Short abstract
Objective
We evaluated the effectiveness of carbon nanoparticle suspension injection combined with parathyroid gland vasculature preservation to identify and preserve the parathyroid gland to reduce postoperative hypoparathyroidism following thyroidectomy.
Material and Methods
Fifty patients with thyroid tumors received carbon nanoparticle suspension injection combined with parathyroid gland vasculature preservation (group A). Serum calcium and PTH levels were recorded and compared with those of 50 control patients who underwent a standard thyroidectomy procedure (group B).
Results
The serum calcium level in group A was significantly higher than that in group B at day 1 (2.20 ± 0.02 vs 2.11 ± 0.03 mmol/L) and day 30 (2.27 ± 0.01 vs 2.21 ± 0.02 mmol/L) after surgery. The PTH level in group A was also significantly higher than that in group B at day 1 (33.5 ± 2.36 vs. 25.31 ± 2.98 pg/mL) after surgery. The incidence of hypoparathyroidism was significantly higher in group B than in group A at day 1 after surgery (19 vs. 7 patients).
Conclusion
When combined with parathyroid gland vasculature preservation, carbon nanoparticle suspension injection can effectively reduce the incidence of temporary parathyroidism following thyroid surgery.
Keywords: Carbon nanoparticle suspension, parathyroid gland, thyroidectomy, hypoparathyroidism, hypocalcemia, parathyroid vasculature preservation technique
Introduction
Thyroid surgery is among the most frequently performed procedures in iodine-deficient regions.1 Hypoparathyroidism is a serious complication after thyroid surgery, and the incidence of permanent hypoparathyroidism is 1.4% to 14.3% while hypocalcemia caused by hypoparathyroidism has a reported incidence of 9.9%.2,3 Postoperative hypocalcemia causes patient discomfort, with effects such as perioral numbness/tingling, seizures, laryngeal spasm, and long-term hospitalization. The etiologies of this condition include iatrogenic injury to the blood supply or inadvertent resection of parathyroid glands (PGs) caused by surgical error.4 Minimizing surgical complications is thus important to preserve the quality of life of patients, and careful identification and preservation of PGs with the blood supply in situ is accepted as necessary to protect PG function. In 2015, Wang Bin et al. reported that the PGs showed negative carbon nanoparticle suspension (CNs) staining during thyroid surgery.5 Several clinical studies have confirmed that CNs can facilitate the identification of PGs,6–8 but few studies to date have examined whether CNs combined with parathyroid vasculature preservation technique (PVPT) can accelerate the recovery of parathyroid function. Therefore, we evaluated the efficacy of CNs combined with PVPT in preserving the function of PGs to reduce the incidence of transient hypoparathyroidism following thyroid surgery.
Material and methods
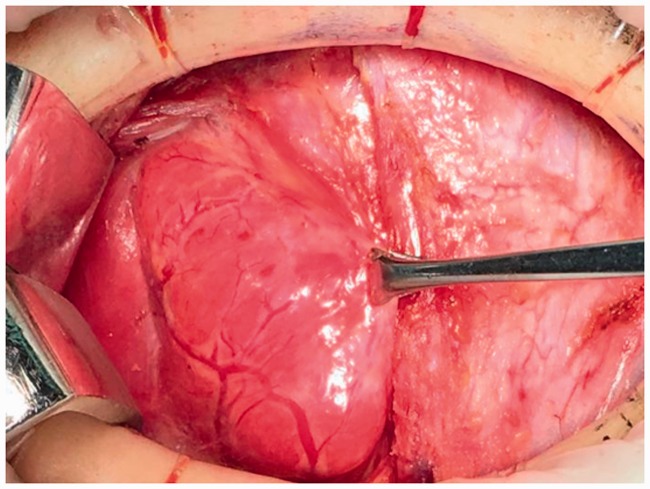
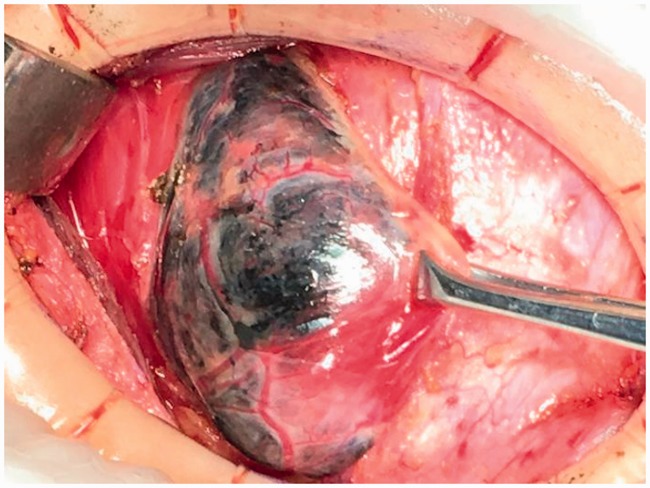
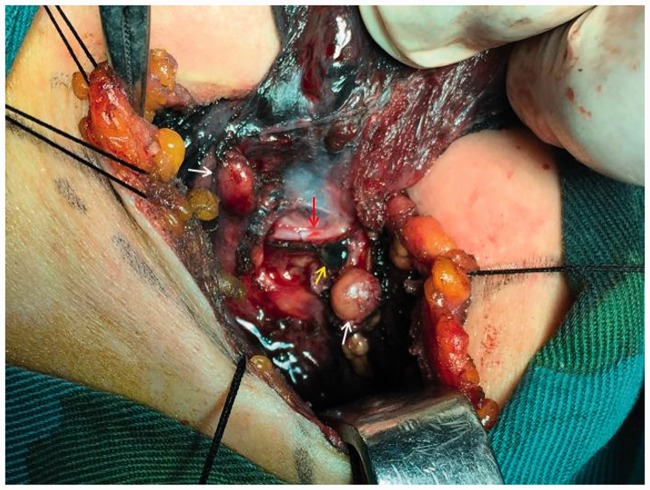
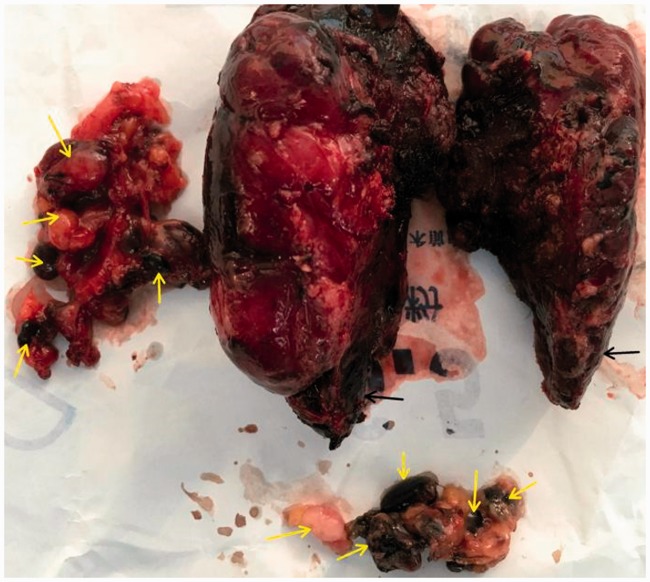
The study protocol was approved by the ethics committee of The First Affiliated Hospital, School of Medicine, Yangtza University (approval number: TH2016-1). All patients provided written informed consent to participate. From January 2016 to April 2018, 100 patients with benign or malignant thyroid disease underwent a total or subtotal thyroidectomy at the First Affiliated Hospital of Yangtze University for the following indications: multinodular goiter (28 cases), solitary adenoma (5 cases), and thyroid carcinoma (67 cases). The exclusion criteria were reoperation, preoperative hypocalcemia or hypoparathyroidism, preoperative cord dysfunction, Grave’s disease, history of radiotherapy or neck surgery, and PG autotransplantation during thyroidectomy. All surgeries were carried out by an experienced surgeon using the same general anesthesia. In all patients, preoperative direct blood testing of serum calcium and intact parathyroid hormone (PTH) levels was performed to determine the presence of hypocalcemia and hypoparathyroidism, respectively. Clinical symptoms of hypocalcemia such as perioral numbness/tingling, seizure, and laryngospasm were noted. Patients were randomized to group A or group B using a random number table until the required number of patients were enrolled. Following a standard low-collar incision, total or subtotal thyroidectomy was performed in group A. The anterior capsule of the thyroid was carefully dissociated and the surgical lobe exposed following dissection of the skin flap layer and strap muscles (Figure 1). Next, 0.1 to 0.2 mL of CNs (0.5 mL ampoule, Lai Mei Pharmaceutical Co, Chongqing, China) was slowly injected into the upper and lower point of the abnormal thyroid lobe (only one injection point was used in patients with a smaller abnormal thyroid). The syringe was withdrawn to avoid erroneous injection into the blood vessels (Figure 1), and the puncture point was gently pressed for 3 to 5 minutes until the thyroid gland showed complete development (i.e., black staining) after CNs injection (Figure 2). After 5 minutes, lymph nodes were also stained black (Figures 5 and 6), whereas PGs with vascular pedicles were negatively stained (Figures 3–5). The recurrent laryngeal nerve remained white, PGs showed the primary yellow color, and the blood vessels of PGs remained red or pink (Figures 3–5, 7, 8). Next, the superior pole was ligated and resected close to the thyroid capsule by preserving the posterior branches of the superior thyroid vein and artery (STV/STA) (Figure 7). During surgery, the terminal branches of the inferior thyroid artery/vein (ITA/ITV, Figure 7) were exposed and preserved. PG blood supply in the thyroid capsule was preserved in situ by meticulous capsular dissection, and particular care was taken to preserve the arch structure from the inferior thyroid vein trunk surrounding the PGs (Figure 8). Following intraoperative confirmation of the frozen-section diagnosis of thyroid tumor, total or subtotal thyroidectomy with/without central lymph node clearance was performed (Figure 6). No autotransplantation or PG impairment or incidental removal was observed during thyroidectomy in group A. Patients in group B underwent standard thyroidectomy. The normal serum PTH range is 15 to 68.3 pg/mL, and clinical hypoparathyroidism was defined as postoperative serum PTH <15 pg/mL accompanied by hypocalcemic symptoms.9 The normal serum calcium range is 2.00 to 2.80 mmol/L, and hypocalcemia was defined as postoperative serum calcium <2.00 mmol/L and/or neuromuscular symptoms (e.g., perioral numbness/tingling, seizure, laryngospasm, positive Trousseau’s sign, or tetany).10 Patients with clinical symptoms of hypocalcemia received oral vitamin D and calcium supplements. Permanent hypoparathyroidism was defined as postoperative serum PTH <15 pg/mL at >6 months after surgery. The clinical manifestations of hypocalcemia and PG pathology results were recorded independently. No patients reported significant complications, and all were discharged within 7 days after surgery. The serum PTH and calcium levels of patients in both groups were measured at day 1, day 30, and day 180 after thyroidectomy.
Figure 1.
Prior to carbon nanoparticles suspension injection.
Figure 2.
Five minutes after carbon nanoparticle suspension injection.
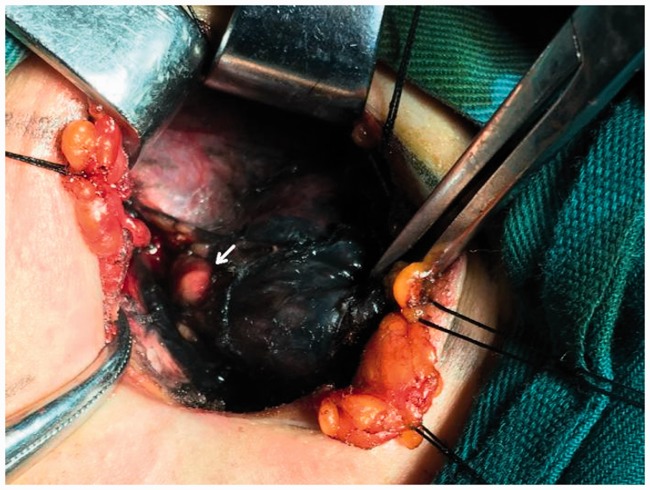
Figure 5.
Development of lymph node (yellow arrow). Non-developed left parathyroid glands (white arrow) and non-developed recurrent laryngeal nerve (red arrow).
Figure 6.
Development of lymph node (yellow arrow) and developed thyroid glands (black arrow).
Figure 3.
After carbon nanoparticle suspension injection (decreased non-development of the left superior parathyroid gland).
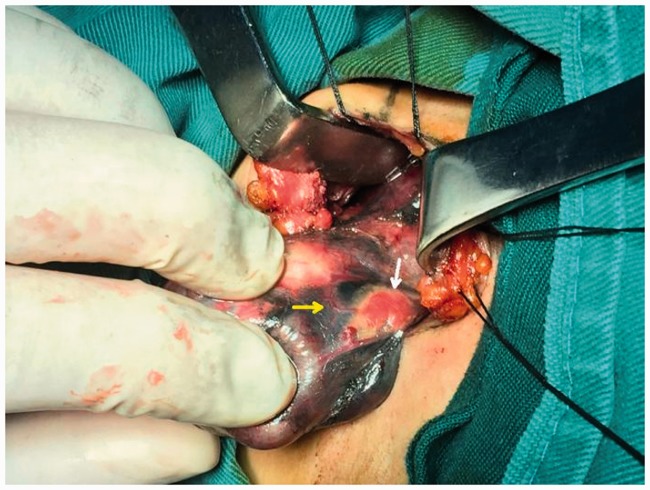
Figure 4.
Non-developed left inferior parathyroid gland (white arrow) and non-developed parathyroid gland vasculature (yellow arrow).
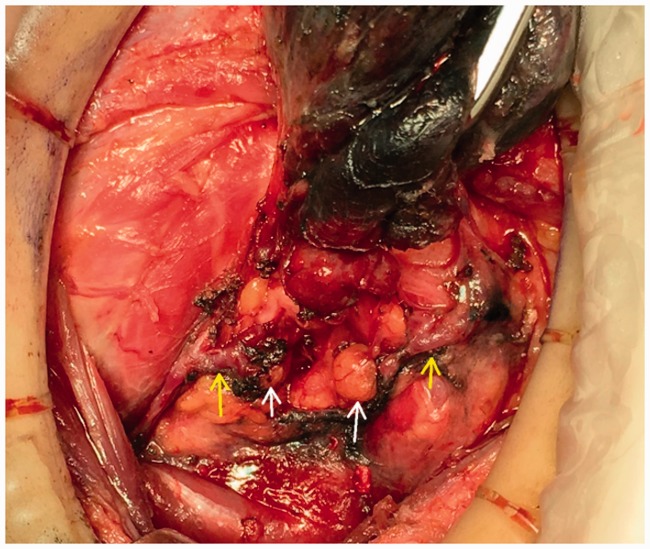
Figure 7.
Non-developed right superior and inferior parathyroid gland (white arrow),non-developed parathyroid gland vasculature (yellow arrow), and developed thyroid glands (black).
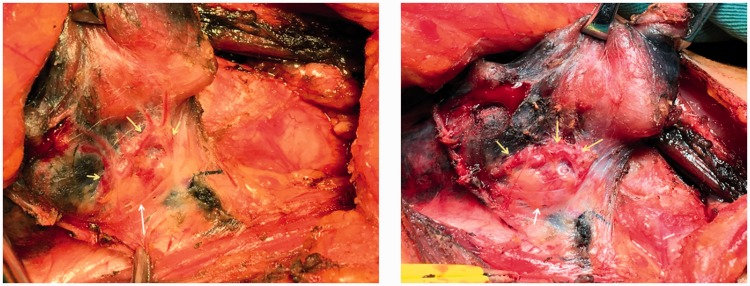
Figure 8.
Non-developed parathyroid gland (white arrow), non-developed right arcuate parathyroid gland vasculature (before and after branch excision, yellow arrow), and developed thyroid glands (black).
SPSS 23.0 software (IBM Corp., Armonk, NY, USA) was used for the statistical comparison of serum PTH and calcium levels. Data were presented as mean ± standard deviation (X ± s). Student’s t-test and the χ2 test were used for comparison of count data between group A and group B. Values of P < 0.05 were considered statistically significant.
Results
One hundred patients underwent total or subtotal thyroidectomy for thyroid disease at our department between January 2016 and April 2018, of which 50 patients in group A underwent combination treatment with CNs and PVPT to identify PGs and preserve their blood supply, while 50 patients in group B underwent standard thyroidectomy. The demographic features, histological manifestations, types of surgery, and number of cases of postoperative hypoparathyroidism are reported in Table 1. Group A consisted of 13 (26%) men and 37 (74%) women with an average age of 46.06 ± 1.70 years. There were also 13 men and 37 women in group B, with an average age of 48.92 + 2.13 years. Total thyroidectomy was performed in 34 cases in group A and 29 cases in group B. Subtotal thyroidectomy was performed in 16 patients in group A and 21 patients in group B. The pathological types consisted of cancer (35:32, group A vs. group B) and non-cancer (15:18) (Table 1). The preoperative mean values of PTH and calcium were similar between the two groups (Table 2). However, serum calcium levels in group A were significantly higher than those in group B at day 1 (2.20 ± 0.02 vs. 2.11 ± 0.03, P < 0.05) and day 30 (2.27 ± 0.01 vs. 2.21 ± 0.02, P < 0.05) after surgery. There was no statistically significant difference in serum calcium level between the two groups at day 180 after surgery (Table 2). Twelve patients in group B (24%) and three patients in group A (6%) showed hypocalcemia at day 1 after surgery. Postoperative hypocalcemia was significantly more frequent in group B than in group A (24% vs. 6%, P < 0.05). In addition, the serum PTH level in group A was significantly higher than in group B at day 1 after surgery (33.50 ± 2.36 vs. 25.31 ± 2.98, P < 0.05). However, there was no statistically significant difference in serum PTH level between the two groups at day 30 and day 180 after surgery (Table 2). Postoperative hypoparathyroidism was observed in 26 patients (26%) at day 1 after thyroidectomy in all patients from both groups. These 26 patients included 8 men and 18 women with an average age of 46.42 years, 17:9 cancer and non-cancer, and 16:10 total thyroidectomy and subtotal thyroidectomy. However, the patients in group B had a higher incidence of transient postoperative hypoparathyroidism at day 1 after surgery than those in group A (19:7), the difference was statistically significant. Permanent postoperative hypoparathyroidism at day 180 after surgery occurred in two patients in group B and no patients in groups A (Table 1). The serum calcium level in group A was significantly higher preoperatively than at day 1 after surgery (2.28 ± 0.01 vs. 2.20 ± 0.02, P < 0.05). There was no statistically significant difference between preoperative and postoperative serum calcium levels in group A on postoperative day 30 and day 180 (Table 3). The preoperative serum calcium level in group B was significantly higher than that at day 1 (2.28 ± 0.01 vs. 2.11 ± 0.03, P < 0.05), day 30 (2.28 ± 0.01 vs. 2.21 ± 0.02, P < 0.05) and day 180 (2.28 ± 0.01 vs. 2.21 ± 0.02, P < 0.05) after surgery (Table 3). The mean preoperative serum PTH level of group A was significantly higher than that in group A patients at day 1 after surgery (59.10 ± 4.33 vs. 33.50 ± 2.36, P < 0.05). However, no statistical difference was observed between preoperative and day 30 and day 180 postoperative serum PTH levels in group A (Table 3). Finally, in group B, the mean preoperative serum PTH level was significantly higher than that at day 1 (63.03 ± 3.75 vs. 25.31 ± 2.98, P < 0.05), day 30 (63.03 ± 3.75 vs. 46.97 ± 3.54, P < 0.05), and day 180 (63.03 ± 3.75 vs. 49.11 ± 4.02, P < 0.05) after surgery (Table 3). These results indicate the patients who underwent CNs injection combined with PVPT showed a safer and more rapid recovery of parathyroid function. In the present study, no patients died or reported vocal cord paralysis after surgery.
Table 1.
Clinical characteristics and hypoparathyroidism status of patients.
| Group A | Group B | P-value | |
|---|---|---|---|
| Age (years) | 46.06 ± 1.70 | 48.92 ± 2.13 | 0.296 |
| Gender | 1.000 | ||
| Female | 37 | 37 | |
| Male | 13 | 13 | |
| Type of surgery | 0.300 | ||
| Total thyroidectomy | 34 | 29 | |
| Subtotal thyroidectomy | 16 | 21 | |
| Surgical cause | 0.523 | ||
| Cancer | 35 | 32 | |
| Non-cancer | 15 | 18 | |
| Hypoparathyroidism | |||
| Transient | 7 | 19 | 0.006 |
| Permanent | 0 | 2 | 0.475 |
Table 2.
Mean preoperative and postoperative serum calcium (mmol/L) and parathyroid hormone (pg/mL) levels.
| Preoperative |
Day 1 |
Day 30 |
Day 180 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | n | Ca2+ | PTH | Ca2+ | PTH | Ca2+ | PTH | Ca2+ | PTH |
| Group A | 50 | 2.28 ± 0.01 | 59.10 ± 4.33 | 2.20 ± 0.02 | 33.50 ± 2.36 | 2.27 ± 0.01 | 50.50 ± 4.66 | 2.27 ± 0.02 | 56.99 ± 4.50 |
| Group B | 50 | 2.28 ± 0.01 | 63.03 ± 3.75 | 2.11 ± 0.03 | 25.31 ± 2.98 | 2.21 ± 0.02 | 46.97 ± 3.54 | 2.21 ± 0.02 | 49.11 ± 4.02 |
| P-value | >0.05 | >0.05 | <0.05 | <0.05 | <0.05 | >0.05 | >0.05 | >0.05 | |
Ca2+, calcium; PTH, parathyroid hormone
Table 3.
Mean preoperative and postoperative serum calcium (mmol/L) and parathyroid hormone (pg/mL) levels in each group
| Preoperative | Day 1 | P-value | Day 30 | P-value | Day 180 | P-value | |
|---|---|---|---|---|---|---|---|
| Group A | |||||||
| PTH | 59.10 ± 4.33 | 33.50 ± 2.36 | <0.01 | 50.50 ± 4.66 | >0.05 | 56.99 ± 4.50 | >0.05 |
| Ca2+ | 2.28 ± 0.01 | 2.20 ± 0.02 | <0.01 | 2.27 ± 0.01 | >0.05 | 2.27 ± 0.02 | >0.05 |
| Group B | |||||||
| PTH | 63.03 ± 3.75 | 25.31 ± 2.98 | <0.01 | 46.97 ± 3.54 | <0.05 | 49.11 ± 4.02 | <0.05 |
| Ca2+ | 2.28 ± 0.01 | 2.11 ± 0.03 | <0.01 | 2.21 ± 0.02 | <0.05 | 2.21 ± 0.02 | <0.05 |
Ca2+, calcium; PTH, parathyroid hormone
Discussion
Hypoparathyroidism and hypocalcemia caused by accidental removal of or injury to the PGs during thyroidectomy is a relatively common occurrence that can affect patient quality of life.4 Hypoparathyroidism may be attributed to injury, accidental removal, or devascularization of the PGs during thyroid surgery. Postoperative hypoparathyroidism may cause hypocalcemic tetany, such as tingling in the toes, fingers, and around the lips, as well as anxiety, depression, and other mental conditions that can prolong hospitalization and require life-long treatment with vitamin D and calcium oral supplementation.6 In previous studies, the incidence of transit hypoparathyroidism varied from 14% to 31%11 while a rate of 0.4% to 13.8% has been reported for permanent hypoparathyroidism.12 In the present study, the incidence of transient hypoparathyroidism and permanent hypoparathyroidism tended to be lower in patients in group A who underwent CNs injection combined with PVPT compared with patients in group B who underwent standard thyroidectomy. A statistically significant difference in postoperative serum calcium and PTH levels was observed between group A and group B at day 1 after surgery. This result may be attributable to the facilitation of PG identification by CNs and the preservation of PG blood supply in situ by PVPT, both of which are essential to the recovery of PG function.
Methylene blue, a heterocyclic aromatic compound, has historically been widely used in sentinel lymph node biopsy.13 However, Sari et al.14 showed that PG staining faded within 3 minutes after the application of methylene blue solution and that both lymph nodes and PGs were stained, potentially leading to the accidental removal of PGs. Following reports of potential teratogenic effects of methylene blue, CNs gradually replaced methylene blue for the identification of PGs during thyroid surgery. Hao et al.15 examined sentinel lymph node biopsies following CNs injection compared with methylene blue injection in Chinese patients with papillary thyroid microcancer and found that CNs injection had higher sensitivity and accuracy and a lower false negative rate than methylene blue injection.
CNs is the only lymph tracer approved to date for clinical use. CNs consists of carbon nanoparticles with an average diameter of 150 nm that can readily enter the lymphatic capillaries (120–500 nm in diameter) rather than blood vessel capillaries (20–50 nm in diameter). Therefore, CNs do not enter the blood vessels following injection into the thyroid gland tissue surrounding the tumor but instead rapidly enter the lymphatic vessels via macrophage pinocytosis and subsequently accumulate in lympho-vascular tissues such as thyroid tissue and lymph nodes, resulting in black staining. In the present study, recurrent laryngeal nerve (RLN) and PGs were observed as unstained, owing to an absence of lympho-vascular tissue (Figures 4 and 5), and could thus be distinguished from adjacent black-stained lymph nodes and thyroid glands. Identification of PGs using negative development technique does not effectively prevent injury to the parathyroid blood supply or accidental removal of PGs, meaning that preservation of the vasculature surrounding PGs is important to protect their function. We thus conclude that visual identification of PGs and preservation of blood supply in situ is the gold standard approach to preserving PG function during thyroid surgery. According to recent studies15–17 and our clinical experience, CNs injection does not present any safety issues following injection at 1 to 2 points around the tumor at injection doses of no more than 0.1 ml, with an interval of 3 to 5 minutes after each injection. Each injection point must be pressed or cauterized to prevent bleeding and CNs leakage. As a lymph node tracer, CNs flow can be impaired when tumor cells or inflammation block lymphatic channels. After intraoperative injection of CNs, thyroid glands and some lymph nodes, but not PGs with blood supply and RLN, were completely stained.16 CNs injection is simple, safe, and inexpensive to use, even by an inexperienced surgeon. Some previous studies have reported that CNs play an important role in preserving the physiologic function of PGs during thyroid surgery,17 although other studies showed no significant difference in complications between thyroidectomy with or without CNs. The present study is the first to use CNs injection to identify PGs with blood supply combined with PVPT to protect PG function.
Careful preservation of PGs with blood supply in situ, particularly meticulous dissection with preservation of the tertiary branches of ITA, is advocated to protect PG function.18 A previous study reported that the incidence of hypocalcemia decreased after preservation of the inferior thyroid vein during thyroidectomy.19 In our experience, blood supply to PGs is not from a single source, but from the complex vascular system surrounding the PGs and may be attributable to the following factors: (1) formation of terminal branches of PG vessels, which partly originate from the posterior branches of the superior thyroid vein and artery (STV/STA); (2) most of the PG blood supply coming from the terminal branches of the inferior thyroid artery and vein (ITA/ITV, Figure 7); (3) some vessels coming from the thyroid capsule; (4) in rare cases, PG blood supply coming from the thyroid gland parenchyma, which is less likely to be dissected and is protected by the thyroid gland; (5) the arch structure of the inferior thyroidal vein trunk being considered the “golden arch”, which should be preserved intact to maintain terminal blood flow to the PGs (Figure 8). Therefore, our principles for the preservation of PGs with blood supply are as follows: (1) the identification and location of normal PGs by CNs in thyroidectomy is critical for preserving PG blood supply (Figures 3–5); (2) the blood supply of PGs is compensated from the diverse original vasculature around the thyroid gland (Figures 3, 7, and 8); (3) the posterior branches of the STV/STA and the ITA/ITV terminal branches of parathyroid side must be preserved to the maximum extent possible (Figure 7); (4) the thyroid capsule supplying vessels to the PGs must be carefully preserved by meticulous capsular dissection; (5) the arch structure of the ITV trunk must be preserved intact by dissecting and removing terminal branches on the thyroid side (Figure 8); (6) devascularized PGs within the thyroid gland parenchyma should be immediately autotransplanted to sternocleidomastoid muscle or other muscle tissues.
In the present study, the incidence of transit hypoparathyroidism was 14% and no cases of permanent hypoparathyroidism were reported in group A. Consequently, the combination of CNs and PVPT was associated with higher mean postoperative serum PTH and calcium levels. Additionally, we verified the effectiveness of CNs and PVPT for identifying PGs and preserving their blood supply.
Conclusion
Protection of the structure and function of the recurrent laryngeal nerve and PGs during thyroidectomy is an important surgical consideration. The earlier RLN and PGs are identified, the safer the thyroidectomy procedure becomes. CNs injection to identify PGs in combination with PVPT to preserve PGs with blood supply in situ may decrease the incidence of postoperative hypoparathyroidism after thyroid surgery. This combined approach represents an effective method by which less experienced surgeons in particular can identify and preserve PG function. However, further research of the use of CNs injection with PVPT in more thyroidectomies is required to confirm its effectiveness.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Gurrado A, Bellantone R, Cavallaro G, et al. Can total thyroidectomy be safely performed by residents? Medicine (Baltimore) 2016; 95: e3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergenfelz A, Jansson S, Kristoffersson A, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg 2008; 393: 667–673. 10.1007/s00423-008-0366-7. [DOI] [PubMed] [Google Scholar]
- 3.White ML, Gauger PG, Doherty GM. Central lymph node dissection in differentiated thyroid cancer. World J Surg 2007; 31: 895–904. [DOI] [PubMed] [Google Scholar]
- 4.Gourgiotis S, Moustafellos P, Dimopoulos N, et al. Inadvertent parathyroidectomy during thyroid surgery: the incidence of a complication of thyroidectomy. Langenbecks Arch Surg 2006; 391: 557–560. 10.1007/s00423-006-0079-8. [DOI] [PubMed] [Google Scholar]
- 5.Wang B, Qiu NC, Zhang W, et al. The role of carbon nanoparticles in identifying lymph nodes and preserving parathyroid in total endoscopic surgery of thyroid carcinoma. Surg Endosc 2015; 29: 2914–2920. [DOI] [PubMed] [Google Scholar]
- 6.Tian W, Jiang Y, Gao B, et al. Application of nano-carbon in lymph node dissection for thyroid cancer and protection of parathyroid glands. Med Sci Monit 2014; 20: 1925–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park I, Rhu J, Woo JW, et al. Preserving parathyroid gland vasculature to reduce post-thyroidectomy hypocalcemia. World J Surg 2016; 40: 1382–1389. [DOI] [PubMed] [Google Scholar]
- 8.Gu J, Wang J, Nie X, et al. Potential role for carbon nanoparticles identification and preservation in situ of parathyroid glands during total thyroidectomy and central compartment node dissection. Int J Clin Exp Med 2015; 8: 9640–9648. [PMC free article] [PubMed] [Google Scholar]
- 9.Stack BC, Jr, Bimston DN, Bodenner DL, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Disease State Clinical Review: postoperative hypoparathyroidism – definitions and management. Endocr Pract 2015; 21: 674–685. [DOI] [PubMed] [Google Scholar]
- 10.Shoback DM, Bilezikian JP, Costa AG, et al. Presentation of hypoparathyroidism: etiologies and clinical features. J Clin Endocrinol Metab 2016; 101: 2300–2312. [DOI] [PubMed] [Google Scholar]
- 11.Moo TA, McGill J, Allendorf J, et al. Impact of prophylactic central neck lymph node dissection on early recurrence in papillary thyroid carcinoma. World J Surg 2010; 34: 1187–1191. [DOI] [PubMed] [Google Scholar]
- 12.Trupka A, Sienel W. Autotransplantation of at least one parathyroid gland during thyroidectomy in benign thyroid disease minimizes the risk of permanent hypoparathyroidism. Zentralbl Chir 2002; 127: 439–442. [DOI] [PubMed] [Google Scholar]
- 13.Nour A. Efficacy of methylene blue dye in localization of sentinel lymph node in breast cancer patients. Breast J 2015; 1: 388–391. [DOI] [PubMed] [Google Scholar]
- 14.Sari S, Aysan E, Muslumanoglu M, et al. Safe thyroidectomy with intraoperative methylene blue spraying. Thyroid Res 2012; 5: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao RT, Chen J, Zhao LH, et al. Sentinel lymph node biopsy using carbon nanoparticles for Chinese patients with papillary thyroid microcarcinoma. Eur J Surg Oncol 2012; 38: 718–724. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Jian WH, Guo ZM, et al. A meta-analysis of carbon nanoparticles for identifying lymph nodes and protecting parathyroid glands during surgery. Otolaryngol Head Neck Surg 2015; 152: 1007–1016. [DOI] [PubMed] [Google Scholar]
- 17.Gao B, Tian W, Jiang Y, et al. Application of carbon nanoparticles for parathyroid protection in reoperation of thyroid diseases. Int J Clin Exp Med 2015; 8: 22254. [PMC free article] [PubMed] [Google Scholar]
- 18.Reev T, Thompson NW. Complications of thyroid surgery: how to avoid them, how to manage them, and observations on their possible effect on the whole patient. World J Surg 2000; 24: 971–975. [DOI] [PubMed] [Google Scholar]
- 19.Lee DY, Cha W, Jeong WJ, et al. Preservation of the inferior thyroidal vein reduces post-thyroidectomy hypocalcemia. Laryngoscope 2014; 124: 1272–1277. [DOI] [PubMed] [Google Scholar]