Abstract
The Disrupted-in-Schizophrenia 1 (DISC1) protein has been implicated in a range of biological mechanisms underlying chronic mental disorders such as schizophrenia. Schizophrenia is associated with abnormal striatal dopamine signalling, and all antipsychotic drugs block striatal dopamine 2/3 receptors (D2/3Rs). Importantly, the DISC1 protein directly interacts and forms a protein complex with the dopamine D2 receptor (D2R) that inhibits agonist-induced D2R internalization. Moreover, animal studies have found large striatal increases in the proportion of D2R receptors in a high affinity state (D2highR) in DISC1 rodent models. Here, we investigated the relationship between the three most common polymorphisms altering the amino-acid sequence of the DISC1 protein (Ser704Cys (rs821616), Leu607Phe (rs6675281) and Arg264Gln (rs3738401)) and striatal D2/3R availability in 41 healthy human volunteers, using [11C]-(+)-PHNO positron emission tomography. We found no association between DISC1 polymorphisms and D2/3R availability in the striatum and D2R availability in the caudate and putamen. Therefore, despite a direct interaction between DISC1 and the D2R, none of its main functional polymorphisms impact striatal D2/3R binding potential, suggesting DISC1 variants act through other mechanisms.
Introduction
The Disrupted-in-Schizophrenia 1 (DISC1) gene was originally identified at the breakpoint of a balanced t(1;11) (q42;q14.3) translocation in a Scottish family with a high-prevalence of psychiatric disorder 1–3. Evidence for a link between DISC1 and mental disorders such as psychotic and affective disorders emerged from the follow-up of families displaying rare DISC1 mutations in large family-based studies and association studies 4–9. DISC1 protein may be a useful molecule for investigating biological mechanisms underlying mental disorders 10,11. Among its neuronal functions, DISC1 is a scaffold protein involved in neuro-signaling and signal transduction, through a wide range of protein interactions 12,13.
The DISC1 protein is known to form a protein complex with the dopamine D2 receptor (D2R) 14. The DISC1-D2R complex, which formation is induced by D2R stimulation, is involved in the regulation of D2R internalization and downstream behavioural effects of D2 signaling 14,15. It has been shown that the DISC1-D2R complex inhibits agonist-induced D2R internalization, whilst its disruption prevents amphetamine-induced locomotor hyperactivity see in an artificial DISC1 model with a point mutation in exon 2 (Disc1-L100P model) 14. The D2R exists in two interconverting states, a low-affinity (μM) and a high-affinity (nM) state 16. A switch from D2 low-affinity to D2 high-affinity has been described in schizophrenia 17,18, with recent evidence showing a higher proportion of D2 high-affinity receptors (D2highR) in the putamen of antipsychotic-naïve patients 19. Interestingly, two animal studies investigated the effect of DISC1 on the D2highR using [3H]-domperidone binding challenged with dopamine 20,21. Both studies found large striatal D2highR increases compared to wild-type controls: 113% in the Disc1 L100P model 20 and 80% in a full-length DISC1-overexpressing rat model 21. This increase in proportionD2highR levels has been suggested as a putative mechanism for the increased locomotor sensitivity to amphetamine seen in the two DISC1 models, and also consistently reported in other DISC1 models 17,20–22. However, it is noteworthy that divergent findings have been found in D2/3R availability and dopamine D1 and D2 receptor levels in rodent studies 22. Therefore, the question of whether DISC1 variants are associated with alterations in striatal D2R or D2/3R availability remains unanswered, and to our knowledge no study has yet investigated it in humans.
This study examined whether three DISC1 single nucleotide polymorphisms (Ser704Cys (rs821616), Leu607Phe (rs6675281) and Arg264Gln (rs3738401)) are associated with altered D2R and/or D2/3R availability in humans. We focused on the Ser704Cys, Leu607Phe and Arg264Gln as they are the most common DISC1 polymorphisms altering the DISC1 amino-acid sequence and therefore the DISC1 protein itself. As non-synonymous missense variants, the polymorphisms result in amino acids changes of respectively serine (A) to cysteine (T) at codon 704 in exon 11 (Minor Allele Frequency (MAF) for cysteine 0.31-0.33), leucine (C) to phenylalanine (T) at codon 607 in exon 9 (MAF for phenylalanine 0.12-0.14) and arginine (G) by glutamine (A) at codon 264 in exon 2 (MAF for glutamine 0.28) 23–25. Ser704Cys, Leu607Ph and Arg264Gln have all been shown to have biological impacts on cellular signaling transduction pathways such as extracellular signal-regulated protein Kinases 1 and 2 (ERK1/2) and Wnt signaling 26,27. All three polymorphisms have also been associated with an increased risk for psychosis and also with the severity of positive psychotic symptoms in some studies 23,28–41. However it should be acknowledged that the involvement of the DISC1 gene in schizophrenia is debated 10,42,43 and that these DISC1 polymorphisms have not been linked to schizophrenia 44–46 or any other psychiatric disorder 47,48 in Psychiatric Genomics Consortium Genome-Wide Association Studies. We hypothesized that the serine (rs821616), leucine (rs6675281) and arginine (rs3738401) alleles would be associated with increased striatal D2R availability, in accordance with the alleles expressed on the DISC1 protein in the full-length human DISC1-overexpressing model 21. We used Positron Emission Tomography (PET) to measure D2/3R availability in 41 healthy participants using the high-affinity D2/3R ligand [11C]-(+)-4-propyl-9-hydroxynaphthoxazin (PHNO). The PET ligand [11C]-(+)-PHNO measures the non-displaceable binding potentials (BPND) of D2/3R in the brain 49,50. As a full agonist, [11C]-(+)-PHNO may preferentially bind to D2high compared with D2low receptors 51. Moreover, although the relative D2R:D3R binding fraction of [11C]-(+)-PHNO in the striatum varies between sub-regions, [11C]-(+)-PHNO BPND has been shown to be largely due to binding to D2 receptors in the caudate and putamen, allowing us to selectively quantify D2R availability in addition to striatum D2/3R availability 52–54. We first examined the striatum in accordance with the findings from the rodent studies 20,21, and then its caudate and putamen subdivisions 52 in order to focus on D2 rich striatal regions.
Materials and Methods
Participants
The study was approved by the institutional review board and local research ethics committee (15/LO/0011 and 12/LO/1955). Participants were recruited via online advertisement and in the newspaper. All participants gave informed written consent to take part in the study after its full description. The inclusion criteria for the study were 1) age above 18 years; 2) capacity to give written informed consent. The exclusion criteria were 1) any current medical conditions, or history of medical condition (past minor self-limiting conditions were permitted); 2) history of a psychiatric disorder as determined by the Structured Clinical Interview for DSM-IV Axis 1 Disorders, Clinician Version (SCID-CV) 55; 3) history of substance abuse/dependence as determined by the Structured Clinical Interview for DSM-IV Axis 1 Disorders, Clinician Version (SCID-CV)55; 4) history of head injury with a loss of consciousness; 5) a family history of any psychiatric disorder in first- or second-degree relatives; 6) contraindications to positron emission tomography (PET) scanning (significant prior exposure to radiation, pregnancy or breast feeding); 7) positive urine drug screen for cannabis as the drug has been shown to influence D2/3 availability 56,57. All participants provided urine samples to screen for a drug use and pregnancy test in women prior to the scan.
Data acquisition
[11C]-PHNO PET data acquisition
PET images were acquired using a Siemens Biograph HiRez XVI PET scanner (Siemens Healthcare, Erlangen, Germany). A low-dose computed tomography scan was first administered for attenuation and model-based scatter correction followed by the injection of a single intravenous bolus of 0.020-0.029 micrograms/kg [11C]-(+)-PHNO. Dynamic emission data were acquired continuously for 90 minutes after the administration of the radiotracer. The dynamic images were then reconstructed using a filtered back-projection algorithm into 31 frames (8x15 seconds, 3x60 seconds, 5x120 seconds, 15x300 seconds) with a 128 matrix, a zoom of 2.6 and a transaxial Gaussian filter of 5mm.
Structural MRI acquisition
The PET spatial pre-processing pipeline required a high resolution structural magnetic resonance imaging (MRI) scan for each subject. MR images were acquired on a Siemens MAGNETOM Verio 3T MRI scanner and a 32-channel phased-array head-coil. A high-resolution T1-weighted volume was acquired for PET coregistration using a Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence with parameters from the Alzheimer’s Disease Research Network (ADNI-GO; 160 slices x240x256, TR=2300ms, TE=2.98ms, flip angle=9°, 1mm isotropic voxels, bandwidth=240Hz/pixel, parallel imaging (PI) factor=2) 58.
PET analysis
PET images were analysed using MATLAB version 2015B and an automatic analysis pipeline implemented in MIAKAT (MIAKAT release 4.2.6, www.miakat.org) 59. The ICBM152 high-resolution structural MRI template in Montréal Neurologic Institute (MNI) space was non-linearly warped to the high-resolution T1-weighted MRI of each participant using Statistical Parametric Mapping (SPM8) (Wellcome Trust Centre for Neuroimaging). The deformation parameters were applied to the CIC atlas which defines the anatomical extents of the striatum, caudate and putamen and a whole cerebellum ROI in MNI space 53. The application of deformation parameters brings the ROIs into the native space of each subject’s MRI scan. The MRI and ROIs were then downsampled to the PET resolution (2 mm). A frame-by-frame registration process on a single frame of reference was used for motion correction for dynamic PET images. Individual averaged PET images were then co-registered to their respective MRIs using rigid body co-registration.Regional time activity curves (TAC) were obtained by applying individual parcellations to the realigned dynamic images. Our outcome measure of interest was non-displaceable binding (BPND) of [11C]-(+)-PHNO:
where Bavail is the proportion of D2/3Rs available to be bound by PHNO (i.e. the fraction of receptors not bound by endogenous synaptic dopamine), fND is the free fraction of PHNO in the brain and 1/KD the affinity of ligand for the target. BPND was obtained by kinetic modelling with a simplified reference tissue model 60,61, using the whole cerebellum as a reference region due its low content in dopaminergic neurons 62,63.
Genetic analysis
Genomic DNA was extracted from blood using standard methods 64. Genotyping of the rs821616 A>T, rs6675281 A>G, rs3738401 A>G polymorphisms was performed using the Illumina Infinium™ CoreExome-24 PsychArray version 1.1 BeadChip Kit (https://emea.illumina.com). The genotype frequencies did not significantly deviate from Hardy–Weinberg equilibrium for the rs821616 SNP (χ2=1.636, p=0.200), the rs3738401 SNP (χ2=0.573, p=0.449) and the rs6675281 SNP (χ2 =0.124, p=0.724).
Statistical analysis
Statistical Package for the Social Sciences SPSS version 24 was used for all statistical analysis (IBM, Armonk, N.Y.). Independent t test and Mann-Whitney test were used to compare age and injected dose respectively. Participants were divided into two groups for each polymorphism. For rs821616 (Ser704Cys), serine homozygotes (AA) were compared to cysteine homozygotes (TT) and heterozygotes (AT). For rs6675281 (Leu607Phe), leucine homozygotes (CC) were compared to phenylalanine homozygotes (TT) and heterozygotes (TC). For rs3738401 (Arg264Gln), arginine homozygotes (GG) were compared to glutamine homozygotes (AG) and heterozygotes (AA). LDlink 65 was used to map linkage disequilibrium for the three polymorphisms based on the 1000 genomes phase 3 (version 5) data 66. Planned-independent t-tests were used to test for an effect of the DISC1 polymorphisms rs821616 (Ser704Cys) and rs3738401 (Arg264Gln) on mean D2/3R availability in the striatum, caudate and putamen. As age has been shown to affect [11C]-(+)-PHNO signal 67 and subjects were not matched for age for rs6675281 (Leu607Phe), univariate analysis of covariance (ANCOVAs) were used for this polymorphism, with the rs6675281 (Leu607Phe) genotype as the independent variable, D2/3R binding potential for the striatum, the caudate and putamen respectively as the dependent variables, and age entered as covariate. Effect sizes are reported as corrected Cohen’s d and partial η2. An alpha threshold was set at a 0.05 (two-tailed) for significance for all statistical comparisons.
Results
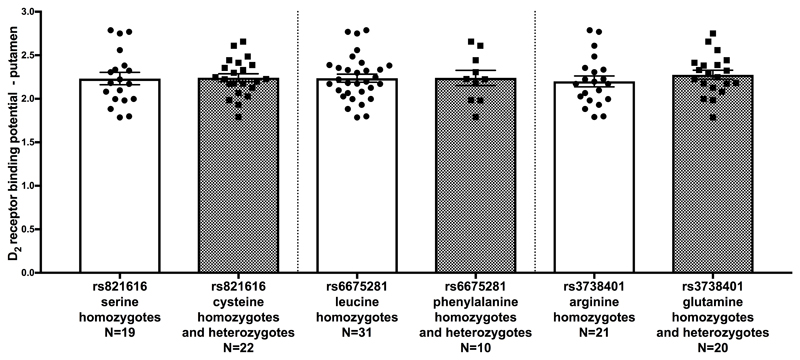
Forty-two subjects underwent a [11C]-(+)-PHNO PET scan and a structural Magnetic Resonance Imaging scan. One subject was excluded due to positive urine drug screen result for cannabis. This resulted in the final inclusion of 41 subjects (16 females, mean age/year (SD)=25.51 (6.58)). Demographics, scans parameters and PET results are shown in Table 1, with the mean D2/3R binding potentials for the DISC1 Ser704Cys (rs821616), Leu607Phe (rs6675281) and Arg264Gln (rs3738401) polymorphisms for the striatum caudate, and putamen respectively. The polymorphisms have all been reported to be in linkage equilibrium (χ2=0.48, p=0.493 for rs821616 and rs6675281), (χ2=0.46, p=0.496 for rs821616 and rs3738401) and (χ2=1.84, p=0.175 for rs6675281 and rs3738401). The T carriers and CC homozygotes of the rs6675281 group were not matched for age (mean age (SD) respectively 30.10 (10.08) and 24.03 (4.25), p=0.009). Age was therefore included as a covariate for this polymorphism as age has been shown to be linearly related to [11C]-(+)-PHNO signal 67. There was no effect of rs821616 (t(39)=-0.204, p=0.839, corrected Cohen’s d =0.06), rs6675281 (F(1,38)=1.166, p=0.287), partial η2=0.03) or rs3738401 (t(39)=0.971, p=0.338, corrected Cohen’s d=0.30) genotype on D2/3R binding potential in the striatum (Figure 1). To avoid a masking effect by the D3R, we also examined the caudate and putamen sub-regions, as these regions have negligible D2R/D3R fraction 53. There was no effect of rs821616 (t(39)=-1.022, p=0.313, corrected Cohen’s d =0.32), rs6675281 (F(1,38)=0.352, p=0.557, partial η2=0.01) or rs3738401 (t(39)=0.767, p=0.448, corrected Cohen’s d =0.24) genotypes on D2R binding potential in the caudate (Figure 2). There was no effect of rs821616 (t(39)=0.121, p=0.905, corrected Cohen’s d =0.04), rs6675281 (F(1,38)=1.217, p=0.277, partial η2=0.03) and rs3738401 (t(39)=0.935, p=0.355, corrected Cohen’s d =0.29) genotypes on D2R binding potential in the putamen (Figure 3).
Table 1. Demographics and PET parameters.
| DISC1 Ser704Cys (rs821616) | DISC1 Leu607Phe (rs6675281) | DISC1 Arg264Gln (rs3738401) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Serine homozygotes AA carriers | Cysteine homozygotes and heterozygotes (TT or AT carriers) | p value | Leucine homozygotes (CC carriers) | Phenylalanine homozygotes and heterozygotes (TC or TT carriers) | p value | Arginine homozygotes GG carriers | Glutamine homozygotes and heterozygotes (AG or AA carriers) | p value | |
| Demographics | ||||||||||
| Males, n (%) | 25 | 9 (36%) | 16 (64%) | 0.097ii | 18 (72%) | 7 (28%) | 0.501ii | 14 (56%) | 9 (34%) | 0.444ii |
| Females, n (%) | 16 | 10 (62.5%) | 6 (37.5%) | 13 (81.3%) | 3 (18.7%) | 7 (43.8%) | 11 (56.2%) | |||
| Total n (%) | 41 | 19 (46.3%) | 22 (7 TT) (53.7%) | 31 (75.6%) | 10 (1 TT) (24.4%) | 21 (51.2%) | 20 (2 AA) (48.8%) | |||
| Age, mean (SD) | 25.51 (6.58) | 25.32 (5.06) | 25.7 (7.79) | 0.862i | 24.03 (4.25) | 30.10 (10.08) | 0.009i | 25.19 (5.57) | 25.85 (7.64) | 0.753i |
| White Caucasian, n (%) | 26 | 11 (42.3%) | 15 (57.7%) | 0.586ii | 19 (73.1%) | 7 (26.9%) | 0.588ii | 11 (42.3%) | 15 (57.7%) | 0.140ii |
| Black British, n (%) | 12 (Black Africans n=11; Black Caribean n=1) | 7 (58.3%) | 5 (41.7%) | 9 (75%) | 3 (25%) | 9 (75%) | 3 (25%) | |||
| Mixed, n (%) | 3 (East Asian: n=1; Mixed White Caucasian/South Asian n=1; Mixed White Caucasian/Central Asian n=1) | 1 (33%) | 2 (66%) | 3 (100%) | 0 (0%) | 1 (33%) | 2 (66%) | |||
| PET parameters | ||||||||||
| Radioactivity injected (MBq), mean (SD) | 177.16 (47.36) | 176.98 (51.31) | 177.32 (44.90) | 0.983i | 179.28 (48.02) | 170.60 (47.13) | 0.620i | 176.89 (51.68) | 177.44 (43.72) | 0.971i |
| Mass Injected (μg), mean (SD) | 1.57 (0.32) | 1.56 (0.31) | 1.58 (0.34) | 0.792i | 1.57 (0.35) | 1.58 (0.23) | 0.881i | 1.57 (0.33) | 1.58 (0.33) | 0.939i |
| D2/3R BPND striatum, mean (SD) | 2.05 (0.27) | 2.05 (0.31) | 2.04 (0.24) | 0.839i | 2.05 (0.26) | 2.05 (0.32) | 0.287iii | 2.01 (0.31) | 2.09 (0.23) | 0.338i |
| D2R BPND caudate, mean (SD) | 1.48 (0.30) | 1.53 (0.31) | 1.43 (0.29) | 0.313i | 1.49 (0.29) | 1.45 (0.36) | 0.557iii | 1.44 (0.34) | 1.52 (0.26) | 0.448i |
| D2R BPND putamen, mean (SD) | 2.24 (0.26) | 2.23 (0.31) | 2.24 (0.21) | 0.905i | 2.23 (0.26) | 2.24 (0.28) | 0.277iii | 2.20 (0.29) | 2.28 (0.23) | 0.355i |
Independent t test
Pearson Chi-Square
ANCOVA
D2/3R BPND: dopamine D2/3 receptor non-displaceable binding potential
MBq: megabecquerel
SD: standard deviation
μg: microgram
Figure 1.
Ser704Cys, Leu607Phe and Arg264Gln and dopamine D2/3 receptor binding potentials in the striatum
Figure 2.
Ser704Cys, Leu607Phe and Arg264Gln and dopamine D2 receptor binding potentials in the caudate
Figure 3.
Ser704Cys, Leu607Phe and Arg264Gln and dopamine D2 receptor binding potentials in the putamen
Discussion
This study examined for the first time whether the three most common missense variants of the DISC1 gene Ser704Cys, Leu607Phe and Arg264Gln have an effect on D2/3R availability in the striatum, or D2R availability in the caudate and putamen, using [11C]-(+)-PHNO PET in 41 healthy participants. Our results showed that none of these polymorphisms were associated with significant alterations of [11C]-(+)-PHNO signal in any of these regions of interest in humans.
Our results are not in line with the rodent studies showing 1) increased D2R availability in the artificial point mutation Disc1 model and DISC1-overexpressing rat model using [3H]domperidone binding challenged with dopamine 20,21; and 2) increased striatal D2/3R availability using [11C]-raclopride, increased D2R availability in the medial part of the right rostral striatum using [3H]-spiperone autoradiography and increased D2R levels using real-time PCR in the hDISC1 model 68. However, our results are consistent with other rodent studies. For example, no significant differences in striatal D2R levels were found in the DISC1-overexpressing model using [3H]-raclopride autoradiography (D2/3R) 21, in the hDISC1 model using [3H]-spiperone autoradiography (D2R) (lateral part of the right rostral striatum) 68 or [11C]-raclopride autoradiography (D2/3R) 69, and in the Disc1Δ2–3 model using real-time polymerase chain reaction 70. The variable effects on D2 highlights that DISC1’s interactions with D2 are complex.
The differences observed with the rodent studies could be due to the three human variants having different biological effects compared to the DISC1 models in which significant effects on D2 levels were found. Among the DISC1 models used, only the short interfering RNA knockdown or knockout models (hDISC1 model) should have loss of function phenotypes whereas all others could have either loss of function, gain of function or combined phenotypes at the same time. Notwithstanding this, as highlighted above, there is still inconsistency amongst the animal loss of function models (hDISC1 model). In summary, the DISC1 rodent models are not the same between one another, and not the same as the DISC1 human variants which could explain the discrepancies between the results observed in the literature. The point mutations associated with the variants might also not encompass the binding site with the D2R. Despite the description of the direct interaction between DISC1 and D2R 14, its biophysical characterization and the exact site(s) of the DISC1 protein involved remain unknown.
Limitations
This study has several limitations. First, although our sample size is relatively large for human PET studies (N=41), a type II error could account for the observed results, although our sample had more than 80% power to detect a main effect of Cohen’s d=1 with alpha=0.05 (two-tailed), corresponding to a percent difference between groups of 13% for the striatum. Nevertheless our effect size estimates indicate that, if there is an effect it is likely to be small (Cohen’s d values from 0.01 to 0.32), and, as such, an effect is unlikely to be clinically significant on its own if it is present. Increased D2R binding potential have been found with large effect sizes (Cohen’s d>2) in the artificial point mutation Disc1 model and DISC1-overexpressing rat model 20,21. However, it should be noted that human variants are likely to have much smaller effects. Second, while some studies report that [11C]-(+)-PHNO binds specifically to D2highR as opposed to D2lowR 51, other studies present opposing results (Seeman, 2012). Therefore, [11C]-(+)-PHNO PET imaging may be unable to adequately distinguish between high vs low variants of D2Rs, further diminishing our power to detect a specific effect of DISC1 polymorphisms on D2highR. Third, the heterogeneous ancestry of the sample should also be acknowledged as a limitation since genetic ancestry has been associated with striatal dopamine D2/3R availability 71. However, the groups for each polymorphism were matched for the different ethnicities (p=0.586 for Ser704Cys, p=0.588 for Leu607Phe and p=0.140 for Arg264, Table 1).
Implications
Our results did not show an effect of the Ser704Cys, Leu607Phe and Arg264Gln polymorphisms on availability of D2/3Rs in the striatum, or D2Rs in the caudate and putamen regions, with no indication of difference between groups. We therefore have not found evidence that the associations between these polymorphisms and psychotic and other mental disorders (Ser704Cys 28–36, Leu607Phe 37–40, and Arg264Gln 23,29,41) are likely mediated by altered striatal D2/3R availability.
However, statistical epistasis between the DISC1 polymorphisms and other genes involved in the D2R signaling pathway affecting D2R cannot be ruled out, as well as effects of other DISC1 variants on the D2R. Likewise, environmental factors such as exposure to psychosocial stress may also interact with the polymorphisms to affect dopamine function and mediate risk for schizophrenia and other mental illnesses 72. The DISC1 Ser704Cys, Leu607Phe and Arg264Gln polymorphisms could increase risk of psychotic and others disorders through effect on prefrontal or hippocampal structure and function 25, other neurotransmitters such as glutamate 73, or alterations in other components of the dopamine system such as the dopamine receptor (DAT) 21 or presynaptic dopamine synthesis capacity and release. We have recently shown association between the serine allele of Ser704Cys and increased striatal dopamine synthesis capacity in healthy participants 74. Interestingly, recent studies indicate the t(1;11) translocation may increase the risk of psychosis through various other mechanisms, including altered DNA methylation 75, regulation of N-methyl-D-aspartate receptors (NMDAR) motility 76 and/or 76added effects of the translocation and a variable subset of potential phenotypic polymorphisms 77. Future studies should aim at clarifying how the DISC1 protein interacts with the D2R and whether the DISC1 Ser704Cys, Leu607Phe and Arg264Gln polymorphisms affect D2R availability in clinical populations.
Conclusions
The three most common DISC1 polymorphisms Ser704Cys, Leu607Phe and Arg264Gln are not associated with significant alterations in striatal D2/3R or D2R availability in healthy volunteers. This indicates the mechanism mediating associations between these variants and psychotic disorders is unlikely to involve altered D2 availability.
Acknowledgements
T.D., O.H., and C.K. were supported by a EU-FP7 MC-ITN IN-SENS grant (grant number 607616). T.D. was supported by the National Institute for Health Research (NIHR) at Oxford Health NHS Foundation Trust. O.H was supported by Medical Research Council-UK (no. MC-A656-5QD30), Maudsley Charity (no. 666), Brain and Behavior Research Foundation, and Wellcome Trust (no. 094849/Z/10/Z) grants and the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. We acknowledge the contribution of the NIHR BRC BioResource and Genomics & Biomarker Core Facility for genotyping. M.N. was supported by the National Institute for Health Research (UK). R.A.A. is supported by the Academy of Medical Sciences (AMS-SGCL13-Adams), the National Institute of Health Research (CL-2013-18-003), and the NIHR UCLH Biomedical Research Centre. This study presents independent research supported by the National Institute for Health Research NIHR BioResource Centre Maudsley at South London and Maudsley NHS Foundation Trust and King's College London. C.C and S.H.L gratefully acknowledge capital equipment funding from the Maudsley Charity (Grant Ref. 980) and Guy’s and St Thomas’s Charity (Grant Ref. STR130505).
O.H. has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organised by Astra-Zeneca, Autifony, BMS, Eli Lilly, Heptares, Jansenn, Lundbeck, Lyden-Delta, Otsuka, serinevier, Sunovion, Rand and Roche. Neither Dr Howes nor his family have been employed by or have holdings/ a financial stake in any biomedical company. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. All other authors do not declare any conflict of interest. We thank participants and all the imaging staff for their assistance with this study.
References
- 1.St Clair D, Blackwood D, Muir W, et al. Association within a family of a balanced autosomal translocation with major mental illness. Lancet. 1990;336(8706):13–16. doi: 10.1016/0140-6736(90)91520-k. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs P, Brunton M, Frackiewicz A, Newton M, Cook P, Robson E. Studies on a family with three cytogenetic markers. Annals of Human Genetics (Lond) 1970;33:325–336. [Google Scholar]
- 3.Millar JK, Wilson-Annan JC, Anderson S, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9(9):1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- 4.Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Molecular psychiatry. 2008;13(1):36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- 5.Blackwood DH, Fordyce A, Walker MT, St Clair DM, Porteous DJ, Muir WJ. Schizophrenia and affective disorders--cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. American journal of human genetics. 2001;69(2):428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sachs NA, Sawa A, Holmes SE, Ross CA, DeLisi LE, Margolis RL. A frameshift mutation in Disrupted in Schizophrenia 1 in an American family with schizophrenia and schizoaffective disorder. Molecular psychiatry. 2005;10(8):758–764. doi: 10.1038/sj.mp.4001667. [DOI] [PubMed] [Google Scholar]
- 7.Ekelund J, Hennah W, Hiekkalinna T, et al. Replication of 1q42 linkage in Finnish schizophrenia pedigrees. Molecular psychiatry. 2004;9(11):1037–1041. doi: 10.1038/sj.mp.4001536. [DOI] [PubMed] [Google Scholar]
- 8.Ekelund J, Hovatta I, Parker A, et al. Chromosome 1 loci in Finnish schizophrenia families. Hum Mol Genet. 2001;10(15):1611–1617. doi: 10.1093/hmg/10.15.1611. [DOI] [PubMed] [Google Scholar]
- 9.Hennah W, Varilo T, Kestila M, et al. Haplotype transmission analysis provides evidence of association for DISC1 to schizophrenia and suggests sex-dependent effects. Hum Mol Genet. 2003;12(23):3151–3159. doi: 10.1093/hmg/ddg341. [DOI] [PubMed] [Google Scholar]
- 10.Niwa M, Cash-Padgett T, Kubo KI, et al. DISC1 a key molecular lead in psychiatry and neurodevelopment: No-More Disrupted-in-Schizophrenia 1. Molecular psychiatry. 2016;21(11):1488–1489. doi: 10.1038/mp.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawa A, Ishizuka K, Katsanis N. The potential of DISC1 protein as a therapeutic target for mental illness. Expert opinion on therapeutic targets. 2016:1–3. doi: 10.1517/14728222.2016.1146694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nature reviews Neuroscience. 2011;12(12):707–722. doi: 10.1038/nrn3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porteous DJ, Millar JK, Brandon NJ, Sawa A. DISC1 at 10: connecting psychiatric genetics and neuroscience. Trends in molecular medicine. 2011;17(12):699–706. doi: 10.1016/j.molmed.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su P, Li S, Chen S, et al. A dopamine D2 receptor-DISC1 protein complex may contribute to antipsychotic-like effects. Neuron. 2014;84(6):1302–1316. doi: 10.1016/j.neuron.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Zheng P, Hu M, Xie Y, Yu Y, Jaaro-Peled H, Huang XF. Aripiprazole and haloperidol protect neurite lesions via reducing excessive D2R-DISC1 complex formation. Progress in neuro-psychopharmacology & biological psychiatry. 2018 doi: 10.1016/j.pnpbp.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Seeman P. Nomenclature of central and peripheral dopaminergic sites and receptors. Biochem Pharmacol. 1982;31(16):2563–2569. doi: 10.1016/0006-2952(82)90700-6. [DOI] [PubMed] [Google Scholar]
- 17.Seeman P, Schwarz J, Chen JF, et al. Psychosis pathways converge via D2high dopamine receptors. Synapse. 2006;60(4):319–346. doi: 10.1002/syn.20303. [DOI] [PubMed] [Google Scholar]
- 18.Seeman P. Schizophrenia and dopamine receptors. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2013;23(9):999–1009. doi: 10.1016/j.euroneuro.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Kubota M, Nagashima T, Takano H, et al. Affinity States of Striatal Dopamine D2 Receptors in Antipsychotic-Free Patients with Schizophrenia. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2017;20(11):928–935. doi: 10.1093/ijnp/pyx063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipina TV, Niwa M, Jaaro-Peled H, et al. Enhanced dopamine function in DISC1-L100P mutant mice: implications for schizophrenia. Genes Brain and Behavior. 2010;9(7):777–789. doi: 10.1111/j.1601-183X.2010.00615.x. [DOI] [PubMed] [Google Scholar]
- 21.Trossbach SV, Bader V, Hecher L, et al. Misassembly of full-length Disrupted-in-Schizophrenia 1 protein is linked to altered dopamine homeostasis and behavioral deficits. Molecular psychiatry. 2016 doi: 10.1038/mp.2015.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahoun T, Trossbach SV, Brandon NJ, Korth C, Howes OD. The impact of Disrupted-in-Schizophrenia 1 (DISC1) on the dopaminergic system: a systematic review. Translational psychiatry. 2017;7(1):e1015. doi: 10.1038/tp.2016.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palo OM, Antila M, Silander K, et al. Association of distinct allelic haplotypes of DISC1 with psychotic and bipolar spectrum disorders and with underlying cognitive impairments. Hum Mol Genet. 2007;16(20):2517–2528. doi: 10.1093/hmg/ddm207. [DOI] [PubMed] [Google Scholar]
- 24.Raznahan A, Lee Y, Long R, et al. Common functional polymorphisms of DISC1 and cortical maturation in typically developing children and adolescents. Molecular psychiatry. 2011;16(9):917–926. doi: 10.1038/mp.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duff BJ, Macritchie KA, Moorhead TW, Lawrie SM, Blackwood DH. Human brain imaging studies of DISC1 in schizophrenia, bipolar disorder and depression: a systematic review. Schizophr Res. 2013;147(1):1–13. doi: 10.1016/j.schres.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Singh KK, De Rienzo G, Drane L, et al. Common DISC1 polymorphisms disrupt Wnt/GSK3beta signaling and brain development. Neuron. 2011;72(4):545–558. doi: 10.1016/j.neuron.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashimoto R, Numakawa T, Ohnishi T, et al. Impact of the DISC1 Ser704Cys polymorphism on risk for major depression, brain morphology and ERK signaling. Hum Mol Genet. 2006;15(20):3024–3033. doi: 10.1093/hmg/ddl244. [DOI] [PubMed] [Google Scholar]
- 28.Callicott JH, Straub RE, Pezawas L, et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(24):8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song W, Li W, Feng J, Heston LL, Scaringe WA, Sommer SS. Identification of high risk DISC1 structural variants with a 2% attributable risk for schizophrenia. Biochemical and biophysical research communications. 2008;367(3):700–706. doi: 10.1016/j.bbrc.2007.12.117. [DOI] [PubMed] [Google Scholar]
- 30.Qu M, Tang F, Yue W, et al. Positive association of the Disrupted-in-Schizophrenia-1 gene (DISC1) with schizophrenia in the Chinese Han population. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2007;144B(3):266–270. doi: 10.1002/ajmg.b.30322. [DOI] [PubMed] [Google Scholar]
- 31.Luo X, Jin C, Zhou Z, et al. New findings support the association of DISC1 genetic variants with susceptibility to schizophrenia in the Han Chinese population. Psychiatry research. 2015;228(3):966–968. doi: 10.1016/j.psychres.2015.05.115. [DOI] [PubMed] [Google Scholar]
- 32.He BS, Zhang LY, Pan YQ, et al. Association of the DISC1 and NRG1 genetic polymorphisms with schizophrenia in a Chinese population. Gene. 2016;590(2):293–297. doi: 10.1016/j.gene.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 33.Wang HY, Liu Y, Yan JW, et al. Gene polymorphisms of DISC1 is associated with schizophrenia: Evidence from a meta-analysis. Progress in neuro-psychopharmacology & biological psychiatry. 2017;81:64–73. doi: 10.1016/j.pnpbp.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Kim HJ, Park HJ, Jung KH, et al. Association study of polymorphisms between DISC1 and schizophrenia in a Korean population. Neuroscience letters. 2008;430(1):60–63. doi: 10.1016/j.neulet.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Vazquez-Bourgon J, Mata I, Roiz-Santianez R, et al. A Disrupted-in-Schizophrenia 1 Gene Variant is Associated with Clinical Symptomatology in Patients with First-Episode Psychosis. Psychiatry investigation. 2014;11(2):186–191. doi: 10.4306/pi.2014.11.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeRosse P, Hodgkinson CA, Lencz T, et al. Disrupted in schizophrenia 1 genotype and positive symptoms in schizophrenia. Biological psychiatry. 2007;61(10):1208–1210. doi: 10.1016/j.biopsych.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 37.Hodgkinson CA, Goldman D, Jaeger J, et al. Disrupted in schizophrenia 1 (DISC1): association with schizophrenia, schizoaffective disorder, and bipolar disorder. American journal of human genetics. 2004;75(5):862–872. doi: 10.1086/425586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rastogi A, Zai C, Likhodi O, Kennedy JL, Wong AH. Genetic association and post-mortem brain mRNA analysis of DISC1 and related genes in schizophrenia. Schizophr Res. 2009;114(1-3):39–49. doi: 10.1016/j.schres.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Lepagnol-Bestel AM, Dubertret C, Benmessaoud D, et al. Association of DISC1 gene with schizophrenia in families from two distinct French and Algerian populations. Psychiatric genetics. 2010;20(6):298–303. doi: 10.1097/YPG.0b013e32833aa5c4. [DOI] [PubMed] [Google Scholar]
- 40.Szeszko PR, Hodgkinson CA, Robinson DG, et al. DISC1 is associated with prefrontal cortical gray matter and positive symptoms in schizophrenia. Biological psychology. 2008;79(1):103–110. doi: 10.1016/j.biopsycho.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mouaffak F, Kebir O, Chayet M, et al. Association of Disrupted in Schizophrenia 1 (DISC1) missense variants with ultra-resistant schizophrenia. The pharmacogenomics journal. 2011;11(4):267–273. doi: 10.1038/tpj.2010.40. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan PF. Questions about DISC1 as a genetic risk factor for schizophrenia. Molecular psychiatry. 2013;18(10):1050–1052. doi: 10.1038/mp.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porteous DJ, Thomson PA, Millar JK, et al. DISC1 as a genetic risk factor for schizophrenia and related major mental illness: response to Sullivan. Molecular psychiatry. 2014;19(2):141–143. doi: 10.1038/mp.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pardinas AF, Holmans P, Pocklington AJ, et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nature genetics. 2018;50(3):381–389. doi: 10.1038/s41588-018-0059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bipolar D. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Electronic address drve, Bipolar D, Schizophrenia Working Group of the Psychiatric Genomics C. Genomic Dissection of Bipolar Disorder and Schizophrenia, Including 28 Subphenotypes. Cell. 2018;173(7):1705–1715. doi: 10.1016/j.cell.2018.05.046. e1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathieson I, Munafo MR, Flint J. Meta-analysis indicates that common variants at the DISC1 locus are not associated with schizophrenia. Molecular psychiatry. 2012;17(6):634–641. doi: 10.1038/mp.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cross-Disorder Group of the Psychiatric Genomics C. Lee SH, Ripke S, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nature genetics. 2013;45(9):984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grove J, Ripke S, Als TD, et al. Identification of common genetic risk variants for autism spectrum disorder. Nature genetics. 2019;51(3):431–444. doi: 10.1038/s41588-019-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levant B, Grigoriadis DE, De Souza EB. Relative affinities of dopaminergic drugs at dopamine D2 and D3 receptors. European journal of pharmacology. 1995;278(3):243–247. doi: 10.1016/0014-2999(95)00160-m. [DOI] [PubMed] [Google Scholar]
- 50.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000;20(3):423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Willeit M, Ginovart N, Kapur S, et al. High-affinity states of human brain dopamine D2/3 receptors imaged by the agonist [11C]-(+)-PHNO. Biological psychiatry. 2006;59(5):389–394. doi: 10.1016/j.biopsych.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 52.Nakajima S, Caravaggio F, Boileau I, et al. Lack of age-dependent decrease in dopamine D3 receptor availability: a [(11)C]-(+)-PHNO and [(11)C]-raclopride positron emission tomography study. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015;35(11):1812–1818. doi: 10.1038/jcbfm.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tziortzi AC, Searle GE, Tzimopoulou S, et al. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. NeuroImage. 2011;54(1):264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 54.Searle G, Beaver JD, Comley RA, et al. Imaging dopamine D3 receptors in the human brain with positron emission tomography, [11C]PHNO, and a selective D3 receptor antagonist. Biological psychiatry. 2010;68(4):392–399. doi: 10.1016/j.biopsych.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 55.First Michael B, Spitzer Robert L, Gibbon Miriam, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. American Psychiatric Press, Inc; 1996. [Google Scholar]
- 56.Bloomfield MA, Ashok AH, Volkow ND, Howes OD. The effects of Delta(9)-tetrahydrocannabinol on the dopamine system. Nature. 2016;539(7629):369–377. doi: 10.1038/nature20153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bossong MG, Mehta MA, van Berckel BN, Howes OD, Kahn RS, Stokes PR. Further human evidence for striatal dopamine release induced by administration of 9-tetrahydrocannabinol (THC): selectivity to limbic striatum. Psychopharmacology. 2015;232(15):2723–2729. doi: 10.1007/s00213-015-3915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27(4):685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gunn RN, Coello C, Searle G. Molecular Imaging And Kinetic Analysis Toolbox (MIAKAT) - A Quantitative Software Package for the Analysis of PET Neuroimaging Data. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016;57(supplement 2):1928. [Google Scholar]
- 60.Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. NeuroImage. 1997;6(4):279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- 61.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. NeuroImage. 1996;4(3 Pt 1):153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 62.Kumakura Y, Cumming P. PET studies of cerebral levodopa metabolism: a review of clinical findings and modeling approaches. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2009;15(6):635–650. doi: 10.1177/1073858409338217. [DOI] [PubMed] [Google Scholar]
- 63.Egerton A, Demjaha A, McGuire P, Mehta MA, Howes OD. The test-retest reliability of 18F-DOPA PET in assessing striatal and extrastriatal presynaptic dopaminergic function. NeuroImage. 2010;50(2):524–531. doi: 10.1016/j.neuroimage.2009.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Freeman B, Smith N, Curtis C, Huckett L, Mill J, Craig IW. DNA from buccal swabs recruited by mail: evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behavior genetics. 2003;33(1):67–72. doi: 10.1023/a:1021055617738. [DOI] [PubMed] [Google Scholar]
- 65.Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31(21):3555–3557. doi: 10.1093/bioinformatics/btv402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Genomes Project C. Abecasis GR, Altshuler D, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467(7319):1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matuskey D, Worhunksy P, Correa E, et al. Age-related changes in binding of the D2/3 receptor radioligand [(11)C](+)PHNO in healthy volunteers. NeuroImage. 2016;130:241–247. doi: 10.1016/j.neuroimage.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jaaro-Peled H, Niwa M, Foss CA, et al. Subcortical dopaminergic deficits in a DISC1 mutant model: a study in direct reference to human molecular brain imaging. Hum Mol Genet. 2013;22(8):1574–1580. doi: 10.1093/hmg/ddt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pogorelov VM, Nomura J, Kim J, et al. Mutant DISC1 affects methamphetamine-induced sensitization and conditioned place preference: a comorbidity model. Neuropharmacology. 2012;62(3):1242–1251. doi: 10.1016/j.neuropharm.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakai T, Nagai T, Wang R, et al. Alterations of GABAergic and dopaminergic systems in mutant mice with disruption of exons 2 and 3 of the Disc1 gene. Neurochemistry international. 2014;74:74–83. doi: 10.1016/j.neuint.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 71.Wiers CE, Towb PC, Hodgkinson CA, et al. Association of genetic ancestry with striatal dopamine D2/D3 receptor availability. Molecular psychiatry. 2017 doi: 10.1038/mp.2017.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Howes OD, McCutcheon R, Owen MJ, Murray RM. The Role of Genes, Stress, and Dopamine in the Development of Schizophrenia. Biological psychiatry. 2017;81(1):9–20. doi: 10.1016/j.biopsych.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thomson PA, Duff B, Blackwood DH, et al. Balanced translocation linked to psychiatric disorder, glutamate, and cortical structure/function. NPJ Schizophr. 2016;2 doi: 10.1038/npjschz.2016.24. 16024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dahoun T, Pardinas AF, Veronese M, et al. The effect of the DISC1 Ser704Cys polymorphism on striatal dopamine synthesis capacity: an [18F]-DOPA PET study. Hum Mol Genet. 2018 doi: 10.1093/hmg/ddy242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCartney DL, Walker RM, Morris SW, et al. Altered DNA methylation associated with a translocation linked to major mental illness. NPJ Schizophr. 2018;4(1):5. doi: 10.1038/s41537-018-0047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malavasi ELV, Economides KD, Grunewald E, et al. DISC1 regulates N-methyl-D-aspartate receptor dynamics: abnormalities induced by a Disc1 mutation modelling a translocation linked to major mental illness. Translational psychiatry. 2018;8(1):184. doi: 10.1038/s41398-018-0228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ryan NM, Lihm J, Kramer M, et al. DNA sequence-level analyses reveal potential phenotypic modifiers in a large family with psychiatric disorders. Molecular psychiatry. 2018;23(12):2254–2265. doi: 10.1038/s41380-018-0087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]