Abstract
Mosaic embryos have the potential to implant and develop into healthy babies. The transfer of mosaic embryos is now considered to be a possible option for women undergoing ART with preimplantation genetic testing for aneuploidies and in the absence of euploid embryos, particularly those with diminished ovarian reserve and/or advanced maternal age. It can aid in avoiding the discard of potentially viable embryos, which might otherwise result in healthy babies. In over 500 studies on mosaicism, there have been no reports of mosaicism in babies born following the transfer of mosaic embryos. Here, we present a case report of a 39-year-old woman with diminished ovarian reserve with only one blastocyst available for trophectoderm biopsy. The transfer of the embryo, which showed 35% mosaicism of monosomy 2, resulted in pregnancy. Amniocentesis revealed a mosaic trisomic mos46,XX(98)/47,XX,+2(2) karyotype. There were no pathological findings in detailed ultrasonography, and the fetus showed a normal fetal growth with no evidence of intrauterine growth retardation. A healthy female baby was born at Week 37. The peripheral blood chromosome analysis validated with fluorescence in situ hybridization showed 2% mosaic monosomy 2 [mos45,XX,-2(2)/46,XX(98)]. This is the first reported case of true fetal mosaicism resulting in a live birth following the transfer of a known mosaic embryo. Worldwide, prenatal diagnosis has shown the depletion of mosaicism in embryos transferred after they have been reported as mosaics. Our case demonstrates the need for close prenatal monitoring and diagnosis by early amniocentesis, preferably at >14 weeks gestation.
Keywords: preimplantation genetic testing, mosaic embryo transfer, next-generation sequencing, true fetal mosaicism, baby with mosaicism
Introduction
Chromosomal mosaicism is defined as two or more distinct cell lines within an embryo and is a relatively common finding in IVF-derived human embryos. Mosaicism arises from mitotic errors occurring after fertilization, usually after the first three cleavage divisions (Baart et al., 2006; Fragouli et al. 2011; Taylor et al., 2014). The incidence of mosaicism in trophectoderm biopsies is variously reported as between 4 and 32% (Fragouli et al., 2011; Capalbo et al., 2013; Fragouli et al., 2017; Harton et al., 2017; Vera-Rodriguez and Rubio, 2017). With the introduction of next-generation sequencing (NGS), it is now possible to detect chromosomal mosaicism at levels between 20 and 80%. The term mosaicism covers a broad range. The extent (low, moderate or high) and the type of mosaicism (segmental, single chromosome, double chromosome or complex mosaicism) may affect ART success rates. Also, the percentage of mosaicism in the biopsied sample may not always be a direct indication of the percentage of mosaicism in the whole embryo. Sub-chromosomal variations detected by NGS are mainly post-zygotic events, with high variation between different centers, and are not dependent on maternal age (Munné et al., 2002; McCoy et al., 2015).
The variables for the risk stratification of mosaic embryos have been defined as the percentage of mosaicism, specific chromosomes involved, monosomy versus trisomy and inclusion of complete or segmental chromosome mosaicism (Grati et al., 2018; Preimplantation Genetic Diagnosis International Society (PGDIS) Position Statement 2016, 2019; COGEN Position Statement 2017).
The impact of mosaicism on implantation and the developmental potential of embryos is not fully known, but it is thought that the mosaicism is likely to influence the implantation rate. The percentage of abnormal cells within a euploid/aneuploid mosaic embryo is influenced by the cleavage stage at which the chromosomal segregation error occurs. For example, errors occurring at the time of the second cleavage may result in a greater proportion of abnormal cells than errors occurring during the third cleavage (Spinella et al., 2018). Higher levels of mosaicism may decrease implantation potential and increase the risk of miscarriage. A statistically significant reduction in rates of clinical pregnancy/embryo transfer, implantation and live births compared to mosaic embryos with a lower aneuploidy percentage has been reported (Fragouli et al., 2017; Spinella et al., 2018).
Mosaic embryos have the potential to implant and develop into healthy babies. From centers worldwide, there is an increasing number of reports of live births following the transfer of mosaic embryos (Greco et al. 2015; Fragouli et al. 2017; Munné et al., 2017; Spinella et al., 2018; Victor et al., 2019). However, in most of these studies, there is a lack of information about the follow-up results during the prenatal and postnatal periods. Very few of these reports mention results of prenatal genetic tests, such as amniocentesis, or the karyotype of the resulting babies (Greco et al., 2015; Spinella et al., 2018; Victor et al., 2019).
One of the main problems in preimplantation genetic testing for aneuploidies (PGT-A) is the frequent occurrence of mosaicism, which may lead to difficulties in correctly diagnosing the true chromosomal status of the embryo. Although there are some reports in the literature proposing risk scoring systems for mosaic embryo transfers (COGEN Position Statement, 2017; Grati et al., 2018), it is still not known to what extent mosaicism in trophectoderm cells reflects true fetal mosaicism. This raises the question of which mosaic embryos can safely be transferred. This question has led the PGDIS to report Position Statements in 2016 and 2019 (PGDIS Position Statements, 2016, 2019). The purpose of these documents is to review current information and update recommendations regarding the transfer of mosaic embryos.
In natural pregnancies, mosaicism affects 2% of all gestations in the form of confined placental mosaicism (Taylor et al., 2014). There are very few reports of cases of mosaic monosomy of chromosome 2 either in natural pregnancies or in transferred embryos (Greco et al., 2015; Victor et al., 2019). Furthermore, the only paper to report live births with mosaic monosomy 2 did not report a confirmatory diagnostic amniocentesis or a peripheral karyotype analysis but confirmed a normal karyotype by chorionic villus sampling (CVS) in two cases of mosaic monosomy 2 (Greco et al., 2015).
As far as we know, our case is the first reported in the literature of a healthy live birth after the transfer of a mosaic monosomy 2 (35%) embryo presenting mosaic trisomy 2 in amniocentesis and true fetal mosaicism (2% mosaic monosomy 2) in the karyotype of the resulting baby.
Case report
Here we present the first report of a successful pregnancy and a healthy live birth with true fetal mosaicism of monosomy 2 after the transfer of an embryo with mosaic monosomy of chromosome 2. Ethical approval (2019/006) from the local ethical board and permission from the family was obtained prior to this report.
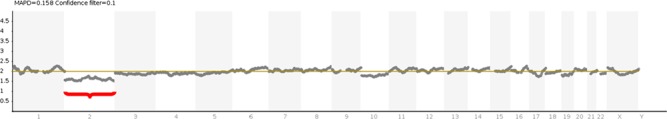
A couple with 2 years of infertility presented with advanced maternal age, poor ovarian reserve and normal female karyotype (46,XX). Anti-Mullerian Hormone level was 0.75 ng/ml, and antral follicle count showed few follicles in both ovaries. She was classified as having poor ovarian reserve according to the Bologna Criteria (Ferraretti et al., 2011). A short antagonist protocol was used, and a total 2025 IU dosage of recombinant FSH (Gonal F, Merck Serono, Switzerland) with a daily dosage of 225 IU was administered for 9 days. Ovulation was triggered with one ampoule recombinant hCG (rhCG: Ovitrelle®, Merck Serono, Switzerland). After controlled ovarian stimulation, only one follicle developed and only one oocyte was retrieved. This developed into a blastocyst which was biopsied. Before trophectoderm biopsy, on Day 3, a hole was made in the zona pellucida using a diode laser (RI Saturn 3, England). On Day 5 after fertilization, between five and eight cells were excised using a laser, without loss of the inner cell mass. One hour after the biopsy, blastocysts were vitrified using Kitazato vitrification media (Kitazato, Japan) according to the manufacturer’s instructions, using a Cryotop® as carrier. The biopsied material was prepared for DNA extraction and whole genome amplification (WGA) with a Ion Torrent Ion SingleSeq™ 96 kit (Thermo Fisher Scientific, USA). The NGS procedure was completed using the Ion Chef System (Thermo Fisher Scientific, USA) and Ion GeneStudio S5 (Thermo Fisher Scientific, USA), and the data was analyzed with Ion Reporter Software v5.6 (Thermo Fisher Scientific, USA). NGS revealed a mosaic monosomy 2 embryo with 35% mosaicism (Fig. 1). After PGT-A, the blastocyst, mosaic for the monosomy of chromosome 2, was thawed with Kitazato warming media according to manufacturer’s instructions.
Figure 1. The result of next-generation sequencing revealed 35% mosaicism of monosomy 2. No mosaicism was reported for chromosome 10 since the ratio of divergence from the baseline was below 20%.
Following genetic counseling and detailed discussion of the option of a new ART cycle, the couple decided to proceed with a mosaic embryo transfer. An informed consent form was signed by the couple. This included information regarding the risk of a mosaic embryo transfer and the necessity of close follow-up in the case of pregnancy. The frozen embryo transfer was performed after endometrial preparation using a modified natural cycle with rhCG trigger (Ovitrelle®, Merck Serono, Switzerland). Vaginal progesterone gel with 90 mg (8%) Crinone® (Merck Serono, Switzerland) administered once a day after rHCG as a luteal phase support. The mosaic embryo transfer resulted in a pregnancy.
An amniocentesis was performed in the 17th week of the pregnancy. Amniocyte culture was performed using standard methods (Howe, et al. 2014). The karyotype was observed to be mos46,XX(98)/47,XX,+2(2) in two separate culture flasks (Fig. 2). Fluorescence in situ hybridization (FISH) analysis of amniocytes was also performed to confirm the mosaic karyotype result (Fig. 3).
Figure 2.
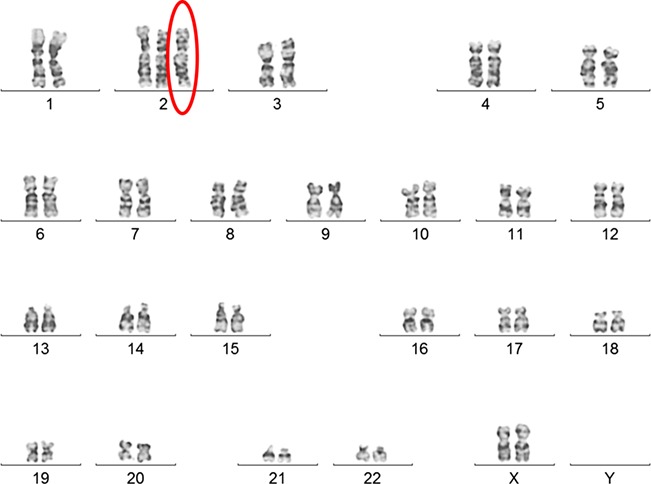
Two different cultures revealed a mos 46,XX(98)/47,XX,+2(2) karyotype.
Figure 3. Fluorescence in situ hybridization analysis of amniocytes. Red dots represent the centromeric probe and green represents telomeric probe for chromosome 2. After fluorescence in situ hybridization (FISH), the right picture shows two cells, one diploid normal and the other trisomic. The left picture shows one normal cell and three trisomic cells in the same frame (Vysis CEP2 SpectrumOrange, Vysis Tel2p SpectrumGreen, Abbott Molecular Inc. USA).
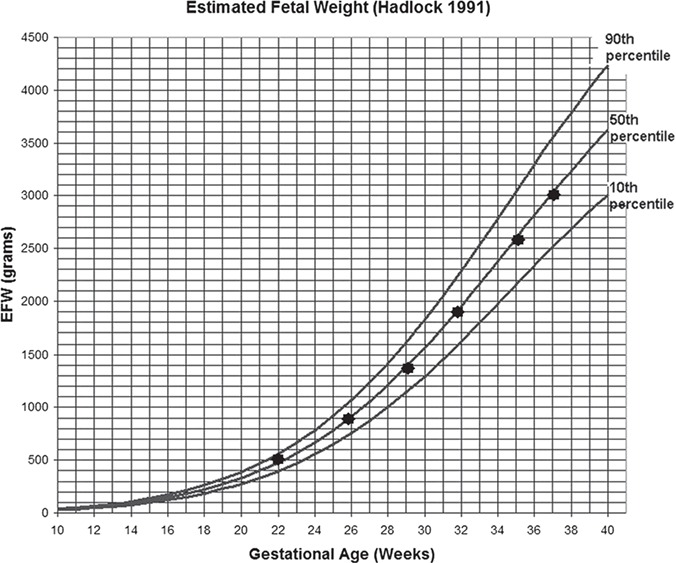
After comprehensive counseling with the team comprising an IVF specialist, perinatologist and geneticist, the couple decided to continue their pregnancy to term. The development of the fetus was monitored very closely, and the couple was extensively counseled. Counseling included the possibility of intrauterine growth retardation (IUGR). There were no pathological findings in detailed ultrasonography at Week 20 of pregnancy. There was no sign of limb, genitourinary, craniofacial, cardiac, spinal or renal abnormalities or hydronephrosis. The fetus showed normal growth around the 50th percentile in the ultrasonographic follow-up with no signs of fetal growth retardation (Fig. 4). After early rupture of the membranes, a healthy 2880-g, female baby 48 cm in length and with a 34.5-cm head circumference with no phenotypic abnormalities was born at Week 37 of gestation.
Figure 4. Intrauterine fetal growth chart. The black dots represent the estimated fetal birthweight calculations of the fetus in the corresponding week of gestation.
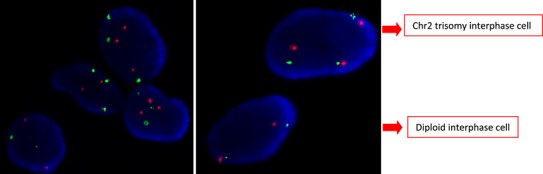
The peripheral karyotype was found to be 2% mosaic monosomy 2 [mos45,XX,-2(2)/46,XX(98)] (Fig. 5). Once again, this result was validated using FISH analysis, this time of 400 interphase nuclei (Fig. 6). A buccal smear sample from the baby, analyzed using FISH, showed no monosomy or trisomy in the 250 cells evaluated (Fig. 7).
Figure 5. The Giemsa banding from peripheral blood lymphocytes of the newborn. (A) 45,XX,-2, (B) 46,XX from the same sample.
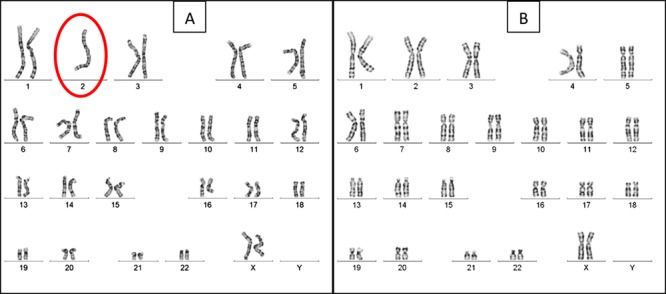
Figure 6. FISH analysis of chromosome 2 monosomic peripheral blood lymphocytes of the newborn. Red dots represent the centromeric probe and green represents telomeric probe for chromosome 2 (Vysis CEP2 SpectrumOrange, Vysis Tel2p SpectrumGreen, Abbott Molecular Inc. USA).
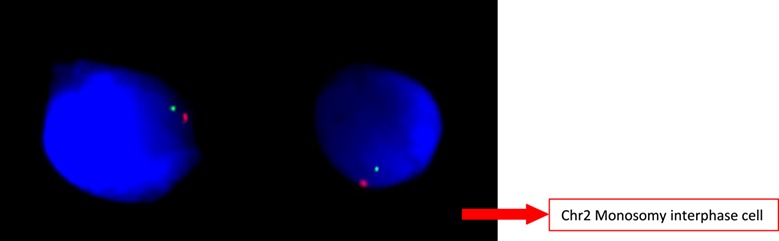
Figure 7. FISH analysis of the buccal smear. Red dots represent the centromeric probe and green represents telomeric probe for chromosome 2. Both pictures show diploid normal cells (Vysis CEP2 SpectrumOrange, Vysis Tel2p SpectrumGreen, Abbott Molecular Inc. USA).
Discussion
This case is unique; we present a case report of a 39-year-old woman with diminished ovarian reserve with only one blastocyst available for trophectoderm biopsy. The transfer of the embryo, which showed 35% mosaicism of monosomy 2, resulted in pregnancy. Amniocentesis revealed a mosaic trisomic [mos46,XX(98)/47,XX,+2(2)] karyotype. There were no pathological findings in detailed ultrasonography and the fetus showed a normal fetal growth with no evidence of IUGR. A healthy female baby was born at Week 37. The peripheral blood chromosome analysis validated with FISH showed 2% mosaic monosomy 2 [mos 45,XX,-2(2)/46,XX(98)].
Although in the literature there are cases with variable phenotypic abnormalities associated with prenatally detected mosaic trisomy 2 or non-mosaic partial deletions of chromosome 2 (Sifakis et al., 2004; Falk and Casas, 2007; Chen et al., 2012; Leroy et al., 2013; Fisch et al., 2016; Tuğ et al., 2017), as far as we know no case of mosaic monosomy 2 has ever been reported in a live birth after the transfer of a known mosaic embryo.
Chromosome 2 is the second largest human chromosome, containing 1200–1300 genes (Genetics Home Reference). Partial deletions of this chromosome have been linked to a variety of syndromes causing intellectual disability, skeletal or craniofacial abnormalities and developmental delays (Falk and Casas, 2007; Leroy et al., 2013; Fisch et al., 2016). A wide spectrum of limb, genitourinary, craniofacial, cardiac and spinal abnormalities, hydronephrosis and fetal growth restriction have been reported in the ultrasonographic follow-up of cases of prenatally detected mosaic aneuploidies of chromosome 2 (Sifakis et al., 2004; Chen et al., 2012; Tuğ et al., 2017).
The follow-up through gestation went uneventfully with no signs of morphological abnormalities. The amniotic fluid volume and intrauterine growth was around the 50th percentile with no signs of oligohydramnios or fetal growth restriction. There was also no sign of morphological abnormalities in the newborn.
Grati et al. (2018) have published CVS results in natural pregnancies, showing to what extent a mosaicism (aneuploid/normal) detected in the cytotrophoblast is likely to be found in the fetus. Accordingly, they devised a risk scoring system for prioritizing mosaic embryos by comparing the results of CVS with further amniocentesis to assess the incidence of true fetal mosaicism. They also conducted a uniparental disomy investigation reviewing mosaic aneuploidies likely to be associated with miscarriage. It was concluded that mosaicism involving chromosomes 13, 14, 15, 16, 18 and 21 and monosomy X were all high risk. Eight trisomy 2 abnormal cell lines and eight confined placental mosaicisms with no true fetal mosaicism or fetal involvement were identified. In products of conception in three trisomy 2 cases, the percentage of mosaicism was 5%. Furthermore, an evidence-based scoring system for prioritizing mosaic embryos for transfer following PGT noted two cases of miscarriage (Grati et al., 2018).
Three main mechanisms are proposed in mosaicism of the human embryos: anaphase lagging, mitotic non-disjunction and endoreplication. However, the effect of each on the chromosomal constitution of the cells is different, thus leading to a distinct aneuploidy in the blastocyst. In anaphase lagging, a single chromatid fails to incorporate in the nucleus and, as a result, one daughter cell will be disomic whereas the other will be monosomic for this ‘lagging’ chromosome since it is lost in the process. The blastocyst derived from this embryo will therefore be mosaic, with two distinct cell lines, one disomic and one monosomic. In the mitotic non-disjunction event, the sister chromatids fail to separate during mitosis, creating two distinct cell lines, one with a monosomy and another with a trisomy of the chromosome which has failed to split. The incidence of this type of mitotic error in embryos is not known but is believed to be dependent on the embryo developmental stage (Taylor et al., 2014): interestingly, sex chromosome malsegregation in early cleavages is mainly caused by this type of error (Bean et al., 2001, 2002). Finally, endoreplication occurs when a chromosome replicates itself, but this replication is not followed by a cell division. As a result, this cell gains one extra chromosome, thus becoming trisomic, whereas the adjacent cells remain disomic. Endoreplication is thought to be mainly due to cell cycle checkpoint failures (Taylor et al., 2014).
The only mechanism out of these three main causes of mosaicism that can lead to the occurrence of both monosomic and trisomic cell lines for a specific chromosome is the non-disjunction error. A mitotic non-disjunction error taking place during the cleavage stage and before the differentiation of the inner cell mass and the trophoblast could explain the mirroring mosaicisms observed in our patient for chromosome 2. The trophectoderm biopsy was mosaic for the monosomy of chromosome 2, whereas the amniocentesis revealed a mosaic trisomy 2. Finally, the peripheral blood chromosome analysis was mosaic for monosomy 2 postnatally, and the epithelial cells in a buccal smear were diploid.
The lower ratio of mosaicism observed in amniocentesis and the peripheral blood chromosome analysis when compared to the trophectoderm biopsy may be the result of one of the two following proposed mechanisms: the preferential localization of abnormal cells in trophectoderm or the preferential growth of euploid cells (Harton et al., 2017). The placenta was not available for testing.
The prioritization of mosaic embryos is not very straightforward. There are several hypotheses regarding the declining incidence of mosaicism from the cleavage to blastocyst stages of preimplantation development: the embryonic mortality model, the clonal depletion model and the trisomic/monosomic rescue model. The embryonic mortality model indicates that embryos with high mosaicism or full aneuploidy do not survive to implant; however, fully euploid and low-level mosaic embryos have a chance to implant. In other words, the embryonic mortality models invoke selection against embryos based on the proportion of aneuploid cells. This is the most commonly accepted model for implantation of mosaic embryos, and it is based on the fact that the aneuploid cell lines produced after a mitotic error during cleavage do not survive and are lost during implantation, so the fetus is fully composed of euploid cells. The clonal depletion model describes apoptosis or reduced propagation of aneuploid cells within a mosaic embryo (Bolton et al., 2016). The trisomic/monosomic rescue model proposes that aneuploid cells can give rise to diploid cells through mitotic chromosome gain or loss, respectively. In other words, this shows the self-correction of cell lines that are initially aneuploid but which, through rescue mechanisms, evolve into euploid cells (McCoy, 2017).
Patient counseling regarding mosaic embryo transfer is extremely important. Detailed genetic counseling prior to the transfer of a mosaic embryo and referral to a perinatologist as well as an early amniocentesis at >14th weeks of gestation are strongly recommended. Considering the mechanisms of mosaicism, it must be remembered that discrepancies between embryo chromosomal status and prenatal or postnatal chromosomal evaluations, such as between monosomy and trisomy, can also occur.
Our case report illustrates that mosaicism in human embryos arising from a mitotic non-disjunction error leads to both monosomic and trisomic cell lines. However, because of the nature of the trophectoderm biopsy itself, regarding the random site of biopsy and the number of cells excised, the trisomy may not be observed. Therefore, when extrapolating the result of our case to the clinical dilemma of transferring mosaic embryos, the ‘safer’ choice of transferring mosaic monosomic embryos must be approached carefully. Additional reports and data including postpartum karyotype analysis of the newborns are necessary to provide conclusive decisions.
The transfer of mosaic embryos marks a new era in ART and future studies and reports of cases are needed to help guide clinicians to make safe decisions regarding mosaic embryos. Ideally, future studies would include analysis of more cells, such as placental tissue and skin cells in the postpartum period. PGT-A is widely used for a number of indications and with the introduction of NGS into the field and increased identification of mosaicism, clinicians require more informative data to guide them to make safe decisions when considering transfer of mosaic embryos. Meanwhile, clinicians are strongly advised to give comprehensive counseling to patients regarding the possible risks of mosaic embryo transfer, including true fetal mosaicism.
Authors’ roles
Semra Kahraman, Prof.: manuscript draft and editing, critical discussion. Murat Cetinkaya, MD, PhD: manuscript draft and editing, genetic data evaluation. Beril Yuksel, Assoc. Prof.: manuscript draft and editing. Mesut Yesil, MSc.: fish analyses. Caroline Pirkevi Cetinkaya, PhD: manuscript editing.
Funding
No external funding was received for this case report.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- Baart EB, Martini E, van den Berg I, Macklon NS, Galjaard RJ, Fauser BC, Van Opstal D. Preimplantation genetic screening reveals a high incidence of aneuploidy and mosaicism in embryos from young women undergoing IVF. Hum Reprod 2006 Jan;21(1):223–233. [DOI] [PubMed] [Google Scholar]
- Bean CJ, Hunt PA, Millie EA, Hassold TJ. Analysis of a malsegregating mouse Y chromosome: evidence that the earliest cleavage divisions of the mammalian embryo are non-disjunction-prone. Hum Mol Genet 2001 Apr 15;10(9):963–972. [DOI] [PubMed] [Google Scholar]
- Bean CJ, Hassold TJ, Judis L, Hunt PA. Fertilization in vitro increases non-disjunction during early cleavage divisions in a mouse model system. Hum Reprod 2002 Sep;17(9):2362–2367. [DOI] [PubMed] [Google Scholar]
- Bolton H, Graham SJL, Van der Aa N, Kumar P, Theunis K, Fernandez Gallardo E, Voet T, Zernicka-Goetz M. Mouse model of chromosome mosaicism reveals lineage-specific depletion of aneuploid cells and normal developmental potential. Nat Commun 2016 Mar 29;7:11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capalbo A, Wright G, Elliott T, Ubaldi FM, Rienzi L, Nagy ZP. FISH reanalysis of inner cell mass and trophectoderm samples of previously array-CGH screened blastocysts shows high accuracy of diagnosis and no major diagnostic impact of mosaicism at the blastocyst stage. Hum Reprod 2013 Aug;28(8):2298–2307. [DOI] [PubMed] [Google Scholar]
- Chen CP, Su YN, Chern SR, Chen YT, Wu PS, Su JW, Pan CW, Wang W. Mosaic trisomy 2 at amniocentesis: prenatal diagnosis and molecular genetic analysis. Taiwan J Obstet Gynecol 2012 Dec;51(4):603–611. [DOI] [PubMed] [Google Scholar]
- COGEN position statement on chromosomal mosaicism detected in preimplantation blastocyst biopsies 2017. Available at: https://ivf-worldwide.com/cogen/oep/publications/cogen-position-statement-on-chromosomal-mosaicism-detected-in-preimplantation-blastocyst-biopsies.html
- Falk RE, Casas KA. Chromosome 2q37 deletion: clinical and molecular aspects. Am J Med Genet C Semin Med Genet 2007 Nov 15;145C(4):357–371. Review. [DOI] [PubMed] [Google Scholar]
- Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L; ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod 2011 Jul;26(7):1616–1624. [DOI] [PubMed] [Google Scholar]
- Fisch GS, Falk RE, Carey JC, Imitola J, Sederberg M, Caravalho KS, South S. Deletion 2q37 syndrome: cognitive-behavioral trajectories and autistic features related to breakpoint and deletion size. Am J Med Genet A 2016 Sep;170(9):2282–2291. [DOI] [PubMed] [Google Scholar]
- Fragouli E, Alfarawati S, Daphnis DD, Goodall NN, Mania A, Griffiths T, Gordon A, Wells D. Cytogenetic analysis of human blastocysts with the use of FISH, CGH and aCGH: scientific data and technical evaluation. Hum Reprod 2011 Feb;26(2):480–490. [DOI] [PubMed] [Google Scholar]
- Fragouli E, Alfarawati S, Spath K, Babariya D, Tarozzi N, Borini A, Wells D. Analysis of implantation and ongoing pregnancy rates following the transfer of mosaic diploid-aneuploid blastocysts. Hum Genet 2017 Jul;136(7):805–819. [DOI] [PubMed] [Google Scholar]
- Genetics Home Reference Available at: https://ghr.nlm.nih.gov/chromosome/2
- Grati FR, Gallazzi G, Branca L, Maggi F, Simoni G, Yaron Y. An evidence-based scoring system for prioritizing mosaic aneuploid embryos following preimplantation genetic screening. Reprod Biomed Online 2018 Apr;36(4):442–449. [DOI] [PubMed] [Google Scholar]
- Greco E, Minasi MG, Fiorentino F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N Engl J Med 2015 Nov 19;373(21):2089–2090. [DOI] [PubMed] [Google Scholar]
- Harton GL, Cinnioglu C, Fiorentino F. Current experience concerning mosaic embryos diagnosed during preimplantation genetic screening. Fertil Steril 2017;107(5):1113–1119. [DOI] [PubMed] [Google Scholar]
- Howe B, Umrigar A, Tsien F. Chromosome preparation from cultured cells. J Vis Exp. 2014;(83):e50203. [DOI] [PMC free article] [PubMed]
- Leroy C, Landais E, Briault S, David A, Tassy O, Gruchy N, Delobel B, Grégoire MJ, Leheup B, Taine L, et al. The 2q37-deletion syndrome: an update of the clinical spectrum including overweight, brachydactyly and behavioural features in 14 new patients. Eur J Hum Genet 2013 Jun;21(6):602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy Mosaicism in preimplantation human embryos: when chromosomal abnormalities are the norm Trends Genet 2017; 33(7):448–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy RC, Demko ZP, Ryan A, Banjevic M, Hill M, Sigurjonsson S, Rabinowitz M, Petrov DA. Evidence of selection against complex mitotic-origin aneuploidy during preimplantation development. PLoS Genet 2015 Oct 22;11(10):e1005601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné S, Sandalinas M, Escudero T, Márquez C, Cohen J. Chromosome mosaicism in cleavage-stage human embryos: evidence of a maternal age effect. Reprod Biomed Online 2002;4(3):223–232. [DOI] [PubMed] [Google Scholar]
- Munné S, Blazek J, Large M, Martinez-Ortiz PA, Nisson H, Liu E, Tarozzi N, Borini A, Becker A, Zhang J, et al. Detailed investigation into the cytogenetic constitution and pregnancy outcome of replacing mosaic blastocysts detected with the use of high-resolution next-generation sequencing. Fertil Steril 2017 Jul;108(1):62–71.e8. [DOI] [PubMed] [Google Scholar]
- PGDIS PGDIS position statement on chromosome mosaicism and preimplantation aneuploidy testing at the blastocyst stage.2016. Available at: http://www.pgdis.org/docs/newsletter_071816.html.
- PGDIS Position Statement On The Transfer Of Mosaic Embryos In Preimplantatıon Genetic Testing For Aneuploidy (PGT-A)- Based on Materials of 18th International Conference On Preimplantatıon Genetics, Geneva, Switzerland, 2019. Available at:http://pgdis.org/docs/newsletter_052719.pdf [Google Scholar]
- Sifakis S, Velissariou V, Papadopoulou E, Petersen MB, Koumantakis E. Prenatal diagnosis of trisomy 2 mosaicism: a case report. Fetal Diagn Ther 2004;19(6):488–490. [DOI] [PubMed] [Google Scholar]
- Spinella F, Fiorentino F, Biricik A, Bono S, Ruberti A, Cotroneo E, Baldi M, Cursio E, Minasi MG, Greco E. Extent of chromosomal mosaicism influences the clinical outcome of in vitro fertilization treatments. Fertil Steril 2018 Jan;109(1):77–83. [DOI] [PubMed] [Google Scholar]
- Taylor TH, Gitlin SA, Patrick JL, Crain JL, Wilson JM, Griffin DK. The origin,mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum Reprod Update 2014;20(4):571–581. [DOI] [PubMed] [Google Scholar]
- Tuğ E, Karcaaltincaba D, Yirmibeş Karaoğuz M, Saat H, Özek A. Confirmation of the prenatal mosaic trisomy 2 via fetal USG and cytogenetic analyses. J Matern Fetal Neonatal Med 2017 Jul;30(13):1579–1583. [DOI] [PubMed] [Google Scholar]
- Vera-Rodriguez M, Rubio C. Assessing the true incidence of mosaicism in preimplantation embryos. Fertil Steril 2017 May;107(5):1107–1112. [DOI] [PubMed] [Google Scholar]
- Victor AR, Tyndall JC, Brake AJ, Lepkowsky LT, Murphy AE, Griffin DK, RC McCoy, Barnes FL, Zouves CG, Viotti M. One hundred mosaic embryos transferred prospectively in a single clinic: exploring when and why they result in healthy pregnancies. Fertil Steril 2019 Feb;111(2):280–293. [DOI] [PubMed] [Google Scholar]


