Abstract
Purpose
The objective of the present retrospective analysis was to describe the experience of intraperitoneal (IP) paclitaxel and systemic chemotherapy in patients with peritoneal metastasis (PM) of advanced gastric cancer (AGC) in a multicenter setting in Korea.
Materials and Methods
The medical records of patients with AGC, who were diagnosed with PM between January 2015 and December 2018, were reviewed. IP catheter was placed in the pouch of Douglas and was used for the administration of IP paclitaxel chemotherapy.
Results
We reviewed the clinical outcomes of IP paclitaxel and systemic chemotherapy administration in 82 patients at six institutions in Korea. Mean number of IP chemotherapy cycles was 6.6. The mean peritoneal cancer index (PCI) was 21.9. Postoperative complications related to IP catheter and port were observed in 15 patients. The overall median survival was 20.0 months. A significant difference was observed in the survival rate according to the ascites grade (grade I and II, 24.1 months; grade III and IV, 15.3 months; P=0.014) and PCI grade (grade I, 25.6 months; grade II and III, 16.3 months; P=0.023).
Conclusions
The feasibility of IP paclitaxel and systemic chemotherapy administration was demonstrated in this experience-based retrospective analysis suggesting that the procedure is beneficial in patients with PM of AGC.
Keywords: Advanced gastric cancer, Peritoneal metastasis, Intraperitoneal chemotherapy
INTRODUCTION
Stage IV gastric cancer has a heterogeneous phenotype with a mixture of distant metastasis, including hematogenous, lymph nodal, and peritoneal metastasis (PM). In particular, PM is the most frequent type of metastasis with high recurrence in patients with advanced gastric cancer (AGC) [1]. The guidelines in Korea and Japan recommend palliative systemic chemotherapy for the treatment of AGC with PM, similar to patients with other distant metastasis [2,3]. However, despite recent advances in systemic chemotherapy, the prognosis of AGC with PM remains poor, with a median survival of 6–14 months [4,5,6,7]. Various treatment strategies such as combination chemotherapy, cytoreductive surgery, hyperthermic intraperitoneal chemotherapy (HIPEC), and immunotherapy have been investigated without much success in providing satisfactory clinical outcomes [8]. Consequently, in the absence of a standard treatment for patients with AGC exhibiting PM, it is often considered a terminal disease in general practice.
Nevertheless, even a slight improvement in the prognosis is a much sought-after goal; therefore, it is crucial to seek effective methods to control the progression of PM. Among several approaches, intraperitoneal (IP) chemotherapy has credibly demonstrated its effectiveness against AGC with PM in clinical trials [9,10,11]. In Japan, Ishigami et al. [12] designed the PHOENIX-GC randomized phase III trial that assessed the safety and efficacy of IP paclitaxel and systemic chemotherapy and suggested the potential clinical benefits of IP paclitaxel. One of our ongoing multicenter phase I/II study (Perioperative Intra-Peritoneal & Systemic Chemotherapy for Gastric Cancer; PIPS-GC) is investigating the safety and efficacy of IP paclitaxel and oral S-1 plus intravenous oxaliplatin combination therapy in patients with gastric cancer. In the present study, we conducted a retrospective analysis of IP chemotherapy administered to patients in PIPS-GC study.
MATERIALS AND METHODS
Patients
Between January 2015 and December 2018, the medical records of patients with gastric cancer who received IP chemotherapy at 6 institutions in Korea were reviewed. All patients were diagnosed with PM, confirmed using abdominal computed tomography (CT) or laparoscopic examination and laparotomy. Synchronous PM was defined as an initial detection of PM that was diagnosed with gastric cancer. Metachronous PM was defined as a pattern of recurrence in patients who had previously received curative gastrectomy with adjuvant chemotherapy (TS-1 monotherapy or XELOX: capecitabine/oxaliplatin). Exclusion criteria included presence of other types of distant metastasis, poor general health condition (Eastern Cooperative Oncology Group performance status >2), or administration of IP chemotherapy other than paclitaxel. The present study was approved by the Institutional Review Board (IRB) of each institution from where the data was collected (approval number: 201907017 at the institution of the principal investigator) and was conducted in line with the Declaration of Helsinki. Written informed consent was waived by the IRB.
Treatment strategy and disease evaluation
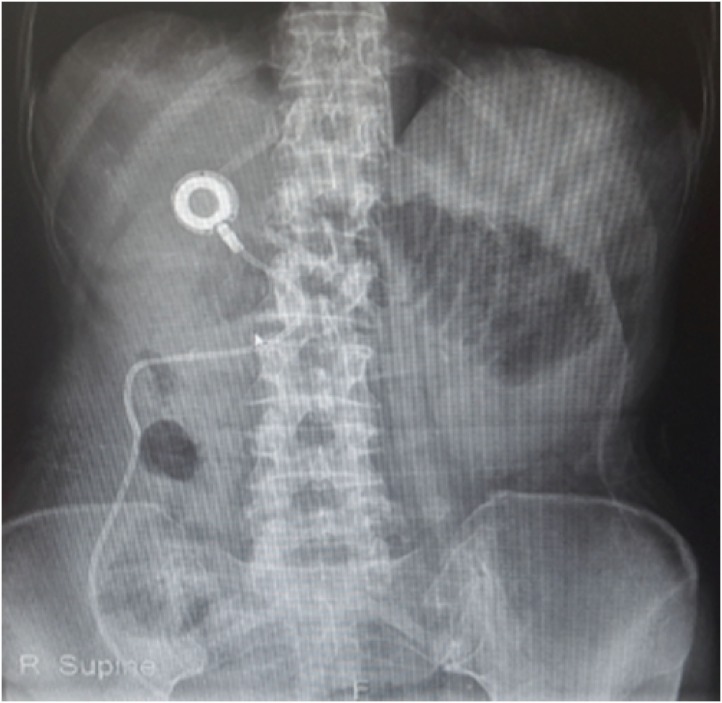
Most patients underwent IP paclitaxel chemotherapy via catheter and port system, with the catheter placed in the pouch of Douglas and paclitaxel (20–60 mg/m2) dissolved in 500–1000 mL normal saline, administered over 60 min. The port system for IP chemotherapy, similar to that used for intravenous chemotherapy, was implanted in the subcutaneous fat tissue at the right subcostal area (Fig. 1). Patients received IP chemotherapy in 3-week cycles until disease progression, severe toxicity, or postoperative complications. The amount of ascites was evaluated using CT and categorized as follows: none (grade I), minimal (grade II, limited to pelvis), moderate (grade III, beyond the pelvic cavity), and massive (grade IV, entire abdomen). Measurable lesions were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [13]. The extent of PM was measured using the peritoneal cancer index (PCI), which evaluates cancer distribution and implant size, according to the Sugarbaker classification [14]. Three PCI grades (0–10, 11–20, and 21–39) were used for analysis.
Fig. 1. X-ray of intraperitoneal port and catheter.
Statistical analysis
Statistical differences were assessed using the χ2 or Fisher's exact tests for categorical variables. In addition, the Student's t-test or Mann-Whitney U test was used to assess continuous variables. Survival outcomes were analyzed using the Kaplan-Meier method and log-rank test. Univariable and multivariable survival analyses were performed using Cox proportional hazards regression model to generate a hazard ratio. A P-value lower than 0.05 (<0.05) was considered statistically significant. All statistical analyses were performed using the SPSS software package for Windows, version 22.0 (IBM Inc., Chicago, IL, USA).
RESULTS
We examined 82 patients (50 male and 32 female patients) at 6 institutions in Korea. The baseline characteristics of the patients are summarized in Table 1. The median age of the patients was 56.0 (25–82) years. Synchronous and metachronous PM were identified in 51 (62.2%) and 31 (37.8%) patients, respectively. Mean number of IP chemotherapy cycles was 6.6 (range, 1–41). Depending on the disease status and insurance coverage, IP chemotherapy was combined with several intravenous chemotherapies. The amount of ascites measured was as follows: none (I) 30.5%, minimal (II) 31.7%, moderate (III) 19.5%, and massive (IV) 18.3%.
Table 1. Patients demographics and baseline characteristics (n=82).
| Characteristics | Values | |
|---|---|---|
| Age (yr) | 56.0 (25–82) | |
| Sex | ||
| Male | 50 (61.0) | |
| Female | 32 (39.0) | |
| Histological subtypes | ||
| Well differentiated | 1 (1.2) | |
| Moderate differentiated | 12 (14.6) | |
| Poorly differentiated | 44 (53.7) | |
| Poorly cohesive carcinoma | 25 (30.5) | |
| Disease presentation | ||
| Synchronous | 51 (62.2) | |
| Metachronous | 31 (37.8) | |
| Palliative systemic chemotherapy | ||
| XELOX | 20 (24.4) | |
| FOLFOX | 19 (23.2) | |
| SP | 6 (7.3) | |
| FOLFIRI | 16 (19.5) | |
| Others* | 21 (25.6) | |
| Mean No. of systemic chemotherapy cycles | 9.1 (1–21) | |
| Mean No. of intraperitoneal chemotherapy cycles | 6.6 (1–41) | |
| Ascites grade | ||
| I (none) | 25 (30.5) | |
| II (minimal) | 26 (31.7) | |
| III (moderate) | 16 (19.5) | |
| IV (massive) | 15 (18.3) | |
Values are expressed as median (range) or number (%).
XELOX = capecitabine and oxaliplatin; FOLFOX = 5-fluorouracil, folinic acid, and oxaliplatin; SP = S-1 (tegafur/gimeracil/oteracil) and cisplatin; FOLFIRI = 5-fluorouracil, folinic acid, and irinotecan; DFP = docetaxel, 5-fluorouracil, and capecitabine; DCF = docetaxel, cisplatin, and 5-fluorouracil.
*Weekly paclitaxel, S-1 monotherapy, DFP, and DCF.
Palliative surgeries were simultaneously performed according to disease presentation, while 32 patients received only IP catheter insertion. The surgical results (Table 2) showed a mean PCI of 21.9 (grade I, 29.3%; grade II, 32.9%; and grade III, 37.8%). Among the 32 patients with measurable lesions, the response rate measured by RECIST criteria was 18.8% (complete response, 1; partial response, 5).
Table 2. Surgical result and tumor response.
| Type of surgery | Values | |
|---|---|---|
| Synchronous (n=51) | ||
| IP catheter insertion+palliative gastrectomy | 34 (66.7) | |
| IP catheter insertion+palliative gastrojejunostomy | 3 (5.9) | |
| Only IP catheter insertion | 14 (27.4) | |
| Metachronous (n=31) | ||
| IP catheter insertion+palliative surgery | 13 (41.9) | |
| Only IP catheter insertion | 18 (58.1) | |
| T staging (n=82) | ||
| T1, T2 | 5 (6.1) | |
| T3 | 8 (9.8) | |
| T4a | 45 (54.9) | |
| T4b | 7 (8.5) | |
| Unknown | 17 (20.7) | |
| N staging (n=82) | ||
| N0 | 8 (9.8) | |
| N1 | 6 (7.3) | |
| N2 | 13 (15.9) | |
| N3a | 16 (19.5) | |
| N3b | 22 (26.8) | |
| Unknown | 17 (20.7) | |
| RECIST criteria (n=32) | ||
| Complete response | 1 (3.1) | |
| Partial response | 5 (15.6) | |
| Stable disease | 9 (28.1) | |
| Progressive disease | 17 (53.2) | |
| PCI grade (n=82) | 21.9±12.2 | |
| I (1–10) | 24 (29.3) | |
| II (11–20) | 27 (32.9) | |
| III (21–39) | 31 (37.8) | |
Values are expressed as number (%) or mean±standard deviation.
IP = intraperitoneal; RECIST = Response Evaluation Criteria in Solid Tumors; PCI = peritoneal cancer index.
Surgical morbidity was 29.3%. Postoperative complications related to IP catheter and port were observed in 15 patients (18.3%)—catheter malposition in 4 patients, catheter obstruction in 3, catheter fistula in 2, catheter infection in 5, and port site infection in 1. Recovery in patients was observed after the removal of the IP catheter and port (n=12), or following conservative managements (n=2). There were 2 (2.4%) postoperative mortalities due to pneumonia and catheter site infection (Table 3).
Table 3. Postoperative morbidity and mortality.
| Variables | Values | |
|---|---|---|
| No. of postoperative morbidities | 24 (29.3%) | |
| IP catheter- and port-related complications | ||
| IP catheter malposition | 4 | |
| IP catheter obstruction | 3 | |
| IP catheter fistula | 2 | |
| IP catheter infection | 5 | |
| IP port infection | 1 | |
| Intraabdominal complications | ||
| Bleeding | 1 | |
| Intestinal obstruction/ileus | 1 | |
| Wound complication | 2 | |
| Medical complication | ||
| Respiratory | 2 | |
| Hepatic | 1 | |
| No. of postoperative mortalities | 2 (2.4%)* | |
IP = intraperitoneal.
*Pneumonia and catheter infection.
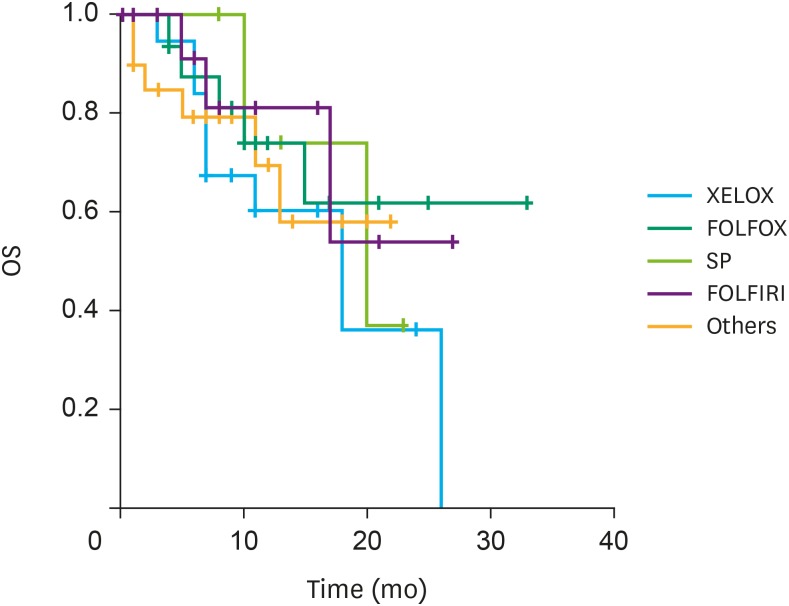
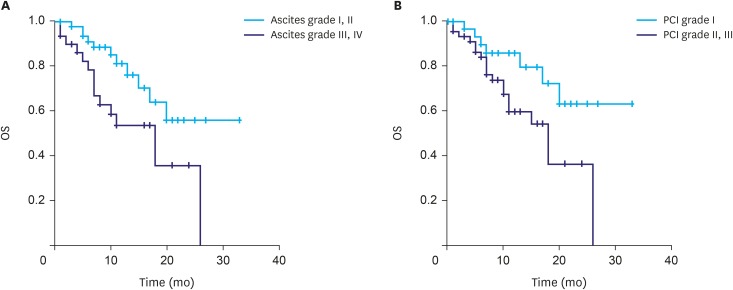
The median overall survival (OS) was 20.0 months (synchronous PM, 24.0 months; metachronous PM, 15.0 months; P=0.128). No differences in OS, according to the type of systemic chemotherapy, were observed (Fig. 2). Patients with ascites graded I and II showed a significantly higher survival rate (24.1 months) compared to those with ascites graded III and IV (15.3 months; P=0.014; Fig. 3A). Similarly, a significant difference in survival rate was observed in patients according to the PCI grade (grade I, 25.6 months; grade II and III, 16.3 months; P=0.023; Fig. 3B). Multivariable analysis showed that PCI grades were positive independent prognostic factors for OS (Table 4).
Fig. 2. OS according to the types of systemic chemotherapy.
OS = overall survival; XELOX = capecitabine and oxaliplatin; FOLFOX = 5-fluorouracil, folinic acid, and oxaliplatin; SP = S-1 (tegafur/gimeracil/oteracil) and cisplatin; FOLFIRI = 5-fluorouracil, folinic acid, and irinotecan.
Fig. 3. OS according to (A) ascites grade and (B) PCI grade.
OS = overall survival; PCI = peritoneal cancer index.
Table 4. Univariable and multivariable analysis of prognostic factors for overall survival.
| Variables | No. | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |||
| Sex | ||||||||
| Male | 50 | 1 | 0.31–1.53 | 0.353 | 1 | 0.29–1.70 | 0.433 | |
| Female | 32 | 0.68 | 0.70 | |||||
| Age (yr) | ||||||||
| <60 | 50 | 1 | 0.95–4.57 | 0.069 | 1 | 0.84–4.42 | 0.124 | |
| ≥60 | 32 | 2.08 | 1.92 | |||||
| Histology | ||||||||
| WD, MD | 13 | 1 | 0.15–2.14 | 0.650 | 1 | 0.34–2.81 | 0.968 | |
| PD, PCC | 69 | 1.26 | 0.98 | |||||
| Presentation | ||||||||
| Synchronous | 51 | 1 | 0.82–3.85 | 0.142 | 1 | 0.99–5.17 | 0.051 | |
| Metachronous | 31 | 1.78 | 2.27 | |||||
| Ascites | ||||||||
| I, II | 51 | 1 | 1.16–5.40 | 0.020 | 1 | 0.46–4.72 | 0.510 | |
| III, IV | 31 | 2.50 | 1.48 | |||||
| PCI | ||||||||
| I | 24 | 1 | 1.09–6.18 | 0.032 | 1 | 1.33–8.35 | 0.010 | |
| II, III | 58 | 2.59 | 3.33 | |||||
HR = hazard ratio; CI = confidence interval; WD = well differentiated; MD = moderate differentiated; PD = poorly differentiated; PCC = poorly cohesive carcinoma; PCI = peritoneal cancer index.
DISCUSSION
Patients with AGC that has metastasized to the peritoneum have poor survival rates. Although trans-mesothelial, trans-lymphatic, and superficially growing PM have been proposed [15], there is little evidence. The clinical presentations of PM, including bowel obstruction, hydronephrosis, and massive ascites; lead to poor treatment compliance [16]. In cases where numerous seeding nodules are detected during surgery, surgeons in several institutions choose peritoneal biopsy for pathological confirmation via a process termed as “open and closure” that involves limited wound management and patient referral to a medical oncologist for palliative systemic chemotherapy. Therefore, patients and surgeons alike, consider PM a difficult issue that requires a collaborative effort to tackle.
The advantages offered by IP chemotherapy render it an ideal option for patients with PM because the effect of systemic chemotherapy on PM is limited. Compared with the intravenous route, IP route causes higher concentrations of chemotherapeutic agents to be absorbed through the peritoneal surface [17]. Furthermore, direct application of chemotherapeutic agents to the peritoneal metastatic nodules avoids the peritoneum–plasma barrier that prevents drug delivery [18]. Low morbidity and mild systemic toxicity are essential criteria favoring IP chemotherapy. Although port- and catheter-related issues could occur, as in our experience, other complications are relatively rare, including grade III/IV adverse events [9,12]. The feasibility of repeatedly performing IP chemotherapy without requiring general anesthesia is probably a significant advantage. Implanting the IP catheter and port facilitates the repeated administration of the drug to the patient as an outpatient, thereby reducing hospital resources [19]. Moreover, since most patients with PM exhibit poor general health, the burden on patients upon IP chemotherapy is less compared to that upon other treatment methods.
The study group, which was first established in August 2017 for conducting clinical trials that investigated treatment modalities for AGC with PM, currently includes 17 surgical oncologists from 13 institutions. Although Japan initiated IP chemotherapy studies in the early 2000s [19,20], only a few have been reported from Korea, thereby justifying the need for IP chemotherapy trials. Initially, it was necessary to investigate the tendency of IP chemotherapy in Korea. Paclitaxel was selected for IP chemotherapy because taxanes have demonstrated desirable pharmaceutical characteristics [21,22]. Taxanes are hydrophobic and high molecular weight compounds that are gradually drained from the peritoneum via the lymphatic system [19]. These properties ensure the feasibility and benefit of IP chemotherapy in patients with PM of AGC. Although there are several other variables to consider, including various types of surgery and systemic chemotherapy, it is considered worthwhile to share the experiences of IP chemotherapy with multiple institutions for the first time in Korea.
Patient selection is a challenging issue in the study of stage IV gastric cancer because there are several variables determining patient survival rate. In the present study, except for PCI grade, no significant information was obtained from the multivariable analysis. Although the retrospective nature of the data is a limitation, the characteristics of PM should be considered. The responsiveness to drugs is limited in peritoneal disease, owing to the lack of lesions that are measurable by radiology. This limitation was evident from the present study that found only 32 (39.0%) patients with measurable lesions. Response rate is a crucial endpoint in phase II studies that evaluate drug effects; however, patients with PM are ineligible [16] in several cases.
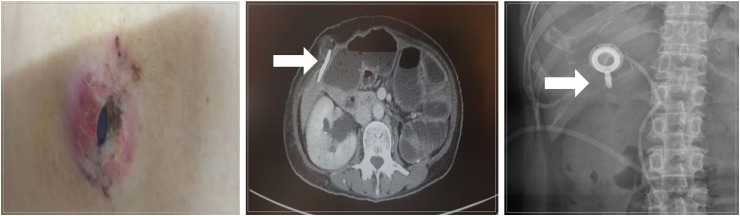
Complications related to IP catheter and port were also observed in the present study. The effectiveness of IP chemotherapy depends on constant distribution of the drug in the entire abdomen [19], which in turn relies on the proper functioning of IP catheter and port; any failure in this regard can be a serious complication in IP chemotherapy [23]. In the present study, 15 patients (18.3%) demonstrated complications associated with an implanted access port system (Table 3). Following much deliberations, we decided to implant the IP port on the right subcostal area instead of the abdomen to avoid the possibility of the port being flipped upside down or moved and buried in the subcutaneous tissue of the abdomen. Except for a lean patient, who developed port site infection accompanied by skin necrosis (Fig. 4A), no port-related complications were observed. However, significant catheter-related complications occurred in 14 patients as shown in Fig. 4B and C. Inflow obstruction and infection were the main complications that were inevitable in some cases. Malposition, that occurred in four patients, was attributable to technical issues that originated from lack of experience. Therefore, precise procedure should be an essential component of patient management in IP chemotherapy. Moreover, the overall morbidity in this study was substantially high compared with that reported in other studies on IP chemotherapy [12,20]. These results may be due to the large number of patients receiving palliative surgery, which is considered to increase postoperative complications (Table 2). Further safety issues should be re-considered in future prospective studies.
Fig. 4. Complications related to intraperitoneal approach. (A) Skin necrosis of the port site, (B) abscess of the catheter site, and (C) malposition of catheter due to kinking.
The diagnosis and confirmation of PM are essential for patients with AGC. Accurate radiologic imaging must be performed in the preoperative diagnosis of PM. Patients with PM often have locoregional or distant metastasis; Lee et al. [1] reported that 16% of patients who were diagnosed with pure PM had mixed type of metastasis. Inaccurate imaging can result in poor prognosis and ineffective IP chemotherapy compounded by the presence of hidden incurable factors [24]. To prevent this situation, a multidisciplinary approach involving radiologists and medical oncologists is essential.
The limitations associated with the scoring of PM can impair treatment strategies. Although the PCI scoring system can meticulously measure the degree of PM, accurate scoring is difficult. Jacquet and Sugarbaker [14] first described the PCI scoring system as a record of the value observed in laparotomy, which is considered a highly accurate diagnostic modality for the determination of PM in AGC. Recently, laparoscopic surgery has been demonstrated in IP chemotherapy treatment, such as HIPEC and pressurized intraperitoneal aerosol chemotherapy. In the present study, laparoscopy was conducted in most patients who did not undergo gastrectomy; therefore, PCI scoring was performed via laparoscopy. Because the detection of small nodules hidden in the corners of the organs may be a tedious process in laparoscopy, there may be an underestimation of the PCI score. Therefore, improvements in laparoscopic measurements are warranted in the field of peritoneal cancer.
The present study has several limitations. First, it was a retrospective study affected by unrecognized biases. The heterogeneity in systemic chemotherapy and palliative surgery received by patients in this study can affect their survival rate. In addition, the dosages of paclitaxel administered at each institution was different (20–60 mg/m2). Identifying the exact date of PM diagnosis, particularly in patients with metachronous presentation who received second- or third-line chemotherapy in each institution, was also a challenge. Second, this study comprised a small sample size and the follow-up period was short. Despite these limitations, this study suggested that patients with PM of AGC who received IP paclitaxel and systemic chemotherapy had better prognosis (median OS, 20 months) compared with those who did not.
In conclusion, the present retrospective analysis discussing the experience of IP chemotherapy for patients with PM of AGC in Korea, has paved the way for our investigation of the safety and efficacy of IP paclitaxel and oral S-1 plus intravenous oxaliplatin combination therapy in a multicenter phase I/II study (PIPS-GC). IP chemotherapy may be beneficial for improving OS of well-selected patients with PM of AGC. The strengths and limitations presented here will be of immense importance for future applications of IP chemotherapy.
Footnotes
- Conceptualization: K.J.H., J.Y.S.
- Data curation: K.D.W., K.C.H., P.S., K.J.J., C.S.I., P.J.M., K.J.H.
- Formal analysis: K.D.W.
- Writing - original draft: K.D.W.
- Writing - review & editing: J.Y.S., K.J.H.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Lee JH, Son SY, Lee CM, Ahn SH, Park DJ, Kim HH. Factors predicting peritoneal recurrence in advanced gastric cancer: implication for adjuvant intraperitoneal chemotherapy. Gastric Cancer. 2014;17:529–536. doi: 10.1007/s10120-013-0306-2. [DOI] [PubMed] [Google Scholar]
- 2.Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean Practice Guideline for Gastric Cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer. 2019;19:1–48. doi: 10.5230/jgc.2019.19.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 5.Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–673. doi: 10.1093/annonc/mdn717. [DOI] [PubMed] [Google Scholar]
- 6.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 7.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 8.Kitayama J, Ishigami H, Yamaguchi H, Sakuma Y, Horie H, Hosoya Y, et al. Treatment of patients with peritoneal metastases from gastric cancer. Ann Gastroenterol Surg. 2018;2:116–123. doi: 10.1002/ags3.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishigami H, Kitayama J, Kaisaki S, Hidemura A, Kato M, Otani K, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010;21:67–70. doi: 10.1093/annonc/mdp260. [DOI] [PubMed] [Google Scholar]
- 10.Imano M, Yasuda A, Itoh T, Satou T, Peng YF, Kato H, et al. Phase II study of single intraperitoneal chemotherapy followed by systemic chemotherapy for gastric cancer with peritoneal metastasis. J Gastrointest Surg. 2012;16:2190–2196. doi: 10.1007/s11605-012-2059-3. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi H, Kitayama J, Ishigami H, Emoto S, Yamashita H, Watanabe T. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer. 2013;119:3354–3358. doi: 10.1002/cncr.28204. [DOI] [PubMed] [Google Scholar]
- 12.Ishigami H, Fujiwara Y, Fukushima R, Nashimoto A, Yabusaki H, Imano M, et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol. 2018;36:1922–1929. doi: 10.1200/JCO.2018.77.8613. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 15.Yonemura Y, Sako S, Wakama S, Ishibashi H, Mizumoto A, Takao N, et al. History of peritoneal surface malignancy treatment in Japan. Indian J Surg Oncol. 2019;10:3–11. doi: 10.1007/s13193-019-00893-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kodera Y. Surgery with curative intent for stage IV gastric cancer: is it a reality of illusion? Ann Gastroenterol Surg. 2018;2:339–347. doi: 10.1002/ags3.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dedrick RL, Myers CE, Bungay PM, DeVita VT., Jr Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep. 1978;62:1–11. [PubMed] [Google Scholar]
- 18.Jacquet P, Sugarbaker PH. Peritoneal-plasma barrier. Cancer Treat Res. 1996;82:53–63. doi: 10.1007/978-1-4613-1247-5_4. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi D, Kodera Y. Intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Gastric Cancer. 2017;20:111–121. doi: 10.1007/s10120-016-0662-9. [DOI] [PubMed] [Google Scholar]
- 20.Cho H, Ryu MH, Kim KP, Ryoo BY, Park SR, Kim BS, et al. Phase I/II study of a combination of capecitabine, cisplatin, and intraperitoneal docetaxel (XP ID) in advanced gastric cancer patients with peritoneal metastasis. Gastric Cancer. 2017;20:970–977. doi: 10.1007/s10120-017-0710-0. [DOI] [PubMed] [Google Scholar]
- 21.Fushida S, Kinoshita J, Yagi Y, Funaki H, Kinami S, Ninomiya I, et al. Dual anti-cancer effects of weekly intraperitoneal docetaxel in treatment of advanced gastric cancer patients with peritoneal carcinomatosis: a feasibility and pharmacokinetic study. Oncol Rep. 2008;19:1305–1310. [PubMed] [Google Scholar]
- 22.Imano M, Peng YF, Itoh T, Nishikawa M, Satou T, Yasuda A, et al. A preliminary study of single intraperitoneal administration of paclitaxel followed by sequential systemic chemotherapy with S-1 plus paclitaxel for advanced gastric cancer with peritoneal metastasis. Anticancer Res. 2012;32:4071–4075. [PubMed] [Google Scholar]
- 23.Emoto S, Ishigami H, Hidemura A, Yamaguchi H, Yamashita H, Kitayama J, et al. Complications and management of an implanted intraperitoneal access port system for intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Jpn J Clin Oncol. 2012;42:1013–1019. doi: 10.1093/jjco/hys129. [DOI] [PubMed] [Google Scholar]
- 24.Fukuchi M, Ishiguro T, Ogata K, Suzuki O, Kumagai Y, Ishibashi K, et al. Prognostic role of conversion surgery for unresectable gastric cancer. Ann Surg Oncol. 2015;22:3618–3624. doi: 10.1245/s10434-015-4422-6. [DOI] [PubMed] [Google Scholar]