Abstract
Introduction
Tofacitinib is an oral Janus kinase inhibitor for the treatment of psoriatic arthritis (PsA).
Objective
Our objective was to compare the incidence rates (IRs) of adverse events in tofacitinib clinical trials and real-world observational data for alternative treatments.
Methods
The tofacitinib “dose-comparison cohort” included months 0–12 of two phase III studies (tofacitinib 5 [n = 238] and 10 [n = 236] mg twice daily [BID]); the “all-tofacitinib comparison cohort” (n = 783) included two phase III and one ongoing long-term extension study (data cutoff May 2016). An “observational comparison cohort” (n = 5799) comprised patients initiating a conventional synthetic disease-modifying antirheumatic drug (DMARD), biologic DMARD, or apremilast in the US Truven MarketScan database from 2010 to 2015. IRs for serious infections (SIEs; requiring hospitalization), herpes zoster (HZ), malignancies (excluding non-melanoma skin cancer [NMSC]), NMSC, and major adverse cardiovascular events (MACE) across cohorts were qualitatively compared.
Results
IRs (patients with events/100 patient-years) for SIEs were similar between the tofacitinib dose-comparison cohort (5 mg BID: 1.3; 10 mg BID: 2.0) and the observational comparison cohort (1.1–7.9; treatment dependent). The tofacitinib dose-comparison cohort had a higher rate of HZ (5 mg BID: 2.0; 10 mg BID: 2.7) than did the observational comparison cohort (0.8–2.0). IRs for NMSC were generally lower in the all-tofacitinib comparison cohort (0.5) than in the observational comparison cohort (0.4–6.0). IRs for MACE, malignancies excluding NMSC, and NMSC were similar between cohorts.
Conclusion
In patients with PsA, tofacitinib had a safety profile similar to that of other systemic therapies in real-world settings, except for the risk of HZ, a known risk of tofacitinib.
Trial Registration
ClinicalTrials.gov: NCT01877668; NCT01882439; NCT01976364.
Electronic supplementary material
The online version of this article (10.1007/s40264-020-00904-9) contains supplementary material, which is available to authorized users.
Key Points
| In patients with active psoriatic arthritis (PsA), the safety profile of tofacitinib was generally consistent with that of other therapies in real-world settings. |
| Tofacitinib was associated with a higher risk for herpes zoster than were most other PsA therapies. |
| No new risks were identified compared with those already observed with tofacitinib treatment of rheumatoid arthritis. |
Introduction
Psoriatic arthritis (PsA) is an immune-mediated systemic inflammatory disease with multiple disease manifestations, including peripheral arthritis, enthesitis, dactylitis, spondylitis, and skin and nail psoriasis [1]. Treatment recommendations for patients with PsA from the European League Against Rheumatism [2] and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis [1] vary according to adverse prognostic risk factors, disease manifestations, and responsiveness to prior treatment. Current approved treatments for PsA include nonsteroidal anti-inflammatory drugs, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), biologic DMARDs (bDMARDs), and the targeted synthetic DMARD (tsDMARD) apremilast [1–3]. There are safety concerns with most established therapies for PsA [4], including gastrointestinal adverse events (AEs), hepatotoxicity, opportunistic infections (OIs) including tuberculosis, serious infections (SIEs), malignancy, and—in rare instances—bone marrow toxicity [5–9].
Tofacitinib is an oral Janus kinase inhibitor for the treatment of PsA. We describe the safety profile of tofacitinib in PsA, using pooled data from two phase III [10, 11] and one ongoing long-term extension (LTE) study [12], and compare the incidence rates (IRs) for AEs of special interest from the tofacitinib PsA clinical program with those from observational data for other PsA treatments using data from the US Truven MarketScan database.
Methods
Study Design
Safety data for patients in two global phase III studies (OPAL Broaden [NCT01877668] [10] and OPAL Beyond [NCT01882439] [11]) and one LTE study (OPAL Balance [NCT01976364] [12]) were pooled for analysis.
OPAL Broaden and OPAL Beyond, studies of 12- or 6-months duration, respectively, have been described previously [11, 12]. Briefly, both were double-blind, placebo-controlled, parallel-group studies in patients with active PsA. Patients in OPAL Broaden were tumor necrosis factor inhibitor (TNFi) naïve and had an inadequate prior response to one or more csDMARD. Patients in OPAL Beyond had an inadequate response to one or more prior TNFi. In both studies, patients were randomized to receive tofacitinib 5 mg twice daily (BID), tofacitinib 10 mg BID, placebo advancing to tofacitinib 5 mg BID after 3 months, or placebo advancing to tofacitinib 10 mg BID after 3 months. In both studies, patients received one background csDMARD. In OPAL Broaden, patients were also randomized to receive adalimumab 40 mg subcutaneously every 2 weeks (Q2W).
OPAL Balance [12] is an ongoing, open-label LTE study (database not locked) that enrolled patients who had participated in OPAL Broaden or OPAL Beyond. Data up to 10 May 2016 were included in the current analysis, including up to 3 years of tofacitinib exposure per patient. Upon entry, all patients received tofacitinib 5 mg BID. Tofacitinib dose could be increased to 10 mg BID at the investigator’s discretion after 1 month and could be decreased from 10 mg BID to 5 mg BID for safety reasons at any time.
Tofacitinib Clinical Trial Cohorts
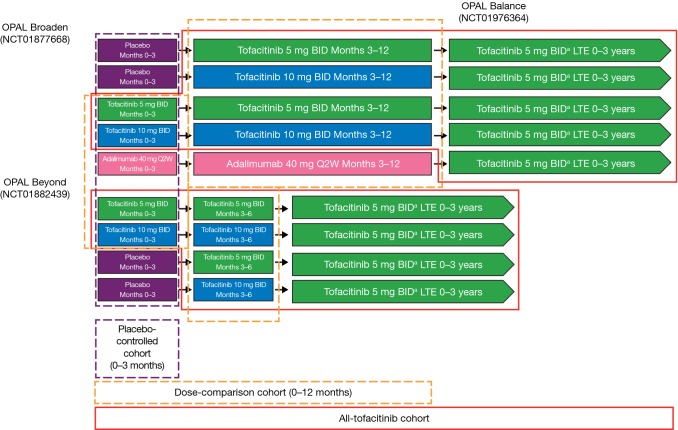
Three analysis cohorts were defined (Fig. 1): (1) the tofacitinib “placebo-controlled cohort” comprised data from the placebo-controlled portion (months 0–3) of the phase III studies (all treatment groups); (2) the “tofacitinib dose-comparison cohort” included the same tofacitinib-treated patients at baseline as in the placebo-controlled cohort but included the entire length of both studies in patients randomized to tofacitinib 5 or 10 mg BID (OPAL Broaden, months 0–12; OPAL Beyond, months 0–6); and (3) the “all-tofacitinib comparison cohort” comprised data from all patients who received one or more dose of tofacitinib in the phase III or LTE studies (including patients in the placebo–controlled and dose-comparison cohorts and patients who advanced from placebo to tofacitinib after their first dose of tofacitinib). Baseline demographics and characteristics were shared for cohorts 1 and 2 and were separate for cohort 3.
Fig. 1.
Schematic of clinical trial cohorts. aAll patients received tofacitinib 5 mg BID upon entry into OPAL Balance; the tofacitinib dose could be increased to 10 mg BID at the investigator’s discretion if it was believed that a patient would benefit from a higher dose and was not experiencing any tofacitinib-related AEs, including abnormalities in laboratory test results that were judged to be related to tofacitinib. The dose could be decreased from 10 to 5 mg BID for safety reasons at any time. AEs adverse events, BID twice daily, LTE long-term extension, Q2W every 2 weeks
Observational Comparison Cohort
The “observational comparison cohort” included real-world data from adult patients receiving approved PsA therapies in the US Truven MarketScan database, comprising data from privately and publicly insured US patients obtained from employers and health plans. Patients had a diagnosis of PsA, defined by either one or more inpatient, or two or more outpatients (provided on two unique calendar days, 1 January 2010–30 September 2015) International Classification of Disease (ICD) diagnosis codes of 696.0 (psoriatic arthropathy); at least one code had to be assigned by a rheumatologist. Patients must have been aged ≥ 18 years, have initiated therapy with a systemic agent for PsA (csDMARD, bDMARD, or tsDMARD; as a proxy definition for active moderate to severe disease), and have been enrolled in the database for ≥ 12 months before the index date (date of first prescription or administration for PsA treatment, or first procedure date following confirmation of PsA diagnosis for infusion therapies), with no data gap > 30 days. Patient exclusion criteria reflecting those of the phase III tofacitinib studies were applied where possible (see Online Resource 1). Patients receiving tofacitinib were not included in the observational cohort because tofacitinib was not yet approved at the time of the analysis.
Patients were classified by initiation of approved PsA therapies, in nonmutually exclusive categories: (1) bDMARD (adalimumab, etanercept, infliximab, golimumab, certolizumab pegol, ustekinumab, secukinumab); (2) bDMARD + csDMARD (methotrexate, leflunomide, sulfasalazine); (3) TNFi (adalimumab, etanercept, infliximab, golimumab, certolizumab pegol); (4) TNFi + csDMARD; and (5) individual therapies (adalimumab, etanercept, infliximab, golimumab, certolizumab pegol, apremilast).
Outcomes and Analyses
Tofacitinib Clinical Trial Cohorts
Events analyzed included AEs, deaths, serious AEs (SAEs), and AEs leading to discontinuation. AEs of special interest were SIEs (infections requiring parenteral antimicrobials in an emergency department setting or infections resulting in hospitalization or prolonging an existing hospitalization), herpes zoster (HZ), OIs (excluding tuberculosis) [13], tuberculosis, major adverse cardiovascular events (MACE), malignancies (excluding non-melanoma skin cancer [NMSC]), and NMSC. Adjudication of AEs is detailed in Online Resource 1.
Common AEs (occurring in ≥ 2% of patients in any group) were analyzed in the placebo-controlled tofacitinib cohort, including data up to 3 months. SAEs, discontinuations due to AEs, and infections were analyzed in the tofacitinib dose-comparison cohort, including data up to 12 months. MACE, malignancies (excluding NMSC), NMSC, and deaths were evaluated in the all-tofacitinib comparison cohort because of the lower frequency of these events and the longer latency of MACE, malignancies (excluding NMSC), and NMSC. Tofacitinib exposure in patient-years was calculated based on the total follow-up time to the day of the first event within the event-counting period for patients with events or until 28 days after the last study drug dose (or to the end of the study) for patients without events. IRs were defined as the number of patients with one or more events/100 patient-years of treatment exposure along with 95% confidence intervals (CIs) [14]; IRs and the number of patients with an AE included AEs occurring up to 28 days beyond the last dose of study treatment (or to the data cutoff date for OPAL Balance). A 28-day risk period was applied to prevent inflated IR estimations due to potential differences between elapsed time and exposure time. Analyses were descriptive, with no formal statistical testing of differences between groups.
Observational Comparison Cohort
Outcomes in the observational comparison cohort were defined via ICD-9 codes, with algorithms validated in medical claims databases [13, 15–30], and included all incident events occurring from the index date until the time of first occurrence of AEs of each type, the earliest date of death, loss of medical or pharmacy coverage, date of switch to another bDMARD or apremilast, discontinuation of the specific PsA treatment, or the end of the study (30 September 2015). Outcomes were weighted by previous TNFi use, concomitant methotrexate use, and concomitant steroid use to control for observed differences in patient population characteristics between the tofacitinib global clinical studies and the observational comparison cohort (further details in Online Resource 1).
As several PsA therapies and combinations of therapies were studied, patients were permitted to contribute person–time data to one or more exposure category if they switched PsA treatments. IRs reflected the time to first event of each type, e.g., if a patient initiated etanercept and experienced an SIE (defined as infections occurring during hospitalization; see Online Resource 1) and then subsequently initiated adalimumab and experienced another SIE, both exposures and infection events were deemed to have contributed to the respective IRs for etanercept and adalimumab and to the composite exposure categories for TNFi and bDMARDs.
IRs were calculated for SIEs, OIs, HZ, malignancies (excluding NMSC), NMSC, and MACE from the observational comparison cohort. IRs were calculated for events occurring during treatment or up to predefined intervals beyond the estimated last dose of PsA treatment, which considered the variable latency period for developing each type of AE: 30 days for SIEs and HZ, 90 days for MACE, and all available follow-up time for malignancies.
Results
Patients
Placebo-Controlled and Tofacitinib Dose-Comparison Cohorts
A total of 474 tofacitinib-treated, 106 adalimumab-treated, and 236 placebo-treated patients were included in the placebo-controlled cohort (Table 1). The tofacitinib dose-comparison cohort included the same 474 tofacitinib-treated patients as the placebo-controlled cohort (Table 1).
Table 1.
Demographics and baseline characteristics (placebo-controlled cohort and dose-comparison cohort)
| Characteristics | Tofacitinib 5 mg BID (n = 238) | Tofacitinib 10 mg BID (n = 236) | Placebo (n = 236) | Adalimumab 40 mg Q2Wa (n = 106) |
|---|---|---|---|---|
| Age (years) | 49.5 ± 12.4 | 49.4 ± 11.7 | 48.4 ± 12.5 | 47.4 ± 11.3 |
| Female | 121 (51) | 136 (58) | 136 (58) | 50 (47) |
| BMI (kg/m2) | 29.8 ± 6.3 | 30.2 ± 6.3 | 29.2 ± 5.6 | 28.8 ± 5.3 |
| Race | ||||
| White | 226 (95.0) | 221 (93.6) | 222 (94.1) | 103 (97.2) |
| Black | 1 (0.4) | 1 (0.4) | 1 (0.4) | 0 (0.0) |
| Asian | 2 (0.8) | 10 (4.2) | 9 (3.8) | 2 (1.9) |
| Other | 9 (3.8) | 4 (1.7) | 4 (1.7) | 1 (0.9) |
| Geographical regionb | ||||
| USA/Canada | 58 (24.4) | 56 (23.7) | 45 (19.1) | 11 (10.4) |
| Eastern Europe/Russia | 93 (39.1) | 100 (42.4) | 112 (47.5) | 72 (67.9) |
| Western Europe/Australia | 58 (24.4) | 59 (25.0) | 49 (20.8) | 17 (16.0) |
| Asia | 1 (0.4) | 7 (3.0) | 6 (2.5) | 1 (0.9) |
| Latin America | 28 (11.8) | 14 (5.9) | 24 (10.2) | 5 (4.7) |
| PsA duration (years) | 8.6 ± 7.9 | 7.5 ± 6.6 | 8.1 ± 7.5 | 5.3 ± 5.3 |
| Corticosteroid usec | 67 (28.2) | 37 (15.7) | 49 (20.8) | 23 (21.7) |
| Concomitant MTX | 190 (79.8) | 184 (78.0) | 193 (81.8) | 80 (75.5) |
Data are presented as mean ± standard deviation or N (%) unless otherwise indicated
BID twice daily, BMI body mass index, MTX methotrexate, PsA psoriatic arthritis, Q2W once every 2 weeks
aPatients from OPAL Broaden receiving adalimumab 40 mg Q2W subcutaneously
bEastern Europe includes Bulgaria, Czech Republic, Hungary, Poland, and Slovakia. Western Europe includes Belgium, France, Germany, Spain, and the UK. Asia includes Taiwan. Latin America includes Brazil and Mexico
cOral systemic corticosteroid use at baseline (maximum dose 10 mg/day prednisone equivalent)
Patient demographics were generally similar across tofacitinib and placebo groups (Table 1). The adalimumab group included a smaller proportion of patients from the USA and Canada and a larger proportion from Eastern Europe and Russia than the other groups and also had a shorter duration of PsA. This reflected the fact that all patients treated with adalimumab were from OPAL Broaden only, which had a different geographical spread from that in OPAL Beyond and required patients to be TNFi naïve.
The lowest proportion of patients taking corticosteroids at baseline was in the tofacitinib 10 mg BID group (15.7%), and the highest proportion was in the tofacitinib 5 mg BID group (28.2%). All patients were receiving one background csDMARD, with most patients receiving methotrexate (75.5–81.8%).
All-Tofacitinib Comparison Cohort
The all-tofacitinib comparison cohort included 783 tofacitinib-treated patients, with a total of 776 patient-years of tofacitinib exposure (all doses). Table 2 presents demographic data and baseline characteristics for the all-tofacitinib comparison cohort.
Table 2.
Demographics and baseline characteristics for the all-tofacitinib comparison cohort and the observational comparison cohort
| Characteristics | All-tofacitinib comparison cohort (n = 783) | Observational comparison cohort (n = 5799) |
|---|---|---|
| Age (years) | 48.7 ± 12.0 | 48.9 ± 11.5 |
| Female | 428 (54.7) | 3127 (53.9) |
| Race | ||
| White | 739 (94.4) | NA |
| Black | 3 (0.4) | NA |
| Asian | 23 (2.9) | NA |
| Other | 18 (2.3) | NA |
| Geographical regiona | ||
| USA/Canada | 158 (20.2) | 5799 (100.0) |
| Eastern Europe/Russia | 369 (47.1) | 0 (0.0) |
| Western Europe/Australia | 173 (22.1) | 0 (0.0) |
| Asia | 15 (1.9) | 0 (0.0) |
| Latin America | 68 (8.7) | 0 (0.0) |
| PsA duration (years) | 7.7 ± 7.2 | NA |
| Diabetes | 107 (13.7) | 706 (12.2) |
| Prior TNFi experience | 377 (48.1) | 2125 (36.6) |
| Corticosteroid useb | 170 (21.7) | 856 (11.9) |
| Concomitant MTX | 609 (77.8) | 2202 (38.0) |
Data are presented as mean ± standard deviation or N (%) unless otherwise indicated
MTX methotrexate, NA not available, PsA psoriatic arthritis, TNFi tumor necrosis factor inhibitor
aEastern Europe includes Bulgaria, Czech Republic, Hungary, and Poland. Western Europe includes Belgium, France, Germany, Spain, and the UK. Asia includes Taiwan. Latin America includes Brazil and Mexico
bOral systemic corticosteroid use at baseline (maximum dose 10 mg/day prednisone equivalent)
Observational Comparison Cohort
A total of 5799 patients meeting the selection criteria were identified in the US Truven MarketScan database. All patients were from the USA, with mean age, sex, and diabetes history comparable to those of the all-tofacitinib comparison cohort (Table 2). However, more patients in the all-tofacitinib comparison cohort had prior experience with TNFi and methotrexate and were taking corticosteroids at baseline than in the observational comparison cohort.
Outcomes
Overview of Adverse Events
In the placebo-controlled tofacitinib cohort, headache (tofacitinib 5 mg BID: 3.8%; 10 mg BID: 8.5%), nasopharyngitis (tofacitinib 5 mg BID: 5.9%; 10 mg BID: 5.5%), and upper respiratory tract infection (tofacitinib 5 mg BID: 5.0%; 10 mg BID: 4.7%) were the most commonly reported AEs in patients receiving tofacitinib over 3 months (see the table in Online Resource 1). IRs for SAEs over 12 months for tofacitinib 5 and 10 mg BID in the tofacitinib dose-comparison cohort were 7.9 (95% CI 4.1–13.8) and 8.1 (95% CI 4.2–14.2), respectively. Discontinuations due to AEs occurred in 11 patients randomized to each of tofacitinib 5 and 10 mg BID, with IRs of 7.2 (95% CI 3.6–12.8) and 7.3 (95% CI 3.7–13.1), respectively (a further three discontinuations occurred in the tofacitinib 10 mg BID group because of AEs reported outside the 28-day risk period).
Serious Infections
In the tofacitinib dose-comparison cohort (12 months), SIEs (infections requiring parenteral antimicrobials in an outpatient or emergency department setting or resulting in hospitalization) were experienced by two patients receiving tofacitinib 5 mg BID (IR 1.3; 95% CI 0.2–4.7) and three receiving tofacitinib 10 mg BID (IR 2.0; 95% CI 0.4–5.8) (Fig. 2a). Of these, only pneumonia occurred in one or more patient (n = 2) and was resolved with conventional therapy. All SIEs in the tofacitinib dose-comparison cohort were the result of hospitalization; no outpatient infections requiring parenteral antibiotics were reported. Across all tofacitinib-treated patients in the phase III and LTE studies (the all-tofacitinib comparison cohort), SIEs occurred in 11 patients during treatment or within 28 days of the last dose of tofacitinib (IR 1.4; 95% CI 0.7–2.5); SIEs were reported in two additional patients > 28 days after their last dose of tofacitinib.
Fig. 2.
IRs for SIEs a resulting in hospitalization or b requiring parenteral antimicrobials in an emergency department setting or resulting in hospitalization over 12 months across cohorts. The tofacitinib dose-comparison cohort included patients who were randomized to receive either tofacitinib 5 mg BID or 10 mg BID (n = 474) in the two phase III studies (12 or 6 months’ duration). For the observational comparison cohort, follow-up was truncated at 1 year because of the possible time-varying hazard between PsA treatments and infections to ensure equal follow-up time. Observational comparison cohort outcomes were weighted based on previous TNFi use (identified using all available data: TNFi naïve vs. TNFi experienced), concomitant MTX use (identified using data from the index date to 90 days before the index date: MTX only vs. no MTX or with other csDMARDs), and concomitant steroid use (identified on the index date: steroid use vs. no steroid use); the weights were derived using the all-tofacitinib comparison cohort data. For bDMARD, bDMARD + csDMARD, TNFi, and TNFi + csDMARD, n refers to “treatment episodes” rather than patients, as the patients in these groups may have initiated more than one drug in the given class. bDMARD biologic DMARD, BID twice daily, CI confidence interval, csDMARD conventional synthetic DMARD, DMARD disease-modifying antirheumatic drug, IR incidence rate (patients with event/100 PY), MTX methotrexate, PsA psoriatic arthritis, PY patient-years, SIEs serious infections, TNFi tumor necrosis factor inhibitor, tofa tofacitinib
For patients in the observational comparison cohort, the IR for SIEs (defined as infections resulting in hospitalization) was 2.2 (95% CI 1.4–3.2) for bDMARDs, ranging from 1.1 (95% CI 0.5–2.2) to 7.9 (95% CI 0.8–30.0) across all treatments (Fig. 2a). When considering SIEs defined as infection events requiring parenteral antimicrobial treatment in an emergency department setting or resulting in hospitalization, similar IRs for SIEs were reported for the observational comparison cohort (ranging from 1.3 [95% CI 0.6–2.5] to 8.1 [95% CI 0.9–29.9]) (Fig. 2b) as the tofacitinib dose-comparison cohort.
Herpes Zoster
In the tofacitinib dose-comparison cohort, HZ events were reported for three patients receiving tofacitinib 5 mg BID (IR 2.0; 95% CI 0.4–5.7) and four receiving tofacitinib 10 mg BID (IR 2.7; 95% CI 0.7–6.8) (Fig. 3a). HZ events were reported in 16 patients receiving tofacitinib in the all-tofacitinib cohort (IR 2.1; 95% CI 1.2–3.3). For patients in the observational comparison cohort, IRs for HZ ranged from 0.8 (95% CI 0.3–1.5) to 2.0 (95% CI 0.8–4.0) across treatments, with the IR for infliximab (2.0; 95% CI 0.8–4.0) being most similar to that reported for tofacitinib 5 mg BID in the tofacitinib dose-comparison cohort (Fig. 3a).
Fig. 3.
IRs for a HZ and b OI over 12 months across cohorts. The tofacitinib dose-comparison cohort included patients who were randomized to receive either tofacitinib 5 mg BID or 10 mg BID (n = 474) in the two phase III studies (12 or 6 months’ duration). OI included HZ and excluded tuberculosis. Observational comparison cohort outcomes were weighted based on previous TNFi use (identified using all available data: TNFi naïve vs. TNFi experienced), concomitant MTX use (identified using data from the index date to 90 days before the index date: MTX only vs. no MTX or with other csDMARDs), and concomitant steroid use (identified on the index date: steroid use vs. no steroid use); the weights were derived using the all-tofacitinib comparison cohort data. For bDMARD, bDMARD + csDMARD, TNFi, and TNFi + csDMARD, n refers to “treatment episodes” rather than patients, as the patients in these groups may have initiated more than one drug in the given class. bDMARD biologic DMARD, BID twice daily, CI confidence interval, csDMARD conventional synthetic DMARD, DMARD disease-modifying antirheumatic drug, HZ herpes zoster, IR incidence rate (patients with event/100 PY), MTX methotrexate, OI opportunistic infection, PY patient-years, TNFi tumor necrosis factor inhibitor, tofa tofacitinib
Opportunistic Infections
In total, three adjudicated OIs occurred in the all-tofacitinib comparison cohort (two patients receiving tofacitinib 5 mg BID and one receiving tofacitinib 10 mg BID; IR 0.4; 95% CI 0.1–1.1) (Fig. 3b). All were cases of multidermatomal HZ and were resolved. A case of HZ with two adjacent dermatomes in a patient receiving tofacitinib 5 mg BID was not included in the IR calculation for OI, as it was classified as a special interest infection. No cases of active tuberculosis were reported in tofacitinib-treated patients. For patients in the observational comparison cohort, IRs for OI ranged from 0.9 (95% CI 0.4–1.7) to 3.8 (95% CI 0.7–11.6) across treatments (Fig. 3b).
Major Adverse Cardiovascular Events
MACE were reported in three patients in the all-tofacitinib comparison cohort (IR 0.4; 95% CI 0.1–1.1) (Fig. 4a) and included sudden cardiac death (after advancing from placebo to tofacitinib 5 mg BID for 57 days), myocardial infarction (after receiving tofacitinib 5 mg BID for 197 days), and ischemic stroke (after receiving tofacitinib 10 mg BID for 80 days). For patients in the observational comparison cohort, IRs for MACE ranged from 0.0 (95% CI 0.0–1.6) to 0.7 (95% CI 0.1–2.4) across treatments (Fig. 4a).
Fig. 4.
IRs for a MACE, b malignancies (excluding NMSC), and c NMSC across cohorts. The all-tofacitinib comparison cohort included patients who received at least one dose of either tofacitinib 5 mg BID or 10 mg BID (n = 783) in either of the two phase III studies or the LTE. Observational comparison cohort outcomes were weighted based on previous TNFi use (identified using all available data: TNFi naïve vs. TNFi experienced), concomitant MTX use (identified using data from the index date to 90 days before the index date: MTX only vs. no MTX or with other csDMARDs), and concomitant steroid use (identified on the index date: steroid use vs. no steroid use); the weights were derived using the all-tofacitinib comparison cohort data. For bDMARD, bDMARD + csDMARD, TNFi, and TNFi + csDMARD, n refers to “treatment episodes” rather than patients, as the patients in these groups may have initiated more than one drug in the given class. bDMARD biologic DMARD, BID twice daily, CI confidence interval, csDMARD conventional synthetic DMARD, DMARD disease-modifying antirheumatic drug, IR incidence rate (patients with event/100 PY), LTE long-term extension, MACE major adverse cardiovascular event, MTX methotrexate, NMSC non-melanoma skin cancer, PY patient-years, TNFi tumor necrosis factor inhibitor, tofa tofacitinib
Malignancies
Malignancies (excluding NMSC) were reported in five patients in the all-tofacitinib comparison cohort (IR 0.6; 95% CI 0.2–1.5) (Fig. 4b). These were bladder transitional cell carcinoma (after receiving tofacitinib 5 mg BID for 48 days); renal cell carcinoma (after receiving adalimumab 40 mg Q2W for 342 days followed by tofacitinib 5 mg BID for 32 days); metastatic pancreatic cell carcinoma (after receiving adalimumab 40 mg Q2W for 353 days followed by tofacitinib 5 mg BID for 84 days; resulted in death); squamous cell carcinoma of the vulva (after receiving tofacitinib 5 mg BID for 65 days); and invasive ductal breast carcinoma (after receiving tofacitinib 5 mg BID for 244 days). For patients in the observational comparison cohort, IRs for malignancies (excluding NMSC) ranged from 0.0 (95% CI 0.0–0.9) to 1.1 (95% CI 0.3–2.8) across treatments (Fig. 4b).
NMSC was reported in four patients in the all-tofacitinib comparison cohort (IR 0.5; 95% CI 0.1–1.3) and included two basal cell and two squamous cell carcinomas. All NMSC were reported in White patients: two from Australia and one each from Belgium and the USA. For patients in the observational comparison cohort, IRs for NMSC ranged from 0.4 (95% CI 0.1–1.1) to 6.0 (95% CI 1.7–14.9) across treatments (Fig. 4c).
Deaths
Four deaths occurred in the tofacitinib PsA clinical program. Two occurred in the all-tofacitinib comparison cohort during treatment or within 28 days of the last tofacitinib dose (IR 0.3; 95% CI 0.0–0.9); both patients were receiving tofacitinib 5 mg BID at the time of death. The causes were cardiac arrest (described in Sect. 3.2.5) and pulmonary embolism (PE; after receiving tofacitinib 5 mg BID for 346 days). Two further deaths occurred outside the 28-day risk period and were not included in the mortality IR analyses. The causes were metastatic pancreatic cell carcinoma (described in Sect. 3.2.6) and acute cardiac failure (after receiving tofacitinib 5 mg BID for 274 days). All deaths were considered by the investigator to be unrelated to the study drug.
Clinical Laboratory Findings
Overall, clinical laboratory findings were in line with those observed in other clinical programs for tofacitinib (see Online Resource 1).
Discussion
This post hoc analysis of data from two phase III studies and one LTE study examined the safety profile of tofacitinib in patients with active PsA and compared IRs for AEs of special interest with observational data for other PsA treatments.
The types and rates of AEs were similar to those observed with tofacitinib in clinical programs for other indications [31–33], with nasopharyngitis, headache, and upper respiratory tract infections the most commonly reported AEs during the first 3 months of tofacitinib treatment. AEs in the placebo group may reflect in part the fact that all patients were receiving background csDMARD treatment. IRs for SAEs in patients receiving tofacitinib 5 and 10 mg BID for up to 12 months were 7.9 and 8.1, respectively, and were similar to the IR of 9.4 reported for all tofacitinib doses across phase I, II, III, and LTE studies in rheumatoid arthritis (RA) [31]. IRs for treatment discontinuation due to AEs in the PsA dose-comparison cohort for patients receiving tofacitinib 5 and 10 mg BID were 7.2 and 7.3, respectively, and were also similar to the IR of 7.5 reported for all tofacitinib doses in patients with RA [31].
Increased rates of SIEs are an acknowledged risk of medications that have an immunomodulatory effect, including tofacitinib and bDMARDs [31, 34, 35]. The IRs for SIEs in the tofacitinib dose-comparison cohort (1.3 and 2.0 for patients with PsA receiving tofacitinib 5 and 10 mg BID, respectively) and in the all-tofacitinib comparison cohort (1.4) were consistent with the IR of 2.7 reported for tofacitinib in patients with RA who participated in phase I, II, III, and LTE studies [31] and 1.9 in patients with psoriasis in phase III and LTE studies [32]. The IRs for SIEs with tofacitinib in patients with PsA were also within the range of 1.7–4.7 reported for other systemic PsA therapies in the observational comparison cohort.
Analyses of data from the tofacitinib RA and psoriasis clinical programs identified increased rates of HZ infection with tofacitinib versus placebo [36, 37]. In the current analysis, the IRs for HZ in patients with PsA were 2.0, 2.7, and 2.1 for patients with PsA receiving tofacitinib 5 and 10 mg BID in the tofacitinib dose-comparison cohort and those in the all-tofacitinib comparison cohort, respectively. These IRs were somewhat higher than the IRs of 1.1 (95% CI 0.5–2.4) and 2.0 (95% CI 1.1–3.4) reported for tofacitinib 5 and 10 mg BID, respectively, in two 12-month studies in patients with chronic plaque psoriasis [32] but lower than the IR of 3.9 (95% CI 3.6–4.2) reported for the pooled analysis of data from phase I, II, III, and LTE studies in the RA clinical program (all tofacitinib doses) [31]. As noted earlier in this section, this may reflect the fact that tofacitinib was administered as monotherapy in the psoriasis studies (i.e., without corticosteroids or other background immunomodulators) but was more frequently administered with (56.0%) than without concomitant corticosteroids in the RA studies [31], which has been shown to increase the risk of HZ [38]. Although using corticosteroids in combination with csDMARDs has also been associated with higher rates of HZ, csDMARDs have not been identified as an independent risk factor for HZ [38], and their use in the PsA studies was actually higher than that in the RA studies [31]. OIs are also considered a risk with tofacitinib [31, 39], as well as with biologic therapies [13], but in this analysis—other than three cases of multidermatomal HZ (out of 16 total cases)—no infections were adjudicated to be OIs. By comparison, IRs for HZ in the observational comparison cohort were generally lower, ranging from 0.8 to 2.0.
The IR for malignancies (excluding NMSC) in patients with PsA in the all-tofacitinib comparison cohort (0.6) was within the range reported for other PsA treatments in the observational comparison cohort (0.0–1.1). Of the five malignancy events, four occurred within the first 3 months of tofacitinib treatment, and all were different types of malignancy. The IR for NMSC in patients with PsA in the all-tofacitinib comparison cohort (0.5) appeared lower than the range observed in the observational comparison cohort for other agents (0.8−6.0), except for golimumab (0.4). However, the higher IRs for NMSC in the observational comparison cohort may be reflective of clinical trial populations receiving more intense follow-up, even during the trial screening process. Although misclassifications of outcomes in claims data are possible, the approach used here mirrors that of previous high-quality validation studies that have demonstrated high positive predictive values compared with the gold standard or medical record review [13, 15–30]. In addition, differences in incidence of NMSC have been reported based on geographical region [40] and, while the tofacitinib clinical studies were conducted at centers worldwide, the observational data cohort was based on patients from the USA only. However, the IRs for patients with PsA in the tofacitinib studies were similar to those reported for bDMARD exposures in external sources [41–43] and were consistent with results from pooled analyses of patients receiving tofacitinib in the RA [31] and psoriasis [32] clinical programs.
Patients with PsA have an increased risk of cardiovascular morbidity and mortality compared with the general population [44–46]. Modest, dose-dependent changes in lipid profile were observed in tofacitinib-treated patients with PsA [10, 11]; however, this did not appear to correlate with increased cardiovascular risk, and the incidence of MACE was low in the tofacitinib clinical studies. The IR for MACE in patients with PsA in the all-tofacitinib comparison cohort was 0.4, which is the same as that reported for tofacitinib in clinical trials in patients with RA (0.4) [47] or psoriasis (0.4) [48] and within the range reported for other PsA therapies in the observational comparison cohort (0.0–0.7).
In February 2019, a safety analysis of the ongoing study A3921133 (NCT02092467), completed by the external, independent tofacitinib Rheumatology Data Safety Monitoring Board, reported that the incidence of PE events was higher in patients receiving tofacitinib 10 mg BID than in patients receiving a TNFi. Patients in the A3921133 randomized, endpoint-driven postauthorization safety study had a diagnosis of RA, were aged ≥ 50 years, and had one or more cardiovascular risk factor. Based on the safety analysis of study A3921133 and knowledge of the safety profile of other Janus kinase inhibitors [49, 50], venous thromboembolism events (including deep vein thrombosis and PE) were identified as an important risk for treatment with tofacitinib, irrespective of dose. Subsequently, thromboembolism was added as a warning and as an adverse drug reaction to the current product labeling for tofacitinib. Specifically, the updated US prescribing information includes thrombosis as a boxed warning and recommends that tofacitinib be avoided in patients at risk of thrombosis (including PE, deep vein thrombosis, and arterial thrombosis) [51], whereas the European Medicines Agency Committee for Medicinal Products for Human Use (CHMP) states that tofacitinib should be used with caution in patients with known risk factors for venous thromboembolism, regardless of indication and dosage [52].
A number of limitations of this analysis are acknowledged. Comparisons with placebo (with background csDMARDs) in this analysis were limited to the 3-month placebo-controlled portion of the phase III studies; the extent and length of exposure to placebo was therefore less. The design of the LTE study, with the optional dose adjustments between tofacitinib 5 and 10 mg BID allowed at the investigators’ discretion, prevented long-term comparison of doses. The evaluation of safety events over time was also limited by the sample size and extent of exposure; this is common in clinical trials of limited duration, and longer-term follow-up may be required. Care must be taken in interpreting data from the observational comparison cohort and comparisons of these data with the tofacitinib global clinical studies, as the comparison cohort was derived from an observational claims database, including only US-insured patients (including Medicaid). Claims reflect dispensing of medication, not necessarily actual use; medication adherence is generally higher in clinical trials than in clinical practice [53], although adherence was not confirmed by testing for drug levels in the tofacitinib clinical program. In addition, for some individual drugs such as certolizumab and apremilast, the overall patient-years of exposure for this analysis were relatively small compared with other therapies. Differences in AE reporting between the tofacitinib clinical trials and the observational comparison cohort must be noted. In the tofacitinib clinical trials, the investigator was to pursue and obtain information regarding the outcome and causality of an AE, potentially underestimating outpatient infections requiring parenteral antibiotics. In contrast, in the US Truven MarketScan database, all events were captured using administrative codes, limiting clinical detail. These differences should be taken into account when comparing the IRs of AEs between the cohorts.
Conclusions
In patients with active PsA, tofacitinib had a safety profile that was generally consistent with patients with PsA receiving other therapies in real-world settings. No new risks were identified compared with those observed with tofacitinib treatment of RA. As when used in RA, tofacitinib was associated with a higher risk for HZ than are most other PsA therapies. Longer-term follow-up and larger patient populations will provide further information on the safety profile of tofacitinib in patients with PsA.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank the patients who participated in the OPAL Broaden, OPAL Beyond, and OPAL Balance clinical studies.
Author Contributions
All authors were involved in the analysis and interpretation of data and critically revising the manuscript for important intellectual content. All authors agree to be accountable for all aspects of the work and read and approved the final manuscript to be published.
Availability of Data and Materials
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the USA and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Compliance with Ethical Standards
Funding
This study was funded by Pfizer Inc. Medical writing support, under the guidance of the authors, was provided by Carole Evans, PhD, and Richard Knight, PhD, CMC Connect, McCann Health Medical Communications Ltd, and was funded by Pfizer Inc, New York, NY, USA, in accordance with good publication practice (GPP3) guidelines (Ann Intern Med 2015;163:461–4).
Conflicts of Interest
Gerd R. Burmester has received consulting fees from AbbVie, Gilead, Lilly, and Pfizer Inc and lecture fees from AbbVie, Lilly, and Pfizer Inc. Jeffrey R. Curtis has received research grants and consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Eli Lilly, Janssen, Myriad, Pfizer Inc, Regeneron, Roche, and UCB. Huifeng Yun has received grant/research support from Pfizer Inc. Oliver FitzGerald has received research grants and honoraria from AbbVie, Celgene, Janssen, Lilly, Novartis, Pfizer Inc, and UCB. Kevin L. Winthrop has received consulting fees from AbbVie, Galapagos, Gilead, GSK, Lilly, Pfizer Inc, Roche, and UCB. Valderilio F. Azevedo has received grant/research support and consulting fees from AbbVie, Lilly, Novartis, and Pfizer Inc and lecture fees from AbbVie, Boehringer Ingelheim, Celltrion, Janssen, Lilly, Novartis, and Pfizer Inc. William F.C. Rigby has received consulting fees for work unrelated to this manuscript. Keith S. Kanik, Cunshan Wang, Pinaki Biswas, Thomas Jones, Thijs Hendrikx, and Ricardo Rojo are shareholders and employees of Pfizer Inc. Sujatha Menon is an employee of Pfizer Inc. Niki Palmetto was an employee of Pfizer Inc at the time of the analysis.
Research involving Human Participants and/or Animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board or independent ethics committee at each investigational site and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Patient data from the US Truven MarketScan database were de-identified and complied with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act. Therefore, institutional review board approval was not required.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Acosta-Felquer ML, Armstrong AW, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68(5):1060–1071. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 2.Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510. doi: 10.1136/annrheumdis-2015-208337. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb A, Korman NJ, Gordon KB, Feldman SR, Lebwohl M, Koo JY, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol. 2008;58(5):851–864. doi: 10.1016/j.jaad.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 4.Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;74(4):423–441. doi: 10.1007/s40265-014-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadarajan P, Fabre A, Kelly E. Sulfasalazine: a rare cause of acute eosinophilic pneumonia. Respir Med Case Rep. 2016;18:35–36. doi: 10.1016/j.rmcr.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceponis A, Kavanaugh A. Use of methotrexate in patients with psoriatic arthritis. Clin Exp Rheumatol. 2010;28(5 Suppl 61):S132–S137. [PubMed] [Google Scholar]
- 7.Sevilla-Mantilla C, Ortega L, Agúndez JA, Fernández-Gutiérrez B, Ladero JM, Díaz-Rubio M. Leflunomide-induced acute hepatitis. Dig Liver Dis. 2004;36(1):82–84. doi: 10.1016/j.dld.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Krathen MS, Gottlieb AB, Mease PJ. Pharmacologic immunomodulation and cutaneous malignancy in rheumatoid arthritis, psoriasis, and psoriatic arthritis. J Rheumatol. 2010;37(11):2205–2215. doi: 10.3899/jrheum.100041. [DOI] [PubMed] [Google Scholar]
- 9.Minozzi S, Bonovas S, Lytras T, Pecoraro V, González-Lorenzo M, Bastiampillai AJ, et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf. 2016;15(sup1):11–34. doi: 10.1080/14740338.2016.1240783. [DOI] [PubMed] [Google Scholar]
- 10.Mease P, Hall S, FitzGerald O, van der Heijde D, Merola JF, Avila-Zapata F, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377(16):1537–1550. doi: 10.1056/NEJMoa1615975. [DOI] [PubMed] [Google Scholar]
- 11.Gladman D, Rigby W, Azevedo VF, Behrens F, Blanco R, Kaszuba A, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377(16):1525–1536. doi: 10.1056/NEJMoa1615977. [DOI] [PubMed] [Google Scholar]
- 12.Nash P, Coates LC, Kivitz AJ, Mease PJ, Gladman DD, Covarrubias-Cobos JA, et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, up to 24 months in patients with active psoriatic arthritis: interim data from OPAL Balance, an open-label, long-term extension study. Ann Rheum Dis. 2017;76:682. [Google Scholar]
- 13.Winthrop KL, Novosad SA, Baddley JW, Calabrese L, Chiller T, Polgreen P, et al. Opportunistic infections and biologic therapies in immune-mediated inflammatory diseases: consensus recommendations for infection reporting during clinical trials and postmarketing surveillance. Ann Rheum Dis. 2015;74(12):2107–2116. doi: 10.1136/annrheumdis-2015-207841. [DOI] [PubMed] [Google Scholar]
- 14.Daly L. Simple SAS macros for the calculation of exact binomial and Poisson confidence limits. Comput Biol Med. 1992;22(5):351–361. doi: 10.1016/0010-4825(92)90023-g. [DOI] [PubMed] [Google Scholar]
- 15.Baddley JW, Winthrop KL, Chen L, Liu L, Grijalva CG, Delzell E, et al. Non-viral opportunistic infections in new users of tumour necrosis factor inhibitor therapy: results of the SAfety Assessment of Biologic ThERapy (SABER) study. Ann Rheum Dis. 2014;73(11):1942–1948. doi: 10.1136/annrheumdis-2013-203407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bharat A, Xie F, Baddley JW, Beukelman T, Chen L, Calabrese L, et al. Incidence and risk factors for progressive multifocal leukoencephalopathy among patients with selected rheumatic diseases. Arthritis Care Res (Hoboken). 2012;64(4):612–615. doi: 10.1002/acr.21564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curtis JR, Chen SY, Werther W, John A, Johnson DA. Validation of ICD-9-CM codes to identify gastrointestinal perforation events in administrative claims data among hospitalized rheumatoid arthritis patients. Pharmacoepidemiol Drug Saf. 2011;20(11):1150–1158. doi: 10.1002/pds.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtis JR, Lanas A, John A, Johnson DA, Schulman KL. Factors associated with gastrointestinal perforation in a cohort of patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64(12):1819–1828. doi: 10.1002/acr.21764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis JR, Sarsour K, Napalkov P, Costa LA, Schulman KL. Incidence and complications of interstitial lung disease in users of tocilizumab, rituximab, abatacept and anti-tumor necrosis factor alpha agents, a retrospective cohort study. Arthritis Res Ther. 2015;17:319. doi: 10.1186/s13075-015-0835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haynes K, Beukelman T, Curtis JR, Newcomb C, Herrinton LJ, Graham DJ, et al. Tumor necrosis factor α inhibitor therapy and cancer risk in chronic immune-mediated diseases. Arthritis Rheum. 2013;65(1):48–58. doi: 10.1002/art.37740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148(1):99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Kumamaru H, Judd SE, Curtis JR, Ramachandran R, Hardy NC, Rhodes JD, et al. Validity of claims-based stroke algorithms in contemporary Medicare data: reasons for geographic and racial differences in stroke (REGARDS) study linked with medicare claims. Circ Cardiovasc Qual Outcomes. 2014;7(4):611–619. doi: 10.1161/CIRCOUTCOMES.113.000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patkar NM, Curtis JR, Teng GG, Allison JJ, Saag M, Martin C, et al. Administrative codes combined with medical records based criteria accurately identified bacterial infections among rheumatoid arthritis patients. J Clin Epidemiol. 2009;62(3):321–327. doi: 10.1016/j.jclinepi.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott FI, Mamtani R, Brensinger CM, Haynes K, Chiesa-Fuxench ZC, Zhang J, et al. Risk of nonmelanoma skin cancer associated with the use of immunosuppressant and biologic agents in patients with a history of autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol. 2016;152(2):164–172. doi: 10.1001/jamadermatol.2015.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Setoguchi S, Solomon DH, Glynn RJ, Cook EF, Levin R, Schneeweiss S. Agreement of diagnosis and its date for hematologic malignancies and solid tumors between medicare claims and cancer registry data. Cancer Causes Control. 2007;18(5):561–569. doi: 10.1007/s10552-007-0131-1. [DOI] [PubMed] [Google Scholar]
- 26.Winthrop KL, Baxter R, Liu L, McFarland B, Austin D, Varley C, et al. The reliability of diagnostic coding and laboratory data to identify tuberculosis and nontuberculous mycobacterial disease among rheumatoid arthritis patients using anti-tumor necrosis factor therapy. Pharmacoepidemiol Drug Saf. 2011;20(3):229–235. doi: 10.1002/pds.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winthrop KL, Neal J, Hrycaj P, Soma K, Wilkinson B, Hodge J, et al. Evaluation of influenza and pneumococcal vaccine responses in rheumatoid arthritis patients using tofacitinib. Ann Rheum Dis. 2013;72:A107–A108. doi: 10.1136/annrheumdis-2014-207191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie F, Yun H, Bernatsky S, Curtis JR. Brief report: risk of gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologic treatments. Arthritis Rheumatol. 2016;68(11):2612–2617. doi: 10.1002/art.39761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yawn BP, Wollan P, St Sauver J. Comparing shingles incidence and complication rates from medical record review and administrative database estimates: how close are they? Am J Epidemiol. 2011;174(9):1054–1061. doi: 10.1093/aje/kwr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yun H, Xie F, Delzell E, Chen L, Levitan EB, Lewis JD, et al. Risks of herpes zoster in patients with rheumatoid arthritis according to biologic disease-modifying therapy. Arthritis Care Res (Hoboken). 2015;67(5):731–736. doi: 10.1002/acr.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen SB, Tanaka Y, Mariette X, Curtis JR, Lee EB, Nash P, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis. 2017;76(7):1253–1262. doi: 10.1136/annrheumdis-2016-210457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papp KA, Krueger JG, Feldman SR, Langley RG, Thaci D, Torii H, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: long-term efficacy and safety results from 2 randomized phase-III studies and 1 open-label long-term extension study. J Am Acad Dermatol. 2016;74(5):841–850. doi: 10.1016/j.jaad.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Sandborn WJ, Ghosh S, Panés J, Vranic I, Su C, Rousell S, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367(7):616–624. doi: 10.1056/NEJMoa1112168. [DOI] [PubMed] [Google Scholar]
- 34.Ramiro S, Sepriano A, Chatzidionysiou K, Nam JL, Smolen JS, van der Heijde D, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis. 2017;76(6):1101–1136. doi: 10.1136/annrheumdis-2016-210708. [DOI] [PubMed] [Google Scholar]
- 35.Desai RJ, Thaler KJ, Mahlknecht P, Gartlehner G, McDonagh MS, Mesgarpour B, et al. Comparative risk of harm associated with the use of targeted immunomodulators: a systematic review. Arthritis Care Res (Hoboken). 2016;68(8):1078–1088. doi: 10.1002/acr.22815. [DOI] [PubMed] [Google Scholar]
- 36.Winthrop KL, Yamanaka H, Valdez H, Mortensen E, Chew R, Krishnaswami S, et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(Suppl 10):2675–2684. doi: 10.1002/art.38745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winthrop KL, Lebwohl M, Cohen AD, Weinberg JM, Tyring SK, Rottinghaus ST, et al. Herpes zoster in psoriasis patients treated with tofacitinib. J Am Acad Dermatol. 2017;77(2):302–309. doi: 10.1016/j.jaad.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 38.Winthrop KL, Curtis JR, Lindsey S, Tanaka Y, Yamaoka K, Valdez H, et al. Herpes zoster and tofacitinib: clinical outcomes and the risk of concomitant therapy. Arthritis Rheumatol. 2017;69(10):1960–1968. doi: 10.1002/art.40189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winthrop KL, Park SH, Gul A, Cardiel MH, Gomez-Reino JJ, Tanaka Y, et al. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75(6):1133–1138. doi: 10.1136/annrheumdis-2015-207319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leiter U, Garbe C. Epidemiology of melanoma and nonmelanoma skin cancer–the role of sunlight. Adv Exp Med Biol. 2008;624:89–103. doi: 10.1007/978-0-387-77574-6_8. [DOI] [PubMed] [Google Scholar]
- 41.Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72(4):517–524. doi: 10.1136/annrheumdis-2011-201244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinblatt ME, Moreland LW, Westhovens R, Cohen RB, Kelly SM, Khan N, et al. Safety of abatacept administered intravenously in treatment of rheumatoid arthritis: integrated analyses of up to 8 years of treatment from the abatacept clinical trial program. J Rheumatol. 2013;40(6):787–797. doi: 10.3899/jrheum.120906. [DOI] [PubMed] [Google Scholar]
- 43.Bykerk VP, Cush J, Winthrop K, Calabrese L, Lortholary O, de Longueville M, et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann Rheum Dis. 2015;74(1):96–103. doi: 10.1136/annrheumdis-2013-203660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gladman DD, Ang M, Su L, Tom BD, Schentag CT, Farewell VT. Cardiovascular morbidity in psoriatic arthritis. Ann Rheum Dis. 2009;68(7):1131–1135. doi: 10.1136/ard.2008.094839. [DOI] [PubMed] [Google Scholar]
- 45.Ogdie A, Yu Y, Haynes K, Love TJ, Maliha S, Jiang Y, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74(2):326–332. doi: 10.1136/annrheumdis-2014-205675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polachek A, Touma Z, Anderson M, Eder L. Risk of cardiovascular morbidity in patients with psoriatic arthritis: a meta-analysis of observational studies. Arthritis Care Res (Hoboken). 2017;69(1):67–74. doi: 10.1002/acr.22926. [DOI] [PubMed] [Google Scholar]
- 47.Charles-Schoeman C, Wicker P, Gonzalez-Gay MA, Boy M, Zuckerman A, Soma K, et al. Cardiovascular safety findings in patients with rheumatoid arthritis treated with tofacitinib, an oral Janus kinase inhibitor. Semin Arthritis Rheum. 2016;46(3):261–271. doi: 10.1016/j.semarthrit.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 48.Wu JJ, Strober BE, Hansen PR, Ahlehoff O, Egeberg A, Qureshi A, et al. Effects of tofacitinib on cardiovascular risk factors and cardiovascular outcomes based on phase III and long-term extension data in patients with plaque psoriasis. J Am Acad Dermatol. 2016;75(5):897–905. doi: 10.1016/j.jaad.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 49.Taylor PC, Weinblatt ME, Burmester GR, Rooney TP, Witt S, Walls CD, et al. Cardiovascular safety during treatment with baricitinib in rheumatoid arthritis. Arthritis Rheumatol. 2019;71(7):1042–1055. doi: 10.1002/art.40841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Genovese MC, Fleischmann R, Combe B, Hall S, Rubbert-Roth A, Zhang Y, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391(10139):2513–2524. doi: 10.1016/S0140-6736(18)31116-4. [DOI] [PubMed] [Google Scholar]
- 51.US Food and Drug Administration. XELJANZ® (tofacitinib): Highlights of prescribing information. 2019. https://labeling.pfizer.com/ShowLabeling.aspx?id=959. Accessed 29 Oct 2019.
- 52.European Medicines Agency. EMA confirms Xeljanz to be used with caution in patients at high risk of blood clots. 2019. https://www.ema.europa.eu/en/documents/referral/xeljanz-article-20-procedure-ema-confirms-xeljanz-be-used-caution-patients-high-risk-blood-clots_en.pdf. Accessed 15 Nov 2019.
- 53.Matsui D. Strategies to measure and improve patient adherence in clinical trials. Pharm Med. 2009;23(5–6):289–297. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the USA and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.