Abstract
The hypothalamus is a key homeostatic brain region and the primary effector of neuroendocrine signaling. Recent studies show that early embryonic developmental disruption of this region can lead to neuroendocrine conditions later in life, suggesting that hypothalamic progenitors might be sensitive to exogenous challenges. To study the behavior of hypothalamic neural progenitors, we developed a novel dissection methodology to isolate murine hypothalamic neural stem and progenitor cells at the early timepoint of embryonic day 12.5, which coincides with peak hypothalamic neurogenesis. Additionally, we established and optimized a culturing protocol to maintain multipotent hypothalamic neurospheres that are capable of sustained proliferation or differentiation into neurons, oligodendrocytes, and astrocytes. We characterized media requirements, appropriate cell seeding density, and the role of growth factors and sonic hedgehog (Shh) supplementation. Finally, we validated the use of fluorescence activated cell sorting of either Sox2GFPKI or Nkx2.1GFPKI transgenic mice as an alternate cellular isolation approach to enable enriched selection of hypothalamic progenitors for growth into neurospheres. Combined, we present a new technique that yields reliable culturing of hypothalamic neural stem and progenitor cells that can be used to study hypothalamic development in a controlled environment.
Keywords: hypothalamus, neurogenesis, neural stem cells, radial glial cells, embryogenesis, neurodevelopment
The hypothalamus is a key regulatory brain region that controls basic functions essential for survival, including homeostatic regulation of the neuroendocrine system, autonomic nervous system, immune system, and behavioral functions (1). Specifically, the hypothalamus is involved in regulating processes such as the release of hormones, thermoregulation, hunger and thirst, sexual drive, circadian rhythm, energy metabolism, and the mediation of emotional responses (2). Importantly, the hypothalamus links the nervous and endocrine systems through the release of trophic hormones that act on the pituitary, resulting in stimulation or inhibition of other target endocrine glands (3), as well as creating complex feedback loops that finely control endocrine interactions (4, 5).
Hypothalamic development can be divided into 5 broad embryonic stages based on embryonic day (E), as characterized in mice (6, 7): early regionalization (< E9.5); cell fate specification (neurogenesis: E9.5-E16.5; gliogenesis: E13.5 to early postnatal); neuronal migration (E12.5-E16.5); coalescence of neurons into a mature nuclei (E16.5- E18.5); and circuitry formation (late embryonic to early postnatal). Early patterning genes such as SIX homeobox 3 (Six3), Ventral anterior homeobox 1 (Vax1), Retinal and anterior homeobox (Rax), Sonic hedgehog (Shh), and NK2 homeobox 1 (Nkx2.1) are involved in the development of the hypothalamic region (6–11). Six3, Vax1, and Rax are expressed in the ventral and rostral forebrain, contributing to the regional specification of rostral hypothalamic progenitors (12). Shh (expressed E8.5–9.5) induces specification of ventral diencephalon progenitors along the ventricular zone and combine with Nodal signaling to drive expression of Nkx2.1, a classic pan-hypothalamic marker (13–16). Shh inhibits retinal cell fates in hypothalamic regions and a secondary Shh peak at E12.5 contributes to the progression of distinct hypothalamic progenitor subdomains (13–15). Rax is also required for both activation and repression of gene expression programs, particularly in the rostral ventral hypothalamus (10).
Following early regionalization and patterning, the hypothalamus first becomes populated with neurons and second glia, which arise from the neural stem and progenitor cells (NSPCs) that reside in the ventricular zone (VZ) surrounding the third ventricle (3,17). NSPCs are pluripotent and serve as the progenitor pool for the production of neurons, astrocytes, and oligodendrocytes through 2 temporally distinct processes that only slightly overlap: neurogenesis and gliogenesis (17). In both neurogenesis and gliogenesis, NSPC division can be symmetric proliferative (i.e., producing multipotent progenitors to increase the progenitor pool), asymmetric neurogenic/gliogenic (i.e., producing both a daughter progenitor cell and a neuron or glia), or symmetric neurogenic/gliogenic (i.e., producing 2 daughter neurons or glia, which serves to exhaust the progenitor pool). Following neurogenesis, newly-born neurons migrate away from the VZ to their appropriate positions in the mantle zone, whereby the cell bodies form a dense nuclear cluster and their neuronal dendrites and projections create a cell body-sparse shell zone that surround the nuclei (18). Many of the precise mechanisms that induce proper neuronal specification, migration, and sorting remain unidentified.
Neurospheres are nonadherent spherical cell clusters that derive from proliferating neural stem cells or neural stem-like progenitors that are cultured to enable the study of neural precursor cell behavior in vitro (19). As neural precursor division shifts from symmetrical to asymmetrical, a heterogeneous sphere composed of NSPCs and postmitotic neurons and glia is created (19). These growing neurospheres are influenced by growth factor and media components, as well as cell density and changes in the culturing method, such as timing and frequency of media replenishment and passages (20–22). Neurospheres can be expanded, passaged, and/or differentiated, providing valuable insight into 3 fundamental characteristics of NSPCs: proliferation, self-renewal, and multipotency (23). Given the relative lack of understanding of the mechanisms regulating hypothalamic development, the neurosphere assay can be used to examine the response of isolated progenitors to morphogens, growth factors, and/or exogenous compounds that influence hypothalamic development.
To date, hypothalamic neurospheres have been developed from fetal rats (24–26), but no prenatal murine equivalent to the technique exists, although a description of hypothalamic progenitors from late neurogenic (E14.5) mouse embryos was presented as part of the generation of a cell line (27). Here we develop and validate dissection and culturing conditions for the establishment of multipotent hypothalamic neurospheres that can be repeatedly passaged while maintaining potency. To our knowledge, our method generates neurospheres from the earliest embryonic timepoint reported to date. Given the ability to combine the usefulness and shorter generation time of the neurosphere assay with the benefits of readily-available mouse transgenic lines, while using progenitors from peak hypothalamic neurogenesis, we propose that this methodology may provide researchers with a valuable tool to further explore hypothalamic progenitor behaviors in primary culture.
Methods
Animals
Timed-pregnant mice were bred to obtain embryonic brain samples. For development of dissection and culturing techniques, CD1 mice (Jackson Labs) were used. For fluorescence activated cell sorting (FACS), Sox2GFPKI or NKX2.1GFPKI were used and have been previously presented in the literature (28, 29). For embryonic staging, female mice were plug-checked in the morning and those with a positive vaginal plug were assigned embryonic day (E) 0.5. Gravid females received our standard chow (Labdiet Rodent Diet 20) and water ad libitum but were fasted overnight prior to sacrifice for use in neurosphere experiments. Fasting was employed to minimize variability in maternal environment due to feeding-related hormonal changes. All experiments were approved by the University of Calgary Animal Care Committee and in accordance with the Guidelines of the Canadian Council of Animal Care.
Microdissection
Pregnant dams were sacrificed via cervical dislocation and embryos were collected at E12.5 for neurosphere cultures. Embryos were removed and placed in sterile 1X phosphate-buffered saline (PBS) + penicillin-streptomycin (100U/mL) (PBS + PS) at 37°C. E12.5 embryos were microdissected for presumptive hypothalamic tissue under sterile conditions and all embryo samples were pooled per dam (seeFigs. 1A-D for microdissection methodology and region of interest, and below in results for detailed explanation). Dissected tissue was collected in sterile PBS + PS. For immunohistochemistry, samples were fixed overnight with 4% paraformaldehyde in 1X PBS, washed in 1X PBS, and treated overnight with 20% sucrose before being embedded in Tissue-Tek optimum cutting temperature (O.C.T.) compound for cryosectioning (10 μm sections).
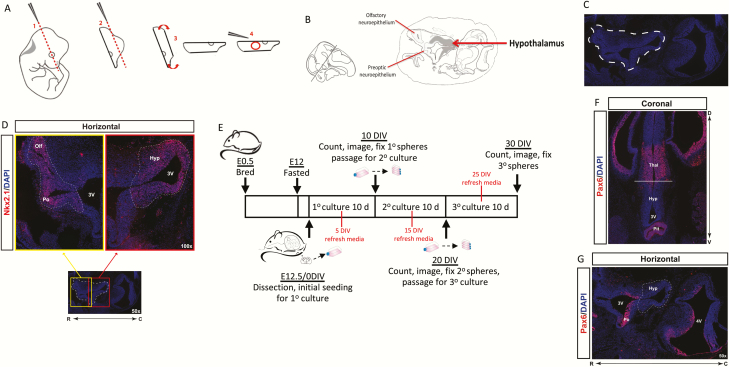
Figure 1.
Dissection methodology and culturing timeline.
Schematic diagram of dissection methodology for hypothalamic neurospheres (A) and specific regions of interest (B, C) as identified by endogenous Nkx2.1 expression (D) in horizontal sections of the developing E12.5 murine hypothalamus and surrounding regions. Timeline of breeding, dissection, and neurosphere culturing protocol (E). Assessment of Pax6 expression in coronal (F) and horizontal (G) sections of the developing E12.5 hypothalamus (Hyp) and surrounding regions including the thalamus (thal), pituitary (pit), and preoptic neuroepithelium (Po). Solid white line (F) defines hypothalamus/thalamus border, dashed white line (G) marks target dissection region.
Neurosphere cultures and passaging
Microdissected hypothalamic tissue was mechanically dissociated in sterile PBS + PS by pipetting up and down 40 times with a 1 mL pipette. Dissociated samples were centrifuged at 300g for 10 minutes at room temperature with acceleration and brake set to 0, then the supernatant was decanted, the cells were resuspended in 4 mL of media, and again mechanically separated and centrifuged as before. The supernatant was again discarded and the cell pellet was resuspended in 1 mL of media and passed through a 40 μm cell strainer to be collected in a 50 mL falcon tube. An additional 1 mL of media was used to remove any remaining cells and maximize the collection. Cells were counted, then plated and cultured at specified densities. All cultures were incubated for 10 days at 37°C in a 5% CO2 atmosphere and refreshed with 50% initial volume of corresponding media and treatments every 5 days (unless otherwise noted). For passaging, primary neurospheres were collected after 10 days in culture, mechanically dissociated by pipetting 40 times, and centrifuged at 300g for 10 minutes as described above. The supernatant was removed and cells were resuspended in 1 mL of media. Total cells were counted and replated to generate secondary neurospheres that are formed from the NSPCs from dissociated primary neurospheres. Passaged cells were replated in media at a density of 1000 cells/mL (unless otherwise specified) in 1 mL initial volume (24-well flat bottom plate) or 5000 cells/mL in 5 mL initial volume (T25 flask). All cultures were incubated for 10 days at 37°C in a 5% CO2 atmosphere and refreshed with 50% of corresponding media and treatments every 5 days. Subsequently, after another 10 days of incubation, secondary neurospheres were collected, passaged exactly as with primary neurospheres, and cultured at 1000 cells/mL for the generation of tertiary neurospheres. See Fig. 1E for a schematic timeline of culturing experiments and endpoints.
Media preparation
Complete insulin-supplemented neurosphere media (ISM) contains: 20 ng/mL human recombinant EGF (Cedarlane) and human recombinant bFGF (R&D Systems), 100 U/mL penicillin-streptomycin, 1 mg/mL heparan sulfate sodium salt, 0.6% glucose, 0.1125% bicarbonate, 1× DMEM/F12, 0.005M HEPES, 2 mM glutamine, 20 μg/mL apo-transferrin (human, Sigma), 2.5 μg/mL insulin, 1 μg/mL putrescine dihydrochloride, and 3 mM sodium selenite. A hormone-free version of the media (HF) was made as described above, with the exception that no insulin was added. For growth factor experiments, media was manipulated to contain one of the following: 20 ng/mL of EGF only, 20 ng/mL bFGF only, 2 ng/mL each of EGF and bFGF (2 ng EGF + bFGF), or 20 ng/mL each of EGF and bFGF (20 ng EGF + bFGF). As specified, 1 μg/mL Fungizone (FZ) antimycotic was included in optimization experiments. NeuroCult NS-A proliferation (Stemcell Technologies) media was also used with 20 ng/mL EGF and bFGF, and heparin solution (Stemcell Technologies; 2 μg/mL) added.
Sphere assessment
Neurospheres were quantified and imaged for size measurements at 10 days in vitro (DIV). For neurosphere number quantifications, spheres were live counted using a Zeiss Axiovert 40 CFL inverted microscope and images were captured with an Axiocam MRc camera. Adobe Photoshop CC 2017 counting software was used to manually measure individual sphere diameters and measurements were converted to their respective size. Sphere diameters were binned into 2 categories ≤ 200 μm or > 200 μm for all size comparisons. These bin sizes were selected for being the common size range in the literature after ~10 days in culture (30, 31). Neurospheres < 30 μm were excluded from quantification and size analyses, as they consist mainly of single or paired cells.
Immunohistochemistry
Neurosphere and primary cell staining was performed by allowing spheres and cells to lightly adhere to substrates on glass cover slips. Prior to preparation, circular micro glass cover slips were sterilized by soaking overnight in 95% ethanol followed by autoclaving. Geltrex LDEV-reduced factor basement membrane matrix (Geltrex, Thermofisher) was diluted 1:100 in DMEM/F-12 (1:1 ratio) medium. Slips were covered with this working solution and coated for 1 hour at 37°C, then refrigerated for storage at 4°C. Prior to use, slips were washed with NeuroCult media. 10 DIV spheres were transferred to slips at 8 DIV and cultured for an additional 48 hours. Slips coated with poly-ornithine and laminin were also tested and no differences in expression or differentiation were seen with any of the markers tested, as conducted over multiple staining trials; however, we observed that spheres tended to adhere best to geltrex slips which allowed for ease of staining without loss of spheres during antibody applications or washes. Cells were then fixed with 4% paraformaldehyde (PFA) for 20 minutes, washed with 1X PBS (3 × 10 minutes), and processed for immunostaining with the following washes: 1X PBS (1 × 5 minutes), 1X PBT (0.1% Triton in PBS) (3 × 5 minutes), and 1X PBS + 1% Triton (1 × 30 minutes). Samples were blocked in 5% normal donkey serum (NDS) for 1 hour and primary antibodies were applied overnight at 4°C in blocking solution (5% NDS, PBS + 0.1% Triton). Primary antibodies were as follows: rabbit anti-Nkx2.1 (Santa Cruz; 1:400 (32)), rabbit anti-Sox2 (Millipore; 1:500 (33)), goat anti-Sox3 (R&D systems, 1:20 (34)), mouse anti-Tuj1 (Millipore; 1:400 (35)), goat anti-PdgfRα (R&D Systems; 1:150 (36)), rabbit anti-GFAP (Dako; 1:500 (37)), rabbit anti-Vax1 (Novus Biochem; 1:50 (38)), rabbit anti-Six3 (Rockland; 1:100 (39)), rabbit anti-Rax (Abcam; 1:500 (40)), mouse anti-Pax6 (Abcam; 1:100 (41)). These antibodies have been established previously in our lab and in the literature. Prior to secondary antibody application, cells were washed with 1X PBS (2 × 30min). Secondary antibodies employed were Alexa Fluor-conjugated donkey anti-IgG (1:500) applied for 2 hours at room temperature in darkness. Slips were then washed with PBT (3 × 5 minutes) and counterstained with DAPI nuclear stain (1:1000), washed with PBT (3 × 5 minutes) and mounted on slides for imaging. Whole brain sections were processed and stained as outlined above, with the exception that post-antibody (primary and secondary) washes were 4 × 10 minutes. No-primary and no-secondary control stains were performed to verify antibody specificity. Immunostained samples were analyzed using a Zeiss Axioplan 2 manual compound microscope with a Lumen Dynamics X-Cite Series 120 Q fluorescent module and Zeiss Axiocam HRc camera and processed using Zeiss Zen microscope software. Images captured with an objective lens of 100× objective magnification required an oil immersion application.
FACS
Embryos isolated from Sox2GFPKI and NKX2GFPKI were first sorted into GFP+ and GFP− pups by examination under a compound microscope. GFP− embryos were used as a fluorescence control to set the sorting threshold. Embryos were then dissected as described above and the resulting single-cell suspension was then processed through FACS for GFP fluorescence using a Sony SH800 machine with a 488 nm blue laser to excite GFP. A fluorescein isothiocyanate (FITC) emission filter collected GFP+ cells into heat-inactivated fetal bovine serum, which were then diluted and cultured at 10 000 cells/mL. Cells were first sorted by fluorescence threshold, then by size to ensure only single cells were sorted.
Statistical analysis
Statistical differences between treatments were assessed using an ordinary or repeated measures one-way ANOVA with Tukey post-hoc analysis or a paired or unpaired Student t test when applicable. Statistics were calculated using the Graphpad Prism software package. P values of less than 0.05 were considered statistically significant. Results were displayed as mean ± standard error of mean.
Results
Microdissection of embryonic hypothalamic cells
To specifically target hypothalamic cells for isolation, we developed a microdissection protocol, designed for ease of replicability and maximal isolation of progenitors, that also minimizes the inclusion of contaminating cells from nonhypothalamic regions. We selected E12.5 murine embryos, primarily because this timepoint represents the peak of hypothalamic neurogenesis (7). We considered earlier timepoints that would enable the study of early neurogenic stages, but the dissection strategies were difficult to replicate and prone to error due to the tissue being friable; thus, we settled on E12.5 as the earliest reproducible timepoint for this procedure. The microdissection process is summarized in Fig. 1A. All work was conducted within a sterile biosafety cabinet after the embryos were removed from the dam and washed using sterile PBS + P/S. Briefly, an individual embryo was placed in a petri dish and viewed under a dissecting microscope. Two cuts using a razor blade were made through the head of the embryo to enable dissection of the hypothalamus. The first cut required placement of the razor blade at a tangential angle across the head, using the developing eye as a landmark (Fig. 1A, Step 1). This cut exposes the developing hypothalamus on the ventral side of the slice. To produce a solid base against which the hypothalamus can be isolated, a secondary cut was made at the top of the slice, near the developing cortex (Fig. 1A, Step 2). The slice was then placed dorsal-side down (Fig. 1A, Step 3) and the open third ventricle was readily visualized under the microscope. It is critical to have good lighting for this step, which will allow for full visualization of the ventricle and surrounding tissue that consists of the developing olfactory neuroepithelium on the rostral-lateral side, preoptic neuroepithelium on the rostral side, and the hypothalamus across the caudal region (Fig. 1B and 1C). The developing hypothalamic region was identified by its raised, opaque collection of cells surrounding the caudal-lateral regions of the third ventricle. To confirm the identity of these key regions following the crude cuts to the brain, Nkx2.1 immunostaining in a horizontal section through the E12.5 developing hypothalamus was conducted. Specifically, Nkx2.1-positive cells were localized in the preoptic and olfactory neuroepithelium (Fig. 1D, yellow box), as well as the hypothalamus (Fig. 1D, red box). To isolate the hypothalamic region (the region outlined in Fig. 1D, red box), fine-tip #5 Dumont forceps were held at a shallow angle to the tissue section (Fig. 1A, Step 4) to avoid taking thalamic tissue too deep (dorsally) to the section, and brought from the caudal direction to avoid taking olfactory or preoptic tissue located more rostral. The hypothalamic tissue surrounding the caudal third ventricle was removed and placed in a separate small petri dish with PBS + P/S. This process was repeated for each embryo and the collected tissue was pooled for all embryos from a single dam. On average, ~3 million hypothalamic cells were isolated per litter of approximately 10 to 12 pups. The timeline of culturing, passaging, other measurements, and experimental endpoints is summarized (Fig. 1E) and discussed above in “Methods” and below in the “Results.”
Once the dissection methodology was established, we assayed for a marker that could identify contamination from unwanted nearby regions. We identified Paired box 6 (Pax6), a transcription factor that is present in the developing thalamus (42, 43) and cortex (44), and previous reports used the absence of Pax6 to further distinguish hypothalamic progenitors (11). Here, Pax6 immunohistochemistry was conducted in coronal and horizontal sections of the E12.5 hypothalamus (Fig. 1F and 1G). In the coronal section, Pax6 positive cells were detected in the developing pituitary (Fig. 1F, pit) and thalamus (Fig. 1F, thal) and clearly marked the hypothalamus-thalamus border (Fig. 1F, white line). In the horizontal section, Pax6 positive cells were also located in regions neighboring our target dissection area (Fig. 1G, dashed line), specifically in the preoptic region just caudal to the rostral third ventricle (Fig. 1G, Po). Based on these Pax6 expression patterns, an accurate dissection technique omits the Pax6+ region in the developing pituitary as per the initial cut (Fig. 1A, Step 1), and avoids the thalamic or preoptic tissue with proper angling of the forceps during collection (Fig. 1A, Step 4). Therefore, we consider Pax6 a useful marker to control for contamination from nonhypothalamic regions.
Validating multipotency and hypothalamic identity of neurospheres
To start, we validated the hypothalamic identity and progenitor purity of these neurospheres using the expression of known markers. Since hypothalamic NSPCs that reside in the VZ express Sox2 and Sox3 (28, 45, 46), we first examined the expression of these markers in our neurosphere cultures. Primary, secondary, and tertiary neurospheres were nearly ubiquitous for both Sox2 (Fig. 2A-C′′) and Sox3 (Fig. 2D-2F′′). Given that Nkx2.1 expression defines the hypothalamic boundary as outlined above (Fig. 1D), patterns the presumptive hypothalamic region (6), and is required for specifying hypothalamic neuronal identity (16), we also confirmed the presence of Nkx2.1+ cells in the E12.5 hypothalamic neurosphere cultures. Primary, secondary, and tertiary neurospheres showed strong expression of Nkx2.1 (Fig. 2G and 2I). Since Nkx2.1 is a transcription factor localized in the nucleus, further analysis at high magnification on dissociated tertiary neurospheres cells showed high levels of Nkx2.1 within the nuclei (Fig. 2J-2J′′). We further validated our isolation method by analyzing the expression of hypothalamic specification markers. We utilized 3 markers that are involved in patterning and specification of hypothalamic neurons, specifically Rax, Vax1, and Six3, which have previously been used to validate cultured hypothalamic progenitors induced from embryonic stem cells (11). Rax is a marker for tuberal and mamillary hypothalamic progenitors (10), Vax1 is a rostroventral forebrain marker and Vax1 mutants exhibit reduced developmental Nkx2.1 expression indicating altered hypothalamic patterning (47,48), and Six3 drives early Shh dorsoventral forebrain patterning. All three markers (Fig. 2K-2M′′) were expressed in our primary neurospheres, to varying levels. Rax was strongly and near ubiquitously expressed (Fig. 2K- 2K′′), whereas Six3 exhibited varying intensity across a given neurosphere, with stronger staining at the exterior edges (Fig. 2L-2L′′), indicating that it is more strongly expressed in newly proliferating cells as opposed to the older cells in the middle of the sphere. Vax1 was also detected in neurospheres but was sparsely expressed within a given sphere (Fig. 2M-2M′′). To further validate the purity and specificity of our cultured neurospheres, we co-stained primary neurospheres for both Nkx2.1 and Pax6 (Fig. 2N-N′′′) and did not observe any expression of Pax6 in primary hypothalamic neurospheres despite strong Nkx2.1 expression, indicating that our dissection technique prevents contamination from either the thalamus or the preoptic neuroepithelium, as well as the pituitary. When learning this protocol, we recommend staining for Pax6 as a negative control to demonstrate proficiency of the dissection techniques.
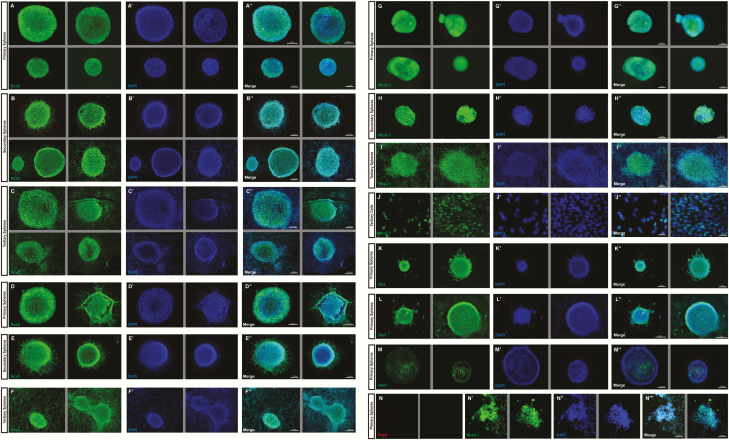
Figure 2.
Validation of progenitor and hypothalamic identity of cultured neurospheres.
The progenitor marker Sox2 is strongly expressed in primary (A-A′′), secondary (B-B′′), and tertiary (C-C′′) neurospheres. The progenitor marker Sox3 is strongly expressed in primary (D-D′′), secondary (E-E′′), and tertiary (F-F′′) neurospheres. The pan-hypothalamic marker Nkx2.1 is also near-ubiquitously expressed in primary (G-G′′), secondary (H-H′′), and tertiary (I-I′′) neurospheres, as well as in the nuclei of tertiary cells (J-J′′). Primary neurospheres also express additional hypothalamic markers Rax (K-K′′), Six3 (L-L′′), and Vax1 (M-M′′). Pax6 is not expressed in primary Nkx2.1+ hypothalamic neurospheres (N-N′′′). (2–4 representative fields are presented, grey lines divide different fields of exposure).
To ensure that our hypothalamic cultures contained multipotent NSPCs, we tested for the presence of postmitotic neurons and glia. Specifically, we assayed for neurons using Tuj1 and oligodendrocyte precursor cells (OPCs) using PdgfR□. We also co-labeled with GFAP, which is expressed as early as E15.5 in radial glia cells (RGCs) that will give rise to astrocytes. We detected expression of all three markers within primary (Fig. 3A-3C′′) and secondary (Fig. 3D-3F′′) neurospheres, as demonstrated by the combination of dual-staining for each marker pair. Expression of these proteins was variable within a given neurosphere, with GFAP and PdgfRα more strongly expressed than Tuj1, indicating that glial fates might be more common than neuronal fates in hypothalamic differentiating neurospheres. Of the roughly 200 spheres examined to date on approximately 10 different immunostained slips, all have contained some level of Tuj1+ and/or GFAP+ and/or PdgfRα+ cells, indicating that neurospheres remain multipotent for all three cell types. Finally, the expression of these differentiation markers varied between neurospheres, with some neurospheres containing more postmitotic neurons than glial cells or vice versa, for example. This shift may be dependent on the initial sphere-forming cell or other unknown factors. We have attempted to document this range of postmitotic cell identity in the representative images (e.g. Fig. 3C top vs bottom, Fig. 3F′ top vs bottom).
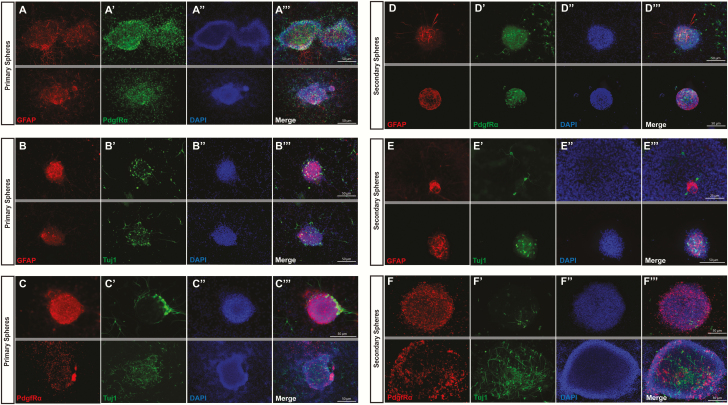
Figure 3.
Verification of multipotency in cultured neurospheres.
Markers for neurons (Tuj1), oligodendrocytes (GFAP), and astrocytes (PdgfRα) were measured in primary and secondary neurospheres in pairs to confirm multipotency. Primary neurospheres were simultaneously positive for oligodendrocytes/astrocytes (A-A′′), oligodendrocytes/neurons (B-B′′), and astrocytes/neurons (C-C′′). Similarly, secondary neurospheres were also simultaneously positive for oligodendrocytes/astrocytes (D-D′′), oligodendrocytes/neurons (E-E′′), and astrocytes/neurons (F-F′′). (2 representative fields are presented, grey lines divide different fields of exposure).
Optimization of culturing protocol
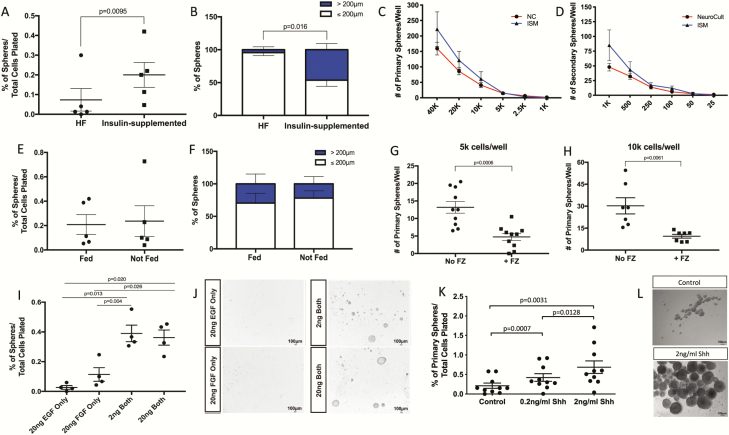
Typically, neurospheres are grown in media supplemented with progesterone, which is an upstream precursor in sex steroid biosynthesis that could potentially cause local estrogen synthesis and the activation (or even downregulation) of endogenous estrogen receptors found in hypothalamic progenitors (49, 50). Here, we tested a modified media formulation lacking sex steroids and all precursors in order to remove these confounding variables when studying hormones in the developing hypothalamus. Two standard culturing medias were tested: a complete hormone-free media (HF; lacking insulin and progesterone) and an insulin-supplemented media (ISM; lacking only progesterone). After 10DIV, E12.5 primary hypothalamic neurospheres were analyzed by quantifying sphere numbers and size (by measuring their diameter). ISM had a significantly higher percentage of primary neurosphere colonies compared with HF media cultures (~35% increase; P = 0.0095; n = 5; Fig. 4A). Neurospheres cultured in ISM also produced a significantly greater percentage of large spheres (~46% > 200μm, Fig. 4B) compared to HF media sphere size proportions (~4% > 200μm) (P = 0.016; n = 3; Fig. 4B).
Figure 4.
Testing of culturing factors.
Insulin supplementation yields more (A) and larger (B) primary neurospheres. Clonal density is achieved by seedings cells at 5000 (primary neurospheres, C) and 250 (secondary neurospheres, C) cells/well. Feeding cells at 5DIV did not significantly alter the resultant amount (E) or size (F) of neurospheres. Fungizone treatment significantly decreased the number of resultant neurospheres at both 5000 (G) or 10 000 (H) cells/well initial density. Treatment with both EGF and FGF (either 2 or 20 ng/mL) significantly increased the number of resultant neurospheres compared with treatment of either factor alone (I), representative images are included (J). Shh treatment (0.2 or 2 ng/mL) significantly increased the number of resultant neurospheres (K), representative images are included (L).
After choosing ISM as the preferred culture media, limiting dilution assays (LDA) were employed to determine the appropriate cell density for growing clonal E12.5 hypothalamic neurospheres. NeuroCult was also tested since it is the gold-standard commercial media, even though it contains both progesterone and insulin. The following broad range of serial dilutions for cell density were plated for primary sphere growth: 40 000, 20 000, 10 000, 5000, 2500, and 1000 cells/well in 1 mL volume in a standard 24-well plate (n = 10–12, Fig. 4C). According to the LDA criteria, clonality is reached at densities that produce 10 or fewer spheres (20,30). Given the need for released secreted paracrine signals to cue the growth of neurospheres, we categorized our clonal density as the lowest seeding density needed to produce 10 spheres; however, a lower density often still generated spheres but the risk of a complete lack of sphere growth increases as density is reduced. At 10DIV neurosphere colonies were quantified and the optimal primary clonal seeding density for the two sample culture medias was approximately 5000 cells/well, producing on average 12 spheres/well in NC media and 14 spheres/well in ISM media (Fig. 4C). An LDA was also performed on secondary spheres at cell densities of: 1000, 500, 250, 100, 50, and 25 cells/well (n = 7–8, Fig. 4D) in 1 mL volume in a standard 24-well plate. Similarly, both culture medias displayed the same optimal clonal seeding density (250 cells/well) for secondary neurosphere cultures forming on average 13 spheres/well in NC media and 14 spheres/well in ISM media.
The clonality of spheres is also dependent on the frequency of movement-induced aggregation, whereby the movement of plates needed to refresh the media or observe the cells causes dissociation of neurospheres as they are starting to form (51). However, since exogenously supplemented hormones can be degraded during culturing, replenishment (e.g., “feeding”) is a necessary step. To determine the impact of movement on the growth of hypothalamic neurospheres, we tested whether feeding (e.g., movement) or not feeding (e.g., no movement) differentially affected E12.5 hypothalamic neurospheres grown in ISM at primary clonal density (5000 cells/well). After 10DIV, sphere number and size showed no significant differences between cells that were refreshed with media and cells that were left undisturbed (Fig. 4E). Specifically, for both treatment conditions ~0.2% of colonies formed from the initial 5000 cells seeded per well (n = 5; Fig. 4E) and ~25% of these spheres were > 200μm (n = 5; Fig. 4F). Given that no detrimental effect of movement on clonality was observed, as well as the documented benefits to replenishing the media, supplements, and treatments (22, 51), we incorporated feeding cells at 5DIV (or 15DIV/25DIV for secondary/tertiary culturing) into our standard protocol.
Due to the presence of fungal contamination sometimes harbored in incubators and the long culturing time needed to grow tertiary neurospheres (~30 DIV), we initially added the anti-fungal Fungizone (FZ) to our standard media. However, we noticed a potential inhibitory effect in some of our cultures whereby neurosphere growth seemed to be stunted, which we hypothesized might be due to FZ in the media. In particular, ISM and ISM + FZ culture medias showed significant differences in E12.5 hypothalamic neurosphere numbers (Fig. 4G-H). The number of neurospheres formed in ISM + FZ was consistently lower than ISM media: ~68% reduction at 10 000 cells/well (P = 0.0139) and ~62% reduction at 5000 cells/well (P = 0.0005) (n = 7-1; Fig. 4G and 4H), demonstrating that FZ does indeed have a negative effect on the growth of hypothalamic neurospheres. Thus, unless fungus was a persistent problem, we removed this agent from our standard media recipe.
In other brain regions, growth factor supplementation is necessary for the induction of robust neurospheres (52). To determine the optimal components and concentrations for hypothalamic neurospheres, a growth factor (GF) test was performed. E12.5 hypothalamic cells were isolated and plated in ISM (5000 cells/well) in the following conditions: 20 ng EGF only, 20 ng bFGF only, 2 ng of both, or 20 ng of both. Neurospheres grew best when both GFs were supplemented, showing no significant differences between 2 ng/mL and 20 ng/mL concentrations (Fig. 4I, 4J). ISM with EGF only or bFGF only failed to support neurosphere growth, generating very few spheres (Fig. 4I). Specifically, in the hypothalamus the percent of spheres that grew with 2 ng/mL both GFs (0.28%) was significantly higher than those cultured with EGF only (0.016%; P = 0.030) and bFGF only (0.073%; P = 0.0164) (n = 4; Fig. 4I). As well, the percentage of hypothalamic spheres with 20 ng/mL both GFs (0.39%) grew significantly more than EGF (0.016%, P = 0.02) bFGF only cultures (0.073%; P = 0.049) (n = 4, Fig. 4I). Representative images illustrate that supplementation with both EGF and bFGF give maximal growth (Fig. 4J).
Finally, to determine if morphogen supplementation increased the growth of hypothalamic neurospheres, we tested the addition of Shh, a morphogen known to affect hypothalamic development prior and during neurogenesis (13,15). E12.5 neurospheres were plated at 10 000 cells/well and assessed after 10DIV (Fig. 4K). We observed a dose-dependent response to Shh, with a significant increase in the number of spheres grown in the presence of Shh (Fig. 4K). Primary spheres treated with Shh (0.2 ng/mL) generated significantly more neurospheres (~100% increase) compared with untreated controls (0.42% vs 0.21%; P = 0.0007; n = 10; Fig. 4K), and there was a dose-dependent increase (~63%) with 2 ng/mL treatment versus 0.2 ng/mL (0.687% vs 0.42%, P = 0.0128, n = 10, Fig. 4K). Representative images illustrate the influence of Shh on the growth of embryonic hypothalamic neurospheres (Fig. 4L) and demonstrated that these Shh-treated spheres were also larger in size.
Validating FACS to generate a purer population
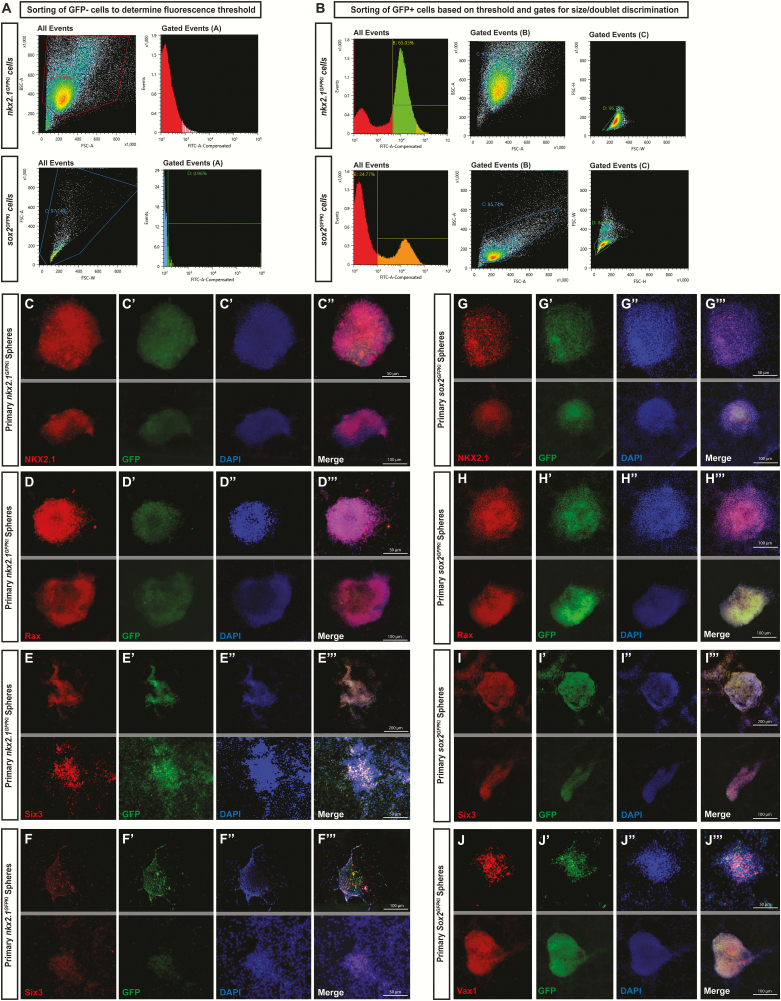
A drawback of our dissection approach arises from the fact that both NSPCs and postmitotic cells are captured using this methodology. Although only the progenitors survive in suspension culture across multiple passages, it is possible that experiments conducted on primary and secondary neurospheres include outcomes from interactions with postmitotic cells. For this reason, we also validated a FACS method to sort cells postdissection to generate a purer progenitor population. We utilized 2 strategies for this FACS: a Sox2GFPKI that nearly ubiquitously labels neural progenitors (28, 45), but has the caveat that nonhypothalamic cells might also be captured, and Nkx2.1GFPKI that specifically marks hypothalamic progenitors (53), but is less ubiquitous and key subpopulations of hypothalamic progenitors might be missed. In addition, Nkx2.1GFPKI also labels some postmitotic neurons starting at E12.5, which might contaminate the neurosphere. In both cases we utilized a fluorescent dissecting microscope to visualize GFP+ versus GFP− embryos prior to dissection, with cells from GFP- embryos used as a negative control to set the fluorescence threshold when sorting (Fig. 5A). Sorting on GFP+ hypothalamic tissue was first conducted using a gate for GFP fluorescence as determined from the negative control (Fig. 5B, left column), followed by sorting for size to remove nonviable cells as well as doublets and clusters (Fig. 5B, centre and right columns). Postsort cell viability was 66.4 ± 3.25% (n = 6) for Sox2+ samples and 64.1 ± 3.06% (n = 5) for Nkx2.1+ samples. Sorting cells into a small volume (~500 μL) of heat-inactivated fetal bovine serum helped viability and recovery, and as long as cells were promptly diluted and serum percentage reduced to under 5%, there was no effect of this step on culturing. However, FACS is a relatively lengthy (~60 minutes) and traumatic procedure, and in experiments when the dissection process was lengthened by even 15 to 20 minutes, we observed a dramatic drop in postsort viability (approximately 30% viability or less).
Figure 5.
Validation of hypothalamic identity of FACS-generated neurospheres.
FACS methodology is displayed for sorting GFP- cells to establish a fluorescence threshold (A) and for sorting GFP+ cells to isolate single cells for culturing (B). Neurospheres cultured from tissue dissected from NKX2.1GFPKI transgenic mice express the hypothalamic markers Nkx2.1 (C-C′′′), Rax (D-D′′′), Six3 (E-E′′′), and Vax1 (F-F′′′). Similarly, neurospheres cultured from tissue dissected from Sox2GFPKI also express Nkx2.1 (G-G′′′), Rax (H-H′′′), Six3 (I-I′′′), and Vax1 (J-J′′′). (2 representative fields are presented; grey lines divide different fields of exposure).
To characterize these FACS-generated neurospheres, we assessed their hypothalamic identity by conducting immunohistochemistry for Nkx2.1, Rax, Six3, and Vax1. Qualitatively, spheres generated from Nkx2.1GFPKI FAC sorted-cells (Fig. 5C-5F′′′) phenocopied neurospheres originating from our dissection technique (Fig. 2G-2M′′′), with strong near-ubiquitous expression of Nkx2.1 (Fig. 5C-5C′′′) and Rax (Fig. 5D-5D′′′). Six3 expression was more variable across spheres (Fig. 5E-5E′′′) and as with non-FACS clones, Vax1 was only sparsely expressed (Fig. 5F-5F′′′). Overall, all 4 hypothalamic markers were expressed in > 99% of spheres from Nkx2.1GFPKI. Neurospheres generated from cells labeled with Sox2GFPKI also expressed all 4 hypothalamic markers assessed (Fig. 5G-5J′′′), with Vax1 expression more widespread in these spheres (Fig. 5J-5J′′′) than Nkx2.1GFPKI (Fig. 5F-5F′′′) or non-FACS spheres (Fig. 2M-2M′′′). Combined, these data suggest that Nkx2.1GFPKI transgenic line might be best to isolate high quality, enriched hypothalamic neurospheres.
Discussion
Although hypothalamic neurospheres have been previously generated from fetal and adult rat and postnatal and adult murine tissue (24–27, 54, 55), no study has established and validated this technique for embryonic murine studies. Additionally, these previous papers use later developmental timepoints that fall after peak neurogenesis and when progenitors might be undergoing neurogenic symmetric division, and also fail to demonstrate that the resultant primary neurospheres are of hypothalamic origin, which is essential to relate these in vitro findings back to in vivo developmental mechanisms. Here we provide a method for murine researchers to reliably isolate and culture NSPCs at the peak of neurogenesis. This technique can be utilized in concert with the wide variety of existing mouse genetic lines, including knockout, knockin, and reporters, as well as other established molecular techniques to better characterize hypothalamic progenitor behavior during this sensitive timepoint of embryonic development. To our knowledge, this method is the earliest isolation, expansion, and validation of hypothalamic neurospheres from fetal mouse brains.
In our cultures, neurospheres expressed multiple markers required for hypothalamic specification and patterning. Our findings are in agreement with previous reports that mouse embryonic stem cells can be induced to differentiate into Rax+/Six3+/Vax1+ hypothalamic progenitors that are further promoted by Shh treatment (11). The early hypothalamic specification marker Vax1 was less ubiquitous in our hypothalamic neurospheres compared with the other proteins, indicating a potential loss of Vax expression as these hypothalamic cells become specified into neuronal phenotypes. These data were further supported by the broad expression of the hypothalamic marker Nkx2.1, whose progenitor expression peaks in the mouse brain at E12.5 (15), a time point that coincides with the collection of hypothalamic neural progenitors. We also observed strong expression of Sox2 and Sox3, indicating that our spheres consist primarily of progenitor cells that remain multipotent through multiple passages, further reinforced by the limited number of cells that express glial makers (e.g., PdgfRα, GFAP) and the postmitotic neuronal marker Tuj1. Additionally, the expression of these postmitotic markers further demonstrates that these hypothalamic neurospheres comprise cells capable of differentiating into neurons and glia. Prior work in neurospheres shows that cellular differentiation can be dramatically accelerated by the removal of growth factors from the media (56), and our data herein confirms that without growth factors hypothalamic neurospheres differentiate much more rapidly. Combined, the presence of these hypothalamic markers confirms that our method yields a population enriched in hypothalamic multipotent progenitors capable of proliferating and/or differentiating into neuron, astrocytes, or oligodendrocytes.
Given that our microdissection technique alone cannot be guaranteed to exclude nonhypothalamic precursors or nonproliferative postmitotic cells, we employed Sox2GFPKI (28) and Nkx2.1GFPKI (29) lines and FACS to isolate the GFP+ cells needed to generate the hypothalamic neurospheres. Since Sox2 is highly expressed in NSPCs throughout the brain (28, 45), this line enabled us to isolate an enriched stem cell population but has the caveat that nonhypothalamic cells might be captured as well, whereas Nkx2.1-GFP+ mark hypothalamic neural progenitors, but might also collect hypothalamic postmitotic neurons in the FAC sort. Consistently, Nkx2.1GFPKI neurospheres were > 99% positive for hypothalamic markers, while Sox2GFPKI neurospheres had a higher number of cells but the resultant neurospheres were only ~90% hypothalamic, indicating that some contamination from nearby brain regions (likely the thalamus) occurred. Ideally, a Sox2-GFP/Nkx2.1-mCherry (for example) double transgenic line could be especially helpful to yield a cellular population that is uniformly hypothalamic and NPSCs. Of note, we found the FACS process stressful to the cells and it required extensive troubleshooting to optimize. Successful FACS requires speed and care in the dissection, as we observed a clear association between longer dissection times and reduced post-FACS cell survival. Given that isolated postmitotic cells are unlikely to survive the dissection process or in the suspension culture that the neurospheres grow in, we suggest that unless a pure progenitor pool is expressly required to answer experimental questions, the FACS process may be an unnecessary complication.
Given that neurogenic murine hypothalamic neurospheres have yet to be defined, we characterized the parameters and conditions necessary for their growth, summarized in Table 1. Classically, the neurosphere assay is performed using commercially available media, but these premade solutions contain hormones, such as insulin and progesterone, an upstream sex steroid precursor that could lead to local sex steroid synthesis, producing estrogen that may then act on fetal neural progenitors. In contrast, insulin is a common ingredient in NSPC culturing that acts as a growth factor and mediates metabolic, mitotic, and anti-apoptotic actions (57,58). Furthermore, the withdrawal of insulin supplementation can result in significant cell death and/or delayed cell growth (57). Thus, a pure hormone-free media was not desirable. To circumvent the concern of progesterone, we created hormone-free (HF) and insulin-supplemented (ISM) variation in-house media protocol that was adapted from Reynolds and Weiss (23). Our results showed that ISM promotes healthier and improved neurosphere growth compared with a completely HF version, as expected, and we employed ISM as the most supportive media for hypothalamic neurosphere growth. We also assessed clonal density, a fundamental concept for accurate quantifications of NSPCs. Clonal density indicates an initial cell seeding value that ensures a 1:1 ratio of NSPC to neurosphere. However, since these numbers were not previously determined for the hypothalamus, we used an LDA to define clonal density. It became apparent that a fine balance between obtaining clonality and still maintaining the appropriate niche environment for interactions and optimal growth must be kept. According to the LDA criteria, clonality is reached at densities that produce a single digit number of spheres (20,30) and we found that our clonal density (primary 5000 cells/well; secondary/tertiary 250 cells/well) to be somewhat lower but in the same range as literature values (51). Finally, movement-induced aggregation can occur when spheres are disturbed during their growth phase, negatively affecting clonality (51). We saw no aggregation of spheres by the minor disturbances needed to remove cells for feeding, and instead saw marked benefits in media condition and cell quality over the culturing period, so replenishing media at the halfway point of each 10-day culturing window was deemed the best compromise between limiting disturbances and maintaining a nutrient-rich environment. Combined, here we establish the optimal conditions for growing hypothalamic neurospheres.
Table 1.
Summary of Hypothalamic Neurosphere Culturing Protocol
| Culture Media | Insulin-supplemented media (ISM) provides required environment for strong growth without introducing exogenous hormones. Ingredients: 1x DMEM/F12 100 U/mL penicillin-streptomycin, 1 mg/mL heparan sulfate sodium salt 0.6% glucose 0.1125% bicarbonate 0.005M HEPES 2mM glutamine 20 μg/mL apo-transferrin 2.5 μg/mL insulin 1 μg/mL putrescine dihydrochloride 3mM sodium selenite |
| Clonal Density | Primary cells: 5000 cells/well Secondary cells: 250 cells/well (each in 1mL volume in standard 24-well plate) |
| Refreshment Routine | Refresh 50% media and treatment components at 5DIV |
| Growth Factors | ISM—Require both EGF & bFGF (2ng/mL or 20ng/mL) |
| Shh Supplement | Recommended to enhance neurosphere growth (2ng/mL) |
| Fungizone Supplement | Not recommended, strongly inhibits neurosphere growth |
Fungal contamination can be an issue in primary or heterologous cultures that are maintained for long periods of time, and antifungals have been used in some neurosphere studies from adult murine brain tissue and from cell lines (59, 60). This led us to test the effects of addition of FZ to our ISM. Interestingly, FZ caused a reduction in the number of primary neurospheres formed, indicating that FZ inhibits the ability of NSPCs to generate neurospheres. Fungizone is an Amphotericin B antifungal that mechanistically acts by binding egosterol, a component of fungal cell membranes, forming a pore and causing rapid leakage of monovalent ions and ultimately death (61). FZ also causes oxidative stress within cells and/or potentially causes cellular toxicity by attacking sterols in host membranes (61). Therefore, it is likely that FZ targets primary NSPCs, particularly those with high cholesterol content in their membrane, resulting in a reduced amount of viable sphere forming cells. It is unclear whether embryonic NSPCs are more susceptible to this result compared with adult NSPCs, but we found a clear loss in sphere-forming capacity in the presence of FZ. Thus, we do not recommend the use of FZ in these neurosphere cultures. Therefore, extra care for appropriate aseptic techniques is critical if contamination issues are encountered.
In order to provide a culture that would best sustain hypothalamic identity, the necessity of intrinsic and/or extrinsic signals was considered. The addition of GF mitogens EGF and/or bFGF is a defining feature of neurosphere media, as they allow for the maintenance of a stem-cell like environment. The responsiveness to GFs is region- and cell type-specific, which prompted us to investigate the GF requirements for the embryonic hypothalamus (62). In keeping with findings from other regions, sphere growth in ISM required the presence of both EGF and bFGF. Given that Shh induces the pan-hypothalamic marker Nkx2.1 and is a morphogen known to influence hypothalamic precursor cells (15), we tested its influence on our neurosphere cultures and observed a robust increase in size and numbers of primary neurospheres in the presence of Shh. This indicates the presence of more activated sphere forming cells and more proliferation, suggesting an alteration in cell cycle length and/or cellular division. Recently, Shh was shown to play an important role in controlling the interplay between quiescent and activated adult neural stem cells in the ventricular-subventricular zone of the hippocampus, resulting in shortened G1 and S-G2/M phases in adult activated neural stem cells (63). Since the developing hypothalamus also contains quiescent NSPCs reserved for adult life (64,65), this could be a plausible mechanism underlying the observed Shh effect.
Compared with other brain regions, the use of fetal hypothalamic neurospheres is relatively new in the literature, with no extensive optimization procedures described. As such, we identify and verify a microdissection method for the isolation of hypothalamic NSPCs during peak neurogenesis, as well as a supplementary FACS option for isolation of a purer progenitor pool. Significantly, our data highlight key components of the neurosphere assay consisting of media conditions, clonal density, growth factor requirements, and the influence of Shh. Altogether, we provide a working protocol and an important basis for the standardization of NSPC conditions for embryonic hypothalamic neurospheres during peak neurogenesis.
Acknowledgements
We thank Rozina Hassam and Dr. Samuel Weiss from the University of Calgary for helpful instruction on initial neurosphere culturing techniques. Sox2GFPKI mice were generously donated by Dr. Jeff Biernaskie at the University of Calgary. Nkx2.1GFPKI mice were generously shared by Dr. Darrell Kotton at Boston University.
Financial Disclosure: This study was supported by a University of Calgary Cumming School of Medicine graduate scholarship to H.T; postdoctoral fellowships to D.N. from the Canadian Institutes of Health Research (201611MFE-381713-245522), Alberta Innovates Health Solutions (201610151), and the University of Calgary Eyes High Competition; and a Natural Sciences and Engineering Research Council of Canada Discovery grant (DG386445) to D.M.K.
Glossary
Abbreviations
- bFGF
fibroblast growth factor basic
- DIV
days in vitro
- E
embryonic day
- EGF
epidermal growth factor
- FZ
Fungizone
- GF
growth factor
- GFP
green fluorescent protein
- HF
hormone free
- ISM
insulin-supplemented neurosphere media
- LDA
limiting dilution assay
- NSPCs
neural stem and progenitor cells
- PBS
phosphate-buffered saline
- PS
penicillin-streptomycin
- VZ
ventricular zone
Additional Information
Disclosure Summary: The authors have no competing financial interests in this work. H.F.T., D.N., L.C.S. have nothing to declare. D.M.K. is a cofounder of Path Therapeutics, focused on epilepsy drug discovery.
Data Availability. The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Markakis EA. Development of the neuroendocrine hypothalamus. Front Neuroendocrinol. 2002;23(3):257–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biran J, Tahor M, Wircer E, Levkowitz G. Role of developmental factors in hypothalamic function. Front Neuroanat. 2015;9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maggi R, Zasso J, Conti L. Neurodevelopmental origin and adult neurogenesis of the neuroendocrine hypothalamus. Front Cell Neurosci. 2014;8:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vadakkadath Meethal S, Atwood CS. The role of hypothalamic-pituitary-gonadal hormones in the normal structure and functioning of the brain. Cell Mol Life Sci. 2005;62(3):257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bedont JL, Newman EA, Blackshaw S. Patterning, specification, and differentiation in the developing hypothalamus. Wiley Interdiscip Rev Dev Biol. 2015;4(5):445–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nesan D, Kurrasch DM. Genetic programs of the developing tuberal hypothalamus and potential mechanisms of their disruption by environmental factors. Mol Cell Endocrinol. 2016;438:3–17. [DOI] [PubMed] [Google Scholar]
- 8. Blackshaw S, Scholpp S, Placzek M, Ingraham H, Simerly R, Shimogori T. Molecular pathways controlling development of thalamus and hypothalamus: from neural specification to circuit formation. J Neurosci. 2010;30(45):14925–14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao Y, Sun T. Molecular regulation of hypothalamic development and physiological functions. Mol Neurobiol. 2016;53(7):4275–4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu F, Kar D, Gruenig N, et al. Rax is a selector gene for mediobasal hypothalamic cell types. J Neurosci. 2013;33(1):259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wataya T, Ando S, Muguruma K, et al. Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. Proc Natl Acad Sci U S A. 2008;105(33):11796–11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Plata-Salamán CR. Epidermal growth factor and the nervous system. Peptides. 1991;12(3):653–663. [DOI] [PubMed] [Google Scholar]
- 13. Szabó NE, Zhao T, Cankaya M, Theil T, Zhou X, Alvarez-Bolado G. Role of neuroepithelial Sonic hedgehog in hypothalamic patterning. J Neurosci. 2009;29(21):6989–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Szarek E, Cheah PS, Schwartz J, Thomas P. Molecular genetics of the developing neuroendocrine hypothalamus. Mol Cell Endocrinol. 2010;323(1):115–123. [DOI] [PubMed] [Google Scholar]
- 15. Alvarez-Bolado G, Paul FA, Blaess S. Sonic hedgehog lineage in the mouse hypothalamus: from progenitor domains to hypothalamic regions. Neural Dev. 2012;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126(15):3359–3370. [DOI] [PubMed] [Google Scholar]
- 17. Marsters CM, Rosin JM, Thornton HF, et al. Oligodendrocyte development in the embryonic tuberal hypothalamus and the influence of Ascl1. Neural Dev. 2016;11(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rakic P, Cameron RS, Komuro H. Recognition, adhesion, transmembrane signaling and cell motility in guided neuronal migration. Curr Opin Neurobiol. 1994;4(1):63–69. [DOI] [PubMed] [Google Scholar]
- 19. Bez A, Corsini E, Curti D, et al. Neurosphere and neurosphere-forming cells: morphological and ultrastructural characterization. Brain Res. 2003;993(1-2):18–29. [DOI] [PubMed] [Google Scholar]
- 20. Singec I, Knoth R, Meyer RP, et al. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat Methods. 2006;3(10):801–806. [DOI] [PubMed] [Google Scholar]
- 21. Jensen JB, Parmar M. Strengths and limitations of the neurosphere culture system. Mol Neurobiol. 2006;34(3):153–161. [DOI] [PubMed] [Google Scholar]
- 22. Xiong F, Gao H, Zhen Y, et al. Optimal time for passaging neurospheres based on primary neural stem cell cultures. Cytotechnology. 2011;63(6):621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. [DOI] [PubMed] [Google Scholar]
- 24. Desai M, Li T, Ross MG. Hypothalamic neurosphere progenitor cells in low birth-weight rat newborns: neurotrophic effects of leptin and insulin. Brain Res. 2011;1378:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salvi R, Arsenijevic Y, Giacomini M, et al. The fetal hypothalamus has the potential to generate cells with a gonadotropin releasing hormone (GnRH) phenotype. Plos One. 2009;4(2):e4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sousa-Ferreira L, Álvaro AR, Aveleira C, et al. Proliferative hypothalamic neurospheres express NPY, AGRP, POMC, CART and Orexin-A and differentiate to functional neurons. Plos One. 2011;6(5):e19745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cariboni A, Conti L, Andrè V, Aprile D, Zasso J, Maggi R. Establishment of a radial glia-like mouse fetal hypothalamic neural stem cell line (AC1) able to differentiate into neuroendocrine cells. Neurogenesis (Austin). 2014;1(1):e29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ellis P, Fagan BM, Magness ST, et al. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26(2-4):148–165. [DOI] [PubMed] [Google Scholar]
- 29. Longmire TA, Ikonomou L, Hawkins F, et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10(4):398–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mori H, Ninomiya K, Kino-oka M, et al. Effect of neurosphere size on the growth rate of human neural stem/progenitor cells. J Neurosci Res. 2006;84(8):1682–1691. [DOI] [PubMed] [Google Scholar]
- 31. Azari H, Sharififar S, Rahman M, Ansari S, Reynolds BA. Establishing Embryonic Mouse Neural Stem Cell Culture Using the Neurosphere Assay. J. Vis. Exp. 2011;(47):e2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.RRID:AB_793532. https://scicrunch.org/resolver/RRID:AB_793532.
- 33.RRID:AB_2286686. https://scicrunch.org/resolver/RRID:AB_2286686.
- 34.RRID:AB_2239933. https://scicrunch.org/resolver/RRID:AB_2239933.
- 35.RRID:AB_2728521. https://scicrunch.org/resolver/RRID:AB_2728521.
- 36.RRID:AB_2236897. https://scicrunch.org/resolver/RRID:AB_2236897.
- 37.RRID:AB_10013382. https://scicrunch.org/resolver/RRID:AB_10013382.
- 38.RRID:AB_11028612. https://scicrunch.org/resolver/RRID:AB_11028612.
- 39.RRID:AB_11181864. https://scicrunch.org/resolver/RRID:AB_11181864.
- 40.RRID:AB_447379. https://scicrunch.org/resolver/RRID:AB_447379.
- 41.RRID:AB_1566562. https://scicrunch.org/resolver/RRID:AB_1566562.
- 42. Simpson TI, Pratt T, Mason JO, Price DJ. Normal ventral telencephalic expression of Pax6 is required for normal development of thalamocortical axons in embryonic mice. Neural Dev. 2009;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Robertshaw E, Matsumoto K, Lumsden A, Kiecker C. Irx3 and Pax6 establish differential competence for Shh-mediated induction of GABAergic and glutamatergic neurons of the thalamus. Proc Natl Acad Sci U S A. 2013;110(41):E3919–E3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Manuel MN, Mi D, Mason JO, Price DJ. Regulation of cerebral cortical neurogenesis by the Pax6 transcription factor. Front Cell Neurosci. 2015;9:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pevny LH, Nicolis SK. Sox2 roles in neural stem cells. Int J Biochem Cell Biol. 2010;42(3):421–424. [DOI] [PubMed] [Google Scholar]
- 46. Rogers N, Cheah PS, Szarek E, Banerjee K, Schwartz J, Thomas P. Expression of the murine transcription factor SOX3 during embryonic and adult neurogenesis. Gene Expr Patterns. 2013;13(7):240–248. [DOI] [PubMed] [Google Scholar]
- 47. Hallonet M, Hollemann T, Wehr R, et al. Vax1 is a novel homeobox-containing gene expressed in the developing anterior ventral forebrain. Development. 1998;125(14):2599–2610. [DOI] [PubMed] [Google Scholar]
- 48. Hallonet M, Hollemann T, Pieler T, Gruss P. Vax1, a novel homeobox-containing gene, directs development of the basal forebrain and visual system. Genes Dev. 1999;13(23):3106–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88(1):91–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu MV, Manoli DS, Fraser EJ, et al. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139(1):61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Coles-Takabe BL, Brain I, Purpura KA, et al. Don’t look: growing clonal versus nonclonal neural stem cell colonies. Stem Cells. 2008;26(11):2938–2944. [DOI] [PubMed] [Google Scholar]
- 52. Caldwell MA, He X, Wilkie N, et al. Growth factors regulate the survival and fate of cells derived from human neurospheres. Nat Biotechnol. 2001;19(5):475–479. [DOI] [PubMed] [Google Scholar]
- 53. Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506(1):16–29. [DOI] [PubMed] [Google Scholar]
- 54. Chipperfield H, Cool SM, Bedi K, Nurcombe V. Adult CNS explants as a source of neural progenitors. Brain Res Brain Res Protoc. 2005;14(3):146–153. [DOI] [PubMed] [Google Scholar]
- 55. Biehl MJ, Kaylan KB, Thompson RJ, et al. Cellular fate decisions in the developing female anteroventral periventricular nucleus are regulated by canonical Notch signaling. Dev Biol. 2018;442(1):87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Deleyrolle LP, Reynolds BA. Isolation, expansion, and differentiation of adult Mammalian neural stem and progenitor cells using the neurosphere assay. Methods Mol Biol. 2009;549:91–101. [DOI] [PubMed] [Google Scholar]
- 57. De Meyts P. Insulin and its receptor: structure, function and evolution. Bioessays. 2004;26(12):1351–1362. [DOI] [PubMed] [Google Scholar]
- 58. Kim JJ, Accili D. Signalling through IGF-I and insulin receptors: where is the specificity? Growth Horm IGF Res. 2002;12(2):84–90. [DOI] [PubMed] [Google Scholar]
- 59. Jessberger S, Clemenson GD Jr, Gage FH. Spontaneous fusion and nonclonal growth of adult neural stem cells. Stem Cells. 2007;25(4):871–874. [DOI] [PubMed] [Google Scholar]
- 60. Nör C, Sassi FA, de Farias CB, et al. The histone deacetylase inhibitor sodium butyrate promotes cell death and differentiation and reduces neurosphere formation in human medulloblastoma cells. Mol Neurobiol. 2013;48(3):533–543. [DOI] [PubMed] [Google Scholar]
- 61. Baginski M, Czub J. Amphotericin B and its new derivatives - mode of action. Curr Drug Metab. 2009;10(5):459–469. [DOI] [PubMed] [Google Scholar]
- 62. Nieto-Estévez V, Pignatelli J, Araúzo-Bravo MJ, Hurtado-Chong A, Vicario-Abejón C. A global transcriptome analysis reveals molecular hallmarks of neural stem cell death, survival, and differentiation in response to partial FGF-2 and EGF deprivation. Plos One. 2013;8(1):e53594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Daynac M, Tirou L, Faure H, et al. Hedgehog controls quiescence and activation of neural stem cells in the adult ventricular-subventricular zone. Stem Cell Reports. 2016;7(4):735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kokoeva MV, Yin H, Flier JS. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol. 2007;505(2):209–220. [DOI] [PubMed] [Google Scholar]
- 65. Migaud M, Batailler M, Segura S, Duittoz A, Franceschini I, Pillon D. Emerging new sites for adult neurogenesis in the mammalian brain: a comparative study between the hypothalamus and the classical neurogenic zones. Eur J Neurosci. 2010;32(12):2042–2052. [DOI] [PubMed] [Google Scholar]