.Corne J.M., Marshall C., Smith S., Schreiber J., Sanderson G., Holgate S.T. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359(9309):831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 20.Wark P.A., Johnston S.L., Bucchieri F., Powell R., Puddicombe S., Laza-Stanca V. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201(6):937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hament J.M., Kimpen J.L., Fleer A., Wolfs T.F. Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS Immunol Med Microbiol. 1999;26(3–4):189–195. doi: 10.1111/j.1574-695X.1999.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 22.Bartelt M.A., Duncan J.L. Adherence of group A streptococci to human epithelial cells. Infect Immun. 1978;20(1):200–208. doi: 10.1128/iai.20.1.200-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raza M.W., El Ahmer O.R., Ogilvie M.M., Blackwell C.C., Saadi A.T., Elton R.A. Infection with respiratory syncytial virus enhances expression of native receptors for non-pilate Neisseria meningitidis on HEp-2 cells. FEMS Immunol Med Microbiol. 1999;23(2):115–124. doi: 10.1111/j.1574-695X.1999.tb01230.x. [DOI] [PubMed] [Google Scholar]
- 24.Sanford B.A., Shelokov A., Ramsay M.A. Bacterial adherence to virus-infected cells: a cell culture model of bacterial superinfection. J Infect Dis. 1978;137(2):176–181. doi: 10.1093/infdis/137.2.176. [DOI] [PubMed] [Google Scholar]
- 25.Colamussi M.L., White M.R., Crouch E., Hartshorn K.L. Influenza A virus accelerates neutrophil apoptosis and markedly potentiates apoptotic effects of bacteria. Blood. 1999;93(7):2395–2403. [PubMed] [Google Scholar]
- 26.Nickerson C.L., Jakab G.J. Pulmonary antibacterial defenses during mild and severe influenza virus infection. Infect Immun. 1990;58(9):2809–2814. doi: 10.1128/iai.58.9.2809-2814.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abramson J.S., Mills E.L. Depression of neutrophil function induced by viruses and its role in secondary microbial infections. Rev Infect Dis. 1988;10(2):326–341. doi: 10.1093/clinids/10.2.326. [DOI] [PubMed] [Google Scholar]
- 28.Ramphal R., Small P.M., Shands J.W., Jr., Fischlschweiger W., Small P.A., Jr. Adherence of Pseudomonas aeruginosa to tracheal cells injured by influenza infection or by endotracheal intubation. Infect Immun. 1980;27(2):614–619. doi: 10.1128/iai.27.2.614-619.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Punch G., Syrmis M.W., Rose B.R., Harbour C., Bye P.T., Nissen M.D. Method for detection of respiratory viruses in the sputa of patients with cystic fibrosis. Eur J Clin Microbiol Infect Dis. 2005;24(1):54–57. doi: 10.1007/s10096-004-1273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan A., Bhalla P., Smyth R. Vaccines for preventing influenza in people with cystic fibrosis. Cochrane Database Syst Rev. 2000;2:CD001753. doi: 10.1002/14651858.CD001753. [DOI] [PubMed] [Google Scholar]
- 31.Piedra P.A., Cron S.G., Jewell A., Hamblett N., McBride R., Palacio M.A. Immunogenicity of a new purified fusion protein vaccine to respiratory syncytial virus: a multi-center trial in children with cystic fibrosis. Vaccine. 2003;21(19–20):2448–2460. doi: 10.1016/s0264-410x(03)00098-7. [DOI] [PubMed] [Google Scholar]
- 32.Dolin R., Reichman R.C., Madore H.P., Maynard R., Linton P.N., Webber-Jones J. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza A infection. N Engl J Med. 1982;307(10):580–584. doi: 10.1056/NEJM198209023071002. [DOI] [PubMed] [Google Scholar]
- 33.Hayden F.G., Gubareva L.V., Monto A.S., Klein T.C., Elliot M.J., Hammond J.M. Inhaled zanamivir for the prevention of influenza in families. Zanamivir Family Study Group. N Engl J Med. 2000;343(18):1282–1289. doi: 10.1056/NEJM200011023431801. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson K.G., Aoki F.Y., Osterhaus A.D., Trottier S., Carewicz O., Mercier C.H. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000;355(9218):1845–1850. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- 35.McCullers J.A. Effect of antiviral treatment on the outcome of secondary bacterial pneumonia after influenza. J Infect Dis. 2004;190(3):519–526. doi: 10.1086/421525. [DOI] [PubMed] [Google Scholar]
- 36.Hall C.B., McBride J.T., Walsh E.E., Bell D.M., Gala C.L., Hildreth S. Aerosolized ribavirin treatment of infants with respiratory syncytial viral infection. A randomized double-blind study. N Engl J Med. 1983;308(24):1443–1447. doi: 10.1056/NEJM198306163082403. [DOI] [PubMed] [Google Scholar]
- 37.Hayden F.G., Herrington D.T., Coats T.L., Kim K., Cooper E.C., Villano S.A. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin Infect Dis. 2003;36(12):1523–1532. doi: 10.1086/375069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kreiselmeier N.E., Kraynack N.C., Corey D.A., Kelley T.J. Statin-mediated correction of STAT1 signaling and inducible nitric oxide synthase expression in cystic fibrosis epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285(6):L1286–L1295. doi: 10.1152/ajplung.00127.2003. [DOI] [PubMed] [Google Scholar]


