Abstract
We describe an outbreak of rabies in a pack of African wild dogs (Lycaon pictus) in the Limpopo-Lipadi Private Game and Wilderness Reserve in the Tuli region of south-eastern Botswana. We define the pack’s behavioural response to the disease, clinical signs, and management interventions undertaken and make recommendations to mitigate against future disease outbreaks of this nature. The outbreak, which occurred in late 2014 and early 2015, resulted in the death or disappearance of 29 individuals out of a pack of 35 wild dogs. The disruption to the social structure within the pack, the behaviour of the animals and clinical signs were similar to that documented during previous rabies outbreaks amongst African wild dogs in Southern and East Africa in recent years. Management interventions taken during the outbreak were aimed at preventing extirpation of the pack and reducing the risk of further disease spread to other mammals in the reserve.
Keywords: Rabies, Outbreak, Clinical signs, Behaviour, Intervention, Mitigation
1. Introduction
The endangered African wild dog (Lycaon pictus) has disappeared from much of its historical range. Their population is estimated at approximately 6600 adults in 39 subpopulations, of which only 1400 are mature individuals with the largest subpopulations remaining in southern Africa and East Africa (Woodroffe & Sillero-Zubiri, 2012). Infectious diseases are a leading cause of mortality amongst wild dogs and population decline and local extinctions have been linked to the viral rabies pathogen (Gascoyne, Laurenson, Lelo, & Borner, 1993; Hofmeyr, Bingham, Lane, Ide, & Nel, 2000; Davies-Mostert et al., 2016). Rabies related deaths and local extinctions have been reported in the Maasai Mara National Reserve, Kenya (Kat, Alexander, Smith, Richardson, & Munson, 1996; Woodroffe, Ginsberg, Macdonald, & the IUCN/SSC Canid Specialist Group, 1997), Serengeti National Park, Tanzania, (Burrows, Hofer, & East, 1994; Gascoyne et al., 1993), Etosha National Park, Namibia (Scheepers & Venske, 1995), Madikwe Game Reserve, South Africa (Davies-Mostert et al., 2016; Hofmeyr, Hofmeyr, Nel, & Bingham, 2004) and the western boundary of Kruger National Park, South Africa (Davies-Mostert et al., 2016). Domestic dog populations serve as reservoirs of rabies infection (Alexander & Appel, 1994; Cleaveland, Hess, Dobson, Laurenson, & McCallum, 2002; Prager, Mazet, Dubovi et al., 2012) and it is known that domestic dogs transmit canine pathogens to African wild dogs (Woodroffe, Prager, Munson, Conrad, & Dubovi, 2012) with a clear link between domestic dog density and pathogen exposure (Woodroffe et al., 2012).
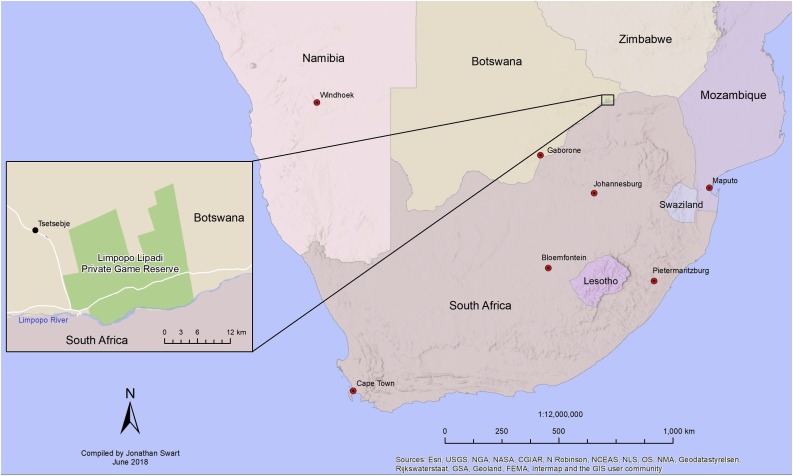
Limpopo-Lipadi Private Game and Wilderness Reserve (22°34´59.24´´S, 28°30´32.61´´E, 710 m ASL) is a privately owned 20 700 ha fenced game reserve located in the Tuli region of south-eastern Botswana (Fig. 1 ). The reserve was previously used for agricultural purposes and adjacent land consists of agricultural and community land. The largest human settlement is Tsetsebjwe, approximately 25 km from the reserve boundary. Domestic and feral dogs occur in surrounding communities and are occasionally found on the reserve either by themselves or accompanying human poachers and it is likely that this outbreak was as a result of contact with domestic dogs.
Fig. 1.
Location of Limpopo-Lipadi Private Game and Wilderness Reserve, Tuli, Botswana.
In August 2008 a litter of nine, wild-caught African wild dog pups (four females and five males) was introduced to a holding facility on the reserve. Serum samples collected from the litter shortly prior to their translocation to the reserve were sent to the Department of Veterinary Tropical Diseases, Faculty of Veterinary Science at the University of Pretoria, South Africa for serological testing using an indirect fluorescent antibody test for immunoglobulin G (IgG) against Canine Distemper virus, Canine Parvovirus, Canine Coronavirus, Canine Adenovirus and Canine Parainfluenza virus. Rabies serology was not performed as expert opinion concluded a very low likelihood of rabies virus exposure at such a young age. Test results revealed that the litter was immunologically naïve to all pathogens tested. Between 2008 and 2010 the dogs were subject to routine veterinary interventions that included chemical immobilisation with Zoletil© (Virbac) and prophylactic vaccinations against the major canine viral pathogens of Canine Distemper virus, Canine Parvovirus, Canine Adenovirus, Canine Parainfluenza virus and Rabies virus using Recombitec RDACPP© (Merial) and Rabisin© (Merial). All animals were routinely treated with anthelmintic and antibiotic treatments during immobilisation as part of their veterinary management plan.
In March 2010 the five males were exchanged for two wild-caught, adult males from Venetia Limpopo Nature Reserve in neighbouring Limpopo Province, South Africa. The introduced adult males were bonded with the resident females in the holding facility and in May 2010 the pack was released onto the reserve. In late 2010 five free-ranging male wild dogs entered the reserve, leading to conflict with the resident dogs and a disruption to the pack structure. The resident dogs, with the exception of the alpha female (which bonded with the five newly arrived males), were not seen again and it is assumed they were either killed or departed from the reserve. Between 2011 and 2014 the pack denned and produced pups annually. In June 2013 the alpha male and a female from the 2012 litter were immobilized and VHF collars were placed on them for monitoring purposes, however no vaccinations were administered. By December 2014 the pack consisted of 35 individuals including the alpha pair and a beta female.
2. Field observations and management intervention
On 26 November 2014 an emaciated pup from the 2014 litter was observed displaying signs of illness and on 27 November the pup was found dead. On inspection of the carcass, bite marks were observed around the facial area, particularly on the cheeks and lips and there were two puncture wounds on the top of the head. The carcass was collected by the Department of Veterinary Services for necropsy and samples of brain tissue were sent to the National Veterinary Laboratory of the Ministry of Agriculture in Gaborone, Botswana. On 11 December a partial test report was obtained with the results that a direct fluorescent antibody test (FAT) performed on a sample of brain tissue preserved in 50% glycerol indicated a positive result for the rabies antigen, which provided a definitive diagnosis of infection. No further information was provided subsequent to this initial laboratory report. Further brain samples were collected from two other wild dog carcasses, the second sample was collected on 5 December 2014 from a two and a half year old male and the third sample was collected from a six month old female on 6 December 2014, although this carcass was in an advanced state of decomposition and autolysis of tissues would likely have diminished the diagnostic quality of the samples. Requests to the Department of Veterinary Services for additional information, including the testing of additional samples collected were denied. Requests to transport samples for further testing (for genetic typing of the outbreak virus using PCR amplification and gene sequencing) to the OIE Rabies reference laboratory at the Onderstepoort Veterinary Institute in neighbouring South Africa were also denied. Between 27 November and 4 January 2015 (39 days) ten more carcasses were found and 19 animals disappeared and were presumed dead (Appendix A). During the outbreak no carcasses of any other carnivores were discovered; this does not imply that other species were not affected, but rather that any other carcasses were possibly scavenged or simply not found.
Daily monitoring between 7 December 2014 and 8 January 2015 using radio telemetry indicated a disruption to the composition of the pack, resulting in a change in social dynamics manifesting as increased aggression between the members of the pack. The alpha pair as well as the beta female bore the brunt of sustained aggressive behaviour with all three individuals being repeatedly and violently attacked by pack members. Although the beta female was not mauled as severely as the alpha pair, all three individuals had, at times, severe lacerations and bite marks to the head, face and neck area resulting in severe swelling and secondary infection (Figs. 2 and 3 ). Clinically ill wild dogs were attacked and mauled by other members of the pack not displaying signs of the disease. Younger individuals and pups that displayed signs of illness were particularly aggressively attacked by individuals of the same age; older individuals were not seen to attack younger wild dogs. Once attacked, individuals would retaliate; submissive behaviour on the part of the attacked individual, pre-empted further attacks by the cohort and licking of the afflicted dog in the facial area followed. On numerous occasions, merely standing up and moving elicited an attack from other cohort members.
Figs. 2 and 3.
Alpha male displaying lacerations and swelling around head region.
Small groups of wild dogs of mixed sex and age withdrew from the pack for a few hours or days before returning. A group of four females of the same age split from the pack for two days before returning and then dispersed again at a later date and did not return (Appendix A). From 21 December 2014 hunting success declined and unsuccessful hunts were common. Alpha females often lead hunts (McNutt; Rasmussen cited in Courchamp & Macdonald, 2001); however the vicious attacks on the alpha female prevented her from doing so. Intraspecific aggression was routine on kills and clinically ill dogs were prevented from feeding by healthy dogs. During this period the pack moved an average of 3.6 km per day, whereas prior to the outbreak they moved an average of 10.3 km per day. On numerous occasions the pack remained in the same location for between two and three days and on at least two occasions the pack remained with ill pups for two days until the pups died. On 23 December a pup died and individuals of the same age remained with the carcass, even after the rest of the pack had moved off. The pups moved off after the carcass was removed for disposal.
Moderating disease threats is challenging, partly due to uncertainty about disease dynamics, making it difficult to identify the best management approaches (Woodroffe et al., 2012). Hofmeyr et al. (2004) propose that post-exposure prophylaxis may reduce virus shedding and the potential for the spread of infection to healthy pack members. For this reason a decision was made by reserve management that the wild dogs would be vaccinated as an emergency measure once a diagnosis of disease had been laboratory confirmed. Vaccination of the wild dogs commenced on 15 December with the intramuscular administration of one ml Rabisin© (Merial) per dog via a remote darting system. A total of 21 wild dogs (out of 30 still alive) were darted over three days. The alpha male was vaccinated, along with the beta female and the majority of juveniles; however the alpha female was not vaccinated due to her remaining wary of vehicles and out of range of the remote darting system. On 26 December a management intervention decision was taken to feed the wild dogs in an effort to reduce stress and limit intraspecific conflict which was promulgating disease spread within the pack. If tracking the pack in the morning provided evidence of unsuccessful hunts, an impala (Aepyceros melampus) was shot and provided for the pack. This was done in the afternoon as well, until the pack size declined to a size where one impala a day was sufficient to feed them. For the first two days after initiating feeding, the carcass was fed on in a manner uncharacteristic of wild dog feeding behaviour. After two days of feeding they started feeding in a manner more characteristic of their normal feeding behaviour with no aggressive interactions whilst feeding. Wild dogs previously prevented from feeding on the carcass were allowed to feed. Feeding continued until 26 January, well after the end of the outbreak and after cohesion and the dominance hierarchy had been restored. From 27 January the dominant wild dogs were in a much improved condition and the recovery of wounds progressed until fully healed. From this date the wild dogs started moving longer distances, consistent with the distances moved prior to the disease outbreak.
After permission to test additional samples following the initial diagnosis was denied by the authorities, all carcasses discovered were retrieved and disposed of through burning by reserve employees wearing personal protective equipment.
3. Clinical signs
An acute onset of illness generally occurred within 24 h; all individuals displayed similar clinical signs, although of variable severity. No furious signs were observed and affected animals lost condition rapidly with death following within two to three days of the first visible signs. Adult wild dogs had swollen heads and necks, likely as a result of inflammation and secondary infection due to bite wounds sustained through fighting with other pack members. Other clinical signs included restlessness, apparent hydrophobia, discoordination, stiff gait, listlessness, diarrhoea and progressive ataxia. Excessive licking of ill animals by healthy companions was observed, particularly in the facial area and mouth. Adult and sub-adult wild dogs tended to die away from the pack, whilst pups died close to the pack. Certain pups vocalised (howled) in the terminal stages of the disease and signs on the ground around carcasses indicated agonal convulsions or thrashing of the limbs. Restlessness, a hunched posture and hindquarter weakness were further signs observed in all clinically ill individuals.
4. Discussion
All sex and age classes succumbed to the disease and determining a temporal pattern to the mortalities was not possible given that the numbers of animals observed on a day-to-day basis during the course of the outbreak was somewhat variable; a number of animals simply disappeared, presumed dead but with the exact date of onset of illness and death unknown. Six individuals survived to remain in the pack at the conclusion of the outbreak by mid-January 2015. The wild dogs that survived comprised of the alpha female, the alpha male, the beta female, two sub-adult females and one female pup. The alpha female was the only surviving individual that was not vaccinated during the outbreak, although she had previously been vaccinated four times against rabies (in 2008, twice in 2009 and in 2010) and was the only individual that had previously been vaccinated. At the time of the outbreak the population consisted of 35 individuals comprising six mature animals and four annual litters. African wild dogs generally live in packs of up to 20 adults and their dependent offspring (Childes, 1988; Creel & Creel, 1995; Fuller et al., 1992; Maddock & Mills, 1994). The density of wild dogs on the reserve was 0.175 / km², whilst mean wild dog densities in similar habitats have been found by Lindsey, du Toit, and Mills, (2004) to be approximately 0.019 / km². This unnaturally high density of wild dogs may be attributed to a number of factors. Lions (Panthera leo), being a significant cause of wild dog mortality (Creel & Creel, 1996; Mills & Gorman, 1997; Woodroffe, Davies-Mostert, Ginsberg, Graf, & Leigh, 2007; Woodroffe, McNutt, & Mills, 2004) were until 2015, absent from the reserve, and the lack of interspecific competition allowed the wild dog population to increase significantly. Spotted hyaenas (Crocuta crocuta) also pose a substantial threat by stealing wild dog kills (Creel & Creel, 1996; Fanshawe & FitzGibbon, 1993; Mills & Gorman, 1997) but the low densities and small clan sizes of spotted hyaenas on the reserve precluded this threat. Survival rate of pups to adulthood was 100% prior to the rabies outbreak, whereas Creel, Mills, and McNutt, (2004) determined that pup survival rate to adulthood varied between 16% in Kruger National Park, South Africa, 35% in the north-eastern corner of the Okavango Delta in Botswana and 63% in Selous Game Reserve, southern Tanzania. Fences around the reserve limited the natural dispersal of sexually mature wild dogs that would normally occur under free range conditions. Collectively, these factors contributed to the growth of the population of the wild dogs to reach an abnormal level before the outbreak of rabies significantly reduced the population. Prior to the incident, reserve management had on several occasions cautioned that the pack had reached an unnatural size and structure, creating various risks including disease outbreaks, inbreeding and depletion of suitable prey; however, executive management policy conflicts prevented implementation of risk mitigation measures including relocation. Prior to the occurrence of the outbreak in late 2014, no wild dogs on the reserve had received vaccinations since 2010; therefore all animals from litters born from 2011 to 2014 were unvaccinated and fully susceptible to rabies and other diseases. High contact rates which occur within social groups elevate the prevalence of directly transmitted infections and exposure might be especially high in large wild dog packs after pathogen introduction (Woodroffe et al., 2012). The large number of fully susceptible animals, combined with the close-knit and highly social nature of African wild dogs, which manifests as frequent close contact, resulted in a high effective contact rate which ensured that the disease was able to rapidly spread through the population. Dispersal events, albeit temporary in some cases, were a common occurrence, although four females that dispersed were at the age when wild dogs would normally disperse (Burrows, 1995; Fuller et al., 1992). Dispersal events of mixed age and sex groups occurred and the unnatural composition of the pack may have played a role in this, although there is no empirical evidence to support this supposition. Both Hofmeyr et al. (2004) and Kat et al. (1996) observed similar behaviour in rabies outbreaks in Madikwe Game Reserve, South Africa and Maasai Mara National Reserve, Kenya. Aggression towards the alpha pair and the resulting social discord and stress likely contributed to the pack’s diminished social cohesion and significantly decreased hunting success.
Clinical signs observed in the population resemble signs observed in other wild dog populations afflicted by rabies (Hofmeyr et al., 2004; Kat et al., 1996). During previous outbreaks of rabies amongst African wild dogs, survival of some unvaccinated pack members has been documented, albeit in low numbers (Kat et al., 1996). Scheepers and Venske (1995) and Hofmeyr et al. (2000) have also found that vaccination has not protected all animals and vaccinated wild dogs have succumbed to the disease. It has been reported that in captive wild dogs neutralising antibody titres decreased to insignificant levels after primary vaccination and that high and prolonged titres were maintained only after first boosters (Van Heerden, Bingham, van Vuuren, Burroughs, & Stylianides, 2002), implying that boosters are an essential component of post-exposure prophylaxis. It is therefore difficult to make any conclusive statement that the emergency vaccination of individuals in the Limpopo-Lipadi pack was in any way effective in protecting the survivors against rabies infection. It is highly likely that most of the pack members had already been exposed to the virus; however, the fact that the six surviving individuals, other than the alpha female, had all been vaccinated during the outbreak suggests that unvaccinated wild dogs may have an improved likelihood of survival if they are vaccinated as an emergency intervention during a rabies outbreak.
The inability to submit samples to a laboratory facility which could have provided additional virological information is unfortunate. Due to the lack of genetic sequencing and virus typing which may have yielded additional epidemiological data through phylogenetic studies, it is impossible to confirm the virus biotype, and thus the maintenance host species that was the likely source of the disease. Circumstantial evidence indicates that the disease was likely caused through contact with domestic dogs. Information was received from a source in the local community in mid-January 2015 that an attempted poaching incident had occurred on the reserve in mid-November 2014 and that domestic dogs used to hunt had contact with the resident wild dogs. Further anecdotal information from the source, although unconfirmed and subject to reporting bias, indicates that these domestic dogs died towards the end of November 2014. Unfortunately no further information was forthcoming from the source due to a reluctance to speak to reserve management or local authorities. Considering that the outbreak started with one individual and not with numerous wild dogs displaying signs simultaneously, it is possible that exposure of one or more animals in the pack occurred through a bite from a rabid vector. Pathogens associated with domestic dog contact have all been linked to wild dog mortality, leading to the potential to undermine the viability of wild dog populations (Woodroffe et al., 2012). Rabies is considered endemic to southern Africa; the disease does not always maintain itself indefinitely in local populations of known maintenance host species (including domestic dogs, jackal, and occasionally mongoose species) in a given area, but may rather occur as intermittent epidemics when infected individuals enter the local subpopulation through migration from infected local subpopulations in other areas. Through this mechanism the long-term persistence of the pathogen in the canid meta-population (domestic and wild) in the wider sub-region occurs. African wild dogs are generally considered spill-over hosts and do not appear to support extended virus cycles independent of other maintenance host species (Bingham, 2005).
In African wild dog ecology, pack numbers can fluctuate dramatically due to stochastic mortality events, pack fission or dispersal events; therefore long-term survival of the pack as a core unit is considered a more objective measure of long-term conservation success than the survival of individual animals at any given point in time (Whittington-Jones, 2015). According to Courchamp and Macdonald (2001) the break point between success and failure of pack survival hovers around a pack size of five adults, implying that the persistence of the Limpopo-Lipadi pack was potentially very close to the survival threshold. The survival of the alpha pair was highly fortuitous in that it maintained a dominant reproductive unit and the birth of a litter of 12 pups in June 2015 following the severe depletion of pack numbers may help to ensure the longer term persistence of the Limpopo-Lipadi pack, provided that lessons learnt from the outbreak serve to trigger continuous and regular veterinary management.
5. Conclusion
A disease outbreak of this nature and the ensuing high mortality rate resulting in near extirpation of the pack highlights the necessity for rigorous and continuous veterinary and ecological management of geographically isolated subpopulations of African wild dogs in fenced reserves, particularly if nearby or adjacent to human habitation. The survival of one of Africa’s most endangered carnivores would benefit from correct management protocols being implemented by both reserve management and government departments responsible for their conservation, management and welfare. Accurate records of veterinary or management interventions (including preventative vaccination programme schedules) need to be maintained and all immobilisation of endangered African wild dogs should be used as an opportunity to implement preventative healthcare, particularly preventative vaccination against common viral pathogens of sympatric or nearby domestic dog populations. The parenteral vaccination of all new litters should occur and should include at least one booster at a young age, preferably administered intramuscularly (Hofmeyr et al., 2004) and an oral bait vaccine for younger pups should be considered (Knobel, du Toit, & Bingham, 2002).
Serious impacts on susceptible populations frequently occur when generalist pathogens are maintained within populations of abundant, and often domestic, reservoir hosts and spill over into less abundant host species (Cleaveland et al., 2002). It is therefore imperative that domestic dogs are prevented as far as possible from entering conservation areas that provide a refuge for wild and endangered canids; however it is not always possible to prevent incursions by domestic dogs into such areas. Evidence suggests that rabies risks to people and wild carnivores might be controlled by targeted vaccination of the reservoir host (Prager, Mazet, Dubovi et al., 2012, Prager, Mazet, Munson et al., 2012).
Hampson et al. (2009) showed that 70% annual vaccination coverage is recommended as the target to ensure long term elimination of rabies in almost all demographic settings, including those with high population turnover; however data did show successful control of the disease at low levels of coverage (30–50%) for short term control, but turnover of domestic dogs in rural areas is high and therefore where vaccinations are carried out in pulses this turnover reduces herd immunity. Regular preventative vaccination programmes of domestic animal populations should be considered in communities surrounding conservation areas that are vulnerable to edge effects and anthropogenic pressures. This is the most sustainable, practical and cost effective long-term approach to protect wild dogs from rabies infection at the source (Flacke et al., 2013; Taylor & Nel, 2015). Proactive preventative vaccination programmes are aligned with the One Health concept; the outcome being to protect both domestic and wild animal populations, thereby improving the health of endangered wildlife, as well as the health, welfare and economic resilience of communities surrounding protected areas. Fenced, managed reserves that host endangered species have a responsibility to ensure the welfare of these endangered and threatened species. The management of these species should include a disease management plan; which should incorporate records of all veterinary interventions and a timetable of planned interventions. The plan needs to be maintained, reviewed and revised on an ongoing basis.
Being a highly mobile species, African wild dogs do not recognise international borders and dispersal events occasionally result in the long-distance movement of transient animals between suitable habitats in various countries, particularly where free-ranging subpopulations occur in border regions. This could potentially lead to the spread of disease to adjacent geographical locations. Given the strategic locality of the Tuli region where three range states for African wild dogs converge (the Republics of Botswana, Zimbabwe and South Africa) it may be argued there is a clear imperative for proactive management policies at public and private stakeholder level in all countries in the region. This would enable individual fenced reserves that provide a refuge for geographically isolated subpopulations of African wild dogs to participate in the larger meta-population management programme currently operational in South Africa (Gusset et al., 2008; Gusset, Slotow, & Somers, 2006). Such a proactive management approach may facilitate the periodic cross-border translocation of animals between subpopulations and may create opportunities for the re-introduction of packs into suitable conservation areas.
Appendix A.
Timeline of events from initial discovery of clinically ill wild dog through veterinary testing of carcass, deaths due to rabies and mitigation interventions to survival of core group of six wild dogs in Limpopo-Lipadi Private Game & Wilderness Reserve in Tuli region, Botswana. Observed individuals varied due to their splitting from pack and later returning until their disappearance and presumed deaths.
| Date | Event | Surviving / observed individuals |
|---|---|---|
| 26 November | Juvenile displaying signs of illness | 35 |
| 27 November | Juvenile discovered dead, collected for veterinary testing | 34 |
| 5 December | Dead sub-adult discovered, brain sample collected | 33 |
| 6 December | Juvenile female found dead, necropsy conducted, samples collected | 32 |
| 7 December | 22 individuals seen together, no sign of rest of pack | 22 |
| 9 December | 22 individuals seen together, no sign of rest of pack | 22 |
| 11 December | Results obtained from State Veterinarian - FAT test positive for rabies | 18 |
| 12 December | Juvenile found dead | 30 |
| 15 December | Inoculation commenced, seven juveniles darted | 30 |
| 16 December | Inoculation ongoing, 12 individuals darted, all age groups, including alpha male and beta female | 20 |
| 17 December | Two adult males inoculated | 20 |
| 18 December | Juvenile aggressively attacked by juvenile cohort in the morning, found dead in the afternoon | 16 |
| 21 December | Juvenile individual displaying signs of illness | 16 |
| 22 December | Juvenile individual displaying signs of illness aggressively attacked by juvenile cohort | 16 |
| 23 December | Juvenile displaying signs of illness on 21 and 22 December found dead. Four sub-adult females split from pack | 15 |
| 26 December | Decision made to feed pack, pack split | 15 |
| 27 December | Pack regrouped; adult, male impala fed to pack | 15 |
| 28 December | Adult, male impala fed to pack | 15 |
| 29 December | Adult, male impala fed to pack | 15 |
| 30 December | Sub-adult female found dead | 14 |
| 31 December | Sub-adult male found dead; adult, male impala fed to pack | 15 |
| 1 January 2015 | Four sub-adult females split from pack, one additional individual returned to pack, juvenile found dead; adult, male impala fed to pack | 15 |
| 2 January | 11 individuals seen together | 11 |
| 3 January | Four sub-adult females observed together, group of 11 observed, one juvenile displaying signs of illness; adult, male impala fed to each group | 15 |
| 4 January | Juvenile found dead | 10 |
| 5 January | Juvenile observed displaying signs of illness; adult, male impala fed to pack | 8 |
| 6 January | Two adult males observed together, one adult female observed alone displaying signs of illness with injuries (bite marks) to facial area (from group of four sub-adults that split on 1 January) | 3 |
| 8 January | Alpha male observed with injuries (bite marks) to facial area; adult, male impala fed to pack | 6 |
| 9 January | Adult, male impala fed to pack | 6 |
| 10 January | Adult, male impala fed to pack | 6 |
| 12 January | Alpha female and beta female not observed with pack | 4 |
| 14 January | Two separate groups observed - three sub-adult females that split from pack on 1 January together, alpha female, beta female (both with fresh facial injuries) and sub-adult female seen together, adult, male impala fed to second group | 6 |
| 15 January | Two groups observed as per 14 January; adult, male impala fed to both groups | 6 |
| 17 January | Five individuals observed together, group of sub-adult females not seen; adult, male impala fed to pack | 5 |
| 18 January | Two groups observed, three sub-adult females appear to have fed. Second group with five individuals, alpha male with fresh facial injuries; adult, male impala fed to second group | 8 |
| 19 January | Alpha male observed alone, calling; no sig n of other dogs | 1 |
| 20 January | Six individuals observed together (alpha male, alpha female, beta female, three sub-adult females); adult, male impala fed to pack | 6 |
| 21 January | Group of six attempted hunt, unsuccessful; adult, male impala fed to pack. Unconfirmed report of three sub-adult females; not seen again | 6 |
| 22 January | Group of six observed, alpha male, alpha female, beta female injuries healing | 6 |
| 23 January | Group of six observed, all appear in good condition with all injuries healing; adult, male impala fed to pack | 6 |
| 25 January | Group of six observed, in good condition; adult, male impala fed to pack | 6 |
| 26 January | Group of six observed, condition of all individuals greatly improved; adult, male impala fed to pack | 6 |
| 27 - 31 January | Pack seen every day, moving longer distances, successfully hunting | 6 |
References
- Alexander K.A., Appel M.J. African wild dogs (Lycaon pictus) endangered by a canine distemper epizootic among domestic dogs near the Masai Mara National Reserve, Kenya. Journal of Wildlife Diseases. 1994;30(4):481–485. doi: 10.7589/0090-3558-30.4.481. [DOI] [PubMed] [Google Scholar]
- Bingham J. Canine rabies ecology in southern Africa. Emerging Infectious Diseases. 2005;11.9:1337–1342. doi: 10.3201/eid1109.050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows R. Demographic changes and social consequences in wild dogs, 1964–1992. In: Sinclair A.R.E., Arcese P., editors. Serengeti II: Dynamics, management and conservation of an ecosystem. University of Chicago Press; Chicago, Illinois: 1995. pp. 400–420. [Google Scholar]
- Burrows R., Hofer H., East M.L. Demography, extinction and intervention in a small population: The case of the Serengeti wild dogs. Proceedings of the Royal Society of London. 1994;256:281–292. doi: 10.1098/rspb.1994.0082. [DOI] [PubMed] [Google Scholar]
- Childes S.L. The past history, present status and distribution of the hunting dog Lycaon pictus in Zimbabwe. Biological Conservation. 1988;44:301–316. [Google Scholar]
- Cleaveland S., Hess G.R., Dobson A.P., Laurenson M.K., McCallum H.I. The role of pathogens in biological conservation. In: Hudson P.J., Rizzoli A., Grenfell B., Heesterbeck H., editors. The ecology of wildlife diseases. Oxford University Press; Oxford: 2002. pp. 139–150. [Google Scholar]
- Courchamp F., Macdonald D.W. Crucial importance of pack size in the African wild dog Lycaon pictus. Animal Conservation. 2001;4:169–174. [Google Scholar]
- Creel S., Creel N.M. Communal hunting and pack size in African wild dogs, Lycaon pictus. Animal Behaviour. 1995;50:1325–1339. [Google Scholar]
- Creel S., Creel N.M. Limitation of African wild dogs by competition with larger carnivores. Conservation Biology. 1996;10(2):526–538. [Google Scholar]
- Creel S., Mills M.G., McNutt J.W. Biology and conservation of wild canids. Oxford University Press; Oxford: 2004. Demography and population dynamics of African wild dogs in three critical populations; pp. 337–350. [Google Scholar]
- Davies-Mostert H.T., Page-Nicholson S., Marneweck D.G., Marnewick K., Cilliers D., Whittington-Jones B. A conservation assessment of lycaon pictus. In: Child M.F., Roxburgh L., Do Linh San E., Raimondo D., Davies-Mostert H.T., editors. The Red list of mammals of South Africa, Swaziland and Lesotho. South Africa. South African National Biodiversity Institute and Endangered Wildlife Trust.; 2016. [Google Scholar]
- Fanshawe J.H., FitzGibbon C.D. Factors influencing the hunting success of an African wild dog pack. Animal Behaviour. 1993;45:479–490. [Google Scholar]
- Flacke G., Becker P., Cooper D., Gunther M.S., Robertson I., Holyoake C. An infectious disease and mortality survey in a population of free-ranging african wild dogs and sympatric domestic dogs. International Journal of Biodiversity. 2013 doi: 10.1155/2013/497623. 9 p. [DOI] [Google Scholar]
- Fuller T.K., Kat P.W., Bulger J.B., Maddock A.H., Ginsberg J.R., Burrows R. Population dynamics of African wild dogs. In: McCullough D.R., Barrett R.H., editors. Wildlife 2001: Populations. Elsevier Applied Science; London: 1992. [Google Scholar]
- Gascoyne S.C., Laurenson M.K., Lelo S., Borner M. Rabies in African wild dogs (Lycaon pictus) in the Serengeti region, Tanzania. Journal of Wildlife Diseases. 1993;29(3):396–402. doi: 10.7589/0090-3558-29.3.396. [DOI] [PubMed] [Google Scholar]
- Gusset M., Slotow R., Somers M.J. Divided we fail: The importance of social integration for the re‐introduction of endangered African wild dogs (Lycaon pictus) Journal of Zoology. 2006;270(3):502–511. [Google Scholar]
- Gusset M., Ryan S.J., Hofmeyr M., Van Dyk G., Davies-Mostert H.T., Graf J.A. Efforts going to the dogs? Evaluating attempts to re-introduce endangered wild dogs in South Africa. The Journal of Applied Ecology. 2008;45:100–108. [Google Scholar]
- Hampson K., Dushoff J., Cleaveland S., Haydon D.T., Kaare M., Packer C. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biology. 2009;7(3) doi: 10.1371/journal.pbio.1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeyr M., Bingham J., Lane E.P., Ide A., Nel L. Rabies in African wild dogs (Lycaon pictus) in the Madikwe Game Reserve, South Africa. The Veterinary Record. 2000;146:50–52. doi: 10.1136/vr.146.2.50. [DOI] [PubMed] [Google Scholar]
- Hofmeyr M., Hofmeyr D., Nel L., Bingham J. A second outbreak of rabies in African wild dogs (Lycaon pictus) in Madikwe Game Reserve, South Africa, demonstrating the efficacy of vaccination against natural rabies challenge. Animal Conservation. 2004;7(2):193–198. [Google Scholar]
- Kat P.W., Alexander K.A., Smith J.S., Richardson J.D., Munson L. Rabies among African wild dogs (Lycaon pictus) in the Masai Mara, Kenya. Journal of Veterinary Diagnostic Investigation. 1996;8:420–426. doi: 10.1177/104063879600800403. [DOI] [PubMed] [Google Scholar]
- Knobel D.L., du Toit J.T., Bingham J. Development of a bait and baiting system for delivery of oral rabies vaccine to free-ranging African wild dogs (Lycaon pictus) Journal of Wildlife Diseases. 2002;38(2):352–362. doi: 10.7589/0090-3558-38.2.352. [DOI] [PubMed] [Google Scholar]
- Lindsey P.A., du Toit J.T., Mills M.G.L. Area and prey requirements of African wild dogs under varying habitat conditions: Implications for reintroductions. South African Journal of Wildlife Research. 2004;34(1):77–86. [Google Scholar]
- Maddock A.H., Mills M.G.L. Population characteristics of African wild dogs Lycaon pictus in the eastern Transvaal Lowveld, South-Africa, as revealed through photographic records. Biological Conservation. 1994;67:57–62. [Google Scholar]
- Mills M.G.L., Gorman M.L. Factors affecting the density and distribution of wild dogs in the Kruger National Park. Conservation Biology. 1997;11(6):1397–1406. [Google Scholar]
- Prager K.C., Mazet J.A.K., Dubovi E.J., Frank L.G., Munson L., Wagner A.P. Rabies Virus and Canine Distemper Virus in Wild and Domestic Carnivores in Northern Kenya: Are Domestic Dogs the Reservoir? EcoHealth. 2012;9(4):483–498. doi: 10.1007/s10393-013-0815-9. [DOI] [PubMed] [Google Scholar]
- Prager K.C., Mazet J.A.K., Munson L., Cleaveland S., Donnelly C.A., Dubovi E.J. The effect of protected areas on pathogen exposure in endangered African wild dog (Lycaon pictus) populations. Biological Conservation. 2012;150(1):15–22. doi: 10.1016/j.biocon.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheepers J.L., Venske K.A.E. Attempts to reintroduce African Wild Dogs Lycaon pictus into Etosha National Park, Namibia. South African Journal of Wildlife Research. 1995;25:138–140. [Google Scholar]
- Taylor L.H., Nel L.H. Global epidemiology of canine rabies: Past, present, and future prospects. Veterinary Medicine Research and Reports. 2015;(6):361–371. doi: 10.2147/VMRR.S51147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heerden J., Bingham J., van Vuuren M., Burroughs R.E., Stylianides E. Clinical and serological response of wild dog (Lycaon pictus) to vaccination against canine distemper, canine parvovirus infections and rabies. Journal of the South African Veterinary Association. 2002;73:8–12. doi: 10.4102/jsava.v73i1.541. [DOI] [PubMed] [Google Scholar]
- Whittington-Jones B. Jacana Media; Johannesburg: 2015. African wild dogs: On the front line. [Google Scholar]
- Woodroffe R., Sillero-Zubiri C. 2012. Lycaon pictus. The IUCN red list of threatened species 2012. e.T12436A16711116. Accessed 05 October 2015. [DOI] [Google Scholar]
- Woodroffe R., Ginsberg J., Macdonald D., the IUCN/SSC Canid Specialist Group . IUCN; Gland, Switzerland: 1997. The african wild dog – Status survey and conservation action plan. [Google Scholar]
- Woodroffe R., McNutt J.W., Mills M.G.L. African wild dog lycaon pictus. In: Sillero-Zubiri, Hoffmann M., Macdonald D.W., editors. Canids: Foxes, wolves, jackals and dogs: Status survey and action plan. IUCN/SSC Canid Specialist Group; Gland, Switzerland, & Cambridge, United Kingdom: 2004. [Google Scholar]
- Woodroffe R., Davies-Mostert H., Ginsberg J., Graf J., Leigh K. Rates and causes of mortality in endangered African wild dogs Lycaon pictus: Lessons for management and monitoring. Oryx. 2007;41:215–223. [Google Scholar]
- Woodroffe R., Prager K.C., Munson L., Conrad P.A., Dubovi E.J. Contact with domestic dogs increases pathogen exposure in endangered african wild dogs (Lycaon pictus) PloS One. 2012;7(1) doi: 10.1371/journal.pone.0030099. [DOI] [PMC free article] [PubMed] [Google Scholar]