Fig. 1.
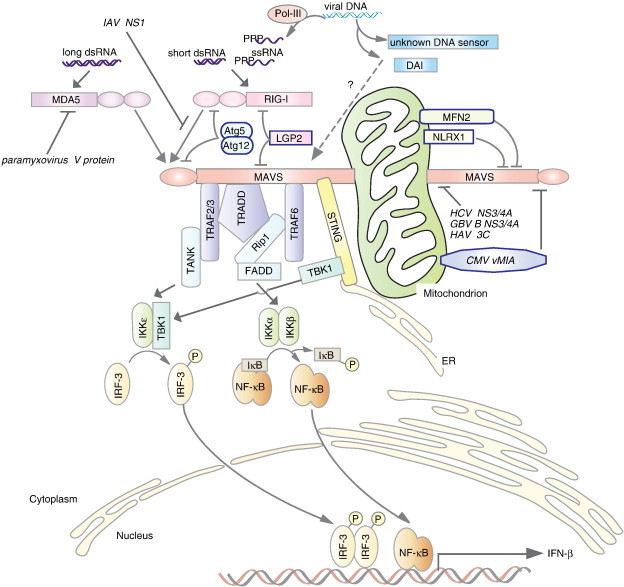
RLR-mediated viral recognition and pathways leading to IFN-β production. Cytosolic viral RNA and DNA are recognized by RIG-I, MDA5, DAI, or other unknown DNA sensors. Cytosolic double-strand DNA is recognized by cytosolic DNA receptors such as DAI, or, in another pathway, cytosolic DNA-dependent RNA polymerase III (Pol-III) synthesizes 5′-triphosphate single-stranded RNA, which is recognized by RIG-I. RIG-I and MDA5 recognize long or short viral double-stranded RNA (dsRNA) or 5′-triphosphate single-stranded RNA (ssRNA) via their C-terminal RNA helicase domain. The two N-terminal caspase recruitment domains (CARDs) then interact with an N-terminal CARD from mitochondrial antiviral signaling protein (MAVS; also referred to as IPS-1, Cardif, and VISA). Anchored to mitochondria via a C-terminal transmembrane domain, MAVS receives signals from RLRs via a CARD and transduces the signal to downstream proteins via other regions including a proline-rich region that interacts with tumor necrosis factor receptor-associated factor 2 (TRAF2) and TRAF3 and a central region that interacts with tumor necrosis factor receptor associated-death domain (TRADD) and TRAF6. TRAF2 and TRAF3 interact with TRAF family member-associated NF-κB activator (TANK). TANK recruits a kinase complex composed of TANK-binding kinase 1 (TBK1) and IKKε, which phosphorylates IRF3 and activation of IFN-β transcription. TRADD interacts with TRAF3 and Rip1, whereas TRAF6 interacts with Rip1. Rip1 mediates signals between Fas-associated protein with death domain (FADD) and IKKα and IKKβ, leading to activation of the transcription factor NF-κB. Negative regulators of MAVS-mediated signals are also shown. Interactions between laboratory of genetics and physiology 2 (LGP2) and MAVS or RIG-I antagonize RIG-I/MAVS-mediated signaling. NLRX1 localizes to mitochondria and interacts with MAVS, leading to inhibition of signaling. Mitofusin 2 (MFN2), a mitochondrial tethering GTPase, interacts with MAVS via the heptad region of MFN2, which interferes with interactions between MAVS and various downstream molecules. The autophagic regulator protein conjugate Atg5/Atg12 also inhibits MAVS signaling. Several viral proteins inhibit MAVS-mediated signals. Nonstructural protein 1 (NS1) from influenza A virus, for instance, may interfere with the interaction between RIG-I and MAVS. MAVS is cleaved by viral proteases, including NS3/4A from hepatitis C virus and GB virus B, and 3C protein from hepatitis A virus. Cleaved MAVS does not transmit signals downstream or localize to mitochondrial membranes.
