Abstract
Purpose
Patients with pathological stage T1N+ or T2–3N0 gastric cancer may experience disease recurrence following curative gastrectomy. However, the current Japanese Gastric Cancer Treatment Guidelines do not recommend postoperative adjuvant chemotherapy for such patients. This study aimed to identify the prognostic factors for patients with pT1N+ or pT2–3N0 gastric cancer using a multi-institutional dataset.
Materials and Methods
We retrospectively analyzed the data obtained from 401 patients with pT1N+ or pT2–3N0 gastric cancer who underwent curative gastrectomy at 9 institutions between 2010 and 2014.
Results
Of the 401 patients assessed, 24 (6.0%) experienced postoperative disease recurrence. Multivariate analysis revealed that age ≥70 years (hazard ratio [HR], 2.62; 95% confidence interval [CI], 1.09–7.23; P=0.030) and lymphatic and/or venous invasion (lymphovascular invasion (LVI): HR, 7.88; 95% CI, 1.66–140.9; P=0.005) were independent prognostic factors for poor recurrence-free survival. There was no significant association between LVI and the site of initial recurrence.
Conclusions
LVI is an indicator of poor prognosis in patients with pT1N+ or pT2–3N0 gastric cancer.
Keywords: Gastric cancer, Recurrence, Risk factor
INTRODUCTION
Gastric cancer continues to be one of the leading causes of cancer-related deaths worldwide [1]. In Japan, the mortality from gastric cancer has significantly decreased over the last 2 decades, largely due to various therapeutic advancements, including surgery and chemotherapy [2]. Adjuvant chemotherapy with the oral fluoropyrimidine derivative S-1 or capecitabine plus oxaliplatin following curative surgery is recommended by the Japanese Gastric Cancer Treatment Guidelines for patients with pathological stage II or III gastric cancer [3,4,5]. In contrast, patients with pathological stage IA (T1N0) cancers are likely to have an excellent prognosis, and there is broad consensus that adjuvant therapy is unnecessary for patients with this stage. However, some patients with stage IB (T1N1 or T2N0) or stage II (T1N2–3 or T3N0) disease experience recurrence following curative gastrectomy, and yet the current Japanese Gastric Cancer Treatment Guidelines do not recommend adjuvant chemotherapy for these subpopulations [4,6].
Analysis of the data pertaining to more than 100,000 patients from the Japanese Gastric Cancer Association nationwide registry revealed that the postoperative 5-year disease-specific survival (DSS) rates of patients with pT1N0 gastric cancer is 99.0%, compared to 91.9%, 94.0%, 84.8%, and 44.2% for patients with pT2N0, pT1N1, pT1N2, and pT1N3 disease, respectively [6]. Thus, the ability to identify patients within these subgroups who are at high risk for recurrence would help improve the prognosis of patients with gastric cancer.
To this end, we conducted a retrospective analysis of a multi-institutional dataset for identifying the prognostic factors for patients with pT1N+ or pT2–3N0 gastric cancer.
MATERIALS AND METHODS
Patients
Clinical data pertaining to 3,484 patients who underwent gastrectomy for gastric cancer between January 2010 and December 2014 were retrospectively collected from the medical records of 9 institutions [7]. Written informed consent for surgery and the use of clinical data was obtained from the patients, as required by the Institutional Review Board of Nagoya University (approval number 2017-0104) and all the participating institutions. As we had aimed to conduct clinical research using only retrospective clinical data without intervention, we applied the opt-out recruitment strategy, according to the policy adopted by the Japanese Government. The purpose, design, and objectives of the study have been published on our homepage (https://www.med.nagoya-u.ac.jp/medical_J/ethics/rinsyoukansatsu.html) to provide an opportunity to the patients for declining to participate in our study. Out of the 3,484 patients, data pertaining to 401 patients were considered for analysis based on the following inclusion criteria: R0 resection with systematic lymphadenectomy performed in accordance with the Japanese Gastric Cancer Treatment Guidelines, pathological examination showing pT1N+ or pT2–3N0 gastric cancer based on the 3rd English edition of the Japanese Classification of Gastric Carcinoma [8] that corresponds to the tumor-node-metastasis (TNM) system of classification of the International Union Against Cancer Classification of Malignant Tumors, 8th edition [9], and the availability of sufficient data for analysis (Fig. 1) [8,9]. We excluded patients with gastric stump cancer, with cancers having histologies other than the common histological classification types, such as carcinoid tumor and carcinoma with lymphoid stroma, who underwent extended surgery, such as pancreaticoduodenectomy and Appleby's procedure, who received neoadjuvant or adjuvant chemotherapy, and those with a history of other primary cancers.
Fig. 1. Flow diagram of the study.
Data collection
The following data were collected from the medical records of the 9 institutions: demographic and clinical data, including sex, age, tumor location, tumor diameter, and macroscopic type; surgical details, such as type of procedure, grade of lymphadenectomy, concomitant resection of other organs, duration of operation, intraoperative blood loss, and postoperative complications; and histological tumor findings, including tumor location, size, macroscopic type, histological type, lymphatic invasion, and venous invasion (classified according to 3rd English edition of the Japanese Classification of Gastric Carcinoma) [8]. The tumors that were classified as differentiated included well-differentiated tubular, moderately differentiated tubular, and papillary adenocarcinomas. The tumors that were classified as undifferentiated included poorly differentiated tubular adenocarcinoma, signet ring cell carcinoma, and mucinous adenocarcinoma [10]. Recurrence-free survival (RFS) was defined as the date of surgery to the date when recurrence was first detected. Only gastric cancer-related deaths were considered for calculating DSS, and subjects who died of other causes were not considered.
Patient management
The patients underwent postoperative follow-up for 5 years or until recurrence. The follow-up included physical examinations and laboratory tests, including tests for serum tumor markers every 3 months, enhanced computed tomography of the chest and abdominal cavity every 6 months, and upper gastrointestinal endoscopy at 1, 3, and 5 years, as described in the Japanese Gastric Cancer Treatment Guidelines [4]. Treatment after recurrence was based on the patient's consent and condition, and the evidence available at that point of time.
Statistical analyses
Survival curves were calculated using the Kaplan-Meier method and compared using the log-rank test. Cox proportional hazard models were used to perform univariate and multivariate analyses. A χ2 test was performed for analyzing the categorical variables. All the statistical analyses were performed using JMP 13 software (SAS Institute, Cary, NC, USA). A P-value of <0.05 was considered to be statistically significant.
RESULTS
Patient characteristics
The patient characteristics are enlisted in Table 1. The median age of the 401 patients was 70 years, and the male:female ratio was approximately 2.2:1. The most frequently performed surgical procedure was distal gastrectomy (73%) followed by total gastrectomy (22%). More than half (54%) of the patients underwent D2 lymphadenectomy. Approximately 20%–30% of the patients underwent non-D2 lymphadenectomy, and included those with clinical T2–4 gastric cancer (Supplementary Fig. 1). There were 78 patients who underwent non-D2 lymphadenectomy despite having clinical T2–4 gastric cancer (74 patients) and clinical T1N+ (4 patients). Of these 78 patients, 64 (82%) were aged 70 years or older. Of the total 401 patients studied, 85 (21%) experienced postoperative complications of grade II or higher, according to the Clavien-Dindo system of classification, and 40 patients (10%) had postoperative complications of grade III or higher. There were 2 operative deaths (0.5%), and both the patients died of pneumonia. Lymphatic and venous invasions were detected in 279 (69.6%) and 169 patients (42.1%), respectively. The patients were categorized into the lymphovascular invasion (LVI)-positive group (n=300) and the LVI-negative group (n=101) based on the presence and absence of LVI, respectively. Most patients with pT1N+ disease (86%) were at the pN1 stage (1 or 2 lymph node metastases). It was observed that the number of patients with pT2N0 disease was greater than the number of patients with pT3N0 disease. The median follow-up time for the 401-patient cohort was 48.9 months.
Table 1. Patient characteristics and clinicopathological features.
| Variables | No. of patients (n=401) | |
|---|---|---|
| Age (yr) | 70 (28–89) | |
| Sex ratio (male:female) | 275:126 | |
| cT category | ||
| cT1 | 139 | |
| cT2 | 150 | |
| cT3 | 77 | |
| cT4 | 35 | |
| cN category | ||
| cN0 | 308 | |
| cN+ | 93 | |
| Tumor location | ||
| Upper one-third of stomach | 74 | |
| Middle one-third of stomach | 180 | |
| Lower one-third of stomach | 143 | |
| Whole stomach | 4 | |
| Diameter (mm) | 30 (7–125) | |
| Macroscopic type | ||
| 0 | 177 | |
| 1 | 27 | |
| 2 | 117 | |
| 3 | 77 | |
| 4 | 1 | |
| 5 | 2 | |
| Surgical procedure | ||
| Total gastrectomy | 88 | |
| Proximal gastrectomy | 14 | |
| Pylorus-preserving gastrectomy | 7 | |
| Distal gastrectomy | 292 | |
| Lymphadenectomy | ||
| D2 | 216 | |
| Non-D2 | 185 | |
| Operative time (min) | 244 (113–817) | |
| Intraoperative blood loss (mL) | 170 (0–1,475) | |
| Postoperative complication | ||
| CD ≥2 | 85 | |
| CD ≥3 | 40 | |
| Histology | ||
| Differentiated type | 226 | |
| Undifferentiated type | 175 | |
| Lymphatic invasion | ||
| − | 122 | |
| + | 279 | |
| Venous invasion | ||
| − | 232 | |
| + | 169 | |
| Lymphovascular invasion | ||
| − | 101 | |
| + | 300 | |
| pT pN category | ||
| pT1 pN1 | 118 | |
| pT1 pN2 | 18 | |
| pT1 pN3 | 2 | |
| pT2 pN0 | 166 | |
| pT3 pN0 | 97 | |
Values represent the median (range) or number.
CD = Clavien-Dindo classification.
Analysis of prognostic factors
The 5-year overall survival, DSS, and RFS rates for the 401 patients were 84.3%, 95.2%, and 93.1%, respectively (Supplementary Fig. 2). Univariate analysis revealed that age ≥70 years and the presence of LVI were significantly associated with poor RFS (hazard ratio [HR], 2.81; 95% confidence intervals [CIs], 1.18–7.75; P=0.019 and HR, 7.82; 95% CI, 1.65–139.9; P=0.005, respectively). In the multivariate analysis, age ≥70 years and the presence of LVI remained the independent prognostic factors for poor RFS (HR, 2.62; 95% CI, 1.09–7.23; P=0.030 and HR, 7.88; 95% CI, 1.66–140.9; P=0.005, respectively) (Table 2).
Table 2. Univariable and multivariable analyses for identifying the predictors of recurrence-free survival.
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| Hazard ratio | P | Hazard ratio | P | |
| Age (≥70 yr) | 2.81 (1.18–7.75) | 0.019 | 2.62 (1.09–7.23) | 0.030 |
| Sex (male) | 2.39 (0.90–8.21) | 0.082 | 2.33 (0.88–8.02) | 0.093 |
| BMI (<25 kg/m2) | 2.06 (0.61–12.8) | 0.280 | ||
| Histology (undifferentiated) | 0.71 (0.30–1.60) | 0.418 | ||
| Tumor location (lower one-third) | 0.97 (0.39–2.20) | 0.942 | ||
| Tumor diameter (≥30 mm) | 1.21 (0.53–2.97) | 0.663 | ||
| Multiple lesions | 1.89 (0.55–4.99) | 0.280 | ||
| Lymph node dissection (non-D2) | 0.61 (0.25–1.39) | 0.244 | ||
| Operative time (≥240 min) | 0.67 (0.30–1.51) | 0.337 | ||
| Intraoperative blood loss (≥200 mL) | 1.73 (0.78–4.02) | 0.180 | ||
| Type of gastrectomy (total) | 1.85 (0.75–4.22) | 0.171 | ||
| Postoperative complication (CD ≥II) | 0.36 (0.06–1.21) | 0.107 | ||
| pTN (pT2–3N0) | 0.61 (0.27–1.39) | 0.236 | ||
| LVI (LVI-positive) | 7.82 (1.65–139.9) | 0.005 | 7.88 (1.66–140.9) | 0.005 |
BMI = body mass index; CD = Clavien-Dindo classification; LVI = lymphovascular invasion.
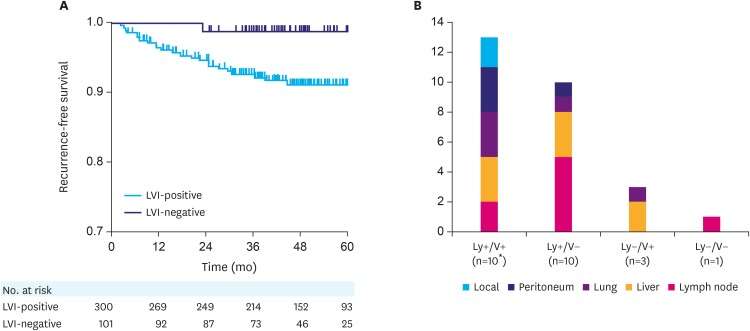
LVI and recurrence following curative gastrectomy
The LVI-positive group had significantly worse RFS in comparison to that of the LVI-negative group (P=0.017) (Fig. 2A). In total, 24 (6.0%) patients experienced recurrence following curative gastrectomy, of which 23 patients had LVI. The most frequently observed sites of initial recurrence were the liver and lymph nodes (8 patients each), followed by the lung (n=5), peritoneum (n=4), and local recurrence (n=2). Multiple sites of recurrence were observed for 2 patients at the time of initial detection. The distribution of the sites of recurrence according to the LVI status is depicted in Fig. 2B. There were no significant associations between lymph node recurrence and lymphatic invasion (P=0.700) or between hematogenous recurrence (liver and lung metastasis) and venous invasion (P=0.110) (Supplementary Table 1).
Fig. 2. Association between LVI and disease recurrence. (A) Recurrence-free survival of patients with pT1N+ or pT2–3 N0 gastric cancer according to the LVI status. (B) Distribution of initial recurrence sites.
LVI = lymphovascular invasion.
*Two patients had multiple sites of first recurrence.
DISCUSSION
The current Japanese Gastric Cancer Treatment Guidelines do not recommend adjuvant therapy for patients with pT1N+ or pT2–3N0 gastric cancer [4]. However, a certain proportion of these patients do experience disease recurrence. Therefore, identifying the risk factors for recurrence could improve prognosis. Tokunaga et al. [11] analyzed the data pertaining to 1,442 patients with pT1N+ or pT2–3N0 gastric cancer who underwent curative gastrectomy at 5 high-volume, specialized cancer centers between 2000 and 2008. They reported that the histology, tumor location, age, sex, and clinical T stage were important prognostic factors for RFS [12]. In the present study, we found that age (<70 years vs. ≥70 years) and LVI (positive vs. negative) were the only independent prognostic factors for RFS. Of the 401 patients examined in our study, 78 underwent non-D2 lymphadenectomy despite having clinical T2–4 gastric cancer (74 patients) and clinical T1N+ (4 patients). As 82% of these patients were aged 70 years or older, surgeons might refrain from D2 lymphadenectomy owing to the age-related vulnerabilities. The recurrence rate of these 78 patients was only 2.6%, while that of the whole patient cohort (n=401) was 6.0%. Thus, although the extent of lymphadenectomy was lesser than that recommended in the Japanese Treatment Guidelines, it had minimal adverse effect on disease recurrence in the present study.
Our results are consistent with those of previous studies that indicated that LVI is a prognostic factor for patients at various stages of gastric cancer [13,14,15,16,17]. In the present study, we observed that 23 of the 24 patients who had postoperative recurrence also had LVI, and there was a significant difference in RFS between the LVI-positive and LVI-negative groups. LVI is considered to be an early step in the process of lymph node or hematogenous metastasis. Therefore, LVI-positive patients may have undetectable micrometastases even if there is no evidence of pathological lymph node or distant metastases on imaging [17]. Risk stratification solely based on the TNM system of classification is not always satisfactory and there is room for improvement. Some studies have demonstrated that the TNM system in combination with independent risk factors may provide a more comprehensive and accurate evaluation of patient prognosis than that achieved using the TNM system alone. For instance, in the study conducted by Lu et al. [16], the retrospective analysis of 2,102 gastric cancer patients undergoing curative gastrectomy revealed that the inclusion of LVI improved the accuracy of the TNM staging system (8th edition) [17]. Our findings support this concept and further suggest that LVI may be used to identify patients with pT1N+ or pT2–3N0 gastric cancer who are at a high risk of recurrence.
Previous studies have revealed that there is a close association between lymphatic invasion and lymph node metastasis, as well as between vascular invasion and hematogenous metastasis. In the present study, we found that there were no significant associations between these subgroups, possibly due to the fact that there were a limited number of patients with recurrence in this study. However, some researchers are of the opinion that lymphatic invasion and vascular invasion do not occur independently, and that either can be included for the purpose of identifying patients who are at a high risk for disease recurrence [15].
We employed a multi-institutional dataset with a relatively large number of patients; however, the retrospective nature of the study remains a limitation. Detailed information on the degree and extent of LVI might aid in assessing its potential utility as a prognostic indicator.
Most of the patients in the LVI-positive group did not experience disease recurrence. Therefore, it is not reasonable to conclude that postoperative adjuvant chemotherapy should be recommended for all patients in the LVI-positive group. Further studies with a larger patient pool are necessary to evaluate the prognostic potential of LVI and determine whether LVI can be a useful factor for identifying patients who may benefit from adjuvant chemotherapy.
In conclusion, we found that LVI is an independent prognostic factor for patients with pT1N+ or pT2–3N0 gastric cancer.
ACKNOWLEDGEMENTS
We thank Anne M. O'Rourke, PhD, from the Edanz Group (www.edanzediting.com/ac) for editing the draft of this manuscript.
Footnotes
- Conceptualization: F.K., K.M.
- Data curation: M.T.
- Formal analysis: M.K.
- Methodology: M.K.
- Resources: T.H., I.K., A.T., I.A., M.H.
- Supervision: K.Y.
- Writing - original draft: F.K., M.T.
- Writing - review & editing: K.M., I.S., M.Y., T.C., K.D., F.M.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
SUPPLEMENTARY MATERIALS
Relationship between lymphatic and venous invasion and the recurrence pattern in 24 patients
Proportion of D2 and non-D2 lymphadenectomy, according to the clinical T stage.
Disease-specific (A) and recurrence-free (B) survival curves of the patients with pT1N+ or pT2–3N0 gastric cancer.
References
- 1.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 2.Shum H, Rajdev L. Multimodality management of resectable gastric cancer: a review. World J Gastrointest Oncol. 2014;6:393–402. doi: 10.4251/wjgo.v6.i10.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanda M, Murotani K, Kobayashi D, Tanaka C, Yamada S, Fujii T, et al. Postoperative adjuvant chemotherapy with S-1 alters recurrence patterns and prognostic factors among patients with stage II/III gastric cancer: a propensity score matching analysis. Surgery. 2015;158:1573–1580. doi: 10.1016/j.surg.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, et al. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14:e535–e547. doi: 10.1016/S1470-2045(13)70436-4. [DOI] [PubMed] [Google Scholar]
- 6.Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001–2007) Gastric Cancer. 2018;21:144–154. doi: 10.1007/s10120-017-0716-7. [DOI] [PubMed] [Google Scholar]
- 7.Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, Murai T, et al. Multi-institutional analysis of the prognostic significance of postoperative complications after curative resection for gastric cancer. Cancer Med. 2019;8:5194–5201. doi: 10.1002/cam4.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 9.Liu JY, Peng CW, Yang XJ, Huang CQ, Li Y. The prognosis role of AJCC/UICC 8th edition staging system in gastric cancer, a retrospective analysis. Am J Transl Res. 2018;10:292–303. [PMC free article] [PubMed] [Google Scholar]
- 10.Kanda M, Mizuno A, Fujii T, Shimoyama Y, Yamada S, Tanaka C, et al. Tumor infiltrative pattern predicts sites of recurrence after curative gastrectomy for stages 2 and 3 gastric cancer. Ann Surg Oncol. 2016;23:1934–1940. doi: 10.1245/s10434-016-5102-x. [DOI] [PubMed] [Google Scholar]
- 11.Tokunaga M, Ito S, Yoshikawa T, Nunobe S, Fukagawa T, Misawa K, et al. Prognostic factors for survival in patients with pT1 N+ or T2–3 N0 gastric cancer in Japan. Br J Surg. 2017;104:885–890. doi: 10.1002/bjs.10509. [DOI] [PubMed] [Google Scholar]
- 12.Kunisaki C, Makino H, Kimura J, Takagawa R, Kosaka T, Ono HA, et al. Impact of lymphovascular invasion in patients with stage I gastric cancer. Surgery. 2010;147:204–211. doi: 10.1016/j.surg.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Du CY, Chen JG, Zhou Y, Zhao GF, Fu H, Zhou XK, et al. Impact of lymphatic and/or blood vessel invasion in stage II gastric cancer. World J Gastroenterol. 2012;18:3610–3616. doi: 10.3748/wjg.v18.i27.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.del Casar JM, Corte MD, Alvarez A, García I, Bongera M, González LO, et al. Lymphatic and/or blood vessel invasion in gastric cancer: relationship with clinicopathological parameters, biological factors and prognostic significance. J Cancer Res Clin Oncol. 2008;134:153–161. doi: 10.1007/s00432-007-0264-3. [DOI] [PubMed] [Google Scholar]
- 15.Dicken BJ, Graham K, Hamilton SM, Andrews S, Lai R, Listgarten J, et al. Lymphovascular invasion is associated with poor survival in gastric cancer: an application of gene-expression and tissue array techniques. Ann Surg. 2006;243:64–73. doi: 10.1097/01.sla.0000194087.96582.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu J, Dai Y, Xie JW, Wang JB, Lin JX, Chen QY, et al. Combination of lymphovascular invasion and the AJCC TNM staging system improves prediction of prognosis in N0 stage gastric cancer: results from a high-volume institution. BMC Cancer. 2019;19:216. doi: 10.1186/s12885-019-5416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao B, Huang X, Zhang J, Luo R, Lu H, Xu H, et al. Clinicopathologic factors associated with recurrence and long-term survival in node-negative advanced gastric cancer patients. Rev Esp Enferm Dig. 2019;111:111–120. doi: 10.17235/reed.2018.5829/2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relationship between lymphatic and venous invasion and the recurrence pattern in 24 patients
Proportion of D2 and non-D2 lymphadenectomy, according to the clinical T stage.
Disease-specific (A) and recurrence-free (B) survival curves of the patients with pT1N+ or pT2–3N0 gastric cancer.