Abstract
The hormone leptin regulates fat storage and metabolism by signaling through the brain and peripheral tissues. Lipids delivered to peripheral tissues originate mostly from the intestine and liver via synthesis and secretion of apolipoprotein B (apoB)-containing lipoproteins. An intracellular chaperone, microsomal triglyceride transfer protein (MTP), is required for the biosynthesis of these lipoproteins, and its regulation determines fat mobilization to different tissues. Using cell culture and animal models, here we sought to identify the effects of leptin on MTP expression in the intestine and liver. Leptin decreased MTP expression in differentiated intestinal Caco-2 cells, but increased expression in hepatic Huh7 cells. Similarly, acute and chronic leptin treatment of chow diet-fed WT mice decreased MTP expression in the intestine, increased it in the liver, and lowered plasma triglyceride levels. These leptin effects required the presence of leptin receptors (LEPRs). Further experiments also suggested that leptin interacted with long-form LEPR (ObRb), highly expressed in the intestine, to down-regulate MTP. In contrast, in the liver, leptin interacted with short-form LEPR (ObRa) to increase MTP expression. Mechanistic experiments disclosed that leptin activates signal transducer and activator of transcription 3 (STAT3) and mitogen-activated protein kinase (MAPK) signaling pathways in intestinal and hepatic cells, respectively, and thereby regulates divergent MTP expression. Our results also indicated that leptin-mediated MTP regulation in the intestine affects plasma lipid levels. In summary, our findings suggest that leptin regulates MTP expression differentially by engaging with different LEPR types and activating distinct signaling pathways in intestinal and hepatic cells.
Keywords: leptin, lipid, lipid absorption, lipid metabolism, lipid transport, lipoprotein, lipoprotein secretion
Introduction
Assimilation and redistribution of fat, a major source of energy, in the body requires assembly of apolipoprotein B (apoB)3-containing lipoproteins by the intestine and liver that, in turn, is critically dependent on the activity of microsomal triglyceride transfer protein (MTP) that mainly resides in the endoplasmic reticulum lumen. Regulation of MTP expression at the transcriptional and post-transcriptional levels is a major determinant of hepatic and intestinal fat mobilization (1, 2). Evidence indicates that MTP expression is differentially regulated in the liver (3) and intestine (4, 5). For example, hepatic, but not intestinal, MTP is increased by peroxisome proliferator-activated receptor α agonists (3). We have previously shown that in the intestine, but not in the liver, inositol-requiring enzyme 1β post-transcriptionally degrades MTP mRNA (4). Lin et al. (6) showed that a high-fat diet increases MTP expression in the liver and intestine, but a high-sucrose diet increases MTP expression only in the liver and not in the intestine. Oleoylethanolamide has been shown to increase MTP in the intestine and decrease in the liver (5). Specific intestinal inhibition of MTP is considered a viable therapeutic approach to lower plasma lipids (7), as hepatic MTP inhibition promotes hepatosteatosis and increases plasma transaminases (8, 9). Identification of novel tissue-specific regulatory mechanisms might be beneficial in targeting intestinal MTP to lower plasma lipids and avoid unwanted hepatic side effects associated with its inhibition.
Leptin is released by the adipose tissue into the circulation in proportion to the amount of lipids stored (10–12), and plays an important role in the control of metabolism. Besides adipose tissue, leptin is also produced by other tissues (13, 14) and has a wide repertoire of peripheral effects, mediated indirectly through the central nervous system as well as via direct actions on target tissues. Leptin has been shown to be secreted by chief epithelial cells in the lower half of the gastric mucosa fundus and reaches the intestinal lumen (15). Food triggers a rapid decline in the levels of gastric leptin (13, 16, 17) due to its release into blood circulation. Feeding of high-fat diet to mice also decrease the gastric leptin levels (18). Gastric leptin has been shown to display a remarkable resistance to proteolytic conditions in the gastric lumen (19) by binding to a high molecular weight protein that protects it from proteolytic degradation (20). The role of leptin in the direct regulation of intestinal functions is further implicated due to the presence of leptin receptors (LEPRs) in the enterocytes (21–23). Leptin exerts its effects by binding to cell-surface LEPR and activating downstream signaling mediators (24–26). Several different alternatively spliced leptin receptor isoforms have been reported (27–31) that have in common an extracellular leptin-binding domain of 816 amino acids, a transmembrane domain of 34 amino acids, and a variable cytoplasmic domain that is characteristic for each of the isoforms. These isoforms are classified into short, long, and secreted LEPR. The short forms of the receptor consist of 30–40 amino acids in their cytoplasmic domain. The major short-form (ObRa) that is highly expressed in the liver has a 34-residue cytoplasmic domain and contains one Janus kinase 2 (JAK2)-binding site. The long-form (ObRb), highly expressed in the intestine, has a 302-amino acid long cytoplasmic domain containing both JAK2 and signal transducer and activator of transcription (STAT)-binding sites. The secreted isoform (ObRe), which lacks the transmembrane and cytoplasmic domains, represents an alternate splicing or proteolytic cleavage product of membrane-bound LEPR. Leptin binding to the extracellular domain of ObRb mediates the activation of the cytoplasmic, leptin receptor-associated JAK2 tyrosine kinase (32), resulting in JAK2 autophosphorylation as well as the phosphorylation of tyrosine residues Tyr985, Tyr1077, and Tyr1138 present only on the cytoplasmic domain of ObRb (33, 34). Phosphorylated Tyr1138 residue recruits STAT3 and phosphorylates it. Phosphorylated STAT3 dimerizes and translocates to the nucleus to mediate transcriptional activation (35–37). ObRa, which is ubiquitously expressed, lacks the cytoplasmic domain necessary for STAT signaling (29). However, this receptor contains the conserved box 1 motif present in cytokine receptors (38), which is required for the association of JAK2 with cytokine receptor proteins (39) and has also been shown to perform leptin signaling (35, 40, 41). Hence, binding of leptin to ObRa results in activation of JAK2 but not STAT3. ObRa signals through extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) or phosphatidylinositol 3-kinase (PI3K) pathways by direct phosphorylation of growth factor receptor-bound protein 2 or insulin receptor substrate 1 by JAK2, respectively (42, 43).
We have previously shown that MTP expression is reduced in the intestine of Leprdb/db mice, but not in the liver (23). Furthermore, we showed that gut-brain innervation is not required for the down-regulation of intestinal MTP in these mice. This led to the suggestion that there exist an intrinsic intestine-specific leptin-leptin receptor signaling pathway that modulates intestinal MTP expression. Here, we explored molecular mechanisms for differential regulation of MTP by leptin in the intestine and liver. We show that leptin signals through STAT3 and MAPK pathways in the intestinal and liver cells, respectively, after binding to different types of LEPR expressed in these cells to regulate differential MTP expression in these tissues.
Results
Leptin decreases MTP in intestine-derived cells but increases in liver-derived cells
Previously we published that global ablation of leptin receptors decreases the expression of MTP in the intestine but not in the liver (23). Here, we aimed to explain mechanisms involved in differential, tissue-specific regulation of MTP by leptin in the intestine and liver. To test that leptin directly regulates MTP expression in cells, we incubated human colon carcinoma Caco-2 cells differentiated on a Transwell with 60 nm exogenous leptin for 24 h on the apical side. Leptin treatment decreased basolateral secretion of apoB by 33% in Caco-2 cells (Fig. 1A) but had no effect on apoA1 secretion (Fig. 1B). Furthermore, leptin treatment decreased the MTP activity and mRNA levels by 30 and 44%, respectively (Fig. 1, C and D).
Figure 1.
Effect of leptin on apoB secretion and MTP expression in Caco-2 and Huh7 cells. Differentiated Caco-2 cells (A–D) and Huh7 cells (E–H) were incubated in triplicate with 60 nm leptin for 24 h in serum-free media. Conditioned media from these cells were used to measure the secreted levels of apoB (A and E) and apoA1 (B and F) by ELISA. Cells were used to measure the activity (C and G) and the mRNA (D and H) levels of MTP. Data are plotted as mean ± S.D. *, p < 0.05 and **, p < 0.01 were determined by Student's t test. The data are representation of 3 independent experiments.
Next, we studied the effect of leptin in liver-derived cells. Treatment of human hepatoma Huh7 cells with leptin increased secretion of apoB by 70% (Fig. 1E) with no change in apoA1 levels (Fig. 1F). Moreover, it increased MTP activity and mRNA levels by 26 and 64%, respectively, in Huh7 cells (Fig. 1, G and H). These studies indicate a direct and differential effect of leptin on MTP expression in intestinal and hepatic cells in which leptin decreases intestinal MTP but increases hepatic MTP expression.
Leptin injected in intestinal loops reduces intestinal MTP
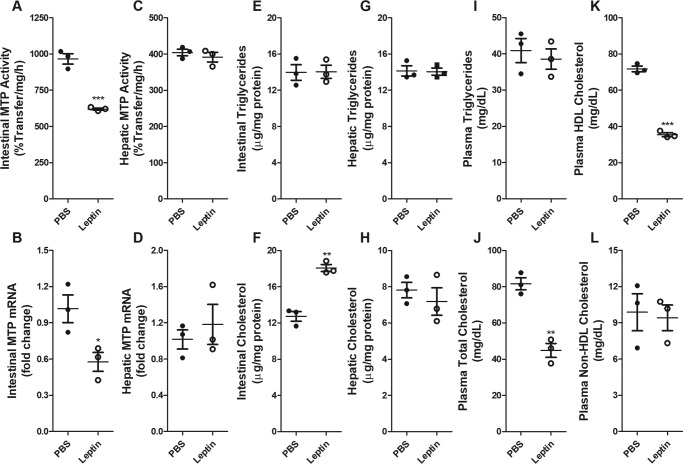
To study further the effect of lumenal leptin on intestinal MTP regulation, leptin was injected into intestinal loops (Fig. 2). This treatment significantly reduced intestinal MTP activity and mRNA levels (Fig. 2, A and B), but had no effect on hepatic MTP (Fig. 2, C and D). Lipid analysis in tissues showed that leptin had no effect on intestinal triglycerides, but increased intestinal cholesterol levels (Fig. 2, E and F). Hepatic tissue lipids were unaltered in these mice (Fig. 2, G and H). Analysis of plasma lipids showed that leptin had no effect on plasma triglyceride (Fig. 2I) but reduced total cholesterol levels (Fig. 2J). The reduction in cholesterol was due to decrease in plasma HDL cholesterol and not in non-HDL cholesterol levels (Fig. 2, K and L). These studies show that lumenal leptin reduces intestinal MTP expression. Surprisingly, it increased intestinal cholesterol and decreased plasma HDL cholesterol levels.
Figure 2.
Effect of intestinal in situ loop leptin treatment on lipids and MTP expression in the intestine and liver of WT mice. In situ loops were prepared from 4-h fasted 10-week old-male C57BL/6J mice on chow diet (n = 3) and were injected with either PBS or 0.1 mg of leptin. Mice were sacrificed after 1 h. Intestinal and hepatic tissues were also used to measure the activity (A and C) and the mRNA (B and D) levels of MTP. Triglycerides (E and G) and cholesterol (F and H) were also measured in the intestines (E and F) and livers (G and H) of these mice. Plasma was used to measure the levels of triglycerides (I), total cholesterol (J), HDL cholesterol (K), and non-HDL cholesterol (L). Data are plotted as mean ± S.D. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 were determined by Student's t test. The data are representative of 2 independent studies.
Acute and chronic leptin treatments have differential effects on intestinal and hepatic MTP in chow-fed mice
To study the effect of leptin in animals, we injected chow-fed WT C57BL/6J mice intraperitoneally with 10 mg/kg body weight of leptin (acute) and sacrificed them after 4 h of fasting. Injection of leptin reduced plasma levels of triglycerides and cholesterol by 36 and 16%, respectively (Fig. 3, A and B). There was a decrease of 19% in the levels of HDL cholesterol with no change in non-HDL cholesterol (Fig. 3, C and D). Next, we measured tissue lipid levels and found that intestinal triglycerides were increased by 40% after leptin treatment without any significant change in intestinal cholesterol levels (Fig. 3, E and F). On the other hand, leptin treatment decreased the levels of triglycerides by 24% in the hepatic cells (Fig. 3G). Similar to intestinal cholesterol, we did not see any significant change in the hepatic cholesterol levels after leptin treatment (Fig. 3H). To determine whether the changes in lipid levels were associated with changes in MTP expression, we measured MTP activity and mRNA levels in the intestines and livers of these mice. MTP activity and mRNA levels decreased in the intestine and increased in the liver tissues after leptin injection (Fig. 3, I–L). These data indicate that acute treatment of leptin has differential effects on MTP expression in the intestine and liver. Moreover, leptin reduces plasma and hepatic triglycerides, increases intestinal triglycerides, and has no effect on tissue cholesterol.
Figure 3.
Acute effect of leptin on lipids and MTP expression in the intestine and liver of WT mice. Ten-week-old male C57BL/6J mice on chow diet (n = 5) were injected intraperitoneally with 10 mg/kg body weight of leptin and fasted for 4 h after the injection. Plasma was used to measure the levels of triglycerides (A), total cholesterol (B), HDL cholesterol (C), and non-HDL cholesterol (D). Triglycerides (E and G) and cholesterol (F and H) were also measured in the intestines (E and F) and livers (G and H) of these mice. Intestinal and hepatic tissues were also used to measure the activity (I and K) and the mRNA (J and L) levels of MTP. Data are plotted as mean ± S.D. *, p < 0.05 and **, p < 0.01 were determined by Student's t test. The data are representative of 2 independent studies.
To determine whether chronic treatment of leptin also differentially affects MTP expression in the intestine and liver, we treated chow-fed WT mice with a daily intraperitoneal injection of 0.05 mg/kg body weight of leptin for 2 months and looked at the changes in lipids and MTP expression. Chronic treatment of leptin decreased plasma triglycerides, total cholesterol, HDL cholesterol, and non-HDL cholesterol (Fig. 4, A–C) and increased intestinal triglycerides and cholesterol levels (Fig. 4E). Contrary to decreased hepatic triglycerides in acute treatment, we observed increased levels of triglycerides and cholesterol in the livers of these mice after chronic leptin treatment (Fig. 4, G and H). However, chronic leptin treatment had a similar effect on the activity and mRNA levels of MTP in the intestine and liver as we observed after acute treatment, i.e. the MTP expression decreased in the intestine and increased in the liver (Fig. 4, I–L). Thus, both acute and chronic leptin treatments differentially affect expression of MTP in the liver and intestine.
Figure 4.
Chronic effect of leptin on lipids and MTP expression in the intestine and liver of chow-fed WT mice for 2 months. C57BL/6J mice on chow diet (n = 4) were injected daily with 0. 05 mg/kg body weight of leptin for 2 months. Mice were fasted for 4 h after the last injection. Plasma was used to measure the levels of triglycerides (A), total cholesterol (B), HDL cholesterol (C), and non-HDL cholesterol (D). Triglycerides (E and G) and cholesterol (F and H) were also measured in the intestines (E and F) and livers (G and H) of these mice. Intestinal and hepatic tissues were also used to measure the activity (I and K) and the mRNA (J and L) levels of MTP. Data are plotted as mean ± S.D. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 were determined by Student's t test.
Chronic leptin treatment in Western diet-fed mice has no effect on hepatic MTP expression
To determine whether chronic treatment of leptin in Western diet-fed mice has a similar effect on lipid levels and MTP expression as in chow-fed mice, Western diet-fed mice were injected daily with 0.05 mg/kg body weight of leptin. Chronic leptin injection in Western diet-fed mice reduced plasma total triglyceride, total cholesterol, and non-HDL cholesterol without affecting HDL cholesterol (Fig. 5, A–D). Hepatic and intestinal lipids were not different in PBS- and leptin-injected groups (Fig. 5, E–H). Intestinal MTP mRNA and activity were reduced in leptin-treated mice (Fig. 5, I and J). In contrast, leptin had no effect on hepatic MTP expression in Western diet-fed mice (Fig. 5, K and L). These data suggest that the Western diet has no effect on intestinal regulation of MTP but it abrogates the hepatic regulation of MTP by leptin.
Figure 5.
Chronic effect of leptin on lipids and MTP expression in the intestine and liver of WT mice fed a Western diet for 2 months. C57BL/6J mice on a Western diet (n = 4) were injected daily with 0.05 mg/kg body weight of leptin for 2 months. Mice were fasted for 4 h after the last injection. Plasma was used to measure the levels of triglycerides (A), total cholesterol (B), HDL cholesterol (C), and non-HDL cholesterol (D). Triglycerides (E and G) and cholesterol (F and H) were also measured in the intestines (E and F) and livers (G and H) of these mice. Intestinal and hepatic tissues were also used to measure the activity (I and K) and the mRNA (J and L) levels of MTP. Data are plotted as mean ± S.D. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 were determined by Student's t test.
Modulation of MTP expression by leptin requires expression of leptin receptors
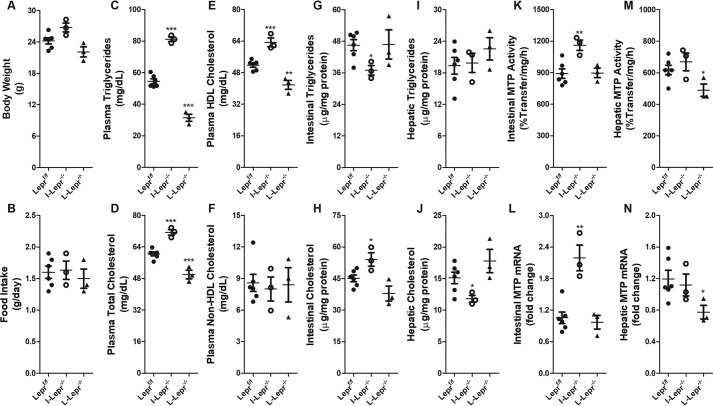
To determine whether tissue-specific regulation of MTP by leptin requires leptin receptors, we studied MTP expression in various tissue-specific leptin receptor-deficient mouse models. Ablation of LEPR in the intestine and liver of mice had no effect on body weight and food intake (Fig. 6, A and B). Intestine-specific ablation of LEPR increased, whereas liver-specific ablation of LEPR decreased plasma triglycerides, total cholesterol, and HDL cholesterol levels compared with controls (Fig. 6, C–E). No change was observed in plasma non-HDL cholesterol levels (Fig. 6F). Intestinal triglycerides were reduced, whereas cholesterol levels increased in I-Lepr−/− mice (Fig. 6, G and H). Intestine-specific ablation of LEPR had no effect on hepatic triglyceride but reduced hepatic cholesterol (Fig. 6, I and J). Liver-specific ablation of LEPR had no effect on intestinal and hepatic triglyceride and cholesterol (Fig. 6, G–J). Intestine-specific ablation of LEPR significantly increased MTP activity and mRNA levels in the intestine, but liver-specific LEPR ablation had no effect on intestinal MTP (Fig. 6, K and L). Intestine-specific ablation of LEPR had no effect on MTP activity and mRNA levels in the liver; however, liver-specific ablation of LEPR reduced hepatic MTP expression (Fig. 6, M and N). These studies show that intestine-specific ablation of LEPR increases intestinal MTP expression and plasma lipids. In contrast, hepatic-specific ablation of LEPR reduces hepatic MTP expression and plasma lipids. Thus, tissue-specific LEPR are involved in differential regulation of MTP in the intestine and liver and changes in plasma lipids follow changes in intestinal MTP expression.
Figure 6.
Tissue-specific deletion of leptin receptors has differential effect on lipids and MTP expression in chow-fed mice. Body weight (A) and average daily food intake (B) of 10-week-old male C57BL/6J Leprf/f (n = 6), I-Lepr−/− (n = 3), and L-Lepr−/− (n = 3) mice on a chow diet were recorded. Mice were sacrificed after 4 h of fasting. Plasma was used to measure the levels of triglycerides (C), total cholesterol (D), HDL cholesterol (E), and non-HDL cholesterol (F). Triglycerides (G and I) and cholesterol (H and J) were also measured in the intestines (G and H) and livers (I and J) of these mice. Intestinal and hepatic tissues were also used to measure the activity (K and M) and the mRNA (L and N) levels of MTP. Data are plotted as mean ± S.D. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 were determined by Student's t test.
Differential regulation of MTP expression through the short- and long-forms of leptin receptors
Attempts were then made to address molecular mechanisms in tissue-specific differential regulation of MTP by leptin. Intestine expresses both ObRb and ObRa (44, 45), whereas hepatocytes mainly express ObRa (46). To test the hypothesis that different isoforms of LEPR may be involved in the differential effects of leptin on MTP expression, we isolated enterocytes from overnight fasted mice expressing both long- and short-form (WT Lepr+/+ and Leprdb/+), short-form (Leprdb/db), or no membrane bound (Leprdb3j/db3j) LEPR and incubated them for 3 h with PBS or 60 nm leptin. Leptin treatment significantly decreased MTP mRNA levels in WT and Leprdb/+ enterocytes (Fig. 7, A and B). However, incubation of enterocytes from Leprdb/db mice that express ObRa with leptin increased MTP expression by 71% compared with PBS-treated Leprdb/db enterocytes (Fig. 7C). Conversely, incubation of enterocytes from Leprdb3j/db3j mice lacking all membrane-bound receptors with leptin had no effect on MTP expression (Fig. 7D) indicating that membrane-bound LEPR is required for leptin action. These results suggest that leptin decreases intestinal MTP expression in enterocytes expressing both ObRb and ObRa but increases MTP expression in Leprdb/db enterocytes that express only ObRa.
Figure 7.
Differential regulation of MTP expression by leptin in enterocytes from Lepr+/+, Leprdb/+, Leprdb/db, and Leprdb3j/db3j mice. Enterocytes were isolated from either WT Lepr+/+ (A), Leprdb/+ (B), Leprdb/db (C), or Leprdb3j/db3j mice (D) (n = 3) and incubated without (PBS) or with leptin (60 nm) for 3 h and used for MTP mRNA quantification. All the expression values were normalized to the related values of Lepr+/+ PBS-treated enterocytes. Data are plotted as mean ± S.D. *, p < 0.05 were determined by Student's t test. The data are representative of 2 independent experiments.
Comparison of MTP mRNA in control PBS-treated enterocytes revealed that MTP mRNA levels were similar in Lepr+/+ and Leprdb/+ enterocytes (Fig. 7, A and B). In contrast, Leprdb/db and Leprdb3j/db3j enterocytes (Fig. 7, C and D) that express ObRb or no receptors had ∼60% reduced levels of MTP mRNA compared with controls (Fig. 7, A and B) suggesting that Lepr does play a role in maintaining higher steady-state levels of MTP.
Leptin decreases MTP expression in Huh7 cells expressing long-form LEPR
To test the hypothesis that the expression of ObRb will decrease MTP expression, we overexpressed (∼11-fold) human ObRb (hObRb) in Huh7 cells that mostly express ObRa (Fig. 8A). Empty vector control and hObRb overexpressing cells were incubated with or without 60 nm leptin for 24 h (Fig. 8, B–D). As expected, incubation of Huh7 cells transfected with empty vector (control) with leptin resulted in a significant 62% increase in the expression of MTP mRNA levels. However, when these cells were overexpressing hObRb, leptin significantly decreased MTP mRNA levels by 44% (Fig. 8B). These results suggest that ObRb signals to down-regulate MTP expression. As biological output of changes in MTP mRNA, we measured the secretion of apoB in the media of these cells (Fig. 8C). Treatment with leptin increased the secretion of apoB by 39% in control Huh7 cells transfected with empty vector. On the other hand, overexpression of hObRb in these cells decreased the secretion of apoB by 21% after leptin treatment. Because MTP is involved only in the assembly and secretion of apoB-containing lipoproteins, treatment with leptin did not have any effect on the secretion of apoA1 (Fig. 8D). These gain-of-function studies show that leptin decreases MTP expression and apoB secretion in Huh7 cells expressing ObRb. Thus, differential regulation of MTP expression in intestinal and hepatic cells is related to the presence of different leptin receptors in these cells.
Figure 8.
Leptin decreases MTP expression and apoB secretion in Huh7 cells overexpressing the long-form of leptin receptor. Human long-form of leptin receptor (hObRb) was overexpressed in Huh7 cells for 48 h (A) and then incubated in triplicate with 60 nm leptin for 24 h in serum-free media. Cells were used to measure the mRNA levels of MTP (B). Conditioned media from these cells were used to measure the secreted levels of apoB (C) and apoA1 (D) by ELISA. Data are plotted as mean ± S.D. *, p < 0.05 and ***, p < 0.001 were determined by Student's t test. The data are representative of 2 independent experiments.
Leptin regulates MTP expression through STAT3 signaling in intestinal cells and MAPK signaling in liver cells
To identify the signaling pathways responsible for differential MTP regulation, we used specific chemical inhibitors to disrupt these signaling pathways in Caco-2 and Huh7 cells. First, differentiated Caco-2 cells were incubated without or with 60 nm leptin in the presence or absence of 5 μm 5,15-DPP (STAT3 inhibitor), U0126 (MAPK inhibitor), or LY294002 (PI3K inhibitor) for 3 h (Fig. 9A). Leptin significantly decreased MTP mRNA levels in Caco-2 cells treated with vehicle, MAPK, and PI3K inhibitor, but not in cells treated with STAT3 inhibitor (Fig. 9A). None of these inhibitors had an effect on MTP expression in cells not treated with leptin. Next, we studied the signaling pathways involved in MTP regulation by leptin in Huh7 cells. Treatment of Huh7 cells with leptin increased MTP expression (Fig. 9B) and this increase was prevented in the presence of MAPK inhibitor, but not in the presence of STAT3 or PI3K signaling inhibitors. These results suggest that leptin might be signaling through the STAT3 pathway in intestinal cells expressing predominantly ObRb and through the MAPK pathway in liver cells that express mainly ObRa.
Figure 9.

Leptin differentially regulates MTP expression through STAT3 and MAPK pathways in Caco-2 and Huh7 cells, respectively. Differentiated Caco-2 cells and Huh7 cells were treated in triplicate with 5 μm 5,15-DPP (STAT3 inhibitor), U0126 (MAPK inhibitor), and LY294002 (PI3K inhibitor) for 3 h in the absence and presence of 60 nm leptin. Caco-2 (A) and Huh7 (B) cells were used to measure the mRNA levels of MTP. Data are plotted as mean ± S.D. *, p < 0.05 and ***, p < 0.001 were determined by Student's t test. The data are representative of 2 independent experiments.
Discussion
Here we show that leptin differentially regulates MTP expression in the intestine and liver. In the intestinal cells, leptin down-regulates MTP expression and apoB secretion; whereas in the liver cells, leptin up-regulates MTP expression and apoB secretion (Fig. 1). These effects require the presence of LEPR in these tissues, as ablation of LEPR in the intestine increases intestinal MTP expression and plasma triglyceride; and hepatic ablation of LEPR decreases hepatic MTP expression (Fig. 6). We provide evidence that differential tissue-specific regulation of MTP in the intestine and liver is due to expression of different types of LEPR in these tissues and engagement of two different signaling pathways by these receptors (Figs. 8 and 9). Our data indicate that leptin reduces MTP expression in enterocytes expressing LEPR with long intracellular domain (ObRb), whereas it increases MTP expression in enterocytes expressing ObRa (Fig. 7). Leptin has no effect on MTP expression in enterocytes if both ObRb and ObRa are absent (Fig. 7). Thus, cells expressing ObRb respond to leptin by down-regulating MTP expression. This was substantiated further by overexpressing ObRb in liver cells that usually express ObRa (Fig. 8). Hepatic cells express ObRa and leptin increases MTP expression in these cells. Overexpression of ObRb in these cells reduced MTP expression after leptin treatment. We further show that leptin interacts with ObRa and elicits signaling through the MAPK pathway to increase MTP expression (Fig. 9). In contrast, leptin engages with ObRb in intestinal cells to induce the STAT3 signaling pathway leading to reduced MTP expression. Thus, we provide evidence for novel molecular mechanisms involved in differential tissue-specific regulation of MTP by leptin involving different types of leptin receptors and different signaling pathways.
Leptin is secreted by the stomach and adipose tissue to modulate function through LEPRs (15). We tried to address which of these two sources of leptin might be regulating intestinal and hepatic MTP. Acute injection of leptin in the lumen of the intestine reduced MTP expression (Fig. 2). Similarly, leptin provided apically to differentiated Caco-2 cells reduced MTP expression. Thus, leptin can interact with apically expressed LEPR (45) to modulate MTP expression in enterocytes. Surprisingly, acute and chronic intraperitoneal leptin injections also reduced intestinal MTP. It is unlikely that intraperitoneally injected leptin reaches intestinal lumen. There is evidence that LEPR is also present on the basolateral side of enterocytes (15, 21). We speculate that intraperitoneally injected leptin might interact with LEPR expressed on the basolateral side of enterocytes. Thus, leptin originating from adipose tissue may also regulate intestinal MTP. We speculate that gastric leptin may be involved in short-term regulation of intestinal MTP after a meal, whereas adipocyte leptin may exert long-term control over intestinal MTP expression.
Leptin is a satiety hormone. Leptin regulates food intake via neuronal signaling in the hypothalamus involving, among many neuron types, proopiomelanocortin (POMC) and agouti gene-related peptide (AGRP) neurons (48). We showed previously that loss of leptin signaling in global ObRb-deficient Leprdb/db mice resulted in reduced MTP expression in the intestine (23). Our current studies (Fig. 7) are consistent with these studies. Furthermore, ablation of LEPR in POMC and AGRP neurons reduced intestinal MTP (23). These previous studies provided evidence for the regulation of intestinal MTP involving neuronal signaling. In the current study, leptin decreased intestinal MTP expression in situ in intestinal loops. Furthermore, leptin reduced MTP expression and apoB secretion in differentiated Caco-2 cells. These studies point to local effects of leptin at the cellular level. Thus, leptin may use both central and cellular mechanisms to reduce MTP expression and lipid transport in enterocytes.
Leptin treatment increased hepatic MTP expression in chow-fed mice but not in Western diet-fed mice (Figs. 3 and 4). In contrast, leptin reduced intestinal MTP in both chow- and Western diet-fed mice. These data suggest that Western diet feeding interferes with hepatic leptin signaling and regulation of MTP expression but has no effect on intestinal MTP regulation.
It is likely that leptin secreted by adipocytes is the major determinant of hepatic MTP regulation for the following reasons. When injected into intestinal loops, leptin had no effect on hepatic MTP. Acute and chronic leptin injections in chow-fed mice increased hepatic MTP. Similar increases in MTP expression were observed when Huh7 cells were treated with leptin.
Acute and chronic leptin injections consistently reduced intestinal MTP and increased hepatic MTP in chow-fed mice. These acute and chronic treatments reduced plasma triglyceride. In the absence of intestinal LEPR, plasma triglyceride levels increased. Thus, plasma triglyceride levels correlate with changes in intestinal MTP and not with changes in hepatic MTP. Therefore, leptin appears to modulate plasma triglyceride levels via intestinal MTP.
The effect of leptin on plasma cholesterol levels was variable and appeared independent of changes in MTP expression. Acute lumenal injections of leptin in intestinal loops increased intestinal cholesterol but decreased plasma cholesterol levels (Fig. 2). Acute and chronic injections of leptin in chow-fed mice reduced plasma cholesterol levels (Figs. 3 and 4). We observed that changes in plasma cholesterol after acute leptin injections were mainly due to a decrease in HDL cholesterol. However, chronic leptin injection decreased both HDL and non-HDL cholesterol. Plasma cholesterol levels increased in intestine-specific LEPR-deficient mice but they were reduced in liver-specific LEPR-deficient mice (Fig. 6). These changes were again due to changes in HDL cholesterol only. These studies point to a complex effect of leptin on HDL cholesterol metabolism and require further investigations.
It is well-known that global deficiency of LEPR in Leprdb/db mice increases body weight gain and food intake (49–51). However, in the present study, tissue-specific loss of LEPR in the intestine or liver was not associated with changes in body weight and food intake. Our data are consistent with the study by Huynh et al. (52), in which they showed that liver-specific deletion of LEPR does not affect the body weight gain on both chow- and high-fat diets. In their study, Huynh et al. (52) further showed that circulating plasma leptin levels were not different in either group in both male and female mice.
Studies in enterocytes lacking LEPR point to the importance of these receptors in leptin modulation of MTP. Studies in intestine- and liver-specific LEPR mice corroborate the importance of these receptors in tissue-specific regulation of MTP. Overexpression of human ObRb in Huh7 cells resulted in reduced expression of MTP after leptin exposure as is seen in enterocytes. These studies suggest that the presence of different forms of LEPR is critical in determining the leptin effects on cellular expression of MTP.
Mechanistically, our data indicate that the reciprocal effect of leptin on MTP expression in the intestine and liver may be due to differential signaling through STAT3 and MAPK pathways, respectively (Fig. 10). We speculate that two different sources of leptin, gut and adipocytes, may be activating these signaling pathways through different isoforms of LEPR in the intestine and liver to regulate MTP expression and lipid levels. It is possible that the gut leptin regulates MTP expression and lipid absorption in the intestine via the STAT3 signaling pathway through the activation of ObRb (Fig. 10A). However, in the hepatic cells, adipocyte-derived leptin may be activating the MAPK signaling pathway through ObRa to regulate MTP expression (Fig. 10B). Differential regulation of MTP expression by leptin in the intestine and liver may contribute to lipid metabolism in the postprandial and fasting conditions. It is well-known that intestine increases chylomicron production in the postprandial state, whereas liver increases VLDL production in fasting conditions. Food intake leads to rapid depletion of gastric leptin levels (13, 16, 17), which is exacerbated in the presence of a high-fat diet (18). This decline in leptin levels may trigger an increase in the activity of intestinal MTP during the postprandial state leading to the formation of lipid-rich lipoprotein particles (Fig. 11A). On the other hand, there is a several hour delay in the release of leptin from adipose tissues after the meal (15). The delayed release of leptin from the adipose tissues may be an important factor in increasing the activity of hepatic MTP during the late postprandial state to maintain the steady-state levels of plasma lipids (Fig. 11B).
Figure 10.

Schematic diagram of leptin-mediated MTP regulation through long- (ObRb) and short- (ObRa) forms of leptin receptors. Leptin exerts its effects by binding to cell-surface receptors and activating its downstream signaling mediators. Upon binding to the long-form of leptin receptor (panel A), which is abundantly expressed in the enterocytes, leptin activates the receptor-associated JAK2 tyrosine kinase resulting in JAK2 autophosphorylation as well as the phosphorylation of tyrosine residue Tyr1138 of the receptor. pY1138 recruits STAT3, phosphorylates it resulting in the dimerization of phosphorylated STAT3 and translocation to the nucleus to mediate transcriptional events that result in decreased expression of MTP by unknown mechanisms. The short-form leptin receptor, which is ubiquitously expressed, lacks the cytoplasmic domain necessary for STAT3 signaling (panel B). Short-form leptin receptor in the liver may signal through the ERK-MAPK pathway to activate downstream signaling transcription factors that increase the expression of MTP.
Figure 11.
Schematic diagram of leptin regulation of plasma lipids during immediate and late postprandial states. Intake of food depletes the gastric leptin levels (panel A). During this state, the levels of adipose tissue-secreted leptin is also low. A decline in the gastric leptin levels may lead to increased synthesis and secretion of chylomicron-rich lipoproteins due to increased intestinal MTP expression. During the late postprandial state, there is an increase in secretion of leptin by the adipose tissues that may activate the hepatic MTP levels to increase the synthesis and secretion of VLDL particles (panel B).
It is likely that MAPK and STAT3 signaling pathways are affecting MTP expression at the transcriptional level. It has been shown that insulin reduces MTP expression in HepG2 cells via the MAPK pathway (53). In addition, suppression of FOXO1 has been implicated in the hepatic suppression of MTP expression (2). Thus, it remains to be determined how leptin can increase hepatic MTP expression via MAPK pathway. To our knowledge, the regulation of intestinal MTP by STAT3 pathway has not been studied. Thus, tissue-specific regulation of MTP by leptin involves novel unexplained molecular mechanisms.
In summary, we show for the first time that leptin regulates lipid homeostasis by differentially modulating the expression of MTP in the intestine and liver via signaling through STAT3 and MAPK pathways, respectively, involving different isoforms of LEPR. We speculate that gastric leptin may be involved in short-term regulation of lipid absorption in the intestine, whereas adipocyte leptin may control long-term lipid homeostasis by modulating the hepatic MTP activity. De-regulation of intestinal leptin signaling may increase MTP expression and lipid absorption and contribute to obesity associated with leptin deficiency.
Experimental procedures
Materials
Recombinant human leptin was purchased from PeproTech (Rocky Hill, NJ, catalogue number 300-27). Human long-form LEPR (hObRb) plasmid (pRP.ExBi-CMV-LeptinReceptorB-T2A-Luc) was designed through VectorBuilder Inc. (Chicago, IL) using the pRP.Des2d as vector backbone under the CMV promoter. STAT3 inhibitor 5,15-DPP (catalogue number 16090) and PI3K inhibitor LY294002 (catalogue number 70920) were purchased from Cayman Chemicals (Ann Arbor, MI). MAPK inhibitor U0126 was purchased from Sigma-Aldrich (catalogue number 19-147). All other chemicals and solvents were obtained from Fisher Scientific (Pittsburgh, PA).
Animals
We crossed Leprf/f (exon 17 floxed) mice (54) with Vil-CRE transgenic mice (55) to obtain heterozygous I-Lepr+/−. These mice were crossed to generate homozygous I-Lepr−/− mice. We also crossed Leprf/f mice with Alb-CRE mice (56) (Jackson Lab) to generate liver-specific Lepr-deficient mice (L-Lepr−/−). The Leprf/f mice deleted with either Vil- or Alb-CRE have neither long- or short-form LEPRs and express a frameshift-mutated membrane-bound LEPR from exon 16 transmembrane domain with no signaling capacity (54). Leprdb/db (29, 49) and Leprdb3j/db3j (57) mice have been described previously. To avoid any confounding effects of hormonal changes in female mice, we only used male mice in this study. All studies were approved by the Institutional Animal Care and Use Committee of the State University of New York Downstate Medical Center.
Cell culture studies
Caco-2 (human colon carcinoma) cells obtained from the American Type Culture Collection (ATCC, Manassas, VA, catalogue number HTB-37) were cultured (75-cm2 flasks, Corning Glassworks, Corning, NY, catalogue number 3276) in Dulbecco's modified Eagle's medium containing high glucose supplemented with l-glutamine and antibiotic/antimycotic mixture (Dulbecco's modified Eagle's medium) and 20% fetal bovine serum. For experiments, cells from 70 to 80% confluent flasks were seeded on polycarbonate micropore membrane inserts (Transwells®, 12-well plates, 12-mm diameter, 3-μm pore size, Corning Costar Corp., Cambridge, MA, catalogue number 3462) at a density of 1 × 105 cells/cm2. To induce differentiation of these cells, media were changed every other day for 21 days (58). Huh7 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, l-glutamine, and antibiotic/antimycotic mixture, and experiments were done in 12-well-cell culture plates. Differentiated Caco-2 cells and Huh7 cells were incubated with 60 nm recombinant human leptin for 24 h in serum-free media. Basolateral conditioned media from Caco-2 cells or conditioned media from Huh7 cells was collected and used to determine apoB and apolipoprotein A1 (apoA1) levels by ELISA (59). Cells were also collected and used to measure the activity and mRNA levels of MTP as described below. In some experiments, differentiated Caco-2 cells or Huh7 cells in a 12-well-plate were serum starved for 16 h. Cells were then incubated without or with 60 nm leptin in the presence or absence of 5 μm of either STAT3 inhibitor 5,15-DPP, or MAPK inhibitor U0126, or PI3K inhibitor LY294002 for 3 h in serum-free media. After the incubation, cells were used to measure mRNA levels by quantitative PCR to determine MTP expression. To study the effect of the long-form of LEPR on MTP regulation, Huh7 cells were transfected with either empty plasmid or a plasmid expressing human long-form LEPR (hObRb) using Lipofectamine 2000 (Life Technologies, catalogue number 11668019) as per the manufacturer's instructions. After transfection, cells were incubated in the presence or absence of 60 nm leptin for 24 h in serum-free media.
Acute and chronic treatment of mice with leptin
Ten-week-old chow fed WT C57BL/6J mice were injected intraperitoneally with 10 mg/kg body weight of leptin (acute) and sacrificed after 4 h of fasting. For chronic treatment, chow-fed or Western diet-fed WT mice were intraperitoneally injected daily with 0.05 mg/kg body weight of leptin for 2 months. Mice were fasted for 4 h after the last injection and sacrificed. Plasma and tissues were collected and stored at −80 °C for different measurements later.
In situ loop technique
In situ loops were prepared from 10-week-old male C57BL/6J mice on chow diet fasted for 4 h. Briefly, proximal small intestines were opened by making two small incisions at both ends and flushed with PBS. A loop (5 cm) was made by tying with strings (60). PBS (0.5 ml) with or without leptin (0.1 mg) was introduced into the loop with a microsyringe. After 1 h, proximal intestinal loops, liver, and plasma were collected for various biochemical measurements as described below.
Plasma and tissue lipid measurements
Total cholesterol and triglyceride levels in the plasma and tissues were measured using commercially available kits from Thermo Scientific (Middletown, VA) as described previously (61). Cholesterol in HDL was measured after the precipitation of apoB lipoproteins with sodium phosphate and magnesium chloride (62).
Determination of MTP activity
Differentiated Caco-2 cells, Huh7 cells, small pieces (0.1 g) of liver or proximal small intestine (∼1 cm) were homogenized in low salt buffer (1 mm Tris-HCl, pH 7.6, 1 mm EGTA, and 1 mm MgCl2) and centrifuged, and supernatants were used for protein determination and MTP assay (63).
mRNA quantification
Total RNA from tissues was isolated using TRIzolTM (Life Technologies, catalogue number 15596018). The purity of RNA was assessed by the A260/A280 ratio. RNA preparations with A260/A280 ratios more than 1.7 were used for cDNA synthesis. The first strand cDNA was synthesized using the OmniScript RT (Qiagen, Germantown, MD, catalogue number 205113) kit. Each reaction of quantitative PCR was carried out in a volume of 20 μl, consisting of 5 μl of cDNA sample (1:100 dilution of the first strand cDNA sample) and 15 μl of PCR master mix solution containing 1× PCR buffer from the qPCRTM core kit for SYBR Green I (Eurogentec, San Diego, CA, catalogue number 10-SN10-05). The PCR was carried out by incubating the reaction mixture first for 10 min at 95 °C followed by 40 cycles of 15-s incubations at 95 °C and 1 min at 60 °C in an ABI 7000 SDS PCR machine. Data were analyzed using the ΔΔCT method, according to the manufacturer's instructions, and presented as arbitrary units that were normalized to ARPp0 mRNA.
Study of differential regulation of MTP by leptin in enterocytes
To study the differential effect of leptin on MTP regulation through ObRb and ObRa, we isolated enterocytes as previously described (47) from overnight fasted mice expressing either both long- and short-form (WT Lepr+/+ and Leprdb/+) or short-form (Leprdb/db) (29, 49) or no membrane-bound (Leprdb3j/db3j) (57) LEPR and incubated them for 3 h with 60 nm leptin. At the end of the incubation, cells were washed and used to determine the expression of MTP by real-time quantitative PCR as described above.
Statistics
Data are presented as mean ± S.D. Statistical significance (p < 0.05) was determined using Student's t test (GraphPad Prism 5).
Author contributions
J. I. conceptualization; J. I. data curation; J. I. and M. M. H. formal analysis; J. I. investigation; J. I. methodology; J. I. writing-original draft; E. M. and S. C. resources; E. M., S. C., and M. M. H. writing-review and editing; M. M. H. supervision; M. M. H. funding acquisition.
This work was supported by American Heart Association Grant-in-Aid 12GRNT9690010 (to J. I.), National Institutes of Health Grants HL95924, DK046900, and HL137202, and United States Department of Health Affairs Merit Awards BX001728 and BX004113 (to M. M. H.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the American Heart Association, the National Institutes of Health, the U.S. Dept. of Veteran Affairs, or the United States Government.
- apoB
- apolipoprotein B
- AGRP
- agouti gene-related peptide
- apoA1
- apolipoprotein A1
- ERK
- extracellular signal-regulated kinase
- hObRb
- human long-form leptin receptor
- IRS-1
- insulin receptor substrate 1
- JAK2
- Janus kinase 2
- LEPR
- leptin receptors
- MAPK
- mitogen-activated protein kinase
- MTP
- microsomal triglyceride transfer protein
- ObRa
- short-form leptin receptor
- ObRb
- long-form leptin receptor
- ObRe
- secreted isoform of lepr
- PI3K
- phosphatidylinositol 3-kinase
- POMC
- proopiomelanocortin
- STAT
- signal transducer and activator of transcription
- 5/15-DPP
- 5,15-diphenylporphyrin
- HDL
- high density lipoprotein
- CMV
- cytomegalovirus.
References
- 1. Hussain M. M., Rava P., Pan X., Dai K., Dougan S. K., Iqbal J., Lazare F., and Khatun I. (2008) Microsomal triglyceride transfer protein in plasma and cellular lipid metabolism. Curr. Opin. Lipidol. 19, 277–284 10.1097/MOL.0b013e3282feea85 [DOI] [PubMed] [Google Scholar]
- 2. Hussain M. M., Nijstad N., and Franceschini L. (2011) Regulation of microsomal triglyceride transfer protein. Clin. Lipidol. 6, 293–303 10.2217/clp.11.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Améen C., Edvardsson U., Ljungberg A., Asp L., Akerblad P., Tuneld A., Olofsson S. O., Lindén D., and Oscarsson J. (2005) Activation of peroxisome proliferator-activated receptor α increases the expression and activity of microsomal triglyceride transfer protein in the liver. J. Biol. Chem. 280, 1224–1229 10.1074/jbc.M412107200 [DOI] [PubMed] [Google Scholar]
- 4. Iqbal J., Dai K., Seimon T., Jungreis R., Oyadomari M., Kuriakose G., Ron D., Tabas I., and Hussain M. M. (2008) IRE1β inhibits chylomicron production by selectively degrading MTP mRNA. Cell Metab. 7, 445–455 10.1016/j.cmet.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pan X., Schwartz G. J., and Hussain M. M. (2018) Oleoylethanolamide differentially regulates glycerolipid synthesis and lipoprotein secretion in intestine and liver. J. Lipid Res. 59, 2349–2359 10.1194/jlr.M089250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin M. C., Arbeeny C., Bergquist K., Kienzle B., Gordon D. A., and Wetterau J. R. (1994) Cloning and regulation of hamster microsomal triglyceride transfer protein. The regulation is independent from that of other hepatic and intestinal proteins which participate in the transport of fatty acids and triglycerides. J. Biol. Chem. 269, 29138–29145 [PubMed] [Google Scholar]
- 7. Aggarwal D., West K. L., Zern T. L., Shrestha S., Vergara-Jimenez M., and Fernandez M. L. (2005) JTT-130, a microsomal triglyceride transfer protein (MTP) inhibitor lowers plasma triglycerides and LDL cholesterol concentrations without increasing hepatic triglycerides in guinea pigs. BMC Cardiovasc. Disord. 5, 30 10.1186/1471-2261-5-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cuchel M., Bloedon L. T., Szapary P. O., Kolansky D. M., Wolfe M. L., Sarkis A., Millar J. S., Ikewaki K., Siegelman E. S., Gregg R. E., and Rader D. J. (2007) Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N. Engl. J. Med. 356, 148–156 10.1056/NEJMoa061189 [DOI] [PubMed] [Google Scholar]
- 9. Josekutty J., Iqbal J., Iwawaki T., Kohno K., and Hussain M. M. (2013) Microsomal triglyceride transfer protein inhibition induces endoplasmic reticulum stress and increases gene transcription via Ire1α/cJun to enhance plasma ALT/AST. J. Biol. Chem. 288, 14372–14383 10.1074/jbc.M113.459602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahima R. S., and Flier J. S. (2000) Leptin. Annu. Rev. Physiol. 62, 413–437 10.1146/annurev.physiol.62.1.413 [DOI] [PubMed] [Google Scholar]
- 11. Himms-Hagen J. (1999) Physiological roles of the leptin endocrine system: differences between mice and humans. Crit. Rev. Clin. Lab. Sci. 36, 575–655 10.1080/10408369991239259 [DOI] [PubMed] [Google Scholar]
- 12. Palou A., Serra F., Bonet M. L., and Picó C. (2000) Obesity: molecular bases of a multifactorial problem. Eur. J. Nutr. 39, 127–144 10.1007/s003940070017 [DOI] [PubMed] [Google Scholar]
- 13. Bado A., Levasseur S., Attoub S., Kermorgant S., Laigneau J. P., Bortoluzzi M. N., Moizo L., Lehy T., Guerre-Millo M., Le Marchand-Brustel Y., and Lewin M. J. (1998) The stomach is a source of leptin. Nature 394, 790–793 10.1038/29547 [DOI] [PubMed] [Google Scholar]
- 14. Cinti S., Matteis R. D., Picó C., Ceresi E., Obrador A., Maffeis C., Oliver J., and Palou A. (2000) Secretory granules of endocrine and chief cells of human stomach mucosa contain leptin. Int. J. Obes. Relat. Metab. Disord. 24, 789–793 10.1038/sj.ijo.0801228 [DOI] [PubMed] [Google Scholar]
- 15. Cammisotto P. G., and Bendayan M. (2007) Leptin secretion by white adipose tissue and gastric mucosa. Histol. Histopathol. 22, 199–210 [DOI] [PubMed] [Google Scholar]
- 16. Picó C., Sánchez J., Oliver P., and Palou A. (2002) Leptin production by the stomach is up-regulated in obese (fa/fa) Zucker rats. Obes. Res. 10, 932–938 10.1038/oby.2002.127 [DOI] [PubMed] [Google Scholar]
- 17. Sanchez J., Oliver P., Palou A., and Pico C. (2004) The inhibition of gastric ghrelin production by food intake in rats is dependent on the type of macronutrient. Endocrinology 145, 5049–5055 10.1210/en.2004-0493 [DOI] [PubMed] [Google Scholar]
- 18. Le B. J., Pelletier A. L., Arapis K., Hourseau M., Cluzeaud F., Descatoire V., Ducroc R., Aparicio T., Joly F., Couvelard A., Marmuse J. P., Le Gall M., and Bado A. (2014) Overexpression of gastric leptin precedes adipocyte leptin during high-fat diet and is linked to 5HT-containing enterochromaffin cells. Int. J. Obes. 38, 1357–1364 10.1038/ijo.2014.14 [DOI] [PubMed] [Google Scholar]
- 19. Sobhani I., Buyse M., Goiot H., Weber N., Laigneau J. P., Henin D., Soul J. C., and Bado A. (2002) Vagal stimulation rapidly increases leptin secretion in human stomach Gastroenterology 122, 259–263 10.1053/gast.2002.31385 [DOI] [PubMed] [Google Scholar]
- 20. Guilmeau S., Buyse M., Tsocas A., Laigneau J. P., and Bado A. (2003) Duodenal leptin stimulates cholecystokinin secretion: evidence of a positive leptin-cholecystokinin feedback loop. Diabetes 52, 1664–1672 10.2337/diabetes.52.7.1664 [DOI] [PubMed] [Google Scholar]
- 21. Barrenetxe J., Villaro A. C., Guembe L., Pascual I., Muñoz-Navas M., Barber A., and Lostao M. P. (2002) Distribution of the long leptin receptor isoform in brush border, basolateral membrane, and cytoplasm of enterocytes. Gut 50, 797–802 10.1136/gut.50.6.797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cammisotto P. G., Renaud C., Gingras D., Delvin E., Levy E., and Bendayan M. (2005) Endocrine and exocrine secretion of leptin by the gastric mucosa. J. Histochem. Cytochem. 53, 851–860 10.1369/jhc.5A6620.2005 [DOI] [PubMed] [Google Scholar]
- 23. Iqbal J., Li X., Chang B. H., Chan L., Schwartz G. J., Chua S. C. Jr., and Hussain M. M. (2010) An intrinsic gut leptin-melanocortin pathway modulates intestinal microsomal triglyceride transfer protein and lipid absorption J. Lipid Res. 51, 1929–1942 10.1194/jlr.M005744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Israel D., and Chua S. Jr. (2010) Leptin receptor modulation of adiposity and fertility. Trends Endocrinol. Metab. 21, 10–16 10.1016/j.tem.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frühbeck G. (2006) Intracellular signalling pathways activated by leptin. Biochem. J. 393, 7–20 10.1042/BJ20051578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morris D. L., and Rui L. (2009) Recent advances in understanding leptin signaling and leptin resistance. Am. J. Physiol. Endocrinol. Metab. 297, E1247–E1259 10.1152/ajpendo.00274.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tartaglia L. A., Dembski M., Weng X., Deng N., Culpepper J., Devos R., Richards G. J., Campfield L. A., Clark F. T., Deeds J., Muir C., Sanker S., Moriarty A., Moore K. J., Smutko J. S., et al. (1995) Identification and expression cloning of a leptin receptor, OB-R. Cell 83, 1263–1271 [DOI] [PubMed] [Google Scholar]
- 28. Lee G. H., Proenca R., Montez J. M., Carroll K. M., Darvishzadeh J. G., Lee J. I., and Friedman J. M. (1996) Abnormal splicing of the leptin receptor in diabetic mice. Nature 379, 632–635 10.1038/379632a0 [DOI] [PubMed] [Google Scholar]
- 29. Ghilardi N., Ziegler S., Wiestner A., Stoffel R., Heim M. H., and Skoda R. C. (1996) Defective STAT signaling by the leptin receptor in diabetic mice. Proc. Natl. Acad. Sci. U.S.A. 93, 6231–6235 10.1073/pnas.93.13.6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bjorbaek C., Elmquist J. K., Michl P., Ahima R. S., van B. A., McCall A. L., and Flier J. S. (1998) Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology 139, 3485–3491 10.1210/endo.139.8.6154 [DOI] [PubMed] [Google Scholar]
- 31. Elmquist J. K., Bjørbaek C., Ahima R. S., Flier J. S., and Saper C. B. (1998) Distributions of leptin receptor mRNA isoforms in the rat brain. J. Comp Neurol. 395, 535–547 [DOI] [PubMed] [Google Scholar]
- 32. Ghilardi N., and Skoda R. C. (1997) The leptin receptor activates Janus kinase 2 and signals for proliferation in a factor-dependent cell line. Mol. Endocrinol. 11, 393–399 10.1210/mend.11.4.9907 [DOI] [PubMed] [Google Scholar]
- 33. White D. W., Kuropatwinski K. K., Devos R., Baumann H., and Tartaglia L. A. (1997) Leptin receptor (OB-R) signaling: cytoplasmic domain mutational analysis and evidence for receptor homo-oligomerization. J. Biol. Chem. 272, 4065–4071 10.1074/jbc.272.7.4065 [DOI] [PubMed] [Google Scholar]
- 34. Banks A. S., Davis S. M., Bates S. H., and Myers M. G. Jr. (2000) Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 275, 14563–14572 10.1074/jbc.275.19.14563 [DOI] [PubMed] [Google Scholar]
- 35. Bjorbaek C., Uotani S., da Silva B., and Flier J. S. (1997) Divergent signaling capacities of the long and short isoforms of the leptin receptor. J. Biol. Chem. 272, 32686–32695 10.1074/jbc.272.51.32686 [DOI] [PubMed] [Google Scholar]
- 36. Bjorbaek C., and Kahn B. B. (2004) Leptin signaling in the central nervous system and the periphery. Recent Prog. Horm. Res. 59, 305–331 [DOI] [PubMed] [Google Scholar]
- 37. Myers M. G., Jr. (2004) Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog. Horm. Res. 59, 287–304 10.1210/rp.59.1.287 [DOI] [PubMed] [Google Scholar]
- 38. Tanner J. W., Chen W., Young R. L., Longmore G. D., and Shaw A. S. (1995) The conserved box 1 motif of cytokine receptors is required for association with JAK kinases. J. Biol. Chem. 270, 6523–6530 10.1074/jbc.270.12.6523 [DOI] [PubMed] [Google Scholar]
- 39. Tsuchiya T., Shimizu H., Horie T., and Mori M. (1999) Expression of leptin receptor in lung: leptin as a growth factor. Eur. J. Pharmacol. 365, 273–279 10.1016/S0014-2999(98)00884-X [DOI] [PubMed] [Google Scholar]
- 40. Madiehe A. M., Hebert S., Mitchell T. D., and Harris R. B. (2002) Strain-dependent stimulation of growth in leptin-treated obese db/db mice. Endocrinology 143, 3875–3883 10.1210/en.2002-220362 [DOI] [PubMed] [Google Scholar]
- 41. Cohen B., Novick D., and Rubinstein M. (1996) Modulation of insulin activities by leptin. Science 274, 1185–1188 10.1126/science.274.5290.1185 [DOI] [PubMed] [Google Scholar]
- 42. Chauhan D., Kharbanda S. M., Ogata A., Urashima M., Frank D., Malik N., Kufe D. W., and Anderson K. C. (1995) Oncostatin M induces association of Grb2 with Janus kinase JAK2 in multiple myeloma cells. J. Exp. Med. 182, 1801–1806 10.1084/jem.182.6.1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thirone A. C., JeBailey L., Bilan P. J., and Klip A. (2006) Opposite effect of JAK2 on insulin-dependent activation of mitogen-activated protein kinases and Akt in muscle cells: possible target to ameliorate insulin resistance. Diabetes 55, 942–951 10.2337/diabetes.55.04.06.db05-1265 [DOI] [PubMed] [Google Scholar]
- 44. Buyse M., Berlioz F., Guilmeau S., Tsocas A., Voisin T., Péranzi G., Merlin D., Laburthe M., Lewin M. J., Rozé C., and Bado A. (2001) PepT1-mediated epithelial transport of dipeptides and cephalexin is enhanced by luminal leptin in the small intestine. J. Clin. Invest. 108, 1483–1494 10.1172/JCI13219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cammisotto P. G., Bendayan M., and Levy E. (2012) Regulation of leptin receptor expression in human polarized Caco-2/15 cells. Endocr. Metab. Immune Disord. Drug Targets. 12, 57–70 10.2174/187153012799279027 [DOI] [PubMed] [Google Scholar]
- 46. Zhao A. Z., Shinohara M. M., Huang D., Shimizu M., Eldar-Finkelman H., Krebs E. G., Beavo J. A., and Bornfeldt K. E. (2000) Leptin induces insulin-like signaling that antagonizes cAMP elevation by glucagon in hepatocytes. J. Biol. Chem. 275, 11348–11354 10.1074/jbc.275.15.11348 [DOI] [PubMed] [Google Scholar]
- 47. Iqbal J., and Hussain M. M. (2005) Evidence for multiple complementary pathways for efficient cholesterol absorption in mice. J. Lipid Res. 46, 1491–1501 10.1194/jlr.M500023-JLR200 [DOI] [PubMed] [Google Scholar]
- 48. Sohn J. W., Elmquist J. K., and Williams K. W. (2013) Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 36, 504–512 10.1016/j.tins.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen H., Charlat O., Tartaglia L. A., Woolf E. A., Weng X., Ellis S. J., Lakey N. D., Culpepper J., Moore K. J., Breitbart R. E., Duyk G. M., Tepper R. I., and Morgenstern J. P. (1996) Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84, 491–495 10.1016/S0092-8674(00)81294-5 [DOI] [PubMed] [Google Scholar]
- 50. Chua S. C. Jr., Chung W. K., Wu-Peng X. S., Zhang Y., Liu S. M., Tartaglia L., and Leibel R. L. (1996) Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 271, 994–996 [DOI] [PubMed] [Google Scholar]
- 51. Cohen P., Zhao C., Cai X., Montez J. M., Rohani S. C., Feinstein P., Mombaerts P., and Friedman J. M. (2001) Selective deletion of leptin receptor in neurons leads to obesity. J. Clin. Invest. 108, 1113–1121 10.1172/JCI200113914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huynh F. K., Levi J., Denroche H. C., Gray S. L., Voshol P. J., Neumann U. H., Speck M., Chua S. C., Covey S. D., and Kieffer T. J. (2010) Disruption of hepatic leptin signaling protects mice from age- and diet-related glucose intolerance. Diabetes 59, 3032–3040 10.2337/db10-0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Au W. S., Kung H. F., and Lin M. C. (2003) Regulation of microsomal triglyceride transfer protein gene by insulin in HepG2 cells: roles of MAPKerk and MAPKp38. Diabetes 52, 1073–1080 10.2337/diabetes.52.5.1073 [DOI] [PubMed] [Google Scholar]
- 54. McMinn J. E., Liu S. M., Dragatsis I., Dietrich P., Ludwig T., Eiden S., and Chua S. C. Jr. (2004) An allelic series for the leptin receptor gene generated by CRE and FLP recombinase. Mamm. Genome 15, 677–685 10.1007/s00335-004-2340-1 [DOI] [PubMed] [Google Scholar]
- 55. Madison B. B., Dunbar L., Qiao X. T., Braunstein K., Braunstein E., and Gumucio D. L. (2002) Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 277, 33275–33283 10.1074/jbc.M204935200 [DOI] [PubMed] [Google Scholar]
- 56. Postic C., Shiota M., Niswender K. D., Jetton T. L., Chen Y., Moates J. M., Shelton K. D., Lindner J., Cherrington A. D., and Magnuson M. A. (1999) Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β cell-specific gene knock-outs using Cre recombinase. J. Biol. Chem. 274, 305–315 10.1074/jbc.274.1.305 [DOI] [PubMed] [Google Scholar]
- 57. Kowalski T. J., Liu S. M., Leibel R. L., and Chua S. C. Jr. (2001) Transgenic complementation of leptin-receptor deficiency: I. rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes 50, 425–435 10.2337/diabetes.50.2.425 [DOI] [PubMed] [Google Scholar]
- 58. Luchoomun J., and Hussain M. M. (1999) Assembly and secretion of chylomicrons by differentiated Caco-2 cells: nascent triglycerides and preformed phospholipids are preferentially used for lipoprotein assembly. J. Biol. Chem. 274, 19565–19572 10.1074/jbc.274.28.19565 [DOI] [PubMed] [Google Scholar]
- 59. Bakillah A., Zhou Z., Luchoomun J., and Hussain M. M. (1997) Measurement of apolipoprotein B in various cell lines: correlation between intracellular levels and rates of secretion. Lipids 32, 1113–1118 10.1007/s11745-997-0143-8 [DOI] [PubMed] [Google Scholar]
- 60. Pan X., Terada T., Irie M., Saito H., and Inui K. (2002) Diurnal rhythm of H+-peptide cotransporter in rat small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G57–G64 10.1152/ajpgi.00545.2001 [DOI] [PubMed] [Google Scholar]
- 61. Iqbal J., Rudel L. L., and Hussain M. M. (2008) Microsomal triglyceride transfer protein enhances cellular cholesteryl esterification by relieving product inhibition. J. Biol. Chem. 283, 19967–19980 10.1074/jbc.M800398200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Seigler L., and Wu W. T. (1981) Separation of serum high-density lipoprotein for cholesterol determination: ultracentrifugation vs precipitation with sodium phosphotungstate and magnesium chloride. Clin. Chem. 27, 838–841 10.1093/clinchem/27.6.838 [DOI] [PubMed] [Google Scholar]
- 63. Athar H., Iqbal J., Jiang X. C., and Hussain M. M. (2004) A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J. Lipid Res. 45, 764–772 10.1194/jlr.D300026-JLR200 [DOI] [PubMed] [Google Scholar]