Abstract
Purpose
Osteoarthritis (OA) is one of the most common degenerative joint diseases in the world, characterized primarily by the progressive degradation of articular cartilage. Accumulating evidence has shown that Morusin, a flavonoid derived from the root bark of Morus alba (mulberry) plants, exerts unique protective properties in several diseases. However, its effects on OA, specifically, have not yet been characterized.
Methods
In this study, we evaluated the anti-inflammatory effect of Morusin on mouse chondrocytes and its underlying mechanism in vitro. In addition, the protective effect of Morusin on destabilization of the medial meniscus (DMM) model was also explored in vivo.
Results
In vitro, IL-1β-induced activation of inflammatory factors (TNF-α, IL-6, INOS and COX2) was dramatically suppressed by Morusin. Further, Morusin treatment inhibited the expression of ADAMTS5 and metalloproteinase (MMPs), both of which regulate extracellular matrix degradation. Morusin also decreased IL-1β-induced p65 phosphorylation and IκBα degradation. In vivo, degradation of the articular cartilage following surgical DMM, which mimicked OA pathology, was abrogated following treatment with Morusin, thus demonstrating a protective effect in the DMM model.
Conclusion
Herein, we demonstrate that Morusin reduces the OA inflammatory response in vitro and protects against articular cartilage degradation in vivo potentially via regulation of the NF-κB pathway. Hence, Morusin may prove to be an effective candidate for novel OA therapeutic strategies.
Keywords: osteoarthritis, chondrocytes, Morusin, IL-1β, NF-κB pathway
Introduction
Osteoarthritis (OA) is one of the most common degenerative joint disease in the world, affecting more than 25% of the global population.1 Chronic OA induces pain and joint dysfunction causing it to gradually become a serious socioeconomic burden.2
It has been well documented that the primary characteristic of OA is the destruction of the structure and function of articular cartilage.3 Although the underlying mechanism of OA remains poorly understood, inflammatory responses have been proposed to contribute significantly to the initiation and development of OA.4 Furthermore, IL-1β has been described as a vital element responsible for the initiation and progression of OA via induction of proinflammatory and procatabolic responses.4–7 Chondrocytes account for the only cell type constituting articular cartilage, and hence, play an important role in maintaining the synthesis of normal tissues and renewal of aged extracellular matrix (ECM).8,9 The IL-1β-enhanced catabolic response serves to suppress the expression of cartilage-related genes and promote that of matrix-degrading related genes, including collagenases and matrix metalloproteinases (MMPs), which were previously reported to increase the anabolic activities of chondrocytes via promoting ECM degradation.5,10,11 Moreover, IL-1β promotes chondrocytes to express inflammatory-related genes and proteins, such as INOS and COX-2, which reduce aggrecan and collagen II production, the primary components of cartilage ECM.12–14 Thus, a candidate compound capable of targeting IL-1β-induced inflammation may prove effective as a novel OA therapeutic strategy.
Recent studies have highlighted the ability of naturally derived products to elicit anti-inflammatory effects without inducing significant adverse side effects. We have, therefore, chosen to also focus on a natural product, namely Morusin (Mor), a prenylated flavonoid isolated from the root bark of Morus australis (Moraceae).15 This compound has been found to engage in a myriad of biological functions, including anti-inflammatory, anti-oxidative and antitumor activities.16–18 For instance, Morusin was reported to attenuate LPS-induced proinflammatory responses in RAW264.7 cells.19 Additionally, an in vivo study revealed that Morusin ameliorates 2, 4, 6-trinitrobenzene sulfonic acid sodium salt (TNBS)-induced colitis in rats.20 However, the precise effect and mechanism elicited by Morusin in OA remain unclear.
Herein, we examined the effects of Morusin in OA and its underlying mechanism in vitro and in vivo, in an attempt to determine whether Morusin has the potential to be a novel candidate for use in future OA treatment.
Materials and Methods
Reagents
Morusin was obtained from MCE (New Jersey, USA), dissolved in dimethylsulfoxide (DMSO), and diluted in cell culture medium so that DMSO < 0.1% of the total volume. Recombinant human IL-1β, obtained from Peprotech (New Jersey, USA), was dissolved in water, and diluted in cell culture medium to a concentration of 10 ng/m for use in the study. Dulbecco’s Modified Eagle Medium (DMEM/F12), fetal bovine serum (FBS), penicillin and streptomycin were purchased from Gibco (Rockville, MD, USA). Primary antibodies against actin, Type II Collagen and ADAMTS5 were purchased from Abcam (Cambridge, MA, USA), iNOS, COX-2, aggrecan, MMP-3, MMP-13, p65, P-p65, IκBα, P-erk, erk, P-JNK, JNK, P-p38, p38, PI3K, P-AKT, AKT were purchased from CST (Cambridge, MA, USA). RNAiso plus and SYBR Green Master Mix were purchased from Takara (Japan); and QuantiTect Reverse Transcription kit was purchased from Vazyme (Nanjing, China). All other reagents were purchased from Sigma-Aldrich (St Louis, MO, USA) unless otherwise stated.
Isolation and Culture of Chondrocytes
Ten 5-day-old C57BL/6 mice (five males and five females) were euthanized using an overdose of sodium pentobarbital, and cartilage was removed from the knee and hip joints. Cartilage was then minced and washed with phosphate-buffered saline (PBS), and centrifuged at 1000 rpm for 3 min. A total of 10 mL of 0.2% type II collagenase was added to the tissue and digestion was performed for 6–8 h in an incubator maintained at 5% CO2 and 37°C. Detached cells were collected, centrifuged at 1000 RPM for 3 min, transferred to a culture flask and incubated (37°C, 5% CO2) for a further 24 h. Once 80% 0 – 90% confluency was achieved, cells were harvested using 0.25% Trypsin-EDTA (Gibco, Invitrogen) and centrifuged at 1000 rpm for 5 min, after which the supernatant was discarded. The inner cell mass was collected and resuspended in DMEM/F12 supplemented with 10% FBS and 1% antibiotic mixture (penicillin and streptomycin). Finally, cells were plated at a density of 1 × 105 cells/mL in 6-well plates and incubated in a humidified atmosphere of 5% CO2 at 37°C. The media were changed every 2–3 days. Cells were passaged when 80% to 90% confluence was observed, using 0.25% trypsin-EDTA solution. Only passages 1 and 2 were used in our study to avoid phenotype loss.
Cytotoxicity Assays
Chondrocytes were seeded in 96-well plates at a density of 8000 cells/well. After culturing for 24 h, the medium was replaced with fresh culture medium containing various concentrations of Morusin (0, 1, 2, 4, 8, 16, 32 and 64 μM) for 24 and 48 h. Each Morusin concentration was repeated in 6 wells. At each time point, 10 μL CCK-8 reagent was added to each well, and cells were incubated for 4 h at 37°C. Next, the optical densities (ODs) were measured at 450 nm (Thermo Scientific, Multiskan GO, Waltham, MA, USA).
Western Blot Analysis
Total proteins were isolated from cultured chondrocytes using RIPA lysis buffer with 1 mM PMSF (Phenylmethanesulfonyl fluoride). The samples were incubated on ice for 30 min, and centrifuged for 10 min at 12,000 rpm and 4°C. The supernatant containing protein was collected, and the protein concentration was measured using a bicinchoninic acid (BCA) protein assay kit according to manufacturer’s instructions. The protein was then mixed with sodium dodecyl sulfate-sampling buffer, followed by incubation at 95°C for 5 min. A total of 40 ng protein from each sample was loaded onto sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) and separated by electrophoresis. Proteins were then transferred to PVDF membranes (Bio-Rad, USA), membranes were blocked with 5% nonfat milk for 2 h and incubated overnight with primary antibodies directed against collagen II (1:1000), iNOS (1:1000), COX-2 (1:1000), MMP-13 (1:1000), MMP-3 (1:1000), ADAMTS5 (1:1000), aggrecan (1:1000), p65 (1:1000), P-p65 (1:1000), IκBα (1:1000), P-erk (1:1000), erk (1:1000), P-JNK (1:1000), JNK (1:1000), P-p38 (1:1000), p38 (1:1000), PI3K (1:1000), P-AKT (1:1000), AKT (1:1000), actin (1:1000) at 4°C. Next, membranes were incubated with appropriate enzyme-linked secondary antibodies for 2 h at room temperature. To visualize immunoblots, enhanced chemiluminescence (ECL) solution was added according to the manufacturer’s instructions.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction
Chondrocytes were seeded in DMEM/F-12 in 6-well plates at a density of 3 × 105 cells/mL and incubated for 24 h. Following stimulation with IL-1β and ALT at various concentrations (0, 0.5, 1, and 2 μM), total RNA was isolated from the monolayer of cultured chondrocytes using RNAiso plus (Takara, Japan). Total RNA (≤1000 ng) was reverse-transcribed into complementary DNA (cDNA) in a 20 μL-reaction volume using a Double-Strand cDNA Synthesis Kit (Takara, Japan). The PCR reaction was performed with 1 μL cDNA as template in triplicate using the Power SYBR® Green PCR Master Mix (Takara, Japan) on the ABI StepOnePlus System (Applied Biosystems, Warrington, UK). The total volume (20 μL) for each PCR reaction consisted of 10 μL SYBR Green QPCR Master Mix, 8 μL ddH2O, 1 μL cDNA, and 10 μM each of forward and reverse primers. The cycling conditions were as follows: 95°C for 10 min (activation), followed by 40 cycles at 95°C for 10 s, 60°C for 20 s, and 72°C for 90 s. β-actin was utilized as the housekeeping gene, and independent experiments were repeated for a minimum of three times. The specific primers (based on mouse sequences) used are shown in Table 1.
Table 1.
Sequences of Primers Used in Real-Time Polymerase Chain Reaction (Real-Time PCR)
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| TNF- α | 5′-CAGGCGGTGCCTATGTCTC-3′ | 5′-CGATCACCCCGAAGTTCAGTAG-3′ |
| IL-6 | 5′-TAGTCCTTCCTACCCCAATTTCC-3′ | 5′-TTGGTCCTTAGCCACTCCTTC-3′ |
| INOS | 5′-GTTCTCAGCCCAACAATACAAGA-3′ | 5′-GTGGACGGGTCGATGTCAC-3′ |
| MMP3 | 5′-ACATGGAGACTTTGTCCCTTTTG-3′ | 5′-TTGGCTGAGTGGTAGAGTCCC-3′ |
| MMP9 | 5′-GCAGAGGCATACTTGTACCG-3′ | 5′-TGATGTTATGATGGTCCCACTTG-3′ |
| MMP13 | 5′-TGTTTGCAGAGCACTACTTGAA-3′ | 5′-CAGTCACCTCTAAGCCAAAGAAA-3′ |
| β-actin | 5′-AGC CAT GTA CGT AGC CAT CC-3′ | 5′-CTC TCA GCA GTG GTG GTG AA-3′ |
Immunofluorescence Microscopy
Chondrocytes were seeded on glass coverslips in a 12-well plate at a density of 2 × 105 cells/mL and treated under indicated conditions. At the end of each experiment, glass coverslips with chondrocyte monolayers were rinsed thrice in PBS. Cells were fixed with 4% paraformaldehyde for 30 min, and permeabilized in Triton X-100 for 10 min at room temperature. Following blocking with 5% BSA for 60 min, chondrocytes were incubated with primary antibodies against collagen II (1:500), MMP3 (1:500), MMP13 (1:500) and P65 (1:500) at 4°C overnight; after which they were incubated with Alexa Fluor 647-conjugated anti-IgG secondary antibodies for 1 h and with DAPI for 5 min. Finally, images were observed using a Nikon ECLIPSE Ti microscope (Nikon, Japan).
Mice DMM Model
Thirty 10-week-old C57BL/6 female mice were purchased from the Animal Center of the Chinese Academy of Sciences (Shanghai, China). The protocol for animal care and use of laboratory animals was conducted in accordance with National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Pub No 85–23, revised 1996), and approved by the Zhejiang University Institutional Animal Care and Use Committee. Mice were randomly divided into three groups (Sham group, DMM group and Morusin treated group), and were anesthetized, at which the mice knee joints were carefully, and aseptically, exposed with a scalpel, followed by resectioning of the medial meniscuses. The wound was later sutured carefully, and penicillin was injected intramuscularly for the following 3 days. The Mourusin-treated group was administered Morusin, dissolved in saline (40 mg/kg), intragastrically once every 2 days for 8 weeks; while mice in the Sham and DMM groups received 5% DMSO (dimethylsulfoxide) in saline once every 2 days for 8 weeks.
Histological Analysis
At the completion of the study, all knee joints were collected from mice, and fixed in 4% formaldehyde. Next, decalcifying solution was added for approximately 21 days to decalcify the calcium in knee joints. The knee joints were then embedded in paraffin, and sectioned coronally. Safranin O and hematoxylin and eosin (H & E) staining were performed to measure the extend of cartilage destruction.
Statistical Analysis
All experiments were performed a minimum of three times independently. Data are expressed as mean ± standard deviation (SD). Statistical analyses were conducted using Student’s t-tests, one way ANOVA, or two-way ANOVA; p < 0.05 and p < 0.01 were considered statistically significant.
Results
Potential Cytotoxicity of Morusin on Chondrocytes
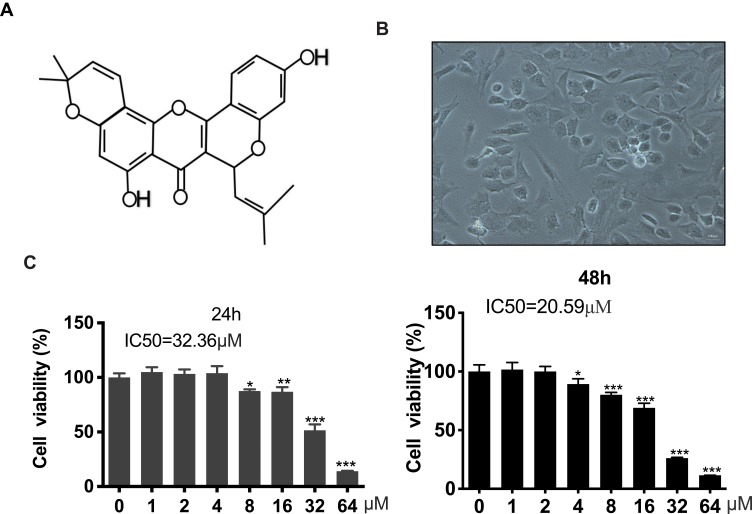
The chemical structure of Morusin is shown in Figure 1A, and the cell morphology of chondrocytes is provided in Figure 1B. A CCK-8 assay was performed to evaluate the cytotoxicity of different concentrations (0, 1, 2, 4, 8, 16, 32 and 64 μM) of Morusin on chondrocytes. Results revealed that Morusin treatment at specific concentrations (0~2 μM) did not induce significant differences in cell viability (Figure 1C). Therefore, Morusin at concentration of 0, 0.5, 1 and 2 μM was used in subsequent experiments.
Figure 1.
Potential cytotoxicity of Morusin on chondrocytes. (A) Chemical structure of Morusin. (B) Cell morphology of chondrocytes. (C) A CCK-8 assay was performed to evaluate the cytotoxicity of different concentrations of Morusin (0, 1, 2, 4, 8, 16, 32 and 64 μM) on chondrocytes. All data are presented as mean ± SD. Significant differences among the Morusin treatment groups are presented as *P < 0.05, **P < 0.01, ***P < 0.001 vs T group, n = 3.
The Protective Effect of Morusin on IL-1β-Induced Inflammatory Reaction
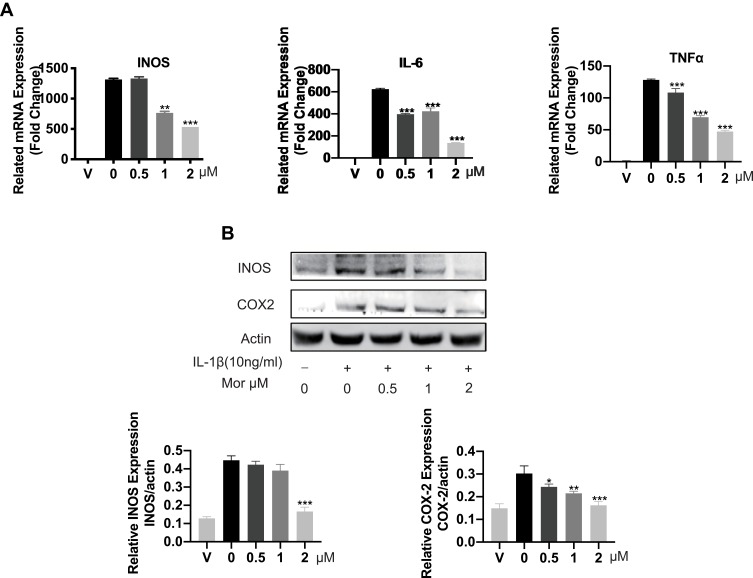
As IL-1β was previously reported to contribute to the initiations and progression of OA via induction of proinflammatory and procatabolic responses. We sought to further explore the protective effect of Morusin on IL-1β-induced chondrocytes inflammatory response. Results from qPCR analysis revealed treatment with IL-1β (10 ng/mL) dramatically increased the mRNA expression of inflammatory-related factors, such as TNF-α, IL-6, and INOS, while Morusin protected against these inflammatory responses (Figure 2A). Similarly, Western blot analysis indicated that Morusin significantly attenuated the protein expression of inflammatory-related makers: INOS and COX-2, which were up-regulated following stimulation by IL-1β (Figure 2B).
Figure 2.
The protective effect of Morusin on IL-1β-induced inflammation reaction. (A) Genes of IL-1β-induced inflammation-related markers were suppressed by the treatment of Morusin. qPCR was carried on to assess the expression of inflammation-related genes: TNF-α, IL-6, and INOS in chondrocytes. (B) IL-1β-induced inflammation-related proteins were measured by Western blot analysis. All genes were normalized to β-actin. “V” in the histogram represents chondrocytes without stimulation of IL-1β or Morusin treatment. Significant differences among the Morusin treatment groups are presented as *P < 0.05, **P < 0.01, ***P < 0.001 vs IL-1β treatment group, n = 3.
Morusin Alleviates IL-1β-Induced ECM Degradation
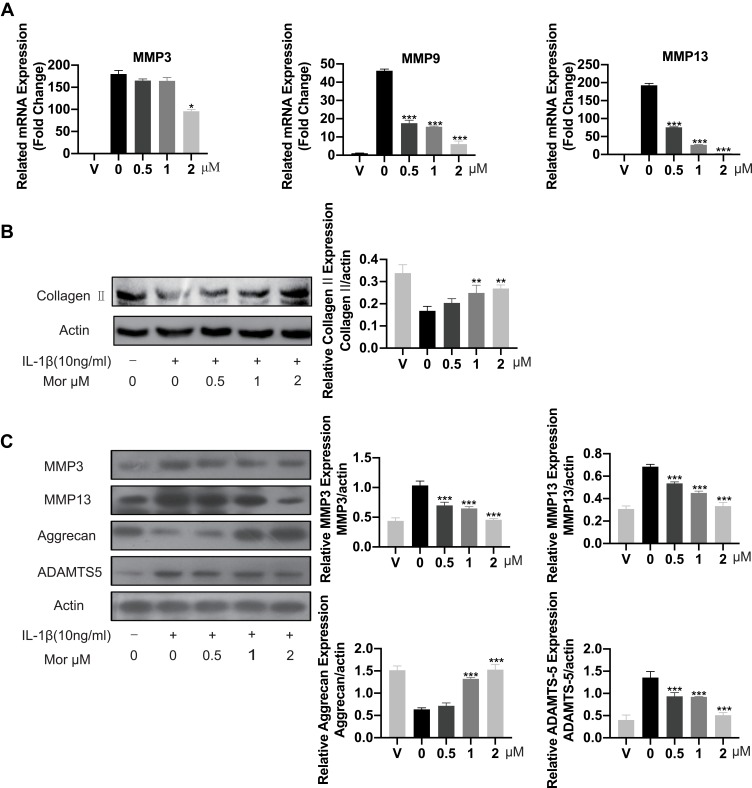
Collagen II was previously reported as a primary component of ECM, where it contributes significantly to supporting the cartilaginous structure.21 Thus, we next explored the effect of Morusin on collagen II. The results from Western blot (Figure 3B) and immunofluorescence (Figure 4A and B, respectively) analysis indicated that Morusin protects against IL-1β-induced degradation of collagen II.
Figure 3.
Morusin inhibited ECM degradation in mouse chondrocytes cells. (A) The mRNA expression level of MMP3, MMP9 and MMP13 was detected by qPCR. (B) and (C) ECM related proteins: Collagen II, MMP3, MMP13, Aggrecan, ADAMTS5 were detected by Western blot. “V” in the histogram represents chondrocytes without stimulation of IL-1β or Morusin treatment. Significant differences among the Morusin treatment groups are presented as *P < 0.05, **P < 0.01, ***P < 0.001 vs IL-1β alone treatment group, n = 3.
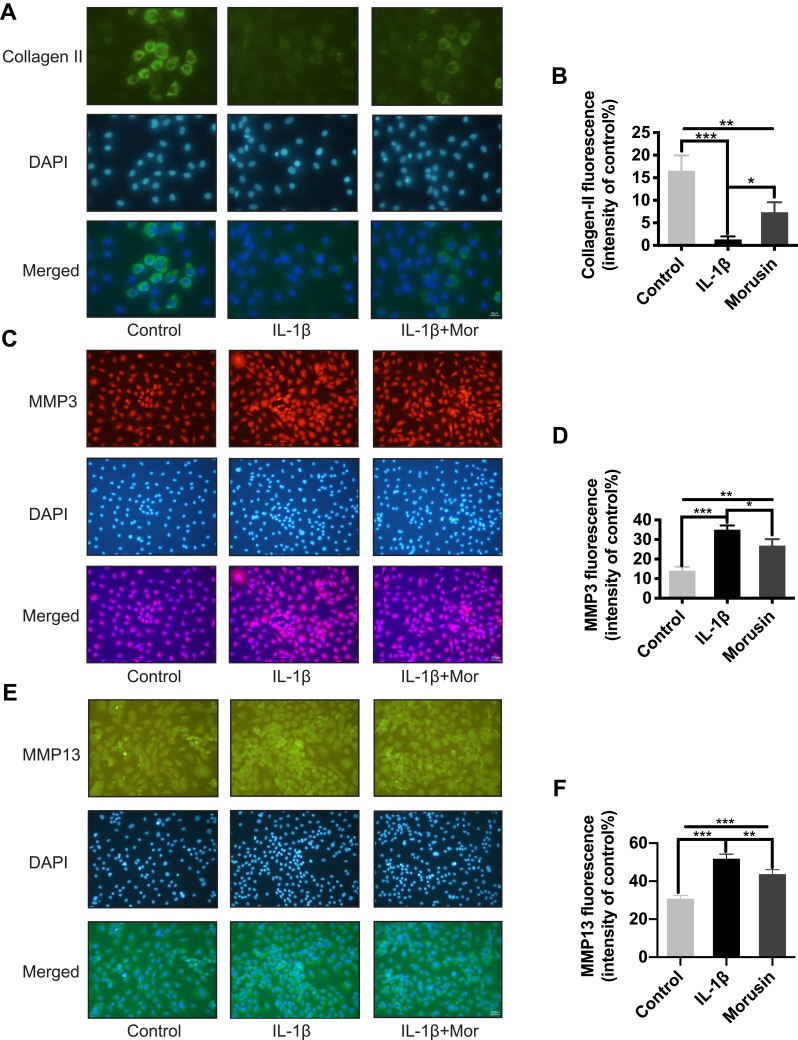
Figure 4.
Morusin inhibited ECM degradation in mouse chondrocytes cells. (A), (C), (E) Representative (A) collagen II, (B) MMP3 and (C) MMP13 were explored by immunofluorescent staining. (B), (D), (F) The fluorescence intensity of collagen II, MMP3 and MMP13 was determined using ImageJ software. Significant differences among the Morusin treatment groups are presented as *P < 0.05, **P < 0.01, ***P < 0.001 vs IL-1β treatment group, n = 3.
The destruction of ECM structural integrity leads to OA pathology. Treatment with IL-1β induces this ECM degradation, thus promoting the process of OA. Similarly, MMPs are proteolytic enzymes that mediate ECM destruction during OA.22–24 We, therefore, sought to explore the potential effect of Morusin on MMPs. qPCR results demonstrated that IL-1β up-regulated the expression of MMP-3, MMP-9 and MMP13, however, treatment with Morusin significantly attenuated this effect in a dose-dependent manner (Figure 3A). These results were confirmed via Western blot for MMP3 and MMP13 (Figure 3C). Furthermore, immunofluorescence analysis also demonstrated that Morusin protected against IL-1β-induced expression of MMP3 and MMP13 (Figure 4C and E), providing comprehensive evidence that Morusin significantly inhibits the expression of MMP3 and MMP13. The statistical analysis for the fluorescence assay is presented in Figure 4D and F.
Aggrecan has been defined as a major component of ECM, endowing articular cartilage with the ability to withstand compressive loads.25 Hence, the decrement of aggrecan has been shown to contribute to OA development. Our results demonstrate that IL-1β reduced aggrecan, while Morusin recovered its expression (Figure 3C). Furthermore, the ADAMTS family of metalloproteases cleaves aggrecan at a specific “aggrecanase” site. The absence of active ADAMTS5 was shown to prevent cartilage degradation in mouse OA.26 Therefore, we investigated the effect of Morusin on IL-1β-induced expression of ADAMTS5, and found that Morusin significantly inhibited the expression of ADAMTS5 (Figure 3C).
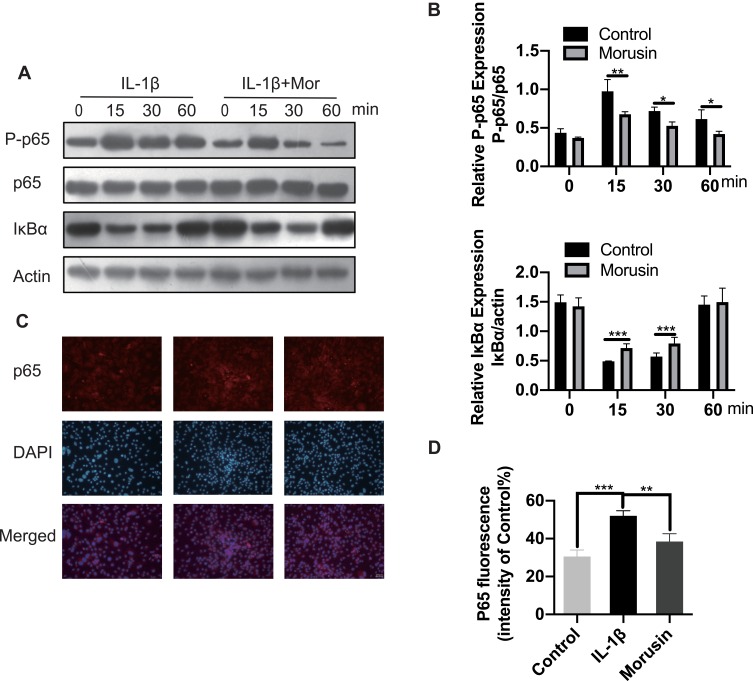
Morusin Suppresses IL-1β-Induced Phosphorylation of the NF-κB Signaling Pathway
The NF-κB signaling pathway was previously reported to play a vital role in modulating matrix degradation, ECM synthesis as well as proinflammation.27 We, therefore, further investigated the protective effect of Morusin on IL-1β activation of the NF-κB signaling pathway in chondrocytes. Results show that IL-1β significantly promoted the phosphorylation of p65, while Morusin dramatically attenuated phosphorylation in a time-dependent manner (Figure 5A and B). Moreover, IL-1β strongly increased the degradation of Iκβα; however, Morusin reversed this effect (Figure 5A and B). The immunofluorescence microscopy analysis also indicated that Morusin significantly decreased IL-1β-induced translocation of p65 from the cytoplasm to nucleus (Figure 5C). Statistical analysis for fluorescence data is presented in Figure 5D.
Figure 5.
Effect of Morusin on IL-1β-induced NF-κB activation on mouse chondrocytes. Chondrocytes cells were pretreated with Morusin (2 μM) for 2 h, followed by stimulation with or without IL-1β for the indicated time (0, 15, 30, 60 min). (A) and (B) Western blot was performed to measure the protein expression level of P-p65, p65, IκBα and actin. (C) and (D) Representative p65 was explored by immunofluorescent staining. The fluorescence intensity of p65 was determined using ImageJ software. Significant differences among the Morusin treatment groups are presented as *P < 0.05, **P < 0.01, ***P < 0.001 vs IL-1β treatment group, n = 3.
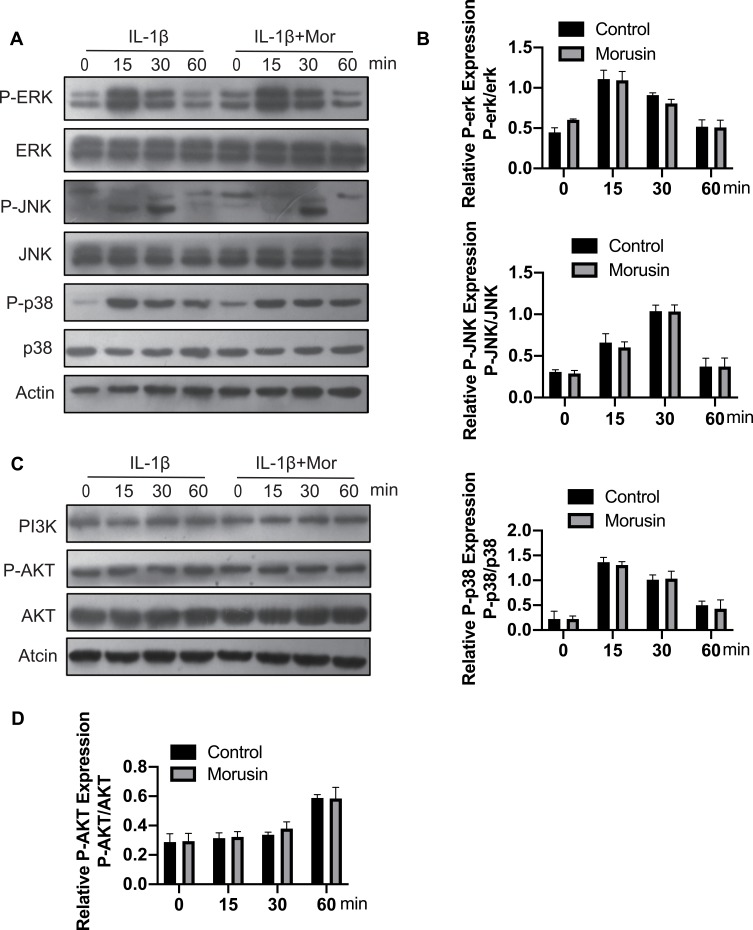
Morusin did not impact IL-1β-Induced activation of MAPK and PI3K/AKT pathways.
MAPK and PI3K/AKT signaling pathways have also been shown to be vital for IL-1β-induced inflammation in OA pathology.28–31 Extracellular signal-regulated kinase p44/42 MAPK, c-Jun N-terminal kinase and p38 MAPK were demonstrated to be the main components of the MAPK signaling pathway. Further, a previous animal study reported that inhibition of p38 MAPK served to significantly protect against cartilage degradation.32 JNK was also contributed to regulating the expression of MMP3;23 while downregulating ERK decreased the expression of MMP13.33 Otherwise, previous research revealed the critical role of the PI3K/AKT pathway in regulating the production of MMPs by chondrocytes.31 Cumulatively, these previous studies demonstrate the significant role of MAPKs and PI3K/AKT signaling pathways in L-1β-induced inflammation during OA pathology. We, therefore, sought to explore the effect of Morusin on these two pathways. Results revealed that Morusin treatment did not impact the phosphorylation of p38, ERK and JNK (Figure 6A and B). Moreover, the activation of AKT was not influenced by Morusin. Our data demonstrated that Morusin did not inhibit IL-1β-induced phosphorylation of MAPKs and PI3K/AKT signaling pathways (Figure 6C and D).
Figure 6.
Effect of Morusin on IL-1β-induced MAPKs and PI3K-AKT signaling pathways activation on mouse chondrocytes. Chondrocytes cells were pretreated with Morusin (2 μM) for 2 h, followed by stimulation with or without IL-1β for the indicated time (0, 15, 30, 60 min). Western blot was performed to measure the protein expression level of p-ERK, ERK, P-JNK, JNK, P-p38, p38, PI3K, P-AKT, AKT and actin (A–D).
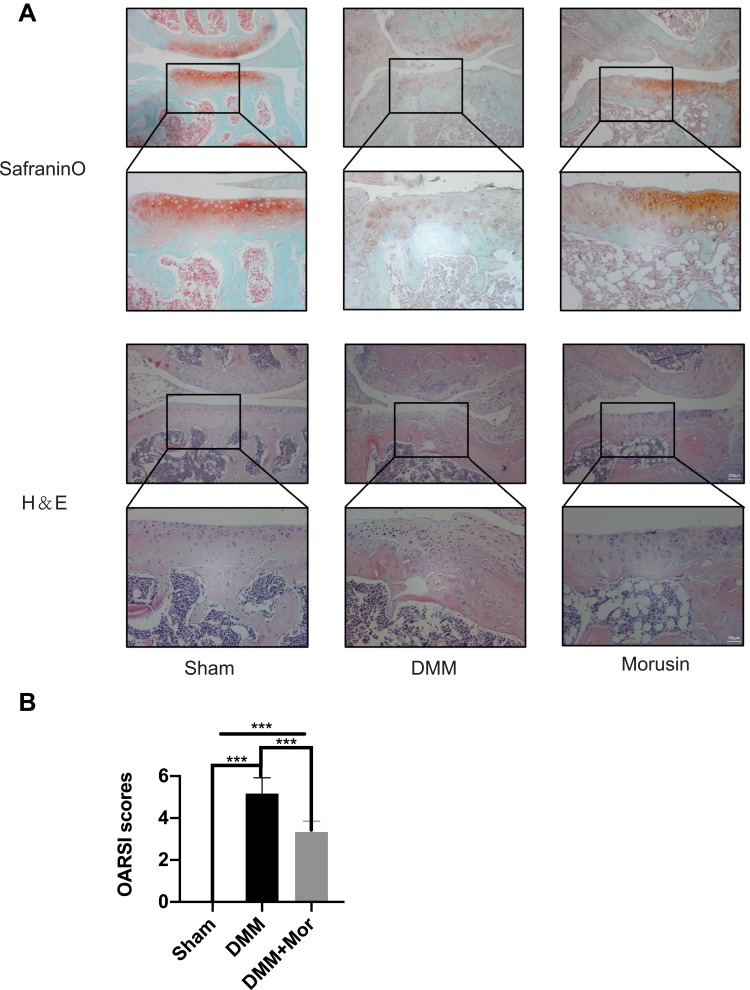
Morusin Decreases Cartilage Degeneration in a DMM OA Model
Since the in vitro data revealed a protective effect of Morusin on the inflammatory reaction associated with OA pathology, a surgically induced DMM model was established to verify whether Morusin protects against the development of OA in vivo. Ten-week-old mice were randomly divided into three groups (Sham group, DMM group, DMM+ Morusin treated group), and surgical DMM OA models were established, followed by intragastric administration of 40 mg/kg Morusin once every 2 days for 8 weeks in the treatment group; meanwhile, the Sham and DMM groups were intragastrically administrated 5% DMSO as a control. The intragastric administration continued until the end of study. Results from the Safranin O staining and H&E staining showed that compared with the Sham group, severe cartilage erosion and cartilage destruction were observed in DMM group (Figure 7A); however, treatment with Morusin reversed the destruction. Further, the OARSI scores were much higher in the OA compared to the Sham group. Similarly, treatment with Morusin reduced the OARSI scores (Figure 7B). Cumulatively, these results demonstrate that Morusin may serve as a potential candidate for the treatment of OA.
Figure 7.
Morusin ameliorates OA development in mouse DMM model in vivo. (A) Representative Safranin O and H&E staining of cartilage and synovitis from different experimental groups. (B) Diagrams show the cartilage OARSI scores. Significant differences among the animal groups are presented as ***P < 0.001.
Discussion
Osteoarthritis (OA) is a chronic bone disease characterized by degeneration of articular cartilage.34 In late stages of osteoarthritis, intensive cartilage ECM degradation leads to development of fissures, fibrillation and narrow joint spaces, aggrandizing joint friction, structural remodeling and functional impairment. Current therapeutic strategies including physical therapy and pain-relieving drugs only relieve the symptoms and often elicit adverse side effects.35 Therefore, a valid agent is urgently required to attenuate the initiation and development of OA. Previous studies had revealed the important therapeutic potential of Morusin, a Chinese herbal medicine, in inflammatory processes. In fact, Morusin was believed to reduce the inflammatory reaction in several diseases.17,36,37 We, therefore, explored whether it possessed anti-inflammatory properties in osteoarthritis. Our data demonstrate that Morusin significantly inhibits the IL-1β-induced inflammatory reaction in chondrocytes; potentially via suppression of the NF-κB signaling pathway.
Previously, inflammation has been shown to contribute significantly to the initiation and development of OA.38 During the pathological process of OA, inflammatory mediators, most notably IL-1β, induce the release of other proinflammatory cytokines, thereby promoting the chondrocytes catabolic response and destroying the structure of articular cartilage.39 Candidate molecules capable of targeting IL-1-induced inflammation were, therefore, postulated to be potential novel therapeutic strategies for OA. IL-1β promotes the expression of INOS and COX-2.40 INOS synthesizes the inflammatory mediator NO,41,42 and COX-2 is a major mediator of inflammation and pain in the pathological process of OA.43 Hence, specific inhibitors of COX-2 are now used clinically to treat OA.44 Our data demonstrate that Morusin significantly inhibited IL-1β-induced expression of INOS and COX-2, further supporting its anti-inflammatory properties in vitro.
Since the articular cartilage of ECM maintains mechanical load distribution generated by weight, its degradation directly leads to joint morbidity.45 Complex enzyme systems lead to the breakdown of ECM,46 with metalloproteinases (MMPs) reported to be responsible for the cataplasia involved in ECM degradation.47 IL-1β also enhances the secretion of MMP3 and MMP13,48,49 disrupts the synthesis/degradation equilibrium of ECM.50 In fact, accumulating evidence has shown that MMP3 and MMP13 downregulation may function as a therapeutic target for OA.51,52 In addition, type II collagen and aggrecan comprise the primary components of ECM, meeting the demands of weight-bearing mechanical stress.21,53 IL-1β inhibits the expression of type II collagen and aggrecan,54,55 thus disrupting the stability of articular cartilage and promoting the initiation of OA. Our data indicate that Morusin significantly suppresses the expression of MMP3 and MMP13, while rescuing the degeneration of type II collagen and aggrecan, thus protecting against ECM degradation, and delaying the process of OA. Furthermore, convincing evidence has demonstrated the vital role of ADAMTS-5 in modulating degradation of aggrecan. Stimulation with IL-1β up-regulated ADAMTS-5 expression, which then functions to cleave aggrecan.55,56 We thus explored the effect of Morusin on ADAMTS-5 and found that Morusin protects against IL-1β-induced stimulation of ADAMTS-5.
Activation of the NF-κB signaling pathway is reported to stimulate the expression of inflammatory cytokines and ECM degradation products, leading to initiation of OA.57 Under normal conditions, NF-κB is localized in the cytoplasm where it binds IκBα. Once chondrocytes become simulated by IL-1β, NF-κB is freed from the cytoplasm and translocated to the nucleus,27 where it triggers the expression of inflammatory-related genes, including COX-2, INOS, and IL-6.57 Agents that target NF-κB signaling may protect against IL-1β induced chondrocyte inflammatory reactions.58,59 We, therefore, determined the effect of Morusin on IL-1β-induced p65 phosphorylation and IκBα degradation. Results show that Morusin significantly inhibited the activation of p65 and induced the degradation of IκBα in chondrocytes in a time-dependent manner.
Following confirmation of the protective effect of Morusin in vitro, we performed surgical DMM in a mouse model to further explore whether Morusin could protect against OA in vivo. This DMM model was previously shown to serve as a mature model that mimics OA pathology.60 OARSI scoring was also performed to evaluate the degree of cartilage degeneration. Results revealed that Morusin ameliorated cartilage erosion in vivo. These data along, with that from the in vitro study, suggest Morusin as a potential therapy drug for future OA treatment.
Conclusion
In conclusion, our study indicates that Morusin may serve as a potential agent in treating IL-1β-induced chondrocyte inflammatory reaction in vitro as well as DMM-induced cartilage degeneration in vivo. The suppression of Il-1β- induced activation of NF-κB and degradation of IκB α is considered to be the primary mechanism of action for Morusin. However, their certain limitations were noted in this study. First, the effect of Morusin on other important factors such as IKKα and P-IκBα in the NF-κB signaling pathway was not examined and thus, remain unclear. Second, the immunohistochemical staining of IL-1β in articular cartilage was not performed. We would like to explore these areas in our future studies.
Funding Statement
This study was funded by the National Natural Science Foundation of China [grant number 81572126,81871801]; the Natural Science Foundation of Zhejiang Province [grant number LY15H060005]; Natural Science Foundation of Zhejiang Province [grant number LQ16H160013]; and Zhejiang Basic Public Welfare Research Project [grand number LGF18H060010].
Abbreviations
OA, osteoarthritis; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-alpha; INOS, inducible nitric oxide synthase; COX-2, cyclooxygenase-2; MMP, matrix metalloproteinase; ADAMTS-5, A disintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif; DMM, destabilization of the medial meniscus; MCE, MedChemExpress; ECM, extracellular matrix; NF-κB, nuclear factor kappa-B; BCA, bicinchoninic acid; CCK-8, cell counting kit-8; MAPK, mitogen-activated protein kinase; PI3K, The phosphatidylinositol 3ʹ -kinase.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
Yewei Jia and Wei He are co-first authors for this study. The authors report no conflicts of interest in this work.
References
- 1.Chen D, Shen J, Zhao W, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5(1):16044. doi: 10.1038/boneres.2016.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glyn-Jones S, Palmer AJR, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376–387. doi: 10.1016/S0140-6736(14)60802-3 [DOI] [PubMed] [Google Scholar]
- 3.Marchev AS, Dimitrova PA, Burns AJ, Kostov RV, Dinkova-Kostova AT, Georgiev MI. Oxidative stress and chronic inflammation in osteoarthritis: can NRF2 counteract these partners in crime? Ann N Y Acad Sci. 2017;1401(1):114–135. doi: 10.1111/nyas.2017.1401.issue-1 [DOI] [PubMed] [Google Scholar]
- 4.Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology. 2005;44(1):7–16. doi: 10.1093/rheumatology/keh344 [DOI] [PubMed] [Google Scholar]
- 5.Santangelo KS, Nuovo GJ, Bertone AL. In vivo reduction or blockade of interleukin-1β in primary osteoarthritis influences expression of mediators implicated in pathogenesis. Osteoarthritis Cartilage. 2012;20(12):1610–1618. doi: 10.1016/j.joca.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier J-P, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2010;7(1):33. doi: 10.1038/nrrheum.2010.196 [DOI] [PubMed] [Google Scholar]
- 7.Charlier E, Relic B, Deroyer C, et al. Insights on molecular mechanisms of chondrocytes death in osteoarthritis. Int J Mol Sci. 2016;17(12):2146. doi: 10.3390/ijms17122146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasi S, Ea H-K, So A, Busso N. Revisiting the role of interleukin-1 pathway in osteoarthritis: interleukin-1α and −1β, and NLRP3 inflammasome are not involved in the pathological features of the murine meniscectomy model of osteoarthritis. Front Pharmacol. 2017;8:282. doi: 10.3389/fphar.2017.00282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habouri L, El Mansouri FE, Ouhaddi Y, et al. Deletion of 12/15-lipoxygenase accelerates the development of aging-associated and instability-induced osteoarthritis. Osteoarthritis Cartilage. 2017;25(10):1719–1728. doi: 10.1016/j.joca.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-κB: its role in health and disease. J Mol Med. 2004;82(7):434–448. doi: 10.1007/s00109-004-0555-y [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Liu B, Zhao D, et al. Omentin-1 prevents cartilage matrix destruction by regulating matrix metalloproteinases. Biomed Pharmacother. 2017;92:265–269. doi: 10.1016/j.biopha.2017.05.059 [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Shen J, Jin H, Im H-J, Sandy J, Chen D. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann N Y Acad Sci. 2011;1240(1):61–69. doi: 10.1111/j.1749-6632.2011.06258.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fioravanti A, Tinti L, Pascarelli NA, et al. In vitro effects of VA441, a new selective cyclooxygenase-2 inhibitor, on human osteoarthritic chondrocytes exposed to IL-1β. J Pharmacol Sci. 2012;120(1):6–14. doi: 10.1254/jphs.12016FP [DOI] [PubMed] [Google Scholar]
- 14.Tchetina EV. Developmental mechanisms in articular cartilage degradation in osteoarthritis. Arthritis. 2011;2011:1–16. doi: 10.1155/2011/683970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng T-H, Chuang S-K, Hu -C-C, et al. The synthesis of Morusin as a potent antitumor agent. Tetrahedron. 2010;66(6):1335–1340. doi: 10.1016/j.tet.2009.12.002 [DOI] [Google Scholar]
- 16.Kim C, Kim JH, Oh EY, et al. Blockage of STAT3 signaling pathway by morusin induces apoptosis and inhibits invasion in human pancreatic tumor cells. Pancreas. 2016;45(3):409–419. doi: 10.1097/MPA.0000000000000496 [DOI] [PubMed] [Google Scholar]
- 17.Jin SE, Ha H, Shin HK, Seo CS. Anti-allergic and anti-inflammatory effects of Kuwanon G and Morusin on MC/9 mast cells and HaCaT keratinocytes. Molecules. 2019;24(2):265 undefined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue J, Li R, Zhao X, et al. Morusin induces paraptosis-like cell death through mitochondrial calcium overload and dysfunction in epithelial ovarian cancer. Chem Biol Interact. 2018;283:59–74. doi: 10.1016/j.cbi.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 19.Tseng T-H, Lin W-L, Chang C-K, Lee K-C, Tung S-Y, Kuo H-C. Protective effects of Morus Root Extract (MRE) against lipopolysaccharide-activated RAW264. 7 cells and CCl4-induced mouse hepatic damage. Cell Physiol Biochem. 2018;51(3):1376–1388. doi: 10.1159/000495555 [DOI] [PubMed] [Google Scholar]
- 20.Vochyanova Z, Pokorna M, Rotrekl D, et al. Prenylated flavonoid Morusin protects against TNBS-induced colitis in rats. PLoS One. 2017;12(8):e0182464. doi: 10.1371/journal.pone.0182464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poole AR, Kobayashi M, Yasuda T, et al. Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann Rheum Dis. 2002;61(suppl2):ii78. doi: 10.1136/ard.61.suppl_2.ii78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahmoud RK, El-Ansary AK, El-Eishi HH, Kamal HM, El-Saeed NH. Matrix metalloproteinases MMP-3 and MMP-1 levels in sera and synovial fluids in patients with rheumatoid arthritis and osteoarthritis. Ital J Biochem. 2005;54(3–4):248–257. [PubMed] [Google Scholar]
- 23.Shi J, Zhang C, Yi Z, Lan C. Explore the variation of MMP3, JNK, p38 MAPKs, and autophagy at the early stage of osteoarthritis. IUBMB Life. 2016;68(4):293–302. doi: 10.1002/iub.v68.4 [DOI] [PubMed] [Google Scholar]
- 24.Chen -J-J, Huang J-F, Du W-X, Tong P-J. Expression and significance of MMP3 in synovium of knee joint at different stage in osteoarthritis patients. Asian Pac J Trop Med. 2014;7(4):297–300. doi: 10.1016/S1995-7645(14)60042-0 [DOI] [PubMed] [Google Scholar]
- 25.Roughley PJ, Mort JS. The role of aggrecan in normal and osteoarthritic cartilage. J Exp Orthop. 2014;1(1):8. doi: 10.1186/s40634-014-0008-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glasson SS, Askew R, Sheppard B, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–658. doi: 10.1038/nature03369 [DOI] [PubMed] [Google Scholar]
- 27.Roman-Blas JA, Jimenez SA. NF-κB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14(9):839–848. doi: 10.1016/j.joca.2006.04.008 [DOI] [PubMed] [Google Scholar]
- 28.Prasadam I, Friis T, Shi W, van Gennip S, Crawford R, Xiao Y. Osteoarthritic cartilage chondrocytes alter subchondral bone osteoblast differentiation via MAPK signalling pathway involving ERK1/2. Bone. 2010;46(1):226–235. doi: 10.1016/j.bone.2009.10.014 [DOI] [PubMed] [Google Scholar]
- 29.Gao S-C, Yin H-B, Liu H-X, Sui Y-H. [Research progress on MAPK signal pathway in the pathogenesis of osteoarthritis]. Zhongguo Gu Shang. 2014;27(5):441–444. Chinese. [PubMed] [Google Scholar]
- 30.Zhao H, Zhang T, Xia C, et al. Berberine ameliorates cartilage degeneration in interleukin-1β-stimulated rat chondrocytes and in a rat model of osteoarthritis via Akt signalling. J Cell Mol Med. 2014;18(2):283–292. doi: 10.1111/jcmm.2014.18.issue-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Crawford R, Xiao Y. Vertical inhibition of the PI3K/Akt/mTOR pathway for the treatment of osteoarthritis. J Cell Biochem. 2013;114(2):245–249. doi: 10.1002/jcb.v114.2 [DOI] [PubMed] [Google Scholar]
- 32.Kong D, Zheng T, Zhang M, et al. Static mechanical stress induces apoptosis in rat endplate chondrocytes through MAPK and mitochondria-dependent caspase activation signaling pathways. PLoS One. 2013;8(7):e69403. doi: 10.1371/journal.pone.0069403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z, Cai H, Zheng X, Zhang B, Xia C. The involvement of mutual inhibition of ERK and mTOR in PLCγ1-mediated MMP-13 expression in human osteoarthritis chondrocytes. Int J Mol Sci. 2015;16(8):17857–17869. doi: 10.3390/ijms160817857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 2006;20(1):3–25. doi: 10.1016/j.berh.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 35.Ge Z, Hu Y, Heng BC, et al. Osteoarthritis and therapy. Arthritis Care Res. 2006;55(3):493–500. doi: 10.1002/art.21994 [DOI] [PubMed] [Google Scholar]
- 36.Wan L-Z, Ma B, Zhang Y-Q. Preparation of Morusin from Ramulus mori and its effects on mice with transplanted H22 hepatocarcinoma. BioFactors. 2014;40(6):636–645. doi: 10.1002/biof.1191 [DOI] [PubMed] [Google Scholar]
- 37.Lim HJ, Jin H-G, Woo E-R, Lee SK, Kim HP. The root barks of Morus alba and the flavonoid constituents inhibit airway inflammation. J Ethnopharmacol. 2013;149(1):169–175. doi: 10.1016/j.jep.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 38.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013;21(1):16–21. doi: 10.1016/j.joca.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 39.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23(5):471–478. doi: 10.1097/BOR.0b013e328349c2b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chowdhury TT, Bader DL, Lee DA. Dynamic compression counteracts IL-1β induced iNOS and COX-2 activity by human chondrocytes cultured in agarose constructs. Biorheology. 2006;43(3, 4):413–429. [PubMed] [Google Scholar]
- 41.Amin AR, Dave M, Attur M, Abramson SB. COX-2, NO, and cartilage damage and repair. Curr Rheumatol Rep. 2000;2(6):447–453. doi: 10.1007/s11926-000-0019-5 [DOI] [PubMed] [Google Scholar]
- 42.Arasapam G, Scherer M, Cool JC, Foster BK, Xian CJ. Roles of COX‐2 and iNOS in the bony repair of the injured growth plate cartilage. J Cell Biochem. 2006;99(2):450–461. doi: 10.1002/(ISSN)1097-4644 [DOI] [PubMed] [Google Scholar]
- 43.Abramson SB. The role of COX-2 produced by cartilage in arthritis. Osteoarthritis Cartilage. 1999;7(4):380–381. doi: 10.1053/joca.1998.0217 [DOI] [PubMed] [Google Scholar]
- 44.Ehrich EW, Schnitzer TJ, McIlwain H, et al. Effect of specific COX-2 inhibition in osteoarthritis of the knee: a 6 week double blind, placebo controlled pilot study of rofecoxib. Rofecoxib Osteoarthritis Pilot Study Group. J Rheumatol. 1999;26(11):2438–2447. [PubMed] [Google Scholar]
- 45.Smith RL. Degradative enzymes in osteoarthritis. Front Biosci. 1999;4(4):D704–D712. doi: 10.2741/A388 [DOI] [PubMed] [Google Scholar]
- 46.Iannone F, Lapadula G. The pathophysiology of osteoarthritis. Aging Clin Exp Res. 2003;15(5):364–372. doi: 10.1007/BF03327357 [DOI] [PubMed] [Google Scholar]
- 47.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajshankar D, Downey GP, McCulloch CA. IL-1β enhances cell adhesion to degraded fibronectin. FASEB J. 2012;26(11):4429–4444. doi: 10.1096/fsb2.v26.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsuzaki M, Guyton G, Garrett W, et al. IL‐1β induces COX2, MMP‐1,‐3 and‐13, ADAMTS‐4, IL‐1β and IL‐6 in human tendon cells. J Orthop Res. 2003;21(2):256–264. doi: 10.1016/S0736-0266(02)00141-9 [DOI] [PubMed] [Google Scholar]
- 50.Lee YJ, Lee EB, Kwon YE, et al. Effect of estrogen on the expression of matrix metalloproteinase (MMP)-1, MMP-3, and MMP-13 and tissue inhibitor of metalloproteinase-1 in osteoarthritis chondrocytes. Rheumatol Int. 2003;23(6):282–288. doi: 10.1007/s00296-003-0312-5 [DOI] [PubMed] [Google Scholar]
- 51.Bigg HF, Rowan AD. The inhibition of metalloproteinases as a therapeutic target in rheumatoid arthritis and osteoarthritis. Curr Opin Pharmacol. 2001;1(3):314–320. doi: 10.1016/S1471-4892(01)00055-8 [DOI] [PubMed] [Google Scholar]
- 52.Sabatini M, Lesur C, Thomas M, Chomel A, Pastoureau P. Effect of inhibition of matrix metalloproteinases on cartilage loss in vitro and in a guinea pig model of osteoarthritis. Arthritis Rheumatol. 2005;52(1):171–180. doi: 10.1002/art.20900 [DOI] [PubMed] [Google Scholar]
- 53.Dudhia J. Aggrecan, aging and assembly in articular cartilage. Cell Mol Life Sci CMLS. 2005;62(19–20):2241–2256. doi: 10.1007/s00018-005-5217-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding J, Ghali O, Lencel P, et al. TNF-α and IL-1β inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 2009;84(15):499–504. doi: 10.1016/j.lfs.2009.01.013 [DOI] [PubMed] [Google Scholar]
- 55.Sun Z, Yin Z, Liu C, Liang H, Jiang M, Tian J. IL-1β promotes ADAMTS enzyme-mediated aggrecan degradation through NF-κB in human intervertebral disc. J Orthop Surg Res. 2015;10(1):159. doi: 10.1186/s13018-015-0296-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song R-H, Tortorella MD, Malfait A-M, et al. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheumatism. 2007;56(2):575–585. doi: 10.1002/(ISSN)1529-0131 [DOI] [PubMed] [Google Scholar]
- 57.Rigoglou S, Papavassiliou AG. The NF-κB signalling pathway in osteoarthritis. Int J Biochem Cell Biol. 2013;45(11):2580–2584. doi: 10.1016/j.biocel.2013.08.018 [DOI] [PubMed] [Google Scholar]
- 58.Ma Z, Piao T, Wang Y, Liu J. Astragalin inhibits IL-1β-induced inflammatory mediators production in human osteoarthritis chondrocyte by inhibiting NF-κB and MAPK activation. Int Immunopharmacol. 2015;25(1):83–87. doi: 10.1016/j.intimp.2015.01.018 [DOI] [PubMed] [Google Scholar]
- 59.Jia Y, Pang C, Zhao K, et al. Garcinol suppresses IL-1β-induced chondrocyte inflammation and osteoarthritis via inhibition of the NF-κB signaling pathway. Inflammation. 2019;42(5):1–13. doi: 10.1007/s10753-019-01037-7 [DOI] [PubMed] [Google Scholar]
- 60.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15(9):1061–1069. doi: 10.1016/j.joca.2007.03.006 [DOI] [PubMed] [Google Scholar]