Abstract
Peripheral neuropathy is one of the most common, dose limiting, and long-lasting disabling adverse events of chemotherapy treatment. Unfortunately, no treatment has proven efficacy to prevent this adverse effect in patients or improve the nerve regeneration, once it is established. Experimental models, particularly using rats and mice, are useful to investigate the mechanisms related to axonal or neuronal degeneration and target loss of function induced by neurotoxic drugs, as well as to test new strategies to prevent the development of neuropathy and to improve functional restitution. Therefore, objective and reliable methods should be applied for the assessment of function and innervation in adequately designed in vivo studies of CIPN, taking into account the impact of age, sex and species/strains features. This review gives an overview of the most useful methods to assess sensory, motor and autonomic functions, electrophysiological and morphological tests in rodent models of peripheral neuropathy, focused on CIPN. We include as well a proposal of protocols that may improve the quality and comparability of studies undertaken in different laboratories. It is recommended to apply more than one functional method for each type of function, and to perform parallel morphological studies in the same targets and models.
Keywords: peripheral neuropathy, functional evaluation, electrophysiology, pain testing, locomotion, rat, mouse, chemotherapy, neurotoxicity, histology, immunohistochemistry
INTRODUCTION
Peripheral neuropathy is one of the most common, not modifiable dose-limiting, and disabling adverse event of chemotherapy treatment in cancer patients. Chemotherapy-induced damage to the neurons and peripheral nerve fibers can cause the progressive loss of sensory, and even the motor and autonomic functions in the body, resulting in substantial functional deficits and decreased quality of life of the affected subjects (Cavaletti and Marmiroli, 2015). Depending on the cytostatic mechanism of action, the clinical features and the type of nerve fibers involved can vary among these neurotoxic drugs, ranging from pure sensory neuropathies as with platinum, proteasome inhibitors and thalidomide compounds to sensory-motor peripheral nerve involvement, induced by vinka alkaloids and taxane drugs. In addition, positive symptoms often appear, particularly in the sensory sphere, related to neuropathic pain and paresthesia (Argyriou et al., 2012).
This paper focuses on animal experimental models that allow the detailed study of variables influencing the progression of neuropathy and recovery, and the evaluation of new preventive and therapeutic approaches for chemotherapy-induced peripheral neuropathy (CIPN). For obtaining relevant conclusions in preclinical research, appropriate and objective methods have to be conducted rigorously. This paper aims to provide a critical review of the most useful methods for the assessment of peripheral neuropathies in vivo in mammalian models, their application to CIPN studies and their limitations, as well as general considerations for the adequate design of in vivo CIPN experimental studies. Information on particular animal models and results obtained can be found in other articles included in this Special Issue of Experimental Neurology. Functional evaluation methods to apply in those models are preferably no or minimally invasive, so quantitative results along the evolution of the disease can be derived, even repeatedly in the same animals. It is also important to use several functional and electrophysiological tests for the investigation of the different types of functions (motor, sensory, autonomic) conveyed by the peripheral nerve fibers. Histological techniques, importantly including light and electron microscopy observations of the peripheral nerves, with morphometrical analysis, are relevant to assess the proportion and class of nerve fibers that are lost. Immunohistochemical methods have great interest in providing information on functional types of nerve fibers affected and also on the degree and pattern of tissue denervation. Most studies referred have been done using the sciatic nerve and the caudal nerves of rodents, mostly mouse and rat, although the same methods can be adapted to other nerves and animal models of interest. The majority of studies published on rodent models of CIPN so far have used only a subset of available methods (Hoke and Ray, 2012), thus making it only a partial characterization of the pathology and limiting the comparisons between studies. Indeed, previous reviews on evaluation of experimental models of CIPN focused only on assessment of the pain component (Fricker et al., 2008; Hama and Takamatsu, 2016).
General considerations on animal models
An in vivo approach in laboratory mammals allows an experimental setting as similar as possible to the clinical setting: behavioral, neurophysiological and neuropathological observations can be obtained and correlated. In the field of CIPN, rodent models have been extensively devised and studied in the last 30 years (Carozzi et al., 2015; Alberti, 2017). Many advantages are typical of these models: small size and prolific nature of the animals, easiness for handling, pathology and physiology similar to humans, as well as the availability of reliable methods for peripheral nerve assessment (Rodríguez et al., 2004; Radaelli et al., 2019).
Impact of age
Most CIPN rodent studies have used young adult animals as test subjects. Aside from possible species differences between humans and rodents, this assumption is contradicted by epidemiological evidence, since the median age of many common solid cancers (breast, colorectal, lung, prostate cancer) at diagnosis is between 60 to 70 years for both men and women, with the exception of leukaemia and some central nervous system neoplasms, less prevalent in the whole population. Thus, the generalizability of findings from rodent studies of CIPN would be improved by the inclusion of groups of older animals. There are well known age-related changes in the function and structure of the peripheral nerves (Verdú et al., 1996; Ceballos et al., 1999; Canta et al., 2016). Moreover, the response to insults and toxic events tends to decline and vary with age. Evidence provided from other pathological conditions warns about this phenomenon. After traumatic nerve injury, Wallerian degeneration is delayed, the rate of axonal regeneration slower and the amount of regenerating axons decreased in aged animals (reviewed in Verdú et al., 2000). Aging also determines a reduction in terminal and collateral sprouting of regenerated fibers, further limiting the capabilities for compensatory reinnervation. Neurophysiological abnormalities that appear in aged animals are enhanced and appear earlier when a neuropathy is induced, as it has been shown for diabetes (Garcia-Perez et al., 2018), and aged rodents become more sensitive to the neuropathy-induction (Wang-Fischer and Garyantes, 2018). On the other hand, induction of diabetes in prepubertal rats produced effects on peripheral nerve fibers which differ from those observed in adult animals (Thomas et al., 1990).
Impact of sex
Although epidemiological data show roughly a similar prevalence between men and women for a large number of cancers, male rodents have been used in the majority of studies on models of CIPN. Recently, an increasing number of studies report the use of both sexes. However, the heterogeneity of the studies, in terms of number of subjects, dose and duration of treatment, makes difficult to determine sex differences from the literature. Comprehensive sensory, electrophysiological, behavioral and morphological comparison of male and female rodents in models of CIPN is lacking. A few studies have measured sex differences in chemotherapy-induced mechanical and thermal hypersensitivity. In general, female mice and rats were reported to be more sensitive to cold allodynia than males after intraperitoneal injection of paclitaxel (Ward et al., 2011; Hwang et al., 2012; Naji-Esfahani et al., 2016; Brewer et al., 2019). However, no differences in mechanical allodynia were seen after paclitaxel administration. Interestingly, no sex-related differences were observed in cisplatin-induced cold hypersensitivity in mice (Naji-Esfahani et al., 2016).
Impact of species and strain
In a recent systematic review and meta-analysis of animal models of CIPN described in the literature, it was reported that over 85% of the studies conducted used mice and rats (Currie et al., 2019). While early studies were largely done in rats, the availability of transgenic technology in mice has caused a significant rise in the use of mice for CIPN studies. Indeed, the mouse is gaining increasing attention over the rat, since mouse genome and immune response are well-characterized, and they can be manipulated with advanced genetic technologies (Bryda, 2013; Justice and Dhillon, 2016). No systematic comparisons are available between rat and mouse in CIPN. As a note of caution, mouse strains were found to be more sensitive to cisplatin toxicity than rat using a dorsal root ganglia (DRG) neurite outgrowth assay (Podratz et al., 2016).
In the case of CIPN, the use of immunodeficient strains, in which human cancer cells can be inoculated and grown, is strongly relevant for a translational approach (Carozzi et al., 2015). Thereby, neuroprotective or preventive agents against CIPN can be tested in animals also bearing the same tumor the chemotherapy agent is intended to cure, enabling also a non-interference study.
The genetic differences between commonly used mouse and rat strains may reduce the generalizability of basic findings in CIPN. This is an important issue since a number of human studies have reported the potential influence of genetic factors in determining the risk of developing CIPN (Cliff et al., 2017). Differences in strains of rat and mouse have been observed among experimental CIPN studies. For instance, most rat studies used outbred animals, in particular the outbred Sprague-Dawley and Wistar strains. While such use is encouraging, a note of caution is worth mentioning since a recent study found that the genetic profile of two Sprague-Dawley colonies from different providers are highly divergent (Gileta et al., 2018). While the implication of these findings on rat CIPN outcomes is still too early to know, future studies using Sprague-Dawley rats should not be presented without noting the vendor.
A large number of studies use inbred mice on a C57BL/6 genetic background. This is an important issue to consider as C57Bl/6 mice are hardly representative of genetic diversity of laboratory mice or their pain sensitivity (Mogil et al., 2009). Strain differences in chemotherapy-induced mechanical and cold hypersensitivity have been reported in a small number of comparative studies. Thus, a study of the responses of 10 inbred mouse strains (129P3, A, AKR, C3H/He, C57BL/6, C57BL/10, CBA, DBA/2, RIIIS, SM) to paclitaxel showed that almost all mouse strains developed significant mechanical and cold hypersensitivity, with DBA/2J being the most sensitive and C57BL/6J the least sensitive (Smith et al., 2004). A more recent study compared the susceptibility of different mouse strains (Balb-c, C57BL/6, DBA/2J, AJ, FVB and CD1 from Envigo) to oxaliplatin i.v. administration (Marmiroli et al., 2017). Surprisingly, C57BL/6 mice did not develop cold hypersensitivity but did show mechanical hypersensitivity. In addition, C57BL/6 strain did not show significant changes in morphometry, electrophysiological and intraepidermal nerve fiber density after oxaliplatin administration. In contrast, the most sensitive strain on most measures of CIPN was the Balb-c mouse strain.
Finally, an emerging issue that can contribute to the differences between mouse studies is the use of inbred mice substrains that are related but not genetically identical and for which behavioral differences have been reported. The most common example is the inbred mouse strain C57BL/6 which has been widely used in CIPN studies because it is the preferred choice for the generation of transgenic and knockout mice for disease modeling. There are two major substrains of C57BL/6 mice, known as C57BL/6J and C57BL/6N (see Zurita et al., 2011 for a review on the genetic and phenotypic differences). The importance of the genetic and phenotypic differences among these substrains has not been recognized yet by CIPN researchers. Indeed, C57BL/6J and C57BL/6N differ in their pain-related behavior in acute and chronic pain models (Bryant et al., 2019). Similarly, BALB/c mouse substrains show differences in several behavioral measures between J and ByJ substrains (Sittig et al., 2014). This is very relevant since recent studies on CIPN used BALB/c mice as test subjects (Marmiroli et al., 2017; Wozniak et al., 2017; Cook et al., 2018) without identifying the substrain employed. Researchers using inbred mouse models of CIPN must also consider the substrain of C57BL/6, BALB/c and other strains when investigating CIPN.
Route of administration
Current neurotoxic anticancer drugs are administered by intravenous route in patients. However, in the developing of CIPN animal models both intravenous and intraperitoneal routes have been used. The intraperitoneal drug delivery predominates in rodent models because it is safe for the animals (Davis et al., 2014) and an easier method without need of much training (Hirota and Shimizu, 2012). However, the accuracy of intraperitoneal administration has been questioned; in one old study, almost 20% of injections conducted by trained personnel failed to deliver the drug in the peritoneal cavity, being injected in the gastrointestinal tract, retroperitoneally, subcutaneously or into the bladder (Lewis et al., 1966). On the other hand, repeated intravenous injection become more difficult for the deterioration of the tail veins, commonly used in this route. Besides to have well trained researchers, when choosing the administration route, it is important to consider the chemical properties of the compound, as the capacity to induce local irritation, and the pH, because low pH solutions can precipitate when mixed with blood, resulting in vascular occlusion (Turner et al., 2011).
Another critical factor related with the administration is its influence on the drug pharmacokinetics. These parameters are well determined and reported in human patients (Liston and Davis, 2017). However, it is unknown if the neurotoxic effect of chemotherapy drugs is depending on the peak concentration or on the AUC achieved, a problem approached in teratogenesis studies (Nau, 1986). In this regard, the intraperitoneal route can add variability to the pharmacokinetic of interest, and difficulty for extrapolating findings between animal models and patients. The parietal peritoneum drains into the inferior cava vein while the visceral peritoneum drains to the portal vein; therefore, an uncertain amount of drug administered may pass to the liver and be biotransformed prior to reach the systemic circulation. In addition, small amounts of drug may pass directly into the thoracic lymph across the diaphragm (Abu-Hijleh et al., 1995).
FUNCTIONAL EVALUATION METHODS
Electrophysiological tests
Nerve conduction tests in vivo
Nerve conduction tests reliably assess the functional state of sensory and motor myelinated nerve fibers. Methods are minimally invasive (percutaneous small needle electrodes), and thus have the advantage of allowing serial evaluation at desired intervals without killing the animals or disturbing the neuropathy establishment and the further degenerating/regenerating processes. For comparisons over time and between studies, it is important to place the anesthetized animals at regulated body temperature, and use standardized needle electrodes placed under microscope to ensure the same location. The possibility of testing in the same session proximal and distal (nerve and muscle) targets increases the information acquired regarding progression of the neuropathy. The most sensitive tests to detect differences are the conduction tests for distal targets, such as foot muscles and digital or caudal nerves. It is advisable when a new drug-induced neuropathy model is implemented or new drug schedules are tested, to perform both sensory and motor nerve conductions tests, to ensure an accurate characterization of nerve involvement, and to resemble the examination protocols most used in patients, thus facilitating the correlation of results from basic to clinical studies.
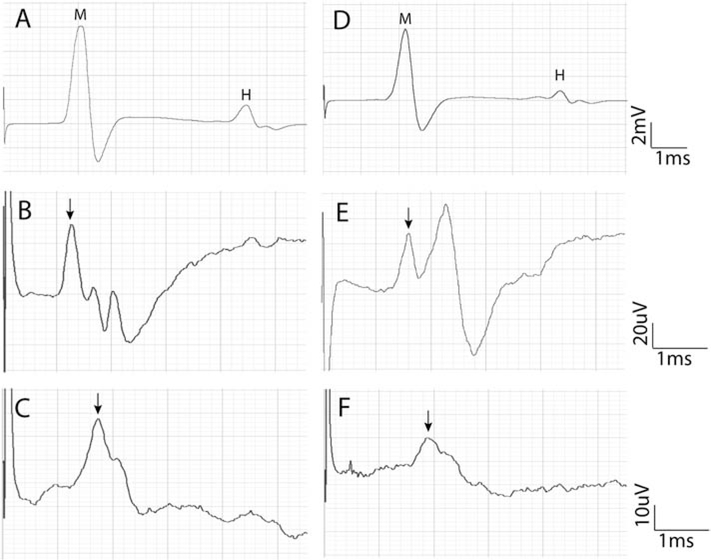
For nerve conduction tests, an invasive approach with dissection of the tested nerve can be used, but non-invasive methods are preferable since they allow repeated testing the same animals over time (Navarro and Udina, 2009; Bruna et al., 2011; Boehmerle et al., 2014). The nerve of interest is directly stimulated with short duration (50–100 μs) electrical pulses of progressively increasing intensity until the maximal response is recorded, usually delivered with small needle electrodes inserted at a proximal site of the assessed nerve (for instance, sciatic notch and ankle for the sciatic nerve), and the evoked compound nerve action potential (CNAP) may be recorded with small needle electrodes placed also near the distal segment of the nerve. The biphasic potential recorded represents the CNAP of myelinated fibers. Recording needle electrodes are preferred to surface electrodes because they allow for higher resolution to detect the small CNAPs, in the range of microvolts. Small recording needle electrodes can be placed near the tibial nerve at the ankle, the plantar nerves of the hindpaw and the digital nerves of toes for evaluation of the sciatic nerve, near digital nerves of fingers for the median and ulnar nerves, or near the caudal nerves in the tail. The CNAP amplitude correlates with the number of large (Aαβ) myelinated fibers. The latency time, from the stimulus artifact to the onset of the CNAP, is used to derive the conduction velocity of the fastest axons in each nerve segment tested. CNAPs recorded from digital and caudal nerves give a good assessment of the innervation of sensory axons to the most distal skin (Fig. 1), and are among the most sensitive measures to detect neuropathic changes (Verdú et al., 1999; Bruna et al., 2010). Orthodromic or antidromic techniques can be used; the latter have the advantage of usually giving larger amplitude of the CNAP. It is worth to note that pure sensory CNAPs can be recorded only from the digital nerves, whereas the caudal nerves are mixed sensory-motor nerves in most of their length except at the very tip. Nevertheless, both motor and sensory caudal nerve conductions can be estimated, since stimulation of the tail root evokes a muscle response, which is preceded by a smaller nerve potential, due to the fastest sensory fibers conducting antidromically (Leandri et al., 2007). Detailed information on technical issues and electrophysiological methods can be found in previous texts (Loeb and Gans, 1986; Navarro and Udina, 2009).
Figure 1.
Electrophysiological recordings of the CMAP from the interosseus plantar muscle (A, D), the CNAPs from the fourth digital nerve (B, E) and from the tail nerve (C, F) in a control mouse (A, B, C) and in a mouse treated with cisplatin (D, E, F). Note the longer latency and the smaller amplitude of the potentials, most evident in the nerve recordings, in the cisplatin-treated mouse compared with the untreated mouse. A,D: letters signal the M wave and the H reflex wave; B,C,E,F: the arrow points to the peak of the CNAP.
Motor nerve conduction tests
Motor nerve conduction tests are easier to perform, because of the larger amplitude of the recorded potentials from skeletal muscles, than direct nerve conduction tests, and can be considered as a key method for assessing motor axon degeneration and muscle denervation. The nerve is similarly stimulated with electrical pulses of increasing intensity, and the evoked compound muscle action potential (CMAP) is recorded with needle electrodes placed percutaneously on the target muscles, the active electrode on the muscle belly near the motor point and the reference electrode at the distal tendon. Different muscles located proximal or distal in the limbs and tail can be tested for assessing progression of the motor neuropathy. For example, gastrocnemius and interosseus muscles for the tibial nerve, tibialis anterior and extensor digitorum brevis muscles for the peroneal nerve, flexor carpi and interosseus for the median nerve, tail muscles for the caudal nerves. The amplitude of the CMAP is determined by the number of muscle fibers innervated, and it is the most useful indicator for motor neuropathy assessment. The latency to the CMAP includes the conduction time of the impulse along the nerve and also the transmission time in the neuromuscular junction (Fig. 1). Therefore, the motor nerve conduction velocity can be calculated only in the nerve segment between two sites of stimulation, for example, at the sciatic notch and the ankle for the sciatic-tibial nerve (Navarro and Udina, 2009).
Motor unit number estimation (MUNE)
Despite this is a test not much used in CIPN studies, due to the predominant or exclusive sensory nerve impairment caused by most neurotoxic cytostatic drugs, for some compounds as vincristine and taxanes that induce also motor impairment the MUNE might be useful (Taleb et al., 2017). An accurate estimation of the number of functional motor units in a muscle can be obtained by using the incremental stimulation technique with the motor nerve conduction setting (Shefner, 2001; Mancuso et al., 2011). Starting from subthreshold intensity, the nerve is stimulated with single electrical pulses of gradually increased intensity until the first response appears, representing the first motor unit recruited. After following stimuli, quantal increases in the response are recorded. The estimated number of motor units results from the equation: MUNE = maximal CMAP / mean amplitude of single motor unit action potential. These methods give good information on the number of functional motoneurons innervating the studied muscle, and their capacity for collateral sprouting that increases the size of reinnervated motor units over normal values. MUNE requires an accurate methodology and some time for testing, but it may add valuable information on models of motor neuropathies and motoneuron diseases.
Microneurography
Conventional nerve conduction studies assess mainly the functional state of large myelinated fibers; thus, small fibers involved in nociception, thermal sensation and autonomic functions cannot be assessed, even though they are frequently affected by chemotherapy agents (Grisold et al., 2012). Microneurography allows overcoming this pitfall. It was first developed in human setting in the 1960s (Hagbarth and Vallbo, 1968; Vallbo and Hagbarth, 1968); despite it never reached a widespread use, given its technical complexity and time-consuming features, it has relevant applications for research and a few groups have translated this technique to the preclinical setting. Serra et al. (2010) proposed a detailed method to assess unmyelinated C-fibers in rats, targeting alterations underlying neuropathic pain and dysautonomia. Briefly, the anesthetized rat is subjected to sciatic nerve exposure and a microneurography tungsten needle inserted within the nerve. Recording of neural activity, spontaneous and evoked by stimulation under specific protocols, allows to identify different functional types of small nerve fibers and their potential dysfunctions (Serra et al., 2012). The microneurography procedure can be carried on for 3–5 hours if it is a one-time procedure, but if longitudinal monitoring is sought for, less than 3 hours recording allows animal recovery for subsequent procedures. Specialized equipment is required (a shielded cage, micromanipulator for intraneural electrode positioning, special amplifier, appropriate hardware and software settings). The advantage of microneurography in rodents is that it is an in vivo technique and it does not damage axons during the recordings, as happens with single unit nerve recordings (Fleischer et al., 1983; Koltzenburg et al., 1997). This technique is far more stable than in humans provided that animals are anesthetized, and an open-field setting is possible. The natural situation of the tested nerve is maintained during the recording (i.e., temperature, vascular supply, humidity), and the exposure to active drugs is similar to clinical practice (i.e., the compound active on small fibers is administered per os, intravenous or intraperitoneal, better mimicking the route of administration in clinical practice).
Nerve Excitability Testing
A technique to test axonal excitability, Nerve Excitability Testing (NET), was developed in the clinical setting in the 1990s (Bostock et al., 1998). NET provides complementary data to nerve conduction studies; in fact, nerve excitability properties reflect many different elements involved in impulse conduction: ion channels and ion pumps activation and ion exchanges (Burke et al., 2001; Kiernan et al., 2000; Krishnan et al., 2008). Therefore, NET allows to gain inferences on axonal properties, detection of early functional changes that can precede neuropathy detection by conventional nerve conduction studies. It is not a widely used technique since it has been introduced in the preclinical setting quite recently and it requires specialized equipment (hardware and setting), software and a trained examiner. For NET recordings a non-invasive montage is required, similar to that of conventional nerve conduction tests, apart from the use of non-polarizable electrodes for the stimulating dipole. A dedicated software and hardware are used; the TROND protocol, a semi-automated computer-controlled protocol, is available in the Qtrac® software (Kiernan et al., 2000, 2001). TROND protocol allows to obtain multiple excitability parameters that can be used to define both early neuropathy signs and to identify pathogenetic mechanisms, useful to drive future drug discovery in this field. Regarding CIPN, NET has been mainly used to test patients with alterations due to oxaliplatin administration. These studies showed alterations compatible with axonal hyperexcitability (Park et al., 2009) and slowed sodium voltage operated channels activation (Heide et al., 2018), thus confirming the potential role of NET in CIPN pathogenetic studies. NET has been successfully performed both in rats (Yang et al., 2000; George and Bostock, 2007; Arnold et al., 2017) and in mice (Boërio et al., 2009, 2010) in models of neuropathy other than CIPN. Recently Alberti et al. (2019) used NET in a rat model of oxaliplatin neuropathy, demonstrating not only the presence of axonal hyperexcitability but also that modulating alterations seen in NET resulted in neuroprotection.
Electrophysiological evaluation of reflex circuits
Electrophysiological methods are useful for the quantitative assessment of the spinal reflex circuits and their dysfunctions. Testing monosynaptic H and polysynaptic withdrawal reflexes are valuable for the investigation of secondary alterations to peripheral nerve injuries, such as neuropathic pain and neuronal hyperexcitability. The H reflex represents the equivalent electrophysiological response to the functional stretch reflex. The H wave can be easily recorded when performing motor nerve conduction tests. Following stimulation to a motor or mixed nerve, the recording on the target muscle shows a short-latency direct CMAP or M wave, elicited by activation of the Aα motor fibers at the site of stimulation, followed by a late response, the reflex H wave, due to stimulation of the large Ia proprioceptive sensory fibers that conduct the impulse to the spinal cord where they synapse with the α-motoneurons and part of them are excited to conduct impulses to the muscle. In rodents the H wave is evoked at low threshold similar to the M wave and, unlike in humans, it is consistently maintained even when delivering high intensity stimuli, although it may slightly decrease in amplitude at supramaximal stimulus for the M wave (Meinck, 1976; Valero-Cabré and Navarro, 2001). The H wave amplitude provides an indirect measure of the efficacy of transmission between Ia sensory fibers and α-motoneurons, and of the excitability of the motoneuron pool (Valero-Cabré and Navarro, 2001; English et al., 2007). The H wave latency may be used to assess the central conduction time (H latency – M latency), and to derive the conduction velocity of sensory Ia proprioceptive fibers. This neurophysiological parameter has been used in the characterization and to assess neuroprotective strategies in cisplatin, vincristine and taxanes models (De Koning et al., 1987; Cliffer et al., 1998; Ja’afer et al., 2006; Callizot et al., 2008). Evaluation of the full stretch reflex by stretch applied to the muscle tendons can be also of interest in CIPN experimental paradigms, but it is quite more complex to apply (Goldstein et al., 1981).
The functional status of the facial and trigeminal nerves can be evaluated using the blink reflex (Terrell and Terzis, 1994), paralleling the electrophysiological test utilized in human patients. There is increasing evidence of impairment of corneal innervation in patients under chemotherapy treatment (Argyriou et al., 2019) and also in paclitaxel animal model (Ferrari et al., 2013) assessed by corneal confocal microscopy. Thus, blink reflex can be an alternative to monitor the functional involvement of trigeminal nerve fibers in CIPN.
Functional motor tests
Rotarod test
The rotarod test is a broadly used method that allows to assess general sensory-motor function and coordination. The animals are placed on the rod of a rotarod apparatus, which is made to rotate either at a fixed rate (5–20 rpm) or at accelerating rate (0 to 40 rpm), until the animals are unable to maintain themselves on the rod and fall off. The time or the maximal rate of maintenance on the turning rod is measured. Animals have to be trained for several days before starting the study, and then tested at regular intervals to ensure consistent performance, since performance in the test is affected by learning during initial trials. To prevent visual information and thus to enhance the role of proprioceptive input, the rotarod test may be performed in the dark (Apfel et al., 1992). However, the rotarod is not a specific test, since abnormalities can be due to sensory or motor nerve dysfunction but also to central alterations of coordination and strength. The rotarod has been shown useful for the study of animal models of peripheral neuropathies (Apfel et al., 1992; Rustay et al., 2003; Callizot et al., 2008; Bruna et al., 2010; Bruna et al., 2011) and is commonly applied in motoneuron disease models (Miana-Mena et al., 2005). It has been noted that certain animal strains are not testable on the rotarod due to their propensity to jump from the rod instead of running on it. Variable conditions have been reported in the literature, and the optimal parameters (e.g. rotation speed and acceleration) need to be defined for each condition (Rustay et al., 2003).
Gait and kinematic analyses
The analysis of the walking pattern by recording the footprints is a well-established and widely employed method for the assessment of motor recovery after nerve injuries, mostly of the sciatic nerve. The plantar surface of the hindpaws is painted (with ink or other means) and the animal allowed to walk along a narrow track. Then, measures of the footprints length and toes spreading are taken to derive the sciatic functional index (SFI) (De Medinaceli et al., 1982; Bain et al., 1989). However, its use in the CIPN field is anecdotal (Tassler et al., 2000). Taken into account the disappointing results obtained in the traumatic or surgical nerve injury models (Varejao et al., 2004; Navarro, 2016) and the lack of reports, the usefulness of this test in the evaluation of CIPN models is more than uncertain.
In order to overcome some of the frequent problems found in hard prints of the rat/mouse feet, video recordings of the walking animal were introduced, and analysis expanded to the gait kinematics. Rats are recorded walking in a transparent runway with a camera placed under the runway or at the side. The video recordings enable analysis of quantitative gait parameters (stride length, stance and swing phase duration, ankle angle) as well as kinematic reconstructions of paw excursion. Commercially computerized systems (CatWalk®, DigiGait®) are available allowing a quantitative assessment of both dynamic and static gait changes associated with nerve injuries and diseases. The most relevant disturbances observed in CIPN models are increases in the swing walk phase and decreases in the stance walk phase and the print area of hind paws (Huehnchen et al., 2013; Boehmerle et al., 2014). Discriminant models using kinematics data of ankle, knee and hip joints revealed high sensitivity and specificity to identify denervated versus reinnervated and control animals although such analysis has not been applied to neuropathies (Amado et al., 2011).
Functional sensory tests
Pinprick test for assessing innervation of the skin
Recovery of pain sensitivity can be tested on awake animals by light pricking with a blunt needle at several distal points on the skin of the projection territory of the nerve under study. For example, after injuries to the sciatic nerve, six delineated areas of the plantar skin are tested, from the ankle to the tip of the toes (Navarro et al., 1994; Cobianchi et al., 2014). Each site is stimulated two or three times, and responses are recorded as positive only when clear reaction of limb withdrawal and vocalization appear. A pinprick score can be constructed by addition of the graded response in each delimited area (Navarro et al., 1994). Given the discrete area that is stimulated for each pinprick, this test is useful to assess hyperalgesia, progression of skin denervation with time, and to map the denervated territory (Cobianchi et al., 2014; Vashistha et al., 2017; Kaur and Muthuraman, 2019).
Algesimetry tests
Algesimetry tests are mainly used to assess hypersensitivity in neuropathic or inflammatory pain models. However, the same behavioral tests are useful to evaluate the decrease of innervation by different modalities of nociceptive sensory neurons in neuropathies.
Mechanical algesimetry is mostly tested using von Frey filaments (Chaplan et al., 1994) or von Frey electronic devices (Casals-Diaz et al., 2009). Animals are placed on a wire net platform in plastic chambers and habituated. Then, von Frey calibrated filaments or the probe of the electronic device is gently applied to a test site in the plantar surface of the fore- or hind-paw, increasing the pressure (Fig. 2A–B). The nociceptive threshold is expressed as the force at which the animal withdraws the paw in response to the stimulus. The modified Randall-Selitto test can be also applied to test pressure-induced pain. An increasing amount of pressure with a pointed probe is exerted to the animal paw and the amount of force needed for withdrawal or vocalization is the withdrawal threshold (in grams) (Anseloni et al., 2003; Santos-Nogueira et al., 2012). This test may assess nociceptors in deeper tissues.
Figure 2.
Pain sensibility tests. A. An electronic Von Frey device for the mechanical algesimetry test. B. Image of the stimulation with the tip of the Von Frey probe pressing the lateral side of the sole. Note the different position of the intact paw (top) and the denervated paw (bottom) of the rat. C. Plantar algesimetry apparatus for the hot algesimetry test. D. Image of rat being stimulated with the hot light beam under the paw. Reproduced with permission from Navarro, Eur. J. Neurosci. 2016; 43:271–286.
Thermal algesimetry tests use a radiant heat source (Hargreaves et al., 1988). The animal is placed into a plastic box with an elevated glass floor. The beam of the lamp probe is focused on the paw plantar surface, until the animal withdraws the paw, and sensors in the device shut off the lamp (Fig. 2D–E). The threshold is defined as the latency time until withdrawal. Other devices that can be also used for the same type of evaluation include the hot plate and tail-flick tests.
For cold algesimetry the animal is placed into a similar box on a wire mesh platform. A small pointed ice probe is applied directly on the plantar surface of both hindpaws (Lindsey et al., 2000) or a drop of acetone deposited, and the withdrawal latency measured with a time-meter. The cold plate can be also used, although it exposes the four paws to cool, and it has been pointed out in some studies that cold temperature exposure does not appear to evoke “pain” in uninjured animals, making difficult the interpretation of hyperalgesia (Ta et al., 2009). Responses to cold result more variable than those to heat and pressure in rodents as well as in human subjects (Navarro and Kennedy, 1991; Galtrey and Fawcett, 2007; Casals-Diaz et al., 2009) so the cold testing has more problems in interpretation (Hama and Takamatsu, 2016).
To reduce the variability in testing conditions, the withdrawal responses are recorded at least in triplicate, with minutes between stimuli, for one or both paws at each testing day, and the mean of the values used for calculating the percentage of the experimental vs the control group tested in parallel. Using these tests, the accompanying vocalization or paw lick should be monitored in order to further assess whether foot withdrawal is due to a painful stimulus (Kemp et al., 2011). It is important to note that algesimetry results are indicative of a reduced withdrawal threshold, and not directly of pain, which is a subjective sensation. The lowered withdrawal response in models of CIPN is attributed to sensitization of nociceptors to mechanical and thermal stimuli, although an important part of these reports use short or very short schedules of drug administration. The most commonly described results in rats and mice receiving different chemotherapeutic agents have been mechanical and thermal hyperalgesia (Hama and Takamatsu, 2016). Nevertheless, later in the course increased thresholds evidencing hypoalgesia due to skin denervation may appear. Thus, controversial results are expected in the literature depending upon drug exposure and time, but also biased testing. The mechanical algesimetry and the thermal algesimetry methods can give similar results or sometimes diverging results, depending on the particular involvement of the different populations of primary nociceptive neurons (Verdú et al., 1999; Authier et al., 2000; Dina et al., 2001; Ta et al., 2009).
Conditioned Place Preference
The ability to measure the affective component of pain and pain relief using the Conditioned Place Preference (CPP) procedure is an interesting recent progress in the evaluation of animal models of pain. CPP allows to measure the non-evoked spontaneous or ongoing component of neuropathic pain. Pairing a rewarding experience with a distinctive environment increases the time spent in that environment (Navratilova and Porreca, 2014). This is an important procedure for testing potential treatments that alleviate pain in CIPN rodent models. Drugs that are not rewarding in the absence of pain (or at least at doses that do not activate reward pathways) become rewarding in the presence of CIPN since they improve pain sensation (Park et al., 2013; Toma et al., 2019).
Functional autonomic tests
Sweat gland function tests
These tests allow evaluating the sympathetic sudomotor function non-invasively in diverse animal models. Sweating is stimulated by cholinergic stimulation with pilocarpine or by thermal stimulation heating the animal body. Then, a silicone material is spread over the plantar surface of the paw; as the silicone hardens, it retains the impressions caused by the sweat droplets emerging from each sweat duct. The silicone mold is viewed under a dissecting microscope using transmitted light to count the number of sweat impressions (Navarro and Kennedy, 1989). Denervated sweat glands do not secrete, so the number and volume of the sweat impressions are reliable measures of loss of sympathetic axons (Bharali et al., 1988, Navarro and Kennedy, 1989). Despite its use is not much extended in the CIPN assessment, it has been employed in the characterization of cisplatin (Vilches et al., 1998) and bortezomib (Bruna et al., 2010) induced neuropathies in mice. A variation using iodine paper to record the sweat secretion has been also used in diabetic neuropathy studies (Liu et al., 2017).
Heart rate variability tests
Heart rate variability, which is influenced by the activity and balance between the sympathetic and parasympathetic cardiac sinus innervation, is an extended non-invasive measure to assess autonomic neuropathy in patients. However, its use in rodent models carry important difficulties, such as the high heart rate and frequent recording artifacts. Most reports come from the cardiology field and show discrepancies about the analysis and recording methodology (Thireau et al., 2007). Most works use 24h continuous electrocardiogram recordings, and the telemetry device requires surgery for subcutaneous implanting teletransmitters. More practical for testing in CIPN models are the protocols involving short time recording periods in the study of rodent R-R variability (Gehrmann et al., 2000). After propranolol and atropine-induced conduction blocks, the parasympathetic innervation is the main responsible of the R-R variability in mice. Thus, this technique could be useful to study the parasympathetic branch in autonomic neuropathy. However, this type of tests has been scarcely attempted in CIPN experimental models (Bruna et al., 2010).
HISTOLOGICAL METHODS
Light microscopy and nerve morphometry
The morphological examination of peripheral nerves provides the needed information about structural characteristics of nerve fibers, both axonal and myelin sheath compartments, to complement the functional studies for understanding the pathological process involving peripheral nerves (Marmiroli et al., 2012). The different techniques developed allow either qualitative assessments, as the observation of Wallerian degeneration phenomena and macrophage infiltration, and quantitative assessments, including quantification of the number of nerve fibers and measurements of axonal and fiber perimeters and myelin thickness.
The nerves most commonly used for histological evaluation of peripheral neuropathy are the sciatic nerve and its tributaries. In CIPNs, probably the most distal nerves are more likely to present the most marked abnormalities, although these nerve segments are not usually studied or reported. The nerve has to be harvested carefully, after fixation by cardiac perfusion or local immersion (Kasukurthi et al., 2009). Fixation is preferred with a mixture of glutaraldehyde and paraformaldehyde, at appropriate osmolarity. Specific details on using fixatives for light microscopy or immunohistochemical procedures have been published (Fix and Garman, 2000). Then the nerve is segmented and embedded in resin; paraffin embedding does not allow thin sectioning for optimal viewing of the nerve. Toluidine blue staining of resin embedded semi-thin nerve sections is the standard method to visualize myelinated nerve fibers, that can be enhanced by the use of osmium tetroxide after the fixation. Other stains show suboptimal resolution in comparison to toluidine blue for morphometric purposes. Transverse semithin sections (0.5–1 μm) are obtained with an ultracryotome and inspected (Ghnenis et al., 2018).
Data on the number of axons, myelin thickness, axon diameter, and the relationship between axon diameter and myelinated fiber diameter (g-ratio) can be extracted from morphometric analysis. These data are useful to demonstrate the neuropathy and assess its severity, through fiber quantification, and also to provide information about the predominant nature of the neuropathy (axonal and/or demyelinating), by measuring g-ratio and size of axons and myelin sheaths. Quantitative morphometry is done by measuring the whole nerve cross sectional area and then analyzing systematic randomly selected fields at 1000–2000x magnification, covering a representative part of the nerve (at least 15% of the total area, containing 500 myelinated fibers) (Gomez et al., 1996), using stereological methodology (Coggeshall and Lekan, 1996). For morphometrical measures, the manual method is more time-consuming and tedious, but the measurements are more accurate than using automated approaches (Bilego-neto et al., 2013). For this reason, some groups have developed semi-automated methods with good results (Urso-Baiarda and Grobbelaar, 2006).
Ultra-thin sections can be also obtained, counterstained with lead citrate and processed for electron microscopy observation. Under higher magnification, it is possible to assess changes in organelles of axons and Schwann cells, to refine observations on myelin lamellae and alterations. For example, swollen and vacuolated mitochondria in myelinated and unmyelinated axons in sensory nerves have been described as a relevant feature after systemic administration of different cytotherapy drugs (Flatters and Bennett, 2006; Bruna et al., 2010; Xiao et al., 2012). Electron microscopy also allows a quantitative approximation to the number of unmyelinated fibers and the microtubule configuration (Jenq and Coggeshall, 1985; Tanner et al., 1998; Topp et al., 2000).
Skin immunohistochemistry
In the early 1990s the immunohistochemical methods started to be applied in the morphologic study of skin innervation in neuropathic conditions, both in patients and rodent models, after the availability of the pan-axonal marker protein gene product (PGP) 9.5 (Kennedy and Wendelschafer-Crabb, 1993; Navarro et al., 1995). Later, their use was extended, refined and the assessment method systematized for clinical studies (Lauria et al., 2009; Lauria et al., 2010). Immunohistochemical labeling of skin samples makes possible the visualization of unmyelinated, small myelinated and the endings of large myelinated fibers from sensory and sympathetic neurons within the epidermis and dermis layers and in visceral appendices (sweat glands, blood vessels, piloerector muscles). Thus, this technique can be used to study the peripheral innervation of all types of nerve fibers, and of particular interest small nerve fibers that are commonly not assessed by classic histological techniques and conventional nerve conduction tests.
Unlike in human patient studies, the repeated collection of samples along the time is generally not used in animal models, although it may be done if adequate care of the biopsy wound is taken. Most often the plantar pads, and sometimes the dorsal foot skin of mice or rats are harvested by micro-dissection. The skin sample is immediately fixed in paraformaldehyde (Lauria et al., 1999) and then cryopreserved. Serial sections perpendicular to the epidermis, about 40 to 80 μm thick, can be obtained using freezing microtome or cryostat, and incubated with the primary antibody of interest. Then, two methods can be used for detection, the bright-field (McCarthy et al., 1995) and the indirect immunofluorescence (Kennedy and Wendelschafer-Crabb, 1993), showing both similar efficiency results for diagnostic purposes (Nolano et al., 2015). The indirect immunofluorescence offers the possibility of identifying multiple antigens in the same tissue section, turning this method more interesting for research purposes.
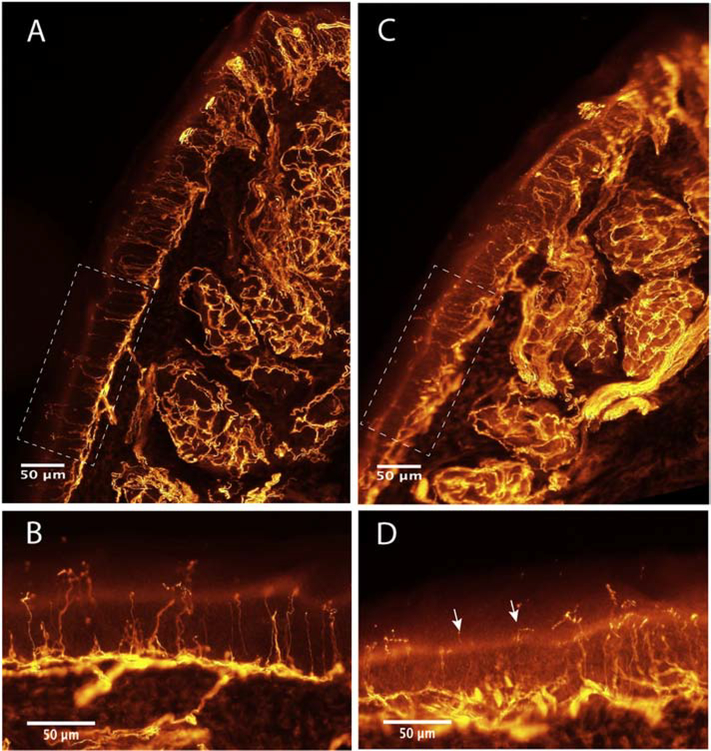
For overall innervation assessment PGP 9.5 is the marker of choice (Figure 3). To evaluate the unmyelinated intraepidermal nerve fibers, PGP 9.5 positive profiles crossing the dermal-epidermal junction are counted, and the linear density calculated (Verdú et al., 1999; Lauria et al., 2005). Despite having received less attention, the same immunohistochemical labeling allows the quantification of additional features, such as density of innervated Meissner corpuscles (Verdú et al., 1999; Provitera et al., 2007), length of dermal fibers (Lauria et al., 2011), the relative sweat gland innervated area (Verdú et al., 1999; Vilches et al., 2002), and the semi-quantitative assessment of structural fiber abnormalities like swellings and fragmentations. Likewise, another potentially useful measure for CIPN models allowed by the immunohistochemical methods, is the quantification of muscle spindle receptors from Ia sensory fibers (Verdú and Navarro, 1997).
Figure 3.
Immunohistochemical images of the plantar pad of a control mouse (A, B) and a mouse treated with cisplatin (C, D), labeled against PGP9.5. The images show densely innervated sweat glands in the dermis, the subepidermal nerve plexus underlying the epidermis, the Meissner corpuscles at the papillae of the pad tip (top part of the pad), and the numerous intraepidermal nerve fibers (IENF) crossing the epidermis. Boxes in A,C are the area of epidermis magnified in B,D. Note the reduction in number of IENF and the degenerating appearance of some IENF (arrows) in the skin of the cisplatin-treated mouse.
Other markers besides PGP 9.5 can be used to study specific components of interest, such as calcitonin gene related peptide (CGRP), substance P, somatostatin, transient receptor potential (TRP) family, cannabinoid and purinergic receptors for sensory nerve endings in pain-related studies, or vasoactive intestinal peptide (VIP), neuropeptide Y, tyrosine and dopamine β hydroxylase, dynorphin, choline acetyl-transferase, nitric oxide synthase type I, and also CGRP for the study of different populations of autonomic fibers. Of interest, the immunolabeling for neuropeptides, such as CGRP and VIP, may detect a reduction that appears earlier than signs of fiber degeneration, becoming early markers of dysfunction at low levels of intoxication in certain CIPN (Verdú et al., 1999; Gracias et al., 2011).
DRG histological evaluation
Sensory neurons present in the DRG are one of the main cells of interest in the study of CIPN, especially for compounds that accumulate in the cell soma and nucleus, like platinum drugs (Tredici et al., 1998). For morphological studies, DRG have to be carefully dissected, fixed in paraformaldehyde and cryopreserved, and embedded in resin or paraffin depending on the type of technique. Then thin sections can be examined under light or electron microscopy (Cavaletti et al., 1992; Jamieson et al., 2007). DRG morphometrical analysis provides data about neuron cell body, nuclear and nucleolar size and eccentricity. Changes in neuronal counting and morphology provides information about the severity of the neuropathy and even about the predominant type of neurons involved (small, medium or large size). To estimate a total cells count the dissector principle is used (Pover and Coggeshall, 1991). Immunohistochemical analyses provide a means of assessing the different functional populations of neurons, by labeling against CGRP and substance P for peptidergic nociceptive neurons, against IB4 for non-peptidergic neurons, against parvalbumin for proprioceptive neurons, and against Npy2r for innocuous mechanosensitive neurons; or labeling receptors related with the pathway of interest (Barajon et al., 1996; Joseph et al., 2008; Bruna et al., 2010). The histological study of DRGs also enables assessing the participation of the satellite cells in CIPN models, of especial interest in neuropathies with painful component (Warwick and Hanani, 2013).
FLUID BIOMARKERS
Fluid biomarkers can be of a great value in the clinical setting to predict the neurological outcome of the patient following treatment and to stratify patients in risk classes, particularly if they can be measured on blood samples. Potentially useful blood biomarker can be tested for a proof of concept of their utility. A good example is Neurofilament Light Chain (NfL), that can be determined in body fluids using the Simoa Nfl assay (Rohrer et al., 2016). NfL is a neuron-specific protein of the cytoskeleton involved in cell structural stability. Damage to axons results in release and increased levels of NfL into the interstitial fluid and, therefore, it can be detected in blood and cerebrospinal fluid samples. Recently, increased NfL concentration has been demonstrated in a cross-sectional study performed in inherited peripheral neuropathies, and their levels correlated with the severity of nerve impairment (Sandelius et al., 2018). So far, NfL levels have not been tested at a clinical level in CIPN, but there are promising preclinical evidence of increased NfL levels in a rodent models of vincristine CIPN. Therefore, it would be interesting to evaluate NfL levels in other animal models as well as in CIPN patients to evaluate their usefulness as a predictive biomarker.
ETHICAL CONSIDERATIONS
Animal models are a precious tool in the study of peripheral neuropathies, including CIPN, since they allow to evaluate molecular and cellular processes at a level that is not possible in human patients. Importantly, exploration of novel treatments requires thorough preclinical testing of drugs, compounds or gene therapy vectors in adequate animal models, most often done in rodent models. This is of particular attention for the assessment of possible adverse events, that requires a live animal capable of generating behavioral and neurophysiological signs and symptoms, and therefore cannot be achieved with in-vitro or ex-vivo methods. However, when performing preclinical research in vivo, ethical considerations have to be followed. First concerns against animal vivisection were raised long ago, in 1875 by the Society for the Protection of Animals Liable to Vivisection (MacArthur Clark et al., 2019) and in the second half of the 20th century many regulatory laws were approved. A relevant event was the release of the 3Rs (Replacement, Reduction and Refinement) principle of Russels and Burch, which then evolved in fundamental rules underlying the use of animals in experimental research and the need for developing alternative models (MacArthur Clark, 2018). However, a balance must be found between safeguarding animal welfare and performing high-quality research. In the case of peripheral neuropathies, animal models are still a precious cornerstone for anatomical, pharmacokinetics and pharmacodynamics considerations. The animal models developed accurately replicate the main CIPN conditions, including similar histopathology, sensory, motor and autonomic impairments, making them appropriate for studies that are relevant for the human diseases. The use of in vivo preparations, evaluated by functional, behavioral and morphological analyses, is mandatory, since in vitro and cell culture studies cannot fully reproduce the complex aspects of diseases and injuries affecting the nervous system. Moreover, only in vivo settings allow a similar administration compared to the “bed-side” when testing compounds to modify peripheral nerve damage (Cavaletti, et al., 2008; Cavaletti and Marmiroli, 2015).
As pointed out by Brabb et al. (2014), the institutional Animal Care and Use or Ethical Committees should consider the same issues which are in common with other disease models when evaluating a model for peripheral nerve damage (i.e., if the general rules are met, and if there is a sound research design and scientific approach), but some specific issues arise. In general, pain is a condition which should be avoided in in vivo research; however, in CIPN models, neuropathic pain is one of the features that should be reproduced, since it is frequent in human patients. Thus, the researcher as well as the responsible veterinarian face the challenge to address animal welfare balancing it against the need to obtain high quality and translational data (Carbone, 2011). Moreover, as recently pointed out (Bayne and Turner, 2019), a harmonized, conscientious care and use of animals is necessary to mitigate potential confounding factors and to obtain high standard inferences; a simple example is animal husbandry and veterinarian care. Several organization are contributing to this goal, such as the Federation of European Laboratory Animal Science Association (FELASA, for more information see: www.felasa.eu) in Europe and the Institute for Laboratory Animal Research (ILAR, www.ilar.org) in the USA. Regarding study design and communication, the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments; www.nc3rs.org.uk/arrive-guidelines) are intended to improve the performance, reporting and publication of research using animals, maximizing the information published and thus minimizing unnecessary and repeated studies. Last, but not least, it is important to appropriately communicate the results of preclinical research to the general public and to the stakeholders, to educate the general public of the potential of a well-conducted, respectful animal research as addressed in a recent ILAR review (MacArthur Clark et al., 2019).
CONSIDERATIONS FOR OPTIMIZING PRECLINICAL STUDIES
The comparison of the functional state of different types of peripheral nerve fibers is possible by using several neurophysiological methods that allow evaluation of responses in sensory and motor nerves, skin receptors, skeletal muscle and glands in the same animal model. By using several complementary methods, qualitative differences between types of nerve fibers have been described, allowing a phenotypic characterization of the different types of CIPN models. Therefore, variable results between different methods applied to evaluate peripheral nerve function in the same animals are not unexpected. The variability may be due to the type of fibers assessed, the complexity of the functional response, and the sensibility of the methods to detect the response. Indeed, some of the reported differences between experimental studies might be influenced by variations in the animal model, schedules of treatment, methodological changes between laboratories, and to discrepancies between morphological and functional investigations.
Comparisons between experimental studies may be hampered due to the variable susceptibility of different species and strains of rodents (Tomiwa et al., 1986; Podratz et al., 2016; Marmiroli et al., 2017), and the variable time and intensity of effects of different drug administration schedules as demonstrated in human patients (Cavaletti et al., 1992). The generalizability of findings from rodent studies of CIPN has to be improved by the inclusion of both sexes in experimental studies. In addition, the age of the animals should be considered, since there are age-related changes in the function and structure of the peripheral nerves (Verdú et al., 2000; Canta et al., 2016). In order to make comparisons of data from different laboratories more valid, it has been previously proposed that a consensus should be reached on choice of animals (similar sex, age, strain and genetic background), mode and schedule of drug delivery, periodicity of testing, and also on outcome measures used in evaluation (Höke and Ray, 2014).
A relevant point when studying novel strategies to prevent or improve CIPN is that the methods selected allow serial evaluation of innervation at desired intervals without having to kill the animals, and that are not or minimally invasive in order to not disturb the nerve environment during the test. The selection of methods has to be in accordance with the objectives of the study. Therefore, different methods should be considered to assess different types of nerve fibers from a functional and a morphological perspective. For a multimodal analysis approach, it would be recommended to combine electrophysiological tests (nerve conduction tests for sensory and motor fibers) and behavioral tests (algesimetry, rotarod) that can be repeatedly performed along follow-up (Table 1), with the histological study of the peripheral nerve (fiber count, morphometrical analysis) and immunohistochemical study of the skin innervation. It has been previously proposed that histology and electrophysiology should be used in concert, since they offer complementary views of neurotoxic damage (Arezzo et al., 2011). The results of each test may not necessarily correlate with those of other tests, but all together allow for a comprehensive quantitative evaluation of the complex processes that may develop in CIPN. Indeed, a consensus has been reached on a unified approach to define diabetic neuropathy phenotype in rodents by the presence of statistically different values between diabetic and control animals in 2 of 3 assessments including nociceptive behavioral test, nerve conduction tests, and nerve structure (Biessels et al., 2014). A similar proposal has been raised for the evaluation of nerve regeneration after traumatic injuries (Navarro, 2016). Such a consensus will improve the capability for different research groups to compare and share preclinical data relevant for investigating the mechanisms underlying CIPN and preventive therapies.
Table 1.
Tests classified upon the nerve function assessed, and the main outcome parameters.
| Test | Useful measure | Type of neurons/axons |
|---|---|---|
| Nerve conduction in situ | CNAP and conduction velocity | All classes |
| Sensory Nerve conduction | CNAP amplitude and SCV | Myelinated Sensory |
| Motor Nerve conduction | CMAP amplitude and MCV | Motor |
| Motor Unit number estimation | Number of motor units innervated | Motor |
| Reflex tests | Spinal reflex circuit. H wave amplitude and latency | Sensory - Motor |
| Pinprick test | Skin innervation. Extension of denervation | Sensory nociceptive |
| Algesimetry, mechanical | Withdrawal threshold. Mechanical allodynia | Sensory nociceptive |
| Algesimetry, thermal heat | Withdrawal threshold. Hot hyperalgesia | Sensory nociceptive |
| Algesimetry, thermal cold | Withdrawal threshold. Cold hyperalgesia | Sensory nociceptive |
| Silicone mold test | Number of innervated sweat glands | Sympathetic sudomotor |
| R-R variability | Heart rate variability | Parasympathetic cardiac |
| Grasping test | Grasp strength | Motor |
| Rotarod | Time in the rod | Motor – Sensory, Coordination |
| Walking track | Foot prints during locomotion. SFI | Motor |
| Video analysis of gait | Pattern of locomotion | Motor – Sensory, Integration |
| Somatosensory Evoked potentials | Amplitude and latency of SSEP | Sensory central pathway |
CNAP: compound nerve action potential; CMAP: compound muscle action potential; SCV: Sensory Nerve Conduction Velocity; MCV: Motor Nerve Conduction Velocity; SFI: Sciatic Functional Index; SSEP: Somatosensory Evoked Potential.
Acknowledgments
The authors research was supported by funds from CIBERNED and TERCEL networks to XN, PI1501303 grant from the Instituto de Salud Carlos III of Spain to JB, and FEDER funds, grants 1R01CA206028-01and R01CA219637-01 from the National Institutes of Health (NIH) to M.I.D.. JB has received support of grant number SLT008/18/00028 from the Department of Health of the Government of Catalonia CERCA Program.
Footnotes
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Hijleh MF, Habbal OA, Moqattash ST, 1995. The role of the diaphragm in lymphatic absorption from the peritoneal cavity. J. Anat. 186, 453–467. [PMC free article] [PubMed] [Google Scholar]
- Alberti P, 2017. Chemotherapy-induced peripheral neurotoxicity - outcome measures: the issue. Expert Opin. Drug Metab. Toxicol. 13, 241–243. [DOI] [PubMed] [Google Scholar]
- Alberti P, Canta A, Chiorazzi A, Fumagalli G, Meregalli C, Monza L, Pozzi E, Ballarini E, Rodriguez-Menendez V, Oggioni N, Sancini G, Marmiroli P, Cavaletti G, 2019. Topiramate prevents oxaliplatin-related axonal hyperexcitability and oxaliplatin induced peripheral neurotoxicity. Neuropharmacology doi.org/ 10.1016/j.neuropharm.2019.107905 [DOI] [PubMed] [Google Scholar]
- Amado S, Armada-da-Silva PA, João F, Maurício AC, Luís AL, Simões MJ, Veloso AP, 2011. The sensitivity of two-dimensional hindlimb joint kinematics analysis in assessing functional recovery in rats after sciatic nerve crush. Behav. Brain Res. 225, 562–573. [DOI] [PubMed] [Google Scholar]
- Anseloni VC, Ennis M, Lidow MS, 2003. Optimization of the mechanical nociceptive threshold testing with the Randall-Selitto assay. J. Neurosci. Methods 131, 93–97. [DOI] [PubMed] [Google Scholar]
- Apfel SC, Arezzo JC, Lipson L, Kessler JA, 1992. Nerve growth factor prevents experimental cisplatin neuropathy. Ann. Neurol. 31, 76–80. [DOI] [PubMed] [Google Scholar]
- Arezzo JC, Litwak MS, Zotova EG, 2011. Correlation and dissociation of electrophysiology and histopathology in the assessment of toxic neuropathy. Toxicol. Pathol. 39, 46–51. [DOI] [PubMed] [Google Scholar]
- Argyriou AA, Bruna J, Marmiroli P, Cavaletti G, 2012. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit. Rev. Oncol. Hematol. 82, 51–77. [DOI] [PubMed] [Google Scholar]
- Argyriou AA, Bruna J, Genazzani AA, Cavaletti G, 2017. Chemotherapy-induced peripheral neurotoxicity: management informed by pharmacogenetics. Nat. Rev. Neurol. 13, 492–504. [DOI] [PubMed] [Google Scholar]
- Argyriou AA, Park SB, Islam B, Tamburin S, Velasco R, Alberti P, Bruna J, Psimaras D, Cavaletti G, Cornblath DR, 2019. Neurophysiological, nerve imaging and other techniques to assess chemotherapy-induced peripheral neurotoxicity in the clinical and research settings. J. Neurol. Neurosurg. Psychiat. doi: 10.1136/jnnp-2019-320969. [DOI] [PubMed] [Google Scholar]
- Arnold R, Moldovan M, Rosberg MR, Krishnan AV, Morris R, Krarup C, 2017. Nerve excitability in the rat forelimb: a technique to improve translational utility. J. Neurosci. Methods 275, 19–24. [DOI] [PubMed] [Google Scholar]
- Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F, 2000. Description of a short-term taxol-induced nociceptive neuropathy in rats. Brain Res. 887, 239–249. [DOI] [PubMed] [Google Scholar]
- Bain JR, Mackinnon SE, Hunter DA, 1989. Functional evaluation of complete sciatic, peroneal and posterior tibial nerve lesions in the rat. Plast. Reconstr. Surg. 83, 129–136. [DOI] [PubMed] [Google Scholar]
- Barajon I, Bersani M, Quartu M, Del Fiacco M, Cavaletti G, Holst JJ, Tredici G, 1996. Neuropeptides and morphological changes in cisplatin-induced dorsal root ganglion neuronopathy. Exp. Neurol. 138, 93–104. [DOI] [PubMed] [Google Scholar]
- Bayne K, Turner PV, 2019. Animal welfare standards and international collaborations. ILAR J. doi: 10.1093/ilar/ily024. [DOI] [PubMed] [Google Scholar]
- Bharali LAM, Burgess SA, Lisney SJW, Pearson D, 1988. Reinnervation of sweat glands in the rat hind paw following peripheral nerve injury. J. Auton. Nerv. Syst. 23, 125–129. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Bril V, Calcutt NA, Cameron NE, Cotter MA, Dobrowsky R, Feldman EL, Fernyhough P, Jakobsen J, Malik RA, Mizisin AP, Oates PJ, Obrosova IG, Pop-Busui R, Russell JW, Sima AA, Stevens MJ, Schmidt RE, Tesfaye S, Veves A, Vinik AI, Wright DE, Yagihashi S, Yorek MA, Ziegler D, Zochodne DW, 2014. Phenotyping animal models of diabetic neuropathy: a consensus statement of the diabetic neuropathy study group of the EASD (Neurodiab). J. Peripher. Nerv. Syst. 19, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilego Neto AP, Silveira FB, Rodrigues da Silva GA, Sanada LS, Fazan VP, 2013. Reproducibility in nerve morphometry: comparison between methods and among observers. Biomed. Res. Int. 2013, 682849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmerle W, Huehnchen P, Peruzzaro S, Balkaya M, Endres M, 2014. Electrophysiological, behavioral and histological characterization of paclitaxel, cisplatin, vincristine and bortezomib-induced neuropathy in C57Bl/6 mice. Sci. Rep. 4, 6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boërio D, Greensmith L, Bostock H, 2009. Excitability properties of motor axons in the maturing mouse. J. Peripher. Nerv. Syst. 14, 45–53. [DOI] [PubMed] [Google Scholar]
- Boërio D, Kalmar B, Greensmith L, Bostock H, 2010. Excitability properties of mouse motor axons in the mutant SOD1(G93A) model of amyotrophic lateral sclerosis. Muscle Nerve 41, 774–784. [DOI] [PubMed] [Google Scholar]
- Bostock H, Cikurel K, Burke D, 1998. Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve 21,137–158. [DOI] [PubMed] [Google Scholar]
- Brabb T, Carbone L, Snyder J, Phillips N, 2014. Institutional animal care and use committee considerations for animal models of peripheral neuropathy. ILAR J. 54, 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer AL, Shirachi DY, Quock RM, Craft RM, 2019. Effect of hyperbaric oxygen on chemotherapy-induced neuropathy in male and female rats. Behav. Pharmacol doi.org/ 10.1097/FBP.0000000000000497 [DOI] [PubMed] [Google Scholar]
- Bruna J, Udina E, Alé A, Vilches JJ, Vynckier A, Monbaliu J, Silverman L, Navarro X, 2010. Neurophysiological, histological and immunohistochemical characterization of bortezomib neuropathy in mice. Exp. Neurol. 223, 599–608. [DOI] [PubMed] [Google Scholar]
- Bruna J, Alé A, Velasco R, Jaramillo J, Navarro X, Udina E, 2011. Evaluation of pre-existing neuropathy and bortezomib retreatment as risk factors to develop severe neuropathy in a mouse model. J. Peripher. Nerv. Syst. 16, 199–212. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Bagdas D, Goldberg LR, Khalefa T, Reed ER, Kirkpatrick SL, Kelliher JC, Chen MM, Johnson WE, Mulligan MK, Imad Damaj M, 2019. C57BL/6 substrain differences in inflammatory and neuropathic nociception and genetic mapping of a major quantitative trait locus underlying acute thermal nociception. Mol. Pain 15, 1744806918825046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryda EC, 2013. The Mighty Mouse: the impact of rodents on advances in biomedical research. Mol. Med. 110, 207–211. [PMC free article] [PubMed] [Google Scholar]
- Burke D, Kiernan MC, Bostock H, 2001. Excitability of human axons. Clin. Neurophysiol. 112, 1575–1585. [DOI] [PubMed] [Google Scholar]
- Callizot N, Andriambeloson E, Glass J, Revel M, Ferro P, Cirillo R, Vitte PA, Dreano M, 2008. Interleukin-6 protects against paclitaxel, cisplatin and vincristine-induced neuropathies without impairing chemotherapeutic activity. Cancer Chemother. Pharmacol. 62, 995–1007. [DOI] [PubMed] [Google Scholar]
- Canta A, Chiorazzi A, Carozzi VA, Meregalli C, Oggioni N, Bossi M, Rodriguez-Menendez V, Avezza F, Crippa L, Lombardi R, de Vito G, Piazza V, Cavaletti G, Marmiroli P, 2016. Age-related changes in the function and structure of the peripheral sensory pathway in mice. Neurobiol. Aging 45, 136–148. [DOI] [PubMed] [Google Scholar]
- Carbone L, 2011. Pain in laboratory animals: the ethical and regulatory imperatives. PLoS One 6, e21578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carozzi VA, Chiorazzi A, Canta A, Meregalli C, Oggioni N, Cavaletti G, Marmiroli P, 2015. Chemotherapy-induced peripheral neurotoxicity in immune-deficient mice: new useful ready-to-use animal models. Exp. Neurol. 264, 92–102. [DOI] [PubMed] [Google Scholar]
- Casals-Díaz L, Vivó M, Navarro X, 2009. Nociceptive responses and spinal plastic changes of afferent C-fibers in three neuropathic pain models induced by sciatic nerve injury in the rat. Exp. Neurol. 217, 84–95. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Marzorati L, Bogliun G, Colombo N, Marzola M, Pittelli MR, Tredici G, 1992. Cisplatin-induced peripheral neurotoxicity is dependent on total-dose intensity and single-dose intensity. Cancer 69, 203–207. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Nicolini G, Marmiroli P, 2008. Neurotoxic effects of antineoplastic drugs: the lesson of pre-clinical studies. Front. Biosci. 13, 3506–3524. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Marmiroli P, 2015. Chemotherapy-induced peripheral neurotoxicity. Curr. Opin. Neurol. 28, 500–507. [DOI] [PubMed] [Google Scholar]
- Ceballos D, Cuadras J, Verdú E, Navarro X. 1999. Morphometrical and ultrastructural changes with aging in the mouse peripheral nerve. J. Anat. 195,563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL, 1994. Quantitative assessment of allodynia in the rat paw. J. Neurosci. Methods 53, 55–63. [DOI] [PubMed] [Google Scholar]
- Cliff J, Jorgensen AL, Lord R, Azam F, Cossar L, Carr DF, Pirmohamed M, 2017. The molecular genetics of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 120, 127–140. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, Siuciak JA, Carson SR, Radley HE, Park JS, Lewis DR, Zlotchenko E, Nguyen T, Garcia K, Tonra JR, Stambler N, Cedarbaum JM, Bodine SC, Lindsay RM, DiStefano PS, 1998. Physiological characterization of Taxol-induced large-fiber sensory neuropathy in the rat. Ann. Neurol. 43, 46–55. [DOI] [PubMed] [Google Scholar]
- Cobianchi S, de Cruz J, Navarro X, 2014. Assessment of sensory thresholds and nociceptive fiber growth after sciatic nerve injury reveals the differential contribution of collateral reinnervation and nerve regeneration to neuropathic pain. Exp. Neurol. 255, 1–11. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA, 1996. Methods for determining numbers of cells and synapses: A case for more uniform standards of review. J. Comp. Neurol. 364, 6–15. [DOI] [PubMed] [Google Scholar]
- Cook BM, Wozniak KM, Proctor DA, Bromberg RB, Wu Y, Slusher BS, Littlefield BA, Jordan MA, Wilson L, Feinstein SC, 2018. Differential morphological and biochemical recovery from chemotherapy-induced peripheral neuropathy following paclitaxel, ixabepilone, or eribulin treatment in mouse sciatic nerves. Neurotox. Res. 34, 677–692. [DOI] [PubMed] [Google Scholar]
- Currie GL, Angel-Scott HN, Colvin L, Cramond F, Hair K, Khandoker L, Liao J, Macleod M, McCann SK, Morland R, Sherratt N, Stewart R, Tanriver-Ayder E, Thomas J, Wang Q, Wodarski R, Xiong R, Rice ASC, Sena ES, 2019. Animal models of chemotherapy-induced peripheral neuropathy: A machine-assisted systematic review and meta-analysis. PLoS Biol. 17, e3000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JN, Courtney CL, Superak H, Taylor DK, 2014. Behavioral, clinical and pathological effects of multiple daily intraperitoneal injections on fema le mice. Lab. Anim. 43, 131–139. [DOI] [PubMed] [Google Scholar]
- De Medinaceli L, Freed WJ, Wyatt RJ, 1982. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp. Neurol. 77, 634–643. [DOI] [PubMed] [Google Scholar]
- De Koning P, Neijt JP, Jennekens FG, Gispen WH, 1987. Org.2766 protects from cisplatin-induced neurotoxicity in rats. Exp. Neurol. 97, 746–750. [DOI] [PubMed] [Google Scholar]
- Dina OA, Chen X, Reichling D, Levine JD, 2001. Role of protein kinase C and protein kinase A in a model of paclitaxel-induced painful peripheral neuropathy in the rat. Neuroscience 108, 507–515. [DOI] [PubMed] [Google Scholar]
- English AW, Chen Y, Carp JS, Wolpaw JR, Chen XY, 2007. Recovery of electromyographic activity after transection and surgical repair of the rat sciatic nerve. J. Neurophysiol. 97, 1127–1134. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Nallasamy N, Downs H, Dana R, Oaklander AL, 2013. Corneal innervation as a window to peripheral neuropathies. Exp. Eye Res. 113, 148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fix AS, Garman RH, 2000. Practical aspects of neuropathology: a technical guide for working with the nervous system. Toxicol. Pathol. 28, 122–131. [DOI] [PubMed] [Google Scholar]
- Flatters SJL, Bennett GJ, 2006. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain 122, 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer E, Handwerker HO, Joukhadar S, 1983. Unmyelinated nociceptive units in two skin areas of the rat. Brain Res. 267,81–92. [DOI] [PubMed] [Google Scholar]
- Fricker B, Muller A, René F, 2008. Evaluation tools and animal models of peripheral neuropathies. Neurodegener. Dis. 5, 72–108. [DOI] [PubMed] [Google Scholar]
- Galtrey CM, Fawcett JW, 2007. Characterization of tests of functional recovery after median and ulnar nerve injury and repair in the rat forelimb. J. Peripher. Nerv. Syst. 12, 11–27. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez E, Schönberger T, Sumalla M, Stierstorfer B, Solà R, Doods H, Serra J, Gorodetskaya N, 2018. Behavioural, morphological and electrophysiological assessment of the effects of type 2 diabetes mellitus on large and small nerve fibres in Zucker diabetic fatty, Zucker lean and Wistar rats. Eur. J. Pain 22, 1457–1472. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Hammer PE, Maguire CT, Wakimoto H, Triedman JK, Berul CI, 2000. Phenotypic screening for heart rate variability in the mouse. Am. J. Physiol. Heart Circ. Physiol. 279(2), H733–740. [DOI] [PubMed] [Google Scholar]
- George A, Bostock H, 2007. Multiple measures of axonal excitability in peripheral sensory nerves: an in vivo rat model. Muscle Nerve 36, 628–636. [DOI] [PubMed] [Google Scholar]
- Ghnenis AB, Czaikowski RE, Zhang ZJ, Bushman JS, 2018. Toluidine Blue Staining of Resin-Embedded Sections for Evaluation of Peripheral Nerve Morphology. J. Vis. Exp. 137, e58031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gileta AF, Fitzpatrick CJ, Chitre AS, St Pierre CL, Joyce EV, Maguire RJ, McLeod AM, Gonzales NM, Williams AE, Morrow JD, Robinson TE, Flagel SB, Palmer AA, 2018. Genetic characterization of outbred Sprague Dawley rats and utility for genome-wide association studies. bioRxiv preprint posted online Sep 10, 2018 Doi: 10.1101/412924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BD, Lowndes HE, Cho E, 1981. Neurotoxicology of vincristine in the cat. Electrophysiological studies. Arch. Toxicol. 48, 253–264. [DOI] [PubMed] [Google Scholar]
- Gómez N, Cuadras J. Butí M. Navarro X, 1996. Histologic assessment of sciatic nerve regeneration following resection and graft or tube repair in the mouse. Restor. Neurol. Neurosci. 10, 187–196. [DOI] [PubMed] [Google Scholar]
- Gracias NG, Cummins TR, Kelley MR, Basile DP, Iqbal T, Vasko MR, 2011. Vasodilatation in the rat dorsal hindpaw induced by activation of sensory neurons is reduced by Paclitaxel. Neurotoxicology 32, 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisold W, Cavaletti G, Windebank AJ, 2012. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol. 14 Suppl 4, iv45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth KE, Vallbo AB, 1968. Discharge characteristics of human muscle afferents during muscle stretch and contraction. Exp. Neurol. 22, 674–694. [DOI] [PubMed] [Google Scholar]
- Hama A, Takamatsu Y, 2016. Chemotherapy-induced peripheral neuropathic pain and rodent models. CNS Neurol. Disord. Drug Targets 15, 7–19. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J, 1988. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77–88. [DOI] [PubMed] [Google Scholar]
- Heide R, Bostock H, Ventzel L, Grafe P, Bergmans J, Fuglsang-Frederiksen A, Finnerup NB, Tankisi H, 2018. Axonal excitability changes and acute symptoms of oxaliplatin treatment: In vivo evidence for slowed sodium channel inactivation. Clin. Neurophysiol. 129, 694–706. [DOI] [PubMed] [Google Scholar]
- Hirota J, Shimizu S, 2012. Routes of Administration, in: Hedrich H (Ed), The Laboratory Mouse (2nd Edition). Elsevier Ltd, London, pp. 709–725. [Google Scholar]
- Hoke A, Ray M, 2014. Rodent models of chemotherapy-induced peripheral neuropathy. ILAR J. 54, 273–281. [DOI] [PubMed] [Google Scholar]
- Huehnchen P, Boehmerle W, Endres M, 2013. Assessment of paclitaxel induced sensory polyneuropathy with “Catwalk” automated gait analysis in mice. PLoS One 8, e76772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang B-Y, Kim E-S, Kim C-H, Kwon J-Y, Kim H-K, 2012. Gender differences in paclitaxel-induced neuropathic pain behavior and analgesic response in rats. Korean J. Anesthesiol. 62, 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ja’afer FM, Hamdan FB, Mohammed FH, 2006. Vincristine-induced neuropathy in rat: electrophysiological and histological study. Exp. Brain Res. 173, 334–45. [DOI] [PubMed] [Google Scholar]
- Jamieson SM, Liu JJ, Connor B, Dragunow M, McKeage MJ, 2007. Nucleolar enlargement, nuclear eccentricity and altered cell body immunostaining characteristics of large-sized sensory neurons following treatment of rats with paclitaxel. Neurotoxicology 28, 1092–1098. [DOI] [PubMed] [Google Scholar]
- Jenq CB, Coggeshall RE, 1985. Numbers of regenerating axons in parent and tributary peripheral nerves in the rat. Brain Res. 326, 27–40. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Chen X, Bogen O, Levine JD, 2008. Oxaliplatin acts on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy. J. Pain 9, 463–472. [DOI] [PubMed] [Google Scholar]
- Justice MJ, Dhillon P, 2016. Using the mouse to model human disease: increasing validity and reproducibility. Dis. Model. Mech. 9, 101–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasukurthi R, Brenner MJ, Moore AM, Moradzadeh A, Ray WZ, Santosa KB, Mackinnon SE, Hunter DA, 2009. Transcardial perfusion versus immersion fixation for assessment of peripheral nerve regeneration. J. Neurosci. Methods 184, 303–309. [DOI] [PubMed] [Google Scholar]
- Kaur S, Muthuraman A, 2019. Ameliorative effect of gallic acid in paclitaxel-induced neuropathic pain in mice. Toxicol. Rep. 6, 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp SW, Webb AA, Dhaliwal S, Syed S, Walsh SK, Midha R, 2011. Dose and duration of nerve growth factor (NGF) administration determine the extent of behavioral recovery following peripheral nerve injury in the rat. Exp. Neurol. 229, 460–470. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Wendelschafer-Crabb G, 1993. The innervation of human epidermis. J. Neurol. Sci. 115, 184–190. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Burke D, Andersen KV, Bostock H, 2000. Multiple measures of axonal excitability: a new approach in clinical testing. Muscle Nerve 23, 399–409. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Lin CS, Andersen KV, Murray NM, Bostock H, 2001. Clinical evaluation of excitability measures in sensory nerve. Muscle Nerve 24, 883–892. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Stucky CL, Lewin GR, 1997. Receptive properties of mouse sensory neurons innervating hairy skin. J. Neurophysiol. 78, 1841–1850. [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Lin CS, Park SB, Kiernan MC, 2008. Assessment of nerve excitability in toxic and metabolic neuropathies. J. Peripher. Nerv. Syst. 13, 7–26. [DOI] [PubMed] [Google Scholar]
- Lauria G, Holland N, Hauer P, Cornblath DR, Griffin JW, McArthur JC, 1999. Epidermal innervation: changes with aging, topographic location, and in sensory neuropathy. J. Neurol. Sci. 164, 172–178. [DOI] [PubMed] [Google Scholar]
- Lauria G, Lombardi R, Borgna M, Penza P, Bianchi R, Savino C, Canta A, Nicolini G, Marmiroli P, Cavaletti G, 2005. Intraepidermal nerve fiber density in rat foot pad: neuropathologic-neurophysiologic correlation. J. Peripher. Nerv. Syst. 10, 202–208. [DOI] [PubMed] [Google Scholar]
- Lauria G, Lombardi R, Camozzi F, Devigili G, 2009. Skin biopsy for the diagnosis of peripheral neuropathy. Histopathology 54, 273–285. [DOI] [PubMed] [Google Scholar]
- Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, Nolano M, Merkies IS, Polydefkis M, Smith AG, Sommer C, Valls-Solé J, 2010. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur. J. Neurol. 17, 903–912. [DOI] [PubMed] [Google Scholar]
- Lauria G, Cazzato D, Porretta-Serapiglia C, Casanova-Molla J, Taiana M, Penza P, Lombardi R, Faber CG, Merkies IS, 2011. Morphometry of dermal nerve fibers in human skin. Neurology 77, 242–249. [DOI] [PubMed] [Google Scholar]
- Leandri M, Saturno M, Cilli M, Bisaglia M, Lunardi G, 2007. Compound action potential of sensory tail nerves in the rat. Exp. Neurol. 203, 148–57. [DOI] [PubMed] [Google Scholar]
- Lewis RE, Kunz AL, Bell RE, 1966. Error of intraperitoneal injections in rats. Lab. Anim. Care 16, 505–509. [PubMed] [Google Scholar]
- Lindsey AE, LoVerso RL, Tovar CA, Hill CE, Beattie MS, Bresnahan JC, 2000. An analysis of changes in sensory thresholds to mild tactile and cold stimuli after experimental spinal cord injury in the rat. Neurorehabil. Neural. Repair 14, 287–300. [DOI] [PubMed] [Google Scholar]
- Liston DR, Davis M, 2017. Clinically relevant concentrations of anticancer drugs: a guide for nonclinical studies. Clin. Cancer Res. 23, 3489–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sebastian B, Liu B, Zhang Y, Fissel JA, Pan B, Polydefkis M, Farah MH, 2017. Sensory and autonomic function and structure in footpads of a diabetic mouse model. Sci. Rep. 7, 41401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb GE, Gans C, 1986. Electromyography for experimentalists. University of Chicago Press, Chicago. [Google Scholar]
- MacArthur Clark J, 2018. The 3Rs in research: a contemporary approach to replacement, reduction and refinement. Br. J. Nutr. 120, S1–S7. [DOI] [PubMed] [Google Scholar]
- MacArthur Clark J, Clifford P, Jarrett W, Pekow C, 2019. Communicating about animal research with the public. ILAR J. doi: 10.1093/ilar/ilz007 [DOI] [PubMed] [Google Scholar]
- Mancuso R, Santos-Nogueira E, Osta R, Navarro X, 2011. Electrophysiological analysis of a murine model of motoneuron disease. Clin. Neurophysiol. 122, 1660–1670. [DOI] [PubMed] [Google Scholar]
- Marmiroli P, Nicolini G, Miloso M, Scuteri A, Cavaletti G, 2012. The fundamental role of morphology in experimental neurotoxicology: the example of chemotherapy-induced peripheral neurotoxicity. Ital. J. Anat. Embryol. 117, 75–97. [PubMed] [Google Scholar]
- Marmiroli P, Riva B, Pozzi E, Ballarini E, Lim D, Chiorazzi A, Meregalli C, Distasi C, Renn CL, Semperboni S, Morosi L, Ruffinatti FA, Zucchetti M, Dorsey SG, Cavaletti G, Genazzani A, Carozzi VA, 2017. Susceptibility of different mouse strains to oxaliplatin peripheral neurotoxicity: Phenotypic and genotypic insights. PLoS One 12, e0186250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, Griffin JW, McArthur JC, 1995. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology 45, 1848–1855. [DOI] [PubMed] [Google Scholar]
- Meinck HM, 1976. Occurrence of the H reflex and the F wave in the rat. Electroencephalogr. Clin. Neurophysiol. 41, 530–533. [DOI] [PubMed] [Google Scholar]
- Meregalli C, Fumagalli G, Alberti P, et al. 2018. Neurofilament light chain as disease biomarker in a rodent model of chemotherapy induced peripheral neuropathy. Exp Neurol 307, 129–132. [DOI] [PubMed] [Google Scholar]
- Miana-Mena FJ, Muñoz MJ, Yagüe G, Mendez M, Moreno M, Ciriza J, Zaragoza P, Osta R, 2005. Optimal methods to characterize the G93A mouse model of ALS. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 6, 55–62. [DOI] [PubMed] [Google Scholar]
- Mogil JS, 2009. Animal models of pain: progress and challenges. Nat. Rev. Neurosci. 10, 283–294. [DOI] [PubMed] [Google Scholar]
- Naji-Esfahani H, Vaseghi G, Safaeian L, Pilehvarian A-A, Abed A, Rafieian-Kopaei M, 2016. Gender differences in a mouse model of chemotherapy-induced neuropathic pain. Lab. Anim. 50, 15–20. [DOI] [PubMed] [Google Scholar]
- Nau H, 1986. Species differences in pharmacokinetics and drug teratogenesis. Environ. Health Perspect. 70, 113–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro X, Kennedy WR, 1989. Sweat gland reinnervation by sudomotor regeneration after different types of lesions and graft repairs. Exp. Neurol. 104, 229–234. [DOI] [PubMed] [Google Scholar]
- Navarro X, Kennedy WR, 1991. Evaluation of thermal and pain sensitivity in type I diabetic patients. J, Neurol, Neurosurg, Psychiat. 54, 60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro X, Verdú E, Butí M, 1994. Comparison of regenerative and reinnervating capabilities of different functional types of nerve fibers. Exp. Neurol. 129, 217–224. [DOI] [PubMed] [Google Scholar]
- Navarro X, Verdú E, Wendelscafer-Crabb G, Kennedy WR, 1995. Innervation of cutaneous structures in the mouse hind paw: a confocal microscopy immunohistochemical study. J. Neurosci. Res. 41, 111–120. [DOI] [PubMed] [Google Scholar]
- Navarro X, Udina E, 2009Methods and protocols in peripheral nerve regeneration experimental research. Part III – Electrophysiological evaluation. Int. Rev. Neurobiol. 87, 105–126. [DOI] [PubMed] [Google Scholar]
- Navarro X, 2016. Functional evaluation of peripheral nerve regeneration and target reinnervation in animal models. A critical overview. Eur. J. Neurosci. 43, 271–286. [DOI] [PubMed] [Google Scholar]
- Navratilova E, Porreca F, 2014. Reward and motivation in pain and pain relief. Nat. Neurosci. 17, 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolano M, Biasiotta A, Lombardi R, Provitera V, Stancanelli A, Caporaso G, Santoro L, Merkies IS, Truini A, Porretta-Serapiglia C, Cazzato D, Dacci P, Vitale DF, Lauria G, 2015. Epidermal innervation morphometry by immunofluorescence and bright-field microscopy. J. Peripher. Nerv. Syst. 20, 387–391. [DOI] [PubMed] [Google Scholar]
- Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC, 2009. Oxaliplatin-induced neurotoxicity: changes in axonal excitability precede development of neuropathy. Brain 132, 2712–2723. [DOI] [PubMed] [Google Scholar]
- Park HJ, Stokes JA, Pirie E, Skahen J, Shtaerman Y, Yaksh TL, 2013. Persistent hyperalgesia in the cisplatin-treated mouse as defined by threshold measures, the conditioned place preference paradigm, and changes in dorsal root ganglia activated transcription factor 3: the effects of gabapentin, ketorolac, and etanercept. Anesth. Analg. 116, 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podratz JL, Kulkarni A, Pleticha J, Kanwar R, Beutler AS, Staff NP, Windebank AJ, 2016. Neurotoxicity to DRG neurons varies between rodent strains treated with cisplatin and bortezomib. J. Neurol. Sci. 362, 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pover CM, Coggeshall RE, 1991. Verification of the disector method for counting neurons, with comments on the empirical method. Anat. Rec. 231, 573–578. [DOI] [PubMed] [Google Scholar]
- Provitera V, Nolano M, Pagano A, Caporaso G, Stancanelli A, Santoro L, 2007. Myelinated nerve endings in human skin. Muscle Nerve 35, 767–775. [DOI] [PubMed] [Google Scholar]
- Radaelli E, Santagostino SF, Sellers RS, Brayton CF, 2019. Immune relevant and immune deficient mice: options and opportunities in translational research. ILAR J. doi: 10.1093/ilar/ily026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez FJ, Valero-Cabré A, Navarro X, 2004. Regeneration and functional recovery following peripheral nerve injuries. Drug Discovery Today: Dis. Models 1, 177–185. [Google Scholar]
- Rohrer JD, Woollacott IO, Dick KM, et al. , 2016. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology 87, 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustay NR, Wahlsten D, Crabbe JC, 2003. Influence of task parameters on rotarod performance and sensitivity to ethanol in mice. Behav. Brain Res. 141, 237–249. [DOI] [PubMed] [Google Scholar]
- Sandelius Å, Zetterberg H, Blennow K, et al. 2018. Plasma neurofilament light chain concentration in the inherited peripheral neuropathies. Neurology 90, e518–e524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Nogueira E, Redondo-Castro E, Mancuso R, Navarro X, 2012. Randall-Selitto test: a new approach for the detection of neuropathic pain after spinal cord injury. J. Neurotrauma 29, 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra J, Bostock H, Navarro X, 2010. Microneurography in rats: a minimally invasive method to record single C-fiber action potentials from peripheral nerves in vivo. Neurosci. Lett. 470, 168–174. [DOI] [PubMed] [Google Scholar]
- Serra J, Bostock H, Solà R, Aleu J, García E, Cokic B, Navarro X, Quiles C, 2012. Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states of humans and rats. Pain 153, 42–55. [DOI] [PubMed] [Google Scholar]
- Shefner JM, 2001. Motor unit number estimation in human neurological diseases and animal models. Clin. Neurophysiol. 112, 955–964. [DOI] [PubMed] [Google Scholar]
- Sittig LJ, Jeong C, Tixier E, Davis J, Barrios-Camacho CM, Palmer AA, 2014. Phenotypic instability between the near isogenic substrains BALB/cJ and BALB/cByJ. Mamm. Genome 25, 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, Crager SE, Mogil JS, 2004. Paclitaxel-induced neuropathic hypersensitivity in mice: responses in 10 inbred mouse strains. Life Sci. 74, 2593–2604. [DOI] [PubMed] [Google Scholar]
- Ta LE, Low PA, Windebank AJ, 2009. Mice with cisplatin and oxaliplatin-induced painful neuropathy develop distinct early responses to thermal stimuli. Mol. Pain 5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleb O, Bouzobra F, Tekin-Pala H, Meyer L, Mensah-Nyagan AG, Patte-Mensah C, 2017. Behavioral and electromyographic assessment of oxaliplatin-induced motor dysfunctions: Evidence for a therapeutic effect of allopregnanolone. Behav. Brain Res. 320, 440–449. [DOI] [PubMed] [Google Scholar]
- Tanner KD, Levine JD, Topp KS, 1998. Microtubule disorientation and axonal swelling in unmyelinated sensory axons during vincristine-induced painful neuropathy in rat. J. Comp. Neurol. 395, 481–92. [PubMed] [Google Scholar]
- Tassler P, Dellon AL, Lesser GJ, Grossman S, 2000. Utility of decompressive surgery in the prophylaxis and treatment of cisplatin neuropathy in adult rats. J. Reconstr. Microsurg. 16, 457–463. [DOI] [PubMed] [Google Scholar]
- Terrell GS, Terzis JK, 1994. An experimental model to study the blink reflex. J. Reconstr. Microsurg. 10, 175–183. [DOI] [PubMed] [Google Scholar]
- Thireau J, Zhang BL, Poisson D, Babuty D, 2008. Heart rate variability in mice: a theoretical and practical guide. Exp. Physiol. 93, 83–94. [DOI] [PubMed] [Google Scholar]
- Thomas PK, Fraher JP, O’Leary D, Moran MA, Cole M, King RH, 1990. Relative growth and maturation of axon size and myelin thickness in the tibial nerve of the rat. 2. Effect of streptozotocin-induced diabetes. Acta Neuropathol. 79, 375–386. [DOI] [PubMed] [Google Scholar]
- Toma W, Kyte SL, Bagdas D, Jackson A, Meade JA, Rahman F, Chen ZJ, Del Fabbro E, Cantwell L, Kulkarni A, Thakur GA, Papke RL, Bigbee JW, Gewirtz DA, Damaj MI, 2019. The α7 nicotinic receptor silent agonist R-47 prevents and reverses paclitaxel-induced peripheral neuropathy in mice without tolerance or altering nicotine reward and withdrawal. Exp. Neurol. doi: 10.1016/j.expneurol.2019.113010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiwa K, Nolan C, Cavanagh JB, 1986. The effects of cisplatin on rat spinal ganglia: a study by light and electron microscopy and by morphometry. Acta Neuropathol. (Berlin) 69, 295–308. [DOI] [PubMed] [Google Scholar]
- Topp KS, Tanner KD, Levine JD, 2000. Damage to the cytoskeleton of large diameter sensory neurons and myelinated axons in vincristine-induced painful peripheral neuropathy in the rat. J. Comp. Neurol. 424, 563–576. [PubMed] [Google Scholar]
- Tredici G, Tredici S, Fabbrica D, Minoia C, Cavaletti G, 1998. Experimental cisplatin neuronopathy in rats and the effect of retinoic acid administration. J. Neurooncol. 36, 31–40. [DOI] [PubMed] [Google Scholar]
- Turner PV, Brabb T, Pekow C, Vasbinder MA, 2011. Administration of substances to laboratory animals: routes of administration and factors toconsider. J. Am. Assoc. Lab. Anim. Sci. 50, 600–613. [PMC free article] [PubMed] [Google Scholar]
- Urso-Baiarda F, Grobbelaar AO, 2006. Practical nerve morphometry. J. Neurosci. Methods 156, 333–341. [DOI] [PubMed] [Google Scholar]
- Valero-Cabré A, Navarro X, 2001. H reflex restitution and facilitation after different types of peripheral nerve injury and repair. Brain Res. 919, 302–312. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, 1968. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Exp. Neurol. 21, 270–289. [DOI] [PubMed] [Google Scholar]
- Varejao A, Melo-Pinto P, Meek M, Filipe VM, Bulas-Cruz J, 2004. Methods for the experimental functional assessment of rat sciatic nerve regeneration. Neurol. Res. 26, 186–194. [DOI] [PubMed] [Google Scholar]
- Vashistha B, Sharma A, Jain V, 2017. Ameliorative potential of ferulic acid in vincristine-induced painful neuropathy in rats: An evidence of behavioral and biochemical examination. Nutr. Neurosci. 20, 60–70. [DOI] [PubMed] [Google Scholar]
- Verdú E, Butí M, Navarro X, 1996. Functional changes of the peripheral nervous system with aging in the mouse. Neurobiol. Aging 17, 73–77. [DOI] [PubMed] [Google Scholar]
- Verdú E, Navarro X, 1997. Comparison of immunohistochemical and functional reinnervation of skin and muscle after peripheral nerve injury. Exp. Neurol. 146, 187–198. [DOI] [PubMed] [Google Scholar]
- Verdú E, Vilches JJ, Rodríguez FJ, Ceballos D, Valero A, Navarro X, 1999. Physiological and immunohistochemical characterization of cisplatin-induced neuropathy in mice. Muscle Nerve 22, 329–340. [DOI] [PubMed] [Google Scholar]
- Verdú E, Ceballos D, Vilches JJ, Navarro X, 2000. Influence of aging on peripheral nerve function and regeneration. J. Peripher. Nerv. Syst. 5, 191–208. [DOI] [PubMed] [Google Scholar]
- Vilches JJ, Rodríguez FJ, Verdú E, Valero A, Navarro X, 1998. Changes in cholinergic responses of sweat glands during denervation and reinnervation. J. Auton. Nerv. Syst. 74, 134–142. [DOI] [PubMed] [Google Scholar]
- Vilches JJ, Ceballos D, Verdú E, Navarro X, 2002. Changes in mouse sudomotor function and sweat gland innervation with ageing. Auton. Neurosci. 95, 80–87. [DOI] [PubMed] [Google Scholar]
- Wang-Fischer Y, Garyantes T, 2018. Improving the reliability and utility of streptozotocin-induced rat diabetic model. J. Diabetes Res. 2018, 8054073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Ramirez MD, Neelakantan H, Walker EA, 2011. Cannabidiol prevents the development of cold and mechanical allodynia in paclitaxel-treated female C57Bl6 mice. Anesth. Analg. 113, 947–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick RA, Hanani M, 2013. The contribution of satellite glial cells to chemotherapy-induced neuropathic pain. Eur. J. Pain 17, 571–580. [DOI] [PubMed] [Google Scholar]
- Wozniak KM, Vornov JJ, Wu Y, Liu Y, Carozzi VA, Rodriguez-Menendez V, Ballarini E, Alberti P, Pozzi E, Semperboni S, Cook BM, Littlefield BA, Nomoto K, Condon K, Eckley S, DesJardins C, Wilson L, Jordan MA, Feinstein SC, Cavaletti G, Polydefkis M, Slusher BS, 2018. Peripheral neuropathy induced by microtubule-targeted chemotherapies: insights into acute injury and long-term recovery. Cancer Res. 78,817–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao WH, Zheng H, Bennett GJ, 2012. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience 203,194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Kaji R, Hirota N, Kojima Y, Takagi T, Kohara N, Kimura J, Shibasaki H, Bostock H, 2000. Effect of maturation on nerve excitability in an experimental model of threshold electrotonus. Muscle Nerve 23, 498–506. [DOI] [PubMed] [Google Scholar]
- Zurita E, Chagoyen M, Cantero M, Alonso R, González-Neira A, López-Jiménez A, López-Moreno JA, Landel CP, Benítez J, Pazos F, Montoliu L, 2011. Genetic polymorphisms among C57BL/6 mouse inbred strains. Transgenic Res. 20, 481–489. [DOI] [PubMed] [Google Scholar]