Abstract
STUDY QUESTION
Do seminal plasma (SP) and its constituents affect the decidualization capacity and transcriptome of human primary endometrial stromal fibroblasts (eSFs)?
SUMMARY ANSWER
SP promotes decidualization of eSFs from women with and without inflammatory disorders (polycystic ovary syndrome (PCOS), endometriosis) in a manner that is not mediated through semen amyloids and that is associated with a potent transcriptional response, including the induction of interleukin (IL)-11, a cytokine important for SP-induced decidualization.
WHAT IS KNOWN ALREADY
Clinical studies have suggested that SP can promote implantation, and studies in vitro have demonstrated that SP can promote decidualization, a steroid hormone-driven program of eSF differentiation that is essential for embryo implantation and that is compromised in women with the inflammatory disorders PCOS and endometriosis.
STUDY DESIGN, SIZE, DURATION
This is a cross-sectional study involving samples treated with vehicle alone versus treatment with SP or SP constituents. SP was tested for the ability to promote decidualization in vitro in eSFs from women with or without PCOS or endometriosis (n = 9). The role of semen amyloids and fractionated SP in mediating this effect and in eliciting transcriptional changes in eSFs was then studied. Finally, the role of IL-11, a cytokine with a key role in implantation and decidualization, was assessed as a mediator of the SP-facilitated decidualization.
PARTICIPANTS/MATERIALS, SETTING, METHODS
eSFs and endometrial epithelial cells (eECs) were isolated from endometrial biopsies from women of reproductive age undergoing benign gynecologic procedures and maintained in vitro. Assays were conducted to assess whether the treatment of eSFs with SP or SP constituents affects the rate and extent of decidualization in women with and without inflammatory disorders. To characterize the response of the endometrium to SP and SP constituents, RNA was isolated from treated eSFs or eECs and analyzed by RNA sequencing (RNAseq). Secreted factors in conditioned media from treated cells were analyzed by Luminex and ELISA. The role of IL-11 in SP-induced decidualization was assessed through Clustered regularly interspaced short palindromic repeats (CRISPR)/Cas-9-mediated knockout experiments in primary eSFs.
MAIN RESULTS AND THE ROLE OF CHANCE
SP promoted decidualization both in the absence and presence of steroid hormones (P < 0.05 versus vehicle) in a manner that required seminal proteins. Semen amyloids did not promote decidualization and induced weak transcriptomic and secretomic responses in eSFs. In contrast, fractionated SP enriched for seminal microvesicles (MVs) promoted decidualization. IL-11 was one of the most potently SP-induced genes in eSFs and was important for SP-facilitated decidualization.
LARGE SCALE DATA
RNAseq data were deposited in the Gene Expression Omnibus repository under series accession number GSE135640.
LIMITATIONS, REASONS FOR CAUTION
This study is limited to in vitro analyses.
WIDER IMPLICATIONS OF THE FINDINGS
Our results support the notion that SP promotes decidualization, including within eSFs from women with inflammatory disorders. Despite the general ability of amyloids to induce cytokines known to be important for implantation, semen amyloids poorly signaled to eSFs and did not promote their decidualization. In contrast, fractionated SP enriched for MVs promoted decidualization and induced a transcriptional response in eSFs that overlapped with that of SP. Our results suggest that SP constituents, possibly those associated with MVs, can promote decidualization of eSFs in an IL-11-dependent manner in preparation for implantation.
STUDY FUNDING/COMPETING INTEREST(S)
This project was supported by NIH (R21AI116252, R21AI122821 and R01AI127219) to N.R.R. and (P50HD055764) to L.C.G. The authors declare no conflict of interest.
Keywords: reproduction, semen, endometrium, stromal fibroblast, decidualization, RNAseq, interleukin-11, extracellular vesicles, CRISPR/Cas-9
Introduction
Seminal plasma (SP) is the liquid fraction of semen produced by the male accessory sex organs. In addition to serving as a vehicle to transport sperm, SP can also elicit responses in the female reproductive tract (FRT) that promote conception, in particular implantation, as shown in mice (Bromfield et al., 2014) and humans (Bellinge et al., 1986; Coulam et al., 1995; Tremellen et al., 2000) and as confirmed in a recent meta-analysis (Crawford et al., 2015). Rodent models indicate that SP components may promote implantation, in part, through the induction of regulatory T cells that mediate maternal immune tolerance of newly formed semi-allogenic embryos (Robertson et al., 1996; Johansson et al., 2004; Moldenhauer et al., 2009; Robertson et al., 2009; Balandya et al., 2012; Meuleman et al., 2015) and through enhancing secretion of embryotrophic cytokines and chemokines by FRT epithelial cells (Robertson et al., 1992; Sharkey et al., 2012a; 2012b; 2018).
Implantation occurs in the endometrium, and successful pregnancy requires activity of the endometrial stromal fibroblasts (eSFs). During the secretory phase of the menstrual cycle, eSFs undergo decidualization, a progesterone (P4)-driven differentiation process that is essential for embryo implantation and subsequent maintenance of pregnancy (Cha et al., 2012). In vitro, eSFs can be induced to decidualize by treatment with estradiol (E2) together with P4 (Irwin et al., 1989). Endometrial SFs from women with inflammatory disorders associated with suboptimal implantation (e.g. endometriosis or polycystic ovary syndrome (PCOS)) have reduced decidualization capacity (Klemmt et al., 2006; Piltonen et al., 2015), suggesting that aberrations in eSF function may be an underlying cause of female infertility.
Interestingly, SP can accelerate and increase P4-induced decidualization (Doyle et al., 2012), providing another potential explanation for its ability to improve implantation rates. However, the components of SP and the mechanisms responsible for this effect are unknown. Furthermore, to what extent SP can promote decidualization of eSFs from women with inflammatory disorders and comprised decidualization has not been explored. A recent microarray study conducted by our group revealed that eSFs respond dramatically to SP exposure and that one of the most highly SP-induced genes is interleukin (IL)-11 (~15-fold induction relative to vehicle treatment) (Chen et al., 2014). IL-11 belongs to the gp130 family of cytokines, which includes IL-6 and leukemia inhibitory factor (LIF); both of which are also induced by eSFs upon SP exposure (Gutsche et al., 2003; Chen et al., 2014). IL-11, IL-6 and LIF play important roles in implantation—LIF and IL-11 as essential factors (Stewart et al., 1992; Robb et al., 1998; Menkhorst et al., 2009) and IL-6 in a supporting role (Jasper et al., 2007). Furthermore, IL-11 promotes P4-induced decidualization of eSFs in vitro and eSFs from patients with infertility have diminished IL-11 production and decidualization capacity (Dimitriadis et al., 2002; Karpovich et al., 2005).
We sought to better understand how SP can improve implantation rates by investigating the effects of SP and SP components on decidualization and on global gene expression in eSFs. SP contains a variety of bioactive agents that are good candidates for the mediators of decidualization. Because the SP factor(s) promoting decidualization is >3500 Da (Doyle et al., 2012), SP proteins (which are typically >3500 Da) are attractive candidates. Proteins in SP may exist in a soluble form or may be associated with extracellular vesicles (EVs) that are highly abundant in semen (Machtinger et al., 2016). Among SP proteins, semen amyloids seemed particularly promising candidates. Initially identified for their ability to enhance HIV infection (Munch et al., 2007; Roan et al., 2011; Usmani et al., 2014) and more recently implicated in reproduction via sperm selection (Roan et al., 2017), semen amyloids are structurally similar to amyloids associated with neurodegenerative diseases. In these diseases, amyloids are generally pro-inflammatory and induce IL-6 (Griffin et al., 2016) and LIF (Rensink et al., 2002). Furthermore, IL-11 levels in cerebrospinal fluid are associated with the levels of Aβ42 amyloids (Galimberti et al., 2008). We therefore postulated that semen amyloids may also induce these IL-6 family members in the FRT, thereby promoting a receptive state. We first assessed the ability of SP to promote decidualization of eSFs from healthy women and from women with inflammatory disorders with comprised decidualization. We then carried out a detailed global transcriptomic analysis of eSFs exposed to semen amyloids and other constituents of SP. Our findings revealed that unlike other SP constituents, SP amyloids are not potent signaling agents in the FRT. Furthermore, we demonstrate that the ability of SP to promote decidualization is dependent on IL-11.
Materials and Methods
Ethical approval
Research procedures were performed in accordance with the Declaration of Helsinki. All donors were confirmed not to be pregnant and provided written, informed consent.
Tissue processing and cell culture
Human endometrial tissues were obtained from the Women’s Health Clinic of Naval Medical Center Portsmouth (NMCP) in Virginia (CIP no. NMCP.2016.0068) as well as the University of California, San Francisco (UCSF) Endometrial Tissue Bank (IRB no. 14-15 361), under established operating procedures (Fassbender et al., 2014). Details regarding the age, cycle phase and clinical diagnosis (if available) of each donor is included in Supplementary Table SI.
Endometrial epithelial cells (eECs) and eSFs were isolated as previously reported (Chen et al., 2014). Briefly, endometrial tissue was digested with 6.4 mg/ml collagenase type I (Worthington Biochemical Corporation, Lakewood, NJ, USA) and 100 U/ml hyaluronidase (Sigma-Aldrich, St. Louis, MO, USA) in Hanks’ buffered salt solution with Ca++ and Mg++ (Life Technologies, Carlsbad, CA, USA). To isolate single cells from glandular fragments and luminal epithelial sheets, digested cells were passed through a Falcon 40-μm cell strainer (Fisher Scientific, Waltham, MA, USA). The eSFs were then further purified using selective attachment, as previously described (Chen et al., 2014, 2015.
Following isolation, eSFs were cultured in serum-containing fibroblast growth medium (SCM; 75% phenol red-free Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies) and 25% MCDB-105 (Sigma-Aldrich) supplemented with 10% charcoal-stripped fetal bovine serum (FBS; BenchMark from Gemini), 1-mM sodium pyruvate (Sigma-Aldrich) and 5-μg/ml insulin) until confluent. eSFs were passaged serially as previously described (Irwin et al., 1989). The eECs were then plated in 24-well plates coated with Matrigel (VWR Scientific, Radnor, PA, USA) with defined keratinocyte serum-free medium (KSFM; Life Technologies). The eEC cultures were monitored for epithelial morphology and the absence of eSF contamination. When an epithelial monolayer with dome-like folding structures (Chen and Roan, 2015) was visible, eEC cultures were considered ready for treatments. Images were captured by bright field microscopy (×100 magnification) using an Olympus CKX41 microscope (Olympus, Tokyo, Japan) with a QIClick CCD Camera and QCapture Suite Plus software (QImaging, Surrey, British Columbia).
SP preparation
Individual semen samples from 20 de-identified donors obtained from the UCSF Center for Reproductive Health (IRB no. 11-06115) were liquefied at room temperature for 2 h and frozen. The samples were then all simultaneously thawed, pooled and centrifuged at 828g for 10 min at room temperature to remove spermatozoa and other cells. Supernatant containing SP was collected, aliquoted and frozen at −80°C and served as the SP stock. Endotoxin levels in SP were quantified using the ToxinSensor Chromogenic LAL Endotoxin Assay Kit (GenScript, Piscataway, NJ, USA).
SP protein degradation
For assessing the role of SP proteins in SP-induced changes in eSFs, SP proteins were denatured by heating at 100°C for 1 h or degraded by treatment with Proteinase K (200 μg/ml) (Sigma-Aldrich) for 18 h at 37°C followed by the addition of phenylmethylsulphonyl fluoride at a final concentration of 5 mM (Roche, Basel, Switzerland) to inactivate the Proteinase K. Protein degradation by Proteinase K was confirmed by running samples at 100 V for 1 h on a Bis-Tris 4–12% gradient protein gel (ThermoFisher) followed by staining with Bio-Safe™ Coomassie (Bio-Rad, Hercules, CA, USA) following the manufacturer’s protocol. To degrade the HIV infection-enhancing amyloids (Roan et al., 2011, 2014), SP was incubated for 37°C for 20 h as previously described (Roan et al., 2014).
Preparation of reconstituted SP and seminal microvesicles
SP was first centrifuged on 0.65-μm centrifugal filters (Millipore, Burlington, MA, USA) at 860g for 10 min at room temperature or until the majority of supernatant had passed through filter. The filtrate was then collected and transferred to 0.22-μm centrifugal filter units (Millipore) and further centrifuged at 860g for 10 min. The retenate on the 0.22-μm centrifugal filter was resuspended with PBS and this solution was used as the microvesicle (MV)-enriched fraction. In order to generate reconstituted SP, the MV-enriched fraction was resuspended in the filtrate from both the 0.65- and 0.22-μm centrifugal filters.
Generation of pure seminal amyloids
As it is not possible to purify endogenous semen amyloids (Usmani et al., 2014), human SEM1(86-107) (sequence: DLNALHKTTKSQRHLGGSQQLL) was custom-synthesized to generate semen amyloids (Celtek Bioscience, Franklin, TN, USA). The peptide was resuspended in PBS to a concentration of 2.5 mg/ml, and amyloid fibrils were formed by agitating overnight at 37°C at 1400 rpm using an Eppendorf Thermomixer (Eppendorf, Hamburg, Germany). Fibrillation was confirmed by quantifying the binding of thioflavin T (ThT) (5 μM) to the agitated peptides (125 μg/ml) using a PerkinElmer Enspire (excitation 440 nm, emission 482 nm; PerkinElmer, Waltham, MA, USA), as described (Roan et al., 2014). Monomeric peptide, corresponding to freshly resuspended SEM1(86-107), was confirmed not to be fibrillar by ThT binding.
TZM-bl infectivity assay
To assess for functional activity of the amyloid fibrils, a TZM-bl infectivity assay was conducted as previously described (Roan et al., 2011, 2014). The TZM-bl cell line was generated from HeLa cells engineered to express CD4, CCR5 and CXCR4, as well as firefly luciferase and Escherichia coli β-galactosidase reporter genes under the control of an HIV-1 long terminal repeat (Wei et al., 2002). A total of 5 × 103 TZM-bl cells/well were seeded in 100-μL DMEM (Life Technologies) supplemented with 10% FBS (BenchMark cat 100–106 from Gemini Bioproducts, West Sacramento, CA, USA) and 1% Pen-Strep (Gemini Bioproducts). The day after seeding, 180 μl of additional media was added to each well. Peptides, fibrils and SP were serially diluted and then pre-treated with CCR5-tropic 81A HIV-1 (100 ng/ml p24Gag) obtained from 293T-transfected cells using methods previously described (Roan et al., 2014). A total of 20 μl of the pre-treated 81A was added to each well and infection was allowed to proceed for 2 h. Next, media were replaced with fresh DMEM (Life Technologies) supplemented with 10% FBS, 1% Pen-Strep and 50-μg/ml gentamicin (Gibco, Waltham, MA, USA) and cells were cultured for additional 3 days. Infection was assessed by the way of beta-galactosidase activity (Tropix, Bedford, MA, USA) by quantifying luminescence on a PerkinElmer Enspire (PerkinElmer). Viability was quantified in parallel using the Cell Titer Glo assay (Promega, Madison, WI, USA).
Decidualization assay
Endometrial SFs were treated with ovarian steroids as described (Aghajanova et al., 2009). This treatment protocol allows eSFs to differentiate in response to E2 and P4 (Aghajanova et al., 2009; Piltonen et al., 2015), including the induction of decidualization. eSFs were seeded into 24-well plates and cultured in SCM until confluent, and then cultured in SFM media, which consists of SCM media made with 2% instead of 10% FBS. To test the ability of hormones, SP or SP components to promote decidualization, eSFs were treated for 6 h at 37°C with various combinations of the following: 0.1% ethanol (EtOH) as a vehicle control, 10 nM E2 (17β-estradiol; Sigma-Aldrich) + 1-μM P4 (E2P4; Sigma-Aldrich), 10% SP (a concentration previously used by Sharkey et al., 2012a, 2015, 2018), MV-enriched fraction (equivalent to that present in 10% SP), MV-enriched fraction + E2P4, 100-μg/ml amyloids (equivalent to the average physiological concentration in semen; Roan et al., 2014) or 10% SP + E2P4. After 6 h, media were replaced with fresh SFM containing hormones as appropriate for the treatment condition and cultured for another 42 h, at which time another cycle of treatment was initiated. Hormone and SP treatments were repeated every 2–3 days for 14 days and supernatants were collected immediately prior to each subsequent round of treatment. Decidualization was assessed by quantifying levels of human insulin-like growth factor binding protein 1 (IGFBP1) and prolactin in the cell culture supernatant by ELISA (Abcam), according to the manufacturer’s protocol. Samples were assayed in triplicates and a standard curve was run for each assay. Images of eSFs (×100 magnification) were captured by bright-field microscopy using an Olympus CKX41 microscope with a QIClick CCD Camera and QCapture Suite Plus software (QImaging). Viability of cells was assessed by the Cell Titer Glo assay (Promega).
Treatment of eSFs and eECs with SP and SP components for RNAseq
The responses of eSFs and eECs to exposure to SP and SP components were examined by RNA sequencing (RNAseq). Of note, similar to our prior microarray study (Chen et al., 2014), we tested the transcriptional response of eECs and eSFs in the absence of hormones. This was because in order to have a system where the response of eSFs and eECs to SP can be assessed in the presence of ovarian hormones, the cells must be co-cultured since eECs require paracrine support from eSFs to differentiate in response to ovarian hormones (Cunha, 1976; Cunha et al., 1983). Because this system is technically challenging as cells are less viable in co-culture than in monoculture conditions (Chen et al., 2013), we generated our RNAseq datasets in the absence of hormones. eSFs were seeded into 24-well plates and cultured in SCM until confluent, while eEC glands were seeded into 24-well, thin layer Corning Matrigel Matrix Plates (VWR Scientific) and cultured in KSFM until domes were apparent. Cells were treated for 6 h at 37°C as follows: with media alone, with 1% SP (a concentration previously used in microarray studies; Chen et al., 2014), with 1% SP pretreated for 20 h at 37°C, with 100-μg/ml peptides, with 100-μg/ml amyloids or with 0.1-Eu/ml lipopolysaccharides (LPS) from E. coli O111:B4 (Sigma-Aldrich). Note the amount of LPS used was equivalent to the levels of LPS present in 1% SP, as determined by limulus amebocyte lysate (LAL) endotoxin analysis. In a separate set of experiments, cells were treated with as follows: with media alone, with 1% reconstituted SP or with 1% of the MV-enriched fraction. Cultured cells were harvested and RNA was isolated, as detailed below.
RNA extraction and library preparation
Total RNA was extracted from eSF or eEC cultures (1 μg of total RNA from each sample) using the High Pure RNA Isolation kit (Roche) according to the manufacturer’s protocol. Quality of isolated RNA was confirmed using Nanodrop for RNA purity (OD 260/280) and Bioanalyzer (Agilent, Santa Clara, CA, USA) for RNA integrity. RNA samples with an RNA integrity number > 6 were used for library preparation for mRNA sequencing.
All RNAseq procedures were performed by Novogene. Library preparation was performed using 1 μg of total RNA from each sample with the NEBNext Ultra RNA Library Prep Kit (New England BioLabs, Inc., Ipswich, MA, USA). Briefly, polyadenylated (polyA) RNAs were selected using NEBNext Magnetic Oligo d(T)25 Beads (New England BioLabs, Inc.). Following polyA selection, RNA was fragmented and primed for first-strand cDNA synthesis, followed by second-strand synthesis. Double-stranded cDNA was purified using Agencourt AMPure XP Beads (Beckman Coulter, Brea, CA, USA), the fragments were end-repaired, 3′ adenylated and NEBNext Adaptor with hairpin loop structure (Illumina, San Diego, CA) was ligated to prepare for hybridization. cDNA fragments ~350 bp in length were selected using the Agencourt AMPure XP system (Beckman Coulter) and PCR amplification was performed to create the final cDNA library.
RNAseq and data analysis
Paired-end RNAseq at >20 million reads per sample was sequenced on an Illumina HiSeq platform (Illumina), as per the manufacturer’s instructions. Sequencing data were deposited in the Gene Expression Omnibus repository under series accession number GSE135640. Trimmed reads were aligned to the Genome Reference Consortium Human Build 38 reference genome (GenBank assembly accession: GCA_000001405.15) using TopHat2 v2.1.0 with the following parameters: global alignment, no mismatch in the 20 base-pair seed, up to two mismatches in the read, library-type fr-unstranded and mate inner distance 50. Aligned reads were filtered by removing reads with low mapping quality (below 20) and keeping singletons. Filtered reads were annotated to the Genome Reference Consortium Human Build 38 reference transcriptome (RefSeq Assembly Accession: GCF_000001405.26) using the Partek E/M annotation model (Partek Flow, build version 5.0.16.1128; Partek, St Louis, MO, USA) with the following parameters: junction reads required to match introns (set to true), strict paired-end compatibility not required (set to false) and strand specificity not required (set to false). Filtered reads were normalized using transcripts per kilobase million. Low-expressing transcripts were removed, with the lowest maximum coverage set to 1.0. Differential gene expression was calculated by ANOVA in a paired test (paired on donor ID), by using treatment as the fixed factor and ID as the random factor. Differentially expressed transcripts were identified based on fold change (≥ ±1.5), with significance level set at P < 0.05. Principal component analysis (PCA) and hierarchical clustering was performed using Partek Flow software. Ingenuity pathway analysis (IPA) software (Qiagen, Hilden, Germany) was used for pathway analysis through the core analysis function to determine biological context of differentially expressed genes.
Luminex multiplex assay
Culture supernatants from samples analyzed by RNAseq were analyzed by the Luminex multiplex assay (ThermoFisher) to assess the effects of SP and SP components on the secretion of cytokines/soluble factors by eSFs and eECs. Supernatants were analyzed by Luminex using a 30-plex human cytokine panel (ThermoFisher) consisting of epidermal growth factor (EGF), eotaxin, basic fibroblast growth factor (FGFβ), granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), hepatocyte growth factor (HGF), interferon alpha (IFN)-α, IFN-γ, IL-10, IL-12, IL-13, IL-15, IL-17, IL-1β, IL-1 receptor antagonist (IL-1RA), IL-2, IL-2 receptor (IL-2R), IL-4, IL-5, IL-6, IL-7, IL-8, IFN gamma-induced protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), monokine induced by gamma (MIG), macrophage inflammatory protein (MIP)-1α, MIP-1β, regulated upon activation normal T cell expressed and secreted (RANTES), tumor necrosis factor (TNF)-α and vascular endothelial growth factor (VEGF). The assay was performed by the Endocrine Technologies Support Core (ETSC) at the Oregon National Primate Research Center (ONPRC) according to the manufacturer’s instructions (ThermoFisher). Briefly, 50 μl of each serum sample was diluted in assay diluent and incubated overnight with antibody-coated, fluorescent-dye capture microspheres specific for each analyte, followed by detection antibodies and streptavidin-phycoerythrin. Washed microspheres with bound analytes were resuspended in reading buffer and analyzed on a Milliplex LX-200 Analyzer (EMD Millipore, Burlington, MA, USA) bead sorter with XPonent Software version 3.1 (Luminex, Austin, TX, USA). Data were calculated using Milliplex Analyst software version 5.1 (EMD Millipore). An in-house generated rhesus macaque serum pool was run in quadruplicate as a quality control (QC). Intra-assay coefficients of variation (CVs) were <11% for all targets.
Enzyme-linked immunosorbent assay
Supernatants from samples analyzed by RNAseq were additionally analyzed for LIF by the ETSC at the ONPRC by ELISA following the manufacturer’s instructions (ThermoFisher). Two vendor-supplied QCs were run in each assay (Ctrl 1: 1179 ± 295 pg/ml; Ctrl 2: 2420 ± 606 pg/ml). The intra- and inter-assay CVs were 1.7% and 4.6%, respectively, for Ctrl 1; and 0.5% and 0.5%, respectively, for Ctrl 2. An in-house generated rhesus macaque serum pool spiked with LIF was used as a QC. Intra-assay CV was 6.9%. The assay dynamic was 280–7100 pg/ml LIF. Where indicated, IL-8 and IL-11 levels were quantified by ELISA (R&D Systems, Minneapolis, MN, USA) using similar methods, following the manufacturer’s directions.
CRISPR/Cas-9-mediated editing of IL-11RA in primary eSFs
Synthetic guide RNAs (sgRNAs) targeting the human IL-11 receptor (IL-11RA) were designed using the Synthego CRISPR Gene Knockout (KO) design tool (Synthego, Menlo Park, CA, USA). IL-11RA sgRNA (sequence: 5′-CCAUCCCGAAACCAGGACAC-3′) and negative control scrambled sgRNA (sequence: 5′-GCACUACCAGAGCUAACUCA-3′) were chemically synthesized by Synthego. For each reaction, the sgRNA (30 pmol/μl; Synthego) and Cas9 protein (20 pmol/μl; Synthego) were added at a 6:1 ratio in 23 μl P2 buffer (Amaxa P2 Primary Cell 96-well 4D-Nucleofector Kit, Lonza, Basel, Switzerland) and incubated for 20 min at 37°C. eSFs (250 000 cells/reaction, three reactions per condition) were washed with PBS, resuspended with Cas9/sgRNA RNP complex and transferred to a 16-well reaction cuvette. Electroporation was performed with the 4D-Nucleofector (Lonza; program DT-130). Following electroporation, cells were incubated in the reaction cuvette for 15 min at 37°C. Nucleofected cells were then gently collected and cultured in T-75 flasks at 37°C for 6 days and passaged for further expansion. On Day 10 post-nucleofection, eSFs were harvested for decidualization assays (see Materials and Methods above) or for DNA extraction and evaluation of editing efficiency.
PCR amplification of IL-11RA and ICE analysis
To assess KO efficiency, genomic DNA was extracted from eSFs using the QuickExtract DNA Extraction Solution as per manufacturer’s protocol (Epicentre, Madison, WI, USA). Genomic primers flanking the proposed editing site (forward: 5′-GGGGAGAGAGATAGGAGCTG-3′; reverse: 5′-CCCTATGTGTCATCAGTGCC-3′) were designed using the Primer3 online tool (http://bioinfo.ut.ee/primer3/), chemically synthesized (ELIM Biopharmaceuticals, Inc., Hayward, CA, USA) and suspended at 100 μM in water. The PCR amplification of the IL-11RA gene using Phusion High Fidelity Hot Start Polymerase x2 Mastermix (ThermoFisher) was conducted according to the manufacturer’s instructions. The thermocycler setting included one step at 98°C for 30 sec, followed by 35 cycles at 98°C for 10 sec, 68°C for 30 sec, 72°C for 30 sec, with one final step at 72°C for 5 min. PCR cleanup and capillary sequencing was performed by ELIM Biopharmaceuticals, Inc. Synthego’s ICE CRISPR Analysis Tool was utilized to determine the KO efficiency and percentage of indels from sequencing traces.
Statistical analysis
Two-tailed Student’s t-tests were conducted with Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA), with mean and SD reported. Differential gene expression analysis of RNAseq data was calculated by ANOVA in a paired test, using treatment as the fixed factor and ID as the random factor (Partek Flow, build version 5.0.16.1128). Statistical significance was established at P < 0.05. For pathway analysis of RNAseq data, IPA was used to identify pathways affected by differentially expressed genes (>1.5-fold change) and Z-scores >1.5 fold were included in the analyses similar to methods previously implemented (Chen et al., 2014).
Results
SP enhances decidualization of eSF from women with and without inflammatory disorders
To characterize the effects of SP on decidualization, we treated eSFs from endometrial biopsies (Supplementary Table SI) with 10% SP, in the presence or absence of E2P4, for up to 12 days and monitored decidualization by quantifying the production of prolactin and IGFBP1 in the conditioned media, as previously described (Chen et al., 2014). Endometrial SFs from a woman without an inflammatory disorder (‘Donor A’) were capable of decidualizing upon treatment with E2P4, but addition of SP further increased the levels of secreted prolactin (Fig. 1A, left) and decreased the time at which IGFBP1 levels peaked (Fig. 1A, right). By contrast, eSFs from a second donor without an inflammatory disorder (‘Donor B’) could not decidualize with E2P4 alone but their decidualization was rescued upon the addition of SP (Fig. 1B, left). Morphological assessment of Donor B cultures on Day 12 revealed that the combination of SP and E2P4 induced a change from spindle-like to polygonal/cobblestone cell shape, further confirming their decidualization (Zhu et al., 2014) (Fig. 1B, right).
Figure 1.
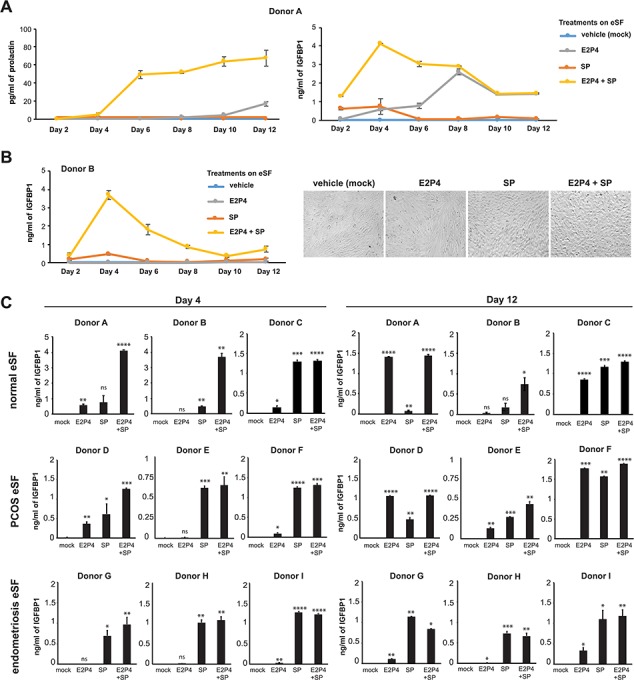
Seminal plasma enhances decidualization of endometrial stromal fibroblasts from women with and without inflammatory diseases. (A) Kinetic assessment of prolactin and insulin-like growth factor binding protein 1 (IGFBP1) secretion by endometrial stromal fibroblasts (eSFs) from a woman without inflammatory disease following treatment with media alone, estradiol with progesterone (E2P4), seminal plasma (SP) or E2P4 + SP. In this donor, SP increased the kinetics and extent of E2P4-induced decidualization. Each value corresponds to the mean ± SD of cultures set up in triplicates. (B) Kinetic and morphologic analysis of eSFs from a woman without inflammatory disease. Left: kinetic assessment of IGFBP1 secretion. Each value corresponds to the mean ± SD of triplicate cultures. Right: representative images (×100 magnification) of eSFs from Donor B after 10 days of treatment with media alone, E2P4, SP or E2P4 + SP. In this donor, decidualization only occurred in the presence of SP. (C) eSFs from women without inflammatory disease (Donors A–C), women with polycystic ovary syndrome (PCOS) (Donors D–F) or women with endometriosis (Donors G–I) were treated with media alone, E2P4, SP or E2P4 + SP. After 4 or 12 days of treatment, culture supernatants were assessed for decidualization by measuring IGFBP1 levels by ELISA. Each value corresponds to the mean ± SD of cultures set up in triplicates. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 as determined using a two-tailed Student’s t-test comparing each treatment condition (E2P4, SP and E2P4 + SP) to the mock-treated samples. ns indicates non-significant.
We next analyzed the ability of SP to promote decidualization in eSFs from multiple donors, including healthy women, women with PCOS and women with endometriosis (Supplementary Table SI). eSFs were treated with vehicle alone, E2P4 alone, 10% SP alone or E2P4 together with 10% SP for 4 or 12 days. After 4 days of treatment, the combination of SP with E2P4 led to higher levels of IGFBP1 than E2P4 treatment alone in all eSF samples, including those from normal, PCOS and endometriosis donors (Fig. 1C, left). Furthermore, data from Day 12 of treatment demonstrated that eSFs from women that failed to decidualize in response to E2P4 alone (Donors B, G, H and I) could be induced to decidualize when the E2P4 was supplemented with SP (Fig. 1C, right). Collectively, these data suggest that SP promotes decidualization of eSFs from healthy women as well as those with inflammatory disorders, including those where E2P4 alone could not initiate a decidualization response.
Degradation or denaturation of seminal proteins eliminates SP-enhanced decidualization
Since the SP factor(s) promoting decidualization is >3500 Da (Doyle et al., 2012), we hypothesized it was a protein. To test this, we treated SP for 18 h with Proteinase K, which degraded the majority of seminal proteins (Fig. 2A) and then tested the ability of the Proteinase K-treated SP to promote decidualization in the presence of E2P4. Proteinase K treatment completely abrogated SP-enhanced decidualization in eSFs (Fig. 2B). The lack of activity was not due to cellular toxicity (Fig. 2C). To further demonstrate that the decidualization-promoting factor is a protein, we denatured SP proteins by boiling. Consistent with the Proteinase K treatment data, boiled SP combined with E2P4 poorly enhanced decidualization of eSFs (Fig. 2D). Boiled SP was also minimally cytotoxic to eSFs (Fig. 2E).
Figure 2.
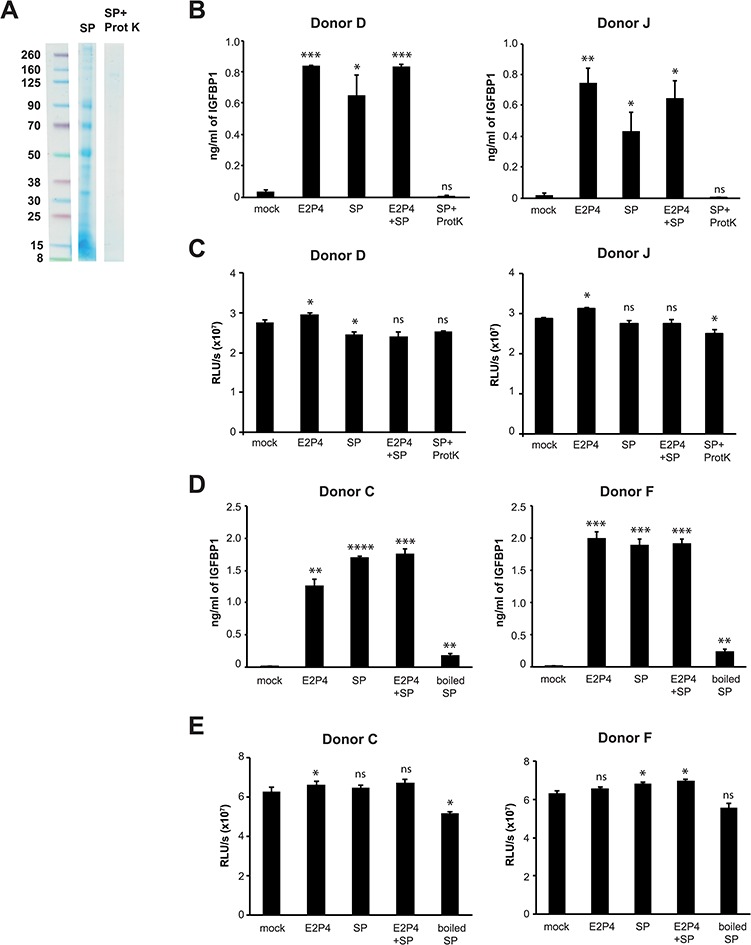
Degradation or denaturation of seminal proteins eliminates SP-enhanced decidualization. (A) SP was mock-treated or incubated with Proteinase K (200 μg/ml) for 18 h at 37°C, followed by the addition of phenylmethylsulphonyl fluoride to inactivate the Proteinase K. Protein content was then analyzed by Coomassie staining. (B) Proteinase K treatment abrogates SP-enhanced decidualization. IGFBP1 levels were measured in supernatants of eSF cultures (Donors D and J) after 14 days of treatment with media alone, E2P4, SP, E2P4 + SP or E2P4 + Proteinase K-treated SP. *P < 0.05, **P < 0.01 and ***P < 0.001, as determined using a two-tailed Student’s t-test comparing each treatment condition (E2P4, SP, E2P4 + SP and E2P4 + Proteinase K-treated SP) to the corresponding mock-treated samples. (C) Proteinase K-treated SP is not cytotoxic to eSFs. eSF cultures set up as described in panel B were assessed for cellular viability using the CellTiter-Glo luminescent assay. (D) Denaturation of seminal proteins by boiling diminishes SP-enhanced decidualization. IGFBP1 levels were measured in supernatants of eSF cultures (Donors C and F) after 14 days of treatment with media alone, E2P4, SP, E2P4 + SP or E2P4 + boiled SP. **P < 0.01, ***P < 0.001 and ****P < 0.0001 as determined using a two-tailed Student’s t-test comparing each treatment condition (E2P4, SP, E2P4 + SP and E2P4 + boiled SP) to the corresponding mock-treated samples. (E) Boiled SP is not cytotoxic to eSFs. eSF cultures set up as described in panel D were assessed for cellular viability using the CellTiter-Glo luminescent assay.
Semen amyloids do not promote eSF decidualization and do not induce a potent transcriptional response in eSFs or eECs
Semen amyloids are protein-derived fibrils that have recently been suggested to play a role in sperm selection during fertilization (Roan et al., 2017) and are known to enhance HIV infection (Munch et al., 2007; Roan et al., 2011; Usmani et al., 2014). To test whether semen amyloids might be responsible for SP-mediated decidualization, we chemically synthesized the highly abundant semenogelin amyloids (Roan et al., 2011, 2014). After confirming the functional activity of the synthetic amyloids by demonstrating their ability to enhance HIV infection (Supplementary Fig. S1), we tested their ability to promote decidualization of eSFs. We found that they did not (Fig. 3A) and that this was not a result of SP toxicity (Fig. 3B).
Figure 3.
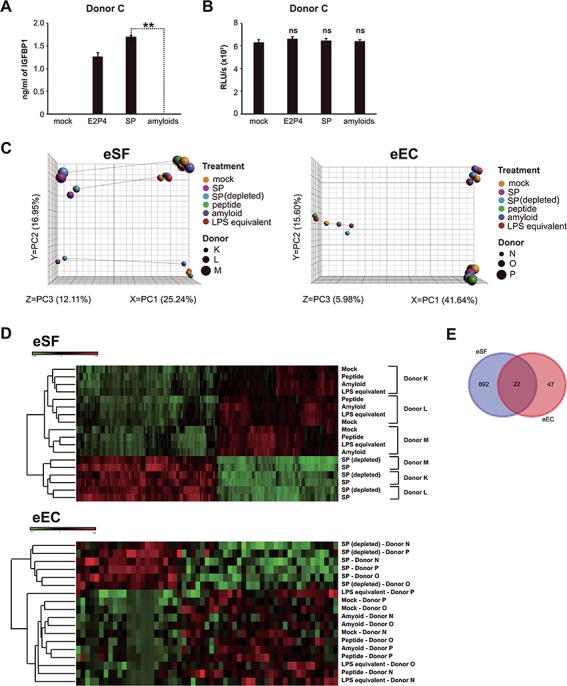
Semen amyloids do not promote eSF decidualization and induce a limited transcriptional response in eSFs and endometrial epithelial cells. (A) Purified SP amyloids do not enhance decidualization. IGFBP1 levels were measured in supernatants of eSFs treated for 14 days with media alone, E2P4, SP or purified amyloids, for which HIV infection-promoting activity was confirmed. **P < 0.01 as determined by a two-tailed Student’s t-test. (B) Purified SP amyloids are not cytotoxic. eSF cultures were prepared as described in panel A and were assessed for cellular viability using the CellTiter-Glo luminescent assay. (C) Semen amyloids, unlike SP, do not induce a potent transcriptional response in eSFs or endometrial epithelial cells (eECs). eSFs or eECs were treated for 6 h with SP, SP depleted of HIV-enhancing amyloids, purified SP amyloids or the monomeric control peptide. Because SP contains a low amount of contaminating lipopolysaccharides (LPS), a negative control of LPS matching the levels measured in SP stock was also included. After treatment, samples were harvested for RNAseq analysis. Left: Principal component analysis (PCA) of eSFs treated as indicated. Right: PCA of eECs treated as indicated. (D) Heatmap illustrating hierarchical clustering of eSF and eEC samples treated as indicated. Top: Heatmap showing the most differentially expressed genes following treatment of eSFs with the indicated factors, based on RNAseq analysis. Bottom: Heatmap showing the most differentially expressed genes following treatment of eECs with the indicated factors. The hierarchical clustering was based on differentially expressed genes across treatment (2467 and 57 genes for eSFs and eECs, respectively), not taking donor ID into account. (E) Venn diagram showing the number of SP-induced genes in eSFs and eECs.
To confirm the inability of the synthetic amyloids to promote decidualization, we assessed whether they elicited any transcriptional changes in eSFs associated with decidualization. To this end, we performed global RNAseq analysis of eSFs treated for 6 h with purified SP amyloids, control monomeric peptide or SP. As another control, we also tested SP depleted of HIV-enhancing amyloids (Supplementary Fig. S1). Furthermore, because low amounts of bacteria are sometimes present in SP, we quantified the amount of LPS in our SP stock by the LAL endotoxin assay and tested the effect of an equivalent amount of pure LPS as an additional negative control. We found no evidence that the amyloids induced transcription of any genes associated with decidualization. In fact, PCA of treated eSFs revealed that while SP and SP depleted of HIV-enhancing amyloids induce a potent transcriptional response, purified amyloids, like the negative control peptide and the LPS control, did not (Fig. 3C, left).
Because eECs are also a major cell type in the endometrium and one of the main cells exposed to the SP that enters this tissue, we also assessed the effects of SP and the SP amyloids on eECs. The transcriptional response of eECs to SP and SP depleted of HIV-enhancing amyloids was attenuated relative to that observed in eSFs, with the majority of the variance due to donor (Fig. 3C, right). In both eECs and eSFs, hierarchical clustering analyses of gene expression profiles identified two major subdivisions (Fig. 3D): SP and SP depleted of HIV-enhancing amyloids and amyloids and controls (mock, monomeric peptide, LPS equivalent). Subsequent branches separated samples by donor. Treated eECs showed reduced differential gene expression to SP in comparison with eSFs (69 versus 914 genes; Fig. 3E; Supplementary Table SII), suggesting that SP induces a more potent response in eSFs than in eECs and confirming our previous microarray study (Chen et al., 2014).
To understand how decidualization may be enhanced by SP, we conducted IPA on genes that were differentially expressed between mock and SP-treated eSFs (Table I). SP treatment of eSFs induced activation of pathways related to cell survival, cell morphology, cellular development, cellular movement and gene expression. Concomitantly, pathways related to cell death were inhibited. In eECs, SP activated pathways related to cellular movement and inhibited pathways related to cell death (Table II). Selections of eSF and eEC genes that are differentially expressed in response to SP are presented in Table III, along with their associated pathways.
Table I.
Selected pathways significantly affected by SP in eSFs.
| Category | Functions annotation | Activation State | Z-score |
|---|---|---|---|
| Cell Death and Survival | Cell survival | Increased | 2.89 |
| Cell Death and Survival | Cell viability | Increased | 2.696 |
| Cell Death and Survival | Necrosis | Decreased | 3.215 |
| Cell Death and Survival | Cell death | Decreased | 3.072 |
| Cell Death and Survival | Apoptosis | Decreased | 2.118 |
| Cell Death and Survival | Cell death of connective tissue cells | Decreased | 1.928 |
| Cell Morphology | Tubulation of endothelial cells | Increased | 1.935 |
| Cell Morphology | Morphogenesis of endothelial cells | Increased | 1.664 |
| Cell Morphology | Tubulation of cells | Increased | 1.656 |
| Cellular Development | Proliferation of tumor cells | Increased | 2.311 |
| Cellular Development | Differentiation of stromal cells | Increased | 2.19 |
| Cellular Development | Proliferation of cancer cells | Increased | 1.852 |
| Cellular Development | Endothelial cell development | Increased | 1.775 |
| Cellular Development | Differentiation of endothelial cells | Increased | 1.709 |
| Cellular Development | Proliferation of endothelial cells | Increased | 1.656 |
| Cellular Movement | Migration of vascular endothelial cells | Increased | 2.564 |
| Cellular Movement | Movement of vascular endothelial cells | Increased | 2.547 |
| Cellular Movement | Invasion of endothelial cells | Increased | 2.537 |
| Cellular Movement | Cell movement of endothelial cells | Increased | 2.443 |
| Cellular Movement | Migration of endothelial cells | Increased | 2.293 |
| Cellular Movement | Cell movement | Increased | 2.273 |
| Cellular Movement | Migration of cells | Increased | 2.055 |
| Cellular Movement | Chemotaxis of endothelial cells | Increased | 2.012 |
| Increased | |||
| Gene Expression | Activation of DNA endogenous promoter | Increased | 2.107 |
| Gene Expression | Binding of DNA | Increased | 1.709 |
P < 0.05; Z-score > |1.5|.
SP indicates seminal plasma; eSFs, endometrial stromal fibroblast.
Table II.
Selected pathways significantly affected by SP in endometrial epithelial.
| Category | Functions annotation | Activation State | Z-score |
|---|---|---|---|
| Cell Death and Survival | Cell Death | Decreased | 1.698 |
| Cellular Movement | Migration of cells | Increased | 2.978 |
| Cellular Movement | Chemotaxis | Increased | 2.122 |
| Cellular Movement | Migration of endothelial cells | Increased | 1.751 |
| Cellular Movement | Movement of vascular endothelial cells | Increased | 1.526 |
P < 0.05; Z-score > |1.5|.
Table III.
Selected genes regulated in eSFs and eECs.
| eSF SP versus eSF Mock | eEC SP versus eEC Mock | ||||
|---|---|---|---|---|---|
| Category | Gene | Fold Change | Category | Gene | Fold Change |
| Cell Death and Survival | IL33 | 74.78 | Cell Death and Survival | MT1X | 4.29 |
| CXCR4 | 16.81 | NR4A1 | 3.23 | ||
| CXCL8 | 11.00 | IL11 | 1.93 | ||
| PTGS2 | 9.65 | PTGS2 | 1.77 | ||
| LIF | 8.18 | CXCL8 | 1.62 | ||
| STC1 | 7.53 | IGFBP3 | 1.55 | ||
| VEGFA | 3.96 | IL1RN | 1.51 | ||
| DUSP1 | 3.67 | FOSL1 | 1.51 | ||
| FOXO1 | 3.61 | ZNF43 | −2.43 | ||
| HIF1A | 2.7 | ||||
| FGF2 | 2.65 | ||||
| WNT5A | 2.61 | ||||
| BMP2 | 2.55 | ||||
| EGFR | 2.33 | ||||
| IGF1R | 2.21 | ||||
| TP53 | −2.07 | ||||
| IRF1 | −2.16 | ||||
| JAG1 | −2.18 | ||||
| JUN | −2.48 | ||||
| IL24 | −2.74 | ||||
| HEY1 | −2.97 | ||||
| KLF11 | −3.27 | ||||
| TLR4 | −3.36 | ||||
| PDGFB | −5.18 | ||||
| IFIT3 | −5.18 | ||||
| IFIT2 | −7.72 | ||||
| Category | Gene | Fold Change | Category | Gene | Fold Change |
| Cellular Movement | IL33 | 74.78 | Cellular Movement | NR4A1 | 3.23 |
| IL11 | 58.38 | NR4A2 | 2.68 | ||
| CXCR4 | 16.81 | CXCL3 | 2.37 | ||
| CXCL8 | 11.00 | IL11 | 1.93 | ||
| PTGS2 | 9.65 | CXCL2 | 1.81 | ||
| LIF | 8.18 | PTGS2 | 1.77 | ||
| STC1 | 7.53 | CCL2 | 1.81 | ||
| TP53 | −2.07 | CXCL8 | 1.62 | ||
| HEY1 | −2.97 | IGFBP3 | 1.55 | ||
| TLR4 | −3.36 | ||||
| PDGFB | −5.18 | ||||
| IFIT2 | −7.72 | ||||
P < 0.05.
SP: seminal plasma, eSF: endometrial stromal fibroblasts, eEC: endometrial epithelial cells.
In summary, our decidualization and RNAseq analyses reveal that semen amyloids do not induce decidualization, semen amyloids signal poorly to both eSFs and eECs and SP induces a more potent transcriptional response in eSFs than in eECs.
Secretome analyses of SP-exposed eSFs and eECs
Given the importance of cytokines in decidualization and implantation (Stewart et al., 1992; Robb et al., 1998; Jasper et al., 2007), we also characterized the effects of SP and SP components on the secretion of 32 different growth factors and cytokines by eSFs. To distinguish between cytokines secreted by eSFs in response to SP from endogenous cytokines present in SP, empty wells (without cells) were treated in parallel with SP (‘SP alone’) as a negative control. Secreted growth factors and cytokines were quantified through a combination of Luminex and ELISA. We found that treatment of eSFs with SP and SP depleted of HIV-enhancing amyloids increased (P < 0.05) the secretion of EGF, FGFβ, HGF, IFN-γ, IL-8, MCP-1, MIP-1β and VEGF (Table IV). In addition, we also found SP upregulated secretion of LIF, IL-6 and IL-11 (Table IV; Fig. 4A, B), which have known roles in implantation (Stewart et al., 1992; Robb et al., 1998; Jasper et al., 2007).
Table IV.
The effect of SP components on the production of cytokines in eSF.
| Analytes | Vehicle | SP | SP(depleted) a | Peptide b | Amyloid c | LPS equivalent | SP, no eSF |
|---|---|---|---|---|---|---|---|
| EGF | 2.97 ± 0.61 | 42.52 ± 3.52 * | 43.67 ± 4.11 * | 3.56 ± 0.41 | 3.32 ± 0.71 | ND | ND |
| Eotaxin | ND | ND | ND | ND | ND | ND | 160.23 |
| FGFβ | 6.15 ± 1.21 | 21.00 ± 0.71 * | 21.68 ± 3.47 * | ND | ND | 6.97 ± 1.86 | ND |
| G-CSF | ND | ND | ND | ND | ND | ND | 15.16 |
| GM-CSF | ND | ND | ND | ND | ND | ND | ND |
| HGF | 9.64 ± 2.04 | 61.80 ± 21.01 * | 46.52 ± 11.38 * | 8.46 ± 2.044 | 8.46 ± 2.04 | 7.85 ± 0.99 | ND |
| IFN-⍺ | ND | 11.39 ± 2.76 | 10.19 ± 2.08 | ND | ND | ND | 23.79 |
| IFN-γ | 3.49 | 18.94 ± 3.30 * | 20.71 ± 0.89 * | 3.49 | 3.49 | ND | ND |
| IL-10 | ND | 4.92 ± 1.13 | 5.23 ± 1.17 | ND | ND | ND | 16.63 |
| IL-11 | 206.70 ± 111.57 | 4520.96 ± 821.96 * | 4480.81 ± 591.74 * | ND | ND | ND | 86 |
| IL-12 | ND | 7.43 ± 0.82 | 7.19 ± 0.71 | ND | ND | ND | 3.3 |
| IL-13 | ND | 19.16 ± 4.28 | 20.13 ± 2.34 | ND | ND | ND | 6.48 |
| IL-15 | ND | 48.24 ± 6.27 | 50.77 ± 7.63 | ND | ND | ND | 16.69 |
| IL-17 | ND | ND | ND | ND | ND | ND | 52.36 |
| IL-1β | ND | 13.12 ± 1.72 | 11.99 ± 1.56 | ND | ND | ND | ND |
| IL-1RA | ND | 38.37 ± 1.89 | 34.04 ± 1.86 | ND | ND | ND | 7.57 |
| IL-2 | ND | ND | ND | ND | ND | ND | 42.76 |
| IL-2R | ND | 28.05 | 24.92 ± 3.13 | ND | ND | ND | ND |
| IL-4 | ND | ND | ND | ND | ND | ND | ND |
| IL-5 | ND | ND | ND | ND | ND | ND | ND |
| IL-6 | ND | 87.50 ± 13.06 | 106.73 ± 45.92 | ND | ND | ND | ND |
| IL-7 | ND | 143.13 ± 48.24 | 72.03 ± 7.98 | ND | 7.61 | 8.63 ± 3.33 | 19.78 |
| IL-8 | 516.51 ± 457.14 | 6497.67 ± 2236.15 * | 4654.00 ± 1298.72 * | 78.56 ± 238.6 | 349.19 ± 292.53 | 411.83 ± 314.94 | 421.71 |
| IP-10 | ND | ND | ND | ND | ND | ND | 49.34 |
| LIF | ND | 1310.66 ± 344.29 | 1160.45 ± 567.06 | ND | ND | ND | ND |
| MCP-1 | 198.17 ± 248.15 | 572.59 ± 575.39 * | 399.31 ± 17.53 * | 145.45 ± 180 | 139.06 ± 169.34 | 177.75 ± 224.34 | 113.08 |
| MIG | ND | 74.01 ± 15.74 | 49.21 ± 3.38 | ND | ND | ND | 81.52 |
| MIP-1⍺ | ND | 22.88 ± 1.74 | 23.27 | ND | ND | ND | 18.62 |
| MIP-1β | 2.60 ± 0.38 | 20.06 ± 4.29 * | 17.28 * | 2.40 ± 0.722 | 2.82 | 2.82 | 11.32 |
| RANTES | ND | ND | ND | ND | ND | ND | ND |
| TNF-⍺ | ND | ND | ND | ND | ND | ND | ND |
| VEGF | 6.03 ± 3.70 | 213.57 ± 36.40 * | 239.80 ± 39.50 * | 5.49 ± 3.356 | 6.09 ± 4.01 | 6.26 ± 3.42 | 196.82 |
aSeminal plasma devoid of amyloids.
bMonomeric peptide.
cSynthesized human SEM1(86–107) amyloid fibrils.
LPS: lipopolysaccharide*P < 0.05eSF (pg/ml)
Figure 4.
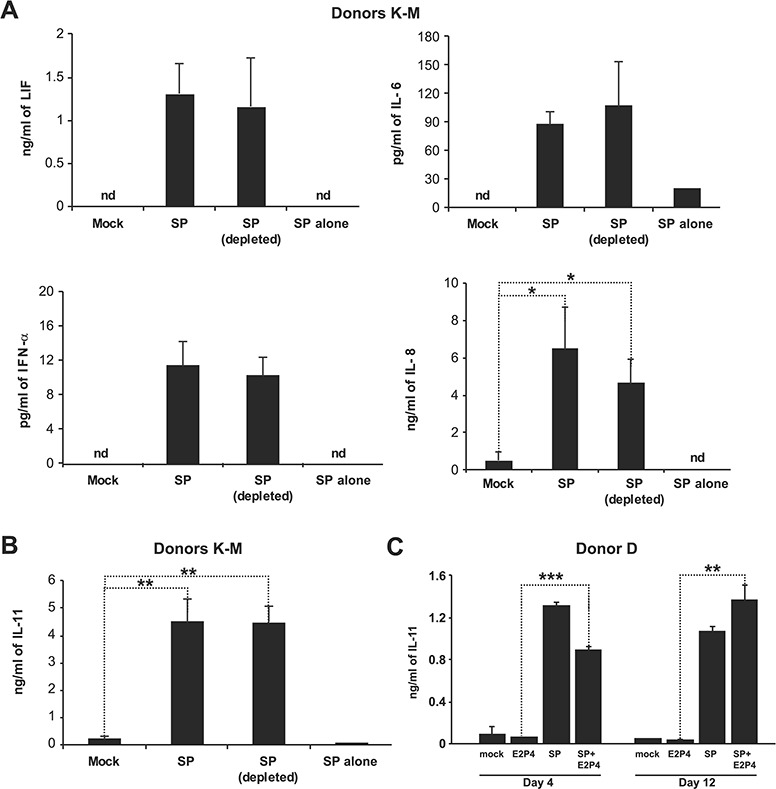
Secretome analysis of eSFs following SP treatment reveals a potent upregulation of interleukin-11, a cytokine crucial for implantation. (A) eSFs from three donors (Donors K–M) were treated for 6 h with media alone, SP or SP depleted of HIV-enhancing amyloids, after which cell supernatants were collected for Luminex analysis. Both SP and SP depleted of HIV-enhancing amyloids induced secretion of multiple cytokines in eSFs (see Table IV for full list). To control for endogenous levels of the cytokines, the equivalent concentration of SP (‘SP alone’) was also assayed in parallel. *P < 0.05 as determined using a two-tailed Student’s t-test comparing each treatment condition (SP and SP(depleted)) to the corresponding mock-treated samples. nd indicates not detectable. (B) eSF culture supernatants set up as described in panel A were assayed for interleukin (IL)-11 levels by ELISA. **P < 0.01 as determined using a two-tailed Student’s t-test comparing each treatment condition (SP and SP(depleted)) to the mock-treated samples. (C) eSFs were treated with media alone, estradiol with progesterone (E2P4), SP or E2 + SP. After 4 or 12 days of treatment, culture supernatants were assessed for decidualization by measuring IGFBP1 levels by ELISA. **P < 0.01 and ***P < 0.001 as determined using a two-tailed Student’s t-test comparing the E2P4 and E2P4 + SP treatment conditions.
Given the particularly important role of IL-11 in both decidualization and implantation (Robb et al., 1998; Dimitriadis et al., 2002; Karpovich et al., 2005; Menkhorst et al., 2009), we focused on it further by investigating whether SP-induced secretion of IL-11 occurs during E2P4-mediated decidualization. As shown in Fig. 4C, E2P4 alone induced only low levels of IL-11 production consistent with a prior report (Dimitriadis et al., 2002). However, SP in the presence of E2P4 potently stimulated eSF secretion of IL-11, both at 4 (P < 0.001 versus E2P4) and 12 days (P < 0.01 versus E2P4; Fig. 4C) post-treatment.
Induction of IL-11 by SP is mediated by a proteinaceous component associated with MVs
We postulated that SP-induced IL-11 secretion by eSF played a critical role in SP’s ability to promote decidualization. If this were true, then SP that had been devoid of proteins, which lack the ability to promote decidualization (Fig. 2), should not induce IL-11. To test this, we measured IL-11 levels in culture supernatants of Proteinase K-treated SP. SP devoid of proteins was unable to elicit IL-11 secretion (Fig. 5A), consistent with IL-11 signaling as an important pathway in SP-enhanced decidualization.
Figure 5.
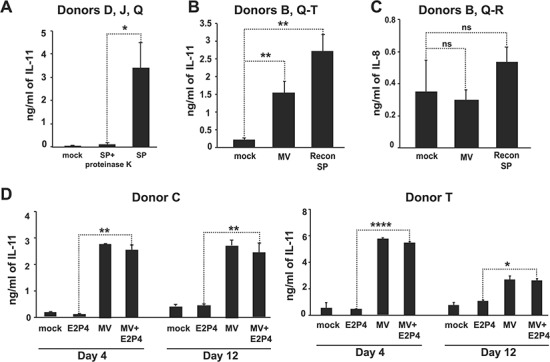
SP-mediated induction of IL-11 is mediated by proteins associated with microvesicles. (A) eSFs from three donors (Donors D, J and Q) were treated for 6 h with vehicle, SP or SP treated with Proteinase K to determine whether seminal proteins are necessary for IL-11 induction. IL-11 levels in culture supernatants were quantified by ELISA. *P < 0.05 as determined using a two-tailed Student’s t-test comparing the SP and the SP + Proteinase K conditions. (B) eSFs from five donors (Donors B and Q–T) were treated for 6 h with vehicle, the microvesicle (MV)-enriched fraction, or reconstituted SP, and cell supernatants were assessed for IL-11 levels by ELISA. **P < 0.01 as determined using a two-tailed Student’s t-test comparing the MV-enriched treatment to the mock-treated samples. (C) eSFs from three donors (Donors B, Q and R) were treated for 6 h with vehicle, the MV-enriched fraction, or reconstituted SP, and cell supernatants were assessed for IL-8 levels by Luminex. (D) eSFs from two donors (Donors C and T) were treated with media alone, E2P4, the MV-enriched fraction or E2P4 + the MV-enriched fraction. After 4 or 12 days of treatment, culture supernatants were assayed for IL-11 levels by ELISA. *P < 0.05, **P < 0.01 and ****P < 0.0001 as determined using a two-tailed Student’s t-test comparing treatment condition E2P4 to E2P4 + the MV-enriched fraction.
Proteins in SP can exist in either a soluble form or associated with EVs, including large MVs (typically 50–1000 nm in diameter) (Machtinger et al., 2016). To determine whether the SP proteins with decidualization capacity may be MV-associated, we assessed whether SP enriched for large MVs (220–650 nm) induce IL-11 secretion by eSF. Indeed, fractionated SP enriched for MVs induced IL-11 secretion by eSF at a level of ~57% of that induced by SP (Fig. 5B). This effect was specific for IL-11 since other cytokines, such as IL-8, were highly induced by SP but not by the MV-enriched fraction (compare Figs 4A and 5C). We further confirmed that the ability of the MV-enriched fraction to upregulate IL-11 secretion by eSF also occurred in the presence of E2P4 (Fig. 5D).
The MV-enriched fraction of SP induces decidualization and an intermediate transcriptional response in eSFs
In addition to upregulating IL-11 in the presence of E2P4, the MV-enriched fraction also promoted E2P4-mediated eSF decidualization (Fig. 6A). We then assessed the global effects of the MV-enriched fraction on eSFs. eSFs were treated for 6 h with vehicle, the MV-enriched fraction, or reconstituted SP (comprised a solution where the MV-enriched and non-MV fractions were added back together to reconstitute the SP; this was used instead of SP to account for any effect fractionation may have had on the signaling effects of SP). RNAseq analysis was then conducted on the specimens. PCA revealed that the MV-enriched fraction elicited a transcriptional response intermediate between that of vehicle and reconstituted SP (Fig. 6B). Hierarchical clustering analyses indicated that cells treated with the MV-enriched fraction clustered more closely with cells exposed to reconstituted SP than to vehicle-treated eSFs (Fig. 6C). Furthermore, the majority of transcripts induced by the MV-enriched fraction overlapped with those induced by reconstituted SP (347 genes in common; Fig. 6D; Supplementary Table SIII).
Figure 6.
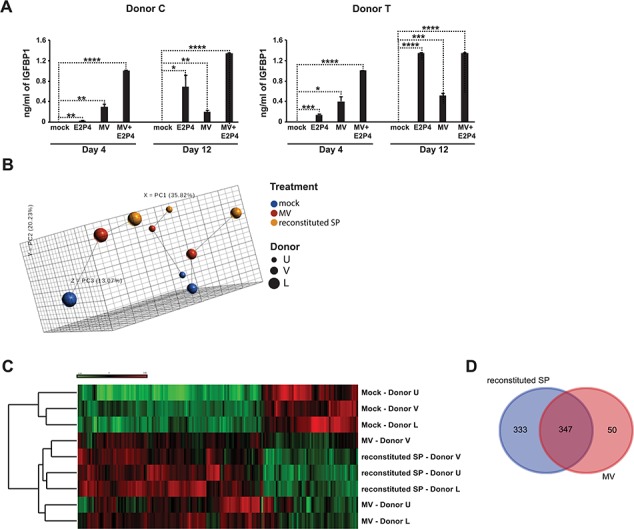
MV-enriched fraction from SP induces a potent transcriptional response and decidualization in eSFs. (A) eSFs were treated with media alone, E2P4, the MV-enriched fraction or E2P4 + the MV-enriched fraction. After 4 or 12 days of treatment, culture supernatants were assessed for decidualization by measuring IGFBP1 levels by ELISA. Each value corresponds to the mean ± SD of cultures set up in triplicates. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 as determined using a two-tailed Student’s t-test comparing each treatment condition (E2P4, the MV-enriched fraction or E2P4 + the MV-enriched fraction) to the mock-treated samples. (B) PCA of RNAseq data from eSFs treated for 6 h with vehicle, the MV-enriched fraction or reconstituted SP. (C) Heatmap illustrating hierarchical clustering of eSFs treated with vehicle, the MV-enriched fraction, or reconstituted SP, based on the RNAseq datasets. Hierarchical clustering was based on differentially expressed genes across treatment (281 genes), not taking donor ID into account. (D) Venn diagram showing the number of transcripts induced by the MV-enriched fraction and reconstituted SP.
IPA analysis of differentially expressed genes revealed similar regulated biological pathways between cells treated with the MV-enriched fraction versus reconstituted SP (P < 0.05, Z-score > 1.5; Table V). Following both treatments, pathways related to cell survival, cell movement and cellular development increased while pathways related to cell death decreased (Table V). Selected differentially expressed genes and their associated pathways affected by the MV-enriched fraction and reconstituted SP in eSFs are presented in Table VI.
Table V.
Selected pathways significantly affected by microvesicles in eSF.
| Reconsituted SP versus Mock | MV versus Mock | ||||||
|---|---|---|---|---|---|---|---|
| Category | Functions annotation | Activation State | Z-score | Category | Functions annotation | Activation State | Z-score |
| Cell Death and Survival | Cell survival | Increased | 2.485 | Cell Death and Survival | Cell survival | Increased | 2.703 |
| Cell Death and Survival | Cell viability | Increased | 2.125 | Cell Death and Survival | Cell viability | Increased | 2.186 |
| Cell Death and Survival | Cell viability of endothelial cells | Increased | 2.101 | Cell Death and Survival | Cell viability of endothelial cells |
Increased | 1.869 |
| Cell Death and Survival | Cell death | Decreased | 3.45 | Cell Death and Survival | Cell death | Decreased | 2.143 |
| Cell Death and Survival | Necrosis | Decreased | 3.141 | Cell Death and Survival | Necrosis | Decreased | 2.292 |
| Cell Death and Survival | Apoptosis | Decreased | 2.564 | Cell Death and Survival | Apoptosis | Decreased | 1.893 |
| Cell Death and Survival | Cell death of tumor cell lines | Decreased | 2.232 | ||||
| Cellular Movement | Cell movement | Increased | 2.964 | Cellular Movement | Cell movement | Increased | 2.212 |
| Cellular Movement | Migration of cells | Increased | 2.886 | Cellular Movement | Migration of cells | Increased | 2.033 |
| Cellular Movement | Chemotaxis of endothelial cells | Increased | 1.952 | Cellular Movement | Chemotaxis of endothelial cells |
Increased | 1.952 |
| Cellular Movement | Migration of tumor cell lines | Increased | 1.859 | Cellular Movement | Chemotaxis | Increased | 1.658 |
| Cellular Movement | Invasion of endothelial cells | Increased | 1.756 | ||||
| Cellular Movement | Movement of vascular endothelial cells | Increased | 1.573 | ||||
| Cellular Movement | Chemotaxis | Increased | 1.509 | ||||
| Cellular Development | Proliferation of tumor cells | Increased | 2.499 | Cellular Development | Proliferation of tumor cells | Increased | 1.634 |
| Cellular Development | Differentiation of stromal cells | Increased | 1.96 | Cellular Development | Tubulation of endothelial cells | Increased | 1.585 |
| Cellular Development | Tubulation of endothelial cells | Increased | 1.79 | Cellular Development | Differemtiation of endothelial cells | Increased | 1.709 |
| Cellular Development | Differentiation of endothelial cells | Increased | 1.709 | ||||
| Cellular Development | Proliferation of cancer cells | Increased | 1.649 | ||||
| Cellular Development | Differentiation of epithelial tissues | Increased | 1.51 | ||||
| Cellular Growth and Proliferation | Colony formation of cells | Increased | 3.527 | ||||
| Gene Expression | Binding of DNA | Increased | 2.239 | ||||
P < 0.05; Z-score > |1.5|.
MV indicates microvesicles.
Table VI.
Selected genes regulated in eSF by MV.
| Reconstituted SP versus Mock | MV versus Mock | ||||
|---|---|---|---|---|---|
| Category | Gene | Fold Change | Category | Gene | Fold Change |
| Cell Death and Survival | SST | 379.6 | Cell Death and Survival | SST | 385.22 |
| IL11 | 24.65 | IL11 | 12.98 | ||
| IL33 | 21.19 | CCL7 | 11.99 | ||
| CCL7 | 14.06 | IL33 | 7.49 | ||
| NR4A2 | 5.14 | CCL2 | 4.17 | ||
| CCL2 | 5.05 | PTGS2 | 3.00 | ||
| PTGS2 | 4.21 | FGF7 | 2.83 | ||
| FGF7 | 3.42 | FOXO1 | 2.13 | ||
| HIF1A | 2.58 | HIF1A | 2.01 | ||
| STC1 | 2.51 | WNT5A | 1.98 | ||
| VEGFA | 2.45 | IFIT3 | −2.05 | ||
| DUSP1 | 2.37 | ICAM1 | −2.07 | ||
| FOXO1 | 2.37 | TLR4 | −2.35 | ||
| EGFR | 2.35 | JUN | −2.46 | ||
| WNT5A | 2.28 | IFIT2 | −3.1 | ||
| FGF2 | 2.07 | IL24 | −3.21 | ||
| IRF1 | −1.95 | EGR3 | −4.79 | ||
| PDGFB | −2.16 | ||||
| ICAM1 | −2.21 | ||||
| KLF11 | −2.25 | ||||
| IFIT3 | −2.84 | ||||
| JUN | −3.07 | ||||
| IL24 | −3.31 | ||||
| BMF | −3.34 | ||||
| EGR3 | −5.58 | ||||
| IFIT2 | −5.16 | ||||
| Cellular Movement | IL11 | 24.65 | Cellular Movement | IL11 | 12.98 |
| IL33 | 21.19 | CCL7 | 11.99 | ||
| CCL7 | 14.06 | IGFBP1 | 8.53 | ||
| IGFBP1 | 7.07 | IL33 | 7.49 | ||
| CCL2 | 5.05 | CCL2 | 4.17 | ||
| FGF7 | 3.42 | PTGS2 | 3.00 | ||
| VEGFA | 2.45 | FGF7 | 2.83 | ||
| FOXO1 | 2.37 | ICAM1 | −2.07 | ||
| EGFR | 2.35 | KLF2 | −2.14 | ||
| WNT5A | 2.27 | TLR4 | −2.35 | ||
| PDGFB | −2.16 | IFIT2 | −3.1 | ||
| IFIT2 | −5.16 | RGS4 | −6.84 | ||
P < 0.05.
IL-11 signaling in eSFs is required for SP-promoted decidualization
The data presented thus far suggest that proteinaceous factors associated with seminal MVs promote decidualization of eSFs, possibly through the induction of IL-11 secretion. To directly assess a role for IL-11, we used CRISPR gene-editing technology to knock out the IL-11RA in eSFs and disrupt the cells’ ability to respond to IL-11 signaling. Using specific guide RNAs for IL-11RA, we knocked out IL-11RA in eSFs from two donors with PCOS (Donors W and X). Efficient gene editing (average 95.5% indel and 92% knockout efficiency) was demonstrated by inference of CRISPR edit (ICE) analysis (Fig. 7A). Control eSFs (treated with non-targeting guides) and IL-11RA-knockout eSFs were treated with SP, in the presence or absence of E2P4, and decidualization was monitored by the production of IGFBP1 and morphological characterization. As expected, vehicle-treated eSFs as well as eSFs treated with scrambled guides decidualized in response to 10% SP and 10% SP + E2P4 (Fig. 7B). In comparison, in the presence of either 10% SP or 10% SP + E2P4, IL-11RA eSF knockout lines secreted lower levels of IGFBP1 on both Days 4 and 12 post-treatment (Fig. 7B). Similar results were observed when treatment was performed with 1% SP (Supplementary Fig. S2). These results suggest that IL-11 signaling in eSFs is necessary for SP-enhanced decidualization.
Figure 7.
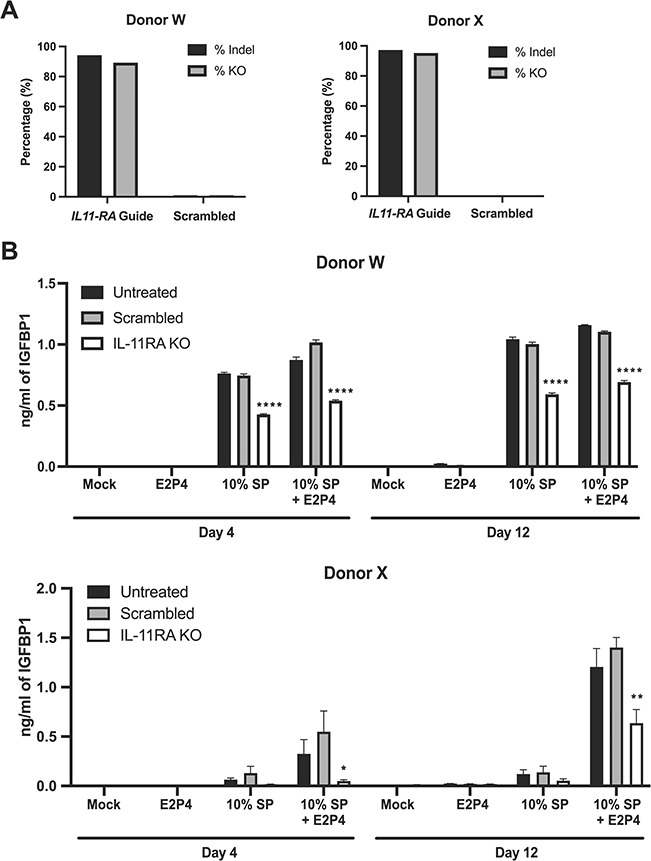
CRISPR/Cas-9 mediated knockout of IL-11 receptor reveals IL-11 signaling is important for SP-enhanced decidualization. (A) Average percentage of indels and knockout (KO) efficiency at the interleukin-11 receptor (IL-11RA) locus in eSFs treated with the IL-11RA guide or the scrambled control, as determined by ICE analyses following Clustered regularly interspaced short palindromic repeats (CRISPR)/Cas-9-mediated gene editing. (B) IL-11RA KO eSFs, scrambled negative control eSFs and unedited (untreated) eSFs were treated with media alone, E2P4, 10% SP or E2P4 + 10% SP. After 4 or 12 days of treatment, culture supernatants were assessed for decidualization by measuring IGFBP1 levels by ELISA. Each value corresponds to the mean ± SD of cultures set up in triplicates. *P < 0.05, **P < 0.01 and ****P < 0.0001, as determined by two-tailed Student’s t-test, indicate a significant decrease in IGFBP1 levels in the IL-11RA KO as compared with the scrambled negative control.
Discussion
While beneficial effects of SP on implantation are recognized (Robertson et al., 2016), the underlying molecular mechanisms are not well understood. In this study, we have used in vitro assays to characterize how SP signals to eSFs. Our major findings are as follows: SP promotes decidualization of eSFs from healthy women as well as those with PCOS and endometriosis; MV-associated SP proteins are, in part, responsible for this decidualization-promoting activity; the SP proteins promoting decidualization induce transcriptional responses in eSFs associated with cellular differentiation, including potent induction of IL-11; and the ability of SP to support decidualization is dependent on IL-11 signaling. These data support the notion that SP may promote implantation by either initiating or increasing the efficiency of decidualization.
SP promotes ex vivo eSF decidualization
Doyle et al. (2012) previously reported that dialyzed SP accelerates and increases the extent of P4-induced decidualization in eSFs, providing a possible explanation for how SP may increase implantation rates. In this study, we used both E2 and P4, as this more closely mimics the in vivo development of a receptive endometrium during the luteal phase, which requires the concerted action of both E2 and P4 (Cha et al., 2012). Our findings confirm that SP promotes decidualization and further demonstrate that this effect also holds true in eSFs from women with PCOS and endometriosis whose eSFs are more commonly resistant to E2P4-mediated decidualization. Furthermore, we found multiple patterns of SP effects on E2P4-mediated decidualization, including enhanced kinetics and instances where eSFs that normally do not decidualize with E2P4 can be rendered capable to do so in the presence of SP. These results demonstrate that SP can both enhance and, in some cases, potentiate E2P4-mediated eSF decidualization.
What in SP is promoting decidualization?
Given that the factor(s) in SP promoting decidualization is >3500 Da (Doyle et al., 2012) and SP proteins are typically this size, it was perhaps not surprising to find that protein degradation or denaturation eliminated SP-enhanced decidualization in eSFs. On the other hand, the fact that amyloids, a very abundant class of proteins in SP, did not mediate this effect was somewhat unexpected since other amyloids have been reported to induce IL-6, whose family members have been associated with the decidualization response (Rensink et al., 2002; Galimberti et al., 2008; Griffin et al., 2016). Unlike other amyloids that signal potently to cells (Meda et al., 1995; Hu et al., 1998), semen amyloids are distinct in that they are produced in a non-disease state (Usmani et al., 2014) and, as demonstrated in this study, are relatively inert with regards to signaling a transcriptional response in both eSFs and eECs. It is possible that the role of semen amyloids in the FRT is more structural in nature and limited to promoting phagocytosis of excess and/or defective sperm, as recently suggested (Roan et al., 2017).
The proteinaceous moiety responsible for SP-induced decidualization seems, at least in part, associated with MVs. SP proteins can exist in a soluble form but many are also packaged in EVs, which consist of larger MVs (typically 50–1000 nm in diameter) (Machtinger et al., 2016) and smaller exosomes (typically 40–100 nm in diameter) (Vojtech et al., 2014). SP EVs originate from several cell types in the male reproductive tract (Renneberg et al., 1997; Vojtech et al., 2014) and have been documented to fuse with the sperm membrane to assist with survival in the acidic vaginal environment (Arienti et al., 1997). In this study, we focused on larger MVs and found that an SP fraction enriched for MVs promotes eSF decidualization. Whether MVs fuse with eSFs to deliver a decidualization signal is not known but would be of interest to examine in future studies.
Molecular pathways underlying SP-enhanced decidualization
To better understand the mechanisms by which SP promotes decidualization, we assessed how eSFs respond to treatment with SP and SP components with or without decidualization-promoting activity. To characterize the signaling pathways in eSFs that are elicited upon treatment with SP or SP constituents, we conducted global gene expression analysis and Luminex assays. Consistent with our prior microarray study (Chen et al., 2014), SP treatment resulted in a potent transcriptional response in eSFs, eliciting signaling pathways related to cell death and survival and cellular development and movement. We also assessed the effects of the MV-enriched fraction on eSFs and observed an intermediary transcriptional response as compared with SP. Since the MV-enriched fraction was sufficient to promote decidualization, the signaling pathways associated with transcripts induced by the MV-enriched fraction may be the ones responsible for enhancing the decidualization response. These pathways include those related to cell survival and cellular movement.
The RNAseq datasets generated in this study of how eSFs respond to SP and specific SP constituents provide a useful resource for the field. In comparison with the previous microarray datasets examining how eSFs respond to SP exposure (Chen et al., 2014), the RNAseq datasets generated here provide a more unbiased assessment of transcripts, since RNAseq technology is independent of probe availability and intensity of transcript expression (Wang et al., 2009). Furthermore, RNAseq has a larger dynamic range and can detect low abundance transcripts, transcript variants and novel transcripts, which is not possible with probe hybridization in microarrays. Compared with our prior microarray study (Chen et al., 2014), we identified additional transcripts affected by unfractionated SP and MV-enriched SP in eSFs, including those with essential roles in uterine receptivity and implantation. For example, RNAseq demonstrated that both unfractionated and MV-enriched SP stimulated the expression of FOXO1 in eSFs, which has recently been defined as critical for embryo implantation through downregulation of progesterone receptor (PGR) in the uterine epithelium (Vasquez et al., 2018). The FOXO1-PGR interaction has also been documented as important for in vitro eSF decidualization (Labied et al., 2006; Takano et al., 2007; Vasquez et al., 2015). Another transcript induced by SP and uniquely identified by RNAseq was Stanniocalcin-1 (STC1), which is upregulated in endometrial fluid during the mid-secretory phase in fertile women and increases during ex vivo eSF decidualization but is downregulated in endometriosis (Aghajanova et al., 2016).
We also identified differentially expressed transcripts that were shared between the microarray and RNAseq datasets, thus confirming our prior analyses. One transcript upregulated by SP in both the microarray and RNAseq datasets was IL-33, which has previously been associated with the decidual response (Salker et al., 2012). Furthermore, altered IL-33 signaling is observed in decidualizing eSFs from women with recurrent pregnancy loss and pretreatment of mice uteri with conditioned media from those cultures results in pregnancy failure (Salker et al., 2012), supporting the notion that SP promotes eSFs decidualization and can potentially facilitate implantation.
Of most interest in our microarray and RNAseq datasets, however, was the potent induction of IL-11 transcription (3.9-fold as assessed by microarray, >74-fold as assessed by RNAseq) by SP in eSFs. Consistent with the transcriptomic datasets, IL-11 protein was secreted by eSF at significantly higher levels following treatment with SP in both the presence (>25-fold) and absence (>32-fold) of E2P4 at 12 days post-treatment. Moreover, it was also induced by the MV-enriched fraction of SP that is sufficient to promote decidualization. In contrast, semen amyloids, which did not promote decidualization, did not induce IL-11 secretion in eSFs (data not shown). To directly examine the role of IL-11 signaling in SP-enhanced decidualization, we abrogated the ability of eSFs to respond to IL-11 signaling by mutating IL-11RA via CRISPR/Cas9 technology. To our knowledge, this is the first reported instance of successful application of CRISPR/Cas9 technology to disrupt genes in primary eSFs. We found that knockout of IL-11RA in eSFs diminishes SP-induced decidualization, suggesting that SP does not overcome the requirement for IL-11 signaling in eSF decidualization. Future studies should establish if SP exposure after coitus induces similar effects in vivo and the identity of the seminal protein(s) responsible for such effects.
Inflammation and SP-induced decidualization
The mechanism by which SP restores endometrial decidualizing ability in women with inflammatory disorders is unclear, and at face value may seem at odds with the notion that SP harbors pro-inflammatory cytokines and generally elicits an inflammatory response in cells of the FRT. We speculate that the reason SP can elicit a decidual response is because pro-inflammatory factors including cytokines are pleiotrophic in their actions and can be pro-inflammatory in one setting yet anti-inflammatory in another. Furthermore, decidualization is biphasic and involves an acute-phase inflammatory response followed by an anti-inflammatory response required for embryo implantation (Salker et al., 2012). For example, both IL-11 and IL-33 are important for initiating decidualization (Dimitriadis et al., 2002, 2006; Menkhorst et al., 2009; Salker, et al., 2012), but if these cytokines are elevated later in pregnancy, complications can occur (Salker et al., 2012; Winship, et al., 2015). Furthermore, while PCOS eSFs that do not decidualize in response to steroid hormones secrete increased levels of IL-6 and IL-8 relative to eSFs from control women, exogenously added IL-6 and/or IL-8 do not inhibit decidualization in the control eSFs (Piltonen et al., 2015). These results suggest that high levels of these inflammatory cytokines in non-decidualizing PCOS samples are likely not the cause for impaired decidualization. We postulate that SP does not amplify the inflammation already present in eSFs from women with PCOS or endometriosis but rather elicits a different type of response that promotes decidualization.
Under what in vivo conditions might SP be able to access the endometrium and promote decidualization?
For SP to induce decidualization of the endometrium in vivo, semen and SP components must reach the upper FRT, which may occur through peristalsis (Egli et al., 1961; Leyendecker et al., 1996; Kunz et al., 1997; Wildt et al., 1998; Barnhart et al., 2001). Once semen components reach the uterine lumen, they would need to access the underlying eSFs to promote decidualization. This could occur during P4-dominant hormonal conditions of the secretory phase when permeability of the endometrial epithelium increases and luminal contents can gain increased access to the stroma (Someya et al., 2013; Goddard et al., 2014; Neidleman et al., 2017). Another possibility is that SP proteins deposited in the vagina access eSFs via countercurrent exchange from vaginal venous blood into uterine arterial blood, as has been shown with steroid hormones (Cicinelli et al., 1999, 2000, 2001, 2003).
The timing when eSFs encounter SP may also be important. Morphological changes associated with decidualization are evident ~9 days post-ovulation in humans (Gellersen et al., 2014). Because SP components are largely cleared from the lower FRT (Pandya et al., 1985) and few sperm are present in the uterus (Rubenstein et al., 1951; Moyer et al., 1970) by 24 h post-coitus, it is unlikely that the SP carrying the fertilizing sperm is also the source of SP that promotes in vivo decidualization; rather, the latter SP would likely arise from a subsequent coital event. Thus, we speculate that repeated SP exposure during the secretory phase will increase the efficiency of decidualization, but the extent to which this occurs in vivo remains to be determined. Alternatively, it is also possible that SP proteins could be sequestered within the endometrial epithelial glycocalyx to act days after a coital event. However, it remains to be determined whether coital events in the proliferative phase results in premature decidualization of the eSFs.
Limitations
One limitation of this study is that our in vitro culture system did not incorporate paracrine interactions between eECs and eSFs, which eECs require to differentiate in response to ovarian hormones that vary throughout the menstrual cycle (Cunha, 1976; Cunha et al., 1983). This could explain why in our experimental conditions, SP treatment in the absence of E2P4 did not induce as many transcriptional changes in eECs as in eSFs. Nonetheless, SP in the absence of steroid hormones did induce transcriptional changes in eECs (69 genes), with MT1G (metallothionein 1G) as one of the most highly upregulated genes. MT1G is a P4-regulated gene that is highly increased in the secretory-phase endometrium of women without disease (Talbi et al., 2006) and is decreased in the early secretory-phase endometrium of women with endometriosis (Burney et al., 2007). Thus, eECs do respond to SP in a manner that is likely to support decidualization and endometrial receptivity, and future studies to better characterize the effects of SP on eECs are warranted.
Conclusion
In conclusion, our results suggest that MV-associated proteins actively signal to cells of the endometrium and can promote decidualization of eSFs from women with and without inflammatory disorders through induction of IL-11.
Future directions
Future studies should focus on determining the identity of the protein(s) in SP responsible for initiating and potentiating decidualization in eSFs. Because some proteins/peptides with previously described direct or indirect roles in decidualization—including transforming growth factor-β (Cork et al., 2001), relaxin (Huang et al., 1987), activin A (Jones et al., 2002), EGF (Irwin et al., 1994), FSH (Tang et al., 1993) and laminin (Brar et al., 1995)—are present in semen; one or more of these proteins may be responsible for SP-induced decidualization.
It will also be important to more precisely define the cellular mechanisms of SP-induced decidualization, particularly in the context of endometrial disorders and compromised decidualization. One candidate pathway downstream of IL-11/IL-11RA signaling is the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling cascade (Negahdaripour et al., 2016), since the activation of JAK results in STAT3 phosphorylation and activation, which is required for decidualization and implantation (Catalano et al., 2005; Dimitriadis et al., 2006; Wang et al., 2012; Pawar et al., 2013; Sun et al., 2013).
While SP may not be required to achieve fertilization or implantation, our results suggest it may enhance reproductive success, particularly in women with decidualization deficiencies. Indeed, women with infertility have been found to have compromised IL-11 production and eSF decidualization in vitro (Karpovich et al., 2005). Additionally, even if women with decidualization deficiencies manage pregnancy with ART, abnormalities in decidualization can lead to miscarriage, aberrant placentation and preterm birth (Cha et al., 2012; Schatz et al., 2016). Should follow-up studies demonstrate similar effects of SP on decidualization in vivo, it would support the notion that exposure of the endometrium of women to SP or SP components prior to standard IVF procedures may rescue compromised endogenous eSF decidualization disorders and enhance pregnancy establishment and success.
Supplementary Material
Acknowledgements
The authors thank Dr David Erikson and the team at the Endocrine Technologies Support Core at the Oregon National Primate Research Center (supported by NIH grant P51OD011092) for conducting Luminex multiplex and LIF immunoassays. The authors would also like to acknowledge Liza Jalalian and UCSF Fertility Clinic for providing seminal plasma, Kim Chi Vo and Michelle Shaw for the assistance with tissue acquisition, the NIH Specialized Cooperative Centers Program in Reproduction and Infertility Research Human Endometrial Tissue and DNA Bank and the Women’s Health Clinic at the Naval Medical Center in Portsmouth, VA, USA. The authors also thank Maryam Nafissi, Ethan Brookes and Jessica Roginsky from Synthego for technical guidance. The authors also thank Francoise Chanut for editorial assistance.
Authors’ roles
A.F.G.: study design, sample preparation and experiments, data analysis, figure preparation and manuscript preparation; K.S.J.: study design, sample preparation and experiments, data analysis and figure preparation; M.N.: data analysis and manuscript revision; J.N.: study design and manuscript revision; T.S.: sample collection; G.X.: sample preparation, technical troubleshooting and manuscript revision; J.C.: study design, data interpretation and manuscript revision; E.H.: experiments; A.L.: technical troubleshooting and manuscript revision; E.G.M.: sample preparation and manuscript revision; S.H.: sample selection and collection; C.D.P.: sample selection and collection; P.J.N.: study design; M.R.J.: study design; W.C.G.: study design, technical troubleshooting and manuscript revision; L.C.G.: study design, technical troubleshooting, clinical interpretations and manuscript revision; N.R.R.: corresponding author, study design, technical troubleshooting, data analysis and interpretation and manuscript preparation.
Funding
National Institutes of Health (R21AI116252, R21AI122821 and R01AI127219) to N.R.R. and (P50HD055764) to L.C.G.
Conflict of interest
None declared.
The views expressed in this article reflect the results of research conducted by the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government. Coauthor T.S. is a military service member, and her contribution to this work was prepared as part of her official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the US Government.” Title 17 U.S.C. 101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties.
References
- Aghajanova L, Altmae S, Kasvandik S, Salumets A, Stavreus-Evers A, Giudice LC. Stanniocalcin-1 expression in normal human endometrium and dysregulation in endometriosis. Fertil Steril 2016;106:681–691e681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC. Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol Reprod 2009;80:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arienti G, Carlini E, Palmerini CA. Fusion of human sperm to prostasomes at acidic pH. J Membr Biol 1997;155:89–94. [DOI] [PubMed] [Google Scholar]
- Balandya E, Wieland-Alter W, Sanders K, Lahey T. Human seminal plasma fosters CD4(+) regulatory T-cell phenotype and transforming growth factor-beta1 expression. Am J Reprod Immunol 2012;68:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart KT, Stolpen A, Pretorius ES, Malamud D. Distribution of a spermicide containing nonoxynol-9 in the vaginal canal and the upper female reproductive tract. Hum Reprod 2001;16:1151–1154. [DOI] [PubMed] [Google Scholar]
- Bellinge BS, Copeland CM, Thomas TD, Mazzucchelli RE, O’Neil G, Cohen MJ. The influence of patient insemination on the implantation rate in an in vitro fertilization and embryo transfer program. Fertil Steril 1986;46:252–256. [DOI] [PubMed] [Google Scholar]
- Brar AK, Frank GR, Richards RG, Meyer AJ, Kessler CA, Cedars MI, Klein DJ, Handwerger S. Laminin decreases PRL and IGFBP-1 expression during in vitro decidualization of human endometrial stromal cells. J Cell Physiol 1995;163:30–37. [DOI] [PubMed] [Google Scholar]
- Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ, Robertson SA. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proc Natl Acad Sci USA 2014;111:2200–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 2007;148:3814–3826. [DOI] [PubMed] [Google Scholar]
- Catalano RD, Johnson MH, Campbell EA, Charnock-Jones DS, Smith SK, Sharkey AM. Inhibition of Stat3 activation in the endometrium prevents implantation: a nonsteroidal approach to contraception. Proc Natl Acad Sci USA 2005;102:8585–8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med 2012;18:1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Erikson DW, Piltonen TT, Meyer MR, Barragan F, McIntire RH, Tamaresis JS, Vo KC, Giudice LC, Irwin JC. Coculturing human endometrial epithelial cells and stromal fibroblasts alters cell-specific gene expression and cytokine production. Fertil Steril 2013;100:1132–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Johnson BA, Erikson DW, Piltonen TT, Barragan F, Chu S, Kohgadai N, Irwin JC, Greene WC, Giudice LC et al. Seminal plasma induces global transcriptomic changes associated with cell migration, proliferation and viability in endometrial epithelial cells and stromal fibroblasts. Hum Reprod 2014;29:1255–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Roan NR. Isolation and culture of human endometrial epithelial cells and stromal fibroblasts. Bioanalysis 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicinelli E, de Ziegler D. Transvaginal progesterone: evidence for a new functional ‘portal system’ flowing from the vagina to the uterus. Hum Reprod Update 1999;5:365–372. [DOI] [PubMed] [Google Scholar]
- Cicinelli E, de Ziegler D, Bulletti C, Matteo MG, Schonauer LM, Galantino P. Direct transport of progesterone from vagina to uterus. Obstet Gynecol 2000;95:403–406. [DOI] [PubMed] [Google Scholar]
- Cicinelli E, Di Naro E, De Ziegler D, Matteo M, Morgese S, Galantino P, Brioschi PA, Schonauer A. Placement of the vaginal 17beta-estradiol tablets in the inner or outer one third of the vagina affects the preferential delivery of 17beta-estradiol toward the uterus or periurethral areas, thereby modifying efficacy and endometrial safety. Am J Obstet Gynecol 2003;189:55–58. [DOI] [PubMed] [Google Scholar]
- Cicinelli E, Einer-Jensen N, Galantino P, Pinto V, Barba B, Tartagni M. Model of counter-current transfer from vagina to urethra in postmenopausal women. Hum Reprod 2001;16:2496–2500. [DOI] [PubMed] [Google Scholar]
- Cork BA, Li TC, Warren MA, Laird SM. Interleukin-11 (IL-11) in human endometrium: expression throughout the menstrual cycle and the effects of cytokines on endometrial IL-11 production in vitro. J Reprod Immunol 2001;50:3–17. [DOI] [PubMed] [Google Scholar]
- Coulam CB, Stern JJ. Effect of seminal plasma on implantation rates. Early Pregnancy 1995;1:33–36. [PubMed] [Google Scholar]
- Crawford G, Ray A, Gudi A, Shah A, Homburg R. The role of seminal plasma for improved outcomes during in vitro fertilization treatment: review of the literature and meta-analysis. Hum Reprod Update 2015;21:275–284. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Stromal induction and specification of morphogenesis and cytodifferentiation of the epithelia of the mullerian ducts and urogenital sinus during development of the uterus and vagina in mice. J Exp Zool 1976;196:361–370. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Chung LW, Shannon JM, Taguchi O, Fujii H. Hormone-induced morphogenesis and growth: role of mesenchymal-epithelial interactions. Recent Prog Horm Res 1983;39:559–598. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, Robb L, Salamonsen LA. Interleukin 11 advances progesterone-induced decidualization of human endometrial stromal cells. Mol Hum Reprod 2002;8:636–643. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, Stoikos C, Tan YL, Salamonsen LA. Interleukin 11 signaling components signal transducer and activator of transcription 3 (STAT3) and suppressor of cytokine signaling 3 (SOCS3) regulate human endometrial stromal cell differentiation. Endocrinology 2006;147:3809–3817. [DOI] [PubMed] [Google Scholar]
- Doyle U, Sampson N, Zenzmaier C, Schwarzler P, Berger P. Seminal plasma enhances and accelerates progesterone-induced decidualisation of human endometrial stromal cells. Reprod Fertil Dev 2012;24:517–522. [DOI] [PubMed] [Google Scholar]
- Egli GE, Newton M. The transport of carbon particles in the human female reproductive tract. Fertil Steril 1961;12:151–155. [DOI] [PubMed] [Google Scholar]
- Fassbender A, Rahmioglu N, Vitonis AF, Vigano P, Giudice LC, D’Hooghe TM, Hummelshoj L, Adamson GD, Becker CM, Missmer SA et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: IV. Tissue collection, processing, and storage in endometriosis research. Fertil Steril 2014;102:1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti D, Venturelli E, Fenoglio C, Guidi I, Villa C, Bergamaschini L, Cortini F, Scalabrini D, Baron P, Vergani C et al. Intrathecal levels of IL-6, IL-11 and LIF in Alzheimer’s disease and frontotemporal lobar degeneration. J Neurol 2008;255:539–544. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev 2014;35:851–905. [DOI] [PubMed] [Google Scholar]
- Goddard LM, Murphy TJ, Org T, Enciso JM, Hashimoto-Partyka MK, Warren CM, Domigan CK, McDonald AI, He H, Sanchez LA et al. Progesterone receptor in the vascular endothelium triggers physiological uterine permeability preimplantation. Cell 2014;156:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JM, Kho D, Graham ES, Nicholson LF, O’Carroll SJ. Statins inhibit Fibrillary beta-amyloid induced inflammation in a model of the human blood brain barrier. PLoS One 2016;11:e0157483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutsche S, von Wolff M, Strowitzki T, Thaler CJ. Seminal plasma induces mRNA expression of IL-1beta, IL-6 and LIF in endometrial epithelial cells in vitro. Mol Hum Reprod 2003;9:785–791. [DOI] [PubMed] [Google Scholar]
- Hu J, Akama KT, Krafft GA, Chromy BA, Van Eldik LJ. Amyloid-beta peptide activates cultured astrocytes: morphological alterations, cytokine induction and nitric oxide release. Brain Res 1998;785:195–206. [DOI] [PubMed] [Google Scholar]
- Huang JR, Tseng L, Bischof P, Janne OA. Regulation of prolactin production by progestin, estrogen, and relaxin in human endometrial stromal cells. Endocrinology 1987;121:2011–2017. [DOI] [PubMed] [Google Scholar]
- Irwin JC, de las Fuentes L, Giudice LC. Growth factors and decidualization in vitro. Ann N Y Acad Sci 1994;734:7–18. [DOI] [PubMed] [Google Scholar]
- Irwin JC, Kirk D, King RJ, Quigley MM, Gwatkin RB. Hormonal regulation of human endometrial stromal cells in culture: an in vitro model for decidualization. Fertil Steril 1989;52:761–768. [DOI] [PubMed] [Google Scholar]
- Jasper MJ, Tremellen KP, Robertson SA. Reduced expression of IL-6 and IL-1alpha mRNAs in secretory phase endometrium of women with recurrent miscarriage. J Reprod Immunol 2007;73:74–84. [DOI] [PubMed] [Google Scholar]
- Johansson M, Bromfield JJ, Jasper MJ, Robertson SA. Semen activates the female immune response during early pregnancy in mice. Immunology 2004;112:290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL, Salamonsen LA, Findlay JK. Activin A promotes human endometrial stromal cell decidualization in vitro. J Clin Endocrinol Metab 2002;87:4001–4004. [DOI] [PubMed] [Google Scholar]
- Karpovich N, Klemmt P, Hwang JH, McVeigh JE, Heath JK, Barlow DH, Mardon HJ. The production of interleukin-11 and decidualization are compromised in endometrial stromal cells derived from patients with infertility. J Clin Endocrinol Metab 2005;90:1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril 2006;85:564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz G, Beil D, Deiniger H, Einspanier A, Mall G, Leyendecker G. The uterine peristaltic pump. Normal and impeded sperm transport within the female genital tract. Adv Exp Med Biol 1997;424:267–277. [PubMed] [Google Scholar]
- Labied S, Kajihara T, Madureira PA, Fusi L, Jones MC, Higham JM, Varshochi R, Francis JM, Zoumpoulidou G, Essafi A et al. Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol 2006;20:35–44. [DOI] [PubMed] [Google Scholar]
- Leyendecker G, Kunz G, Wildt L, Beil D, Deininger H. Uterine hyperperistalsis and dysperistalsis as dysfunctions of the mechanism of rapid sperm transport in patients with endometriosis and infertility. Hum Reprod 1996;11:1542–1551. [DOI] [PubMed] [Google Scholar]
- Machtinger R, Laurent LC, Baccarelli AA. Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum Reprod Update 2016;22:182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda L, Cassatella MA, Szendrei GI, Otvos L Jr, Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature 1995;374:647–650. [DOI] [PubMed] [Google Scholar]
- Menkhorst E, Salamonsen L, Robb L, Dimitriadis E. IL11 antagonist inhibits uterine stromal differentiation, causing pregnancy failure in mice. Biol Reprod 2009;80:920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuleman T, Snaterse G, van Beelen E, Anholts JD, Pilgram GS, van der Westerlaken LA, Eikmans M, Claas FH. The immunomodulating effect of seminal plasma on T cells. J Reprod Immunol 2015;110:109–116. [DOI] [PubMed] [Google Scholar]
- Moldenhauer LM, Diener KR, Thring DM, Brown MP, Hayball JD, Robertson SA. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol 2009;182:8080–8093. [DOI] [PubMed] [Google Scholar]
- Moyer DL, Rimdusit S, Mishell DR Jr. Sperm distribution and degradation in the human female reproductive tract. Obstet Gynecol 1970;35:831–840. [PubMed] [Google Scholar]
- Munch J, Rucker E, Standker L, Adermann K, Goffinet C, Schindler M, Wildum S, Chinnadurai R, Rajan D, Specht A et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 2007;131:1059–1071. [DOI] [PubMed] [Google Scholar]
- Negahdaripour M, Nezafat N, Ghasemi Y. A panoramic review and in silico analysis of IL-11 structure and function. Cytokine Growth Factor Rev 2016;32:41–61. [DOI] [PubMed] [Google Scholar]
- Neidleman JA, Chen JC, Kohgadai N, Muller JA, Laustsen A, Thavachelvam K, Jang KS, Sturzel CM, Jones JJ, Ochsenbauer C et al. Mucosal stromal fibroblasts markedly enhance HIV infection of CD4+ T cells. PLoS Pathog 2017;13:e1006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya IJ, Cohen J. The leukocytic reaction of the human uterine cervix to spermatozoa. Fertil Steril 1985;43:417–421. [DOI] [PubMed] [Google Scholar]
- Pawar S, Starosvetsky E, Orvis GD, Behringer RR, Bagchi IC, Bagchi MK. STAT3 regulates uterine epithelial remodeling and epithelial-stromal crosstalk during implantation. Mol Endocrinol 2013;27:1996–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piltonen TT, Chen JC, Khatun M, Kangasniemi M, Liakka A, Spitzer T, Tran N, Huddleston H, Irwin JC, Giudice LC. Endometrial stromal fibroblasts from women with polycystic ovary syndrome have impaired progesterone-mediated decidualization, aberrant cytokine profiles and promote enhanced immune cell migration in vitro. Hum Reprod 2015;30:1203–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renneberg H, Wennemuth G, Konrad L, Aumuller G. Immunohistochemistry of a prostate membrane specific protein during development and maturation of the human prostate. J Anat 1997;190:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensink AA, Gellekink H, Otte-Holler I, ten Donkelaar HJ, de Waal RM, Verbeek MM, Kremer B. Expression of the cytokine leukemia inhibitory factor and pro-apoptotic insulin-like growth factor binding protein-3 in Alzheimer’s disease. Acta Neuropathol 2002;104:525–533. [DOI] [PubMed] [Google Scholar]
- Roan NR, Liu H, Usmani SM, Neidleman J, Muller JA, Avila-Herrera A, Gawanbacht A, Zirafi O, Chu S, Dong M et al. Liquefaction of semen generates and later degrades a conserved semenogelin peptide that enhances HIV infection. J Virol 2014;88:7221–7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roan NR, Muller JA, Liu H, Chu S, Arnold F, Sturzel CM, Walther P, Dong M, Witkowska HE, Kirchhoff F et al. Peptides released by physiological cleavage of semen coagulum proteins form amyloids that enhance HIV infection. Cell Host Microbe 2011;10:541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roan NR, Sandi-Monroy N, Kohgadai N, Usmani SM, Hamil KG, Neidleman J, Montano M, Standker L, Rocker A, Cavrois M et al. Semen amyloids participate in spermatozoa selection and clearance. Elife 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med 1998;4:303–308. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlstrom AC, Care AS. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod 2009;80:1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA, Mau VJ, Tremellen KP, Seamark RF. Role of high molecular weight seminal vesicle proteins in eliciting the uterine inflammatory response to semen in mice. J Reprod Fertil 1996;107:265–277. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Mayrhofer G, Seamark RF. Uterine epithelial cells synthesize granulocyte-macrophage colony-stimulating factor and interleukin-6 in pregnant and nonpregnant mice. Biol Reprod 1992;46:1069–1079. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Sharkey DJ. Seminal fluid and fertility in women. Fertil Steril 2016;106:511–519. [DOI] [PubMed] [Google Scholar]
- Rubenstein BB, Strauss H, Lazarus ML, Hankin H. Sperm survival in women; motile sperm in fundus and tubes of surgical cases. Fertil Steril 1951;2:15–19. [PubMed] [Google Scholar]
- Salker MS, Nautiyal J, Steel JH, Webster Z, Sucurovic S, Nicou M, Singh Y, Lucas ES, Murakami K, Chan YW et al. Disordered IL-33/ST2 activation in decidualizing stromal cells prolongs uterine receptivity in women with recurrent pregnancy loss. PLoS One 2012;7:e52252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz F, Guzeloglu-Kayisli O, Arlier S, Kayisli UA, Lockwood CJ. The role of decidual cells in uterine hemostasis, menstruation, inflammation, adverse pregnancy outcomes and abnormal uterine bleeding. Hum Reprod Update 2016;22:497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey DJ, Glynn DJ, Schjenken JE, Tremellen KP, Robertson SA. Interferon-gamma inhibits seminal plasma induction of colony-stimulating factor 2 in mouse and human reproductive tract epithelial cells. Biol Reprod 2018;99:514–526. [DOI] [PubMed] [Google Scholar]
- Sharkey DJ, Macpherson AM, Tremellen KP, Mottershead DG, Gilchrist RB, Robertson SA. TGF-beta mediates proinflammatory seminal fluid signaling in human cervical epithelial cells. J Immunol 2012a;189:1024–1035. [DOI] [PubMed] [Google Scholar]
- Sharkey DJ, Schjenken JE, Mottershead DG, Robertson SA. Seminal fluid factors regulate activin A and follistatin synthesis in female cervical epithelial cells. Mol Cell Endocrinol 2015;417:178–190. [DOI] [PubMed] [Google Scholar]
- Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol 2012b;188:2445–2454. [DOI] [PubMed] [Google Scholar]
- Someya M, Kojima T, Ogawa M, Ninomiya T, Nomura K, Takasawa A, Murata M, Tanaka S, Saito T, Sawada N. Regulation of tight junctions by sex hormones in normal human endometrial epithelial cells and uterus cancer cell line Sawano. Cell Tissue Res 2013;354:481–494. [DOI] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 1992;359:76–79. [DOI] [PubMed] [Google Scholar]
- Sun X, Bartos A, Whitsett JA, Dey SK. Uterine deletion of Gp130 or Stat3 shows implantation failure with increased estrogenic responses. Mol Endocrinol 2013;27:1492–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano M, Lu Z, Goto T, Fusi L, Higham J, Francis J, Withey A, Hardt J, Cloke B, Stavropoulou AV et al. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol 2007;21:2334–2349. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 2006;147:1097–1121. [DOI] [PubMed] [Google Scholar]
- Tang B, Gurpide E. Direct effect of gonadotropins on decidualization of human endometrial stroma cells. J Steroid Biochem Mol Biol 1993;47:115–121. [DOI] [PubMed] [Google Scholar]
- Tremellen KP, Valbuena D, Landeras J, Ballesteros A, Martinez J, Mendoza S, Norman RJ, Robertson SA, Simon C. The effect of intercourse on pregnancy rates during assisted human reproduction. Hum Reprod 2000;15:2653–2658. [DOI] [PubMed] [Google Scholar]
- Usmani SM, Zirafi O, Muller JA, Sandi-Monroy NL, Yadav JK, Meier C, Weil T, Roan NR, Greene WC, Walther P et al. Direct visualization of HIV-enhancing endogenous amyloid fibrils in human semen. Nat Commun 2014;5:3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez YM, Mazur EC, Li X, Kommagani R, Jiang L, Chen R, Lanz RB, Kovanci E, Gibbons WE, DeMayo FJ. FOXO1 is required for binding of PR on IRF4, novel transcriptional regulator of endometrial stromal decidualization. Mol Endocrinol 2015;29:421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez YM, Wang X, Wetendorf M, Franco HL, Mo Q, Wang T, Lanz RB, Young SL, Lessey BA, Spencer TE et al. FOXO1 regulates uterine epithelial integrity and progesterone receptor expression critical for embryo implantation. PLoS Genet 2018;14:e1007787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtech L, Woo S, Hughes S, Levy C, Ballweber L, Sauteraud RP, Strobl J, Westerberg K, Gottardo R, Tewari M et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res 2014;42:7290–7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Taylor RN, Bagchi IC, Bagchi MK. Regulation of human endometrial stromal proliferation and differentiation by C/EBPbeta involves cyclin E-cdk 2 and STAT3. Mol Endocrinol 2012;26:2016–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gerstein M, Snyder M. RNA-seq: a revolutionary tool for transcriptomics. Nat Rev Genet 2009;10:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother 2002;46:1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildt L, Kissler S, Licht P, Becker W. Sperm transport in the human female genital tract and its modulation by oxytocin as assessed by hysterosalpingoscintigraphy, hysterotonography, electrohysterography and Doppler sonography. Hum Reprod Update 1998;4:655–666. [DOI] [PubMed] [Google Scholar]
- Winship AL, Koga K, Menkhorst E, Van Sinderen M, Rainczuk K, Nagai M, Cuman C, Yap J, Zhang JG, Simmons D et al. Interleukin-11 alters placentation and causes preeclampsia features in mice. Proc Natl Acad Sci USA 2015;112:15928–15933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Hou CC, Luo LF, Hu YJ, Yang WX. Endometrial stromal cells and decidualized stromal cells: origins, transformation and functions. Gene 2014;551:1–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


