Abstract
BACKGROUND:
Extracellular trap formation (ETosis) by various blood cells has been reported. This trap contains DNA, histones and granular proteins which can elicit an innate immune response by entrapping microorganisms. The trap thus formed has been reported to have an involvement in various pathogenic conditions as well. This review focusses on the trap formation by different blood cells, the immune response associated with trap formation and also its role in various clinical conditions.
METHOD:
An extensive literature survey on ETosis by blood cells from 2003 to 2019 has been done. After going through the literature throughly, in this review we focuses on the trap formation by different blood cell types such as neutrophils, macrophages, eosinophils, basophils, mast cells, plasmacytoid dentritic cells, and monocytes. The mechanism with which it releases trap, the immune response it elicits and ultimately its involvement in various pathogenic conditions are described here. This article extensively covered all the above aspects and finally comprehends in nutshell the various stimuli that are currently known in trigerring the ETosis, its effect and ultimately its role in disease process.
RESULTS:
A clarity about the extracellular trap formation by various blood cells, mechanism of ETosis, role of Etosis in microbial invasion and in various pathogenic situations by various blood cells have been described here.
CONCLUSION:
The current understanding about the process of ETosis and its effects has been extensively described here. Along with lot of favourable outcomes, the process of ETosis will lead to lot of pathogenic situations including thrombosis, tumour metastasis and sepsis. Current understanding about ETosis is limited. Indepth understanding of ETosis may have great therapeutic potential in the diagnosis, guiding of therapy and prognostication in various pathogenic situations including infectious conditions, autoimmune disorders and tumors.
Keywords: Neutrophil, Netosis, Extracellular trap, Monocytes, METosis, Macrophage
Introduction
The release of extracellular trap, termed as ETosis, is a cell death mechanism found to have significant role in antimicrobial host responses [1]. Extracellular Trap is a three-dimensional web like structure composed of DNA, histones and granular proteins which can elicit an innate immune response by entrapping microorganisms. The 3D network localizes cellular proteins and enzymes which can otherwise inflict damage to host tissue and creates a distributed structure which can destroy the microbes getting trapped inside it [2]. ETosis have major involvement in pathogenic conditions like autoimmune diseases, skin diseases, thrombosis, etc. Role of cytoplasmic and granular proteins in disease scenario varies in each cell type. Also, the involvement of extracellular histones in pathogenic environment affects the disease outcome. This mechanism has been reported in blood cells such as neutrophils, macrophages, eosinophils, basophils, mast cells, plasmacytoid dentritic cells and monocytes. Here in this review we are focussing on the ETosis in these cell types and its clinical implications.
Neutrophil extracellular traps (NETs)
The formation of Neutrophil Extracellular Traps (NETs), known as NETosis, is a novel mechanism of neutrophil-mediated host defence experimentally identified in 2004 [3], which is different from both apoptotic and necrotic mechanisms [1]. Neutrophils release a three-dimensional web like structure composed of DNA and histones together with granular proteins, known as NETs [1]. The major proteins embedded in the trap include histones, neutrophil elastase and myeloperoxidase. There are also other peptides and enzymes, namely lactoferrin, lysozyme C, neutrophil defensins, cathepsin G, gelatinase, cathelicidins, leukocyte proteinase 3 and calprotectin [4]. Most of these biomolecules can cause irreversible damage to organelles of the host cell and because they are restricted by the web-like structure, it can cause damage only to those microbes getting trapped inside the 3D network.
Several stimuli triggers NET formation. A team of Japanese scientists reported for the first time that by the addition of phorbol esters like phorbolmyristate acetate (PMA) in culture media can induce NETosis, even though the process behind it was not known. In a physiological system an infection, activation of platelets, cytokines and auto-antibodies can triger NET formation. During an infection when neutrophils are exposed to bacterial products such as endotoxins and formyl-methionyl-leucyl-phenylalanine (fMLP), NETosis occurs. Also, the physical interaction of neutrophils with activated platelets (in a pathologic condition) or certain cytokines (in tumour environment), is reported to induce NETosis [5].
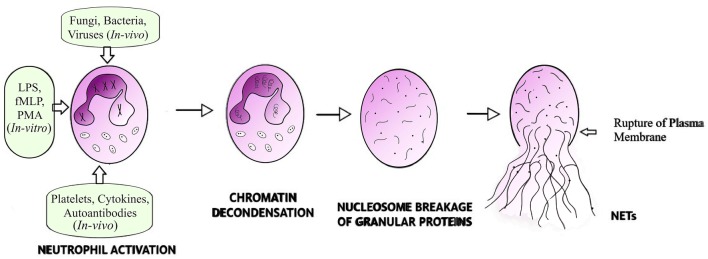
It has been reported that an expression of Peptidyl Arginine Diminase 4 (PAD4) enzyme is a primary requirement for NETosis, to mediate the post-translational modification of histones and for the conversion of arginine residues to the amino acid citrulline in a process termed ‘citrullination’. Decondensation of DNA results due to the charge neutrality of citrulline, which replace the positively charged arginine and subsequently DNA starts unfolding and unwinding [6]. The dissolution of nuclear membrane exposes the unfolded DNA to the cytoplasmic granules and this mixing creates a fibrous structure studded with granular and cytoplasmic proteins, which is then expelled by rupturing the cell membrane, as illustrated in Fig. 1.
Fig. 1.
Cartoon showing extracellular trap formation by neutrophils. Neutrophils are primed for NETosis by different stimuli which initially leads to decondensation of chromatin fibres. The dissolution of nuclear membrane permits proper blending of cytoplasmic and nuclear content, which results in studding the chromatin fibres with cytoplasmic granular proteins within the intact plasma membrane, which finally disrupts to release the 3D web-like fibrous structure called extracellular trap
NETs create a high local concentration of anti-microbial substances and therefore amplify the effectiveness of these toxic enzymes. This can kill the micro-organism, inactivate the bacterial virulence factors and enhance the efficacy of phagocytosis by recruiting more leukocytes towards the trap site. NET is effective against all types of bacteria, fungi, protozoa and viruses. It is also reported to have pro-thrombotic properties [7].
Mechanism of NETosis
There are two mechanisms of NET formation reported so far. (a) Vital NETosis and (b) Suicidal or Classical NETosis. But how far these biological processes are dissimilar is still questioned. Since the neutrophils have relatively very small life span, in order to perform their intended duties (i.e., anti-microbial activities) even beyond their life span, the neutrophils undergo ‘Suicidal’ or ‘Classical’ NETosis. In suicidal or classical NETosis there will be a release of nuclear DNA from the neutrophils to the extracellular space leading to the death of the cell. Where as in vital NETosis only partial release of DNA to extracellular space occurs without rupturing the cell membrane, after which the neutrophils will survive and continue to carry out phagocytosis and chemotaxis even without a distinct nucleus. It is reported that in in vitro condition, vital NETosis was observed when neutrophils were incubated with Staphylococcus aureus for just 10 min [8]. In this process, the double-layer structure of nuclear membrane of neutrophils undergo morphological changes resulting in (a) nucleus become spherical in shape by the disappearance of polymorphonuclear structure (b) blebbing of the nuclear membrane followed by the formation of small vesicles with DNA getting trapped inside (c) vesicles getting transported efficiently, independent of conventional exocytosis mechanism. These vesicles are then released to the extracellular mileu from discrete portions of the cell membrane [8]. The released vesicles are seen intact in the extracellular space and they release the DNA content directly rather than fusing with the plasma membrane as in conventional vesicular exocytosis. The released DNA form extracellular traps, will capture the bacteria and kill it. This mode of NETosis is activated through microbial antigen or toxin detection by pattern recognition receptors like TLRs, complement proteins and/or protein components during platelet activation [2, 9, 10].
Neutrophil cell death in the classical or suicidal NETosis is quite different from apoptosis or necrosis. This mechanism occurs after a long time exposure (3–4 h) to stimulants including microbes, IL-8, LPS and PMA leading to the production of ROS by NADPH oxidase in neutrophils [1]. During the exposure to stimulants the following events occur (a) nuclear membrane layers separate from each other and DNA decondensation starts, (b) nuclear membrane disintegrates and release the chromatin into the cytoplasm, (c) mixing of nuclear material with the cytoplasmic contents, (d) disappearance of cytoplasmic granular membranes and mixing of cytoplasmic, granular and nuclear material within the cell membrane, (e) pore formation on plasma membrane and release of cellular contents to extracellular space. In this process, the entire contents of the granules of the cell are studded in the fibrous network, which can trigger intense proteolytic activity against microbes and its toxic products. The role of ROS in NETosis is understood to be important in membrane disruptions and formation of superoxide anions and hydrogen peroxide [1, 11, 12] which further results in enzymatic activity leading to DNA decondensation and release of extracellular traps. Suicidal NETosis can be considered as a different strategy executed by neutrophils against severe and lasting infections by sacrificing itself to defend the host, extending the battle against microbes beyond its life span. Another mechanism involving ROS-mediated NETosis is the release of mitochondrial DNA to the extracellular space to form the traps rather than nuclear DNA [13]. This also relieves the cell from death and permits it to continue the other cellular functions.
A close observation of these mechanisms will reveal the fact that all the three mechanisms are different strategies of neutrophil defence, based on the type and extend of stimuli or infection. If the stimuli are from severely virulent pathogens like S. aureus, the Vital NETosis will be initiated, which can clear the pathogen before it develops adaptive mechanism to evade host defence. This has to happen rapidly. If the infection exists further, ROS-mediated release of mitochondrial DNA can cause further damage to the pathogen and save the neutrophil from undergoing a lytic process. If the infection is severe and long-lasting, the neutrophils will resort to the suicidal mission to protect the host.
It should also be kept in mind that many pathogens have inherent physiological and molecular mechanisms which can interfere with the NETosis mechanisms described above and evade this host defence strategy. Also, extracellular traps shall be removed effectively from circulation as it can stimulate an inflammatory response and thus can inflict damage to the host itself. It is observed in all experiments done with neutrophils that only a small percentage and not all neutrophils produce NETs. A justification for this finding is suggested in recent literature describing existence of neutrophil sub-populations based on functional characteristics and cellular machinery [14]. More detailed understanding of neutrophil subsets is warranted to provide any conclusive remarks.
Role of NETs in various pathogenic conditions
NET is an efficient anti-microbial mechanism which protects the host from several infectious diseases. At the same time this is a double-edged sword of innate immune system in the sense that, if produced in excess or if not removed from the system in a timely manner, can instigate a plethora of diseases including autoimmune disorders, coagulation chaos and even cancer metastases. A brief description of the involvement of NETs in various pathogenic conditions is described here:
NETs in microbial invasions
Neutrophils are evolutionarily adapted for chemotaxis and rapid crawling across the tissues to reach a site of injury and evoke the first line of host immune defence mechanism. The formation of NETs at the site of injury is therefore an effective mechanism which controls the infection at the entry point and preventing its further spread inside the host. The three-dimensional structure set the boundary for pathogens to access internal tissues as well as it limits the spread of powerful biomolecules embedded in it from damaging host tissues. It directly kills both gram-positive and gram-negative bacteria and a broad spectrum of other microorganisms through proteolytic action of embedded enzymes and other peptides in the trap [1]. Some organisms escape the trapping by NETs. Certain species of bacteria, especially S. aureus is capable of escaping from these traps. DNA is the backbone of NET and its degradation caused by DNases secreted by certain species of bacteria will affect its structural integrity. S. aureus is capable of deriving deoxyadenosine from NET structure and thereby escapes the trapping by NETs. The response of neutrophils to fungi is also interesting. The formation of NETs is more pronounced with hyphae forming Candida albicans, whereas yeast-locked fungi are cleared mainly by phagocytic mechanism. IL-1 signalling is found to be crucial for this size-dependant anti-fungal response by neutrophils [15]. NETs are also effective against viruses and parasites but the stimuli for activation and signalling mechanisms are not clearly understood [16]. Microorganisms have also developed other sophisticated mechanisms to evade entrapment by NETs. Creation of a polysaccharide capsule or manipulating the electric charge on cell wall are similar such mechanisms. Even though there are suggestions on the role of NETs in immune dysfunction associated with sepsis and septic shock, there is so far no experimental validation of the same.
NETs in thrombosis
NETs concatenate innate immunity and blood coagulation. The presence of NETs in circulation is a stimulus for platelet adhesion and subsequent activation of the coagulation cascade. The interaction of NETs with coagulation factors affect thrombolysis [17]. In Deep Vein Thrombotic (DVT) animal models, this association is observed where colocalizations of NETs occur with Von Willibrant Factor (VWF). When DNases are applied, it prevents the formation of DVT.
Endothelial cell dysfunction induces NETosis, which in turn becomes toxic to the neighbouring endothelial cells and the catastrophic loop checks in. This injury in the endothelial lining of arteries causes atherosclerosis. The impact of the injury induces changes in the sub endothelial layer where lipoproteins start accumulating. Sensing tissue damage, the migrated macrophages start feeding on these lipoproteins and cholesterol, creating a small plaque to protrude towards the lumen of the artery. This plaque will obstruct the blood flow and if at all the plaque ruptures, it will cause immediate clotting and thereby complete obstruction of the blood vessel. This causes hypoxia to the end organs served by the vessels and thus results in organ dysfunction. The NETotic potential of cholesterol crystals and the potential of plaque formation has been reported in mouse models where it was observed that NETosis is spontaneous when neutrophils interact with cholesterol [18]. This association also influence the macrophages to secrete proinflammatory cytokines and further elicit a systemic inflammatory response in addition to progression of plaques in blood vessels. In acute situations like sepsis, the haematopoietic machinery increases the number of circulating neutrophils thereby increasing the probability of immense production of NETs, which is not degraded rapidly. This can lead to the formation of intravascular clots which results in multiple organ dysfunction and septic shock leading to death [10].
The association of platelets with neutrophils will trigger the production of ROS. ROS productions by endothelial cells also stimulate NETosis [10]. The formed NETs concentrate coagulation factors and blood cells and thus provide a scaffold for thrombus formation. The presence of VWF and fibrin in this scaffold further provide stability to the thrombus formed. This can lead to the formation of DVT [19]. Since NETs can be degraded by DNases, the thrombolytic capability of DNase-based drugs against DVT needs to be experimented. The relationship between the commencement of flow restriction in vessels and the onset and mechanism of NETosis is not determined in venous thrombogenesis. If a flow restriction is identified by the immune system as an inflammatory stimulus, NETosis will be rapid and thrombogenesis may proceed very fast. The presence of NETs as an interconnection between the lining of lumen and the thrombus body is indicative of the fact that NETs help adherence of thrombus to the blood vessels. Also as the thrombus gets aged, NETs facilitate reshaping of the thrombus with newly recruited progenitor cells. Thrombolysis is an inherent mechanism of human body to disintegrate inappropriate thrombus formation. The colocalization of chromatin, VWF and fibrin in NETs adversely affect the action of many thrombolytic enzymes and each of the above components are inactivated by different enzymes, which makes the presence of NETs a tough target for thrombolytic activity. Also other phagocytic cells like monocytes are recruited for the degradation of thrombus at the site of its formation. So far it is not proven whether this polarization of monocytes is affected by the presence of components of NETs in the thrombus, even though they are able to degrade DNA and other coagulation mediators. Tissue Factor (TF) -containing microparticles are another important DVT component. The interactions of NETs with these particles need to be studied further.
NETs in malignancy
Cancer, a group of diseases showcasing uncontrolled cell division and invasion of malignant cells, shows an increasing trend globally [20]. Metastasis is a distinct characteristic of cancer where many host immune barricades are breached by the invading cells and thus forms the major cause of death in patients with malignancy. Cancer-associated thrombosis is another complication which increases the mortality. At the cellular level, neutrophils are the prominent first line of host defence against any disease, but their role in cancer is not clearly understood. A few recent studies have revealed the role of neutrophils as a mediator between immune system and cancer. Infiltration of neutrophils to the tumour at the primary site as well as metastatic lesions is observed. They tend to modify the tumour microenvironment which in turn facilitates metastasis [21]. This can be correlated with the poor prognosis in cancer patients having neutrophilia. It has been suggested that tumour-infiltrating neutrophils produce NETs in the tumour microenvironment, which helps tumour progression whereas NETs formed at the metastatic sites will persuade circulating tumour cells to get attached to remote sites leading to formation of micrometastasis and thus promoting metastasis [22]. Tumour cells are also reported to prime neutrophils towards NETosis.
Cancer-associated thrombosis is a major factor for mortality in metastatic cancer patients and NETs favour systemic thrombus formation. The detrimental effect of NETs in tumour progression is attributed to the inherent capability of neutrophils to express Tissue Factor. This biomolecule via signalling mediated by thrombin will cause thrombosis as well as facilitate neoangiogenesis, both resulting in poor prognosis. Recent studies report the presence of NETs in metastatic lymphadenopathy and primary tumour both studded with Tissue Factor [23]. In another experiment, an elevated level of H3Cit, typical of NET formation is observed in serum, which correlated with poor clinical outcome [5]. In contrast, there are also experimental results demonstrating the tumour suppressor capabilities of NETs, where it is observed that NETs are able to induce apoptosis in cancer cells in vitro [23].
In vitro studies reported the role of NETs in promoting metastasis in several carcinoma cells and the degradation of NETs by DNase-I, attenuated metastatic ability of the circulating tumour cells [22]. The interaction of platelets with NETs also promotes metastasis as platelets are known to facilitate early metastatic events and tumour progression in many malignancies. It is important to understand through further research, the role of NETs and the proteomic profile of traps in metastasis, hypothesizing that the involvement is much beyond providing a mechanical support for metastasis. This is because NETs are able to support a pre-metastatic niche and facilitate interaction of cancer cells with immune complexes and coagulation cascade. Whether this process facilitates or disrupts metastasis, which is a highly complex multi-step process, is not clearly understood so far. It also needs to be identified whether cancer progression from benign tumour towards a metastatic malignancy is initiated following a systemic or local inflammatory response, where NETosis which helped in eradicating a microbial infection is unfavourably launching a metastatic disease progression. A clear understanding demands detailed experimental studies, the implications of which may prevent metastasis by inhibiting or promoting NETosis.
NETs in metabolic diseases
Metabolic diseases occur due to impairment of normal biochemical processes typically due to enzymatic deficiencies at the molecular level. Diabetes mellitus, cardiovascular disorders, renal and liver diseases are some of the most common metabolic disorders. These diseases are associated with low grade inflammation and activation of the innate immune system [24]. Neutrophils are the primary participants in these chronic inflammatory events. The exposure of neutrophils to hyperglycaemic condition triggers NETosis through various biochemical pathways [25, 26]. The constituents of the traps, particularly double-stranded DNAs, circulating in blood will further worsen the inflammation and fuel further trap formation. NETs cause endothelial cell damage and activated endothelial cells further induce NETosis [27, 28]. The endothelial dysfunction stimulate thrombosis and artherosclerotic lesions and thus causes cardiovascular diseases and renal diseases [24, 29–31]. Human and animal studies reported increased NETosis due to hypofibrinolysis and hypercoagulation associated with diabetes [29]. Recent studies showed that neutrophils are primed for NETosis in non-alcoholic steatohepatitis (NASH) mouse models, which forms the instigating event in the accelerated progression of the metabolic syndrome towards hepatocellular carcinoma [32]. The role of NETs in gallstone formation is also identified recently [33].
In patients with impaired wound healing, there are reports of aberrant signaling pathways mediated by PAD4 protein [34] and gonadotropin releasing hormone (GnRH) [35]. DNase treatment, inhibition of NETosis by hydrogen sulphide [34, 36] and inhibition of PAD4 [34] showed improvement in diabetic wound healing, suggesting a role of NETosis. NETosis is also linked to pancreatic β cell destruction [37] and forms a vicious circle with hyperglycemia [25] and ROS production [26]. Patients with Diabetic Foot Ulcers (DFU) showed elevated levels of NET components such as H3Cit, neutrophil gelatinase-associated lipocalin, neutrophil elastase and proteinase-3 in their wound microenvironment. Those patients with increased H3Cit in circulation, reported a higher risk for amputation [38, 39]. These findings open up potential strategies of managing several metabolic diseases and complications associated with such diseases.
Macrophage extracellular traps (METs)
Macrophages include group of cells which is present in all tissues and is responsible for diverse functions. These tissue macrophages are derived either from bone marrow via dispersing blood monocytes or embryonic precursor present within the tissues [40]. The macrophages clear all undesirable particles like dead cells and cellular debris by engulfing them. These cells have key role in chronic inflammation by adequately clearing the threat made by neutrophils and dendritic cells [41]. In addition to the known functions of macrophages, recent studies have reported that macrophages are also capable of forming extracellular traps. MET is a peculiar mechanism of macrophage-mediated host defence involving the release of nuclear components and citrullinated histones with granular proteins. It can elicit innate immune response by entrapping the bacteria, fungi and viruses. Recent preclinical observations suggested that all macrophages are not capable of producing ETs, but are able to eliminate ETs and also control the ET-involved tissue damage and inflammation [41]. ETosis by macrophages is confirmed by the release of extracellular DNA with the combination of other elements such as citrullinated histones, peptidyl arginase deiminase (PAD) and granular proteases. METs were visualized using confocal microscopy, by identifying the released components that burst out after trap formation [42, 43]. Recently METs were detected and quantified using flowcytometry by utilizing the molecular expressions such as histones H2 and H3, elastase, myeloperoxidase and lactoferrin [44].
Role of METs in pathogenic conditions
METosis is a cell death mechanism identical to classic pathways in neutrophils and which triggers macrophages to form ETs. Macrophages can be triggered by several stimuli such as microbes, fungi, etc. and get involved in different pathogenic conditions due to MET formation. The involvement of METs in various pathogenic conditions are discussed here.
METs in obesity
In mature macrophages the presence of PAD4 and PAD2 facilitates ET formation through PAD-mediated hyper-citrullination. Primary functions of macrophages in adipose tissues are believed to be cleaning of cytoplasmic debris, dead/dying adipocytes by lipid phagocytosis. It is also identified that in obese women, these cells release proinflammatory substances in circulation, which might lead to breast cancer lesions. The dead adipocytes are surrounded by macrophages which form the characteristic “crown-like structures” (CLS) and these lesions are formed due to the increased level of inflammatory mediators including TNF-α, COX-2 and IL-1β. It is shown that TNF-α can promote NETs and METs in CLS lesions. Recent study reported that PADs which are expressed in CLS-macrophages stimulate the formation of METs. In these CLS-macrophages, PAD2 shows higher expression compared to PAD4. Extracellular histones are detected within CLS lesions and these histones modulate the H4Cit3 modification. The obesity mediated adipose tissue inflammation induces ET formation in macrophages within CLS lesions through histone hyper-citrullination controlled by PADs in the mammary gland [43].
METs in infections
METosis in human macrophages is regulated by elastase activity and Interferon-γ (IFN-γ), and these factors can promote trap formation as observed during severe M. tuberculosis infection [45]. E. coli and C. albicans also stimulate MET formation. Microbes are restricted at the site of infection by ET formation, preventing the occurrence of systemic transmission and limiting invasive infections, particularly by fungal pathogens. Macrophages cannot engulf C. albicans hyphae effectively due to linear structure. Therefore phagocytocis is not favoured. A longer exposure of hyphae triggers METosis. PMA, LPS and hydrogen peroxide are more powerful inducers of trap formation from macrophages. Unlike in neutrophils, mitochondrial DNA release is the most prominent trap formation mechanism in macrophages, which is not mediated by ROS production [46]. This permits the macrophages to survive longer at the site of infection and carry out other immunomodulatory and tissue regenerative functions. Tissue macrophages are capable of modifying the innate and adaptive immune responses through chemical mediators. Since their number is also limited, they are not that expendable at occasions.
Eosinophil extracellular traps (EETs)
Eosinophils are those granulocytes in circulation, responsible for fighting against specific infections, particularly due to multicellular parasites. These cells are known to be involved in the pathophysiology of asthma and allergy as well. Current evidences re-define their role in defensive mechanisms against HIV infection, bacterial and fungal infections. Some biomolecules produced by eosinophils can cause toxicity to host tissues and can lead to the improper regulation of haemostasis [47].
The release of granular proteins and DNA to extracellular space through cytolytic degranulation followed by exocytosis is called as EETs. EETosis is stimulated by activators like cytokines, PMA, calcium ionophore, adhesion receptors, platelet activating factors and immunoglobulins like IgG, IgA. In in vitro, the stimulation occurs in 3–4 h in a reduced NADPH oxidase dependent activity [47, 48]. EETs also comprised of network of DNA fibres and granule proteases including ECP (Eosinophil Cationic Protein) and MBP (Major Basic Protein). Mitochondrial DNA is released through the bursting of mitochondria, that permits eosinophil survival after EETosis [48].
Role of EETs in pathogenic conditions
Eosinophils attain an extra property of killing bacteria through the formation of extracellular traps. TSLP (Thymic stromal lymphopoietin) can trigger the production of EETs consisting of mitochondrial DNA and granule proteins. Sinus exudative secretions from patients with Eosinophilic Otitis Media (EOM) shows the presence of nuclear debris and cytolytic eosinophil extracellular granules. The EETs are comparatively more in secretions of ear and sinus and produce functional mesh to entrap bacteria [48]. EETs are reported in various pathological conditions such as skin disease, asthma and also in both bacterial and fungal infections.
EETs in skin diseases
Eosinophils play an important role in a wide range of skin diseases. EETs have huge involvement in infectious skin diseases like ectoparasitoses and larva migrans, in addition to autoimmune diseases and allergic conditions. Due to the elevated expression of IFN-γ, IL-5 and eotaxins, EET formation is stimulated in skin tissues. But EETs are not observed with skin tissue infiltrating eosinophils present in some skin diseases like skin neoplasms. Acute dermatoses (AD) and acute exacerbation diseases like Drug Hypersensitivity (DHS), Allergic Contact Dermatitis (ACD), infectious diseases, Hyper Eosinophilic Syndrome (HES), Wells Syndrome (WS) and also lesional AD skin were found to have extracellular trap formation by eosinophils. Except acute diseases like HES and WS, many of the EETs related diseases are activated by endogenous and exogenous pathogens like allergen, drug, autoantigen and parasite. Eosinophils forming EETs are morphologically identical to tissue eosinophils. Involvement of eosinophils and EETs are suspected in the apoptotic keratinocytes of patients with Bullous pemphigoid (BP), which comprises of keratinocyte damage and blister formation [49].
EETs in asthma
Eosinophils cause the symptoms of asthma and it often damage tissues. Airway inflammation occurring due to eosinophilia is directly linked with the asthma exacerbation in Severe Eosinophilic Asthma (SEA). EETs which release DNA complex and granule proteins in turn cause asthma. Higher expression of granule proteins like eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN) and major basic protein illustrate degranulation and activation of eosinophils related with SEA. The involvement of EETs in SEA and non-severe asthma (NSA) is entirely different. EET formation through stimulation of IL-5 and LPS is comparatively high in SEA patients than those in NSA patients, suggesting phenotype differences in the two eosinophils.
The airway epithelial cells act as the first line of defense and barrier in the lungs, responding to pollutants, viruses and allergens which can cause asthma. Patients with asthma, chronic pulmonary diseases and viral respiratory infection produce large amount of IL-8 in the inflammatory sites. EET production is enhanced with IL-8 secretion from airway epithelial cells causing airway inflammation and obstruction in SEA patients. Immune responses in airway epithelium are obstructed by cytotoxic granules released by eosinophils. EETs have a vital role in the pathogenesis of airway inflammation due to the favourable stimulation occurring in the airway epithelial cells, which hints that control of EET formation and its activity can give innovative treatment methodologies for asthma patients [50].
EETs in infection
Bacterial products like LPS trigger eosinophils to produce extracellular traps with DNA and granule proteins. Also, eosinophils get primed by IL-5, eotaxin and IFN-γ, forming EETs upon bacterial infection by E. coli and S. aureus. Eosinophils release its mitochondrial DNA and granule proteins which forms its antibacterial defense machinery in GI tract inflammatory diseases. Fungal associated asthma and allergic bronchopulmonary mycosis leads to the formation of extracellular traps by eosinophils in infectious condition, but the mode of interaction, pathological role and inflammatory conditions of fungal infected EETs are still unknown completely. It is recently reported that allergic bronchopulmonary aspergillosis (ABPA) patients show the presence of chromatolytic nuclei, histone and granule proteins in the bronchial secretions, which confirms the presence of EETs. Candida albicans are also capable of stimulating the eosinophils and produce traps. Aspergillus fumigatus activates eosinophils and form EETs by ROS production via fungal antigen receptors. The presence of large amount of extracellular histones is seen in the nasal lavage from patients having pulmonary microvascular thrombosis and lung infections caused by influenza virus, even though the role of EETs is not elucidated in viral infections. It is interesting to note that the infiltration to lungs caused under the influence of A. fumigatus leads to the degranulation of eosinophils, which provides a protective cover from lethal respiratory virus infections [51].
Basophil extracellular traps (BETs)
Basophils have a major role in inflammation and microbial infection. The factors that supervise the survival and lifespan of cells will control the compliances that occur due to the inflammatory responses. The improper functioning of death pathway in basophils which is associated with inflammation, affects the lifespan of basophils and also leads to the pathological dysfunctioning [52]. Basophils are also able to form extracellular traps upon cytokine release and pathogen associated cell damage known as Basophil Extracellular Traps [BETs]. Basophils form an extracellular cluster containing externalized DNA upon 2 h contact with Monosodium urate crystals (MSU). In the basophils, instead of uptaking the crystals, they deregulate CD203c activation marker after the stimulation with MSU crystals [53]. In BET formation, mitochondrial DNA and granule proteins are released to form a network structure, and not the nuclear DNA. Basophil releases it DNA to its extracellular space due to the activation with chemokines, lipid mediator receptors, IgE, Toll-Like Receptors and cytokines within minutes. Basophils get primed with IL-3, forming extracellular trap via mitochondrial reactive oxygen species. Basophils contain large number of mitochondria, which generate more ROS needed for extracellular trap formation. BET is a means by which basophils might be able to exert direct innate immune effector function against pathogens [54]. BETs are reported to show antimicrobial activity.
BETs and its anti-microbial functions
Basophils are mainly involved with immune-mediated responses in parasitic infections and allergic diseases. Functional NADPH oxidase is absent in basophils and these cells are not able to kill intracellular bacteria. Basophils usually act as immunomodulatory cells and do not show any phagocytic activity. Activated basophils release BETs which is associated with antibacterial killing processes. Because BETs are able to entrap and kill bacteria, basophils are also effector cells against pathogens. BET mediated antibacterial property is demonstrated against E. coli and S. aureus by activation with inflammatory mediators [55].
Mast cell extracellular traps (MCETs)
Mast cells (MCs) are commonly found in tissues which have regular contact with the external environment like skin, gastrointestinal mucosa and respiratory tract. MCs are capable of producing cytokines, chemokines, proteases and inflammatory mediators that in turn stimulate neutrophils to act in the area of infection. Recent reports suggest the antimicrobial activity of MCs. The killing of bacteria can be done by non-oxidative and oxidative mechanisms in these cells, and therefore can eliminate even intracellular bacteria. MCs are also able to form extracellular traps under certain circumstances similar to NETs. Upon stimulation with H2O2, PMA and bacterial pathogens, MCs get activated to release 3D web like structure which entraps pathogen. The formations of extracellular trap by MCs are called MCETs, which contains histones, DNA and granular proteins like tryptase. MCs kill bacteria by entrapping them in the extracellular mesh like structure similar to NETs. Antimicrobial peptides present in MC granules are β-defensins, piscidins and cathelicidin LL-37 which get released upon stimulation with different pathogens. High expressions of MCETs are mediated by ROS-dependent mechanism which results in the degradation of nuclear envelope and plasma membrane [56]. These MCETs have major involvement in pathogenic conditions and microbial environment, but are not fully understood till date.
MCETs in infection
MCs play an important role in immune responses, mainly in parasitic infections and allergic conditions. They are one among the first immune cell that respond to fungal and bacterial infections. MCs have major role in recruiting the effector cells to the site of infection mainly neutrophils and macrophages, releasing TNF-α. MCET formation results in the disintegration of nuclear membrane, followed by the mixing of granular and nuclear components which leads to the death of cells. The trap formation is an immune effector function against pathogens [57]. Visceral Leishmaniasis and Cutaneous Leishmaniasis, caused by Leishmania donovani and Leishmania tropica, can stimulate the generation of extracellular traps by MCs. MCs which generate ROS due to the presence of Leishmania will eventually release the DNA and granular proteins forming the extracellular trap. Thus MCs which are involved in host defence to Leishmania can actively entrap and also surround pathogen by effector mediators to clear them extracellularly [58]. Upon stimulation with C.albicans, extracellular trap get released from MCs, as an anti-fungal response [59].
Mast Cells play a major role in controlling Group A Streptococcus (GAS) infections in the skin. MC granule proteases are highly cytotoxic to GAS but are not released in response to GAS infection. One of the major MC granule protease, LL-37 is found embedded in MCETs and thus controls the GAS infection [60]. MCETs have major involvement in Tuberculosis, which is resistant to immune responses for years. Mycobacterium tuberculosis (Mtb) stimulates mast cells by activating degranulation, releasing inflammatory cytokines and internalizing the bacteria. It is experimentally verified that heat-killed Mtb (HK-Mtb) can promote the release of MCETs, whereas live Mtb prevents the release of MCETs. Thus it is confirmed that live Mtb produces virulence factors, which can prevent MCET production particularly by interfering the production of hydrogen peroxide in mast cells [61]. MCs get accumulated in the clump at bacterial infection sites and release large amount of enzymes through extracellular traps. The clumps are thus effectively controlled at the site and prevented from further internal infections, since clumps are too huge to be removed by other phagocytic cells [56]. On another note, involvement of MCETs in coronary thrombosis is detected at various stages of thrombus progression and maturation as well [62].
Plasmacytoid dendritic cell extracellular traps (pETs)
Plasmacytoid Dendritic cells (pDCs) are immune cells that play an important role in protection from viruses and tumors. pDCs produce Type I interferons (IFN1), mainly IFN-β and IFN-α, when activated by Toll-Like Receptors, TLR9 and TLR7. Interferons inhibit viral replication [63]. This production of IFN1 by pDCs is found to be triggered with the presence of NETs. pDCs have major involvement in autoimmune and autoinflammatory diseases like systemic lupus erythematosus (SLE) and psoriasis. SLE is a human incurable autoimmune disease which is common and influenced by the chronic activation of pDCs finally releasing autoantibodies. In this disease, large excess of NET is observed in patient samples, which in turn stimulates pDCs by TLR9 and thereby activating the adaptive immune system to release auto antibodies [64]. pDCs are also capable of releasing DNA and citrullinated histone H3 to the extracellular space upon stimulation and forms a mesh like structure similar to NETs called pDC Extracellular Traps (pETs). The antifungal activity of pETs is observed in inhibiting the production of Aspergillus fumigatus hyphae. Hyphae which stimulates the pDCs via Dectin-2, will prime the pDCs to release its DNA and H3Cit in a suicidal ETosis pathway [65].
Monocyte extracellular traps
Monocytes are a group of multi-faceted blood cells which are circulating in peripheral blood and are able to differentiate into multiple cell-types depending on the requirements of the host. These cells can differentiate into macrophages, myeloid-derived dendritic cells and poly-morpho nuclear neutrophils under specific stimuli. This progenitor capability makes these cells different from other leukocytes and thus shows involvement in diverse physiological and diseases processes [66]. Similar to neutrophils, monocytes also form ETs [67] through oxidative burst and independent of MPO activity. These traps are also degraded by DNase I activity and through inhibition of NADPH oxidase [68]. The understanding behind the mechanism, inflammatory properties and pro-coagulant role of the monocyte extracellular traps are still in its infancy. But the participation of monocytes in inflammatory disorders, infection and thrombosis is well-known and how far these are affected by the METosis, still need to be investigated. METosis and its activation will be able to launch a rapid, discrete and targeted response in the host which will be much more complex than NETosis in the sense that monocytes and its terminal differentiation cells have additional capability of antigen presentation to T cells which can trigger an acute adaptive immune response within the host. Whether such responses are beneficial or detrimental to the host is a much bigger question to be answered, necessitating rigorous research in this perspective.
Etosis in nutshell
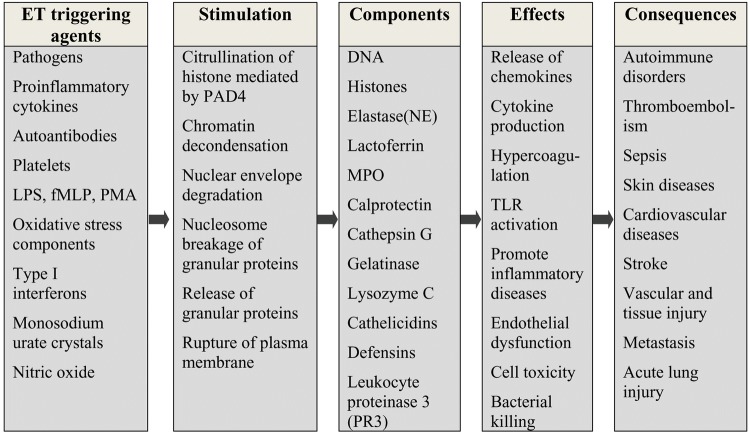
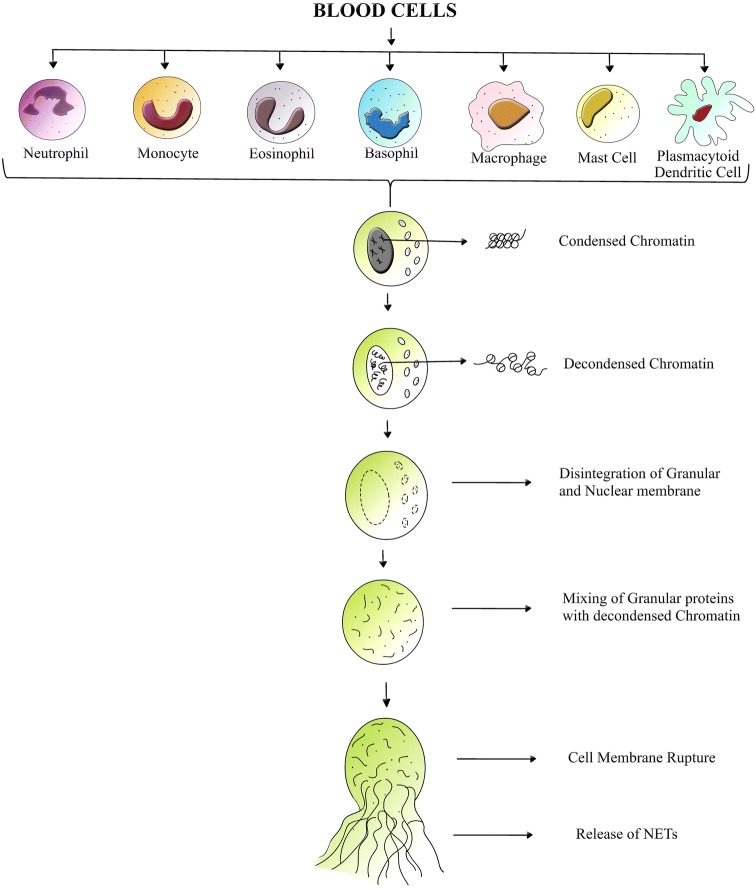
Extracellular trap formation is observed with all leukocytes of myeloid origin. The various agents inducing ETosis and its consequences are listed in Fig. 2. Each cell type show varying degree of stimulation with the same inducer. Many of the cell types follow a common mechanism of ETosis, as shown in Fig. 3, whereas exclusive activation pathways exist in diverse cell types under different conditions and different agents of stimulation.
Fig. 2.
Various stimuli triggering ETosis, their effects and their role in disease processes
Fig. 3.
Cartoon showing the mechanism of extracellular trap formation in blood cells. Lytic ETosis reported in blood cells like neutrophils, monocytes, eosinophils, basophils, mast cells, macrophages and plasmacytoid
Conclusion
ETosis is a known mechanism by which anti-microbial effector functions are performed by many blood cells. The understanding behind the stimulation, activation, inflammatory properties and pathogenic responses due to extracellular trap formation is still not understood fully. But its effect and involvement in disease patho-physiology is unquestionable. The release of chromatin to the extracellular matrix acts as a double-edged sword. Along with favourable outcome, this process can also fuel thrombosis, sepsis, tumor metastasis, etc. It is also observed that ETosis is a highly regulated multi-step process and is a crucial cell-death mechanism. The genetic predisposition of derailment of this highly conserved process is not experimentally identified. Exploration into ETosis mechanism and its cellular machinery stemmed the identification of many phenotypic subsets of cell types, which were not known before. Further understanding of this process is essential, as it may have great therapeutic potential especially in the diagnosis, guiding of therapy and prognostication and thus may inturn help to tackle many infectious conditions, autoimmune disorders and tumors.
Acknowledgement
The authors are grateful for the support and infrastructure provided by the Centre for Nanoscience and Molecular Medicine, Amrita Vishwa Vidyapeetham, Kochi, India
Compliance with ethical standards
Conflict of interest
All authors declare that there is no conflict of interest
Ethical statement
There are no animal experiments carried out for this article
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
R. J. Nija and S. Sanju equally contributed.
Contributor Information
Neeraj Sidharthan, Email: neerajsidh@gmail.com.
Ullas Mony, Email: ullas19351@aims.amrita.edu.
References
- 1.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18:1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 4.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci USA. 2012;109:13076–13081. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boone BA, Orlichenko L, Schapiro NE, Loughran P, Gianfrate GC, Ellis JT, et al. The receptor for advanced glycation end products (RAGE) enhances autophagy and neutrophil extracellular traps in pancreatic cancer. Cancer Gene Ther. 2015;22:326–334. doi: 10.1038/cgt.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cedervall J, Zhang Y, Huang H, Zhang L, Femel J, Dimberg A, et al. Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumor-bearing animals. Cancer Res. 2015;75:2653–2662. doi: 10.1158/0008-5472.CAN-14-3299. [DOI] [PubMed] [Google Scholar]
- 8.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 9.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol. 2008;180:1895–1902. doi: 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 10.Carestia A, Kaufman T, Rivadeneyra L, Landoni VI, Pozner RG, Negrotto S, et al. Mediators and molecular pathways involved in the regulation of neutrophil extracellular trap formation mediated by activated platelets. J Leukoc Biol. 2016;99:153–162. doi: 10.1189/jlb.3A0415-161R. [DOI] [PubMed] [Google Scholar]
- 11.Palmer LJ, Cooper PR, Ling MR, Wright HJ, Huissoon A, Chapple IL. Hypochlorous acid regulates neutrophil extracellular trap release in humans. Clin Exp Immunol. 2012;167:261–268. doi: 10.1111/j.1365-2249.2011.04518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamam HJ, Khan MA, Palaniyar N. Histone acetylation promotes neutrophil extracellular trap formation. Biomolecules. 2019;9:E32. doi: 10.3390/biom9010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 14.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altmeier S, Toska A, Sparber F, Teijeira A, Halin C, LeibundGut-Landmann S. IL-1 coordinates the neutrophil response to C. albicans in the oral mucosa. PLoS Pathog. 2016;12:e1005882. doi: 10.1371/journal.ppat.1005882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appelgren D, Enocsson H, Skogman BH, Nordberg M, Perander L, Nyman D, et al. Neutrophil extracellular traps (NETs) in the cerebrospinal fluid samples from children and adults with central nervous system infections. Cells. 2019;9:E43. doi: 10.3390/cells9010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 18.Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. J Thromb Haemost. 2015;349:316–320. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–144. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 21.Berger-Achituv S, Brinkmann V, Abed UA, Kühn LI, Ben-Ezra J, Elhasid R, et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol. 2013;4:48. doi: 10.3389/fimmu.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123:3446–3458. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arelaki S, Arampatzioglou A, Kambas K, Papagoras C, Miltiades P, Angelidou I, et al. Gradient infiltration of neutrophil extracellular traps in colon cancer and evidence for their involvement in tumour growth. PLoS One. 2016;11:e0154484. doi: 10.1371/journal.pone.0154484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadini GP, Menegazzo L, Scattolini V, Gintoli M, Albiero M, Avogaro A. A perspective on NETosis in diabetes and cardiometabolic disorders. Nutr Metab Cardiovasc Dis. 2016;26:1–8. doi: 10.1016/j.numecd.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Menegazzo L, Ciciliot S, Poncina N, Mazzucato M, Persano M, Bonora B, et al. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol. 2015;52:497–503. doi: 10.1007/s00592-014-0676-x. [DOI] [PubMed] [Google Scholar]
- 26.Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res. 2016;118:1808–1829. doi: 10.1161/CIRCRESAHA.116.306923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis. 2015;74:1417–1424. doi: 10.1136/annrheumdis-2013-204837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, et al. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010;584:3193–3197. doi: 10.1016/j.febslet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Bryk AH, Prior SM, Plens K, Konieczynska M, Hohendorff J, Malecki MT, et al. Predictors of neutrophil extracellular traps markers in type 2 diabetes mellitus: associations with a prothrombotic state and hypofibrinolysis. Cardiovasc Diabetol. 2019;18:49. doi: 10.1186/s12933-019-0850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta S, Kaplan MJ. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat Rev Nephrol. 2016;12:402–413. doi: 10.1038/nrneph.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JK, Lee HW, Joo N, Lee HS, Song YR, Kim HJ, et al. Prognostic role of circulating neutrophil extracellular traps levels for long-term mortality in new end-stage renal disease patients. Clin Immunol. 2020;210:108263. doi: 10.1016/j.clim.2019.108263. [DOI] [PubMed] [Google Scholar]
- 32.van der Windt DJ, Sud V, Zhang H, Varley PR, Goswami J, Yazdani HO, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68:1347–1360. doi: 10.1002/hep.29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muñoz LE, Boeltz S, Bilyy R, Schauer C, Mahajan A, Widulin N, et al. Neutrophil extracellular traps initiate gallstone formation. Immunity. 2019;51:443–450.e4. doi: 10.1016/j.immuni.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21:815–819. doi: 10.1038/nm.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee YS, Kang SU, Lee MH, Kim HJ, Han CH, Won HR, et al. GnRH impairs diabetic wound healing through enhanced NETosis. Cell Mol Immunol. 2019. https://www.nature.com/articles/s41423-019-0252-y. [DOI] [PMC free article] [PubMed]
- 36.Yang C, Chen L, Chen WL, Li N, Chen MJ, Li X, et al. Hydrogen sulfide primes diabetic wound to close through inhibition of NETosis. Mol Cell Endocrinol. 2019;480:74–82. doi: 10.1016/j.mce.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Xiao Y, Zhong L, Ye D, Zhang J, Tu Y, et al. Increased neutrophil elastase and proteinase 3 and augmented NETosis are closely associated with β-cell autoimmunity in patients with type 1 diabetes. Diabetes. 2014;63:4239–4248. doi: 10.2337/db14-0480. [DOI] [PubMed] [Google Scholar]
- 38.Yang S, Gu Z, Lu C, Zhang T, Guo X, Xue G, et al. Neutrophil extracellular traps are markers of wound healing impairment in patients with diabetic foot ulcers treated in a multidisciplinary setting. Adv Wound Care (New Rochelle) 2020;9:16–27. doi: 10.1089/wound.2019.0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fadini GP, Menegazzo L, Rigato M, Scattolini V, Poncina N, Bruttocao A, et al. NETosis delays diabetic wound healing in mice and humans. Diabetes. 2016;65:1061–1071. doi: 10.2337/db15-0863. [DOI] [PubMed] [Google Scholar]
- 40.Gautiar EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farrera C, Fadeel B. Macrophage clearance of neutrophil extracellular traps is a silent process. J Immunol. 2013;191:2647–2656. doi: 10.4049/jimmunol.1300436. [DOI] [PubMed] [Google Scholar]
- 42.Sharma R, O’Sullivan KM, Holdsworth SR, Bardin PG, King PT. Visualizing macrophage extracellular traps using confocal microscopy. J Vis Exp. 2017;2017:e56459. doi: 10.3791/56459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohanan S, Horibata S, McElwee JL, Dannenberg AJ, Coonrod SA. Identification of macrophage extracellular trap-like structures in mammary gland adipose tissue: a preliminary study. Front Immunol. 2013;4:67. doi: 10.3389/fimmu.2013.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donis-Maturano L, Sánchez-Torres LE, Cerbulo-Vázquez A, Chacón-Salinas R, García-Romo GS, Orozco-Uribe MC, et al. Prolonged exposure to neutrophil extracellular traps can induce mitochondrial damage in macrophages and dendritic cells. Springerplus. 2015;4:161. doi: 10.1186/s40064-015-0932-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong KW, Jacobs WR. Mycobacterium tuberculosis exploits human interferon γ to stimulate macrophage extracellular trap formation and necrosis. J Infect Dis. 2013;208:109–119. doi: 10.1093/infdis/jit097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu P, Wu X, Liao C, Liu X, Du J, Shi H, et al. Escherichia coli and Candida albicans induced macrophage extracellular trap-like structures with limited microbicidal activity. PLoS One. 2014;9:e90042. doi: 10.1371/journal.pone.0090042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ueki S, Melo RC, Ghiran I, Spencer LA, Dvorak AM, Weller PF. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood. 2013;121:2074–2083. doi: 10.1182/blood-2012-05-432088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ueki S, Konno Y, Takeda M, Moritoki Y, Hirokawa M, Matsuwaki Y, et al. Eosinophil extracellular trap cell death-derived DNA traps: their presence in secretions and functional attributes. J Allergy Clin Immunol. 2016;137:258–267. doi: 10.1016/j.jaci.2015.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon D, Hoesli S, Roth N, Staedler S, Yousefi S, Simon HU. Eosinophil extracellular DNA traps in skin diseases. J Allergy Clin Immunol. 2011;127:194–199. doi: 10.1016/j.jaci.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Choi Y, Le Pham D, Lee DH, Lee SH, Kim SH, Park HS. Biological function of eosinophil extracellular traps in patients with severe eosinophilic asthma. Exp Mol Med. 2018;50:104. doi: 10.1038/s12276-018-0136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muniz VS, Silva JC, Braga YAV, Melo RCN, Ueki S, Takeda M, et al. Eosinophils release extracellular DNA traps in response to Aspergillus fumigatus. J Allergy Clin Immunol. 2018;141:571–585.e7. doi: 10.1016/j.jaci.2017.07.048. [DOI] [PubMed] [Google Scholar]
- 52.Didichenko SA, Spiegl N, Brunner T, Dahinden CA. IL-3 induces a Pim1-dependent antiapoptotic pathway in primary human basophils. Blood. 2008;112:3949–3958. doi: 10.1182/blood-2008-04-149419. [DOI] [PubMed] [Google Scholar]
- 53.Schorn C, Janko C, Latzko M, Chaurio R, Schett G, Herrmann M. Monosodium urate crystals induce extracellular DNA traps in neutrophils, eosinophils, and basophils but not in mononuclear cells. Front Immunol. 2012;3:277. doi: 10.3389/fimmu.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morshed M, Hlushchuk R, Simon D, Walls AF, Obata-Ninomiya K, Karasuyama H, et al. NADPH oxidase-independent formation of extracellular DNA traps by basophils. J Immunol. 2014;192:5314–5323. doi: 10.4049/jimmunol.1303418. [DOI] [PubMed] [Google Scholar]
- 55.Yousefi S, Morshed M, Amini P, Stojkov D, Simon D, von Gunten S, et al. Basophils exhibit antibacterial activity through extracellular trap formation. Allergy. 2015;70:1184–1188. doi: 10.1111/all.12662. [DOI] [PubMed] [Google Scholar]
- 56.van Köckritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, et al. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 57.Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170:2274–2278. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- 58.Naqvi N, Ahuja K, Selvapandiyan A, Dey R, Nakhasi H, Puri N. Role of mast cells in clearance of Leishmania through extracellular trap formation. Sci Rep. 2017;7:13240. doi: 10.1038/s41598-017-12753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopes JP, Stylianou M, Nilsson G, Urban CF. Opportunistic pathogen Candida albicans elicits a temporal response in primary human mast cells. Sci Rep. 2015;5:12287. doi: 10.1038/srep12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clark M, Kim J, Etesami N, Shimamoto J, Whalen RV, Martin G, et al. Group A Streptococcus prevents mast cell degranulation to promote extracellular trap formation. Front Immunol. 2018;9:1–11. doi: 10.3389/fimmu.2018.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campillo-Navarro M, Leyva-Paredes K, Donis-Maturano L, Rodríguez-López GM, Soria-Castro R, García-Pérez BE, et al. Mycobacterium tuberculosis catalase inhibits the formation of mast cell extracellular traps. Front Immunol. 2018;9:327. doi: 10.3389/fimmu.2018.01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pertiwi KR, de Boer OJ, Mackaaij C, Pabittei DR, de Winter RJ, Li X, et al. Extracellular traps derived from macrophages, mast cells, eosinophils and neutrophils are generated in a time-dependent manner during atherothrombosis. J Pathol. 2019;247:505–512. doi: 10.1002/path.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skrzeczynska-Moncznik J, Wlodarczyk A, Banas M, Kwitniewski M, Zabieglo K, Kapinska-Mrowiecka M, et al. DNA structures decorated with cathepsin G/secretory leukocyte proteinase inhibitor stimulate IFNI production by plasmacytoid dendritic cells. Am J Clin Exp Immunol. 2013;2:186–194. [PMC free article] [PubMed] [Google Scholar]
- 64.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loures FV, Röhm M, Lee CK, Santos E, Wang JP, Specht CA, et al. Recognition of Aspergillus fumigatus hyphae by human plasmacytoid dendritic cells is mediated by Dectin-2 and results in formation of extracellular traps. PLoS Pathog. 2015;11:e1004643. doi: 10.1371/journal.ppat.1004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol. 2007;178:2000–2007. doi: 10.4049/jimmunol.178.4.2000. [DOI] [PubMed] [Google Scholar]
- 67.Granger V, Faille D, Marani V, Noël B, Gallais Y, Szely N, et al. Human blood monocytes are able to form extracellular traps. J Leukoc Biol. 2017;102:775–781. doi: 10.1189/jlb.3MA0916-411R. [DOI] [PubMed] [Google Scholar]
- 68.Muñoz-Caro T, Silva LM, Ritter C, Taubert A, Hermosilla C. Besnoitia besnoiti tachyzoites induce monocyte extracellular trap formation. Parasitol Res. 2014;113:4189–4197. doi: 10.1007/s00436-014-4094-3. [DOI] [PubMed] [Google Scholar]