Abstract
The 32nd International Conference on Antiviral Research (ICAR), sponsored by the International Society for Antiviral Research (ISAR), was held in Baltimore, Maryland, USA, on May 12–15, 2019. This report gives an overview of the conference on behalf of the Society. It provides a general review of the meeting and awardees, summarizing the presentations, and their main conclusions from the perspective of researchers active in many different areas of antiviral research and development. As in past years, ICAR promoted and showcased the most recent progress in antiviral research, and continued to foster collaborations and interactions in drug discovery and development. The 33rd ICAR will be held in Seattle, Washington, USA, March 30th-April 3rd, 2020.
1. Introduction
The International Society for Antiviral Research (ISAR) organizes and sponsors an annual international meeting, the International Conference on Antiviral Research (ICAR). The 32nd annual ICAR was held in Baltimore, MD, USA on May 12–15, 2019. The goal of these meetings is to foster and nurture progress in antiviral research, so as to accelerate the development of new drugs and vaccines to help prevent and treat the many viral diseases that affect humans and animals. As in previous years, the 32nd ICAR provided a most collegiate environment in which the most recent advances in antiviral research and development, as well as historical perspectives and current situation analyses of the worldwide landscape of viral disease and therapy, were presented and discussed. As in previous years (Andrei et al., 2017; Bray et al., 2018; Vere Hodge, 2013; 2014, 2015; 2017), the following pages provide an overview of the meeting, The ISAR awards were again one of the highlights of the meeting. This year, ISAR bestowed the fourth-ever Award for Outstanding Contributions to the Society to Mark Prichard, who has since passed away. Many of us have used Mark's MacSynergy™ software series to analyze drug-drug interactions. The fifth-ever ISAR Award of Excellence was granted to Dr. Robert Gallo, who discussed the challenges in the discovery of HTLVs, the impact of these discoveries in the later discovery of HIV, and the current challenges posed by HTLVs. He also introduced the Global Virus Network. The Gertrude Elion Memorial awardee, David Evans, discussed the challenges and opportunities in developing antivirals against viruses that do not currently infect humans. The Antony Holý Memorial awardee, Richard Mackman, presented a lecture on the development and activities of remdesivir and its ongoing clinical testing against Ebola virus. This year's William Prusoff Young Investigator awardee, Marnix Van Loock, disserted on dengue virus and the development of anti-dengue drugs from a pharmaceutical perspective. Our second Women in Science awardee, Grace Zhou, gave her excellent perspectives on a woman's most successful career in academy and industry in the USA and China. The Keynote address was delivered by Diane Griffin, who spoke on measles virus persistence and immunity. Her lecture was partly focused on the requirement for very high vaccine coverage to curtail measles epidemiology, and the different challenges to achieve this coverage in developed and less developed parts of the world.
ICAR 2019's four symposia focused on influenza, emerging viruses, retroviruses, and medicinal chemistry. Two sessions on “What's new in antiviral research” covered a range of topics and new challenges and progress in antiviral research and development. The short talks were organized in sessions dedicated to specific viruses or diseases, such as DNA viruses and respiratory or hepatotropic viruses, or areas such as medicinal chemistry. The poster presentations further expanded in all areas of antiviral research and development. As in all previous years, the symposia, sessions, short talks, shotgun talks, and poster presentations altogether gave an excellent background and update on the challenges posed by viruses and on the virological and chemical aspects of antiviral drug discovery and development in academia, industry, health care, and other national, international, governmental, or non-for-profit organizations. This year's ICAR thus continued to reinforce one of the major strengths of the society: bringing together people working in all areas of antiviral discovery and development, in all different settings. Also highlighting its international presence were the many talks by researchers from the USA, Europe, China, Japan, Australia, India, Singapore, South America and other parts of the world.
The program of the 32nd ICAR can be accessed at https://www.isar-icar.com/. In the following pages, we provide a general overview of the meeting and the topics presented and discussed. The 32nd ICAR was once again an excellent opportunity for people at all stages of their careers in a variety of professional settings. The usual mix of attendees from academia, industry, government, non-governmental, national and international organizations and other settings continues to facilitate the prompt discovery and development of the so much needed antiviral treatments.
2. The ISAR awards
2.1. ISAR Award for outstanding contributions to the society: Dr. Mark Neal Prichard, department of pediatrics, university of Alabama at Birmingham, Birmingham, AB USA
Mark Prichard
During the 32nd ICAR, the society conferred upon Mark Neal Prichard the ISAR Award for Outstanding Contributions to the Society. Mark is only the fourth recipient of this major society Award, after Earl Kern (2004), George Gallaso (2007) and Erik De Clercq (2012). The presentation was preceded by a Laudatio by Prof. John Drach, with Mark joining in via video link from Birmingham, Alabama. Sadly, Mark passed away on Thursday June 13th. A symposium on DNA viruses in his memory will be published by Antiviral Research.
John started by providing the definition of laudatio - to honor and praise the individual - highlighting how appropriate it was for ISAR to honor and praise Mark, not only for his work and contributions to the society but also for his human qualities. John then proceed to highlight Mark's contribution to the profession and the society, and Mark's life.
Mark completed his B.S. in Microbiology in the University of Minnesota in 1987 and an M.Sc., also in Microbiology, at the University of Michigan before starting his Ph.D. at the same institution under the mentorship of Charlie Shipman. John met Mark at that time, as John and Charlie worked together, and even shared a common lab space, and became a mentor for Mark. After completing his Ph.D. in 1992, Mark did postdoctoral training with Ed Mocarski until 1996, when he moved to the pharmaceutical and biotech industry, with stints at Hybridon, Iconix Pharmaceuticals and MedImmune Vaccines, before moving back to Academia in 2003 to take a position as Associate Professor at the University of Alabama at Birmingham, Department of Pediatrics. He quickly climbed the ranks, becoming a full professor in 2008 and the Director of the Molecular Diagnostics laboratory in 2012. John reminisced that Rich Whitley not only helped recruit Mark back into academia, but he also mentored him in the academic “publish or perish” world.
John briefly summarized the many contributions of Mark to antiviral research and development, including contributions to the invention of new drugs for herpesvirus, orthopoxviruses, polyomaviruses, papillomaviruses and influenza virus, and the analyses of resistance to antiviral drugs from specimens obtained in pediatric clinical trials. Mark has published an impressive 120 original research papers and four books or book chapters, and has presented more than 150 scientific abstracts at major meetings. He is also a co-inventor on issued patents and the developer of the MacSynergy™ software to analyze drug-drug interactions. He was the principal investigator in contracts from NIAID to help academia and industry in identifying new antivirals and their mechanisms of action. Overall, he secured an impressive ~$15M in research support. Appropriately, Mark was recognized in 2009 by our Society as the William Prusoff Young Investigator Awardee. Mark has also been a most active member of the Society, generously giving his time in the posters award committee from 2006 till 2012, and as its chair since 2007, as an elected (in 2009) member of the Board of Directors, and as the Chair or Co-Chair of the program committee between 2013 and 2017.
John highlighted that Mark was not just an outstanding scientist, but also an excellent colleague and friend, who enjoyed re-connecting with his colleagues at ICARs and NIH Annual Antiviral Contractors’ meetings, and a very loving and dedicated husband and father. He also had artistic talents, from playing the tuba in Menomonie Wisconsin to computer art, which he put to good use while in the Drach and Shipman labs re-creating Calvin and Hobbles into the science arena with “some resemblances” between Calvin and Leroy Townsend.
Then, ISAR's president Johan Neyts proceeded to present the award to Mark, thank him for “all (his) contributions to the society”, and wish him all the best. Mark then thanked his wife Lynn and his son Brian via the video link, before thanking all the attendees, too, and wishing them to enjoy their lunch. In summary, the attendees were treated to an emotive and appropriate panegyric and award ceremony for a long-term outstanding member of ISAR.
2.2. ISAR Award of Excellence: Dr. Robert Gallo, Homer & Martha Gudelsky distinguished professor of Medicine, Co- founder & director of the institute of human virology at the University of Maryland school of Medicine and co-founder and scientific director of the Global Virus Network
Robert Gallo
At this year's ICAR, ISAR introduced its fifth Award of Excellence, granted to Dr. Robert Gallo (the four previous awardees are Drs. Erik De Clercq, Gertrude Elion, William Prusoff, and Richard J. Whitley). Dr. Robert Gallo's award dissertation focused mostly on HTLVs, and then HIV, but it also introduced the Global Viral Network, which would be later further discussed by its president, Christian Bréchot, MD, PhD.
Dr. Gallo started his lecture discussing the discovery of HTLV-I in 1979, HTLV-II in 1982, and HIV in the following years. HTLVs and HIV are the two the major types of human pathogenic retroviruses. Dr. Gallo recounted that the concept of oncogenic human viruses in general and retroviruses in particular faced some stiff resistance at the time of the HTLV-1 discovery, and the potential existence of human retroviruses was not considered of high interest as they were not considered likely to be pathogenic. Dr. Gallo discussed how the discovery of HTLVs started changing the appreciation for human retroviruses, supporting the then not so widely accepted model that AIDS could be caused by a retrovirus, and provided the technologies required to detect and analyze human retroviruses, thus facilitating the discovery of HIV. Both these discoveries, of HTLVs and HIVs, became possible by the then new capacity to grow T cells using interleukin (Il)-2 and the development of sensitive methods to detect reverse transcriptase activity, differentiating it from that of the human DNA polymerases.
HTLV-1 produces adult-cell leukemia (ATL), and also HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP), inflammatory disorders and immunodeficiencies. HTLVs are far more conserved than HIVs, but nonetheless the relatively minor differences, particularly at the 3’ end, determine different HTLV- 1 subtypes. These variations are associated with differences in pathogenicities. HTLV-1 A and B are associated with ATL and paralytic CNS disease (HAM/TSP), and HTLV-1 C with immune disorders and bronchiectasis; HTLV-2 is basically non-pathogenic.
No vaccine or anti-viral therapy for HTLV-1 is available, and the infection cannot be cured. Dr. Gallo discussed that no current evidence indicates antiviral drugs would be too useful against the pathologies induced by these viruses, as the pathologies happen much too late, but much can be done to prevent infection. HTLV-1 is highly oncogenic, with approximately 3–5% of those infected, including approximately 30% of those infected at birth, eventually developing ATL. The global incidence of HTLV is around 0.1%, but the actual incidence is truly unknown yet as there are large regional variations and the epidemiology is still not completely known in many regions, including several highly populated ones such as China and India. For example, it has only recently been appreciated that HTLV-I causes high morbidity and mortality in the indigenous Australian population, with incidences as high as 45%. Dr. Gallo expressed his thoughts that HTLV-1 remains an important pathogen but research and development in the area are extremely underfunded and consequently the field is still not adequately developed to succeed at eradicating HTLV-induced disease.
In contrast to HTLVs, HIVs replicate much faster and more efficiently, and were thus good target for antiviral development. It was the use of AZT that first demonstrated that specific antivirals could control a systemic retrovirus infection with objective data showing declines in viremia. Although AZT was not too good at controlling disease, the evidence collected with its use eventually culminated in the development and use of combination therapy, which has so vastly improved the lives of the infected patients. Despite these successes, however, HIV infections cannot yet be cured. There are three main goals in HIV prevention/therapeutics. The first one is the development of an effective vaccine. Unfortunately, progress towards this goal has been limited. The second would be a complete cure - eliminating the virus. Although much progress has been made at controlling the disease, there is no major progress yet toward curing the infection. There is, however, a third goal towards controlling HIVs, prevention by antiviral drugs used as pre-exposure prophylaxis. This latter approach is already showing great potential and it is being tested for its potential for regional eradication of the virus.
Dr. Gallo then focused on introducing the Global Virus Network (GVN), of which he is a founding member. The GVN is an independent global organization bringing together expertise in human virology across the world and making this expertise broadly available. It is cross-national and not focused on any single virus or disease, but rather bringing together expertise in all areas of human virology. It consists of 45 centers of excellence, and 7 affiliated organizations in 29 countries in the 6 continents. It currently includes researchers working on 51 viruses and 26 different fields.
2.3. Gertrude Elion Memorial award: Dr. David Evans, department of medical Microbiology & immunology, Li ka shing institute of virology, university of alberta, Edmonton, AB, Canada
David Evans
The Gertrude Elion Memorial Awardee, Dr. David Evans, introduced the poxviruses, a large family of DNA viruses infecting many animals. The most famous one is variola virus, the causative agent of smallpox, which only infects humans. Smallpox, a highly contagious and sometimes fatal disease, was declared eradicated by the WHO in 1980 as a result of a massive worldwide vaccination campaign.
The first successful antivirals against DNA viruses were developed to treat herpesvirus infections. There are several challenges for treating smallpox with antivirals: it is a unique human disease for which there are no good animal models, there are no “at-risk” populations to support field-based trials, and ethics precludes human experimentation. Perhaps most critically, there was little perceived need until the raised concerns about bioterrorism developed in the early 2000's.
Tecovirimat (ST-246, TPOXX®), developed by Siga Technologies, was, in 2018, the first drug approved under FDA's ‘Animal Rule’ (Hoy, 2018). It targets the F13 protein orthologues inhibiting virus exit. Tecovirimat has a low resistance barrier with certain amino acid substitutions in the F13L gene conferring high levels of resistance (Yang et al., 2005).
The Orthopoxviruses DNA polymerases (pol) are highly conserved and thus represent another antiviral target. The structure of vaccinia virus (VACV) DNA pol was reported in 2017 (Tarbouriech et al., 2017). VACV (200 kb), the prototypic orthopoxvirus, replicates to high titers in cytoplasmic structures called factories. Virus recombination is linked inextricably to replication. Recombination requires VACV DNA pol, as shown by the recombination frequency in cells infected with a temperature sensitive DNA polymerase mutant (Willer et al., 1999) and by the in vitro catalysis of recombination by the purified VACV DNA pol (Gammon and Evans, 2009).
Next, David Evans reported on his collaboration with G. Andrei and R. Snoeck from the Rega Institute, Leuven, Belgium, concerning the genetic analysis of cidofovir-resistant (CDVR) VACV. Nucleoside phosphonate drugs, like CDV (HPMPC) and HPMPA are potent orthopoxvirus DNA pol inhibitors. CDVR virus was isolated during >40 rounds of passage. Resistance mapped to two mutations (A314T and A684V) in the VACV DNA pol (Andrei et al., 2006). Marker rescue transferred A314T or A684V alleles into a vaccinia virus Western Reserve strain. Each mutation alone conferred drug resistance; though the level of resistance conferred by the single mutants was lower than that conferred by both together. Encouragingly, CDVR attenuated the pathogenicity of VACV WR strain in an intranasal mouse model and CDV was still protective against a lethal challenge by CDVR strains.
He then highlighted a collaboration with K. Hostetler (University of California, San Diego, La Jolla) who synthesized 1-O-hexadecyloxypropyl-CDV (CMX-001/Brincidofovir) and was interested in understanding the biochemical effects of CDV diphosphate (CDVpp) on VACV DNA pol (Magee et al., 2005, 2008, 2011). Steady state primer extension kinetics with VACV DNA pol showed that CDVpp causes polymerase pausing at one nucleotide past the site of CDV incorporation (CDV+1). Stepwise analysis of the extension and excision kinetics showed that (i) CDVpp is incorporated into DNA by the E9 DNA pol, (ii) a CDV-terminated primer is still a polymerase substrate, and (iii) the CDV+1 product is extended poorly and resists exonucleases. CDVpp behaves generally like a chain terminator and its effects on VACV DNA pol resembles those described on HCMV DNA pol.
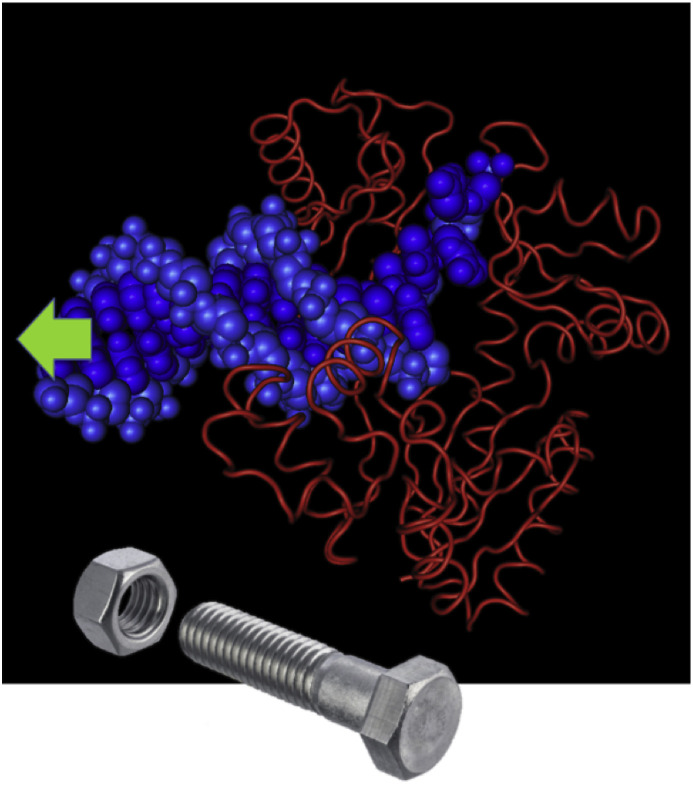
Subsequently, David Evans discussed how HPMPA, which is more bioactive in vitro, inhibits VACV replication. As HPMPApp is a less efficient chain terminator than CDVpp, more HPMPA is expected to accumulate in the replicated DNA in what would later become the template strand. VACV DNA pol faithfully incorporates a nucleotide opposite a drug residue when either CDVpp or HPMPApp were incorporated in the template rather than the primer strand. However, template-encoded HPMPA or CDV strongly inhibited further primer extension. David Evans speculated how these biochemical data might relate to the mutations responsible for CDVR. The A314T substitution maps to the exonuclease domain of E9 DNA pol and results in differential exonuclease activity that can excise CDV from the end of the primer strand. The A684V mutation maps to a highly conserved α-helix of the DNA pol and may help dNTP versus CDVpp selection. A third class of mutation (S851Y), at the base of the thumb of the DNA pol, confers resistance to the purine analogue HPMPDAP and results in a highly attenuated virus. David Evans suggested that these drugs in the template strand might inhibit transit of the DNA through a polymerase molecule and that the S851Y mutation may also alter the way in which DNA is enclosed within the thumb and palm domains. Thus, nucleoside phosphonates may act by inhibiting virus polymerases with what is essentially damaged DNA (Fig. 1 ).
Fig. 1.
Poisoning virus polymerases with damaged DNA. The presence of these drugs in the template strand might inhibit transit of the DNA through a polymerase. The mechanism is like the one when a stripped thread blocks a bolt passage through a nut.
In support to this hypothesis, NMR structures of a dodecamer DNA duplex containing a CDV molecule at position 7 show that CDV is well accommodated within the dodecamer DNA duplex (Julien et al., 2011), but the duplex is destabilized, suggesting increased dynamics around CDV. Thus, template-bearing drug adducts create a barrier to poxviruses replication. Whereas cells bypass template damage with the specialized error-prone DNA polymerases, poxviruses replicate in the cytoplasm with just their own highly faithful DNA polymerase.
David finalized his talk by reviewing whether nucleoside phosphonate drugs meet the challenge posed by disease eradication. These drugs offer promise as a second smallpox therapeutic when considering that they have a high barrier to resistance and resistant mutants have reduced fitness, mutants are still drug sensitive with only a modest increase in EC50, and the drugs have shown efficacy in several animal models. These attributes may all result from the complex ways in which these drugs inhibit multiple steps in the DNA polymerization reaction. David suggested that a DNA damage model deserves more attention in that it may be the bases for both the selectivity of these drugs and their somewhat surprising anti-cancer activity. The later could result from the DNA repair/bypass systems in normal cells being tolerant to drug damage, whereas repair defects in cancer cells may sensitize them to these agents (Andrei et al., 2015).
2.4. Antonín Holý Memorial award: Dr. Richard Mackman, Gilead Sciences, foster city, CA USA
Richard Mackman
The 2019 Antonín Holý Memorial Award was given to Dr. Richard Mackman, Vice President of medicinal chemistry at Gilead Sciences. Richard Mackman has numerous publications focused on antiviral research, as well as being named a co-inventor on >40 patents. Most recently he was elected to Fellow of the Royal Society of Chemistry for his achievements. Richard Mackman's lecture focused on the development of remdesivir, a broad-spectrum antiviral.
Remdesivir, initially known as GS-5734, is a broadly active antiviral nucleotide RNA polymerase inhibitor that has shown potent activity against Ebola and Marburg, and also against coronaviruses, paramyxoviruses, some flaviviruses, and to a lesser extent, arenaviruses. Prior to the major Ebola outbreak that occurred in West Africa claiming >11,000 lives (40% mortality), including 512 health care workers, a focused library of nucleoside analogues to target viral RNA polymerases had been assembled from >10,000 nucleoside analogues within Gilead's library. Shortly after the Ebola outbreak began, Remdesivir was identified as an effective anti-EBOV agent through a focused screening of this library followed by prodrug optimization. The discovery and profiling of remdesivir was accomplished by a unique public-private partnership with many different agencies including NIH (NIAID), USAMRIID, CDC, Vanderbilt, UAB, UNC and other organizations.
Remdesivir's structure features a novel 1′-CN connection on a C-nucleoside adenosine analogue. Limited phosphorylation of the parent nucleoside required the utilization of ProTide prodrugs for facile delivery of the monophosphate form into the cell. The monophosphate is then rapidly converted by kinases to the active form – the triphosphate polymerase inhibitor. As detailed by Richard Mackman, Gilead pursued additional structural modifications and structure activity relationship studies. Despite significant efforts, exploring all aspects of the nucleoside scaffold, remdesivir remained the most active prodrug.
Due to the extreme urgency of the global situation, Gilead set a goal of delivering 200 g of remdesivir in a couple of months to carry out the preliminary in vivo studies to support an IND. This was successfully achieved, but the synthesis required further optimization for advancing the program clinically. A highly impractical cyanation reaction, which required full body suit protection for the process chemists, required that Gilead develop a novel flow reactor system to perform the reaction. This allowed for kilograms of compound to be generated safely. Additional advances included the development of a formulation that could eliminate cold chain requirements making it more practical for use in the African continent.
Notably, remdesivir has been used successfully, in combination with standard of care, in two compassionate cases of Ebola infection - the famous Scottish nurse who was infected while treating patients, and an infant born with Ebola transmitted from the mother. Currently remdesivir is in a randomized clinical trial in the most recent Ebola outbreak in the Democratic Republic of Congo – three sites were originally open for enrollment and enrolled 166 patients as of April 26, 2019. Unfortunately, one of the sites was recently closed due to civil unrest, underscoring the difficulties in developing a drug in this setting. Despite this challenge, remdesivir remains one of the few small molecule drugs currently under clinical development for Ebola, and has the potential to treat other viral pathogens given its broad spectrum of activity.
2.5. The Women in Science award: Grace Zhou, Shenzhen international institute for Biomedical research, Shenzhen, China
Grace Zhou
The Women in Science Awardee, Grace Zhou, was selected by the Women in Science committee and other ISAR members. The awardees exemplify women scientists who have made significant contributions to the field of antiviral research. The aim of Grace Zhou's talk was to discuss her career progression and highlight a subset of her current research.
Grace Zhou began by describing her transition from scientist to entrepreneur, noting the contrasts between academic research and commercial industry, such as the considerations in manufacturing and timelines. However, she found she could apply her basic science training to industry. She emphasized that she was driven at challenging times by her belief that there is “no good excuse (age, gender, education) not to continue learning.” She found that success in running a business (or lab) relies heavily on focusing on the people with whom she works. She shared some advice with the audience: (1) make sure you select wisely the team working with you; (2) recruit them not to work for you, but to work for themselves; (3) encourage and motivate your team instead of pushing them; and (4) provide a big enough stage for young people to develop their careers. Overall, her approach to management centered on her finding that when “you free their spirit, they work very hard.” She emphasized needing to allow employees or mentees to make mistakes while taking herself the responsibility for them. She illustrated her philosophy for both research and industry by encouraging the audience to “play violin on the stage and make sure the audience dances with you.” Finally, she discussed recognizing and being grateful for unplanned situations. She narrated the story of a funder who sought her out and reached her unexpectedly to then invest millions to support her efforts.
Grace Zhou continued by speaking about an oncolytic platform, product line, and drug formulation, with an emphasis on the challenges in manufacturing oncolytic viruses. In brief, these viruses demonstrate robust oncolytic activity because of a specialized backbone that expresses IL-12. Upon a single injection into a tumor model in nude mice, the virus suppressed the tumor in a dose-dependent manner. This product would be applied to the treatment of melanoma. Grace Zhou continued by describing some of her group's recent investigations into HSV-1, in particular the function, activation, and control of innate immune networks in response to HSV infection. Her objective was to identify constitutively expressed cellular genes that limit viral replication. Her group screened a subset of cellular genes and found that HSV-1 relies on the functions of constitutively expressed and inducible cellular genes to suppress innate immunity genes inimical to viral replication. To date, they have identified three recruited genes: LGP2, HDAC4, and GADD45?? Their data suggest that the cellular genes recruited by HSV-1 act independently on the same (or at least on an overlapping set of) innate immunity genes. The targets of recruited genes identified so far include IFI16, IFIH1 (MDA5), IFIT1, and RIG-I. In cells depleted of LGP2 and GADD45?? HSV-1 demonstrated impaired ability to suppress the innate immune response, resulting in reduced viral replication.
Grace Zhou finished her talk by sharing her mentor's philosophy – “whenever you find yourself in times of great difficulty that make you cry, it's time to laugh”. She then concluded, “I have not learned to laugh, but I have learned to smile.”
2.6. William Prusoff Young Investigator award: Marnix Van Loock, Ph.D. Janssen Pharmaceutical NV (J&J), global public health research & development, Beerse, Belgium
Marnix Van Loock
The William Prusoff Young Investigator Awardee. Marnix Van Loock, discussed an Industry perspective on developing dengue antiviral small molecules.
Dengue fever is caused by infection with dengue virus, which is transmitted to humans through the bite of Aedes aegypti or Aedes albopictus mosquitoes. Dengue disease has a major social-economical impact and is listed by the WHO in the top 10 of global health treats for 2019. Most primary dengue infections are asymptomatic. However, approximately 15–25% lead to febrile illness (dengue fever), characterized by a rapid onset of high fever combined with a plethora of other symptoms (e.g. rash, retro-orbital pain, joint and muscle pain, back pain, etc.) Unfortunately, a small fraction of patients progresses to more severe disease, termed dengue hemorrhagic disease or dengue shock syndrome. There are four serotypes of dengue virus that co-circulate in tropical and subtropical regions of the world. Infection with one serotype produces long-lasting immunity to that serotype, but also generates non-neutralizing antibodies to the other three. These non-neutralizing antibodies can enhance infection in people infected with any of those different serotypes by binding to the virion and increasing entry into permissive cells through Fc-mediated uptake of antibody virus complexes, which results in higher viral load and increased risk of developing more severe dengue.
The therapeutic options for treatment and prevention of dengue fever are limited. Currently, no dengue-specific treatment is available. Development of a small molecule antiviral agent to treat dengue is challenging due to a potentially small treatment window as viremia is close to the at a peak when symptoms start to develop. Therefore, it remains an open question if treatment with a direct antiviral resulting in a faster clearance of viremia will translate in clinical benefit for the patients. A quadrivalent dengue vaccine (Dengvaxia™) was recently licensed but its use has been restricted to people who had already had a dengue fever virus infection, which has to be proven with a point of care diagnostic test prior to vaccination.
Marnix Van Loock described the approach taken by Janssen to develop of a potent, pan-serotypic, first-in-class dengue antiviral small molecule for the prevention or treatment of dengue, both for vulnerable populations, including travelers, who live in or visit dengue-endemic areas. This prophylactic approach is more likely to have a larger clinical impact as opposed to treating symptomatic individuals where replication may no longer drive disease. The target product profile described a small molecule inhibitor of all four dengue fever serotypes for the prevention or treatment of dengue. The drug would have to be safe for adults and children, well-tolerated without side effects, and delivered orally once daily. The formulation would have to provide long term stability for stockpiling. The value in such a product for the payers would be reduced health care costs, hospitalization frequency and duration.
Marnix Van Loock also described the standard methodologies used in industry to identify drug candidates, including high throughput screening, lead identification, and lead optimization. More importantly, private-public partnerships are critical to address neglected tropical diseases, illustrated by the collaboration with academia through a partnership with KU Leuven and the Wellcome Trust which were key for the program discussed. Through partnerships, win-win scenarios are developed by combining the strength of industry (e.g. pharmacokinetics, ADME, preclinical data package necessary for regulatory filings, clinical development, etc.) and academia (basic research to identify new targets, mechanism of action studies, resistance evaluation, in vivo models, etc.). Overall, their ultimate goal is to actively help build healthy communities worldwide through innovative and impactful healthcare solutions and partnerships; specifically, in this program to stop or eliminate dengue.
3. ICAR keynote address
3.1. Measles: a role for virus persistence? Dr. Diane Griffin, a university distinguished service professor and former chair of the W. Harry Feinstone department of molecular Microbiology and immunology, John Hopkins Bloomberg school of public health, Baltimore, MD USA
The Keynote speaker of the conference, Diane Griffin, presented a lecture topically focused on the pathogenesis and immunity of measles virus (MeV), which is nowadays producing several major outbreaks as a result of insufficient vaccine coverage. Dr. Griffin started analyzing how strict human viruses (those that do not infect another host) persist in the human population. Many, such as HIV, herpes viruses or hepatitis C virus, persist in the population through long persistent or latent infections. However, most RNA viruses such as MeV produce only acute infections that fully resolve, are transmissible for short periods and the infected hosts develop long-lasting immunity. And yet, MeV remains one of the top 10 causes of mortality among infectious diseases in the world.
The long immunity to MeV was discovered even before the disease was known to result from a viral infection, or viruses were even known to exist. During an outbreak in 1846 in the Faroe Islands, Peter Ludvig Panum, a Danish physician, observed that measles was contagious. He found the incubation time to be 14 days, and the attack rate to be 100% for those who had not been alive at the time of the previous outbreak in the islands some 60 years before. The lifelong immunity to the disease thus became clear. We now know that antibody levels remain high in 60–65 years old people infected as children. The extremely high attack rate of MeV is a consequence of the very high R0 (a descriptor of the number of people who get infected from each case), which for MeV is 12–18 (in comparison to mumps: 10–12, polio: 10–15, smallpox: 5–7, or flu: 3–4).
MeV is transmitted by the respiratory route and it first infects the respiratory tract and then lymphoid cells which distribute the virus through the body before the onset of the clinical symptoms. This pre-symptomatic acute infection is difficult to study in humans, but in a macaque model lymphocytes are the predominantly infected cells. Although a higher percentage of B than T lymphocytes are infected, both cell populations contribute equally to viral spread as there are more T than B lymphocytes. The infection is mostly in memory cells, which express the lymphocyte MeV receptor CD150/SLAM (CD46 is the receptor for the vaccine strain). The peripheral blood mononuclear cells (PBMC) then distribute the virus to the skin, where the typical rash occurs. Infected CD4 and CD8 cells are present in the rash lesions and immunosuppressed patients have basically no rash- but they also fail to clear the infection. MeV also infects the epithelial cells, using Nectin-4 as the receptor.
Measles is immunosuppressive to the point that deaths result from complicating infections, mainly diarrhea, otitis media, or pneumonia (9,500, 6,500, or 5,000 cases per 100,000 MeV infections, respectively). Encephalomyelitis and subacute sclerosing panencephalitis (SSPE) are far less common but very serious complications. SSPE is a fatal neurological slow disease occurring some 7–10 years after the acute infection.
Innate immune responses are not effective at controlling infection. MeV (as other paramyxoviruses) inhibits IFN induction, mostly through the action of the nonstructural proteins V and C, and Type I IFN is not produced. Inhibition of innate immune responses is not total, however, and other cytokines such as IL-1, IL-18, TNFα, and IFNγ are still produced.
MeV cannot be cultured from any site after rash has cleared, but the viral RNA is detectable in the children for at least 6 months afterward, both in HIV+ and HIV- individuals; longer term persistence has not been evaluated. The persistence of viral RNA is reproduced in macaques, in which the viral RNA linearly declines for up to 14 months after infection. There is measurable immune suppression through the time of detection of viral RNA, and the enhanced susceptibility to infections persists for much longer - some 2–3 years. The genomic variability of the persistent genomes has not been analyzed. The persistent RNA includes messenger RNA and ongoing immune responses suggest that MeV proteins are expressed. Regardless of the persistence of viral and messenger RNA, there is no evidence that these long-term infections ever result in transmission.
The antibody responses are critical for protection, in particular the avidity of the antibodies. Neutralizing antibodies protect against disease at 8-fold lower titres than they protect against infection. The neutralizing antibodies bind to the same regions of the MeV H protein as its receptor, which minimizes any antigenic drift. Moreover, MeV is a truly monotypic virus. The vaccine in use today was developed in the ‘60's from a 1953 isolate by John Elders from the blood of a child named Edmonston. All current vaccines are derived from this original isolate with slightly different passage histories. It is used in two doses, the first, either by subcutaneous or intramuscular injection at 12–15 months.
The levels of plasma MeV antibody raise fast during the rash, but the maturation of the antibody response is rather slow and the quality of the antibody is critical for protection. Moreover, the number of meV antibody secreting cells (in blood) increases after the rash is cleared. Likewise, the number of IFNγ secreting cells increases quickly after infection in blood, while those of IL17 peak only later. In the germinal centers, however, the number of meV responsive cells continue to increase late after infection.
The vaccine replicates better than the WT virus in most cell types but not in PBMC, in which the WT produces 100–1,000 infectious units/ml whereas the vaccine produces none. Not surprisingly, WT MeV infection induces much better antibody responses, which do not decrease much with time whereas those resulting from the vaccine do. MeV antibody responses after MMR vaccination are still at around 40% of the original titres 15 years after vaccination. Consequently, secondary vaccine failure is very infrequent (it is estimated at around 10–15% after 15–20 years).
The vaccine is very effective, to the point that thanks to it there were basically no domestic cases in the USA from 1996 till 2013; elimination was declared in 2001. From then till 2013, there were only some 50–100 imported cases per year, which produced only small domestic outbreaks. However, the decrease in vaccination has resulted in a re-emergence, with the first peak of some 650 cases in 2014, and a second one ongoing right now with almost 900 cases in the first four months of this year. The problem is not limited to the US: the number of cases quadrupled worldwide from 2018 Q1 to 2019 Q1. The vast majority of the current cases of MeV occur in unvaccinated people, in particular those who have not been vaccinated yet because of young age. Only less than 1% of cases occur in patients who have received the two doses. As a result of the high R0, however, a very high vaccination coverage (around 92–95% or higher) is required to interrupt transmission and prevent outbreaks. The challenges to vaccination differ. In developing countries, the biggest challenge to achieving these essential high coverages lies in the health infrastructure, the cold chain, the availability of needles and syringes, and the need for sufficient number of skilled trained health workers, which are all compounded by the requirement for two doses. In developed countries, there is a growing challenge regarding vaccine acceptance, compounded by safety worries and the considerations to the balance between the individual rights and public health. As there is no evidence that secondary vaccine failure is any major contributing factor to the ongoing recurrent outbreaks, restoring vaccine coverage is paramount to stop the current ongoing measles outbreaks.
4. Influenza symposium
4.1. 101 years of influenza: lessons from the 1918 pandemic. Pandemic. Dr. Jeffery Taubenberger, M.D., Ph.D. NIAID, NIH, Bethesda, MD USA
Jeffery Taubenberger described the 1918 influenza pandemic as the “worst natural disaster in history”. Although clinical samples were not collected at the time of the pandemic, autopsy specimens have been used to sequence and reconstruct the virus genome. He described the 1918 influenza A virus as a novel “founder virus” that initiated the current “pandemic era”, as all influenza A pandemics and seasonal epidemics since then have been caused by descendants of the 1918 virus. The most important lessons learned from the 1918 pandemic may be the need to produce better antivirals, and broadly protective ‘universal’ influenza vaccines, which will necessitate a better understanding of the correlates of immunity and pathogenicity between humans and animal models of infection.
4.2. Influenza antivirals: recent developments. Dr. Frederick Hayden, M.D., university of Virginia school of Medicine, charlottesville, VA USA
Frederick Hayden described recent advancements in the use of antivirals to treat influenza. He discussed the use of convalescent serum and intravenous immunoglobulins, in addition to evaluation of the intravenous route of administration for zanamivir, identifying a potential threshold of what is possible with neuraminidase inhibitors. He also described the three orally available influenza virus inhibitors favipiravir, pimodivir, and baloxavir marboxil, that target components of the influenza polymerase complex (PB1, PB2, and PA endonuclease, respectively). These antiviral drugs in combination drug studies with neuraminidase inhibitors show synergy in pre-clinical models, and such combinations are currently being studied in hospitalized influenza patients. In addition, he emphasized that antiviral combinations offer the best strategy to enhance potency and reduce selection for resistance when treating higher-risk influenza patients and those hospitalized with serious illness.
4.3. A hemagglutinin stalk-based universal influenza virus vaccine. Florian Krammer, Ph.D. icahn school of Medicine at Mount sinai, New York, NY, USA
Florian Krammer described an influenza hemagglutinin (HA)-stalk based universal influenza vaccine. The membrane-proximal stalk domain of the viral HA exhibits a high degree of conservation across influenza virus subtypes, and monoclonal antibodies directed against this region typically show broad neutralizing activity. He used chimeric HA that combine the H1 or H3 stalk with the globular head of avian influenza viruses to provide cross-protection between phylogenetic subtypes. By sequential vaccination of mice and ferrets with chimeric HA constructs that share the same stalk domain, but divergent head domains, they successfully boosted broadly neutralizing antibody titers against conserved epitopes in the HA stalk, which also interfered with virus transmission between animals. His data suggest that this vaccine strategy has the potential to provide broad influenza virus protection in humans.
5. Emerging virus symposium
5.1. WHO's global program on MERS: improving global preparedness and response to high threat emerging respiratory pathogens with significant public health and economic consequences. Dr. Maria Van Kerkhove, World Health Organization, Geneva, Switzerland
The opening lecture of the Emerging Viruses symposium by Maria Van Kerkhove reviewed the World Health Organization's program on Middle East respiratory syndrome (MERS). The etiologic agent, MERS Coronavirus (MERS-CoV), is an important zoonotic pathogen that since 2012 has caused repeated outbreaks from mostly animal-to-human spread, and with limited human-to-human transmission. Dromedary camels are the natural host, but in some human cases there is no reported direct or indirect link to camels. Overall, the basic reproduction rate (R0), which is a measure of transmission potential of an infectious disease, is less than one. However, Maria Van Kerkhove noted that there can be higher attack rates in health care settings with R0 reaching as high as six. She also highlighted the high case fatality rate (CFR) of approximately 35% since 2012, but indicated that this number is likely an overestimate since mild cases are likely missed by current surveillance systems.
MERS has a global risk with high public health, security and economic impacts: 27 countries have reported cases to date. Although >80% of cases have been reported from Saudi Arabia, the South Korean outbreak had an estimated economic cost of eight billion dollars. Maria Van Kerkhove pointed out that the global risk is associated with travel from areas where MERS-CoV is circulating in dromedary camels and subsequent human-to-human transmission. Risk can be reduced with better surveillance in camel and human populations in areas where the virus is known to be circulating, with more aggressive contact tracing to identify more people with sub-clinical infection, fit enough to travel and serve as potential sources for exposure. Since 2012, the major peaks in case numbers are the result of nosocomial outbreaks, with CFRs reaching 60–70% in specific health care settings.
The WHO MERS global work plan is based on improving preparedness, prevention and response to high threat respiratory pathogens like MERS. MERS is on the WHO's list of Blueprint Priority Pathogens due to its epidemic potential and the current lack of available countermeasures. There is an urgent need for research to better understand transmission of MERS-CoV in dromedaries and at the animal-human interface. Maria Van Kerkhove finished by discussing the potential therapeutics for MERS. One clinical trial is ongoing, the MIRACLE clinical trial in Saudi Arabia, which is exploring the combination of lopinavir/ritonavir and interferon-β1b as the first controlled trial of a potential therapy for MERS. At the time of the meeting, 62 individuals had been enrolled in the MIRACLE trial. In addition, efforts to develop vaccines for camels and humans are being funded by several institutions, including the Coalition for Epidemic Preparedness Innovations (CEPI). Maria Van Kerkhove also mentioned that a MERS app developed by WHO is currently available on Apple and Google Play. The application provides updated MERS-specific guidance materials and investigation tools based on the latest scientific knowledge and experience from Member States dealing with MERS. She concluded with remarks on the WHO's valuable impact in reducing the number of human cases and deaths since 2016.
5.2. Development of medical countermeasures against Nipah virus: a field perspective. Dr. Emily Gurley, Johns Hopkins Bloomberg school of public health, Baltimore, MD USA
For the second lecture of the Emerging Viruses symposium, Emily Gurley provided her perspective and insights into Nipah virus (NiV), considered by the WHO as one of the most dangerous agents. Nipah CFRs are in the range of 40% in Malaysia to 75% in Bangladesh. NiV is a zoonotic pathogen maintained in Pteropus bats, which have a wide geographic distribution reaching eastern Australia where the related Hendra virus causes equine and human cases of severe respiratory and neurologic disease. Fortunately, as with the MERS-CoV, there is relatively inefficient NiV person-to-person transmission; however, 90% of those infected progress to acute encephalitis with respiratory complications, and 30% of those who survive experience neurological sequelae. A contact survey that was conducted showed that asymptomatic infections have likely been rare in Bangladesh (Nikolay et al., 2019).
Emily Gurley also discussed surveillance efforts, transmission data, and poor infection control measures in often overcrowded hospital settings in Bangladesh. Models combining healthcare utilization surveys and active NiV case finding efforts at three Bangladesh hospitals suggest that only half of the actual cases are being detected (Hegde et al., 2019). In terms of transmission, males experiencing breathing difficulty were found to be the most efficient at person-to-person transmission (Nikolay et al., 2019). As reviewed by Emily Gurley, caregivers are at greatest risk of infection. In the understaffed medical centers, and based on Bangladeshi culture, caregivers are largely family members. Spouses are especially at risk and their exposure to body fluids containing infectious virus facilitates transmission.
As with most emerging viral diseases, there are no available treatments, although promising new preclinical data have been recently published suggesting that remdesivir may be useful in treating NiV infection and disease (Caskey et al., 2019). Emily Gurley highlighted that the individuals exposed to infectious virus contaminating date palm sap have a shorter incubation period and more rapid disease progression compared to patients infected through person-to-person transmission, and that their diagnosis generally occurs only on the day of death, under the best possible circumstances (case fatality ratio - CFR of approximately 90% by this exposure route). Therapeutic intervention may be feasible in cases acquired by person-to-person transmission, with longer incubation times, slower evolution to severe disease, and earlier diagnosis (by a few days), which all improve the prospects for survival (approximately 50% CFR with person-to-person transmission), and opportunities for antiviral treatment. In addition to effective antivirals, rapid diagnostics were the highest priority on Emily Gurley's wish list as they are much needed to be able to initiate treatment (when a good treatment is one day available) and infection control interventions earlier. She concluded that, ultimately, mitigating the pandemic risk associated with NiV will require a combined approach that includes prophylactic measures, such personal protective equipment for the care of patients, implementation of sound infection control measures, improved surveillance and education, vaccines for at-risk individuals (such as healthcare workers and those that consume date palm sap), and cost-effective, shelf-stable and easy to administer antivirals.
5.3. Can we predict arbovirus epidemics? Dr. Scott Weaver, Department of Microbiology and immunology, university of Texas medical branch, Galveston, TX, USA
For the third lecture of the Emerging Viruses session, Scott Weaver, a recognized leader in the field of arbovirology, tackled the subject of predicting arboviral epidemics. He began by reviewing that outbreaks of arboviral disease result from of enzootic and epizootic spillover events leading to human infection, although for some, such as Zika and dengue viruses, a human amplification and transmission cycle is involved. He highlighted the importance of the vector and its vectorial capacity, with Aedes aegypti being superior in this regard. The evolution of A. aegypti in close association with humans has led to its major role in the transmission of urban arboviruses, including yellow fever and chikungunya viruses. He discussed how proactive approaches employing deep sequencing of nonhuman primate (NHP) and arthropod vector samples can identify new emerging and re-emerging arboviruses with potential to cause human disease and emphasized the need to develop cell culture, rodent and NHP models to learn about their human impact potential. In parallel, vector competence studies in critical Aedes species such as A. aegypti and A. albopticus are needed to inform potential risks.
Scott Weaver went over a number of important arboviruses; he discussed the public health concerns posed by Mayaro virus (MAYV), which is not well recognized despite the human cases regularly detected in dengue-endemic regions of South and Central America. MAYV has great urban emergence potential, but the viremia may not be high enough for transmission to urban mosquitoes. The MAYV enzootic region overlaps considerably with that of Venezuelan equine encephalitis virus (VEEV) which has outbreak potential based on mutations leading to major epizootics with spillover to humans, as occurred in 1995 in Columbia and Venezuela, as well as high levels of human viremia. He also noted the potential risk of importing Rift Valley fever virus, which like VEEV and Japanese encephalitis virus is amplified in domestic animals. Availability of competent mosquitoes for RVFV in the United States is well documented but evidence of vectorial capacity is lacking. He also referred to recent experimental infection and modeling studies (Althouse et al., 2016; Vanchiere et al., 2018) that support concerns that a Zika virus (ZIKV) sylvatic cycle can be established in areas where susceptible NHP hosts are present. ZIKV spillbacks into enzootic cycles would make elimination near to impossible.
Finally, Scott Weaver discussed the stochastic nature of arboviral emergence and suggested that founder effects such as reduced fitness and epistasis constrains the evolution process. The New World chikungunya virus lineages are excellent examples and predicted that epistatic interactions will likely prevent their adaptation to A. albopictus, as occurred with the African lineage (Tsetsarkin et al., 2007). Founder effects also appear to be at play with ZIKV. Scott Weaver also indicated that there is uncertainty regarding how global warming will affect arboviral diseases. In addition to altering the current range of vector species, increasing temperatures are sometimes harmful to the virus and mosquitoes. Moreover, hotter weather may limit outdoor activities thereby reducing human exposure risk.
6. Retrovirus symposium
6.1. Effects of broadly neutralizing antibody combinations in HIV-1 infection. Dr. Marina Caskey, Rockefeller university, New York, NY USA
Marina Caskey reviewed the preclinical and clinical evidence supporting the potency, efficacy and use of passive immunotherapy with broadly neutralizing antibodies (bNAbs) to treat HIV + individuals.
bNAbs are unique in that they often target conserved viral epitopes in otherwise highly variable antigenic regions such as the HIV-1 envelope. Combination of antiretroviral therapy (ART) is highly successful in suppressing viral replication, preventing disease and virus transmission. However, it cannot eradicate latent infection or the viral reservoir. Immunotherapy with bNAbs offers the advantage of antibodies being safe, having a relatively long half-life, allowing for longer dosing schemes.
A number of bNAbs have been identified to date, owed to the vulnerability of the HIV-1 envelope glycoproteins (gp120/gp41) (Mouquet, 2014). Second-generation bNAbs target various epitopes on the HIV-1 envelope trimer, including antibodies against the CD4 binding site (CD4bs) (e.g. 3BNC117, VRC01, VRC07–523, N6), the gp120 V1/V2 loop (PDGM1400, CAP256), the V3-stem (e.g. 10–1074, PGT121) or the gp41 membrane proximal region (MPER; e.g. 10E8V). Some of these antibodies are currently being investigated in clinical studies (Caskey et al., 2019). For example, 3BNC117 and 10–1074 show exceptional breadth and potency in vitro, and protect against or suppress infection in animal models.
As single agents, bNAbs such as 3BNC117, 10–1074, or VRC01 are safe and effective in reducing HIV-1 viremia. Combination of 3BNC117 and 10–1074 was tested in the SHIVAD8-infected macaques model, in which it led to prolonged control of infection with no evidence of escape from either antibody (Caskey et al., 2019). In humans, combination of two or more antibodies conferred 89–98% protection (double combinations) (Bar-On et al., 2018; Mendoza et al., 2018). Greater than 98% protection may be achieved by triple combinations.
Importantly, bNAbs may boost effective T-cell mediated cytotoxic immune responses leading to the elimination of infected cells and reduction or clearance of the viral reservoir, an effect that is not achieved with antiretroviral therapy. Current efforts focus on identifying sterilizing (to completely eliminate the virus) or functional cures (suppress viral load below the limit of detection without the need of antiretroviral therapy) to HIV-1 infection. bNAbs may be used in combination with virus latency reversal agents and vaccines and that effectively induce cytotoxic T lymphocyte (CTL)- mediated killing of infected cells.
In conclusion, in addition to preventing new infections anti-HIV-1 bNAbs may clear the virus, directly kill infected cells and produce immune complexes that can enhance host immunity to the virus aiming to achieve long-term viral remission.
6.2. Long acting antiretrovirals. Dr. Howard Gendelman, university of Nebraska medical center, Omaha, NE USA
Howard Gendelman focused on the potential use of long-acting antiretrovirals as a step towards improving treatment outcomes, highlighting some recent advances (Dash et al., 2019).
Despite the great success of current antiretroviral regimens in reducing virus transmission and transforming HIV infection in a manageable chronic infection, toxicities and adherence remain causes of concern. Long-acting formulations of antiretrovirals may improve regimen adherence and reduce viral drug resistance.
Howard Gendelman introduced the concept of long-acting slow effective release antiretroviral therapy (LASER-ART) (Dash et al., 2019), that is, hydrophobic lipophilic nanocrystals that serve as agents capable of slowly releasing antiretrovirals prodrugs. The antiretrovirals are modified to improve drug potency, enhance cell membrane permeability, and facilitate encapsulation into nanocrystals that may be rapidly taken up by cells and distributed into the target tissues (Gendelman et al., 2019). One of the examples presented was the nano-formulation of cabotegravir, a HIV integrase inhibitor, that has been packaged into stable particles with high drug loading capacity and capable of targeting the monocyte–macrophages (Zhou et al., 2018) susceptible to HIV infection. A second example, also with an integrase inhibitor -dolutegravir, is a hydrophobic and lipophilic modified prodrug encapsulated into a poloxamer nano-formulation (Sillman et al., 2018) that confer long biological half-life cell and tissue drug penetration, and antiretroviral potency.
Current and future efforts in new drug formulations may lead to a change in paradigm in antiretroviral treatment, improving drug efficacy and patient outcomes. Theranostic nanoparticles, that is, multifunctional nanosystems combined into a single nanoparticle, may provide unprecedented advantages over current treatment strategies.
6.3. The advancement of HIV NRTTIs for extended-duration dosing. Dr. Izzat Raheem, Merck, west point, PA USA
Izzat Raheem discussed Merck's current efforts to tackle what remains one of the major hurdles in successful daily oral antiretroviral regimens: adherence.
Long-acting drug formulations requiring less-frequent dosing offer an opportunity to improve adherence, providing patients with a valuable alternative in treatment forgiveness and convenience. Antiretroviral drugs may be formulated for extended duration dosing (ExDD) options, leading to prolonged (>6 months) dosing intervals (Barrett et al., 2018).
MK-8591 (4′-ethynyl-2-fluoro-2′-deoxyadenosine, EFdA) is a novel nucleoside analogue that displays a differentiated mechanism of action as a nucleoside reverse transcriptase translocation inhibitor (NRTTI) compared to approved nucleoside reverse transcriptase inhibitors (NRTI) (Alexandre et al., 2018). Reverse transcriptase can use EFdA-5′-triphosphate (EFdA-TP, MK-8591-TP) as a substrate more efficiently than the natural substrate, dATP (Acosta-Hoyos and Scott, 2010). MK-8591-TP inhibits the reverse transcriptase by first being incorporated at the 3′-primer terminus, and after its incorporation it prevents further addition of nucleotides by blocking the translocation of the primer strand on the viral polymerase (Michailidis et al., 2014). MK-8591-TP exhibits prolonged intracellular persistence and excellent potency. MK-8591 has been evaluated preclinically for both HIV treatment and pre-exposure prophylaxis (PrEP), demonstrating robust protection in a rhesus macaque (RM)-simian/human immunodeficiency virus (SHIV) model (Markowitz et al., 2019). MK-8591 has been shown to be generally well-tolerated when administered as a one-time dose (0.5–30 mg) to HIV-1-infected adult subjects. Further, it has a half-life in peripheral blood mononuclear cells (PBMCs) from HIV-1-infected subjects of 78.5–128 h, which renders is compatible with a variety of potential dosing regimens. Single-dose treatment with MK-8591 at doses as low as 0.5 mg leads to robust VL decline in treatment-naïve HIV-1-infected subjects (Barrett et al., 2018; Matthews et al., 2007). As a result of its exquisite potency, long-acting PK profile, and robust efficacy, MK-8591 is a candidate for ExDD formulations. Drugs such as MK-8591 may be dispersed within polymers that exhibit controlled degradation (bioerodible and nonerodible) to generate monolithic matrix implants of dimensions suitable for subcutaneous administration. Implants are designed to achieve a broad range of drug release characteristics and durations, achieved through optimization of drug loading and polymer composition of the implants (Barrett et al., 2018). The presentation highlighted back-up medicinal chemistry efforts to MK-8591, novel nucleoside chemistry development, as well as optimization of implant formulations.
7. Medicinal chemistry symposium
In this symposium, three presentations were given by outstanding scientist in the field. The goal of this mini-symposium was to bring Medicinal Chemistry closer to a broad audience describing the methods and the approaches chemists use to develop new potential antiviral agents. The first lecture was given by Katherine Seley-Radtke (Chemistry), the second lecture was presented by Andrea Brancale (Molecular Modeling) and the third lecture was given by Anthony Keefe (compound library development).
7.1. Rational (and sometimes irrational!!!) strategies in nucleoside drug design. Katherine Seley-Radtke, University of Maryland Baltimore County, Baltimore, MD, USA
The content of Katherine Seley-Radtke's lecture was based on two very recent review articles published by her and one of her Ph.D. students, Mary Yates (Seley-Radtke and Yates, 2018; Yates and Seley-Radtke, 2019). These articles reviewed the development of nucleoside analogue antiviral drugs, and were written for a target audience of virologists and other non-chemists, as well as chemists who may not be the most familiar with the history of the field. As a result of their important role in the field of medicinal chemistry for several decades, nucleosides remain a key focus for antiviral research efforts. The naturally occurring nucleosides represent a unique starting point for drug design due to their involvement in numerous critical biological processes as well as their serving as essential building blocks for both DNA and RNA synthesis. Because nucleoside/tide analogues mimic the structure of the natural nucleosides such that they are recognized by cellular or viral enzymes, modifications to their structure typically lead to disruption or termination of replication or other biological processes. Rather than providing a simple chronological account, she walked us through the thought processes, the advances in synthetic chemistry and the lessons learned from antiviral testing that led to several molecules moving forward clinically and eventually approved for human therapy, while others were discarded. There are more than 30 nucleoside/tide analogues on the market approved for use in treating viruses, cancers, and other conditions, with many more in clinical and preclinical trials. In her lecture Katherine Seley-Radtke focused first on early, relatively simplistic changes made to the nucleoside scaffold, beginning with modifications of the nucleoside sugars of Ara-C and other arabinose-derived nucleoside analogues in the 1960s. She then extended the review to more recent developments, focusing particularly on more complex modifications, particularly those involving multiple changes to the nucleoside scaffold as, e.g., in sofosbuvir or remdesivir, a new broad-spectrum antiviral developed from Gilead Sciences. She explained why certain drugs were successfully developed, while the majority of candidate compounds encountered barriers from low-yielding synthetic routes, toxicity or other problems that led to their abandonment. Concurrently, she also discussed some of the hurdles that these types of compounds must overcome, e.g., in activation by lack of phosphorylation that can now be overcome by using monophosphate or triphosphate prodrug forms of the parent nucleoside analogues. Her lecture served to provide an informative and useful overview of the importance of nucleoside analogues in antiviral research, as well as the issues and solutions surrounding their development.
7.2. In search of novel antivirals using structure-based drug design approaches. Andrea Brancale, Welsh school of pharmacy, cardiff UK
Andrea Brancale focused his presentation on the use of computer-aided techniques, in particular structure-based drug design methods, in the early stages of antiviral drug discovery. He supported his discussion by providing few examples from his published work. He started with a summary of the work on the identification of a novel CHIKV nsP2 protease inhibitor, describing that no structure was available for the viral protein when they started the project, and hence a homology model was built to be used in a virtual screening protocol of a library of commercially available compounds. The best ranked compounds resulting from the in silico results were then purchased and tested in an antiviral assay. The biological evaluation resulted in the identification of a promising compound with low micromolar antiviral activity. Interestingly, the hit optimization that followed was carried out using a more traditional systematic structure activity exploration, without support from computer-aided methods. Andrea at this point highlighted that at that time a direct assay for nsP2 activity was not available. Hence, direct evidence that these compounds were active on the protease was missing. However, shortly after the publication of these results, another group tested a selection of the molecules reported by Andrea's group and they reported an inhibitory effect on the CHIKV nsP2 for this chemical scaffold. He then moved to a second example taken from his current work. The modeling approach was similar to the previous one, but in this case, there was more emphasis on the different ligand-based filters used in the virtual screening. The biological target in this case was the norovirus polymerase. The structure of the enzyme has been resolved, also in complex with an inhibitor. The group used this information to filter the compound library using a shape-based approach to select the compounds with a similar volume of the crystallized ligand. The compounds resulting from this procedure were then docked into the polymerase and the most promising ones were tested in a biochemical assay against the purified enzyme. Two interesting hits were identified with a low micromolar inhibitory activity; however the compounds did not show a good antiviral activity in the cell-based assay.
Finally, Andrea talked about the development of an interactive, haptic-driven, molecular docking simulator. In particular he focused the discussion on how he is trying to develop a system that takes into account the induced-fit effect that a small molecule has on the target upon binding. Although this remains a complex problem to solve, Andrea showed how the current tool is able to generate some accurate results, at least for relatively simple systems.
7.3. Drug discovery using DNA-encoded chemical libraries. Anthony D. Keefe, VP discovery technology, X-Chem pharmaceutical, waltham, MA USA
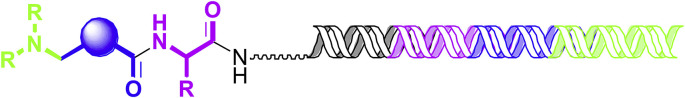
Anthony Keefe from X-Chem, a company which over the last eight years has licensed over sixty programs to a range of partners from large pharmaceutical companies to small biotechs, reported on a new strategy in drug discovery using DNA-encoded chemical libraries. Traditional drug discovery project initiation using HTS is limited to the number of compounds one can screen in a one-compound-one-well paradigm, typically no more than the single-digit millions. DNA-encoded chemical libraries allow the screening of combinatorially-generated libraries of compounds that exceed the numerical size of HTS collections by up to five orders of magnitude. Each compound in a DNA-encoded chemical library is covalently attached to a unique DNA barcode that, when sequenced, can be used to understand the chemical history of the encoded small-molecule to which it is attached and thereby to elucidate its identity. DNA-encoded chemical libraries are generated in solution by split-and-pool chemical synthesis in which individual isolated compartments are used to contain building block installation reactions along with corresponding ligations of short DNA tags, the sequences of which encode the building block identities (Fig. 2 ). These libraries are utilized in affinity-mediated screening in which rare individual library members that bind to protein targets are separated from non-binders by protein capture and stringent washing to remove non-binders. These protein-binding small-molecules are then identified by PCR amplification and sequencing. Re-synthesis and biochemical or biophysical assays are then used to determine if the identified small-molecules bind to the target in a manner that appropriately moderates the target activity.
Fig. 2.
Cartoon representation of a DNA-encoded chemical library compound. A schematic representation of an individual member of a DNA-encoded. The different colors indicate the relationship between building block identity and encoding sequence tag.
The approach offers the potential for discovery of entirely new chemical matter to inhibit or otherwise modulate targets of interest from either a 120-billion compound DNA-encoded chemical library collection, or covalent irreversible inhibitors from a 100-billion compound DNA-encoded electrophile-capped covalent library (www.x-chemrx.com, akeefe@x-chemrx.com ).
8. What's new in antiviral research
This year's meeting included two sessions focused not on a particular virus, disease, discipline or approach, but rather on providing general views of new directions and challenges in the general field of antiviral research.
8.1. Antiviral treatment for patients with yellow fever – a new Frontier. Dr. Michael Jacobs, M.D., Ph.D. Royal free London NHS foundation trust, London, UK
Michael Jacobs described three case studies of patients with severe yellow fever virus (YFV) disease and the first clinical use of the experimental antiviral nucleoside analogue, galidesivir (BCX4430, BioCryst Pharmaceuticals). The first case study was a 33-year-old German traveler returning from Brazil who presented with fever, shivering, prostration, conjunctival suffusion, jaundice and hepatomegaly. The patient had not received the yellow fever vaccine. The patient was positive for yellow fever virus by PCR detection and rapidly progressed to acute liver failure with acute renal failure and coma. Dr. Jacobs described the epidemiology and natural history of yellow fever virus infection. Yellow fever is classically described as having two phases, characterized by an initial viremia associated with an acute febrile illness that lasts for up to 6 days followed by a remission of 2–24 h. Following this brief remission, there is a period of intoxication that lasts 3–8 days with symptoms that include gastroinestional disturbances, liver involvement (jaundice) and increasingly severe clinical presentation leading to death in 30–60% of patients. Although historical descriptions report that infectious virus cannot be isolated at this stage, PCR detects viral RNA in abundance. Approximately 1 in 7 infections progress to the intoxication phase of disease. The pros and cons of liver transplantation or use of antiviral interventions was discussed.
The second case study described a 47-year-old man with a history of thymectomy for thymoma 4 years prior. He experienced an abrupt onset of fever 5 days after yellow fever virus vaccination. He presented 4 days post-illness onset with fever, tachycardia and hypotension and jaundice. He was yellow fever virus positive by PCR detection. He subsequently developed hemophagocytic lymphohistiocytosis and progressive multi-organ failure. Yellow fever virus vaccination has been associated with vaccine-induced viscerotropic disease. By 2004, there had been 23 cases described in the literature, 14 of which resulted in fatal outcomes. A total of 4 of these patients had a history of thymoma, which is a very rare disease. The patient received optimal supportive intensive care and treatments directed at treating hemophagocytic lymphohistiocytosis. The patient also received an experimental antiviral compound, galidesivir, a nucleoside analogue with broad spectrum antiviral activity, which has shown efficacy in a hamster model of yellow fever virus infection. Treatment with galidesivir was associated with a mild, progressive decrease in yellow fever virus RNA in the patient's plasma. Unfortunately, the patient succumbed to multi-organ failure.
The third case study was a 74-year-old man who had also underwent thymectomy for thymoma in 2016. He received the yellow fever vaccine in error as he was planning a trip to Central America. While the patient was clinically well at 48 h post-vaccination, the treatment team decided to administer galidesivir as a post-exposure prophylactic therapy to reduce the risk of vaccine related complications. While there were spikes of viremia and yellow fever virus RNA in the urine measured by RT-qPCR after vaccination and during galdiesivir treatment, the patient resolved the infection without significant clinical consequences and developed antibodies to yellow fever virus.
8.2. Novel utilization of smallpox medical countermeasures – challenges to vaccination against endemic orthopoxvirus disease (monkeypox). Dr. Victoria Olson, Ph.D. chief, poxvirus and rabies branch, Centers for Disease Control and Prevention. Atlanta, GA, USA
Victoria Olson presented on the challenges to vaccination against endemic Orthopoxvirus disease (Monkeypox) and a potential approach to overcome them.
Monkeypox is an orthopoxvirus that causes zoonotic infections in humans leading to a disease that clinically resembles smallpox. The virus is endemic in western and central Africa causing small outbreaks localized to a few individuals. There are two distinct clades of virus, with isolates from central Africa associated with a mortality rate of 11% and an attack rate of 5 per 10,000 in the general population or 17 per 10,000 in healthcare workers. Patients are treated with supportive care and treatment of symptoms since there is no licensed vaccine or therapeutic agent against monkeypox.
The vaccine that was originally developed for prevention of smallpox, and used to eradicate it through the intensified smallpox vaccination campaign led by the WHO, has also shown activity against monkeypox infection. The vaccine is composed of live vaccinia virus, a closely related orthopoxvirus that replicates locally at the site of inoculation and generates robust cellular and humoral immune responses. The vaccine is contraindicated for people with eczema and those with immune-compromising conditions due to the high risk of severe side effects, some life-threatening. Although effective, this vaccine is not recommended for prevention of monkeypox due to concerns over safety.
Imvamune is a third-generation smallpox vaccine derived from an attenuated modified vaccinia Ankara (MVA) strain. The vaccine has been tested in over 7,000 human subjects, including 400 HIV + individuals and 380 individuals with atopic dermatitis and is being stockpiled in the Strategic National Stockpile. No severe adverse events were observed during clinical trials. Since smallpox is eradicated, however, the efficacy of this vaccine can only be evaluated in animals with other orthopoxvirus infections.
The goal of the CDC poxvirus and rabies branch is to protect the public from (re)emerging viral threats such as monkeypox. To this end, the CDC has initiated a surveillance program in Tshuapa province, Democratic Republic of Congo (DRC), in collaboration with Kinshasa School of Public Health, INRB, and the DRC Ministry of Health. The surveillance program has identified approximately 300 confirmed cases per year from the Tshuapa province. This program provides a platform for understanding the effectiveness of countermeasures for treatment of orthopoxvirus infections in a real-world setting. A study of the third generation smallpox vaccine, Imvamune, enrolled a cohort of 1,000 adult healthcare workers at risk for monkeypox in the DRC. The objectives of the study are to evaluate safety, immunogenicity and effectiveness of Imvamune to prevent human monkeypox. The ethical, regulatory and logistical challenges of delivering a vaccine product requiring a cold chain to remote areas of the DRC, and how they were overcome, were discussed.
8.3. Antivirals against chikungunya virus from a medchem perspective: challenges and lessons learned. Dr. Maria-Jesus perez-perez, Ph.D. Instituo de Quimica medica (IQM, CSIC), Madrid, Spain
Chikungunya virus causes a spectrum of illness, ranging from asymptomatic infection to a severe febrile illness with marked (incapacitating) arthralgia. Several strategies were discussed for identifying drug candidates to treat chikungunya infections. The use of approved or investigational drugs (drug repurposing) reduces time and costs and some candidates can be developed with abbreviated clinical studies. Broad-spectrum antivirals have been tested against chikungunya replication, including ribavirin, 4-hydroxycytidine, favipiravir, and sofosbuvir, which have all shown some level of efficacy in vitro. Host targeting agents are another pathway for therapeutic development that can reduce the selection for resistance and may result in broad spectrum activity. However, host pathways can also result in use limiting toxicities. Phenotypic screens can be used to identify chemical matter that inhibit virus replication by high throughput screening. While this methodology can identify novel compounds, hit optimization and target identification are not straightforward. Finally, target-based assays can be developed to identify specific inhibitors of viral enzymes necessary for virus replication. Selected examples were presented to illustrate these strategies.
The compound MADTP314, a triazolopyrimidine, was identified by phenotypic screening. The compound was active against chikungunya in cell-based replication assays with and EC50 value of 19 μM and a CC50 value of 743 μM. Structure activity relationships guided medicinal chemistry efforts to improve its potency. These results identified several areas on the molecule that can be chemically modified to potentially improve activity. This approach led to the identification of several novel MADTP compounds with EC50 values in cell-based replication assays of approximately 1–3 μM. Resistance mapping identified a mutation (P34S) in the nsP1 gene that correlated with reduced susceptibility to the MADTP compounds. The compounds were shown to inhibit the guanylylation activity of the nsP1 protein. Virus variants containing the P34S mutation were resistant to the inhibitory effects of the MADTP compounds. The resulting optimization campaign identified a compound (MADTP411) with EC50 values ranging from 0.3 to 1.2 μM and CC50 values > 70 μM.
8.4. Small molecules for big problems in large animals. Nesya Goris, Ph.D.ViroVet, Leuven, Belgium
In this lecture, Nesya Goris of ViroVet in Leuven, Belgium reviewed the concept of using small-molecule drugs to halt the spread of viral diseases in livestock. She began by noting that some 65 billion animals are consumed for food each year, and by 2050 the demand for animal protein is expected to increase by 75%. Despite the availability of numerous vaccines, however, 20% of livestock production is still being lost to disease and death. Given their success in humans, small-molecule antivirals are gaining attention as alternatives for the prevention and treatment of livestock diseases. Her talk highlighted the potential of antiviral therapy to contain outbreaks of exotic infections, using classical swine fever and the compound BPIP as an example. In experimental studies in piglets, BPIP reduced the viremic period by 74% and the viral load by 1,000-fold, and was highly effective in preventing transmission of infection to untreated sentinels (Vrancken et al., 2009a, 2009b). Modeling studies have shown this to be an economically viable control policy (Backer et al., 2013). Small-molecule antivirals may also have a role to play in endemic disease control, to cost-effectively reduce production losses and improve animal health and welfare.
8.5. Polio eradication: need for antivirals and progress to date. Marc Collett, Ph.D. ViroDefense, inc., Rockville, MD, USA
Marc Collett of ViroDefense in Chevy Chase, MD, USA, reviewed the expected need for antiviral therapies in the “polio endgame.” Three decades of infant immunization campaigns have brought the world close to the goal of eradication, as only 136 cases of paralytic disease were recognized, and in only three countries, in 2018. However, the use of the live attenuated oral vaccine (OPV) has resulted in unanticipated consequences: in under-immunized populations, the vaccine virus can spread, occasionally reverting to virulence. Moreover, in persons with immunodeficiency disorders, the virus may establish a persistent infection and be shed for years. The planned cessation of OPV use will address the former, but eradication will require treatment of chronically infected persons. it is currently accepted that two antivirals with different mechanisms of action will be needed. Marc described the current status of two lead compounds: pocapavir, which acts by stabilizing the viral capsid, preventing disassembly, and V-7404, an inhibitor of the enterovirus 3C protease. Both are highly active against all three strains of vaccine virus, and show synergistic activity, with minimal toxicity. To date, only pocapavir has been tested for efficacy in human volunteers (Collett et al., 2017). The results suggest that combination therapy will be required to block the evolution of resistant virus.
8.6. Update and challenges in research and development of dengue and Zika antivirals. Subhash Vasudevan, Ph.D. Duke NUS Medical School, Singapore, Singapore
In the third presentation, Subhash Vasudevan of Duke-National University of Singapore reviewed the development of antivirals against dengue and Zika viruses. He noted in particular the efforts of the Novartis Institute for Tropical Diseases and highlighted a current article in Antiviral Research that summarizes 15 years of dengue studies (Lim, 2019). He noted that a compound currently under development at Janssen Pharmaceuticals targets the NS4B of all four dengue virus serotypes, while some other candidate molecules are not active against all serotypes. A recent paper from the Bartenschlager group validates the dengue NS1 protein as a “Swiss army knife” that could be an ideal antiviral target’, especially given its interaction with precursor versions of NS4B (Plaszczyca et al., 2019). Opportunities for targeting dengue and Zika virus with nucleoside-analogue inhibitors such as remdesivir (Gilead) were also suggested, based on extensive structural and functional similarities of the NS5 protein with the replication mechanisms of other RNA viruses. He also briefly reviewed the work of small biotech companies attempting to develop therapeutic antibodies against dengue, Zika and yellow fever virus.
9. Developing new antiviral therapies
9.1. Therapeutic strategies to combat cytomegalovirus infection. Dr. Rhonda Cardin, Louisiana state university school of veterinary Medicine, Baton Rouge, LA USA
Rhonda Cardin began by introducing human cytomegalovirus (HCMV), a β-herpesvirus with a 230 kbp genome encoding >200 ORFs. As all herpesviruses, HCMV establishes lifelong infections, characterized by different stages: lytic replication (with viral gene expression and production of infectious virus), latency (with limited gene expression, presence of viral DNA but no infectious virus), and reactivation (when signals stimulate exit from latency into the lytic cycle).
HCMV has a complex lifestyle to escape immune surveillance and to persist in the host, infecting numerous tissues and various cell types. This herpesvirus infects 50–90% of the world population. In immunocompetent individuals, the infection is generally asymptomatic, though the virus is associated with some chronic diseases (e.g., cardiovascular, cancer). The virus also causes severe widespread tissue infection among immunocompromised patients (AIDS, transplant, neonates), requiring lengthy therapeutic regimens, which can lead to drug-resistance and toxicity. HCMV is a leading cause of morbidity and mortality among transplant recipients, resulting in multi-organ HCMV disease. In HSCT or SOT transplantation, the highest risk is for (D+/R-, HCMV seropositive donor and seronegative recipient) followed by D-/R+ and D+/R+. The incidence of congenital cytomegalovirus (cCMV) infection is 0.5–2% of all births, being the most common congenital infection. The majority of babies with cCMV infection (~90%) have no clinical findings at birth (asymptomatic infection). The remaining 10% are born with clinically apparent (symptomatic) infection and about 10% of symptomatic infants die in the newborn period. In symptomatic cCMV, disease manifestations can range from mild nonspecific findings to multiple organ system involvement, with particular damage of the reticuloendothelial and central nervous system. About 60% of symptomatic, and 7–25% of asymptomatic, cCMV babies will develop hearing loss.
An antiviral approach (either by drug or vaccine) should impede or limit virus replication, prevent or limit establishment of latency or reactivation from latency and reduce recurrent disease, thus, diminishing horizontal and vertical transmission. Ideally, in vivo models used for analysis of antivirals and vaccines should mimic aspects of human disease for acute infection, latency, and viral pathogenesis.
She then discussed the approved anti-herpesvirus drugs and their targets as well as some agents under investigation (Table 1, Table 2 ), though new targets, including cellular targets, may be developed (Britt and Prichard, 2018). Treatment with ganciclovir, discovered almost 30 years ago, remains the first line therapy, although it is associated with dose limiting toxicities and resistance (5–20%). Second line therapy with cidofovir and foscarnet is associated with nephropathy. Early but limited antiviral intervention with intravenous (IV) ganciclovir and oral valganciclovir in newborns has demonstrated that neural damage and degree of hearing loss be reduced (Kimberlin et al., 2003, 2008, 2015). New anti-HCMV drugs without toxic side effects are needed.
Table 1.
Approved anti-herpesvirus drugs.
| DNA polymerasea |
Terminasea |
|||||
|---|---|---|---|---|---|---|
| Acyclovir | Penciclovir | Ganciclovir | Cidofovir | Foscavir | Letermovir | |
| HSV-1 (HHV-1) | choice | approved | resistance | resistance | ||
| HSV-2 (HHV-2)rowhead | choice | approved | resistance | resistance | ||
| VZV (HHV-3) | choice | approved | resistance | resistance | ||
| EBV (HHV-4) | off-label | off-label | off-label | |||
| HCMV (HHV-5) | choice | approved | approved | approvedb | ||
| HHV-6A | off-label | off-label | off-label | |||
| HHV-6B | off-label | off-label | off-label | |||
| HHV-7 | off-label | off-label | off-label | |||
| HSHV (HHV-8) | off-label | off-label | off-label | |||
Drug target.
Approved for prophylaxis.
Table 2.
Investigational anti-HCMV drugs.
| Step inhibited | Target enzyme | Drug |
|---|---|---|
| DNA synthesis | DNA polymerase | Brincidofovir (BCV, CMX-001) Filociclovir (FCV, cyclopropavir) |
| Cleavage & packaging | Terminase | BDCRB Tomeglovir (BAY-38-4766) |
| Maturation | UL97 protein kinase | Maribavir (MBV) |
Different models of HCMV infection have been developed (Table 3 ), including some in which the virus is able to cross the placenta resulting in hearing loss in the newborns, thus mimicking human cCMV infection. Hearing loss is assessed by auditory-evoked brainstem response (ABR) that measures brainstem responses to auditory stimuli. These models are useful to evaluate anti-HCMV drugs for their potential in both transplant patients and cCMV.
Table 3.
Models of HCMV infection.
| Animal | CMV | Viral genome | Virus crosses placenta | Disease in newborns |
|---|---|---|---|---|
| Chimpanzee | CCMV | ~241 kbp | ??? | Not studied |
| Rhesus macaque | RhCMV | ~221 kbp | Yesa | Yes |
| Pig | PCMV | ~200 kbp | Yes | Not studied |
| Rat | RCMV | ~230 kbp | Yes | ??? |
| Mouse | MCMV | ~230 kbp | No | Yes (hearing loss)b |
| Guinea pig | GPCMV | ~231 kbp | Yes | Yes (hearing loss)c |
| SCID-hu/mouse HCMV | Not applicable | Not applicable | ||
| Human | HCMV | ~230 kbp | Yes | Yes (hearing loss) |
Depletion of CD44+ cells allows transfer via the placenta.
MCMV models of viral dissemination & replication in adult mice: - intraperitoneal (ip) infection: virus replication in peritoneal cavity mesenthelial cells, spleen sinusoidal lining cells (48 hpi) - intranasal infection (in): virus replication in nasal mucosa, lungsMCMV model of hearing loss: - ip inoculation of newborns
Two models GPCMV-infected newborns - congenital: virus crosses placenta, similar to HCMV - neonatal: mimics widespread infection of newborns Dose and route determines the extent of infection
Rhonda Cardin also presented a model of HCMV latency in myeloid lineage cells. The main sites of HCMV latency are the progenitor cells of the myeloid lineage such as CD34 + cells and their CD14 + derivatives (Sinclair and Reeves, 2014). Isolation and culture of these primary cells in vitro enables reactivating the virus from sorted cells. Differentiation of latently infected primary myeloid cells to dendritic cells and macrophages results in reactivation of latent virus. Whereas primary lytic infection or reactivation is characterized by a regulated cascade of expression of all viral genes, latent infection is associated with a much more restricted viral transcription program in which only a small number of viral genes are expressed. In the murine model, in vivo antibody depletion of T cells leads to reactivation of MCMV, or alternatively, modulation of T cells by specific regulatory T lymphocyte (Treg) depletion decreases MCMV latency in some tissues whereas it reactivates virus in the salivary glands.
There are several challenges to develop new HCMV therapies. Clinical viral isolates need to be tested in additional primary cell types. Reinfection with multiple HCMV strains in both immunocompetent and immunocompromised hosts needs to be considered. Studies with rather complex in vivo models of infection (MCMV, GPCMV, RhCMV, HCMV in tissue implants in SCID mice) should be performed. Safety should be evaluated for the immunocompromised setting and for chronic HCMV-associated diseases. It is also important to consider candidate combination therapies, for instance, ganciclovir with letermovir for therapy of HCMV infections and letermovir with acyclovir for longer-term prophylaxis. Maribavir could also be considered for combinatorial anti-HCMV therapy.
In conclusion, the biology of HCMV is complex, including infection with multiple HCMV strains, latency and reactivation, infection of multiple cell types and tissues, and disease associations in both immunosuppressed and immunocompetent individuals. There is a need for relevant animal models that reflect various aspects of HCMV disease to evaluate antivirals. By targeting both HCMV replication and latency, this will lead to the continued discovery of new inhibitors of HCMV with novel molecular targets to combat HCMV infection.
9.2. Investigational therapies for chronic hepatitis B: does anything really work? Dr. Tim Block, Baruch S. Blumberg institute, Doylestown, Pennsylvania USA
Tim Block discussed the global distribution of the more than 260 million HBV surface Ag positive people, which overlaps that of hepatocellular carcinoma (HCC). HCC accounts for 700,000 to 1 million deaths annually worldwide, but with large regional variations such as the exceedingly high HCC mortality rates in East/Southeast Asia and several areas of Africa (El-Serag and Rudolph, 2007). Chronic hepatitis B, due to persistence of the covalently closed circular DNA (cccDNA) and HBV ability to evade the immune system, is considered a major cause of HCC.
Two classes of antiviral agents are approved for chronic hepatitis B, interferon (IFN) and seven nucleoside/tide analogues [i.e. lamivudine, adefovir, entecavir, PEG-INF, telbivudine, tenofovir disoproxil fumarate (TDF) and TAF (tenofovir alafenamide)]. These analogues are direct-acting antivirals (DAAs) that competitively inhibit the viral reverse transcriptase. Health records from 2,671 adult participants (diagnosed with chronic HBV infection) in the Chronic Hepatitis Cohort Study show that antiviral treatment leads to a decrease in viral load and a lower risk of HCC (Gordon et al., 2014).
Current antiviral therapies are effective at reducing viral replication and decreasing the complications resulting from chronic HBV infection. However, they have no impact on the cccDNA reservoir and, thus, are not curative. There is an unmet need for new HBV treatments to suppress HBsAg. The main goal of new anti-HBV treatments is to reduce (or eliminate) the clinical consequences of chronic hepatitis B (i.e. sustained loss of HBsAg with or without development of hepatitis B surface antibody - anti-HBs) without the need for ongoing therapy. Several surrogate endpoints, including elimination of detectable viremia, normalization of circulating levels of liver derived enzymes (ALT, AST), reduction of HBs antigenemia, and sustained off drug beneficial antiviral effects, can be used. The FINITE study demonstrated the potential for HBsAg loss, or sustained virological response, in non-cirrhotic HBeAg-negative patients stopping long-term TDF therapy (Berg et al., 2017).
The HBV life cycle presents various targets for intervention. Tim Block discussed the current HBV therapeutic development landscape, with a growing number of drugs in clinical development (Table 4 ). There are more than 30 experimental therapies for the management of chronic hepatitis B in the pipeline with several candidates in Phase III, and with high expectations. These new HBV agents target specific viral gene products (DAAs) or host targets (indirect-acting antiviral agents, IAAAs).
Table 4.
The HBV therapeutic development landscape as of 2019.
| Target/strategy | Preclinical | Human Phase trials | |
|---|---|---|---|
| DDA | Entry | Myrcludex B (MyrB) | |
| DNA pol | Tenofovir exalidex, CMX-157, hexadecyloxypropyl-tenofovir (Contravir Pharmaceuticals) ─ TAF (GS) ─ Clevudine (Korea) | ||
| siRNA/antisense | Ben BBHB 331 ─ DCR HBs ─ RG6004 ─ LunarHBV Janssen | ARO HBV RNAi ─ Aln Vir HBV ─ ARB 467 RNAi ─ IONIS-HBV-LRx IONIS-HBVRx | |
| sAg | BSBI edi2 ─ BSBI 259 sAg ─ TTP sAg | REP 2139 ─ REP 2165* | |
| Capsid | Benza capsid ─ CpAMS capsid | AB 423 capsid ─ ABI H2158 ─ ABI H0731 ─ RG7907 ─ AB 503 ─ QL 007 ─ RG7048 ─ GLS-4 HEC ─ ABI 7031 ─ J&J 6379 | |
| RNA destabilizers | EBT 106 ─ Roche DHQ ─ AB 452 | ||
| cccDNA | CRISPCAS (intel)ia ─ CRIPR CoCrystal ─ BSBI (Jt Guo) | ALN-HBV ─ ARB-1467 ─ ARC RNAi | |
| Host | Non immunomodulators | EYP001 FXR Enyo ─ GS 5801 [inhibitor of histone demethylase (lysine demethylase 5 (KDM5)] | EYP001 ─ Lonafarnib* EI |
| Innate Immune modulators | GS 9688 (TLR 8 agonist) | SB 9200 (RIG 1+, di-nuc) ─ Poly IC (interferon pathway) ─ HBV inf den cells (Ag presentation) ─ Inarigivir (RIG I/STING) ─ GS-9620 (vesatolimod - TLR 7 agonist) ─ Nivolumab (anti-PD-1) | |
| Adaptive immune modulators/vaccines | Imm TAV TCR IC ─ Tomega Vax ─ MVA-VIP GV | CVIHBV002 ─ ABX203 ─ Ino 1800 ─ DV601 ─ HB110 Ichor ─ INO 1800 ─ TG 1050 ─ IFN + IL2 + vaccine |
AB, ARB: Arbutus; ABI, Aln, Alnylam; Alt, Altimmune; Apg, Ascentage; BSBI, Blumberg; Ben, Benitech; DCR, Dicerna; EBT, Excision; EI, Eiger, EY, Enyo, GS, Gilead; INO, Innovio; Rep, Replicor; GV, Genvax; GSK, Glaxo; IC, Immunocore; RG, Roche; SB, Springbank; TG, Transgene.
The understanding of the HBV entry process led to the development of inhibitors of de novo hepatocyte infection. This process requires the coordinated attachment to heparan sulfate proteoglycans (a low-affinity receptor) followed by a high-affinity binding to sodium taurocholate cotransporting polypeptide (NTCP). Myrcludex B (MyrB), a myristoylated peptide, binds to NTCP and inhibits HBV entry. This strategy is clinically validated, also has anti-HDV effect, and it stops the replication cycle at the start. However, current HBV NTCP entry inhibitors could interfere with bile acid uptake, as HBV and bile acids interact with the same NTCP site. Entry inhibitors are also unable to affect an established infection.
Several programs from Assembly, Blumberg/Arbutus, Novartis, Novira, Roche and Sunshine focus on disruption of, or interference with, nucleocapsid assembly or disassembly via small molecular core protein allosteric modulators (CpAMs). CpAMs target multiple essential viral functions, are clinically validated, and escape mutants are rare. The Class I CpAMs comprise the heteroaryldihydropyrimidines (HAPs), such as Bay 41–4109 and GLS4, which lead to aberrant core protein aggregates which are subsequently degraded. The Class II CpAMs encompasses the sulfamoylbenzamides (SBAs), such as ENAN-34017, and the phenylpropenamides (PPAs), represented by AT-61, which induce the formation of morphologically ‘normal’ empty capsids with distinct quaternary or tertiary structural changes preventing viral DNA replication. HAPs and SBAs, but not PPAs, also induce disassembly of nucleocapsids from both virions and double-stranded DNA-containing cytoplasmic progeny nucleocapsids, thus interfering with cccDNA biosynthesis during both de novo infection and from the intracellular amplification pathways (Guo et al., 2017).
Blumberg Institute, Arbutus, Contravir, and Replicor are developing inhibitors of HBV Ag, such as AB452 (which causes degradation of HBs RNA), TTP sAg, BSBI 259 sAg, and Rep2139 sAg. Preclinical data with the siRNA ARB-1467 in a mouse model demonstrated an HBV DNA reduction of 1.4 log10 at 100 mg/kg. Clinical data from an ongoing Phase 1/2 study of JNJ-3989 (formerly ARO-HBV), a subcutaneously administered RNA interference (RNAi) therapeutic candidate, are encouraging in that JNJ-3989 afforded a durable reduction of HBsAg (100% of patients achieved greater-than or equal to 1.0 Log10 IU/mL HBsAg reduction), HBeAg and serum HBV DNA.
Combinatorial RNAi and vaccination therapy for chronic hepatitis B achieved long-term functional cure in a preclinical mouse model, via induction of virus-specific CD8+ T cell response. HBsAg mRNA degradation induced by a dihydroquinolizinone compound (DHQ-1), in development by Roche, depends on the HBV posttranscriptional regulatory element (Zhou et al., 2018). DHQ-1 mediated HBV RNA reduction resulted from accelerated nuclear viral RNA degradation, rather than from inhibition of transcription initiation. HBV mRNA biogenesis may thus subvert the normal cell mRNA degradation machinery. There is also a growing interest in targeting the cccDNA, with programs ongoing at Gilead, Arbutus, Assembly, Blumberg, Fox Chase, Duke, and Rockefeller.
IAAAs include non-immunomodulating and immune modulators (programs from Akshaya, Arbutus/Blumberg, BMS, Dynavax, Gilead Sciences, HepTcell, Inovio, Roche, Springbank, Tomegvax). Among the non-immunomodulating agents being developed, Tim Block discussed GS 5801 [histone lysine demethylase 5 (KDM5) inhibitor], innate host activators, Springbank's putative RIGI/STING acting small molecule inarigivir, the TLR 7 agonist GS-9620 (vesatolimod), and the checkpoint inhibitor nivolumab (anti-PD-1).
The combination therapies are being investigated with the purpose of achieving a better Ag suppression rather than to repress resistance. Repression of viremia and antigenemia with two complementary DAAs could suffice for a large percentage of infected people. Immune modulators could be used after antigen control to enhance host immune mediated antiviral response. Current regimens result in about 5% HBs loss or sustained off drug benefits. The introduction of each new drug in combination therapy was proposed to step up clinical benefit (better DNA suppression, superior sAg loss and enhanced sAb/T cell function gain).
10. DNA, respiratory and other viruses
This session had several outstanding talks in three distinct topics. The first topic within the symposium focused on enteroviruses. The first talk by Bart Tarbet, Utah State University, Logan, UT, USA, described the development of a mouse model for neurological disease associated with infection by enterovirus 71. For use within this model, interferon alpha/gamma knockouts were employed with immune globulin as a control. The second talk by Jun Wang, University of Arizona, Tucson, AZ, USA, explored the viral 2A protease of enterovirus D68 as a potential target for antiviral therapy. Telapravir, an HCV NS3 protease inhibitor, demonstrated activity against enterovirus and resistance development studies confirmed the 2A protease as the target.
The second topic within the symposium dealt with modeling and treating the herpes family of viruses. The first talk by Megan Lloyd, SUNY Upstate University, Syracuse, NY, USA, described the development of skin tissue model for the testing of anti-HCMV compounds. This system is done concurrently with an organ culture system to measure the effectiveness of said antiviral compounds. The second talk by Luis Schang, Cornell University, Ithaca, NY, USA, described his work in identifying commonalities between unrelated viruses as a means for developing small molecule inhibitors that would have broad-spectrum antiviral activity. Toward that end, glycan binding by HSV-1 or influenza A virus and inhibition of this binding by di-galloyl esters was described. The final herpesvirus talk by Leor Weinberger, Gladstone Institute, Sand Francisco, CA, USA, described the disruption of IE86 as a means of producing virus-infected cell toxicity. IE86, a key protein necessary for herpes viral replication, self-regulates and loss of control over the production of this protein leads to cytotoxic consequences within the virus-infected cell.
The final topic focused on the delivery of an immunogenic antigen to influenza infected cells by linking it to zanamivir, a virus-specific neuraminidase inhibitor. This presentation by Xin Liu, Purdue University, West Lafayette, IN, USA, demonstrated that the immune system can be better directed to combat the infection by linking a highly specific antigen to a drug designed to inhibit a virus-specific protein. This interaction was demonstrated by the further reduction of infection when compared to the administration of zanamivir alone.
11. Emerging infections
This session included nine short talks discussing new antiviral approaches against emerging viral pathogens.
Brian Gowen, Utah State University, Logan, UT, USA, presented the in vitro and in vivo activities of an exciting new arenavirus fusion inhibitor developed by Arisan Therapeutics, ARN75039. Five New World arenaviruses (NWAs) cause life-threatening viral hemorrhagic fever in the Americas, and others such as Lassa in the Old World. With only supportive therapy, ribavirin and immune plasma – against only Junin virus, these viruses are considered high priority pathogens by the NIAID, the CDC and WHO. The envelope glycoprotein (GPC) mediates arenavirus entry through a process of pH-dependent fusion with the endosomal membrane. Brian Gowen discussed the antiviral activity against Tacaribe virus (TCRV) of a novel fusion inhibitor, ARN75039 and on its preclinical development. TCRV is closely related to Junin and Machupo viruses, pathogenic NWAs. Dose-response analyses showed ARN75039 to be highly active against TCRV with sub-nM potency and a selectivity index (SI) > 1,000 (CC50 was not achieved at the highest concentration of 100 nM). The compound's half-life in mice was 13.2 h, allowing for once-daily dosing, and no clinical adverse effects observed at 100 mg/kg (highest dose tested). At 10 or 35 mg/kg, ARN75039 treatment started prophylactically 2 h before infection protected against mortality (~80% survival at 27 days vs 20% for the placebo treated animals) and weight loss. Viremia and liver and spleen titres were between 2.5 and 5 orders of magnitude lower in the treated animals. Therapeutic treatment could be started up to 5 days post infection (dpi) and still achieve full protection against mortality and weight loss, albeit viremia, serum and spleen viral loads were only partially inhibited. Treatment started at 7 dpi still protected 50% of the mice against a lethal TCRV challenge dose. Treatment was therefore effective when started after the onset of clinical signs, as it is required to support a potential clinical use for ARN75039.
Tim Sheahan, University of North Carolina at Chapel Hill, NC, USA, presented an exciting project about the effects of remdesivir against MERS-CoV in vitro and in vivo, resulting from a collaboration between his university, Gilead Sciences and Vanderbilt University Medical Center. Coronaviruses (CoVs) have a natural predilection for expansion into new host species giving rise to novel human diseases, as exemplified by the recent emergence of severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV). MERS causes a severe respiratory syndrome, which has been associated with more than 2,200 cases and 800 deaths in 27 countries since 2012. Zoonotic CoVs similar to SARS and MERS-CoV circulate in animal reservoirs. Thus, is it possible that novel CoV may emerge in the future. Currently, there are no approved therapeutics for any human CoV. Remdesivir (RDV, GS-5734) is a monophosphoramidate prodrug of an adenosine analogue that targets the viral RNA-dependent RNA polymerase (RdRp) and has been shown to inhibit replication of viruses in multiple families. This presentation showed RDV and IFNβ had superior antiviral activity to LPV in vitro. RDV showed potent inhibition of MERS-CoV replication with an EC50 of 0.09 μM and no observable cytotoxicity up to 10 μM in a human lung epithelial cell line (Calu-3). The EC50 values were 100-fold lower than those of LPV (11.6 μM) or RTV (24.9 μM). The EC50 for IFNβ against MERS-CoV in Calu-3, 175 IU/mL (CC50 > 2800 IU/mL), did not change significantly (160 IU/mL) when coupled with a clinically relevant LPV/RTV concentration (5 μM:1 μM). Humanized DPP4 mice deficient in Ces1c expression (C57BL/6J Ces1c−/− hDPP4) were generated. In this model, MERS-CoV lung viral loads were significantly reduced, respiratory function was improved, and mice exhibited less weight loss with prophylactic or therapeutic administration of RDV (25 mg/kg, BID, subcutaneously), as compared to vehicle. Prophylactic and therapeutic combinations of LPV/RTV (LPV:160 mg/kg and RTV: 40 mg/kg, QD, PO) and IFNβ (1x human equivalent dose 1.6 MIU/kg or 25x human equivalent dose 40 MIU/kg, every other day, subcutaneously) slightly improved pulmonary function, but only prophylactic LPV/RTV- IFNβ had modest impacts on viral load. Prophylactic treatment with IFNβ alone failed to reduce viral loads and moreover it exacerbated disease.
Mike Flint, CDC Atlanta, GA, USA, presented a genome-wide screen using the CRISPR/Cas9 system that identified the gene GNPTAB, which encodes the alpha and beta subunits of N-acetylglucosamine-1-phosphate transferase, to be required for Ebola virus (EBOV) replication. The validity of the screen was confirmed by its detection of NPC1, the EBOV receptor, and the hit was confirmed by independent knockout and re-expression. GNPTAB, which is activated following cleavage by SKI-1/S1P protease, participates in the trafficking of lysosomal hydrolases; GNPTAB mutations result in mucolipidosis II (MLII). Fibroblasts from MLII patients were far less susceptible to EBOV than cells from their healthy parents or other healthy people. GNPTAB knockout results in lower levels of the EBOV entry factor cathepsin B (CatB), and inhibition of the SKI-1/S1P protease with PF-429242 inhibited EBOV entry and infection. GNPTAB is a potential target for antiviral intervention against EBOV, and is also required for entry of Marburg and Nipah viruses. Lack of GNPTAB activity results in mucolipidosis, however. The consequences of interfering with GNPTAB as part of an EBOV therapy would need to be carefully evaluated.
Colleen Jonsson, of the University of Tennessee, Knoxville, TN, USA discussed a family of inhibitors of Venezuelan and Eastern Equine encephalitis viruses. No antivirals are approved for the treatment of the encephalitic infections produced by these viruses. Alphavirus epidemics are unpredictable and occur only sporadically, and whereas endemic disease is common it is mostly undiagnosed. It is thus difficult to identify the populations in which a vaccination campaign would be effective and sufficient. An alternative to curtail outbreaks is to develop effective post-exposure treatments. The talk discussed the evaluation of four ML336 analogues in culture and of three of them, BDGR-4, BDGR-69 and BDGR-70, in a mouse model. Best protection was achieved at 12.5 mg/kg/day, twice daily for 8 days. BDGR-4 protected all mice challenged with VEEV Trinidad Donkey (TrD) from lethal disease when treatment started at 24 h and 90% when treatment started at 48 h post-infection, respectively. It also protected mice infected with Eastern equine encephalitis virus by 90. Doses of up to of 25 mg/kg/day over 4 days resulted in no observable clinical or clinical chemistry toxicity, or interferon induction. Resistance was selected in culture and was mapped to mutations in nsP4, the RdRp. The most critical mutations were in glutamine 210.
Giselle Barbosa-Lima, FIOCRUZ, Rio de Janeiro, Brazil, presented on the potential repurposing of sofosbuvir against Zika virus (ZIKV). The aim is to be able to move an antiviral against ZIKV as promptly as possible, taking advantage that sofosbuvir is clinically approved (for hepatitis C virus) and that it targets the most conserved flavivirus protein, RdRp. Sofosbuvir tri-phosphate inhibited ZIKV RdRp, as did ribavirin tri-phosphate. Sofosbuvir inhibited ZIKV replication in iPS derived neuronal stem cells and brain organoids. In a neonatal mice model, 20 mg/kg/day sofosbuvir resulted in lower levels of ZIKV RNA, to approximately 50 to 10% in plasma, kidney, and brain, and to 60 to 35% in spleen. Pre-treatment of mice before infection conferred modest protection against lethality and treatment after infection provided marginal protection. In the survivors, sofosbuvir had some effect on the acute neuromotor impairment triggered by ZIKV and the long-term loss of hippocampal- and amygdala-dependent memory.
Jessica Spengler, CDC, Atlanta, GA, USA, described a non-replicating viral replicon particle (VRP)-based platform for vaccines against Lassa virus (LASV) and Crimean-Congo hemorrhagic fever virus (CCHFV). The LASV and CCHFV VRP lack the glycoprotein gene and therefore cannot produce infectious virions after the first round of replication although they replicate in the primarily infected cells as a result of trans-complementation during production. A single-dose subcutaneous vaccination with 1 × 107 FFU LASV VRP protected all guinea pigs against all clinical signs after lethal challenge with LASV (Josiah strain). VRP gamma irradiation resulted in loss of clinical protection, and 40% lethality, indicating that the replication in the primarily infected cells plays a major role in providing protection. Vaccination one day after challenge (post-exposure) still protected against lethality, although no against clinical signs. CCHFV VRP were also effective at protecting IFNΑR−/- mice against lethal CCHFV (IbAr10200 strain) challenge. High (4.4 × 105 TCID50) single dose subcutaneous vaccination prevented clinical signs of disease in all animals, whereas a low dose (4.6 × 103 TCID50) protected 7 of 9 (78%) from fatal outcome. Most critically CCHFV VRP conferred heterologous protection against diverse CCHF strains.
Leen Delang, the Rega Institute for Medical Research, KU Leuven, Belgium, discussed a new class of CHIKV inhibitors, the CHVB-series. From the initial hit, 67 analogues were synthesized, of which 62 were active and 18 more potent than the original hit (EC50 values reaching the low μM range and 4–5 log10 inhibition of replication) and showed no adverse cellular effects. The compounds did not inhibit entry, but acted early during infection. CHVB-resistant variants were selected in less than 10 passages. They had two mutations in the non-structural protein 1 (nsP1) gene, and one in each nsP2 and nsP3. The four mutations together conferred 20-fold resistance, half of which was conferred by both nsP1 mutations together. The mutant virus was cross-resistant to the MADTP series, which are CHIKV capping inhibitors and the most important mutations mapped to nsP1, which is involved in capping. Although functional CHIKV nsP1 is not available, CHVB inhibited the methyltransferase and guanylyltransferase activities of VEEV nsP1. Optimization of the series is ongoing towards improving PK properties, with the goal to next assess activity in mouse models.
Randall Lanier, Chimerix, Durham, NC, USA, presented data on the activity of CMX521 against noroviruses. Despite the high medical and economic impact, with approximately 200,000 deaths and an estimated 60 billion USD costs per year, there are no indicated vaccines or antivirals for noroviruses. One of the major challenges to develop vaccines or drugs is the high genetic variability. However, as shown in an earlier talk for ZIKV, the RdRp gene is highly conserved and presents an attractive target for drug discovery. The RdRp inhibitor CMX521, a ribonucleoside analogue that inhibits NoV RdRp (IC50s 1–2 μM) by competing with GTP, has pan-genotypic norovirus activity (EC50, 0.12–5 μM). 150 mg/kg CMX521 twice a day reduced fecal MNV shedding by approximately 10-fold in immunocompetent mice and extended survival in a lethal immunocompromised mouse model. CMX521was evaluated in a Phase 1 double-blind, single-dose escalation clinical phase I safety and pharmacokinetics study. Thirty-eight healthy adults divided into 5 cohorts were randomized to receive single oral doses of CMX521 (200 mg–2400 mg) or placebo. All doses were generally well-tolerated and no clinically significant safety concerns were identified. In a second part of the trial, the concentration of the active form (CMX521-triphosphate) was evaluated 4 h after a single dose (2,400 mg, orally) in seven duodenal biopsies for each of three subjects. Although CMX521-TP was detected in all samples, the concentration was below target based on in vitro efficacy and it was concluded that a prodrug or optimized formulation is likely needed to reach effective concentrations of the active compound in the target cells. This talk represented work from Chimerix in collaboration with the University of Cambridge, Georgetown University, the University of Michigan, St Louis University and NIH.
Durbadal Ojha, from the NIAID, Hamilton, MN, USA, presented a study on Rottlerin, which inhibits neuronal damage resulting from La Crosse Virus (LACV), a member of the California Orthobunyavirus serogroup. LACV induces rare life-threatening neurological disease in children, for which no vaccine nor FDA approved therapy is available. A screen of more than 3,500 FDA approved and bio-active compounds, acquired from the National Center for Advancement of Transnational Science (NCATS), identified 38 compounds that appeared to inhibit LACV-induced death in human neuronal-type SHSY5Y cells. Of them, four were selected for further analyses. Only one, rottlerin, a protein kinase inhibitor that also decouples mitochondrial respiration from oxidative phosphorylation, inhibited LACV-induced apoptosis in neuronal-type N2a, C17.2, hNSC and SHSY5Y cells, and primary murine neurons. The EC50 values were 0.16–0.38 μg/mL and the selectivity index, of 5–37.8. Rottlerin treatment reduced virus release by these cell lines by up to 1,000-fold, suggesting suppression of virus infection or replication. Rottlerin was effective when added up to 12 h post infection. Rottlerin inhibited virus release from the Golgi, by a PKC-∂ independent mechanism (rottlerin has been described also as a PKC delta inhibitor). Used intraperitoneally or intracerebrally., rottlerin inhibited LACV-induced disease in mice by 48% or 66%, respectively.
12. Medicinal chemistry
The Medicinal Chemistry short session included six talks on new chemical matter and analyses of mechanisms of actions of previously disclosed compounds.
Filip Kalčic, Institute of Organic Chemistry and Biochemistry CAS and Charles University, Czech Republic, reported the development of a new type of ProTide-like prodrugs for delivery of acyclic nucleoside phosphonates. Tenofovir (TNF), (R)-PMPA, was selected as a model compound and isopropyl ester L-phenylalanine and suitably protected L-tyrosine derivatives as suitable masking groups. The prepared TNF prodrugs were evaluated for their antiviral potency in comparison to recently approved TNF prodrug - tenofovir alafenamide fumarate (TAF). Regarding the anti-HIV-1 activity in MT-4 cells, the most potent derivative (as a mixture of epimers) was almost one order of magnitude more potent than TAF. Although it had about 3-fold higher cytotoxicity than TFA, the selectivity index of the new prodrug was still 3-fold higher. Another TNF prodrug (as a single epimer isolated using chiral HPLC) exhibited the same potency as TAF against HBV in HEPG2hNTCP cells but with no observed cytotoxicity and, thus, had a higher selectivity index.
Favipiravir (T-705) is an important anti-influenza agent that requires intracellular multi-step metabolic conversion to its ribonucleoside triphosphate (RTP), T-705-RTP, to exert antiviral activity. Johanna Huchting, University of Hamburg, Germany, presented a parallel study with T-705 and its non-fluorinated analogue T-1105 using three different RNA viruses (influenza A, parainfluenza-3, and Punta Toro) and various cells. While T-1105 exhibited higher antiviral potency than T-705 in MDCK cells, it was less active in A549, Vero, and HEK293T cells. This trend was also reflected in the observed cell line-dependency of T-705/T-1105-RTP-levels and was proposed to indicate T-1105-RTP's superior antiviral properties. Unlike for T-705, T-1105-RMP was observed as main metabolite in A549, Vero, and HEK293T cells. The conversion of RMP to RDP was suggested as the bottleneck that limits T-1105 antiviral potency.
Nora Constanze Fohrmann, University of Hamburg, Germany, informed the audience about the discovery and development of potent antiviral agents against bunyaviruses (negative-sense single-stranded RNA viruses). High-throughput screening (in Toscana virus infected Vero E6 cells) and subsequent structural modification of the hit (T-13) led to an identification of anthranilic acid based inhibitors potent against various RNA viruses with EC50 values as low as single-digit nanomolar range. Dihydroorotate dehydrogenase (DHODH) was identified and fully validated using enzyme assays and X-ray co-crystal structures as the target. The intrinsic low metabolic stability of the compounds, due to hydrolysis of an amide bond in hepatic microsomes, was improved by replacement of the labile amide group for more stable carbamate moiety.
Ge Meng, Fudan University, Shanghai, China, reported the development of a novel series of non-nucleoside reverse transcriptase inhibitors (NNRTIs) based on the diarylbenzopyrimidine (DABP) scaffold and bearing the 4-cyanovinyl-2,6-disubstitued phenyl motif. The most potent analogue exhibited single-digit nanomolar activities against HIV-1, wild type (WT) or several drug-resistant mutants, with EC50 values of 1.5 nM (WT), 1.6 nM (K103N), 1.9 nM (E138K), 3.5 nM (Y181C), 8 nM (L100I). Molecular docking and SAR studies provided further insights into the interactions between DABPs and the active binding pocket of HIV-1 RT.
Nucleoside and nucleotide analogue drugs represent an important class of antiviral agents in which the nucleobase or the ribose moiety are generally modified. Kalyan Das, Rega Institute for Medical Research, KU Leuven, Belgium, described another approach, where the triphosphate (TP) can be substituted by a smaller chelating group (e.g. α-carboxy phosphonate) that mimics the TP moiety. Additional NTP/dNTP mimics were synthesized by conjugating dAMP or tenofovir to a variety of amino acids, namely L- or D-glutamate, histidine, methionine, and arginine. Crystal structures revealed a conserved mode of binding for the nucleotide moieties, whereas different amino-acid substitutions interact with the binding pocket differently. These results suggest the potential for improving the target-specificity of NTP/dNTP analogues by introducing additional substitutions beyond the TP substations.
NS2B-NS3 protease plays an essential role in Flavivirus replication and it is therefore considered an ideal antiviral drug target, especially for treatment of dengue (DENV) and Zika virus (ZIKV) infections. Dahai Luo, Nanyang Technological University, Singapore, presented high-resolution co-crystal structures of NS2B-NS3 protease from ZIKV (bZiPro) with peptidomimetic inhibitors. The observed binding mode interactions of inhibitors within bZiPro active site resulted in subsequent structure-activity relationship study leading to the design of more effective cyclic peptidomimetic analogues as NS2B-NS3 protease inhibitors with improved potency and bioavailability.
13. Hepatitis viruses and retroviruses; clinical evaluations etc
This session included seven talks centered on hepatitis B virus, HIV, and clinical analyses of antiviral activity and drug resistant viruses.
Jinhong Chang of Baruch Blumberg Institute, Doylestown, PA, USA, presented the identification of a new small molecule human STING agonist, 6,bromo-N-(naphthalen-1-yl)benzo[d][1,3]dioxole-5-carboxamide (BNBC), which was identified in an HTS screening of about 17,000 compounds. BNBC activates a human-STING dependent cytokine response in primary human fibroblast cells. Pretreatment of cells with BNBC established an antiviral state against flaviviruses. Furthermore, BNBC also induces a robust type I and III interferon dominated cytokine response in PBMC and PBMC-derived macrophages and dendritic cells, and promotes dendritic cell maturation. Importantly, BNBC also inhibited cccDNA transcription and viral replication in HBV-infected Hep-G2 cells overexpressing NTCP. The work provides evidence supporting the development of STING agonists to treat viral infections in the context of a dysfunctional immune response, such as that by HBV.
Following up on a published work showing that TLR1/2 ligand (Pam3CSK4) was the most efficient amongst all TLR agonists at directly inhibiting HBV replication in infected hepatocytes in vitro, David Durantel (INSERM, Lyon, Fr) updated their progresses in terms of its preclinical development in the context of immune-therapeutic strategies. He reported the improved efficacy of poly-lactic-acid (PLA)-nanoparticulated-Pam3CSK4 (NP-Pam3SCK4) in vitro and in vivo (AAV-HBV transduced mice). In vivo, NP-Pam3SCK4 was far more efficient than free-Pam3CSK4 to inhibit HBV, with a negativation of viremia and a significant decrease of viral episomes in the liver. Hence, the data demonstrate the potential of innate immune stimulation of TLR2 in vivo as anti-HBV agents, provided liver delivery is optimized.
Junjun Cheng of Baruch Blumberg Institute, Doylestown, PA, USA, described a loss-of-function genetic screening of individual IFN-stimulated genes that allowed the identification of 7 genes that significantly increased viral RNA levels without altering cccDNA levels. Further mechanistic studies on STAT1, SMCHD1 and PML showed that these three cellular proteins are recruited to the cccDNA minichromosome and induce histone modifications partially mimicking the ones induced by IFNα, thus mediating (at least in part) the suppressive effect of IFNα on hepadnaviral cccDNA transcription.
Tiffany Edwards of Saint Louis University, Saint Louis, MO, USA, presented the characterization of HBV RNase H inhibitors identified through a screening of around 3,000 compounds that led to the identification of 130 inhibitors, which were classified into four chemotypes. Compounds were shown to be selective for HBV RNase H versus human RNase H1 or growth of bacterial pathogens. Kinetic studies showed that some of the active-site chelating compounds in different chemotypes are noncompetitive inhibitors while others are mixed-type inhibitors.
Cole Schonhofer of Simon Fraser University, Burnaby, BC, Canada, reported the investigation of novel HIV-1 Tat inhibitors as pro-latency agents (PLAs) as an approach to suppress expression from HIV reservoirs. Screening 97,000 compounds by high-throughput microscopy to identify Tat-dependent transcriptional inhibitor lead to 96 hits. Two compounds with different mode of actions suppressed HIV latency reversal in J-Lat cells, a cell line model of HIV latency. One of them was shown to inhibit activity of cyclin-dependent kinase 9, a component of the host p-TEFb complex required for Tat-mediated transcription. These compounds can be used to probe mechanisms of HIV transcription and inform ongoing “block-and-lock” strategies to permanently silence latent HIV reservoirs in people living with HIV/AIDS.
Hanna Schalkwijk of KU Leuven, Leuven, Belgium, reported a case study of HSV-1 infection in a hematopoietic stem cell transplant patient treated consecutively with acyclovir, foscarnet and cidofovir. In a four-month period, five viral isolates were obtained. Most isolates showed population heterogeneity of HSV-1 variants. Genotyping of two isolates demonstrated the presence of the A189V mutation in the thymidine kinase (TK) resulting in acyclovir resistance. Other isolates presented different mutations in the TK (A189V, R222H) and DNA polymerase (L778M, L802F) that led to resistance to both acyclovir and foscarnet. The TK T183P is a novel polymorphism that does not affect drug susceptibility. Two isolates obtained the same day from different body sites showed differences in heterogeneity and frequency of viral variants. The study demonstrates that typing of drug-resistance at multiple time points and at multiple body sites is a useful approach to adjust antiviral therapy.
Elaine Thomas of ReViral Ltd, Stevenage, UK, described studies on sisunatovir (RV521), an inhibitor of respiratory syncytial virus (RSV). RSV is a major cause of lower respiratory tract infections, especially in infants, for which no drug is available. A phase 1 study showed sisunatovir to be safe while a phase 2 RSV challenge study showed that sisunatovir was able to reduce viral load and disease severity compared to placebo group. In vitro characterization of sisunatovir resistance showed that its target is the viral F protein. The F-protein gene selected mutation D489Y increased EC50 value by 76-fold. However, the mutant virus has reduced in vitro fitness and inability to compete with wt RSV. Only two RSV F protein variants were detected in 34 sisunatovir treated subjects, an L141F and an D453G mutation, which resulted in no emergence of clinical resistance.
14. Presentation awards
As customary, ICAR 2019 included the poster award and the shot gun presentations by the authors of selected posters. Continuing with last year's success, ICAR 2019 held the second Pechakucha competition as well. The winners of the Chu Family Foundation Award were also announced at the meeting.
14.1. Poster awards-shotgun presentations
The shotgun presentations were co-chaired by Justin Julander and Ila Nimgaonkar (the PechaKucha Event winner).
-
•
“Chikungunya Virus is Susceptible to Sofosbuvir both in vitro and in vivo” by Carolina Sacramento, FIOCRUZ, Rio de Janeiro, Brazil.
-
•
“Structural Investigation of the Effect of Pyrimidine Functional Groups of Fleximer Analogues on Antiviral Activity” by Mary Yates, University of Maryland, Baltimore County, USA.
-
•
“Identification of Sterol Regulatory Element Binding Protein-Dependent Lipidomic Reprogramming as a Broad-Spectrum Antiviral Target” by Jasper Chan, The University of Hong Kong, Hong Kong.
-
•
“Evaluation of Sex as a Variable for Influenza Virus Infection and Treatment with Oseltamivir in Mice” by Brett Hurst, Utah State University, Logan, Utah, USA.
-
•
“From Oxetane to Thietane: Extending the Antiviral Spectrum of 2′-Deoxy-2'spirocyclic Uridine Derivatives by Substituting Oxygen for Sulfur” by Tim Jonckers., Janssen Pharmaceutica N.V., Beerse, Antwerpen, Belgium.
14.2. Poster awards- prizes
-
•
Young Investigator ($1,000 and a publication fee waiver to Antiviral Chemistry and Chemotherapy for one publication within the next year): “Identification of Sterol Regulatory Element Binding Protein-Dependent Lipidomic Reprogramming as a Broad-Spectrum Antiviral Target” by Jasper Chan, The University of Hong Kong, Hong Kong.
-
•
Post-Doctoral: First prize ($1,000): “Chikungunya Virus is Susceptible to Sofosbuvir both in vitro and in vivo” by Carolina Sacramento, FIOCRUZ, Rio de Janeiro, Brazil. Second place ($750): “Studies towards Pan-Flaviviral Protease Inhibitors” by Crystall Swarbrick, Duke-NUS Medical School, Singapore. Third prize ($500): “Mercaptobenziamide Thioesters as HIV Inactivators: SAR Evaluation, Computational Modeling, and Thermodynamic Studies” by Marco Robello, NIDDK, NIH, Bethesda, MD, USA.
-
•
Graduate Student: First prize ($1,000): “Neuraminidase-Targeted Immunotherapy of Influenza: Repurposing Zanamivir as a Targeting Ligand for Delivery of an Attached Immunogenic Hapten to Virus/Virus-Infected Cells” by Xin Liu, Purdue University, West Lafayette, IN, USA. Second place ($750): “Development of Novel hDHODH Inhibitors as Potent Broad Spectrum Antiviral Agents” by Nora Constanze Fohrmann, University of Hamburg, Hamburg, Germany. Third place ($500): Tie– “Identification and Characterization of Inhibitors of Hepatitis E Virus Replication” by Ila Nimgaonkar, Princeton University, Princeton, NJ, USA, and “Advances in HBV Ribonuclease H Drug Development” by Tiffany Edwards, Saint Louis University, St. Louis, MO, USA.
14.3. Pechakucha competition
Finalists: Julia Ma, Dhruvkumar Soni, Florencia Torti, Ila Nimgoankar, Juli Tohme, Tiffany Edwards, Sonali Chatuvedi, Cole Schonhofer and Li-hsin Li, from US, India, Canada, Argentina, and Belgium. First prize ($250) Ila Nimgaonkar, Princeton University, Princeton, NJ, USA. Second place ($150): Florencia Torti, University of Buenos Aires- IQUIBICEN- CONICET, Buenos Aires, CABA, Argentina. Third place ($75): Tiffany Edwards, Saint Louis University, St. Louis, MO, USA.
14.4. Chu Family Foundation Awards
First and second prize ($3,000): Bo Kyeong Yoon (first prize; postdoctoral fellow, University of Singapore, Singapore) and Erofili Giannakopouli (second prize; graduate student, University of Athens, Athens, Greece). Tied third prize ($2,000): Megan Gribble Lloyd (postdoctoral fellow, SUNY Upstate University, Syracuse, NY, USA) and Mary Yates (graduate student, University of Maryland, Baltimore County, USA).
15. Concluding remarks
Besides the excellent oral and poster presentations, which updated the audiences on the most current topics in antiviral research and development and started many an excellent discussion, the meeting also provided many excellent opportunities for interactions and collaborations. The networking opportunities were further supported by the very well attended and liked Women in Science and Careers workshops. The Pechakucha approach provided an outstanding opportunity to develop skills to present science in a precise but also accessible and entertaining manner. The attendees therefore greatly benefited from the science, the opportunities to network and collaborate, the different perspectives about career development, and the many interactions with colleagues working in different countries in different areas of antiviral development in different settings. We are all already looking forward to the 33rd ICAR in Seattle, March 30-April 3rd, 2020.
Acknowledgements
We thank all of the speakers, presenters, participants, organizers, and sponsors of the 32nd ICAR for their contributions to such a successful meeting. Special recognition goes to all those who participated in preparing this report.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.antiviral.2019.104550.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Acosta-Hoyos A.J., Scott W.A. The role of nucleotide excision by reverse transcriptase in HIV drug resistance. Viruses. 2010;2:372–394. doi: 10.3390/v2020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre F.R., Rahali R., Rahali H., Guillon S., Convard T., Fillgrove K., Lai M.T., Meillon J.C., Xu M., Small J., Dousson C.B., Raheem I.T. Synthesis and antiviral evaluation of carbocyclic nucleoside analogs of nucleoside reverse transcriptase translocation inhibitor MK-8591 (4'-Ethynyl-2-fluoro-2'-deoxyadenosine) J. Med. Chem. 2018;61:9218–9228. doi: 10.1021/acs.jmedchem.8b00141. [DOI] [PubMed] [Google Scholar]
- Althouse B.M., Vasilakis N., Sall A.A., Diallo M., Weaver S.C., Hanley K.A. Potential for Zika virus to establish a sylvatic transmission cycle in the Americas. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei G., Carter K., Janeba Z., Sampath A., Schang L.M., Tarbet E.B., Vere Hodge R.A., Bray M., Este J.A. Highlights of the 30th international conference on antiviral research. Antivir. Res. 2017;145:184–196. doi: 10.1016/j.antiviral.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei G., Gammon D.B., Fiten P., De Clercq E., Opdenakker G., Snoeck R., Evans D.H. Cidofovir resistance in vaccinia virus is linked to diminished virulence in mice. J. Virol. 2006;80:9391–9401. doi: 10.1128/JVI.00605-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei G., Topalis D., De Schutter T., Snoeck R. Insights into the mechanism of action of cidofovir and other acyclic nucleoside phosphonates against polyoma- and papillomaviruses and non-viral induced neoplasia. Antivir. Res. 2015;114:21–46. doi: 10.1016/j.antiviral.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Backer J.A., Vrancken R., Neyts J., Goris N. The potential of antiviral agents to control classical swine fever: a modelling study. Antivir. Res. 2013;99:245–250. doi: 10.1016/j.antiviral.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Bar-On Y., Gruell H., Schoofs T., Pai J.A., Nogueira L., Butler A.L., Millard K., Lehmann C., Suarez I., Oliveira T.Y., Karagounis T., Cohen Y.Z., Wyen C., Scholten S., Handl L., Belblidia S., Dizon J.P., Vehreschild J.J., Witmer-Pack M., Shimeliovich I., Jain K., Fiddike K., Seaton K.E., Yates N.L., Horowitz J., Gulick R.M., Pfeifer N., Tomaras G.D., Seaman M.S., Fatkenheuer G., Caskey M., Klein F., Nussenzweig M.C. Safety and antiviral activity of combination HIV-1 broadly neutralizing antibodies in viremic individuals. Nat. Med. 2018;24:1701–1707. doi: 10.1038/s41591-018-0186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett S.E., Teller R.S., Forster S.P., Li L., Mackey M.A., Skomski D., Yang Z., Fillgrove K.L., Doto G.J., Wood S.L., Lebron J., Grobler J.A., Sanchez R.I., Liu Z., Lu B., Niu T., Sun L., Gindy M.E. Extended-duration MK-8591-eluting implant as a candidate for HIV treatment and prevention. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.01058-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg T., Simon K.G., Mauss S., Schott E., Heyne R., Klass D.M., Eisenbach C., Welzel T.M., Zachoval R., Felten G., Schulze-Zur-Wiesch J., Cornberg M., Op den Brouw M.L., Jump B., Reiser H., Gallo L., Warger T., Petersen J., investigators F.C.s. Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients - FINITE study. J. Hepatol. 2017;67:918–924. doi: 10.1016/j.jhep.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Bray M., Andrei G., Ballana E., Carter K., Durantel D., Gentry B., Janeba Z., Moffat J., Oomen C.J., Tarbet B., Riveira-Munoz E., Este J.A., International Society for Antiviral R. Meeting report: 31(st) international conference on antiviral research. Antivir. Res. 2018;158:88–102. doi: 10.1016/j.antiviral.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W.J., Prichard M.N. New therapies for human cytomegalovirus infections. Antivir. Res. 2018;159:153–174. doi: 10.1016/j.antiviral.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Caskey M., Klein F., Nussenzweig M.C. Broadly neutralizing anti-HIV-1 monoclonal antibodies in the clinic. Nat. Med. 2019;25:547–553. doi: 10.1038/s41591-019-0412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M.S., Hincks J.R., Benschop K., Duizer E., van der Avoort H., Rhoden E., Liu H., Oberste M.S., McKinlay M.A., Hartford M. Antiviral activity of pocapavir in a randomized, blinded, placebo-controlled human oral poliovirus vaccine challenge model. J. Infect. Dis. 2017;215:335–343. doi: 10.1093/infdis/jiw542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash P.K., Kaminski R., Bella R., Su H., Mathews S., Ahooyi T.M., Chen C., Mancuso P., Sariyer R., Ferrante P., Donadoni M., Robinson J.A., Sillman B., Lin Z., Hilaire J.R., Banoub M., Elango M., Gautam N., Mosley R.L., Poluektova L.Y., McMillan J., Bade A.N., Gorantla S., Sariyer I.K., Burdo T.H., Young W.-B., Amini S., Gordon J., Jacobson J.M., Edagwa B., Khalili K., Gendelman H.E. Sequential LASER ART and CRISPR treatments eliminate HIV-1 in a subset of infected humanized mice. Nat. Commun. 2019 doi: 10.1038/s41467-019-10366-y. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag H.B., Rudolph K.L. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- Gammon D.B., Evans D.H. The 3'-to-5' exonuclease activity of vaccinia virus DNA polymerase is essential and plays a role in promoting virus genetic recombination. J. Virol. 2009;83:4236–4250. doi: 10.1128/JVI.02255-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S.C., Lamerato L.E., Rupp L.B., Li J., Holmberg S.D., Moorman A.C., Spradling P.R., Teshale E.H., Vijayadeva V., Boscarino J.A., Henkle E.M., Oja-Tebbe N., Lu M., Investigators C.H. Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a US population. Clin. Gastroenterol. Hepatol. 2014;12:885–893. doi: 10.1016/j.cgh.2013.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F., Zhao Q., Sheraz M., Cheng J., Qi Y., Su Q., Cuconati A., Wei L., Du Y., Li W., Chang J., Guo J.T. HBV core protein allosteric modulators differentially alter cccDNA biosynthesis from de novo infection and intracellular amplification pathways. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde S.T., Salje H., Sazzad H.M.S., Hossain M.J., Rahman M., Daszak P., Klena J.D., Nichol S.T., Luby S.P., Gurley E.S. Using healthcare-seeking behaviour to estimate the number of Nipah outbreaks missed by hospital-based surveillance in Bangladesh. Int. J. Epidemiol. 2019 doi: 10.1093/ije/dyz057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy S.M. Tecovirimat: first global approval. Drugs. 2018;78:1377–1382. doi: 10.1007/s40265-018-0967-6. [DOI] [PubMed] [Google Scholar]
- Julien O., Beadle J.R., Magee W.C., Chatterjee S., Hostetler K.Y., Evans D.H., Sykes B.D. Solution structure of a DNA duplex containing the potent anti-poxvirus agent cidofovir. J. Am. Chem. Soc. 2011;133:2264–2274. doi: 10.1021/ja109823e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin D.W., Acosta E.P., Sanchez P.J., Sood S., Agrawal V., Homans J., Jacobs R.F., Lang D., Romero J.R., Griffin J., Cloud G.A., Lakeman F.D., Whitley R.J., National Institute of A., Infectious Diseases Collaborative Antiviral Study G. Pharmacokinetic and pharmacodynamic assessment of oral valganciclovir in the treatment of symptomatic congenital cytomegalovirus disease. J. Infect. Dis. 2008;197:836–845. doi: 10.1086/528376. [DOI] [PubMed] [Google Scholar]
- Kimberlin D.W., Jester P.M., Sanchez P.J., Ahmed A., Arav-Boger R., Michaels M.G., Ashouri N., Englund J.A., Estrada B., Jacobs R.F., Romero J.R., Sood S.K., Whitworth M.S., Abzug M.J., Caserta M.T., Fowler S., Lujan-Zilbermann J., Storch G.A., DeBiasi R.L., Han J.Y., Palmer A., Weiner L.B., Bocchini J.A., Dennehy P.H., Finn A., Griffiths P.D., Luck S., Gutierrez K., Halasa N., Homans J., Shane A.L., Sharland M., Simonsen K., Vanchiere J.A., Woods C.R., Sabo D.L., Aban I., Kuo H., James S.H., Prichard M.N., Griffin J., Giles D., Acosta E.P., Whitley R.J., National Institute of A., Infectious Diseases Collaborative Antiviral Study G. Valganciclovir for symptomatic congenital cytomegalovirus disease. N. Engl. J. Med. 2015;372:933–943. doi: 10.1056/NEJMoa1404599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin D.W., Lin C.Y., Sanchez P.J., Demmler G.J., Dankner W., Shelton M., Jacobs R.F., Vaudry W., Pass R.F., Kiell J.M., Soong S.J., Whitley R.J., National Institute of A., Infectious Diseases Collaborative Antiviral Study G. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J. Pediatr. 2003;143:16–25. doi: 10.1016/s0022-3476(03)00192-6. [DOI] [PubMed] [Google Scholar]
- Lim S.P. Dengue drug discovery: progress, challenges and outlook. Antivir. Res. 2019;163:156–178. doi: 10.1016/j.antiviral.2018.12.016. [DOI] [PubMed] [Google Scholar]
- Magee W.C., Aldern K.A., Hostetler K.Y., Evans D.H. Cidofovir and (S)-9-[3-hydroxy-(2-phosphonomethoxy)propyl]adenine are highly effective inhibitors of vaccinia virus DNA polymerase when incorporated into the template strand. Antimicrob. Agents Chemother. 2008;52:586–597. doi: 10.1128/AAC.01172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee W.C., Hostetler K.Y., Evans D.H. Mechanism of inhibition of vaccinia virus DNA polymerase by cidofovir diphosphate. Antimicrob. Agents Chemother. 2005;49:3153–3162. doi: 10.1128/AAC.49.8.3153-3162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee W.C., Valiaeva N., Beadle J.R., Richman D.D., Hostetler K.Y., Evans D.H. Inhibition of HIV-1 by octadecyloxyethyl esters of (S)-[3-hydroxy-2-(phosphonomethoxy)propyl] nucleosides and evaluation of their mechanism of action. Antimicrob. Agents Chemother. 2011;55:5063–5072. doi: 10.1128/AAC.05161-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz M., Gettie A., St Bernard L., Andrews C.D., Mohri H., Horowitz A., Grasperge B.F., Blanchard J.L., Niu T., Sun L., Fillgrove K., Hazuda D.J., Grobler J.A. Title: once-weekly oral dosing of MK-8591 protects male rhesus macaques from intrarectal SHIV109CP3 challenge. J. Infect. Dis. 2019 doi: 10.1093/infdis/jiz271. [DOI] [PubMed] [Google Scholar]
- Matthews R.P., Schürmann D., Rudd D.J., Levine V., Fox-Bosetti S., Zhang S., Robberechts M., Huser A., Hazuda D.J., Iwamoto M. 9th International AIDS Society Conference on HIV Science. 2007. Single doses as low as 0.5 mg of the novel NRTTI MK-8591 suppress HIV for at least seven days. [Google Scholar]
- Mendoza P., Gruell H., Nogueira L., Pai J.A., Butler A.L., Millard K., Lehmann C., Suarez I., Oliveira T.Y., Lorenzi J.C.C., Cohen Y.Z., Wyen C., Kummerle T., Karagounis T., Lu C.L., Handl L., Unson-O'Brien C., Patel R., Ruping C., Schlotz M., Witmer-Pack M., Shimeliovich I., Kremer G., Thomas E., Seaton K.E., Horowitz J., West A.P., Jr., Bjorkman P.J., Tomaras G.D., Gulick R.M., Pfeifer N., Fatkenheuer G., Seaman M.S., Klein F., Caskey M., Nussenzweig M.C. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature. 2018;561:479–484. doi: 10.1038/s41586-018-0531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailidis E., Huber A.D., Ryan E.M., Ong Y.T., Leslie M.D., Matzek K.B., Singh K., Marchand B., Hagedorn A.N., Kirby K.A., Rohan L.C., Kodama E.N., Mitsuya H., Parniak M.A., Sarafianos S.G. 4'-Ethynyl-2-fluoro-2'-deoxyadenosine (EFdA) inhibits HIV-1 reverse transcriptase with multiple mechanisms. J. Biol. Chem. 2014;289:24533–24548. doi: 10.1074/jbc.M114.562694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouquet H. Antibody B cell responses in HIV-1 infection. Trends Immunol. 2014;35:549–561. doi: 10.1016/j.it.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Nikolay B., Salje H., Hossain M.J., Khan A., Sazzad H.M.S., Rahman M., Daszak P., Stroher U., Pulliam J.R.C., Kilpatrick A.M., Nichol S.T., Klena J.D., Sultana S., Afroj S., Luby S.P., Cauchemez S., Gurley E.S. Transmission of Nipah virus - 14 Years of investigations in Bangladesh. N. Engl. J. Med. 2019;380:1804–1814. doi: 10.1056/NEJMoa1805376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaszczyca A., Scaturro P., Neufeldt C.J., Cortese M., Cerikan B., Ferla S., Brancale A., Pichlmair A., Bartenschlager R. A novel interaction between dengue virus nonstructural protein 1 and the NS4A-2K-4B precursor is required for viral RNA replication but not for formation of the membranous replication organelle. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seley-Radtke K.L., Yates M.K. The evolution of nucleoside analogue antivirals: a review for chemists and non-chemists. Part 1: early structural modifications to the nucleoside scaffold. Antivir. Res. 2018;154:66–86. doi: 10.1016/j.antiviral.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillman B., Bade A.N., Dash P.K., Bhargavan B., Kocher T., Mathews S., Su H., Kanmogne G.D., Poluektova L.Y., Gorantla S., McMillan J., Gautam N., Alnouti Y., Edagwa B., Gendelman H.E. Creation of a long-acting nanoformulated dolutegravir. Nat. Commun. 2018;9:443. doi: 10.1038/s41467-018-02885-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J., Reeves M. The intimate relationship between human cytomegalovirus and the dendritic cell lineage. Front. Microbiol. 2014;5:389. doi: 10.3389/fmicb.2014.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbouriech N., Ducournau C., Hutin S., Mas P.J., Man P., Forest E., Hart D.J., Peyrefitte C.N., Burmeister W.P., Iseni F. The vaccinia virus DNA polymerase structure provides insights into the mode of processivity factor binding. Nat. Commun. 2017;8:1455. doi: 10.1038/s41467-017-01542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsarkin K.A., Vanlandingham D.L., McGee C.E., Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3 doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanchiere J.A., Ruiz J.C., Brady A.G., Kuehl T.J., Williams L.E., Baze W.B., Wilkerson G.K., Nehete P.N., McClure G.B., Rogers D.L., Rossi S.L., Azar S.R., Roundy C.M., Weaver S.C., Vasilakis N., Simmons J.H., Abee C.R. Experimental Zika virus infection of neotropical primates. Am. J. Trop. Med. Hyg. 2018;98:173–177. doi: 10.4269/ajtmh.17-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vere Hodge R.A. Meeting report: 26th international conference on antiviral research. Antivir. Res. 2013;100:276–285. doi: 10.1016/j.antiviral.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vere Hodge R.A. Meeting report: 27th International conference on antiviral research, in Raleigh, NC, USA. Antivir. Res. 2014;111:143–153. doi: 10.1016/j.antiviral.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vere Hodge R.A. Meeting report: 28th international conference on antiviral research in rome, Italy. Antivir. Res. 2015;123:172–187. doi: 10.1016/j.antiviral.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vere Hodge R.A. Meeting report: 29th international conference on antiviral research in La Jolla, CA, USA. Antivir. Res. 2017;137:23–40. doi: 10.1016/j.antiviral.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrancken R., Haegeman A., Dewulf J., Paeshuyse J., Puerstinger G., Tignon M., Le Potier M.F., Neyts J., Koenen F. The reduction of CSFV transmission to untreated pigs by the pestivirus inhibitor BPIP: a proof of concept. Vet. Microbiol. 2009;139:365–368. doi: 10.1016/j.vetmic.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Vrancken R., Haegeman A., Paeshuyse J., Puerstinger G., Rozenski J., Wright M., Tignon M., Le Potier M.F., Neyts J., Koenen F. Proof of concept for the reduction of classical swine fever infection in pigs by a novel viral polymerase inhibitor. J. Gen. Virol. 2009;90:1335–1342. doi: 10.1099/vir.0.008839-0. [DOI] [PubMed] [Google Scholar]
- Willer D.O., Mann M.J., Zhang W., Evans D.H. Vaccinia virus DNA polymerase promotes DNA pairing and strand-transfer reactions. Virology. 1999;257:511–523. doi: 10.1006/viro.1999.9705. [DOI] [PubMed] [Google Scholar]
- Yang G., Pevear D.C., Davies M.H., Collett M.S., Bailey T., Rippen S., Barone L., Burns C., Rhodes G., Tohan S., Huggins J.W., Baker R.O., Buller R.L., Touchette E., Waller K., Schriewer J., Neyts J., DeClercq E., Jones K., Hruby D., Jordan R. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge. J. Virol. 2005;79:13139–13149. doi: 10.1128/JVI.79.20.13139-13149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates M.K., Seley-Radtke K.L. The evolution of antiviral nucleoside analogues: a review for chemists and non-chemists. Part II: complex modifications to the nucleoside scaffold. Antivir. Res. 2019;162:5–21. doi: 10.1016/j.antiviral.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T., Block T., Liu F., Kondratowicz A.S., Sun L., Rawat S., Branson J., Guo F., Steuer H.M., Liang H., Bailey L., Moore C., Wang X., Cuconatti A., Gao M., Lee A.C.H., Harasym T., Chiu T., Gotchev D., Dorsey B., Rijnbrand R., Sofia M.J. HBsAg mRNA degradation induced by a dihydroquinolizinone compound depends on the HBV posttranscriptional regulatory element. Antivir. Res. 2018;149:191–201. doi: 10.1016/j.antiviral.2017.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.