Abstract
Endometriosis is an inflammatory disease that primarily affects women during their reproductive years, and since current hormonal therapies are of concern, new hormone-independent treatment regimens are needed. The orphan nuclear receptor 4A1 (NR4A1, Nur77) is expressed in patient-derived (stromal) endometriotic cells and also epithelial cell lines, and we observed that knockdown of NR4A1 in patient-derived ectopic endometrium-isolated ovarian endometrioma (ESECT)-7 and ESECT-40 cells decreased cell proliferation and induced apoptosis. Moreover, the treatment of these cells with bis-indole derived NR4A1 ligands 1,1-bis(3’-indolyl)-1-(p-hydroxyphenyl)methane (DIM-C-pPhOH) and its buttressed 3-chloro-5-methoxy analog (DIM-C-pPhOH-3-Cl-5-OCH3) inhibited cell growth and induced apoptosis and related genes. The compounds exhibit NR4A1 antagonist activities in both functional and transactivation assays whereas these effects were not observed in normal endometrial cells. We also observed that NR4A1 knockdown and treatment with NR4A1 antagonists decreased fibrosis, α-smooth muscle actin, and related pro-fibrotic genes in ESECT-7 and ESECT-40 cells, and similar results were observed in epithelial-derived endometriotic cell lines. Moreover, in an endometriosis mouse model with auto-transplantation and also in severe combined immune deficiency mice transplanted with human endometriotic cells treatment with 25 mg/kg/day DIM-C-pPhOH-3-Cl-5-OCH3 significantly inhibited growth and expansion of endometriotic lesions. Thus, bis-indole–derived NR4A1 ligands represent a novel class of drugs as nonhormonal therapy for endometriosis.
Keywords: antagonists, endometriosis, fibrosis, growth, NR4A1
Endometriosis is a common but complex inflammatory disease that primarily affects women during their reproductive years, and it is estimated that 5 500 000 women in the United States and 176 000 000 women worldwide exhibit symptoms of endometriosis (1–4). Endometriosis develops when cells lining the uterus are implanted at distal sites, which can include the pelvic area, peritoneal surfaces, ovaries, ligaments, bowel, and bladder. Endometriosis originates, in part, from retrograde menstruation, resulting in endometriotic lesions, which are variable in their severity and pain and overall short-term or chronic adverse health effects. Once diagnosed, the staging of the disease (ie, stage I–IV) and its location (5–7) are important for determining the appropriate treatment regimen, which may include surgical removal of the endometriotic tissues, and hormonal therapies, which include progestins, oral contraceptives, and gonadotropin-releasing hormone antagonists (8–14). There are serious concerns regarding the use of hormonal therapies for treating endometriosis in women of child-bearing age, and less toxic hormone-independent treatments need to be developed. The signaling pathways activated in endometriosis resemble those observed in cancer and include inflammatory-mediated responses associated with macrophage recruitment and activation, activation of growth-promoting and survival genes/pathways, and angiogenic/pro-migration genes/pathways (3,4,15–18). For example, mammalian target of rapamycin (mTOR) signaling is activated in endometriosis. However, the use of mTOR inhibitors for treating this disease is limited due to toxicity concerns.
The orphan nuclear receptors NR4A1, NR4A2, and NR4A3 are immediate early genes induced by multiple stressors, and NR4A receptors play an important role in maintaining cellular homeostasis and disease (17–19). There is increasing evidence for the role of these receptors in metabolic, cardiovascular, and neurological functions as well as in inflammation and inflammatory diseases and in immune functions and cancer. NR4A1 regulates cancer cell proliferation, survival, cell cycle progression, migration, and invasion in lung, melanoma, pancreatic, colon, cervical, ovarian, and gastric cancer and Rhabdomyosarcoma cell lines, and these responses are inhibited by bis-indole derived NR4A1 ligands that act as antagonists in cancer cells (20–30).
There is also evidence that NR4A1 is overexpressed in endometriosis (31–33), and it was reported that after ultrasound-guided ethanol scleropathy in patients with high serum expression of NR4A1, levels of this receptor were significantly decreased after therapy (33). Levels of phosphorylated NR4A1 were higher in ovarian endometriotic tissue compared to normal endometrium; moreover, loss of NR4A1-stimulated fibrogenesis and activation of NR4A1 by the NR4A1 agonist cytosporone β suggested this receptor protected against fibrogenesis. Ishikawa endometrial cancer cells are frequently used as models for endometriotic epithelial cells, and our recent study clearly showed that NR4A1 exhibited pro-endometriotic activities that were inhibited by NR4A1 antagonists (34). In this study, we have used patient-derived stromal endometriotic cells and demonstrate that NR4A1 regulates multiple pro-endometriotic genes/pathways that are inhibited by NR4A1 antagonists.
Materials and Methods
Primary human endometriotic stromal cells from endometriosis patients
Ovarian endometrioma were isolated from endometriosis patients in the proliferative phase following the institutional review board–approved protocol. Isolated ectopic lesions were digested with collagenase type 3 (300 μg/mL) and deoxyribonuclease I (40 μg/mL) for 90 min at 37°C, and then tissues were filtered through 150, 100, and 40 µm sieves to isolate endometrial stromal cells (35). The collected endometrial stromal cells were cultured in DMEM/F12 with 10% fetal bovine serum plus 1 × antibiotic/antimycotic solution and then validated by flow cytometry with a CD90 antibody (a mesenchymal marker). Henceforth, we refer to the stromal cells as ectopic endometrium-isolated ovarian endometrioma (ESECT). As the control, primary normal endometrial stromal cells (NESCs) isolated from the biopsy of the eutopic endometrium of normal women (NEM) in the proliferative phase. The working passage numbers of primary human cells would be less than 10 as these are the most similar to the original line. Therefore, we used primary human endometrial stromal cells from less than 10 passages in our study. All cells were incubated at 37°C in CO2 incubator in an atmosphere of humidified 5% CO2 and 95% air.
Reagents and antibodies
Annexin V Dead Cell Apoptosis Kit (V13241) was purchased from Invitrogen. The primary antibodies used were epidermal growth factor receptor (EGFR; 4267, 1:1000) (36), survivin (2808, 1:1000) (37), cleaved caspase 3 (CL-CSAP3; 9661, 1;1000) (38), and cleaved PARP (9541, 1:1000) (39) from Cell Signaling Technology (Danvers, MA, US); c-Myc (sc-40, 1:500) (40), Bcl-2 (sc-509, 1:500) (41), and Bax (sc-20067, 1:500) (42) from Santacruz Biotechnology (Santacruz, CA, US); NR4A1 (ab109180, 1:3000) (43) and α-smooth muscle actin (SMA; ab32575, 1:3000) (44) from Abcam (Cambridge, MA, US); β-actin (A1978, 1:10000) (45) from Sigma Aldrich Corporation (Milwaukee, WI, US); and COL1A1 (GTX112731, 1:2000) (46), CTGF (GTX124232, 1:2000) (47), and fibronectin (FN; GTX112794, 1:2000) (48) from GeneTex, Inc. (Irvine, CA, US). Secondary antibodies for rabbit (7074) (49), mouse (7076) (50), and anti-rabbit Alexa Fluor® (4412) (51) were purchased from Cell Signaling Technology (Danvers, MA, US). Two siNR4A1 oligonucleotides used in this study were SASI_Hs02_00333289 and SASI_Hs02_00333290, and nontargeted scrambled small interfering ribonucleic acids (siRNA; iGL2) were used as a control from Sigma Aldrich Corporation (The Woodlands, TX, US). The bis-indole–derived NR4A1 ligands 1,1-bis(3’-indolyl)-1-(p-hydroxyphenyl)methane (DIM-C-pPhOH), 1,1-bis(3’-indolyl)-1-3-chloro-5-methoxyphenyl)methane (DIM-C-pPhOH-3-Cl-5-OCH3), 1,1-bis(3’-indolyl-1-(3,5-dibromo-4-hydroxyohenyl)methane (DIMC-C-pPhOH-3,5-Br2), and 1,1-bis(3’-indolyl)-1-(3-chloro-4-hydroxyphenyl)methane (DIM-C-pPhOH) were prepared as described (24,34).
Cell proliferation assay
Patient-derived endometriotic cells ESECT-7 and ESECT-40 were seeded into a 96-well plate, and the cells were then treated for 24 h with either dimethyl sulfoxide (DMSO) or different concentrations of DIM-C-pPhOH and DIM-C-pPhOH-3-Cl-5-OCH3. The ESECT-7 and ESECT-40 were treated with 50 nM of 2 siNR4A1 oligonucleotides to downregulate NR4A1; 50 nM of non-target siRNAs was employed as the control of siRNA. Afterward, the medium was removed, and the MTT solution diluted in phosphate-buffered saline (PBS) was added to cell cultures. After 3-h incubation, the medium was aspirated and washed with PBS. DMSO was added and incubated at 37°C for 10 min, and absorbance was measured at 570 nM.
Western blotting
ESECT-7 and ESECT-40 cells (2 × 105) were seeded and allowed to attach for 24 h, and cells were then treated for 24 h with either DMSO or different concentrations of DIM-C-pPhOH and DIM-C-pPhOH-3-Cl-5-OCH3. Cells were then lysed and whole-cell lysates were resolved in 10% SDS-PAGE gels, and proteins were transferred using polyvinylidene fluoride membrane by wet blotting followed by primary and secondary antibody incubation and detected using enhanced chemiluminescence reagent as previously described (34).
Annexin V staining
ESECT-7 and ESECT-40 cells were seeded in Nunc chambered cover glass followed by various drug treatments. The cells were then washed with ice-cold PBS, and 5 µL Alexa Fluor® 488 Annexin V with 100 µg/mL propidium iodide (as per the manufacturer’s instructions) were added to the cells and incubated for 15 min, and the cells were observed using a Zeiss confocal fluorescence microscope.
Immunofluorescence
ESECT-7 and ESECT-40 cells were seeded in Nunc chambered coverglass followed by various drug treatments. The cells were fixed with 4% paraformaldehyde in PBS for 20 min at 37°C. Cells were then blocked and incubated overnight with primary α-SMA antibody in the buffer (5% bovine serum albumin in PBS) at 4°C, followed by incubation with Alexa Fluor®–conjugated secondary antibody at a dilution of 1:250 for 2 h at room temperature. Finally, cells were observed using a Zeiss confocal fluorescence microscope.
RNA interference
ESECT-7 and ESECT-40 cells were seeded in 6-well plates and allowed to grow to 60% confluence (24 h), and then transfections were performed with Lipofectamine 2000 according to the manufacturer’s protocol. Both siNR4A1 oligonucleotides and nontargeted control siRNAs were used. Six hours after transfection, the medium was replaced with fresh medium and left for 72 h, and then the cells were harvested, and protein expression was determined.
Quantitative real-time polymerase chain reaction
Total ribonucleic acid (RNA) was isolated from cultured cells according to the manufacturer’s instructions (Zymo Research, Irvine, CA, US). The concentration and purity of the RNA samples were determined using a nanodrop spectrophotometer. Total RNA was reverse transcribed using iTaq Universal SYBR Green One-Step Kit (Thermo Fisher Scientific, Grand Island, NY, US) using the manufacturer’s protocol with the CFX384 real-time polymerase chain reaction system (Bio-Rad). The comparative cycle threshold method was used for relative quantitation of samples. Values for each gene were normalized to expression levels of TATA-binding protein. The sequences of the primers used for real-time polymerase chain reaction included the following: α-SMA, 5’-GGC CGA GAT CTC ACT GAC TAC-3′ (sense) and 5′- TTC ATG GAT GCC AGC AGA -3′ (antisense); COL1A1, 5’-CAG CCG CTT CAC CTA CAG C-3’ (sense) and 5’-TTT TGT ATT CAA TCA GTG TCT TGC C-3’ (antisense); CTGF, 5’-TTG GCC CAG ACC CAA CTA TG-3’(sense) and 5’-CAG GAG GCG TTG TCA TTG GT-3’ (antisense); FN, 5’-GGG AGC CTC GAA GAG C-3’ (sense) and 5’-AAC AAG TAC AAA CCA ACG CA-3’ (antisense); and TATA-binding protein, 5′-GAT CAG AAC AAC AGC CTG CC-3′ (sense) and 5′-TTC TGA ATA GGC TGT GGG GT-3′ (antisense).
Luciferase assay
ESECT-7 and ESECT-40 cells were plated on 12-well plates in DMEM/F12 supplemented with 2.5% charcoal-stripped fetal bovine serum. After overnight attachment and growth, various amounts of deoxyribonucleic acid (ie, upstream activation sequence (UAS)x5-Luc [500 ng], GAL4-NR4A1 [50 ng], nerve growth factor β response element [NBRE]x3-Luc [800 ng], NurREx3-Luc [800 ng], and Flag-NR4A1 [80 ng]) were co-transfected into each well using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, US) according to the manufacturer’s protocol. After 6 h of transfection, cells were treated with plating medium (as previously described) containing either solvent (DMSO) or indicated concentrations of the compound for 18 h. Cells were then lysed, and cell extracts were used for chemiluminescence quantification of luciferase activity. Luciferase activity values were normalized against corresponding protein concentration values determined by Bradford assay. Both GAL4- and Flag-NR4A1 constructs contain full-length NR4A1 coding sequence and all the plasmids used in this study were previously described (24).
Endometriosis mouse model with auto-transplantation
One uterine horn was isolated from a female mouse (6 weeks old; C57BL/6J) under anesthesia, and then the uterine horn was longitudinally cut. Using a 2-mm dermal biopsy punch, 1 endometrial fragment was obtained from the isolated uterus and subsequently sutured to the mesenteric membrane attached to the intestine of the same mouse (52). The abdominal incision then was closed by suture. Before harvesting ectopic lesions, the mouse estrous cycle was determined using vaginal cytology (53). At the estrus stage in the third week after endometriosis induction, ectopic lesions were isolated from mice with endometriosis. As the endometriosis control, uteri were isolated from C57BL/6J female mice without endometriosis at the estrus stage at 10 weeks of age.
Endometriosis mouse model by hetero-transplantation with immortalized human endometrial cells
The luciferase stable immortalized human endometrial epithelial cells (LIHEECs) and luciferase stable immortalized human endometrial stromal cells (LIHESCs) were generated from immortalized human endometrial stromal cells isolated from endometria obtained from hysterectomies for benign conditions (54) and immortalized human endometrial epithelial cells (IHEECs) generated from ovarian endometrioma (55) by transducing lentivirus-expressing luciferase and then the expression of luciferase was validated with the luciferase activity assay (56). Matrigel contained the mixture of LIHESCs (1 × 106 cells), and LIHEECs (1 × 106 cells) were injected into the peritoneal cavity of female severe combined immune deficiency (SCID) mice (6 weeks old) to induce endometriosis. Henceforth, we refer to the mixture of LIHEECs and LIHESCs as human endometrial cells (HEC). The ectopic lesions were well developed in ~80% of the HEC-transplanted SCID mice, and immunostaining with antibodies against vimentin and cytokeratin 18 revealed that ectopic lesions were successfully developed from the HECs (57). To noninvasively determine the progression of human ectopic lesions, we injected mice with 150 mg/kg of D-Luciferin intraperitoneally 5 min before imaging and then determined luciferase activities of human ectopic lesions in mice using In Vivo Image System (56).
Endometriosis treatment with C-DIM
Endometriosis was induced to mice with auto- and hetero-transplantation method as previously described. After ectopic lesions were established in mice (third week after endometriosis induction), we randomly divided mice with endometriosis into 2 groups and then interperitoneally injected mice in the experimental group 25 mg/kg of methylene substituted diindolylmethane (C-DIM) for 3 weeks (once a day, daily) and injected mice in the control group with the vehicle (5% DMSO and 10% 2-hydroxypropyl-β-cyclodextrin, once a day) for 3 weeks. In the case of the auto-transplantation model, we isolated mouse ectopic lesions treated with C-DIM versus the vehicle from mice with endometriosis at the estrus stage in the third week after drug treatment and then determined their volume. In the case of the hetero-transplantation model, we determined the luciferase activity of the human ectopic lesions treated with C-DIM versus vehicle in SCID mice with endometriosis during the drug treatment.
Immunohistochemistry
Immunostaining was performed with 10% neutral-buffered, formalin-fixed, and paraffin-embedded sections of mouse tissue, as previously described (58). For immunostaining, sections were dewaxed, rehydrated, and boiled for 10 min in 10 mM citrate buffer, pH 6.0. To reduce nonspecific binding of antibodies, sections were washed in PBS with 0.1% Tween-20 again and preincubated with 10% goat serum in PBS with 0.1% Tween-20 for 1 h at room temperature. We determined NR4A1 levels in the uterus, eutopic endometrium, and ectopic lesions with antibodies against NR4A1(NB100-5674, Novus, 1:300). Also, the apoptosis and fibrosis were detected in ectopic lesions treated with vehicle and C-DIM using antibodies against cleaved capsase 3 (9661, 1;300) and α-SMA (ab32575, 1:300). Normal rabbit immunoglobulin G (Milipore Sigma, 12–370) was used as the control for immunohistochemistry (IHC). The specific antigens were visualized with the DAB (3,3’-diaminobenzidine) substrate kit. The immunostaining intensity was quantified using the ImageJ program (59).
Liver panel assay
C57BL/6J mice (6 weeks old) were treated with C-DIM (25 mg/kg, once a day, daily; n = 3) and the vehicle (5% DMSO and 10% 2-hydroxypropyl-β-cyclodextrin, once a day; n = 3) for 5 days. Whole blood was then collected and allowed to clot by leaving it room temperature for 20 min. The clot by centrifuging at 1000 to 2000 × g for 10 min in a refrigerated centrifuge and then collect the supernatant as serum. In the liver panel assay, levels of total protein, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, total-value bilirubin, direct bilirubin, indirect bilirubin, and albumin–globulin ratio in serum were determined by Clinical Pathology Core in the Center for Comparative Medicine in Baylor College of Medicine (Baylor, TX, US). All animal experiments were carried out according to the principles of Institutional Animal Care and Use Committee of Baylor College of Medicine.
Statistical analysis
All of the experiments were repeated a minimum of 3 times. The data are expressed as the mean ± standard error. One-way analysis of variance was used to determine statistical significance. P values < .05 were considered statistically significant.
Results
Expression of NR4A1 and effects of receptor knockdown
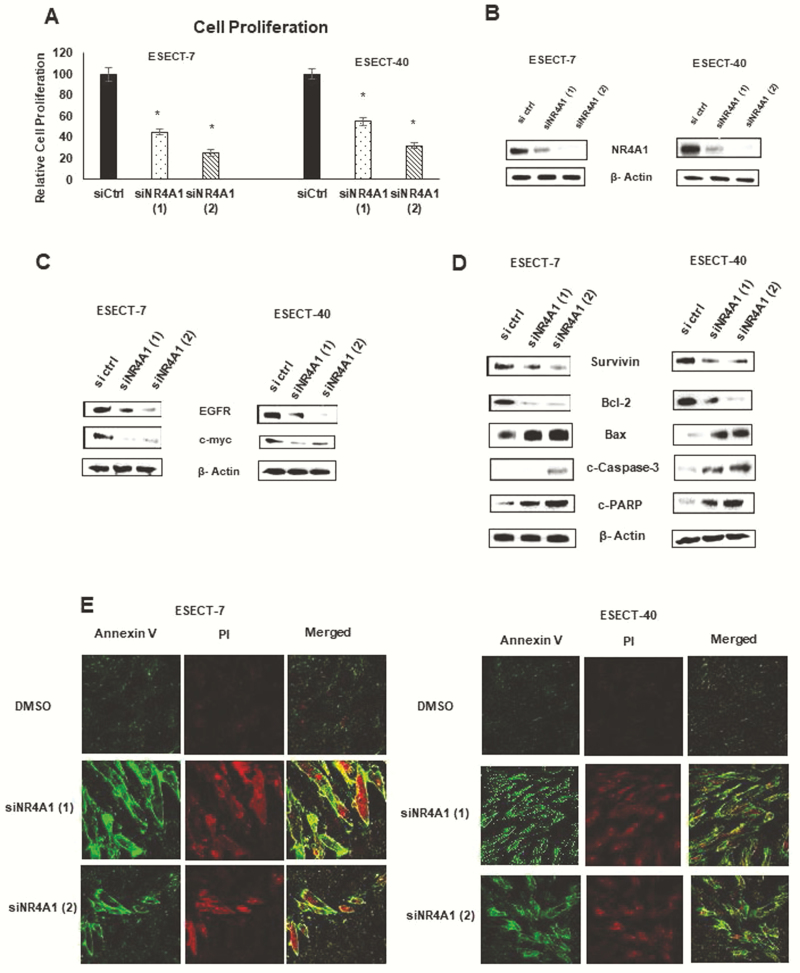
Recent studies showed that NR4A1 is expressed in endometrial cancer cells (Ishikawa and Hec1B) (34) and played an important role in regulating cell growth, survival, migration/invasion, and related genes as previously observed in other solid tumor-derived cancer cells (20–30). Endometriotic cells also express NR4A1 (31–33), and results in Fig. 1A show that knockdown of NR4A1 by RNA interference decreases the growth of patient-derived ESECT-7 and ESECT-40 endometriotic cells. Knockdown efficiency of both oligonucleotides was >85%, as illustrated in Fig. 1B, and loss of NR4A1 was paralleled by decreased expression of growth-promoting genes EGFR and cMyc (Fig. 1C). We also observed that knockdown of NR4A1 in endometriotic cells decreased expression of pro-survival survivin and Bcl-2 gene products, and induced Bax, caspase 3, and PARP cleavages, which are all markers of apoptosis (Fig. 1D). In addition, NR4A1 knockdown also induced Annexin V staining in ESECT-7 and ESECT-40 cells (Fig. 1E), and these results were comparable to those previously observed in endometrial cancer cells (34).
Figure 1.
Effects of NR4A1 knockdown in endometriotic cells. ESECT-7 and ESECT-40 cells were transfected with oligonucleotides targeting downregulation of NR4A1 [siNR4A1 (1) and siNR4A1 (2)] and effects on cell proliferation (A), NR4A1 expression (B), growth-promoting (C) and proapoptotic (D) gene products were determined as outlined in the methods section. (E) Effects of NR4A1 knockdown on Annexin V staining were determined by fluorescence measurements as outlined in the methods section.
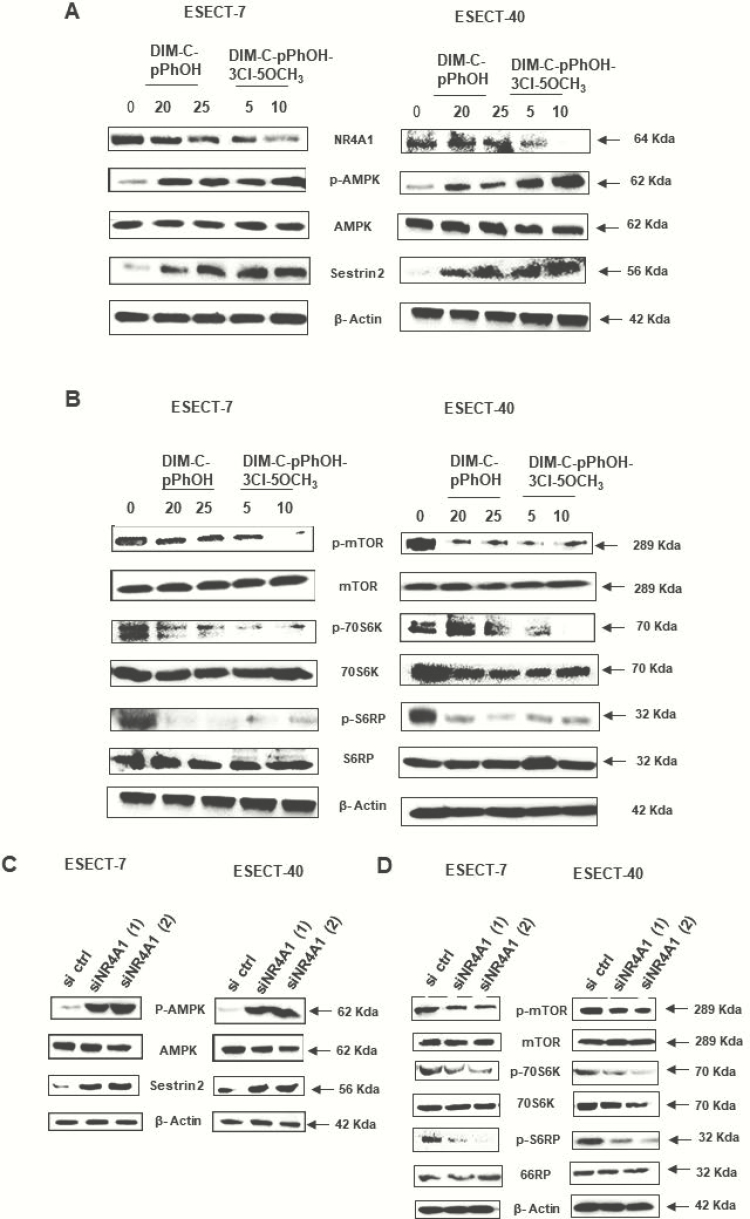
Our recent studies in HEC-1B and Ishikawa endometrial cancer cell lines show that NR4A1 regulates mTOR signaling and treatment with bis-indole derived NR4A1 antagonists-induced reactive oxygen species and sestin2, which, in turn, activated adenosine monophosphate–activated protein kinase C (AMPK) and inhibited mTOR (34). Similar results have previously been observed in breast, renal, lung, and colon cancer cells and in Rhabdomyosarcoma cells (21,24–27), and we extended these studies to patient-derived ESECT-7 and ESECT-40 cells. Treatment of these cells with DIM-C-pPhOH or DIM-C-pPhOH-3-Cl-5-OCH3 induced sestin2 and activated AMPK (Fig. 2A), and this was accompanied by decreased phosphorylation of mTOR, 7056K (p7056K), and S6RP (pS6RP) (Fig. 2B). Similar results were observed after knockdown of NR4A1 (siNR4A1-2 oligonucleotides), which resulted in induction of sestin2 and phosphorylated AMPK (Fig. 2C) and downregulation of phosphorylation of mTOR, p7056K and pS6RP (Fig. 2D). These results confirm that like Ishikawa (epithelial) cells the stromal-derived ESECT-7 and ESECT-40 endometriotic cells exhibited constitutively activated mTOR signaling, which can be inhibited by NR4A1 knockdown or treatment with bis-indole–derived NR4A1 antagonists.
Figure 2.
Role of NR4A1 in mTOR signaling. ESECT-7 and ESECT-40 cells were treated with bis-indole derived NR4A1 antagonists for 24 h, and whole cell lysates were analyzed for activation of AMPK (A) and inhibition of mTOR signaling (B) by western blots. ESECT-7 and ESECT-40 cells were transfected with 2 different oligonucleotides targeting NR4A1 (siNR4A1), and whole cell lysates were analyzed for activation of AMPK (C) and inhibition of mTOR signaling (D).
Bis-indole–derived NR4A1 ligands: transactivation and function
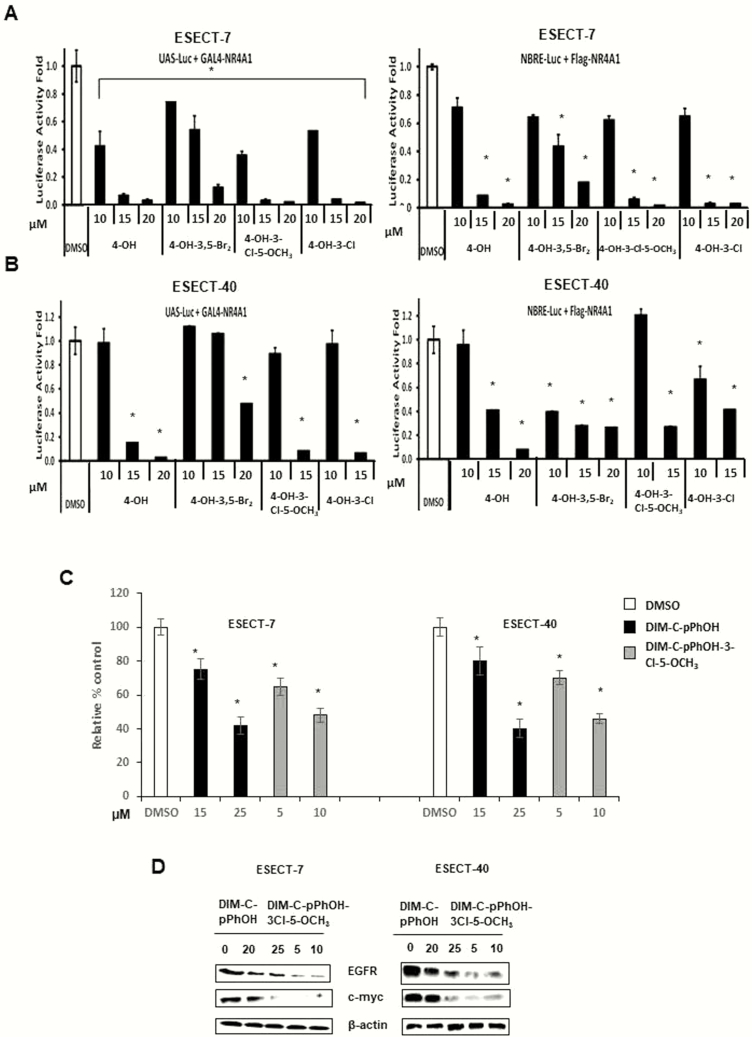
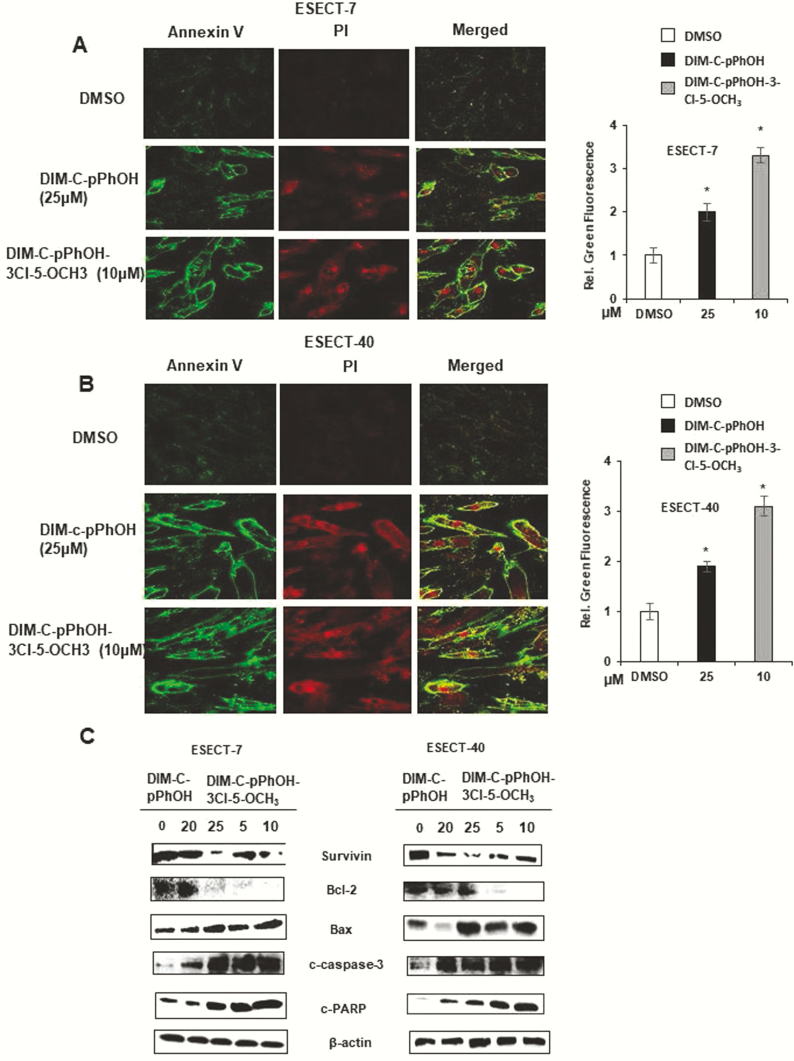
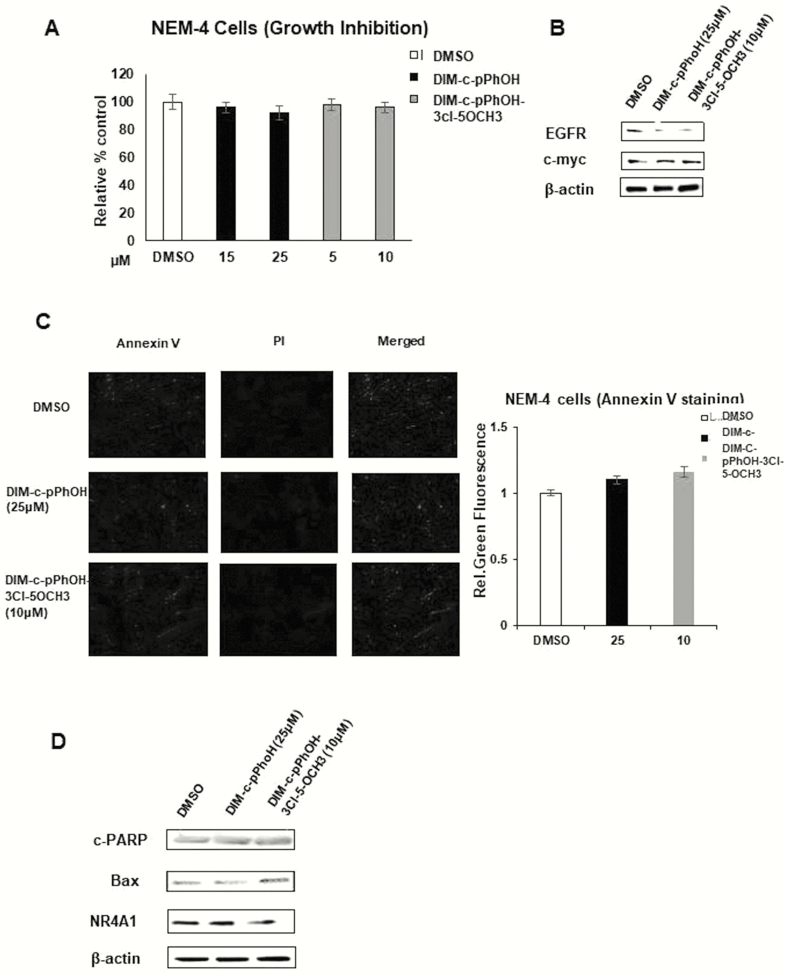
Our previous studies identified 1,1-bis(3’-indolyl)-1-(p-hydroxyphenyl)methane (DIM-C-pPhOH, CDIM8, 4-OH) as an NR4A1 antagonist (24), and we have also developed several buttressed (3,5-substituted) analogs of CDIM8 to decrease the in vivo metabolism (conjugation) at the hydroxyl group and to enhance activity (34). Results in Fig. 3A show that CDIM8 and the 3,5-dibromo (4-OH-3,5-Br2), 3-chloro-5-methoxy (4-OH-3-Cl-3-OCH3), and 3-chloro-(4-OH-3-Cl) buttressed analogs of DIM-C-pPhOH inhibited the intrinsic transcriptional activity of GAL4-NR4A1 and NR4A1 in ESECT-7 cells transfected with GAL4-NR4A1 chimera/UAS-luciferase and an NR4A1/NBRE-luciferase construct. The UAS-luciferase constructs contain 5 tandem GAL4 binding sites, and the NBRE-luciferase construct contains an NBRE site that binds NR4A1. The same set of compounds decreased the intrinsic transcriptional activity of NR4A1 in ESECT-40 cells (Fig. 3B). Therefore, bis-indole–derived NR4A1 antagonists effectively suppressed the intrinsic transcriptional activity of NR4A1 in both human endometriotic cells and endometrial cancer cells (34). The comparative analysis of NR4A1 ligand derivatives revealed that DIM-C-pPhOH-3-Cl-5-OCH3 more effectively suppressed the intrinsic transcriptional activity of NR4A1 as compared to other NR4A1 ligands. For example, 5–10 µM of DIM-C-pPhOH-3-Cl-5-OCH3 effectively inhibited the growth of ESECT-7 and ESECT-40 cells (Fig. 3C) and downregulated the expression of EGFR and cMyc in the same cell lines (Fig. 3D) as compared to 15 to 25 µM of DIM-C-pPhOH. In addition, 10 µM DIM-C-pPhOH-3-Cl-5-OCH3 significantly induced Annexin V staining in ESECT-7 (Fig. 4A) and ESECT-40 (Fig. 4B) cells as compared to 25 µM DIM-C-pPhOH. Five µM DIM-C-pPhOH-3-Cl-5-OCH3 treatment also significantly induced several markers of apoptosis including downregulation of survivin and Bcl-2 and induced Bax and cleaved PARP and caspase 3 in ESECT-7 (Fig. 4C) and ESECT-40 (Fig. 4D) cells compared to 25 µM DIM-C-pPhOH.
Figure 3.
Effects of NR4A1 ligands on transactivation and growth in endometriotic cells. ESECT-7 (A) or ESECT-40 (B) cells were transfected with UAS-luc/GAL4-Luc or NBRE-luc/flag-NR4A1 and treated with DIM-C-pPhOH (OH), DIM-C-pPhOH-3,5-Br2 (4-OH-3,5-Br2), DIM-C-pPhOH-3-Cl-5-OCH3 (4-OH-3-Cl-5-OCH3), or DIM-C-pPhOH-3-Cl (4-OH-3-Cl), and effects on luciferase reporter gene activity were determined as outlined in the methods section. Effects of DIM-C-pPhOH and DIM-C-pPhOH-3-Cl-5-OCH3 on cell growth (C) and growth promoting gene products (D) were determined as outlined in the methods section.
Figure 4.
NR4A1 antagonists induce apoptosis in endometriotic cells. ESECT-7 (A) and ESECT-40 (B) cells were treated with NR4A1 antagonists, and annexin V staining was determined by fluorescence as outlined in the methods section. ESECT-7 (C) and ESECT-40 (D) cells were treated with NR4A1 ligands for 24 h, and whole cell lysates were analyzed by western blots for proapoptotic gene products.
In contrast with human endometriotic stromal cells (ESECT), however, 15 to 25 µM DIM-C-pPhOH and 5 to 10 µM DIM-C-pPhOH-3-Cl-5-OCH3 treatment did not suppress the growth of normal endometrial NEM cells (Fig. 5A) and did not decrease cMyc and EGFR expression (Fig. 5B) as compared to the DMSO control. Also, 25 µM DIM-C-pPhOH and 10 µM DIM-C-pPhOH-3-Cl-5-OCH3 treatment did not enhance Annexin V staining (Fig. 5C) or levels of the cleaved form of PARP and Bax in NEM cells as compared to the DMSO control (Fig. 5D). The NEM cells also expressed NR4A1; however, the NR4A1 antagonists exhibited cell context-dependent effects and did not affect these cells, and this cell-dependent specificity is typically observed for selective receptor modulators, which depend not only on the receptor but also on the expression of specific cofactors, which can differ between cell lines.
Figure 5.
Effects of NR4A1 ligand on normal endometrium (NEM) cells. NEM cells were treated with NR4A1 ligands for 24 h, and effects on cell growth (A), growth promoting gene (B), annexin V staining (C), and proapoptotic gene products (D) were determined as outlined in the methods section.
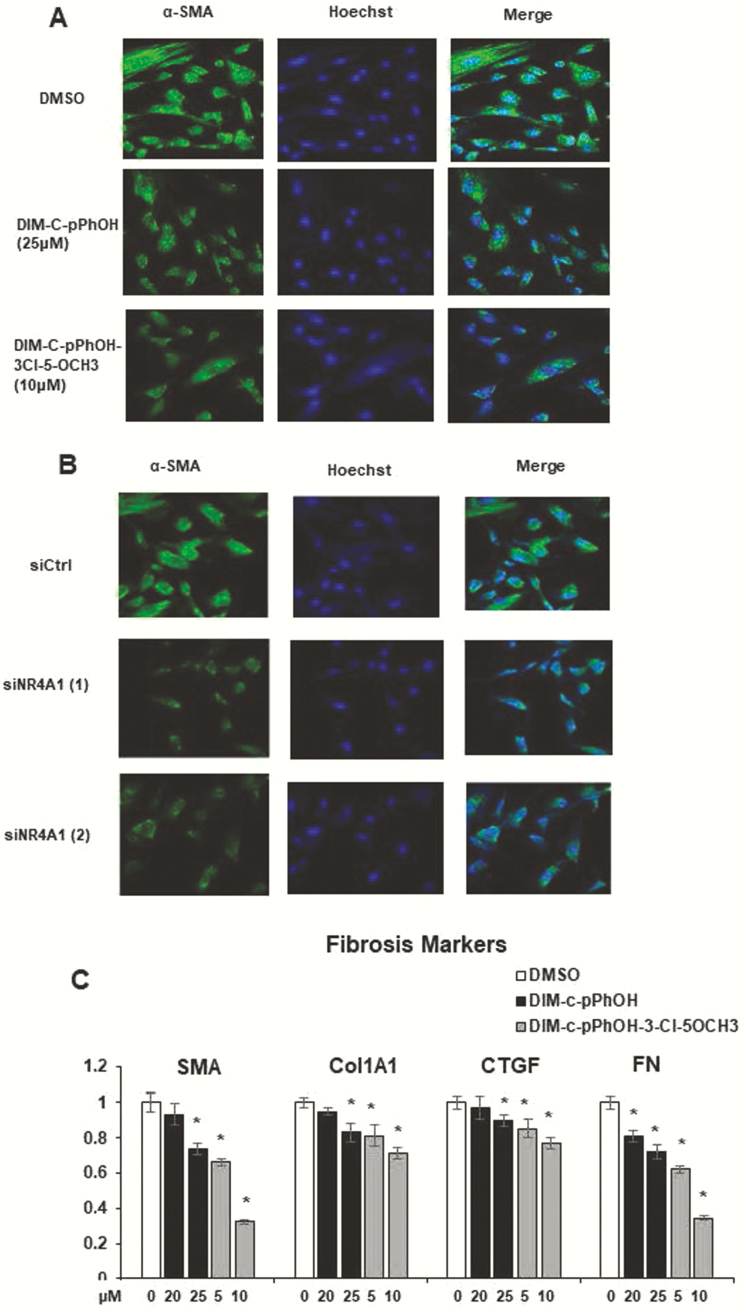
Previous studies in stromal-derived endometriotic cells showed that knockdown of NR4A1 enhanced TGFβ1-induced fibrotic gene expression in human ectopic endometrial stromal cells (EESCs) and NESCs. To validate this observation, we inhibited the intrinsic transcriptional activity of NR4A1 (Fig. 6A) and reduced NR4A1 levels by siNR4A1 (Fig. 6B) in ESECT-7 endometriotic stromal cells and then determine the progression of fibrosis. The suppression of NR4A1 decreased expression of SMA levels in ESECT-7 cells as compared with their control (Figs. 6A and 6B). In addition to SMA, 20 to 25 µM DIM-C-pPhOH and 5 to 10 µM DIM-C-pPhOH-3-Cl-5-OCH3 also decreased mRNA levels of fibrosis makers (such as FN, Col1A1, and CTGF) messenger RNA level in ESECT-7 cells compared to the DMSO control (Fig. 6C).
Figure 6.
Role of NR4A1 on endometriotic cell fibrosis. ESECT-7 cells were treated with NR4A1 antagonists (A) or transfected with siNR4A1 (1) or siNR4A1 (2), and cells were analyzed by immunofluorescence for α-SMA staining. (C) ESECT-7 cells were treated with NR4A1 ligands for 24 h, and effects on gene expression were determined by real-time polymerase chain reaction as outlined in the methods section.
The effect of NR4A1 antagonist in fibrosis of endometriotic cells versus endometrial cancer cells
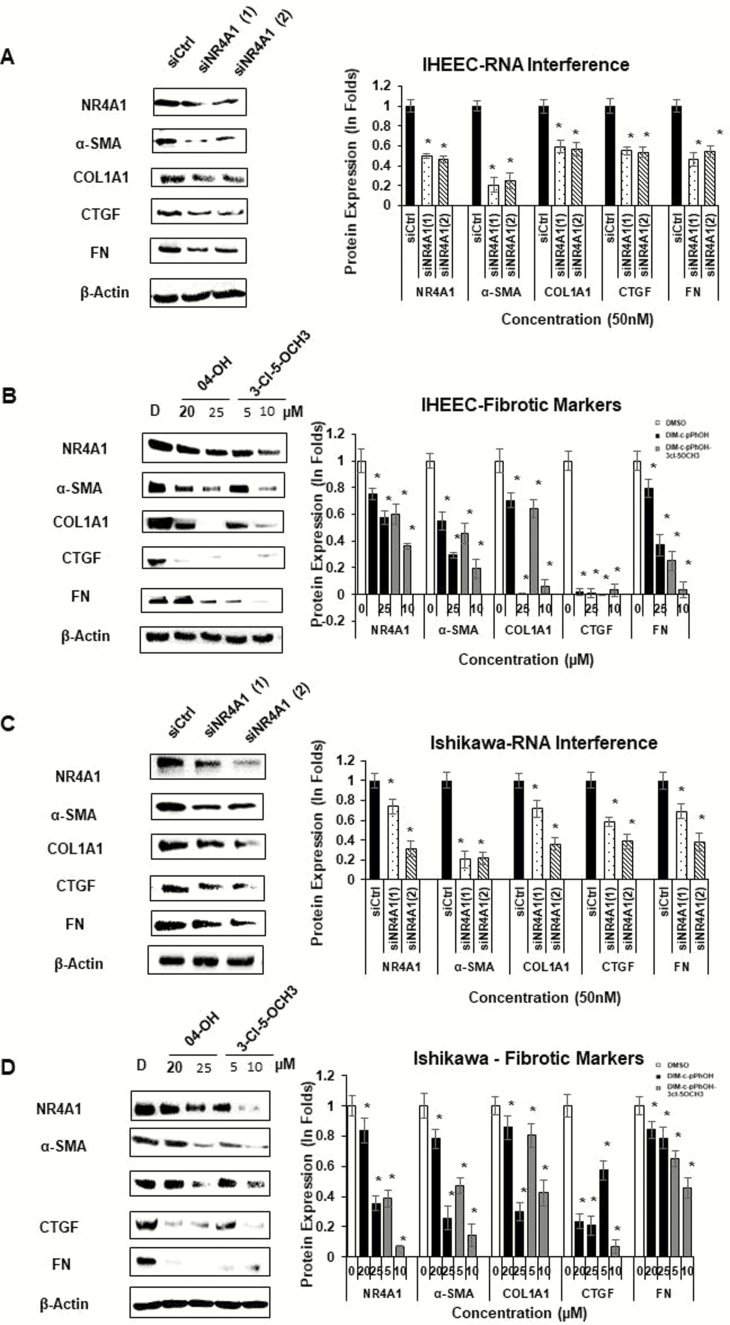
To validate NR4A1 function in fibrosis progression of HECs, we employed IHEECs as normal human endometrial epithelial cells because IHEECs were not transformed in SCID mice (60). As the control, we employed epithelial-derived Ishikawa endometrial cancer cells because the NR4A1 inhibitor prevents growth and survival of Ishikawa cells (34). The knockdown of NR4A1 (Fig. 7A) and DIM-C-pPhOH and DIM-C-pPhOH -3-Cl-5-OCH3 treatment (Fig. 7B) decreased expression of fibrosis markers (α-SMA, COL1A1, FN, and CTGF) in IHEECs as compared to their control. The knockdown of NR4A1 (Fig. 7C) and DIM-C-pPhOH and DIM-C-pPhOH -3-Cl-5-OCH3 (Fig. 7D) also decreased expression of fibrosis markers (α-SMA, COL1A1, FN, and CTGF) in Ishikawa cells, and these results were consistent with the comparable effects of siNR4A1 and NR4A1 antagonists on cell growth and survival (Figs. 1–3). Quantitation of the western blots are summarized in Fig. 7. Therefore, the activation of NR4A1 stimulates the fibrosis progression of endometriosis, as observed in the endometrial epithelial cancer cell line.
Figure 7.
Effects of NR4A1 antagonists and receptor knockdown on IHEEC and Ishikawa cells. IHEEC cells were transfected with siNR4A1 oligonucleotides (A) or treated for 24 h with DMSO (D) and NR4A1 antagonists (4-OH and 3-Cl-5-OCH3); Ishikawa cells received the same treatments (C/D), and whole cell lysates were analyzed by western blots as outlined in the methods section. The western blots were quantitated by determining relative band intensities compared to the controls (set at 1.0) and normalized to β-actin.
Expression levels of Nr4a1 were elevated in endometriotic tissues as compared with the normal uterus
To determine whether the expression levels of NR4A1 are elevated in endometriotic tissues as compared with normal endometrium, we surgically induced endometriosis into mice with the auto-transplantation method (53). At the estrus stage in the third week after endometriosis induction, ectopic lesions and eutopic endometrium were isolated from mice with endometriosis. As the control, we also isolated uterus at the estrus stage of female mice (10-week old) without endometriosis.
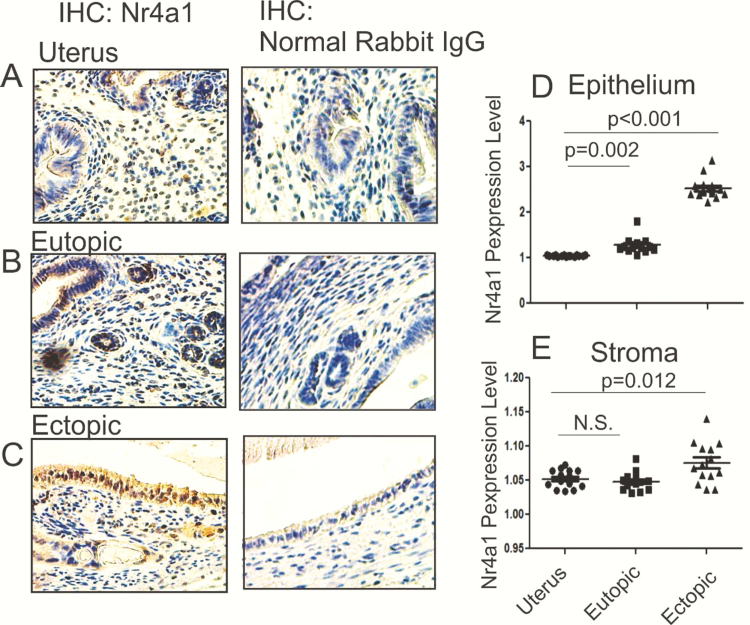
Immunohistochemistry with the NR4A1 antibody revealed that NR4A1 levels were significantly elevated in the epithelium of ectopic lesions (2.6-fold, P < .001) as compared with the epithelium of uterus (Fig. 8A, 8C, and 8D). In addition to ectopic lesions, NR4A1 levels were also elevated in the epithelium of eutopic endometrium as compared with those in the epithelium of normal uterus (1.27-fold, P = .002) (Fig. 8A, 8B, and 8D). Therefore, epithelium of ectopic lesions and eutopic endometrium of mice with endometriosis have higher levels of NR4A1 compared to the epithelium of normal uterus. In addition to the epithelium, levels of NR4A1 in the stroma of ectopic lesions slightly elevated as compared with stroma of uterus (1.1-fold, P = .012) (Fig. 8E). However, the level of NR4A1 was not elevated in the stroma of eutopic endometrium compared to the stroma of normal uterus (Fig. 8E). Collectively, the elevation of NR4A1 levels in endometriotic tissues was associated with endometriosis progression.
Figure 8.
Nr4a1 expression in mouse endometriotic tissues versus normal uterus. Nr4a1 levels were determined in the normal mouse uterus (A), eutopic endometrium, (B) and ectopic lesions (C) isolated from mice with endometriosis at estrus stage in the third week after endometriosis induction using IHC. As the control for IHC, normal rabbit immunoglobulin G (5 ug/mL) was incubated with the normal mouse uterus, eutopic endometrium, and ectopic lesions. IHC signal density of Nr4a1 in the epithelium (D) and stroma (E) of the uterus, eutopic endometrium, and ectopic lesions were quantified by ImageJ program.
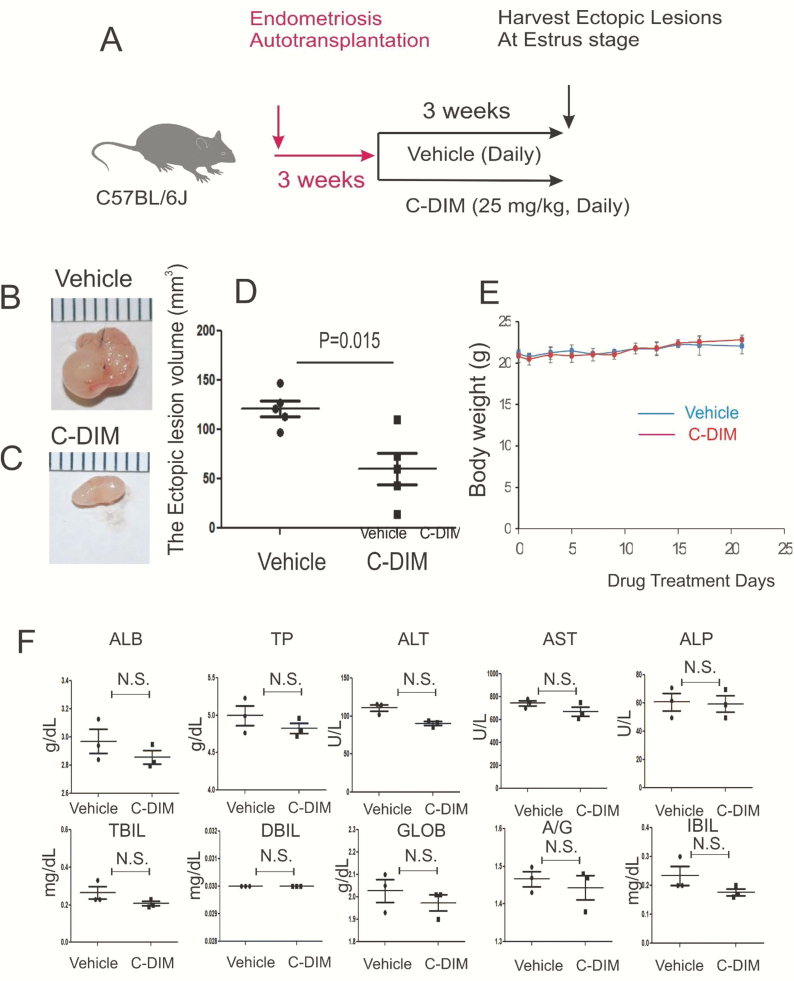
The DIM-C-pPhOH-3-Cl-5-OCH3 treatment effectively suppresses the growth of ectopic lesions in mice with endometriosis
Even though expression levels of NR4A1 were significantly elevated in ectopic lesions compared to the normal uterus, it is not clear whether the elevation of NR4A1 induces endometriosis or is simply a consequence of endometriosis. To address this question, we employed the NR4A1 specific antagonist, DIM-C-pPhOH-3-Cl-5-OCH3, to examine whether inhibition of NR4A1 in ectopic lesions suppresses endometriosis daily progression in mice with endometriosis. Based on our previous study (61), we treated mice with endometriosis with 25 mg/kg of DIM-C-pPhOH-3-Cl-5-OCH3 and vehicle control every day (Fig. 9A). The 25 mg/kg of DIM-C-pPhOH-3-Cl-5-OCH3 treatment significantly reduced (2.4-fold, P = .015) the volume of ectopic lesions compared to the vehicle (Figs. 9B–9D). However, DIM-C-pPhOH-3-Cl-5-OCH3 treatment did not change the body weight of mice as compared to the vehicle (Fig. 9E). To further validate the toxicity of DIM-C-pPhOH-3-Cl-5-OCH3, we determined the liver function using a liver panel assay with serum from control and treated mice. The liver panel assay revealed that the 25 mg/kg dose of DIM-C-pPhOH-3-Cl-5-OCH3 treatment did not damage the liver function as compared with the vehicle (Fig. 9F). Therefore, DIM-C-pPhOH-3-Cl-5-OCH3 treatment effectively suppressed the growth of mouse ectopic lesions in mice with endometriosis without any detectable side effects.
Figure 9.
Suppression of growth of mouse ectopic lesions in mice with endometriosis by DIM-C-pPhOH-3-Cl-5-OCH3 (C-DIM) treatment. Endometriosis was surgically induced in C57BL/6J mouse by implanting one endometrial fragment into the mesentery membrane of the intestine by the auto-transplantation method (n = 10) (A). At the third week after endometriosis induction, mice were randomly divided 2 groups (n = 5/group). Mice in one group were treated with 25 mg/kg of C-DIM (once a day, daily), and mice in the other group were treated with the vehicle (once a day, everyday) for 3 weeks. At the estrus stage in the third week after endometriosis induction, 5 ectopic lesions were isolated from mice with endometriosis treated with the vehicle (B) and C-DIM (C). The volume of isolated 5 ectopic lesions treated with the vehicle versus C-DIM was determined (D). During the drug treatment period, the bodyweight of mice treated with vehicle versus C-DIM was determined (E). Also, serum was isolated from a mouse treated with C-DIM and vehicle after the 3-week drug treatment (n = 3/drug-treated group). Afterward, the liver panel assay was conducted with this isolated serum. The levels of levels of total protein (TP), alanine aminotransferase (ATL), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total-value bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (IBIL), and albumin–globulin ratio (A/G) in serum treated with vehicle versus C-DIM were obtained (F).
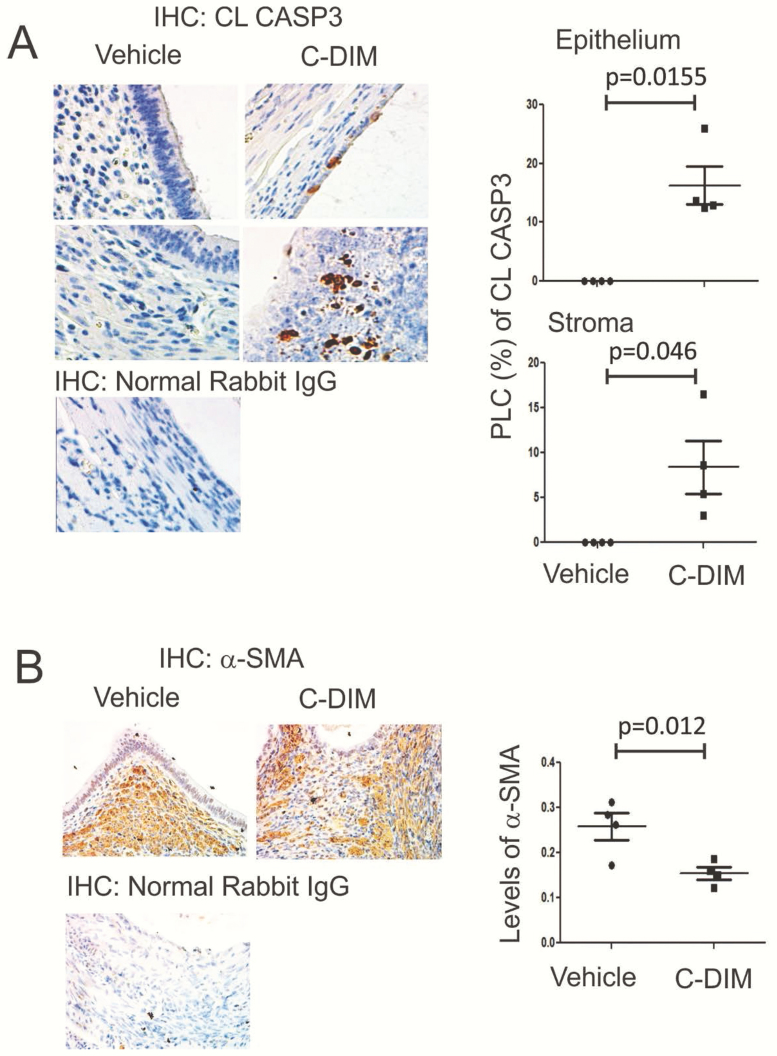
The DIM-C-pPhOH-3-Cl-5-OCH3 treatment increases the apoptosis signaling but reduced the fibrosis of human endometrial stromal cells isolated from endometriosis patients (Figs. 4 and 6). To validate the in vitro effects of DIM-C-pPhOH-3-Cl-5-OCH3, we determined the apoptosis and fibrosis signling in ectopic lesions treated with DIM-C-pPhOH-3-Cl-5-OCH3 as compared with the vehicle (Fig. 10). In the DIM-C-pPhOH-3-Cl-5-OCH3 treatment group elevated levels of CL-CASP3 in both epithelial and stromal cells of mouse ectopic lesions were observed to compare the vehicle control animal (Fig. 10A). Therefore, DIM-C-pPhOH-3-Cl-5-OCH3 treatment induces the apoptosis signaling in ectopic lesions to suppress the endometriosis progression. In addition, the DIM-C-pPhOH-3-Cl-5-OCH3 treatment reduced the levels of α-SMA in ectopic lesions as compared with the vehicle (Fig. 10B). Therefore, DIM-C-pPhOH-3-Cl-5-OCH3 treatment suppressed the fibrosis progression in ectopic lesions to suppress endometriosis progression.
Figure 10.
The C-DIM treatment-activated apoptosis and reduced fibrosis in ectopic lesions. The expression levels of CL-CASP3 (B) and α-SMA (B) were determined in ectopic lesions treated with C-DIM versus vehicle by IHC (n = 4/group). The percentage of labled cells (PCL, %) of CL-CASP3 and levels of α-SMA in ectopic lesions were calculated by Image J.
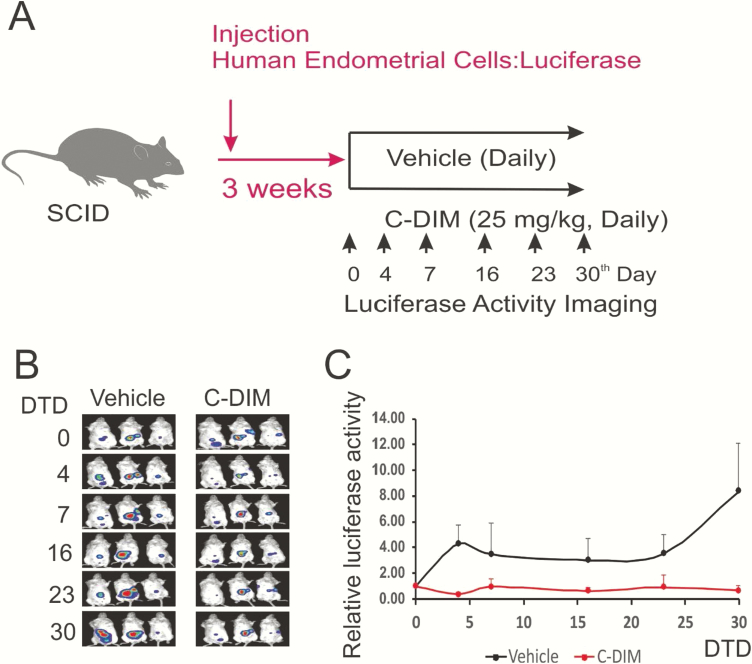
The previous observation raised the question of whether DIM-C-pPhOH-3-Cl-5-OCH3 also suppresses the growth of human ectopic lesions. To address this question, we induced endometriosis into SCID mice with HECs carrying the luciferase gene. Mice with human ectopic lesions were treated with 25 mg/kg of DIM-C-pPhOH-3-Cl-5-OCH3 versus the vehicle as to the control (Fig. 11A). In addition to mouse ectopic lesions, the DIM-C-pPhOH-3-Cl-5-OCH3 treatment also significantly reduced the luciferase activity in ectopic lesions as compared with the vehicle image (13.4-fold, P = .022) (Figs. 11B and 11C). Since the luciferase activity recapitulates the growth of human ectopic lesions in mice, DIM-C-pPhOH-3-Cl-5-OCH3 treatment with the NR4A1 antagonist suppressed the growth of human ectopic lesions in SCID mice compared to the vehicle and demonstrated the in vivo efficacy of NR4A1 antagonists as inhibitors of endometriosis.
Figure 11.
Suppression of the growth of human ectopic lesions in SCID mice by DIM-C-pPhOH-3-Cl-5-OCH3 (C-DIM). The human ectopic lesions were generated by injection of HECs expressing luciferase into SCID mice (A). At the third week after endometriosis induction, mice were randomly divided 2 groups. Mice in one group were treated with 25 mg/kg of C-DIM (once a day), and mice in the other group were treated with the vehicle (once a day) for 30 days. The luciferase activities of human ectopic lesions were determined at 4, 7, 16, 23, and 30 days after drug treatment (B). The luciferase activity of ectopic lesions treated with the vehicle and C-DIM were quantified. Based on these, the relative fold of luciferase activity compared to 0-day drug treatment was calculated (C). Abbreviation: DTD, drug treatment days.
Discussion
NR4A1 and related NR4A receptors are activated by diverse stressors in many cell types, and activation of NR4A1 causes stress-related diseases such as cancer (17–19). For example, the elevation of NR4A1 is detected in breast, lung, colon, pancreatic, and ovarian tumors with the poor prognosis (21–23,62–65). In cancer progression, activation of NR4A1 stimulates growth, survival, invasion, and metastasis of several cancer cell lines, and C-DIM treatment suppressed tumorigenesis by inhibiting NR4A1-regulated pro-oncogenic pathways in cancer cells (20–30). For example, knockdown of NR4A1 suppressed the growth of Ishikawa and Hec1B endometrial cancer cells (34). Interestingly, Ishikawa cells are frequently used as models for epithelial-derived endometriotic cells. Therefore, this observation implies that NR4A1 might have an essential role in endometriosis progression and NR4A1 could be a new target for nonhormonal therapy for endometriosis treatment. Since NR4A1 inhibition by DIM-C-pPhOH-3-Cl-5-OCH3 is the phenocopy of NR4A1 knockdown in human endometriotic cells, DIM-C-pPhOH-3-Cl-5-OCH3 can be employed to treat the endometriosis without impairing estrogen signaling.
How do NR4A1 antagonists suppress the endometriosis progression? Our study revealed that NR4A1 antagonist inhibited mTOR signaling due to reactive oxygen species-dependent induction of sestrin 2, which, in turn, activated AMPK phosphorylation and mTOR inhibition in Ishikawa cells (34). The PI3K–AKT–mTOR axis has a critical role in endometriosis progression, and inhibition of this axis effectively suppressed the endometriosis progression (66). Therefore, NR4A1 antagonists might suppress the endometriosis progression by inhibiting the activated mTOR signaling in endometriotic lesions.
The C-DIM/NR4A1 antagonists suppressed the growth of ESECT-7 and ESECT-40 cells isolated from ovarian endometrioma by activating apoptosis and inhibiting mTOR signaling but do not inhibit the growth of NEM (normal) endometrial cells even though NR4A1 was expressed in NEM cells. In contrast with endometriotic cells, DIM-C-pPhOH and DIM-C-pPhOH-3-Cl-5-OCH3 worked as NR4A1 agonists to induce genes involved in glucose uptake process in C2C12 muscle cells (67). These cellular differences in the activity of CDIM/NR4A1 ligands (eg, antagonist/agonist/inactive) are typically observed for selective receptor modulators that exhibit tissue/cell-specific activities suggesting that the CDIMs are selective NR4A1 modulators. The selectivity of these compounds in their effectiveness in endometriotic, but nonnormal endometrium cells may be due to several reasons, including the differential expression of essential co-factors necessary for activation/repression of NR4A1-dependent genes. This may be attributed to phenotypic differences between normal versus endometriotic cells such as degree of inflammation, and this is currently being investigated.
The development of fibrosis can lead to decreased fertility and pain during endometriosis. Zeng and coworkers revealed that the knockdown of NR4A1 in NESCs and endometriotic tissue (EESCs) enhanced fibrosis and cytosporone β (NR4A1 agonist) treatment inhibited TGFβ-induced fibrosis in NESC and EESC cells (31). In contrast to this observation, the knockdown of NR4A1 or treatment with C-DIM (NR4A1 antagonists) inhibited the fibrosis progression of ESECT-7 cells and IHEECs as compared to their controls. In the endometriosis mouse model, we observed higher levels of NR4A1 in endometriotic tissue compared to normal endometrial tissue, and DIM-C-pPhOH-3-Cl-5-OCH3 treatment suppressed the endometriosis progression in mouse with endometriosis without any changes in body weight, and similar results were observed in SCID mice bearing HECs. Why are their apparently conflicting reports on the role of NR4A1 on endometriosis? First, the Zeng group induced endometriosis into NR4A1 total knock-out (KO) mice by auto-transplantation of NR4A1 KO uterine fragments. Therefore, we cannot determine the function of NR4A1 in ectopic lesions for endometriosis progression due to the interference of whole-body NR4A1 KO female recipients. In the SCID mouse model, the Zeng group treated mice with 17-β estradiol to stimulate endometriosis progression. However, we did not inject 17-β estradiol to recipient mice in our study. The high concentration of exogenous 17-β estradiol exposure significantly changes intracellular signaling in SCID mice compared to nontreated SCID mice. Therefore, this type of experimental difference might change the effects of NR4A1 in endometriosis progression.
In summary, our results reveal that NR4A1 is a new pro-endometriotic transcription factor that stimulates progression of endometriosis, and the bis-indole–derived NR4A1 antagonists could be employed as new nonhormonal therapy for treatment of endometriosis, and this would minimize the side effects of current hormonal therapies.
Acknowledgments
Financial Support: The financial assistance of the National Institutes of Health (P30-ES029067, S. Safe), Texas AgriLife Research (S. Safe), Sid Kyle Chair endowment (S. Safe) and Mike Hogg Research Foundation (SJ Han) is gratefully acknowledged.
Glossary
Abbreviations
- ALB
albumin
- C-DIM
methylene substituted diindolylmethane
- CL-CSAP3
cleaved caspase 3
- DAB
diaminobenzidine
- DIM
3,3’-diindolylmethane
- DIM-C-pPhOH
1,1-bis(3’-indolyl)-1-(4-hydroxyphenyl)methane
- DMSO
dimethyl sulfoxide
- EGFR
epidermal growth factor receptor
- EESCs
human ectopic endometrial stromal cells
- ESECT
ectopic endometrium-isolated ovarian endometrioma
- FN
fibronectin
- HEC
human endometrial cells
- IHC
immunohistochemistry
- IHEECs
immortalized human endometrial epithelial cells
- LIHEECs
luciferase stable immortalized human endometrial epithelial cells
- LIHESCs
luciferase stable immortalized human endometrial cells
- NBRE
nerve growth factor β response element
- NESCs
normal endometrial stromal cells
- NR4A1
nuclear receptor 4A1
- PBS
phosphate-buffered saline
- SCID
severe combined immune deficiency
- SMA
smooth muscle actin
Additional Information
Disclosure Summary: The authors have nothing to disclose, and the authors declare that they have no conflicts of interest with the contents of this article.
Data Availability: Data generated in this study are included in the article.
References
- 1. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24(2):235–258. [DOI] [PubMed] [Google Scholar]
- 2. Buck Louis GM, Hediger ML, Peterson CM, et al. ; ENDO Study Working Group. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. 2011;96(2):360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. 2018;4(1):9. [DOI] [PubMed] [Google Scholar]
- 4. Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA. Endometriosis: where are we and where are we going? Reproduction. 2016;152(3):R63–R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–21. [DOI] [PubMed] [Google Scholar]
- 6. Hirsch M, Begum MR, Paniz É, Barker C, Davis CJ, Duffy J. Diagnosis and management of endometriosis: a systematic review of international and national guidelines. Bjog. 2018;125(5):556–564. [DOI] [PubMed] [Google Scholar]
- 7. Fernando S, Soh PQ, Cooper M, et al. . Reliability of visual diagnosis of endometriosis. J Minim Invasive Gynecol. 2013;20(6):783–789. [DOI] [PubMed] [Google Scholar]
- 8. Angioni S, Cofelice V, Pontis A, Tinelli R, Socolov R. New trends of progestins treatment of endometriosis. Gynecol Endocrinol. 2014;30(11):769–773. [DOI] [PubMed] [Google Scholar]
- 9. Andres Mde P, Lopes LA, Baracat EC, Podgaec S. Dienogest in the treatment of endometriosis: systematic review. Arch Gynecol Obstet. 2015;292(3):523–529. [DOI] [PubMed] [Google Scholar]
- 10. Zito G, Luppi S, Giolo E, et al. . Medical treatments for endometriosis-associated pelvic pain. Biomed Res Int. 2014;2014:191967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Granese R, Perino A, Calagna G, et al. . Gonadotrophin-releasing hormone analogue or dienogest plus estradiol valerate to prevent pain recurrence after laparoscopic surgery for endometriosis: a multi-center randomized trial. Acta Obstet Gynecol Scand. 2015;94(6):637–645. [DOI] [PubMed] [Google Scholar]
- 12. Brown J, Farquhar C. An overview of treatments for endometriosis. JAMA. 2015;313(3):296–297. [DOI] [PubMed] [Google Scholar]
- 13. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. [DOI] [PubMed] [Google Scholar]
- 14. Bulun SE, Monsivais D, Kakinuma T, et al. . Molecular biology of endometriosis: from aromatase to genomic abnormalities. Semin Reprod Med. 2015;33(3):220–4. [DOI] [PubMed] [Google Scholar]
- 15. Klemmt PAB, Starzinski-Powitz A. Molecular and cellular pathogenesis of endometriosis. Curr Womens Health Rev. 2018;14(2):106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferrero S, Barra F, Leone Roberti Maggiore U. Current and emerging therapeutics for the management of endometriosis. Drugs. 2018;78(10):995–1012. [DOI] [PubMed] [Google Scholar]
- 17. Safe S, Jin UH, Hedrick E, Reeder A, Lee SO. Minireview: role of orphan nuclear receptors in cancer and potential as drug targets. Mol Endocrinol. 2014;28(2):157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pearen MA, Muscat GE. Minireview: nuclear hormone receptor 4A signaling: implications for metabolic disease. Mol Endocrinol. 2010;24(10):1891–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurakula K, Koenis DS, van Tiel CM, de Vries CJ. NR4A nuclear receptors are orphans but not lonesome. Biochim Biophys Acta. 2014;1843(11):2543–2555. [DOI] [PubMed] [Google Scholar]
- 20. Lee SO, Abdelrahim M, Yoon K, et al. . Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth. Cancer Res. 2010;70(17):6824–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee SO, Andey T, Jin UH, et al. . The nuclear receptor TR3 regulates mTORC1 signaling in lung cancer cells expressing wild-type p53. Oncogene. 2012;31(27):3265–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cho SD, Yoon K, Chintharlapalli S, et al. . Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and nuclear receptor-independent pathways. Cancer Res. 2007;67(2):674–683. [DOI] [PubMed] [Google Scholar]
- 23. Zhou F, Drabsch Y, Dekker TJ, et al. . Nuclear receptor NR4A1 promotes breast cancer invasion and metastasis by activating TGF-beta signalling. Nat Commun. 2014;5:3388. [DOI] [PubMed] [Google Scholar]
- 24. Lee SO, Li X, Hedrick E, et al. . Diindolylmethane analogs bind NR4A1 and are NR4A1 antagonists in colon cancer cells. Mol Endocrinol. 2014;28(10):1729–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hedrick E, Lee SO, Doddapaneni R, Singh M, Safe S. Nuclear receptor 4A1 as a drug target for breast cancer chemotherapy. Endocr Relat Cancer. 2015;22(5):831–840. [DOI] [PubMed] [Google Scholar]
- 26. Hedrick E, Lee SO, Doddapaneni R, Singh M, Safe S. NR4A1 antagonists inhibit β1-integrin-dependent breast cancer cell migration. Mol Cell Biol. 2016;36(9):1383–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lacey A, Rodrigues-Hoffman A, Safe S. PAX3-FOXO1A expression in rhabdomyosarcoma is driven by the targetable nuclear receptor NR4A1. Cancer Res. 2017;77(3):732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lacey A, Hedrick E, Cheng Y, Mohankumar K, Warren M, Safe S. Interleukin-24 (IL24) is suppressed by PAX3-FOXO1 and is a novel therapy for Rhabdomyosarcoma. Mol Cancer Ther. 2018;17(12):2756–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hedrick E, Safe S. Transforming growth factor beta/NR4A1-inducible breast cancer cell migration and epithelial-to-mesenchymal transition is p38alpha (Mitogen-Activated Protein Kinase 14) dependent. Mol Cell Biol. 2017;37(18). doi: 10.1128/MCB.00306-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hedrick E, Mohankumar K, Safe S. TGFbeta-induced lung cancer cell migration is NR4A1-dependent. Molecular Cancer Research. 2018;16(12):1991-2002. doi: 10.1158/1541-7786.MCR-18-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zeng X, Yue Z, Gao Y, et al. . NR4A1 is involved in fibrogenesis in ovarian endometriosis. Cell Physiol Biochem. 2018;46(3):1078–1090. [DOI] [PubMed] [Google Scholar]
- 32. Han SJ, O’Malley BW. The dynamics of nuclear receptors and nuclear receptor coregulators in the pathogenesis of endometriosis. Hum Reprod Update. 2014;20(4):467–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang LL, Cai HQ, Dong XQ, et al. . Differentially expressed gene profiles in the serum before and after the ultrasound-guided ethanol sclerotherapy in patients with ovarian endometriomas. Clin Biochem. 2015;48(16-17):1131–1137. [DOI] [PubMed] [Google Scholar]
- 34. Mohankumar K, Li X, Sridharan S, Karki K, Safe S. Nuclear receptor 4A1 (NR4A1) antagonists induce ROS-dependent inhibition of mTOR signaling in endometrial cancer. Gynecol Oncol. 2019;154(1):218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fayazi M, Salehnia M, Ziaei S. Differentiation of human CD146-positive endometrial stem cells to adipogenic-, osteogenic-, neural progenitor-, and glial-like cells. In Vitro Cell Dev Biol Anim. 2015;51(4):408–414. [DOI] [PubMed] [Google Scholar]
- 36. RRID: AB_2246311 ProMED-mail website. https://scicrunch.org/resolver/AB_2246311
- 37. RRID: AB_2063948 ProMED-mail website. https://scicrunch.org/resolver/AB_2063948.
- 38. RRID: AB_2341188 ProMED-mail website. https://scicrunch.org/resolver/AB_2341188.
- 39. RRID: AB_331426 ProMED-mail website. https://scicrunch.org/resolver/AB_331426.
- 40. RRID: AB_627268 ProMED-mail website. https://scicrunch.org/resolver/AB_627268.
- 41. RRID: AB_626733 ProMED-mail website. https://scicrunch.org/resolver/AB_626733.
- 42. RRID: AB_626726 ProMED-mail website. https://scicrunch.org/resolver/AB_626726.
- 43. RRID: AB_10861258 ProMED-mail website. https://scicrunch.org/resolver/AB_10861258.
- 44. RRID: AB_722538 ProMED-mail website. https://scicrunch.org/resolver/AB_722538.
- 45. RRID: AB_476692 ProMED-mail website. https://scicrunch.org/resolver/AB_476692.
- 46. RRID: AB_10721155 ProMED-mail website. https://scicrunch.org/resolver/AB_10721155.
- 47. RRID: AB_11169640 ProMED-mail website. https://scicrunch.org/resolver/AB_11169640.
- 48. RRID: AB_1950298 ProMED-mail website. https://scicrunch.org/resolver/AB_1950298.
- 49. RRID: AB_2099233 ProMED-mail website. https://scicrunch.org/resolver/AB_2099233.
- 50. RRID: AB_330924 ProMED-mail website. https://scicrunch.org/resolver/AB_330924.
- 51. RRID: AB_1904025 ProMED-mail website. https://scicrunch.org/resolver/AB_1904025.
- 52. Pelch KE, Sharpe-Timms KL, Nagel SC. Mouse model of surgically-induced endometriosis by auto-transplantation of uterine tissue. J Vis Exp. 2012;(59):e3396. doi: 10.3791/3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS One. 2012;7(4):e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Krikun G, Mor G, Alvero A, et al. . A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology. 2004;145(5):2291–2296. [DOI] [PubMed] [Google Scholar]
- 55. Bono Y, Kyo S, Takakura M, et al. . Creation of immortalised epithelial cells from ovarian endometrioma. Br J Cancer. 2012;106(6):1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Han SJ, Jung SY, Wu SP, et al. . Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell. 2015;163(4):960–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Han SJ, Hawkins SM, Begum K, et al. . A new isoform of steroid receptor coactivator-1 is crucial for pathogenic progression of endometriosis. Nat Med. 2012;18(7):1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Han SJ, Jeong J, Demayo FJ, et al. . Dynamic cell type specificity of SRC-1 coactivator in modulating uterine progesterone receptor function in mice. Mol Cell Biol. 2005;25(18):8150–8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rueden CT, Schindelin J, Hiner MC, et al. . ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 2017;18(1):529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kyo S, Nakamura M, Kiyono T, Maida Y, Kanaya T, Tanaka M, Yatabe N, Inoue M. Successful immortalization of endometrial glandular cells with normal structural and functional characteristics. Am J Pathol. 2003;163(6):2259–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lacey A, Hedrick E, Li X, et al. . Nuclear receptor 4A1 (NR4A1) as a drug target for treating rhabdomyosarcoma (RMS). Oncotarget. 2016;7(21):31257–31269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang JR, Gan WJ, Li XM, et al. . Orphan nuclear receptor Nur77 promotes colorectal cancer invasion and metastasis by regulating MMP-9 and E-cadherin. Carcinogenesis. 2014;35(11):2474–2484. [DOI] [PubMed] [Google Scholar]
- 63. Muscat GE, Eriksson NA, Byth K, et al. . Research resource: nuclear receptors as transcriptome: discriminant and prognostic value in breast cancer. Mol Endocrinol. 2013;27(2): 350–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhu B, Yang JR, Jia Y, et al. . Overexpression of NR4A1 is associated with tumor recurrence and poor survival in non-small-cell lung carcinoma. Oncotarget. 2017;8(69): 113977–113986. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65. Xie L, Jiang F, Zhang X, et al. . Honokiol sensitizes breast cancer cells to TNF-α induction of apoptosis by inhibiting Nur77 expression. Br J Pharmacol. 2016;173(2):344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Barra F, Ferro Desideri L, Ferrero S. Inhibition of PI3K/AKT/mTOR pathway for the treatment of endometriosis. Br J Pharmacol. 2018;66(17):3626–3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mohankumar K, Lee J, Wu CS, Sun Y, Safe S. Bis-indole-derived NR4A1 ligands and metformin exhibit NR4A1-dependent glucose metabolism and uptake in C2C12 cells. Endocrinology. 2018;159(5):1950–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]