Abstract
Purpose:
A critical biological function of retina pigment epithelium (RPE) cells is phagocytosis of photoreceptor outer segment (POS) disc membranes. Mitochondrial damage and dysfunction are associated with RPE cells of age-related macular degeneration (AMD) retinas. In this study, we use a transmitochondrial cybrid model to compare the phagocytic properties of RPE cells that contain AMD mitochondria versus age-matched normal mitochondria, and their response to treatment with anti-vascular endothelial growth factor (VEGF) drugs: bevacizumab, ranibizumab, and aflibercept.
Methods:
Cybrids, which are cell lines with identical nuclei but mitochondria (mt) from different subjects, are created by fusing mtDNA depleted ARPE-19 cells with platelets from AMD or age-matched normal patients. AMD (n = 5) and normal (n = 5) cybrids were treated with 1 μm fluorescent latex beads (1.52 × 107 beads/mL) and either 2.09 μM of bevacizumab, 2.59 μM of ranibizumab, or 5.16 μM of aflibercept. These doses of anti-VEGF drugs are equivalent to intravitreal injections given to AMD patients with choroidal neovascularization. Flow cytometry was performed using the ImageStreamX Mark II to assess phagocytic bead-uptake. The average fold values for bead-uptake and SEM were calculated using GraphPad Prism software.
Results:
Normal cybrids showed decreased bead-uptake with a fold value of 0.65 ± 0.103 (p=0.001) after treatment with bevacizumab, 0.80 ± 0.034 (p=0.0003) with ranibizumab, and 0.81 ± 0.053 (p=0.007) with aflibercept compared to the untreated normal cybrids (baseline fold of 1). The bevacizumab-treated, ranibizumab-treated, and aflibercept-treated AMD cybrids had decreased bead-uptake with a fold value of 0.71 ± 0.061 (p=0.001), 0.70 ± 0.101 (p=0.02), and 0.74 ± 0.125 (p=0.07), respectively, compared to the untreated AMD cybrids (baseline fold of 1).
Conclusions:
Our initial findings showed that when treated with bevacizumab and ranibizumab, both AMD cybrids and age-matched normal cybrids had a significant decrease in bead-uptake. A similar decrease in bead-uptake was found in normal cybrids treated with aflibercept and while the AMD values trended lower, they were not significant. This data suggests that anti-VEGF drugs can cause loss of phagocytic function.
Keywords: Phagocytosis, latex beads, bevacizumab, ranibizumab, aflibercept, anti-VEGF, cybrids, retinal pigment epithelium, mitochondria, age-related macular degeneration
1. Introduction
Age-related macular degeneration (AMD) is a progressive retinal condition that causes damage to the macula, the region of the retina responsible for sharp, central vision. Two types of AMD exist. Dry AMD, which accounts for 90% of AMD patients, is characterized by the accumulation of cellular debris, called drusen, between the retinal and choroid layers. As AMD progresses to geographic atrophy (GA), significant loss of RPE cells and overlying photoreceptors can occur, resulting in decreased visual acuity. Wet AMD, which accounts for the approximately 10% of patients, involves the proliferation of abnormal choroidal blood vessels causing bleeding and scarring, resulting in damage to the macula. At this time, there are no proven treatments for dry AMD, whereas anti-angiogenic drugs exist for treating wet AMD.1 Researchers, however, are now concerned that extensive anti-VEGF treatment may increase the risk of progressing to GA.2,3
Mitochondria, organelles that function in energy production, metabolism, and nuclear signaling, have been studied as a key contributor in the development of AMD. In 2006, Feher et al. demonstrated through transmission microscopy that the mitochondria of RPE cells were damaged in AMD patients.4 In 2009, our lab found that AMD retinas are characterized by higher levels of damaged DNA and decreased mitochondrial function.5 It was observed that individuals with particular haplogroups, such as those with haplogroup J, are at higher risk for developing AMD whereas those with haplogroup H are protected. The transmitochondrial cybrid model, human RPE cells with identical nuclei but containing mitochondria from different individuals, was used to further investigate the role of mitochondria in AMD. This unique model allows for comparisons of gene expression, response to drugs, and even biological functions between individuals with different mtDNA haplogroups and disease states. Our previous studies have demonstrated that AMD cybrids behave differently from non-AMD cybrids with respect to levels of gene expression and bioenergetics.6–8 In other studies, molecular analyses of RPE cell mtDNA demonstrated a correlation between higher levels of mtDNA damage and increased AMD severity.9,10 Increased levels of mtDNA damage also lead to decreased cell viability and ultimately loss of biological functions, which includes phagocytic functions.
Phagocytosis of photoreceptor outer segments (POS) disc membranes is an important biological function of RPE cells. Lipofuscin N-retinylidene-N-retinylethanolamine (A2E) granules, which are photo-inducible generators of reaction oxygen species (ROS), can accumulate if RPE cells are unable to completely digest these discs and work synergistically with mitochondrial dysfunction to reduce phagocytic function.11,12 Researchers have argued that RPE phagocytosis plays a critical role in retinal health. Some have proposed that the failure of RPE cells to phagocytose POS disc membranes contributes to accumulation of drusen apically, atrophy of the subretinal retinal pigment layer, and loss of macular photoreceptors causing vision loss.13–16 This hypothesis is further supported by Zhao et al., who found in vitro, that RPE cells can migrate towards photoreceptors, resulting in translocation of non-phagocytosized debris from the apical side to the basal side.17 A separate study by Inana et al. (2018) demonstrated that RPE phagocytic function was indeed reduced in AMD.18
The current treatment of wet AMD is the anti-vascular endothelial growth factor (anti-VEGF) class of drugs. The effects of these drugs on the phagocytic properties of RPE cells in AMD patients have not been well characterized and are poorly understood.19–21 Three anti-VEGF drugs used regularly in the treatment of AMD are ranibizumab (Lucentis®), bevacizumab (Avastin®), and aflibercept (Eylea®). While their antagonistic targets are very similar, they differ in binding affinities, molecular sizes and weights, and their metabolic pathways in RPE cells. Klettner et al. describes that bevacizumab, but not ranibizumab, is taken up by RPE cells in vitro and stored in the cells.19–21 This may in turn, negatively affect the ability of the RPE cells to phagocytose POS disc membranes as more space is occupied by the phagocytosed drug resulting in a sense of “fullness”.21,22
In this study, we investigate the effects of bevacizumab, ranibizumab, and aflibercept on phagocytic properties of cybrids, human RPE cells with identical nuclei but containing either AMD mitochondria or age-matched normal mitochondria. . We hypothesize that while all three drugs target VEGF, their effects on the physiologic properties of RPE cells possessing AMD versus normal mitochondria may differ in vitro.
2. Materials and Methods
2.1. Cybrid cell culture
Institutional review board approval was obtained from the University of California, Irvine (#2003–3131). For this study, 10 ml of peripheral blood was collected via venipuncture in tubes containing 3.2% sodium citrate from AMD patients (n = 5) and age-matched non-AMD patients (n = 5). The baseline characteristics of the subjects are presented in Table 1. The platelets were isolated through a series of centrifugation steps and the final pellet suspended in Tris buffer saline. ARPE-19 cells, a cell line derived from human retinal pigment epithelia (ATCC, Manassa, VA, USA), are frequently used in retinal research due to similar structural and functional properties to RPE cells. The ARPE-19 cells were converted to Rho0 (deficient of mtDNA) by serial passage in low-dose ethidium bromide.23 The cybrids were produced by polyethylene glycol fusion of platelets with Rho0 ARPE-19 cells based on a modified procedure of Chomyn.24 The resulting cytoplasmic hybrids (cybrids) have unique mtDNA but shared nuclear DNA.
Table 1.
Baseline characteristics of patients from which the cybrids were created.
| AMD Cybrids (n = 5) | Normal Cybrids (n = 5) | |
|---|---|---|
| Average Age | 83.4 (range, 75–90) | 72.6 (range, 69–78) |
| Male | 1 | 2 |
| Female | 4 | 3 |
| Caucasian | 5 | 5 |
| Wet AMD | 5 | N/A |
| Dry AMD | 0 | N/A |
Cybrid cells were cultured in cybrid media containing a mixture of DMEM/F12 (Mediatech/Cellgro), with 10% fetal bovine serum, 1% penicillin G, 1% fungizone, and 0.1% gentamicin. Cybrids were then incubated at 37° C in a humidified environment (relative humidity 85%) consisting of 5% CO2 and 95% air. Cybrid media were changed every other day to remove any non-viable cells. If the cells reached 90% confluence, they were passaged by dissociation in 0.05% trypsin/0.02% EDTA and re-plated at a split ratio of either 1:3 or 1:4. Only cells from the fifth passage were used for experimentation. Cybrid cells were plated evenly onto 6-well plates at a density of 500,000 cells per well and allowed to incubate for 24 hours at 37° C before initiating treatments. Experiments were performed in duplicate.
2.2. Treatment of cybrids with ranibizumab, bevacizumab, and aflibercept
Following the initial 24 hour incubation, cells were treated with cybrid media solutions containing either 2.59 μM of ranibizumab, 2.09 μM of bevacizumab, or 5.16 μM of aflibercept. These doses of anti-VEGF drugs are equivalent to intravitreal injections given to AMD patients with choroidal neovascularization. The cybrids were then incubated for a second 24hour interval at 37° C before addition of the fluorescent latex beads.
2.3. Incubation of cybrids with latex beads
The use of fluorescent latex beads to emulate POS disc membranes is based on a modified protocol from Boochoon et al.14 The latex bead solution was created by adding 3.33 μL of yellow-green latex fluorescent beads (Polysciences Inc, diameter 1-μm, initially packaged as a 2.5% aqueous suspension) for every 10 mL of the anti-VEGF cybrid media described above. This solution reduces the stock concentration of 4.55 × 1010 beads/mL to a working concentration of 1.52 × 107 beads/mL. 2 mL of the solution containing beads was added to each well. Once cultures were treated with the fluorescent beads, care was taken to avoid light exposure to minimize any possible photo-bleaching. Following 24 hour incubation at 37° C and 5% CO2, the cells were washed 3 times with PBS-EDTA to remove unbound beads and then detached using 0.05% trypsin/0.02% EDTA. Cells were washed twice with cybrid media before being passed through a 35 μm nylon mesh of a Corning Falcon Test Tube (Thermo Fisher Scientific, Waltham, MA) to remove debris. Samples were then centrifuged at 1000 RPM for 5 minutes and resuspended in 50 μl of PBS-EDTA for flow cytometry.
2.4. Flow cytometry
The yellow-green beads use a spectrum with an excitation max of 441 nm and emission max of 486 nm. Flow cytometry was performed with the ImageStreamX Mark II Imaging Flow Cytometer (EMD Millapore), which offers real time photo acquisition of cells at 60x magnification (Figure 1). The combination of photos and data collected allow accurate assessment of bead internalization for thousands of cells.
Fig. 1.

Representative images of one cybrid cell line taken with the ImageStreamX Mark II demonstrating internalization of the fluorescent latex beads (colored green). These images are then used by the ImageStream X software for analysis of bead uptake.
2.5. Statistical analysis
The ImageStream X software and GraphPad Prism 7.0 (GraphPad Software Inc., La Jolla, CA) were used to process the data gathered from flow cytometry. To determine the gated population that internalized the fluorescent latex beads, the ImageStream X software first uses the initial gated population, which consists of clumped and single RPE cells, to isolate the population of only single cells. After identifying the population of single cells, the population is gated to identify the population containing a measurable degree of yellow-green fluorescence. This population is once again gated to identify the population of cells that demonstrate internalization of the fluorescent latex bead within the RPE cells. This is achieved by “masking”; where the software precisely identifies the location of the bead in the photo relative to the position of the cell. The various levels of population gating and masking can be finely adjusted by the user with the ImageStream X software; ensuring accurate assessment of all cell samples. To ensure consistent analysis, the ImageStream X software offers batch analysis, allowing each experiment to be analyzed under identical parameters. The raw gated values for cells demonstrating internalization of beads were converted to a percentage of bead-uptake by comparing it to the raw gated values for the single cell population. This was done to help normalize the values across different cell lines. The bead-uptake fold was calculated by comparing the bead-uptake percentage in the treatment arms against the control of the same cell line. The GraphPad Prism software was used to calculate mean ± standard error of the mean (SEM) of the bead-uptake folds and to perform statistical testing. An unpaired t-test was used to compare the mean fold change between sample groups, with the controls set to a baseline fold of 1.00. Statistical significance was set to a cutoff of p < 0.05.
3. Results
The normal cybrids showed a bead-uptake fold value of 0.65 ± 0.103 (p = 0.001) after treatment with bevacizumab, 0.80 ± 0.034 (p = 0.0003) with ranibizumab, and 0.81 ± 0.053 (p = 0.007) with aflibercept compared to the untreated normal cybrids (Figure 2). The bevacizumab-treated, ranibizumab-treated, and aflibercept-treated AMD cybrids had a bead-uptake fold value of 0.71 ± 0.061 (p = 0.001), 0.70 ± 0.101, (p = 0.02), and 0.74 ± 0.125 (p = 0.07), respectively, compared to the untreated AMD cybrids (Figure 3). No statistically significant difference was found when comparing bead-uptake fold for AMD versus normal cybrids treated with bevacizumab (p = 0.65), ranibizumab (p = 0.39), and aflibercept (p = 0.60) (Figure 4).
Figure 2.

(a) Summary of bead-uptake fold for the age-matched normal cybrids when treated with bevacizumab, ranibizumab, and aflibercept compared to the untreated control normal cybrids. (NL, normal). (b) Illustration of bead-uptake fold for the AMD cybrids when treated with bevacizumab, ranibizumab, and aflibercept compared to the untreated control AMD cybrids. (AMD, age-related macular degeneration). * p < 0.05, ** p < 0.01, *** p < 0.001
Figure 3.
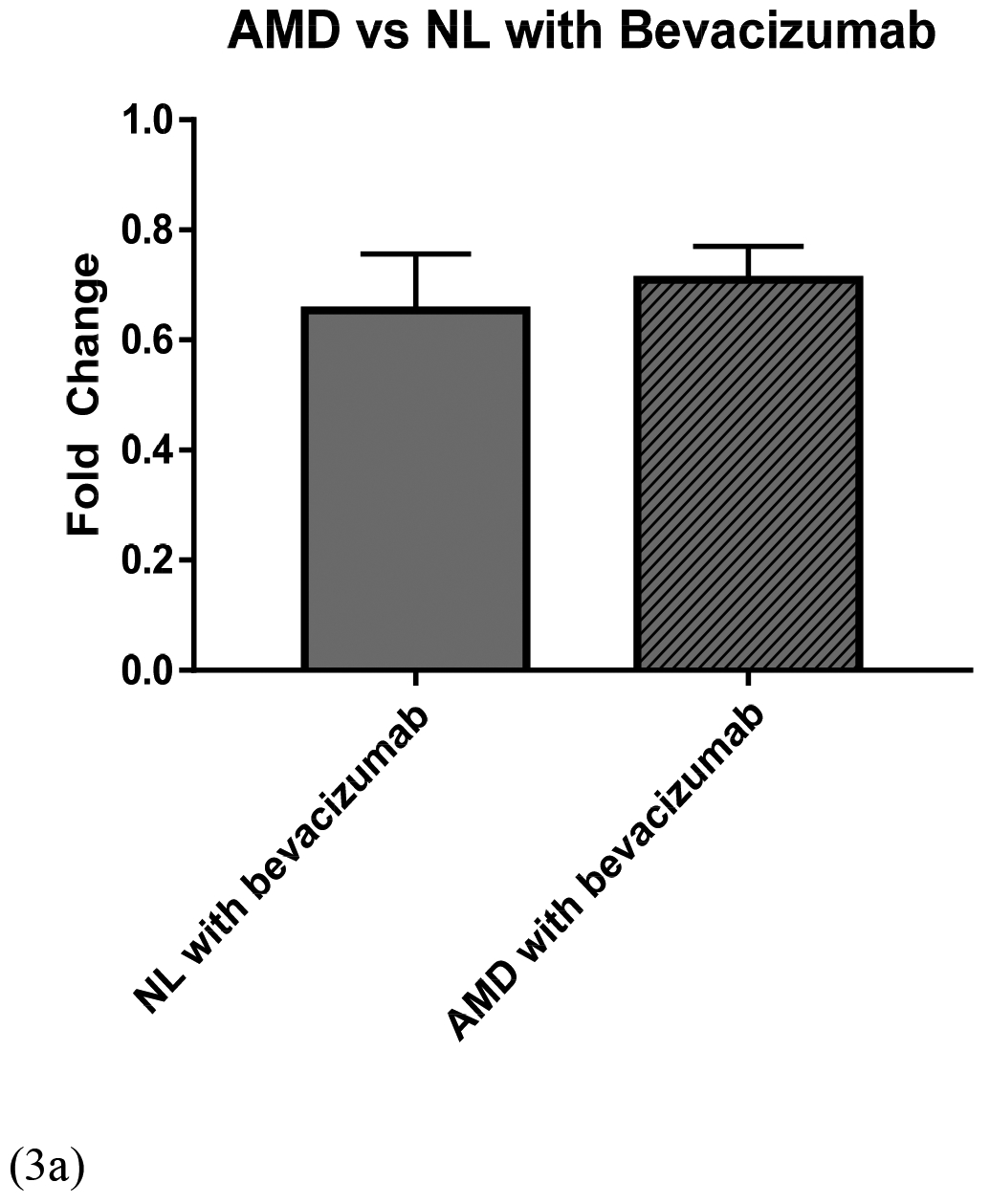

Comparison of bead-uptake fold for AMD versus age-matched normal (NL) cybrids when treated with (a) bevacizumab (p = 0.65), (b) ranibizumab (p = 0.39), and (c) aflibercept (p = 0.60). (NL, normal; AMD, age-related macular degeneration).
4. Discussion
Damage to the mitochondria and mtDNA play important roles in the development of AMD.4,5,9,10 As loss of cell vitality is increased, there is also impairment of essential biological functions. For example, impaired digestion of POS can result in lipofuscin granule accumulation and subsequently ROS that can damage RPE cells, attract macrophages, and thereby stimulate localized inflammation.25–29 These events in turn, can result in decreased phagocytic capacity.29 Some authors have argued that the insufficient phagocytosis of POS disc membranes contributes to the development of dry AMD through the accumulation of drusen and other processes that damage the retinal pigment layer.13,14,25
In our study, we observed decreased phagocytosis when both the AMD cybrids and age-matched normal cybrids were treated with bevacizumab and ranibizumab, with bevacizumab having the most prominent effect in the normal cybrids. A decrease in phagocytosis was also observed when the normal cybrids were treated with aflibercept. The observed decrease in phagocytosis with bevacizumab and aflibercept treatment is consistent with the findings by Klettner et al.21,30Although the underlying mechanisms for this observation are not fully understood, Klettner et al. suggests that the difference in phagocytic capacity maybe due to the pathway in which RPE cells process the drugs. Bevacizumab and aflibercept, but not ranibizumab, are taken up and stored by the RPE cells.19–21,30 This may contribute to RPE cells having less space available for the uptake of beads and an overall sense of “fullness”.21,22 Similarly, bevacizumab and aflibercept may utilize the receptors on the cell surfaces in a way that ranibizumab does not.21,30 Interestingly, Sheu et al. (2015) found that while bevacizumab negatively affected phagocytosis, ranibizumab and aflibercept did not. Sheu et al. also found that when RPE cells are pretreated with acrolein, an oxidative cytotoxic agent that lowers phagocytic activity, bevacizumab is protective in regards to maintaining the cell’s phagocytic capacity.28 The different results observed in these studies may be attributed to the different cell models (e.g., AMD cybrids versus primary porcine RPE cells) being tested or the varying methods being used to assess phagocytosis.
Ranjbar et al. showed that the Fc receptor (FcR) plays an important role in the uptake of bevacizumab but not aflibercept.31 When pre-treated with FcR inhibitors, increased levels of bevacizumab, but not aflibercept, were observed extracellularly, suggesting that FcR’s play a key role in uptake of bevacizumab. It is possible that if the uptake of fluorescent latex beads relies on the FcR, then bevacizumab may act as a competitive antagonist to the fluorescent beads. Naga et al. also studied the intracellular pathway for bevacizumab uptake in RPE cells and proposed a different pathway.32 Their data suggested an association between bevacizumab and Rab5, a GTPase involved in the formation, fusion, and sorting of early endosome that plays an important factor for phagosome formation in RPE cells.33–35 Their study additionally suggested that actin filaments via myosin7a may also play a role in the intracellular transport of the anti-VEGF agent.32 However, Naga et al. acknowledged that Rab5 may not be the only pathway involved in bevacizumab uptake. Less is understood about the exact pathways in which ranibizumab and aflibercept are processed by RPE cells. This lack of knowledge highlights the need for further studies as it may reveal if one anti-VEGF agent should be preferred over another given either its safety profile or uptake pathway.
We also suspect that the safety profiles of bevacizumab, ranibizumab, and aflibercept, which were previously investigated by our laboratory, play a role in the phagocytic changes observed.36 At clinical doses of intravitreal injections, bevacizumab produced mild mitochondrial toxicity, while neither ranibizumab nor aflibercept demonstrated such effects.36 Since mitochondria are responsible for energy production and metabolism, it is plausible that bevacizumab negatively effects phagocytosis through decreased mitochondrial bioenergetics.
The use of fluorescent latex beads provides an easy and rapid method for assessing the phagocytic activity of RPE cells, but several limitations should be noted. First, our study assessed what percentage of cells demonstrates internalization of beads and did not distinguish between the total numbers of beads phagocytosed by each cell. Secondly, the ImageStream X software does not differentiate between internalized beads versus beads attached to the cell surface and for that reason all cultures were washed repeatedly with PBS in order to minimize the chances that beads were just attached to cell surfaces.
5. Conclusion
Phagocytosis of photoreceptor outer segments (POS) is an important biological function of RPE cells and incomplete digestion of POS may contribute to the accumulation of harmful cellular debris, an event associated with development of AMD. The anti-VEGF drugs bevacizumab, ranibizumab, and aflibercept have been shown to be effective in treating patients with wet AMD. However, the effects that these drugs have on functional capacity of RPE cells are not fully understood and their biological pathways not well characterized. In this study, we examine three commonly used anti-VEGF agents and their effects on phagocytosis in cybrids (cell lines with identical nuclei but containing either AMD or normal mitochondria). Our findings suggest that when treated with anti-VEGF drugs, both AMD cybrids and age-matched normal cybrids have loss in phagocytic function.
Acknowledgements
This work was supported by the Research to Prevent Blindness (RPB) Medical Student Eye Research Fellowship (awarded to TAV) and the Gavin Herbert Eye Institute, which is an institutional RPB grant recipient. The research was supported by the Discovery Eye Foundation, Polly and Michael Smith, Iris and B. Gerald Cantor Foundation and Max Factor Family Foundation. Supported in part by an Unrestricted Grant from Research to Prevent Blindness to Gavin Herbert Eye Institute. We acknowledge the support of the Institute for Clinical and Translational Science (ICTS) at University of California Irvine.
Footnotes
Declarations of Interest: None
References
- 1.Singer M. Advances in the management of macular degeneration. F1000Prime Rep 2014;6 Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4017905/ [Accessed June 6, 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gemenetzi M, Lotery AJ, Patel PJ. Risk of geographic atrophy in age-related macular degeneration patients treated with intravitreal anti-VEGF agents. Eye 2017;31:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enslow R, Bhuvanagiri S, Vegunta S, et al. Association of Anti-VEGF Injections with Progression of Geographic Atrophy. Ophthalmol Eye Dis 2016;8:31–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feher J, Kovacs I, Artico M, et al. Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol Aging 2006;27:983–993. [DOI] [PubMed] [Google Scholar]
- 5.Udar N, Atilano SR, Memarzadeh M, et al. Mitochondrial DNA haplogroups associated with age-related macular degeneration. Invest Ophthalmol Vis Sci 2009;50:2966–2974. [DOI] [PubMed] [Google Scholar]
- 6.Nashine S, Chwa M, Kazemian M, et al. Differential Expression of Complement Markers in Normal and AMD Transmitochondrial Cybrids. PLOS ONE 2016;11:e0159828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenney MC, Chwa M, Atilano SR, et al. Inherited mitochondrial DNA variants can affect complement, inflammation and apoptosis pathways: insights into mitochondrial-nuclear interactions. Hum Mol Genet 2014;23:3537–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenney MC, Chwa M, Atilano SR, et al. Mitochondrial DNA variants mediate energy production and expression levels for CFH, C3 and EFEMP1 genes: implications for age-related macular degeneration. PloS One 2013;8:e54339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karunadharma PP, Nordgaard CL, Olsen TW, Ferrington DA. Mitochondrial DNA Damage as a Potential Mechanism for Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 2010;51:5470–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terluk MR, Kapphahn RJ, Soukup LM, et al. Investigating mitochondria as a target for treating age-related macular degeneration. J Neurosci Off J Soc Neurosci 2015;35:7304–7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wassell J, Davies S, Bardsley W, Boulton M. The photoreactivity of the retinal age pigment lipofuscin. J Biol Chem 1999;274:23828–23832. [DOI] [PubMed] [Google Scholar]
- 12.Vives-Bauza C, Anand M, Shirazi AK, et al. The Age Lipid A2E and Mitochondrial Dysfunction Synergistically Impair Phagocytosis by Retinal Pigment Epithelial Cells. J Biol Chem 2008;283:24770–24780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green WR. Histopathology of age-related macular degeneration. Mol Vis 1999;5:27. [PubMed] [Google Scholar]
- 14.Boochoon KS, Manarang JC, Davis JT, et al. The influence of substrate elastic modulus on retinal pigment epithelial cell phagocytosis. J Biomech 2014;47:3237–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W. Phagocyte dysfunction, tissue aging and degeneration. Ageing Res Rev 2013;12:1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzoni F, Safa H, Finnemann SC. Understanding photoreceptor outer segment phagocytosis: Use and utility of RPE cells in culture. Exp Eye Res 2014;0:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao L, Wang Z, Liu Y, et al. Translocation of the retinal pigment epithelium and formation of subretinal pigment epithelium deposit induced by subretinal deposit. Mol Vis 2007;13:873–880. [PMC free article] [PubMed] [Google Scholar]
- 18.Inana G, Murat C, An W, et al. RPE phagocytic function declines in age-related macular degeneration and is rescued by human umbilical tissue derived cells. J Transl Med 2018;16:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klettner AK, Kruse M-L, Meyer T, et al. Different properties of VEGF-antagonists: Bevacizumab but not Ranibizumab accumulates in RPE cells. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol 2009;247:1601–1608. [DOI] [PubMed] [Google Scholar]
- 20.Klettner A, Roider J. Comparison of bevacizumab, ranibizumab, and pegaptanib in vitro: efficiency and possible additional pathways. Invest Ophthalmol Vis Sci 2008;49:4523–4527. [DOI] [PubMed] [Google Scholar]
- 21.Klettner A, Möhle F, Roider J. Intracellular bevacizumab reduces phagocytotic uptake in RPE cells. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol 2010;248:819–824. [DOI] [PubMed] [Google Scholar]
- 22.Weisman RA, Korn ED. Phagocytosis of Latex Beads by Acanthamoeba. I. Biochemical Properties*. Biochemistry (Mosc) 1967;6:485–497. [DOI] [PubMed] [Google Scholar]
- 23.Miceli MV, Jazwinski SM. Nuclear Gene Expression Changes Due to Mitochondrial Dysfunction in ARPE-19 Cells: Implications for Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 2005;46:1765–1773. [DOI] [PubMed] [Google Scholar]
- 24.Chomyn A. Platelet-mediated transformation of human mitochondrial DNA-less cells. Methods Enzymol 1996;264:334–339. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser PK. Ranibizumab: the evidence of its therapeutic value in neovascular age-related macular degeneration. Core Evid 2008;2:273–294. [PMC free article] [PubMed] [Google Scholar]
- 26.de Jong PTVM. Age-related macular degeneration. N Engl J Med 2006;355:1474–1485. [DOI] [PubMed] [Google Scholar]
- 27.Nowak JZ. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep PR 2006;58:353–363. [PubMed] [Google Scholar]
- 28.Sheu S-J, Chao Y-M, Liu N-C, Chan JYH. Differential effects of bevacizumab, ranibizumab and aflibercept on cell viability, phagocytosis and mitochondrial bioenergetics of retinal pigment epithelial cell. Acta Ophthalmol (Copenh) 2015;93:e631–643. [DOI] [PubMed] [Google Scholar]
- 29.Sundelin S, Wihlmark U, Nilsson SE, Brunk UT. Lipofuscin accumulation in cultured retinal pigment epithelial cells reduces their phagocytic capacity. Curr Eye Res 1998;17:851–857. [PubMed] [Google Scholar]
- 30.Klettner A, Tahmaz N, Dithmer M, et al. Effects of aflibercept on primary RPE cells: toxicity, wound healing, uptake and phagocytosis. Br J Ophthalmol 2014;98:1448–1452. [DOI] [PubMed] [Google Scholar]
- 31.Ranjbar M, Brinkmann MP, Zapf D, et al. Fc Receptor Inhibition Reduces Susceptibility to Oxidative Stress in Human RPE Cells Treated with Bevacizumab, but not Aflibercept. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 2016;38:737–747. [DOI] [PubMed] [Google Scholar]
- 32.Aboul Naga SH, Dithmer M, Chitadze G, et al. Intracellular pathways following uptake of bevacizumab in RPE cells. Exp Eye Res 2015;131:29–41. [DOI] [PubMed] [Google Scholar]
- 33.Bucci C, Thomsen P, Nicoziani P, et al. Rab7: A Key to Lysosome Biogenesis. Mol Biol Cell 2000;11:467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoppe G, Marmorstein AD, Pennock EA, Hoff HF. Oxidized Low Density Lipoprotein–Induced Inhibition of Processing of Photoreceptor Outer Segments by RPE. Invest Ophthalmol Vis Sci 2001;42:2714–2720. [PubMed] [Google Scholar]
- 35.Woodman PG. Biogenesis of the sorting endosome: the role of Rab5. Traffic Cph Den 2000;1:695–701. [DOI] [PubMed] [Google Scholar]
- 36.Malik D, Tarek M, del Carpio JC, et al. Safety profiles of anti-VEGF drugs: bevacizumab, ranibizumab, aflibercept and ziv-aflibercept on human retinal pigment epithelium cells in culture. Br J Ophthalmol 2014;98:i11–i16. [DOI] [PMC free article] [PubMed] [Google Scholar]


