Abstract
STUDY QUESTION
Does a previous Caesarean section affect reproductive outcomes, including live birth, in women after IVF or ICSI?
SUMMARY ANSWER
A previous Caesarean section impairs live birth rates after IVF or ICSI compared to a previous vaginal delivery.
WHAT IS KNOWN ALREADY
Rates of Caesarean sections are rising worldwide. Late sequelae of a Caesarean section related to a niche (Caesarean scar defect) include gynaecological symptoms and obstetric complications. A systematic review reported a lower pregnancy rate after a previous Caesarean section (RR 0.91 CI 0.87–0.95) compared to a previous vaginal delivery. So far, studies have been unable to causally differentiate between problems with fertilisation, and the transportation or implantation of an embryo. Studying an IVF population allows us to identify the effect of a previous Caesarean section on the implantation of embryos in relation to a previous vaginal delivery.
STUDY DESIGN, SIZE, DURATION
We retrospectively studied the live birth rate in women who had an IVF or ICSI treatment at the IVF Centre, Amsterdam UMC, location VUmc, Amsterdam, the Netherlands, between 2006 and 2016 with one previous delivery. In total, 1317 women were included, of whom 334 had a previous caesarean section and 983 had previously delivered vaginally.
PARTICIPANTS/MATERIALS, SETTING, METHODS
All secondary infertile women, with only one previous delivery either by caesarean section or vaginal delivery, were included. If applicable, only the first fresh embryo transfer was included in the analyses. Patients who did not intend to undergo embryo transfer were excluded. The primary outcome was live birth. Multivariate logistic regression analyses were used with adjustment for possible confounders ((i) age; (ii) pre-pregnancy BMI; (iii) pre-pregnancy smoking; (iv) previous fertility treatment; (v) indication for current fertility treatment: (a) tubal, (b) male factor and (c) endometriosis; (vi) embryo quality; and (vii) endometrial thickness), if applicable. Analysis was by intention to treat (ITT).
MAIN RESULTS AND THE ROLE OF CHANCE
Baseline characteristics of both groups were comparable. Live birth rates were significantly lower in women with a previous caesarean section than in women with a previous vaginal delivery, 15.9% (51/320) versus 23.3% (219/941) (OR 0.63 95% CI 0.45–0.87) in the ITT analyses. The rates were also lower for ongoing pregnancy (20.1 versus 28.1% (OR 0.64 95% CI 0.48–0.87)), clinical pregnancy (25.7 versus 33.8% (OR 0.68 95% CI 0.52–0.90)) and biochemical test (36.2 versus 45.5% (OR 0.68 95% CI 0.53–0.88)). The per protocol analyses showed the same differences (live birth rate OR 0.66 95% CI 0.47–0.93 and clinical pregnancy rate OR 0.72 95% CI 0.54–0.96).
LIMITATIONS, REASONS FOR CAUTION
This study is limited by its retrospective design. Furthermore, 56 (16.3%) cases lacked data regarding delivery outcomes, but these were equally distributed between the two groups.
WIDER IMPLICATIONS OF THE FINDINGS
The lower clinical pregnancy rates per embryo transfer indicate that implantation is hampered after a caesarean section. Its relation with a possible niche (caesarean scar defect) in the uterine caesarean scar needs further study. Our results should be discussed with clinicians and patients who consider an elective caesarean section.
STUDY FUNDING/COMPETING INTEREST(S)
Not applicable.
TRIAL REGISTRATION NUMBER
This study has been registered in the Dutch Trial Register (Ref. No. NL7631 http://www.trialregister.nl).
Keywords: caesarean section, subfertility, implantation, IVF, niche
Introduction
Caesarean section rates are rising worldwide. The rise is mainly due to an increase in primary Caesarean section. In particular, a sharp increase in repeat Caesarean section after a previous Caesarean section has been identified (Elliot et al., 1998) and in many western countries Caesarean section on maternal request have increased (Wortman and Alexander, 2013; Nilstun et al., 2008; Mylonas and Friese, 2015).
Although a Caesarean section is sometimes a lifesaving intervention, it is of major importance to investigate its further influence on women’s health. The well-known complications associated with a Caesarean section are infection, an increase in haemorrhage and increased risk of several obstetric complications in subsequent pregnancies, including malplacentation, niche pregnancies and uterine rupture (Diaz et al., 2002; Silver, 2010; Clark and Silver, 2011; D'Antonio et al., 2018).
In addition, an adverse effect of Caesarean section, reported in some studies is reduced fertility. A population cohort study reported that women who underwent a Caesarean section had a lower pregnancy rate (4–19%) compared to women who had a vaginal delivery (Gurol-Urganci et al., 2014). Moreover, a previous Caesarean section is associated with a greater median time to next pregnancy (Murphy et al., 2002; Mollison et al., 2005).
Various explanations for subfertility after Caesarean section have been proposed, ranging from uterine pathology, placental bed disruption and pelvic adhesions influencing tubal ovum pick-up (OPU) (Murphy et al., 2002) to women’s reproductive choices (Porter et al., 2003; Oral and Elter, 2007). Women with a higher maternal age have a higher risk of having a Caesarean delivery than women who are younger (Jolly et al., 1999; Khalil et al., 2013).
Earlier studies suggest an overall lower chance of pregnancy after a Caesarean section. A retrospective analysis performed in China (n = 310) reported lower pregnancy (40.3 versus 54.2%) and implantation rates (24.0 versus 34.7%) after IVF—embryo transfer (IVF-ET). This study included women with one or more previous Caesarean sections or vaginal deliveries. The majority of these patients (97%) received a double embryo transfer (DET) (Wang et al., 2017).
The underlying causes of the lower pregnancy rates after a previous Caesarean section are unclear but may relate to problems with implantation or an increased risk of miscarriage. This can be specifically studied by comparing the outcome of IVF between women who delivered by Caesarean section and those who had a previous vaginal delivery. We hypothesize that the main cause of subfertility after a Caesarean section is impaired implantation due to changes in the uterine environment in the presence of a uterine scar.
The aim of the current study was to investigate whether a previous Caesarean section compared to a previous vaginal delivery affects reproductive outcomes, primarily live births, in women undergoing their first IVF or ICSI cycle in a large retrospective cohort study.
Materials and Methods
Patients
In this retrospective cohort study, all secondary infertile women with only one previous delivery who underwent an IVF or ICSI treatment at the IVF centre, Amsterdam UMC, location VU university, the Netherlands, between 2006 and 2016 were included. Only the first fresh embryo transfer was included for analysis. Patients without an intention for embryo transfer were excluded. This study was exempt from approval of the Medical Research Involving Human Subjects Act (WMO) because it involved analysis of an existing data set. Approval for the use of this database was obtained as part of the standard approval process of the Medical Research Involving Human Subjects Act (WMO) of the Medical Ethics Committee (METC) of the VU University Medical Centre (no. 2019/057).
Stimulation protocol fresh IVF/ICSI cycles
Patients underwent controlled ovarian hyperstimulation with either a standard long or short GnRH agonist (triptorelin; Decapeptyl®; Ferring, Denmark) or short GnRH antagonist (Cetrorelix; Cetrotide®; Merck Serono, Germany) protocol as outlined by (Vergouw et al., 2012). Ovarian stimulation was performed with individually determined dosages of recombinant FSH (Gonal-F®; Merck Serono, Germany) or highly purified human menopausal gonadotropin (Menopur®; Ferring, Denmark). Treatment cycles were monitored using transvaginal ultrasonography and serum oestradiol determinations. A minimum of 1 follicle of >17 mm or 3 follicles of ≥16 mm were required to subcutaneously administer 10 000 IU of human chorionic gonadotropin (hCG; Pregnyl®; Organon, The Netherlands) or 6500 IU recombinant chorionic gonadotropin (Ovitrelle®; Merck Serono, Germany). Oocyte retrieval was performed 36 h after administration of hCG. Luteal phase support (intra-vaginal progesterone 200 mg three times daily, Utrogestan®; Besins Health Care, Belgium) was started on the day of oocyte retrieval. On Day 0 (oocyte retrieval), IVF and ICSI were performed in accordance with the IVF centre’s standard insemination procedures.
Laboratory protocol until December 2008
Fertilization was checked 16–18 h after insemination. Embryos were individually cultured in 25-μl pre-equilibrium medium drops (HTF; Lonza, Belgium; GPO (containing 4 mg/ml HSA); Sanguin, the Netherlands) with oil in incubators at 37°C, 5% CO2 and atmospheric O2 concentration. Embryo development was recorded daily at 25–27, 44–48 and 68–72 h after insemination. Transfer was carried out 73–75 h after insemination.
Laboratory protocol from January 2009 onwards
On day 0 (oocyte retrieval), IVF oocytes were placed in a fertilization medium (Sage®; Quinn’s advantage protein plus fertilization medium; Cooper Surgical, USA) and checked 18–20 h after insemination. IVF zygotes were then transferred into 25-μl pre-equilibrium cleavage medium drops (Sage®; Cooper Surgical, USA). ICSI oocytes were placed directly into 25-μl pre-equilibrium cleavage medium drops after injection. Embryos were cultured individually and kept under the same conditions, with their development recorded daily as described above. On the morning of Day 3, embryos were transferred to a new culture dish with blastocyst medium (Sage®; Quinn’s advantage protein plus blastocyst medium; Cooper Surgical, USA). For both IVF and ICSI embryos, embryo transfer was carried out 73–75 h after insemination.
Embryo selection
Prior to transfer on Day 3, embryo morphology was checked according to standard laboratory procedures (Vergouw et al., 2012). According to the count and regularity of blastomeres and the degree of fragmentation, an embryo quality score was calculated and categorized as ‘good’, ‘medium’ or ‘poor’ (Table I). An embryo transfer was often described as difficult if additional equipment and/or time was necessary to complete a transfer. Predominantly, one embryo was transferred under ultrasound guidance by an experienced reproductive specialist. In our centre, we prefer to perform a single embryo transfer (SET) in order to prevent iatrogenic multiple births. Exceptionally, we perform a DET in patients older than 38 years or undergoing a third treatment.
Table I. Definition of Day 3 embryo quality as ‘Good Quality Embryo’ (1), ‘Medium Quality Embryo’ (2) or ‘Poor Quality Embryo’ (3).
| Fragmentation (%) | Embryo stage | ||
|---|---|---|---|
| 7, 8, 9 or 10 cells | 12, 11, 5, 6 cells, compaction, B-blastocyst, morula | 1, 2, 3 or 4 cells | |
| <10 | 1 | 2 | 3 |
| 10–50 | 2 | 2 | 3 |
| >50 | 3 | 3 | 3 |
Pregnancy protocol
Patients took a biochemical pregnancy test 4 weeks after OPU. If needed, this test was repeated after several days. In the scenario of a positive test, an ultrasound was made 2 to 4 weeks later (six to 8 weeks’ gestation) to determine the presence of an amniotic sac (defined as a clinical pregnancy if present). If an amniotic sac was present, an ultrasound scan was repeated three to 5 weeks later (9 to 11 weeks’ gestation) to determine continued beating heart action (defined as an ongoing pregnancy if present).
Data collection
Data were obtained from the electronic patient files stored in the database of the department. Patients without an intention of embryo transfer (n = 13) were excluded for the following reasons: six (06) cases were either high-technological surrogate mothers (n = 4) or oocyte donors (n = 2); seven (n = 7) due to cryopreservation before cancer treatment (for a full record of patient selection and exclusion see Fig. 1).
Figure 1.
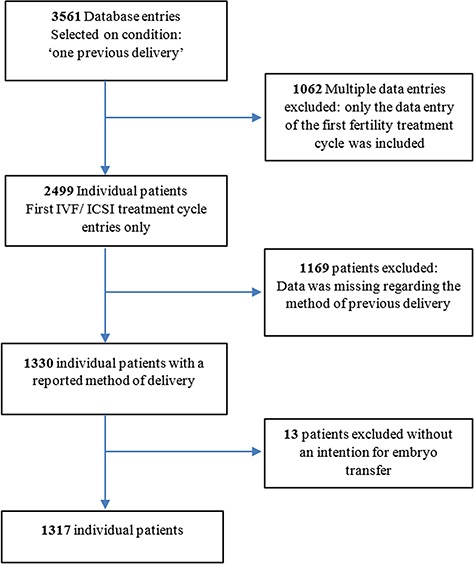
Flowchart of patient selection and exclusion criteria.
Outcomes
The primary outcome was live birth rate. Secondary outcomes were (i) clinical pregnancy; (ii) biochemical pregnancy test; (iii) mean number of amniotic sacs; (iv) mean implantation rate as the average of all individual implantation rates (implantation rate is defined as the number of amniotic sacs per patient/number of embryos transferred per patient); (v) miscarriage rate; (vi) ectopic pregnancy rate; (vii) ongoing pregnancy rate; and (viii) difficult embryo transfer (Table II).
Table II. Definitions of pregnancy outcomes.
| Definitions | |
|---|---|
| Biochemical pregnancy | HCG >5 |
| Clinical pregnancy | HCG >5, amniotic sac visible on ultrasound 6–8 weeks of gestation |
| Ongoing pregnancy | Amniotic sac present, beating heart 9–11 weeks of gestation |
| Implantation | Amniotic sac present (clinical pregnancy) |
| Implantation rate | Number of amniotic sacs per patient/number of embryos transferred per patient |
| Miscarriage | Amniotic sac present at 6–8 weeks, no beating heart at 9–11 weeks |
| Birth rate | All births |
| Live birth rate | All live births |
Sample size calculations
Assuming 10% difference in live birth rate between women with a previous Caesarean section and women with a previous vaginal delivery, we calculated that 773 patients needed to be included, with an alpha of 0.05, considering a 15% incomplete follow-up, to achieve a power of 90%.
Statistical analysis
Primary analyses were based on an ITT principle: all women who actually started an IVF or ICSI treatment were taken into account in this analysis. We performed subgroup analysis including only women who actually received embryo transfer (per protocol analyses) and those who received a SET. Data were tested for normality prior to the use of t test. Otherwise, non-parametric tests were used. Then, Student’s t tests (continuous variables) and Pearson’s X2 test or, if necessary, Fisher’s exact test (binary and categorical variables) were used to compare baseline characteristics between the two groups. Logistic regression analysis was used to test the relationship between the method of previous delivery and the rates of live births, clinical pregnancy and secondary outcomes. Possible predefined confounding factors were (i) age at the start of IVF or ICSI treatment; (ii) pre-pregnancy body mass index; (iii) pre-pregnancy smoking; (iv) previous fertility treatment; (v) reasons for current fertility treatment: (a) tubal factor, (b) male factor and (c) endometriosis; and (vi) two effect-modifying factors: (a) embryo quality and (b) endometrial thickness. These potential confounding and/or effect-modifying factors were tested, using multivariate analysis, and if necessary, results were adjusted. Statistical analysis was performed with SPSS 22.0 (Statistical Package for the Social Sciences: SPSS Inc., Chicago, IL, USA). A two-sided P value of 0.05 or less was considered statistically significant.
Results
Baseline characteristics are presented in Table III. A total of 1317 patients were included in this study: 334 women with a previous Caesarean section and 983 women with a previous vaginal delivery. Male factor and—in relation to the latter—ICSI therapy were more frequently reported in the previous vaginal delivery group. Endometriosis as the reason for fertility treatment was more reported in the Caesarean section group. We observed no further differences between the two groups (i.e. maternal age, BMI, smoking, duration of subfertility, previous fertility treatment, endometrium thickness at OPU, fertilisation rates and quality of embryos). The ratio between the number of Caesarean section and vaginal deliveries was similar in the period before 2009 (n = 196, Caesarean section 24.4% versus vaginal delivery 75.8%) and after 2009 (Caesarean section 25.6% versus 74.7%). Women with a previous Caesarean section more often did not receive an embryo (13.2%) compared to those with a previous vaginal delivery (8.2%). In total, 77% of the women received a SET, equally distributed between the groups, (75.7 versus 77.8%, respectively). In total, 37 (11.1%) women with a previous Caesarean section versus 137 (13.9%) with a previous vaginal delivery received a DET, which was not a statistically significant difference.
Table III.
Baseline characteristics.
| Parameter | Caesarean section n = 334 | Vaginal delivery n = 983 | P value |
|---|---|---|---|
| Age (years) | 36.6 ± 3.6 | 36.2 ± 3.8 | .051 |
| BMI kg/m2 | 24.9 ± 10.1 | 24.2 ± 4.4 | .251 |
| Smoking, n (%) | 49/305 (16.1) | 119/907 (13.1) | .198 |
| Alcohol use, n (%) | 161/304 (53.0) | 471/906 (52.0) | .769 |
| Duration of subfertility (years) | 2.7 ± 1.9 | 2.9 ± 2.2 | .145 |
| Current fertility treatment, n (%) | .003 | ||
| - IVF | 211 (63.2) | 528 (53.7) | |
| - ICSI | 123 (36.8) | 455 (46.3) | |
| Previous fertility treatment, n (%) | .834 | ||
| - None (spontaneous/OI) | 150/323 (46.4) | 441/936 (47.1) | |
| - IVF/ICSI/IUI | 173/323 (53.6) | 495/936 (52.9) | |
| Reason for fertility treatment, n (%) | |||
| - Tubal factor | 55 (16.5) | 122 (12.4) | .060 |
| - Male factor | 106 (31.7) | 408 (41.5) | .002 |
| - Endometriosis | 51 (15.3) | 107 (10.9) | .033 |
| - Other | 122 (36.5) | 346 (35.2) | .661 |
| Uterus factors, n (%) | |||
| - DES exposure | 3 (0.9) | 3 (0.3) | .221 |
| - Fibroids | 15 (4.5) | 37 (3.8) | |
| - Congenital/structural abnormalitiesa | 12 (3.6) | 10 (1.0) | |
| - Adenomyosis | 4 (1.2) | 11 (1.1) | |
| - Asherman syndrome | 3 (0.9) | 4 (0.4) | |
| Endometrium thickness on OPU (mm) | 11.9 ± 12.9 | 11.7 ± 10.7 | .715 |
| Number of follicles ≥14 | 8.1 ± 5.2 | 8.6 ± 5.5 | .152 |
| Total number of oocytes retrieved | 9.8 ± 6.0 | 10.6 ± 6.5 | .071 |
| Number of embryos transferred, n (%) | .018 | ||
| - 0 | 44 (13.2) | 81 (8.2) | |
| - 1 | 253 (75.7) | 765 (77.8) | |
| - 2 | 37 (11.1) | 137 (13.9) | |
| Embryo qualityb, n (%) | .447 | ||
| - 1 | 110/286 (38.5) | 374/893 (41.9) | |
| - 2 | 134/286 (46.9) | 409/893 (45.8) | |
| - 3 | 42/286 (14.7) | 110/893 (12.3) | |
| Fertilisation rate (IVF) | 63.5 ± 22.6 | 68.4 ± 19.8 | .010 |
| Fertilisation rate (ICSI) | 71.0 ± 20.0 | 70.8 ± 18.5 | .923 |
| Amniotic sacs, n (%) | |||
| - 0 | 248 (74.3) | 651 (66.2) | .007 |
| - 1 | 79 (23.7) | 213 (31.7) | .005 |
| - 2 | 7 (2.1) | 20 (2.0) |
Data are mean ± SD unless stated otherwise.
aStructural abnormalities e.g.: uterus bicornis/unicornis/didelphys/arcuatus/duplex/septum. bSee Table VI.
OI, ovulation induction; DES, diethyl stilbesterol.
Reproductive outcomes
The various reproductive outcomes of both groups are shown in Figure 2.
Figure 2.
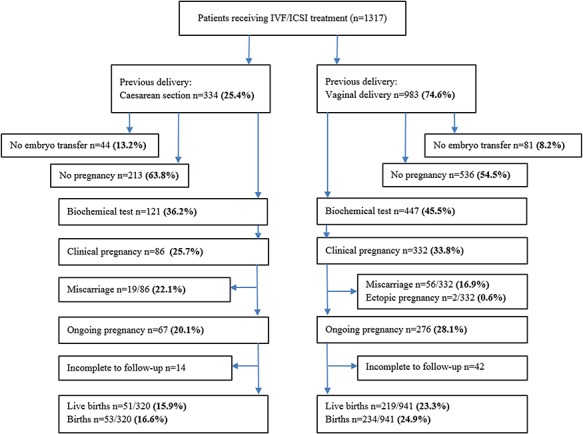
Flowchart of reproductive outcomes after embryo transfer (IVF/ICSI) for previous Caesarean section versus previous vaginal delivery.
The unadjusted reproductive outcomes after the ITT analysis are shown in Figure 2. Live birth rates were statistically significantly lower in women with a previous Caesarean section versus women with a previous vaginal delivery (15.9 versus 23.3% respectively, [OR 0.63, 95% CI 0.45–0.87]). Clinical pregnancy rates were also lower after Caesarean section (25.7 versus 33.8% respectively, [OR 0.68, 95% CI 0.52–0.90]). The mean implantation rate (the average of all individual implantation rates) was significantly lower after a previous Caesarean section (0.25 ± 0.43 versus 0.32 ± 0.46, P = 021). Difficulty concerning embryo transfer was more frequently reported after a previous Caesarean section than after a previous vaginal delivery (9.3 versus 1.0%, respectively [OR 10.0 95% CI 4.61–21.54]).
In Table IV, we present the results of both the crude analyses and the analyses adjusted for (i) age; (ii) BMI; (iii) smoking; (iv) previous fertility treatment; (v) indication for current fertility treatment (tubal, male factor or endometriosis); and (vi) two effect-modifying factors, being (a) embryo quality and (b) endometrial thickness.
Table IV. Logistic regression and multivariate regression analysis (adjusted for (1) age, (2) BMI, (3) smoking, (4) pervious fertility treatment, (5) indication for current fertility treatment (tubal, male factor or endometriosis) and for two effect-modifying factors being (1) embryo quality and (2) endometrial thickness).
| Parameter | Caesarean section n (%) n = 334 | Vaginal delivery n (%) n = 983 | OR (CI 95%) | P value | Adjusted OR (CI 95%) | P value |
| Primary outcome | ||||||
| Live birth | 52/320 (15.9) | 219/941 (23.3) | 0.63 (0.45–0.87) | .006 | 0.68 (0.47–0.97) | .035 |
| Secondary outcomes | ||||||
| Clinical pregnancy | 86 (25.7) | 332 (33.8) | 0.68 (0.52–0.90) | .007 | 0.76 (0.56–1.03) | .073 |
| No pregnancy | 213 (63.8) | 536 (54.5) | 1.47 (1.14–1.90) | .003 | 1.32 (1.01–1.75) | .050 |
| Miscarriages | 19/86 (22.1) | 56/332 (16.9) | 1.40 (0.78–2.51) | .260 | .310 | |
| Ectopic pregnancy | 0 | 2 (0.2) | ||||
| Transfer difficulty | 26/280 (9.3) | 9/885 (1.0) | 9.96 (4.61–21.54) | <.001 | 8.69 (4.61–19.27) | <.001 |
| Implantation rate Mean ± SD | 0.25 ± 0.43 | 0.32 ± 0.46 | 0.62 (0.46–0.86) | 0.06 | 0.64 (0.48–0.95) | .021 |
OR, odds ratio
Subgroup analysis
Delivery outcomes
The delivery outcomes of patients with an ongoing pregnancy are shown in Table V. We obtained information concerning delivery outcomes of 343 women. In 56 (16.3%) cases, the delivery outcomes of patients with an ongoing pregnancy were missing. With regard to ongoing pregnancies, delivery outcomes such as full-term and preterm delivery, gestational age at the time of delivery, twin birth, stillbirth and birth weight did not differ between the two groups. In the group of women who delivered by Caesarean section, one woman delivered at 25 weeks of gestation. This infant died due to prematurity. The other women delivered after 31 weeks. In the group of women who delivered vaginally, five delivered after an unexplained intra-uterine fetal death at a gestational age of 16 (two patients), 18, 19 and 30 weeks, respectively. One woman, pregnant with a twin pregnancy, delivered her first infant at 16 weeks and her second infant at 26 weeks of gestation. The infant born after 26 weeks of gestation died 2 months after birth due to prematurity. Information about the other three intrauterine fetal deaths is lacking. Most women received obstetric care by a midwife or by obstetricians in other hospitals closer to their homes. We were not always able to obtain detailed pregnancy or delivery data.
Table V. Delivery outcomes after IVF/ICSI treatment.
| Parameter ongoing pregnancy |
Previous caesarean section
n = 67 |
Previous vaginal delivery
n = 276 |
P value |
|---|---|---|---|
| Full-term birth (>37 weeks gestation) | 44/52 (84.6) | 199/224 (88.8) | .40 |
| Pre-term birth (<37 weeks gestation) | 8/52 (15.4) | 25/224 (11.2) | .40 |
| Gestation at delivery, weeks Mean ± SD | 37.7 ± 3.1 | 38.1 ± 4.8 | .61 |
| Still birth | 2/53 (3.8) | 10/229 (4.3) | .91 |
| Birth weight, g ± SD | 3308 (858) | 3375 (780.2) | .60 |
| Twin-births | 1/52 (2) | 3/224 (1) | |
| Lost to follow-up | 12 (17.9) | 44 (16.0) | .69 |
Data are n (%) unless stated otherwise
Per protocol analysis
This subgroup analysis only included patients who actually received an embryo transfer (Caesarean section n = 290 and vaginal delivery n = 902). The differences in reproductive outcomes between a previous Caesarean section and previous vaginal delivery were comparable to the ITT analysis (see Table VI).
Table VI. Subgroup analysis of per-protocol analysis.
| Parameter | Caesarean section n (%) n = 290 | Vaginal delivery n (%) n = 902 | OR (CI 95%) | P value | Adjusted OR (CI 95%) | P value |
|---|---|---|---|---|---|---|
| Primary outcomes | ||||||
| - Live birth | 51/276 (18.5) | 219/860 (25.5) | 0.66 (0.47–0.93) | .018 | 0.69 (0.48–0.98) | .041 |
| Secondary outcomes | ||||||
| - Clinical pregnancy | 86 (29.7) | 332 (36.8) | 0.72 (0.54–0.96) | .027 | 0.75 (0.57–1.03) | .080 |
| - Ongoing pregnancy | 67 (23.1) | 276 (30.6) | 0.68 (0.50–0.93) | .015 | 0.71 (0.53–0.99) | .004 |
| - No pregnancy | 169 (58.3) | 455 (50.4) | 1.37 (1.05–1.79) | .020 | 1.34 (1.02–1.78) | .051 |
| - Ectopic pregnancy | 0 | 0 | ||||
| - Mean implantation rate | 0.28 ± 0.45 | 0.35 ± 0.47 | 0.64 (0.46–0.91) | 0.03 | 0.66 (0.47–0.99) | .024 |
Logistic regression and multivariate regression analysis (adjusted for (1) age, (2) BMI, (3) smoking, (4) pervious fertility treatment, (5) indication for current fertility treatment (tubal, male factor or endometriosis) and for two effect-modifying factors being (1) embryo quality and (2) endometrial thickness)
SET
A total of 1018 patients received one embryo (Caesarean section n = 253 and vaginal delivery n = 765). Reproductive outcomes were also comparable to the ITT analysis (Table VII).
Table VII. Subgroup analysis of single embryo transfer.
| Parameter | Caesarean section n (%) n = 253 | Vaginal delivery n = 765 n (%) | P value | OR (CI 95%) | Adjusted OR (CI 95%) | P value | |
|---|---|---|---|---|---|---|---|
| Primary outcomes | |||||||
| - Live birth | 40/239 (16.7) | 182/724 (25.1) | .008 | 0.60 (.41;.87) | 0.62 (.43;.89) | .012 | |
| Secondary outcomes | |||||||
| - Clinical pregnancy | 71 (28.1) | 282 (36.9) | .011 | 0.67 (.49;.91) | 0.69 (.51;.92) | .020 | |
| - Ongoing pregnancy | 56 (22.1) | 237 (31.0) | .007 | 0.63 (.45;.88) | 0.65 (.49;.91) | .010 | |
| - No pregnancy | 155 (61.3) | 382 (49.9) | .002 | 1.59 (1.19;2.12) | 1.62 (1.20;2.14) | .006 | |
| - Mean implantation rate | 0.28 ± 0.46 | 0.37 ± 0.49 | .011 | 0.61 (.42;.89) | 0.63 (.43;.90) | .019 | |
Logistic regression and multivariate regression analysis (adjusted for (1) age, (2) BMI, (3) smoking, (4) pervious fertility treatment, (5) indication for current fertility treatment (tubal, male factor or endometriosis) and for two effect-modifying factors being (1) embryo quality and (2) endometrial thickness)
Discussion
Main findings
A previous Caesarean section in women undergoing their first IVF or ICSI cycle significantly impairs the chances of subsequent pregnancy. Live birth rates were significantly lower after a previous Caesarean section (15.9 versus 23.3% respectively [OR 0.63, 95% CI 0.45–0.87]) both in the entire study population and in the subgroup of women who received an embryo transfer. This did not change when we adjusted for possible confounders and effect-modifying factors.
This is the one of the largest cohort studies to investigate live birth outcomes after IVF with respect to the previous method of delivery in women with one previous delivery. We used a database in which all IVF cycles and pregnancy rates were registered prospectively, which reduced the risk of selection bias. Including only women with one previous delivery provides a good opportunity to compare the effect of one previous Caesarean section with one previous vaginal delivery on implantation. Studying an IVF population meant that other potential factors that could impair pregnancy rates, such as psychological effects affecting the desire to become pregnant or intra-abdominal adhesion impairing tubal transportation, are not included. Additionally, there is the advantage that we have qualitative and quantitative information of the achieved embryos in the two groups.
Differences in pregnancy and implantation rates were observed in ITT analysis including all women undergoing their first cycle but also in all predefined subgroup analyses including (i) only women with an actual embryo transfer; (ii) the large group of women with a SET; and (iii) women with a registered niche (Caesarean scar defect). This implies that, in particular, implantation may be impaired by a previous Caesarean section.
Although IVF and pregnancy outcomes were registered prospectively, the retrospective analysis of these results is a limitation. In 56 (16.3%) cases, data were missing regarding the delivery outcomes; fortunately, these cases were equally distributed between the two groups. An explanation for this is that the majority of women were referred to our hospital for IVF/ICSI whereas they delivered in their local hospital, so we were not able to obtain detailed information on the mode of delivery of the second pregnancy in all patients.
Unfortunately, data regarding previous single or twin deliveries were also missing because the majority of the patients were referred to our centre for IVF/ICSI treatment.
Another limitation is the difference in baseline characteristics between the two groups. In the previous vaginal delivery group, the male factor was more frequently reported than in the previous Caesarean section group, resulting in imbalanced percentages of ICSI therapies between the two groups. The latter did not influence the quality of embryos and is not expected to affect the implantation itself; however, this cannot be ruled out entirely. The same accounts for the higher proportion of women with endometriosis in the Caesarean section. We do not know if endometriosis is related to a higher risk of Caesarean section or that it is more prevalent after a Caesarean section, but it may impair implantation. However, adjusting for these possible confounders in a multivariate analysis did not change the results.
Comparison with previous studies
A large meta-analysis of 16 studies reported that a Caesarean section on average reduced the chance of a subsequent pregnancy by 9% in comparison to a vaginal delivery (Gurol-Urganci et al., 2013). Studies that adjusted for maternal age showed smaller effects. In a population cohort study (n = 14 541) marginally lower hazard ratios (HR) for time to live birth after a Caesarean section versus vaginal delivery if indicated for a breech position (adjusted HR 0.96, 95% CI 0.94–0.98), elective Caesarean section for other indications (adjusted HR 0.81, 95% CI 0.78–0.83) and emergency Caesarean section (adjusted HR 0.91, 95% CI 0.90–0.93) (Gurol-Urganci et al., 2014). These results suggest a 4–19% reduction in birth rates after a Caesarean section.
In our study, conducted in IVF population, even lower outcomes for live birth rates and clinical pregnancy rates were found than those reported by (Gurol-Urganci et al., 2014), whose database included women with one or more CS or vaginal deliveries and the results were not adjusted for applied fertility therapies, making the group much more heterogeneous than in our study.
Our data suggest that, the early implantation phase is affected by a previous Caesarean section. Once implantation is achieved, as detected by a biochemical test, the differences between the two groups do not additionally change in clinical, ongoing and live birth rates. Consequently, all rates are 8–9% lower in the previous Caesarean section group compared to the previous vaginal delivery group.
The detrimental effect of a Caesarean section on implantation is in line with the findings of a retrospective case control study of 310 IVF patients (Wang et al., 2017). Lower pregnancy rates were found in women with a previous Caesarean section, in particular if a post-Caesarean scar defect (also called a niche) in combination with endometrial fluid was present, compared to after a previous vaginal delivery. The authors reported reduced clinical pregnancy rates after a Caesarean section in comparison to a vaginal delivery (40.3 versus 54.8%, respectively (P < 0.05)). In women with a post-Caesarean scar defect or with endometrial fluid, clinical pregnancy rates reduced to 12.5%. In our study, women with a previous Caesarean section more often did not receive an embryo (13.2%) compared to those with a previous vaginal delivery (8.2%). Furthermore, the study of Wang et al. (2017) also reported a lower implantation rate after a Caesarean section (24.0 versus 34.7% (P < 0.05)). The difference in outcomes can be explained by different definitions of implantation rate. In our study, implantation rate is defined as the number of amniotic sacs per patient/number of embryos transferred per patient. In the study of Wang et al. (2017), implantation is defined as the number of pregnancies present divided by the number of embryos transferred. We believe that their definition would overestimate the implantation rate. Another difference compared to our study is that they included women with one or more Caesarean sections or vaginal delivery were included. Furthermore, in 97% of the cases, a DET was performed, while we performed SET in the majority of the patients (77%).
The underlying cause of lower pregnancy rates after a Caesarean section remains to be elucidated. Some studies suggest that incomplete uterine healing and post-operative infection may play a key role (Hurry et al., 1984). Other studies do suggest that there is evidence for impaired tubal transportation due to intra-abdominal adhesions (Wolf et al., 1990; Kendrick et al., 1996; Bider et al., 1998; Barnhart et al., 2006; Saraswat et al., 2008). Limited evidence for the psychological effects affecting the desire to become pregnant after a Caesarean section has been reported (Gurol-Urganci et al., 2013; Evers et al., 2014).
Several studies suggested that implantation near or into the niche may result in a higher miscarriage rate (Hemminki, 1986; Hemminki, 1996; Naji et al., 2013). Apart from changes in the endometrial ability to implant, a difficult embryo transfer due to the niche may also play a role. In a systematic review and meta-analysis (including five studies) reported that lower clinical pregnancy rates following a non-easy embryo transfer (RR = 0.75; 95% CI = 0.66–0.86) (Phillips et al. 2013).When a large niche is present, most women have a retroverted uterus, possibly due to a lack of myometrial support at the site of the niche. In our study, a difficult embryo transfer was more frequently reported in the previous Caesarean section group compared to the previous vaginal delivery group (OR 10.0 95% CI 4.61–21.54). A transfer was described as difficult if extra equipment (obturator and/or hand-pliers instead of only a catheter to successfully transfer an embryo) and/or longer time was necessary to complete a transfer. These results are in line with the results of (Wang et al., 2017). When a large niche was present, in combination with an extremely retroverted uterus, extensive manipulation of the catheter was needed to pass the niche and to enter the uterine cavity. This manipulation contributes to uterine irritation and could have a negative effect on embryo implantation (Moragianni et al., 2010; Phillips et al., 2013). In women with a history of a Caesarean section ET took longer and there is more likely to be blood or mucus on the catheter (Alvero et al., 2003; Patounakis et al., 2016).
In the study of Wang et al. (2017), even lower pregnancy rates after a niche’ were mentioned. If we focus on the very small subgroup of women with a registered niche in our database, due to the retrospective design this will be an underestimation of the total women with a niche, and if we compare these outcomes, the differences become more prominent: 10.7% of women with a Caesarean section and a registered niche with live birth versus 23.3% of women with a vaginal delivery (OR 0.40 (0.12–1.32)). This indicates that a uterine niche may have a detrimental effect on implantation. However, we need to be very cautious about the interpretation of this finding, because the sample size of this subgroup is too small for effective statistical analysis. Therefore, larger prospective studies evaluating the relation between a niche and implantation are recommended to elucidate the underlying causes of lower implantation (Vervoort et al., 2018).
Future perspectives
Prospective research is needed to investigate the role of a niche in implantation. Our data suggest that a previous Caesarean section affects early implantation in pregnancy. Additionally, it would be of value to evaluate the long-term outcomes, including mode of delivery, maternal and neonatal outcomes, such as malplacentation, severe haemorrhage and uterine rupture, in future prospective studies.
Conclusion
In an IVF/ICSI population, clinical pregnancy rates and implantation are decreased after one Caesarean section compared to a previous vaginal delivery. Its relation with a niche (Caesarean scar defect) in the uterine Caesarean scar needs to be studied. Our results should be discussed with clinicians and patients who consider an elective Caesarean section.
Acknowledgements
We thank S. Mahri for her help with collecting data.
Authors’ roles
The study was designed by J.V., T.S., C.D., C.B.L. and J.H.. J.V., T.S. and C.D. collected the data. Interpretation and analysis of data was performed by J.V., T.S., C.D., C.B.L., J.H. and J.T. The first draft was written by J.V., T.S. and J.H. and critically reviewed by C.D., R.S., C.G., J.W. and C.B.L. All co-authors approved the final version of the manuscript to be published.
Funding
This study used no external funding.
Conflict of interest
None.
References
- Alvero R, Hearns-Stokes RM, Catherino WH, Leondires MP, Segars JH. The presence of blood in the transfer catheter negatively influences outcome at embryo transfer. Hum Reprod 2003;18:1848–1852. [DOI] [PubMed] [Google Scholar]
- Barnhart KT, Sammel MD, Gracia CR, Chittams J, Hummel AC, Shaunik A. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril 2006;86:36–43. [DOI] [PubMed] [Google Scholar]
- Bider D, Blankstein J, Tur-Kaspa I. Fertility in anovulatory patients after primary cesarean section. J Reprod Med 1998;43:869–871. [PubMed] [Google Scholar]
- Clark EA, Silver RM. Long-term maternal morbidity associated with repeat cesarean delivery. Am J Obstet Gynecol 2011;205:S2–S10. [DOI] [PubMed] [Google Scholar]
- D'Antonio F, Timor-Tritsch IE, Palacios-Jaraquemada J, Monteagudo A, Buca D, Forlani F, Minneci G, Foti F, Manzoli L, Liberati M et al. First-trimester detection of abnormally invasive placenta in high-risk women: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018;51:176–183. [DOI] [PubMed] [Google Scholar]
- Diaz SD, Jones JE, Seryakov M, Mann WJ. Uterine rupture and dehiscence: ten-year review and case-control study. South Med J 2002;95:431–435. [PubMed] [Google Scholar]
- Evers EC, McDermott KC, Blomquist JL, Handa VL. Mode of delivery and subsequent fertility. Hum Reprod 2014;29:2569–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurol-Urganci I, Bou-Antoun S, Lim CP, Cromwell DA, Mahmood TA, Templeton A, Meulen JH. Impact of caesarean section on subsequent fertility: a systematic review and meta-analysis. Hum Reprod 2013;28:1943–1952. [DOI] [PubMed] [Google Scholar]
- Gurol-Urganci I, Cromwell DA, Mahmood TA, Meulen JH, Templeton A. A population-based cohort study of the effect of caesarean section on subsequent fertility. Hum Reprod 2014;29:1320–1326. [DOI] [PubMed] [Google Scholar]
- Hemminki E. Effects of cesarean section on fertility and abortions. J Reprod Med 1986;31:620–624. [PubMed] [Google Scholar]
- Hemminki E. Impact of caesarean section on future pregnancy--a review of cohort studies. Paediatr Perinat Epidemiol 1996;10:366–379. [DOI] [PubMed] [Google Scholar]
- Hurry DJ, Larsen B, Charles D. Effects of postcesarean section febrile morbidity on subsequent fertility. Obstet Gynecol 1984;64:256–260. [PubMed] [Google Scholar]
- Jolly J, Walker J, Bhabra K. Subsequent obstetric performance related to primary mode of delivery. Br J Obstet Gynaecol 1999;106:227–232. [DOI] [PubMed] [Google Scholar]
- Kendrick JS, Tierney EF, Lawson HW, Strauss LT, Klein L, Atrash HK. Previous cesarean delivery and the risk of ectopic pregnancy. Obstet Gynecol 1996;87:297–301. [DOI] [PubMed] [Google Scholar]
- Khalil A, Syngelaki A, Maiz N, Zinevich Y, Nicolaides KH. Maternal age and adverse pregnancy outcome: a cohort study. Ultrasound in Obstetrics & Gynecology: the Official Journal of the International Society of Ultrasound in. Obstet Gynecol 2013;42:634–643. [Google Scholar]
- Mollison J, Porter M, Campbell D, Bhattacharya S. Primary mode of delivery and subsequent pregnancy. BJOG 2005;112:1061–1065. [DOI] [PubMed] [Google Scholar]
- Moragianni VA, Cohen JD, Smith SE, Schinfeld JS, Somkuti SG, Lee A, Barmat LI. Effect of macroscopic or microscopic blood and mucus on the success rates of embryo transfers. Fertil Steril 2010;93:570–573. [DOI] [PubMed] [Google Scholar]
- Murphy DJ, Stirrat GM, Heron J. The relationship between caesarean section and subfertility in a population-based sample of 14 541 pregnancies. Hum Reprod 2002;17:1914–1917. [DOI] [PubMed] [Google Scholar]
- Mylonas I, Friese K. Indications for and risks of elective cesarean section. Dtsch Arztebl Int 2015;112:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naji O, Wynants L, Smith A, Abdallah Y, Stalder C, Sayasneh A, McIndoe A, Ghaem-Maghami S, Van Huffel S, Van Calster B et al. Predicting successful vaginal birth after cesarean section using a model based on cesarean scar features examined by transvaginal sonography. Ultrasound Obstetrics Gynecol 2013;41:672–678. [DOI] [PubMed] [Google Scholar]
- Nilstun T, Habiba M, Lingman G, Saracci R, Da Fre M, Cuttini M. Cesarean delivery on maternal request: can the ethical problem be solved by the principlist approach? BMC Med Ethics 2008;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oral E, Elter K. The impact of cesarean birth on subsequent fertility. Curr Opin Obstet Gynecol 2007;19:238–243. [DOI] [PubMed] [Google Scholar]
- Patounakis G, Ozcan MC, Chason RJ, Norian JM, Payson M, DeCherney AH, Yauger BJ. Impact of a prior cesarean delivery on embryo transfer: a prospective study. Fertil Steril 2016;106:311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JA, Martins WP, Nastri CO, Raine-Fenning NJ. Difficult embryo transfers or blood on catheter and assisted reproductive outcomes: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2013;168:121–128. [DOI] [PubMed] [Google Scholar]
- Porter M, Bhattacharya S, Teijlingen E, Templeton A. Does caesarean section cause infertility? Hum Reprod 2003;18:1983–1986. [DOI] [PubMed] [Google Scholar]
- Saraswat L, Porter M, Bhattacharya S, Bhattacharya S. Caesarean section and tubal infertility: is there an association? Reprod Biomed Online 2008;17:259–264. [DOI] [PubMed] [Google Scholar]
- Sewell EJ. 1998Cesarean Section - A Brief History. The American College of Obstetricians and Gynecologists in cooperation with the National Library of Medicine. Available:www.nlm.nih.gov
- Silver RM. Delivery after previous cesarean: long-term maternal outcomes. Semin Perinatol 2010;34:258–266. [DOI] [PubMed] [Google Scholar]
- Vergouw CG, Kostelijk EH, Doejaaren E, Hompes PG, Lambalk CB, Schats R. The influence of the type of embryo culture medium on neonatal birthweight after single embryo transfer in IVF. Hum Reprod 2012;27:2619–2626. [DOI] [PubMed] [Google Scholar]
- Vervoort A, Vissers J, Hehenkamp W, Brolmann H, Huirne J. The effect of laparoscopic resection of large niches in the uterine caesarean scar on symptoms, ultrasound findings and quality of life: a prospective cohort study. BJOG 2018;125:317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YQ, Yin TL, Xu WM, Qi QR, Wang XC, Yang J. Reproductive outcomes in women with prior cesarean section undergoing in vitro fertilization: a retrospective case-control study. J Huazhong Univ Sci Technolog Med Sci 2017;37:922–927. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Daling JR, Voigt LF. Prior cesarean delivery in women with secondary tubal infertility. Am J Public Health 1990;80:1382–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortman AC, Alexander JM. Placenta accreta, increta, and percreta. Obstet Gynecol Clin North Am 2013;40:137–154. [DOI] [PubMed] [Google Scholar]


